Abstract
APOBEC3G (APO3G) is a host cytidine deaminase that is incorporated into human immunodeficiency virus type 1 (HIV-1) particles. We report here that viral RNA promotes stable association of APO3G with HIV-1 nucleoprotein complexes (NPC). A target sequence located within the 5′-untranslated region of the HIV-1 RNA was identified to be necessary and sufficient for efficient APO3G packaging. Fine mapping revealed a sequence normally involved in viral genomic RNA dimerization and Gag binding to be important for APO3G packaging and association with viral NPC. Our data suggest that packaging of APO3G into HIV-1 NPC is enhanced by viral RNA.
Replication of human immunodeficiency virus type 1 (HIV-1) in primary cells is dependent on the expression of Vif protein, which counteracts the activity of the host cytidine deaminases APOBEC3G (APO3G) and APOBEC3F (4, 25, 29, 32). In the absence of Vif, APO3G is incorporated into virus particles (11, 16, 19, 20, 26, 27, 30), resulting in hypermutation of the viral genome (19) or degradation of mutated cDNA (14, 18, 31) via a DNA repair mechanism (reviewed in references 3 and 12). Interestingly, human APO3G is not only packaged into human immunodeficiency viruses but also incorporated into simian immunodeficiency viruses and murine leukemia virus (9, 18, 19). Packaging of APO3G into such diverse viruses suggests that it either is a relatively nonspecific process or involves signals shared by these viruses. APO3G can bind RNA in vitro (10). Indeed, several reports have noted that the presence of viral RNA enhanced APO3G encapsidation (28); however, others (17, 23) suggested that viral RNA was not essential for APO3G packaging (2, 5, 8, 17, 23, 28).
To further study the role of viral RNA in the packaging of APO3G into HIV-1 virions, we first compared the packaging of APO3G into either the wild-type infectious NL4-3 virus or a helper virus (C-Help) whose RNA genome is not packaged due to a 33-base deletion in the putative RNA packaging signal (21). Virus stocks were prepared by transient cotransfection of HeLa cells with either the pNL4-3 plasmid (1) or the C-Help vector DNA and the APO3G-expressing plasmid pcDNA-APO3G (11). Viruses were collected 48 h after transfection and purified by two rounds of sucrose gradient centrifugation. Cell lysates and concentrated virus preparations were analyzed by immunoblotting (Fig. 1A). We found that packaging of APO3G into helper virus was reduced by >3.5-fold compared to packaging into NL4-3 virus (Fig. 1B). Thus, viral RNA contributes to the specific packaging of APO3G into HIV-1 virions, consistent with data reported by Svarovskaia et al. (28).
FIG. 1.
Virus incorporation of APO3G protein requires packaging of viral RNA. HeLa cells were co-transfected with either the full-length molecular clone pNL4-3 (WT) or a helper virus construct (C-Help) along with pcDNA-APO3G vector DNA. Cells and virus-containing supernatants were harvested 48 h posttransfection. (A) Whole-cell lysates and purified, concentrated virus preparations were analyzed by immunoblotting using an anti-APO3G antibody (APO3G) or an HIV-positive patient serum (CA). (B) Packaging of APO3G into virions was quantified by densitometric analysis of the bands shown in panel A. Results were corrected for variations in CA protein. APO3G levels in NL4-3 preparations were defined as 100%. (C) Schematic presentation of the modifications introduced into the pHR′CMVGFP packaging vector. Vector ΔC contains both HIV-1 LTRs, the 5′-untranslated region plus the first 350 nucleotides of the gag gene, an 880-nucleotide fragment containing the HIV-1 RRE element, and a GFP expression cassette. This vector is identical to pHR′CMVGFP except for a deletion in the cytomegalovirus promoter to prevent GFP protein expression. Variants lacking the LTR regions (ΔL), the RRE domain (ΔR), or both (ΔL/R) are described in the text. SV40 and AAA symbolize simian virus 40 promoter and terminator elements, respectively. SD, splice donor site; CMV, cytomegalovirus; SA, splice acceptor site; Opt. Gag, codon-optimized Gag. (D) Helper virus (C-Help) DNA was transfected into HeLa cells together with either an empty vector (−) or one of the packaging vectors shown in panel C. Cells and viruses were harvested 48 h after transfection. Packaging of APO3G was assessed by immunoblotting as described in the legend to panel A, and the level of APO3G packaging was quantified as described in the legend to panel B. APO3G packaging efficiency is expressed as fold increase over the level observed with helper virus alone (−). Values were corrected for variations in CA protein. Error bars reflect the standard deviations calculated from three independent experiments.
If encapsidation of APO3G and viral RNA are linked, the APO3G packaging defect observed with the helper virus (Fig. 1A and B) should be overcome by the coexpression of packaging-competent vector-derived RNA. To test this hypothesis, several packaging vectors were constructed based on the lentiviral packaging vector pHR′CMVGFP (15). This vector contains both HIV-1 long terminal repeats (LTRs), the 5′-untranslated region, 350 nucleotides of gag, and 880 nucleotides of an env segment encompassing the Rev-responsive element (RRE) region. Vector ΔC (Fig. 1C) is similar to pHR′CMVGFP except for a 650-bp deletion in the cytomegalovirus promoter region, which prevents expression of the green fluorescent protein (GFP) reporter gene. This deletion is present in all other constructs tested here. Vector ΔL lacks the LTR regions and is under the transcriptional control of an SV40 promoter. Vector ΔR lacks the RRE-containing env sequence and contains a codon-optimized gag coding sequence (24). A vector containing a deletion of both LTRs and RRE (ΔL/R) was also constructed (Fig. 1C). As illustrated in Fig. 1D, coexpression of the packaging vector (ΔC) resulted in a sixfold increase in the packaging of APO3G compared to the helper virus alone. Similarly, cotransfection of the ΔL, ΔR, and ΔL/R variant constructs increased packaging of APO3G by six- to sevenfold. These results suggest that the 5′-untranslated region and the first 350 nucleotides of gag were sufficient for APO3G encapsidation into HIV-1 virions.
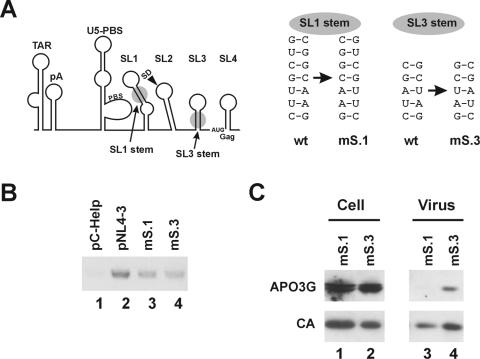
The 5′-untranslated region contains numerous secondary structures required for virus replication (Fig. 2A) (reviewed in reference 22). To further map the sequences and RNA secondary structures required for APO3G packaging, we tested two stem-loop mutants, mS.1 and mS.3 (Fig. 2A), located in a region that has been shown to be important for viral RNA dimerization and encapsidation. The sequences located on both strands of the individual helical structures of stem-loop 1 (SL1) or stem-loop 3 (SL3) were swapped in mS.1 and mS.3 mutants, respectively, thus changing the primary sequences of the stems without perturbing formation of the hairpin structures. These mutants were selected because they package viral RNA at or near wild-type levels and were found to produce fully infectious viruses (7). Reverse transcription (RT)-PCR analysis showed similar levels of virus-associated RNA in the mS.1 and mS.3 viruses while C-Help virus did not contain detectable levels of viral RNA (Fig. 2B). Packaging of APO3G into mS.1 and mS.3 particles was analyzed by immunoblotting (Fig. 2C). While APO3G was expressed at similar levels intracellularly (Fig. 2C, lanes 1 and 2), APO3G packaging into mS.1 was reduced by >3-fold compared to mS.3 virus (Fig. 2C, compare lanes 3 and 4). While our data do not rule out the possible involvement of other regions in the 5′-untranslated region, they clearly identify SL1 as a region important for packaging of APO3G into HIV-1 virions and hence strongly argue for a specific involvement of viral RNA in APO3G packaging.
FIG. 2.
The SL1 hairpin structure located in the 5′-untranslated region is important for APO3G packaging. (A) Schematic representation of the RNA structure predicted for the 5′-untranslated region (left panel) (6). TAR, transactivation response region; PBS, primer binding site; SD, splice donor site. The right panel depicts the nucleotide changes introduced into the stem regions of SL1 and SL3 in the mS.1 and mS.3 mutants, respectively. wt, wild type. (B) Packaging of viral RNA was analyzed by RT-PCR of the NL4-3mS.1 and NL4-3mS.3 virus stocks using a Vif-specific primer set. Helper virus (pC-Help) and NL4-3 virus stocks were analyzed in parallel. All virus preparations were normalized by RT activity. (C) HeLa cells were transfected with plasmids encoding the variants carrying mutations in stem-loop 1 (mS.1) or stem-loop 3 (mS.3) together with the APO3G expression vector. Cells and virus-containing supernatants were collected 24 h after transfection. Cell lysates and purified, concentrated viral pellets were analyzed by immunoblotting as described for Fig. 1.
We have previously established that components of the viral core are resistant to detergent extraction, whereas other viral components, such as matrix (MA) or envelope protein, are detergent sensitive and can be separated from core-associated proteins by sucrose step gradient centrifugation (13). In this assay, intact viruses accumulate at the 20%/60% interphase of the step gradient column (Fig. 3B, S3) as evidenced by the enrichment of CA and MA proteins in the S3 fraction (Fig. 3A, lane 3). In contrast, detergent treatment resulted in the partitioning of MA into the soluble S1 fraction (Fig. 3A, lane 4). As observed previously (13), CA remained partially resistant to detergent treatment. Overall, the detergent sensitivity of these viral components was very similar for all samples tested. Interestingly, NL4-3-associated APO3G was largely resistant to detergent extraction (Fig. 3A). In fact, >70% of the virus-associated APO3G copurified with the viral core fraction S3 (Fig. 3C). These results suggest for the first time that APO3G is packaged into virus particles as a stable complex with the viral core. Similar results were observed for Vif-defective NL4-3 (Fig. 3D). Thus, the presence or absence of Vif did not affect the relative affinity of APO3G to the viral core but merely altered the absolute amounts of virus-associated APO3G. In contrast, detergent treatment of C-Help virus extracted about two-thirds of virus-associated APO3G from the core fraction (Fig. 3E). Similarly, APO3G packaged into mS.1 particles was highly detergent sensitive (Fig. 3F). Despite the presence of viral RNA, APO3G association with the mS.1 core was significantly reduced, suggesting an important role for the sequences surrounding stem-loop 1 in core association of APO3G. These biochemical studies therefore support the genetic data provided in Fig. 2 and strongly argue for a role of viral RNA in APO3G packaging.
FIG. 3.
Viral RNA stabilizes the association of APO3G with viral nucleoprotein complexes. (A) Immunoblot analysis of NL4-3 virus. Virus stocks were made in HeLa cells by cotransfection of pNL4-3 plasmid DNA with pcDNA-APO3G DNA. Virus was collected 24 h posttransfection, filtered to remove cellular debris, and purified by pelleting twice through 20% sucrose. To assess the detergent sensitivity of the viral protein components, 50% of the virus preparation was treated with Triton X-100 (0.1%) prior to step gradient centrifugation (lanes 4 to 6). The remaining virus was left untreated (lanes 1 to 3). Three fractions containing soluble proteins (S1), a buffer fraction (S2), and the virus-containing fraction (S3) were harvested. Individual gradient fractions were subjected to immunoblot analysis using an HIV-positive human serum (CA or MA) or an APO3G-specific antibody (APO3G). (B) Schematic representation of the step gradient procedure. (C) APO3G-specific bands depicted in panel A were quantified as described for Fig. 1, and the amounts of APO3G present in each of the gradient fractions were calculated as percentages of the total APO3G present in the three fractions combined. (D to F) NL4-3vif(−) (D), C-Help (E), and NL4-3mS.1 viruses (F) were analyzed for APO3G packaging and core association as described for NL4-3.
While our data clearly demonstrate that APO3G packaging into virus particles is enhanced by viral RNA packaging, the presence of viral RNA per se is not absolutely essential as low levels of APO3G are packaged even in the absence of viral RNA (Fig. 1). These results are consistent with previous studies demonstrating that APO3G can be packaged into viruslike particles in the absence of viral RNA through an interaction with the viral NC protein (5, 8, 17, 23, 28). Although APO3G has RNA binding properties, it is conceivable that APO3G recognizes a unique RNA-protein complex that forms during viral assembly as part of the RNA dimerization/encapsidation process that has been shown to involve the SL1 hairpin as well as other hairpin structures and sequences (7). Such a mechanism could explain the efficient packaging of APO3G into diverse viruses, such as HIV-2, simian immunodeficiency virus, or murine leukemia virus and the sensitivity of APO3G packaging to mutations in NC protein, which has been shown to participate in both dimerization and encapsidation of viral RNA (22).
Acknowledgments
We thank George Pavlakis and Antonio Valentin for the codon-optimized Gag expression vector p55BM1-10PO-2SD+. We thank Xiao-Fang Yu and Bryan Cullen for sharing unpublished data. We gratefully acknowledge Alicia Buckler-White and Ron Plishka for oligonucleotide synthesis and sequence analysis.
Part of this work was supported by a grant from the NIH Intramural AIDS Targeted Antiviral Program to K.S.
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alce, T. M., and W. Popik. 2004. APOBEC3G is incorporated into virus-like particles by a direct interaction with HIV-1 Gag nucleocapsid protein. J. Biol. Chem. 279:34083-34086. [DOI] [PubMed] [Google Scholar]
- 3.Beard, B. C., S. H. Wilson, and M. J. Smerdon. 2003. Suppressed catalytic activity of base excision repair enzymes on rotationally positioned uracil in nucleosomes. Proc. Natl. Acad. Sci. USA 100:7465-7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop, K. N., R. K. Holmes, A. M. Sheehy, N. O. Davidson, S. J. Cho, and M. H. Malim. 2004. Cytidine deamination of retroviral DNA by diverse APOBEC proteins. Curr. Biol. 14:1392-1396. [DOI] [PubMed] [Google Scholar]
- 5.Cen, S., F. Guo, M. Niu, J. Saadatmand, J. Deflassieux, and L. Kleiman. 2004. The interaction between HIV-1 Gag and APOBEC3G. J. Biol. Chem. 279:33177-33184. [DOI] [PubMed] [Google Scholar]
- 6.Clever, J. L., D. Mirandar, Jr., and T. G. Parslow. 2002. RNA structure and packaging signals in the 5′ leader region of the human immunodeficiency virus type 1 genome. J. Virol. 76:12381-12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clever, J. L., and T. G. Parslow. 1997. Mutant human immunodeficiency virus type 1 genomes with defects in RNA dimerization or encapsidation. J. Virol. 71:3407-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douaisi, M., S. Dussart, M. Courcoul, G. Bessou, R. Vigne, and E. Decroly. 2004. HIV-1 and MLV Gag proteins are sufficient to recruit APOBEC3G into virus-like particles. Biochem. Biophys. Res. Commun. 321:566-573. [DOI] [PubMed] [Google Scholar]
- 9.Harris, R. S., K. N. Bishop, A. M. Sheehy, H. M. Craig, S. K. Petersen-Mahrt, I. N. Watt, M. S. Neuberger, and M. H. Malim. 2003. DNA deamination mediates innate immunity to retroviral infection. Cell 113:803-809. [DOI] [PubMed] [Google Scholar]
- 10.Jarmuz, A., A. Chester, J. Bayliss, J. Gisbourne, I. Dunham, J. Scott, and N. Navaratnam. 2002. An anthropoid-specific locus of orphan C to U RNA-editing enzymes on chromosome 22. Genomics 79:285-296. [DOI] [PubMed] [Google Scholar]
- 11.Kao, S., M. A. Khan, E. Miyagi, R. Plishka, A. Buckler-White, and K. Strebel. 2003. The human immunodeficiency virus type 1 Vif protein reduces intracellular expression and inhibits packaging of APOBEC3G (CEM15), a cellular inhibitor of virus infectivity. J. Virol. 77:11398-11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kavli, B., O. Sundheim, M. Akbari, M. Otterlei, H. Nilsen, F. Skorpen, P. A. Aas, L. Hagen, H. E. Krokan, and G. Slupphaug. 2002. hUNG2 is the major repair enzyme for removal of uracil from U:A matches, U:G mismatches, and U in single-stranded DNA, with hSMUG1 as a broad specificity backup. J. Biol. Chem. 277:39926-39936 [DOI] [PubMed] [Google Scholar]
- 13.Khan, M. A., C. Aberham, S. Kao, H. Akari, R. Gorelick, S. Bour, and K. Strebel. 2001. Human immunodeficiency virus type 1 Vif protein is packaged into the nucleoprotein complex through an interaction with viral genomic RNA. J. Virol. 75:7252-7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lecossier, D., F. Bouchonnet, F. Clavel, and A. J. Hance. 2003. Hypermutation of HIV-1 DNA in the absence of the Vif protein. Science 300:1112. [DOI] [PubMed] [Google Scholar]
- 15.Li, S. L., X. Y. Zhang, H. Ling, J. Ikeda, K. Shirato, and T. Hattori. 2000. A VSV-G pseudotyped HIV vector mediates efficient transduction of human pulmonary artery smooth muscle cells. Microbiol. Immunol. 44:1019-1025. [DOI] [PubMed] [Google Scholar]
- 16.Liu, B., X. Yu, K. Luo, Y. Yu, and X. F. Yu. 2004. Influence of primate lentiviral Vif and proteasome inhibitors on human immunodeficiency virus type 1 virion packaging of APOBEC3G. J. Virol. 78:2072-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo, K., B. Liu, Z. Xiao, Y. Yu, X. Yu, R. Gorelick, and X. F. Yu. 2004. Amino-terminal region of the human immunodeficiency virus type 1 nucleocapsid is required for human APOBEC3G packaging. J. Virol. 78:11841-11852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mangeat, B., P. Turelli, G. Caron, M. Friedli, L. Perrin, and D. Trono. 2003. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature 424:99-103. [DOI] [PubMed] [Google Scholar]
- 19.Mariani, R., D. Chen, B. Schrofelbauer, F. Navarro, R. Konig, B. Bollman, C. Munk, H. Nymark-McMahon, and N. R. Landau. 2003. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell 114:21-31. [DOI] [PubMed] [Google Scholar]
- 20.Marin, M., K. M. Rose, S. L. Kozak, and D. Kabat. 2003. HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nat. Med. 9:1398-1403. [DOI] [PubMed] [Google Scholar]
- 21.Mochizuki, H., J. P. Schwartz, K. Tanaka, R. O. Brady, and J. Reiser. 1998. High-titer human immunodeficiency virus type 1-based vector systems for gene delivery into nondividing cells. J. Virol. 72:8873-8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paillart, J. C., R. Marquet, E. Skripkin, C. Ehresmann, and B. Ehresmann. 1996. Dimerization of retroviral genomic RNAs: structural and functional implications. Biochimie 78:639-653. [DOI] [PubMed] [Google Scholar]
- 23.Schafer, A., H. P. Bogerd, and B. R. Cullen. 2004. Specific packaging of APOBEC3G into HIV-1 virions is mediated by the nucleocapsid domain of the Gag polyprotein precursor. Virology 328:163-168. [DOI] [PubMed] [Google Scholar]
- 24.Schneider, R., M. Campbell, G. Nasioulas, B. K. Felber, and G. N. Pavlakis. 1997. Inactivation of the human immunodeficiency virus type 1 inhibitory elements allows Rev-independent expression of Gag and Gag/protease and particle formation. J. Virol. 71:4892-4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheehy, A. M., N. C. Gaddis, J. D. Choi, and M. H. Malim. 2002. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418:646-650. [DOI] [PubMed] [Google Scholar]
- 26.Sheehy, A. M., N. C. Gaddis, and M. H. Malim. 2003. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat. Med. 9:1404-1407. [DOI] [PubMed] [Google Scholar]
- 27.Stopak, K., C. de Noronha, W. Yonemoto, and W. C. Greene. 2003. HIV-1 Vif blocks the antiviral activity of APOBEC3G by impairing both its translation and intracellular stability. Mol. Cell 12:591-601. [DOI] [PubMed] [Google Scholar]
- 28.Svarovskaia, E. S., H. Xu, J. L. Mbisa, R. Barr, R. J. Gorelick, A. Ono, E. O. Freed, W. S. Hu, and V. K. Pathak. 2004. Human apolipoprotein B mRNA-editing enzyme-catalytic polypeptide-like 3G (APOBEC3G) is incorporated into HIV-1 virions through interactions with viral and nonviral RNAs. J. Biol. Chem. 279:35822-35828. [DOI] [PubMed] [Google Scholar]
- 29.Wiegand, H. L., B. P. Doehle, H. P. Bogerd, and B. R. Cullen. 2004. A second human antiretroviral factor, APOBEC3F, is suppressed by the HIV-1 and HIV-2 Vif proteins. EMBO J. 23:2451-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu, X., Y. Yu, B. Liu, K. Luo, W. Kong, P. Mao, and X. F. Yu. 2003. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science 302:1056-1060. [DOI] [PubMed] [Google Scholar]
- 31.Zhang, H., B. Yang, R. J. Pomerantz, C. Zhang, S. C. Arunachalam, and L. Gao. 2003. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature 424:94-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng, Y. H., D. Irwin, T. Kurosu, K. Tokunaga, T. Sata, and B. M. Peterlin. 2004. Human APOBEC3F is another host factor that blocks human immunodeficiency virus type 1 replication. J. Virol. 78:6073-6076. [DOI] [PMC free article] [PubMed] [Google Scholar]