Abstract
There are approximately 5 million pregnancies per year in the USA, with 1 million ending in miscarriage (a loss occurring prior to 20 weeks of gestation) and over 20,000 ending in stillbirth at or beyond 20 weeks of gestation. As many as 50% of these losses are unexplained. Our objective was to evaluate the effect of expanding the placental pathology diagnostic categories to include the explicit categories of (1) dysmorphic chorionic villi and (2) small placenta in examining previously unexplained losses. Using a clinical database of 1256 previously unexplained losses at 6–43 weeks of gestation, the most prevalent abnormality associated with each loss was determined through examination of its placental pathology slides. Of 1256 cases analyzed from 922 patients, there were 878 (69.9%) miscarriages and 378 (30.1%) antepartum stillbirths. We determined the pathologic diagnoses for 1150/1256 (91.6%) of the entire series, 777/878 (88.5%) of the miscarriages (< 20 weeks’ gestation), and 373/378 (98.7%) of the stillbirths (≥ 20 weeks’ gestation). The most common pathologic feature observed in unexplained miscarriages was dysmorphic chorionic villi (757 cases; 86.2%), a marker associated with genetic abnormalities. The most common pathologic feature observed in unexplained stillbirths was a small placenta (128 cases; 33.9%). Our classification system reinforced the utility of placental examination for elucidating potential mechanisms behind pregnancy loss. The improved rate of diagnosis appeared to be the result of filling a gap in previous pregnancy loss classification systems via inclusion of the categories of dysmorphic chorionic villi and small placenta.
Graphical Abstract
Keywords: Pregnancy loss, Miscarriage, Stillbirth, Placenta, Trophoblast inclusion, Small placenta
Introduction
Miscarriage rates based on life table analysis reveal that the cumulative risk of pregnancy loss between 5 and 20 weeks of gestation ranges between 11 and 22% [1]. Although pregnancy loss rates decrease after 20 weeks of gestation, there are approximately 2 million stillbirths globally per year [2], with over 20,000 losses occurring annually in the USA [3, 4]. Up to 60–70% of miscarriages are caused by aneuploidies [5–7], and although many of these cases were historically classified as unexplained, recent detailed studies have steadily increased the genetic fraction [8–10]. Despite these advances, the pressing clinical issue remains identifying the cause of the loss and employing methods of preventing future losses when possible [11–14].
Current pregnancy loss classification systems require improved consistency to more accurately determine the potential causes of each pregnancy loss [11, 15]. A 2009 systemic review found a large variability in the rates of unexplained stillbirths when various classification systems were applied to the same cohort of stillbirths, ranging from 9.5 to 50.4% [15]. Consistent with these findings, the Centers for Disease Control and Prevention’s 2015–2017 Cause of Fetal Death report found that the most frequent cause for fetal death was “Unspecified.” [16].
The key may be the placenta, as placental abnormalities are commonly detected in adverse pregnancy outcomes [11, 17–20], and have been associated with potentially preventable types of losses [21–23]. One systemic review reported that up to 65% of stillbirths are attributable to placental abnormalities [24]. However, absent in these abovementioned classification systems are the categories of dysmorphic chorionic villi, represented by trophoblast inclusions [20, 25–37], and the consistent inclusion of the category of a small placenta, which is clearly associated with pregnancy loss [38–41]. We thus hypothesized that expanding the placental pathology diagnostic categories to include the two explicit categories of dysmorphic chorionic villi and small placenta in examining previously unexplained losses could decrease the number of cases that remained “Unspecified” [16].
Materials and Methods
Cases
A case series of 1527 singleton pregnancies that ended in loss were identified from our tertiary-care consult service. Cases were excluded if the cause of loss could be elucidated from the clinical records alone, such as the presence of aneuploidies. Available demographic, clinical data, and gross description were abstracted from the clinical records when submitted with the consult request. Hematoxylin and eosin placental slides (no autopsy slides) were reviewed by the senior author (HJK). The analysis of this retrospective case series was approved by the Yale University Human Research Protection Program Institutional Review Board (protocol ID 2000029781).
Excluded Cases
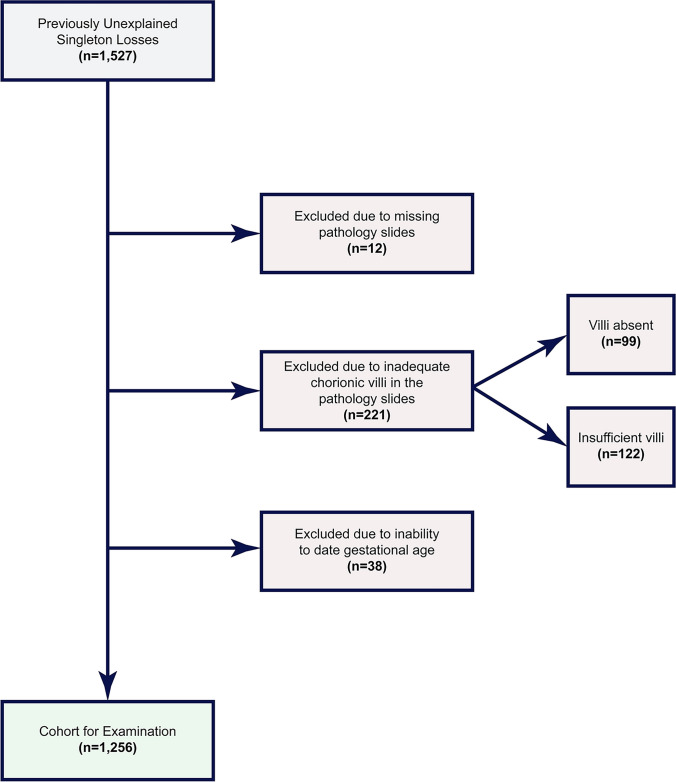
Cases with missing pathology slides, or an absence or insufficient number (fewer than five cross sections) of chorionic villi in the placental sample (Fig. 1) were excluded. The second exclusion criterion was an inability to date the clinical gestational age (GA), determined by the patient’s last menstrual period (LMP). In the absence of an LMP, the GA was approximated by chorionic villus histologic criteria [42–44]. The remaining cases in which gestational age could not be reliably estimated were excluded from further analysis. All subsequent references to GA are related to LMP dating.
Fig. 1.
Flowchart demonstrating the selection and exclusion criteria of the eligible cases
Pathologic Evaluation
The placental pathology of included cases was re-reviewed following the Amsterdam Placental Workshop Group Consensus Statement [45], with the following modifications. This statement does not include the diagnostic categories of dysmorphic chorionic villi, trophoblast inclusions (TIs), and/or invaginations (Fig. 2). TIs were first described by Boyd and Hamilton in 1964 [46], and later linked specifically to placentas from triploid losses in 1969 [47, 48]. Over time other investigators found that TIs were not a specific marker of triploidy but rather were seen in a wide range of karyotypic and non-karyotypic genetic abnormalities [25, 27–30, 49, 50], and adverse pregnancy outcomes, including stillbirth [20]. Importantly, the frequency of TIs in normal control placentas is very low [51–53]. Therefore, we added dysmorphic chorionic villi (not to be confused with villous dysmaturity [45]) as a diagnostic category, defined as identification of at least one TI and/or multiple invaginations in the examined slides. Additionally, based on normative curves developed by Pinar et al. [54], we added the explicit category of small placenta, defined as fixed trimmed disk weight below the 10th percentile for cases ≥ 20 weeks. Values below the 10th percentile were mathematically extrapolated from the primary Pinar data.
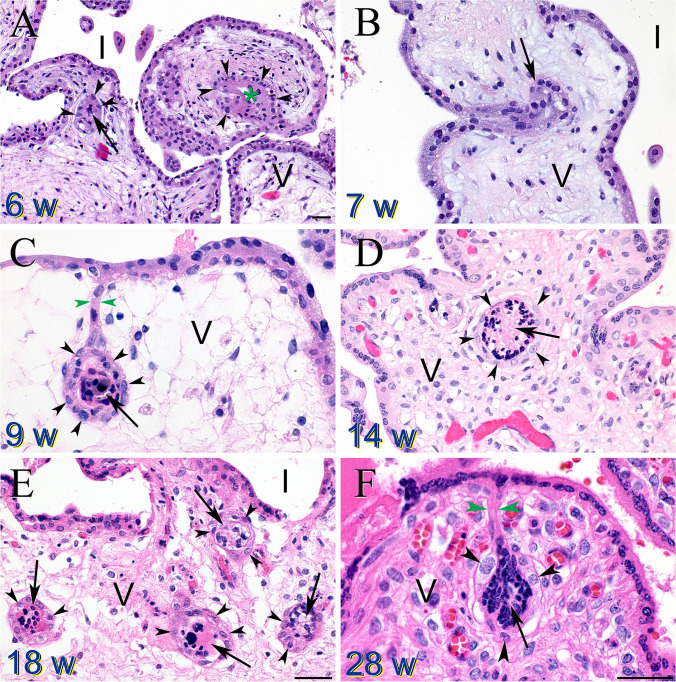
Fig. 2.
Trophoblast invaginations and inclusions (TIs). A Chorionic villi from a 6-week loss revealed both a trophoblast inclusion (TI) (arrow) and invagination (green asterisk), with surrounding cytotrophoblasts (black arrowheads). Intervillous space (I) and villus core (V). B Trophoblast invagination (arrow) in a villus from a 7-week loss. C Trophoblast invagination (green arrowheads) forming a TI (arrow) with surrounding cytotrophoblasts (black arrowheads). D Cross section of a large TI (arrow) surrounded by cytotrophoblasts (black arrowheads) in a 14-week villus. E Multiple TIs (arrows) with surrounding cytotrophoblasts (black arrowheads) in an 18-week villus. F Trophoblast invagination (green arrowheads) forming a TI (arrow) with surrounding cytotrophoblasts (black arrowheads). Magnification bars all represent 50 μM. Panels B–E are all at the same magnification
Identifying a nonacute cord accident required evidence of cord compression, as manifested by (1) the presence of squamous metaplasia [55–57] on the umbilical cord surface (Fig. 3A); (2) fetal hypoxia defined as an abnormal increase in fetal nucleated RBCs [58]; (3) and thrombosis within the fetal circulation [59]. A loss was only identified as being caused by an infection when a fetal inflammatory response was observed, evidenced by either fetal neutrophil migration through the fetal chorionic plate vessels and/or through the umbilical cord vessels (funisitis) (Fig. 3B) [60]. A maternal inflammatory response alone, as evidenced by maternal neutrophils migrating into and through either the chorionic plate or external membranes, was not sufficient to identify a loss as being caused by an infection. Maternal immunologic rejection was identified when significant numbers of maternal T-cells infiltrated the chorionic villi (chronic villitis, Fig. 3C) [61–64], or monocytes filled the intervillous space (chronic histiocytic intervillositis; CHI) [65–67]. Abruption occurred when a clear, well-developed fibrin clot was adherent to the maternal surface of the placenta [68]. Fetal maternal hemorrhage was identified when intervillous fibrin forming layered lines of Zahn (indicative of blood clot formation in flowing blood [69]) was admixed with blood containing nucleated red blood cells (indicative of a fetal bleeding source) (Fig. 3E) [70, 71]. In contrast, massive perivillous fibrin (a manifestation of maternal intervillous blood thrombosis [72–74]) was identified when the intervillous space was largely filled with fibrin (Fig. 3F).
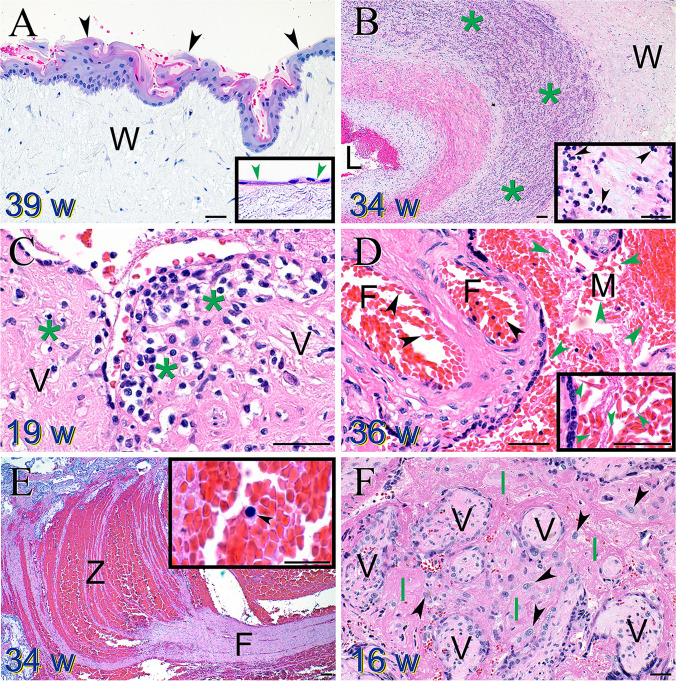
Fig. 3.
Select placental pathology findings in pregnancy losses. A Squamous metaplasia of the umbilical cord surface epithelium (black arrowheads) confirms compression in this 39-week gestation. Compare to the normal umbilical cord surface epithelium (green arrowheads) in the inset. Wharton’s jelly (W). Magnification bar = 50 μM. B Severe fetal inflammatory response observed as a large wave of neutrophils (green asterisks) migrating through and into the Wharton’s jelly (W) of a 34-week gestation umbilical vein. Magnification bar = 100 μM. Inset reveals individual neutrophils (black arrowheads). Magnification bar = 50 μM. C Maternal T-cells (green asterisks) infiltrating into a 19-week chorionic villus (V). Magnification bar = 50 μM. D Sickled erythrocytes observed in both the fetal (F) (black arrowheads) and maternal (M) circulations (green arrowheads) in a 36-week gestation. Magnification bars = 50 μM. E Fetal maternal hemorrhage evidenced by a large area of intervillous thrombosis with characteristic regions of fibrin (F) and lines of Zahn (Z). Magnification bar = 100 μM. Inset reveals a nucleated fetal erythrocyte (black arrowhead). Magnification bar = 25 μM. F Villi (V) from a 16-week gestation are totally enmeshed in intervillous fibrin (referred to as massive perivillous fibrin)—a result of maternal intervillous blood thrombosis (I). Detached villus trophoblasts (black arrowheads) can be seen migrating through the fibrin. Magnification bar = 50 μM
Classification System
After pathologic examination, we identified the most prevalent abnormality associated with the loss according to the following classification system. First, any clear and marked case of abruption, cord accident, or fetal bleed was assigned. Next, we identified all cases with evidence of thrombosis or fetal inflammatory response.
After losses associated with the above five abnormalities were identified, the remaining cases with a placental weight < 10th percentile for the corresponding gestational age were categorized as a small placenta and sorted into four etiologic sub-categories: small placenta with evidence of maternal immunologic rejection, small placenta with dysmorphic chorionic villi, small placenta with evidence of uteroplacental insufficiency (evidenced by findings of increased syncytial knots and accelerated maturation of the chorionic villi), or small placenta with no other pathologic findings.
Next, remaining cases with indication of maternal immunologic rejection were classified. Cases that showed dysmorphic chorionic villi with no other etiology were then assigned. The remaining “other” defined abnormalities included viral stigmata revealed on pathologic examination [75, 76], uteroplacental insufficiency without a concomitantly small placenta [77], maternal and/or fetal sickle cell disease (Fig. 3D) [78], premature inappropriate maternal perfusion prior to 8 weeks of gestation [79], complete mole [27], and severe intraamniotic fluid infection without apparent fetal inflammatory responses [80]. Cases revealing no pathologic findings remained unexplained.
Statistical Analysis
We displayed the distribution of pregnancy losses across gestational age and associated abnormalities using kernel density estimation [81, 82]. This smoothed version of a histogram that replaces each individual data point is replaced with a Gaussian and the total density plot is the sum of all such Gaussians. For each individual category, all corresponding gestational ages were used to create a density estimate of that associated abnormality. Then, to account for how some abnormalities occur more frequently than others, we multiplied the density of each cause by the proportion of cases with that associated abnormality.
To analyze the frequencies of small and large placentas in our series, we converted placental weight percentiles to z-scores, allowing us to visualize this loss cohort against the standard z-score distribution of placentas from normal term or uncomplicated preterm deliveries [54].
We conducted an analysis of patients with multiple losses to investigate whether their associated abnormalities were correlated. More precisely, the null hypothesis to be tested was that the abnormality identified in the second loss was not related to that of the first loss. We tested this against the alternative hypothesis that the abnormality identified in the second loss was the same as that of the first. To perform this hypothesis test, we used a permutation test [83]. Specifically, we randomly shuffled the order of all second losses and calculated what proportion of them matched the findings in the unshuffled first loss causes. Repeating this 500,000 times via computer algorithm gave an estimate of the distribution for the proportion of matching abnormalities when the null hypothesis was true.
Statistical analysis was performed using R version 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria) and the Python packages of Scikit-learn [84] and Matplotlib [85].
Results
Of the original 1527 cases, 12 cases were excluded due to an absence of any placental pathology slides (Fig. 1). Two hundred twenty-one cases could not be classified due to absence (n = 99) or lack of (n = 122) chorionic villi in the placental sample. We estimated the gestational ages of 178 losses. Including these 178 cases did not lead to any visually identifiable change in the violin plot distributions of gestational age for any category of pregnancy loss (Fig. 4). Thirty-eight cases were excluded due to an inability to date the GA at loss by any means. The demographics of the final case series are presented in Table 1.
Fig. 4.
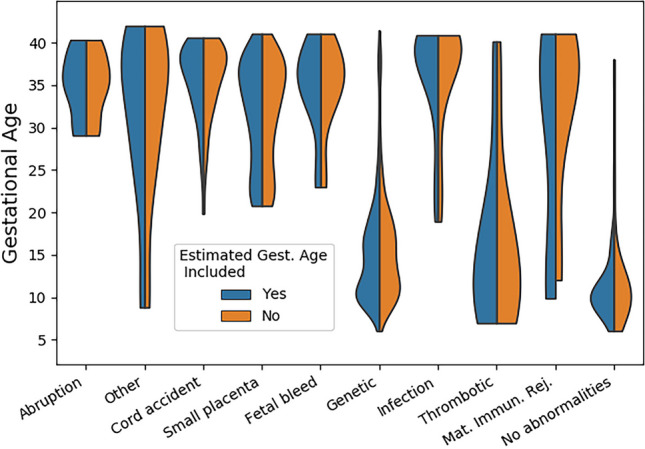
Violin plot of pregnancy pathologies with and without estimated gestational age (GA) cases. The frequencies of the different pathologies remained similar whether cases with estimated GA were included or not in the GA distributions
Table 1.
Case series demographics
| Characteristic | Count (%) |
|---|---|
| Maternal age | |
| < 20 years | 10 (0.8%) |
| 20– < 30 years | 210 (17%) |
| 30– < 40 years | 841 (67%) |
| > = 40 years | 93 (7.4%) |
| Data missing | 102 (8.1%) |
| Maternal BMI | |
| Underweight (< 18 kg/m2) | 11 (0.9%) |
| Normal (18– < 25 kg/m2) | 498 (40%) |
| Overweight (25– < 30 kg/m2) | 205 (16%) |
| Obese (> = 30 mg/m2) | 140 (11%) |
| Data missing | 402 (32%) |
| Sex of fetus | |
| Male | 235 (19%) |
| Female | 212 (17%) |
| Data missing | 809 (65%) |
Of the 1256 cases analyzed from 922 patients, there were 878 (69.9%) miscarriages and 378 (30.1%) antepartum stillbirths. The average maternal age of these cases at delivery was 33.7 ± 4.8 years (range 14.8 to 48.3 years). A total of 102 cases had no maternal age at delivery in the clinical record. Most pregnancy losses occurred in the first trimester (44.8%) as compared to the second (35.0%) and third trimesters (20.2%), as defined by American College of Obstetricians and Gynecologists (ACOG) criteria [86].
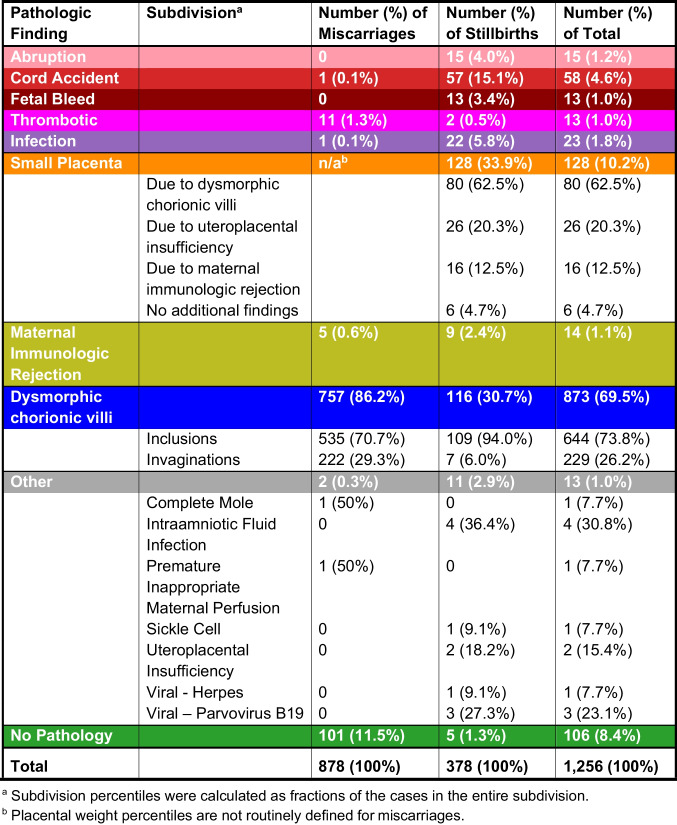
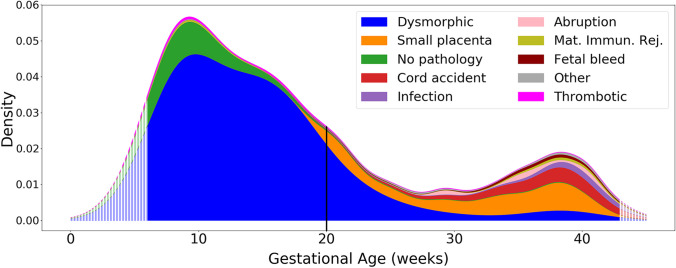
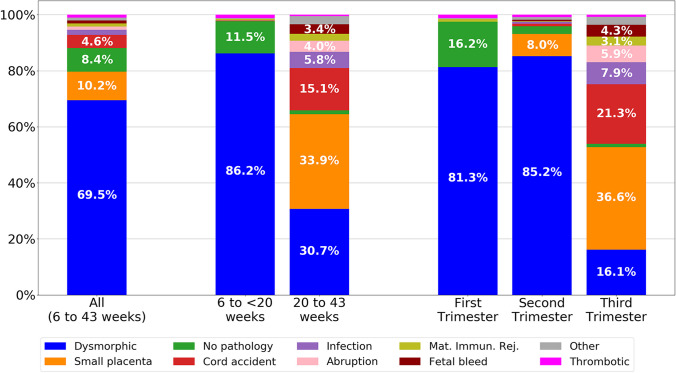
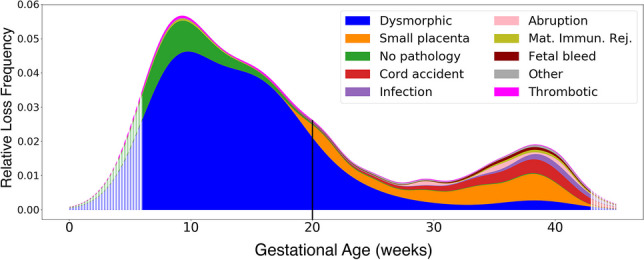
The tabulation of percentages of each type of abnormality following the order of our classification system is displayed in Table 2, and a graphical density plot of this data is presented in Fig. 5. Abnormalities were identified in 777/878 (88.5%) of miscarriages (losses prior to 20 weeks of gestation). Seven hundred fifty-seven out of 878 (86.2%) of miscarriages were marked by dysmorphic chorionic villi (Fig. 2), while 111/878 (11.5%) revealed no pathologic findings. In contrast, abnormalities were identified in 373/378 (98.7%) of analyzed stillbirths. The most prevalent abnormalities associated with our 378 cases of antepartum stillbirth were small placenta (128, 33.9%), dysmorphic chorionic villi (116, 30.7%), and cord accidents (57, 15.1%) (Fig. 6). Of the 873 total cases of losses with dysmorphic chorionic villi, 644 (73.8%) cases showed TIs, while 229 (26.2%) showed only trophoblast invaginations.
Table 2.
Placental pathologies identified (n = 1256)
Fig. 5.
Density plot of pregnancy loss pathologies from 6 to 43 weeks of gestation. Hatched edges represent mathematical extrapolations of the density plot beyond the primary data. The vertical line at 20 weeks represents the demarcation between miscarriages and stillbirths. Mat. Immun. Rej. = maternal immunologic rejection
Fig. 6.
Stacked bar charts of placental pathologies in pregnancy losses. All cases (left bar); miscarriages and stillbirths (middle pair); by trimester (right three bars). Mat. Immun. Rej. = maternal immunologic rejection
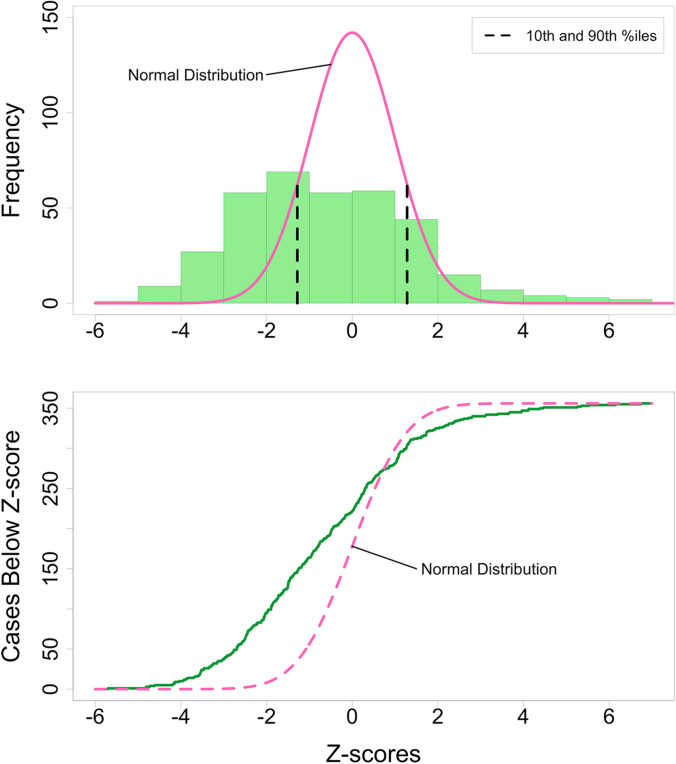
Placental weights were available in 355/378 (93.9%) of stillbirth cases. Converting percentiles to z-scores enabled the visualization of this case series against the standard z-score distribution of placentas from uncomplicated term or preterm livebirths [54]. The normal z-score distribution of placental weights (pink curve, upper panel Fig. 7) differed from our cases’ stillbirth placental weight distribution (green bars, upper panel Fig. 7), as 47/355 (13.2%) stillbirth cases fell below the normal distribution and 20/355 (5.6%) above (q-q plot, lower panel Fig. 7). The probability that the stillbirths in this case series would demonstrate this degree of dispersion beyond the normal distribution by chance was less than 2 × 10−16.
Fig. 7.
Normal versus loss case series weight distributions. (Upper) Normal placenta z-score weight distribution (pink line) compared to this loss case series (green columns). Tenth and 90th percentiles (black dashed lines) are indicated for reference. (Lower) q-q plot to illustrate excess number of small (47 cases) and large (20 cases) placentas (green line) compared to the normal placenta z-score weight distribution (pink dashed line). Two large outliers with z-scores of + 9 and + 16.6 were not plotted
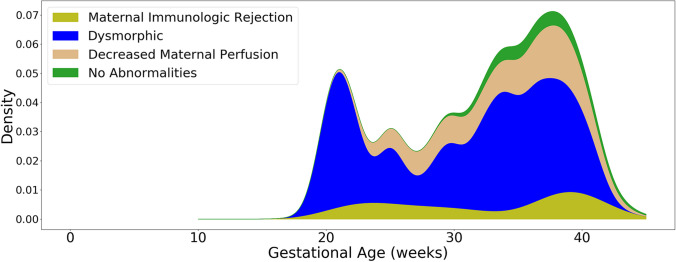
Within the group of 128 stillbirths with a small placenta, 80 (62.5%) were associated with dysmorphic chorionic villi, 26 (20.3%) with uteroplacental insufficiency, and 16 (12.5%) with maternal immunologic rejection (Fig. 8). Six cases (4.7%) demonstrated no additional pathologic findings. One hundred nine out of 128 (85%) of stillbirths with a small placenta had placental weights that were at or less than the 1st percentile. Within the group of 44 stillbirths with a large placenta (trimmed fixed disk weight greater than the 90th percentile), 20 (45.5%) were associated with dysmorphic chorionic villi, while the remainder were associated other miscellaneous abnormalities.
Fig. 8.
Density plot of small placenta associated pathologies
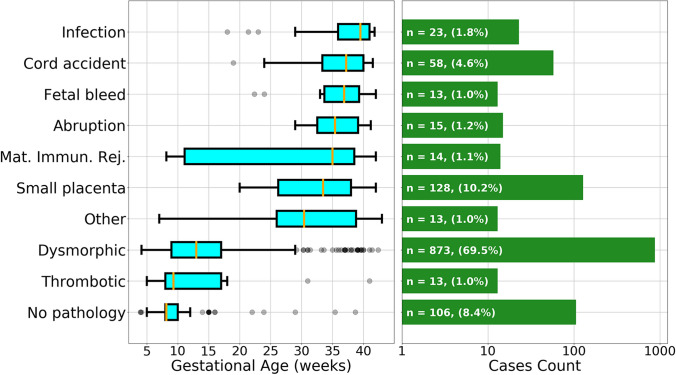
Compared to losses occurring in the first two trimesters, third trimester stillbirths demonstrated increasingly varied abnormalities, with the highest percentage of cases with small placentas (36.2%) and cord accidents (21.2%) (Fig. 6). The median gestational age of loss for each pathologic finding is displayed in Fig. 9. Thrombotic and dysmorphic chorionic villi were the most prevalent associated findings in early pregnancy losses, with medians at 9.3 and 13.0 weeks, respectively. Most other abnormalities were seen later in gestation, such as small placenta at 33.5 weeks, maternal immunologic rejection at 35.0 weeks, abruptions at 35.4 weeks, fetal maternal hemorrhages at 36.9 weeks, cord accidents at 37.2 weeks, and infections at 39.5 weeks.
Fig. 9.
Box plot of pathologic findings in pregnancy loss cases. Gestational age median (gold line), interquartile (25th to 75th percentile) range (teal box), minimum without outliers (lower bar), maximum without outliers (upper bar), and outliers (grey circles) (left panel). Number and percentages of pregnancy loss cases for each pathologic finding (green bars) (right panel). Mat. Immun. Rej. = maternal immunologic rejection
Two hundred thirty-one out of 922 (25%) patients had more than one loss included in the case series, ranging between 2 (16.8%) and 6 (0.2%) losses. One hundred ninety-one out of 231 patients (82.7%) with more than one loss had two or more losses with the same pathologic findings. The most prevalent recurrent findings were dysmorphic chorionic villi (94.8%), followed by no abnormalities (3.14%).
Discussion
Utilizing the presented classification system, we identified a pathologic finding in 91.6% of pregnancy losses ranging between 6 and 43 weeks of gestation and 98.7% of stillbirths, underscoring the utility of placental pathologic examination for elucidating potential mechanisms underlying pregnancy loss. As placental pathological examination is already the recommended standard of care following stillbirth [23, 87–92], our expanded methodology may aid clinicians in analyzing previously unexplained or challenging cases.
Our study’s finding that a mechanism for almost 99% of stillbirths could be elucidated by placental examination is a significant improvement compared to other studies. Blythe et al. examined 258 clinically unexplained stillbirths (CUS) using ReCoDe criteria, finding that 60.5% of CUS were due to “placental insufficiency” and/or fetal growth restriction [90]. Specifically, their results showed that ReCoDe category C4 (placenta, “other placental insufficiency”) and C5 (placenta, “other”) were present in 146 (56.5%) cases. Importantly, category C5 included the diagnosis of a small placenta, which was similarly defined as < 10th percentile placental weight for gestational age. Man et al. analyzed the placental pathology of 931 intrauterine fetal demises from 13 to 40 weeks of gestational age and found that 32% of stillbirths were due to abnormalities of the placenta [87]. Another study from the Stillbirth Collaborative Research Network determined that 12.7% of all stillbirths were due to “placental insufficiency” and were, therefore, potentially preventable [93]. A study using TULIP criteria deemed that 27% of stillbirths fell in their placenta cause category [94].
The 20-week marker in the density plot of placental pathologies (Fig. 5) reveals the often-identified U-shaped curve for stillbirth rates [95–97]. Viewing these losses as a continuum, rather than starting at 20 weeks, suggests a more nuanced and improved understanding of the epidemiology of pregnancy losses.
Our finding that a third of previously unexplained stillbirths were associated with a small placenta may be of clinical utility, as prenatal identification of a small placenta may reveal important growth discordance between the fetus and its primary supporting organ [98, 99]. While the Amsterdam criteria defines a placenta with a weight less than the 10th percentile as “placental hypoplasia due to maternal malperfusion,” [45] our data suggest that a placenta can be small for this and other reasons. While placental size alone may not predict stillbirth, we observed an increased number of small placentas in our case series. These results support Hutcheon et al.’s findings that the probability of stillbirth increased significantly with a placental weight more than one standard deviation below the mean [41]. Furthermore, our data contained a significant proportion of extremely small placentas weighing less than the 1st percentile for their gestational age.
Placental size evaluation could provide clinicians with additional data and tools to identify high-risk pregnancies and help determine when to deliver [98–100]. Our case series demonstrated a peak of losses at full term, in line with other studies that demonstrate the prevalence of full-term stillbirths [90, 101]. Although not currently clinically validated, the identification of a fetus with a small placenta, when balanced with other clinical risk factors, may support an earlier delivery to potentially prevent antenatal stillbirth.
TIs and invaginations have been shown to be associated with abnormal genetics, including cases of triploidy, trisomies, and other genetic conditions [27–30, 47, 49]. Therefore, the identification of TIs in most miscarriages suggests a genetic mechanism for these losses [20, 25]. Support for the strong association of developmental anomalies [102] and genetic abnormalities as the basis of pregnancy loss also comes from detailed genetic studies of loss cases [103–110]. However, validation of the specific genetic bases of TIs awaits further, more detailed, genetic analysis of these loss cases.
An increased frequency of TIs and invaginations have been observed in cases of placenta accreta, increta, and percreta [18] and intrauterine growth restriction [20], but not in cases of gestational diabetes, gestational hypertension, or preeclampsia [51]. There is no data relating TI frequencies to other common obstetrical pathologies, such as placenta previa, or to the method of conception. Investigating the relationship between TIs and method of conception, such as in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI), would be worthy of future studies.
Our study does not directly address mechanisms underlying the association of TIs with either miscarriages or small placentas, but previous studies on the relationship between cytotrophoblasts and syncytiotrophoblasts may shed some light on this issue [26, 111]. First, syncytiotrophoblasts are created by the fusion of cytotrophoblasts [111]. Second, alterations in the rates of cytotrophoblast proliferation and fusion into the syncytial layer determines the bending of the trophoblast bilayer [26], and increased cytotrophoblast proliferation or decreased fusion leads to inward bending (invagination) of the trophoblast bilayer. Cross sections of chorionic villi through these invaginations create TIs. It should be noted that TIs are epithelial islands within a chorionic villus cross section, not a body within the syncytiotrophoblast cytoplasm (see Fig. 2). Therefore, genetic abnormalities that lead to increased cell proliferation or decreased cell differentiation may lead to increased trophoblast invaginations and inclusions. Although this alteration in the placenta alone may not be deleterious to placental function, other organs in the embryo and fetus may be very susceptible to alterations in branching morphogenesis and infolding, such as the heart [110, 112, 113]. Therefore, further molecular and genetic understanding of the formation of trophoblast invaginations and inclusions may elucidate specific mutations that lead to pregnancy loss.
Although a priori we did not define a separate causal category for stillbirth with a large placenta, we also observed that there was an increased number of large placentas in our case series, indicating a potentially unexplored research avenue.
Our paper’s strengths included the large number of cases examined spanning over a wide gestational age range, as well as the utilization of a classification system for losses that may be elusive to prior classification measures. Although our study was limited by selection bias from the nature of our specialized consultation service, our findings aligned with US national pregnancy loss distributions across the course of pregnancy [6, 16]. In addition, the large number of cases with a small placenta suggests the potential benefit of further research examining the utility of estimated placental volume measurements during clinical care [41, 98, 99].
Our study’s greatest weaknesses were that the sample population was a non-random series of consultative cases, the data was analyzed by a single pathologist at one institution, and gross pathology descriptions relied on materials supplied by referring pathologists. In addition, the gestational age for 178 out of 1256 total cases (14.2%) was approximated. However, this approximation did not appear to significantly affect the results (see Fig. 4). Another limitation was the lack of a comparison group of placentas from livebirths, although pathologic findings in normal placentas have been well studied [114, 115]. We also lacked robust data on maternal demographic and clinical characteristics. For instance, we did not have data on maternal race or ethnicity, which significantly limited us from analyzing this important mediating factor, as, for example, non-Hispanic Black patients have consistently higher rates of fetal demise [16, 116]. While lack of time of death data might also have led to placental weight changes after stillbirth, placental weights for intrapartum versus antepartum stillbirths have not been shown to vary significantly [41]. Lastly, assigning a single abnormality has potential limitations. Incidental findings surely play a contributing and compounding role to the mechanism of any given loss.
Conclusions
Prior research estimates that up to one-fourth of stillbirths are potentially preventable, most of whose etiology originates in the placenta [88, 93, 117]. Hutcheon et al. concluded their seminal 2012 paper with a clarion call that placental volume measurement may “improve the prenatal identification of fetuses at increased risk of developing adverse perinatal outcomes.” [41]. Our research reinforces this insight with the finding that one-third of previously unexplained stillbirths were associated with a small placenta. We also suggest that these small placentas could have been detected in utero and flagged as high risk prior to the loss. Additionally, we highlight that the identification of dysmorphic chorionic villi containing trophoblast inclusions may be one way to potentially identify genetic abnormalities for further exploration. Adding these two diagnostic categories appears to have eliminated most remaining unexplained loss cases, supporting their adoption and inclusion in pregnancy loss evaluations.
Acknowledgements
This study was supported by the Department of Obstetrics, Gynecology and Reproductive Sciences, Reproductive and Placental Research Unit, Yale University School of Medicine. We wish to thank Colleen Furlow MSN, CNM for her excellent organizational skills in cataloguing and entering the data for many of the cases in our series and Kristin M. Milano for her help in organizing the glass slides from specific cases for further analysis. Finally, we wish to thank the many patients who sought our help at one of the most difficult times of their lives and whose losses may help other patients and couples understand their own losses.
Author Contribution
Conceptualization: Harvey Kliman. Data curation: Beatrix Thompson, and Harvey Kliman. Formal analysis: Parker Holzer. Funding acquisition: Harvey Kliman. Methodology: Beatrix Thompson and Parker Holzer. Visualization: Beatrix Thompson, Parker Holzer, and Harvey Kliman. Writing—original draft: Beatrix Thompson. Writing—review and editing: Beatrix Thompson, Parker Holzer, and Harvey Kliman.
Data Availability
The data underlying the results presented in the study are available from the Dryad Digital Repository database (https://doi.org/10.5061/dryad.3xsj3txks).
Code Availability
Not applicable.
Declarations
Ethics Approval
Ethical clearance was obtained from the Yale University Human Research Protection Program Institutional Review Board (protocol ID 2000029781).
Consent to Participate
Not applicable.
Consent for Publication
All authors have read the manuscript and agreed to submit it to Reproductive Sciences.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ammon Avalos L, Galindo C, Li DK. A systematic review to calculate background miscarriage rates using life table analysis. Birth Defects Res A Clin Mol Teratol. 2012;94(6):417–23. doi: 10.1002/bdra.23014. [DOI] [PubMed] [Google Scholar]
- 2.Hug L, You D, Blencowe H, Mishra A, Wang Z, Fix MJ, Wakefield J, Moran AC, Gaigbe-Togbe V, Suzuki E, Blau DM, Cousens S, Creanga A, Croft T, Hill K, Joseph KS, Maswime S, McClure EM, Pattinson R, Pedersen J, Smith LK, Zeitlin J, Alkema L, as members of the UN Inter-agency Group for Child Mortality Estimation and its Core Stillbirth Estimation Group. Global, regional, and national estimates and trends in stillbirths from 2000 to 2019: a systematic assessment. Lancet. 2021;398(10302):772–85. 10.1016/S0140-6736(21)01112-0 [DOI] [PMC free article] [PubMed]
- 3.MacDorman MF, Gregory EC. Fetal and Perinatal Mortality: United States, 2013. Natl Vital Stat Rep. 2015;64(8):1–24. [PubMed] [Google Scholar]
- 4.Gregory ECW, Valenzuela CP, Hoyert DL. Fetal mortality: United States, 2020. National Vital Statistics Reports, (vol 71 no 4). National Center for Health Statistics, Hyattsville, MD; 2022. 10.15620/cdc:118420. [PubMed]
- 5.McQueen DB, Lathi RB. Miscarriage chromosome testing: indications, benefits and methodologies. Semin Perinatol. 2019;43(2):101–4. doi: 10.1053/j.semperi.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Pinar MH, Gibbins K, He M, Kostadinov S, Silver R. Early pregnancy losses: review of nomenclature, histopathology, and possible etiologies. Fetal Pediatr Pathol. 2018;37(3):191–209. doi: 10.1080/15513815.2018.1455775. [DOI] [PubMed] [Google Scholar]
- 7.Larsen EC, Christiansen OB, Kolte AM, Macklon N. New insights into mechanisms behind miscarriage. BMC Med. 2013;11:154. doi: 10.1186/1741-7015-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woolner AMF, Raja EA, Bhattacharya S, Danielian P, Bhattacharya S. Inherited susceptibility to miscarriage: a nested case-control study of 31,565 women from an intergenerational cohort. Am J Obstet Gynecol. 2020;222(2):168.e1–e8. doi: 10.1016/j.ajog.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Colley E, Hamilton S, Smith P, Morgan NV, Coomarasamy A, Allen S. Potential genetic causes of miscarriage in euploid pregnancies: a systematic review. Hum Reprod Update. 2019;25(4):452–72. doi: 10.1093/humupd/dmz015. [DOI] [PubMed] [Google Scholar]
- 10.Laisk T, Soares ALG, Ferreira T, Painter JN, Censin JC, Laber S, Bacelis J, Chen CY, Lepamets M, Lin K, Liu S, Millwood IY, Ramu A, Southcombe J, Andersen MS, Yang L, Becker CM, Borglum AD, Gordon SD, Bybjerg-Grauholm J, Helgeland O, Hougaard DM, Jin X, Johansson S, Juodakis J, Kartsonaki C, Kukushkina V, Lind PA, Metspalu A, Montgomery GW, Morris AP, Mors O, Mortensen PB, Njolstad PR, Nordentoft M, Nyholt DR, Lippincott M, Seminara S, Salumets A, Snieder H, Zondervan K, Werge T, Chen Z, Conrad DF, Jacobsson B, Li L, Martin NG, Neale BM, Nielsen R, Walters RG, Granne I, Medland SE, Magi R, Lawlor DA, Lindgren CM. The genetic architecture of sporadic and multiple consecutive miscarriage. Nat Commun. 2020;11(1):5980. doi: 10.1038/s41467-020-19742-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldenberg RL, McClure EM. Thoughts on assigning cause of death for stillbirth and neonatal death: a commentary. BJOG. 2020;127(5):532–5. doi: 10.1111/1471-0528.16007. [DOI] [PubMed] [Google Scholar]
- 12.Hirst JE, Villar J, Victora CG, Papageorghiou AT, Finkton D, Barros FC, Gravett MG, Giuliani F, Purwar M, Frederick IO, Pang R, Cheikh Ismail L, Lambert A, Stones W, Jaffer YA, Altman DG, Noble JA, Ohuma EO, Kennedy SH, Bhutta ZA, for the International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st). The antepartum stillbirth syndrome: risk factors and pregnancy conditions identified from the INTERGROWTH-21st Project. BJOG. 2018;125(9):1145–53. 10.1111/1471-0528.14463. [DOI] [PMC free article] [PubMed]
- 13.Townsend R, Manji A, Allotey J, Heazell A, Jorgensen L, Magee LA, Mol BW, Snell K, Riley RD, Sandall J, Smith G, Patel M, Thilaganathan B, von Dadelszen P, Thangaratinam S, Khalil A. Can risk prediction models help us individualise stillbirth prevention? A systematic review and critical appraisal of published risk models. BJOG. 2021;128(2):214–24. doi: 10.1111/1471-0528.16487. [DOI] [PubMed] [Google Scholar]
- 14.Townsend R, Sileo FG, Allotey J, Dodds J, Heazell A, Jorgensen L, Kim VB, Magee L, Mol B, Sandall J, Smith G, Thilaganathan B, von Dadelszen P, Thangaratinam S, Khalil A. Prediction of stillbirth: an umbrella review of evaluation of prognostic variables. BJOG. 2021;128(2):238–50. doi: 10.1111/1471-0528.16510. [DOI] [PubMed] [Google Scholar]
- 15.Flenady V, Froen JF, Pinar H, Torabi R, Saastad E, Guyon G, Russell L, Charles A, Harrison C, Chauke L, Pattinson R, Koshy R, Bahrin S, Gardener G, Day K, Petersson K, Gordon A, Gilshenan K. An evaluation of classification systems for stillbirth. BMC Pregnancy Childbirth. 2009;9:24. doi: 10.1186/1471-2393-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoyert DL, Gregory ECW. Cause-of-death data from the fetal death file, 2015–2017. Natl Vital Stat Rep. 2020;69(4):1–20. [PubMed] [Google Scholar]
- 17.Genest DR. Partial hydatidiform mole: clinicopathological features, differential diagnosis, ploidy and molecular studies, and gold standards for diagnosis. Int J Gynecol Pathol. 2001;20(4):315–22. doi: 10.1097/00004347-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Adler E, Madankumar R, Rosner M, Reznik SE. Increased placental trophoblast inclusions in placenta accreta. Placenta. 2014;35(12):1075–8. doi: 10.1016/j.placenta.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Firestein MR, Abellar R, Myers MM, Welch MG. Increased trophoblast inclusions in placentas from prematurely born infants: a potential marker of risk for preterm neurodevelopmental outcomes. Placenta. 2017;60:61–3. doi: 10.1016/j.placenta.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Firestein MR, Kliman HJ, Sania A, Brink LT, Holzer PH, Hofmann KM, Milano KM, Pini N, Shuffrey LC, Odendaal HJ, Fifer WP. Trophoblast inclusions and adverse birth outcomes. PLoS One. 2022;17(3):e0264733. doi: 10.1371/journal.pone.0264733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freedman AA, Silver RM, Gibbins KJ, Hogue CJ, Goldenberg RL, Dudley DJ, Pinar H, Drews-Botsch C. The association of stillbirth with placental abnormalities in growth-restricted and normally grown fetuses. Paediatr Perinat Epidemiol. 2019;33(4):274–383. doi: 10.1111/ppe.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korteweg FJ, Erwich JJ, Timmer A, van der Meer J, Ravise JM, Veeger NJ, Holm JP. Evaluation of 1025 fetal deaths: proposed diagnostic workup. Am J Obstet Gynecol. 2012;206(1):53.e1–e12. doi: 10.1016/j.ajog.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Heazell AE, Martindale EA. Can post-mortem examination of the placenta help determine the cause of stillbirth? J Obstet Gynaecol. 2009;29(3):225–8. doi: 10.1080/01443610802716042. [DOI] [PubMed] [Google Scholar]
- 24.Ptacek I, Sebire NJ, Man JA, Brownbill P, Heazell AE. Systematic review of placental pathology reported in association with stillbirth. Placenta. 2014;35(8):552–62. doi: 10.1016/j.placenta.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Kliman HJ, Firestein MR, Hofmann KM, Milano KM, Holzer PH, Brink LT, Odendaal HJ, Fifer WP. Trophoblast inclusions in the human placenta: identification, characterization, quantification, and interrelations of subtypes. Placenta. 2021;103:172–6. doi: 10.1016/j.placenta.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kliman HJ, Segel L. The placenta may predict the baby. J Theor Biol. 2003;225(1):143–5. doi: 10.1016/s0022-5193(03)00248-0. [DOI] [PubMed] [Google Scholar]
- 27.Szulman AE, Surti U. The syndromes of hydatidiform mole. II. Morphologic evolution of the complete and partial mole. Am J Obstet Gynecol. 1978;132(1):20–7. doi: 10.1016/0002-9378(78)90792-5. [DOI] [PubMed] [Google Scholar]
- 28.Honore LH, Dill FJ, Poland BJ. The association of hydatidiform mole and trisomy 2. Obstet Gynecol. 1974;43(2):232–237. [PubMed] [Google Scholar]
- 29.Novak R, Agamanolis D, Dasu S, Igel H, Platt M, Robinson H, Shehata B. Histologic analysis of placental tissue in first trimester abortions. Pediatr Pathol. 1988;8(5):477–82. doi: 10.3109/15513818809022303. [DOI] [PubMed] [Google Scholar]
- 30.Silvestre E, Cusi V, Borras M, Antich J. Cytogenetic and morphologic findings in chorionic villi from spontaneous abortions. Birth Defects Orig Artic Ser. 1996;30(1):353–357. [PubMed] [Google Scholar]
- 31.Mall FP, Meyer AW. Volume 12 of studies on abortuses: a survey of pathologic ova in the carnegie embryological collection, (pp. 364). Carnegie Institution of Washington; 1921. https://books.google.com/books/about/Contributions_to_Embryology.html?id=lIvRyAEACAAJ.
- 32.Rushton DI. Examination of products of conception from previable human pregnancies. J Clin Pathol. 1981;34(8):819–35. doi: 10.1136/jcp.34.8.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conran RM, Hitchcock CL, Popek EJ, Norris HJ, Griffin JL, Geissel A, McCarthy WF. Diagnostic considerations in molar gestations. Hum Pathol. 1993;24(1):41–8. doi: 10.1016/0046-8177(93)90061-k. [DOI] [PubMed] [Google Scholar]
- 34.Vejerslev LO, Sunde L, Hansen BF, Larsen JK, Christensen IJ, Larsen G. Hydatidiform mole and fetus with normal karyotype: support of a separate entity. Obstet Gynecol. 1991;77(6):868–874. [PubMed] [Google Scholar]
- 35.Fukunaga M. Histopathologic study of partial hydatidiform moles and DNA triploid placentas. Pathol Int. 1994;44(7):528–34. doi: 10.1111/j.1440-1827.1994.tb02603.x. [DOI] [PubMed] [Google Scholar]
- 36.Lorenzato M, Visseaux-Coletto B, Lallemand A, Masure M, Gaillard D. Determination of reliable histological features associated with early triploidy using DNA image cytometry. Pathol Res Pract. 1995;191(12):1179–85. doi: 10.1016/S0344-0338(11)81123-4. [DOI] [PubMed] [Google Scholar]
- 37.Sumithran E, Cheah PL, Susil BJ, Looi LM. Problems in the histological assessment of hydatidiform moles: a study on consensus diagnosis and ploidy status by fluorescent in situ hybridisation. Pathology. 1996;28(4):311–5. doi: 10.1080/00313029600169254. [DOI] [PubMed] [Google Scholar]
- 38.Dypvik J, Larsen S, Haavaldsen C, Saugstad OD, Eskild A. Placental weight and risk of neonatal death. JAMA Pediatr. 2020;174(2):197–9. doi: 10.1001/jamapediatrics.2019.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haavaldsen C, Samuelsen SO, Eskild A. Fetal death and placental weight/birthweight ratio: a population study. Acta Obstet Gynecol Scand. 2013;92(5):583–90. doi: 10.1111/aogs.12105. [DOI] [PubMed] [Google Scholar]
- 40.Voskamp BJ, Peelen M, Ravelli ACJ, van der Lee R, Mol BWJ, Pajkrt E, Ganzevoort W, Kazemier BM. Association between fetal sex, birthweight percentile and adverse pregnancy outcome. Acta Obstet Gynecol Scand. 2020;99(1):48–58. doi: 10.1111/aogs.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hutcheon JA, McNamara H, Platt RW, Benjamin A, Kramer MS. Placental weight for gestational age and adverse perinatal outcomes. Obstet Gynecol. 2012;119(6):1251–8. doi: 10.1097/AOG.0b013e318253d3df. [DOI] [PubMed] [Google Scholar]
- 42.Baergen RN. Chorionic villi: histology and villous development. In: Baergen RN, editor. Manual of pathology of the human placenta. 2. Boston: Springer US; 2011. pp. 69–83. [Google Scholar]
- 43.Roberts L, Sebire NJ, Fowler D, Nicolaides KH. Histomorphological features of chorionic villi at 10–14 weeks of gestation in trisomic and chromosomally normal pregnancies. Placenta. 2000;21(7):678–683. doi: 10.1053/plac.2000.0553. [DOI] [PubMed] [Google Scholar]
- 44.Hakvoort RA, Lisman BA, Boer K, Bleker OP, van Groningen K, van Wely M, Exalto N. Histological classification of chorionic villous vascularization in early pregnancy. Hum Reprod. 2006;21(5):1291–4. doi: 10.1093/humrep/dei456. [DOI] [PubMed] [Google Scholar]
- 45.Khong TY, Mooney EE, Ariel I, Balmus NC, Boyd TK, Brundler MA, Derricott H, Evans MJ, Faye-Petersen OM, Gillan JE, Heazell AE, Heller DS, Jacques SM, Keating S, Kelehan P, Maes A, McKay EM, Morgan TK, Nikkels PG, Parks WT, Redline RW, Scheimberg I, Schoots MH, Sebire NJ, Timmer A, Turowski G, van der Voorn JP, van Lijnschoten I, Gordijn SJ. Sampling and definitions of placental lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch Pathol Lab Med. 2016;140(7):698–713. doi: 10.5858/arpa.2015-0225-CC. [DOI] [PubMed] [Google Scholar]
- 46.Boyd JD, Hamilton WJ. Stromal trophoblastic buds. J Obstet Gynaecol Br Commonw. 1964;71:1–10. doi: 10.1111/j.1471-0528.1964.tb04235.x. [DOI] [PubMed] [Google Scholar]
- 47.Philippe E, Boue JG. The placenta in lethal chromosome aberrations. Ann Anat Pathol (Paris) 1969;14(3):249–266. [PubMed] [Google Scholar]
- 48.Philippe E, Boue J. Anatomo-pathologic character of human placentas according to chromosome anomalies. C R Acad Hebd Seances Acad Sci D. 1969;269(4):535–537. [PubMed] [Google Scholar]
- 49.Philippe E, Boue JG. Placenta and chromosome aberrations in spontaneous abortion. Presse Med. 1970;78(14):641–646. [PubMed] [Google Scholar]
- 50.Katz J, Holzer PH, Kliman HJ. Genetics, not the uterine environment, drive the formation of trophoblast inclusions: insights from a twin study. Placenta. 2021 doi: 10.1016/j.placenta.2021.04.010. [DOI] [PubMed] [Google Scholar]
- 51.Walker CK, Anderson KW, Milano KM, Ye S, Tancredi DJ, Pessah IN, Hertz-Picciotto I, Kliman HJ. Trophoblast inclusions are significantly increased in the placentas of children in families at risk for autism. Biol Psychiatry. 2013;74(3):204–11. doi: 10.1016/j.biopsych.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szulman AE, Philippe E, Boue JG, Boue A. Human triploidy: association with partial hydatidiform moles and nonmolar conceptuses. Hum Pathol. 1981;12(11):1016–21. doi: 10.1016/s0046-8177(81)80259-6. [DOI] [PubMed] [Google Scholar]
- 53.Kliman HJ, McSweet JC, Franco A, Ying X, Zhao Y, Stetten G. Trophoblast inclusions are rare in elective terminations and normal deliveries, but common in cases with karyotypic abnormalities. Fertil Steril. 2003;80:88. doi: 10.1016/S0015-0282(03)02035-1. [DOI] [Google Scholar]
- 54.Pinar H, Sung CJ, Oyer CE, Singer DB. Reference values for singleton and twin placental weights. Pediatr Pathol Lab Med. 1996;16(6):901–7. doi: 10.1080/15513819609168713. [DOI] [PubMed] [Google Scholar]
- 55.Mrklic I, Bendic A, Pogorelic Z, Karaman I, Glavina Durdov M, Druzijanic N, Tomic S. Squamous metaplasia of the peritoneum: report of a case. Int J Surg Pathol. 2013;21(1):82–84. doi: 10.1177/1066896912450316. [DOI] [PubMed] [Google Scholar]
- 56.Jongyotha K, Sriphrapradang C. Squamous cell carcinoma of the renal pelvis as a result of long-standing Staghorn calculi. Case Rep. 2015;8(3):399–404. doi: 10.1159/000440764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schatz JE, Colgan TJ. Squamous metaplasia of the peritoneum. Arch Pathol Lab Med. 1991;115(4):397–398. [PubMed] [Google Scholar]
- 58.Christensen RD, Lambert DK, Richards DS. Estimating the nucleated red blood cell ‘emergence time’ in neonates. J Perinatol. 2014;34(2):116–9. doi: 10.1038/jp.2013.113. [DOI] [PubMed] [Google Scholar]
- 59.Tantbirojn P, Saleemuddin A, Sirois K, Crum CP, Boyd TK, Tworoger S, Parast MM. Gross abnormalities of the umbilical cord: related placental histology and clinical significance. Placenta. 2009;30(12):1083–8. doi: 10.1016/j.placenta.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 60.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015;213(4 Suppl):S29–52. doi: 10.1016/j.ajog.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Redline RW, Patterson P. Villitis of unknown etiology is associated with major infiltration of fetal tissue by maternal inflammatory cells. Am J Pathol. 1993;143(2):473–9. [PMC free article] [PubMed] [Google Scholar]
- 62.Yusuf K, Kliman HJ. The fetus, not the mother, elicits maternal immunologic rejection: lessons from discordant dizygotic twin placentas. J Perinat Med. 2008;36(4):291–6. doi: 10.1515/JPM.2008.054. [DOI] [PubMed] [Google Scholar]
- 63.Redline RW. Villitis of unknown etiology: noninfectious chronic villitis in the placenta. Hum Pathol. 2007;38(10):1439–46. doi: 10.1016/j.humpath.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 64.Kim CJ, Romero R, Chaemsaithong P, Kim JS. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol. 2015;213(4 Suppl):S53–69. doi: 10.1016/j.ajog.2015.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marchaudon V, Devisme L, Petit S, Ansart-Franquet H, Vaast P, Subtil D. Chronic histiocytic intervillositis of unknown etiology: clinical features in a consecutive series of 69 cases. Placenta. 2011;32(2):140–5. doi: 10.1016/j.placenta.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 66.Traeder J, Jonigk D, Feist H, Brocker V, Langer F, Kreipe H, Hussein K. Pathological characteristics of a series of rare chronic histiocytic intervillositis of the placenta. Placenta. 2010;31(12):1116–9. doi: 10.1016/j.placenta.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 67.Boyd TK, Redline RW. Chronic histiocytic intervillositis: a placental lesion associated with recurrent reproductive loss. Hum Pathol. 2000;31(11):1389–1396. doi: 10.1016/S0046-8177(00)80009-X. [DOI] [PubMed] [Google Scholar]
- 68.Elsasser DA, Ananth CV, Prasad V, Vintzileos AM. New Jersey-Placental Abruption Study I. Diagnosis of placental abruption: relationship between clinical and histopathological findings. Eur J Obstet Gynecol Reprod Biol. 2010;148(2):125–30. doi: 10.1016/j.ejogrb.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rashidi A, Gilles S, Linden MA. Lines of Zahn in the splenic vein. Thromb Haemost. 2018;118(6):957–8. doi: 10.1055/s-0038-1642610. [DOI] [PubMed] [Google Scholar]
- 70.Biankin SA, Arbuckle SM, Graf NS. Autopsy findings in a series of five cases of fetomaternal haemorrhages. Pathology. 2003;35(4):319–324. doi: 10.1080/0031302031000150515. [DOI] [PubMed] [Google Scholar]
- 71.Ravishankar S, Migliori A, Struminsky J, Has P, Sung CJ, He M. Placental findings in feto-maternal hemorrhage in livebirth and stillbirth. Pathol Res Pract. 2017;213(4):301–304. doi: 10.1016/j.prp.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 72.Redline RW. Extending the spectrum of massive perivillous fibrin deposition (maternal floor infarction) Pediatr Dev Pathol. 2021;24(1):10–11. doi: 10.1177/1093526620964353. [DOI] [PubMed] [Google Scholar]
- 73.Kim EN, Lee JY, Shim JY, Hwang D, Kim KC, Kim SR, Kim CJ. Clinicopathological characteristics of miscarriages featuring placental massive perivillous fibrin deposition. Placenta. 2019;86:45–51. doi: 10.1016/j.placenta.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 74.Romero R, Whitten A, Korzeniewski SJ, Than NG, Chaemsaithong P, Miranda J, Dong Z, Hassan SS, Chaiworapongsa T. Maternal floor infarction/massive perivillous fibrin deposition: a manifestation of maternal antifetal rejection? Am J Reprod Immunol. 2013;70(4):285–98. doi: 10.1111/aji.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heifetz SA, Bauman M. Necrotizing funisitis and herpes simplex infection of placental and decidual tissues: study of four cases. Hum Pathol. 1994;25(7):715–22. doi: 10.1016/0046-8177(94)90306-9. [DOI] [PubMed] [Google Scholar]
- 76.Silingardi E, Santunione AL, Rivasi F, Gasser B, Zago S, Garagnani L. Unexpected intrauterine fetal death in parvovirus B19 fetal infection. Am J Forensic Med Pathol. 2009;30(4):394–7. doi: 10.1097/PAF.0b013e3181c17b2e. [DOI] [PubMed] [Google Scholar]
- 77.Salafia CM, Minior VK, Pezzullo JC, Popek EJ, Rosenkrantz TS, Vintzileos AM. Intrauterine growth restriction in infants of less than thirty-two weeks’ gestation: associated placental pathologic features. Am J Obstet Gynecol. 1995;173(4):1049–57. doi: 10.1016/0002-9378(95)91325-4. [DOI] [PubMed] [Google Scholar]
- 78.Bloomfield RD, Suarez JR, Malangit AC. The placenta: a diagnostic tool in sickle cell disorders. J Natl Med Assoc. 1978;70(2):87–8. [PMC free article] [PubMed] [Google Scholar]
- 79.Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol. 2000;157(6):2111–22. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lau J, Magee F, Qiu Z, Hoube J, Von Dadelszen P, Lee SK. Chorioamnionitis with a fetal inflammatory response is associated with higher neonatal mortality, morbidity, and resource use than chorioamnionitis displaying a maternal inflammatory response only. Am J Obstet Gynecol. 2005;193(3 Pt 1):708–13. Epub 2005/09/10. 10.1016/j.ajog.2005.01.017. PubMed PMID: 16150264. [DOI] [PubMed]
- 81.Parzen E. On estimation of a probability density function and mode. Ann Math Stat. 1962;33(3):1065–1076. doi: 10.1214/aoms/1177704472. [DOI] [Google Scholar]
- 82.Davis RA, Lii K-S, Politis DN. Remarks on some nonparametric estimates of a density function. In: Davis RA, Lii K-S, Politis DN, editors. Selected Works of Murray Rosenblatt. New York: Springer New York; 2011. pp. 95–100. [Google Scholar]
- 83.Anderson MJ. Permutation tests for univariate or multivariate analysis of variance and regression. Can J Fish Aquat Sci. 2001;58(3):626–639. doi: 10.1139/f01-004. [DOI] [Google Scholar]
- 84.Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, Blondel M, Prettenhofer P, Weiss R, Dubourg V, Vanderplas J, Passos A, Cournapeau D, Brucher M, Perrot M, Duchesnay E. Scikit-learn: Machine learning in Python. J Mach Learn Res. 2011;12:2825–2830. [Google Scholar]
- 85.Hunter JD. Matplotlib: A 2D graphics environment. Comput Sci Eng. 2007;9(3):90–95. doi: 10.1109/MCSE.2007.55. [DOI] [Google Scholar]
- 86.Committee Opinion No 700: Methods for estimating the due date. Obstet Gynecol. 2017;129(5):e150-e154. 10.1097/AOG.0000000000002046 [DOI] [PubMed]
- 87.Man J, Hutchinson JC, Heazell AE, Ashworth M, Jeffrey I, Sebire NJ. Stillbirth and intrauterine fetal death: role of routine histopathological placental findings to determine cause of death. Ultrasound Obstet Gynecol. 2016;48(5):579–84. doi: 10.1002/uog.16019. [DOI] [PubMed] [Google Scholar]
- 88.Page JM, Christiansen-Lindquist L, Thorsten V, Parker CB, Reddy UM, Dudley DJ, Saade GR, Coustan D, Rowland Hogue CJ, Conway D, Bukowski R, Pinar H, Heuser CC, Gibbins KJ, Goldenberg RL, Silver RM. Diagnostic tests for evaluation of stillbirth: results from the stillbirth collaborative research network. Obstet Gynecol. 2017;129(4):699–706. doi: 10.1097/AOG.0000000000001937. [DOI] [PubMed] [Google Scholar]
- 89.Ananthan A, Nanavati R, Sathe P, Balasubramanian H. Placental Findings in singleton stillbirths: a case-control study. J Trop Pediatr. 2019;65(1):21–8. doi: 10.1093/tropej/fmy006. [DOI] [PubMed] [Google Scholar]
- 90.Blythe C, Vazquez REZ, Cabrera MS, Zekic Tomas S, Oc Anumba D, Cohen MC. Results of full postmortem examination in a cohort of clinically unexplained stillbirths: undetected fetal growth restriction and placental insufficiency are prevalent findings. J Perinatol. 2019;39(9):1196–203. doi: 10.1038/s41372-019-0412-z. [DOI] [PubMed] [Google Scholar]
- 91.Graham N, Stephens L, Johnstone ED, Heazell AEP. Can information regarding the index stillbirth determine risk of adverse outcome in a subsequent pregnancy? findings from a single centre cohort study. Acta Obstet Gynecol Scand. 2020 doi: 10.1111/aogs.14076. [DOI] [PubMed] [Google Scholar]
- 92.Heazell AE, Worton SA, Higgins LE, Ingram E, Johnstone ED, Jones RL, Sibley CP. IFPA Gabor than award lecture: recognition of placental failure is key to saving babies' lives. Placenta. 2015;36(Suppl 1):S20–8. doi: 10.1016/j.placenta.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 93.Page JM, Thorsten V, Reddy UM, Dudley DJ, Hogue CJR, Saade GR, Pinar H, Parker CB, Conway D, Stoll BJ, Coustan D, Bukowski R, Varner MW, Goldenberg RL, Gibbins K, Silver RM. Potentially preventable stillbirth in a diverse U.S. cohort. Obstet Gynecol. 2018;131(2):336–43. doi: 10.1097/AOG.0000000000002421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Korteweg FJ, Gordijn SJ, Timmer A, Erwich JJ, Bergman KA, Bouman K, Ravise JM, Heringa MP, Holm JP. The Tulip classification of perinatal mortality: introduction and multidisciplinary inter-rater agreement. BJOG. 2006;113(4):393–401. doi: 10.1111/j.1471-0528.2006.00881.x. [DOI] [PubMed] [Google Scholar]
- 95.MacDorman MF, Reddy UM, Silver RM. Trends in stillbirth by gestational age in the United States, 2006–2012. Obstet Gynecol. 2015;126(6):1146–1150. doi: 10.1097/AOG.0000000000001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ray JG, Urquia ML. Risk of stillbirth at extremes of birth weight between 20 to 41 weeks gestation. J Perinatol. 2012;32(11):829–836. doi: 10.1038/jp.2012.60. [DOI] [PubMed] [Google Scholar]
- 97.Management of Stillbirth: Obstetric Care Consensus No, 10 Summary. Obstet Gynecol. 2020;135(3):747–51. 10.1097/AOG.0000000000003720. [DOI] [PubMed]
- 98.Arleo EK, Troiano RN, da Silva R, Greenbaum D, Kliman HJ. Utilizing two-dimensional ultrasound to develop normative curves for estimated placental volume. Am J Perinatol. 2014;31(8):683–8. doi: 10.1055/s-0033-1357265. [DOI] [PubMed] [Google Scholar]
- 99.Isakov KMM, Emerson JW, Campbell KH, Galerneau F, Anders AM, Lee YK, Subramanyam P, Roberts AE, Kliman HJ. Estimated placental volume and gestational age. Am J Perinatol. 2018;35(8):748–57. doi: 10.1055/s-0037-1615285. [DOI] [PubMed] [Google Scholar]
- 100.Azpurua H, Funai EF, Coraluzzi LM, Doherty LF, Sasson IE, Kliman M, Kliman HJ. Determination of placental weight using two-dimensional sonography and volumetric mathematic modeling. Am J Perinatol. 2010;27(2):151–5. doi: 10.1055/s-0029-1234034. [DOI] [PubMed] [Google Scholar]
- 101.Management of Stillbirth: Obstetric Care Consensus No, 10. Obstet Gynecol. 2020;135(3):e110–e32. 10.1097/AOG.0000000000003719. [DOI] [PubMed]
- 102.Son SL, Allshouse AA, Page JM, Debbink MP, Pinar H, Reddy U, Gibbins KJ, Stoll BJ, Parker CB, Dudley DJ, Varner MW, Silver RM. Stillbirth and fetal anomalies: secondary analysis of a case-control study. BJOG. 2021;128(2):252–8. doi: 10.1111/1471-0528.16517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yates CL, Monaghan KG, Copenheaver D, Retterer K, Scuffins J, Kucera CR, Friedman B, Richard G, Juusola J. Whole-exome sequencing on deceased fetuses with ultrasound anomalies: expanding our knowledge of genetic disease during fetal development. Genet Med. 2017;19(10):1171–1178. doi: 10.1038/gim.2017.31. [DOI] [PubMed] [Google Scholar]
- 104.Sahlin E, Gustavsson P, Lieden A, Papadogiannakis N, Bjareborn L, Pettersson K, Nordenskjold M, Iwarsson E. Molecular and cytogenetic analysis in stillbirth: results from 481 consecutive cases. Fetal Diagn Ther. 2014;36(4):326–332. doi: 10.1159/000361017. [DOI] [PubMed] [Google Scholar]
- 105.Martinez-Portilla RJ, Pauta M, Hawkins-Villarreal A, Rial-Crestelo M, Paz YMF, Madrigal I, Figueras F, Borrell A. Added value of chromosomal microarray analysis over conventional karyotyping in stillbirth work-up: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2019;53(5):590–597. doi: 10.1002/uog.20198. [DOI] [PubMed] [Google Scholar]
- 106.Zhao C, Chai H, Zhou Q, Wen J, Reddy UM, Kastury R, Jiang Y, Mak W, Bale AE, Zhang H, Li P. Exome sequencing analysis on products of conception: a cohort study to evaluate clinical utility and genetic etiology for pregnancy loss. Genet Med. 2020;26:26. doi: 10.1038/s41436-020-01008-6. [DOI] [PubMed] [Google Scholar]
- 107.Wilkins-Haug L. Genetic innovations and our understanding of stillbirth. Hum Genet. 2020;139(9):1161–1172. doi: 10.1007/s00439-020-02146-2. [DOI] [PubMed] [Google Scholar]
- 108.Shehab O, Tester DJ, Ackerman NC, Cowchock FS, Ackerman MJ. Whole genome sequencing identifies etiology of recurrent male intrauterine fetal death. Prenat Diagn. 2017;37(10):1040–1045. doi: 10.1002/pd.5142. [DOI] [PubMed] [Google Scholar]
- 109.Stanley KE, Giordano J, Thorsten V, Buchovecky C, Thomas A, Ganapathi M, Liao J, Dharmadhikari AV, Revah-Politi A, Ernst M, Lippa N, Holmes H, Povysil G, Hostyk J, Parker CB, Goldenberg R, Saade GR, Dudley DJ, Pinar H, Hogue C, Reddy UM, Silver RM, Aggarwal V, Allen AS, Wapner RJ, Goldstein DB. Causal genetic variants in stillbirth. N Engl J Med. 2020;383(12):1107–16. doi: 10.1056/NEJMoa1908753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Perez-Garcia V, Fineberg E, Wilson R, Murray A, Mazzeo CI, Tudor C, Sienerth A, White JK, Tuck E, Ryder EJ, Gleeson D, Siragher E, Wardle-Jones H, Staudt N, Wali N, Collins J, Geyer S, Busch-Nentwich EM, Galli A, Smith JC, Robertson E, Adams DJ, Weninger WJ, Mohun T, Hemberger M. Placentation defects are highly prevalent in embryonic lethal mouse mutants. Nature. 2018;555(7697):463–8. doi: 10.1038/nature26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF., 3rd Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118(4):1567–82. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- 112.Zhao X, Radford BN, Ungrin M, Dean W, Hemberger M. The trophoblast compartment helps maintain embryonic pluripotency and delays differentiation towards cardiomyocytes. Int J Mol Sci. 2023;24(15). 10.3390/ijms241512423. [DOI] [PMC free article] [PubMed]
- 113.Radford BN, Zhao X, Glazer T, Eaton M, Blackwell D, Mohammad S, Lo Vercio LD, Devine J, Shalom-Barak T, Hallgrimsson B, Cross JC, Sucov HM, Barak Y, Dean W, Hemberger M. Defects in placental syncytiotrophoblast cells are a common cause of developmental heart disease. Nat Commun. 2023;14(1):1174. doi: 10.1038/s41467-023-36740-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huppertz B. The anatomy of the normal placenta. J Clin Pathol. 2008;61(12):1296–302. doi: 10.1136/jcp.2008.055277. [DOI] [PubMed] [Google Scholar]
- 115.Gude NM, Roberts CT, Kalionis B, King RG. Growth and function of the normal human placenta. Thromb Res. 2004;114(5–6):397–407. doi: 10.1016/j.thromres.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 116.Hoyert DL, Gregory EC. Cause of fetal death: data from the fetal death report, 2014. Natl Vital Stat Rep. 2016;65(7):1–25. [PubMed] [Google Scholar]
- 117.The Stillbirth Collaborative Research Network Writing Group. Causes of death among stillbirths. JAMA. 2011;306(22):2459–68. 10.1001/jama.2011.1823 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying the results presented in the study are available from the Dryad Digital Repository database (https://doi.org/10.5061/dryad.3xsj3txks).
Not applicable.