Abstract
Melioidosis, caused by the gram-negative saprophyte Burkholderia pseudomallei, is a disease of public health importance in southeast Asia and northern Australia that is associated with high case-fatality rates in animals and humans. It has the potential for epidemic spread to areas where it is not endemic, and sporadic case reports elsewhere in the world suggest that as-yet-unrecognized foci of infection may exist. Environmental determinants of this infection, apart from a close association with rainfall, are yet to be elucidated. The sequencing of the genome of a strain of B. pseudomallei has recently been completed and will help in the further identification of virulence factors. The presence of specific risk factors for infection, such as diabetes, suggests that functional neutrophil defects are important in the pathogenesis of melioidosis; other studies have defined virulence factors (including a type III secretion system) that allow evasion of killing mechanisms by phagocytes. There is a possible role for cell-mediated immunity, but repeated environmental exposure does not elicit protective humoral or cellular immunity. A vaccine is under development, but economic constraints may make vaccination an unrealistic option for many regions of endemicity. Disease manifestations are protean, and no inexpensive, practical, and accurate rapid diagnostic tests are commercially available; diagnosis relies on culture of the organism. Despite the introduction of ceftazidime- and carbapenem-based intravenous treatments, melioidosis is still associated with a significant mortality attributable to severe sepsis and its complications. A long course of oral eradication therapy is required to prevent relapse. Studies exploring the role of preventative measures, earlier clinical identification, and better management of severe sepsis are required to reduce the burden of this disease.
INTRODUCTION
Background and History
The pathologist Alfred Whitmore and his assistant C. S. Krishnaswami first described melioidosis as a “glanders-like” disease among morphia addicts in Rangoon, Burma, in 1911 (474, 475). They recognized a new organism that fulfilled Koch's postulates for causation of disease. This bacterium, which could be isolated from autopsy specimens on peptone agar and potato slopes, could be distinguished from the organism causing glanders by its relatively rapid growth, its motility, and the lack of the Strauss reaction when it was injected into guinea pigs. Based on these characteristics, “… sufficiently peculiar to distinguish it from all pathogenic bacteria previously known to us” (475), they correctly surmised that this new bacterium was closely related to that which caused glanders, a finding that has only recently been confirmed by molecular studies (170, 197, 313).
This disease, now termed melioidosis, was named from the Greek “melis” (distemper of asses) and “eidos” (resemblance) by Stanton and Fletcher in 1932 (405). During the last century this gram-negative environmental bacterium has been variously known as Bacillus pseudomallei, Bacillus whitmorii (or Bacille de Whitmore), Malleomyces pseudomallei, Pseudomonas pseudomallei, and, since 1992, Burkholderia pseudomallei (496).
In the latter half of the 20th century, melioidosis emerged as an infectious disease of major public health importance in southeast Asia and northern Australia. In Ubon Ratchathani, Thailand, B. pseudomallei accounts for up to approximately 20% of community-acquired bacteremias (74). At the Royal Darwin Hospital, Australia, it has been the most common cause of fatal community-acquired bacteremic pneumonia (112, 147).
Largely due to clinical trials in Thailand, significant improvements have been made in defining the optimal antibiotic therapy for melioidosis. However, the choice of antibiotic regimen has not been shown to have an impact on mortality within the first 48 h of admission (473), and severe melioidosis in Thailand is still associated with a case fatality rate of approximately 50% (472). In Australia, the mortality rate is still significant and approaches 20% among all patients with melioidosis (111).
EPIDEMIOLOGY
Melioidosis is regarded as endemic to southeast Asia and northern Australia, corresponding approximately to the tropical latitudes between 20°N and 20°S. The worldwide epidemiology of melioidosis has been comprehensively reviewed by David Dance (118, 130); data from those and other, more recent reports are summarized in Table 1 and Fig. 1.
TABLE 1.
Worldwide distribution of melioidosis based on reported cases
| Level of evidence | Country (reference[s]) |
|---|---|
| Endemic; multiple case series described | Northern Australia (24, 111), Thailand (74, 267), Singapore (191, 272, 424), Malaysia (345), Burma (475), Vietnam (97, 204) |
| Possibly endemic; multiple cases, significant numbers of | Southern China (499, 501), Hong Kong Special Administrative Region (170, |
| exported cases | 397, 398), Brunei (130), PDR Laos (329; P. Newton, personal communication), Cambodia (9, 68), Taiwan (40, 205, 262-264) |
| Epidemic; limited outbreak | Aruba (Netherland Antilles) (421), France (130), Brisbane River Valley (Queensland, Australia) (236, 374) |
| Sporadic case reports | Asia |
| India (43, 44, 122, 224, 231, 348), Indonesia (39, 120, 341, 370), Bangladesh (122, 201, 239, 300, 414), Japan (16), Philippines (120), Pakistan (122), Sri Lanka (452) | |
| Americas and Caribbean | |
| Guadeloupe (327), Martinique (320), Puerto Rico (94, 146), Equador (45), Panama (296), El Salvador (45, 130), Haiti (130), Brazil (301, 378), Costa Rica and Colombia (O. Dance, personal communication), Venezuela (M. C. Redondo, Abstr. 11th Int. Conf. Infect. Dis. abstr. 58.026, 2004) | |
| Pacific | |
| Guam (302), Fiji (100; B. J. Currie and M. Lowe, unpublished data), Papua New Guinea (112, 132, 240, 362, 469), New Caledonia (S. Hello, personal communication) | |
| Africa and Middle East | |
| Iran (131, 339), Uganda (130), Sierra Leone (130), The Gambia (130), Madagascar (130), Kenya (49) | |
| Unconfirmed identification, uncertain travel history, or | Asia-Pacific |
| serological evidence only | Korea (130), Hawaii (323), East Timor (P. Armstrong, personal communication) |
| Europe | |
| Spain (130), Germany (130) | |
| Africa and Middle East | |
| United Arab Emirates (118), Saudi Arabia (118), Egypt (130), South Africa (451), Turkey (130), Egypt (130), Niger (153), Burkina Faso (Upper Volta) (130) | |
| Americas | |
| Mexico (35), United States (38, 150, 164, 228, 295) | |
| Isolates from environment only | Italy (504), Peru (130), Côte d'Ivoire (130), Reunion Island (130), Haiti (130) |
FIG. 1.
Worldwide distribution of melioidosis.
Writing over 10 years ago, Dance (130) noted that published case reports and series are likely to represent only the “tip of the iceberg,” as culture facilities are not available in most of the rural tropics where the infection is likely to be prevalent. This is also evident in the apparent changing epidemiology of the infection; despite Krishnaswami documenting melioidosis in 5% of all autopsy deaths in 1917, the only reported cases from Burma since 1945 have been in travelers (205, 268, 472). Similarly, Thailand and Australia, where the highest rates of disease are currently noted, did not record cases until 1947 and 1950, respectively (130, 358).
Other anomalies that may be related to incomplete ascertainment include a high serological prevalence (7%) of melioidosis in returning American troops stationed in Vietnam (97) but a low rate of disease in the indigenous population (324, 453). In addition, the high prevalence of melioidosis in the Issan region of northeastern Thailand contrasts with the low prevalence in the People's Democratic Republic of Laos (PDR Laos) to the east of the Mekong River and in Cambodia further south (9, 68, 329).
A caveat to this paradox is the uncertainty associated with the seropositivity rates in southeast Asia, which may represent exposure to the less pathogenic Burkholderia thailandensis (472). The worldwide distribution of B. thailandensis is yet to be clearly defined, but it is clear that it comprises the most common soil isolates in northeast Thailand (437); it has not been found in Australia (108).
Melioidosis in the Australia-Pacific Region
Melioidosis was first recognized within Australia from an outbreak in sheep in 1949 in Winton, northern Queensland (101). The first human case described was in a diabetic who died from septicemic melioidosis in Townsville in 1950 (358), and the first case reported from the Northern Territory occurred in 1960 (102). This apparently late emergence of such an important infectious disease in northern Australia led to suggestions that B. pseudomallei may have colonized Australia from southeast Asia (157), although the molecular diversity of isolates contrasts with foci in areas where the organism is not endemic (107).
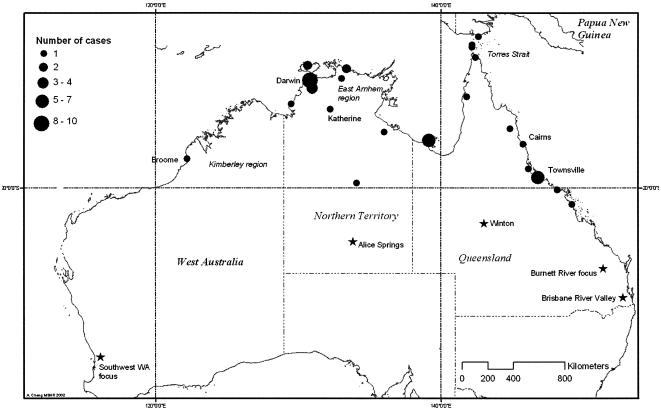
Although the area where melioidosis is endemic has generally been regarded as restricted to the latitudes 20oS and 20oN in southeast Asia and northern Asia (266), large outbreaks have occurred outside this area in Australia, including the first case in Winton (22oS) and 159 cases of melioidosis in pigs over 3 years in the Burnett River region (25.5oS), which were attributed to a contaminated water supply (237). Autochthonous cases have also occurred outside this area in southwest Western Australia (107, 171), the Brisbane River Valley in Queensland (27oS) (236, 374), Alice Springs, and Mackay. The distribution of endemic cases in 2001 and 2002 and previously reported foci outside of the area of endemicity is shown in Fig. 2.
FIG. 2.
Regional variation in incidence rate in northern Australia based on data for notifiable diseases in 2001 and 2002 (80). Previous autochthonous cases outside the area of endemicity are indicated by stars.
Epidemiological studies have defined an annual incidence rate in the Top End of the Northern Territory as 16.5 per 100,000 between 1989 and 1999 (112), with rates as high as 41.7 per 100,000 in 1998, which were associated with two severe weather events and high annual rainfall (114). There have been few other population-based rates described previously for Australia, but in a geographically restricted area within the Torres Strait in northern Queensland in the 2000 to 2002 seasons (151), a rate of 40 cases per 100,000 was documented. In contrast to many other countries where the disease is endemic, in Australia most patients are from remote locations but are transported to referral hospitals in the Top End region of the Northern Territory, the Kimberley region of Western Australia, and far north Queensland and the Torres Strait for management.
Environmental sampling has revealed widespread isolation of samples from soil, mud, and pooled surface water in northern Australia, including Queensland (427), around Darwin (58, 299), and remote communities in the Northern Territory and Western Australia (107, 215). Two outbreaks have been linked to contamination of the drinking water supply, where disease control measures, such as cleaning of the water supply and pipes, led to a cessation of the outbreaks (115, 212, 213).
Although serological tests have been demonstrated to have poor sensitivity and specificity in clinical situations, seroprevalance is likely to reflect background exposure to B. pseudomallei on a population basis. Serosurveys of populations in northern Australia have demonstrated relatively low rates of seropositivity compared to the rates seen in northeastern Thailand. This was reflected in a study in Queensland, where seropositivity in urban populations (up to 5%) was lower than that in patients residing in rural locations or in patients of Aboriginal or South Pacific origin (up to 10%) (24), which were similar to those found in the primarily indigenous population of Arnhem Land in the Northern Territory (12.8%) (112). These seroprevalences contrast with the much higher rates in immigrants from southeast Asia to Queensland (29%) (24).
At least six cases of melioidosis have been reported from Port Moresby in Papua New Guinea (104, 132, 260, 362), as has one additional case in an ex-serviceman living in Brisbane (240), for whom the place of exposure was not clear. Small serological surveys in the Port Moresby region have not demonstrated antibodies to B. pseudomallei (33, 362). However, a series of clinical cases in Balimo, Western Province, has led to the suggestion that melioidosis may occur elsewhere in the country (112, 469).
Distribution of Melioidosis in Asia
Thailand.
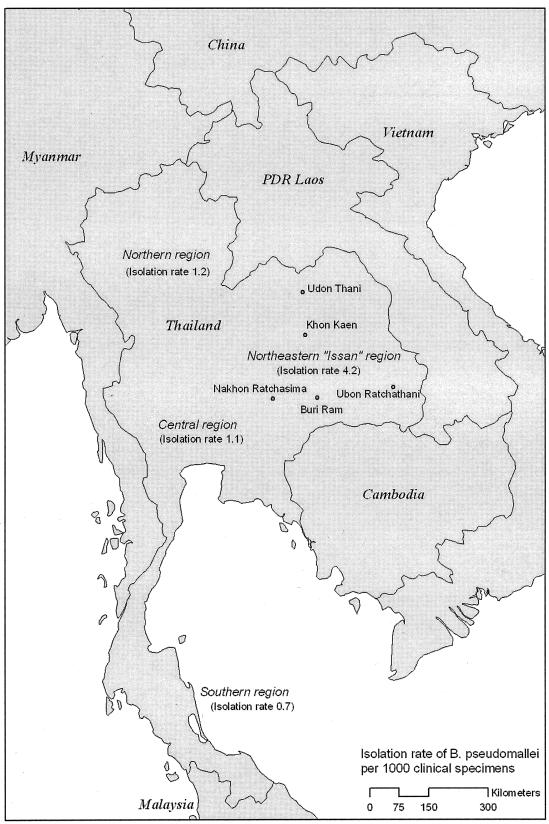
The high rates documented in northern Australia compare to the annual incidence of 4.4 cases per 100,000 in Ubon Ratchathani province in northeast Thailand (417). Other centers in northeast Thailand, such as Khon Kaen, Nakhon Ratchasima, Buri Ram, and Udon Thani, also see large numbers of patients. In a national survey, 30 of the 125 hospitals did not have microbiological facilities, and during 1994 to 1995, the annual number of isolates was over 1,100; this probably represents a conservative estimate of the number of cases of melioidosis in Thailand (267). Results from this survey and the locations of hospitals reporting large numbers of cases in Thailand are shown in Fig. 3.
FIG. 3.
Regional variation in rates of isolation of B. pseudomallei from clinical specimens (per 1,000 specimens) in Thai hospitals based on a hospital survey in 1995 (267). Provinces with highest numbers of isolates are highlighted.
In Thailand, B. pseudomallei is widely distributed in soil and, more particularly, in pooled surface water such as in rice paddies (154, 306, 492). However, the rate of the closely related but less virulent B. thailandensis, which had previously been recognized as B. pseudomallei, may account for the variation in disease throughout the country (437); the ratio of B. pseudomallei to B. thailandensis found in soil, which is highest in the northeast, matches rates of clinical B. pseudomallei isolation throughout Thailand (267, 393).
These findings and the possibility of the existence of other, less virulent strains of Burkholderia spp. may also account for the much higher rates of seropositivity seen in Thailand (230), compared to the areas of endemicity of northern Australia (24).
PDR Laos.
Despite the fact that the highest rates in Thailand have been documented in the northeast Issan region, relatively few cases have been reported in adjacent Laos (329). Mahosot Hospital in Vientiane recognized a handful of cases each year, constituting 2% of blood culture isolates at this referral center between 2000 and 2002 (P. Newton, personal communication), despite recovery of B. pseudomallei from soil isolates in the Vientiane region (494a). Outside of Vientiane, microbiological facilities are limited and the epidemiology is undefined.
Vietnam.
Melioidosis was first noted in what is now southern Vietnam by Pons and Advier (336), and the first descriptions of the saprophytic niche of B. pseudomallei were made by Vaucel in Hanoi and Chambon in Saigon (now Ho Chi Minh City) (67, 130). With the large numbers of French and later American troops based in Vietnam, with exposure to environmental B. pseudomallei and access to modern clinical and laboratory services, large numbers of cases were described from the 1940s to the 1970s (130). Cases have continued to be observed in returning servicemen for up to 29 years following exposure (93, 173, 285), as have sporadic cases in Vietnamese emigrants and returned travelers to other countries (192, 489).
However, recent attempts at systematic surveillance have not found significant proportions of B. pseudomallei in blood culture isolates or soil around Ho Chi Minh City (324), although it is likely to be found elsewhere in the country. This may be analogous to the situation in Papua New Guinea, with the patchy distribution of B. pseudomallei in the environment associated with clinical cases.
Malaysia and Indonesia.
Stanton and Fletcher noted animal cases at the Institute of Medical Research of the Federated Malay States as far back as 1913, and they published reports of these and subsequent human and animal cases in 1932 (404).
Cases from both peninsular and east Malaysia have continued to be described, and most recently, Puthucheary et al. reviewed 50 septicemic cases of melioidosis in 1992 at a single referral center in Kuala Lumpur and noted a total of 85 cases from June 1976 to June 1991 (346). A serosurvey conducted in Malaysia in 1964 to 1966 revealed a 7.3% seropositivity (indirect hemagglutination assay [IHA] titers of >1:40), with the highest rates in recruits from Kedah (peninsular Malaysia) and Sabah (east Malaysia) (412).
Sporadic cases of melioidosis from Indonesia have been reported in the Dutch literature for many years (39, 395), in addition to more recent cases exported to Australia (177) the United Kingdom (120), and the United States (370).
Singapore.
In Singapore, melioidosis has been a notifiable disease since 1989; an annual rate of 1.7 cases of melioidosis was documented between 1989 and 1996, with the majority (89%; 337 cases) being culture-confirmed cases (191). More recently, higher numbers have been noted since 1998, when 104 patients were reported (41). During early 2004, 57 cases have been reported, with an unusually higher case-fatality rate (40%), which were attributed to abnormally heavy rains and flooding (O. P. Lim, Abstr. 4th World Melioidosis Congr., abstr. 3, 2004). The case-fatality rate for patients with severe melioidosis otherwise appears in line with those in more developed countries (69).
Serosurveys have consistently demonstrated a low rate of seropositivity in Singapore (0.2% in the general population and 1.6% in construction workers), except in immigrants from Thailand or Malaysia (191, 272). Isolation of B. pseudomallei from surface water appears to be less common than it was in the 1960s (425) and less common than it is in other countries in the region. Unusually, a seasonal pattern or an association with rainfall has not been noted in Singapore, in contrast to the case for most other series (191).
China, Hong Kong Special Administrative Region, and Taiwan.
Small numbers of cases of locally acquired melioidosis have been observed in Hong Kong (397, 398, 440, 483), and a seroprevalence of 14% was demonstrated by IHA in a tuberculosis sanatorium (396). An ongoing outbreak in marine mammals in an ocean park has been described (193). In Taiwan, a case series and several sporadic cases, mostly autochthonous, have been described (40, 205, 262-264, 283).
On mainland China, B. pseudomallei has been isolated from 4.2% of soil and water specimens in Hainan Island and adjoining coastal provinces as far north as 25°N (500), as confirmed by human cases and seroprevalences (IHA titers of >1:40) of up to 34% in farmers in the region (499, 501). In the seven isolates from culture-positive cases tested, a high rate of ceftazidime resistance (57%) was observed (501).
Other parts of Asia.
Multiple cases have been reported from disparate regions of India but have been largely restricted to a few large medical centers, presumably where identification is possible (87, 223, 225, 231, 292, 348); some of these, including an apparent outbreak of a bubonic plague-like illness attributed to B. pseudomallei (44), have been disputed (43, 121). Cases in travelers returning to Europe from the Indian subcontinent have been reported, suggesting poor ascertainment of cases locally (122, 431). In addition, one serosurvey revealed a seroprevalence of 7% in a rural rice-growing area near Vellore (231).
Although few cases in Sri Lanka have been described (452), a report described a case that was believed to have been acquired in that country (326). Sporadic cases of melioidosis in travelers returning from Bangladesh have also been reported (122, 239, 300), including a series of three patients presenting with septic arthritis following travel to Syhet (201). One case from within the country has been described (414). Although the disease was reported in up to 10% of autopsy deaths in Rangoon, Burma, in the original series, since 1945 the only cases reported were one in a Dutch traveler (268) and a second possible exported case in a Taiwanese traveler (205).
Cases imported into the United Kingdom probably reflect the prevalence of melioidosis within the countries of origin, distorted by the magnitude of immigration from those countries. Human and animal cases have been imported from Bangladesh, Pakistan, India, Indonesia, and the Philippines (120, 122, 205, 370). Serological studies in East Timor suggest exposure to B. pseudomallei, but no culture-confirmed cases have been reported (P. Armstrong, personal communication).
Areas outside Asia
Most cases reported outside southeast Asia are from travelers to areas of endemicity, requiring an awareness of this disease by clinicians worldwide (65, 122, 326, 373, 436, 458). However, sporadic autochthonous cases have been reported throughout the world, including west and east Africa, the Caribbean, Central and South America, and the Middle East (118, 130).
Much debate has focused on the question of indigenous B. pseudomallei in the Americas. In North America, early reported cases were associated with travel (150), had poorly documented travel histories (38, 228), or are disputed (164).
Possibly the most intensely studied organism was the “Oklahoma isolate” from a soil-contaminated wound infection following a farming accident (295), which was identified by the authors as B. pseudomallei but with atypical characteristics that were felt to put this identification in doubt (130). Subsequent molecular studies have been conflicting (495), with phylogenetic analysis placing this isolate in a distinct group apart from both B. thailandensis and B. pseudomallei (170).
Animals and Melioidosis
A wide variety of animal species have been shown to be susceptible to melioidosis, including camels, horses, sheep, cattle, goats, pigs, and kangaroos (156, 237, 252, 426, 429); koalas (253); alpacas (220a); deers, cats, and dogs (277); and captive marine animals (193). Cattle, water buffalo, and crocodiles are considered to be relatively resistant to melioidosis despite their constant exposure to mud (53, 277). Birds are also considered to be relatively resistant to melioidosis (258), although cases have been reported (428, 430).
A variety of animals have been used in experimental models, including inbred mouse strains (456), chickens (457), and rats and guinea pigs (474). Most recently, the susceptible BALB/c and more resistant C57BL/6 inbred mouse strains have been used extensively in studies of host responses to B. pseudomallei (200).
Epizoontic outbreaks have also been reported among imported animals from areas of endemicity. A cluster of infections is sheep, goats, and pigs living on Aruba (Dutch Antilles) was attributed to B. pseudomallei in 1957 (421). An outbreak in a Paris zoo in the 1970s resulted in the spread to other zoos and equestrian clubs throughout France and to the deaths of at least two humans and a number of animals. This outbreak, referred to as “l’affaire du jardin des plantes,” was thought to be due to either the importation of horses from Iran or an infected panda donated by Mao Tse-Tung (130, 472).
BACTERIOLOGY AND PATHOGENESIS
General Bacteriology
B. pseudomallei is visualized as a gram-negative bacillus with bipolar staining and is vacuolated and slender and has rounded ends; it is often described as having a “safety pin” appearance. It is oxidase positive and can be distinguished from the closely related but less pathogenic B. thailandensis by its ability to assimilate arabinose (271, 390). Whitmore distinguished it from Burkholderia mallei by its motility on a hanging drop, but in semisolid media this finding is less reliable (154).
On culture, the organism demonstrates differing colonial morphology, with mostly smooth colonies initially and dry or wrinkled colonies on further incubation. The clinical significance of the various colony types, including small-colony variants, is being prospectively investigated in Thailand (N. Chantratita, personal communication).
Environmental Microbiology and Epidemiology
B. pseudomallei is a resilient organism that is capable of surviving hostile environmental conditions, including prolonged nutrient deficiency (493) (of durations of up to 10 years [V. Wuthiekanun, personal communication]), antiseptic and detergent solutions (161, 277, 401), acidic environments (pH 4.5 for up to 70 days) (133), and a wide temperature range (24o to 32°C) and dehydration (soil water content of <10% for up to 70 days) (77, 434) but not exposure to UV light (434). It is likely that harsh environmental conditions may confer a selective advantage for the growth of B. pseudomallei.
The saprophytic nature of B. pseudomallei was first recognized in 1955 in French Indochina (67). Some early studies implicated the aerosolization of dry dusts as a route of acquisition for American servicemen in Vietnam, based on the high incidence in helicopter crews (204). However, further studies have demonstrated highest yields from moist soils and pooled surface water (306, 413, 492).
The association between surface water and melioidosis is supported by the strong association with monsoonal rains (74, 112, 113, 266) and with occupational and recreational exposure to surface water and mud (74, 112, 266), particularly with flooding of rice paddies and planting at the commencement of the monsoonal season (417). The finding that higher rainfall is significantly associated with sepsis and pneumonia suggests that environmental conditions during the monsoonal season may be associated with inhalation rather than inoculation as the primary mode of acquisition (113).
In particular, moist clay soils seem to be favored by the organism (427), and populations residing on these soil types in Darwin, Australia, have a higher rate of disease (343). Sampling studies in Australia have suggested that bacterial counts increase to a depth of 60 to 90 cm (427, 492), but the finding that dry, shallower soils may be culture negative yet PCR positive has led to the suggestion that the organism may persist in a viable but nonculturable state (58). Although cleared, irrigated areas have been shown to be favored by the organism in Malaysia and Thailand (306, 413, 492), these are not found in the Top End region of Australia; a sampling study of the site of an aborted attempt to grow rice in the Northern Territory at Fogg Dam failed to recover B. pseudomallei (B. Currie, unpublished data).
In a Western Australia outbreak, both B. pseudomallei and the parasite Acanthamoeba sp. were isolated from a potable water source and the environment. Subsequent studies suggested that B. pseudomallei may infect Acanthamoeba trophozoites by coiling phagocytosis, a process described for other pathogens such as Legionella pneumophila (217). The significance of this interaction and those with plant-associated organisms as a reservoir for persistent environmental contamination is being assessed (A. Levy and T. Inglis, personal communication).
Contamination of drinking water supplies, rather than soil, has also been implicated in other outbreaks in Australia (115, 237). Chlorination of the water supply was associated with the termination of one of these outbreaks and appears to be effective against B. pseudomallei in vitro (A. D. Thomas, unpublished data). However, sensitive techniques using flow cytometry do demonstrate the possible presence of viable bacteria in small numbers despite free chlorine concentrations of up to 1,000 ppm (202). The presence of chlorinators does not affect community rates of melioidosis in areas of endemicity (82).
Factors that may influence the distribution of B. pseudomallei in the environment may include physical factors such as rainfall, humidity, UV radiation, and temperature; chemical factors such as soil composition, other vegetation, and the use of fertilizers; and recent soil disturbances such as excavation and plowing (215). The implications of global climate change for the epidemiology of melioidosis are as yet unknown (103).
There has been some interest in the interaction between species in the Burkholderia genus (99, 167). As Burkholderia cepacia can degrade toxic compounds in pesticides and is active against many soilborne pathogens, there has been interest in its use as a crop biological control agent (199). However, numerous insertion sequences within B. cepacia, including for some strains sequences identical to B. pseudomallei insertion sequences (284), and transposable genetic elements in B. pseudomallei (61) have been identified. This justifies concerns that widespread agricultural use of B. cepacia may be a hazard to human health, with the potential for more virulent B. cepacia bacteria to emerge following horizontal transmission of genetic elements (199).
Bacterial Virulence Factors
Like many saprophytic organisms, B. pseudomallei is a resilient bacterium that can survive in a variety of hostile conditions, including nutrient deficiency, acid and alkali pH, disinfectant and antiseptic solutions (including detergents and chlorine), exposure to many antibiotics, and extremes of temperature. B. pseudomallei is also well adapted to its many hosts, producing proteases, lipases, lechithinase, catalase, perioxidase, superoxide dismutase, hemolysins, a cytotoxic exolipid, and a siderophore. It is resistant to complement lysosomal defensins and cationic peptidases and can survive within many eukaryotic cell lines, including professional phagocytes such as neutrophils and macrophages (472).
B. pseudomallei produces a glycocalyx polysaccharide capsule that is probably an important virulence determinant (407). This capsule (biofilm, or “slime”) allows for the formation of microcolonies in a protective environment in which the organism is phenotypically altered, resulting in significant antibiotic resistance (461). In other bacteria, such as B. cepacia, it is believed that biofilm formation is stimulated by bacterial quorum-sensing mediators such as N-acylhomoserine lactones (468); early work has defined putative signaling pathways in B. pseudomallei that may be virulence factors (E. Valede and Y. Song, Abstr. 4th World Melioidosis Congr., abstr 30 and 31, 2004).
Altered phenotypes such as slow-growing small-colony variants can be observed on primary plates from clinical specimens (V. Wuthiekanun, personal communication) or can be induced by passaging in vivo or in vitro and are also associated with significant antibiotic resistance. These variants may subsequently revert spontaneously to their normal morphology and antibiotic susceptibility (188). The significance of other mutant forms of the organism, such as cell wall-deficient L-forms that can be induced only in vitro by passage through rabbit alveolar cells (241), remains uncertain. This suggests that unusual mechanisms may mediate the survival of B. pseudomallei within the body, such as the “globi” observed within macrophages and giant cells in autopsy specimens (477).
Ultrastructural studies using electron microscopy have shown multiplication within the vacuoles of phagocytes following internalization and subsequent endosome lysis (185). When Acanthamoeba trophozoites are infected with B. pseudomallei, bacterial escape from vacuoles is mediated by coiling phagocytosis, a process described for other pathogens such as L. pneumophila, as discussed above (217). Movement of the bacterium towards one pole of the cell occurs by means of continuous actin polymerization into a “comet-tail” formation similar to that observed with other pathogens such as Listeria (48, 234, 410). Direct cell-to-cell spread is thought to occur by the induction of these cellular protrusions and fusion of cell membranes to form multinucleated giant cells (184, 234, 410), which have also been observed in human tissue (477).
Secretory antigens.
The role of secreted antigens is unclear. Many, including proteases (261, 376), phospholipase C (246), and hemolysin, lecithinase, and lipase (26) are probably secreted via the general secretory pathway (type II secretion system) (137). Transposon mutations in the general secretory pathway, resulting in a failure to secrete protease, lipase, or lecithinase, do not appear to result in an attenuation of virulence in an animal model (53). However, the finding that the relationship between the density of bacteremia and mortality is similar in melioidosis and other gram-negative bacteremias suggests that exotoxins do not play a significant role in determining outcome (472).
However, a number of type III secretion systems (TTSS) have been described (349). TTSS in other organisms, such as Salmonella enterica, are activated under specific conditions to allow delivery of effector molecules to host cells in order to facilitate invasion and survival in phagosomes (162, 503). This presumed function in B. pseudomallei is supported by the finding of homology between the SPI-1 pathogenicity island of Salmonella enterica (Inv/Spa/Prg) and TTSS3 of B. pseudomallei (349, 408, 410). Bacterial products secreted by this TTSS (termed Bsa, or Burkholderia secretion apparatus), including BopE, are thought to result in cytoskeletal rearrangements facilitating host cell invasion (408). B. pseudomallei organisms with mutations in the Bsa and BopE system are also confined to the endosome and are unable to gain access to cell actin, suggesting that this system is also important in mediating endosomal membrane lysis (410).
In addition, less virulent B. thailandensis strains do not contain some TTSS (349), and mutations involving the TTSS3 system (as opposed to TTSS1 and TTSS2) attenuate virulence in a hamster model (467). However, B. pseudomallei strains with mutations involving known putative TTSS3 effectors are not significantly attenuated (409, 467). Microarray studies have determined that growth of B. thailandensis in the presence of arabinose results in downregulation of TTSS3 via the putative positive regulator bsaN, suggesting that the loss of the ability to assimilate arabinose is linked to the increased virulence of B. pseudomallei in humans and animals (304).
Cell-associated antigens.
A number of cell-associated antigens have been demonstrated to be immunogenic in patients with melioidosis, including capsular polysaccharide (CPS), lipopolysaccharide (LPS) (formerly O-PS II) (195), and flagellin proteins (75, 138, 290, 328).
Antibodies to LPS have been shown to be protective against severe disease in humans (75) and in animals (63). The important role of LPS is supported by studies examining laboratory-inducted mutations in the gene coding for LPS, which demonstrate a susceptibility to alternative complement pathway (139, 486) and an attenuation in virulence in a mouse diabetes model (139, 487).
Capsular polysaccharide appears to have a role in environmental protection (229), immune system evasion (347), and attachment to epithelial cells (3). Capsular polysaccharide appears to provide protection within the phagosomal environment (342, 389), and strains with mutations in this antigen are less virulent than wild-type strains (28). In addition, passive immunization against an exopolysaccharide provided protection against high-dose challenge in a mouse model (227).
Antigenic differences in CPS or other surface proteins may account for the lack of epithelial attachment and pathogenicity of B. thailandensis (233, 356). The ability of B. pseudomallei to attach to and invade epithelial cell lines appears to be growth phase and temperature dependent; the mechanisms underlying this in vitro phenomenon and its clinical relevance are yet to be determined (60). A number of genes for different CPSs, termed CPS I to IV, have recently been described. CPS I (previously thought to represent a component of LPS and known as O-PS I) is found only in B. pseudomallei and is a virulence determinant; CPS II is downregulated in vivo and is thought to be involved in environmental survival (S. Reckseilder-Zenteno, Abstr. 4th World Melioidosis Congr., abstr. 37, 2004).
Outer membrane proteins, such as a protein tyrosine phosphatase, have been defined (245). Although bacteria do not contain the substrate tyrosine phosphate, analogous enzymes in other bacteria such as Yersinia have alternative substrates that are believed to be important in signal transduction (229). However, acid phosphatases do not appear to be a major virulence determinant, as strains with mutations of acpA, resulting in loss of phosphatase activity, retain their virulence (64). Other outer membrane proteins, with molecular masses of 70, 38, 31, 24, and 17 kDa, have also been identified and used in diagnostic tests; the 38-kDa peptidoglycan-associated protein appears to form aggregates and to function as a porin (174, 388).
Other cell-associated antigens include type I pili (encoded by fimA, fimC, and fimD) and a putative type IV pilus complex, and other antigens with strong homology to the pilB, pilC, and pilD products of Pseudomonas aeruginosa have been defined. Flagellin proteins may be important in pathogenesis; flagellin-specific antiserum passively protected diabetic infant rats against B. pseudomallei challenge (52). However, conflicting results have been reported with nonmotile fliC mutants which were less virulent in BALB/c mice (96) but not in Syrian hamsters or diabetic mice (138).
A largely unexplored area has been the role of iron metabolism in determining virulence. It is known that a siderophore, malleobactin, is elaborated by B. pseudomallei and is efficient at acquiring iron at acidic pH (498). This siderophore is regulated by the fur gene, which also regulates superoxide dismutase and peroxidase (275). In other organisms, such as Vibrio parahaemolyticus, iron-restricted conditions result in the induction of siderophore production, which parallels increased virulence (117). The virulence of B. pseudomallei in a mouse model appears to be attenuated by iron-enriched media (441). Although clinical conditions with iron overload, such as hemosiderosis and thalassemia, appear to be associated with increased rates of melioidosis (286, 363), like infections with Vibrio and Salmonella spp, this may suggest that iron plays a more important role in pathogenesis than has been recognized.
It is important to note that these putative virulence factors are mechanisms developed (in evolutionary terms) by the organism to survive in its as-yet-unidentified ecological niche(s). They also happen to allow the bacterium to avoid the host immune responses of humans and animals. However, infection of these hosts is accidental and is not likely to provide an evolutionary advantage for an otherwise environmental organism. This fact is reflected in the poor characterization of bacterial products as being truly virulent in animal studies and in the organism's primary affinity for hosts with impaired immunity. In addition B. pseudomallei generally has low disease-causing potential in healthy hosts despite its ubiquity in the environment. This stands in contrast to other organisms whose ecological niche is in humans and animals, such as Staphylococcus aureus, which can affect otherwise healthy individuals and where virulence factors such as Panton-Valentine leukocidin correlate with severe disseminated disease.
Molecular Epidemiology
A variety of molecular tools have been used to infer genetic relatedness between isolates of B. pseudomallei. These have included pulsed-field gel electrophoresis (PFGE), random amplified polymorphic DNA (RAPD) analysis, and ribotyping. These studies have demonstrated that environmental isolates can be identical to epidemiologically related human or animal strains (107, 115, 179, 180), that recurrent infection is usually due to relapse with the same strain rather than reinfection with a different strain (109, 140, 448), and that outbreaks of infection may be clonal (107, 115).
These and other studies have demonstrated considerable diversity in isolates (333). This suggests that introduction of B. pseudomallei into these regions is not a recent event, in contrast to clonal outbreaks in regions where the organism is not endemic (107). Comparisons of typing methods demonstrate that RAPD analysis and PFGE are more discriminatory than ribotyping (180, 216, 449). Multilocus sequence typing appears to have a discriminatory ability similar to that of PFGE (79, 170).
The finding that the antigenically similar B. thailandensis was relatively avirulent led to a suggestion that different strains of B. pseudomallei may have differing virulence. Molecular typing of Australian isolates by PFGE and ribotyping have shown three clusters, termed A, B, and C (107, 180, 441). There was stability in the levels of virulence within two of these clone types, but many isolates in this study did not fall into defined groups (441).
Two other studies have suggested that clinical presentation or outcome may depend on the strain type. Certain ribotypes appeared to be associated with a higher mortality or risk of relapse in one study (333). A second small study (n = 18) using multilocus enzyme electrophoresis and RAPD analysis suggested that soft tissue infections were restricted to one cluster and respiratory and neurological infections were seen in another cluster (317).
More recently, a sequence-based successor to multilocus enzyme electrophoresis, multilocus sequence typing, has been developed for B. pseudomallei. This technique, based on allelic differences present at seven housekeeping genes, is ideal for genetic analysis beyond the outbreak situation due to its reproducibility and the low rate of genetic change in the allelic sites and allows for comparisons between strains typed at different laboratories though an internet-based database (http://www.mlst.net/). This study confirmed the diversity seen in isolates worldwide and placed B. mallei within this wider B. pseudomallei group, both being distinct from B. thailandensis (170). Furthermore, Australian isolates appear to be clustered separately from those from the other countries of southeast Asia where the disease is endemic, suggesting ancient origins that predate the transient land bridges between these regions in the Miocene period (79).
Genome Sequence of B. pseudomallei and Comparison with That of B. mallei
A major recent advance has the completion of sequencing and the annotation of the genomes of both B. mallei (strain ATCC 23344) and B. pseudomallei (clinical strain K92643) (197, 313). The genome of B. pseudomallei is composed of two large chromosomes (of 4.07 and 3.17 Mb, respectively) that demonstrate functional partitioning (197). The larger chromosome carries many genes required for cell metabolism and growth, and the smaller tends to carry genes required for survival and virulence factors. A summary of some of the key genes identified is given in Table 2. The findings that the gene order is conserved on the larger chromosome and has a greater number of orthologous matches compared to other bacteria suggest that they may not have a common ancestry. A striking finding was the demonstration of genomic islands, identified by anomalies in GC content or dinucleotide signatures together with accompanying coding sequences resembling those of mobile genetic elements. These are likely to represent DNA that has been recently horizontally acquired and are variably found in clinical and environmental strains of B. pseudomallei.
TABLE 2.
Genes associated with survival and virulence identified in the B. pseudomallei genomea
| Survival | Virulence | |
|---|---|---|
| Secondary metabolism: possible antibiotic, surfactant, siderophore biosynthetic pathways (including fur) | Secretion: type I, II, III, and V secretion systems, including three type III secretion systems (including bsa, bip, and bop) | |
| Drug resistance: Ambler class A, B, and D beta-lactamases (including oxa and penA); multidrug efflux pumps (including amr); aminoglycoside acetyltransferase | Surface components: lipopolysaccharide, capsular polysaccharide, and potential surface polysaccharide biosynthesis (including waa, rfb, and wcb) | |
| Intracellular stress: superoxide and nitric oxide detoxification enzymes (including sod) | Exoproteins: phospholipase C (including plc), metalloprotease A and other proteases (including mpr), collegenase | |
| Motility and chemotaxis: flagellum system (including fli and flg), chemotaxis-associated proteins (including che) | Adhesins: surface proteins that may modulate host-cell interaction | |
| Fimbriae and pili: type I and IV pili and tad-type pili (including pil) |
In contrast, B. mallei does not contain any of these genomic islands, suggesting that horizontal acquisition of DNA is not an important source of genetic variation as it is in B. pseudomallei (313). Gene deletion, probably as a result of mammalian host restriction and a consequent reduction in selective pressure, is suggested by the absence of many of the genes required for environmental survival in B. pseudomallei. Rather, numerous insertion sequences and simple sequence repeats point to an alternative mechanism for genetic variability in B. mallei.
Role of Host Immune Responses
Although many studies have observed and defined immune responses believed to be important in pathogenesis, several points require explanation by any comprehensive model of pathogenesis: (i) the specific comorbidities present in patients susceptible to melioidosis, particularly patients with diabetes, thalassemia, alcoholism, and renal impairment; (ii) the fact that repeated exposure, which is sufficient in some patients to provoke an antibody response to a highly conserved lipopolysaccharide antigen, is insufficient protection against infection, although there is some evidence that high-titer antibodies against LPS may be protective against severe infection; (iii) the fact that although gamma interferon (IFN-γ) appears to be vital in resistance, as might be expected for an intracellular bacterium, the role of adaptive CD4-mediated immunity remains uncertain, particularly as human immunodeficiency virus (HIV) infection does not seem to be a risk factor for disease; (iv) the contributions of the various bacterial products to infection and, more specifically, the relative contributions of endotoxin and exotoxins to pathogenesis and evasion of the immune system (the finding that the relationship between bacterial counts in blood and mortality is similar to that with other gram-negative organisms suggests that exotoxins do not contribute directly to outcome); and (v) the site from which latent infection may reactivate, as relapse after apparently successful treatment and an extended incubation period after exposure suggest a dormant state similar to that seen in tuberculosis.
Innate immunity.
B. pseudomallei appears to be resistant to serum bactericidal components (220); although the alternative complement pathway is activated, resulting in phagocytosis, it is resistant to the effects of the terminal complement membrane attack complex (149). Phagocytosis was increased by the addition of specific antibodies and complement (148).
Studies of the role of phagocytes in melioidosis have demonstrated conflicting results. It is established that B. pseudomallei can survive and multiply within professional phagocytes, including macrophage/monocyte and neutrophil cell lines (226, 342). It appears to be able to evade phagosome-lysosome fusion and destroy the phagosome membrane as soon as 15 min after ingestion (185). This is consistent with functional studies demonstrating poor early bactericidal activity in most (149, 195, 289) but not all (354) studies.
The comorbidities recognized as risk factors for melioidosis also may be operating by impairing neutrophil function. Diabetes mellitus has been demonstrated to result in impaired chemotaxis, phagocytosis, oxidative burst, and killing activity (30-32, 166, 287, 288, 305, 353). Similar defects have been observed in association with high alcohol consumption (27, 46, 47, 168, 183, 309, 312, 325, 411, 505), chronic renal failure (190, 194, 207, 338, 366), and thalassemia (476). Reports of patients with chronic granulomatous disease (146, 422) support the role of neutrophils in resistance to melioidosis.
Of further interest is the possibility of reversing these functional defects with granulocyte colony-stimulating factor (G-CSF). Multiple studies of G-CSF in nonneutropenic animal models have demonstrated improvements in outcome (172, 276, 312, 322, 433). In human studies, improvements in laboratory markers are seen following G-CSF use (186, 242, 360), and patients with diabetic foot infection may benefit from the use of G-CSF (134, 175). However, large multicenter clinical trials have failed to demonstrate benefits in pneumonia and severe sepsis, possibly due to late administration of G-CSF in the course of the illness (85, 310, 311, 361). The use of G-CSF has been associated with a fall in mortality in patients with septic shock due to B. pseudomallei; a clinical trial has commenced recently (84).
Role of macrophages in immunity.
As mentioned above, B. pseudomallei appears to be able to survive and multiply within professional phagocytes, including those of the macrophage/monocyte lineage (185, 226). Although this is assumed to play a role in the site of latent infection, little is known regarding the precise localization of latent intracellular B. pseudomallei after early host-bacterium interaction and the bactericidal ability of macrophages.
Macrophages exposed to B. pseudomallei do not appear to respond in the same way as they do to other pathogens; in one study, lower levels and slower production of inducible nitric oxide synthase and tumor necrosis factor alpha (TNF-α) were seen in a macrophage cell line compared to those after exposure to Escherichia coli and S. enterica serovar Typhi (446, 447). However, the responsiveness of these cells, as well as their bactericidal activity, was increased by priming with IFN-γ, providing an explanation for the important role of this cytokine in mediating resistance (447). Similarly, the low levels of IFN-β production by macrophages may also mediate poor intracellular control by inducible nitric oxide synthase-dependent mechanisms (444).
In addition, ultrastructural studies of macrophage-B. pseudomallei interactions have compared responses in patients with melioidosis and healthy controls. They suggest that there is less early phagolysosome fusion in macrophages from melioidosis patients, resulting in higher intracellular bacterial concentrations (S. Puthucheary, Abstr. 4th World Melioidosis Congr., abstr. 40, 2004).
TNF-α, produced primarily by macrophages but also by B cells, T cells, and fibroblasts, is an early and potent proinflammatory cytokine. High systemic levels are associated with septic shock, including in patients with melioidosis, where high TNF-α levels were associated with mortality. However, TNF-α is required for the containment of infection; neutralization of TNF-α in a mouse model increased susceptibility to melioidosis (369).
The TNF2 allele, representing a stable mutation in the TNF-α promoter (G→A substitution at base −308), is associated with increased production of TNF-α. It is also associated with increased susceptibility to mucocutaneous leishmaniasis, cerebral malaria, and meningococcal purpura fulminans (249). In 109 of 123 Thai patients with melioidosis, the presence of TNF2 (present in 9% of the study population) was associated with an increased risk of death, septicemia, and multiple and single organ involvement. When the patients were matched to 74 seronegative controls, the presence of TNF2 was associated with a statistically significant relative risk of melioidosis of 2.3 (318).
We have previously speculated that the intracellular niche of this organism and its interaction with hosts with specific comorbidities, such as diabetes, suggest that the innate immune response plays a prime role in the control of this organism (111). Severe impairment of specific cellular immunity, such as with advanced HIV infection, does not appear to be a risk factor for infection. Similarly, the disease may be present even with high antibody titers, indicating that natural humoral immunity does not appear to be protective. The relatively minor role of specific immunity relative to innate immunity in this infection may explain the capacity of this organism for latency as well as the lack of protection against disease despite repeated exposure to the organism.
Role of T cells in immunity.
A classic framework to view specific host responses is the type 1/type 2 dichotomy, in which cell-mediated responses are usually inversely proportional to the levels of antibody response. Type 1 responses are generally adaptive against intracellular pathogens; C57BL mice are more resistant to Leishmania major and IL-10 gene knockout (IL-10−/−) mice are more resistant to Listeria monocytogenes, and their resistance is attributed to their tendency to type 1 responses (403).
In melioidosis, this appears to be supported by the demonstration that type 1 response-prone C57BL6 mice were relatively resistant to B. pseudomallei compared to BALB/c mice (200, 259) and by the demonstration of the key role of IFN-γ, a key Th1 cytokine that stimulates phagocytosis, the oxidative burst, and intracellular bacterial killing (368, 369), and its correlation with the severity of illness in humans (59, 256).
Similarly, the sine qua non of Th1 polarization, IL-12, can be demonstrated in patients with melioidosis and in ex vivo blood simulated with heat-killed B. pseudomallei, and neutralization of IL-12 can be demonstrated to result in impaired IFN-γ production (256) and increased susceptibility in mice (369). In addition, the ratio of immunoglobulin G1 (IgG1) to IgG2 in humans (454) and mice (200) also suggests that a type 1 response may be adaptive.
However, there is evidence that anti-inflammatory Th2 responses are necessary to balance proinflammatory Th1 responses; severe sepsis may represent an unregulated Th1 response. In septic patients, as in melioidosis, poor outcomes are associated with high levels of both IL-6 (a Th1 cytokine) and IL-10 (a Th2 cytokine) (382). This may suggest that the intensities of both pro- and anti-inflammatory responses merely reflect the magnitude of the inflammatory insult. More extensive cytokine characterization of Th1 and Th2 responses in mouse strains with differential susceptibility has now demonstrated a mixed pattern; levels of mRNA for Th1 cytokines (IFN-γ, IL-6, and IL-12)and Th2 cytokines (IL-10) increased in both susceptible BALB/c and resistant C57BL/6 mice (443).
Other studies have implicated effector cells as cytotoxic T lymphocytes and NK cells responding to IFN-γ via the CXC chemokines IP-10 and Mig (257). These cells may act to induce apoptosis in infected cells via granzymes (GrA and GrB) (255). NK cells and, unexpectedly, CD8+ T cells have been demonstrated to be a source of IFN-γ early in the response to infection with B. pseudomallei (270). This process appears to be a “bystander” phenomenon mediated by IL-12 and IL-18 rather than engagement of the T-cell receptor.
The demonstration of a specific cell-mediated immune response in survivors of acute melioidosis suggests that this may be important in the long-term control and prevention of relapse (34, 235). However, most puzzling is the absence of evidence to suggest that HIV infection is a risk factor for melioidosis, despite its prevalence in Thailand (92).
Antibody responses.
As discussed above, many B. pseudomallei components have been demonstrated to be immunogenic in patients with melioidosis, including capsular polysaccharide and lipopolysaccharide (O-PS I and O-PS II) (195), flagellin proteins (75, 138, 290, 328), and other cell wall proteins (386). Of these, antibodies against the LPS component O-PS II and possible an exopolysaccharide and flagellin components have been demonstrated to be protective (52, 75, 227).
Antibodies of all classes against a culture filtrate antigen were demonstrated in patients with prior melioidosis, and the level was highest for IgG, particularly the IgG1 and IgG2 subtypes (86). Antibodies could persist variably for over 3 years (454). The description of a patient with a persistently high IHA titer (>1:5,120) for many years who presented with reactivation in association with staphylococcal endocarditis suggests that persistently high titers may define a group of patients that require close follow-up (355). Evidently, the antibody response resulting from repeated natural exposure to B. pseudomallei and B. thailandensis is insufficient to provoke a protective response for primary infection or relapse.
Other host factors.
A possible association between HLA-DRB1*1602 and severe melioidosis, independent of possible confounders such as diabetes mellitus, was described (145), but this has not been borne out by other studies, placing susceptibility genes between the HLA-Cw and HLA-DQ loci of the HLA-B58 haplotype (141).
Studies examining the role of polymorphisms in Toll-like receptor alleles and mannose-binding lectin genes and their correlation with the severity of disease are under way (W. J. Wiersinga, personal communication). It is likely that recognition of bacterial endotoxin or other bacterial components plays a significant role in adaptive and maladaptive responses in melioidosis, as with other septic states.
A promising therapy is the use of the use of the bacterial genomic sequences in the form of unmethylated CpG oligodeoxynucleotide, either as an immunoprotective agent or as a DNA vaccine adjuvant. In a mouse model, CpG was shown to be strongly protective when administered prior to exposure to B. pseudomallei, possibly by increasing bacterial phagocytosis by macrophages (445, 480). Although no significant adverse reactions have been noted in human studies to date, theoretical concerns focus on the possibility of DNA integration into host cells (although CpG itself does not contain a promoter), the induction of adverse cytokine profiles, and the possibility of triggering autoimmune phenomena in susceptible patients (243).
Prospects for Vaccine
A Canadian group has been exploring potential vaccination strategies, which have recently been reviewed (466). Despite recent work demonstrating some protection in animal models following vaccination with B. thailandensis and other attenuated strains (209, 210), it is unlikely that this strategy alone will provide sufficient protection, given the extensive histories of exposure of patients to both B. thailandensis and B. pseudomallei. Other approaches that have been investigated include the use of DNA vaccines against the fliC flagellin structural gene and the use of attenuated B. pseudomallei mutants. Attenuated strains would have to be demonstrated to be avirulent; a case report suggesting that even ara+ B. thailandensis can cause clinical infection has important implication for vaccine research (271).
A B. mallei candidate conjugate vaccine, linking the capsular and O-PS lipopolysaccharide antigens to exotoxin A of Pseudomonas aeruginosa, is being investigated in a horse model of glanders (53, 54). Given the cost of such vaccines, the relatively low incidence of the disease, the uncertain duration of protection, and the resource-constrained regions in which such a vaccine might be used, it is unlikely that a vaccine for B. mallei or B. pseudomallei would find use outside military settings.
Although protective footwear is often recommended for patients at high risk of melioidosis, the usefulness of this advice has not been evaluated. The observation that case clusters are associated with sudden, heavy, postcyclonic rainfall in Australia has suggested that public health interventions may best be targeted after these events (80).
CLINICAL FEATURES
Risk Factors
A number of risk factors for developing melioidosis have been defined in several studies and are summarized in Table 3. Patients with diabetes mellitus, in particular, have a high incidence of melioidosis, with up to 60% of patients having preexisting or newly diagnosed type 2 diabetes (111, 299, 415, 417). Although it was suggested that insulin may have a direct effect on B. pseudomallei (487), the high incidence of type 2 diabetes, rather than type 1 diabetes, points away from this as the mechanism of action (105, 381). Subsequent studies have attributed the inhibitory effect to a preservative used with insulin (384).
TABLE 3.
Clinical risk factors for melioidosis
| Risk factor | Level of evidence |
|---|---|
| Diabetes mellitus | Between 37 and 60% of patients are diabetic, mainly type 2; case-control and population-based studies in Australia and Thailand give estimated relative risk of 5.9 to 13.1 (111, 114, 299, 415, 417) |
| Thalassaemia | α-Thalassemia trait common in Thailand (44%) but disease less common (8%); case-control studies in Thailand estimate relative risk of 10.2 (415, 417, 464) |
| Aboriginality | Population-based study in Australia estimates relative risk of 2.7 to 8.1, assumed to relate to exposure to soil or water (111, 114, 299) |
| Male gender | All series in Australia, Thailand, Malaysia, and Singapore demonstrate male preponderance (114, 191, 299, 417) |
| Soil/water exposure | Rice farmers constitute 81% of patients in Thailand, relative risk in case-control study estimated at 3.3 (415, 417) |
| Renal disease | Patients with renal impairment or failure comprise 10% of Australian series (111) with relative risk of 3.2 (114); renal disease (renal failure and calculi) associated with increased risk of melioidosis (odds ratio, 2.9) (415, 417) |
| Excessive alcohol consumption | Conflicting evidence; excessive alcohol use documented in 39% of Australian patients, with relative risk of 2.1 to 6.7 in case-control and population-based studies (111, 114, 299), less prevalent in Thai patients (12%) (417) |
| Kava use | Use of Piper methysticum root documented in 8% of Australian series (111) but not associated with pneumonia in case-control study (98) |
| Chronic lung disease | Present in 27% of Australian patients (111), with relative risk of 4.3 (114) |
| Splenectomy | Case studies, often related to thalassemia (196, 334, 464) |
| Aplastic anaemia, febrile neutropenia | Case reports only (273, 417) |
| Chronic granulomatous disease | Two case reports (146, 422) |
| Mycobacterial disease | Case reports of patients with infection with atypical mycobacteria, M. tuberculosis, or M. leprae may suggest common host susceptibility (56, 89, 362, 417) |
| Dengue hemorrhagic fever | Five of 18 pediatric patients in Thailand (334) |
| Neutropaenia | Case report (189) |
| Renal transplantation | Case report, patient also diabetic (224) |
| Systemic lupus erythematosis or steroid use | Case reports, also associated with immunosuppressives (29, 95, 398, 417); steroid-containing herbal remedies documented in up to 10% of Thai patients (415) |
| Glucose-6-phosphatase deficiency | Case reports (417) |
| Hemosiderosis | Case reports (286, 363); one unreported case of pulmonary hemosiderosis secondary to mitral valve disease (B. Currie, unpublished data) |
| Cystic fibrosis | Reports from travelers to areas of endemicity (122, 198, 372, 458) |
| Porphyria cutanea tarda | Subsequent to episode of melioidosis; likely to be an adverse event in response to medication (160) |
Studies have examined risk factors in patients with melioidosis compared with septic and nonseptic hospital controls to estimate a relative risk. In a Thai study, diabetes, thalassemia, renal disease (defined as renal calculi or renal failure), and occupational exposure to surface water were all associated with an increased risk of melioidosis (415). A population-based study in Australia defined adjusted relative risks of 4.0 (3.2 to 5.1) for those aged ≥45 years, 2.4 (1.9 to 3.0) for males, 3.0 (2.3 to 4.0) for Aboriginal Australians, 13.1 (9.4 to 18.1) for diabetics, 2.1 (1.6 to 2.6) for those with excess alcohol consumption, 4.3 (3.4 to 5.5) for those with chronic lung disease, and 3.2 (2.2 to 4.8) for those with chronic renal disease. The reason for these specific risk factors is not clear, but many have implicated the effect of these comorbidities on neutrophil function (111, 417), which is known to be important in the pathogenesis of melioidosis (226).
The use of steroids is associated with an increased risk of melioidosis; this includes steroid-containing herbal remedies (“yaa chud”) in Thailand, the use of which was documented in up to 10% of Thai patients (415). In the Australian series, chronic obstructive pulmonary disease and the consumption of kava and alcohol have also been implicated (111).
Despite cell-mediated immunity being implicated as a mechanism of resistance to melioidosis (34, 235), infection with HIV does not appear to be a major risk factor (92).
A number of case studies have noted an intriguing association with chronic granulomatous disease that may highlight the underrecognized role of neutrophil defects in pathogenesis (146, 422). Similarly, reports of patients with hemosiderosis suggest that impairment of phagocytic cells may be important (286, 363). Case reports of melioidosis and previous or subsequent mycobacterial infection (Mycobacterium tuberculosis, Mycobacterium terrae, or Mycobacterium leprae) may reflect a common host susceptibility to these intracellular pathogens (56, 89, 362, 417).
Clinical Syndromes
In all series, pneumonia is the most common presentation of melioidosis and is involved in approximately half of all cases. It is conventionally thought that lung involvement arises after hematogenous spread following inoculation, based on cases of pneumonia arising following a history of inoculation and the finding that radiographic assessment of pneumonia often lags behind the patient's clinical status. However, early reports implicating inhalation in helicopter crews based in Vietnam (204) and the presence of a marked association of the rates of pneumonia with rainfall (113) suggest that inhalation may be more important than had been previously appreciated.
Important clinical differences have been seen between patients in Australia and Thailand (Table 4): the high incidence of genitourinary infection in Australia, with prostatic abscesses occurring in 18% of males; the absence of suppurative parotitis in Australia, in contrast to a rate of 30 to 40% in Thai children (119); and the distinct but uncommon encephalomyelitis syndrome seen in tropical Australia.
TABLE 4.
Variation in clinical pattern of melioidosis worldwide
| Clinical presentation | % of patients in:
|
||||
|---|---|---|---|---|---|
| Royal Darwin Hospital series (1989-1999; n = 252) (111) | Singapore series (1989-1996; n = 331)a (191) | Kuala Lumpur series (1976-1991; n = 50)b (346) | Infectious Diseases Association of Thailand series (n = 686)c (344) | Sapprasithiprasong Hospital series (1986-1987; n = 63)b (74) | |
| Pneumonia or pleural effusion | 58 | NRd | 58 | 45 | 23 |
| Genitourinary infection | 19 | NR | 10 | 7 | 8 |
| Skin or soft tissue infection | 17 | NR | 24 | 13 | 13 |
| Neurological melioidosis or brain abscess | 4 | NR | 6 | 3 | NR |
| Splenic abscess | 4 | NR | 2 | 2 | NR |
| Liver abscess | 2 | NR | 4 | 7 | NR |
| Other intra-abdominal | 3 | NR | 4 | 5 | NR |
| Prostatic abscess | 18 (of males) | NR | NR | 0.3 | NR |
| Parotid abscess | 0 | NR | NR | 2 | NR |
| Bone or joint | 4 | NR | 12 | 5 | 4 |
| Pericardial effusion | 1 | NR | 2 | 3 | NR |
| No clinical focus | 10 | NR | NR | NR | 51 |
| Septic shock | 20 | NR | 16 | NR | 30 |
| Bacteremia | 46 | 43 | 100b | 58 | 100b |
| Mortality | 19 | 39 | 65 | 38-61 | 68 |
Culture-confirmed cases only.
Bacteremic cases only.
Summary of reported cases presented in 1985 from Khon Kaen Hospital (1982 to 1985), Ubon Ratchathani (1982 to 1985), Srinagarind Hospital (1978 to 1985), Nakorn Ratchsima (1983 to 1985), Chulalongkorn Hospital Bangkok (1980 to 1985), and Nontaburi (1983 to 1985).
NR, not recorded.
Encephalomyelitis, characterized by brain stem encephalitis and flaccid paralysis, is seen in 4% of melioidosis presentations in northern Australia and is associated with considerable morbidity and mortality (110). Small numbers of children with a similar syndrome have been recognized in Thailand (365). Cultures of cerebrospinal fluid were positive in only one of seven cases, with monocytic pleocytosis the most common finding (488). This should be distinguished from more focal suppurative infections involving the central nervous system, which have been well described (55, 62, 66, 332, 438, 459). Some of these may represent direct spread from contiguous sites, such as facial sinuses (66) or orbital cellulitis (365). Primary meningitis has been observed in Thailand (365) but more often results from ruptured cerebral abscesses (472). Neurological involvement has also been observed in animals (252, 258, 321).
Acute suppurative parotiditis accounts for up to 40% of pediatric cases but only small numbers of adult cases in Thailand (119, 282). It seems to arise in patients with no defined risk factors and is generally associated with a good prognosis. It may be bilateral in 10% of patients and may be complicated by rupture or permanent facial nerve palsy. It has been reported only once in Australia (151).
The high proportion of patients with prostatic infection in Australia contrasts with the higher proportions of patients with liver and spleen abscesses seen in Thailand (455). In Australia, the prevalence of prostatic infection (18% of male patients) mandates routine imaging, with drainage commonly required (106, 111). This contrasts with other internal abscesses, which may respond to medical therapy alone (111).
Bone and joint infections are uncommon and may be difficult to differentiate from other causes of infection, except that the systemic features of the illness may be more prominent. Surgical drainage is often required, together with long courses of intravenous antibiotics (337).
Skin and soft tissue infections are a common manifestation of melioidosis and may be the source of systemic infection or result from hematogenous spread. Presentations may be rapidly progressive, similar to necrotizing fasciitis from other organisms (465). Infections involving many other sites have been described, including mycotic aneurysms, mediastinal infection, and thyroid and scrotal abscesses (111). Corneal ulcers were described for a series of three Thai patients following corneal trauma. Extensive ulcers, subconjunctival abscesses, and hypopyon were managed with topical and intravenous ceftazidime with good outcomes (385). Other ocular manifestations include orbital cellulitis with contiguous spread to the sinuses (478). Cardiac involvement is rare; pyopericardium is probably the most common manifestation, but myocardial abscesses (37) and endocarditis (344) have been reported.
Markers of organ dysfunction, including leukopenia (particularly lymphopenia), hepatic dysfunction (raised aspartate aminotransferase, alanine aminotransferase, and bilirubin levels), renal dysfunction (raised urea and creatinine levels), and metabolic derangements (hypoglycemia and acidosis), on admission appear to predict mortality (74, 81, 106).
The response to therapy is often poor, with a mean duration of fever of 9 days documented. Treatment failure, with antibiotic therapy alone, has been defined in studies as fever for longer than 14 days or bacteremia for longer than 7 days (383). Persistently positive cultures from other sites and radiological abnormalities are not uncommon and do not necessarily portend a poorer prognosis (111, 419).
Markers of inflammation such as C-reactive protein (CRP) are often used; a small study demonstrated that CRP levels were elevated in 46 patients with melioidosis, that the CRP level responded within 2 days in patients with uncomplicated treatment courses, and that persistent elevation in four patients was attributed to undiagnosed sites of infection or inadequate treatment. In addition, elevations in CRP levels predicted relapse, even in the absence of fever or leukocytosis (22). However, a review of the use of CRP levels in the larger Darwin prospective series has revealed cases with normal levels or mild elevations on admission with severe or fatal disease, including in relapse (83).
The clinical use of procalcitonin has also been assessed in Thailand; although the level of procalcitonin reflected the clinical severity of illness and the response to therapy, this was not specific to melioidosis. In addition, there was a wide variation in response, with a significant mortality even in patients with low procalcitonin levels (391).
The state of asymptomatic carriage has been suggested (109), but in 1,000 hospitalized children and 4,545 adults (1,011 patients with melioidosis and 3,524 healthy subjects) in Thailand, the positive predictive value of a throat swab for clinical disease was 100% (230, 494). In goats, aymptomatic carriage has been reported (426).
Occasional patients, particularly those with cystic fibrosis, with long-term carriage without signs of overt disease have been described (458), raising the possibility that these strains are behaving more like B. cepacia in this group of patients.
Relapse after apparently successful treatment is well described and is associated with a mortality similar to that for the initial episode. It occurs in 13 to 23% of cases and a median of 6 to 8 months (but up to many years) later (72, 109). Factors associated with a higher risk of relapse included poor adherence to therapy, the use of doxycycline monotherapy or amoxicillin-clavuanate in the eradication phase, severe disease (relative risk of 4.7 compared to localized disease), the use of ampicillin-clavulanate or the four-drug conventional therapy in the intensive phase (relative risk of 2 compared to ceftazidime), and eradication therapy of less than 8 weeks (relative risk of 2.5) (72, 109). In the Royal Darwin Hospital series, only 1 patient of >60 treated with trimethoprim-sulfamethoxazole (TMP-SMX) eradication relapsed (109); improved compliance with this simple eradication regimen, in addition to close follow-up, is likely to be an important factor. There also are differences in the dosing of TMP-SMX; in Australia, a higher dose is given (8/40 mg/kg of body weight twice a day) than in Thailand (5/25 mg/kg twice a day).
In the majority of cases, relapse is due to reactivation of the original infecting strain (demonstrated by restriction fragment length polymorphism or pulsed-field gel electrophoresis). Infection with a different strain was demonstrated in between 4 and 7% of cases in Thailand and Australia (109, 140) and in one of five recurrent cases in Malaysia (448).
Variation in the clinical presentation and severity of melioidosis may be due to one or more of three factors: variation in bacterial strains (including the presence or absence of virulence factors), variation in the host immune response, and variation in acquisition. There is a large body of evidence that suggests that host factors, particularly age and comorbidities, are of prime importance in determining the pattern of disease. Although the presence of regional variation in the pattern of disease (such as the relative absence of parotiditis in Australia) suggests that bacterial variation may be important, molecular studies have failed to document this to date. We believe that variations in the mode and magnitude of acquisition, as discussed above, play an underappreciated role in determining the clinical manifestation of disease.
Modes of Acquisition and Incubation Period
Three modes of acquisition, i.e., inhalation, ingestion, and inoculation, are recognized for B. pseudomallei, but the relative contributions of each are yet to be determined. As with other infectious diseases, it is likely that these factors as well as the size of the inoculum are responsible for the pattern and severity of disease. Situations likely to be associated with a high inoculum, such as near drownings, are associated with a short incubation period, even less than 24 h (262, 417).
Inhalation was initially thought to be the primary mode of acquisition, based on studies of U.S. soldiers in Vietnam, where it was noted that helicopter crews seemed to have a high incidence of the disease (204). This and its long incubation period resulted in melioidosis acquiring the sobriquet “the Vietnamese time bomb” (204); although sporadic cases have continued to surface in the United States from remote exposure (93, 173), the feared epidemic failed to materialize. Work done in the 1950s (315) and more recently, driven by biodefense (221), has defined infectious doses via this mechanism in mice and other animals. The finding that periods of heavy rainfall are associated not only with higher numbers of cases but also pneumonic presentations and cases of increased severity may suggest a shift to inhalation during extreme weather events (113). This is supported by the recent observation that a number of patients in Singapore who presented during a period of heavy rainfall were elderly, nonambulant patients with no history of exposure to soil or surface water (O. P. Lim, unpublished data).
It is now believed that inoculation is the major mode of acquisition. Minor wounds to the feet of rice farmers are common during the planting and harvesting seasons, when farmers spend most of the working day wading in mud and surface water; inoculation at the time of a snake bite has also been described (74). In the Darwin series, 25% of patients gave a history of an inoculation injury prior to presentation; in this subgroup of patients, an incubation period of 1 to 21 days has been defined (112). It is also clear that more chronic presentations are not uncommon, with 13% having symptoms exceeding 2 months at presentation (112). In addition, incubation periods of as long as 24 to 29 years in ex-servicemen who were in Papua New Guinea and Vietnam have been described (93, 240, 293).
Ingestion has been suggested as a mode of infection (129, 204). In animals, this route has been implicated by findings of infected gastrohepatic nodes in pigs (237). These findings have also been noted on occasion in humans, with an Australian having microabscesses of the gastric wall with seeding of the peritoneum from a ruptured gastric ulcer (112). However, the contribution of this route of infection is undefined; although contamination of potable water has been implicated as the point source in two outbreaks (115, 212), this may not necessarily reflect ingestion as the primary mode of transmission.
Two laboratory-acquired cases have been described, one associated with sonication outside a safety hood (371) the other after organisms were spilled during centrifugation (176), highlighting the need for biosafety precautions (20, 123).
Other unusual modes of transmission include person to person, both in a sibling of a child with cystic fibrosis (198) and a possible sexual transmission from a returned serviceman to his partner (294). Although prostatic infection is common in Australia and sexual transmission has been suggested (470), no confirmed cases of sexual transmission are known to have occurred from these cases (Currie, unpublished data).
Reports of neonatal cases suggest perinatal transmission (181, 280, 344), and one case has been attributed to culture-positive breast milk (351). Vertical transmission has been proven on only one occasion in humans (1), and transplacental spread has been documented in goats (277).
Possible epizoonotic human infections have been implicated in at least three cases in humans in Australia (277). Nosocomial transmission to four animals that had attended a single veterinary practice was attributed to contamination of a multidose injectable solution (277). Two cases of suspected nosocomial infection were reported from Srinagarind Hospital in Thailand and were associated with contamination of chlorhexidine-cetramide (Savlon) (344). Contaminated detergent has also been implicated in a cluster of cases in a small, remote Australian community (161). In the original description of melioidosis, intravenous use of illicit opiates was described as a risk factor, but this has not been implicated since then (474).
Reactivation of melioidosis following influenza infection has been described (285), but this probably is not a significant factor in Australia, as the influenza season comes after the melioidosis season (109). Cases of reactivation associated with other illnesses, such as staphylococcal endocarditis, have been noted (355). Malaria and seasonal dietary factors have also been proposed as triggers (417), but these are obviously not applicable in Australia. It is more likely that seasonal agricultural practices and exposure to surface water are more important determinants of the seasonal pattern of disease.
We believe that both inhalation and inoculation may be important in the acquisition of melioidosis. Inhalation or inoculation with a high bacterial load, more likely in the monsoonal season with heavy rain and wind, is characterized by more severe infection and pneumonia. Inoculation is characterized by infection with a longer incubation period and disease of a lesser severity. Although outbreaks have been linked to contamination of drinking water, we do not believe that ingestion plays an important role but rather believe that this simply represents a source of inoculation or inhalation.
Melioidosis as a Potential Bioweapon
Almost a century before the “anthrax letters” incident in the United States, Sir Arthur Conan-Doyle recognized the potential of this infectious agent as a potential bioweapon in his Sherlock Holmes story “The Dying Detective.” In this story, Holmes is sent a box designed to inoculate the recipient with “Tapanuli fever” on opening, and Tapanuli fever is thought by many to represent melioidosis (218, 399).
More recently, B. pseudomallei has been considered an important potential bioweapon, with increasing funding overseas for research into virulence factors and vaccine development (221, 466). It is believed that biological weapons research using B. pseudomallei occurred in the former USSR, although the extent of this effort and the possibility of engineered antibiotic resistant strains remain unknown (6, 269). Other countries with a military interest in B. pseudomallei included the United States and possibly Egypt (247, 377).
Nonprimate animal models of inhalational melioidosis were defined in the 1950s (315), as well as more recently (221), but apart from laboratory accidents (176, 371), no other cases are believed to have occurred outside the natural environment. One study has demonstrated the value of prophylactic or immediate ciprofloxacin or doxycycline (for 5 days following exposure) in increasing the median lethal dose in an animal model of peritoneal inoculation (364).
The potential risk posed by B. pseudomallei as a bioweapon is uncertain. Melioidosis carries a potentially high mortality rate, and its causative agent has intrinsic antibiotic resistance and a wide host range. However, weaponization has not been known to have been performed, the disease does not spread from person to person, and the susceptibility of immunocompetent individuals after inhalation is not clear.
In contrast, the closely related bacterium B. mallei was believed to have been used in the First World War by the Central Powers to infect Russian horses with glanders on the eastern front, with significant effect. Subsequently, deliberate infection of human prisoners of war and animals with B. mallei was done at the Pinfang (China) Institute during the Second World War by the Japanese (247). Further development with B. mallei was also undertaken in the United States, the former Soviet Union, and Egypt (5, 377), and glanders possibly was used in the Afghanistan conflict between 1982 and 1984 (6).
DIAGNOSIS AND MANAGEMENT OF MELIOIDOSIS
Diagnosis
Isolation of B. pseudomallei from bodily fluids of patients remains the “gold standard” in diagnosis and requires the use of selective media for nonsterile specimens. Gram stain and other histopathological stains are not specific for the organism. A number of techniques have been employed to attempt to reduce the time required to achieve a diagnosis, including antigen detection on specimens or on culture supernatant, antibody detection, molecular techniques, and rapid culture techniques (Table 5). Although many of these rapid tests have been developed, few have been extensively tested in the field, and only IHA, latex agglutination, and immunofluorescence are currently used clinically.
TABLE 5.
Sensitivities and specificities of diagnostic tests for melioidosis
| Testa | Study location (n) | Sensitivity (%) | Specificity (%) | Reference |
|---|---|---|---|---|
| Antibody detection | ||||
| Complement fixation test | Unknown (47) | Not recorded | 100 | 314 |
| Serum IHA | Thailand (150) | 71 | 75 | 142 |
| Serum IHA | Thailand (184) | 77 | 92 | 15 |
| Serum IHA (purified antigens) | Thailand (101) | 46-94 | 34-82 | 386 |
| Serum IHA | Thailand (130) | 61.9 | 79.9 | 375 |
| Serum IHA | Thailand (148) | 64 | 93 | 479 |
| Serum IHA | Thailand (299) | 72 | 68 | 490 |
| Serum IHA | Australia (298) | 80 | 91 | 319 |
| Serum IHA (acute-phase serum only) | Australia (191) | 85 | 100 | 25 |
| Serum IFA (IgG, acute-phase serum only) | Australia (191) | 86 | 99 | 25 |
| Serum ELISA (IgG; acute-phase serum only) | Australia (191) | 79 | 99 | 25 |
| Serum ELISA (purified glycolipid antigen) | Japan, Vietnam, Thailand (416) | 100 | 97.8 | 330 |
| Serum ELISA (IgG; immunoaffinity-purified antigen) | Thailand (150) | 88 | 83 | 142 |
| Serum ELISA (IgM) | Thailand (150) | 87.8 | 81.8 | 142 |
| Serum ELISA (various antigens) | Thailand (101) | 74-82 | 75-80 | 386 |
| Serum ELISA (immunoaffinity-purified antigen) | Thailand (130) | 71.4 | 86.2 | 375 |
| Serum ELISA (culture filtrate antigen) | Thailand (148) | 93 | 97 | 479 |
| Serum DOT (culture filtrate antigen) | Thailand (101) | 72 | 64 | 386 |
| Serum DOT (culture filtrate antigen) | Thailand (130) | 85.7 | 85.3 | 375 |
| Serum DOT (culture filtrate antigen) | Thailand (148) | 94.1 | 99.2 | 479 |
| Immunochromogenic card test (IgG) | Australia (298) | 88 | 90 | 319 |
| Immunochromogenic card test (IgM) | Australia (298) | 77 | 69 | 319 |
| Immunochromogenic card test (IgG) | Thailand (299) | 79 | 90 | 490 |
| Immunochromogenic card test (IgM) | Thailand (299) | 67 | 80 | 490 |
| Serum (Western blot) | Thailand (101) | 70 | 91 | 386 |
| Serum (various antibody targets) | Thailand (101) | 23-59 | 56-95 | 386 |
| Serum IFA (whole-cell antigen) | India (22) | 45-63 | 291 | |
| Antigen detection | ||||
| Specimen immunofluoresence | Thailand (272) | 73 | 99 | 462 |
| Serum ELISA (19.5-kDa antigen) | Thailand (147) | 82 | 96 | 14 |
| Direct specimen ELISA (MAb 5F8) | Thailand (114) | 75 | 98 | 10 |
| Urine IFA | Thailand | 81 | 96 | 136 |
| Blood PCR (LPS1, LPS2) | Thailand (130) | 95.2 | 91.7 | 375 |
| Blood PCR (16S rRNA) | Australia (52) | 100 | 67 | 178 |
| Blood PCR (various primers) | Thailand | 31-41 | 47-100 | 251 |
| Blood PCR (16S rRNA) | Thailand (29) | 47 | 100 | 144 |
| Blood PCR | Thailand (7) | 100 | 100 | 352 |
| Blood culture supernatant | ||||
| MAb-LA | Thailand (1, 369) | 95.1 | 99.7 | 13 |
| MAb-LA | Thailand | 100 | 86 | 335 |
| MAb-LA | Thailand | 100 | 96 | 335 |
| MAb-LA | Thailand (88) | 100 | 100 | 143 |
IFA, immunofluorescence assay; DOT, DOT immunoassay; MAb-LA, monoclonal antibody latex agglutination.
Culture-based methods.
Selective media have long been used for the isolation of B. pseudomallei from nonsterile fluids and environmental samples, utilizing the broad antibiotic resistance of the organism (152, 163). Ashdown (17) tested his eponymous medium, containing tryptase soy agar with glycerol, crystal violet, neutral red, and gentamicin (4 mg/liter), on 8,000 clinical specimens in Townsville, Australia, in 1979. B. pseudomallei was isolated in eight specimens, with Klebsiella spp. (n = 73), P. aeruginosa (n = 57), Enterococcus faecalis (n = 23), B. cepacia (n = 17), and Serratia marcescens (n = 14) being the most common contaminants (17). A modified Ashdown medium, with colistin, is now commonly used (128). Recovery from throat, rectal, and wound swabs was increased by the use of a selective broth (491). The use of a recently described B. pseudomallei selective agar (BPSA) (203) and the more available B. cepacia selective agar is currently being evaluated in Thailand (S. Peacock, personal communication).
The time to blood culture positivity, reflecting the density of bacteremia, correlated with mortality; 73.7% of patients died if blood cultures became positive with 24 h, compared to 40.9% of those with a time to detection of >24 h. In that study, using the automatic BacT/Alert system, 62% of positive cultures were detected in the first 24 h and more than 90% were detected within 48 h (432).
Alternative blood culture methods could decrease the time to obtain a positive culture, but at the cost of reduced sensitivity. Compared with conventional broth-based blood culture (median time to positivity, 61.8 h; n = 42), the Isolator lysis centrifugation had 81% sensitivity with a time to positivity of 39.3 h, and the pour plate method had 61% sensitivity with a median time to positivity 45.5 h (380). Cultures of bone marrow have the same sensitivity as blood culture (124).
There are conflicting opinions as to the reliability of the API 20NE test panel; two studies have reported good results with this manual system (128), as with the API 20E system (278). However, another study found that 6 of 50 B. pseudomallei strains at a Western Australian laboratory were misidentified, most commonly as Chromobacterium violaceum, and a further 4 strains gave indeterminate results (211). The Vitek automated system is widely used; the Vitek 1 system, but not the Vitek 2 system, appeared to identify B. pseudomallei reliably (278). These findings have significant implications for laboratories in areas where the organism is not endemic and is only occasionally encountered. Colonial morphology on Ashdown medium and, where available, latex agglutination (13) and immunofluoresence (307, 462) are practical ways to identify B. pseudomallei in areas of endemicity (130).
Antigen detection.
Although a variety of antigen detection methods have been studied, none are yet commercially available. Antigen tests have been developed for use on direct specimens or in blood culture supernatant; of these, latex agglutination for culture identification and direct immunofluoresence from direct specimens (such as sputum, urine, or pus) have been used in research labs in Thailand.
Antigen tests for exotoxin and cell components have shown reasonable sensitivity and specificity in studies, but most have not been field tested. These include two enzyme-linked immunosorbent assays (ELISAs), for exotoxin in culture supernatant (219) and a 40-kDa secreted protein (481, 482), and monoclonal antibodies for cell wall components, including LPS (143), a 30-kDa protein (335), and an exopolysaccharide (406). A fluorescent urinary antigen system has been developed; in initial trials, a sensitivity of 81% and a specificity of 96% were defined (136), but a subsequent evaluation gave poorer results (386).
The only test finding widespread use in Thailand currently is a monoclonal antibody latex agglutination test against the 200-kDa protein that was evaluated in 12 centers in Thailand. It was shown to agglutinate blood culture fluid positive for B. pseudomallei, including strains with atypical LPS patterns, with a sensitivity of 95%. The test was also highly specific and did not agglutinate ara+ B. thailandensis strains, with a specificity of 99.7% (13).
Immunofluoresence from direct specimen (including sputum, urine, and pus) is the most promising way to reduce the time to diagnosis in areas of endemicity; a result can be obtained within an hour, but only where specialized microscopy facilities are available. Reagents are based on antibodies to lipopolysaccharide and protein fractions of B. pseudomallei but are not currently commercially available (308, 462). An evaluation of this technique and potential improvements to even further simplify the methods is under way (V. Wuthiekanun, personal communication). Immunofluoresence, as with latex agglutination, may also be useful in identifying B. pseudomallei from cultures (307) as well as in distinguishing B. pseudomallei from B. thailandensis (387).
Antibody detection.
IHA remains the most widely used test despite its poor sensitivity and specificity. It was first described in 1965 (208) and has been used extensively in serosurveys (19, 502). An older method is the complement fixation test (314).
The use of the IHA is problematic in areas of endemicity, particularly in Thailand, where rates of background seropositivity may be up to 30 to 47% in various populations (238), presumably due to subclinical exposure to B. thailandensis or B. pseudomallei early in life (12, 230). Background seropositivity appears to be less common in Australia, except in immigrants from southeast Asia (24); the lower cutoff titer in Australia reflects this (1:40, compared to 1:160 in Thailand) (265, 308). Although IgM antibodies should be more specific (21, 238), field tests of IgM antibody detection have not reflected this promise (142).
Studies of the clinical performance of IHA are difficult to compare, as different thresholds are used for interpretation (between 1:10 and 1:160 in various studies) and the strains used to formulate the whole-cell antigen are not standardized. However, it is evident that the sensitivity of IHA is limited in patients with acute septic illnesses (15). Furthermore, there is some heterogeneity between strains in LPS, a major component in the crude antigen used in the IHA; antibodies against atypical LPS may not cross-react against the IHA antigens, depending on which organisms are used to prepare the IHA assay reagent (11).
Efforts have been made to refine the antigen targets, including refinement of a 30-kDa exotoxin and 19.5-, 40-, and 200-kDa proteins. ELISAs based on LPS and 30- and 200-kDa proteins have been validated in a clinical context; IgG, but not IgM, appears to be more sensitive (74 to 82%) and specific (75 to 80%) than the IHA but still lack the performance necessary for clinical use (386). Other tests for detection of antibody have had similar results, and none have performed sufficiently well to replace the IHA (375).
Longitudinal studies of antibody titer have revealed an unpredictable response, with responses of IgG, IgM, and IgA to a culture filtrate antigen falling, rising, or persisting over periods of 1 to 6 years in seven patients (454). Similarly, IHA titers, reflecting total antibody levels, persisted over 2 years in 20 of 23 patients (21).
A rapid immunochromogenic test (PanBio Ltd., Brisbane, Queensland, Australia) for IgM and IgG appeared to perform well in a series of 121 patients. However the high sensitivity (IgG, 100%; IgM, 93%) and specificity (both assays, 95%) reported were for comparison against IHA, rather than culture, as a gold standard (116). Similar results were reported from the Northern Territory, demonstrating a good correlation with IHA (159).
Recent work in Thailand suggested that the IgG immunochromogenic test (PanBio Ltd.) may be moderately sensitive (79%) but more specific (90%) than IHA (490); this and the positive predictive value remain to be validated prospectively. However, a parallel study performed in Darwin demonstrated that IgG performed similarly to IHA, whereas IgM had poor specificity compared to culture (319). Nevertheless, a commercial test kit using standardized antigens would be useful for areas where the organism is not endemic, where the positive predictive value of a serological test is much higher.
Molecular methods.
Many tests based on molecular detection of B. pseudomallei have been described, but few have been field tested. Primers targeting regions in the 23S rRNA, the 16S RNA, and the 16S and 23S RNA junction have been evaluated (58, 144, 250, 251, 352, 386, 420). The use of primers to detect a region of the 16S RNA demonstrated a sensitivity of 100% but a low specificity in a small clinical study (178). More recent studies are examining the role of a PCR for the type III secretion system (394) in clinical and environmental specimens (D. Gal, unpublished data), as well as other 16S mRNA-specific primers (165).
16S mRNA sequencing has long been used for the identification of bacterial species; this method has been used for phylogeny (50, 51) as well as clinical identification (458) of Burkholderia spp. Sequencing of the groEL gene may also be useful but may not reliably differentiate B. mallei from B. pseudomallei (484, 485).
Antibiotic Resistance
B. pseudomallei exhibits resistance to diverse groups of antibiotics, including third-generation cephalosporins, penicillins, rifamycins, and aminoglycosides. In addition, its relative resistance to quinolones and macrolides limits therapeutic options for the treatment of melioidosis. The in vitro susceptibility of B. pseudomallei against antibiotics is summarized in Table 6.
TABLE 6.
In vitro activities of selected antibiotics against B. pseudomallei
| Agent | Source (no. of isolates) | MIC (mg/liter)a
|
Reference(s) | ||
|---|---|---|---|---|---|
| 50% | 90% | Range | |||
| Amikacin | United Kingdom (12) | NRb | 64 | 32-64 | 297 |
| Amoxicillin | Various (127) | >64 | >64 | 32-256 | 18, 76 |
| Amoxicillin-clavulanate | Various (406) | 2-8 | 4-8 | 2/0.5->512 | 18, 76, 222, 232, 297, 392 |
| Ampicillin | Various (199) | 32-64 | 32->64 | 0.25->256 | 232, 402 |
| Ampicillin-sulbactam | Thailand (199) | 4 | 8 | 0.25-128 | 402 |
| Azithromycin | United Kingdom collection (unknown) | 64 | >64 | 1->64 | 232 |
| Azlocillin | Australia (100) | 2 | 4 | 2-16 | 18 |
| Aztreonam | Various (423) | 4-16 | 8-32 | 2->256 | 18, 76, 402, 497 |
| Biapenem | Thailand (124) | 0.25-0.5 | 0.25-2 | 0.06-2 | 392 |
| Carbenicillin | Canadian collection (20) | NR | 100 | 50-200 | 158 |
| Carumonam | Thailand, United Kingdom collection (211) | 2 | 4-8 | 0.5-256 | 297, 402 |
| Cefamandole | Australia (100) | >64 | >64 | >64 | 23 |
| Cefazolin | Australia (100) | >64 | >64 | >64 | 23 |
| Cefepime | Thailand (97) | 12.5 | 12.5 | 3.13-50 | 497 |
| Cefixime | Thailand (199) | 2 | 4 | 1-16 | 402 |
| Cefoperazone | Thailand, Hong Kong (27) | 8 | 16 | 8-16 | 76 |
| Cefoperazone-sulbactam | Malaysia (50) | NR | 4 | NR | 244 |
| Cefotaxime | Various (324) | 2-4 | 3.13-8 | 0.78-12.5 | 18, 23, 76, 497 |
| Cefoxitin | Australia (100) | >64 | >64 | >64 | 23 |
| Ceftazidime | Australian clinical (170) | 1-3 | 222 | ||
| Ceftazidime | Various (887) | 0.78-2 | 1-4 | 0.25-256 | 18, 76, 222, 232, 346, 392, 402, 497 |
| Ceftriaxone | Various (127) | 2-4 | 4-8 | 2-8 | 18, 76 |
| Cefuroxime | Various (127) | 16-64 | 32->64 | 16->64 | 23, 76, 232 |
| Cefuzonam | Thailand (97) | 3.13 | 6.25 | 1.56-25 | 497 |
| Cephalexin | Australia (100) | >64 | >64 | >64 | 23 |
| Cephalothin | Australian clinical isolates (100) | >64 | >64 | >64 | 23 |
| Chloramphenicol | Various (409) | 6.3-16 | 6.3-32 | 1.6->200 | 76, 155, 182, 222, 232, 346, 497 |
| Ciprofloxacin | Various (423) | 2-4 | 3.13-8 | 1-16 | 18, 76, 232, 402, 497 |
| Cycloserine | U.S. NIHc collection (51) | 32 | 42 | 8-64 | 182 |
| Doxycycline | Various (277) | 0.5-4 | 1-8 | 0.5-16 | 155, 182, 222, 232 |
| Enoxacin | Thailand (97) | 6.25 | 6.25 | 3.13-25 | 497 |
| Erythromycin | U.S. NIH collection (51) | 128 | 128 | 16-128 | 182 |
| Fosfomycin | Thailand (97) | >200 | >200 | >200 | 497 |
| Gentamicin | Various (63) | 32 | 64 | 0.125-128 | 182, 232, 297 |
| Imipenem | Various (590) | 0.39-0.5 | 0.78-1 | 0.12-8 | 222, 232, 392, 402, 497 |
| Kanamycin | U.S. NIH collection (51) | 8 | 32 | 1-32 | 182 |
| Lomefloxacin | Thailand (97) | 6.25 | 6.25 | 3.13-25 | 497 |
| Meropenem | Australia, Thailand (391) | 0.78-1 | 0.78-1 | 0.25-4 | 222, 392, 497 |
| Minocycline | Thailand (97) | 1.56 | 3.13 | 0.78-3.13 | 497 |
| Moxalactam | Australia (100) | 8 | 16 | 4-16 | 23 |
| Nalidixic acid | Thailand (97) | 25 | 50 | 3.13->200 | 497 |
| Neomycin | U.S. NIH collection (51) | 64 | 128 | 8->128 | 182 |
| Netilmicin | United Kingdom collection (12) | NR | 128 | 16-128 | 297 |
| Norfloxacin | Thailand, Australia (197) | 4-12.5 | 8-12.5 | 1-50 | 18, 497 |
| Novobiocin | U.S. NIH collection (51) | 8 | 16 | 4-32 | 182 |
| Ofloxacin | Various (124) | 2-6.25 | 6.25-8 | 0.78-12.5 | 76, 232, 497 |
| Panipenem | Thailand (124) | 0.5 | 1 | 0.06-16 | 392 |
| Piperacillin | Various (605) | 1-4 | 2-8 | 0.25-16 | 18, 76, 222, 232, 297, 402, 497 |
| Piperacillin-tazobactam | Thailand (199) | 1 | 1 | 0.25-8 | 402 |
| Rifampin | Various (175) | 8-25 | 16-64 | 3.13-128 | 76, 182, 232, 497 |
| Sulfamethoxazole | Various (97) | 16-50 | 50->64 | 12.5->64 | 232, 497 |
| Temafloxacin | Thailand (296) | 3.13-8 | 6.25-16 | 0.25-32 | 402, 497 |
| Tetracycline | Various (175) | 4-6.25 | 8-16 | 0.78-16 | 76, 182, 346, 497 |
| Ticarcillin | Various (326) | 128->256 | 256->256 | 64->256 | 18, 76, 402 |
| Ticarcillin-clavulanate | Thailand (199) | 4-16 | 4-16 | 1->256 | 18, 76, 402 |
| Trimethoprim | Thailand, United Kingdom collection (97) | 16-25 | 25->64 | 1.56->64 | 232, 497 |
| TMP-SMXd | Thailand (97) | 1/19-5/25 | 2/28->64 | 0.625/3.125-20/100 | 76, 222, 232, 331, 346, 497 |
| Tosufloxacin | Thailand (97) | 1.56 | 3.13 | 0.39-6.25 | 497 |
50% and 90%, MICs at which 50 and 90% of isolates are inhibited, respectively.
NR, not recorded.
NIH, National Institutes of Health.
MICs vary with testing method.
Ceftazidime, the carbapenem antibiotics (imipenem and meropenem), and to a lesser degree amoxicillin-clavulanate remain the backbone of current initial treatment. Primary resistance to these agents was not observed in 170 isolates from the Darwin prospective study, and ceftazidime resistance emerged on therapy in only one patient (222). Carbapenem antibiotics appear to be useful even for isolates exhibiting extended beta-lactamase activity (392).
Within the B. pseudomallei genome, seven genes encoding Ambler class A, B, and D beta-lactamases have been identified (197). Functionally, the most important of these is the Bush-Jacoby-Medeiros class 2e beta-lactamase BPS-1, encoded by the gene blaA (or penA; Ambler class A), which hydrolyzes most cephalosporins but is readily inhibited by clavulanate (91, 274). Acquired resistance to beta-lactam antibiotics while on treatment with a beta-lactam-beta-lactamase inhibitor combination or ceftazidime resulted from three distinct phenotypic changes: derepression of the chromosomal enzyme, an insensitivity to inhibition by beta-lactamase inhibitors, and a beta-lactamase specific for ceftazidime (169). These were associated with mutations in the blaA gene (439). Overexpression of the class D beta-lactamases, OXA-42 and OXA-43, may also be responsible for ceftazidime resistance in some isolates (316). Although a class C beta-lactamase was initially identified from the genome sequence (316), it is likely that this represents a class B metallo-beta-lactamase (197); the clinical significance of this beta-lactamase is not yet known, as resistance to carbepenems remains uncommon (392).
Antibiotics with bacteriostatic activity do not appear to be clinically useful in the intensive phase; these include the tetracyclines, chloramphenicol, the quinolones, and ceftriaxone (71, 88, 473). The significance of other in vitro phenomena such as postantibiotic effect (463) and results of time-kill studies (392) are not known but may confer a theoretical advantage to carbepenems.
The oral antibiotic TMP-SMX, with or without doxycycline and chloramphenicol, is used for the prolonged eradication phase. They have been demonstrated to have little activity in the acute phase (473) and are bacteriostatic in vitro (36, 126, 497). Primary resistance to chloramphenicol and doxycycline occurs infrequently (approximately 6%) (383, 497).
Testing for TMP-SMX resistance is problematic, with disk diffusion methods probably overestimating the extent of resistance. Methods that determine the MIC (ɛ-tests, broth microdilution, or agar dilution) are recommended and demonstrate much lower rates of primary resistance (3 to 10%) (331). However, resistance rates appear to be higher in Thailand (281); the clinical significance of this is unknown.
Acquired resistance to doxycycline has been observed when doxycycline has been used as monotherapy (222) and, much less frequently, with TMP-SMX monotherapy (111, 222). Acquired resistance to ceftazidime while on therapy is an uncommon cause of treatment failure, but acquired resistance more frequently occurs with chloramphenicol (126). Relapse attributable to resistance may occur with either oral or intravenous agents used in treatment (222, 435).
The use of combination therapy in the initial intensive phase is routine in the Northern Territory of Australia and in parts of Thailand, with the rationale of protection against the emergence of resistant strains during therapy and the improved intracellular penetration of TMP-SMX (111, 126, 400). However, in vitro studies suggest antagonism (127), although clinical evidence is lacking.
B. pseudomallei is susceptible to kanamycin, although this antibiotic is no longer used in the treatment of clinical melioidosis. Transposon mutation analysis has revealed that the efflux system AmrAB-OprA (encoded by the gene amr [aminoglycoside-macrolide resistance]) confers resistance. Although efflux systems conferring macrolide resistance have been described, this system is a unique mechanism for high-level aminoglycoside resistance (303, 486).
The use of in vivo models to test antibiotic combinations remains a research tool. Inglis et al. used synergy testing in Acanthamoeba trophozoites to guide antibiotic therapy in a patient failing treatment (214). Although successful in this case, this applicability of this remains uncertain, and it is unlikely to gain widespread acceptance. Similarly, the BALB/c inbred mouse model has also been employed to determine candidate interventions, with similar caveats (340, 442).
More recent attention has focused on the role of biofilms in protecting bacteria against antibiotics. In one such study, B. pseudomallei was incubated on a silastic surface for 16 h. Electron microscopy demonstrated that cells in biofilm were still viable after 24 h of exposure, with up to 200 times the MIC for the planktonic cells (800 μg of ceftazidime per ml and 8,000 μg of TMP-SMX per ml) (461). Antibiotic combinations active against B. pseudomallei in biofilm were shown to be ciprofloxacin-clarithromycin, ciprofloxacin-azithromycin, and imipenem-azithromycin; the clinical relevance of these findings is not known (460).
Antibiotic Treatment
Six published randomized controlled trials and one unpublished randomized controlled trial have examined intensive-phase interventions in severe melioidosis and are the basis of the ceftazidime-based regimens used currently (Table 7). For eradication-phase therapy (also pessimistically known as maintenance therapy), three published trials and one unpublished trial have examined the role of oral antibiotics in preventing relapse (Table 8). The recommended intensive and eradication antibiotic regimens used in Thailand and Australia differ significantly; details are presented in Tables 9 and 10, respectively. These interventions were the subject of a systematic review (367).
TABLE 7.
Clinical trials of intensive-phase intravenous antibiotics in severe melioidosis
| Reference | Regimen (dose, mg/kg/day) | Duration (days) | No. of patients
|
Outcome measures (%)
|
||
|---|---|---|---|---|---|---|
| Enrolled | Culture confirmed | Treatment failure | Mortality | |||
| 473 | Ceftazidime (120) vs chloramphenicol (100), doxycycline (4), and TMP-SMX (10/50) | At least 7 | 161 | 34 vs 31 | 37 vs 74 | |
| 400 | Ceftazidime (100) and TMP-SMX (8/40) vs chloramphenicol (100), doxycycline (4), and TMP-SMX (8/40) | 10-14 | 136 | 27 vs 34 | 18.5 vs 47 | |
| 419 | Ceftazidime (120) vs amoxicillin-clavulanate (120/40) | At least 7 | 379 | 106 vs 105 | 39 vs 51 | 47 vs 47 |
| 383 | Ceftazidime (120) vs imipenem (50) | At least 10 | 296 | 106 vs 108 | 41 vs 20 | 38 vs 36 |
| 423 | Ceftazidime (25) and co-trimoxazole (8/40) vs cefoperazone-sulbactam (100) and co-trimoxazole (8/40) | 84 | 20 vs 20 | 21 vs 16 | ||
| 90 | Ceftazidime (100) and TMP-SMX (8/40) vs Cefoperazone-sulbactam (25/25) | 14 | 219 | 51 vs 51 | 14 vs 18 | |
| W. Chierakul et al., unpublished data | Ceftazidime (120) vs ceftazidime (120) and TMP-SMX (10/50) | 10 days | 449 | 118 vs 123 | No significant difference | |
TABLE 8.
Clinical trials of eradication-phase oral antibiotics in treatment of melioidosis
| Reference | Regimen (dose, mg/kg/day) | Duration (wk) | No. of patients | Relapse rate (%) |
|---|---|---|---|---|
| 350 | Amoxicillin-clavulanate (60/15) vs chloramphenicol (40), doxycycline (4), and TMP-SMX (10/50) | 20 vs 20 | 49 vs 52 | 16 vs 4 |
| 70 | Doxycycline (4) vs chloramphenicol (40), doxycycline (4), and TMP-SMX (10/50) | 20 vs 20 | 58 vs 58 | 26 vs 1 |
| 88 | Azithromycin (8) and ciprofloxacin (8) vs doxycycline (4) and TMP-SMX (10/50) | 12 vs 20 | 36 vs 29 | 22 vs 3 |
| W. Chaowagul, unpublished data | Doxycycline (4) and TMP-SMX (10/50) vs chloramphenicol (40), doxycycline (4), and TMP-SMX (10/50) | 12-20 vs 12-20 | 89 vs 91 | No significant differences |
TABLE 9.
Intensive-phase antibiotic regimens used for patients with normal renal function
| Regimen
| ||
|---|---|---|
| Royal Darwin Hospital (111) | Other recommended (472) | |
| (i) TMP-SMX at 8/40 mg/kg (up to 320/1,600 mg) every 12 h and ceftazidime at 50 mg/kg (up to 2 g) intravenously every 6 h or (ii) meropenem at 25 mg/kg (up to 1 g) intravenously every 8 h and G-CSF (filgrastim) 300 μg intravenously for 10 days if patient has septic shock (duration of therapy at least 14 days, and longer (4 to 8 weeks) for deep-seated infection, osteomyelitis, or septic arthritis; patients may be discharged for outpatient administration of ceftazidime if clinically stable) | (i) Ceftazidime at 40 mg/kg intravenously every 8 ha, (ii) ceftazidime at 19 mg/kg intravenously (bolus) followed by 3.5 mg/kg per h as a continuous infusion, (iii) imipenem at 20 mg/kg intravenously every 8 hb, or (iv) ampicillin-clavulanate at 20/4 mg/kg intravenously every 4 hc (duration of therapy at least 10 days or until clear clinical improvement) | |
Without TMP-SMX; first-line regimen at Sapprasithiprasong Hospital, Ubon Ratchathani.
Second-line regimen for treatment failure with ceftazidime at Sapprasithiprasong Hospital.
Second-line regimen for empiric treatment of melioidosis at Sapprasithiprasong Hospital.
TABLE 10.
Eradication-phase antibiotic regimens used for patients with normal renal function
| Regimen
| ||
|---|---|---|
| Royal Darwin Hospital (111) | Other recommended (472) | |
| TMP-SMX at 8/40 mg/kg (up to 320/1,600 mg) every 12 h (duration of therapy at least 3-6 months; close follow-up and monitoring of adherence are important) | (i) Chloramphenicol at 10 mg/kg orally four times a day for 8 weeks, doxycycline at 2 mg/kg orally twice a day for at least 20 weeks, and TMP-SMX at 5/25 mg/kg orally twice a day for at least 20 weeksa or (ii) amoxicillin-clavulanate at 30/15 mg/kg orally tds for 20 weeks and amoxicillin at 30 mg/kg orally tds for 20 weeksb | |
First-line regimen at Sapprasithiprasong Hospital.
Second-line regimen for adults and first-line regimen for children (<14 years) and pregnant women at Sapprasithiprasong Hospital. tds, three times a day.
The only treatment to demonstrate a mortality benefit is ceftazidime, in a sequential open-label randomized trial of ceftazidime against chloramphenicol-doxycycline-TMP-SMX (known as conventional therapy) in severe disease. In Thai adults, the use of ceftazidime was associated with a 50% reduction in mortality, from 74 to 37%. Survival curves suggested that the benefit was seen after 48 h, suggesting irreversible severe disease in those who died (473). There results were replicated in another Thai center, Khon Kaen, where a fall in mortality from 47 to 19% was observed in association with ceftazidime with TMP-SMX (400). In that study, the group that received conventional therapy had more severe disease, which may have partially accounted for the mortality difference observed.
Ceftazidime-based regimens have been used as the control arm for studies of the intensive phase; studies since have failed to demonstrate improved outcomes with amoxicillin-clavulanate, cefoperazone-sulbactam, or imipenem. An important issue in these studies is the wide variation in the baseline mortality rate, ranging from 14 to 47% in ceftazidime-based arms. This is likely to represent differences in the severity of illness due to differences in inclusion criteria. In addition, the timing of enrollment, reflecting in part the use of rapid diagnostics such as immunofluorescence, is likely to affect observed mortality rates, as a lower mortality is seen in patients who survive the first 24 to 48 h of admission. The systematic review highlighted the need for a severity-of-illness scoring system (367).
Intravenous amoxicillin-clavulanate is not available in Australia but is widely used in Thailand for the treatment of empirical sepsis and melioidosis. Pharmacokinetic considerations dictate an increased frequency of dosing (every 4 h) to ensure adequate trough levels of clavulanic acid (125). A clinical trial of this agent in intensive therapy demonstrated a similar mortality but a higher treatment failure rate requiring a change in antibiotic regimen (25 versus 5%) (419). In maintenance therapy, use of oral amoxicillin-clavulanate (with supplemental amoxicillin) was associated with a higher relapse rate than conventional treatment with TMP-SMX, doxycycline, and chloramphenicol (16 versus 4%); however, only 50% of patients adhered to the full 20-week duration of therapy, and this appeared to be the most important factor in determining relapse (350).
Imipenem-cilistatin was compared with ceftazidime in a large trial; this trial was terminated prior to planned enrollment due to the withdrawal of pharmaceutical company support. Mortality was not different overall, even with adjustment for known prognostic factors. The use of imipenem was associated with lower rates of treatment failure in patients surviving for >48 h (20 versus 41%) than the use of ceftazidime; however, this may have been a potentially subjective endpoint in this open-label trial (383). This may explain the high rate of treatment failure in the ceftazidime arm of this trial compared to the ceftazidime arm in the amoxicillin-clavulanate trial conducted at the same center (419), despite similar definitions of treatment failure.
Current studies include a trial of ceftazidime alone compared with ceftazidime with TMP-SMX, where no significant differences in mortality were demonstrated (S. Anunnatsiri, Abstr 4th World Melioidosis Congr., abstr 10, 2004). Future studies of meropenem against ceftazidime, particularly in light of the incomplete carbapenem study, will be of interest, particularly in regard to the treatment of severe sepsis, where a lower MIC, a more favorable time-kill profile, and lower endotoxin release may be of theoretical advantage. This is supported by a retrospective review of meropenem use in Australia, where a lower mortality was seen in meropenem-treated patients with severe sepsis, although this may be confounded by other factors (78).
Although in treatment of uncomplicated melioidosis it is usual for blood cultures to become negative within a few days, defevescence may take somewhat longer. In one trial, the median duration of fever following commencement of antibiotics was 9 days, with an interquartile range of 4.5 to 15 days (range of up to 39 days) (383), similar to that seen with another study (10 to 11 days) (90).
Although patients who survived had lower rates of bacteremia (50 to 63%) than nonsurvivors, 10 to 18% of surviving patients remained bacteremic after 4 days of treatment, and 8 to 21% of surviving patients were bacteremic after 7 days of imipenem or ceftazidime, respectively (383). Patients with a fatal outcome often had cultures that were persistently positive until death (111). In addition, sputum cultures in uncomplicated pneumonia may take more than a week to become negative (111); persistent cultures from sites other than blood do not necessarily portend a poorer prognosis (419).
A method to reduce the cost of antibiotics required for treatment using continuous infusions of ceftazidime was explored in a pharmacokinetic study. In that study a bolus dose of 12 mg/kg intravenously followed by a constant infusion of 4 mg/kg/h was found to provide an adequate dose while allowing for a total lower dose to be delivered than with traditional 8-h bolus dosing (7). These data support the use of elastomeric infusion devices (Baxter, Sydney, Australia) through a peripherally inserted central catheter to complete intensive-phase antibiotic therapy for melioidosis in Australia (111, 206). However, this technology is currently expensive, with infusers and antibiotics costing approximately U.S. $100 per day, excluding staffing costs (206).
The clinical utility of measuring serum antibiotic levels by means of a bioassay was assessed in a Thai study (379). Pre- or postdose serum bactericidal and inhibitory titers did not appear to correlate with outcome in 195 adult patients, reflecting similar studies in other groups of patients with other infections (357).
Fewer studies examining eradication therapy have been performed. In Thailand, the four-drug regimen (chloramphenicol, doxycycline, and TMP-SMX) is commonly used, with a relapse rate of approximately 10% (350). Lower rates have been reported in Australia with TMP-SMX alone (111). Adherence to therapy (only 50% completed the 20-week course of therapy in the Thai study) is the most important factor predicting relapse; in addition, a duration of therapy of 8 weeks was associated with high rates of relapse (350, 416).
Doxycycline alone for eradication therapy is associated with unacceptable rates of relapse (26%) and treatment failure (16%) compared with the conventional regimen of TMP-SMX, doxycyline, and chloramphenicol. However, the latter is associated with high rates of adverse events (32% in this trial) that may limit adherence (70). Similarly, short-course (8-week) ciprofloxacin-azithromycin and longer-course (up to 20 weeks) quinolone monotherapy were also associated with high relapse rates of >20% (73, 88). A trial comparing the four-drug regimen with TMP-SMX and doxycycline has recently been completed in Thailand and suggests that TMP-SMX with doxycycline is associated with relapse rates equivalent to those with the four-drug regimen (W. Chaowagul, Abstr 4th World Melioidosis Congr., abstr. 6, 2004). An analysis of this study suggested that the failure to complete at least 12 weeks of therapy remained the most important determinant of relapse, reinforcing the necessity for adherence to therapy and the need to define a better-tolerated regimen. A future trial comparing TMP-SMX alone with TMP-SMX and doxycycline is planned.
Ceftriaxone has borderline activity against B. pseudomallei in vitro, but whether it is active in vivo is debated. In a retrospective review the initial use of ceftriaxone and cefotaxime was associated with a higher mortality than seen for patients treated empirically (71). However, no attempt was made to control for severity of infection or time to administration of antibiotics. The use of high-dose ceftriaxone (2 g intravenously daily) for the empirical treatment of community-acquired sepsis is widespread in Australia and in Thailand.
A systematic Cochrane review published in 2001 did not identify any further unpublished clinical trials. It supported the use of a ceftazidime- or imipenem-containing intensive therapy phase followed by a long oral eradication phase. It found little data available on the appropriate therapy for mild disease and for eradication therapy. Criticisms of trials identified included the failure to conceal allocation, the failure to use an intent-to-treat analysis, and poor standardization of markers of severity (367).
Animal models of prophylactic antibiotic regimens have been prompted by the potential for intentional release but may also be important when considering accidental laboratory exposure (364). In one study, prophylactic ciprofloxacin or doxycycline was demonstrated to raise the median lethal dose of B. pseudomallei when inoculated intraperitoneally. TMP-SMX would also be theoretically effective, but its use has not been assessed.
Future issues yet to be addressed include the definition of the minimum duration and regimen required for eradication, whether oral therapy without an initial intensive intravenous phase can be used for mild disease, and the optimal regimens for children, where relapse appears to be uncommon. Although the prolonged use of eradication-phase antibiotics is currently the norm, the observation that immunocompetent children with mild disease recover with incision and drainage only may suggest that some low-risk patients with limited disease may not even require antibiotic therapy (279). We also speculate that, analogous to the case for deep-seated staphylococcal infections, prolonged intensive-phase antibiotics may be sufficient without the need for eradication therapy. While appearing radical, these two ideas deserve further examination.
Management of Sepsis Syndrome
A detailed review of the management of sepsis is beyond the scope of this review and has been summarized in recent consensus guidelines (135). It should be noted that few of the interventions discussed are supported by evidence (Table 11), and none have included patients with melioidosis.
TABLE 11.
Interventions demonstrated to reduce mortality in critically ill patients (supported by evidence from clinical trials)
| Intervention | Evidence |
|---|---|
| Goal-directed therapy | Early goal-directed therapy (based on fluid and blood product infusions, inotropes, and ventilation with invasive monitoring) associated with a fall in mortality in a clinical trial (31 vs 47%) (359) |
| Insulin infusion | Intensive glycemic control (4.4 to 6.1 mmol/liter) by means of an insulin infusion associated with a fall in mortality in surgical intensive care patients in a clinical trial (8 vs 5%) (450) |
| Sedation protocol | Use of sedation protocols associated with reduced length of stay and duration of ventilation (57) |
| Polyclonal immunoglobulin | Use of polyclonal immunoglobulin associated with mortality benefit in a meta-analysis (relative risk, 0.64) (4); concerns about quality of the pooled studies |
| Activated protein C | Use of drotrecogin alfa in patients with severe sepsis associated with a fall in mortality in a large clinical trial (31 vs 25%) |
| Limiting tidal volumes | Ventilation with lower tidal volumes (6 ml/kg) in patients with acute lung injury or acute respiratory distress syndrome associated with a fall in mortality (40 vs 31%) (2); other smaller studies show conflicting results (135) |
| Physiological-dose steroids | Low-dose hydrocortisone and fludrocortisone in patients with relative adrenal insufficiency due to severe sepsis associated with a fall in mortality in a clinical trial (63 vs 53%) (8) |
| Deep venous thrombosis prophylaxis | Multiple large studies of heparin and/or mechanical devices in general intensive care unit populations (135) |
| Stress ulcer prophylaxis | Multiple large studies of proton pump inhibitors or histamine receptor antagonists (135) |
Much recent interest has focused on the use of drotrecogin alfa (activated protein C) in severe sepsis (42), where a small but significant mortality benefit has been demonstrated. Although its high cost makes it of little relevance to the majority of patients with severe melioidosis worldwide, it has been used in a small number of patients at the Royal Darwin Hospital (D. P. Stephens, personal communication). Activated protein C levels have been demonstrated to be low in severe melioidosis (254); there is no evidence of any pathophysiological differences between patients with severe sepsis due to B. pseudomallei and to other gram-negative organisms.
A negative trial that did include patients with melioidosis involved lexipafant, a platelet-activating factor receptor antagonist. In a randomized placebo-controlled trial in Thailand involving 131 patients, no significant difference in mortality was observed in patients with severe sepsis, including 36 patients with melioidosis (418).
Although exogenous administration of G-CSF has not been associated with improved outcomes in patients with pneumonia and/or severe sepsis in large clinical trials (310, 311, 361), melioidosis may differ from infections due to other organisms because of the key role of neutrophils in patients with recognized risk factors for neutrophil defects. G-CSF may reverse these defects (312), act to counter inflammatory cytokines (187, 471), and increase intracellular concentrations of antibiotics (131, 248, 298). The use of G-CSF in patients with septic shock due to melioidosis was associated with a large fall in mortality (from 95 to 10%); however, several confounding factors may at least partly account for this effect (84). A randomized, placebo-controlled trial is under way in Thailand.
Intensive glycemic control has been associated with improved outcomes in surgical intensive care patients in Belgium (450). Given the prevalence of diabetes and acute hyperglycemia in patients with melioidosis and its putative role in inducing neutrophil dysfunction, a clinical trial examining the role of insulin infusions for tight glycemic control would be of interest.
CONCLUSIONS
Melioidosis is a disease of public health importance in southeast Asia and northern Australia that has the potential for epidemic spread to areas where it is not endemic. Sporadic case reports elsewhere in the world suggest that as-yet-unrecognized foci of infection may exist. Environmental determinants of this infection, apart from a close association with rainfall, are yet to be elucidated.
Identification of virulence factors has been accelerated by the completion of genome sequencing of a strain of B. pseudomallei, but this information is yet to be translated into accurate, practical, and commercially available diagnostic tests. The presence of specific risk factors such as diabetes suggests that functional neutrophil defects are important in the pathogenesis of melioidosis. Other studies have defined virulence factors that allow evasion of killing mechanisms by phagocytes and a possible role for cell-mediated immunity. Whether a vaccine could prevent infection or severe disease due to B. pseudomallei remains to be seen. Economic constraints may make vaccination an unrealistic option for many regions of endemicity.
Despite improvements in antibiotic therapy, melioidosis is still associated with a significant mortality attributable to severe sepsis and its complications. Studies exploring the role of preventative measures, earlier clinical identification, and better management of severe sepsis are required to reduce the burden of this disease.
Acknowledgments
We are grateful to our colleagues in this field for their generosity in sharing unpublished data and information from ongoing studies. These include Nick White, Wipada Chaowagul, Sharon Peacock, Narisara Anuntagool, Wirongrong Chierakul, Don Woods, Paul Newton, Nick Day, Vicki Krause, Tim Inglis, Robert Norton, Vanaporn Wuthiekanun, Susan Jacups, Gary Lum, and David Dance. We are also grateful to the staff at Royal Darwin Hospital and Sappasithiprasong Hospital for supporting ongoing clinical research.
A.C.C. is supported by an Australian National Health and Medical Research Council Training Scholarship.
REFERENCES
- 1.Abbink, F. C., J. M. Orendi, and A. J. de Beaufort. 2001. Mother-to-child transmission of Burkholderia pseudomallei. N. Engl. J. Med. 344:1171-1172. [DOI] [PubMed] [Google Scholar]
- 2.Acute Respiratory Distress Syndrome Network. 2000. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 342:1301-1308. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed, K., H. D. Enciso, H. Masaki, M. Tao, A. Omori, P. Tharavichikul, and T. Nagatake. 1999. Attachment of Burkholderia pseudomallei to pharyngeal epithelial cells: a highly pathogenic bacteria with low attachment ability. Am. J. Trop. Med. Hyg. 60:90-93. [DOI] [PubMed] [Google Scholar]
- 4.Alejandria, M. M., M. A. Lansang, L. F. Dans, and J. B. Mantaring. 2001. Intravenous immunoglobulin for treating sepsis and septic shock (Cochrane Review). Cochrane Database Syst. Rev. 2:CD001090. [DOI] [PubMed] [Google Scholar]
- 5.Alibek, K. 1998. Statement by Dr. Kenneth Alibek. Terrorist and intelligence operations: potential impact on the U.S. economy. Joint Economic Committee, U.S. Congress, Washington, D.C.
- 6.Alibek, K. 1999. Biohazard. Random House, New York, N.Y.
- 7.Angus, B. J., M. D. Smith, Y. Suputtamongkol, H. Mattie, A. L. Walsh, V. Wuthiekanun, W. Chaowagul, and N. J. White. 2000. Pharmacokinetic-pharmacodynamic evaluation of ceftazidime continuous infusion vs intermittent bolus injection in septicaemic melioidosis. Br. J. Clin. Pharmacol. 50:184-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Annane, D., V. Sebille, C. Charpentier, P. E. Bollaert, B. Francois, J. M. Korach, G. Capellier, Y. Cohen, E. Azoulay, G. Troche, P. Chaumet-Riffaut, and E. Bellissant. 2002. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 288:862-871. [DOI] [PubMed] [Google Scholar]
- 9.Anonymous. 1992. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 40-1992. A 43-year-old Cambodian man with several years of recurrent bouts of fever and abdominal pain. N. Engl. J. Med. 327:1081-1087. [DOI] [PubMed] [Google Scholar]
- 10.Anuntagool, A., P. Intachote, P. Naigowit, and S. Sirisinha. 1996. Rapid antigen detection assay for identification of Burkholderia (Pseudomonas) pseudomallei infection. J. Clin. Microbiol. 34:975-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anuntagool, N., P. Aramsri, T. Panichakul, V. R. Wuthiekanun, R. Kinoshita, N. J. White, and S. Sirisinha. 2000. Antigenic heterogeneity of lipopolysaccharide among Burkholderia pseudomallei clinical isolates. Southeast Asian J. Trop. Med. Public Health 31:146-152. [PubMed] [Google Scholar]
- 12.Anuntagool, N., P. Intachote, V. Wuthiekanun, N. J. White, and S. Sirisinha. 1998. Lipopolysaccharide from nonvirulent Ara+ Burkholderia pseudomallei isolates is immunologically indistinguishable from lipopolysaccharide from virulent Ara− clinical isolates. Clin. Diagn. Lab. Immunol. 5:225-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anuntagool, N., P. Naigowit, V. Petkanchanapong, P. Aramsri, T. Panichakul, and S. Sirisinha. 2000. Monoclonal antibody-based rapid identification of Burkholderia pseudomallei in blood culture fluid from patients with community-acquired septicaemia. J. Med. Microbiol. 49:1075-1078. [DOI] [PubMed] [Google Scholar]
- 14.Anuntagool, N., P. Rugdech, and S. Sirisinha. 1993. Identification of specific antigens of Pseudomonas pseudomallei and evaluation of their efficacies for diagnosis of melioidosis. J. Clin. Microbiol. 31:1232-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Appassakij, H., K. R. Silpapojakul, R. Wansit, and M. Pornpatkul. 1990. Diagnostic value of the indirect hemagglutination test for melioidosis in an endemic area. Am. J. Trop. Med. Hyg. 42:248-253. [DOI] [PubMed] [Google Scholar]
- 16.Arakawa, M., T. Mitsui, R. Miki, and E. Yabuuchi. 1993. Chronic melioidosis: a report of the first case in Japan. Kansenshogaku Zasshi 67:154-162. [DOI] [PubMed] [Google Scholar]
- 17.Ashdown, L. R. 1979. An improved screening technique for isolation of Pseudomonas pseudomallei from clinical specimens. Pathology 11:293-297. [DOI] [PubMed] [Google Scholar]
- 18.Ashdown, L. R. 1988. In vitro activities of the newer β-lactam and quinolone antimicrobial agents against Pseudomonas pseudomallei. Antimicrob. Agents Chemother. 32:1435-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashdown, L. R. 1987. Indirect haemagglutination test for melioidosis. Med. J. Aust. 147:364-365. [DOI] [PubMed] [Google Scholar]
- 20.Ashdown, L. R. 1992. Melioidosis and safety in the clinical laboratory. J. Hosp. Infect. 21:301-306. [DOI] [PubMed] [Google Scholar]
- 21.Ashdown, L. R. 1981. Relationship and significance of specific immunoglobulin M antibody response in clinical and subclinical melioidosis. J. Clin. Microbiol. 14:361-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashdown, L. R. 1992. Serial serum C-reactive protein levels as an aid to the management of melioidosis. Am. J. Trop. Med. Hyg. 46:151-157. [DOI] [PubMed] [Google Scholar]
- 23.Ashdown, L. R., and R. J. Frettingham. 1984. In vitro activity of various cephalosporins against Pseudomonas pseudomallei. J. Infect. Dis. 150:779-780. [DOI] [PubMed] [Google Scholar]
- 24.Ashdown, L. R., and R. W. Guard. 1984. The prevalence of human melioidosis in Northern Queensland. Am. J. Trop. Med. Hyg. 33:474-478. [DOI] [PubMed] [Google Scholar]
- 25.Ashdown, L. R., R. W. Johnson, J. M. Koehler, and C. A. Cooney. 1989. Enzyme-linked immunosorbent assay for the diagnosis of clinical and subclinical melioidosis. J. Infect. Dis. 160:253-260. [DOI] [PubMed] [Google Scholar]
- 26.Ashdown, L. R., and J. M. Koehler. 1990. Production of hemolysin and other extracellular enzymes by clinical isolates of Pseudomonas pseudomallei. J. Clin. Microbiol. 28:2331-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Astry, C. L., G. A. Warr, and G. J. Jakab. 1983. Impairment of polymorphonuclear leukocyte immigration as a mechanism of alcohol-induced suppression of pulmonary antibacterial defenses. Am. Rev. Respir Dis. 128:113-117. [DOI] [PubMed] [Google Scholar]
- 28.Atkins, T., R. G. Prior, K. Mack, P. Russell, M. Nelson, P. C. Oyston, G. Dougan, and R. W. Titball. 2002. A mutant of Burkholderia pseudomallei, auxotrophic in the branched chain amino acid biosynthetic pathway, is attenuated and protective in a murine model of melioidosis. Infect. Immun. 70:5290-5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badsha, H., C. J. Edwards, and H. H. Chng. 2001. Melioidosis in systemic lupus erythematosus: the importance of early diagnosis and treatment in patients from endemic areas. Lupus 10:821-823. [DOI] [PubMed] [Google Scholar]
- 30.Bagdade, J. D., K. L. Nielson, and R. J. Bulger. 1972. Reversible abnormalities in phagocytic function in poorly controlled diabetic patients. Am. J. Med. Sci. 263:451-456. [DOI] [PubMed] [Google Scholar]
- 31.Bagdade, J. D., R. K. Root, and R. J. Bulger. 1974. Impaired leukocyte function in patients with poorly controlled diabetes. Diabetes 23:9-15. [DOI] [PubMed] [Google Scholar]
- 32.Bagdade, J. D., M. Stewart, and E. Walters. 1978. Impaired granulocyte adherence. A reversible defect in host defense in patients with poorly controlled diabetes. Diabetes 27:677-681. [DOI] [PubMed] [Google Scholar]
- 33.Barnes, D. J., T. Gottlieb, S. Naraqi, and R. Benn. 1991. The role of viruses and atypical organisms in the pathogenesis of adult pneumonia in Papua New Guinea. Papua New Guinea Med. J. 34:13-16. [PubMed] [Google Scholar]
- 34.Barnes, J. L., J. Warner, W. Melrose, D. Durrheim, R. Speare, J. C. Reeder, and N. Ketheesan. 2004. Adaptive immunity in melioidosis: a possible role for T cells in determining outcome of infection with Burkholderia pseudomallei. Clin. Immunol. 113:22-28. [DOI] [PubMed] [Google Scholar]
- 35.Barnes, P. F., M. D. Appleman, and M. M. Cosgrove. 1986. A case of melioidosis originating in North America. Am. Rev. Respir. Dis. 134:170-171. [DOI] [PubMed] [Google Scholar]
- 36.Bassett, D. C. 1971. The sensitivity of Pseudomonas pseudomallei to trimethoprim and sulphamethoxazole in vitro. J. Clin. Pathol. 24:798-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baumann, B. B., and E. T. Morita. 1967. Systemic melioidosis presenting as myocardial infarct. Ann. Intern. Med. 67:836-842. [DOI] [PubMed] [Google Scholar]
- 38.Beamer, P. R., P. L. Varney, W. G. Brown, F. McDowell, and B. Eck. 1948. Melioidosis. Report of second case from the Western Hemisphere, with bacteriologic studies on both cases. Am. J. Pathol. 24:717-718. [PubMed] [Google Scholar]
- 39.Beeker, A., K. D. Van de Stadt, and K. Bakker. 1999. Melioidosis. Neth. J. Med. 54:76-79. [DOI] [PubMed] [Google Scholar]
- 40.Ben, R. J., Y. Y. Tsai, J. C. Chen, and N. H. Feng. 2004. Non-septicemic Burkholderia pseudomallei liver abscess in a young man. J. Microbiol. Immunol. Infect. 37:254-257. [PubMed] [Google Scholar]
- 41.Berger, S. 2004. 10 April 2004, posting date. Background information on melioidosis in Singapore. Gideon online. ProMED-mail 20040411.0986. [Online.]
- 42.Bernard, G., J. Vincent, P. Laterre, S. LaRosa, J. Dhainaut, A. Lopez-Rodriguez, J. Steingrub, G. Garber, J. Helterbrand, E. Ely, C. J. Fisher, et al. 2001. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 344:699-709. [DOI] [PubMed] [Google Scholar]
- 43.Bharadwaj, R., A. Kagal, S. K. Deshpandey, S. A. Joshi, P. M. Khare, A. R. Junnarkar, and M. A. Phadke. 1995. Burkholderia pseudomallei and Indian plague-like illness. Lancet 346:975. (Erratum, 346:1172.) [DOI] [PubMed] [Google Scholar]
- 44.Bharadwaj, R., A. Kagal, S. K. Deshpandey, S. A. Joshi, P. M. Khare, A. R. Junnarkar, and M. A. Phadke. 1994. Outbreak of plague-like illness caused by Pseudomonas pseudomallei in Maharashtra, India. Lancet 344:1574. [DOI] [PubMed] [Google Scholar]
- 45.Biegeleisen, J. Z., R. Mosquera, and W. B. Cherry. 1964. A case of human melioidosis: clinical, epidemiological and laboratory findings. Am. J. Trop. Med. Hyg. 13:89-99. [DOI] [PubMed] [Google Scholar]
- 46.Boe, D. M., S. Nelson, P. Zhang, and G. J. Bagby. 2001. Acute ethanol intoxication suppresses lung chemokine production following infection with Streptococcus pneumoniae. J. Infect. Dis. 184:1134-1142. [DOI] [PubMed] [Google Scholar]
- 47.Brayton, R. G., P. E. Stokes, M. S. Schwartz, and D. B. Louria. 1970. Effect of alcohol and various diseases on leukocyte mobilization, phagocytosis and intracellular bacterial killing. N. Engl. J. Med. 282:123-128. [DOI] [PubMed] [Google Scholar]
- 48.Breitbach, K., K. Rottner, S. Klocke, M. Rohde, A. Jenzora, J. Wehland, and I. Steinmetz. 2003. Actin-based motility of Burkholderia pseudomallei involves the Arp 2/3 complex, but not N-WASP and Ena/VASP proteins. Cell Microbiol. 5:385-393. [DOI] [PubMed] [Google Scholar]
- 49.Bremmelgaard, A., I. Bygbjerg, and N. Hoiby. 1982. Microbiological and immunological studies in a case of human melioidosis diagnosed in Denmark. Scand. J. Infect. Dis. 14:271-275. [DOI] [PubMed] [Google Scholar]
- 50.Brett, P. J., D. DeShazer, and D. E. Woods. 1998. Burkholderia thailandensis sp. nov., a Burkholderia pseudomallei-like species. Int. J. Syst. Bacteriol. 48:317-320. [DOI] [PubMed] [Google Scholar]
- 51.Brett, P. J., D. Deshazer, and D. E. Woods. 1997. Characterization of Burkholderia pseudomallei and Burkholderia pseudomallei-like strains. Epidemiol. Infect. 118:137-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brett, P. J., D. C. Mah, and D. E. Woods. 1994. Isolation and characterization of Pseudomonas pseudomallei flagellin proteins. Infect. Immun. 62:1914-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brett, P. J., and D. E. Woods. 2000. Pathogenesis of and immunity to melioidosis. Acta Trop. 74:201-210. [DOI] [PubMed] [Google Scholar]
- 54.Brett, P. J., and D. E. Woods. 1996. Structural and immunological characterization of Burkholderia pseudomallei O-polysaccharide-flagellin protein conjugates. Infect. Immun. 64:2824-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brill, D. R., and J. D. Shoop. 1977. Sensitivity of radionuclide isotope brain scan in cerebral melioidosis: case report. J. Nucl. Med. 18:987-989. [PubMed] [Google Scholar]
- 56.Bromwich, A. F., and J. C. Hargrave. 1969. Melioidosis in an Australian aboriginal with lepromatous leprosy. Med. J. Aust. 1:638-639. [DOI] [PubMed] [Google Scholar]
- 57.Brook, A. D., T. S. Ahrens, R. Schaiff, D. Prentice, G. Sherman, W. Shannon, and M. H. Kollef. 1999. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit. Care Med. 27:2609-2615. [DOI] [PubMed] [Google Scholar]
- 58.Brook, M. D., B. Currie, and P. M. Desmarchelier. 1997. Isolation and identification of Burkholderia pseudomallei from soil using selective culture techniques and the polymerase chain reaction. J. Appl. Microbiol. 82:589-596. [PubMed] [Google Scholar]
- 59.Brown, A. E., D. A. Dance, Y. Suputtamongkol, W. Chaowagul, S. Kongchareon, H. K. Webster, and N. J. White. 1991. Immune cell activation in melioidosis: increased serum levels of interferon-gamma and soluble interleukin-2 receptors without change in soluble CD8 protein. J. Infect. Dis. 163:1145-1148. [DOI] [PubMed] [Google Scholar]
- 60.Brown, N. F., J. A. Boddey, C. P. Flegg, and I. R. Beacham. 2002. Adherence of Burkholderia pseudomallei cells to cultured human epithelial cell lines is regulated by growth temperature. Infect. Immun. 70:974-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown, N. F., A. E. Lew, and I. R. Beacham. 2000. Identification of new transposable genetic elements in Burkholderia pseudomallei using subtractive hybridisation. FEMS Microbiol. Lett. 183:73-79. [DOI] [PubMed] [Google Scholar]
- 62.Brundage, W. G., C. J. J. Thuss, and D. C. Walden. 1968. Four fatal cases of melioidosis in U. S. soldiers in Vietnam. Bacteriologic and pathologic characteristics. Am. J. Trop. Med. Hyg. 17:183-191. [DOI] [PubMed] [Google Scholar]
- 63.Bryan, L. E., S. Wong, D. E. Woods, D. A. B. Dance, and W. Chaowagul. 1994. Passive protection of diabetic rats with antisera specific for the polysaccharide portion of the lipopolysaccharide isolated from Pseudomonas pseudomallei. Can. J. Infect. Dis. 5:170-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burtnick, M., A. Bolton, P. Brett, D. Watanabe, and D. Woods. 2001. Identification of the acid phosphatase (acpA) gene homologues in pathogenic and non-pathogenic Burkholderia spp. facilitates TnphoA mutagenesis. Microbiology 147:111-120. [DOI] [PubMed] [Google Scholar]
- 65.Carlson, P., and M. Seppanen. 2000. Melioidosis presenting as urinary tract infection in a previously healthy tourist. Scand. J. Infect. Dis. 32:92-93. [DOI] [PubMed] [Google Scholar]
- 66.Chadwick, D. R., B. Ang, Y. Y. Sitoh, and C. C. Lee. 2002. Cerebral melioidosis in Singapore: a review of five cases. Trans. R. Soc. Trop. Med. Hyg. 96:72-76. [DOI] [PubMed] [Google Scholar]
- 67.Chambon, L. 1955. Isolement du bacille de Whitmore a partir du milieu exterieur. Ann. Inst. Pasteur 89:229-235. [PubMed] [Google Scholar]
- 68.Chan, C. K., R. H. Hyland, W. D. Leers, M. A. Hutcheon, and D. Chang. 1984. Pleuropulmonary melioidosis in a Cambodian refugee. Can. Med. Assoc. J. 131:1365-1367. [PMC free article] [PubMed] [Google Scholar]
- 69.Chan, K., J. Raghuram, J. Low, and A. Kurup. 2003. Epidemiologic characteristics and prognostic determinants of death in severe melioidosis requiring intensive care. Crit. Care Med. 31:A120. [Google Scholar]
- 70.Chaowagul, W., A. J. Simpson, Y. Suputtamongkol, M. D. Smith, B. J. Angus, and N. J. White. 1999. A comparison of chloramphenicol, trimethoprim-sulfamethoxazole, and doxycycline with doxycycline alone as maintenance therapy for melioidosis. Clin. Infect. Dis. 29:375-380. [DOI] [PubMed] [Google Scholar]
- 71.Chaowagul, W., A. J. Simpson, Y. Suputtamongkol, and N. J. White. 1999. Empirical cephalosporin treatment of melioidosis. Clin. Infect. Dis. 28:1328. [DOI] [PubMed] [Google Scholar]
- 72.Chaowagul, W., Y. Suputtamongkol, D. A. Dance, A. Rajchanuvong, J. Pattara-Arechachai, and N. J. White. 1993. Relapse in melioidosis: incidence and risk factors. J. Infect. Dis. 168:1181-1185. [PubMed] [Google Scholar]
- 73.Chaowagul, W., Y. Suputtamongkol, M. D. Smith, and N. J. White. 1997. Oral fluoroquinolones in the maintenance treatment of melioidosis. Trans. R. Soc. Trop. Med. Hyg. 91:599-601. [DOI] [PubMed] [Google Scholar]
- 74.Chaowagul, W., N. J. White, D. A. Dance, Y. Wattanagoon, P. Naigowit, T. M. Davis, S. Looareesuwan, and N. Pitakwatchara. 1989. Melioidosis: a major cause of community-acquired septicemia in northeastern Thailand. J. Infect. Dis. 159:890-899. [DOI] [PubMed] [Google Scholar]
- 75.Charuchaimontri, C., Y. Suputtamongkol, C. Nilakul, W. Chaowagul, P. Chetchotisakd, N. Lertpatanasuwun, S. Intaranongpai, P. J. Brett, and D. E. Woods. 1999. Antilipopolysaccharide II: an antibody protective against fatal melioidosis. Clin. Infect. Dis. 29:813-818. [DOI] [PubMed] [Google Scholar]
- 76.Chau, P. Y., W. S. Ng, Y. K. Leung, and S. Lolekha. 1986. In vitro susceptibility of strains of Pseudomonas pseudomallei isolated in Thailand and Hong Kong to some newer beta-lactam antibiotics and quinolone derivatives. J. Infect. Dis. 153:167-170. [DOI] [PubMed] [Google Scholar]
- 77.Chen, Y. S., S. C. Chen, C. M. Kao, and Y. L. Chen. 2003. Effects of soil pH, temperature and water content on the growth of Burkholderia pseudomallei. Folia Microbiol. (Prague) 48:253-256. [DOI] [PubMed] [Google Scholar]
- 78.Cheng, A. C., D. A. Fisher, N. M. Anstey, D. P. Stephens, S. P. Jacups, and B. J. Currie. 2004. Outcomes of patients with melioidosis treated with meropenem. Antimicrob. Agents Chemother. 48:1763-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheng, A. C., D. Godoy, M. Mayo, D. Gal, B. G. Spratt, and B. J. Currie. 2004. Isolates of Burkholderia pseudomallei from northern Australia are distinct by multilocus sequence typing, but strain types do not correlate with clinical presentation. J. Clin. Microbiol. 42:5477-5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheng, A. C., J. N. Hanna, R. Norton, S. L. Hills, J. Davis, V. L. Krause, G. Dowse, T. J. Inglis, and B. J. Currie. 2003. Melioidosis in northern Australia, 2001-02. Commun. Dis. Intell. 27:272-277. [DOI] [PubMed] [Google Scholar]
- 81.Cheng, A. C., S. P. Jacups, N. M. Anstey, and B. J. Currie. 2003. A proposed scoring system for predicting mortality in melioidosis. Trans. R. Soc. Trop. Med. Hyg. 97:577-581. [DOI] [PubMed] [Google Scholar]
- 82.Cheng, A. C., M. J. Mayo, D. Gal, and B. J. Currie. 2003. Chlorination and pH of drinking water do not correlate with rates of melioidosis in the Northern Territory, Australia. Trans. R. Soc. Trop. Med. Hyg. 97:511-512. [DOI] [PubMed] [Google Scholar]
- 83.Cheng, A. C., M. Obrien, S. P. Jacups, N. M. Anstey, and B. J. Currie. 2004. C-reactive protein in the diagnosis of melioidosis. Am. J. Trop. Med. Hyg. 70:580-582. [PubMed] [Google Scholar]
- 84.Cheng, A. C., D. P. Stephens, N. M. Anstey, and B. J. Currie. 2004. Adjunctive granulocyte colony-stimulating factor for treatment of septic shock due to melioidosis. Clin. Infect. Dis. 38:32-37. [DOI] [PubMed] [Google Scholar]
- 85.Cheng, A. C., D. P. Stephens, and B. J. Currie. 2003. Granulocyte colony stimulating factor (G-CSF) as an adjunct to antibiotics in the treatment of pneumonia in adults. Cochrane Database Syst. Rev. CD004400. [DOI] [PubMed]
- 86.Chenthamarakshan, V., M. V. Kumutha, J. Vadivelu, and S. D. Puthucheary. 2001. Distribution of immunoglobulin classes and IgG subclasses against a culture filtrate antigen of Burkholderia pseudomallei in melioidosis patients. J. Med. Microbiol. 50:55-61. [DOI] [PubMed] [Google Scholar]
- 87.Cherian, T., P. Raghupathy, and T. J. John. 1995. Plague in India. Lancet 345:258-259. [PubMed] [Google Scholar]
- 88.Chetchotisakd, P., W. Chaowagul, P. Mootsikapun, D. Budhsarawong, and B. Thinkamrop. 2001. Maintenance therapy of melioidosis with ciprofloxacin plus azithromycin compared with cotrimoxazole plus doxycycline. Am. J. Trop. Med. Hyg. 64:24-27. [DOI] [PubMed] [Google Scholar]
- 89.Chetchotisakd, P., P. Mootsikapun, S. Anunnatsiri, K. Jirarattanapochai, C. Choonhakarn, A. Chaiprasert, P. N. Ubol, L. J. Wheat, and T. E. Davis. 2000. Disseminated infection due to rapidly growing mycobacteria in immunocompetent hosts presenting with chronic lymphadenopathy: a previously unrecognized clinical entity. Clin. Infect. Dis. 30:29-34. [DOI] [PubMed] [Google Scholar]
- 90.Chetchotisakd, P., S. Porramatikul, P. Mootsikapun, S. Anunnatsiri, and K. Kean. 2001. Randomized, double-blind, controlled study of cefoperazone-sulbactam plus cotrimoxazole verses ceftazidime plus cotrimoxazole for the treatment of severe melioidosis. Clin. Infect. Dis. 33:29-34. [DOI] [PubMed] [Google Scholar]
- 91.Cheung, T. K., P. L. Ho, P. C. Woo, K. Y. Yuen, and P. Y. Chau. 2002. Cloning and expression of class A beta-lactamase gene blaA(BPS) in Burkholderia pseudomallei. Antimicrob. Agents Chemother. 46:1132-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chierakul, W., A. Rajanuwong, V. Wuthiekanun, N. Teerawattanasook, M. Gasiprong, A. Simpson, W. Chaowagul, and N. J. White. 2004. The changing pattern of bloodstream infections associated with the rise in HIV prevalence in northeastern Thailand. Trans. R. Soc. Trop. Med. Hyg. 98:678-686. [DOI] [PubMed] [Google Scholar]
- 93.Chodimella, U., W. L. Hoppes, S. Whalen, A. J. Ognibene, and G. W. Rutecki. 1997. Septicemia and suppuration in a Vietnam veteran. Hosp. Pract. 32:219-221. [DOI] [PubMed] [Google Scholar]
- 94.Christenson, B., Z. Fuxench, J. A. Morales, R. A. Suarez-Villamil, and L. M. Souchet. 2003. Severe community-acquired pneumonia and sepsis caused by Burkholderia pseudomallei associated with flooding in Puerto Rico. Bol. Asoc. Med. P.R. 95:17-20. [PubMed] [Google Scholar]
- 95.Christenson Bravo, B., J. E. Rodriguez, G. Vazquez, and C. H. Ramirez Ronda. 1986. Pseudomonas pseudomallei (melioidosis): acute septicemia and meningitis in patient with systemic lupus erythematosus. Bol. Asoc. Med. P.R. 78:347-349. [PubMed] [Google Scholar]
- 96.Chua, K. L., Y. Y. Chan, and Y. H. Gan. 2003. Flagella are virulence determinants of Burkholderia pseudomallei. Infect. Immun. 71:1622-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Clayton, A. J., R. S. Lisella, and D. G. Martin. 1973. Melioidosis: a serological survey in military personnel. Mil. Med. 138:24-26. [PubMed] [Google Scholar]
- 98.Clough, A. R., Z. Wang, R. S. Bailie, C. B. Burns, and B. J. Currie. 2003. Case-control study of the association between kava use and pneumonia in eastern Arnhem and Aboriginal communities (Northern Territory, Australia). Epidemiol. Infect. 131:627-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Coenye, T., J. J. LiPuma, D. Henry, B. Hoste, K. Vandemeulebroecke, M. Gillis, D. P. Speert, and P. Vandamme. 2001. Burkholderia cepacia genomovar VI, a new member of the Burkholderia cepacia complex isolated from cystic fibrosis patients. Int. J. Syst. Evol. Microbiol. 51:271-279. [DOI] [PubMed] [Google Scholar]
- 100.Corkill, M. M., and B. Cornere. 1987. Melioidosis: a new disease to New Zealand. N.Z. Med. J. 100:106-107. [PubMed] [Google Scholar]
- 101.Cottew, G. S. 1950. Melioidosis in sheep in Queensland. A description of the causal organism. Aust. J. Exp. Biol. Med. Sci. 28:677-683. [PubMed] [Google Scholar]
- 102.Crotty, J. M., A. F. Bromich, and J. V. Quinn. 1963. Melioidosis in the Northern Territory: a report of two cases. Med. J. Aust. i:274-275. [PubMed] [Google Scholar]
- 103.Currie, B. 2001. Environmental change, global warming and infectious diseases in northern Australia. Environ. Health 1:35-44. [Google Scholar]
- 104.Currie, B. 1993. Melioidosis in Papua New Guinea: is it less common than in tropical Australia? Trans. R. Soc. Trop. Med. Hyg. 87:417. [DOI] [PubMed] [Google Scholar]
- 105.Currie, B. 1995. Pseudomonas pseudomallei-insulin interaction. Infect. Immun. 63:3745. (Letter.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Currie, B., D. Howard, V. T. Nguyen, K. Withnall, and A. Merianos. 1993. The 1990-1991 outbreak of melioidosis in the Northern Territory of Australia: clinical aspects. Southeast Asian J. Trop. Med. Public Health 24:436-443. [PubMed] [Google Scholar]
- 107.Currie, B., H. Smith Vaughan, C. Golledge, N. Buller, K. S. Sriprakash, and D. J. Kemp. 1994. Pseudomonas pseudomallei isolates collected over 25 years from a non-tropical endemic focus show clonality on the basis of ribotyping. Epidemiol. Infect. 113:307-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Currie, B. J. 2003. Melioidosis: an important cause of pneumonia in residents of and travellers returned from endemic regions. Eur. Respir. J. 22:542-550. [DOI] [PubMed] [Google Scholar]
- 109.Currie, B. J., D. A. Fisher, N. M. Anstey, and S. P. Jacups. 2000. Melioidosis: acute and chronic disease, relapse and re-activation. Trans. R. Soc. Trop. Med. Hyg. 94:301-304. [DOI] [PubMed] [Google Scholar]
- 110.Currie, B. J., D. A. Fisher, D. M. Howard, and J. N. Burrow. 2000. Neurological melioidosis. Acta Trop. 74:145-151. [DOI] [PubMed] [Google Scholar]
- 111.Currie, B. J., D. A. Fisher, D. M. Howard, J. N. Burrow, D. Lo, S. Selva-Nayagam, N. M. Anstey, S. E. Huffam, P. L. Snelling, P. J. Marks, D. P. Stephens, G. D. Lum, S. P. Jacups, and V. L. Krause. 2000. Endemic melioidosis in tropical northern Australia: a 10-year prospective study and review of the literature. Clin. Infect. Dis. 31:981-986. [DOI] [PubMed] [Google Scholar]
- 112.Currie, B. J., D. A. Fisher, D. M. Howard, J. N. Burrow, S. Selvanayagam, P. L. Snelling, N. M. Anstey, and M. J. Mayo. 2000. The epidemiology of melioidosis in Australia and Papua New Guinea. Acta Trop. 74:121-127. [DOI] [PubMed] [Google Scholar]
- 113.Currie, B. J., and S. P. Jacups. 2003. Intensity of rainfall and severity of melioidosis, Australia. Emerg. Infect. Dis. 9:1538-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Currie, B. J., S. P. Jacups, A. C. Cheng, D. A. Fisher, N. M. Anstey, S. E. Huffam, and V. L. Krause. 2004. Melioidosis epidemiology and risk factors from a prospective whole-population study in northern Australia. Trop. Med. Int. Health 9:1167-1174. [DOI] [PubMed] [Google Scholar]
- 115.Currie, B. J., M. Mayo, N. M. Anstey, P. Donohoe, A. Haase, and D. J. Kemp. 2001. A cluster of melioidosis cases from an endemic region is clonal and is linked to the water supply using molecular typing of Burkholderia pseudomallei isolates. Am. J. Trop. Med. Hyg. 65:177-179. [DOI] [PubMed] [Google Scholar]
- 116.Cuzzubbo, A. J., V. Chenthamarakshan, J. Vadivelu, S. D. Puthucheary, D. Rowland, and P. L. Devine. 2000. Evaluation of a new commercially available immunoglobulin M and immunoglobulin G immunochromatographic test for diagnosis of melioidosis infection. J. Clin. Microbiol. 38:1670-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dai, J. H., Y. S. Lee, and H. C. Wong. 1992. Effects of iron limitation on production of a siderophore, outer membrane proteins, and hemolysin and on hydrophobicity, cell adherence, and lethality for mice of Vibrio parahaemolyticus. Infect. Immun. 60:2952-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dance, D. A. 2000. Melioidosis as an emerging global problem. Acta Trop. 74:115-119. [DOI] [PubMed] [Google Scholar]
- 119.Dance, D. A., T. M. Davis, Y. Wattanagoon, W. Chaowagul, P. Saiphan, S. Looareesuwan, V. Wuthiekanun, and N. J. White. 1989. Acute suppurative parotitis caused by Pseudomonas pseudomallei in children. J. Infect. Dis. 159:654-660. [DOI] [PubMed] [Google Scholar]
- 120.Dance, D. A., C. King, H. Aucken, C. D. Knott, P. G. West, and T. L. Pitt. 1992. An outbreak of melioidosis in imported primates in Britain. Vet. Rec. 130:525-529. [DOI] [PubMed] [Google Scholar]
- 121.Dance, D. A., D. Sanders, T. L. Pitt, and D. C. Speller. 1995. Burkholderia pseudomallei and Indian plague-like illness. Lancet 346:904-905. [DOI] [PubMed] [Google Scholar]
- 122.Dance, D. A., M. D. Smith, H. M. Aucken, and T. L. Pitt. 1999. Imported melioidosis in England and Wales. Lancet 353:208. [DOI] [PubMed] [Google Scholar]
- 123.Dance, D. A., M. D. Smith, V. Wuthiekanun, M. Walsh, and N. J. White. 1992. Melioidosis and laboratory safety. J. Hosp. Infect. 22:333-334. [DOI] [PubMed] [Google Scholar]
- 124.Dance, D. A., N. J. White, Y. Suputtamongkol, Y. Wattanagoon, V. Wuthiekanun, and W. Chaowagul. 1990. The use of bone marrow culture for the diagnosis of melioidosis. Trans. R. Soc. Trop. Med. Hyg. 84:585-587. [DOI] [PubMed] [Google Scholar]
- 125.Dance, D. A., V. Wuthiekanun, W. Chaowagul, and N. J. White. 1989. The activity of amoxycillin/clavulanic acid against Pseudomonas pseudomallei. J. Antimicrob. Chemother. 24:1012-1014. [DOI] [PubMed] [Google Scholar]
- 126.Dance, D. A., V. Wuthiekanun, W. Chaowagul, and N. J. White. 1989. The antimicrobial susceptibility of Pseudomonas pseudomallei. Emergence of resistance in vitro and during treatment. J. Antimicrob. Chemother. 24:295-309. [DOI] [PubMed] [Google Scholar]
- 127.Dance, D. A., V. Wuthiekanun, W. Chaowagul, and N. J. White. 1989. Interactions in vitro between agents used to treat melioidosis. J. Antimicrob. Chemother. 24:311-316. [DOI] [PubMed] [Google Scholar]
- 128.Dance, D. A., V. Wuthiekanun, P. Naigowit, and N. J. White. 1989. Identification of Pseudomonas pseudomallei in clinical practice: use of simple screening tests and API 20NE. J. Clin. Pathol. 42:645-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dance, D. A. B. 1990. Melioidosis. Rev. Med. Microbiol. 1:143-150. [Google Scholar]
- 130.Dance, D. A. B. 1991. Melioidosis: the tip of the iceberg? Clin. Microbiol. Rev. 4:52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Daschner, F. D., H. Grundmann, K. Anding, and S. Lemmen. 1995. Combined effect of human neutrophils, ceftazidime and granulocyte colony-stimulating factor on killing of Escherichia coli. Eur. J. Clin. Microbiol. Infect. Dis. 14:536-539. [DOI] [PubMed] [Google Scholar]
- 132.De Buse, P. J., A. Henderson, and M. White. 1975. Melioidosis in a child in Papua New Guinea successful treatment with kanamycin and trimethoprim-sulphamethoxazole. Med. J. Aust. 2:476-478. [PubMed] [Google Scholar]
- 133.Dejsirilert, S., E. Kondo, D. Chiewslip, and K. Kanai. 1991. Growth and survival of Pseudomonas pseudomallei in acidic environments. Jpn. J. Med. Sci. Biol. 44:63-74. [DOI] [PubMed] [Google Scholar]
- 134.de Lalla, F., G. Pellizzer, M. Strazzabosco, Z. Martini, G. Du Jardin, L. Lora, P. Fabris, P. Benedetti, and G. Erle. 2001. Randomized prospective controlled trial of recombinant granulocyte colony-stimulating factor as adjunctive therapy for limb-threatening diabetic foot infection. Antimicrob. Agents Chemother. 45:1094-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dellinger, R. P., J. M. Carlet, H. Masur, H. Gerlach, T. Calandra, J. Cohen, J. Gea-Banacloche, D. Keh, J. C. Marshall, M. M. Parker, G. Ramsay, J. L. Zimmerman, J. L. Vincent, and M. M. Levy. 2004. Surviving Sepsis Campaign: guidelines for management of severe sepsis and septic shock. Intensive Care Med. 30:536-555. [DOI] [PubMed] [Google Scholar]
- 136.Desakorn, V., M. D. Smith, V. Wuthiekanun, D. A. Dance, H. Aucken, P. Suntharasamai, A. Rajchanuwong, and N. J. White. 1994. Detection of Pseudomonas pseudomallei antigen in urine for the diagnosis of melioidosis. Am. J. Trop. Med. Hyg. 51:627-633. [DOI] [PubMed] [Google Scholar]
- 137.DeShazer, D., P. J. Brett, M. N. Burtnick, and D. E. Woods. 1999. Molecular characterization of genetic loci required for secretion of exoproducts in Burkholderia pseudomallei. J. Bacteriol. 181:4661-4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.DeShazer, D., P. J. Brett, R. Carlyon, and D. E. Woods. 1997. Mutagenesis of Burkholderia pseudomallei with Tn5-OT182: isolation of motility mutants and molecular characterization of the flagellin structural gene. J. Bacteriol. 179:2116-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.DeShazer, D., P. J. Brett, and D. E. Woods. 1998. The type II O-antigenic polysaccharide moiety of Burkholderia pseudomallei lipopolysaccharide is required for serum resistance and virulence. Mol. Microbiol. 30:1081-1100. [DOI] [PubMed] [Google Scholar]
- 140.Desmarchelier, P. M., D. A. Dance, W. Chaowagul, Y. Suputtamongkol, N. J. White, and T. L. Pitt. 1993. Relationships among Pseudomonas pseudomallei isolates from patients with recurrent melioidosis. J. Clin. Microbiol. 31:1592-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dharakul, T., and S. Songsivilai. 1999. The many facets of melioidosis. Trends Microbiol. 7:138-140. [DOI] [PubMed] [Google Scholar]
- 142.Dharakul, T., S. Songsivilai, N. Anuntagool, W. Chaowagul, S. Wongbunnate, P. Intachote, and S. Sirisinha. 1997. Diagnostic value of an antibody enzyme-linked immunosorbent assay using affinity-purified antigen in an area endemic for melioidosis. Am. J. Trop. Med. Hyg. 56:418-423. [DOI] [PubMed] [Google Scholar]
- 143.Dharakul, T., S. Songsivilai, S. Smithikarn, C. Thepthai, and A. Leelaporn. 1999. Rapid identification of Burkholderia pseudomallei in blood cultures by latex agglutination using lipopolysaccharide-specific monoclonal antibody. Am. J. Trop. Med. Hyg. 61:658-662. [DOI] [PubMed] [Google Scholar]
- 144.Dharakul, T., S. Songsivilai, S. Viriyachitra, V. Luangwedchakarn, B. Tassaneetritap, and W. Chaowagul. 1996. Detection of Burkholderia pseudomallei DNA in patients with septicemic melioidosis. J. Clin. Microbiol. 34:609-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dharakul, T., S. Vejbaesya, W. Chaowagul, P. Luangtrakool, H. A. Stephens, and S. Songsivilai. 1998. HLA-DR. and -DQ associations with melioidosis. Hum. Immunol. 59:580-586. [DOI] [PubMed] [Google Scholar]
- 146.Dorman, S. E., V. J. Gill, J. I. Gallin, and S. M. Holland. 1998. Burkholderia pseudomallei infection in a Puerto Rican patient with chronic granulomatous disease: case report and review of occurrences in the Americas. Clin. Infect. Dis. 26:889-894. [DOI] [PubMed] [Google Scholar]
- 147.Douglas, M. W., G. Lum, J. Roy, D. A. Fisher, N. M. Anstey, and B. J. Currie. 2004. Epidemiology of community-acquired and nosocomial bloodstream infections in tropical Australia: a 12-month prospective study. Trop. Med. Int. Health 9:795-804. [DOI] [PubMed] [Google Scholar]
- 148.Egan, A. 1996. Interaction of Burkholderia pseudomallei with opsonins and professional phagocytes. Ph.D. thesis. Flinders University of South Australia, Adelaide, Australia.
- 149.Egan, A. M., and D. L. Gordon. 1996. Burkholderia pseudomallei activates complement and is ingested but not killed by polymorphonuclear leukocytes. Infect. Immun. 64:4952-4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Eickhoff, T. C., J. V. Bennett, P. S. Hayes, and J. Feeley. 1970. Pseudomonas pseudomallei: susceptibility to chemotherapeutic agents. J. Infect. Dis. 121:95-102. [DOI] [PubMed] [Google Scholar]
- 151.Faa, A. G., and P. J. Holt. 2002. Melioidosis in the Torres Strait Islands of Far North Queensland. Commun. Dis. Intell. 26:279-283. [DOI] [PubMed] [Google Scholar]
- 152.Farkas Himsley, H. 1968. Selection and rapid identification of Pseudomonas pseudomallei from other gram-negative bacteria. Am. J. Clin. Pathol. 49:850-856. [DOI] [PubMed] [Google Scholar]
- 153.Ferry, R., B. Poutrel, and F. Bruneau. 1973. Isolation of Whitmore's bacillus from lesions found in pigs from the Niamey slaughterhouse in Niger. Bull. Soc. Pathol. Exot. Filiales 66:42-45. [PubMed] [Google Scholar]
- 154.Finkelstein, R. A., P. Atthasampunna, and M. Chulasamaya. 2000. Pseudomonas (Burkholderia) pseudomallei in Thailand, 1964-1967: geographic distribution of the organism, attempts to identify cases of active infection, and presence of antibody in representative sera. Am. J. Trop. Med. Hyg. 62:232-239. [DOI] [PubMed] [Google Scholar]
- 155.Fisher, M. W., A. B. Hillegas, and P. L. Nazeeri. 1971. Susceptibility in vitro and in vivo of Pseudomonas pseudomallei to rifampin and tetracyclines. Appl. Microbiol. 22:13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Forbes Faulkner, J. C., W. L. Townsend, and A. D. Thomas. 1992. Pseudomonas pseudomallei infection in camels. Aust. Vet. J. 69:148. [DOI] [PubMed] [Google Scholar]
- 157.Fournier, J. 1965. Melioidosis and the Whitmore bacillus. Epidemiological and taxonomic controversies. Bull. Soc. Pathol. Exot. 58:753-765. [PubMed] [Google Scholar]
- 158.Franklin, M. 1974. Inhibition of Pseudomonas pseudomallei by carbenicillin, in vitro. Can. J. Microbiol. 20:1189-1193. [DOI] [PubMed] [Google Scholar]
- 159.Freeman, K., G. Lum, and J. De Boer. 2000. Presented at the Australian Society of Microbiology, Cairns.
- 160.Fung, W. K., S. C. Tam, K. M. Ho, P. Lam, and K. K. Lo. 2001. Porphyria cutanea tarda and melioidosis. Hong Kong Med. J. 7:197-200. [PubMed] [Google Scholar]
- 161.Gal, D., M. Mayo, H. Smith-Vaughan, P. Dasari, M. McKinnon, S. P. Jacups, A. I. Urquhart, M. Hassell, and B. J. Currie. 2004. Contamination of hand wash detergent linked to occupationally acquired melioidosis. Am. J. Trop. Med. Hyg. 71:360-362. [PubMed] [Google Scholar]
- 162.Galan, J. E. 2001. Salmonella interactions with host cells: type III secretion at work. Annu. Rev. Cell Dev. Biol. 17:53-86. [DOI] [PubMed] [Google Scholar]
- 163.Galimand, M., and A. Dodin. 1982. Focus on melioidosis throughout the world. Bull. Soc. Pathol. Exot. Filiales 75:375-383. [PubMed] [Google Scholar]
- 164.Garry, M., and M. Koch. 1951. Chronic melioidosis: bacteriologic and clinical correlation in diagnosis. J. Lab. Clin. Med. 38:374-383. [PubMed] [Google Scholar]
- 165.Gee, J. E., C. T. Sacchi, M. B. Glass, B. K. De, R. S. Weyant, P. N. Levett, A. M. Whitney, A. R. Hoffmaster, and T. Popovic. 2003. Use of 16S rRNA gene sequencing for rapid identification and differentiation of Burkholderia pseudomallei and B. mallei. J. Clin. Microbiol. 41:4647-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Geerlings, S. E., and A. I. Hoepelman. 1999. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol. Med. Microbiol. 26:259-265. [DOI] [PubMed] [Google Scholar]
- 167.Gillis, M., T. V. Van, R. Bardin, M. Goor, P. Hebbar, A. Willems, P. Segers, K. Kersters, T. Heulin, and M. P. Fernandez. 1995. Polyphasic taxonomy in the genus Burkholderia leading to an emended description of the genus and proposition of Burkholderia vietnamensis sp. nov. for N2-fixing isolates from rice in Vietnam. Int. J. Syst. Bacteriol. 45:274-289. [Google Scholar]
- 168.Gluckman, S. J., and R. R. MacGregor. 1978. Effect of acute alcohol intoxication on granulocyte mobilization and kinetics. Blood 52:551-559. [PubMed] [Google Scholar]
- 169.Godfrey, A. J., S. Wong, D. A. Dance, W. Chaowagul, and L. E. Bryan. 1991. Pseudomonas pseudomallei resistance to beta-lactam antibiotics due to alterations in the chromosomally encoded beta-lactamase. Antimicrob. Agents Chemother. 35:1635-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Godoy, D., G. Randle, A. J. Simpson, D. M. Aanensen, T. L. Pitt, R. Kinoshita, and B. G. Spratt. 2003. Multilocus sequence typing and evolutionary relationships among the causative agents of melioidosis and glanders, Burkholderia pseudomallei and Burkholderia mallei. J. Clin. Microbiol. 41:2068-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Golledge, C. L., W. S. Chin, A. E. Tribe, R. J. Condon, and L. R. Ashdown. 1992. A case of human melioidosis originating in south-west Western Australia. Med. J. Aust. 157:332-334. [DOI] [PubMed] [Google Scholar]
- 172.Gorgen, I., T. Hartung, M. Leist, M. Niehorster, G. Tiegs, S. Uhlig, F. Weitzel, and A. Wendel. 1992. Granulocyte colony-stimulating factor treatment protects rodents against lipopolysaccharide-induced toxicity via suppression of systemic tumor necrosis factor-alpha. J. Immunol. 149:918-924. [PubMed] [Google Scholar]
- 173.Goshorn, R. K. 1987. Recrudescent pulmonary melioidosis. A case report involving the so-called ‘Vietnamese time bomb.’ Indiana Med. 80:247-249. [PubMed] [Google Scholar]
- 174.Gotoh, N., N. J. White, W. Chaowagul, and D. E. Woods. 1994. Isolation and characterization of the outer-membrane proteins of Burkholderia (Pseudomonas) pseudomallei. Microbiology 140:797-805. [DOI] [PubMed] [Google Scholar]
- 175.Gough, A., M. Clapperton, N. Rolando, A. V. Foster, J. Philpott-Howard, and M. E. Edmonds. 1997. Randomised placebo-controlled trial of granulocyte-colony stimulating factor in diabetic foot infection. Lancet 350:855-859. [DOI] [PubMed] [Google Scholar]
- 176.Green, R. N., and P. G. Tuffnell. 1968. Laboratory acquired melioidosis. Am. J. Med. 44:599-605. [DOI] [PubMed] [Google Scholar]
- 177.Grosskopf, S. 2000. An unusual cause of pneumonia. Aust. Fam. Physician 29:552-553. [PubMed] [Google Scholar]
- 178.Haase, A., M. Brennan, S. Barrett, Y. Wood, S. Huffam, D. O′Brien, and B. Currie. 1998. Evaluation of PCR for diagnosis of melioidosis. J. Clin. Microbiol. 36:1039-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Haase, A., A. Melder, H. Smith Vaughan, D. Kemp, and B. Currie. 1995. RAPD analysis of isolates of Burkholderia pseudomallei from patients with recurrent melioidosis. Epidemiol. Infect. 115:115-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Haase, A., H. Smith Vaughan, A. Melder, Y. Wood, A. Janmaat, J. Gilfedder, D. Kemp, and B. Currie. 1995. Subdivision of Burkholderia pseudomallei ribotypes into multiple types by random amplified polymorphic DNA analysis provides new insights into epidemiology. J. Clin. Microbiol. 33:1687-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Halder, D., N. Zainal, C. M. Wah, and J. A. Haq. 1998. Neonatal meningitis and septicaemia caused by Burkholderia pseudomallei. Ann. Trop. Paediatr. 18:161-164. [DOI] [PubMed] [Google Scholar]
- 182.Hall, W. H., and R. E. Manion. 1973. Antibiotic susceptibility of Pseudomonas pseudomallei. Antimicrob. Agents Chemother. 4:193-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hallengren, B., and A. Forsgren. 1978. Effect of alcohol on chemotaxis, adherence and phagocytosis of human polymorphonuclear leucocytes. Acta Med. Scand. 204:43-48. [DOI] [PubMed] [Google Scholar]
- 184.Harley, V. S., D. A. Dance, B. S. Drasar, and G. Tovey. 1998. Effects of Burkholderia pseudomallei and other Burkholderia species on eukaryotic cells in tissue culture. Microbios 96:71-93. [PubMed] [Google Scholar]
- 185.Harley, V. S., D. A. Dance, G. Tovey, M. V. McCrossan, and B. S. Drasar. 1998. An ultrastructural study of the phagocytosis of Burkholderia pseudomallei. Microbios 94:35-45. [PubMed] [Google Scholar]
- 186.Hartung, T., W. D. Docke, F. Gantner, G. Krieger, A. Sauer, P. Stevens, H. D. Volk, and A. Wendel. 1995. Effect of granulocyte colony-stimulating factor treatment on ex vivo blood cytokine response in human volunteers. Blood 85:2482-2489. [PubMed] [Google Scholar]
- 187.Hartung, T., W. D. Doecke, D. Bundschuh, M. A. Foote, F. Gantner, C. Hermann, A. Lenz, S. Milwee, B. Rich, B. Simon, H. D. Volk, S. von Aulock, and A. Wendel. 1999. Effect of filgrastim treatment on inflammatory cytokines and lymphocyte functions. Clin. Pharmacol. Ther. 66:415-424. [DOI] [PubMed] [Google Scholar]
- 188.Haussler, S., M. Rohde, and I. Steinmetz. 1999. Highly resistant Burkholderia pseudomallei small colony variants isolated in vitro and in experimental melioidosis. Med. Microbiol. Immunol. 188:91-97. [DOI] [PubMed] [Google Scholar]
- 189.Healey, T., and S. Selva-Nayagam. 2001. Retrospective review of febrile neutropenia in the Royal Darwin Hospital, 1994-99. Intern. Med. J. 31:406-412. [DOI] [PubMed] [Google Scholar]
- 190.Heinzelmann, M., M. A. Mercer-Jones, and J. C. Passmore. 1999. Neutrophils and renal failure. Am. J. Kidney Dis. 34:384-399. [DOI] [PubMed] [Google Scholar]
- 191.Heng, B. H., K. T. Goh, E. H. Yap, H. Loh, and M. Yeo. 1998. Epidemiological surveillance of melioidosis in Singapore. Ann. Acad. Med. Singapore 27:478-484. [PubMed] [Google Scholar]
- 192.Heyse, A. M., J. Dierick, H. Vanhouteghem, F. Ameye, D. Baert, P. Burvenich, and G. Wauters. 2003. A case of imported melioidosis presenting as prostatitis. Infection 31:60-62. [DOI] [PubMed] [Google Scholar]
- 193.Hicks, C. L., R. Kinoshita, and P. W. Ladds. 2000. Pathology of melioidosis in captive marine mammals. Aust. Vet. J. 78:193-195. [DOI] [PubMed] [Google Scholar]
- 194.Hirabayashi, Y., T. Kobayashi, A. Nishikawa, H. Okazaki, T. Aoki, J. Takaya, and Y. Kobayashi. 1988. Oxidative metabolism and phagocytosis of polymorphonuclear leukocytes in patients with chronic renal failure. Nephron 49:305-312. [DOI] [PubMed] [Google Scholar]
- 195.Ho, M., T. Schollaardt, M. D. Smith, M. B. Perry, P. J. Brett, W. Chaowagul, and L. E. Bryan. 1997. Specificity and functional activity of anti-Burkholderia pseudomallei polysaccharide antibodies. Infect. Immun. 65:3648-3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hoc, T. S., C. T. Deng, and R. Khuzaiah. 1993. Melioidosis in a splenectomized boy with beta-thalassemia major. Southeast Asian J. Trop. Med. Public Health 24:601-602. [PubMed] [Google Scholar]
- 197.Holden, M. T., R. W. Titball, S. J. Peacock, A. M. Cerdeno-Tarraga, T. Atkins, L. C. Crossman, T. Pitt, C. Churcher, K. Mungall, S. D. Bentley, M. Sebaihia, N. R. Thomson, N. Bason, I. R. Beacham, K. Brooks, K. A. Brown, N. F. Brown, G. L. Challis, I. Cherevach, T. Chillingworth, A. Cronin, B. Crossett, P. Davis, D. DeShazer, T. Feltwell, A. Fraser, Z. Hance, H. Hauser, S. Holroyd, K. Jagels, K. E. Keith, M. Maddison, S. Moule, C. Price, M. A. Quail, E. Rabbinowitsch, K. Rutherford, M. Sanders, M. Simmonds, S. Songsivilai, K. Stevens, S. Tumapa, M. Vesaratchavest, S. Whitehead, C. Yeats, B. G. Barrell, P. C. Oyston, and J. Parkhill. 2004. Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei. Proc. Natl. Acad. Sci. USA 101:14240-14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Holland, D. J., A. Wesley, D. Drinkovic, and B. J. Currie. 2002. Cystic fibrosis and Burkholderia pseudomallei: an emerging problem? Clin. Infect. Dis. 35:e138-e140. [DOI] [PubMed] [Google Scholar]
- 199.Holmes, A., J. Govan, and R. Goldstein. 1998. Agricultural use of Burkholderia (Pseudomonas) cepacia: a threat to human health? Emerg. Infect. Dis. 4:221-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hoppe, I., B. Brenneke, M. Rohde, A. Kreft, S. Haussler, A. Reganzerowski, and I. Steinmetz. 1999. Characterization of a murine model of melioidosis: comparison of different strains of mice. Infect. Immun. 67:2891-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hoque, S. N., M. Minassian, S. Clipstone, S. J. Lloyd-Owen, E. Sheridan, and M. P. Lessing. 1999. Melioidosis presenting as septic arthritis in Bengali men in east London. Rheumatology (Oxford). 38:1029-1031. [DOI] [PubMed] [Google Scholar]
- 202.Howard, K., and T. J. Inglis. 2003. The effect of free chlorine on Burkholderia pseudomallei in potable water. Water Res. 37:4425-4432. [DOI] [PubMed] [Google Scholar]
- 203.Howard, K., and T. J. Inglis. 2003. Novel selective medium for isolation of Burkholderia pseudomallei. J. Clin. Microbiol. 41:3312-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Howe, C., A. Sampath, and M. Spotnitz. 1971. The pseudomallei group: a review. J. Infect. Dis. 124:598-606. [DOI] [PubMed] [Google Scholar]
- 205.Hsueh, P. R., L. J. Teng, L. N. Lee, C. J. Yu, P. C. Yang, S. W. Ho, and K. T. Luh. 2001. Melioidosis: an emerging infection in Taiwan? Emerg. Infect. Dis. 7:428-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Huffam, S., S. P. Jacups, P. Kittler, and B. J. Currie. 2004. Out of hospital treatment of patients with melioidosis using ceftazidime in 24 h elastomeric infusors, via peripherally inserted central catheters. Trop. Med. Int. Health 9:715-717. [DOI] [PubMed] [Google Scholar]
- 207.Iida, T., K. Umezawa, K. Tanaka, Y. Koga, H. Nakazawa, and T. Satoh. 1997. Polymorphonuclear cells in chronic hemodialysis patients have intact phagocytotic and impaired bactericidal activities. Nephron 75:41-47. [DOI] [PubMed] [Google Scholar]
- 208.Ileri, S. 1965. The indirect haemagglutination tests in the diagnosis of melioidosis in goats. Br. Vet. J. 121:164-170. [DOI] [PubMed] [Google Scholar]
- 209.Iliukhin, V. I., N. N. Kislichkin, L. K. Merinova, L. A. Riapis, I. I. Denisov, S. M. Farber, and O. I. Kislichkina. 1999. The outlook for the development of live vaccines for the prevention of melioidosis. Zh. Mikrobiol. Epidemiol. Immunobiol 1999:52-55. [PubMed] [Google Scholar]
- 210.Iliukhin, V. I., T. V. Senina, N. G. Plekhanova, V. A. Antonov, L. K. Merinova, and I. K. Seimova. 2002. Burkholderia thailandensis: biological properties, identification and taxonomy. Mol. Gen. Mikrobiol. Virusol. 2002:7-11. [PubMed] [Google Scholar]
- 211.Inglis, T. J., D. Chiang, G. S. Lee, and L. Chor-Kiang. 1998. Potential misidentification of Burkholderia pseudomallei by API 20NE. Pathology 30:62-64. [DOI] [PubMed] [Google Scholar]
- 212.Inglis, T. J., S. C. Garrow, C. Adams, M. Henderson, M. Mayo, and B. J. Currie. 1999. Acute melioidosis outbreak in Western Australia. Epidemiol. Infect. 123:437-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Inglis, T. J., S. C. Garrow, M. Henderson, A. Clair, J. Sampson, L. O'Reilly, and B. Cameron. 2000. Burkholderia pseudomallei traced to water treatment plant in Australia. Emerg. Infect. Dis. 6:56-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Inglis, T. J., C. L. Golledge, A. Clair, and J. Harvey. 2001. Case report: recovery from persistent septicemic melioidosis. Am. J. Trop. Med. Hyg. 65:76-82. [DOI] [PubMed] [Google Scholar]
- 215.Inglis, T. J., B. Mee, and B. Chang. 2001. The environmental microbiology of melioidosis. Rev. Med. Microbiol. 12:13-20. [Google Scholar]
- 216.Inglis, T. J., L. O′Reilly, N. Foster, A. Clair, and J. Sampson. 2002. Comparison of rapid, automated ribotyping and DNA macrorestriction analysis of Burkholderia pseudomallei. J. Clin. Microbiol. 40:3198-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Inglis, T. J., P. Rigby, T. A. Robertson, N. S. Dutton, M. Henderson, and B. J. Chang. 2000. Interaction between Burkholderia pseudomallei and Acanthamoeba species results in coiling phagocytosis, endamebic bacterial survival, and escape. Infect. Immun. 68:1681-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ip, M., L. G. Osterberg, P. Y. Chau, and T. A. Raffin. 1995. Pulmonary melioidosis. Chest 108:1420-1424. [DOI] [PubMed] [Google Scholar]
- 219.Ismail, G., M. Noor Embi, O. Omar, J. C. Allen, and C. J. Smith. 1987. A competitive immunosorbent assay for detection of Pseudomonas pseudomallei exotoxin. J. Med. Microbiol. 23:353-357. [DOI] [PubMed] [Google Scholar]
- 220.Ismail, G., N. Razak, R. Mohamed, N. Embi, and O. Omar. 1988. Resistance of Pseudomonas pseudomallei to normal human serum bactericidal action. Microbiol. Immunol. 32:645-652. [DOI] [PubMed] [Google Scholar]
- 220a.Janmaat, A., J. Low Choy, and B. J. Currie. 2004. Melioidosis in an alpaca (Lama pacos). Aust. Vet. J. 82:622-623. [DOI] [PubMed] [Google Scholar]
- 221.Jeddeloh, J. A., D. L. Fritz, D. M. Waag, J. M. Hartings, and G. P. Andrews. 2003. Biodefense-driven murine model of pneumonic melioidosis. Infect. Immun. 71:584-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jenney, A. W., G. Lum, D. A. Fisher, and B. J. Currie. 2001. Antibiotic susceptibility of Burkholderia pseudomallei from tropical northern Australia and implications for therapy of melioidosis. Int. J. Antimicrob. Agents. 17:109-113. [DOI] [PubMed] [Google Scholar]
- 223.Jesudason, M. V., A. Anbarasu, and T. J. John. 2003. Septicaemic melioidosis in a tertiary care hospital in south India. Indian J. Med. Res. 117:119-121. [PubMed] [Google Scholar]
- 224.John, G. T., I. Ahmed, C. K. Jacob, M. V. Jesudason, and M. K. Lalitha. 2003. Melioidosis in a renal transplant recipient. Transplantation 76:262. [DOI] [PubMed] [Google Scholar]
- 225.John, T. J., M. V. Jesudason, M. K. Lalitha, A. Ganesh, V. Mohandas, T. Cherian, D. Mathai, and M. J. Chandy. 1996. Melioidosis in India: the tip of the iceberg? Indian J. Med. Res. 103:62-65. [PubMed] [Google Scholar]
- 226.Jones, A. L., T. J. Beveridge, and D. E. Woods. 1996. Intracellular survival of Burkholderia pseudomallei. Infect. Immun. 64:782-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Jones, S. M., J. F. Ellis, P. Russell, K. F. Griffin, and P. C. Oyston. 2002. Passive protection against Burkholderia pseudomallei infection in mice by monoclonal antibodies against capsular polysaccharide, lipopolysaccharide or proteins. J. Med. Microbiol. 51:1055-1062. [DOI] [PubMed] [Google Scholar]
- 228.Joy, R., R. Scalettar, and D. Sodee. 1960. Optic and peripheral neuritis: probable effect of prolonged chloramphenicol therapy. JAMA 173:1731-1734. [DOI] [PubMed] [Google Scholar]
- 229.Kanai, K., and E. Kondo. 1994. Recent advances in biomedical sciences of Burkholderia pseudomallei (basonym: Pseudomonas pseudomallei). Jpn. J. Med. Sci. Biol. 47:1-45. [DOI] [PubMed] [Google Scholar]
- 230.Kanaphun, P., N. Thirawattanasuk, Y. Suputtamongkol, P. Naigowit, D. A. Dance, M. D. Smith, and N. J. White. 1993. Serology and carriage of Pseudomonas pseudomallei: a prospective study in 1000 hospitalized children in northeast Thailand. J. Infect. Dis. 167:230-233. [DOI] [PubMed] [Google Scholar]
- 231.Kang, G., D. P. Rajan, B. S. Ramakrishna, H. M. Aucken, and D. A. Dance. 1996. Melioidosis in India. Lancet 347:1565-1566. [DOI] [PubMed] [Google Scholar]
- 232.Kenny, D. J., P. Russell, D. Rogers, S. M. Eley, and R. W. Titball. 1999. In vitro susceptibilities of Burkholderia mallei in comparison to those of other pathogenic Burkholderia spp. Antimicrob. Agents Chemother. 43:2773-2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kespichayawattana, W., P. Intachote, P. Utaisincharoen, and S. Sirisinha. 2004. Virulent Burkholderia pseudomallei is more efficient than avirulent Burkholderia thailandensis in invasion of and adherence to cultured human epithelial cells. Microb. Pathog. 36:287-292. [DOI] [PubMed] [Google Scholar]
- 234.Kespichayawattana, W., S. Rattanachetkul, T. Wanun, P. Utaisincharoen, and S. Sirisinha. 2000. Burkholderia pseudomallei induces cell fusion and actin-associated membrane protrusion: a possible mechanism for cell-to-cell spreading. Infect. Immun. 68:5377-5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ketheesan, N., J. L. Barnes, G. C. Ulett, H. J. VanGessel, R. E. Norton, R. G. Hirst, and J. T. LaBrooy. 2002. Demonstration of a cell-mediated immune response in melioidosis. J. Infect. Dis. 186:286-289. [DOI] [PubMed] [Google Scholar]
- 236.Ketterer, P. J., B. Donald, and R. J. Rogers. 1975. Bovine melioidosis in South Eastern Queensland. Aust. Vet. J. 51:395-398. [DOI] [PubMed] [Google Scholar]
- 237.Ketterer, P. J., W. R. Webster, J. Shield, R. J. Arthur, P. J. Blackall, and A. D. Thomas. 1986. Melioidosis in intensive piggeries in South-Eastern Queensland. Aust. Vet. J. 63:146-149. [DOI] [PubMed] [Google Scholar]
- 238.Khupulsup, K., and B. Petchclai. 1986. Application of indirect hemagglutination test and indirect fluorescent antibody test for IgM antibody for diagnosis of melioidosis in Thailand. Am. J. Trop. Med. Hyg. 35:366-369. [DOI] [PubMed] [Google Scholar]
- 239.Kibbler, C. C., C. M. Roberts, G. L. Ridgway, and S. G. Spiro. 1991. Melioidosis in a patient from Bangladesh. Postgrad. Med. J. 67:764-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kingston, C. W. 1971. Chronic or latent melioidosis. Med. J. Aust. 2:618-621. [DOI] [PubMed] [Google Scholar]
- 241.Kishimoto, R. A., and W. C. Eveland. 1975. Induction of microbial variants of Pseudomonas pseudomallei in cultured rabbit alveolar macrophages. Can. J. Microbiol. 21:2112-2115. [DOI] [PubMed] [Google Scholar]
- 242.Kitagawa, S., A. Yuo, L. M. Souza, M. Saito, Y. Miura, and F. Takaku. 1987. Recombinant human granulocyte colony-stimulating factor enhances superoxide release in human granulocytes stimulated by the chemotactic peptide. Biochem. Biophys. Res. Commun. 144:1143-1146. [DOI] [PubMed] [Google Scholar]
- 243.Klinman, D. M., D. Verthelyi, F. Takeshita, and K. J. Ishii. 1999. Immune recognition of foreign DNA: a cure for bioterrorism? Immunity 11:123-129. [DOI] [PubMed] [Google Scholar]
- 244.Koay, A. S., M. Y. Rohani, and Y. M. Cheong. 1997. In-vitro susceptibility of Burkholderia pseudomallei to cefoperazone-sulbactam combination. Med. J. Malaysia 52:158-160. [PubMed] [Google Scholar]
- 245.Kondo, E., V. Petkanchanapong, P. Naigowit, T. Kurata, and K. Kanai. 1991. Demonstration of acid phosphatase activity in antigenic glycoprotein fractions obtained from the culture filtrate of Pseudomonas pseudomallei. Jpn. J. Med. Sci. Biol. 44:213-224. [DOI] [PubMed] [Google Scholar]
- 246.Korbsrisate, S., N. Suwanasai, A. Leelaporn, T. Ezaki, Y. Kawamura, and S. Sarasombath. 1999. Cloning and characterization of a nonhemolytic phospholipase C gene from Burkholderia pseudomallei. J. Clin. Microbiol. 37:3742-3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kortepeter, M. G. Christopher, T. Cieslak, R. Culpepper, R. Darling, J. Pavlin, J. John Rowe, K. Kelly McKee, and E. Eitzen (ed.). 2001. Medical management of biological casualties handbook, p. 23-27. U.S. Army Medical Research Institute of Infectious Diseases, Fort Detrick, Md.
- 248.Kropec, A., S. W. Lemmen, H. J. Grundmann, I. Engels, and F. D. Daschner. 1995. Synergy of simultaneous administration of ofloxacin and granulocyte colony-stimulating factor in killing of Escherichia coli by human neutrophils. Infection 23:298-300. [DOI] [PubMed] [Google Scholar]
- 249.Kumar, A., J. Short, and J. E. Parrillo. 1999. Genetic factors in septic shock. JAMA 282:579-581. [DOI] [PubMed] [Google Scholar]
- 250.Kunakorn, M., and R. B. Markham. 1995. Clinically practical seminested PCR for Burkholderia pseudomallei quantitated by enzyme immunoassay with and without solution hybridization. J. Clin. Microbiol. 33:2131-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kunakorn, M., K. Raksakait, C. Sethaudom, R. W. Sermswan, and T. Dharakul. 2000. Comparison of three PCR primer sets for diagnosis of septicemic melioidosis. Acta Trop. 74:247-251. [DOI] [PubMed] [Google Scholar]
- 252.Ladds, P. W., A. D. Thomas, and B. Pott. 1981. Melioidosis with acute meningoencephalomyelitis in a horse. Aust. Vet. J. 57:36-38. [DOI] [PubMed] [Google Scholar]
- 253.Ladds, P. W., A. D. Thomas, R. Speare, and A. S. Brown. 1990. Melioidosis in a koala. Aust. Vet. J. 67:304-305. [DOI] [PubMed] [Google Scholar]
- 254.Larosa, S., S. Opal, B. Utterback, B. Yan, J. Helterbrand, A. Simpson, N. White, and C. Fisher. 2000. Presented at the 20th International Symposium on Intensive Care and Emergency Medicine, Brussels, Belgium.
- 255.Lauw, F. N., A. J. Simpson, C. E. Hack, J. M. Prins, A. M. Wolbink, S. J. van Deventer, W. Chaowagul, N. J. White, and T. van Der Poll. 2000. Soluble granzymes are released during human endotoxemia and in patients with severe infection due to gram-negative bacteria. J. Infect. Dis. 182:206-213. [DOI] [PubMed] [Google Scholar]
- 256.Lauw, F. N., A. J. Simpson, J. M. Prins, M. D. Smith, M. Kurimoto, S. J. van Deventer, P. Speelman, W. Chaowagul, N. J. White, and T. van der Poll. 1999. Elevated plasma concentrations of interferon (IFN)-gamma and the IFN-gamma-inducing cytokines interleukin (IL)-18, IL-12, and IL-15 in severe melioidosis. J. Infect. Dis. 180:1878-1885. [DOI] [PubMed] [Google Scholar]
- 257.Lauw, F. N., A. J. Simpson, J. M. Prins, S. J. van Deventer, W. Chaowagul, N. J. White, and T. van der Poll. 2000. The CXC chemokines gamma interferon (IFN-γ)-inducible protein 10 and monokine induced by IFN-γ are released during severe melioidosis. Infect. Immun. 68:3888-3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Laws, L., and W. T. K. Hall. 1963. Melioidosis in animals in north Queensland. 1. Incidence and pathology, with special reference to central nervous system lesions. Qeensland J. Agric. Sci. 20:499-513. [Google Scholar]
- 259.Leakey, A. K., G. C. Ulett, and R. G. Hirst. 1998. BALB/c and C57Bl/6 mice infected with virulent Burkholderia pseudomallei provide contrasting animal models for the acute and chronic forms of human melioidosis. Microb. Pathog. 24:269-275. [DOI] [PubMed] [Google Scholar]
- 260.Lee, L., and S. Naraqi. 1980. Primary gram negative pneumonia in adults in Papua New Guinea. P.N.G. Med. J. 23:174-178. [PubMed] [Google Scholar]
- 261.Lee, M., and Y. Liu. 2000. Sequencing and characterization of a novel serine metalloprotease from Burkholderia pseudomallei. FEMS Microbiol. Lett. 192:67-72. [DOI] [PubMed] [Google Scholar]
- 262.Lee, N., J. L. Wu, C. H. Lee, and W. C. Tsai. 1985. Pseudomonas pseudomallei infection from drowning: the first reported case in Taiwan. J. Clin. Microbiol. 22:352-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Lee, S. C., T. S. Ling, J. C. Chen, B. Y. Huang, and W. B. Sheih. 1999. Melioidosis with adrenal gland abscess. Am. J. Trop. Med. Hyg. 61:34-36. [DOI] [PubMed] [Google Scholar]
- 264.Lee, S. S., Y. C. Liu, Y. S. Chen, S. R. Wann, J. H. Wang, M. Y. Yen, H. H. Lin, W. K. Huang, and D. L. Cheng. 1996. Melioidosis: two indigenous cases in Taiwan. J. Formos. Med. Assoc. 95:562-566. [PubMed] [Google Scholar]
- 265.Leelarasamee, A. 1985. Diagnostic value of indirect hemagglutination method for melioidosis in Thailand. J. Infect. Dis. Antimicrob. Agents 2:213-214. [Google Scholar]
- 266.Leelarasamee, A., and S. Bovornkitti. 1989. Melioidosis: review and update. Rev. Infect. Dis. 11:413-425. [DOI] [PubMed] [Google Scholar]
- 267.Leelarasamee, A., S. Trakulsomboon, M. Kusum, and S. Dejsirilert. 1997. Isolation rates of Burkholderia pseudomallei among the four regions in Thailand. Southeast Asian J. Trop. Med. Public Health 28:107-113. [PubMed] [Google Scholar]
- 268.Leeuwenburgh, I., J. T. Driessen, P. H. van Keulen, P. J. Stijnen, and G. P. Verburg. 2002. Melioidosis. Ned. Tijdschr. Geneeskd. 146:723-725. [PubMed] [Google Scholar]
- 269.Leitenberg, M. 2001. Presented at the 7th International Symposium on Protection against Chemical and Biological Warfare, Stockholm, Sweden.
- 270.Lertmemongkolchai, G., G. Cai, C. A. Hunter, and G. J. Bancroft. 2001. Bystander activation of CD8(+) T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. J. Immunol. 166:1097-1105. [DOI] [PubMed] [Google Scholar]
- 271.Lertpatanasuwan, N., K. Sermsri, A. Petkaseam, S. Trakulsomboon, V. Thamlikitkul, and Y. Suputtamongkol. 1999. Arabinose-positive Burkholderia pseudomallei infection in humans: case report. Clin. Infect. Dis. 28:927-928. [DOI] [PubMed] [Google Scholar]
- 272.Lim, M. K., E. H. Tan, and C. S. Soh. 1997. Burkholderia pseudomallei infection in the Singapore armed forces from 1987 to 1994—an epidemiological review. Ann. Acad. Med. Singapore 26:13-17. [PubMed] [Google Scholar]
- 273.Lin, H. P., S. D. Puthucheary, and D. Sinniah. 1980. Acute septicemic melioidosis occurring in a child with acute lymphoblastic leukemia. Clin. Pediatr. (Philadelphia) 19:697-699. [DOI] [PubMed] [Google Scholar]
- 274.Livermore, D. M., P. Y. Chau, A. I. Wong, and Y. K. Leung. 1987. β-Lactamase of Pseudomonas pseudomallei and its contribution to antibiotic resistance. J. Antimicrob. Chemother. 20:313-321. [DOI] [PubMed] [Google Scholar]
- 275.Loprasert, S., R. Sallabhan, W. Whangsuk, and S. Mongkolsuk. 2000. Characterization and mutagenesis of fur gene from Burkholderia pseudomallei. Gene 254:129-137. [DOI] [PubMed] [Google Scholar]
- 276.Lorenz, W., K. P. Reimund, F. Weitzel, I. Celik, M. Kurnatowski, C. Schneider, W. Mannheim, A. Heiske, K. Neumann, H. Sitter, et al. 1994. Granulocyte colony-stimulating factor prophylaxis before operation protects against lethal consequences of postoperative peritonitis. Surgery 116:925-934. [PubMed] [Google Scholar]
- 277.Low Choy, J., M. Mayo, A. Janmaat, and B. J. Currie. 2000. Animal melioidosis in Australia. Acta Trop. 74:153-158. [DOI] [PubMed] [Google Scholar]
- 278.Lowe, P., C. Engler, and R. Norton. 2002. Comparison of automated and nonautomated systems for identification of Burkholderia pseudomallei. J. Clin. Microbiol. 40:4625-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Lumbiganon, P., N. Chotechuangnirun, and P. Kosalaraksa. 2004. Clinical experience with treatment of melioidosis in children. Pediatr. Infect. Dis. J. 23:1165-1166. [PubMed] [Google Scholar]
- 280.Lumbiganon, P., K. Pengsaa, S. Puapermpoonsiri, and A. Puapairoj. 1988. Neonatal melioidosis: a report of 5 cases. Pediatr. Infect. Dis. J. 7:634-636. [DOI] [PubMed] [Google Scholar]
- 281.Lumbiganon, P., U. Tattawasatra, P. Chetchotisakd, S. Wongratanacheewin, and B. Thinkhamrop. 2000. Comparison between the antimicrobial susceptibility of Burkholderia pseudomallei to trimethoprim-sulfamethoxazole by standard disk diffusion method and by minimal inhibitory concentration determination. J. Med. Assoc Thai. 83:856-860. [PubMed] [Google Scholar]
- 282.Lumbiganon, P., and S. Viengnondha. 1995. Clinical manifestations of melioidosis in children. Pediatr. Infect. Dis. J. 14:136-140. [DOI] [PubMed] [Google Scholar]
- 283.Luo, C. Y., W. C. Ko, H. C. Lee, and Y. J. Yang. 2003. Relapsing melioidosis as cause of iliac mycotic aneurysm: an indigenous case in Taiwan. J. Vasc. Surg. 37:882-885. [DOI] [PubMed] [Google Scholar]
- 284.Mack, K., and R. W. Titball. 1998. The detection of insertion sequences within the human pathogen Burkholderia pseudomallei which have been identified previously in Burkholderia cepacia. FEMS Microbiol. Lett. 162:69-74. [DOI] [PubMed] [Google Scholar]
- 285.Mackowiak, P. A., and J. W. Smith. 1978. Septicemic melioidosis. Occurrence following acute influenza A six years after exposure in Vietnam. JAMA 240:764-766. [DOI] [PubMed] [Google Scholar]
- 286.Maegraith, B. G., and C. S. Leithead. 1964. Melioidosis: a case report. Lancet 1:862-863. [DOI] [PubMed] [Google Scholar]
- 287.Marhoffer, W., M. Stein, E. Maeser, and K. Federlin. 1992. Impairment of polymorphonuclear leukocyte function and metabolic control of diabetes. Diabetes Care 15:256-260. [DOI] [PubMed] [Google Scholar]
- 288.Marhoffer, W., M. Stein, L. Schleinkofer, and K. Federlin. 1993. Evidence of ex vivo and in vitro impaired neutrophil oxidative burst and phagocytic capacity in type 1 diabetes mellitus. Diabetes Res. Clin. Pract. 19:183-188. [DOI] [PubMed] [Google Scholar]
- 289.Markova, N., V. Kussovski, and T. Radoucheva. 1998. Killing of Pseudomonas pseudomallei by polymorphonuclear leukocytes and peritoneal macrophages from chicken, sheep, swine and rabbits. Zentralbl. Bakteriol. 288:103-110. [DOI] [PubMed] [Google Scholar]
- 290.Masoud, H., M. Ho, T. Schollaardt, and M. B. Perry. 1997. Characterization of the capsular polysaccharide of Burkholderia (Pseudomonas) pseudomallei 304b. J. Bacteriol. 179:5663-5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Mathai, E., M. V. Jesudason, and A. Anbarasu. 2003. Indirect immunofluorescent antibody test for the rapid diagnosis of melioidosis. Indian J. Med. Res. 118:68-70. [PubMed] [Google Scholar]
- 292.Mathew, S., B. Perakath, G. Mathew, V. Sitaram, A. Nair, M. K. Lalitha, and T. J. John. 1999. Surgical presentation of melioidosis in India. Natl. Med. J. India 12:59-61. [PubMed] [Google Scholar]
- 293.Mays, E. E., and E. A. Ricketts. 1975. Melioidosis: recrudescence associated with bronchogenic carcinoma twenty-six years following initial geographic exposure. Chest 68:261-263. [DOI] [PubMed] [Google Scholar]
- 294.McCormick, J. B., D. J. Sexton, J. G. McMurray, E. Carey, P. Hayes, and R. A. Feldman. 1975. Human-to-human transmission of Pseudomonas pseudomallei. Ann. Intern. Med. 83:512-513. [DOI] [PubMed] [Google Scholar]
- 295.McCormick, J. B., R. E. Weaver, P. S. Hayes, J. M. Boyce, and R. A. Feldman. 1977. Wound infection by an indigenous Pseudomonas pseudomallei-like organism isolated from the soil: case report and epidemiologic study. J. Infect. Dis. 135:103-107. [DOI] [PubMed] [Google Scholar]
- 296.McDowell, F., and P. Varney. 1947. Melioidosis: report of the first case from the Western Hemisphere. JAMA 134:361-362. [DOI] [PubMed] [Google Scholar]
- 297.McEniry, D. W., S. H. Gillespie, and D. Felmingham. 1988. Susceptibility of Pseudomonas pseudomallei to new beta-lactam and aminoglycoside antibiotics. J. Antimicrob. Chemother. 21:171-175. [DOI] [PubMed] [Google Scholar]
- 298.McKenna, P., S. Nelson, and J. Andresen. 1996. Filgrastim (rHuG-CSF) enhances ciprofloxacin uptake and bactericidal activity of human neutrophils in vitro (abstr. 535, American Thoracic Society meeting, May 1996). Am. J. Respir. Crit. Care Med. 153:abstr. 535.
- 299.Merianos, A., M. Patel, J. M. Lane, C. N. Noonan, D. Sharrock, P. A. Mock, and B. Currie. 1993. The 1990-1991 outbreak of melioidosis in the Northern Territory of Australia: epidemiology and environmental studies. Southeast Asian J. Trop. Med. Public Health 24:425-435. [PubMed] [Google Scholar]
- 300.Minassian, M. A., A. Gage, E. Price, and A. M. Sefton. 1999. Imipenem for the treatment of melioidosis. Int. J. Antimicrob. Agents 12:263-265. [DOI] [PubMed] [Google Scholar]
- 301.Miralles, I. S., C. Maciel Mdo, M. R. Angelo, M. M. Gondini, L. H. Frota, C. M. dos Reis, and E. Hofer. 2004. Burkholderia pseudomallei: a case report of a human infection in Ceara, Brazil. Rev. Inst. Med. Trop. Sao Paulo 46:51-54. [DOI] [PubMed] [Google Scholar]
- 302.Mirich, G. S., H. M. Zimmerman, G. D. Maner, and A. A. Humphrey. 1946. Melioidosis on Guam. JAMA 130:1063-1067. [DOI] [PubMed] [Google Scholar]
- 303.Moore, R. A., D. DeShazer, S. Reckseidler, A. Weissman, and D. E. Woods. 1999. Efflux-mediated aminoglycoside and macrolide resistance in Burkholderia pseudomallei. Antimicrob. Agents Chemother. 43:465-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Moore, R. A., S. Reckseidler-Zenteno, H. Kim, W. Nierman, Y. Yu, A. Tuanyok, J. Warawa, D. DeShazer, and D. E. Woods. 2004. Contribution of gene loss to the pathogenic evolution of Burkholderia pseudomallei and Burkholderia mallei. Infect. Immun. 72:4172-4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Mowat, A., and J. Baum. 1971. Chemotaxis of polymorphonuclear leukocytes from patients with diabetes mellitus. N. Engl. J. Med. 284:621-627. [DOI] [PubMed] [Google Scholar]
- 306.Nachiangmai, N., P. Patamasucon, B. Tipayamonthein, A. Kongpon, and S. Nakaviroj. 1985. Pseudomonas pseudomallei in southern Thailand. Southeast Asian J. Trop. Med. Public Health 16:83-87. [PubMed] [Google Scholar]
- 307.Naigowit, P., T. Kurata, P. Wangroongsub, V. Petkanjanapong, E. Kondo, and K. Kanai. 1993. Application of indirect immunofluorescence microscopy to colony identification of Pseudomonas pseudomallei. Asian Pac. J. Allergy Immunol. 11:149-154. [PubMed] [Google Scholar]
- 308.Naigowit, P., W. Petchkanchanapong, W. Mannebungyung, N. Vetchsprsit, and D. Chaeosil. 1989. Indirect hemagglutination test for the diagnosis of melioidosis. Bull. Fac. Med. Tech. Mahidol. U. 13:17-25. [Google Scholar]
- 309.Nelson, S., G. Bagby, J. Andresen, C. Nakamura, J. Shellito, and W. Summer. 1991. The effects of ethanol, tumor necrosis factor, and granulocyte colony-stimulating factor on lung antibacterial defenses. Adv. Exp. Med. Biol. 288:245-253. [DOI] [PubMed] [Google Scholar]
- 310.Nelson, S., S. M. Belknap, R. W. Carlson, D. Dale, B. DeBoisblanc, S. Farkas, N. Fotheringham, H. Ho, T. Marrie, H. Movahhed, R. Root, J. Wilson, et al. 1998. A randomized controlled trial of filgrastim as an adjunct to antibiotics for treatment of hospitalized patients with community-acquired pneumonia. J. Infect. Dis. 178:1075-1080. [DOI] [PubMed] [Google Scholar]
- 311.Nelson, S., A. M. Heyder, J. Stone, M. G. Bergeron, S. Daugherty, G. Peterson, N. Fotheringham, W. Welch, S. Milwee, and R. Root. 2000. A randomized controlled trial of filgrastim for the treatment of hospitalized patients with multilobar pneumonia. J. Infect. Dis. 182:970-973. [DOI] [PubMed] [Google Scholar]
- 312.Nelson, S., W. Summer, G. Bagby, C. Nakamura, L. Stewart, G. Lipscomb, and J. Andresen. 1991. Granulocyte colony-stimulating factor enhances pulmonary host defenses in normal and ethanol-treated rats. J. Infect. Dis. 164:901-906. [DOI] [PubMed] [Google Scholar]
- 313.Nierman, W. C., D. DeShazer, H. S. Kim, H. Tettelin, K. E. Nelson, T. Feldblyum, R. L. Ulrich, C. M. Ronning, L. M. Brinkac, S. C. Daugherty, T. D. Davidsen, R. T. Deboy, G. Dimitrov, R. J. Dodson, A. S. Durkin, M. L. Gwinn, D. H. Haft, H. Khouri, J. F. Kolonay, R. Madupu, Y. Mohammoud, W. C. Nelson, D. Radune, C. M. Romero, S. Sarria, J. Selengut, C. Shamblin, S. A. Sullivan, O. White, Y. Yu, N. Zafar, L. Zhou, and C. M. Fraser. 2004. Structural flexibility in the Burkholderia mallei genome. Proc. Natl. Acad. Sci. USA 101:14246-14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Nigg, C., and M. Johnson. 1961. Complement fixation tests in experimental clinical and subclinical melioidosis. J. Bacteriol. 82:159-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Nigg, C., J. Ruch, E. Scott, and K. Noble. 1956. Enhancement of virulence of Malleomyces pseudomallei. J. Bacteriol. 71:530-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Niumsup, P., and V. Wuthiekanun. 2002. Cloning of the class D beta-lactamase gene from Burkholderia pseudomallei and studies on its expression in ceftazidime-susceptible and -resistant strains. J. Antimicrob. Chemother. 50:445-455. [DOI] [PubMed] [Google Scholar]
- 317.Norton, R., B. Roberts, M. Freeman, M. Wilson, C. Ashhurst-Smith, W. Lock, D. Brookes, and J. La Brooy. 1998. Characterisation and molecular typing of Burkholderia pseudomallei: are disease presentations of melioidosis clonally related? FEMS Immunol. Med. Microbiol. 20:37-44. [DOI] [PubMed] [Google Scholar]
- 318.Nuntayanuwat, S., T. Dharakul, W. Chaowagul, and S. Songsivilai. 1999. Polymorphism in the promoter region of tumor necrosis factor-alpha gene is associated with severe meliodosis. Hum. Immunol. 60:979-983. [DOI] [PubMed] [Google Scholar]
- 319.O'Brien, M., K. Freeman, G. Lum, A. C. Cheng, S. P. Jacups, and B. J. Currie. 2004. Further evaluation of a rapid diagnostic test for melioidosis in an area of endemicity. J. Clin. Microbiol. 42:2239-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Olive, C., G. Loetitia, N. Desbois, B. Roche, J. Jouannelle, and A. Dodin. 1995. Septic pyemic form of human melioidosis: a first case in the French Antilles. Presse Med. 24:1270. [PubMed] [Google Scholar]
- 321.Omar, A. R. 1963. Pathology of melioidosis in pigs, goats and a horse. J. Comp. Pathol. 73:359-372. [DOI] [PubMed] [Google Scholar]
- 322.O′Reilly, M., G. M. Silver, D. G. Greenhalgh, R. L. Gamelli, J. H. Davis, and J. C. Hebert. 1992. Treatment of intra-abdominal infection with granulocyte colony-stimulating factor. J. Trauma 33:679-682. [DOI] [PubMed] [Google Scholar]
- 323.Osteraas, G. R., J. M. Hardman, J. W. Bass, and C. Wilson. 1971. Neonatal melioidosis. Am. J. Dis. Child. 122:446-448. [DOI] [PubMed] [Google Scholar]
- 324.Parry, C. M., V. Wuthiekanun, N. T. Hoa, T. S. Diep, L. T. Thao, P. V. Loc, B. A. Wills, J. Wain, T. T. Hien, N. J. White, and J. J. Farrar. 1999. Melioidosis in Southern Vietnam: clinical surveillance and environmental sampling. Clin. Infect. Dis. 29:1323-1326. [DOI] [PubMed] [Google Scholar]
- 325.Patel, M., A. Keshavarzian, V. Kottapalli, B. Badie, D. Winship, and J. Z. Fields. 1996. Human neutrophil functions are inhibited in vitro by clinically relevant ethanol concentrations. Alcohol Clin. Exp. Res. 20:275-283. [DOI] [PubMed] [Google Scholar]
- 326.Peetermans, W. E., E. Van Wijngaerden, J. Van Eldere, and J. Verhaegen. 1999. Melioidosis brain and lung abscess after travel to Sri Lanka. Clin. Infect. Dis. 28:921-922. [DOI] [PubMed] [Google Scholar]
- 327.Perez, J. M., A. Petiot, C. Adjide, F. Gerry, R. Goursaud, and B. Juminer. 1997. First case report of melioidosis in Guadeloupe, a French West Indies archipelago. Clin. Infect. Dis. 25:164-165. [DOI] [PubMed] [Google Scholar]
- 328.Perry, M. B., L. L. MacLean, T. Schollaardt, L. E. Bryan, and M. Ho. 1995. Structural characterization of the lipopolysaccharide O antigens of Burkholderia pseudomallei. Infect. Immun. 63:3348-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Phetsouvanh, R., S. Phongmany, P. Newton, M. Mayxay, A. Ramsay, V. Wuthiekanun, and N. J. White. 2001. Melioidosis and Pandora's box in the Lao People′s Democratic Republic. Clin. Infect. Dis. 32:653-654. [DOI] [PubMed] [Google Scholar]
- 330.Phung, L. V., Y. Han, S. Oka, H. Hotta, M. D. Smith, P. Theeparakun, E. Yabuuchi, and I. Yano. 1995. Enzyme-linked immunosorbent assay (ELISA) using a glycolipid antigen for the serodiagnosis of melioidosis. FEMS Immunol. Med. Microbiol. 12:259-264. [DOI] [PubMed] [Google Scholar]
- 331.Piliouras, P., G. C. Ulett, C. Ashhurst-Smith, R. G. Hirst, J. Warner, and R. Norton. 2000. Presented at the Australian Society of Antimicrobials Annual Scientific Meeting, Sydney, Australia, 13-16 April 2000.
- 332.Pit, S., F. K. Chea, and F. Jamal. 1988. Melioidosis with brain abscess. Postgrad. Med. J. 64:140-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Pitt, T. L., S. Trakulsomboon, and D. A. Dance. 2000. Molecular phylogeny of Burkholderia pseudomallei. Acta Trop. 74:181-185. [DOI] [PubMed] [Google Scholar]
- 334.Pongrithsukda, V., N. Simakachorn, and J. Pimda. 1988. Childhood melioidosis in northeastern Thailand. Southeast Asian J. Trop. Med. Public Health 19:309-316. [PubMed] [Google Scholar]
- 335.Pongsunk, S., N. Thirawattanasuk, N. Piyasangthong, and P. Ekpo. 1999. Rapid identification of Burkholderia pseudomallei in blood cultures by a monoclonal antibody assay. J. Clin. Microbiol. 37:3662-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Pons, R., and M. Advier. 1927. Melioidosis in Cochin China. J. Hyg. 26:28-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Popoff, I., J. Nagamori, and B. Currie. 1997. Melioidotic osteomyelitis in northern Australia. Aust. N. Z. J. Surg. 67:692-695. [DOI] [PubMed] [Google Scholar]
- 338.Porter, C. J., R. P. Burden, A. G. Morgan, I. Daniels, and J. Fletcher. 1997. Impaired bacterial killing and hydrogen peroxide production by polymorphonuclear neutrophils in end-stage renal failure. Nephron 77:479-481. [DOI] [PubMed] [Google Scholar]
- 339.Pourtaghva, M., A. Dodin, M. Portovi, M. Teherani, and M. Galimand. 1977. First case of human pulmonary melioidosis in Iran. Bull. Soc. Pathol. Exot. Filiales 70:107-109. [PubMed] [Google Scholar]
- 340.Powell, K., G. Ulett, R. Hirst, and R. Norton. 2003. G-CSF immunotherapy for treatment of acute disseminated murine melioidosis. FEMS Microbiol. Lett. 224:315-318. [DOI] [PubMed] [Google Scholar]
- 341.Preston, P. J., N. Lightfoot, and P. Clarke. 1976. A retrospective serological suvey of Royal Marines previously exposed to Pseudomonas pseudomallei in South East Asia. Trans. R. Soc. Trop. Med. Hyg. 70:335-337. [DOI] [PubMed] [Google Scholar]
- 342.Pruksachartvuthi, S., N. Aswapokee, and K. Thankerngpol. 1990. Survival of Pseudomonas pseudomallei in human phagocytes. J. Med. Microbiol. 31:109-114. [DOI] [PubMed] [Google Scholar]
- 343.Puig, P. 2001. Mapping disease. GIS User 45:25-27. [Online.] http://www.gisuser.com.au/GU/gu_frame.html. [Google Scholar]
- 344.Punyagupta, S. 1989. Melioidosis. Review of 686 cases and presentation of a new clinical classification, p. 217-229. In S. Punyagupta, T. Sirisanthana, and B. Stapatayavong (ed.), Melioidosis. Bangkok Medical Publisher, Bangkok, Thailand.
- 345.Puthucheary, S. D., H. P. Lin, and P. K. Yap. 1981. Acute septicaemic melioidosis: a report of seven cases. Trop. Geogr. Med. 33:19-22. [PubMed] [Google Scholar]
- 346.Puthucheary, S. D., N. Parasakthi, and M. K. Lee. 1992. Septicaemic melioidosis: a review of 50 cases from Malaysia. Trans. R. Soc. Trop. Med. Hyg. 86:683-685. [DOI] [PubMed] [Google Scholar]
- 347.Puthucheary, S. D., J. Vadivelu, Ce Cile, C., Kum Thong, W., and G. Ismail. 1996. Electron microscopic demonstration of extracellular structure of Burkholderia pseudomallei. Am. J. Trop. Med. Hyg. 54:313-314. [DOI] [PubMed] [Google Scholar]
- 348.Raghavan, K. R., R. P. Shenoi, F. Zaer, R. Aiyer, P. Ramamoorthy, and M. N. Mehta. 1991. Melioidosis in India. Indian Pediatr. 28:184-188. [PubMed] [Google Scholar]
- 349.Rainbow, L., C. A. Hart, and C. Winstanley. 2002. Distribution of type III secretion gene clusters in Burkholderia pseudomallei, B. thailandensis and B. mallei. J. Med. Microbiol. 51:374-384. [DOI] [PubMed] [Google Scholar]
- 350.Rajchanuvong, A., W. Chaowagul, Y. Suputtamongkol, M. D. Smith, D. A. B. Dance, and N. J. White. 1995. A prospective comparison of co-amoxiclav and the combination of chloramphenicol, doxycycline, and co-trimoxazole for the oral maintenance treatment of melioidosis. Trans. R. Soc. Trop. Med. Hyg. 89:546-549. [DOI] [PubMed] [Google Scholar]
- 351.Ralph, A., J. McBride, and B. J. Currie. 2004. Transmission of Burkholderia pseudomallei via breast milk in northern Australia. Pediatr. Infect. Dis. J. 23:1169-1171. [PubMed] [Google Scholar]
- 352.Rattanathongkom, A., R. W. Sermswan, and S. Wongratanacheewin. 1997. Detection of Burkholderia pseudomallei in blood samples using polymerase chain reaction. Mol. Cell Probes 11:25-31. [DOI] [PubMed] [Google Scholar]
- 353.Rayfield, E. J., M. J. Ault, G. T. Keusch, M. J. Brothers, C. Nechemias, and H. Smith. 1982. Infection and diabetes: the case for glucose control. Am. J. Med. 72:439-450. [DOI] [PubMed] [Google Scholar]
- 354.Razak, N., and G. Ismail. 1982. Interaction of human polymorphonuclear leukocytes with Pseudomonas pseudomallei. J. Gen. Appl. Microbiol. 28:509-518. [Google Scholar]
- 355.Read, K. M., B. Currie, P. McDonald, and D. L. Gordon. 2001. Reactivity of latent melioidosis in association with staphylococcal endocarditis. Intern. Med. J. 31:130-131. [PubMed] [Google Scholar]
- 356.Reckseidler, S. L., D. DeShazer, P. A. Sokol, and D. E. Woods. 2001. Detection of bacterial virulence genes by subtractive hybridization: identification of capsular polysaccharide of Burkholderia pseudomallei as a major virulence determinant. Infect. Immun. 69:34-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Reller, L. B. 1986. The serum bactericidal test. Rev. Infect. Dis. 8:803-808. [DOI] [PubMed] [Google Scholar]
- 358.Rimington, R. A. 1962. Melioidosis in Northern Queensland. Med. J. Aust. 1:50-53. [DOI] [PubMed] [Google Scholar]
- 359.Rivers, E., B. Nguyen, S. Havstad, J. Ressler, A. Muzzin, B. Knoblich, E. Peterson, and M. Tomlanovich. 2001. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N. Engl. J. Med. 345:1368-1377. [DOI] [PubMed] [Google Scholar]
- 360.Roilides, E., T. J. Walsh, P. A. Pizzo, and M. Rubin. 1991. Granulocyte colony-stimulating factor enhances the phagocytic and bactericidal activity of normal and defective human neutrophils. J. Infect. Dis. 163:579-583. [DOI] [PubMed] [Google Scholar]
- 361.Root, R. K., R. F. Lodato, W. Patrick, J. F. Cade, N. Fotheringham, S. Milwee, J. L. Vincent, A. Torres, J. Rello, and S. Nelson. 2003. Multicenter, double-blind, placebo-controlled study of the use of filgrastim in patients hospitalized with pneumonia and severe sepsis. Crit. Care Med. 31:367-373. [DOI] [PubMed] [Google Scholar]
- 362.Rowlands, J. B., and P. G. Curtis. 1965. A case of melioidosis in Papua and New Guinea. Med. J. Aust. 2:494-496. [DOI] [PubMed] [Google Scholar]
- 363.Ruchin, P., J. Robinson, M. Segasothy, and F. Morey. 2000. Melioidosis in a patient with idiopathic pulmonary haemosiderosis resident in Central Australia. Aust. N. Z. J. Med. 30:395-396. [DOI] [PubMed] [Google Scholar]
- 364.Russell, P., S. M. Eley, J. Ellis, M. Green, D. L. Bell, D. J. Kenny, and R. W. Titball. 2000. Comparison of efficacy of ciprofloxacin and doxycycline against experimental melioidosis and glanders. J. Antimicrob. Chemother. 45:813-818. [DOI] [PubMed] [Google Scholar]
- 365.Saipan, P. 1998. Neurological manifestations of melioidosis in children. Southeast Asian J. Trop. Med. Public Health 29:856-859. [PubMed] [Google Scholar]
- 366.Salant, D. J., A. M. Glover, R. Anderson, A. M. Meyers, R. Rabkin, J. A. Myburgh, and A. R. Rabson. 1976. Depressed neutrophil chemotaxis in patients with chronic renal failure and after renal transplantation. J. Lab. Clin. Med. 88:536-545. [PubMed] [Google Scholar]
- 367.Samuel, M., and T. Y. Ti. 2001. Interventions for treating melioidosis. Cochrane Database Syst. Rev. 2:CD001263. [DOI] [PubMed]
- 368.Santanirand, P., V. S. Harley, D. A. Dance, B. S. Drasar, and G. J. Bancroft. 1999. Obligatory role of gamma interferon for host survival in a murine model of infection with Burkholderia pseudomallei. Infect. Immun. 67:3593-3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Santanirand, P., V. S. Harley, D. A. Dance, J. G. Raynes, B. S. Drasar, and G. J. Bancroft. 1997. Interferon-gamma mediates host resistance in a murine model of melioidosis. Biochem. Soc. Trans. 25:287S. [DOI] [PubMed] [Google Scholar]
- 370.Schindler, N., K. D. Calligaro, M. J. Dougherty, J. Diehl, K. H. Modi, and M. N. Braffman. 2002. Melioidosis presenting as an infected intrathoracic subclavian artery pseudoaneurysm treated with femoral vein interposition graft. J. Vasc. Surg. 35:569-572. [DOI] [PubMed] [Google Scholar]
- 371.Schlech, W. F., J. B. Turchik, R. E. J. Westlake, G. C. Klein, J. D. Band, and R. E. Weaver. 1981. Laboratory-acquired infection with Pseudomonas pseudomallei (melioidosis). N. Engl. J. Med. 305:1133-1135. [DOI] [PubMed] [Google Scholar]
- 372.Schulin, T., and I. Steinmetz. 2001. Chronic melioidosis in a patient with cystic fibrosis. J. Clin. Microbiol. 39:1676-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Schwarzmaier, A., F. Riezinger-Geppert, G. Schober, R. Karnik, and A. Valentin. 2000. Fulminant septic melioidosis after a vacation in Thailand. Wien Klin. Wochenschr. 112:892-895. [PubMed] [Google Scholar]
- 374.Scott, I. A., A. M. Bell, and D. R. Staines. 1997. Fatal human melioidosis in south-eastern Queensland. Med. J. Aust. 166:197-199. [DOI] [PubMed] [Google Scholar]
- 375.Sermswan, R. W., S. Wongratanacheewin, N. Anuntagool, and S. Sirisinha. 2000. Comparison of the polymerase chain reaction and serologic tests for diagnosis of septicemic melioidosis. Am. J. Trop. Med. Hyg. 63:146-149. [DOI] [PubMed] [Google Scholar]
- 376.Sexton, M. M., A. L. Jones, W. Chaowagul, and D. E. Woods. 1994. Purification and characterization of a protease from Pseudomonas pseudomallei. Can. J. Microbiol. 40:903-910. [DOI] [PubMed] [Google Scholar]
- 377.Shoham, D. 1998. Chemical and biological weapons in Egypt. Non-Proliferation Rev. 5:48-58. [Google Scholar]
- 378.Silva, S. L., O. G. Macedo, C. A. Damasceno, M. A. de Carvalho, and E. O. Cisalpino. 1991. Bacteriological evaluation of wounds in seriously burned hospitalized patients. Rev. Soc. Bras. Med. Trop. 24:163-168. [DOI] [PubMed] [Google Scholar]
- 379.Simpson, A. J., D. A. Dance, V. Wuthiekanun, and N. J. White. 2000. Serum bactericidal and inhibitory titres in the management of melioidosis. J. Antimicrob. Chemother. 45:123-127. [DOI] [PubMed] [Google Scholar]
- 380.Simpson, A. J., P. A. Howe, V. Wuthiekanun, and N. J. White. 1999. A comparison of lysis centrifugation, pour plate, and conventional blood culture methods in the diagnosis of septicaemic melioidosis. J. Clin. Pathol. 52:616-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Simpson, A. J., P. N. Newton, W. Chierakul, W. Chaowagul, and N. J. White. 2003. Diabetes mellitus, insulin, and melioidosis in Thailand. Clin. Infect. Dis. 36:E71-E72. [DOI] [PubMed] [Google Scholar]
- 382.Simpson, A. J., M. D. Smith, G. J. Weverling, Y. Suputtamongkol, B. J. Angus, W. Chaowagul, N. J. White, S. J. van Deventer, and J. M. Prins. 2000. Prognostic value of cytokine concentrations (tumor necrosis factor-alpha, interleukin-6, and interleukin-10) and clinical parameters in severe melioidosis. J. Infect. Dis. 181:621-625. [DOI] [PubMed] [Google Scholar]
- 383.Simpson, A. J., Y. Suputtamongkol, M. D. Smith, B. J. Angus, A. Rajanuwong, V. Wuthiekanun, P. A. Howe, A. L. Walsh, W. Chaowagul, and N. J. White. 1999. Comparison of imipenem and ceftazidime as therapy for severe melioidosis. Clin. Infect. Dis. 29:381-387. [DOI] [PubMed] [Google Scholar]
- 384.Simpson, A. J. H., and W. Vanaporn. 2000. Interaction of insulin with Burkholderia pseudomallei may be caused by preservative. J. Clin. Pathol. 53:159-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Siripanthong, S., S. Teerapantuwat, W. Prugsanusak, Y. Suputtamongkol, P. Viriyasithavat, W. Chaowagul, D. A. Dance, and N. J. White. 1991. Corneal ulcer caused by Pseudomonas pseudomallei: report of three cases. Rev. Infect. Dis. 13:335-337. [DOI] [PubMed] [Google Scholar]
- 386.Sirisinha, S., N. Anuntagool, T. Dharakul, P. Ekpo, S. Wongratanacheewin, P. Naigowit, B. Petchclai, V. Thamlikitkul, and Y. Suputtamongkol. 2000. Recent developments in laboratory diagnosis of melioidosis. Acta Trop. 74:235-245. [DOI] [PubMed] [Google Scholar]
- 387.Sirisinha, S., N. Anuntagool, P. Intachote, V. Wuthiekanun, S. D. Puthucheary, J. Vadivelu, and N. J. White. 1998. Antigenic differences between clinical and environmental isolates of Burkholderia pseudomallei. Microbiol. Immunol. 42:731-737. [DOI] [PubMed] [Google Scholar]
- 388.Siritapetawee, J., H. Prinz, W. Samosornsuk, R. H. Ashley, and W. Suginta. 2004. Functional reconstitution, gene isolation and topology modelling of porins from Burkholderia pseudomallei and Burkholderia thailandensis. Biochem J. 377:579-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Smith, C., J. Allen, M. Embi, O. Othman, N. Razak, and G. Ismail. 1987. Human melioidosis; an emerging medical problem. MIRCEN J. 3:343-366. [Google Scholar]
- 390.Smith, M. D., B. J. Angus, V. Wuthiekanun, and N. J. White. 1997. Arabinose assimilation defines a nonvirulent biotype of Burkholderia pseudomallei. Infect. Immun. 65:4319-4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Smith, M. D., Y. Suputtamongkol, W. Chaowagul, M. Assicot, C. Bohuon, S. Petitjean, and N. J. White. 1995. Elevated serum procalcitonin levels in patients with melioidosis. Clin. Infect. Dis. 20:641-645. [DOI] [PubMed] [Google Scholar]
- 392.Smith, M. D., V. Wuthiekanun, A. L. Walsh, and N. J. White. 1996. In-vitro activity of carbapenem antibiotics against beta-lactam susceptible and resistant strains of Burkholderia pseudomallei. J. Antimicrob. Chemother. 37:611-615. [DOI] [PubMed] [Google Scholar]
- 393.Smith, M. D., V. Wuthiekanun, A. L. Walsh, and N. J. White. 1995. Quantitative recovery of Burkholderia (Pseudomonas) pseudomallei from soil in Thailand. Trans. R. Soc. Trop. Med. Hyg. 89:488-490. [DOI] [PubMed] [Google Scholar]
- 394.Smith-Vaughan, H. C., D. Gal, P. M. Lawrie, C. Winstanley, K. S. Sriprakash, and B. J. Currie. 2003. Ubiquity of putative type III secretion genes among clinical and environmental Burkholderia pseudomallei isolates in Northern Australia. J. Clin. Microbiol. 41:883-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Snijders, E. P. 1933. Melioidosis op Java. Ned. Tijdschr. Geneeskunde 77:560-561. [Google Scholar]
- 396.So, S. Y., P. Y. Chau, M. Aquinas, M. Gabriel, and W. K. Lam. 1987. Melioidosis: a serological survey in a tuberculosis sanatorium in Hong Kong. Trans. R. Soc. Trop. Med. Hyg. 81:1017-1019. [DOI] [PubMed] [Google Scholar]
- 397.So, S. Y., P. Y. Chau, Y. K. Leung, and W. K. Lam. 1984. First report of septicaemic melioidosis in Hong Kong. Trans. R. Soc. Trop. Med. Hyg. 78:456-459. [DOI] [PubMed] [Google Scholar]
- 398.So, S. Y., P. Y. Chau, Y. K. Leung, W. K. Lam, and D. Y. Yu. 1983. Successful treatment of melioidosis caused by a multiresistant strain in an immunocompromised host with third generation cephalosporins. Am. Rev. Respir. Dis. 127:650-654. [DOI] [PubMed] [Google Scholar]
- 399.Sodeman, W. A. J. 1994. Sherlock Holmes and tropical medicine: a centennial appraisal. Am. J. Trop. Med. Hyg. 50:99-101. [DOI] [PubMed] [Google Scholar]
- 400.Sookpranee, M., P. Boonma, W. Susaengrat, K. Bhuripanyo, and S. Punyagupta. 1992. Multicenter prospective randomized trial comparing ceftazidime plus co-trimoxazole with chloramphenicol plus doxycycline and co-trimoxazole for treatment of severe melioidosis. Antimicrob. Agents Chemother. 36:158-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Sookpranee, M., P. Lumbiganon, S. Puapermpoonsiri, A. Tattawasatra, and J. Nopwinyoovongs. 1989. Contamination of Savlon solution with Pseudomonas pseudomallei at Srinagarind Hospital., p. 211-213. In S. Punyagupta, T. Sirisanthana, and B. Stapatayavong (ed.), Melioidosis. Bangkok Medical Publisher, Bangkok, Thailand.
- 402.Sookpranee, T., M. Sookpranee, M. A. Mellencamp, and L. C. Preheim. 1991. Pseudomonas pseudomallei, a common pathogen in Thailand that is resistant to the bactericidal effects of many antibiotics. Antimicrob. Agents Chemother. 35:484-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Spellberg, B., and J. E. Edwards, Jr. 2001. Type 1/type 2 immunity in infectious diseases. Clin. Infect. Dis. 32:76-102. [DOI] [PubMed] [Google Scholar]
- 404.Stanton, A. T., and W. Fletcher. 1932. Melioidosis, vol. 21. John Bale and Danielson Ltd., London, United Kingdom.
- 405.Stanton, A. T., and W. Fletcher. 1921. Melioidosis, a new disease of the tropics. Trans. Fourth Congr. Far East Assoc. Trop. Med. 2:196-198. [Google Scholar]
- 406.Steinmetz, I., A. Reganzerowski, B. Brenneke, S. Haussler, A. Simpson, and N. J. White. 1999. Rapid identification of Burkholderia pseudomallei by latex agglutination based on an exopolysaccharide-specific monoclonal antibody. J. Clin. Microbiol. 37:225-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Steinmetz, I., M. Rohde, and B. Brenneke. 1995. Purification and characterization of an exopolysaccharide of Burkholderia (Pseudomonas) pseudomallei. Infect. Immun. 63:3959-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Stevens, M. P., A. Friebel, L. A. Taylor, M. W. Wood, P. J. Brown, W. D. Hardt, and E. E. Galyov. 2003. A Burkholderia pseudomallei type III secreted protein, BopE, facilitates bacterial invasion of epithelial cells and exhibits guanine nucleotide exchange factor activity. J. Bacteriol. 185:4992-4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Stevens, M. P., A. Haque, T. Atkins, J. Hill, M. W. Wood, A. Easton, M. Nelson, C. Underwood-Fowler, R. W. Titball, G. J. Bancroft, and E. E. Galyov. 2004. Attenuated virulence and protective efficacy of a Burkholderia pseudomallei bsa type III secretion mutant in murine models of melioidosis. Microbiology 150:2669-2676. [DOI] [PubMed] [Google Scholar]
- 410.Stevens, M. P., M. W. Wood, L. A. Taylor, P. Monaghan, P. Hawes, P. W. Jones, T. S. Wallis, and E. E. Galyov. 2002. An Inv/Mxi-Spa-like type III protein secretion system in Burkholderia pseudomallei modulates intracellular behaviour of the pathogen. Mol. Microbiol. 46:649-659. [DOI] [PubMed] [Google Scholar]
- 411.Stoltz, D. A., P. Zhang, S. Nelson, R. P. Bohm, Jr., M. Murphey-Corb, and G. J. Bagby. 1999. Ethanol suppression of the functional state of polymorphonuclear leukocytes obtained from uninfected and simian immunodeficiency virus infected rhesus macaques. Alcohol Clin. Exp. Res. 23:878-884. [PubMed] [Google Scholar]
- 412.Strauss, J. M., A. D. Alexander, G. Rapmund, E. Gan, and A. E. Dorsey. 1969. Melioidosis in Malaysia. III. Antibodies to Pseudomonas pseudomallei in the human population. Am. J. Trop. Med. Hyg. 18:703-707. [PubMed] [Google Scholar]
- 413.Strauss, J. M., M. G. Groves, M. Mariappan, and D. W. Ellison. 1969. Melioidosis in Malaysia. II. Distribution of Pseudomonas pseudomallei in soil and surface water. Am. J. Trop. Med. Hyg. 18:698-702. [PubMed] [Google Scholar]
- 414.Struelens, M. J., G. Mondol, M. Bennish, and D. A. Dance. 1988. Melioidosis in Bangladesh: a case report. Trans. R. Soc. Trop. Med. Hyg. 82:777-778. [DOI] [PubMed] [Google Scholar]
- 415.Suputtamongkol, Y., W. Chaowagul, P. Chetchotisakd, N. Lertpatanasuwun, S. Intaranongpai, T. Ruchutrakool, D. Budhsarawong, P. Mootsikapun, V. Wuthiekanun, N. Teerawatasook, and A. Lulitanond. 1999. Risk factors for melioidosis and bacteremic melioidosis. Clin. Infect. Dis. 29:408-413. [DOI] [PubMed] [Google Scholar]
- 416.Suputtamongkol, Y., D. A. Dance, W. Chaowagul, Y. Wattanagoon, V. Wuthiekanun, and N. J. White. 1991. Amoxycillin-clavulanic acid treatment of melioidosis. Trans. R. Soc. Trop. Med. Hyg. 85:672-675. [DOI] [PubMed] [Google Scholar]
- 417.Suputtamongkol, Y., A. J. Hall, D. A. Dance, W. Chaowagul, A. Rajchanuvong, M. D. Smith, and N. J. White. 1994. The epidemiology of melioidosis in Ubon Ratchatani, northeast Thailand. Int. J. Epidemiol. 23:1082-1090. [DOI] [PubMed] [Google Scholar]
- 418.Suputtamongkol, Y., S. Intaranongpai, M. D. Smith, B. Angus, W. Chaowagul, C. Permpikul, J. A. Simpson, A. Leelarasamee, L. Curtis, and N. J. White. 2000. A double-blind placebo-controlled study of an infusion of lexipafant (platelet-activating factor receptor antagonist) in patients with severe sepsis. Antimicrob. Agents Chemother. 44:693-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Suputtamongkol, Y., A. Rajchanuwong, W. Chaowagul, D. A. Dance, M. D. Smith, V. Wuthiekanun, A. L. Walsh, S. Pukrittayakamee, and N. J. White. 1994. Ceftazidime vs. amoxicillin/clavulanate in the treatment of severe melioidosis. Clin. Infect. Dis. 19:846-853. [DOI] [PubMed] [Google Scholar]
- 420.Sura, T., M. D. Smith, G. M. Cowan, A. L. Walsh, N. J. White, and S. Krishna. 1997. Polymerase chain reaction for the detection of Burkholderia pseudomallei. Diagn. Microbiol. Infect. Dis. 29:121-127. [DOI] [PubMed] [Google Scholar]
- 421.Sutmoller, P., F. C. Kraneveld, and A. van der Schaaf. 1957. Melioidosis (pseudomalleus) in sheep, goats and pigs on Arubu (Netherland Antilles). J. Am. Vet Med. Assoc. 130:415-417. [PubMed] [Google Scholar]
- 422.Tarlow, M. J., and J. Lloyd. 1971. Melioidosis and chronic granulomatous disease. Proc. R. Soc. Med. 64:19-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Thamprajamchit, S., P. Chetchotisakd, and B. Thinkhamrop. 1998. Cefoperazone/sulbactam + co-trimoxazole vs ceftazidime + co-trimoxazole in the treatment of severe melioidosis: a randomized, double-blind, controlled study. J. Med. Assoc. Thai. 81:265-271. [PubMed] [Google Scholar]
- 424.Thin, R. N., M. Brown, J. B. Stewart, and C. J. Garrett. 1970. Melioidosis: a report of ten cases. Q. J. Med. 39:115-127. [PubMed] [Google Scholar]
- 425.Thin, R. N., M. Groves, G. Rapmund, and M. Mariappan. 1971. Pseudomonas pseudomallei in the surface water of Singapore. Singapore Med. J. 12:181-182. [PubMed] [Google Scholar]
- 426.Thomas, A. D. 1981. Prevalence of melioidosis in animals in northern Queensland. Aust. Vet. J. 57:146-148. [DOI] [PubMed] [Google Scholar]
- 427.Thomas, A. D., J. Forbes Faulkner, and M. Parker. 1979. Isolation of Pseudomonas pseudomallei from clay layers at defined depths. Am. J. Epidemiol. 110:515-521. [DOI] [PubMed] [Google Scholar]
- 428.Thomas, A. D., J. H. Norton, and B. W. Pott. 1980. Melioidosis in a galah (Cacatua roseicapilla). Aust. Vet. J. 56:192-193. [DOI] [PubMed] [Google Scholar]
- 429.Thomas, A. D., G. A. Spinks, T. L. D'Arcy, J. H. Norton, and K. F. Trueman. 1988. Evaluation of four serological tests for the diagnosis of caprine melioidosis. Aust. Vet. J. 65:261-264. [DOI] [PubMed] [Google Scholar]
- 430.Thomas, A. D., A. J. Wilson, and J. N. Aubrey. 1978. Melioidosis in a sulphur-crested cockatoo (Cacatua galerita). Aust. Vet. J. 54:306-307. [DOI] [PubMed] [Google Scholar]
- 431.Thurnheer, U., A. Novak, M. Michel, C. Ruchti, H. Jutzi, and M. Weiss. 1988. Septic melioidosis following a visit to India. Schweiz. Med. Wochenschr. 118:558-564. [PubMed] [Google Scholar]
- 432.Tiangpitayakorn, C., S. Songsivilai, N. Piyasangthong, and T. Dharakul. 1997. Speed of detection of Burkholderia pseudomallei in blood cultures and its correlation with the clinical outcome. Am. J. Trop. Med. Hyg. 57:96-99. [DOI] [PubMed] [Google Scholar]
- 433.Toda, H., A. Murata, N. Matsuura, K. Uda, Y. Oka, N. Tanaka, and T. Mori. 1993. Therapeutic efficacy of granulocyte colony stimulating factor against rat cecal ligation and puncture model. Stem Cells 11:228-234. [DOI] [PubMed] [Google Scholar]
- 434.Tong, S., S. Yang, Z. Lu, and W. He. 1996. Laboratory investigation of ecological factors influencing the environmental presence of Burkholderia pseudomallei. Microbiol. Immunol. 40:451-453. [DOI] [PubMed] [Google Scholar]
- 435.Toohey, M., A. E. Lew, and P. M. Desmarchelier. 1994. Laboratory investigations of Australian isolates of ceftazidime resistant Pseudomonas pseudomallei. Antibiotic Special Interest Group (Australian Society of Microbiology) Newsl. 2:1-3. [Google Scholar]
- 436.Torrens, J. K., P. H. McWhinney, and D. S. Tompkins. 1999. A deadly thorn: a case of imported melioidosis. Lancet 353:1016. [DOI] [PubMed] [Google Scholar]
- 437.Trakulsomboon, S., V. Vuddhakul, P. Tharavichitkul, N. Na-Gnam, Y. Suputtamongkol, and V. Thamlikitkul. 1999. Epidemiology of arabinose assimilation in Burkholderia pseudomallei isolated from patients and soil in Thailand. Southeast Asian J. Trop. Med. Public Health 30:756-759. [PubMed] [Google Scholar]
- 438.Tremonti, L. P., and L. H. Dart. 1971. Focal encephalitis due to Pseudomonas pseudomallei. JAMA 215:112-113. [PubMed] [Google Scholar]
- 439.Tribuddharat, C., R. A. Moore, P. Baker, and D. E. Woods. 2003. Burkholderia pseudomallei class A beta-lactamase mutations that confer selective resistance against ceftazidime or clavulanic acid inhibition. Antimicrob. Agents Chemother. 47:2082-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Tsang, T. Y., and S. T. Lai. 2001. A case of thoracic empyema due to suppurative melioidosis. Hong Kong Med. J. 7:201-204. [PubMed] [Google Scholar]
- 441.Ulett, G. C., B. J. Currie, T. W. Clair, M. Mayo, N. Ketheesan, J. Labrooy, D. Gal, R. Norton, C. A. Smith, J. Barnes, J. Warner, and R. G. Hirst. 2001. Burkholderia pseudomallei virulence: definition, stability and association with clonality. Microbes Infect. 3:621-631. [DOI] [PubMed] [Google Scholar]
- 442.Ulett, G. C., R. Hirst, B. Bowden, K. Powell, and R. Norton. 2003. A comparison of antibiotic regimens in the treatment of acute melioidosis in a mouse model. J. Antimicrob. Chemother. 51:77-81. [DOI] [PubMed] [Google Scholar]
- 443.Ulett, G. C., N. Ketheesan, and R. G. Hirst. 2000. Cytokine gene expression in innately susceptible BALB/c mice and relatively resistant C57BL/6 mice during infection with virulent Burkholderia pseudomallei. Infect. Immun. 68:2034-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Utaisincharoen, P., N. Anuntagool, K. Limposuwan, P. Chaisuriya, and S. Sirisinha. 2003. Involvement of beta interferon in enhancing inducible nitric oxide synthase production and antimicrobial activity of Burkholderia pseudomallei-infected macrophages. Infect. Immun. 71:3053-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Utaisincharoen, P., W. Kespichayawattana, N. Anuntagool, P. Chaisuriya, S. Pichyangkul, A. M. Krieg, and S. Sirisinha. 2003. CpG ODN enhances uptake of bacteria by mouse macrophages. Clin. Exp. Immunol. 132:70-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Utaisincharoen, P., N. Tangthawornchaikul, W. Kespichayawattana, N. Anuntagool, P. Chaisuriya, and S. Sirisinha. 2000. Kinetic studies of the production of nitric oxide (NO) and tumour necrosis factor-alpha (TNF-alpha) in macrophages stimulated with Burkholderia pseudomallei endotoxin. Clin. Exp. Immunol. 122:324-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Utaisincharoen, P., N. Tangthawornchaikul, W. Kespichayawattana, P. Chaisuriya, and S. Sirisinha. 2001. Burkholderia pseudomallei interferes with inducible nitric oxide synthase (iNOS) production: a possible mechanism of evading macrophage killing. Microbiol. Immunol. 45:307-313. [DOI] [PubMed] [Google Scholar]
- 448.Vadivelu, J., S. D. Puthucheary, B. S. Drasar, D. A. Dance, and T. L. Pitt. 1998. Stability of strain genotypes of Burkholderia pseudomallei from patients with single and recurrent episodes of melioidosis. Trop. Med. Int. Health 3:518-521. [DOI] [PubMed] [Google Scholar]
- 449.Vadivelu, J., S. D. Puthucheary, A. Mifsud, B. S. Draser, D. A. B. Dance, and T. L. Pitt. 1997. Ribotyping and DNA macrorestriction analysis of isolates of Burkholderia pseudomallei from cases of melioidosis in Malaysia. Trans. R. Soc. Trop. Med. Hyg. 91:358-360. [DOI] [PubMed] [Google Scholar]
- 450.van den Berghe, G., P. Wouters, F. Weekers, C. Verwaest, F. Bruyninckx, M. Schetz, D. Vlasselaers, P. Ferdinande, P. Lauwers, and R. Bouillon. 2001. Intensive insulin therapy in the critically ill patients. N. Engl. J. Med. 345:1359-1367. [DOI] [PubMed] [Google Scholar]
- 451.Van der Lugt, J. J., and M. M. Henton. 1995. Melioidosis in a goat. J. S. Afr. Vet. Assoc. 66:71-73. [PubMed] [Google Scholar]
- 452.Van Peenen, P. F., R. See, P. E. Soysa, and G. S. Irving. 1976. Seroepidemiological survey of hospital-associated populations in Colombo, Sri Lanka. Southeast Asian J. Trop. Med. Public Health 1976:16-20. [PubMed] [Google Scholar]
- 453.Van Phung, L., H. T. Quynh, E. Yabuuchi, and D. A. Dance. 1993. Pilot study of exposure to Pseudomonas pseudomallei in northern Vietnam. Trans. R. Soc. Trop. Med. Hyg. 87:416. [DOI] [PubMed] [Google Scholar]
- 454.Vasu, C., J. Vadivelu, and S. D. Puthucheary. 2003. The humoral immune response in melioidosis patients during therapy. Infection 31:24-30. [DOI] [PubMed] [Google Scholar]
- 455.Vatcharapreechasakul, T., Y. Suputtamongkol, D. A. Dance, W. Chaowagul, and N. J. White. 1992. Pseudomonas pseudomallei liver abscesses: a clinical, laboratory, and ultrasonographic study. Clin. Infect. Dis. 14:412-417. [DOI] [PubMed] [Google Scholar]
- 456.Veljanov, D., A. Vesselinova, S. Nikolova, H. Najdenski, V. Kussovski, and N. Markova. 1996. Experimental melioidosis in inbred mouse strains. Zentralbl. Bakteriol. 283:351-359. [DOI] [PubMed] [Google Scholar]
- 457.Vesselinova, A., H. Najdenski, S. Nikolova, and V. Kussovski. 1996. Experimental melioidosis in hens. Zentralbl. Veterinarmed. B 43:371-378. [DOI] [PubMed] [Google Scholar]
- 458.Visca, P., G. Cazzola, A. Petrucca, and C. Braggion. 2001. Travel-associated Burkholderia pseudomallei infection (melioidosis) in a patient with cystic fibrosis: a case report. Clin. Infect. Dis. 32:E15-E16. [DOI] [PubMed] [Google Scholar]
- 459.Visudhiphan, P., S. Chiemchanya, and D. Dheandhanoo. 1990. Central nervous system melioidosis in children. Pediatr. Infect. Dis. J. 9:658-661. [PubMed] [Google Scholar]
- 460.Vorachit, M., P. Chongtrakool, S. Arkomsean, and S. Boonsong. 2000. Antimicrobial resistance in Burkholderia pseudomallei. Acta Trop. 74:139-144. [DOI] [PubMed] [Google Scholar]
- 461.Vorachit, M., K. Lam, P. Jayanetra, and J. W. Costerton. 1993. Resistance of Pseudomonas pseudomallei growing as a biofilm on silastic discs to ceftazidime and co-trimoxazole. Antimicrob. Agents Chemother. 37:2000-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Walsh, A. L., M. D. Smith, V. Wuthiekanun, Y. Suputtamongkol, V. Desakorn, W. Chaowagul, and N. J. White. 1994. Immunofluorescence microscopy for the rapid diagnosis of melioidosis. J. Clin. Pathol. 47:377-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Walsh, A. L., M. D. Smith, V. Wuthiekanun, and N. J. White. 1995. Postantibiotic effects and Burkholderia (Pseudomonas) pseudomallei: an evaluation of current treatment. Antimicrob. Agents Chemother. 39:2356-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Wanachiwanawin, W. 2000. Infections in E-beta thalassemia. J. Pediatr. Hematol. Oncol. 22:581-587. [DOI] [PubMed] [Google Scholar]
- 465.Wang, Y. S., C. H. Wong, and A. Kurup. 2003. Cutaneous melioidosis and necrotizing fasciitis caused by Burkholderia pseudomallei. Emerg. Infect. Dis. 9:1484-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Warawa, J., and D. E. Woods. 2002. Melioidosis vaccines. Expert Rev. Vaccines 1:477-482. [DOI] [PubMed] [Google Scholar]
- 467.Warawa, J., and D. E. Woods. 2005. Type III secretion system cluster 3 is required for maximal virulence of Burkholderia pseudomallei in a hamster infection model. FEMS Microbiol. Lett. 242:101-108. [DOI] [PubMed] [Google Scholar]
- 468.Ward, C., M. Camara, I. Forrest, R. Rutherford, G. Pritchard, M. Daykin, A. Hardman, A. de Soyza, A. J. Fisher, P. Williams, and P. A. Corris. 2003. Preliminary findings of quorum signal molecules in clinically stable lung allograft recipients. Thorax 58:444-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Warner, J., D. Learoyd, D. Pelowa, J. Koehler, and R. Hirst. 1998. Presented at the Annual Scientific Meeting, Medical Society of Papua New Guinea, Port Moresby.
- 470.Webling, D. D. 1980. Genito-urinary infections with Pseudomonas pseudomallei in Australian Aboriginals. Trans. R. Soc. Trop. Med. Hyg. 74:138-139. [DOI] [PubMed] [Google Scholar]
- 471.Weiss, M., L. L. Moldawer, and E. M. Schneider. 1999. Granulocyte colony-stimulating factor to prevent the progression of systemic nonresponsiveness in systemic inflammatory response syndrome and sepsis. Blood 93:425-439. [PubMed] [Google Scholar]
- 472.White, N. J. 2003. Melioidosis. Lancet. 361:1715-1722. [DOI] [PubMed] [Google Scholar]
- 473.White, N. J., D. A. Dance, W. Chaowagul, Y. Wattanagoon, V. Wuthiekanun, and N. Pitakwatchara. 1989. Halving of mortality of severe melioidosis by ceftazidime. Lancet ii:697-701. [DOI] [PubMed] [Google Scholar]
- 474.Whitmore, A. 1913. An account of a glanders-like disease occurring in Rangoon. J. Hyg. 13:1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Whitmore, A., and C. S. Krishnaswami. 1912. An account of the discovery of a hitherto underscribed infective disease occurring among the population of Rangoon. Indian Med. Gazette 47:262-267. [PMC free article] [PubMed] [Google Scholar]
- 476.Wiener, E. 2003. Impaired phagocyte antibacterial effector functions in beta-thalassemia: a likely factor in the increased susceptibility to bacterial infections. Hematology 8:35-40. [DOI] [PubMed] [Google Scholar]
- 477.Wong, K. T., S. D. Puthucheary, and J. Vadivelu. 1995. The histopathology of human melioidosis. Histopathology 26:51-55. [DOI] [PubMed] [Google Scholar]
- 478.Wong, P. K., and P. H. Ng. 1996. Melioidosis presenting with orbital cellulitis. Singapore Med. J. 37:220-221. [PubMed] [Google Scholar]
- 479.Wongratanacheewin, S., S. Amornpunt, R. W. Sermswan, U. Tattawasart, and S. Wongwajana. 1995. Use of culture-filtrated antigen in an ELISA and a dot immunoassay for the diagnosis of melioidosis. Southeast Asian J. Trop. Med. Public Health 26:329-334. [PubMed] [Google Scholar]
- 480.Wongratanacheewin, S., W. Kespichayawattana, P. Intachote, S. Pichyangkul, R. W. Sermswan, A. M. Krieg, and S. Sirisinha. 2004. Immunostimulatory CpG oligodeoxynucleotide confers protection in a murine model of infection with Burkholderia pseudomallei. Infect. Immun. 72:4494-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Wongratanacheewin, S., U. Tattawasart, and V. Lulitanond. 1990. An avidin-biotin enzyme-linked immunosorbent assay for the detection of Pseudomonas pseudomallei antigens. Trans. R. Soc. Trop. Med. Hyg. 84:429-430. [DOI] [PubMed] [Google Scholar]
- 482.Wongratanacheewin, S., U. Tattawasart, V. Lulitanond, S. Wongwajana, R. W. Sermswan, M. Sookpranee, and K. Nuntirooj. 1993. Characterization of Pseudomonas pseudomallei antigens by SDS-polyacrylamide gel electrophoresis and Western blot. Southeast Asian J. Trop. Med. Public Health 24:107-113. [PubMed] [Google Scholar]
- 483.Woo, M. L., P. S. Chan, and G. L. French. 1987. A case of melioidosis presenting with prostatic abscess in Hong Kong. J. Urol. 137:120-121. [DOI] [PubMed] [Google Scholar]
- 484.Woo, P. C., S. K. Lau, G. K. Woo, A. M. Fung, A. H. Ngan, W. T. Hui, and K. Y. Yuen. 2003. Seronegative bacteremic melioidosis caused by Burkholderia pseudomallei with ambiguous biochemical profile: clinical importance of accurate identification by 16S rRNA gene and groEL gene sequencing. J. Clin. Microbiol. 41:3973-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Woo, P. C., G. K. Woo, S. K. Lau, S. S. Wong, and K. Yuen. 2002. Single gene target bacterial identification. groEL gene sequencing for discriminating clinical isolates of Burkholderia pseudomallei and Burkholderia thailandensis. Diagn. Microbiol. Infect. Dis. 44:143-149. [DOI] [PubMed] [Google Scholar]
- 486.Woods, D. E., D. DeShazer, R. A. Moore, P. J. Brett, M. N. Burtnick, S. L. Reckseidler, and M. D. Senkiw. 1999. Current studies on the pathogenesis of melioidosis. Microbes Infect. 1:157-162. [DOI] [PubMed] [Google Scholar]
- 487.Woods, D. E., A. L. Jones, and P. J. Hill. 1993. Interaction of insulin with Pseudomonas pseudomallei. Infect. Immun. 61:4045-4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Woods, M. L., B. J. Currie, D. M. Howard, A. Tierney, A. Watson, N. M. Anstey, J. Philpott, V. Asche, and K. Withnall. 1992. Neurological melioidosis: seven cases from the Northern Territory of Australia. Clin. Infect. Dis. 15:163-169. [DOI] [PubMed] [Google Scholar]
- 489.Worthington, M. G., and D. W. McEniry. 1990. Chronic melioidosis in a Vietnamese immigrant. Rev. Infect. Dis. 12:966. [DOI] [PubMed] [Google Scholar]
- 490.Wuthiekanun, V., P. Amornchai, W. Chierakul, A. C. Cheng, N. J. White, S. J. Peacock, and N. P. Day. 2004. Evaluation of immunoglobulin M (IgM) and IgG rapid cassette test kits for diagnosis of melioidosis in an area of endemicity. J. Clin. Microbiol. 42:3435-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Wuthiekanun, V., D. A. Dance, Y. Wattanagoon, Y. Supputtamongkol, W. Chaowagul, and N. J. White. 1990. The use of selective media for the isolation of Pseudomonas pseudomallei in clinical practice. J. Med. Microbiol. 33:121-126. [DOI] [PubMed] [Google Scholar]
- 492.Wuthiekanun, V., M. D. Smith, D. A. Dance, and N. J. White. 1995. Isolation of Pseudomonas pseudomallei from soil in north-eastern Thailand. Trans. R. Soc. Trop. Med. Hyg. 89:41-43. [DOI] [PubMed] [Google Scholar]
- 493.Wuthiekanun, V., M. D. Smith, and N. J. White. 1995. Survival of Burkholderia pseudomallei in the absence of nutrients. Trans. R. Soc. Trop. Med. Hyg. 89:491. [DOI] [PubMed] [Google Scholar]
- 494.Wuthiekanun, V., Y. Suputtamongkol, A. J. Simpson, P. Kanaphun, and N. J. White. 2001. Value of throat swab in diagnosis of melioidosis. J. Clin. Microbiol. 39:3801-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494a.Wuthiekanun, V., M. Mayxay, W. Chierakul, R. Phetsouvanh, A. C. Cheng, N. J. White, N. P. Day, and S. J. Peacock. 2005. Detection of Burkholderia pseudomallei in soil within the Lao People's Democratic Republic. J. Clin. Microbiol. 43:923-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Yabuuchi, E., Y. Kosako, M. Arakawa, H. Hotta, and I. Yano. 1992. Identification of Oklahoma isolate as a strain of Pseudomonas pseudomallei. Microbiol. Immunol. 36:1239-1249. [DOI] [PubMed] [Google Scholar]
- 496.Yabuuchi, E., Y. Kosako, H. Oyaizu, I. Yano, H. Hotta, Y. Hashimoto, T. Ezaki, and M. Arakawa. 1992. Proposal of Burkholderia gen. nov. and transfer of seven species of the genus Pseudomonas homology group II to the new genus, with the type species Burkholderia cepacia (Palleroni and Holmes 1981) comb. nov. Microbiol. Immunol. 36:1251-1275. [DOI] [PubMed] [Google Scholar]
- 497.Yamamoto, T., P. Naigowit, S. Dejsirilert, D. Chiewsilp, E. Kondo, T. Yokota, and K. Kanai. 1990. In vitro susceptibilities of Pseudomonas pseudomallei to 27 antimicrobial agents. Antimicrob. Agents Chemother. 34:2027-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Yang, H., C. D. Kooi, and P. A. Sokol. 1993. Ability of Pseudomonas pseudomallei malleobactin to acquire transferrin-bound, lactoferrin-bound, and cell-derived iron. Infect. Immun. 61:656-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Yang, S. 2000. Melioidosis research in China. Acta Trop. 77:157-165. [DOI] [PubMed] [Google Scholar]
- 500.Yang, S., S. Tong, and Z. Lu. 1995. Geographical distribution of Pseudomonas pseudomallei in China. Southeast Asian J. Trop. Med. Public Health 26:636-638. [PubMed] [Google Scholar]
- 501.Yang, S., S. Tong, C. Mo, Z. Jiang, Y. Ma, and Z. Lu. 1998. Prevalence of human melioidosis on Hainan Island in China. Microbiol. Immunol. 42:651-654. [DOI] [PubMed] [Google Scholar]
- 502.Yap, E. H., Y. C. Chan, T. Y. Ti, T. W. Thong, A. L. Tan, M. Yeo, L. C. Ho, and M. Singh. 1991. Serodiagnosis of melioidosis in Singapore by the indirect haemagglutination test. Singapore Med. J. 32:211-213. [PubMed] [Google Scholar]
- 503.Zaharik, M. L., S. Gruenheid, A. J. Perrin, and B. B. Finlay. 2002. Delivery of dangerous goods: type III secretion in enteric pathogens. Int. J. Med. Microbiol. 291:593-603. [DOI] [PubMed] [Google Scholar]
- 504.Zanetti, F., G. De Luca, and S. Stampi. 2000. Recovery of Burkholderia pseudomallei and B. cepacia from drinking water. Int. J. Food Microbiol. 59:67-72. [DOI] [PubMed] [Google Scholar]
- 505.Zhang, P., S. Nelson, W. R. Summer, and J. A. Spitzer. 1997. Acute ethanol intoxication suppresses the pulmonary inflammatory response in rats challenged with intrapulmonary endotoxin. Alcohol Clin. Exp. Res. 21:773-778. [PubMed] [Google Scholar]