Abstract
Background
In the pursuit of causal insights into neural circuit functionality, various interventions, including electrical, genetic, and pharmacological approaches, have been applied over recent decades. This study employs a comprehensive bibliometric perspective to explore the field of neural circuits.
Methods
Reviews and articles on neural circuits were obtained from the Web of Science Core Collection (WOSCC) database on Apr. 12, 2023. In this article, co-authorship analysis, co-occurrence analysis, citation analysis, bibliographic analysis, and co-citation analysis were used to clarify the authors, journals, institutions, countries, topics, and internal associations between them.
Results
More than 2000 organizations from 52 different countries published 3975 articles in the field of “neural circuit” were used to analysis. Luo liqun emerged as the most prolific author, and Deisseroth Karl garners the highest co-citations (3643). The Journal of Neuroscience leaded in publications, while Nature toped in citations. Chinese Academy of Science recorded the highest article count institutionally, with Stanford University ranking first with 14,350 citations. Since 2020, neurodynamic, anxiety-related mechanisms, and GABAergic neurons have gained prominence, shaping the trajectory of neural circuitry research.
Conclusions
Our investigation has discerned a paradigmatic reorientation towards neurodynamic processes, anxiety-related mechanisms, and GABAergic neurons within the domain of neural circuit research. This identification intimates a prospective trajectory for the field. In the future, it is imperative for research endeavors to accord priority to the translational application of these discernments, with the aim of materializing tangible clinical solutions.
Keywords: Neural circuit, Bibliometric analysis, Topic trends, Visualization, Neurodynamic
1. Introduction
In recent times, there has been an increasing fascination with comprehending the self-regulating capabilities of certain organs, particularly the brain, thanks to advancements in controlling natural phenomena. In April 2013, the United States introduced the Brain Research through Advancing Innovative Neurotechnologies (BRAIN Initiative) with the objective of unraveling the mysteries of the brain [1]. Concurrently, the Human Brain Project (HBP) was initiated within the European Union [2]. Subsequently, countries such as Japan (Brain/MINDS) and China (Brain Science and Brain-like Intelligence Technology) swiftly established their own brain research programs [3]. The BRAIN project can be regarded as an awe-inspiring scientific endeavor, akin to the monumental Human Genome Project (HGP). As an integral part of the BRAIN project, neural circuits have flourished in the context of the brain project. New technologies and methods, such as photoacoustic tomography [4], single cell labeling [5], virtual reality [6], and brain-computer interfaces [7] have also facilitated the exploration of neural circuits.
Neural circuits refer to the interconnected networks of neurons in the nervous system. These circuits are formed by the complex web of connections between individual neurons, enabling the transmission and processing of information within the brain and other parts of the nervous system [8]. In the basic research domain, emerging lines of evidence suggest that discoveries ranging from the delineation of critical periods in the developing brain to the characterization of perceptual and memory processes in the adult brain are involved in the regulation of the neural circuit [9]. For instance, dynamic pruning or elimination of dendritic spines affects development by refining neural circuits [10]. In turn, the modular nature of neuronal circuits also accelerates evolution [11]. The neural circuit of spino-parabrachial aberrations is mainly involved in the regulation of itch [12] and pain [13]. Strikingly, malfunction of the cortico-striatal connection has been implicated in Alzheimer's disease [14]. The dysregulation of neural circuits has been shown to occur in many neurological diseases. Simultaneously, in the clinical realm, newer electromagnetic therapies such as transcranial magnetic stimulation (TMS) and deep brain stimulation (DBS) have been used to target special neural circuits in treatment-resistant depression [15] and Parkinson's disease [16], respectively.
Owing to the ongoing advancements in neuroscience research, there has been a marked escalation in the volume of research literature within the domain of neural circuits. This proliferation of scholarly output presents a formidable challenge for nascent researchers seeking an entry point into this expansive body of work. The application of bibliometrics emerges as a judicious strategy to address this predicament. Bibliometric analysis, a subset of scientometric inquiry [17], operates as a complement to empirical investigations, concurrently furnishing pragmatic recommendations to guide emergent trends and knowledge frameworks within this domain [18]. Notably, the bibliometric scrutiny of extant research offers an objective metric for seminal literature and peer acknowledgment of scholarly contributions, accomplished through the examination of highly cited publications within each respective field [19]. Diversified bibliometric analyses have been previously undertaken across various medical domains [[20], [21], [22]], including cancer disorders [23], psychotic disorders [24], cardiovascular disease [25], endocrine disease [26], cell death [27], and drug addiction [28]. Remarkably, the sphere of neural circuits has hitherto eluded comprehensive bibliometric scrutiny. In the pursuit of filling this lacuna, we undertook the establishment of the intellectual underpinning of neural circuit research spanning the two decades from 2002 to 2022. Employing the dual-core bibliometric software, CiteSpace and VOSviewer, our endeavor sought to delineate the developmental trajectory, research trends, and challenges intrinsic to this field. Methodologically, our inquiry comprised an initial phase wherein a meticulous statistical analysis was conducted across disciplines, journals, authors, countries, institutions, co-cited authors, and co-cited journals to glean foundational insights within this field. Subsequently, a two-fold approach was adopted, encompassing clustering and burstness analyses of keywords, thereby elucidating the prevailing research orientations, focal points, and temporal evolution within the field. Finally, through co-citation analyses of references, we achieved a dual understanding: firstly, an appreciation of the core literature within the field; and secondly, an insight into the dynamic metamorphoses in the knowledge structure of the field over time.
2. Methods
2.1. Data collection
As an important academic platform, the Web of Science contains representative and cutting-edge literature covering fields such as social science, natural science, art, and the humanities, as well as the independent global citation database of the world's most trusted publishers [25]. The Expanded Science Citation Index is a subset of the Web of Science, including more than 12,000 authoritative and high-impact academic journals [29]. Consequently, the Science Citation Index Expanded (SCIE) of the Web of Science Core Collection (WoSCC) has been used to perform this bibliometric analysis (https://www.Webofscience.com/wos/woscc/advanced-search) [30]. The search terms included: TS = “Neural Circuit” OR TS = “Neuronal Circuits”. Articles and reviews from 2002 to 2022 were included, and non-English articles were excluded.
2.2. Data analysis
Full records of publications were collected, including authors, titles, affiliations, countries, document types, languages, keywords, and cited references. Subsequently, a bibliometric analysis was performed using the “bibliometrix” package in the R software (version 4.2.1). A full record and cited references were upload with compression in “bibliometrix” package. The overview of publications, production of authors and countries, the analysis of information flow, trend topic and keywords cloud were visualized in “bibliometrix” after data cleaning.
Next, a network visualization map of authors, journals, institutions, countries, and keywords was created using the VOSviewer program (https://www.vosviewer. com/downloavosviewer). To evaluate the robustness of the results of the network map, we employed more than one strategy in the VOSviewer program, such as bibliographic analysis and co-citation analysis. Finally, CiteSpace (https://sourceforge.net/project/citespace/files/latest/do) was used to identify highly cited references with the strongest citation burst and keyword clustering during a certain period. Appropriate disciplines in neural circuit research were visualized in CiteSpace using a dual-map overlay.
3. Results
3.1. Temporal distribution map of publications
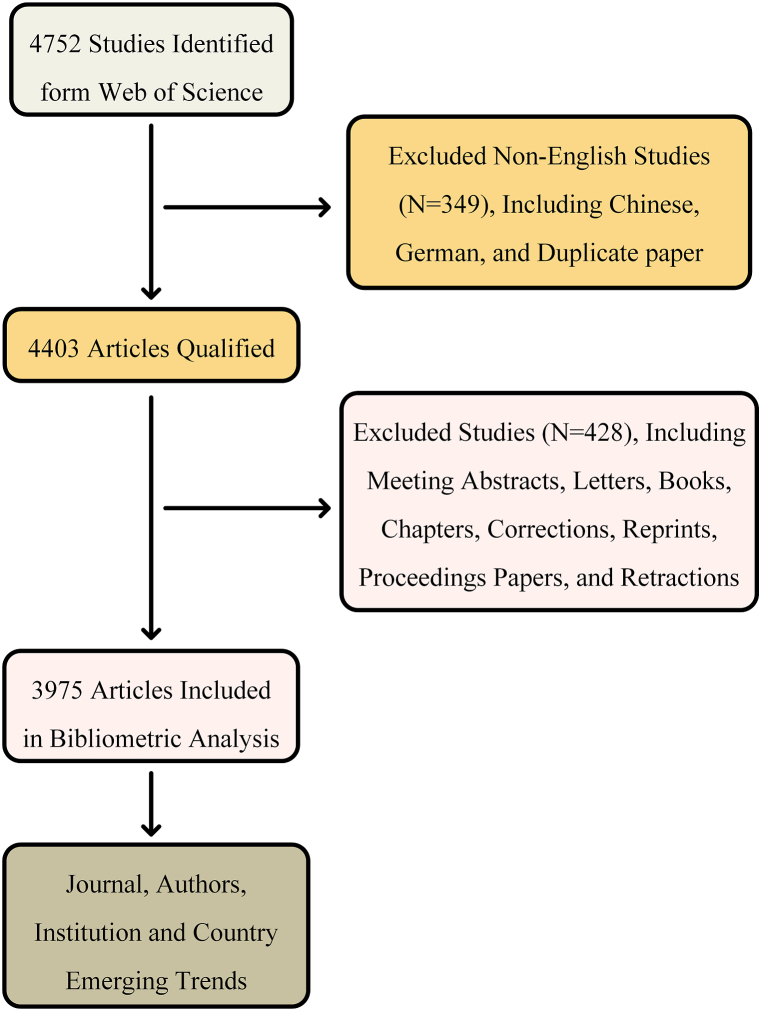
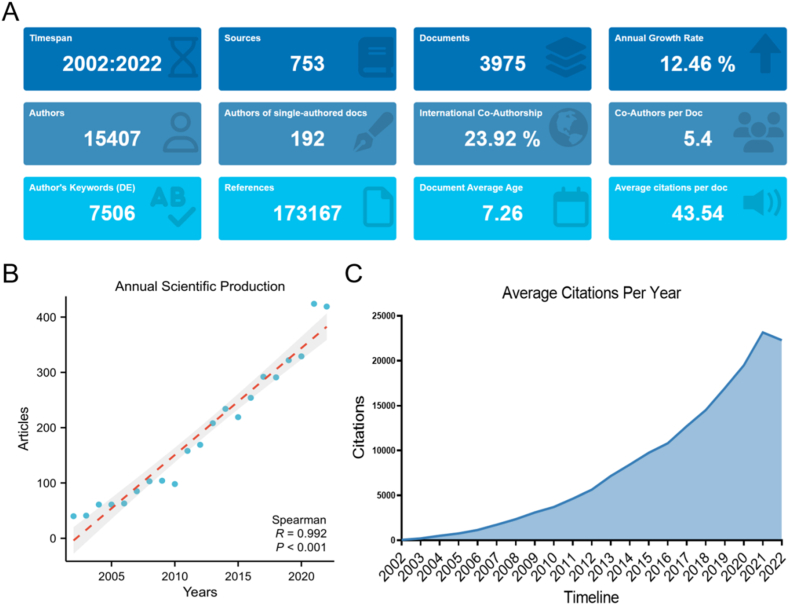
A flowchart of the article selection process from the Web of Science was shown in Fig. 1. We limited the search to articles and reviews published in English from 2002 to 2022. In this research, the number of included publications about neural circuits was 3,975, including 3167 original research articles and 808 reviews, with an average cited frequency per paper of 43.54, and a mean H-index of 133. The descriptive statistics of the included publications were shown in Fig. 2A and Table S1. The number of publications in this period reflected the trend of investigation in the field. Fig. 2B illustrated the trend of annual publications on neural circuits, the annual growth rate in neural circuits reached 12.46 %. There was a clear positive correlation between the annual publications and the publication year, with a correlation coefficient R of 0.992, indicating “neural circuits” has been a topic of interest during the period of assessment. Chronologically, the number of citations in neural circuits has steadily increased, peaking in 2021 (Fig. 2C).
Fig. 1.
Flow diagram for screening.
Fig. 2.
An overview of publications (A) Descriptive statistics of included publications. (B) Annual scientific production of these publications (R = 0.992, P < 0.001). (C) Average citations per year from 2002 to 2022 in this field.
3.2. Analysis of authors and journals
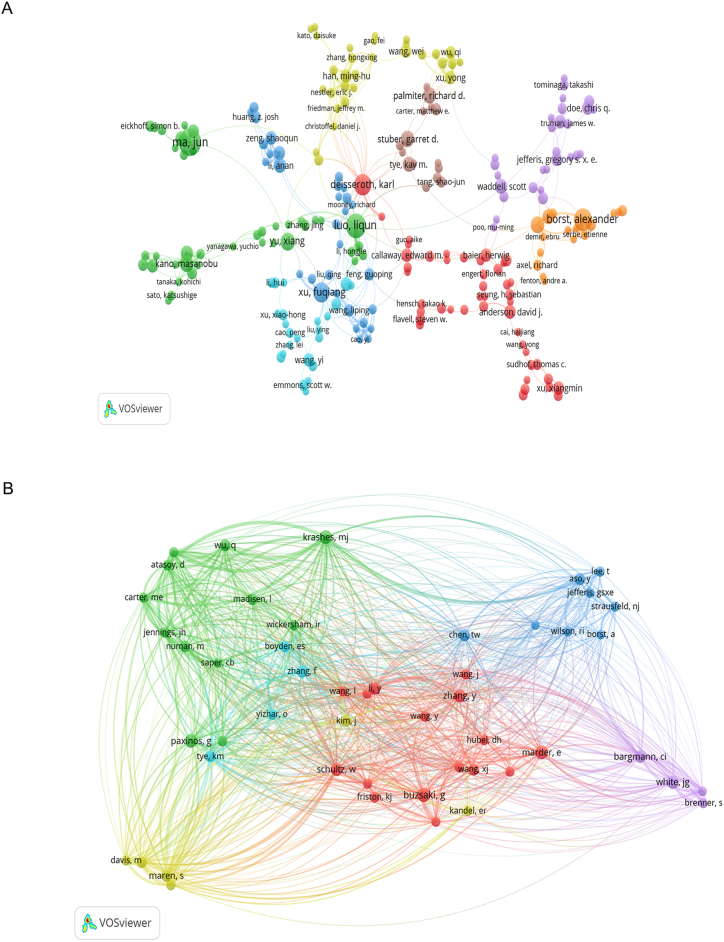
Table 1 displayed the ranking of the most prolific authors, whose collective publication output amounted to 3.92 %. Leading the pack was Luo Liqun with 20 articles, constituting 0.50 % of the total, closely followed by Borst Alexander with 19 articles, representing 0.47 %, and Ma Jun with 18 articles, accounting for 0.45 %. Regarding author citations, the five most frequently referenced individuals were Deisseroth Karl (Citations: 3643), Luo Liqun (Citations: 1577), Dickson Barry J (Citations: 1237), Borst Alexander (Citations: 968), and Mori Ikue (Citations: 721) (Table 1). Fig. 3A showed the co-author network, which included 378 authors with at least four articles for each author. Xu Fuqiang (total link strength, TLS: 32), Ma Jun (TLS: 31), Borst Alexander (TLS: 30), and Luo Liqun (TLS: 30) separately shared the highest collaboration with others. Following this, citation, bibliographic, and co-citation analyses were performed to reveal the authoritativeness of the research in this field and the greatontribution of the authors. The cited bibliographic analysis of authors revealed that Deisseroth Karl, ranking first, was cited 3580 times, with each paper cited 210.6 times on average (Fig. S1). Co-cited articles originated from 16,880 authors. With the top 100-citations reached 50 authors, Krashes Michael J (citations: 229, TLS: 1312), Maren, Stephen (citations: 176, TLS: 1025), and Fanselow Michael S (citations: 132, TLS: 860) had the strongest citations in the neural circuit (Fig. 3B).
Table 1.
The top 10 productive authors.
| Rank | Author | Country | TP | Citations |
|---|---|---|---|---|
| 1 | Luo Liqun | USA | 20 | 1577 |
| 2 | Borst Alexander | Germany | 19 | 968 |
| 3 | Ma Jun | China | 18 | 585 |
| 4 | Deisseroth Karl | USA | 18 | 3643 |
| 5 | Mori Ikue | Japan | 15 | 721 |
| 6 | Stoeckli Esther T | Switzerland | 15 | 434 |
| 7 | Iino Yuichi | Japan | 13 | 598 |
| 8 | Broadie Kendal | USA | 13 | 528 |
| 9 | Lisberger Stephen G | USA | 13 | 353 |
| 10 | Dickson Barry J | Japan | 12 | 1237 |
TP: the number of publications.
Fig. 3.
Author analysis (A) Co-authorship analysis. It was normalized during fractionalization and weighted by the number of documents. (B) Network map of co-citations between authors with minimum citations over 100 times. The size of the circle represents the number of citations in authors, and the lines represent the interaction among authors.
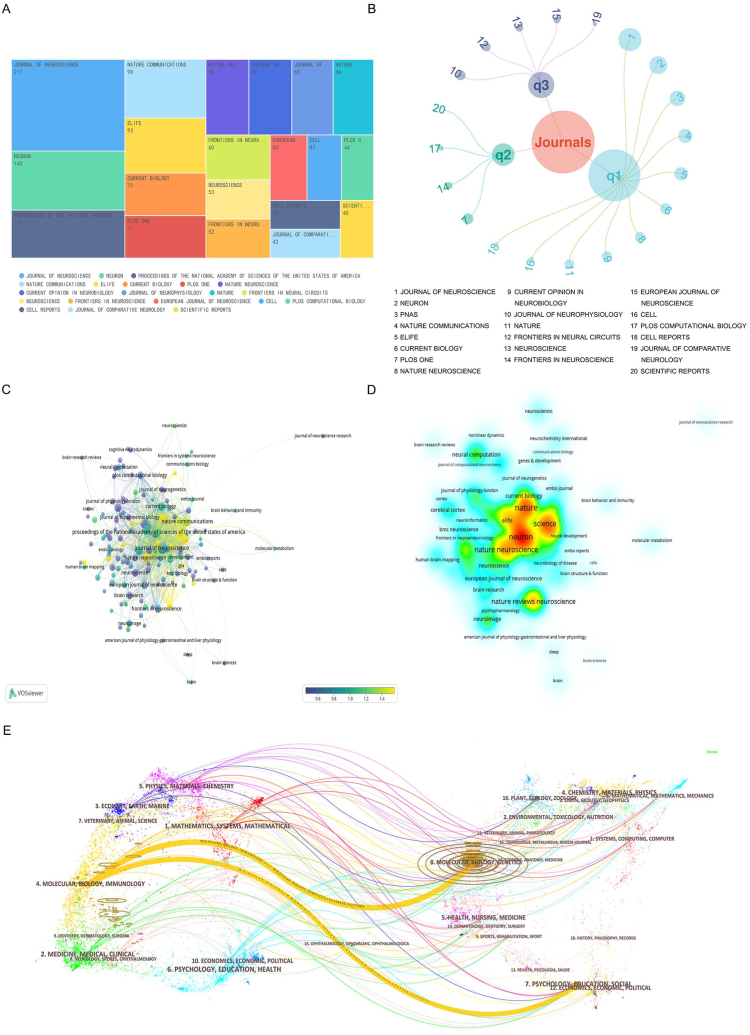
The top 20 journals with the most publications were listed in Fig. 4A. Journal of Neuroscience had the greatest number of publications (217 articles), followed by Neuron (140 articles) and Proceedings of the National Academy of Sciences of the United States of America (113 articles) (Fig. 4A and Table 2). The remaining articles were fragmented into different journals. Hence, research on neural circuits was presumably largely decentralized. Meanwhile, the 2021 impact factor range was 2.97–69.50 and the JCR partition range was Q1-Q3 in this field (Fig. 4B). Of these, most articles (>55 %) were published in Q1, indicating that the overall quality of these research was high. The co-citation relationship of each journal was shown in Fig. 4C. Journals such as Nature, Neuron, Science, etc. as the major intermediaries in the network (Fig. 4D). Relative knowledge flows between disciplines at the journal level were reflected by a dual-map overlay. The results showed that most of papers were published in molecular biology and immunology journal, and these journal mostly cited journals from molecular, biology, genetics, and psychology education social journal (Fig. 4E).
Fig. 4.
Journal analysis of neural circuit (A) Top 20 related sources in this study (B) JCR distribution of the top 20 journals. (C) Co-citation relationship of each journal, weight represents numbers of articles in journals, and score represents numbers of normalized citations. (D) Density visualization of the top 100-citation journals after normalized citations. (E) The dual-map overlay of journals in neural circuit on neural circuit research (left and right sides refer to the citing journal areas and the cited journal areas, respectively).
Table 2.
The top 20 productive journals.
| Rank | Journal | TD | IF (2021) | JCR |
|---|---|---|---|---|
| 1 | Journal of Neuroscience | 217 | 6.709 | Q1 |
| 2 | Neuron | 140 | 18.688 | Q1 |
| 3 | Proceedings of the National Academy of Sciences of the United States of America | 113 | 12.779 | Q1 |
| 4 | Nature Communications | 98 | 14.919 | Q1 |
| 5 | Elife | 93 | 8.713 | Q1 |
| 6 | Current Biology | 72 | 10.9 | Q1 |
| 7 | Plos One | 71 | 3.24 | Q2 |
| 8 | Nature Neuroscience | 68 | 28.771 | Q1 |
| 9 | Current Opinion in Neurobiology | 67 | 7.07 | Q1 |
| 10 | Journal of Neurophysiology | 65 | 2.974 | Q3 |
| 11 | Nature | 64 | 69.504 | Q1 |
| 12 | Frontiers in Neural Circuits | 60 | 3.342 | Q3 |
| 13 | Neuroscience | 53 | 3.708 | Q3 |
| 14 | Frontiers in Neuroscience | 52 | 5.152 | Q2 |
| 15 | European Journal of Neuroscience | 50 | 3.698 | Q3 |
| 16 | Cell | 47 | 66.85 | Q1 |
| 17 | Plos Computational Biology | 44 | 4.779 | Q2 |
| 18 | Cell Reports | 42 | 9.995 | Q1 |
| 19 | Journal of Comparative Neurology | 42 | 3.028 | Q3 |
| 20 | Scientific Reports | 40 | 4.38 | Q2 |
TD: the total number of documents; JCR: journal citation reports.
3.3. Distribution of institutions and countries
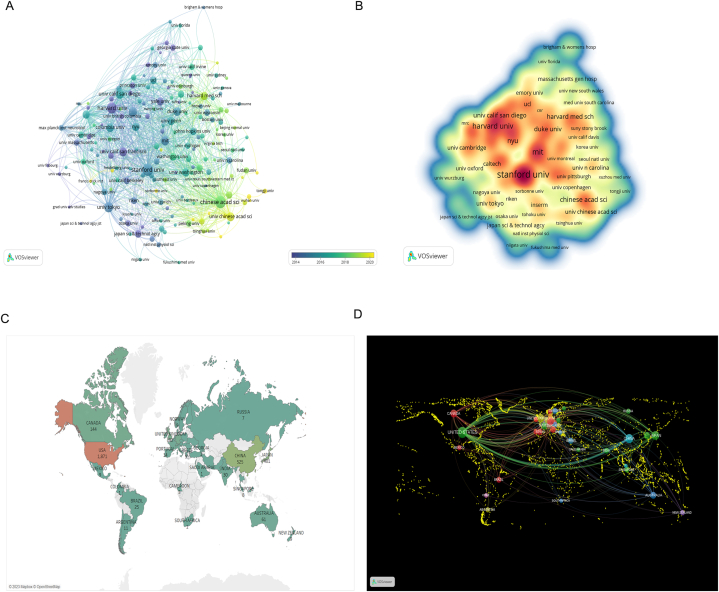
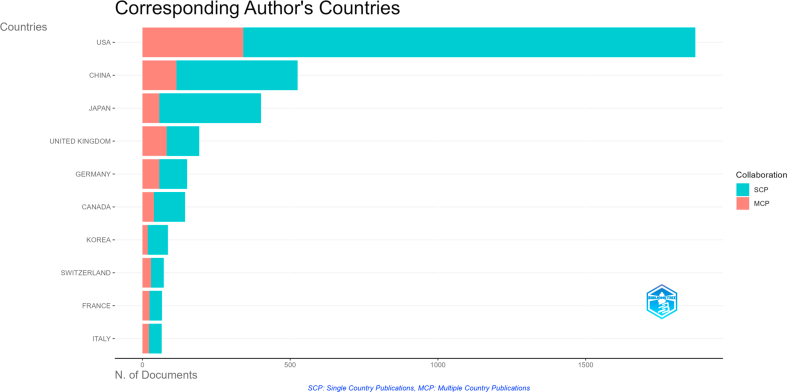
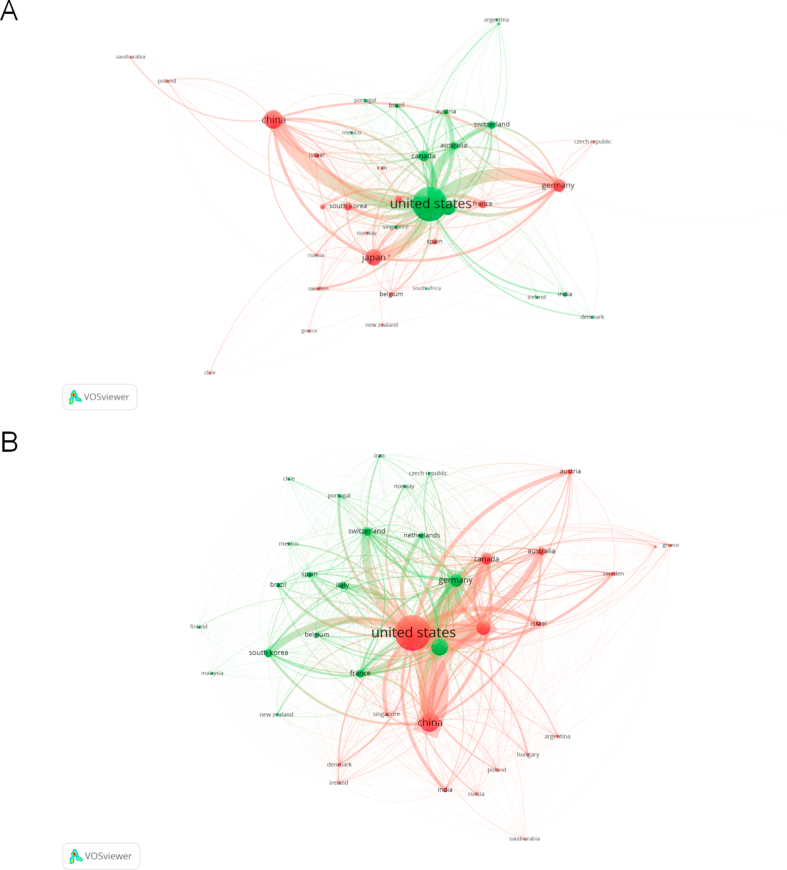
A total of 52 countries and more than 2000 institutions were involved in neural circuit research between 2002 and 2022. Through bibliographic coupling analysis, we retrieved 178 institutions with at least 10 articles published, and found that Chinese Academy of Science had published the most articles (146 articles) and Stanford closely followed up (144 articles) (Fig. 5A). In terms of total citations, Stanford University ranked first with 14,350 citations, while Harvard University ranked second with 10,864 citations (Fig. 5B). Co-authorship (Figs. S2A and B) and citation analysis (Figs. S2C and D) had concordant results with the bibliographic coupling analysis. As shown in Fig. 5C and Table S2, the largest number of published literatures was from the USA, China, and Japan, accounting for 47.1 %, 13.2 %, and 10.1 % of the total articles, respectively. This suggested that there were more researchers in the USA, China, and Japan, focusing on the field of neural circuits. Most articles published in collaboration between different institutions were domestic, but some were international (Fig. S3). Regardless of co-authorship (Fig. 5D), citation (Fig. S4A) or bibliographic coupling analyses (Fig. S4B), collaboration at the national level in the neural circuit of neuroscience was concentrated between the United States and China.
Fig. 5.
The analysis of scientific production and cooperation from different countries and institutions (A) Associations among different institutions through bibliographic coupling. (B) A density map of institutions. The higher the occurrence of citations, the warmer the color becomes in institutions, and the lower the occurrence, the colder the color becomes. (C) Countries' scientific production. (D) Cooperation map of countries involved in neural circuit research. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.4. Trends and thematic analysis
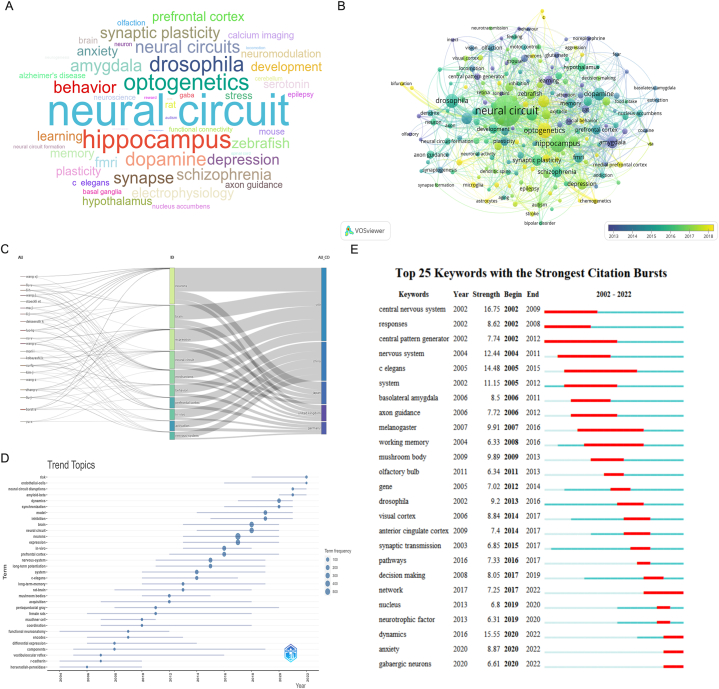
Network analyses of keyword co-occurrences were used to demonstrate frequency of theme concepts and briefly describe the trends of hot spots. The top 50 most frequently used keywords were shown in a Word Cloud by visualization analysis (Fig. 6A). The most frequent keywords were “neural circuit” followed by “hippocampus”, “optogenetics drosophila”, and “dopamine”. A total of 179 keywords appeared more than ten times during the analysis process. Keywords closer to yellow have appeared more frequently in recent years (Fig. 6B). Three components, which consisted of authors, keywords, and countries, were represented by a three-field plot. The connections between the authors (left), keywords (middle), and author nationalities (right) were shown in this plot. The area of each rectangle was proportional to the number of publications. Borst Alexander participated in most of the fields and was significantly associated with the keywords (neurons, brain, etc.). The most described concepts for publications were neuron, in turn, brain, expression, and neural circuit. Additionally, in analyses stratified by nationality, authors from the USA were related to keywords such as neurons, brain, and expression, while Chinese authors preferred to behaviors, prefrontal cortex, and in vivo (Fig. 6C). A temporal distribution of keywords showed that keywords related to neural circuit, such as risk, neural circuit, disruptions, and dynamics, have been used in the past 3 years (Fig. 6D). Fig. 6E showed the top 25 keywords with the strongest citation bursts from 2002 to 2022. During the earlier period, keywords such as “central neuron system (2002–2009)”, “central pattern generator (2002–2012)”, “nervous system (2004–2011)” and “C. elegans (2020–2022)” gained the most sustained attention. In contrast, “dynamics (2020–2022)”, “anxiety (2020–2022)” and “GABAergic neurons (2020–2022)” were the main areas addressed in recent years, indicating that these areas were potential future research frontiers in the field of neural circuits.
Fig. 6.
The keyword mapping of neural circuit (A) Word cloud of Keywords plus. (B) Co-occurrence analysis of keywords over time. (C) Three-field plot of information flow analysis (middle field: keywords; left field: authors; right field: countries). (D) Trend topics over time in neural circuits among local sources. (E) Top 25 keywords with the strongest citation burst over time.
3.5. Co-citation and burst analysis of references
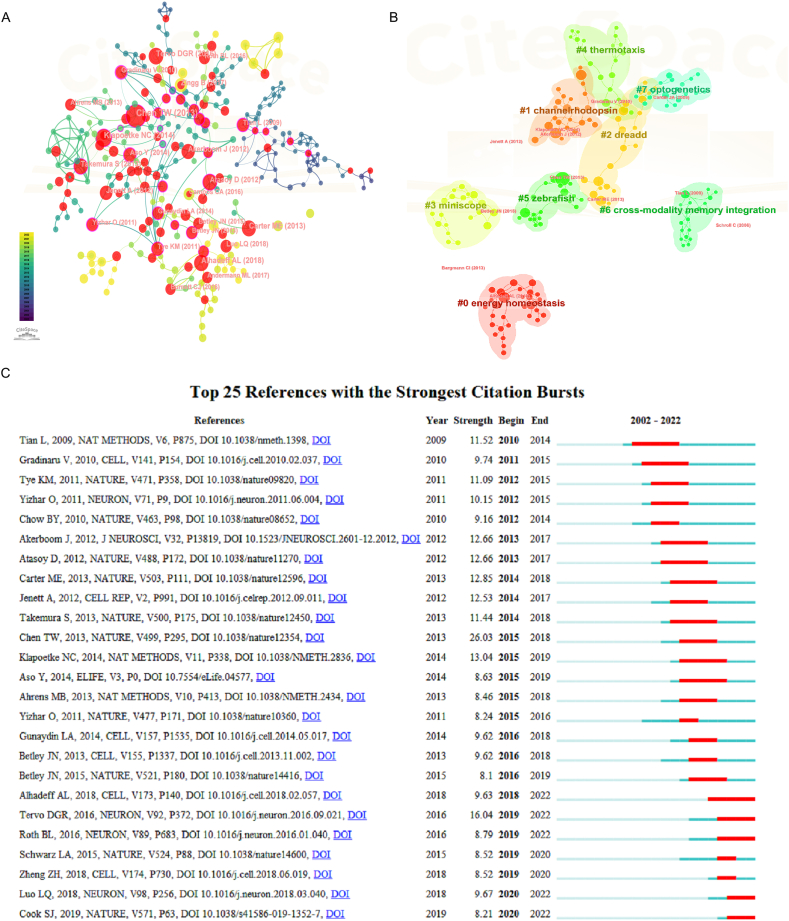
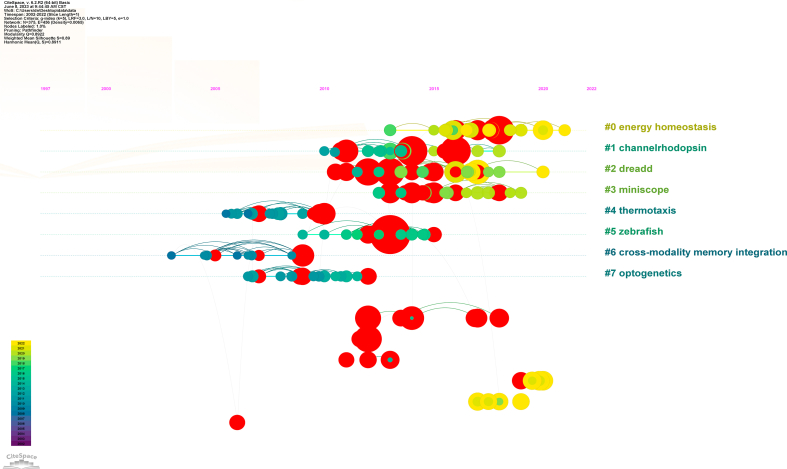
To further illustrate the relationships and interactions among the literature, we conducted an analysis and visualization of the literature data using the visualization network approach. The nodes represented cited references and connecting lines represent co-citations. The node size denoted the number of co-citations, and the red dot indicated bursts. Varying from purple to yellow, different colors indicated different years between 2002 and 2022. As shown in Fig. 7A, the most cited article was written by Tsai-Wen Chen who developed a family of ultrasensitive protein calcium sensors (GCaMP6) that outperformed other sensors in cultured neurons and in zebrafish, flies and mice in vivo [31], which offered novel insights into the structural and functional aspects of neural networks across diverse spatial and temporal dimensions. After clustering the cited networks, 8 clusters were obtained (Fig. 7B and Table S3) and labeled with keywords from the citing articles. These clusters were mainly biological regulation and behavior-related (energy homeostasis, thermotaxis), neuroimaging and techniques-related (channel rhodopsin, miniscope), animal models and behavioral studies-related (zebrafish), and memory and neural regulation-related (DREADD, optogenetics, cross-modality memory integration). We further analyzed this cluster's timeline map, and found that cross-modality memory integration, thermotaxis, and optogenetics began earlier. Then came the zebrafish, followed by channel rhodopsin, DREADD, miniscope, and energy homeostasis (Fig. S5). Referring to citation bursts, these referred to articles with a sudden increase in citations within a certain period, which indicated the latest high-profile research topics in related fields [32]. A total of 85 references with the highest citation bursts were obtained, and the top 25 were selected. The first reference triggering a citation burst that appeared in 2009 reached 11.52 [33], and the most recent citation burst references appeared in 2019 [34] with an intensity of 8.21 (Fig. 7C).
Fig. 7.
Co-citation and burst analysis of references (A) Network Visualization of Cited References. The red dots represent highly cited literature nodes. (B) Clustered network map of neural circuit-related co-cited references. (C) Top 25 references with the strongest citation bursts. The intensity value reflects citation frequency. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
4.1. General overview
Neural circuits are fundamental components of the nervous system and play a crucial role in processing and transmitting information throughout the body. They are composed of interconnected neurons that form complex networks, enabling the brain and spinal cord to receive, process, and respond to various stimuli [35]. Distinct neuron types in the brain work together to process information through specific patterns of synaptic connectivity in a variety of forms that are like series, parallel, feedforward, feedback, positive feedback, and negative feedback, manipulating the behavior of organisms. As noted by the Prof. Liquun Luo, individual neurons are letters in an alphabet used to write an article that is a brain, neural circuit can be considered as words or sentence [11]. Most previous bibliometric analyses have strictly elucidated the mechanism of letters and ignored the relationship between the article and words. Herein, the development of neural circuits can be elaborated from new perspectives of bibliometric analysis.
Over the past half-century, electrical, genetic, pharmacological interventions, and dynamic imaging techniques have been applied to obtain causal insights into the functional significance of neural circuits [9]. Deisseroth et al. demonstrated reliable, millisecond-timescale control of neuronal spiking and excitatory and inhibitory synaptic transmission by inserting light-sensitive proteins into neurons [36]. This lays the foundation for future research on neural circuits. Moreover, Looger et al. achieved long-term tracing of neurons by developing a novel calcium indicator, GCaMP3, in 2009 [33]. In 2010, Deisseroth et al. diversified and extended optogenetics [37]. Subsequently, the cumulative number of publications has steadily increased, and many heavyweight studies continue to emerge, further driving the research fervor for “neural circuits”.
4.2. Various perspectives
From the viewpoint of authors and co-cited authors, Luo Liqun had published 20 documents, ranked first in the fields of “neural circuit,” followed by Borst Alexander (19 documents) and Ma Jun (18 documents) (Fig. 3A). Critically, although Deisseroth karl (3643 times) published fewer articles than the top three authors, the number of citations of their articles was significantly higher than the former, indicating the higher quality of their research and further demonstrating Deisseroth Karl's leadership in “neural circuit” research (Table 1). As the father of optogenetics, Deisseroth Karl has the highest number of citations and occupies a pivotal position in neural circuits.
In the journals and co-cited journals analysis, the most articles on neural circuits were in Journal of neuroscience (217 documents), followed by Neuron (140 documents), and Proceedings of the national academy of sciences of the United States of America (117 documents) (Table 2). Over 50 % of the top 10 journals belonged to Q1, indicating that the field of neural circuits was at the frontier of research and is still a hot research direction that deserves further in-depth exploration in the future. Meanwhile, co-citation analysis revealed Nature, Neuron, Science as the most co-citation sources, which revealed that “neural circuits” were an area of intense interest in the global academic community. The analysis of journals will help future scholars select journals when submitting manuscripts related to neural circuits [25]. In addition, the dual-map overlay of journal analysis found that neural circuits were closely connected with basic subject, and the construction of multidisciplinary management needs to be further strengthened in the future.
Based on the distribution of countries/regions, the USA dominated the output of the neural circuit field in terms of volume and conducted the largest number of cross-border cooperation projects with China. In terms of the most productive research institutes, Chinese Academy of Science had published the most articles and Stanford University ranked first in citations. Although the number of manuscripts in the neural circuit of research in China has grown considerably in recent years, strengthening international multi-institutional exchange and cooperation to improve the quality of manuscripts is just as important.
4.3. Research hotspots, frontiers, and prospects
Neural circuits are fundamental to understanding the functioning of the brain and the basis of cognition, behavior, and various neurological disorders. Research in neural circuits aims to unravel the complex network of interconnected neurons and understand how their activity gives rise to higher-level brain functions. Many neurological and psychiatric disorders arise from altered functioning of neural circuits. Investigating the circuit-level abnormalities associated with conditions like epilepsy, Parkinson's disease, schizophrenia, and depression is a major research focus. Abnormal functioning of neural circuits disrupts the feedback regulation of both electrical and chemical synapses, contributing to the development of psychiatric disorders, such as neurodegenerative diseases (e.g., Alzheimer's disease, Parkinson's disease) [38,39], neurodevelopmental disorders (e.g., autism spectrum disorder, attention deficit hyperactivity disorder) [40,41], and stress-related diseases (e.g., anxiety, depression, and bipolar disorders) [[42], [43], [44]]. The analysis of keywords revealed that the hippocampus, amygdala, and prefrontal cortex have been the most studied brain areas in recent years. These regions play crucial roles in multiple cognitive, perceptual, emotional, and learning processes that shape our perception and understanding of the world. The dysregulation of neural circuits among these areas has been implicated in anxiety [45], Alzheimer's disease [46], and epilepsy [47]. For instance, the amygdala circuitry mediates reversible and bidirectional control of anxiety [48]. A battery of evidence indicated that the disruption of large-scale neuronal circuits and changes in neuronal plasticity and excitability were shown in the neocortex and hippocampus in Alzheimer's disease [38,49]. Furthermore, magnetic resonance imaging and diffusion tensor imaging characterize the pathological changes in the medial septum-hippocampus circuit in epilepsy [50]. There is a growing consensus that most neurological and psychiatric disorders are associated with large-scale dysfunction of brain connectivity [51], and a shift in psychiatric disorder treatment and research strategies from the “molecular” level to the “circuit” level has become a novel therapeutic strategy based on the neural circuit.
Significantly, among co-occurrence network mapping of keywords, optogenetics, chemogenetic, etc. stand out as major methods for exploring alterations in the neural circuit. New tools for mapping neuronal circuit architecture, such as serial electron microscopic (EM) reconstruction, trans-synaptic tracing, and electrophysiological and optical methods, have been used to study the structural organization of the nervous system [11], which interfaces with neural circuits and achieve precise control over their activity. These methods can benefit future studies that focus on manipulating neural circuits underlying psychiatric disorders or that evaluate the efficacy of potential treatments in animal models [52]. The evolution of topics started from nervous systems (e.g., central nervous systems, central pattern generators, nervous systems) in the earlier period to GABAergic neurons, anxiety, and dynamics (2020–2022). Whether it is local keywords or citation keywords burst analysis, “dynamics” have become a hotspot in recent years. Neural circuits process and transmit information through dynamic patterns of activity [53]. Research focuses on deciphering the principles governing the dynamics of neural circuits, such as oscillations [54,55], synchronization [56], and the encoding and decoding of sensory information [57,58]. Theoretical and computational approaches play a crucial role in modeling and predicting the dynamics of neural circuits and their functional consequences [59]. To date, the layer of circuit motifs has been documented, but the neuronal circuit architectures involved in neural activity dynamics have not been clarified. Identifying the most causally relevant circuit dynamical property to be targeted for a specific symptom is a key initial step in interventional therapeutics [9]. Recent studies have suggested that GABAergic interneurons have evolved as a highly heterogeneous collection of cell types that are characterized by their unique spatial and temporal capabilities to influence neuronal circuits [60]. GABAergic neural circuits from the ventral subiculum to the anterior hypothalamic nucleus are essential for anxiety [61]. Multiple populations of GABAergic neurons found throughout the brain, from the cortex to the medulla oblongata, control sleep [62]. GABAergic neurons in the ventral pallidum encode the drive for approach in motivational behavior [63]. According to this, populations of GABAergic neurons from different areas dynamically govern biological behaviors by modulating the input-output of neural circuits.
Burst analysis was employed to detect references with an abrupt increase over time [64]. Among burst analysis of co-cited references, the strongest reference triggered a citation burst appeared reached 26.03 (2015–2018). Chen et al. developed an ultrasensitive protein calcium sensor (GCaMP6) that provided new insights into the organization and dynamics of neural circuits over multiple spatial and temporal scales [31]. Immediately afterward, Klapoetke et al. discovered two channelrhodopsins, Chronos and Chrimson, through sequencing and physiological characterization of opsins. This discovery enabled the independent activation of two distinct neural populations [65]. The second highest burst appeared in 2016, Tervo et al. applied in vivo directed evolution to engineer potent retrograde functionality into the capsid of adeno-associated virus (AAV) to endow AAV with robust retrograde tracers’ functionality and enables sufficient sensor/effector expression [66]. These prominent citations were based on advancements in optogenetic and calcium imaging tools, which were expected to have a lasting and profound impact on the deciphering of neural circuits through further development. Conclusively, iterative updating methods represent significant milestones in the study of neural circuits. After the successful decoding of neural circuits, several refinements can be envisioned to encode the neural loop and invigorate the functionality of the neural interface [67].
In addition to unraveling the intricacies of neural circuitry, the practical applications of these findings hold immense promise for advancing both basic neuroscience research and clinical interventions. Understanding the dysregulation of neural circuits in various neurological and psychiatric disorders, as discussed earlier, not only sheds light on the underlying pathophysiology but also opens avenues for targeted therapeutic strategies. The identified abnormalities in circuit dynamics, such as oscillations, synchronization, and sensory information processing, provide crucial insights into potential intervention points. Recent advancements in tools and methodologies, including optogenetics, chemogenetics, serial electron microscopic reconstruction, and trans-synaptic tracing, empower researchers to manipulate and map neural circuits with unprecedented precision. Optogenetic tools, exemplified by the discoveries of GCaMP6, Chronos, and Chrimson, have enabled real-time monitoring and control of neural activity, fostering a deeper understanding of circuit organization and dynamics. Moreover, the application of in vivo directed evolution, as demonstrated by Tervo et al. has enhanced the capabilities of viral vectors for efficient tracing and manipulation of neural pathways. These technological strides not only contribute to fundamental research but also hold significant translational potential. GABAergic neural circuits, for instance, have emerged as key players in regulating diverse behaviors, from anxiety to sleep control and motivational drive. The heterogeneity of GABAergic interneurons and their specific spatial and temporal capabilities offer a rich landscape for targeted therapeutic interventions. As we progress in decoding neural circuits, the implications extend beyond academic curiosity. The iterative updating methods and refinements in encoding the neural loop represent substantial milestones with the potential to invigorate the functionality of neural interfaces. These refinements are not just theoretical pursuits but pave the way for translating neural circuit research into practical applications that may guide future clinical practices. This shift from understanding the molecular to the circuit level aligns with the growing consensus that effective treatments for neurological and psychiatric disorders require a comprehensive understanding of large-scale brain connectivity.
4.4. Strengths and limitations
Admittedly, limitations of this study exist, such as the omission of literature from other databases, as well as potential bias because of self-citation. Nevertheless, this study is the first bibliometric analysis of neural circuits. We analyzed from different perspectives, including authors, institutions, countries, journals, cited references, and keywords. The results of our study may help researchers explore this promising area of research in greater depth.
5. Conclusions
In the persistent pursuit of unraveling the intricate functionality inherent in neural circuits, a spectrum of interventions, ranging from electrical to genetic and pharmacological approaches, has been employed over recent decades. This research, leveraging an encompassing bibliometric perspective, undertook an in-depth examination of the expansive domain of neural circuits, seeking insights that extend beyond the limitations imposed by conventional experimental methodologies. The investigation, underpinned by data from the WOSCC database as of April 12, 2023, employed diverse analytical methodologies—specifically co-authorship, co-occurrence, citation, bibliographic, and co-citation analyses—to disentangle the complex network of authors, journals, institutions, countries, and topics that defined the landscape of neural circuit research. The substantial dataset, incorporating contributions from over 2000 organizations across 52 countries, yielding 3975 articles, served as the cornerstone for our analytical framework. A pivotal insight derived from our inquiry was the discernible shift, particularly since 2020, towards emphasizing neurodynamic processes, anxiety-related mechanisms, and GABAergic neurons in the realm of neural circuitry research. This paradigmatic reorientation significantly influenced the trajectory of the field, providing a glimpse into the prospective future of neural circuit research. The identified trends not only suggested a potential trajectory for the field but also advocated for a critical directive in future research endeavors. It is imperative for the neural circuit research community to prioritize the translation of these discernments into tangible clinical solutions. By giving precedence to the translational application of our findings, we can effectively bridge the gap between theoretical insights and real-world clinical impact. In conclusion, our comprehensive bibliometric analysis not only illuminates the current state of neural circuit research but also furnishes a roadmap for prospective investigations. The identified trends and influential figures function as guiding beacons for researchers, encouraging them to direct their efforts towards impactful, translational research, thereby holding the promise of converting theoretical knowledge into tangible clinical solutions in the dynamic landscape of neural circuitry.
Funding
This study was supported by grants from National Natural Science Foundation (Young Scientists Fund) Cultivation Project of Taizhou School of Clinical Medicine: TZKY20220107.
Data availability statement
Data included in article/supp. material/referenced in article.
CRediT authorship contribution statement
Yuan Liu: Writing - original draft, Validation, Project administration, Investigation, Data curation, Conceptualization. Wei Lin: Writing - review & editing, Writing - original draft, Validation, Supervision, Formal analysis, Data curation, Conceptualization. Jie Liu: Project administration, Methodology. Haixia Zhu: Funding acquisition, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e24649.
Appendix A. Supplementary data
The following are the supplementary data to this article:
figs1.
figs2.
figs3.
figs4.
figs5.
References
- 1.Bargmann C.I., Newsome W.T. The brain research through advancing Innovative Neurotechnologies (BRAIN) initiative and neurology. JAMA Neurol. 2014;71(6):675–676. doi: 10.1001/jamaneurol.2014.411. [DOI] [PubMed] [Google Scholar]
- 2.Rose N. The Human Brain Project: social and ethical challenges. Neuron. 2014;82(6):1212–1215. doi: 10.1016/j.neuron.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Poo M.M., et al. China brain project: basic neuroscience, brain diseases, and brain-Inspired computing. Neuron. 2016;92(3):591–596. doi: 10.1016/j.neuron.2016.10.050. [DOI] [PubMed] [Google Scholar]
- 4.Olefir I., et al. Spatial and spectral mapping and Decomposition of neural dynamics and organization of the mouse brain with Multispectral Optoacoustic tomography. Cell Rep. 2019;26(10) doi: 10.1016/j.celrep.2019.02.020. 2833-2846.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graybuck L.T., et al. Enhancer viruses for combinatorial cell-subclass-specific labeling. Neuron. 2021;109(9) doi: 10.1016/j.neuron.2021.03.011. 1449-1464.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Runyan C.A., et al. Distinct timescales of population coding across cortex. Nature. 2017;548(7665):92–96. doi: 10.1038/nature23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y., et al. Decoding and synthesizing tonal language speech from brain activity. Sci. Adv. 2023;9(23) doi: 10.1126/sciadv.adh0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang H.H., et al. Subcellular imaging of Voltage and calcium Signals reveals neural processing in vivo. Cell. 2016;166(1):245–257. doi: 10.1016/j.cell.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajasethupathy P., Ferenczi E., Deisseroth K. Targeting neural circuits. Cell. 2016;165(3):524–534. doi: 10.1016/j.cell.2016.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bian W.J., et al. Coordinated spine pruning and Maturation mediated by Inter-Spine Competition for Cadherin/Catenin Complexes. Cell. 2015;162(4):808–822. doi: 10.1016/j.cell.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 11.Luo L. Architectures of neuronal circuits. Science. 2021;373(6559) doi: 10.1126/science.abg7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mu D., et al. A central neural circuit for itch sensation. Science. 2017;357(6352):695–699. doi: 10.1126/science.aaf4918. [DOI] [PubMed] [Google Scholar]
- 13.Kissiwaa S.A., Bagley E.E. Central sensitization of the spino-parabrachial-amygdala pathway that outlasts a brief nociceptive stimulus. J. Physiol. 2018;596(18):4457–4473. doi: 10.1113/JP273976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S.H., et al. Anisotropically organized three-dimensional culture platform for reconstruction of a hippocampal neural network. Nat. Commun. 2017;8 doi: 10.1038/ncomms14346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wise T., et al. Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Mol. Psychiatr. 2017;22(10):1455–1463. doi: 10.1038/mp.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou X.L., et al. Inhibitory gain modulation of defense behaviors by zona incerta. Nat. Commun. 2018;9(1):1151. doi: 10.1038/s41467-018-03581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen P., et al. Brain-gut axis and psychiatric disorders: a perspective from bibliometric and visual analysis. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1047007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fusar-Poli P., et al. Transdiagnostic psychiatry: a systematic review. World Psychiatr. 2019;18(2):192–207. doi: 10.1002/wps.20631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J., Maniruzzaman M. A global bibliometric and visualized analysis of bacteria-mediated cancer therapy. Drug Discov. Today. 2022;27(10) doi: 10.1016/j.drudis.2022.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozsari I. Trend analysis and evaluation of hydrogen energy and hydrogen storage research. Energy Storage. 2023;5:e471. [Google Scholar]
- 21.Ozsari I. Historical research trends and overview about exergy: a comprehensive analysis. 2023;40(1):59–73. [Google Scholar]
- 22.Imran M., et al. Recent research trends in organic Rankine cycle technology: a bibliometric approach. Renew. Sustain. Energy Rev. 2018;81:552–562. [Google Scholar]
- 23.Pei Z., et al. Current perspectives and trend of nanomedicine in cancer: a review and bibliometric analysis. J Control Release. 2022;352:211–241. doi: 10.1016/j.jconrel.2022.10.023. [DOI] [PubMed] [Google Scholar]
- 24.Sun H.L., et al. Schizophrenia and Inflammation research: a bibliometric analysis. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.907851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei N., et al. A bibliometric analysis of T cell and atherosclerosis. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.948314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo K., et al. Emerging trends and focus on the link between gut microbiota and type 1 diabetes: a bibliometric and visualization analysis. Front. Microbiol. 2023;14 doi: 10.3389/fmicb.2023.1137595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miao Y.D., et al. A bibliometric analysis of ferroptosis, necroptosis, pyroptosis, and cuproptosis in cancer from 2012 to 2022. Cell Death Discov. 2023;9(1):129. doi: 10.1038/s41420-023-01421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He T., et al. A bibliometric analysis of research on (R)-ketamine from 2002 to 2021. Neuropharmacology. 2022;218 doi: 10.1016/j.neuropharm.2022.109207. [DOI] [PubMed] [Google Scholar]
- 29.Mu J., et al. Research trends and hotspots in the relationship between outdoor activities and myopia: a bibliometric analysis based on the web of science database from 2006 to 2021. Front. Public Health. 2022;10 doi: 10.3389/fpubh.2022.1047116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao B., et al. Structural and temporal dynamics of mesenchymal stem cells in liver diseases from 2001 to 2021: a bibliometric analysis. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.859972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen T.W., et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499(7458):295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miao L., et al. A bibliometric and knowledge-map analysis of CAR-T cells from 2009 to 2021. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.840956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian L., et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods. 2009;6(12):875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cook S.J., et al. Whole-animal connectomes of both Caenorhabditis elegans sexes. Nature. 2019;571(7763):63–71. doi: 10.1038/s41586-019-1352-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y., et al. The auxiliary calcium channel subunit α2δ4 is required for axonal elaboration, synaptic transmission, and wiring of rod photoreceptors. Neuron. 2017;93(6):1359–1374.e6. doi: 10.1016/j.neuron.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boyden E.S., et al. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005;8(9):1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 37.Gradinaru V., et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141(1):154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zott B., et al. What happens with the circuit in Alzheimer's disease in mice and humans? Annu. Rev. Neurosci. 2018;41:277–297. doi: 10.1146/annurev-neuro-080317-061725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meles S.K., Oertel W.H., Leenders K.L. Circuit imaging biomarkers in preclinical and prodromal Parkinson's disease. Mol Med. 2021;27(1):111. doi: 10.1186/s10020-021-00327-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly E., et al. Regulation of autism-relevant behaviors by cerebellar-prefrontal cortical circuits. Nat. Neurosci. 2020;23(9):1102–1110. doi: 10.1038/s41593-020-0665-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mueller A., et al. Linking ADHD to the neural circuitry of attention. Trends Cognit. Sci. 2017;21(6):474–488. doi: 10.1016/j.tics.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jimenez J.C., et al. Anxiety cells in a hippocampal-hypothalamic circuit. Neuron. 2018;97(3):670–683.e6. doi: 10.1016/j.neuron.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu J., et al. An entorhinal-visual cortical circuit regulates depression-like behaviors. Mol. Psychiatr. 2022;27(9):3807–3820. doi: 10.1038/s41380-022-01540-8. [DOI] [PubMed] [Google Scholar]
- 44.Cotovio G., Oliveira-Maia A.J. Functional neuroanatomy of mania. Transl. Psychiatry. 2022;12(1):29. doi: 10.1038/s41398-022-01786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bian X.L., et al. Anterior cingulate cortex to ventral Hippocampus circuit mediates contextual fear generalization. J. Neurosci. 2019;39(29):5728–5739. doi: 10.1523/JNEUROSCI.2739-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jun H., et al. Disrupted place cell remapping and impaired grid cells in a knockin model of Alzheimer's disease. Neuron. 2020;107(6):1095–1112.e6. doi: 10.1016/j.neuron.2020.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y., et al. Direct septum-Hippocampus cholinergic circuit attenuates seizure through driving somatostatin inhibition. Biol. Psychiatr. 2020;87(9):843–856. doi: 10.1016/j.biopsych.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 48.Tye K.M., et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471(7338):358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buckner R.L., et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J. Neurosci. 2005;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y., Chen Z. An update for epilepsy research and antiepileptic drug development: toward precise circuit therapy. Pharmacol. Ther. 2019;201:77–93. doi: 10.1016/j.pharmthera.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 51.Ghannad-Rezaie M., et al. Engineering brain activity patterns by neuromodulator polytherapy for treatment of disorders. Nat. Commun. 2019;10(1):2620. doi: 10.1038/s41467-019-10541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Broom L., et al. Translational methods to detect asymmetries in temporal and spatial walking metrics in parkinsonian mouse models and human subjects with Parkinson's disease. Sci. Rep. 2019;9(1):2437. doi: 10.1038/s41598-019-38623-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi M.K., et al. NMDAR-mediated modulation of gap junction circuit regulates olfactory learning in C. elegans. Nat. Commun. 2020;11(1):3467. doi: 10.1038/s41467-020-17218-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Girardeau G., Lopes-Dos-Santos V. Brain neural patterns and the memory function of sleep. Science. 2021;374(6567):560–564. doi: 10.1126/science.abi8370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buzsáki G., Wang X.J. Mechanisms of gamma oscillations. Annu. Rev. Neurosci. 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wagner M.J., et al. A neural circuit state change underlying skilled movements. Cell. 2021;184(14) doi: 10.1016/j.cell.2021.06.001. 3731-3747.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hancock R., Pugh K.R., Hoeft F. Neural noise hypothesis of developmental dyslexia. Trends Cognit. Sci. 2017;21(6):434–448. doi: 10.1016/j.tics.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cappotto D., et al. Simultaneous mnemonic and predictive representations in the auditory cortex. Curr. Biol. 2022;32(11) doi: 10.1016/j.cub.2022.04.022. 2548-2555.e5. [DOI] [PubMed] [Google Scholar]
- 59.Luo L., Callaway E.M., Svoboda K. Genetic dissection of neural circuits: a decade of progress. Neuron. 2018;98(2):256–281. doi: 10.1016/j.neuron.2018.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lim L., et al. Development and functional diversification of cortical interneurons. Neuron. 2018;100(2):294–313. doi: 10.1016/j.neuron.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yan J.J., et al. A circuit from the ventral subiculum to anterior hypothalamic nucleus GABAergic neurons essential for anxiety-like behavioral avoidance. Nat. Commun. 2022;13(1):7464. doi: 10.1038/s41467-022-35211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luppi P.H., Peyron C., Fort P. Not a single but multiple populations of GABAergic neurons control sleep. Sleep Med. Rev. 2017;32:85–94. doi: 10.1016/j.smrv.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 63.Stephenson-Jones M., et al. Opposing contributions of GABAergic and glutamatergic ventral pallidal neurons to motivational behaviors. Neuron. 2020;105(5):921–933.e5. doi: 10.1016/j.neuron.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi X., et al. A bibliometric analysis of the innate immune DNA sensing cGAS-STING pathway from 2013 to 2021. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.916383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klapoetke N.C., et al. Independent optical excitation of distinct neural populations. Nat. Methods. 2014;11(3):338–346. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tervo D.G., et al. A designer AAV variant permits efficient retrograde access to projection neurons. Neuron. 2016;92(2):372–382. doi: 10.1016/j.neuron.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Young A.T., Cornwell N., Daniele M.A. Neuro-nano interfaces: utilizing nano-coatings and nanoparticles to enable next-generation electrophysiological recording, neural stimulation, and biochemical modulation. Adv. Funct. Mater. 2018;28(12) doi: 10.1002/adfm.201700239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.