Abstract
This study was conducted to investigate potential differences in vaccine efficacy between patients undergoing palliative chemotherapy and receiving adjuvant chemotherapy. Additionally, the study proved the influence of vaccination timing on vaccine efficacy during active chemotherapy. Anti-receptor-binding domain (RBD) IgG binding antibody assays and surrogate neutralizing antibody assays were performed after BNT162b2 or mRNA-1273 vaccination in 45 solid cancer patients (23 adjuvant and 22 palliative chemotherapy) and in 24 healthy controls before vaccination (baseline), at every two to four weeks after the first (post-dose 1) and the second vaccination (post-dose 2). The levels of anti-RBD IgG and neutralizing antibodies increased significantly from baseline through post-dose 1 to post-dose 2 in all three groups. At the post-dose 1, the anti-RBD IgG and neutralizing antibody levels were significantly lower in cancer patients than in healthy controls. However, by post-dose 2, the seropositivity of anti-RBD IgG and neutralizing antibodies uniformly reached 100% across all groups, with no significant disparity in antibody levels among the three groups. Moreover, the antibody titers were not significantly different between patients with a vaccine and chemotherapy interval of more than 14 days or those with less than 14 days. This study demonstrated that after second doses of mRNA COVID-19 vaccines, humoral immune responses in patients receiving chemotherapy were comparable to those of healthy controls, regardless of whether the purpose of the anti-cancer treatment was palliative or adjuvant. Furthermore, the timing of vaccination did not affect the level of humoral immunity after the second vaccination.
Keywords: COVID-19, Vaccination, Neoplasms, mRNA
INTRODUCTION
Patients with cancer have a higher risk of severe coronavirus disease (COVID-19) and associated mortality than the general population.1 Owing to this increased risk, patients with cancer have been prioritized for COVID-19 vaccination globally, for both primary and booster vaccinations. However, data on the COVID-19 vaccine in cancer populations are still limited. In patients with solid cancers, some studies have found that COVID-19 vaccines have similar immunogenicity relative to the general population,2,3,4 but other studies have demonstrated lower immunogenicity in cancer patients relative to that in healthy controls.5,6,7 Obtaining consistent results for COVID-19 vaccine-related immunogenicity in patients with solid cancers might be difficult because of variations in several factors, such as cancer types, disease status, and anticancer therapy. In terms of therapeutic intent, chemotherapy can be divided into two primary categories: adjuvant and palliative. Adjuvant chemotherapy aims to minimize recurrence by eradicating residual cancer following primary interventions such as surgery. As a result, this modality is administered for a predetermined period of time. Conversely, palliative chemotherapy is used to reduce tumor size with the goal of relieving symptoms in stages that are considered incurable. Such treatment is often characterized by a relatively high tumor burden and prolonged chemotherapy duration. Consequently, patients undergoing palliative chemotherapy are postulated to have more impaired immune function. To date, few studies have compared immunogenicity based on how long chemotherapy lasted or how the cancer progressed.8 Thus, we undertook a study to investigate potential differences in vaccine efficacy between patients undergoing palliative chemotherapy and those receiving adjuvant chemotherapy.
Distinct from conventional vaccines, COVID-19 vaccines are recommended to be given during active chemotherapy regardless of when the chemotherapeutic agents are administered.9 However, there are no studies that have evaluated the optimal timing of COVID-19 vaccination in cancer patients. In addressing this gap, we investigated the effect of vaccination timing on vaccine efficacy in patients undergoing active chemotherapy.
MATERIALS AND METHODS
1. Participants and study design
This prospective study was conducted at three cancer centers and one general hospital in South Korea from July 2021 through February 2022. This study was approved by the Institutional Review Board of Chonnam National University Hwasun Hospital (CNUHH-2021-124), Chonnam National University Bitgoeul Hospital (CNUBH-2021-014), Chungnam National University Sejong Hospital (CNUSH 2021-07-013), and Soonchunhyang University Hospital Cheonan (SUCH 2021-07-039). All participants gave their consent to participate by signing the informed consent form.
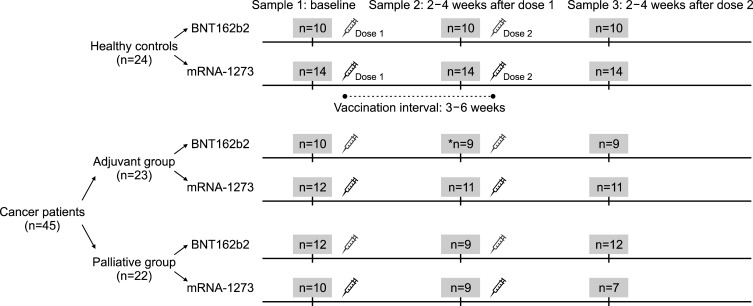
Three groups of participants were enrolled: control individuals without any cancer or other known immunological disorders, patients with solid cancers undergoing adjuvant chemotherapy, and patients with solid cancers undergoing palliative chemotherapy. Adjuvant chemotherapy was defined as chemotherapy given additionally after attempted curative surgery. Palliative chemotherapy was defined as chemotherapy given in the non-curative setting to optimize symptom control, improve quality of life, and extend survival. Cancer patients who received their most recent chemotherapy administration within 4 weeks of mRNA vaccination were enrolled. In all groups, individuals with a history of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection were excluded. After enrollment, participants received two doses of the COVID-19 mRNA vaccines (0.3 mL of BNT162b2 or 0.5 mL of mRNA-1273) 3 to 6 weeks apart. Public healthcare system authorities designed the vaccination program; hence, we could not control the vaccination interval or the timing of vaccine administration within the anticancer therapy schedule. Three blood samples were obtained from each participant: the first sample before vaccination (baseline), the second sample at 2 to 4 weeks after the first vaccination (post-dose 1), and the third sample at 2 to 4 weeks after the second vaccination (post-dose 2) (Fig. 1).
FIG. 1. Study design and schematic diagram of sample collection before and after vaccination. *One patient in the adjuvant group was enrolled after the first vaccination without baseline sampling.
2. Sample size estimation
The primary objective of this study was to evaluate whether the humoral response to the COVID-19 mRNA vaccine in patients with solid tumors receiving palliative or adjuvant chemotherapy was non-inferior to controls. Based on previous reports, the estimated seroconversion rates following the administration of two doses of the BNT162b2 or mRNA-1273 vaccines were 100% in healthy individuals and 95% in cancer patients.2,4 With a non-inferiority margin of 20% and a power of 90% at a two-sided alpha level of 0.05 using Fisher’s exact test, each group required the enrollment of at least 19 subjects. Assuming a dropout rate of approximately 10%, the calculated minimum sample size was 21 subjects in each group.
3. Measurements of humoral antibody responses
1) Binding antibody assay
Elecsys Anti-SARS-CoV-2 is an electrochemiluminescence immunoassay that uses recombinant nucleocapsid (N) protein to detect IgM and IgG antibodies against SARS-CoV-2 with Cobas e801 immunoassay analyzers (Roche Diagnostics, Basel, Switzerland). N-antibody tests were performed on each participant’s first and last samples to rule out resolving or past SARS-CoV-2 infections.10 The manufacturer’s suggested threshold for positivity was a 1.0 cut-off index.
SARS-CoV-2 IgG II Quant, a chemiluminescent microparticle immunoassay, quantitatively detects IgG antibodies that target the receptor-binding domain (RBD) of spike (S) protein using the ARCHITECT i2000 System (Abbott Laboratories, IL, USA). Binding antibody units (BAU)/mL, the standard units proposed by the World Health Organization (WHO) for anti-spike and anti-RBD IgG,11 were calculated by applying the conversion factor as recommended by the manufacturers: BAU/mL=0.142×arbitrary units (AU)/mL. RBD-IgG binding antibody test results were interpreted as positive if the signal value was ≥7.1 BAU/mL (equal to 50.0 AU/mL).
2) Neutralizing antibody assay
The SARS-CoV-2 cPass Surrogate Virus Neutralization Test (sVNT) (Genscript Biotech Co., NJ, USA) was performed using a ThunderBolt ELISA Analyzer (Gold Standard Diagnostics, CA, USA). In this competitive assay, human angiotensin-converting enzyme 2 (ACE-2) was attached to the solid phase, while peroxidase-conjugated RBD was present in the liquid phase. If the human serum contained RBD-targeting neutralizing antibodies, binding of RBD to ACE-2 was inhibited, and the final color reaction was weaker than in RBD-antibody-free serum samples.12 Plates were read at 450 nm, and the% inhibition was calculated using the following formula:% inhibition=[1−(sample optical density value/negative control optical density value)]×100. International units (IU)/mL, the standard units proposed by the WHO for neutralization titers,11 were calculated using the Excel-based conversion tool provided by the manufacturers.13,14 According to the manufacturer’s instructions, test results were interpreted as positive if they showed values ≥28.4 IU/mL (equal to 30% inhibition).
4. Statistical analysis
All statistical analyses were performed using SPSS Statistics for Windows, version 25.0 (IBM Corp., Armonk, NY). Categorical variables are reported as numbers and percentages of participants, and continuous variables are expressed as medians and interquartile ranges (IQRs). Comparisons of categorical variables were performed using chi-square tests or Fisher exact tests, as appropriate. Mann-Whitney or Kruskal-Wallis tests were used for comparisons of continuous variables. A two-sided p<0.05 was considered statistically significant.
RESULTS
1. Demographics and clinical characteristics
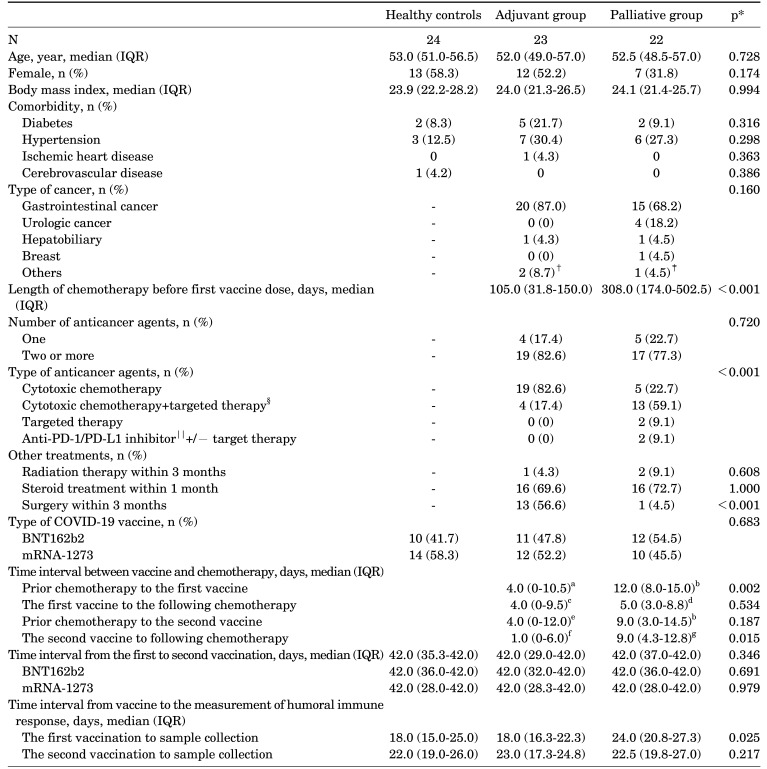
There were 24, 23, and 22 participants in the control, adjuvant, and palliative chemotherapy groups, respectively. Baseline blood samples were drawn for all participants except for one patient in the adjuvant group enrolled after receiving the first vaccine dose without baseline sampling. All participants were SARS-CoV-2-naïve throughout the study, as determined by the negative anti-N antibody tests at the times of the first and last samples. The demographic and clinical characteristics of each group are shown in Table 1. There were no significant differences among the groups regarding age, gender, body mass index, comorbidities other than cancer, or distribution of the COVID-19 mRNA vaccine types. The duration of chemotherapy prior to the first vaccine administration was significantly longer in the palliative group (median days, IQR, 308.0 (174.0-502.5) vs. 10.5 (31.8-150.0), p<0.001). In the palliative group, the most common anticancer treatment was cytotoxic chemotherapy plus targeted therapy (59.1%), whereas cytotoxic chemotherapy alone (82.6%) was the most common in the adjuvant group. The median intervals from the day of administration of any anticancer therapy to the first vaccine were longer in palliative group compared to adjuvant group (median, IQR 4 (0-10.5) vs. 12 (8-15.0), p=0.002). And the interval from the second vaccine to administration of any cancer therapy after vaccine was also longer in palliative group compared to adjuvant group (1 (0-6.0) vs. 9.0 (4.3-12.8), p=0.015). The median days from the first vaccination to sample collection was 24 days (IQR, 20.8-27.3) in the palliative group, which was significantly longer than the 18 days (IQR, 16.3-22.3) in the adjuvant group (p=0.013) and the 18 days (IQR, 15.0-25.0) in the control group (p=0.023).
TABLE 1. Demographic and clinical characteristics of healthy controls and patients with solid cancers receiving adjuvant or palliative chemotherapy.
*Control vs. adjuvant vs. palliative chemotherapy group or adjuvant vs. palliative chemotherapy group. Comparisons of categorical variables were performed using Fisher exact tests or chi-square tests. Mann-Whitney or Kruskal-Wallis tests were used for comparing continuous variables. A two-sided p-value<0.05 was considered statistically significant; †Oropharyngeal cancer and non-small cell lung cancer; ‡nasopharyngeal cancer; §bevacizumab, cetuximab, aflibercept, pazopanib, everolimus; ∥nivolumab, atezolizumab. an=18, one patient received the first vaccine before chemotherapy started. Data of 4 patients for chemotherapeutic agent administration day were not available; bn=21, data of one patient for chemotherapeutic agent administration day were not available; cn=18, one patient no longer received chemotherapy after the first vaccine. Data of 4 patients for chemotherapeutic agent administration day were not available; dn=20, one patient no longer received chemotherapy after the first vaccine. Data of one patient for chemotherapeutic agent administration day were not available; en=19, data of 4 patients for chemotherapeutic agent administration day were not available; fn=16, three patients did not received chemotherapy after the second vaccine. Data of 4 patients for chemotherapeutic agent administration day were not available; gn=20, one patient did not received chemotherapy after the second vaccine. Data of one patient for chemotherapeutic agent administration day were not available. COVID-19: Coronavirus disease 2019, IQR: interquartile range, N, n: number, PD-1: programmed cell death protein 1, PD-L1: programmed death-ligand 1.
2. Humoral antibody responses after mRNA COVID-19 vaccination
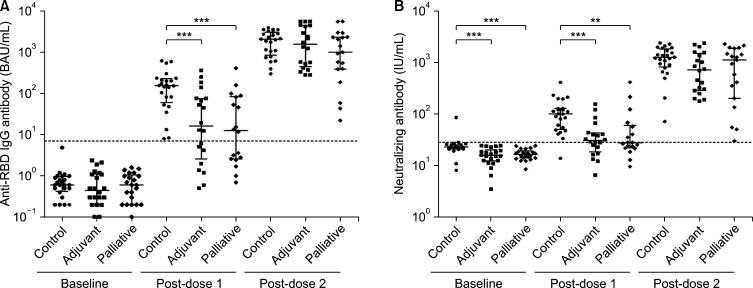
Fig. 2 and Supplementary Table 1 present the antibody levels of cancer patients and healthy controls regarding anti-RBD IgG binding and neutralizing antibody responses after the first and second COVID-19 mRNA vaccine doses.
FIG. 2. The serial changes of (A) anti-RBD IgG binding antibody and (B) neutralizing antibody levels before (baseline) and after the first (post-dose 1) and second (post-dose 2) vaccination of COVID-19 mRNA vaccines in the control, adjuvant, and palliative groups. The levels are on the logarithmic scale and indicated as a scatter dot-plot with median and interquartile ranges. The dotted lines are the threshold for positivity suggested by the manufacturers in conversion to the World Health Organization’s standard units (A: 7.1 BAU/mL, B: 28.4 IU/mL). *p<0.05; **p<0.01; ***p<0.001.
1) Humoral antibody responses after the first COVID-19 mRNA vaccine dose
After the first vaccine dose, serum samples were collected from 24 participants in the control group, 20 in the adjuvant group, and 18 in the palliative group. The levels of anti-RBD IgG antibody and neutralizing antibody significantly increased from baseline to post-dose 1 in healthy controls (median, 0.6 vs. 156.4 BAU/mL, p<0.001; 22.8 vs. 100.3 IU/mL, p<0.001), adjuvant group (0.5 vs. 16.5 BAU/mL, p<0.001; 15.7 vs. 30.5 IU/mL, p<0.001), and palliative group (0.6 vs. 12.6 BAU/mL, p<0.001; 16.4 vs. 27.3 IU/mL, p<0.001). The levels of anti-RBD IgG antibody and neutralizing antibody were significantly lower among cancer patients than controls (median, 13.3 vs. 156.4 BAU/mL, p<0.001; 100.3 vs. 28.1 IU/mL, p<0.001). However, there were no significant differences between the adjuvant and palliative groups in anti-RBD IgG antibody and neutralizing antibody levels. The seroconversion rates of the adjuvant and palliative groups were significantly lower than that of the control group for anti-RBD IgG antibody (60.0% vs. 100%, p<0.001; 55.6% vs. 100%, p<0.001, respectively) and neutralizing antibody (55.0% vs. 95.8%, p=0.003; 38.9% vs. 95.8%, p<0.001, respectively). The mRNA-1273 vaccine was associated with higher titers of anti-RBD IgG and neutralizing antibodies than the BNT162b vaccine in both controls and cancer patients (Supplementary Table 1, Supplementary Fig. 1).
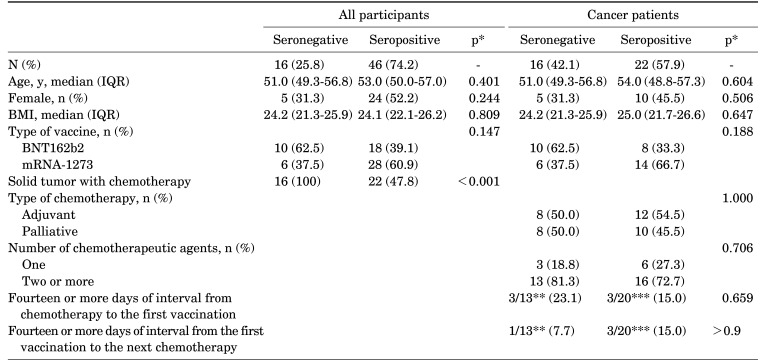
In the additional comparison of the characteristics between the seronegative and seropositive participants after the first mRNA vaccine dose, only the chemotherapy administration was associated with inhibited antibody production (Table 2). The type and number of chemotherapeutic agents were not associated with antibody response in cancer patients. Although the mRNA-1273 vaccine was associated with higher titers of anti-RBD IgG than the BNT162b vaccine, the seropositivity rate was not affected by the type of vaccine. Whether the interval between vaccine administration and chemotherapy was longer than 14 days also did not affect antibody positivity.
TABLE 2. Evaluation of factors associated with seroconversion of anti-RBD IgG after the first COVID-19 mRNA vaccine dose.
*Comparisons of categorical variables were performed using the Fisher exact test. The Mann-Whitney test was used for comparing continuous variables. **Data missing for 3 patients. ***Data missing for 2 patients. BMI: body mass index, IQR: interquartile range, N, n: number.
2)Humoral antibody responses after the second COVID-19 mRNA vaccine dose
After the second vaccine dose, serum samples were collected from 24 participants in the control group, 20 in the adjuvant group, and 19 in the palliative group. The levels of anti-RBD IgG antibody and neutralizing antibody significantly increased from post-dose 1 to post-dose 2 in healthy controls (median, 156.4 vs. 2081.0 BAU/mL, p<0.001; 100.3 vs. 1244.6 IU/mL, p<0.001), adjuvant group (16.5 vs. 1575.0 BAU/mL, p<0.001; 30.5 vs. 721.7 IU/mL, p<0.001), and palliative group (12.6 vs. 1005.0 BAU/mL, p<0.001; 27.3 vs. 1118.0 IU/mL, p<0.001). Anti-RBD IgG and neutralizing antibody seropositivity were observed in all of these participants. The levels of anti-RBD IgG and neutralizing antibodies were comparable between the three groups. Noticeably, the anti-RBD IgG antibody titers in all groups were significantly higher in association with mRNA-1273 vaccination than with BNT162b2 vaccination (Supplementary Table 1, Supplementary Fig. 1). In the control group, the neutralizing antibody level in the mRNA-1273 vaccination was similar to that in the BNT162b2 vaccination. However, among cancer patients, the neutralizing antibody level in mRNA-1273 vaccination was significantly higher than in BNT162b2 vaccination (median, 1460.2 vs. 351.6 IU/mL, p=0.002). Moreover, among participants vaccinated with BNT162b2, the neutralizing antibody titers were significantly lower in the adjuvant group than in healthy controls (median, 322.1 vs. 1073.0 IU/mL, p=0.022).
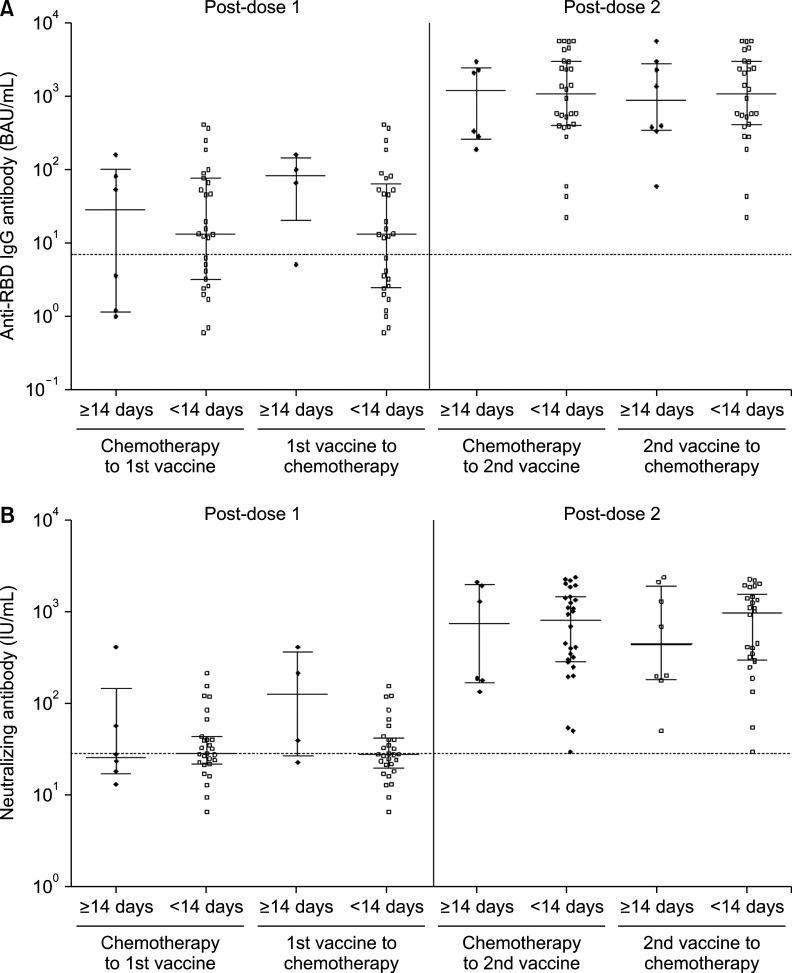
3. Timing of vaccination and humoral antibody responses
In patients receiving chemotherapy, an inactivated vaccine is generally recommended between treatment cycles when immunosuppression from treatment is minimized, generally 2 weeks apart from chemotherapy.15 In the case of COVID-19 vaccines, there has been no study of the appropriate interval between chemotherapy and vaccination, but current guidelines recommend vaccination whenever possible, regardless of the interval between chemotherapy treatments.16 Therefore, we compared antibody responses between patients with a vaccine and chemotherapy interval of more than 14 days and those with less than 14 days. Anti-RBD IgG binding antibody (median, 83.2 vs. 13.1 BAU/mL, p=0.184) and neutralizing antibody levels (127.1 vs. 28.0 IU/mL, p=0.119) tended to be higher in those who started chemotherapy more than 14 days after vaccination, but this was not statistically significant. The interval between the second dose and chemotherapy had no significant effect on antibody levels measured after the second dose (Fig. 3, Supplementary Table 2).
FIG. 3. Comparison of (A) anti-RBD IgG binding antibody and (B) neutralizing antibody levels according to the interval between chemotherapy and vaccine after the first (post-dose 1) and second (post-dose 2) vaccination with COVID-19 mRNA vaccines.
DISCUSSION
The efficacy of mRNA COVID-19 vaccination in cancer patients varies from study to study. A significant number of studies have shown that humoral immunity was comparable to that of healthy controls after two doses, similar to our results.2,17,18 However, other studies have shown lower immunogenicity in cancer patients compared to healthy controls.5,6,7 This difference is due to the heterogeneity of cancer patients, and for a comprehensive understanding of immunogenicity in cancer patients, it is necessary to conduct studies on a wide range of patients. In our study, we evaluated the vaccine immunogenicity in patients receiving palliative chemotherapy, who are presumed to have more compromised immune function due to the relatively high tumor burden and long duration of chemotherapy, and compared it to the adjuvant chemotherapy group and the healthy controls. Intriguingly, the humoral immune reactions showed no statistically significant variance between the three groups after the administration of two mRNA COVID-19 vaccine doses. This finding potentially offers clinicians and patients alike greater confidence in adopting the COVID-19 vaccination strategy.
We noted that anti-RBD IgG and neutralizing antibody levels were significantly lower in some cancer patients after the second vaccination, although the median titers were not statistically different from those of healthy controls. In addition, antibody titers following BNT162b2 vaccination were generally lower than those following mRNA-1273 vaccination, consistent with previous studies,19,20 and the disparity was more accentuated among cancer patients compared to healthy controls in our study. These observations underscore the potential influence of individual immune status and vaccine type on vaccine-induced immunity in cancer patients and suggest the need for more sophisticated vaccine strategies for patients currently receiving chemotherapy.
Because of concerns about effectiveness, it has been advised that inactivated vaccines should be administered at least two weeks prior to any immunosuppression in cancer patients.15 Regarding the COVID-19 vaccine, expert consensus recommends its administration irrespective of chemotherapy schedules.9 However, thus far, no studies have analyzed the effect of the interval between COVID-19 vaccination and chemotherapy on vaccine efficacy. Our study examined post-vaccination antibody titers across two groups: those with an interval exceeding 14 days between vaccine and chemotherapy and those with a shorter gap. Antibody titers measured after the first vaccination tended to be higher when the interval between vaccination and chemotherapy was more than 14 days. However, this trend was not observed after the second dose. Although the small number of patients limits the interpretation of the results, our observation suggests that it is essential to complete the recommended number of vaccinations to compensate for impaired immunogenicity in cancer patients, in addition to determining the optimal timing of vaccination.
This study had several limitations. First, the sample size was small. Even though the sample size was calculated to show non-inferiority of immunogenicity in cancer patients, care must be taken when generalizing these results. Second, given that the participants voluntarily participated in the study, patients in relatively better condition than general cancer patients may have been included. Third, we used humoral response as a surrogate for vaccine immunogenicity, but we did not check cellular immune response against the virus. Other limitations include the lack of data on the effectiveness of vaccination and antibody durability.
In conclusion, this study demonstrated that after second doses of mRNA COVID-19 vaccines, humoral immune responses in patients receiving chemotherapy were comparable to those of healthy controls, regardless of whether the purpose of the anti-cancer treatment is palliative or adjuvant. Furthermore, the level of humoral immunity after the second vaccination was not affected by timing of vaccination. These findings could provide important information to cancer patients and their physicians for the adopting COVID-19 vaccines.
ACKNOWLEDGEMENTS
This work was supported by the Korean South West Oncology Group (KSWOG), National Research Foundation of Korea (NRF) grant (NRF-2020R1A5A2031185, 2021R1A2C2013961) and the Bio & Medical Technology Development Program (NRF-2020M3A9G3080281) funded by the Korean government (MSIT), Chonnam National University (Grant number: 202233960001) and Chonnam National University Hospital Biomedical Research Institute (Grant number BCRI22054, BCRI21020). The reagent ‘SARS-CoV-2 IgG II Quant’ was provided in-kind by Abbott. We are also grateful to the Immunology Research Team of CNUH Laboratory Medicine for their technical support on antibody testing.
Footnotes
CONFLICT OF INTEREST STATEMENT: None declared.
SUPPLEMENTARY MATERIALS
Comparison of anti-RBD IgG binding antibody and neutralizing antibody responses after the first and second doses of COVID-19 mRNA vaccines between healthy controls and patients with solid cancers receiving adjuvant or palliative chemotherapy
Comparison of humoral response by interval between vaccine and chemotherapy
(A) The anti-RBD IgG binding antibody and (B) neutralizing antibody levels before (baseline) and after the first (post-dose 1) and second (post-dose 2) vaccination of each COVID-19 mRNA vaccine, BNT162b2 and mRNA-1273, in the control, adjuvant, and palliative groups. The levels are on the logarithmic scale and indicated as a scatter dot-plot with median and interquartile ranges. The dotted lines are the threshold for positivity suggested by the manufacturers for conversion to the World Health Organization’s standard units (A: 7.1 BAU/mL, B: 28.4 IU/mL). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
References
- 1.Booth A, Reed AB, Ponzo S, Yassaee A, Aral M, Plans D, et al. Population risk factors for severe disease and mortality in COVID-19: a global systematic review and meta-analysis. PLoS One. 2021;16:e0247461. doi: 10.1371/journal.pone.0247461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oosting SF, van der Veldt AAM, GeurtsvanKessel CH, Fehrmann RSN, van Binnendijk RS, Dingemans AC, et al. mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: a prospective, multicentre, non-inferiority trial. Lancet Oncol. 2021;22:1681–1691. doi: 10.1016/S1470-2045(21)00574-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehmsen S, Asmussen A, Jeppesen SS, Nilsson AC, Østerlev S, Vestergaard H, et al. Antibody and T cell immune responses following mRNA COVID-19 vaccination in patients with cancer. Cancer Cell. 2021;39:1034–1036. doi: 10.1016/j.ccell.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monin L, Laing AG, Muñoz-Ruiz M, McKenzie DR, Del Molino Del Barrio I, Alaguthurai T, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peeters M, Verbruggen L, Teuwen L, Vanhoutte G, Vande Kerckhove S, Peeters B, et al. Reduced humoral immune response after BNT162b2 coronavirus disease 2019 messenger RNA vaccination in cancer patients under antineoplastic treatment. ESMO Open. 2021;6:100274. doi: 10.1016/j.esmoop.2021.100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shroff RT, Chalasani P, Wei R, Pennington D, Quirk G, Schoenle MV, et al. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat Med. 2021;27:2002–2011. doi: 10.1038/s41591-021-01542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Funakoshi Y, Yakushijin K, Ohji G, Hojo W, Sakai H, Takai R, et al. Safety and immunogenicity of the COVID-19 vaccine BNT162b2 in patients undergoing chemotherapy for solid cancer. J Infect Chemother. 2022;28:516–520. doi: 10.1016/j.jiac.2021.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wankhede D, Grover S, Hofman P. Determinants of humoral immune response to SARS-CoV-2 vaccines in solid cancer patients: a systematic review and meta-analysis. Vaccine. 2023;41:1791–1798. doi: 10.1016/j.vaccine.2023.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. COVID-19 vaccination guide for people with cancer [Internet] Plymouth Meeting: National Comprehensive Cancer Network; 2022. [cited 2023 Aug 7]. Available from: https://www.logan.org/wp-content/uploads/2023/08/covid-vaccine-and-cancer-05-11.pdf. [Google Scholar]
- 10.Suhandynata RT, Bevins NJ, Tran JT, Huang D, Hoffman MA, Lund K, et al. SARS-CoV-2 serology status detected by commercialized platforms distinguishes previous infection and vaccination adaptive immune responses. J Appl Lab Med. 2021;6:1109–1122. doi: 10.1093/jalm/jfab080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Institute for Biological Standards and Control. First WHO International Reference Panel for anti-SARS-CoV-2 immunoglubulin. NIBSC code: 20/268. Instructions for use (version 3.0) [Internet] Potters Bar: National Institute for Biological Standards and Control; 2020. [cited 2023 Aug 7]. Available from: https://nibsc.org/documents/ifu/20-268.pdf. [Google Scholar]
- 12.Tan CW, Chia WN, Qin X, Liu P, Chen MI, Tiu C, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38:1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 13.GitHub. cPass to IU conversion [Internet] [San Francisco]: GitHub; [2021]. [cited 2023 Aug 7]. Available from: https://github.com/Lelouchzhu/cPass-to-IU_Conversion/ [Google Scholar]
- 14.Zhu F, Althaus T, Tan CW, Costantini A, Chia WN, Van Vinh Chau N, et al. WHO international standard for SARS-CoV-2 antibodies to determine markers of protection. Lancet Microbe. 2022;3:e81–e82. doi: 10.1016/S2666-5247(21)00307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:e44–e100. doi: 10.1093/cid/cit684. Erratum in: Clin Infect Dis 2014;59:144. [DOI] [PubMed] [Google Scholar]
- 16.National Institutes of Health. Special considerations in adults and children with cancer [Internet] Bethesda: National Institutes of Health; 2023. [cited 2023 Aug 7]. Available from: https://www.covid19treatmentguidelines.nih.gov/special-populations/cancer/ [Google Scholar]
- 17.Kim J, Chang E, Park SY, Lee DW, Kang CK, Choe PG, et al. Evaluation of seropositivity after standard doses of vaccination against SARS-CoV-2 in patients with early breast cancer receiving adjuvant treatment. Oncologist. 2022;27:e931–e937. doi: 10.1093/oncolo/oyac196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrière J, Carles M, Audigier-Valette C, Re D, Adjtoutah Z, Seitz-Polski B, et al. Third dose of anti-SARS-CoV-2 vaccine for patients with cancer: should humoral responses be monitored? A position article. Eur J Cancer. 2022;162:182–193. doi: 10.1016/j.ejca.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collier AY, Yu J, McMahan K, Liu J, Chandrashekar A, Maron JS, et al. Differential kinetics of immune responses elicited by Covid-19 vaccines. N Engl J Med. 2021;385:2010–2012. doi: 10.1056/NEJMc2115596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steensels D, Pierlet N, Penders J, Mesotten D, Heylen L. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021;326:1533–1535. doi: 10.1001/jama.2021.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of anti-RBD IgG binding antibody and neutralizing antibody responses after the first and second doses of COVID-19 mRNA vaccines between healthy controls and patients with solid cancers receiving adjuvant or palliative chemotherapy
Comparison of humoral response by interval between vaccine and chemotherapy
(A) The anti-RBD IgG binding antibody and (B) neutralizing antibody levels before (baseline) and after the first (post-dose 1) and second (post-dose 2) vaccination of each COVID-19 mRNA vaccine, BNT162b2 and mRNA-1273, in the control, adjuvant, and palliative groups. The levels are on the logarithmic scale and indicated as a scatter dot-plot with median and interquartile ranges. The dotted lines are the threshold for positivity suggested by the manufacturers for conversion to the World Health Organization’s standard units (A: 7.1 BAU/mL, B: 28.4 IU/mL). *, P < 0.05; **, P < 0.01; ***, P < 0.001.