Abstract
Contingency management is one of the most effective treatments for substance use disorders in not-pregnant people. The most recent quantitative review of its efficacy among pregnant and postpartum women who smoke cigarettes concluded with moderate certainty that those receiving contingent financial incentives were twice as likely to be abstinent compared with controls. We aimed to update and extend previous reviews.
Five databases were systematically searched for randomized controlled trials (RCTs) published before December 2022 that assessed the effectiveness of incentives for abstinence from substance use. Data from trials of smoking abstinence were pooled using a random-effects meta-analysis model (restricted maximum likelihood). Results are reported as risk-ratios (RRs) with 95% confidence intervals (CIs). This study is registered with PROSPERO, CRD42022372291.
Twelve RCTs (3,136) pregnant women) were included. There was high certainty evidence that women receiving incentives were more likely to be abstinent than controls at the last antepartum assessment (12 RCTs; RR=2.43, 95% CI 2.04-2.91, n=2,941, I2=0.0%) and moderate certainty evidence at the longest postpartum assessment while incentives were still available (five RCTs; RR=2.72, 1.47-5.02, n=659, I2=44.5%), and at the longest postpartum follow-up after incentives were discontinued (six RCTs; RR=1.93, 1.08-3.46, n=1,753, I2=51.8%).
Pregnant women receiving incentives are twice as likely to achieve smoking abstinence during pregnancy suggesting this intervention should be standard care for pregnant women who smoke. The results also demonstrate that abstinence continues into the postpartum period, including after incentives are discontinued, but more trials measuring outcomes in the postpartum period are needed to strengthen this conclusion.
Keywords: pregnancy, postpartum, tobacco, cigarettes, smoking, abstinence, contingency management, incentives
Introduction
The 2021 United States National Survey on Drug Use and Health estimated that in the past month approximately 10% of pregnant women (more than 200,000 individuals) smoked tobacco cigarettes (7% daily) [1]. Similar findings have been reported in Europe and data from low- and middle-income countries indicate that smoking among women of reproductive age and during pregnancy is a global and potentially growing problem [2,3].
Smoking during pregnancy increases the risk of poor pregnancy outcomes such as low birth weight, small for gestational age birth, and sudden unexpected infant death [4-6]. Although some women spontaneously abstain from smoking and other substance use during pregnancy [7-10], many are unable to achieve and sustain abstinence without intervention, especially women facing greater psychosocial stressors such as socioeconomic disadvantage and poor mental health [11,12].
One of the most effective treatments for substance use disorders is contingency management, a behavioral intervention wherein patients receive material (often financial) incentives contingent on objectively-verified behavior change [13]. The empirical literature on this topic has grown significantly over time, including an increasing number of studies focused on promoting smoking abstinence among pregnant women. A 2019 meta-analysis of RCTs quantified the effects of incentives on smoking during pregnancy and postpartum reported through July 2018 [14]. In most of the nine trials included, incentives were contingent on smoking abstinence, but one large trial of more than 1,000 participants offered incentives contingent primarily on smoking treatment adherence, such as for completing counseling telephone calls [15]. Two other trials in that 2019 review involved comparisons where the effect of incentives on abstinence could not be or were not isolated [16,17]. Compared to no-incentive conditions, pregnant women who received incentives were nearly three times as likely to be abstinent at the last assessment during pregnancy, which typically took place in the third trimester in the seven trials analyzed. The certainty of evidence for this comparison was deemed moderate. Also deemed of moderate certainty, pregnant women who received incentives were twice as likely to be abstinent at the longest follow-up, based on nine trials analyzed. The timing of this follow-up assessment varied widely, ranging from the 3rd trimester of pregnancy to six months postpartum, and also mixed trials where the longest follow-up occurred while incentives were still available with those where that assessment took place after incentives were discontinued.
Given further additions to the literature on incentives to promote smoking abstinence during pregnancy and postpartum, the importance of reducing peripartum smoking to individual and population health, and recent progress on implementing this intervention into routine prenatal care [18,19], we conducted a systematic review and meta-analysis of the literature to update the results of prior reviews by focusing on RCTs of incentives contingent primarily on abstinence from cigarette smoking and quantifying effects at several key time-points of intervention delivery and follow-up during pregnancy and postpartum.
Methods
Search strategy and selection criteria
The study protocol was registered with PROSPERO (CRD42022372291) [20] and followed Preferred Reporting Items for Systematic reviews and Meta Analyses (PRISMA) guidelines [21].
Using the search strategy outlined in the supplementary appendix, Medline, American Psychological Association PsycInfo, Embase, Cochrane (the Cochrane Central Register of Controlled Trials, the Cochrane Tobacco Addiction Group Specialised Register and the Cochrane Database of Systematic Reviews), and PubMed were searched from their inception until 11/17/2022 for published reports of RCTs or quasi-experimental or pragmatic trials [22] of incentives for abstinence from substance use among pregnant women. Only trials using an experimental design that allowed treatment effects to be attributed to the incentive intervention were included. Trials involving participants who were not pregnant at the time of randomization were excluded. Finally, trials that did not report data on incentive magnitude, treatment duration, or timing of incentive delivery were excluded, as were those where the majority of incentives were contingent on a behavior other than the pregnant participant’s biochemically-confirmed abstinence (e.g., treatment attendance). If data relevant to these inclusion/exclusion criteria were not reported in the published article, trial authors were contacted in an effort to obtain the information. Participant data were extracted from each trial and risk ratios (RRs) with 95% confidence intervals were calculated.
Study selection
LSK conducted the literature search and screened all abstracts. TGE independently screened a random selection of 10% of abstracts. LSK and TGE independently screened all full-text articles. Conflicts over the inclusion of 15 studies were resolved through discussion (five were secondary analyses of included RCTs, four studies did not report the relevant outcome, four were not among the specified designs/interventions, and two were not in the population of interest). All data were extracted by LSK and TGE. Percentage agreement for outcome data was 93% after comparison. Conflicts over inclusion and data extraction were resolved through discussion. LSK and SRMC conducted independent assessments of risk of bias and certainty of evidence using the Cochrane ‘Risk of bias 2’ and the GRADE approach [23].
Data analysis
Data were extracted on recruitment setting, participant characteristics, intervention details and substance use abstinence outcomes (including whether and how it was biochemically verified) in a custom form (https://osf.io/6nm4p/). Self-reported abstinence was only included in the analyses when biochemically verified data were not reported by study authors. All but one of the trials included in the analysis reported results from an intention-to-treat analysis whereby participants lost to follow-up were classified as ‘continued to smoke’. The only exception was Ondersma 2012 [17] wherein the authors excluded nine individuals (8% of the total sample) lost to follow-up from their final analytic sample. The present meta-analysis used existing publicly available summary data, and as such ethical approval by an institutional review board was not necessary.
Changes from pre-registered analysis plan
Regarding changes from the pre-registered analysis plan [20], first, we planned to include RCTs of incentives for abstinence from other substances (see search terms). However, only two trials included in the review involved incentive interventions for substance use other than tobacco [24,25]. Given the small number of trials and the fact that their results have been described in another review [26], we do not discuss them further here. Second, we planned to use meta-regression to examine whether effectiveness was moderated by factors like incentive magnitude and timing of incentive delivery as was observed in our meta-analysis conducted on incentive trials with predominantly not-pregnant participants [27]. However, there was low to no between-study heterogeneity in the effect sizes of trials included in the meta-analysis and values for these specific variables were also very similar between included RCTs, constraining our ability to meaningfully conduct the planned meta-regression [28]. Third, there were substantial reporting differences among the few trials included in the review that included treatment retention rates, leading us to forego exploration of that outcome.
Meta-analyses of trials on incentives for abstinence from cigarette smoking
In a random-effects model (restricted maximum-likelihood method [29]), each RCT was weighted by the inverse of its within- and between-study variance [28]. Pooled risk ratios (RR) with 95% confidence intervals (CIs) for all incentive interventions were calculated as the weighted average of each individual trial’s estimated intervention effect. All computations were done on a log scale using the log RR (including its variance and standard error). Summary effects were then exponentiated for interpretation.
Forest plots were planned to explore the effect of incentive interventions on abstinence from cigarette smoking at the last assessment during pregnancy, at the longest postpartum assessment while incentives were still available, and at the longest postpartum assessment after incentives were discontinued versus passive or active control/usual care. In the previous meta-analysis on this topic [14], a trial could contribute the same outcome data to both the analysis of abstinence at the longest follow-up and at the last assessment during pregnancy, if for that trial the longest follow-up also happened to be the last assessment during pregnancy [17,30,31] (Donatelle 2000b is reported in [30]). In contrast, in the current meta-analysis, outcome data could only be included in an analysis for one time-point. For trials where the last assessment during pregnancy was also the longest follow-up, data were only included in the former analysis. Heterogeneity was explored through review of forest plots, and use of the Chi2 test to assess whether observed differences in results between trials were compatible with chance alone. I2 statistics were calculated to examine the level of inconsistency across trial findings [28]. Where there were sufficient trials, small study bias (including publication bias) was explored using funnel plots and assessed using Egger’s regression test where visual inspection indicated potential asymmetry.
Analyses were conducted in R version 4.2.1 and the packages metafor [32], metameta [33] and tidyverse [34]. Data extraction, risk of bias and GRADE assessment forms are publicly available on the open science framework https://osf.io/6nm4p/.
Sensitivity analyses
Because some trials were described by authors as pilot or feasibility trials, we conducted an additional sensitivity meta-analysis for the last assessment during pregnancy that excluded these trials. For the analysis at the longest follow-up postpartum after incentives were discontinued, a post-hoc sensitivity analysis was conducted excluding the only trial that did not biochemically verify smoking abstinence.
Power analysis
Conforming to the “methods used to assess confidence in the body of evidence” component of the PRISMA [21] guidelines, we calculated the study-level statistical power in the meta-analysis for the outcome at the last assessment during pregnancy under a range of hypothetical effect sizes [33]. Using transformed normally distributed effect sizes (log RRs) and their standard errors, the statistical power of the trial for a hypothetical effect size was calculated using a two-sided Wald test.
Results
From a total of 455 studies identified in the literature search, 46 full text articles were retrieved and screened. Of these, 12 RCTs (3,136 pregnant participants randomized) targeting abstinence from smoking were included in this review (Figure 1, Table 1). Eight trials were conducted in the US [17,30,31,35-39]. Two trials were conducted in the UK [40,41], and one each in France [42] and New Zealand [43]. Of these intervention trials nine were full RCTs [30,35-42], two were feasibility RCTs [31,43], and one was a pilot RCT [17]. Overall, four of the 12 included RCTs were classified as being at low risk of bias, seven as having unclear risk, and one as high risk, on all domains considered in the assessment (Tables S1, S2 and online supplementary material https://osf.io/6nm4p/). Classifications of unclear risk were mostly based on the absence of a registered trial protocol, lack of information about access or uptake of nicotine replacement therapy (NRT), and unbalanced drop-out during the trial, but these concerns were deemed unlikely to seriously alter the results (Table S2).
Figure 1:
PRISMA flow diagram illustrating study selection.
Table 1:
Characteristics of randomized controlled trials (RCTs) testing incentive interventions for smoking abstinence during pregnancy and postpartum.
| Study | Country | Recruitment setting |
Study design |
Number randomized |
Mean weeks gestation at baseline |
Mean cigarettes per day (CPD) at study enrollment |
Control condition |
Incentive condition(s) |
Intervention duration (weeks) a |
Incentive condition maximum possible daily earnings b |
Measure of abstinence outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Berlin 2021 c | France | 18 maternity wards | 2 arm RCT | 460 | Control: 13.7 Intervention: 13.6 |
CPD not reported FTCD score: Control: 4.6 Intervention: 4.6 |
5 monthly visits where participants received a ≥10-minute intervention for smoking cessation, including motivational counseling, support, relapse prevention, and skills training elements for behavioral modifications | Same as control condition plus vouchers contingent on biochemically-verified smoking abstinence (self-report of no smoking in the past 7 days and carbon monoxide (CO) ≤ 8 ppm). | 20 c | Assuming maximum Intervention duration of 5 months, EUR €2.86 | Biochemically-verified (carbon monoxide (CO) ≤ 8 ppm) self-reported 7-day point-prevalence abstinence at 5 monthly visits following randomization |
| Donatelle 2000a | USA | 4 Oregon Women, Infants, and Children (WIC) program sites, which serve women with low-incomes at risk for inadequate nutrition | 2 arm RCT | 220 (13 withdrawn due to pregnancy termination/fetal demise; 207 included in analysis) | Control: 16.4 Intervention: 16.6 |
CPD not reported Mean salivary cotinine (ng/ml): Control: 45.7 Intervention: 45.4 |
Written and verbal information on the importance of smoking cessation provided by WIC or research staff at baseline and biochemical verification of smoking status for those who reported abstinence during monthly monitoring phone calls (up to 8 months until delivery and 2 months postpartum) | Same as control condition plus vouchers contingent on biochemically-verified smoking abstinence(sali vary thiocyanate ≤100μg/ml) delivered to participant and to social supporter | 31.4 | US $1.59 based on incentives delivered to participant, not social supporter | Biochemically-verified (salivary cotinine ≤ 30 ng/ml) self-reported 7-day point-prevalence abstinence at 8 months gestation and 2 months postpartum |
| Donatelle 2000b (data reported in Donatelle 2004) | USA | 8 Oregon WIC program sites | 3 arm RCT d | 186 | Not reported | Neither CPD nor other dependence measure reported | 5A’s (ask, advise, assess, assist, and arrange) intervention | Intervention condition 1: Same as control condition plus vouchers for biochemically-verified smoking abstinence (≤5 CO ppm) Intervention condition 2: Same as intervention condition 1 plus information about risk of harm for specific CO levels For both intervention conditions, abstinence assessed, and incentives provided each month for up to 8 months until delivery |
Not reported | Not reported | Biochemically-verified (salivary cotinine ≤ 30 ng/ml) self-reported 7-day point-prevalence abstinence presumably at 8 months gestation |
| Glover 2015 | New Zealand | Self-identified Māori women recruited from midwife, general practitioner and maternity services in Auckland as well as advertisements in the community and on social media | 3 arm feasibility RCT e | 24 | Control: 17 Intervention condition 1: 18 Intervention condition 2: 12 |
Control: 6 Intervention condition 1: 12 Intervention condition 2: 10 |
Information about different cessation products and services and access to nicotine replacement therapy. Self-reported abstinence assessed weekly; those reporting 2 weeks of abstinence visited up to three times for biochemical verification and delivery of incentives (twice during weeks 2-6 and once at week 8) |
Intervention condition 1: Same as control condition plus retail voucher contingent on biochemically-verified smoking abstinence (CO < 7 ppm) Intervention condition 2: Same as control condition plus retail product worth same amount as voucher contingent on biochemically-verified smoking abstinence For both intervention conditions, self-reported abstinence assessed weekly; those reporting 2 weeks of abstinence visited up to three times for biochemical verification and delivery of incentives (twice during weeks 2-6 and once at week 8) |
8 | NZ $3.57 | Biochemically-verified (CO < 7 ppm) 7-day point-prevalence at monthly assessments and self-reported continuous abstinence weeks 1-8 at 8 weeks following randomization in the antepartum period |
| Heil 2008 | USA | 4 obstetric practices and the WIC program in the Burlington, Vermont area | 2 arm RCT | 82 (5 withdrawn due to pregnancy termination/fetal demise; 77 included in analysis) | Control: 9.5 Intervention: 8.9 |
Control: 9.5 Intervention: 7.9 |
Usual care for smoking cessation through obstetric clinics, additional cessation counselling from study staff at intake and end-of-pregnancy assessments, and vouchers delivered independent of smoking status at daily abstinence monitoring visits for the first 5 days of week 1, then twice weekly during weeks 2-8, once weekly for weeks 9-12, and every other week until delivery, then returning to once weekly for 4 weeks after delivery, then every other week until end of postpartum week 12 | Same as control condition but vouchers contingent on biochemically-verified smoking abstinence (urinary cotinine ≤ 80 ng/ml) | 43.1 | US $4.06 | Biochemically-verified (urinary cotinine ≤ 80 ng/ml) self-reported 7-day point-prevalence abstinence at end of pregnancy (≥28 weeks gestation) and weeks 12 and 24 postpartum |
| Higgins 2014 | USA | Obstetric practices and the WIC program in the Burlington, Vermont area | 3 arm RCT e | 130 (12 withdrawn due to pregnancy termination/fetal demise; 118 included in analysis) | Control: 10.7 Intervention condition 1: 10.1 Intervention condition 2: 10.0 |
Control: 7.8 Intervention condition 1: 8.7 Intervention condition 2: 9.5 |
Usual care for smoking cessation through obstetric clinics, additional cessation counselling from study staff at 8 visits (5 antepartum, 3 postpartum), and vouchers delivered independent of smoking status using the same abstinence monitoring schedule as Heil 2008 above | Intervention condition 1: Same as control condition but vouchers contingent on biochemically-verified smoking abstinence (urinary cotinine ≤ 80 ng/ml) Intervention condition 2: Same as intervention condition 1 but vouchers contingent on biochemically-verified smoking abstinence with potential earnings rescheduled |
42 | US $4.01 | Biochemically-verified (urinary cotinine ≤ 80 ng/ml) self-reported 7-day point-prevalence abstinence at early and late pregnancy (1 month after intake and ≥28 weeks gestation, respectively), weeks 12 and 24 postpartum |
| Higgins 2022 | USA | Obstetric practices in the Burlington, Vermont area | 2 arm RCT | 176 (7 withdrawn due to pregnancy termination/fetal demise: 169 included in analysis) | Control: 11.1 Intervention: 12.4 |
Control: 9.9 Intervention: 9.0 |
Referral to state quitline (maximum of 9 brief phone calls (5 antepartum, 4 postpartum) with quit coach plus up to $65 in incentives for completing calls and access to free nicotine replacement therapy) and smoking cessation counselling from study staff at every assessments (2 antepartum, 6 postpartum) | Same as control condition plus vouchers contingent on biochemically-verified smoking abstinence (urinary cotinine ≤ 80 ng/ml) using the same abstinence monitoring schedule as Heil 2008 | 39.6 | Women smoking <10 cigarettes per day: US $4.10 Women smoking ≥10 cigarettes per day: US $8.19 |
Biochemically-verified (urinary cotinine ≤ 80 ng/ml) self-reported 7-day point-prevalence abstinence at early and late pregnancy (1 month after intake and ≥28 weeks gestation, respectively), weeks 2, 4, 8, 12, 24, and 48 postpartum |
| Kurti 2022 | USA | Social media, obstetric clinics, and WIC offices nationally | 2 arm RCT | 90 (2 withdrawn due to pregnancy termination/fetal demise: 90 reported in analysis) | Control: 17.3 Intervention: 13.7 |
>10 CPD: Control: 60% Intervention: 56% |
Brief smoking cessation counseling by study staff at three points during pregnancy, referral to state quitline (maximum of 9 brief phone calls (5 antepartum, 4 postpartum) and smoking cessation advice that is provided at their obstetric clinic. | Same as control condition plus cash deposited onto a debit card contingent on biochemically-verified smoking abstinence (salivary cotinine < 30 ng/ml) Abstinence assessed and incentives delivered via a smartphone app twice-daily for the first 5 days of intervention week 1,then twice weekly during intervention weeks 2-6, once weekly until delivery, then returning to twice weekly for 4 weeks after delivery, then weekly until the end of postpartum week 12 |
38.3 | us $5.78 | Biochemically-verified self-reported 7-day point-prevalence abstinence (salivary cotinine < 30 ng/ml) at early and late pregnancy (1 month after intake and ≥28 weeks gestation, respectively), weeks 4, 8, 12 and 24 postpartum |
| Ondersma 2012 | USA | 4 prenatal care clinics in Detroit, Michigan | 4 arm pilot RCT d | 110 | Weeks gestation >20 (%): Control: 26.9% Intervention condition 1:26.9% Intervention condition 2: 50.0% Intervention condition 3: 26.7% |
Control: 7.6 Intervention condition 1: 7.6 Intervention condition 2: 8.3 Intervention condition 3: 8.3 |
Treatment as usual from prenatal care providers | Intervention condition 1: Same as control condition plus interactive computer-delivered 5A’s Intervention condition 2: Same as control condition plus invitation to participate in a website-guided contingency management intervention, “CM-Lite”, with biochemical verification of abstinence (urinary cotinine <100) Intervention condition 3: Same as control condition plus combination of interventions 1 and 2 (5A’s plus CM Lite) Participants randomized to intervention conditions 2 & 3 had to ask staff to assess their smoking status and incentives were delivered on a maximum of five occasions at least one week apart over the 10-week intervention period |
10 | US $3.57 | Urinary cotinine <100 at 10 weeks following randomization |
| Tappin 2015 | UK | Stop smoking services serving maternity hospitals in Glasgow, Scotland area | 2 arm RCT | 612 (3 withdrew consent: 609 included in analysis) | Control: 12.6 Intervention: 12.3 |
CPD not reported FTCD: Control: 5.3 Intervention: 4.9 |
Offer of 1 hour of face-to-face support, 4 weekly support calls, and free nicotine replacement therapy for 10 weeks | Same as control condition plus retail vouchers contingent primarily on biochemically-verified smoking abstinence (CO < 10 ppm) Abstinence assessed and incentives delivered 4 and 12 weeks after the quit date and at a random time-point during weeks 34-38 gestation |
25.7 | GB £1.95 | Self-reported abstinence 4 weeks after quit date (set following randomization), biochemicall y-verified (salivary cotinine <14.2 ng/ml or urinary cotinine <44.7 ng/ml) self-reported abstinence over past 8 weeks at 34-38 weeks gestation, and self-reported abstinence at 6 months postpartum |
| Tappin 2022 | UK | 7 stop smoking services serving maternity hospitals in Great Britain | 2 arm RCT | 944 (3 withdrew consent: 941 included in analysis) | Control: 11.3 Intervention: 11.3 |
Control: <11 = 59.1% 11-20 = 35.6% 21-30 = 4.4% >30 = 0.4% Intervention: <11 = 59.7% 11-20 = 35.0% 21-30 = 4.7% >30 = 0.4% |
Withdrawal-oriented therapy and offer of nicotine replacement therapy with variety across services in terms of target population (general population or pregnancy specific), intervention type (smoking only or general health promotion), intervention format (stop smoking advisers with and without midwifery/nur sing backgrounds) and other factors | Same as control condition plus retail vouchers contingent primarily on biochemically-verified smoking abstinence (CO < 10 ppm) Same abstinence monitoring schedule as Tappin 2015 |
26.7 | GB £1.87 | Biochemically-verified (salivary cotinine <10 ng/ml) self-reported abstinence over past 8 weeks at 34-38 weeks gestation and 6 months postpartum and CO < 10 ppm at 4 weeks after quit date with later modification s due to COVID pandemic |
| Tuten 2012 | USA | Patients receiving methadone for the treatment of opioid use disorder at a comprehensive treatment program for pregnant women in Baltimore, Maryland | 3 arm feasibility RCT d | 102 | Control: 17.6 Intervention condition 1: 16.9 Intervention condition 2: 14.9 |
Control: 17.9 Intervention condition 1: 17.1 Intervention condition 2: 19.1 |
Information about the adverse effects of cigarette smoking for the mother and the infant and educational materials about risks of smoking during pregnancy at the first prenatal care appointment, brief Motivational Interviewing-style feedback session, and asked about smoking at subsequent obstetric visits and commended for efforts to abstain | Intervention condition 1: Same as control condition plus vouchers contingent on biochemically-verified smoking abstinence (CO < 4 ppm) Intervention condition 2: Same as control condition plus vouchers earned independent of smoking status For both intervention conditions, abstinence assessed, and incentives delivered thrice weekly for 12 weeks |
12 | US $10.70 | CO < 4 ppm at 1 and 3 months following randomization, and 6 weeks postpartum |
Note. FTCD = Fagerstrom Test for Cigarette Dependence.
For interventions that commence antepartum and end postpartum, intervention duration is calculated as the average pregnancy length (40 weeks) minus the mean weeks gestational age of participants at baseline, plus the number of weeks of the intervention postpartum.
Maximum possible daily earnings for abstinence from smoking calculated as the maximum possible earnings during the intervention period divided by the number of days in the intervention period.
For Berlin 2021 the quit date occurred within 15 days of randomization and for the purpose of maximum daily incentive calculation, the intervention duration is assumed to be the maximum possible intervention period (5 months).
For Donatelle 2000b, Ondersma 2012 and Tuten 2012, only the arm including contingent incentives alone was included in the meta-analysis for comparison with control.
Following Cochrane recommended guidelines for including studies with multiple arms in a meta-analysis [1]: Glover 2015 intervention conditions 1 (voucher incentive) and 2 (product incentive) collapsed into one condition for comparison with control; Higgins 2014 intervention conditions 1 (usual incentive schedule) and 2 (revised incentive schedule) collapsed into one condition for comparison with control.
Summary of included RCTs
Table 1 provides a summary of the included trials. Four RCTs were conducted entirely in the antepartum period [17,30,42,43]. Intervention duration varied from eight to 43 weeks. Estimated maximum possible daily earnings for participants in the incentive groups of included trials ranged from US $1.59 to $10.70 for those conducted in US and up to GB £1.95 (equivalent to US $2.40 in the year the trial was published) in the UK. The respective maximum possible earnings in the New Zealand and France trials were NZD $3.57 (US $2.49 in the year the trial was published) and EUR €2.86 (US $3.43 in the year the trial was published). All trials involved the immediate delivery of the incentive upon biochemical verification of abstinence from smoking. Four trials included access to NRT for all participants in the trial [37,40,41,43]. Most trials recruited from general maternity hospitals or prenatal/obstetric clinics. Two trials recruited solely from Women, Infants, and Children (WIC) programs, which serve women with low incomes at risk for inadequate nutrition [30,35], one trial limited their sample to women receiving methadone for opioid use disorder [31], and another restricted participation to those self-identifying as New Zealand indigenous Māori [43].
Pooled effect at the last assessment during pregnancy
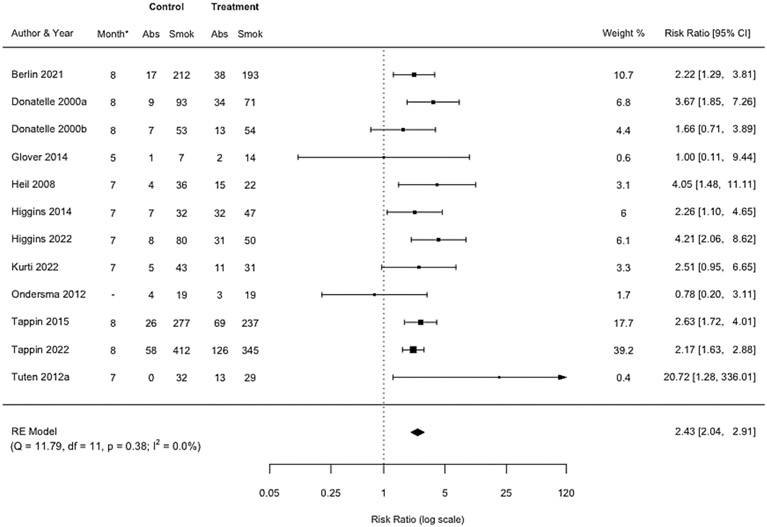
A pooled effect size was estimated based on the 12 RCTs of incentives for smoking abstinence at the last assessment during pregnancy, which in seven trials was also the end of treatment. Smoking abstinence was biochemically verified in all 12 trials. The analytic sample consisted of 2,941 women after trials accounted for drop-out due to fetal demise or withdrawn consent and our exclusion of certain trial arms where it was not possible to separate the effect of incentives from a co-intervention (see Table 1). Women assigned to incentive interventions were more than twice as likely to be abstinent compared with controls (RR=2.43, 95% CI 2.04-2.91) (Figure 2). Trials varied according to when the outcome was measured during pregnancy (range months 5-8 of gestation), but heterogeneity in effect size was low (I2=0.0%). Visual inspection of the funnel plot (Figure S1) and Egger’s regression test (Table S3) suggested there was a low likelihood of small study bias. The certainty of evidence for this comparison was deemed to be high (Table S2).
Figure 2: Forest plot comparing incentive interventions for smoking abstinence at last assessment during pregnancy vs. control or usual care.
*Month of pregnancy when last assessment during pregnancy was completed. Dash (−) indicates could not be calculated.
Similar results were observed in the sensitivity meta-analysis that excluded feasibility and pilot RCTs (nine RCTs; RR=2.47, 95% CI 2.07-2.96, n=2,798; I2=0.0%) (Figure S2). Assuming an alpha of 0.05 (two-tailed), the pre-registered power analysis using standard errors of effect sizes for all RCTs in this meta-analysis indicated that aside from Berlin 2021 [42], Higgins 2022 [37], Tappin 2015 [40], and Tappin 2022 [41], other trials could not reliably detect a range of small hypothetical effect sizes at the last assessment during pregnancy (Table S4 and Figure S3).
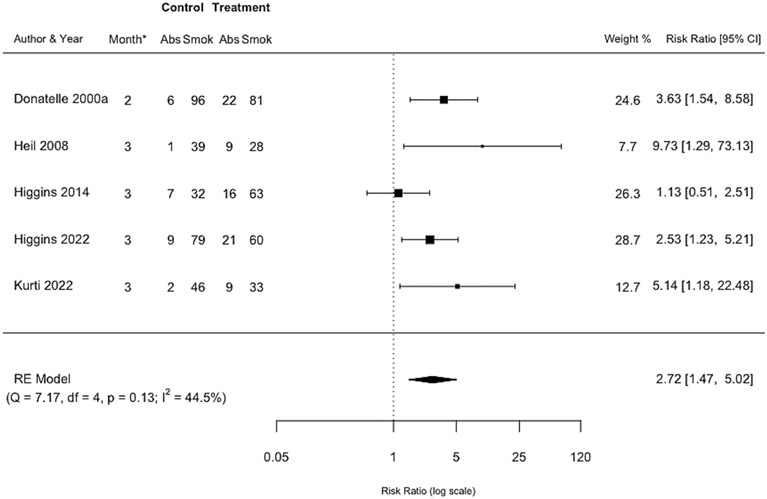
Pooled effect at the longest postpartum assessment while incentives were still available
A pooled effect size was estimated based on five RCTs of incentives for smoking abstinence at the longest postpartum assessment while incentives were still available. Smoking abstinence was biochemically verified in all five trials. Women assigned to incentive interventions were approximately 3 times more likely to be abstinent compared with controls (RR=2.72, 95% CI 1.47-5.02, n=659) (Figure 3). This outcome was measured consistently at 2-3 months postpartum and heterogeneity in the effect size between trials was moderate (I2=44.5%). There were not sufficient studies to examine small study bias. The certainty of evidence for this comparison was deemed to be moderate (Table S2).
Figure 3: Forest plot comparing incentive interventions for smoking abstinence at the longest postpartum assessment while incentives were still available vs. control or usual care.
*Month indicates the month postpartum when this assessment was completed.
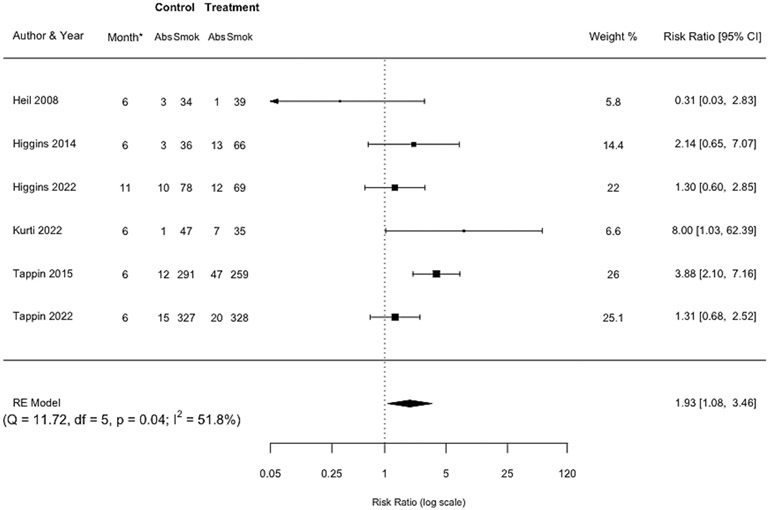
Pooled effect at the longest postpartum assessment after incentives were discontinued
A pooled effect size was estimated based on the six RCTs of incentives for smoking abstinence reported at the longest postpartum assessment after incentives were discontinued. Smoking abstinence was biochemically verified in all six except Tappin 2015 [40] wherein abstinence was based on self-report. Women assigned to incentive interventions were almost twice as likely to be abstinent compared with controls (RR=1.93, 95% CI 1.08-3.46, n=1,753) (Figure 4). This outcome was measured at 6 months postpartum in five of the six trials and at 11 months postpartum in the sixth. Trials also varied somewhat in the time between when incentives were discontinued and when the longest postpartum assessment was administered (range 3-8 months), but heterogeneity in effect size was moderate (I2=51.8%). There were not sufficient studies to examine small study bias. The certainty of evidence was deemed to be moderate (Table S2).
Figure 4: Forest plot comparing incentive interventions for smoking abstinence at the longest postpartum assessment after incentives were discontinued vs. control or usual care.
*Month indicates the month postpartum when this assessment was completed.
In a post-hoc sensitivity meta-analysis that excluded the single study in which smoking abstinence was not biochemically verified, the estimate for abstinence at the longest postpartum assessment after incentives were discontinued was compatible with there being no effect (RR=1.44, 95% CI 0.92-2.23, n=1,144) (Figure S4).
Discussion
Results from the randomized controlled trials included in this review provide high to moderate certainty evidence that pregnant women receiving incentives contingent on biochemically-confirmed smoking abstinence are approximately twice as likely to be abstinent compared to control or usual care conditions. This represents a strengthening in the certainty of the evidence for the last assessment during pregnancy relative to the 2019 Cochrane review, in which certainty was moderate [14]. This upgrade in certainty reflects in part the inclusion of four new RCTs with a combined sample size of nearly 1,700 participants, more than doubling the number of participants assessed during pregnancy. Although some trials in the analysis had limited statistical power to detect a range of small to moderate effect sizes, considering the large and clinically meaningful observed effect size (RR 2.43), these additional trials bolster conclusions about the body of evidence regarding incentives for smoking abstinence.
There was evidence in favor of incentives for smoking abstinence both at the longest postpartum assessment while incentives were still available and at the longest postpartum assessment after incentives were discontinued. However, the evidence at these time-points was graded as moderate due to the observed level of heterogeneity in effect size between the included trials. Data from additional rigorous, well-designed trials of incentives for smoking abstinence will help refine the magnitude of effects in the postpartum period and strengthen the certainty of the evidence for continued abstinence after incentive delivery has ceased. Indeed, data from not-pregnant people suggest benefits will persist. A recent meta-analysis of 23 studies assessed the long-term efficacy of incentives for stimulant, opioid, or polysubstance abstinence among not-pregnant people [45]. Participants in studies in that meta-analysis who received incentives had 22% greater odds of abstinence approximately 24 weeks after treatment ended than participants receiving comparison treatments (OR=1.22, 95% CI 1.03-1.44), which included some high-intensity, evidence-based treatments (e.g., cognitive-behavioral therapy) and intensive outpatient treatment.
In 2021, the National Institute of Health and Care Excellence (NICE) in England added financial incentives to its recommended list of interventions for pregnant women who smoke based on their own economic modelling demonstrating cost-effectiveness [19]. This year, perhaps prompted by subsequent demonstrations of cost-effectiveness in the UK and the US [46,47], the UK government announced that all pregnant women who smoke will be offered incentives by the end of 2024 [18]. The present results should further strengthen this endeavor and prompt questions about why no insurers or public payers in the US, where the majority of the research has been done, have any apparent plans to follow suit.
While developments toward widespread implementation based on the data to date are welcome, there is still more that could be learned through future research. The magnitude of the incentives has been shown to be a significant moderator of effect size in studies targeting abstinence from a wide range of substances with not-pregnant populations, with studies involving maximum possible daily earnings of less than USD $5.00 generating an overall small effect size, while those offering USD $5.00-10.99 and USD $11.00-16.00 generate overall medium effect sizes [27]. We were unable to analyze magnitude in the present report given limited variability across the included studies: eight of the 12 had maximum possible daily earnings of less than USD $5.00 [17,35,36,38,40-43], two were in the USD $5.00-10.99 range [31,39], and a value could not be calculated for another [30]. The twelfth trial, which was conducted by our group, tested magnitude in a unique way [37]. As noted in Table 1, women in that trial who were smoking <10 cigarettes per day at trial intake could earn the same magnitude of incentives offered to all participants in our prior in-person trials, approximately $4.00 per day [36,38,48], but women smoking ≥ 10 cigarettes per day, who are much less likely to achieve abstinence [49,50], could earn double that usual magnitude, or approximately $8.00 per day. As shown in Figure 2 in the present report, incentives in the Higgins et al., 2022 trial collectively produced 4-fold more abstinence than the control condition at the last assessment during pregnancy. Importantly, this overall effect was evident in the abstinence percentages reported in the primary outcomes paper for both the lighter (50% vs. 12%) and heavier (24% vs. 5%) smokers [37]. A subsequent analysis demonstrated the cost-effectiveness of this tailored approach, underscoring its potential utility in addressing a particularly challenging subset of this population [51].
We have also previously encouraged testing the continuation of incentives further into the postpartum period to reduce relapse and protect more infants and children from the adverse consequences of secondhand smoke exposure [37]. Relatedly, a large, ongoing three-arm RCT in the UK is testing the efficacy and cost-effectiveness of one way to approach the problem of postpartum relapse [52]. In this trial, all participants will have been offered incentives contingent on smoking abstinence during pregnancy. Those who are abstinent at the end of pregnancy and intend to stay abstinent postpartum will be randomized to either 1) usual care, 2) the same plus monthly incentives for each participant and a “Significant Other Supporter” for the first three months postpartum, or 3) the same plus additional incentives for participants at 6, 9, and 12 months postpartum. We have also suggested the possibility of combining incentives with other behavioral interventions (e.g., the Community Reinforcement Approach) and pharmacological interventions (e.g., substitution of non-combusted tobacco products for combusted cigarettes) in an effort to help more women initiate abstinence and sustain it longer-term [37].
There are some limitations to the current review. The primary meta-analysis included three feasibility and pilot trials, including one where participants had to ask staff to test their smoking status [17], which less than half of participants did, likely contributing to the lack of efficacy. These pilot and feasibility trials had relatively small weights in the analysis, as demonstrated by the similar effect size in the sensitivity analysis which excluded them. For the analyses involving assessment of abstinence from smoking at the longest postpartum assessment while incentives were still available and at the longest postpartum assessment after incentives were discontinued, the certainty of evidence was rated as moderate due to the relatively small number of trials appraised and the observed levels of heterogeneity in the effect sizes of included trials. Additional trials that collect data at these time-points will help increase confidence that the true effects are similar to what has been estimated. As noted above, there were low levels of heterogeneity between trials with regard to incentive magnitude and also the immediacy of incentive delivery. The consistency of immediate incentive delivery across trials suggests that researchers have heeded clear evidence from studies in not-pregnant populations about the importance of this aspect of incentive schedule design [27].
In conclusion, there is now high certainty evidence that pregnant women receiving incentive interventions are more than twice as likely to achieve smoking abstinence during pregnancy compared to controls, providing a stronger evidence base than any other intervention for this population to date to our knowledge. There is also moderate certainty evidence of greater abstinence at the longest postpartum assessment while incentives were still available and at the longest postpartum assessment after incentives were discontinued. Efforts to implement this intervention in everyday clinical practice should be expanded while research continues to explore ways to optimize effects.
Supplementary Material
Highlights.
Incentives increase smoking cessation during pregnancy.
Incentives for smoking cessation in pregnancy should be part of standard practice.
Incentives for smoking cessation after delivery are promising.
Funding:
Supported in part by National Institutes of Health grants R01DA047867 and P20GM103644 and Health Resources and Services Administration grant UD9RH33633
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests: The authors have no conflicts of interest to disclose.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.The Substance Abuse & Mental Health Data Archive. National Survey on Drug Use and Health (2021): Public-Use Data Analysis System [Internet]. 2023. [cited 2023 Jun 11]. Available from: https://www.datafiles.samhsa.gov/analyze-data [Google Scholar]
- 2.Lange S, Probst C, Rehm J, Popova S. National, regional, and global prevalence of smoking during pregnancy in the general population: a systematic review and meta-analysis. Lancet Glob Health. 2018. Jul 1;6(7):e769–76. [DOI] [PubMed] [Google Scholar]
- 3.Wilson CA, Finch E, Kerr C, Shakespeare J. Alcohol, smoking, and other substance use in the perinatal period. BMJ [Internet]. 2020. May 11 [cited 2022 Oct 26];369. Available from: https://www.bmj.com/content/369/bmj.m1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cnattingius S The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine & Tobacco Research [Internet]. 2004. Apr 1 [cited 2022 Oct 25];6(Suppl_2):S125–40. Available from: https://academic.oup.com/ntr/article/6/Suppl_2/S125/1013941 [DOI] [PubMed] [Google Scholar]
- 5.Gould GS, Havard A, Lim LL, Group PSIPE, Kumar R Exposure to tobacco, environmental tobacco smoke and nicotine in pregnancy: a pragmatic overview of reviews of maternal and child outcomes, effectiveness of interventions and barriers and facilitators to quitting. Int J Environ Res Public Health. 2020;17(6):2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson TM, Lavista Ferres JM, Ren SY, Moon RY, Goldstein RD, Ramirez J-M, et al. Maternal smoking before and during pregnancy and the risk of sudden unexpected infant death. Pediatrics. 2019;143(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurti AN, Redner R, Bunn JY, Tang K, Nighbor T, Lopez AA, et al. Examining the relationship between pregnancy and quitting use of tobacco products in a U.S. national sample of women of reproductive age. Prev Med (Baltim). 2018. Dec 1;117:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forray A, Merry B, Lin H, Ruger JP, Yonkers KA. Perinatal substance use: A prospective evaluation of abstinence and relapse. Drug Alcohol Depend. 2015. May 1;150:147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins PG, Clough DH, Frank B, Wallerstedt C. Changes in Health Behaviors Made by Pregnant Substance Users. http://dx.doi.org/103109/10826089509105137 [Internet]. 2009. [cited 2022 Oct 26];30(10):1323–33. Available from: https://www.tandfonline.com/doi/abs/10.3109/10826089509105137 [DOI] [PubMed] [Google Scholar]
- 10.Forray A Substance use during pregnancy. F1000Res [Internet]. 2016. [cited 2022 Dec 21];5. Available from: /pmc/articles/PMC4870985/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison PA, Sidebottom AC. Alcohol and drug use before and during pregnancy: An examination of use patterns and predictors of cessation. Matern Child Health J [Internet]. 2009. May 3 [cited 2022 Oct 26];13(3):386–94. Available from: https://link.springer.com/article/10.1007/s10995-008-0355-z [DOI] [PubMed] [Google Scholar]
- 12.Solomon LJ, Quinn VP. Spontaneous quitting: Self-initiated smoking cessation in early pregnancy. Nicotine & Tobacco Research [Internet]. 2004. Apr 1 [cited 2023 Apr 30];6(Suppl_2):S203–16. Available from: https://academic.oup.com/ntr/article/6/Suppl_2/S203/1013960 [DOI] [PubMed] [Google Scholar]
- 13.Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. american Journal of psychiatry. 2008;165(2):179–87. [DOI] [PubMed] [Google Scholar]
- 14.Notley C, Gentry S, Livingstone-Banks J, Bauld L, Perera R, Hartmann-Boyce J. Incentives for smoking cessation. Cochrane Database of Systematic Reviews [Internet]. 2019. Jul 17 [cited 2020 Jan 13]; Available from: http://doi.wiley.com/10.1002/14651858.CD004307.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker TB, Fraser DL, Kobinsky K, Adsit R, Smith SS, Khalil L, et al. A randomized controlled trial of financial incentives to low income pregnant women to engage in smoking cessation treatment: Effects on post-birth abstinence. J Consult Clin Psychol. 2018;86(5):464. [DOI] [PubMed] [Google Scholar]
- 16.Harris M, Reynolds B. A pilot study of home-based smoking cessation programs for rural, Appalachian, pregnant smokers. Journal of Obstetric, Gynecologic & Neonatal Nursing. 2015;44(2):236–45. [DOI] [PubMed] [Google Scholar]
- 17.Ondersma SJ, Svikis DS, Lam PK, Connors-Burge VS, Ledgerwood DM, Hopper JA. A randomized trial of computer-delivered brief intervention and low-intensity contingency management for smoking during pregnancy. Nicotine & tobacco research. 2012;14(3):351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.UK Government. Pregnant women will be offered financial incentives to help them quit smoking [Internet]. 2023. [cited 2023 Apr 10]. Available from: https://www.gov.uk/government/news/smokers-urged-to-swap-cigarettes-for-vapes-in-world-first-scheme
- 19.National Institute of Health and Care Excellence. Tobacco: preventing uptake, promoting quitting and treating dependence. Recommendations on treating tobacco dependence in pregnant women. [Internet]. Guidance. 2021. [cited 2023 Mar 13]. Available from: https://www.nice.org.uk/guidance/ng209/chapter/recommendations-on-treating-tobacco-dependence-in-pregnant-women [Google Scholar]
- 20.Kock L, Heil S. Moderators of effectiveness in contingency management interventions for tobacco and substance use disorders in pregnancy: a systematic review and meta-regression. [Internet]. PROSPERO. 2022. [cited 2023 Mar 25]. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022372291 [Google Scholar]
- 21.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. bmj. 2021. ;372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cochranre EPOC. What study designs should be included in an EPOC review and what should they be called? [Internet]. Cochrane EPOC. 2017. [cited 2019 Aug 21]. Available from: https://epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/EPOC Study Designs About.pdf [Google Scholar]
- 23.Schünemann H, Brożek J, Oxman GGA. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. 2013. URL: http://gdtguidelinedevelopment org/app/handbook/handbook html [WebCite Cache ID 6psHGGOvH]. 2017; [Google Scholar]
- 24.Jones HE, Haug NA, Stitzer ML, Svikis DS. Improving treatment outcomes for pregnant drug-dependent women using low-magnitude voucher incentives. Addictive Behaviors. 2000;25(2):263–7. [DOI] [PubMed] [Google Scholar]
- 25.Tuten M, Svikis DS, Keyser-Marcus L, O’Grady KE, Jones HE. Lessons learned from a randomized trial of fixed and escalating contingency management schedules in opioid-dependent pregnant women. Am J Drug Alcohol Abuse. 2012;38(4):286–92. [DOI] [PubMed] [Google Scholar]
- 26.Hand DJ, Ellis JD, Carr MM, Abatemarco DJ, Ledgerwood DM. Contingency management interventions for tobacco and other substance use disorders in pregnancy. Psychol Addict Behav [Internet]. 2017. Jun 22 [cited 2022 Oct 26];31(8):907–21. Available from: https://europepmc.org/articles/PMC5714659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction [Internet]. 2006. Feb 1 [cited 2022 Oct 27];101(2):192–203. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1360-0443.2006.01311.x [DOI] [PubMed] [Google Scholar]
- 28.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. John Wiley & Sons; 2021. [Google Scholar]
- 29.Langan D, Higgins JPT, Jackson D, Bowden J, Veroniki AA, Kontopantelis E, et al. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res Synth Methods. 2019. Mar 6;10(1):83–98. [DOI] [PubMed] [Google Scholar]
- 30.Donatelle RJ, Hudson D, Dobie S, Goodall A, Hunsberger M, Oswald K. Incentives in smoking cessation: status of the field and implications for research and practice with pregnant smokers. Nicotine & Tobacco Research. 2004;6(Suppl_2):S163–79. [DOI] [PubMed] [Google Scholar]
- 31.Tuten M, Fitzsimons H, Chisolm MS, Nuzzo PA, Jones HE. Contingent incentives reduce cigarette smoking among pregnant, methadone-maintained women: results of an initial feasibility and efficacy randomized clinical trial. Addiction. 2012;107(10):1868–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viechtbauer W Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 33.Quintana D A guide for calculating study-level statistical power for meta-analyses. 2022; [Google Scholar]
- 34.Wickham H, Averick M, Bryan J, Chang W, McGowan LD, François R, et al. Welcome to the Tidyverse. J Open Source Softw. 2019;4(43):1686. [Google Scholar]
- 35.Donatelle RJ, Prows SL, Champeau D, Hudson D. Randomised controlled trial using social support and financial incentives for high risk pregnant smokers: Significant Other Supporter (SOS) program. Tob Control. 2000;9(suppl 3):iii67–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heil SH, Higgins ST, Bernstein IM, Solomon LJ, Rogers RE, Thomas CS, et al. Effects of voucher-based incentives on abstinence from cigarette smoking and fetal growth among pregnant women. Addiction. 2008;103(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higgins ST, Nighbor TD, Kurti AN, Heil SH, Slade EP, Shepard DS, et al. Randomized controlled trial examining the efficacy of adding financial incentives to best practices for smoking cessation among pregnant and newly postpartum women. Prev Med (Baltim). 2022;165:107012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins ST, Washio Y, Lopez AA, Heil SH, Solomon LJ, Lynch ME, et al. Examining two different schedules of financial incentives for smoking cessation among pregnant women. Prev Med (Baltim). 2014;68:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurti AN, Nighbor TD, Tang K, Bolívar HA, Evemy CG, Skelly J, et al. Effect of smartphone-based financial incentives on peripartum smoking among pregnant individuals: a randomized clinical trial. JAMA Netw Open. 2022;5(5):e2211889–e2211889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tappin D, Bauld L, Purves D, Boyd K, Sinclair L, MacAskill S, et al. Financial incentives for smoking cessation in pregnancy: randomised controlled trial. Bmj. 2015;350. [DOI] [PubMed] [Google Scholar]
- 41.Tappin D, Sinclair L, Kee F, McFadden M, Robinson-Smith L, Mitchell A, et al. Effect of financial voucher incentives provided with UK stop smoking services on the cessation of smoking in pregnant women (CPIT III): pragmatic, multicentre, single blinded, phase 3, randomised controlled trial. bmj. 2022;379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berlin I, Berlin N, Malecot M, Breton M, Jusot F, Goldzahl L. Financial incentives for smoking cessation in pregnancy: multicentre randomised controlled trial. bmj. 2021;375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glover M, Kira A, Walker N, Bauld L. Using incentives to encourage smoking abstinence among pregnant indigenous women? A feasibility study. Matern Child Health J. 2015;19:1393–9. [DOI] [PubMed] [Google Scholar]
- 44.Shipton D, Tappin DM, Vadiveloo T, Crossley JA, Aitken DA, Chalmers J. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. BMJ [Internet]. 2009. Oct 30 [cited 2021 Jul 5];339(7732):1241. Available from: https://www.bmj.com/content/339/bmj.b4347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ginley MK, Pfund RA, Rash CJ, Zajac K. Long-term efficacy of contingency management treatment based on objective indicators of abstinence from illicit substance use up to 1 year following treatment: A meta-analysis. J Consult Clin Psychol. 2021;89(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McMeekin N, Sinclair L, Robinson-Smith L, Mitchell A, Bauld L, Tappin DM, et al. Financial incentives for quitting smoking in pregnancy: are they cost-effective? medRxiv. 2022;2022–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shepard DS, Slade EP, Nighbor TD, DeSarno MJ, Roemhildt ML, Williams RK, et al. Economic analysis of financial incentives for smoking cessation during pregnancy and postpartum. Prev Med (Baltim). 2022;165:107079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Higgins ST, Heil SH, Solomon LJ, Bernstein IM, Lussier JP, Abel RL, et al. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine & Tobacco Research. 2004;6(6):1015–20. [DOI] [PubMed] [Google Scholar]
- 49.Benowitz NL, Dempsey DA. Pharmacotherapy for smoking cessation during pregnancy. Nicotine & Tobacco Research. 2004;6:S189–202. [DOI] [PubMed] [Google Scholar]
- 50.Lopez AA, Skelly JM, White TJ, Higgins ST. Does impulsiveness moderate response to financial incentives for smoking cessation among pregnant and newly postpartum women? Exp Clin Psychopharmacol. 2015;23(2):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shepard DS, Slade EP, Nighbor TD, DeSarno MJ, Roemhildt ML, Williams RK, et al. Economic analysis of financial incentives for smoking cessation during pregnancy and postpartum. Prev Med (Baltim). 2022. Dec 1;165:107079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ussher M, Best C, Lewis S, McKell J, Coleman T, Cooper S, et al. Financial Incentives for Preventing Postpartum return to Smoking (FIPPS): study protocol for a three-arm randomised controlled trial. Trials. 2021;22:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.