Abstract
Introduction
Patients with moderate-to-severe psoriasis (PsO) treated with interleukin (IL)-inhibitors may require treatment modification to achieve disease control. This study evaluated discontinuation and switching of IL-inhibitors for PsO patients in Japan.
Methods
Japan Medical Data Center claims (1/2005–5/2022) were used to identify patients with PsO diagnosis preceding a first IL-inhibitor claim (index date) with ≥ 6 months of eligibility prior. Treatment switch (claim for another biologic) and discontinuation (gap in care ≥ 150% of the days’ supply of the preceding prescription) were assessed up to 24 months following initiation. Censored Kaplan–Meier time-to-event analyses calculated rates, and Cox proportional hazards models estimated hazard ratios (HRs) adjusting for baseline characteristics.
Results
The study included 1481 unique patients treated with brodalumab (BRO; n = 159), guselkumab (GUS; n = 360), ixekizumab (IXE; n = 279), risankizumab (RIS; n = 327), secukinumab (SEC; n = 366), tildrakizumab (n = 40; excluded due to limited data), and ustekinumab (UST; n = 262). At 12/24 months, 25.9%/38.6% of patients overall had discontinued their index IL-inhibitor and 13.5%/21.2% had switched to another biologic. Discontinuation at 12/24 months was lowest for RIS (11.2%/17.4%), followed by UST (17.9%/32.2%), IXE (27.0%/37.0%), GUS (29.8%/43.0%), SEC (35.6%/53.8%), and BRO (37.2%/47.2%). Switching showed a similar trend: RIS (5.7%/10.7%), UST (11.2%/19.9%), SEC (14.7%/25.7%), IXE (14.8%/21.5%), GUS (16.9%/23.2%), and BRO (19.7%/26.8%). HRs of discontinuation relative to RIS were 2.07 for UST, 2.59 for IXE, 2.70 for GUS, 3.65 for BRO, and 3.69 for SEC (all P ≤ 0.001). HRs of switching relative to RIS were 2.05 for IXE, 2.45 for GUS, 2.67 for SEC, 2.73 for UST, and 2.77 for BRO (all P ≤ 0.01).
Conclusion
Treatment modification of IL-inhibitors for PsO was commonly observed and could indicate insufficient disease control and/or incremental economic burden. Discontinuation and switching rates were lowest for RIS regardless of time point and adjustment for patient characteristics.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-023-01064-1.
Keywords: Biologics, Discontinuation, Interleukin inhibitors, Japan, Persistence, Psoriasis, Switching, Treatment patterns
Key Summary Points
| Why carry out this study? |
| Although several effective treatment options are available for patients with psoriasis (PsO), persistence to therapy is generally poor. |
| Evidence suggest that interleukin (IL)-inhibitors may have improved persistence compared with biologics of other mechanisms of action; however, comparative studies on treatment patterns for IL-inhibitors beyond 12 months are lacking. |
| Our study evaluated discontinuation and switching rates of IL-inhibitors through 2 years in patients with PsO in real-world settings in Japan, expanding upon prior studies by leveraging larger sample sizes and inclusion of more recently approved therapies. |
| What was learned from this study? |
| Treatment modification of IL-inhibitors for treatment of PsO was commonly observed, with 26% and 39% of patients discontinuing their first IL-inhibitor and 14% and 21% switching to another biologic, by 12 and 24 months, respectively. |
| Rates of discontinuation and switching varied across therapies but were lowest in patients who received risankizumab regardless of time point and after adjustment for patient characteristics and prior treatment history. |
| Frequent treatment modification could be indicative of insufficient disease management, and additional research is warranted to evaluate drivers of discontinuation and switching and consequences of the need for changing therapies in PsO to inform treatment decision-making. |
Introduction
Psoriasis (PsO), a chronic autoimmune disease typified by inflammatory plaques of the skin, has a significantly negative impact on patients’ quality of life [1]. PsO affects over half a million individuals in Japan, at a prevalence rate of 0.34% that is expected to increase in future years [2]. Among the population of diagnosed PsO patients in Japan, approximately 14% are estimated to have moderate-to-severe PsO, defined by the percentage of body surface area (BSA) covered by PsO patches [3]. PsO is associated with increased healthcare resource use burden in Japan, including all-cause costs of care and elevated risk of inpatient admission [4].
Many types of treatment are approved for PsO treatment in Japan; however, there are no comprehensive formal guidelines on their use [5]. Treatment commonly begins with topical agents followed by phototherapy, with moderate-to-severe patients often progressing to oral systemic treatments, including a phosphodiesterase-4 (PDE-4) inhibitor (apremilast), immunosuppressants (cyclosporin, methotrexate), or vitamin A (acitretin, etretinate) [6]. Biologics, such as tumor necrosis factor (TNF) inhibitors (adalimumab and infliximab); the interleukin (IL)-12/23 inhibitor ustekinumab (UST) IL-23 inhibitors (guselkumab (GUS), risankizumab (RIS), and tildrakizumab (TIL)); and IL-17 inhibitors [secukinumab (SEC), ixekizumab (IXE), and brodalumab (BRO)], are also used to reduce disease severity and improve patients’ quality of life [7]. In clinical trials, IL-inhibitors have demonstrated superior efficacy relative to other mechanisms of action (MOAs) in reducing BSA; however, it is unknown how well real-world adherence, and thus efficacy, align [8–11].
Despite the availability of effective treatment options, persistence with prescribed therapy is generally poor in PsO. Evidence from Japan suggests that nearly two-thirds of PsO patients discontinue their prescribed treatment within 12 months of initiation, and—though not common—switching to another treatment is associated with increased healthcare costs [6, 12]. Furthermore, persistence on treatment has been found to vary considerably by treatment type, with biologics having substantially longer time on therapy than oral systemic treatments in the first 12 months following initiation [6]. Among biologics, there is evidence that IL-inhibitors, with generally longer dosing intervals, demonstrate improved persistence and lower discontinuation relative to other MOAs [6]. While most persistence studies focus on 12-month outcomes, there is evidence that discontinuation—and switching in particular—increases over time [12, 13]. However, comparative studies on treatment patterns for IL-inhibitors beyond the initial 12 months are lacking but needed to inform treatment decisions with newly available therapy options. This study describes real-world treatment patterns with IL-inhibitors, including discontinuation and switching, for Japanese patients with PsO.
Methods
Study Design and Data Source
This was a non-interventional study using administrative claims from the Japan Medical Data Center Payer-Based Database (JMDC) from 1 January 2005 through 31 May 2022 on beneficiaries with a PsO diagnosis and subsequent dispensing for IL-inhibitor treatment. JMDC is a claims database of approximately 10 million enrollees in contracted health insurance associations (about 8% of the total Japanese population), representing mainly persons of working age (less than 75 years old) employed by middle-to-large-size companies and their dependents. The data include patient-level demographic and plan enrollment information (e.g., start and stop dates of health plan enrollment), date-stamped (month and year) inpatient and outpatient medical claims (e.g., diagnosis codes, procedure codes, provider specialty), and pharmacy claims (e.g., prescription fill/refill dates, drug name/code, dosage, cost).
Cohort Construction
A cohort of IL-inhibitor patients with PsO was identified on the basis of a claim with International Classification of Diseases 10th Revision (ICD-10): L40.X and at least one claim in the same month or after the earliest PsO diagnosis for an IL-inhibitor (including BRO, GUS, IXE, RIS, SEC, TIL, and UST). An index date was assigned as the earliest IL-inhibitor claim for each product following PsO diagnosis. Patients were required to have at least 6 months of continuous enrollment immediately preceding the index date (baseline period). The follow-up period lasted from the index date until the earlier of the end of data availability or 24 months. Patients satisfying the inclusion criteria were included in all IL-inhibitor-specific cohorts for which they had a qualifying index claim. In subgroup analyses, patients were required to have PsO diagnosis with ICD-10 L40.0 (psoriasis vulgaris) and/or L40.9 (psoriasis unspecified) specifically.
Study Measures
Baseline characteristics included demographics assessed as of the first claim for an IL-inhibitor, comorbidities and healthcare resource use during the baseline period, and treatment history using all available data. Demographics included sex, age, and year of index date. Note only birth year and month were available in the data, so all patients were assigned the day of birth as the 15th of the month for the purpose of calculating age. Comorbidities consisted of those included in the Charlson Comorbidity Index [14]. Healthcare resource use was quantified by total charges and stratified by setting of care (outpatient, inpatient, and pharmacy), with US dollar values estimated on the basis of an assumed exchange rate of 1 yen = 0.01 dollars. Inpatient charges included charges associated with both DPC (per diem) and inpatient (fee-for-service) claims. Treatment history was evaluated as any prior use of IL-inhibitors or TNF-inhibitors, with IL-inhibitor experienced versus naïve defined as an IL-inhibitor claim between the first PsO diagnosis and the index date.
Outcomes included treatment switching and discontinuation assessed up to 24 months following the index date. A switch in treatment was defined as a claim for a new (non-index) IL-inhibitor or TNF-inhibitor PsO treatment after the index date. Treatment discontinuation was defined as a gap in care of at least 150% of the days of supply of the preceding prescription (permissible gap; Supplemental Fig. S1: Discontinuation definition). Sensitivities were conducted using 60-, 180-, and 365-day permissible gap lengths.
Statistical Analyses
Baseline characteristics were descriptively summarized as counts and frequencies for categorical measures, and means and standard deviations for continuous measures, overall and by product-specific cohort. No hypothesis testing was conducted. Kaplan–Meier time-to-event analyses censored for loss of follow-up estimated survival to switching and discontinuation up to 24 months following the index date, with descriptive statistics for outcome measures assessed at 12 and 24 months. Among patients who switched, descriptive frequencies summarized the share of patients switching to each MOA. Hazard ratios (HRs) and 95% confidence intervals (CIs) of discontinuation and switching were estimated using Cox proportional hazards models adjusting for patient demographics, biologic treatment history, and baseline comorbidities and healthcare charges. Data analysis was performed using SAS software v9.4 (SAS Institute, Cary, NC).
Compliance with Ethics Guidelines
This article does not contain any studies with human participants or animals performed by any of the authors. Database permission was obtained via a license from the provider; however, the database is otherwise not publicly available. Because deidentification was conducted before providing claims to researchers, and no identifiable protected health information was included in the data used, Institutional Review Board approval was not required. All analyses were conducted in compliance with RECORD-PE guidance and in accordance with the ethical standards in the 1964 Declaration of Helsinki and its subsequent amendments [15].
Results
Study Participants
The study included 1481 unique patients treated with IL-inhibitors: BRO (n = 159), GUS (n = 360), IXE (n = 279), RIS (n = 327), SEC (n = 366), TIL (n = 40), and UST (n = 262; Fig. 1). Note, patient counts by product are not mutually exclusive, as patients are counted in each product cohort for which they qualify. TIL was excluded from treatment-level comparisons due to the small sample and limited follow-up data (Table 1). The sample was predominantly male (70.0%) and aged between 40 and 64 years as of the index date (70.8%). Over 90% of the sample initiated IL-inhibitor treatment in 2017 or later and over 90% were also IL-inhibitor naïve at the time of initiation (over 80% were TNF-inhibitor naïve). Common comorbidities included hypertension (28.3%), hyperlipidemia (27.3%), and liver disease (18.2%). Among treatment-specific cohorts, GUS had the highest mean age (50.1 years) and proportion female (39.2%). Baseline comorbidities and medical costs were similar across cohorts.
Fig. 1.
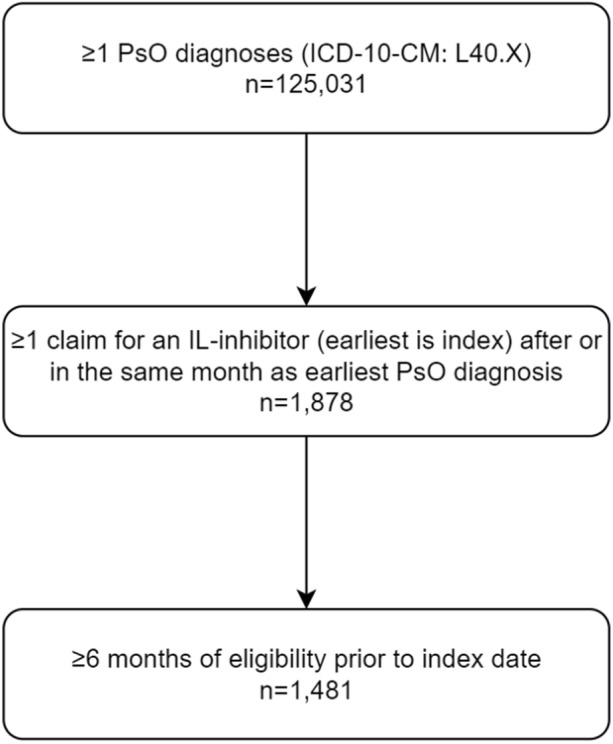
Sample attrition flowchart. Count of patients meeting the study inclusion criteria. ICD-10-CM International Classification of Diseases, 10th Revision, Clinical Modification, IL interleukin, PsO moderate to severe psoriasis
Table 1.
Baseline characteristics
| Characteristic | Overall | Brodalumab | Guselkumab | Ixekizumab | Risankizumab | Secukinumab | Tildrakizumab | Ustekinumab |
|---|---|---|---|---|---|---|---|---|
| N = 1481 | n = 159 | n = 360 | n = 279 | n = 327 | n = 366 | n = 40 | n = 262 | |
| Demographics | ||||||||
| Gender, n (%) | ||||||||
| Male | 1036 (70.0%) | 125 (78.6%) | 219 (60.8%) | 203 (72.8%) | 249 (76.1%) | 241 (65.8%) | 30 (75.0%) | 188 (71.8%) |
| Female | 445 (30.0%) | 34 (21.4%) | 141 (39.2%) | 76 (27.2%) | 78 (23.9%) | 125 (34.2%) | 10 (25.0%) | 74 (28.2%) |
| Age in years, mean (SD)a | 48.1 (11.7) | 48.3 (12.1) | 50.1 (11.4) | 49.3 (10.7) | 47.7 (11.5) | 47.7 (12.1) | 52.4 (7.8) | 46.2 (12.3) |
| 0–17, n (%) | 16 (1.1%) | 1 (0.6%) | 3 (0.8%) | 1 (0.4%) | 2 (0.6%) | 8 (2.2%) | 0 (0.0%) | 2 (0.8%) |
| 18–39, n (%) | 337 (22.8%) | 33 (20.8%) | 64 (17.8%) | 53 (19.0%) | 79 (24.2%) | 76 (20.8%) | 3 (7.5%) | 76 (29.0%) |
| 40–64, n (%) | 1048 (70.8%) | 115 (72.3%) | 267 (74.2%) | 213 (76.3%) | 227 (69.4%) | 263 (71.9%) | 35 (87.5%) | 171 (65.3%) |
| 65+, n (%) | 80 (5.4%) | 10 (6.3%) | 26 (7.2%) | 12 (4.3%) | 19 (5.8%) | 19 (5.2%) | 2 (5.0%) | 13 (5.0%) |
| Year of index date, n (%) | ||||||||
| 2010 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 2011 | 2 (0.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (0.8%) |
| 2012 | 12 (0.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 12 (4.6%) |
| 2013 | 13 (0.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 13 (5.0%) |
| 2014 | 16 (1.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 16 (6.1%) |
| 2015 | 30 (2.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 4 (1.1%) | 0 (0.0%) | 27 (10.3%) |
| 2016 | 74 (5.0%) | 4 (2.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 32 (8.7%) | 0 (0.0%) | 43 (16.4%) |
| 2017 | 128 (8.6%) | 26 (16.4%) | 0 (0.0%) | 28 (10.0%) | 0 (0.0%) | 53 (14.5%) | 0 (0.0%) | 39 (14.9%) |
| 2018 | 201 (13.6%) | 37 (23.3%) | 70 (19.4%) | 56 (20.1%) | 0 (0.0%) | 51 (13.9%) | 0 (0.0%) | 33 (12.6%) |
| 2019 | 309 (20.9%) | 31 (19.5%) | 110 (30.6%) | 71 (25.4%) | 80 (24.5%) | 66 (18.0%) | 0 (0.0%) | 22 (8.4%) |
| 2020 | 272 (18.4%) | 28 (17.6%) | 64 (17.8%) | 57 (20.4%) | 97 (29.7%) | 70 (19.1%) | 3 (7.5%) | 24 (9.2%) |
| 2021 | 303 (20.5%) | 26 (16.4%) | 86 (23.9%) | 43 (15.4%) | 102 (31.2%) | 59 (16.1%) | 29 (72.5%) | 20 (7.6%) |
| 2022 | 121 (8.2%) | 7 (4.4%) | 30 (8.3%) | 24 (8.6%) | 48 (14.7%) | 31 (8.5%) | 8 (20.0%) | 11 (4.2%) |
| Biologic treatment history, n (%)b | ||||||||
| IL-inhibitor naïve | 1339 (90.4%) | 110 (69.2%) | 253 (70.3%) | 186 (66.7%) | 198 (60.6%) | 317 (86.6%) | 31 (77.5%) | 244 (93.1%) |
| IL-inhibitor experienced | 142 (9.6%) | 49 (30.8%) | 107 (29.7%) | 93 (33.3%) | 129 (39.4%) | 49 (13.4%) | 9 (22.5%) | 18 (6.9%) |
| Baseline health | ||||||||
| Comorbidities, n (%) | ||||||||
| Asthma | 136 (9.2%) | 19 (11.9%) | 31 (8.6%) | 25 (9.0%) | 26 (8.0%) | 42 (11.5%) | 4 (10.0%) | 22 (8.4%) |
| Chronic obstructive pulmonary disease | 8 (0.5%) | 0 (0.0%) | 3 (0.8%) | 2 (0.7%) | 1 (0.3%) | 6 (1.6%) | 0 (0.0%) | 0 (0.0%) |
| All cancers | 208 (14.0%) | 15 (9.4%) | 62 (17.2%) | 38 (13.6%) | 42 (12.8%) | 67 (18.3%) | 5 (12.5%) | 27 (10.3%) |
| Lymphoma | 11 (0.7%) | 2 (1.3%) | 6 (1.7%) | 1 (0.4%) | 2 (0.6%) | 4 (1.1%) | 0 (0.0%) | 0 (0.0%) |
| Skin | 1 (0.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.3%) | 0 (0.0%) | 0 (0.0%) |
| Chronic liver disease and cirrhosis | 18 (1.2%) | 3 (1.9%) | 6 (1.7%) | 1 (0.4%) | 4 (1.2%) | 3 (0.8%) | 0 (0.0%) | 4 (1.5%) |
| Liver disease | 270 (18.2%) | 29 (18.2%) | 74 (20.6%) | 42 (15.1%) | 60 (18.3%) | 74 (20.2%) | 10 (25.0%) | 40 (15.3%) |
| Chronic renal disease | 37 (2.5%) | 4 (2.5%) | 12 (3.3%) | 4 (1.4%) | 8 (2.4%) | 11 (3.0%) | 1 (2.5%) | 3 (1.1%) |
| Acute myocardial infarction | 11 (0.7%) | 1 (0.6%) | 7 (1.9%) | 1 (0.4%) | 0 (0.0%) | 1 (0.3%) | 0 (0.0%) | 3 (1.1%) |
| Other ischemic heart disease | 161 (10.9%) | 21 (13.2%) | 39 (10.8%) | 31 (11.1%) | 29 (8.9%) | 46 (12.6%) | 4 (10.0%) | 28 (10.7%) |
| Cerebrovascular disease | 50 (3.4%) | 6 (3.8%) | 9 (2.5%) | 7 (2.5%) | 5 (1.5%) | 17 (4.6%) | 1 (2.5%) | 11 (4.2%) |
| Congestive heart failure | 68 (4.6%) | 10 (6.3%) | 18 (5.0%) | 16 (5.7%) | 12 (3.7%) | 18 (4.9%) | 2 (5.0%) | 10 (3.8%) |
| Hyperlipidemia | 404 (27.3%) | 42 (26.4%) | 108 (30.0%) | 81 (29.0%) | 99 (30.3%) | 109 (29.8%) | 9 (22.5%) | 60 (22.9%) |
| Hypertension | 419 (28.3%) | 42 (26.4%) | 110 (30.6%) | 82 (29.4%) | 101 (30.9%) | 109 (29.8%) | 14 (35.0%) | 71 (27.1%) |
| Peripheral vascular disease | 65 (4.4%) | 5 (3.1%) | 21 (5.8%) | 7 (2.5%) | 9 (2.8%) | 19 (5.2%) | 1 (2.5%) | 12 (4.6%) |
| Atherosclerosis | 37 (2.5%) | 2 (1.3%) | 12 (3.3%) | 5 (1.8%) | 6 (1.8%) | 10 (2.7%) | 1 (2.5%) | 9 (3.4%) |
| Sinusitis | 50 (3.4%) | 6 (3.8%) | 15 (4.2%) | 8 (2.9%) | 13 (4.0%) | 10 (2.7%) | 0 (0.0%) | 8 (3.1%) |
| Tuberculosis | 79 (5.3%) | 11 (6.9%) | 16 (4.4%) | 16 (5.7%) | 14 (4.3%) | 23 (6.3%) | 2 (5.0%) | 22 (8.4%) |
| Diabetes mellitus, type 2 | 162 (10.9%) | 17 (10.7%) | 49 (13.6%) | 30 (10.8%) | 43 (13.1%) | 46 (12.6%) | 3 (7.5%) | 15 (5.7%) |
| Depression | 103 (7.0%) | 3 (1.9%) | 39 (10.8%) | 19 (6.8%) | 17 (5.2%) | 27 (7.4%) | 3 (7.5%) | 15 (5.7%) |
| Baseline healthcare use | ||||||||
| Baseline charges (in USD), mean (SD)c | ||||||||
| Total charges | 6031 (6945) | 7087 (6707) | 6615 (7488) | 8022 (7213) | 6771 (6800) | 6826 (6948) | 4276 (4380) | 6876 (6884) |
| Outpatient | 3435 (5127) | 4564 (6070) | 4093 (5545) | 4933 (5928) | 4835 (5855) | 3996 (5593) | 2665 (4023) | 3668 (4744) |
| Inpatientd | 744 (3954) | 326 (1479) | 839 (5096) | 643 (3113) | 578 (3947) | 560 (2863) | 251 (1207) | 1083 (4086) |
| Pharmacy | 1851 (2977) | 2197 (3262) | 1684 (2831) | 2446 (4247) | 1358 (1870) | 2270 (3466) | 1360 (1813) | 2125 (3081) |
Demographics assessed as of the first prescription for an IL-inhibitor. Comorbidities and baseline resource use assessed in the 6 months prior to the index date
IL interleukin, PsO moderate-to-severe psoriasis, SD standard deviation, USD US dollar
aOnly birth year and month are available in the data. Thus, all patients were assigned the day of birth as the 15th of the month for the purpose of calculating age
bPatients considered treatment experienced if they have a drug claim between first PsO diagnosis and index date
cDollar values were estimated on the basis of the assumed exchange rate of 1 yen = 0.01 dollars
dInpatient charges include charges associated with both DPC (per diem) and inpatient (fee-for-service) claims
Treatment Patterns
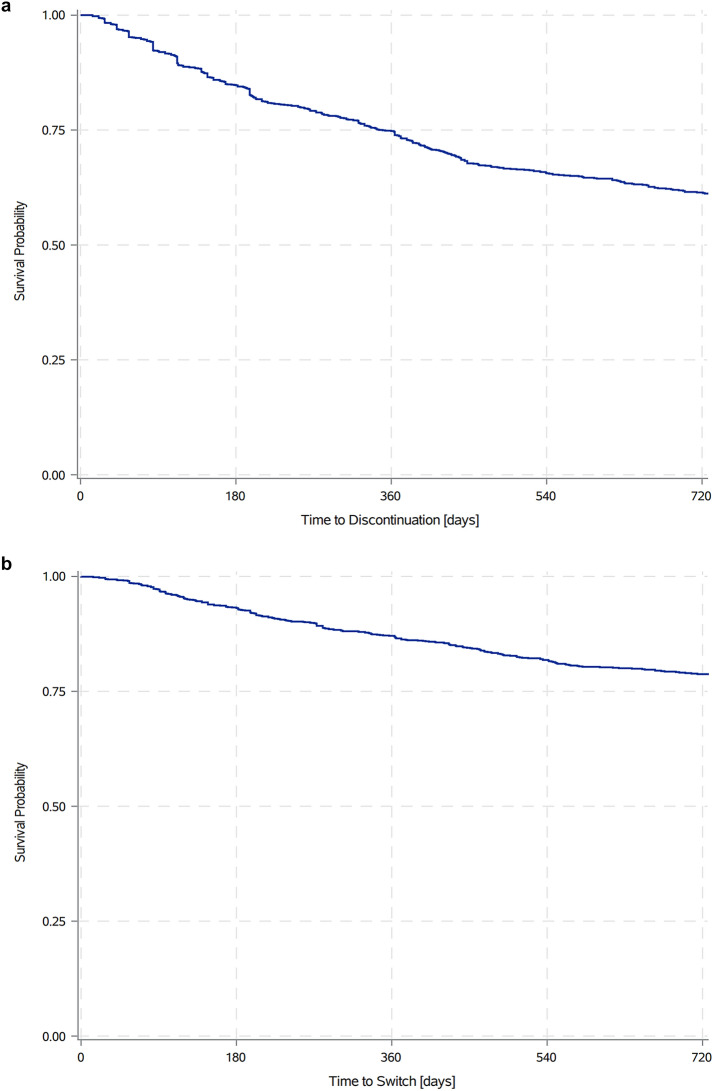
At 12 months following treatment initiation, 25.9% of patients overall had discontinued their index IL-inhibitor; by 24 months following treatment initiation, 38.6% of patients overall had discontinued their index IL-inhibitor (Fig. 2a). At 12 months following treatment initiation, 13.5% of patients overall (and nearly half of discontinuers) had switched to another biologic; by 24 months following treatment initiation, 21.2% of patients overall (and again nearly half of discontinuers) had switched to another biologic (Fig. 2b).
Fig. 2.
a PsO treatment discontinuation rate over time. Kaplan–Meier survival analysis of time to treatment discontinuation following IL-inhibitor initiation. b PsO treatment switch rate over time. Kaplan–Meier survival analysis of time to treatment switch following IL-inhibitor initiation. IL interleukin, PsO moderate-to-severe psoriasis
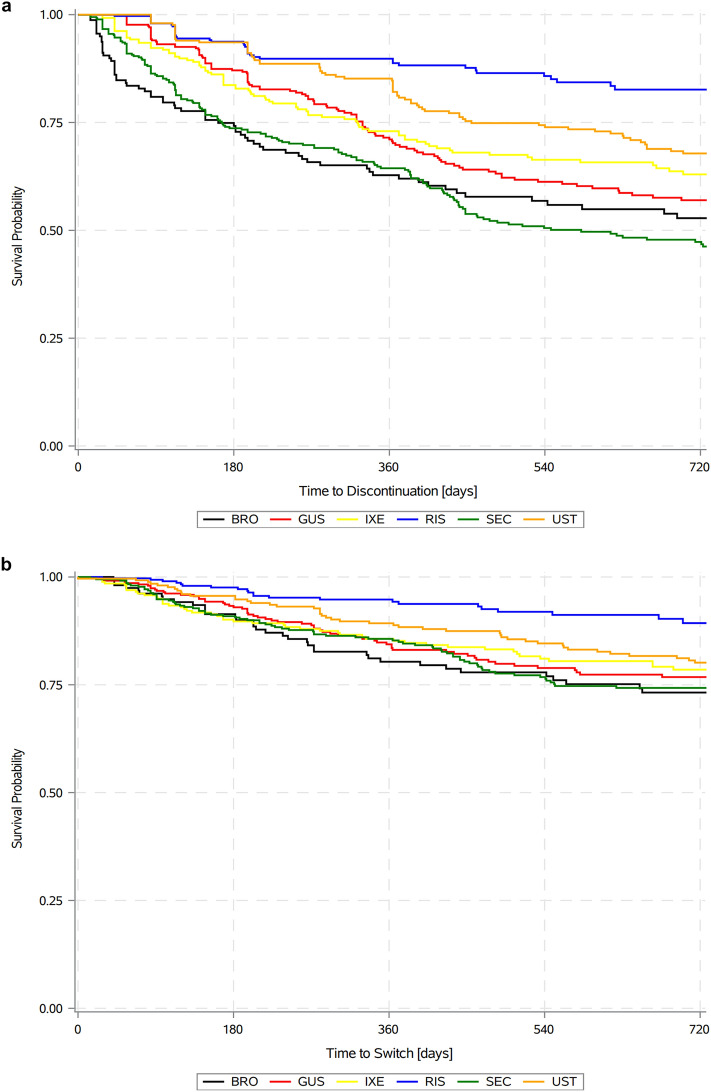
Discontinuation rates at 12 months were lowest for RIS (11.2%), followed by UST (17.9%), IXE (27.0%), GUS (29.8%), SEC (35.6%), and BRO (37.2%); discontinuation rates at 24 months were also lowest for RIS (17.4%), followed by UST (32.2%), IXE (37.0%), GUS (43.0%), BRO (47.2%), and SEC (53.8%; Fig. 3a). Switching rates at 12 months were lowest for RIS (5.7%), followed by UST (11.2%), SEC (14.7%), IXE (14.8%), GUS (16.9%), and BRO (19.7%); switching rates at 24 months were also lowest for RIS (10.7%), followed by UST (19.9%), SEC (25.7%), IXE (21.5%), GUS (23.2%), and BRO (26.8%; Fig. 3b). Among patients who switched following initiation of an index IL-17 treatment, 28.0%, 28.0%, 9.7%, and 34.4% switched to an IL-17, IL-23, IL-12/23, and TNF inhibitor, respectively, and among patients who switched following initiation of an index IL-23 treatment, 46.3%, 18.5%, 7.4%, and 27.8% switched to an IL-17, IL-23, IL-12/23, and TNF inhibitor, respectively (Supplemental Table S1: Descriptive frequency of treatment switches by treatment).
Fig. 3.
a PsO treatment discontinuation rates over time by drug. Kaplan–Meier survival analysis of time to treatment discontinuation following IL-inhibitor initiation. b PsO treatment switch rate over time by drug. Kaplan–Meier survival analysis of time to treatment switch following IL-inhibitor initiation. BRO brodalumab, GUS guselkumab, IXE ixekizumab, RIS risankizumab, SEC secukinumab, UST ustekinumab, IL interleukin, PsO moderate-to-severe psoriasis
Relative trends in discontinuation rates across products were consistent in all sensitivities on permissible gap length (Supplemental Table S2: Sensitivities on permissible gap used to define discontinuation). Defining discontinuation on the basis of 60-, 180-, and 365-day gaps in treatments, RIS continued to have the lowest rates followed by UST, IXE, and GUS, with BRO continuing to have the highest rate using 60-day gaps and SEC having the highest rate using 180- and 365-day gaps.
In adjusted analyses, HRs [CIs] of discontinuation relative to RIS were 2.07 [1.42–3.01] for UST, 2.59 [1.80–3.75] for IXE, 2.70 [1.90–3.86] for GUS, 3.65 [2.47–5.38] for BRO, and 3.69 [2.61–5.23] for SEC (all P ≤ 0.001). HRs [CIs] of switching relative to RIS were 2.05 [1.26–3.33] for IXE, 2.45 [1.54–3.91] for GUS, 2.67 [1.68–4.24] for SEC, 2.73 [1.70–4.40] for UST, and 2.77 [1.67–4.61] for BRO (all P ≤ 0.01; Table 2). For the overall sample, female, asthma, cancers, and baseline pharmacy spending statistically significantly increased the odds of discontinuation (Supplemental Table S3: Covariate estimates from adjusted models).
Table 2.
Adjusted risk of switching/discontinuation by IL-inhibitor
| Drug (reference: risankizumab) | Discontinuation | Switching | ||||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | |||
| Brodalumab | 3.65 | 2.47 | 5.38 | < 0.0001 | 2.77 | 1.67 | 4.61 | < 0.0001 |
| Guselkumab | 2.70 | 1.90 | 3.86 | < 0.0001 | 2.45 | 1.54 | 3.91 | 0.0002 |
| Ixekizumab | 2.59 | 1.80 | 3.75 | < 0.0001 | 2.05 | 1.26 | 3.33 | 0.004 |
| Secukinumab | 3.69 | 2.61 | 5.23 | < 0.0001 | 2.67 | 1.68 | 4.24 | < 0.0001 |
| Ustekinumab | 2.07 | 1.42 | 3.01 | 0.0001 | 2.73 | 1.70 | 4.40 | < 0.0001 |
Values based on Cox multivariable regression model adjusting for demographics, comorbidities, and healthcare resource use
CI confidence interval, IL interleukin
Results were nearly identical in subgroup analyses based on more restrictive diagnosis criteria, as over 90% of the original sample qualified (Supplemental Table S4: Population sensitivity requiring PsO diagnosis codes L40.0 or L40.9).
Discussion
This study provides recent evidence on IL-inhibitor treatment outcomes for patients with PsO in Japan. In the first 24 months of treatment, discontinuation and switching were commonly identified. Rates of each event varied substantially across products but were lowest for RIS regardless of the time point examined and with or without adjustment for patient characteristics and treatment history.
The discontinuation and switching results are generally consistent with prior studies of biologics for PsO in Japan, while the current study employed a different definition of discontinuation, considering different dosing intervals for each biologic. Two earlier studies utilizing the JMDC database found that persistence was highest for IL-inhibitors and for UST in particular (data were not available on RIS in those studies) [6, 12]. One reported a 12-month UST persistence rate of approximately 70% on the basis of a 60-day permissible gap, which is nearly identical to the sensitivity estimate of the current study using the same definition [6]. The other estimated a 12-month persistence of greater than 80% for UST, which dropped to 74% at 24 months using a 150-day permissible gap, slightly above the 68% found in the current study on the basis of 150% of the days of supply (roughly equivalent to a 126-day permissible gap) [12]. Similarly, reviews of clinical records from two Japanese hospitals reported highest treatment persistence with UST relative to other biologics available at the time [16, 17]. Though RIS was not included in these prior studies, the finding of the current analysis that the 12-month switch rate for RIS was 50–70% lower than all other IL-inhibitors is notable, because switching was associated with increased total healthcare costs [6]. At an MOA level, the high rate of TNF-inhibitor switching is consistent with a recent Japanese registry study that noted longer observation periods and more common use of TNF inhibitors in patients with psoriatic arthritis may be influencing this trend [18]. Results presented here build on prior Japanese studies by leveraging larger sample sizes and including more recently approved therapies.
The product-specific persistence rates are also consistent with research outside of Japan showing that IL-inhibitors had longer persistency and lower switching than other MOAs and that IL-23s (and RIS and UST specifically with longer dosing intervals) demonstrated the highest persistency overall [13, 19–22]. Additionally, a recent analysis found lower switch rates with IL-inhibitors to be correlated with reduced all-cause costs [13]. The findings of the current study confirm similar treatment patterns are seen in Japan and provide novel evidence on variation across IL-inhibitor products.
As changes in treatment are associated with increased healthcare resource use, improved persistence found for IL-inhibitors with longer dosing intervals thus has the potential to result in lower spending [6, 12, 13]. Reasons for treatment discontinuation were not observable in the data used for this study; however, the superior persistence seen for RIS is consistent with prior evidence that longer dosing intervals and improved safety and efficacy are associated with reduced discontinuation [11, 23, 24]. Potentially of note, a registry study in Japan found loss of efficacy to be the primary cause of discontinuation of PsO treatment, whereas RIS has shown increasing efficacy in long-term evaluation [18, 25]. A systematic review with meta-analysis of over 50 studies on adherence to biologics in PsO found loss of efficacy and ineffectiveness to be the most common causes of treatment discontinuation [26]. Similarly, structured interviews of PsO patients discontinuing therapy reported insufficient response and loss of response over time as the most common reasons for the biologic therapies included [27]. Retrospective chart reviews, including one in Japan, also found ineffective disease control to be the primary reason for switching of biologics in PsO [28, 29]. Beyond efficacy, adverse events, product attributes (e.g., access and delivery), dosing schedule and location, and medication cost have been associated with treatment persistence and satisfaction [30–34]. Although changes in treatment, including discontinuation, could indicate insufficient disease control, additional research should examine other measures of treatment effectiveness for IL-inhibitors, drivers of switching and discontinuation, and the potential of better persistence to improve clinical and cost outcomes.
This study includes the use of data drawn from a recent, large-scale sample from Japan, with detailed information on demographics, plan enrollment, and pharmacy and medical utilizations. In addition, adjusted models were used to estimate outcomes by IL-inhibitor controlling for observable patient characteristics up to 24 months following treatment initiation. Several limitations of this study should be noted. First, the claims database does not have a specific record of drug discontinuation or switching, or report reasons for treatment changes. Therefore, assessment of dosing patterns is dependent on claims data, which may not accurately reflect actual consumption of the drug, and intended duration of use (i.e., dosing interval or days of supply) was not reported in the database. However, on the basis of sensitivity analyses, consistent treatment patterns were observed regardless of the definition of discontinuation. The pattern did not change even when analyzed with a 365-day permissible gap length. Second, clinical factors such as PsO disease severity and social factors including income were not observable in the dataset; therefore, those characteristics that may impact the outcomes examined were not considered. In addition, physicians may adhere to different treatment guidelines, which are not reported in the data but may affect the observed switching and discontinuation patterns. Third, the data primarily included employed individuals and their dependents for about 8% of the total Japanese population and thus results might not be readily generalizable to the entire population of Japan.
Although the current study was conducted using a recent dataset, newer treatment options such as TIL and bimekizumab were not included due to unavailability of sufficient data at the time of this study to evaluate them. Additionally, this study was limited to IL-inhibitors due to their recency and superior efficacy relative to other MOAs in reducing BSA in clinical trials, and comparison with other treatment options, such as TNF inhibitors, was not conducted. Future research is expected to address this issue and provide the latest evidence to the clinical practices to support treatment decisions for patients and clinicians.
Conclusions
In the first 24 months of treatment with IL-inhibitors for PsO, discontinuation and switching were commonly observed. Rates of switching and discontinuation varied substantially across treatments but were lowest for RIS regardless of the time point examined and with adjustment for patient characteristics. Across IL-inhibitors, rates of switching and discontinuation increased approximately 50% when extending the follow-up period from 12 to 24 months; as evidence continues to accumulate longer-term trends should be examined to provide further insight on switching and discontinuation patterns over several years. As prior studies have found discontinuation or changes in treatment to commonly be caused by insufficient disease control, additional research should examine other measures of treatment effectiveness for IL-inhibitors and drivers of switching and discontinuation, as well as consequences of needing a change in therapy for PsO, to inform treatment decisions.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Medical Writing and Editorial Assistance
Medical writing support was provided by Lufei Tu of Medicus Economics, LLC, and funded by AbbVie Inc.
Author Contributions
Concept and planning of the work described: Ahmed M. Soliman, Luis Puig, Matthew Davis, Dominic Nunag, Andreas Pinter. Acquisition of the data: Ahmed M. Soliman, Matthew Davis, Dominic Nunag. Analysis and interpretation of the data: Yayoi Tada, Ahmed M. Soliman, Kanako Ishii, Ryuta Sakuma, Luis Puig, Matthew Davis, Dominic Nunag, Andreas Pinter and Shinichi Imafuku. Drafting and/or critical revision of the manuscript: Yayoi Tada, Kanako Ishii, Ryuta Sakuma, Ahmed M. Soliman, Luis Puig, Matthew Davis, Dominic Nunag, Andreas Pinter and Shinichi Imafuku.
Funding
Design, study conduct, and financial support for the study, including the Rapid Service Fee for publication, were provided by AbbVie Inc. AbbVie participated in the interpretation of data, review, and approval of the manuscript; all authors contributed to the development of the publication and maintained control over the final content.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to the license agreement with JMDC and cannot be shared with external researchers.
Declarations
Conflict of Interest
Yayoi Tada: Reports grants, personal fees, and nonfinancial support from Kyowa Kirin, Eli Lilly Japan, AbbVie, Maruho, Celgene, Taiho Pharmaceutical, Mitsubishi Tanabe Pharma, Novartis Pharma, Sanofi, UCB Japan, Torii Pharmaceutical, LEO Pharma, Eisai, Kaken Pharmaceutical, Pfizer, Ushio, Meiji Seika Pharma, Nippon Boehringer Ingelheim, JIMRO, Bristol Myers Squibb, Sun Pharma, and TOKIWA Pharmaceutical outside the submitted work; grants and nonfinancial support from Kanebo Cosmetics, MSD, Ono Pharmaceutical, Pola Pharma, Nihon Pharmaceutical, Smith & Nephew, and Sato Pharmaceutical outside the submitted work; personal fees and nonfinancial support from Janssen Pharmaceuticals outside the submitted work; grants from Japan Blood Products Organization, Mochida Healthcare, Oshimatsubaki, and Shionogi outside the submitted work; and personal fees from Chugai Pharmaceutical outside the submitted work. Ahmed M. Soliman, Kanako Ishii, and Ryuta Sakuma: Employees of AbbVie and may own AbbVie stock. Luis Puig: Has received honoraria for serving on advisory boards and speakers’ bureaus or for consulting, and institutional grants for investigator activities sponsored by AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Janssen, Leo-Pharma, Lilly, Novartis, Pfizer, Sandoz, Sanofi, and UCB. Matthew Davis and Dominic Nunag: Employees of Medicus Economics, LLC, which received funding from AbbVie to participate in this research. Andreas Pinter: Received honoraria as an investigator and/or received speakers' honoraria and/or received grants and/or has been an advisor for the following companies: AbbVie, Almirall, Amgen, Biogen Idec, Boehringer Ingelheim, Celgene, Celltrion, GSK, Eli Lilly, Galderma, Hexal, Janssen, LEO Pharma, MC2, Medac, Merck Serono, Mitsubishi, MSD, Novartis, Pascoe, Pfizer, Tigercat Pharma, Regeneron, Roche, Sandoz Biopharmaceuticals, Sanofi-Genzyme, Schering-Plough und UCB Pharma. Shinichi Imafuku: Reports grants and personal fees from AbbVie, Eisai, Kaken Pharmaceutical, Kyowa Kirin, Sato Pharmaceutical, Sanofi, Taiho Pharmaceutical, Mitsubishi Tanabe Pharma, Tsumura, Torii Pharmaceutical, Nippon Zoki Pharmaceutical, Novartis Pharma, Maruho, and LEO Pharma outside the submitted work; grants from Pola Pharma outside the submitted work; and personal fees from Astellas, Eli Lilly Japan, MSD, Otsuka Pharmaceutical, Ono Pharmaceutical, Sun Pharma, GSK, JIMRO, Celgene, Daiichi Sankyo, Sumitomo Dainippon Pharma, Takeda Pharmaceutical, Japan Blood Products Organization, Pfizer, Bristol Myers Squibb, Meiji Seika Pharma, Janssen Pharmaceutical, and UCB Japan outside the submitted work.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors. Database permission was obtained via a license from the provider; however, the database is otherwise not publicly available. Because deidentification was conducted before providing claims to researchers, and no identifiable protected health information was included in the data used, Institutional Review Board approval was not required. All analyses were conducted in compliance with RECORD-PE guidance and in accordance with the ethical standards in the 1964 Declaration of Helsinki and its subsequent amendments [15].
Footnotes
Prior Presentation: A similar analysis based on earlier data was presented at EADV 2022: Puig L, Soliman AM, Davis M, Nunag D, Pinter A. “Real-World Switching and Discontinuation Patterns for Interleukin-Inhibitor Treatments in Patients with Moderate to Severe Psoriasis in Japan.” 31st Congress of the European Academy of Dermatology and Venerology (EADV 2022); Sep 7–10, 2022; Milan, Italy.
References
- 1.Darjani A, Heidarzadeh A, Golchai J, et al. Quality of life in psoriatic patients: a study using the short form-36. Int J Prev Med. 2014;5(9):1146–1152. [PMC free article] [PubMed] [Google Scholar]
- 2.Kubota K, Kamijima Y, Sato T, et al. Epidemiology of psoriasis and palmoplantar pustulosis: a nationwide study using the Japanese national claims database. BMJ Open. 2015;5(1):e006450. doi: 10.1136/bmjopen-2014-006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goto H, Nakatani E, Yagi H, Moriki M, Sano Y, Miyachi Y. Late-onset development of psoriasis in Japan: a population-based cohort study. JAAD Int. 2020;2:51–61. doi: 10.1016/j.jdin.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okubo Y, Kotowsky N, Gao R, Saito K, Morita A. Clinical characteristics and health-care resource utilization in patients with generalized pustular psoriasis using real-world evidence from the Japanese Medical Data Center database. J Dermatol. 2021;48(11):1675–1687. doi: 10.1111/1346-8138.16084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saeki H, Mabuchi T, Asahina A, et al. English version of Japanese guidance for use of biologics for psoriasis (the 2022 version) J Dermatol. 2023;50(2):e41–e68. doi: 10.1111/1346-8138.16691. [DOI] [PubMed] [Google Scholar]
- 6.Tada Y, Kim H, Spanopoulos D, et al. Treatment patterns, healthcare resource utilization, and costs in patients with moderate-to-severe psoriasis treated with systemic therapy in Japan: A retrospective claims database study. J Dermatol. 2022;49(11):1106–1117. doi: 10.1111/1346-8138.16543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iizuka H. Pyramid plan for psoriasis treatment 2017. J Vis Dermatol. 2017;16:850–851. [Google Scholar]
- 8.Yang K, Oak ASW, Elewski BE. Use of IL-23 Inhibitors for the Treatment of Plaque Psoriasis and Psoriatic Arthritis: A Comprehensive Review. Am J Clin Dermatol. 2021;22(2):173–192. doi: 10.1007/s40257-020-00578-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ten Bergen LL, Petrovic A, Krogh Aarebrot A, Appel S. The TNF/IL-23/IL-17 axis-Head-to-head trials comparing different biologics in psoriasis treatment. Scand J Immunol. 2020;92(4):e12946. doi: 10.1111/sji.12946. [DOI] [PubMed] [Google Scholar]
- 10.No DJ, Amin M, Bhutani T, Wu JJ. A systematic review of active comparator controlled clinical trials in patients with moderate-to-severe psoriasis. J Dermatolog Treat. 2018;29(5):467–474. doi: 10.1080/09546634.2017.1402116. [DOI] [PubMed] [Google Scholar]
- 11.Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2021;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sruamsiri R, Iwasaki K, Tang W, Mahlich J. Persistence rates and medical costs of biological therapies for psoriasis treatment in Japan: a real-world data study using a claims database. BMC Dermatol. 2018;18(1):5. doi: 10.1186/s12895-018-0074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu JJ, Wang CA, Jobson G, et al. Treatment patterns and healthcare costs among patients with psoriasis initiating apremilast or biologics: a retrospective claims database cohort analysis. J Dermatol Treat. 2023;34(1):2177095. doi: 10.1080/09546634.2023.2177095. [DOI] [PubMed] [Google Scholar]
- 14.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 15.Langan SM, Schmidt SA, Wing K, Ehrenstein V, Nicholls SG, Filion KB, et al. The reporting of studies conducted using observational routinely collected health data statement for pharmacoepidemiology (RECORD-PE) BMJ. 2018;363:3532. doi: 10.1136/bmj.k3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kishimoto M, Komine M, Kamiya K, Sugai J, Mieno M, Ohtsuki M. Drug survival of biologic agents for psoriatic patients in a real-world setting in Japan. J Dermatol. 2020;47(1):33–40. doi: 10.1111/1346-8138.15146. [DOI] [PubMed] [Google Scholar]
- 17.Bayaraa B, Imafuku S. Sustainability and switching of biologics for psoriasis and psoriatic arthritis at Fukuoka University Psoriasis Registry. J Dermatol. 2019;46(5):389–398. doi: 10.1111/1346-8138.14834. [DOI] [PubMed] [Google Scholar]
- 18.Yanase T, Tsuruta N, Yamaguchi K, et al. Survival rates of systemic interventions for psoriasis in the Western Japan Psoriasis Registry: a multicenter retrospective study. J Dermatol. 2023 doi: 10.1111/1346-8138.16737. [DOI] [PubMed] [Google Scholar]
- 19.Torres T, Puig L, Vender R, et al. Drug survival of interleukin (IL)-17 and IL-23 inhibitors for the treatment of psoriasis: a retrospective multi-country, multicentric cohort study. Am J Clin Dermatol. 2022;23(6):891–904. doi: 10.1007/s40257-022-00722-y. [DOI] [PubMed] [Google Scholar]
- 20.Schmitt-Egenolf M, Freilich J, Stelmaszuk-Zadykowicz NM, Apol E, Hansen JB, Levin LÅ. Drug persistence of biologic treatments in psoriasis: a Swedish national population study. Dermatol Ther (Heidelb) 2021;11(6):2107–2121. doi: 10.1007/s13555-021-00616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pina Vegas L, Penso L, Claudepierre P, Sbidian E. Long-term Persistence of First-line Biologics for Patients With Psoriasis and Psoriatic Arthritis in the French Health Insurance Database. JAMA Dermatol. 2022;158(5):513–522. doi: 10.1001/jamadermatol.2022.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldman SR, Zhang J, Martinez DJ, et al. Real-world biologic and apremilast treatment patterns and healthcare costs in moderate-to-severe plaque psoriasis. Dermatol Online J. 2021;27(1):13030/qt03t0s9j6. [PubMed] [Google Scholar]
- 23.Ruggiero A, Megna M, Fabbrocini G, Ocampo-Garza SS. Anti-IL23 biologic therapies in the treatment of psoriasis: real-world experience versus clinical trials data. Immunol Res. 2023;4:1–28. doi: 10.1007/s12026-022-09356-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armstrong AW, Soliman AM, Betts KA, et al. Long-term benefit-risk profiles of treatments for moderate-to-severe plaque psoriasis: a network meta-analysis. Dermatol Ther (Heidelb) 2022;12(1):167–184. doi: 10.1007/s13555-021-00647-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruggiero A, Picone V, Martora F, Fabbrocini G, Megna M. Guselkumab, risankizumab, and tildrakizumab in the management of psoriasis: a review of the real-world evidence. Clin Cosmet Investig Dermatol. 2022;15:1649–1658. doi: 10.2147/CCID.S364640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piragine E, Petri D, Martelli A, Janowska A, Dini V, Romanelli M, Calderone V, Lucenteforte E. Adherence and persistence to biological drugs for psoriasis: systematic review with meta-analysis. J Clin Med. 2022;11(6):1506. doi: 10.3390/jcm11061506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeung H, Wan J, Van Voorhees AS, Callis Duffin K, Krueger GG, Kalb RE, Weisman JD, Sperber BR, Brod BA, Schleicher SM, Bebo BF, Jr, Shin DB, Troxel AB, Gelfand JM. Patient-reported reasons for the discontinuation of commonly used treatments for moderate to severe psoriasis. J Am Acad Dermatol. 2013;68(1):64–72. doi: 10.1016/j.jaad.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honda H, Umezawa Y, Kikuchi S, Yanaba K, Fukuchi O, Ito T, Nobeyama Y, Asahina A, Nakagawa H. Switching of biologics in psoriasis: reasons and results. J Dermatol. 2017;44(9):1015–1019. doi: 10.1111/1346-8138.13860. [DOI] [PubMed] [Google Scholar]
- 29.Smith JA, Wehausen B, Richardson I, Zhao Y, Li Y, Herrera V, Feldman SR. Treatment changes in patients with moderate to severe psoriasis: a retrospective chart review. J Cutan Med Surg. 2018;22(1):25–30. doi: 10.1177/1203475417724438. [DOI] [PubMed] [Google Scholar]
- 30.Florek AG, Wang CJ, Armstrong AW. Treatment preferences and treatment satisfaction among psoriasis patients: a systematic review. Arch Dermatol Res. 2018;310(4):271–319. doi: 10.1007/s00403-018-1808-x. [DOI] [PubMed] [Google Scholar]
- 31.Kromer C, Schaarschmidt ML, Schmieder A, Herr R, Goerdt S, Peitsch WK. Patient preferences for treatment of psoriasis with biologicals: a discrete choice experiment. PLoS ONE. 2015;10(6):e0129120. doi: 10.1371/journal.pone.0129120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kromer C, Peitsch WK, Herr R, Schmieder A, Sonntag D, Schaarschmidt ML. Treatment preferences for biologicals in psoriasis: experienced patients appreciate sustainability. J Dtsch Dermatol Ges. 2017;15(2):189–200. doi: 10.1111/ddg.12919. [DOI] [PubMed] [Google Scholar]
- 33.Schaarschmidt ML, Umar N, Schmieder A, Terris DD, Goebeler M, Goerdt S, Peitsch WK. Patient preferences for psoriasis treatments: impact of treatment experience. J Eur Acad Dermatol Venereol. 2013;27(2):187–198. doi: 10.1111/j.1468-3083.2011.04440.x. [DOI] [PubMed] [Google Scholar]
- 34.Bolt T, Kobayashi H, Mahlich J. Patient and physician preferences for therapy characteristics for psoriasis: a discrete choice experiment in Japan. Pharmacoecon Open. 2019;3(2):255–264. doi: 10.1007/s41669-018-0104-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to the license agreement with JMDC and cannot be shared with external researchers.