Abstract
Mild cognitive impairment (MCI) has been frequently interpreted as a transitional phase between healthy cognitive aging and dementia, particularly of the Alzheimer's disease (AD) type. Of note, few studies explored that transition from a multifactorial perspective, taking into consideration the effect of basic factors such as biological sex. In the present study 96 subjects with MCI (37 males and 59 females) were followed-up and divided into two subgroups according to their clinical outcome: “progressive” MCI (pMCI = 41), if they fulfilled the diagnostic criteria for AD at the end of follow-up; and “stable” MCI (sMCI = 55), if they remained with the initial diagnosis. Different markers were combined to characterize sex differences between groups, including magnetoencephalography recordings, cognitive performance, and brain volumes derived from magnetic resonance imaging. Results indicated that the pMCI group exhibited higher low-frequency activity, lower scores in neuropsychological tests and reduced brain volumes than the sMCI group, being these measures significantly correlated. When sex was considered, results revealed that this pattern was mainly due to the influence of the females’ sample. Overall, females exhibited lower cognitive scores and reduced brain volumes. More interestingly, females in the pMCI group showed an increased theta activity that correlated with a more abrupt reduction of cognitive and volumetric scores as compared with females in the sMCI group and with males in the pMCI group. These findings suggest that females’ brains might be more vulnerable to the effects of AD pathology, since regardless of age, they showed signs of more pronounced deterioration than males.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-023-01020-z.
Keywords: Mild cognitive impairment, Alzheimer’s disease, Sex differences, Cognition, Brain volumes, Neurophysiological activity
Introduction
Numerous studies that investigate the role of markers to estimate the risk of suffering from a variety of neurological or psychiatric diseases tend to control, rather than to assess, the effect of some basic biological factors such as age or sex. These factors are intentionally balanced along the samples in order to avoid a confounding effect on the results. However, during the last decades more and more data suggested that factors such as biological sex seem to play a crucial role in the vulnerability to some pathologies. For instance, major depression, anorexia nervosa or multiple sclerosis are more prevalent in females; while autism spectrum disorders, dyslexia, schizophrenia, attention deficit/hyperactivity disorder or vascular and Lewy-Body dementias are more prevalent in males [1–7]. Importantly, such discrepancies in the vulnerability to some particular disorders have been associated with a so-called “sexual dimorphism” that, within this background, has been described as “the divergent changes in brain structures that men and women have in response to disease or disease-causing insults” [8]. Moreover, the divergent pattern of changes might derive from constitutive sex differences not only in brain structure, but also at neurochemical and functional levels [9].
A controversial example of this sex-dependent vulnerability is well represented by the Alzheimer’s disease (AD) spectrum [10]. Some epidemiological studies reported a higher prevalence of AD among women, indicating that females might comprise nearly two-thirds of all cases [11]. Usually, this evidence has been interpreted according to two non-incompatible arguments. First, age is the most important risk factor for the development of AD, and it is clearly established that women have a longer life-expectancy. As a consequence, and considering that the incidence of AD doubles every 5 years after the sixth decade of life, more women will be at higher risk of developing the disease and the prevalence will be also necessarily higher [12, 13]. Second, even accepting the plausibility of this argument, when samples of men and women are balanced according to age, the incidence of AD among females is still higher, indicating that some additional factors might be exerting an influence [14]. This argument is based on the previously mentioned idea of a sexual dimorphism. For instance, women seem to be more affected by the ɛ4 allele for the apolipoprotein E (APOE) gene, increasing the risk of conversion to AD and the overall cognitive dysfunction [15, 16]. Women also seem to display more rapid rates of brain atrophy than men [17, 18]. In fact, Barnes et al. [19] demonstrated a clear sex difference in the association between AD pathology and the risk of developing the disease. Authors reported that each additional unit of AD pathology (in terms of amyloid deposits and tau levels) was associated with a threefold increase of the risk of dementia in men, compared with a 22-fold increase in women.
All these evidences highlight the important role of sex differences in the investigation of the AD-spectrum. Particularly, the utilization of markers sensitive to sex-related variations is crucial to attain the goal of a “precision medicine” [20]. In this vein, neurophysiological techniques such as the electroencephalography (EEG) or magnetoencephalography (MEG) have been broadly utilized in the field of AD [21–28] but the effect of sex has been seldom investigated. It is well-recognized that aging exerts an influence on brain’s oscillatory activity, and some EEG investigations indicated that aged females exhibit higher theta power than males [29, 30]. When this research strategy was applied to the AD spectrum, results revealed that the typical increase of delta and theta activity in demented patients was mainly due to the influence of females’ sample [31]. This is a key point that should be carefully considered, as lower frequencies are associated with disease progression, hippocampal atrophy, and poorer cognitive performance [32].
Of note, this line of investigation has been rarely explored until the publication of three very recent studies. Babiloni and coworkers [33] presented results that contradicted Günther et al.’s [31] findings in samples of healthy controls and mild cognitive impairment (MCI) cases. Bruña and coworkers [34] performed a functional connectivity (FC) MEG study in a sample of healthy aged subjects. They reported a pattern of augmented anteroposterior FC in females that has been previously identified as a biomarker for increased risk of developing cognitive impairment. Finally, Chino-Vilca and coworkers [35] failed to find significant differences between sexes in terms of power values in a sample of MCI subjects. Considering this background of contradictory results, our main purpose in the present study was to characterize sex differences in the functional resting state patterns of subjects with MCI who remained stable or progressed to AD. Importantly, given the relevance of including different markers in longitudinal studies, we combined neurophysiological (MEG), cognitive (neuropsychological tests) and volumetric (magnetic resonance imaging, MRI) measures. According to previous literature, we hypothesize that women who progress to AD will show a more pronounced slowing in their MEG activity, along with an increased loss of gray matter (i.e., reduced volumes) and a worse cognitive performance when compared with men.
Materials and methods
Subjects
A total of 96 MCI subjects (37 males and 59 females) were recruited from the Hospital Universitario San Carlos and the Centre for the Prevention of Cognitive Impairment (Madrid, Spain). All of them were native Spanish speakers and right-handed [36]. Demographic and clinical data are shown in Table 1. The diagnostic and follow-up protocol has been exhaustively described in previous articles (see for example [26]) and was based on the National Institute on Aging-Alzheimer's Association (NIA-AA) criteria [36, 37]. Besides meeting the clinical criteria, MCI participants had signs of neurodegeneration (hippocampal atrophy as measured by MRI), and therefore according to NIA-AA criteria, they might be considered as “MCI due to AD” with an intermediate likelihood [36]. All participants were cognitively and clinically followed-up every 6 months for approximately five years. According to their clinical outcome during this follow-up period, they were divided into two subgroups: 1) the “progressive” MCI group (pMCI; n = 41), composed of those subjects that met the criteria for probable AD [37]; and 2) the “stable” MCI group (sMCI; n = 55), comprised of those participants that fulfilled the diagnostic criteria of MCI at the end of follow-up. The time to conversion of the pMCI group was set when the diagnosis changed from MCI to AD (mean = 18,268 months; standard deviation (sd) = 9,940), being of 21,411 ± 11,827 months for males and of 16,041 ± 7,877 months for females.
Table 1.
Demographic and clinical data
| Male | Female | |||
|---|---|---|---|---|
| sMCI (n = 20) | pMCI (n = 17) | sMCI (n = 35) | pMCI (n = 24) | |
| Age | 73.950 ± 5.511 | 74.235 ± 4.507 | 74.429 ± 4.546 | 75.750 ± 5.929 |
| Education (years) | 9.316 ± 5,498 | 9.533 ± 5.208 | 7.939 ± 4.415 | 7.095 ± 3.208 |
| MMSE | 27.105 ± 1.853 | 27.267 ± 2.187 | 26.091 ± 2.441 | 24.833 ± 3.294 |
| CDT | 6.167 ± 0.985 | 6.000 ± 1.309 | 5.355 ± 1.762 | 4.850 ± 2.455 |
| FDS | 6.158 ± 1.537 | 6.467 ± 1.642 | 6.484 ± 1,749 | 7.048 ± 2.397 |
| BDS | 4.263 ± 1.046 | 4.267 ± 1.163 | 3.839 ± 1.214 | 3.952 ± 1.284 |
| IR | 18.000 ± 9.672 | 13.133 ± 9.493 | 14.452 ± 6.233 | 9.476 ± 5.706 |
| DR | 7.421 ± 7.798 | 3.571 ± 6.223 | 4.767 ± 5.697 | 1.190 ± 2.040 |
| PF | 7.421 ± 4.185 | 9.507 ± 4.336 | 8.737 ± 4.578 | 8.062 ± 3.890 |
| SF | 11.158 ± 4.133 | 11.667 ± 4.415 | 12.064 ± 2.294 | 11.024 ± 3.223 |
| IP | 7.444 ± 0.922 | 7.133 ± 1.187 | 7.133 ± 1.167 | 7.048 ± 1.161 |
| RSC | 2.056 ± 1.392 | 1.933 ± 1.580 | 2.034 ± 1.180 | 1.550 ± 0.887 |
| BNT | 49.368 ± 9.593 | 47.933 ± 6.112 | 44.107 ± 7.197 | 41.190 ± 10.759 |
| TMT A_T | 73.684 ± 31.223 | 75.333 ± 31.890 | 96.893 ± 37.832 | 97.550 ± 41.607 |
| TMT B_T | 214.765 ± 106.099 | 191.000 ± 119.547 | 245.696 ± 93.177 | 317.938 ± 103.409 |
| Total_GM_V | 0.365073 ± 0.025935 | 0.351853 ± 0.041776 | 0.377602 ± 0.024385 | 0.360181 ± 0.023012 |
| LH_V | 0.002215 ± 0.000422 | 0.001945 ± 0.000432 | 0.002242 ± 0.000432 | 0.001875 ± 0.000272 |
| RH_V | 0.002169 ± 0.000430 | 0.001946 ± 0.000435 | 0.002258 ± 0.000470 | 0.002008 ± 0.000216 |
| LA_V | 0.000793 ± 0.000134 | 0.000735 ± 0.000150 | 0.000776 ± 0.000186 | 0.000658 ± 0.000129 |
| RA_V | 0.000853 ± 0.000157 | 0.000850 ± 0.000206 | 0.000869 ± 0.000203 | 0.000800 ± 0.000112 |
| LE_V | 0.000670 ± 0.000183 | 0.000589 ± 0.000159 | 0.000637 ± 0.000144 | 0.000541 ± 0.000181 |
| RE_V | 0.000559 ± 0.000121 | 0.000519 ± 0.000161 | 0.000536 ± 0.000139 | 0.000485 ± 0.000093 |
sMCI Stable mild cognitive impairment, pMCI Progressive mild cognitive impairment, MMSE Mini mental state examination, CDT Clock drawing test, FDS Forward digit span test, BDS Backward digit span test, IR Immediate recall, DR Delayed recall, PF Phonemic fluency, SF Semantic fluency, IP Ideomotor praxis, RSC Rule shift cards, BNT Boston naming test, TMTA_ T Trail-making test part A (time score), TMTB_T Trail-making test part B (time score), Total_GM_V Total grey matter volume, LH_V Left hippocampal volume, RH_V Right hippocampal volume, LA_V Left amygdala volume, RA_V Right amygdala volume, LE_V Left entorhinal volume, RE_V Right entorhinal volume
None of the participants exhibited a previous history of psychiatric or neurological disorders, other than MCI. According to our standard protocol, the inclusion criteria included a modified Hachinski score ≤ 4; a short-form Geriatric Depression Scale score ≤ 5 [38]; an T1/ T2- weighted MRI within 12 months and 2 weeks before the MEG recordings without indication of infection, infarction, or focal lesions (rated by two independent experienced radiologists) [39]. In addition, participants were advised to avoid those psychoactive medications that could affect MEG activity 48 h before recordings. The Hospital Universitario San Carlos Ethics Committee (Madrid) approved the study, and all participants or their caregivers signed a written informed consent prior to participation.
Neuropsychological assessment
As in previous studies by our investigation group, all participants were screened by means of standardized diagnostic instruments and received a thorough neuropsychological assessment. The screening procedure has been exhaustively described elsewhere and consisted of a set of standardized tests (for further details see [28] and the supplementary material) which included: clock drawing test (CDT; [40]), forward and backward digit span test (FDS and BDS; Wechsler Memory Scale III, WMS-III; [41], immediate and delayed recall (IR and DR; WMS-III; [41]), phonemic and semantic fluency (PF and SF; controlled oral word association test; [42]), ideomotor praxis (IP) of Barcelona test [43], rule shift cards (RSC; behavioural assessment of the dysexecutive syndrome; [44], Boston naming test (BNT; [45]), and trail-making test (TMT time score), parts A (TMTA_T) and B (TMTB_T) [46]. These cognitive tests, accompanied by the Spanish version of the Mini Mental State Examination (MMSE; [47]) and the years of formal education (henceforth denominated as “education”) were submitted to statistical analyses: education, MMSE, CDT, FDS, BDS, IR, DR, PF, SF, IP, RSC, BNT, TMTA_T and TMTB_T.
Genetic analyses
As firstly described in Cuesta et al. [48], to obtain genomic DNA, 10 ml blood samples were extracted in ethylenediaminetetraacetic acid (EDTA) anticoagulant tubes. Secondly, APOE genotyping (i.e., rs7412 and rs429358 SNPs) was performed by using TaqMan assays on an Applied Biosystems 7500 Fast Real Time PCR machine (Applied Biosystems, Foster City, CA). This resulted in three differentiated APOE gene alleles: ɛ2, ɛ3 and ɛ4. In the present study, participants were classified as carriers (i.e., ɛ3ɛ4) and non-carriers (i.e., ɛ3ɛ3) of the ɛ4 allele for the APOE gene. That is, APOE4 + and APOE4-.
MRI and medial temporal lobe volumes
3D T1 weighted anatomical MRI scans were collected with a General Electric 1.5 T MRI scanner, using a high-resolution antenna and a homogenization PURE filter (Fast Spoiled Gradient Echo (FSPGR) sequence with parameters: TR/TE/TI = 11.2/4.2/ 450 ms; flip angle 12°; 1 mm slice thickness, a 256 × 256 matrix and FOV 25 cm).
Freesurfer software (version 5.1.0.21) was employed to obtain the total grey matter and medial temporal lobe volumes, which were normalized with the overall intracranial volume (ICV) to account for differences in head volume over subjects. Seven variables were submitted to statistical analyses: Total grey matter volume (Total_GM_V), left and right hippocampal volumes (LH_V and RH_V), left and right amygdala volumes (LA_V and RA_V), and left and right entorhinal volumes (LE_V and RE_V).
MEG recordings and preprocessing
MEG data were acquired using a whole-head Elekta Neuromag MEG system with 306 channels (Elekta AB, Stockholm, Sweden) at the Center for Biomedical Technology (Madrid, Spain). Data were collected at a sampling frequency of 1000 Hz and online band-pass filtered between 0.1 and 330 Hz. Each data collection consisted of 5-min resting-state conditions with eyes closed. Participants were accommodated inside the magnetically shielded room where the MEG device is placed. Each subject’s head shape was defined relative to three anatomical locations (nasion and bilateral preauricular points) using a 3D digitizer (Fastrak, Polhemus, VT, USA) and head motion was tracked through four head position indicator (HPI) coils attached to the scalp. These HPI coils continuously monitored the subjects’ head movements, while eye movements were monitored by a vertical electrooculogram (EOG) assembly composed of a pair of bipolar electrodes. As it is established in our standard protocol, the instructions for participants during MEG recording were to be as quiet, still and relaxed as they were able to and to not move the head outside the MEG’s helmet.
The pre-processing procedure started with the application of Maxfilter software (v 2.2, correlation threshold = 0.9, time window = 10 s) to remove external noise using the temporal extension of the signal-space separation method with movement compensation [49]. Then, magnetometers data [50] were automatically examined to detect ocular, muscle, and jump artifacts using Fieldtrip software [51]. These artefacts were visually confirmed by an MEG expert. The remaining artifact-free data was segmented into 4 s epochs. Independent component analysis-based procedure (ICA) was applied to remove heart magnetic field artifacts and electrooculogram components. Only those recordings with at least 20 clean epochs (80 s of brain activity) were utilized in subsequent analyses.
MEG processed time series were band-pass filtered (2 s padding) between 2 and 30 Hz. Source reconstruction was carried out using a regular grid of 1 cm spacing in the Montreal Neurological Institute (MNI) template. The resulting model comprised 2459 sources homogeneously distributed across the brain. This model was linearly transformed to each subject’s space and the leadfield was calculated using a single shell model [52]. Sources time series were reconstructed using a Linearly Constrained Minimum Variance beamformer [53]. The power spectrum of each grid’s node was computed by means of a multitaper method with discrete prolate spheroidal sequences as windowing function and 1 Hz smoothing. For each node, relative power was calculated by normalizing each frequency step by total power over the 2 to 30 Hz range. The grid nodes were anatomically labelled using the automated anatomical labelling atlas (AAL; [54]). Out of the original 2459 nodes, 1202 were included in the analysis by taking those belonging to any region of the AAL (excepting the cerebellum, basal ganglia, thalamus, and olfactory cortices). Epochs were averaged across subjects ending up with a source-reconstructed power matrix of 1202 nodes × 113 frequency steps × 96 participants.
Statistical analyses
As previously advanced in the introductory section, the main aim of this study was to explore the potential sex differences, in terms of resting-state MEG activity, between pMCI and sMCI cases. Notwithstanding, we first evaluated the neuropsychological and volumetric MRI variables with a twofold procedure: 1) we assessed if some basic characteristics such as age, education, time to conversion, and APOE genotype were homogeneously distributed along the sample, particularly when Sex and Group (pMCI vs. sMCI) variables were considered. These analyses were performed by means of two-sample t-test and the Levene’s test to assess the homogeneity of variance, and chi-square test; 2) we assessed the existence of between-groups differences with a series of ANCOVA tests with Group and Sex as fixed factors. The analyses of potential interaction effects and post-hoc mean-group comparisons were accomplished by using Bonferroni method.
The assessment of significant group differences in terms of MEG-power values was based on a cluster-based permutation test (CBPT) [55] that has been previously described (see [56]). According to our analysis protocol, power differences between the two MCI groups (pMCI vs. sMCI) were tested for each pair of nodes using an ANCOVA test. The resulting matrix of F-statistics was binarized by means of applying a critical value computed with a p-value of 0.005 (cluster-defining threshold). Subsequently, the thresholded map was split into two maps corresponding to the voxels with power differences that indicated if sMCI > pMCI or sMCI < pMCI. For each map, a clustering procedure identified groups of adjacent nodes in the volume space, and the mass of each cluster was defined as the sum of the F-values of the nodes comprising each cluster (cluster statistic). As an inclusion criterion to suppress spurious findings, we required the minimum size for each candidate cluster to be equal to 1% of the total nodes in the volume, and those clusters that were smaller than this size were automatically deemed as non-significant. To control for multiple comparisons, the entire analysis pipeline was repeated 10,000 times after shuffling the original group’s labels. At each repetition, the maximum statistic of the surrogate clusters was kept, creating a maximal null distribution that would ensure control of the family-wise error rate (FWER) at the cluster level. Cluster statistics on each cluster in the original data set was compared using the same measure in the randomized data. The CBPT p-value represented the proportion of the permutation distribution with cluster statistic values that were greater or equal to the cluster statistic value of the original data. Only those clusters that survived the CBPT at p < 0.050 were considered for the subsequent analyses as potential “MEG markers”. As descriptive MEG signatures for each significant cluster, we computed the average power (both across all nodes that belonged to the cluster and all significant frequency steps). These MEG signatures were submitted to further analysis that consisted of new between groups ANCOVAs. For the shake of clarity, symbols of the classical frequency bands (i.e. δ, θ, α and β) were used to denominate the resulting clusters when they fulfilled two conditions: 1) the majority of the frequency interval of the cluster should fall within one of the classical bands (i.e. 1–4 Hz for δ, 4–8 Hz for θ, 8–13 Hz for α, and 13–30 Hz for β); and 2) the peak (maximum size) of the cluster should also fall within the limits of the same frequency band.
At last, Spearman correlation tests were carried out to measure the possible association between the MEG markers, the cognitive performance, and the volumetric structural integrity. The correlation results were corrected for multiple comparisons by means of false discovery rate (FDR). The same analysis pipeline was repeated when sMCI and pMCI groups were compared for each sex independently.
Statistical analyses were carried out using Matlab R2021b (Mathworks Inc) and SPSS Statistics version 27.0.
Results
Exploratory analyses of demographic variables
The exploratory analyses indicated that age was homogeneously distributed along the sample, with no significant differences due to Sex (p = 0.409) or Group (p = 0.411). Notably, the analysis of education values showed a marginal effect (p = 0.073) with females exhibiting less years of education as compared with males, while the Group factor had no effect. Considering the importance of education as a proxy of cognitive reserve (CR) [56], further exploratory analyses by means of Spearman correlations were carried out to detect its influence on neuropsychological and volumetric variables. Education was significantly correlated with all cognitive tests except MMSE and DR (all p-values < 0.050), and with the following volumetric measures: Total_GM_V, LH_V, RH_V, and LA_V (all p-values < 0.050). Consequently, education was considered as a covariate in the analyses of neuropsychological and MRI-volumetric data.
Mirroring age results, APOE was also homogeneously distributed along the sample, with no significant differences due to Sex (p = 0.575) or Group (p = 0.406) in the chi-square tests. Similarly, when neuropsychological and volumetric variables were compared between APOE4 + and APOE4-, no significant differences were detected (all p-values > 0.050).
Finally, the influence of Sex on “time to conversion” was also explored. Similar to education, a marginal effect was observed (p = 0.080), with females exhibiting a more rapid time to conversion than males. In addition, the chi-square test revealed that the percentage of progressive and stable cases was homogeneously distributed across sexes (p = 0.612), see Table 1.
Neuropsychological variables
As previously advanced, a series of ANCOVAs with Sex and Group as fixed factors, and education as covariate, were accomplished to investigate the main and interaction effects of these variables on neuropsychological scores. First, results revealed that for nearly 50% of the neuropsychological tests, the only source of significant variation was due to the effect of education (i.e., the covariate): FDS, BDS, PF, IP and RSC (all p values < 0.050). In fact, partial Eta-squared values indicated a large effect of education on these variables (see Table 2). The remaining tests exhibited more complex patterns. For example, the effect of education was in the limit of the significance level (p = 0.050) for MMSE scores, but additionally Sex exerted a significant effect (p = 0.009) with males showing higher scores. The analyses of interaction effects indicated that, within the pMCI group, male converters exhibited greater scores than female converters (p = 0.013). TMTB_T results almost mirrored MMSE, with education showing a clear significant effect in this case (p = 0.003). After controlling for education effects, Sex evidenced a tendency to lower values in males (p = 0.034), and the interaction analyses showed that male pMCIs exhibited reduced times of execution of the task when compared with female converters, thus indicating a better performance (p = 0.014). Interaction analyses also revealed that female sMCIs showed significantly lower scores than female pMCIs (p = 0.047). Similarly, BNT scores were significantly affected by the effects of education (p = 0.001), and after controlling for these effects, Sex still exerted a significant influence with males exhibiting greater scores than females (p = 0.012). Interaction analysis suggested the same tendency to lower scores in female pMCIs, but the effect failed to reach the significance level (p = 0.080).
Table 2.
Contains p-values and estimates of effect sizes in terms of partial Eta-squared values for all the ANCOVA models
| Variables | ANCOVA factors | p-values | Partial Eta- squared-values |
|---|---|---|---|
| MMSE | Education (covariate) | 0.050 | 0.047 |
| Group | 0.391 | 0.012 | |
| Sex | 0.009* | 0.083 | |
| CDT | Education (covariate) | 0.023* | 0.063 |
| Group | 0.453 | 0.007 | |
| Sex | 0.043* | 0.051 | |
| FDS | Education (covariate) | 0.005* | 0.094 |
| Group | 0.219 | 0.019 | |
| Sex | 0.101 | 0.033 | |
| BDS | Education (covariate) | 0.002* | 0.110 |
| Group | 0.693 | 0.002 | |
| Sex | 0.399 | 0.004 | |
| IR | Education (covariate) | 0.060 | 0.043 |
| Group | 0.006* | 0.090 | |
| Sex | 0.080 | 0.037 | |
| DR | Education (covariate) | 0.884 | 0.001 |
| Group | 0.006* | 0.093 | |
| Sex | 0.050 | 0.045 | |
| PF | Education (covariate) | 0.001* | 0.110 |
| Group | 0.261 | 0.016 | |
| Sex | 0.370 | 0.010 | |
| SF | Education (covariate) | 0.318 | 0.012 |
| Group | 0.766 | 0.001 | |
| Sex | 0.720 | 0.002 | |
| IP | Education (covariate) | 0.003* | 0.885 |
| Group | 0.576 | 0.004 | |
| Sex | 0.880 | 0.001 | |
| RSC | Education (covariate) | 0.001* | 0.126 |
| Group | 0.380 | 0.010 | |
| Sex | 0.829 | 0.001 | |
| BNT | Education (covariate) | 0.001* | 0.224 |
| Group | 0.333 | 0.012 | |
| Sex | 0.012* | 0.079 | |
| TMTA_T | Education (covariate) | 0.450 | 0.032 |
| Group | 0.963 | 0.001 | |
| Sex | 0.020* | 0.068 | |
| TMTB_T | Education (covariate) | 0.003* | 0.131 |
| Group | 0.307 | 0.017 | |
| Sex | 0.034* | 0.070 | |
| Total_GM_V | Education (covariate) | 0.020* | 0.064 |
| Group | 0.010* | 0.078 | |
| Sex | 0.261 | 0.015 | |
| LH_V | Education (covariate) | 0.002* | 0.112 |
| Group | 0.001* | 0.160 | |
| Sex | 0.335 | 0.011 | |
| RH_V | Education (covariate) | 0.004* | 0.096 |
| Group | 0.005* | 0.091 | |
| Sex | 0.854 | 0.001 | |
| LA_V | Education (covariate) | 0.003* | 0.100 |
| Group | 0.006* | 0.087 | |
| Sex | 0.045* | 0.048 | |
| RA_V | Education (covariate) | 0.196 | 0.020 |
| Group | 0.327 | 0.012 | |
| Sex | 0.478 | 0.006 | |
| LE_V | Education (covariate) | 0.092 | 0.034 |
| Group | 0.013* | 0.072 | |
| Sex | 0.146 | 0.026 | |
| RE_V | Education (covariate) | 0.137 | 0.027 |
| Group | 0.103 | 0.032 | |
| Sex | 0.193 | 0.021 | |
| θwhole cluster | Education (covariate) | 0.421 | 0.008 |
| Group | 0.011* | 0.099 | |
| Sex | 0.034* | 0.053 | |
| βwhole cluster | Education (covariate) | 0.251 | 0.016 |
| Group | 0.013* | 0.094 | |
| Sex | 0.087 | 0.035 | |
| θfemale cluster | Education (covariate) | 0.310 | 0.020 |
| Group | 0.013* | 0.120 | |
| Sex | – | – | |
| βmale cluster | Education (covariate) | 0.174 | 0.060 |
| Group | 0.046* | 0.127 | |
| Sex | – | – |
MMSE Mini mental state examination, CDT Clock drawing test, FDS Forward digit span test, BDS Backward digit span test, IR Immediate recall, DR Delayed recall, PF Phonemic fluency, SF Semantic fluency, IP Ideomotor praxis, RSC Rule shift cards, BNT Boston naming test, TMTA_ T Trail-making test part A (time score), TMTB_T Trail-making test part B (time score), Total_GM_V Total grey matter volume, LH_V Left hippocampal volume, RH_V Right hippocampal volume, LA_V Left amygdala volume, RA_V Right amygdala volume, LE_V Left entorhinal volume, RE_V Right entorhinal volume
*Significant results (p < 0.05)
Interestingly, IR and DR evidenced a slightly different pattern where education had no significant effect, although it was close to the significance level for IR (p = 0.06). For IR, Group showed a significant influence (p = 0.006) with sMCIs exhibiting greater scores. Sex effect was close to the significance level (p = 0.080), but the analysis of interactions displayed the previously seen tendency to reduced scores in female pMCIs as compared with female sMCIs (p = 0.035). DR results were almost identical but an effect of Sex with p = 0.050 was added to the significant influence of Group (p = 0.006). Again, sMCIs displayed larger scores, while females exhibited a lower performance as compared with males. The interaction analyses indicated the same pattern of significantly lower scores in female pMCIs as compared with female sMCIs (p = 0.022).
At this point, it is very important to emphasize that the repeated evidence of a significant difference between pMCIs and sMCIs, restricted to the female’s sample, is due to the fact that cognitive scores showed a brisker fall within the females that progressed to AD. This fact was further supported by the significant difference between male and female pMCIs observed in MMSE and TMTB_T, that revealed a poorer performance in female converters (see Table 1).
CDT was affected by education effects (p = 0.023) but Sex still showed a significant influence (p = 0.043), with males displaying larger scores. No significant interaction effects emerged. Finally, TMTA_T only showed significant effects of Sex (p = 0.020), with females needing more time to complete the task. No significant interaction effects were detected.
SF scores were not significantly influenced by any of the analysed factors.
Volumetric variables
The analyses of volumetric variables followed the same strategy, and the results revealed a very similar pattern. Total_GM_V was affected by the effect of education (p = 0.020). After controlling for education influence, Group still showed a significant effect (p = 0.010) with sMCIs exhibiting larger volumes. The interaction analyses indicated that female sMCIs displayed larger volumes as compared with female pMCIs (p = 0.015). An identical pattern was found in LH_V (education, p = 0.002; Group, p = 0.001; interaction, p = 0.001), and RH_V (education, p = 0.004; Group, p = 0.005; interaction, p = 0.013). LE_V showed no significant influence of education (p = 0.092) but Group (p = 0.013) and interaction (p = 0.026) effects mirrored those observed in the previously described variables.
LA_V scores depicted a more complex pattern with a significant effect of education (p = 0.003) that was accompanied by the significant influences of Group (p = 0.006) and Sex (p = 0.045). This tendency was further explained by the interaction effect that, once again, evidenced that female sMCIs exhibited larger volumes than female pMCIs (p = 0.030). Paralleling the features found in neuropsychological tests, these effects restricted to females were due to the fact that volume scores show a more abrupt decrease within the females that converted to AD. No significant effects were detected in RA_V and RE_V.
MEG power variables
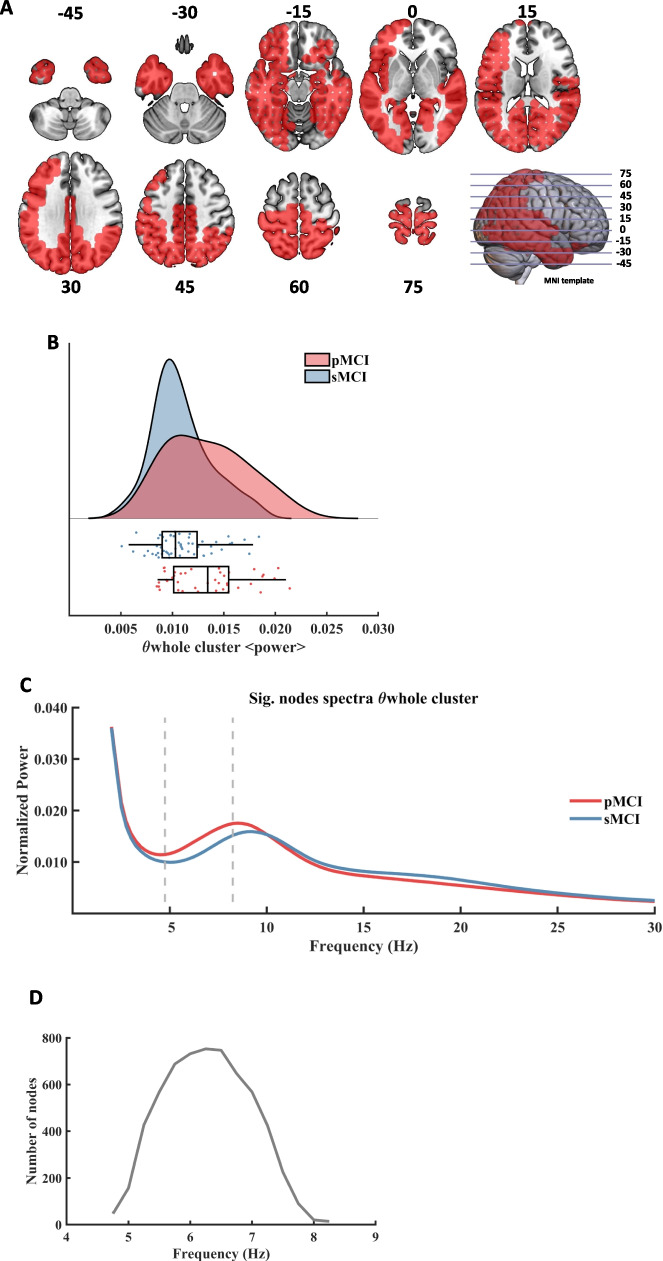
Firstly, the analyses performed in the whole sample (males + females) to search for differences between the pMCI and sMCI groups found two significant clusters. The first significant result (named θwhole cluster, CBPT p value = 0.011) emerged as a widespread posterior cluster (see Fig. 1, A), whose brain oscillatory activity in the 4.75 to 8.25 Hz frequency range (Fig. 1, C) differed between groups (see Fig. 1, B). The power of this cluster, that fell within the classical theta band range, was enhanced in the pMCI group when compared with the sMCI group. The cluster size ranged between 14 nodes up to 753 nodes. The maximum size was reached at the frequency of 6.25 Hz (see Fig. 1, D), while the maximum average F value, across all nodes of the cluster, was found at 6.00 Hz. Sex showed a significant influence on this cluster (p = 0.034), while education had no effect (see Table 2).
Fig. 1.
Significant result found within the theta frequency range in the comparison between pMCI and sMCI groups. A: description of the regions involved in the θwhole cluster at its maximum extension (found at 6.25 Hz). The cluster was found to be significant between 4.75 Hz and 8.25 Hz. Each slice shows the brain below the reference plane defined by the corresponding line on the template. The numbers report the depth in MNI coordinates (mm). B: Violin plots and box plots representing the individual values of the cluster's representative markers. Markers were calculated as the average power across all significant nodes and frequency steps. The sMCI group is marked in blue color and the pMCI group is marked in red color. C: representation of the average spectral power across all significant nodes. The significant frequency region is marked with dashed lines. The sMCI group is marked in blue color and the pMCI group is marked in red color. D: number of grid nodes that are part of the cluster at each frequency step (14 nodes as minimum and 753 as maximum)
An additional significant cluster was found for frequencies belonging to the classical beta frequency band (henceforth called βwhole, CBPT p value = 0.020). This result showed that the pMCI cases exhibited a diminished power in postero-inferior temporal brain regions (See Fig. 2, A) when compared with the sMCI individuals (Fig. 2, B). Specifically, this cluster emerged in the 14.75 Hz to 21.75 Hz frequency range (Fig. 2, C), being the range of its size between 15 and 467 nodes. The maximum size was reached at 16 Hz (Fig. 2, D), and the maximum average F value, across all nodes of the cluster, was found at 17.50 Hz. In this case, Sex showed a marginal influence on this cluster (p = 0.087), while education had no effect.
Fig. 2.
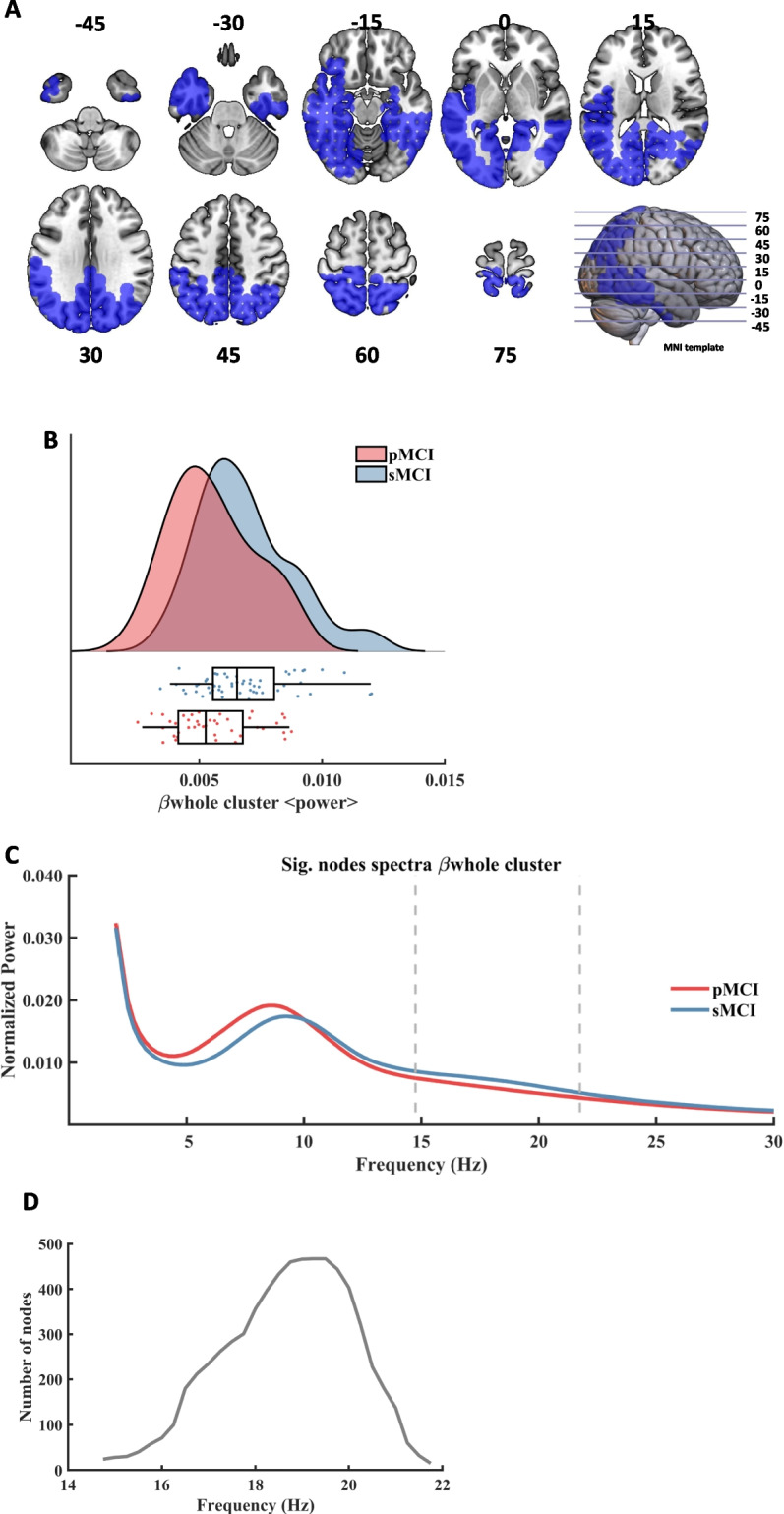
Significant results found within the beta frequency range in the comparison between pMCI and sMCI groups. A: description of the regions involved in the βwhole cluster at its maximum extension (found at 19.25 Hz). The cluster was found to be significant between 14.75 Hz and 21.75 Hz. Each slice shows the brain below the reference plane defined by the corresponding line on the template. The numbers report the depth in MNI coordinates (mm). B: Violin plots and box plots representing the individual values of the cluster's representative markers. Markers were calculated as the average power across all significant nodes and frequency steps. The sMCI group is marked in blue color and the pMCI group is marked in red color. C: representation of the average spectral power across all significant nodes. The significant frequency region is marked with dashed lines. The sMCI group is marked in blue color and the pMCI group is marked in red color. D: number of grid nodes that are part of the cluster at each frequency (minimum number of nodes: 15, and maximum number of nodes: 467)
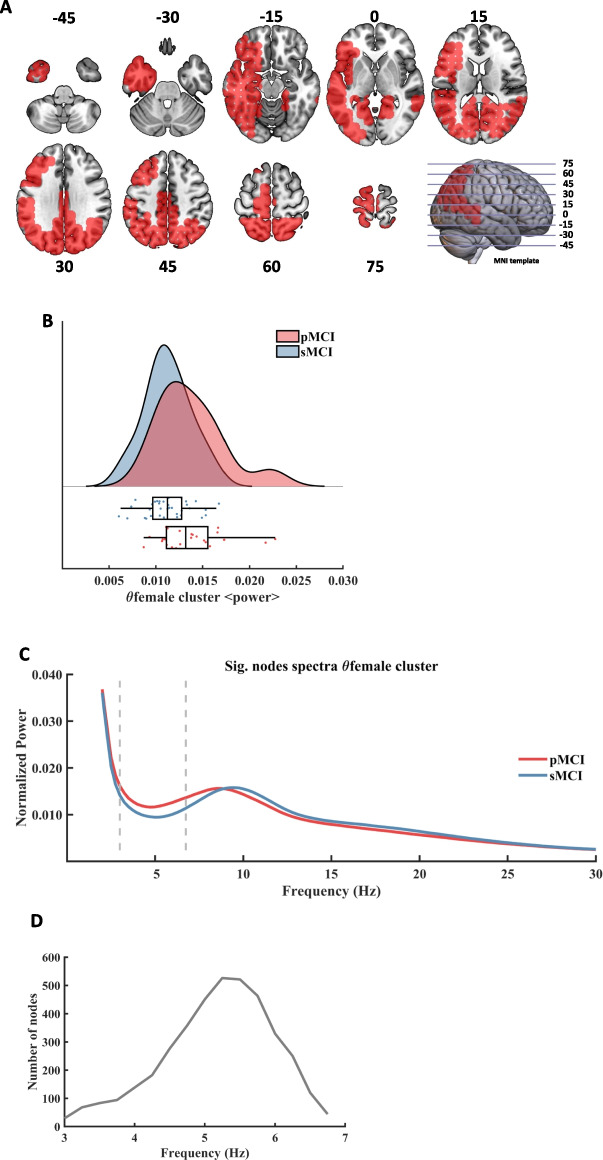
Thus, when the analysis of between-groups power differences was computed considering only the female sample, one significant cluster was found within the theta band (henceforth called θfemale, CBPT p value = 0.013). Females in the pMCI group showed an enhanced power in posterior left-temporal brain regions (See Fig. 3, A) when compared with females in the sMCI group (Fig. 3, B). The θfemale cluster mirrored the result of the θwhole cluster found in both MCIs groups but with a slightly lower frequency range. Thus, θfemale was defined in the 3.00 Hz to 6.75 Hz frequency range (Fig. 3, C). The size of the cluster fluctuated between 29 and 526 nodes, peaking at 5.25 Hz (Fig. 3, D), being the maximum average F value across all its nodes at 3.75 Hz. Education had no influence on this cluster.
Fig. 3.
Spectral power differences within the theta frequency range between female pMCI and sMCI groups. A: description of the regions involved in the θfemale cluster at its maximum extension (found at 5.25 Hz). The cluster was found to be significant between 3.00 Hz and 6.75 Hz. Each slice shows the brain below the reference plane defined by the corresponding line on the template. The numbers report the depth in MNI coordinates (mm). B: Violin plots and box plots representing the individual values of the cluster's representative markers. Markers were calculated as the average power across all significant nodes and frequency steps. The sMCI group is marked in blue color and the pMCI group is marked in red color. C: representation of the average spectral power across all significant nodes. The significant frequency region is marked with dashed lines. The sMCI group is marked in blue color and the pMCI group is marked in red color. D: number of grid nodes (from 29 to 526) that are part of the cluster at each frequency step
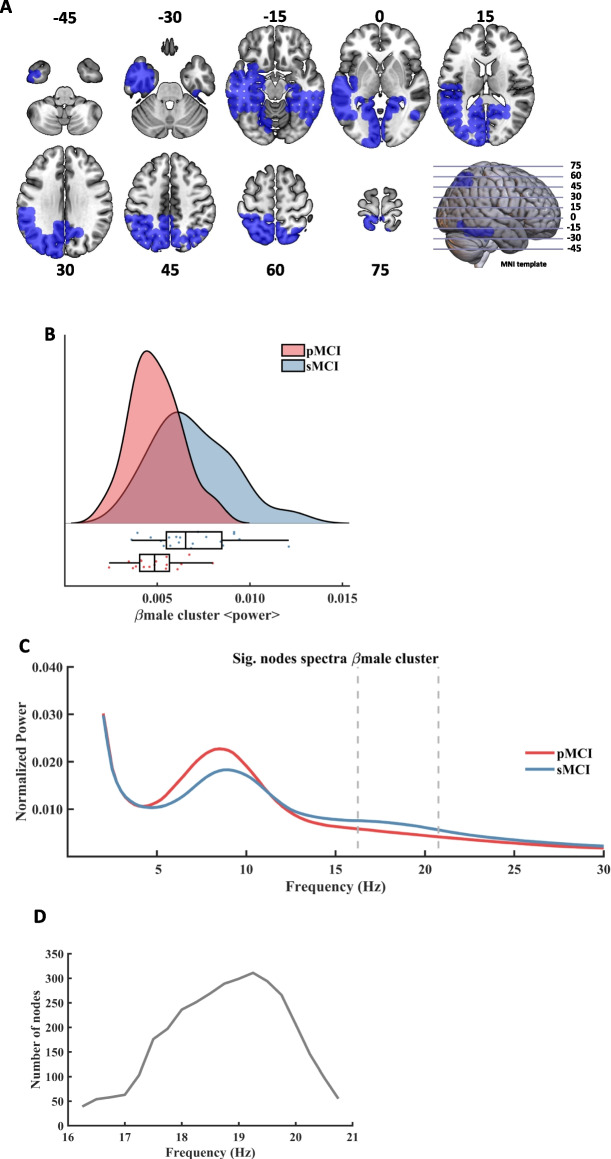
Finally, the last analysis consisted of assessing power differences between pMCI and sMCI groups considering only the male sample. In this case, one significant cluster emerged within the beta frequency band (henceforth called βmale, CBPT p value = 0.046). This result depicted a decreased power in left temporo-occipital regions (See Fig. 4, A) in the male pMCI group when compared with male sMCI group (Fig. 4, B). This cluster could be interpreted as a reflection of the βwhole cluster found in the whole sample. βmale was defined in the frequency range from 16.25 Hz to 20.75 Hz (Fig. 4, C). The size of the cluster fluctuated between 39 and 311 nodes, peaking at 19.25 Hz (Fig. 4, D). The maximum average F value, across all nodes of the cluster, was found at 18.75 Hz. Mirroring θfemale, education had no influence on this cluster.
Fig. 4.
Spectral power differences within the beta frequency range between male pMCI and sMCI groups. A: description of the regions involved in the βmale cluster at its maximum extension (found at 19.25 Hz). The cluster was found to be significant between 16.25 Hz and 20.75 Hz. Each slice shows the brain below the reference plane defined by the corresponding line on the template. The numbers report the depth in MNI coordinates (mm). B: Violin plots and box plots representing the individual values of the cluster's representative markers. Markers were calculated as the average power across all significant nodes and frequency steps. The sMCI group is marked in blue color and the pMCI group is marked in red color. C: representation of the average spectral power across all significant nodes. The significant frequency region is marked with dashed lines. The sMCI group is marked in blue color and the pMCI group is marked in red color. D: number of grid nodes that are part of the cluster at each frequency step, with a minimum of 39 and a maximum of 311 nodes
Table 2 summarizes p-values and effect sizes of all variables submitted to the ANCOVA analyses.
Correlations among MEG, neuropsychological and volumetric variables
With the aim of assisting in the interpretation of the results, additional analyses were carried out by performing Spearman correlation tests between the average power of each cluster and scores of neuropsychological tests and brain volumes. The resulting p-values were corrected for multiple comparisons with a FDR of 0.10 [57]. The p-values of the significant correlations are depicted in Table 3, and the scores included in the analysis are those described in Table 1.
Table 3.
Significant correlations between MEG markers, cognition, and brain volumes
| θwhole | βwhole | θfemale | βmale | |||||
|---|---|---|---|---|---|---|---|---|
| Rho | p value | Rho | p value | Rho | p value | Rho | p value | |
| MMSE | -0.342 | 0.011* | ||||||
| BDS | -0.302 | 0.026* | ||||||
| DR | -0.223 | 0.039 | 0.237 | 0.028 | -0.311 | 0.026* | ||
| SF | 0.209 | 0.048 | ||||||
| TMT_A_T | 0.309 | 0.029* | ||||||
| TMT_B_T | 0.344 | 0.028* | ||||||
| Total_GM_V | -0.302 | 0.003* | 0.286 | 0.006* | -0.336 | 0.011* | ||
| LH_V | -0.226 | 0.029 | 0.206 | 0.047 | ||||
| RH_V | -0.250 | 0.016 | 0.257 | 0.013* | ||||
Spearman correlation analyses between the average power of each corresponding cluster, neuropsychological test, and brain volumes in the whole sample (θwhole and βwhole) and in female (θfemale) and male (βmale) samples. MMSE Mini mental state examination, BDS Backward digit span, DR Delayed recall, SF Semantic fluency, TMTA_ T Trail-making test part A (time score), TMTB_T Trail-making test part B (time score), Total_GM_V Total grey matter volume, LH_V Left hippocampal volume, RH_V Right hippocampal volume. Total_GM_V, LH_V & RH_V were normalized by overall intracranial volume (ICV)
*Values surviving FDR correction
Correlations performed on clusters found in the whole sample revealed a quite consistent pattern. The θwhole cluster exhibited significant negative correlations with Total_GM_V, LH_V, RH_V and DR, indicating that higher power in that frequency range is associated with a worse cognitive performance and more atrophied brain structures. The βwhole cluster showed significant correlations with Total_GM_V, LH_V, RH_V, DR and SF but in this case displaying a positive sign, thus indicating that higher power in that frequency range was associated with a better cognitive performance and less atrophied brain structures.
When correlation analyses were accomplished within females’ sample, the θfemale cluster exhibited significant negative correlations with Total_GM_V, MMSE, BDS and DR; and positive correlations with TMTA_T and TMTB_T. Such correlations suggested that the association between power values, cognitive performance and brain volumes was mainly due to the influence of the female’s group. This affirmation was confirmed by the fact that no significant correlations with βmale emerged within the males’ sample.
Discussion
The results presented in this piece of work basically confirmed our initial hypotheses. The pMCI group exhibited the typical increase of low-frequency activity within the theta band, accompanied by a decrease in high-frequency power, here represented by a frequency range that encompasses most of the classical beta band. When power scores were analysed separately by sex, females in the pMCI group showed a pattern of increased theta power, while males exhibited a decrease of beta power. These findings indicate that the conversion to AD within the females’ group was associated with a pattern of increased low-frequency activity that might be related to accelerated conversion rates and increased AD pathology (see below). In fact, such a notion was confirmed by the results of neuropsychological performance and volumetric data. Overall, females with MCI (i.e., both pMCIs and sMCIs) showed poorer performance in several cognitive tasks than males, but the interaction analyses demonstrated that such difference was mainly due to the more abrupt deterioration of the females that progressed to AD. Volumetric results mirrored these findings, with the pMCI group showing reduced scores in most of the analysed brain regions but, again, this effect was principally attributable to the brisker volume reductions within the female pMCI group. A further confirmation of the close relationship between low-frequency activity, poorer cognitive performance and increased brain atrophy derived from correlation analyses. Both θwhole and βwhole clusters correlated with volumetric data and cognitive performance. Notwithstanding, when correlations were calculated separately, results demonstrated a stronger association among anatomical volumes, cognitive measures, and theta band activity that was restricted to the females’ group.
As Jaušovec and Jaušovec [57] pointed out, the investigations on sex differences in neurophysiological patterns tend to depict contradictory results. For example, some studies reported higher alpha activity in males [58], while others reported higher alpha power in females [59]. Similar contradictions can be found in theta (see for example [60]), and other bands such as delta and beta [61]. When samples with an ample range of ages were studied, higher theta and beta amplitudes were reported in females, but they were not considered of interest for the clinical evaluation [29]. In the few studies that focused on the AD-spectrum, similar controversies can be detected. Previously, we mentioned that Günther and coworkers [31] found that females with AD showed increased delta and theta activity as compared with males, being the females’ group the main responsible for the overall increase of low-frequency activity observed in AD cases. On the contrary, Babiloni et al. [33] reported increased alpha activity in female controls and AD-MCI cases that was independent of factors such as APOE genotype, amyloid and tau accumulation, and MRI neurodegeneration markers. Our results are in line with Günther et al. 's and with the more recent findings by Bruña and coworkers [34] who reported that females would display more signs of a higher predisposition to suffer AD than males.
Augmented low-frequency activity, accompanied by decreased alpha and beta power, is one of the most frequently described signs of increased risk of progression to AD [23, 25, 62–66]. However, a very recent study by Cechetti and coworkers [67] demonstrated that, although AD patients and MCI cases with positive AD markers showed a widespread increase of theta activity and a decrease of beta2, only theta power correlated with Aβ42 levels. Therefore, they considered that theta frequency was the earliest and most sensitive EEG marker of AD pathology. Previously, Stomrud et al. [68] had reported that a combination of CSF biomarkers, theta activity, and cognitive performance could be considered an early marker of AD. Within MEG literature, López et al. [28] confirmed that a mixture of theta power, hippocampal atrophy and a screening test of cognitive performance may predict the conversion to AD with a high sensitivity.
These evidences stress the fact that a widespread increase of theta activity (usually localized in posterior sites) combined with brain atrophy and poorer cognitive performance is associated with elevated risk and earlier progression to AD. Notably, this is the pattern that we observed in our females’ sample, that also showed a faster (though marginally significant) time to conversion. At this point it is worthwhile to further discuss the importance of volumetric and cognitive results. Neuroimaging studies in young and middle-aged subjects revealed that sexual dimorphism is present in several brain structures. Some of the more consistent differences include a greater brain volume in men even after correcting for body size; greater gray matter volume compared to white matter volume and increased cortical depth in women; increased volume in visuospatial association areas in men; increased volume in auditory and language-related regions in women, etc. (see for example [69]). Interestingly, significant differences have been also observed in the medial-temporal lobes, with males showing larger amygdala volumes and females a larger hippocampus [70–72]). These findings are important, since atrophy in medial-temporal structures has been considered as a primary predictor of conversion to AD, a view that is confirmed by our own results, where pMCIs showed significantly reduced volumes than sMCIs [28, 73, 74].
Similarly, differences between men and women in cognitive performance have been described along the aging process (for an exhaustive review, see [75]). Such differences may be of especial relevance when they affect the memory domain, as mnestic problems are usually the first cognitive sign of AD. In this case, females seem to outperform males in memory performance along the adulthood, particularly within the verbal domain, but this feature is attenuated after menopause [76–79]. Nonetheless, the most crucial aspect is to determine if the sexual dimorphism also affects the evolution of structural brain integrity and cognitive performance within the AD-spectrum. Some investigations indicated that women within the AD-spectrum exhibit a worse cognitive performance [80], smaller hippocampal volumes [81], and more pronounced total brain atrophy [75]. More importantly, Koran and coworkers [82] demonstrated in a follow-up study a significant correlation between sex and AD-pathology (i.e., Aβ42 and tau markers). According to their results, females presented greater hippocampal atrophy and longitudinal cognitive decline (both in the memory and executive-function composites of the ADNI database) in presence of positive AD markers. Moreover, the effect was exacerbated by lower levels of education and also was modified by the effect of APOE. Koran et al.’s results represented a further support to previous studies [83–85]; see also Ferretti et al. [20] for a full review on this subject.
Our results also seem to support these findings. Females within the pMCI group tended to show a worse cognitive performance and more pronounced atrophy than males within that group, but perhaps the most consistent finding was that differences between sMCIs and pMCIs were clearly more accentuated within the females’ sample, suggesting a stronger effect of AD pathology in this group. Females also showed a lower level of education attainment that might have played a role, although its effect was statistically controlled (see below further comments on this issue).
From a genetic perspective, sex differences regarding the APOE gene (i.e., APOE4 + carriage) and its role in predicting AD, have been also associated with the female sex [86], especially in the age range of 55–70 years [87]. In contrast, and similar to Babiloni et al. [33], APOE4 + did not exert a significant effect in our sample. Considering the aforementioned age interval, a possible explanation for our results may be due to the fact that our target population has an older average age (i.e., between 74–76 years). Interestingly, recent research aimed at elucidating sex-specific differences in the genetic framework of cognitive resilience to AD’s has also revealed an absence of APOE effects in a cohort with an average age of 77 years (i.e., an age comparable to that of our sample). The authors attributed this finding to the assumption that the effects of APOE on cognition diminishes with age, and in the case of females, this effect is even more noticeable due to the attenuation of circulating oestrogens which have been postulated to modulate APOE effects [88].
When all the findings (i.e., neurophysiological, volumetric, and cognitive) presented in our investigation are considered together, a final matter of discussion emerges. As formerly seen, some investigations reported that adult females may exhibit increased high frequency activity (for example in the beta band), have larger hippocampal volumes and show a better performance in some cognitive domains such as verbal memory. However, some of these features seem to vanish after menopause suggesting a hormonal influence and, in more specific terms, indicating that some type of intrinsic characteristics seem to make females’ brains more sensitive to the harmful effects of AD-pathology. Using Pike’s [14] terminology, female brain might be “inherently” more vulnerable to AD pathogenesis.
Arguably, and as already stated in relation to the APOE effects, sex steroid hormones might play a decisive role in this vulnerability. For instance, some epidemiological studies indicated that women who undergo surgical menopause or had menopause before 47 years of age, and do not receive substitutive hormone treatments, have an increased risk for global cognitive impairment and dementia in later life (see for example [89]). These findings indicated an association between the age of hormone loss and the risk for cognitive impairment, and consequently led to the notion that oestrogens may have a neuroprotective character [90, 91]. However, it seems that not only oestrogens but also testosterone plays that protective role, as reductions on the levels of this hormone are associated with an increased risk of AD [92]. The evidence suggested by epidemiological studies has been confirmed by experimental animal investigations. This line of research demonstrated that ovariectomized female rodents show increased levels of Aβ42, an overall acceleration of Aβ accumulation, and a worsening of cognitive performance [93–95]. Less information is available from animal models in males, but Rosario et al. [94] demonstrated that the age-related decrease in brain levels of androgens significantly correlated with age-related increases in soluble Aβ. In addition, Ramsden et al. [96] reported that orchiectomy significantly increases soluble Aβ in rodent male brains. Then, if oestrogens and testosterone seem to play a parallel role, why are females more vulnerable? The answer is not simple. Perhaps, the most intuitive perspective relies on the fact that menopause represents a brisk and earlier loss of oestrogens that might account for the increased female susceptibility to AD; while the so-called “andropause” is characterized by a gradual decrease of testosterone at a ratio lower than 1% per year [97, 98]. Notwithstanding, Carroll [93] demonstrated in transgenic mice models that neonatal females that were masculinized by testosterone treatment showed a reduction of Aβ accumulation in adulthood; while males that were feminized by pharmacological inhibition of androgen receptors exhibited increased Aβ. These findings support Pike’s [14] idea of an “inherent” vulnerability of females’ brain to the development of AD.
Nevertheless, at this point it is crucial to emphasise that some additional elements should be examined to explain our results. More in detail, we refer to the social determinants of health (SDHs) which are understood by the World Health Organization as nonmedical factors that affect health outcomes [99]. Among these SDHs the most relevant are those associated with socioeconomic status (i.e., income, occupation, community context, neighbourhood, etc.) and educational attainment [100]. Studies devoted to SDHs in the last decades demonstrated a “gradient pattern” by which general health indicators improve as economic/occupational and educational status rise [101, 102]. SDHs may be of special importance in our investigation as participants belong to one of the first generations that were born after the Spanish Civil War. Post-war circumstances, and related socioeconomic conditions, might have exerted an influence on cognitive and general health outcomes, especially in the female sample. Women were particularly affected by such adverse conditions, with a more limited access to educational resources and a significant economic/occupational dependence on men, restricting their role to being caregivers, wives and mothers [103]. Importantly, education and occupational attainment are well-known protective factors that prevent the development of dementia (for a review see [104]), and both are considered as “proxies” of CR [105, 106]. Moreover, some pioneering epidemiological studies indicated that the risk of developing AD seemed to be more pronounced in women with shorter periods of education, while that evidence was not so noticeable in men [107–109]. In fact, our data (see Table 1) showed that women within the pMCI group exhibited an averaged period of education that was 2-years shorter than the education years displayed by men in both MCI groups. Although the effects of education were intended to be statistically controlled, the potential impact of SDHs should be still taken into consideration to explain the between-sexes differences observed in cognitive performance.
Conclusions and limitations
As a concluding remark, it might be claimed that our study provides relevant information on the role of sex to characterize the AD-continuum. In fact, two general conclusions may be drawn from this research: 1) the relevance of multivariate approaches that include factors of different nature to predict the evolution from MCI to AD; and 2) the importance and the multifactorial nature of the role of sex on this evolution. This kind of information might have some clinical applications, such as the personalization of prevention, diagnosis, and pharmacological or non-pharmacological interventions [20]. Nevertheless, the study also presents some limitations that should be addressed. The diagnosis of our MCI sample was based on both clinical criteria and neuronal injury information (measured by MRI), but not in the presence of AD-pathological biomarkers measured by positron emission tomography (PET) or cerebrospinal fluid analysis. Despite this, the inclusion criteria as well as the clinical follow- up of the participants ensured that only MCI cases due to AD were included in the current study. Additionally, information about the onset of the menopause, the number of pregnancies and the possible use of hormonal treatments in females was not available. These data would have been very interesting to explore their impact in the development of AD. Finally, although the sample size was quite ample for a longitudinal and multifactorial study of AD, a larger number of participants would yield more evidence and replicability of the results.
Supplementary Information
(DOCX 16.4 KB)
Author contribution
Alberto Fernández: conceptualization, writing—original draft, writing—review and editing, project administration, supervision. Pablo Cuesta: conceptualization, formal analysis, writing—review and editing. Alberto Marcos and Mercedes Montenegro-Peña: Clinical patients’ recruitment and followed- up, writing—review and editing. Miguel Yus: Acquisition of the MRIs of participants, writing—review and editing. Ricardo Bruña: formal analysis, writing—review and editing. Inmaculada Concepción Rodríguez- Rojo: conceptualization, writing—review and editing. Fernando Maestú: conceptualization, project administration, supervision, writing—review and editing. María Eugenia López: conceptualization, writing—original draft, writing—review and editing, supervision.
Funding
This study was supported by three projects from the Spanish Ministry of Economy and Competitiveness (PSI2009-14415- C03-01, PSI2012-38375-C03-01, and TEC2016-80063-C3- 2-R).
Data Availability
Data are available upon reasonable request, and under the framework of a collaboration agreement.
Declarations
Ethical standards
The Hospital Universitario San Carlos Ethics Committee (Madrid) approved the study, and all participants or their caregivers signed a written informed consent prior to participation.
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Flannery KA, Liederman J, Daly L, Schultz J. Male prevalence for reading disability is found in a large sample of Black and White children free from ascertainment bias. J Int Neuropsychol Soc. 2000;6:433–442. doi: 10.1017/S1355617700644016. [DOI] [PubMed] [Google Scholar]
- 2.Aleman A, Kahn RS, Selten JP. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry. 2003;60:565–571. doi: 10.1001/ARCHPSYC.60.6.565. [DOI] [PubMed] [Google Scholar]
- 3.Bao AM, Swaab DF. Sex differences in the brain, behavior, and neuropsychiatric disorders. Neuroscientist. 2010;16:550–565. doi: 10.1177/1073858410377005. [DOI] [PubMed] [Google Scholar]
- 4.Young CB, Fang DZ, Zisook S. Depression in Asian-American and Caucasian undergraduate students. J Affect Disord. 2010;125:379–382. doi: 10.1016/J.JAD.2010.02.124. [DOI] [PubMed] [Google Scholar]
- 5.Lai DC, Tseng YC, Hou YM, Guo HR. Gender and geographic differences in the prevalence of intellectual disability in children: analysis of data from the national disability registry of Taiwan. Res Dev Disabil. 2012;33:2301–2307. doi: 10.1016/J.RIDD.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Ruigrok ANV, Salimi-Khorshidi G, Lai MC, Baron-Cohen S, Lombardo MV, Tait RJ, et al. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 2014;39:34–50. doi: 10.1016/j.neubiorev.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferencz B, Gerritsen L. Genetics and underlying pathology of dementia. Neuropsychol Rev. 2015;25:113–124. doi: 10.1007/S11065-014-9276-3. [DOI] [PubMed] [Google Scholar]
- 8.Fisher DW, Bennett DA, Dong H. Sexual dimorphism in predisposition to Alzheimer’s disease. Neurobiol Aging. 2018;70:308–324. doi: 10.1016/j.neurobiolaging.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62:847–855. doi: 10.1016/J.BIOPSYCH.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eliot L, Ahmed A, Khan H, Patel J. Dump the “dimorphism”: Comprehensive synthesis of human brain studies reveals few male-female differences beyond size. Neurosci Biobehav Rev. 2021;125:667–697. doi: 10.1016/j.neubiorev.2021.02.026. [DOI] [PubMed] [Google Scholar]
- 11.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0B013E31828726F5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, Schellenberg GD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59:1737–1746. doi: 10.1001/ARCHNEUR.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 13.Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer’s disease. Nat Rev Dis Prim. 2015;1. 10.1038/NRDP.2015.56. [DOI] [PubMed]
- 14.Pike CJ. Sex and the development of Alzheimer’s disease. J Neurosci Res. 2017;95:671–680. doi: 10.1002/jnr.23827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altmann A, Tian L, Henderson VW, Greicius MD. Alzheimer’s disease neuroimaging initiative investigators. Sex modifies the APOE -related risk of developing Alzheimer disease. Ann Neurol. 2014;75:563–73. doi: 10.1002/ana.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beydoun MA, Boueiz A, Abougergi MS, Kitner-Triolo MH, Beydoun HA, Resnick SM, et al. Sex differences in the association of the apolipoprotein E epsilon 4 allele with incidence of dementia, cognitive impairment, and decline. Neurobiol Aging. 2012;33:720–731.e4. doi: 10.1016/J.NEUROBIOLAGING.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holland D, Desikan RS, Dale AM, McEvoy LK. Higher rates of decline for women and apolipoprotein e ε4 carriers. Am J Neuroradiol. 2013;34:2287–2293. doi: 10.3174/ajnr.A3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Paajanen T, Westman E, Wahlund LO, Simmons A, Tunnard C, et al. Effect of APOE ε4 allele on cortical thicknesses and volumes: the AddNeuroMed study. J Alzheimers Dis. 2010;21:947–966. doi: 10.3233/JAD-2010-100201. [DOI] [PubMed] [Google Scholar]
- 19.Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA. Sex differences in the clinical manifestations of alzheimer disease pathology. Arch Gen Psychiatry. 2005;62:685. doi: 10.1001/archpsyc.62.6.685. [DOI] [PubMed] [Google Scholar]
- 20.Ferretti MT, Iulita MF, Cavedo E, Chiesa PA, Dimech AS, Chadha AS, et al. Sex differences in Alzheimer disease — The gateway to precision medicine. Nat Rev Neurol. 2018;14:457–469. doi: 10.1038/s41582-018-0032-9. [DOI] [PubMed] [Google Scholar]
- 21.Aghajani H, Zahedi E, Jalili M, Keikhosravi A, Vahdat BV. Diagnosis of early Alzheimer’s disease based on EEG source localization and a standardized realistic head model. IEEE J Biomed Health Inform. 2013;17:1039–1045. doi: 10.1109/JBHI.2013.2253326. [DOI] [PubMed] [Google Scholar]
- 22.Babiloni C, Del Percio C, Caroli A, Salvatore E, Nicolai E, Marzano N, et al. Cortical sources of resting state EEG rhythms are related to brain hypometabolism in subjects with Alzheimer’s disease: an EEG-PET study. Neurobiol Aging. 2016;48:122–134. doi: 10.1016/J.NEUROBIOLAGING.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 23.Besthorn C, Zerfass R, Geiger-Kabisch C, Sattel H, Daniel S, Schreiter-Gasser U, et al. Discrimination of Alzheimer’s disease and normal aging by EEG data. Electroencephalogr Clin Neurophysiol. 1997;103:241–248. doi: 10.1016/S0013-4694(97)96562-7. [DOI] [PubMed] [Google Scholar]
- 24.Jelic V, Blomberg M, Dierks T, Basun H, Shigeta M, Julin P, et al. EEG slowing and cerebrospinal fluid tau levels in patients with cognitive decline. NeuroReport. 1998;9:157–160. doi: 10.1097/00001756-199801050-00032. [DOI] [PubMed] [Google Scholar]
- 25.Jeong J. EEG dynamics in patients with Alzheimer’s disease. Clin Neurophysiol. 2004;115:1490–1505. doi: 10.1016/j.clinph.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 26.López ME, Turrero A, Cuesta P, Rodríguez-Rojo IC, Barabash A, Marcos A, et al. A multivariate model of time to conversion from mild cognitive impairment to Alzheimer’s disease. GeroScience. 2020;42:1715. doi: 10.1007/S11357-020-00260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.López ME, Turrero A, Delgado ML, Rodríguez-Rojo IC, Arrazola J, Barabash A, et al. APOE ε4 genotype and cognitive reserve effects on the cognitive functioning of healthy elders. Dement Geriatr Cogn Disord. 2018 doi: 10.1159/000481852. [DOI] [PubMed] [Google Scholar]
- 28.López ME, Turrero A, Cuesta P, López-Sanz D, Bruña R, Marcos A, et al. Searching for primary predictors of conversion from mild cognitive impairment to Alzheimer’s Disease: a multivariate follow-up study. J Alzheimer’s Dis. 2016;52(1):133–143. doi: 10.3233/JAD-151034. [DOI] [PubMed] [Google Scholar]
- 29.Duffy FH, McAnulty GB, Albert MS. The pattern of age-related differences in electrophysiological activity of healthy males and females. Neurobiol Aging. 1993;14:73–84. doi: 10.1016/0197-4580(93)90025-7. [DOI] [PubMed] [Google Scholar]
- 30.Veldhuizen RJ, Jonkman EJ, Poortvliet DCJ. Sex differences in age regression parameters of healthy adults–normative data and practical implications. Electroencephalogr Clin Neurophysiol. 1993;86:377–384. doi: 10.1016/0013-4694(93)90133-G. [DOI] [PubMed] [Google Scholar]
- 31.Günther W, Giunta R, Klages U, Haag C, Steinberg R, Satzger W, et al. Findings of electroencephalographic brain mapping in mild to moderate dementia of the Alzheimer type during resting, motor, and music-perception conditions. Psychiatry Res. 1993;50:163–176. doi: 10.1016/0925-4927(93)90028-G. [DOI] [PubMed] [Google Scholar]
- 32.Fernández A, Hornero R, Mayo A, Poza J, Gil-Gregorio P, Ortiz T. MEG spectral profile in Alzheimer’s disease and mild cognitive impairment. Clin Neurophysiol. 2006;117:306–314. doi: 10.1016/j.clinph.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 33.Babiloni C, Noce G, Ferri R, Lizio R, Lopez S, Lorenzo I, et al. Resting state alpha electroencephalographic rhythms are affected by sex in cognitively unimpaired seniors and patients with Alzheimer’s disease and amnesic mild cognitive impairment: a retrospective and exploratory study. Cereb Cortex. 2022;32:2197–2215. doi: 10.1093/CERCOR/BHAB348. [DOI] [PubMed] [Google Scholar]
- 34.Bruña R, Maestú F, López-Sanz D, Bagic A, Cohen AD, Chang YF, et al. Sex differences in magnetoencephalography-identified functional connectivity in the human connectome project connectomics of brain aging and dementia cohort. Brain Connect. 2022;12:561–570. doi: 10.1089/brain.2021.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chino-Vilca B, Rodríguez-Rojo IC, Torres-Simón L, Cuesta P, Vendrell AC, Piñol-Ripoll G, et al. Sex specific EEG signatures associated with cerebrospinal fluid biomarkers in mild cognitive impairment. Clin Neurophysiol. 2022;142:190–198. doi: 10.1016/j.clinph.2022.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 39.Bai F, Shu N, Yuan Y, Shi Y, Yu H, Wu D, et al. Topologically convergent and divergent structural connectivity patterns between patients with remitted geriatric depression and amnestic mild cognitive impairment. J Neurosci. 2012;32:4307–4318. doi: 10.1523/JNEUROSCI.5061-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agrell B, Dehlin O. The clock-drawing test. Age Ageing. 1998;27:399–403. doi: 10.1093/ageing/27.3.399. [DOI] [PubMed] [Google Scholar]
- 41.Wechsler D. Wechsler Memory Scale- Third Edition manual. 1997.
- 42.Benton A, Hamsher K. Multilingual Aphasia Examination. 2nd ed. Iowa City: 1989.
- 43.Peña-Casanova J. Programa Integrado de Exploración Neuropsicológica- Test Barcelona. Protocolo. Barcelona: Masson SA; 1990.
- 44.Norris G, Tate RL. The Behavioural Assessment of the Dysexecutive Syndrome (BADS): ecological concurrent and construct validity. Neuropsychol Rehabil. 2000;10:33–45. doi: 10.1080/096020100389282. [DOI] [Google Scholar]
- 45.Kaplan E, Goodglass H, Weintraub S. The Boston naming test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- 46.Reitan R. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Ski. 1958;8:271–276. doi: 10.2466/pms.1958.8.3.271. [DOI] [Google Scholar]
- 47.Lobo A, Ezquerra J, Gómez Burgada F, Sala JM, Seva Díaz A. [Cognocitive mini-test (a simple practical test to detect intellectual changes in medical patients)] Actas Luso Esp Neurol Psiquiatr Cienc Afines. 1979;7:189–202. [PubMed] [Google Scholar]
- 48.Cuesta P, Garcés P, Castellanos NP, López ME, Aurtenetxe S, Bajo R, et al. Influence of the APOE ε4 allele and mild cognitive impairment diagnosis in the disruption of the MEG resting state functional connectivity in sources space. J Alzheimer’s Dis. 2015;44(2):493–505. doi: 10.3233/JAD-141872. [DOI] [PubMed] [Google Scholar]
- 49.Taulu S, Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol. 2006;51:1759–1768. doi: 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- 50.Garcés P, López-Sanz D, Maestú F, Pereda E. Choice of magnetometers and gradiometers after signal space separation. Sensors (Switzerland) 2017;17:2926. doi: 10.3390/s17122926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oostenveld R, Fries P, Maris E, Schoffelen J-M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nolte G. The magnetic lead field theorem in the quasi-static approximation and its use for magnetoencephalography forward calculation in realistic volume conductors. Phys Med Biol. 2003;48:3637–3652. doi: 10.1088/0031-9155/48/22/002. [DOI] [PubMed] [Google Scholar]
- 53.Van Veen BD, van Drongelen W, Yuchtman M, Suzuki A, Van VBD, Van DW, et al. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng. 1997;44:867–880. doi: 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- 54.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 55.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 56.Nakamura A, Cuesta P, Fernández A, Arahata Y, Iwata K, Kuratsubo I, et al. Electromagnetic signatures of the preclinical and prodromal stages of Alzheimer’s disease. Brain. 2018 doi: 10.1093/brain/awy044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jaušovec N, Jaušovec K. Resting brain activity: differences between genders. Neuropsychologia. 2010;48:3918–3925. doi: 10.1016/J.NEUROPSYCHOLOGIA.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 58.Zappasodi F, Pasqualetti P, Tombini M, Ercolani M, Pizzella V, Rossini PM, et al. Hand cortical representation at rest and during activation: gender and age effects in the two hemispheres. Clin Neurophysiol. 2006;117:1518–1528. doi: 10.1016/J.CLINPH.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 59.Aurlien H, Gjerde IO, Aarseth JH, Eldøen G, Karlsen B, Skeidsvoll H, et al. EEG background activity described by a large computerized database. Clin Neurophysiol. 2004;115:665–673. doi: 10.1016/j.clinph.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 60.Clarke AR, Barry RJ, McCarthy R, Selikowitz M. Age and sex effects in the EEG: development of the normal child. Clin Neurophysiol. 2001;112:806–814. doi: 10.1016/S1388-2457(01)00488-6. [DOI] [PubMed] [Google Scholar]
- 61.Nikulin VV, Brismar T. Long-range temporal correlations in electroencephalographic oscillations: relation to topography, frequency band, age and gender. Neuroscience. 2005;130:549–558. doi: 10.1016/J.NEUROSCIENCE.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 62.Babiloni C, Del Percio C, Lizio R, Marzano N, Infarinato F, Soricelli A, et al. Cortical sources of resting state electroencephalographic alpha rhythms deteriorate across time in subjects with amnesic mild cognitive impairment. Neurobiol Aging. 2014;35:130–142. doi: 10.1016/j.neurobiolaging.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 63.Babiloni C, Lizio R, Marzano N, Capotosto P, Soricelli A, Triggiani AI, et al. Brain neural synchronization and functional coupling in Alzheimer’s disease as revealed by resting state EEG rhythms. Int J Psychophysiol. 2016;103:88–102. doi: 10.1016/J.IJPSYCHO.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 64.Garcés P, Vicente R, Wibral M, Pineda-Pardo JÁ, López ME, Aurtenetxe S, et al. Brain-wide slowing of spontaneous alpha rhythms in mild cognitive impairment. Front Aging Neurosci. 2013;5:1–7. doi: 10.3389/fnagi.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fernández A, Turrero A, Zuluaga P, Gil P, Maestú F, Campo P, et al. Magnetoencephalographic parietal delta dipole density in mild cognitive impairment: preliminary results of a method to estimate the risk of developing Alzheimer disease. Arch Neurol. 2006;63:427–430. doi: 10.1001/ARCHNEUR.63.3.427. [DOI] [PubMed] [Google Scholar]
- 66.Fernández A, Maestú F, Amo C, Gil P, Fehr T, Wienbruch C, et al. Focal temporoparietal slow activity in Alzheimer’s disease revealed by magnetoencephalography. Biol Psychiatry. 2002;52:764–770. doi: 10.1016/S0006-3223(02)01366-5. [DOI] [PubMed] [Google Scholar]
- 67.Cecchetti G, Agosta F, Basaia S, Cividini C, Cursi M, Santangelo R, et al. Resting-state electroencephalographic biomarkers of Alzheimer’s disease. NeuroImage Clin. 2021;31:102711. doi: 10.1016/J.NICL.2021.102711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stomrud E, Hansson O, Minthon L, Blennow K, Rosén I, Londos E. Slowing of EEG correlates with CSF biomarkers and reduced cognitive speed in elderly with normal cognition over 4 years. Neurobiol Aging. 2010;31:215–223. doi: 10.1016/j.neurobiolaging.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 69.Sacher J, Neumann J, Okon-Singer H, Gotowiec S, Villringer A. Sexual dimorphism in the human brain: evidence from neuroimaging. Magn Reson Imaging. 2013;31:366–375. doi: 10.1016/j.mri.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 70.Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, Toga AW, et al. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb Cortex. 2011;21:636–646. doi: 10.1093/CERCOR/BHQ137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koolschijn PCMP, Crone EA. Sex differences and structural brain maturation from childhood to early adulthood. Dev Cogn Neurosci. 2013;5:106–118. doi: 10.1016/J.DCN.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neufang S, Specht K, Hausmann M, Güntürkün O, Herpertz-Dahlmann B, Fink GR, et al. Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex. 2009;19:464–473. doi: 10.1093/CERCOR/BHN100. [DOI] [PubMed] [Google Scholar]
- 73.Ferreira LK, Diniz BS, Forlenza OV, Busatto GF, Zanetti MV. Neurostructural predictors of Alzheimer’s disease: a meta-analysis of VBM studies. Neurobiol Aging. 2011;32:1733–1741. doi: 10.1016/J.NEUROBIOLAGING.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 74.Thompson PM, Moussai J, Zohoori S, Goldkorn A, Khan AA, Mega MS, et al. Cortical variability and asymmetry in normal aging and Alzheimer’s disease. Cereb Cortex. 1998;8:492–509. doi: 10.1093/cercor/8.6.492. [DOI] [PubMed] [Google Scholar]
- 75.Hua X, Hibar DP, Lee S, Toga AW, Jack CR, Weiner MW, et al. Sex and age differences in atrophic rates: an ADNI study with n=1368 MRI scans. Neurobiol Aging. 2010;31:1463–1480. doi: 10.1016/J.NEUROBIOLAGING.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bleecker ML, Bolla-Wilson K, Agnew JMDA. Age-related sex differences in verbal memory. J Clin Psychol. 1988;3:403–411. doi: 10.1002/1097-4679(198805)44:3<403::AID-JCLP2270440315>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 77.Van Hooren SAH, Valentijn AM, Bosma H, Ponds RWHM, Van Boxtel MPJ, Jolles J. Cognitive functioning in healthy older adults aged 64–81: a cohort study into the effects of age, sex, and education. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2007;14:40–54. doi: 10.1080/138255890969483. [DOI] [PubMed] [Google Scholar]
- 78.Weber MT, Rubin LH, Maki PM. Cognition in perimenopause: the effect of transition stage. Menopause. 2013;20:511–517. doi: 10.1097/GME.0B013E31827655E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rentz DM, Weiss BK, Jacobs EG, Cherkerzian S, Klibanski A, Remington A, et al. Sex differences in episodic memory in early midlife: impact of reproductive aging. Menopause. 2017;24:400–408. doi: 10.1097/GME.0000000000000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Henderson VW, Buckwalter JG. Cognitive deficits of men and women with Alzheimer’s disease. Neurology. 1994;44:90–96. doi: 10.1212/WNL.44.1.90. [DOI] [PubMed] [Google Scholar]
- 81.Apostolova LG, Dinov ID, Dutton RA, Hayashi KM, Toga AW, Cummings JL, et al. 3D comparison of hippocampal atrophy in amnestic mild cognitive impairment and Alzheimer’s disease. Brain. 2006;129:2867–2873. doi: 10.1093/BRAIN/AWL274. [DOI] [PubMed] [Google Scholar]
- 82.Koran MEI, Wagener M, Hohman TJ. Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging Behav. 2017;11:205–213. doi: 10.1007/S11682-016-9523-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ardekani BA, Convit A, Bachman AH. Analysis of the MIRIAD data shows sex differences in hippocampal atrophy progression. J Alzheimers Dis. 2016;50:847–857. doi: 10.3233/JAD-150780. [DOI] [PubMed] [Google Scholar]
- 84.Holland D, Desikan RS, Dale AM, McEvoy LK. Higher rates of decline for women and apolipoprotein E epsilon4 carriers. AJNR Am J Neuroradiol. 2013;34:2287–2293. doi: 10.3174/AJNR.A3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin KA, Choudhury KR, Rathakrishnan BG, Marks DM, Petrella JR, Doraiswamy PM. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimer’s Dement (New York, N Y) 2015;1:103–110. doi: 10.1016/J.TRCI.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deming Y, Dumitrescu L, Barnes LL, Thambisetty M, Kunkle B, Gifford KA, et al. Sex-specific genetic predictors of Alzheimer’s disease biomarkers. Acta Neuropathol. 2018;136:857–872. doi: 10.1007/s00401-018-1881-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Neu SC, Pa J, Kukull W, Beekly D, Kuzma A, Gangadharan P, et al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: a meta-analysis. JAMA Neurol. 2017;74:1178–1189. doi: 10.1001/jamaneurol.2017.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eissman JM, Dumitrescu L, Mahoney ER, Smith AN, Mukherjee S, Lee ML, et al. Sex differences in the genetic architecture of cognitive resilience to Alzheimer’s disease. Brain. 2022;145:2541–2554. doi: 10.1093/brain/awac177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hogervorst E. Effects of gonadal hormones on cognitive behaviour in elderly men and women. J Neuroendocrinol. 2013;25:1182–1195. doi: 10.1111/JNE.12080. [DOI] [PubMed] [Google Scholar]
- 90.Bernstein L, Pike MC, Ross RK, Judd HL, Brown JB, Henderson BE. Estrogen and sex hormone-binding globulin levels in nulliparous and parous women. J Natl Cancer Inst. 1985;74:741–745. [PubMed] [Google Scholar]
- 91.Geerlings MI, Ruitenberg A, Witteman JCM, Van Swieten JC, Hofman A, Van Duijn CM, et al. Reproductive period and risk of dementia in postmenopausal women. JAMA. 2001;285:1475–1481. doi: 10.1001/JAMA.285.11.1475. [DOI] [PubMed] [Google Scholar]
- 92.Barron AM, Pike CJ. Sex hormones, aging, and Alzheimer’s disease. Front Biosci (Elite Ed) 2012;4:976–997. doi: 10.2741/E434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, et al. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci. 2007;27:13357–13365. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jayaraman A, Carroll JC, Morgan TE, Lin S, Zhao L, Arimoto JM, et al. 17β-estradiol and progesterone regulate expression of β-amyloid clearance factors in primary neuron cultures and female rat brain. Endocrinology. 2012;153:5467–5479. doi: 10.1210/EN.2012-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao L, Yao J, Mao Z, Chen S, Wang Y, Brinton RD. 17β-Estradiol regulates insulin-degrading enzyme expression via an ERβ/PI3-K pathway in hippocampus: relevance to Alzheimer’s prevention. Neurobiol Aging. 2011;32:1949–1963. doi: 10.1016/j.neurobiolaging.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ramsden M, Nyborg AC, Murphy MP, Chang L, Stanczyk FZ, Golde TE, et al. Androgens modulate beta-amyloid levels in male rat brain. J Neurochem. 2003;87:1052–1055. doi: 10.1046/J.1471-4159.2003.02114.X. [DOI] [PubMed] [Google Scholar]
- 97.Muller M, den Tonkelaar I, Thijssen JHH, Grobbee DE, van der Schouw YT. Endogenous sex hormones in men aged 40–80 years. Eur J Endocrinol. 2003;149:583–589. doi: 10.1530/EJE.0.1490583. [DOI] [PubMed] [Google Scholar]
- 98.Finch CE. The menopause and aging, a comparative perspective. J Steroid Biochem Mol Biol. 2014;142:132–141. doi: 10.1016/J.JSBMB.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marmot M, Friel S, Bell R, Houweling TA, Taylor S. Closing the gap in a generation: health equity through action on the social determinants of health. Lancet. 2008;372:1661–1669. doi: 10.1016/S0140-6736(08)61690-6. [DOI] [PubMed] [Google Scholar]
- 100.Majoka MA, Schimming C. Effect of social determinants of health on cognition and risk of Alzheimer disease and related Dementias. Clin Ther. 2021;43:922–929. doi: 10.1016/j.clinthera.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 101.Braveman P, Gottlieb L. The social determinants of health: it’s time to consider the causes of the causes. Public Health Rep. 2014;129:19–31. doi: 10.1177/00333549141291s206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Preda A, Voigt K. The social determinants of health: why should we care? Am J Bioeth. 2015;15:25–36. doi: 10.1080/15265161.2014.998374. [DOI] [PubMed] [Google Scholar]
- 103.Flecha GC. Education in Spain: close-up of is history in the 20th Century. Anal Reports Int Educ. 2011;1:17–42. doi: 10.3890/1542-3882-4-2. [DOI] [Google Scholar]
- 104.Shirai K, Iso H. Dementia (Chaper 11). In Social determinants of health in non-communicable diseases: Case studies from Japan, vol. 41. Springer Series on Epidemiology and Public Health; 2020. p. 105–123. 10.1007/978-981-15-1831-7_11 .
- 105.Schmand B, Smit JH, Geerlings MI, Lindeboom J. The effects of intelligence and education on the development of dementia. A test of the brain reserve hypothesis. Psychol Med. 1997;27:1337–44. doi: 10.1017/S0033291797005461. [DOI] [PubMed] [Google Scholar]
- 106.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. doi: 10.1017/S1355617702813248. [DOI] [PubMed] [Google Scholar]
- 107.Letenneur L, Launer LJ, Andersen K, Dewey ME, Ott A, Copeland JRM, et al. Education and the risk for Alzheimer’s disease: sex makes a difference EURODEM pooled analyses. Am J Epidemiol. 2000;151:1064–1071. doi: 10.1093/oxfordjournals.aje.a010149. [DOI] [PubMed] [Google Scholar]
- 108.Letenneur L, Gilleron V, Commenges D, Helmer C, Orgogozo JM, Dartigues JF. Are sex and educational level independent predictors of dementia and Alzheimer’s disease? Incidence data from the PAQUID project. J Neurol Neurosurg Psychiatry. 1999;66:177–183. doi: 10.1136/jnnp.66.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ott A, Van Rossum CTM, Van Harskamp F, Van De Mheen H, Hofman A, Breteler MMB. No Education and the incidence of dementia in a large population-based study: the Rotterdam StudyTitle. Neurology. 1999;52:663–666. doi: 10.1212/WNL.52.3.663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 16.4 KB)
Data Availability Statement
Data are available upon reasonable request, and under the framework of a collaboration agreement.