Abstract
Wild-type Escherichia coli grows more slowly on glucosamine (GlcN) than on N-acetylglucosamine (GlcNAc) as a sole source of carbon. Both sugars are transported by the phosphotransferase system, and their 6-phospho derivatives are produced. The subsequent catabolism of the sugars requires the allosteric enzyme glucosamine-6-phosphate (GlcN6P) deaminase, which is encoded by nagB, and degradation of GlcNAc also requires the nagA-encoded enzyme, N-acetylglucosamine-6-phosphate (GlcNAc6P) deacetylase. We investigated various factors which could affect growth on GlcN and GlcNAc, including the rate of GlcN uptake, the level of induction of the nag operon, and differential allosteric activation of GlcN6P deaminase. We found that for strains carrying a wild-type deaminase (nagB) gene, increasing the level of the NagB protein or the rate of GlcN uptake increased the growth rate, which showed that both enzyme induction and sugar transport were limiting. A set of point mutations in nagB that are known to affect the allosteric behavior of GlcN6P deaminase in vitro were transferred to the nagB gene on the Escherichia coli chromosome, and their effects on the growth rates were measured. Mutants in which the substrate-induced positive cooperativity of NagB was reduced or abolished grew even more slowly on GlcN than on GlcNAc or did not grow at all on GlcN. Increasing the amount of the deaminase by using a nagC or nagA mutation to derepress the nag operon improved growth. For some mutants, a nagA mutation, which caused the accumulation of the allosteric activator GlcNAc6P and permitted allosteric activation, had a stronger effect than nagC. The effects of the mutations on growth in vivo are discussed in light of their in vitro kinetics.
Escherichia coli is renowned for its ability to use a diverse array of organic compounds as sources of carbon and energy. However, different carbohydrates do not produce the same growth yield. One of the best carbon sources, after glucose, is N-acetylglucosamine (GlcNAc), an amino sugar. The other common amino sugar, glucosamine (GlcN), is also a source of carbon and nitrogen for E. coli, but use of this sugar results in lower growth rates than use of GlcNAc. Both GlcNAc and GlcN are phosphotransferase system (PTS) sugars (38) in E. coli so that their uptake occurs concomitantly with their phosphorylation, which produces intracellular N-acetylglucosamine 6-phosphate (GlcNAc6P) and glucosamine 6-phosphate (GlcN6P). GlcNAc6P is first deacetylated by the nagA gene product, GlcNAc6P deacetylase (NagA; EC 3.5.1.25); this produces GlcN6P, which is then subject to deamination and isomerization by the nagB-encoded GlcN6P deaminase (NagB; EC 3.5.99.6; formerly GlcN6P isomerase), resulting in fructose 6-phosphate, which enters the glycolytic pathway, and ammonia. Thus, the last amino sugar-specific step in the catabolism of both GlcN and GlcNAc depends on the nagB-encoded enzyme (Fig. 1A).
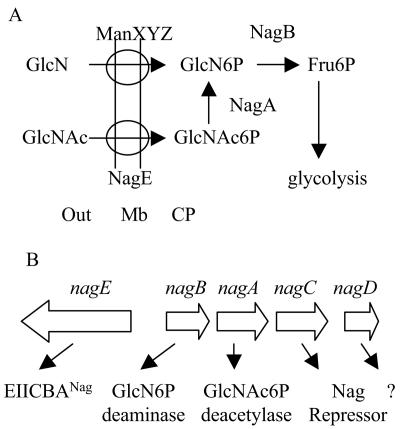
FIG. 1.
(A) Metabolism of GlcN and GlcNAc. Both sugars are PTS sugars, so that their transport across the inner membrane (Mb) into the cytoplasm (CP) by the manXYZ- and nagE-encoded transporters results in their phosphorylation. Subsequent metabolism is via the nagA- and nagB-encoded enzymes, GlcNAc6P deacetylase and GlcN6P deaminase, which results in fructose 6-phosphate. (B) Organization of the divergent nagE-BACD operons. The function of nagD is unknown.
GlcNAc6P has many functions during the metabolism of the amino sugars, both as a metabolite and as a regulator. As well as being the product of GlcNAc transport by the PTS and the substrate of the subsequent enzyme in the degradation pathway (Fig. 1A), it has two key regulatory roles. It is the allosteric activator for GlcN6P deaminase (NagB) (7), and it is also the inducing signal for NagC, the transcriptional repressor of the nag operon (34). The genes for the transport (nagE) and catabolism (nagBA) of GlcNAc are located in the divergent nagE-nagBACD operons (Fig. 1B). Expression of the nagE and nagBA genes is regulated in parallel by NagC binding to two operators, which overlap the promoters, via formation of a DNA repression loop (36). Growth on GlcNAc produces GlcNAc6P, which binds to NagC, displaces it from its operators, and induces expression of the nag genes. GlcN is transported by the rather nonspecific, so-called mannose PTS, EIIABCDMan, which is encoded by manXYZ and is capable of transporting a number of sugars, including glucose and GlcN, and which under normal conditions is the only transporter for GlcN (9, 16, 33, 38) (Fig. 1). Expression of the manXYZ operon is controlled by the Mlc repressor (28). Mlc is primarily involved in controlling utilization of glucose, and the inducing signal for Mlc is generated by growth on glucose and also, to some extent, by growth on other PTS sugars (18, 30, 31).
Growth on GlcN produces intracellular GlcN6P and results in much lower levels of induction of the nagE-nagBA operons than growth on GlcNAc (32). The fact that there is some induction (2- to 4-fold, compared to the 10- to 20-fold for GlcNAc) implies that GlcN6P can be converted to GlcNAc6P by a not-yet-characterized (probably indirect) mechanism. No acetyltransferase that is capable of converting GlcN6P to GlcNAc6P has been identified. Although NagA has been shown to synthesize GlcNAc6P in the presence of high acetate and GlcN6P concentrations in vitro (40), it is unlikely to be the source of GlcNAc6P in vivo, since GlcNAc6P accumulates in a nagA mutant. GlcNAc6P is produced by recycling of peptidoglycan, and NagA appears to have a critical role in the recycling process (27, 41). It is also possible that GlcN6P can act as a secondary inducer of NagC in vivo.
GlcN6P deaminase (NagB) is a homohexameric enzyme whose physiological allosteric activator is GlcNAc6P (7, 20). Like most allosteric enzymes, GlcN6P deaminase has two extreme structural states, one displaying high substrate affinity, which is defined as the R state, and the other displaying low affinity for GlcN6P, the T state. The crystallographic structures of both the R and T forms of NagB have been solved, and the binding sites for GlcNAc6P and GlcN6P have been described (15, 26). The transition from the T state to the R state, which activates the enzyme, can be driven by substrate binding that produces positive cooperativity (homotropic activation) and also by GlcNAc6P binding to the allosteric site (heterotropic activation). The GlcNAc6P binding sites are formed in the subunit interfaces of the hexamer as a consequence of the transition to the R state. The allosteric transition activates the enzyme, increasing its apparent affinity for GlcN6P (Km) without a change in the catalytic constant (kcat) (7). This is called a K system as defined by Monod et al.(22). The affinity for GlcNAc6P is high (Kdis, 0.035 mM), whereas much higher concentrations of GlcN6P (in the millimolar range) are required to produce half-maximum activity of deaminase in vitro in the absence of the allosteric activator (1).
Our initial goal was to try to determine what caused the different growth rates on GlcN and GlcNAc and whether the different growth rates were due to the allosteric regulation of NagB. There are at least three possible rate-limiting steps which could slow growth on GlcN compared to growth on GlcNAc. The following three rate-limiting hypotheses provide nonexclusive explanations for the slower growth on GlcN: (i) the rate of uptake of GlcN is lower than the rate of uptake of GlcNAc; (ii) the amount of the NagB protein, which depends on the level of induction of the nag genes, is smaller with GlcN than with GlcNAc; and (iii) the activity of the NagB enzyme, which depends on the level of allosteric activation of NagB, is lower during growth on GlcN than during growth on GlcNAc. We considered these three hypotheses by testing the effects of (i) an mlc mutation that enhanced the transport of GlcN (via an increase in expression of the manXYZ-encoded transporter), (ii) a nagC mutation that enhanced the level of the nagB-encoded deaminase, and (iii) a nagA mutation which caused the accumulation of GlcNAc6P, the allosteric activator of the deaminase.
The allosteric mechanisms of many allosteric enzymes have been studied in detail by biochemical, biophysical, and crystallographic methods over the years. Allosteric enzymes are often located at the beginning of biochemical pathways, where they are subject to feedback regulation by end products of the pathway (e.g., aspartate transcarbamylase and glutamine synthetase). As first proposed by Monod et al. (21), it is tacitly assumed that the allosteric activation has a function in vivo, adjusting the catalytic activity to the metabolic state of the organism. However, whether the changes in catalytic properties observed in vitro are properly and quantitatively reflected in the physiological properties of the enzyme in vivo has rarely been tested. A notable exception in bacteria is work on glycerol kinase, in which researchers measured the effect of loss of binding of two allosteric effectors, fructose 1,6-bisphosphate and EIIAGlc, on glucose regulation in vivo. For this enzyme it was shown that fructose 1,6-bisphosphate binding was the major factor contributing to glucose control of the enzyme (14).
Several mutants with site-directed mutations in the catalytic or allosteric site of the NagB protein have been constructed and analyzed in vitro. Some of these mutations alter the catalytic constant of the enzyme and modify qualitatively and quantitatively the allosteric properties of the enzyme, in some cases uncoupling the substrate, GlcN6P, induced cooperativity (homotropic effect), and the activation by the allosteric effector, GlcNAc6P (heterotropic effect). The results obtained have allowed a coherent description of the catalytic mechanism and provide some understanding of the allosteric activation mechanism (1, 5, 6, 8, 19, 23, 24).
To better understand the functioning of GlcN6P deaminase in vivo and to determine whether the kinetic data obtained with pure enzymes assayed under standardized pH, ionic strength, and temperature conditions in vitro reflect the behavior of the enzyme in the complex milieu of the cell, we studied in vivo several site-directed mutations in nagB. As deaminase is part of an inducible sugar metabolism operon, it was crucial to have the mutations correctly located on the bacterial chromosome so that their expression was regulated in the same way as the wild-type gene. We took advantage of the RED recombination system (10, 44) to introduce several single-amino-acid mutations into the nagB gene within the nag operon. To assess their effects in vivo, we simply compared their growth rates on the amino sugars GlcN and GlcNAc to those of the wild-type version.
MATERIALS AND METHODS
Bacteriological techniques.
Strains used in this work are listed in Table 1. P1vir transductions were used to introduce the mlc::tc (28), mlc::Tn10Kan, (11), nagC::tc, and nagA::tc mutations (34). Bacteria were grown in minimal morpholineethanesulfonic acid (MOPS) medium (25) containing a carbon source at a concentration of 0.2% (except glycerol, which was added at a concentration of 0.4%). β-Galactosidase activities of the manX-lacZ fusion were measured as described previously (28).
TABLE 1.
Bacterial strains used
| Strain | Genotype | Origin |
|---|---|---|
| MC4100 | araD139 Δ(argF-lac)U139 flb5301 deoC1 relA1 rbsR rpsL150 ptsF25 | Lab stock |
| DY329 | W3110 ΔlacU169 nadA::Tn10 gal490 λcI857 Δ(cro-bioA) | D. Court |
| NC397 | DY329 nad+lac1′kan-cat-sacB′lacZ | D. Court |
| LAA1 | DY329 nagB::cat | This study |
| LAA33 | DY329 asnB::Tn5 | This study |
| LAA41 | DY329 asnB::Tn5 nagB::cat-sacB | This study |
| LAA43 | MC4100 nagB::cat nagC::tc | This study |
| LAA44 | MC4100 nagB::cat nagA::tc | This study |
| LAA20 | MC4100 nagB::cat | This study |
| LAA39 | MC4100 nagB+ | This study |
| LAA34 | MC4100 nagB Tyr121Ser | This study |
| LAA83 | MC4100 nagB Tyr121Thr asnB::Tn5 | This study |
| LAA35 | MC4100 nagB Tyr121Trp | This study |
| LAA85 | MC4100 nagB Tyr254Phe asnB::Tn5 | This study |
| LAA87 | MC4100 nagB Tyr254Trp asnB::Tn5 | This study |
| LAA37 | MC4100 nagB Phe174Ala | This study |
| LAA38 | MC4100 nagB Lys160Glu | This study |
| LAA66 | MC4100 nagB Lys160Glu asnB::Tn5 | This study |
| LAA64 | MC4100 nagB Lys160Ala asnB::Tn5 | This study |
| LAA65 | MC4100 nagB Asp141Asn asnB::Tn5 | This study |
| LAA93 | MC4100 nagB Glu148Gln asnB::Tn5 | This study |
| LAA95 | MC4100 ΔnagEBACD::tc | This study |
Construction of strains carrying single nagB point mutations at the chromosomal locus of the nag operon. (i) General strategy.
The RED recombination system with PCR fragments described by Yu et al. (44) was used in two steps. In the first step the whole nagB gene in DY329 was replaced by a cat-sacB cassette. The resulting strain was chloramphenicol resistant and sucrose sensitive because of the Bacillus subtilis sacB gene (13). In the second step the nagB::cat-sacB cassette was replaced by the mutated nagB allele, with selection for sucrose resistance and screening for Cms. In some cases it was possible to select directly for Nag+ strains in this second step. Finally, the recombinants were transduced out of the RED strain into MC4100 carrying a ΔnagB::cat replacement, either with selection for Nag+ or by using the adjacent asnB::Tn5 mutation and screening for Cms.
(ii) Construction of DY329 nagB::cat and DY329 nagB::cat-sacB.
Approximately 70-nucleotide oligonucleotides having at their 5′ ends 50 nucleotides corresponding to the target gene, nagB, and at their 3′ ends 20 nucleotides corresponding to the cat cassette were synthesized. NagBcm5′ had 50 nucleotides corresponding to amino acids 54 to 70 of the nagB open reading frame joined to the 5′ cat promoter primer, and NagBcm3′ had 50 nucleotides corresponding to amino acid 256 to the nagB stop codon plus 23 nucleotides after the stop codon joined to the 3′ cat primer. NagBsac3′ had the same 3′ nagB sequence joined to a sacB 3′ primer. The nagB::cat fragment (1.03 kb) was amplified by using pACYC184 plasmid DNA as the template and the Pwo enzyme (Roche Diagnostics). The nagB::cat-sacB fragment (3.3 kb) was amplified with chromosomal DNA of strain NC397, which had a cat-sacB insert (a gift from the Don Court lab), by using the Expand High-Fidelity PCR system (Roche Diagnostics). The PCR fragments were purified from agarose gels. The nagB::cat cassette was introduced by electroporation into DY329 after induction of the RED system as described by Yu et al., with selection for Cmr, to obtain LAA1. The nagB::cat-sacB allele was introduced into DY329 carrying the asnB::Tn5 mutation (LAA33), which was adjacent to the nag operon. To ensure that Tn5 (which jumps very frequently during P1 transductions) was adjacent to the nag operon, asnB::Tn5 was introduced into DY329 nagB::cat (LAA1), with selection for Kanr and screening for Cms, to obtain LAA33. Subsequently, the nagB::cat-sacB allele was introduced into LAA33 by electroporation to obtain LAA41 (DY329 asnB::Tn5 nagB::cat-sacB). The sucrose sensitivity produced by the sacB gene was tested on minimal glycerol (0.4%) plates supplemented with nicotinic acid (40 μg/ml), biotin (10 μg/ml), and 4% sucrose at 30°C.
(iii) Replacement of nagB::cat-sacB with nagB point mutations.
DNA fragments having point mutations in nagB were amplified from pTZ(NagB) plasmid DNA containing the mutant alleles by using oligonucleotides Nag52 (5′-TCCAGGTTACGCTTAAAGATGCC-3′) and Nag53 (5′-CAGTGAACGTTGTTCGATCTCTGG-3′). The pTZ(NagB) plasmids have a 1.1-kb FspI-ClaI restriction fragment containing nagB inserted into pTZ18R (1). Oligonucleotides Nag52 and Nag53 were chosen because they hybridized near the extremities of the cloned insert. The Nag52-Nag53 fragments, amplified by Pwo polymerase (Roche Diagnostics), were purified from agarose gels and introduced into LAA41 by electroporation, with selection for sucrose resistance or growth on minimal GlcNAc plates at 30°C. Control experiments showed that significant numbers of apparently sucrose-resistant colonies appeared in the absence of any Nag52-Nag53 DNA (10 to 50% of the number of colonies formed with Nag52-Nag53 DNA). Sucrose-resistant colonies from the electroporation plates were transferred onto grids on the same selection plates with and without 10 μg of chloramphenicol per ml to eliminate spontaneous sucrose-resistant strains when the cat-sacB insertion had not been replaced by the nagB mutant allele. Sucrose-resistant, Cms, or Nag+ Cms colonies were purified and kept. The recombinant nagB point mutations were introduced by P1 transduction into LAA20 (MC4100 carrying the nagB::cat mutation), with selection for asnB::Tn5 (or GlcNAc+) and screening for Cms colonies. To introduce nagA::tc and nagC::tc mutations into the strains having point mutations in nagB, P1 grown on LAA44 (= MC4100 nagB::cat, nagA::tc) and LAA43 (nagB::cat, nagC::tc) were used, with selection for Tcr and screening for colonies which were still Cms and so had retained the mutant nagB allele. To verify correct replacement in the chromosome, the Nag52-Nag53 fragment was amplified by PCR from small cultures of the recombinants, and the entire fragment was sequenced (by MWG-Biotech).
Measurement of bacterial growth rates.
We compared the growth of MC4100 derivatives carrying the mutated nagB alleles on GlcN, GlcNAc, glucose, and glycerol. Bacteria were grown in minimal MOPS medium at 37°C with vigorous aeration. Precultures were usually grown in the same medium, but the strains having mutations which resulted in a defect in growth on GlcN or GlcNAc were also tested by starting with glycerol-grown precultures. Precultures were diluted 100-fold, and bacterial growth was measured by monitoring the optical density at 650 nm for at least 10 to 12 h or until saturation. Measurements were obtained every 30 min. Doubling times (DT) were calculated by linear regression of points in the exponential phase, generally between A650 of 0.1 and 1.0. Slowly growing bacteria were stored at 4°C overnight and, if necessary, rediluted, and growth was monitored for a second day.
Western blotting.
Aliquots of the MC4100 cultures carrying recombinant nagB alleles growing with the different carbon sources were harvested at an A650 of 0.8. Bacterial pellets were resuspended in 10 mM Tris-HCl-1 mM EDTA-10 mM β-mercaptoethanol-10% glycerol and disrupted by sonication. Aliquots (25 μg) were analyzed by sodium dodecyl sulfate-12.5% polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Hybond C) by electroblotting for 1.5 h at 300 mA as described previously (35). The blots were treated with antibodies to NagA and NagB, and the reacting proteins were revealed by treatment with 125I-labeled protein A. The antibodies were not sensitive enough to detect the uninduced chromosomal levels of deaminase and deacetylase. Detection of NagB was particularly difficult because the protein was weakly bound by the membrane and at least 50% passed through the top membrane and could be detected on subsequent membranes (35). These experiments showed that the mutant proteins were present in amounts similar to the wild-type amounts and that their expression was regulated by the nagA and nagC mutations like the expression of the nagB+ protein expressed from the chromosome.
RESULTS AND DISCUSSION
Growth on GlcN compared to growth on GlcNAc.
Growth of a wild-type nagB+ strain (MC4100) on GlcNAc resulted in growth rates (μ) comparable to the growth rates on glucose (μ expressed as doublings per hour, 1.1 to 1.2 h−1, which corresponded to DT of about 50 to 55 min), while growth on GlcN was much slower and the growth rates were less than those on glycerol (μ on Gly, 0.8 h−1 [DT, 75 min]; μ on GlcN, 0.6 h−1 [DT, 100 min]) (Table 2 and Fig. 2) (42). In an attempt to assess what limited growth on GlcN, we examined the effects of introduction of three mutations (mlc, nagC, and nagA) on the growth rate of MC4100 with GlcN as the sole source of carbon.
TABLE 2.
Growth rates of the nagB wild-type and mutant strains on GlcNAc and GlcN
| nagB allele | μ (h−1)a:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GlcNAc
|
GlcN
|
|||||||||
| Wild type | mlc | nagC | nagC mlc | Wild type | mlc | nagC | nagA | nagC mlc | nagA mlc | |
| Wild type | 1.14 ± 0.04 (7)b | 1.08 ± 0.10 (4) | 1.21 ± 0.05 (3) | 1.14 ± 0.02 (2) | 0.61 ± 0.06 (10) | 0.84 ± 0.05 (6) | 0.97 ± 0.02 (5) | 0.96 ± 0.02 (5) | 1.11 ± 0.02 (3) | 1.01 ± 0.02 (2) |
| Mutations affecting GlcNAc6P binding | ||||||||||
| Tyr121Ser | 1.10 ± 0.05 (6) | 1.07 ± 0.05 (4) | 1.09 ± 0.03 (2) | 1.13 ± 0.03 (2) | 0.25 ± 0.05 (6) | 0.70 ± 0.12 (4)c | 0.84 ± 0.04 (5) | 0.95 ± 0.05 (6) | 0.92 ± 0.06 (3) | 1.07 ± 0.04 (3) |
| Tyr121Thr | 1.06 ± 0.05 (3) | 1.08 ± 0.06 (2) | 1.12 ± 0.04 (2) | 0.37 ± 0.03 (3) | 0.67 ± 0.03 (3)c | 0.92 ± 0.07 (2) | 0.92 ± 0.03 (4) | |||
| Tyr121Trp | 1.15 ± 0.02 (3) | 1.11 ± 0.01 (2) | 1.17 (1) | 0.50 ± 0.06 (4) | 0.78 ± 0.04 (7) | 0.90 ± 0.07 (2) | 0.93 ± 0.03 (3) | |||
| Tyr254Phe | 1.11 ± 0.02 (2) | 1.13 ± 0.03 (2) | 1.14 ± 0.02 (2) | 0.40 ± 0.04 (5) | 0.73 ± 0.05 (4) | 0.87 ± 0.04 (3) | 0.95 ± 0.02 (3) | |||
| Tyr254Trp | 1.13 ± 0.02 (2) | 1.16 ± 0.03 (2) | 1.14 ± 0.06 (2) | 0.34 ± 0.06 (6) | 0.84 ± 0.05 (2) | 0.92 (1) | 0.94 ± 0.02 (3) | |||
| Active site lid mutations | ||||||||||
| Phe174Ala | 0.42 ± 0.03 (4) | 0.37 ± 0.05 (3) | 0.72 ± 0.01 (2) | 0.65 ± 0.02 (2) | NG | NG | 0.53 ± 0.05 (4) | 0.80 ± 0.02 (4) | 0.71 ± 0.02 (3) | 0.78 ± 0.04 (3) |
| Lys160Glu | 0.63 ± 0.03 (4) | 0.57 ± 0.06 (4) | 0.97 ± 0.04 (3) | 0.95 ± 0.02 (2) | NG | NG | 0.96 ± 0.04 (6) | 0.95 ± 0.04 (5) | 1.05 ± 0.02 (3) | 1.04 ± 0.01 (3) |
| Lys160Ala | NGd | NG | 0.21 ± 0.01 (3) | NG | NG | 0.42 ± 0.01 (2) | 0.41 ± 0.01 (2) | |||
| Active site mutations | ||||||||||
| Asp141Asn | NG | NG | 0.30 ± 0.01 (4) | NG | NG | 0.51 ± 0.01 (3) | 0.55 ± 0.02 (3) | |||
| Glu148Gln | NG | NG | 0.32 ± 0.06 (3) | NG | NG | 0.52 ± 0.02 (2) | 0.46 ± 0.04 (3) | |||
DT were calculated by linear regression from growth curves of the different strains growing in minimal MOPS medium with either 0.2% GlcNAc or 0.2% GlcN. The possible errors in the values were calculated from the standard deviations of the DT for four or more growth curves or from the range of values obtained for two or three cultures.
The numbers in parentheses are the numbers of growth curves used for calculation of the DT.
There was a long lag phase (μ = 0.3 to 0.35 h−1) before the culture entered the exponential growth phase at an A650 of about 0.2 with the growth rates shown. The reason for this lag phase is not understood, and it occurred whether cultures were pregrown in GlcN or glycerol.
NG, no growth. All strains had similar growth rates on glucose (1.15 h−1).
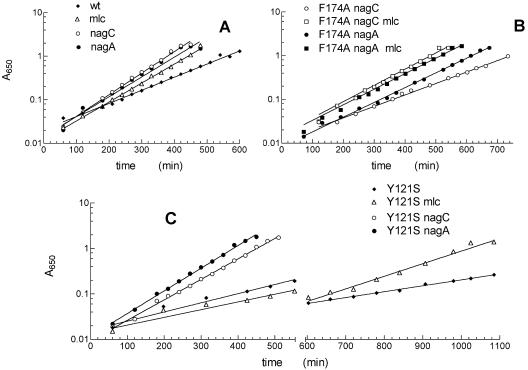
FIG. 2.
Effects of mlc, nagC, and nagA mutations on growth rates on GlcN. Bacteria were grown in minimal MOPS medium with 0.2% GlcN as described in Materials and Methods. (A) Wild-type NagB+; (B) Phe174Ala mutant; (C) Tyr121Ser mutant. Representative growth curves for the nagB strains and their mlc, nagC, and nagA versions are shown. The Phe174Ala mutant did not grow on GlcN in the absence of a nagC or nagA mutation. The slowly growing Tyr121Ser and Tyr121Ser mlc strains were stored overnight at 4°C and then rediluted to an A650 of 0.05 and regrown the next day. wt, wild type.
(i) Rate of transport: effect of an mlc mutation.
The mlc mutation resulted in a 40% increase in the growth rate, giving a μ of 0.84 h−1 corresponding to a decrease in the DT from about 100 to 70 min (Fig. 2A and Table 2). The mlc mutation increased manX-lacZ expression during growth on GlcN (Table 3). Growth on GlcN or Man resulted in very little induction of Mlc-repressed genes, and there was only a twofold increase in manX-lacZ expression during growth on these sugars compared to growth on glycerol. However, the presence of the mlc mutation during growth on GlcN increased the value nearly twofold (Table 3). This increase in manXYZ expression should be reflected in the rate of GlcN uptake. Previously, it was shown that an mlc mutation resulted in a twofold increase in the rate of uptake of N-acetylmannosamine, another sugar substrate of the ManXYZ transporter (37), demonstrating that for this sugar manXYZ expression limits the rate of sugar uptake. In fact, growth on N-acetylmannosamine was only possible in the presence of the mlc mutation (37), which showed that a twofold effect on expression can have a significant physiological effect. In another example, a twofold change in the phosphofructokinase level was found to have appreciable effects on the growth rate and glycolytic flux (3). We concluded that under normal conditions uptake of GlcN is one factor that limits growth on GlcN. Moreover, we could not exclude the possibility that GlcN uptake was still limiting in the mlc mutant strain.
TABLE 3.
Effect of the mlc mutation on induction of the manXYZ operona
| Strain | β-Galactosidase activity (Miller units) with:
|
||||
|---|---|---|---|---|---|
| Gly | Glc | GlcNAc | GlcN | Man | |
| Wild type | 178 | 131 | 203 | 310 | 356 |
| mlc | 703 | 150 | 273 | 594 | 543 |
The β-galactosidase activities of JM101 carrying a manX-lacZ fusion on a λ lysogen (28) were determined during growth in minimal MOPS medium with different carbon sources. The values are the means for three or four independent cultures (the standard deviations were less than 15%).
(ii) Amount of the NagB protein: effect of a nagC mutation.
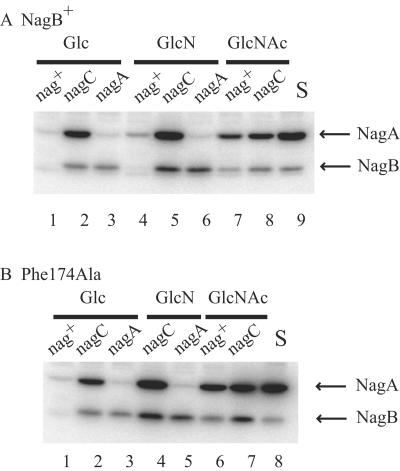
The presence of a nagC mutation resulted in a 60% increase in the growth on GlcN, giving a μ of 0.97 h−1 and a DT of 62 min (Table 2 and Fig. 2). This effect was somewhat stronger than the 40% increase resulting from the mlc mutation. The presence of the nagC mutation resulted in at least a 10-fold increase in nagE-BA expression, and the levels of the NagB and NagA proteins increased similarly, whereas the level of the NagB deaminase during growth of the wild-type strain on GlcN increased only slightly (32, 34, 35) (Fig. 3A). In fact, the level of the NagB protein was even higher in the nagC strain during growth on GlcN than during growth on GlcNAc. This was because growth on GlcNAc generated catabolite repression which reduced the expression of the nagE-BA operons (12, 32). These growth experiments clearly indicated that the amount of the nagB gene product limited growth on GlcN. However, it is interesting that the presence of an mlc mutation further increased the growth rate, to a value nearly equivalent to the growth rate of the wild-type strain on GlcNAc (μ, 1.1 h−1; DT, 55 min) (Table 2 and Fig. 2), showing that increased GlcN uptake in the presence of excess NagB is still advantageous. This is consistent with the notion that increasing all the enzyme levels in a chain of metabolic steps is better than just trying to increase the level of the step that has the highest flux control coefficient (17). Together, these observations strongly suggest that GlcN uptake and enzyme induction are not sufficient for maximum growth of the wild-type strain on GlcN as a sole carbon source.
FIG. 3.
Western blot analysis of cultures of strains carrying a NagB+ allele (A) and Phe174Ala NagB (B). Additional mutations in nagC or nagA were introduced as indicated above the lanes, and bacteria were grown with the carbon sources indicated. Aliquots of sonicated extracts of the cultures were separated by sodium dodecyl sulfate-12.5% polyacrylamide gel electrophoresis, transferred to a Hybond C membrane (Amersham), treated with antibodies to NagA and NagB, and revealed with 125I-labeled protein A. The positions of NagA and NagB on the gel are indicated. S, purified NagA and NagB proteins mixed with an extract of LAA95 (Δnag).
(iii) Enzymatic activity of NagB: effect of a nagA mutation.
The presence of a nagA mutation had an effect on the growth rate on GlcN similar to the effect of the nagC mutation, and as in the case of the nagC mutation, growth was slightly improved by the presence of an mlc mutation. Strains which have a nagA mutation accumulate high (millimolar) concentrations of GlcNAc6P (41, 42; unpublished results), which results in high constitutive expression of the nagE-BA operons via induction of the NagC repressor (34) (Fig. 3A). In addition, the accumulation of GlcNAc6P allows full allosteric activation of the NagB deaminase. A difference in the growth rates of strains having a nagA mutation and strains having a nagC mutation would imply that the accumulated GlcNAc6P contributes to the allosteric activation of deaminase, but no difference was apparent with strains carrying a wild-type nagB allele. Thus, when high levels of the deaminase were present, no effect due to allosteric activation of NagB was detectable. Unfortunately, at the moment we are unable to test the effect of allosteric activation by GlcNAc6P under conditions corresponding to the level of the NagB protein present during growth on GlcN. Increasing the intracellular GlcNAc6P concentration simultaneously derepresses the nag operon and allosterically activates NagB. This is the physiological response when cells grow on GlcNAc; both transcription and enzyme activity are simultaneously increased.
To test whether the allosterically activated form of deaminase, if it is present at the concentrations normally attained during growth on GlcN, allows more rapid growth than the nonactivated form requires a mutant locked in the allosterically activated R form. Such mutants exist (see below), but it is not just their allosteric properties which are changed; their catalytic constants are also significantly lower. Their strongly reduced kcat values mean that the enzymes are not active enough to allow growth unless they are overproduced. However, for some of the mutants described below, the fact that they did grow better on GlcN with a nagA mutation than with a nagC mutation demonstrated that the substrate (homotropic) cooperativity of these mutant forms of the enzyme is not sufficient during growth on GlcN and, at least for these modified forms of the protein, the allosteric activation is functionally significant in vivo.
Finally, it can be noted that the mlc and nagC mutations have no significant effect on growth rates on GlcNAc, implying that during growth of the wild-type strain on this sugar, induction of the nag catabolic genes and transport of the sugar (via NagE) are sufficient. The nagA mutation eliminates growth on GlcNAc. The fact that the nagC and nagA mutations result in similar growth rates on GlcN (or glycerol and glucose [data not shown]) indicates that the intrinsic accumulation of GlcNAc6P in the nagA mutant is not detrimental per se. However, growth of a nagA strain on another carbon source is inhibited when GlcNAc is also added. This is the Nags phenomenon, which is presumably due to accumulation of toxic levels of GlcNAc6P (4, 42). It should also be mentioned that the nagA mutant strains are not very genetically stable; Nagr strains accumulate, and these strains mostly contain noninducible alleles of NagC which keep the nagE-BA genes repressed despite the accumulation of GlcNAc6P (29, 42).
Effects of mutations which affect the allosteric properties of NagB during growth on GlcN. (i) Tyr121 and Tyr254.
Spectroscopic studies implicated two tyrosine residues in GlcNAc6P binding, Tyr121 and Tyr254 (2). Mutants with conservative mutations (Tyr121Thr, Tyr121Ser, Tyr121Trp, Tyr254Phe, and Tyr254Trp) were constructed and were found to have modified kinetics and to be affected both in substrate-induced positive cooperativity (homotropic effect) and in the affinity of the allosteric site for GlcNAc6P (1, 23) (Table 4). However, when the crystal structures were obtained, it was clear that neither Tyr121 nor Tyr254 is directly involved in GlcNAc6P binding (15, 26) and that these amino acids must contribute indirectly to GlcNAc6P binding at the allosteric site. Mutants with substitutions at both positions still displayed substrate (homotropic) cooperativity (although it was reduced for mutants with mutations at Tyr254) and were also allosterically activated by GlcNAc6P, but the effect was different. For the wild-type enzyme, GlcNAc6P binding resulted in only an increase in the affinity for GlcN6P (a K-type system). In the tyrosine mutants, activation by GlcNAc6P also resulted in an increase in the apparent catalytic constant and hence the measured Vmax. This is known as mixed K-V allosteric regulation. The GlcNAc6P-induced increases in kcat were 20- and 7-fold for the Tyr121Ser and Tyr254Phe mutants, respectively. Both of these mutants also had lower affinity for GlcNAc6P. The Kdis for GlcNAc6P was 2 orders of magnitude higher for Tyr254Phe, while the Tyr121Ser and Tyr121Trp mutants produced biphasic binding curves so that the first three GlcNAc6P sites were occupied with the same Kdis as the wild-type enzyme and the remaining three sites were occupied with a Kdis nearly 10 times higher (Table 4) (1, 23).
TABLE 4.
Kinetic properties of the mutant enzymesa
| Allele | Km for GlcN6P (R state) (mM) | Km for GlcN6P (T state) (mM)b | kcat (R state) (s−1)d | Kdis for GlcNAc6P (mM) |
|---|---|---|---|---|
| Wild type | 0.71 ± 0.06 | 32.3 | 158 ± 8 | 0.035 ± 0.006 |
| Mutations affecting GlcNAc6P binding | ||||
| Tyr121Ser | 2.60 ± 0.05 | 130 | 56 ± 1 | Biphasice |
| Tyr121Trp | 0.67 ± 0.08 | Exclusive bindingc | 97 ± 1 | Biphasice |
| Tyr254Phe | 1.00 ± 0.07 | Exclusive bindingc | 75 ± 3 | 3.6 ± 0.4 |
| Tyr254Trp | 0.90 ± 0.10 | 9.0 | 248 ± 18 | 3.0 ± 0.9 |
| Active site lid mutations | ||||
| Phe174Ala | 3.43 ± 0.04 | Exclusive bindingc | 163 ± 7 | 0.139 ± 0.018 |
| Lys160Ala | 0.80 ± 0.09 | NAf | 7.9 ± 0.2 | Not bound |
| Lys160Glu | 1.40 ± 0.10 | NA | 22.8 ± 0.8 | Not bound |
| Active site mutations | ||||
| Asp141Asn | 0.11 ± 0.01 | 1 | 7.7 ± 2 | 0.035 ± 0.002 |
| Glu148Gln | 1.90 ± 0.10 | 6.8 | 6.2 ± 0.2 | 0.105 ± 0.009 |
Data are from references 1, 6, 8, 19, 23, and 24 and unpublished data. Certain data were reevaluated. The mutant previously studied as Tyr121Thr was Tyr121Ser. Ser and Thr replacements produce similar kinetic changes.
Calculated from data fitted to the Monod-Wyman-Changeux equation (22).
Exclusive binding of the substrate to the R state.
Measured in the presence of saturating concentration of GlcNAc6P.
GlcNAc6P binds more strongly to the first three sites than to the second three sites on the hexamer (Kdis = 0.030 and 0.27 mM for Tyr121Trp) (1).
NA, not applicable (enzyme is fixed in the R state).
Consistent with these in vitro results with the purified enzymes, when the mutations were transferred to the E. coli chromosome, none of them had any effect on the growth rate on GlcNAc, which allowed full allosteric activation by GlcNAc6P, but all mutations resulted in lower growth rates on GlcN. The growth rates decreased to between 40 and 60% of the nagB+ value (Table 2 and Fig. 2C). Introducing an mlc mutation, to increase the rate of uptake of GlcN, increased the growth rate for all mutants. In particular, for Tyr121Trp and Tyr254Trp, an mlc mutation was sufficient to produce growth rates that were similar to that of the nagB+ mlc strain.
As observed for the wild-type nagB+ strain, introduction of a nagC or nagA mutation resulted in dramatic increases in the growth rates (Table 2). This demonstrated that an excess of the defective proteins can compensate for their reduced activity. Introduction of a nagA mutation resulted in decreases in the doubling times similar to the decreases produced by nagC, except in the case of Tyr121Ser and Tyr254Phe, where the nagA mutation resulted in higher growth rates than the nagC mutation (Table 2 and Fig. 2C). This was most obvious for Tyr121Ser, where the nagA mutation resulted in a growth rate of 0.95 h−1 (DT, 63 min), while for the nagC mutant the growth rate was 0.84 h−1 (DT, 74 min). This is consistent with the in vitro defects in the allosteric activation of the mutants. The massive accumulation of GlcNAc6P in the nagA strain forced allosteric activation, even though GlcNAc6P binding was deficient and higher concentrations were required for heterotropic activation in vitro for both mutant enzymes (1, 23). In fact, in the presence of the nagA mutation, all five nagB mutant strains had growth rates comparable to the growth rate of the nagB+ nagA strain on GlcN (μ, 0.95 h−1).
Most of the kcat values for the fully activated forms (R forms) of the mutated enzymes in vitro were two- to threefold lower than the value for the wild-type enzyme (Table 4). As strains with these enzymes were capable of normal wild-type growth rates on GlcNAc, this showed that a threefold-lower kcat is still adequate and that the wild-type enzyme, with a kcat of 158 s−1, has unused potential catalytic capacity. As explained above, the Tyr replacements resulted in mixed K-V allosteric activation, and the calculated kcat values for the T forms, in the absence of GlcNAc-6P, for Tyr121Ser and Tyr254Phe were very low (3 and 11 s−1, respectively) (1, 23). This probably accounts for the low growth rates on GlcN in the absence of an adequate supply of the allosteric activator to push the enzyme into the higher-activity R state. In the case of these mutants it is clear that the lack of allosteric activation limited growth on GlcN compared to growth on GlcNAc.
(ii) Mutations in the active site lid: Phe174Ala and Lys160Glu.
The active site of the enzyme is covered by a complex lid motif formed by residues 158 to 187. Arg158 and Lys160 contribute to GlcNAc6P binding in the allosteric site, and Arg172 is involved in GlcN6P binding. This motif exhibits high mobility in the crystal structure when the active site is free of ligands, but it has a fixed position when the active site is occupied. The lid plays a critical role in the functional coupling of allosteric and active sites. It changes position during the allosteric transition, thus transmitting the conformational changes produced by GlcNAc6P binding between the allosteric and active sites (15, 39). Phe174 is involved in anchoring the lid to the rest of the molecule, and the Phe174Ala mutation increases the flexibility of the lid (6). The Phe174Ala mutant enzyme is essentially inactive in vitro in the absence of the allosteric activator, GlcNAc6P. It requires the allosteric activator to restructure the active site (6). Consistent with the in vitro data, the Phe174Ala mutant grew slowly on GlcNAc and not at all on GlcN (Table 2). Growth on GlcNAc was improved by a nagC mutation, implying that the amount of the mutant protein is limiting for growth on GlcNAc. For the Phe174Ala mutant, introduction of the nagC mutation resulted in a 70% increase in the growth rate on GlcNAc. However, the growth rate (0.72 h−1; DT, 83 min) was still distinctly lower than that of the wild-type enzyme (1.14 h−1), implying that the catalytic capacity of the Phe174Ala NagB mutant was not sufficient for maximal growth even when the enzyme was overproduced. This is perhaps surprising, since the kcat in vitro for the fully allosterically activated enzyme was the same as the kcat for the wild-type enzyme, 163 s−1, whereas other mutants with lower kcat values in vitro (Tyr121Ser and Tyr254Phe) had normal growth rates on GlcNAc. The level of production of the Phe174Ala NagB protein during growth on the different carbon sources paralleled the level of production of the nagB+ allele (Fig. 3B), showing that the differences in the growth rate were apparently due to the enzyme activity of the mutated protein and not to the amount of protein. Possibly the F174A protein was less stable and the protein accumulated in vivo in an inactive form. However, unstable or misfolded proteins are usually eliminated by protein degradation machinery (43). Alternatively, the in vitro assay conditions may not have reflected the prevailing conditions in the cell, and the mutant enzyme could be particularly sensitive to ionic conditions or pH.
In the absence of a nagC or nagA mutation that increased the amount of the Phe174Ala protein, there was no growth on GlcN. The nagA mutation with Phe174Ala resulted in distinctly higher growth rates than nagC (0.8 h−1 [DT, 75 min] for nagA, compared to 0.53 h−1 [DT, 113 min] for nagC), showing that, as predicted, the accumulation of GlcNAc6P in the nagA strain allosterically activated the enzyme. We also tested to see if GlcN uptake was still limiting under these conditions. An mlc mutation that enhanced GlcN transport had no effect on the Phe174Ala nagA strain but did seem to slightly improve the growth on GlcN of the Phe174Ala nagC strain (Table 2 and Fig. 2B). Increasing GlcN uptake increases the GlcN6P pool; i.e., it increases the substrate concentration for NagB and allows whatever residual homotropic activation that exists in this enzyme to occur and/or facilitates the intracellular conversion of GlcN6P to GlcNAc6P and hence allows some heterotropic activation.
Mutations in another residue in the active site lid were also tested. Lys160 is at the N terminus of the sequence forming the lid and is also part of the allosteric site cleft involved in binding the phospho group of GlcNAc6P. Mutations at Lys160 eliminated homotropic cooperativity and binding of GlcNAc6P so that the enzyme showed hyperbolic kinetics but a lower kcat (S. Lara-González and M. Calcagno, unpublished results) (Table 4). The Lys160Glu mutation resulted in a pattern of growth similar to the Phe174Ala pattern of growth, but there was no apparent stabilization by GlcNAc6P since the growth rate on GlcN was identical for the nagC or nagA version and was also identical to the growth rate of the nagC derivative on GlcNAc. The Lys160Ala mutation, on the other hand, had a more pronounced detrimental effect on growth than Lys160Glu had. There was no growth on either GlcNAc or GlcN, which correlated with the lower kcat of the Lys160Ala version. In the presence of a nagC mutation, the growth rate of the Lys160Ala mutant strain on GlcNAc was unexpectedly much lower than the growth rate on GlcN (0.2 h−1 [DT, 290 min] for GlcNAc and 0.42 h−1 [DT, 142 min] for GlcN). In this respect Lys160Ala behaved like the Asp141Asn and Glu148Gln catalytic site mutations (see below). Slower growth on GlcNAc could be interpreted to mean that GlcNAc6P is an inhibitor for the enzyme, but this does not seem to be the case since the Lys160Ala nagA strain, which should have contained high concentrations of GlcNAc6P, grew as rapidly on GlcN (0.41 h−1 [DT, 146 min]) as the nagC version and grew more rapidly than the Lys160Ala nagC strain on GlcNAc. We do not have an explanation for this behavior (but see the discussion of Asp141Asn and Glu148Gln below).
(iii) Active site mutations: Asp141Asn and Glu148Gln.
The enzymatic mechanism of GlcN6P deamination includes a catalytic triad consisting of Asp141, His143, and Glu148 involved in the sugar ring-opening step, followed by a base-catalyzed enolization reaction involving Asp72 (20, 24, 26). Mutants with changes at the crucial residues His143 (e.g., His143Gln) and Asp72 (e.g., Asp72Asn) are essentially inactive (their kcat values are only 0.1 and 0.01% of the wild-type values, respectively) (24), and mutations at these positions were not tested in vivo. However, mutants with substitutions that changed the acid residues of Asp141 and Glu148 to their amides were still appreciably active in vitro, although they showed very marked kinetic changes. In these mutant forms the allosteric activator had become (at concentrations of the substrate GlcN6P above a certain critical but low concentration) an allosteric inhibitor so that binding GlcNAc6P drove the enzyme to a less catalytically active form (8).
When present at the nagB locus, neither mutation allowed growth of E. coli on GlcN or GlcNAc in the absence of a nagC mutation that increased the amount of the mutant proteins (Table 2). Significantly, the growth rates of nagC derivatives of Glu148Gln and Asp141Asn mutants were nearly twofold higher on GlcN (0.52 h−1 [DT, 115 min]) than on GlcNAc (0.31 h−1 [DT, 200 min]). This implies that in vivo, as in vitro, the presence of GlcNAc6P formed by growth on GlcNAc inhibits the growth on GlcNAc compared to the growth on GlcN. According to the same rationale, the presence of a nagA mutation, which results in the accumulation of millimolar amounts of GlcNAc6P, should also reduce growth on GlcN compared to the effect of a nagC mutation. However, the presence of the nagA mutation had only minor effects on the growth rates of Glu148Gln and Asp141Asn mutants on GlcN compared to the presence of the nagC mutation. Possibly the intracellular concentration of GlcN6P was lower during growth on GlcN than during growth on GlcNAc in the nagC strain. The nagC mutation resulted in overexpression of the fully active NagE transporter and NagA deacetylase, leading to higher intracellular levels of GlcN6P during growth on GlcNAc than during growth on GlcN. As allosteric inhibition by GlcNAc6P depends on the concentration of the substrate GlcN6P, this could mean that GlcNAc6P was more inhibitory during growth on GlcNAc, when GlcN6P concentrations were high, than it was in the nagA strain during growth on GlcN, when GlcN uptake was still limiting.
Concluding remarks.
At least two factors contribute to the slower growth on GlcN than on GlcNAc: lower induction of the nag genes and limiting GlcN uptake. The major reason appears to be the level of the NagB protein. As measured previously by using nag-lacZ fusions and Western blots (32, 35) and as confirmed here for the wild-type and Phe174Ala mutant strains, GlcN results in much lower levels of induction of the nag genes than GlcNAc (Fig. 3). Either a nagA mutation or a nagC mutation derepresses the nag genes 20-fold so that the levels of the nag operon gene products that are present during growth on GlcN are similar to the levels in a nagA+C+ strain during growth on GlcNAc. Second, the rate of GlcN uptake is also limiting, since a mlc mutation which increased expression of the manXYZ-encoded transporter for GlcN (Table 3) resulted in a nearly 40% increase in the growth rate. For the wild-type enzyme we cannot decide whether a lack of allosteric activation also limits growth on GlcN. Another intriguing question is why in E. coli the metabolism of amino sugars is designed to favor GlcNAc as a carbon source over GlcN.
We tested a series of site-directed mutations in the nagB gene whose effects on the in vitro kinetics made them interesting candidates to study in vivo. The amino acids mutated were picked mostly on the basis of the crystallographic structures, and we chose residues involved in either the allosteric site or the active site. However, in most cases, the amino acid replacements had pleiotropic effects that altered both active site catalysis and allosteric activation. The catalytic constants (which were reflected in the Vmax) were usually decreased, but several mutants were still capable of normal growth on GlcNAc. On the basis of the in vitro kinetic parameters, certain characteristics of the growth pattern could be predicted. For example, mutations that resulted in a defect in homotropic activation (Tyr121Ser) affected only growth on GlcN, and this could be fully compensated for by a nagA mutation, which increased the intracellular GlcNAc6P concentration and produced allosteric activation. Similarly, the Phe174Ala mutant, which was active in vitro only in the presence of GlcNAc6P, grew better on GlcN in the presence of a nagA mutation than in the presence of a nagC mutation, since the accumulation of GlcNAc6P forced allosteric activation. On the other hand, strains having mutations that distorted the active site, so that the allosteric activator behaved as an inhibitor (Asp141Asn and Glu148Gln), grew better on GlcN than on GlcNAc. Although the correlation was not always perfect, these experiments showed that the in vivo growth characteristics of the NagB mutants reflect and can be interpreted in terms of the enzymatic and allosteric properties of the mutant proteins in vitro.
Acknowledgments
We thank the Don Court lab for gifts of strains and advice on the use of the RED recombination system and Samuel Lara-Gonzáles and Ismael Bustos-Jaimes for providing unpublished results and for useful discussions. L.A.-A. is pleased to acknowledge a leave of absence from the UNAM. We are grateful to the following people for their critical comments on the manuscript: Luis Acerenza, Bernard Badet, Henri Buc, Guy Hervé, Annie Kolb, Rosario Muñoz-Clares, Mathias Springer, and the reviewers of the manuscript.
This work was supported by grants from the CNRS and Université Paris 7-Denis Diderot (to UPR9073) and from CONACYT (grant 41328Q) and PAPIIT-UNAM (grant IN228703) (to M.L.C.).
REFERENCES
- 1.Altamirano, A. A., J. A. Plumbridge, E. Horjales, and M. L. Calcagno. 1995. Asymmetric allosteric activation of Escherichia coli glucosamine-6-phosphate deaminase produced by replacements of Tyr121. Biochemistry 34:6074-6082. [DOI] [PubMed] [Google Scholar]
- 2.Altamirano, M. M., A. Hernandez-Arana, S. Tello-Solis, and M. L. Calcagno. 1994. Spectrochemical evidence for the presence of a tyrosine residue in the allosteric site of glucosamine-6-phosphate deaminase from Escherichia coli. Eur. J. Biochem. 220:409-413. [DOI] [PubMed] [Google Scholar]
- 3.Andersen, H., C. Solem, K. Hammer, and P. Jensen. 2001. Twofold reduction of phosphofructokinase activity in Lactococcus lactis results in strong decreases in growth rate and in glycolytic flux. J. Bacteriol. 183:3458-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernheim, N. J., and W. J. Dobrogosz. 1970. Amino sugar sensitivity in Escherichia coli mutants unable to grow on N-acetylglucosamine. J. Bacteriol. 101:384-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bustos-Jaimes, I., M. Ramirez-Costa, L. D. Anda-Aguilar, P. Hinjosa-Ocaña, and M. Calcagno. 2005. Evidence for two different mechanisms triggering the change in quaternary structure of the allosteric enzyme, glucosamine-6-phosphate deaminase. Biochemistry 44:1127-1135. [DOI] [PubMed] [Google Scholar]
- 6.Bustos-Jaimes, I., A. Sosa-Peinado, E. Rudiño-Piñera, E. Horjales, and M. L. Calcagno. 2002. On the role of the conformational flexibility of the active site lid on the allosteric kinetics of glucosamine-6-phosphate deaminase. J. Mol. Biol. 319:183-189. [DOI] [PubMed] [Google Scholar]
- 7.Calcagno, M., P. J. Campos, G. Mulliert, and J. Suastegui. 1984. Purification, molecular and kinetic properties of glucosamine-6-phosphate isomerase (deaminase) from E. coli. Biochim. Biophys. Acta 787:165-173. [DOI] [PubMed] [Google Scholar]
- 8.Cisneros, D., G. M. Montero-Morán, S. Lara-González, and M. L. Calcagno. 2003. Inversion of the allosteric response of Escherichia coli glucosamine-6-P deaminase to N-acetyglucosamine 6P by single amino acid replacements. Arch. Biochem. Biophys. 421:77-84. [DOI] [PubMed] [Google Scholar]
- 9.Curtis, S. J., and W. Epstein. 1975. Phosphorylation of d-glucose in Escherichia coli mutants defective in glucosephosphotransferase, mannosephosphotransferase, and glucokinase. J. Bacteriol. 122:1189-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Datsenko, K. A., and B. L. Wanner. 2000. One step inactivation of chromosomal genes in Escherichia coli K12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Decker, K., J. Plumbridge, and W. Boos. 1998. Negative transcriptional regulation of a positive regulator: the expression of malT, encoding the transcriptional activator of the maltose regulon of Escherichia coli, is negatively controlled by Mlc. Mol. Microbiol. 27:381-390. [DOI] [PubMed] [Google Scholar]
- 12.Dobrogosz, W. J. 1968. Effect of amino sugars on catabolite repression in Escherichia coli. J. Bacteriol. 95:578-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gay, P., D. Le Coq, M. Steinmetz, T. Berkelman, and C. Kado. 1985. Positive selection procedure for entrapment of insertion sequence elements in gram-negative bacteria. J. Bacteriol. 164:918-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holtman, C., A. Pawlyk, N. Meadow, and D. Pettigrew. 2001. Reverse genetics of Escherichia coli glycerol kinase allosteric regulation and glucose control of glycerol utilization in vivo. J. Bacteriol. 183:3336-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horjales, E., M. M. Altamirano, M. L. Calcagno, R. Garratt, and G. Oliva. 1999. The allosteric transition of glucosamine-6-phosphate deaminase: the structure of the T state at 2.3A resolution. Structure 7:527-537. [DOI] [PubMed] [Google Scholar]
- 16.Jones-Mortimer, M. C., and H. L. Kornberg. 1980. Amino-sugar transport systems in Escherichia coli K12. J. Gen. Microbiol. 117:369-376. [DOI] [PubMed] [Google Scholar]
- 17.Kacser, H., and L. Acerenza. 1993. A universal method for achieving increases in metabolite production. Eur. J. Biochem. 270:361-367. [DOI] [PubMed] [Google Scholar]
- 18.Kimata, K., T. Inada, H. Tagami, and H. Aiba. 1998. A global repressor (Mlc) is involved in glucose induction of the ptsG gene encoding major glucose transporter in Escherichia coli. Mol. Microbiol. 29:1509-1519. [DOI] [PubMed] [Google Scholar]
- 19.Lara-González, S., H. B. F. Dixon, G. Mendoza-Hernandez, M. M. Altamirano, and M. L. Calcagno. 2000. On the role of the N-terminal group in the allosteric function of glucosamine-6-phosphate deaminase from Escherichia coli. J. Mol. Biol. 301:219-227. [DOI] [PubMed] [Google Scholar]
- 20.Midelfort, C. F., and I. A. Rose. 1977. Studies on the mechanism of E. coli glucosamine-6-phosphate isomerase. Biochemistry 16:1590-1596. [DOI] [PubMed] [Google Scholar]
- 21.Monod, J., J. Changeux, and F. Jacob. 1963. Allosteric proteins and cellular control systems. J. Mol. Biol. 6:306-329. [DOI] [PubMed] [Google Scholar]
- 22.Monod, J., J. Wyman, and J. Changeux. 1965. On the nature of the allosteric transitions: a plausible model. J. Mol. Biol. 12:88-118. [DOI] [PubMed] [Google Scholar]
- 23.Montero-Morán, G. M., E. Horjales, M. L. Calcagno, and M. M. Altamirano. 1998. Tyr254 hydroxyl group acts as a two-way switch mechanism in the coupling of heterotropic and homotropic effects in Escherichia coli glucosamine-6-phosphate deaminase. Biochemistry 37:7844-7849. [DOI] [PubMed] [Google Scholar]
- 24.Montero-Morán, G. M., S. Lara-González, L. I. Álvarez-Añorve, J. A. Plumbridge, and M. L. Calcagno. 2001. On the multiple functional roles of the active site histidine in catalysis and allosteric regulation of Escherichia coli glucosamine 6-phosphate deaminase. Biochemistry 40:10187-10196. [DOI] [PubMed] [Google Scholar]
- 25.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliva, G., M. Fontes, R. Garratt, M. M. Altamirano, M. L. Calcagno, and E. Horjales. 1995. Structure and catalytic mechanism of glucosamine-6-phosphate deaminase from Escherichia coli at 2.1A resolution. Structure 3:1323-1332. [DOI] [PubMed] [Google Scholar]
- 27.Park, J. T. 2001. Identification of a dedicated recycling pathway for anhydro-N-acetylmuramic acid and N-acetylglucosamine derived from Escherichia coli cell wall murein. J. Bacteriol. 183:3842-3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plumbridge, J. 1998. Control of the expression of the manXYZ operon in Escherichia coli: Mlc is a negative regulator of the mannose PTS. Mol. Microbiol. 27:369-381. [DOI] [PubMed] [Google Scholar]
- 29.Plumbridge, J. 1992. A dominant mutation in the gene for the Nag repressor of Escherichia coli that renders the nag regulon uninducible. J. Gen. Microbiol. 138:1011-1017. [DOI] [PubMed] [Google Scholar]
- 30.Plumbridge, J. 1998. Expression of ptsG, the gene for the major glucose PTS transporter in Escherichia coli, is repressed by Mlc and induced by growth on glucose. Mol. Microbiol. 29:1053-1063. [DOI] [PubMed] [Google Scholar]
- 31.Plumbridge, J. 1999. Expression of the phosphotransferase system (PTS) both mediates and is mediated by Mlc regulation in Escherichia coli. Mol. Microbiol. 33:260-273. [DOI] [PubMed] [Google Scholar]
- 32.Plumbridge, J. 1990. Induction of the nag regulon of Escherichia coli by N-acetylglucosamine and glucosamine: role of the cAMP-catabolite activator protein complex in expression of the regulon. J. Bacteriol. 172:2728-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plumbridge, J. 2000. A mutation which affects both the specificity of PtsG sugar transport and the regulation of ptsG expression by Mlc in Escherichia coli. Microbiology 146:2655-2663. [DOI] [PubMed] [Google Scholar]
- 34.Plumbridge, J. 1991. Repression and induction of the nag regulon of Escherichia coli K12: the roles of nagC and nagA in maintenance of the uninduced state. Mol. Microbiol. 5:2053-2062. [DOI] [PubMed] [Google Scholar]
- 35.Plumbridge, J., O. Cochet, J. M. Souza, M. M. Altamirano, M. L. Calcagno, and B. Badet. 1993. Coordinated regulation of amino sugar-synthesizing and degrading enzymes in Escherichia coli K-12. J. Bacteriol. 175:4951-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plumbridge, J., and A. Kolb. 1993. DNA loop formation between Nag repressor molecules bound to its two operator sites is necessary for repression of the nag regulon of Escherichia coli in vivo. Mol. Microbiol. 10:973-981. [DOI] [PubMed] [Google Scholar]
- 37.Plumbridge, J., and E. Vimr. 1999. Convergent pathways for utilization of the amino sugars N-acetylglucosamine, N-acetylmannosamine, and N-acetylneuraminic acid by Escherichia coli. J. Bacteriol. 181:47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Postma, P. W., J. W. Lengeler, and G. R. Jacobson. 1996. Phosphoenolpyruvate:carbohydrate phosphotransferase systems, p. 1149-1174. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM, Washington, D.C.
- 39.Rudiño-Piñera, E., S. Morales-Arrieta, S. Rojas-Trejo, and E. Horjales. 2002. Structural flexibility, an essential component of the allosteric activation in Escherichia coli glucosamine-6-phosphate deaminase. Acta Crystallogr. Sect. D 58:10-20. [DOI] [PubMed] [Google Scholar]
- 40.Souza, J.-M., J. A. Plumbridge, and M. L. Calcagno. 1997. N-Acetyl-d-glucosamine-6-phosphate deacetylase from Escherichia coli: purification and molecular and kinetic characterization. Arch. Biochem. Biophys. 340:338-346. [DOI] [PubMed] [Google Scholar]
- 41.Uehara, T., and J. T. Park. 2004. The N-acetyl-d-glucosamine kinase of Escherichia coli and its role in murein recycling. J. Bacteriol. 186:7273-7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White, R. J. 1968. Control of amino sugar metabolism in Escherichia coli and isolation of mutants unable to degrade amino sugars. Biochem. J. 106:847-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wickner, S., M. Maurizi, and S. Gottesman. 1999. Posttranslational quality control: folding, refolding and degrading proteins. Science 286:1888-1893. [DOI] [PubMed] [Google Scholar]
- 44.Yu, D., H. M. Ellis, E.-C. Lee, N. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]