Abstract
Whole brain irradiation (WBI), also known as whole brain radiation therapy (WBRT), is a well-established treatment for multiple brain metastases and as a preventive measure to reduce the risk of recurrence after surgical removal of a cerebral metastasis. However, WBI has been found to lead to a gradual decline in neurocognitive function in approximately 50% of patients who survive the treatment, significantly impacting their overall quality of life. Recent preclinical investigations have shed light on the underlying mechanisms of this adverse effect, revealing a complex cerebrovascular injury that involves the induction of cellular senescence in various components of the neurovascular unit, including endothelial cells. The emergence of cellular senescence following WBI has been implicated in the disruption of the blood-brain barrier and impairment of neurovascular coupling responses following irradiation. Building upon these findings, the present study aims to test the hypothesis that WBI-induced endothelial injury promotes endothelial dysfunction, which mimics the aging phenotype. To investigate this hypothesis, we employed a clinically relevant fractionated WBI protocol (5 Gy twice weekly for 4 weeks) on young mice. Both the WBI-treated and control mice were fitted with a cranial window, enabling the assessment of microvascular endothelial function. In order to evaluate the endothelium-dependent, NO-mediated cerebral blood flow (CBF) responses, we topically administered acetylcholine and ATP, and measured the resulting changes using laser Doppler flowmetry. We found that the increases in regional CBF induced by acetylcholine and ATP were significantly diminished in mice subjected to WBI. These findings provide additional preclinical evidence supporting the notion that WBI induces dysfunction in cerebrovascular endothelial cells, which in turn likely contributes to the detrimental long-term effects of the treatment. This endothelial dysfunction resembles an accelerated aging phenotype in the cerebrovascular system and is likely causally linked to the development of cognitive impairment. By integrating these findings with our previous results, we have deepened our understanding of the lasting consequences of WBI. Moreover, our study underscores the critical role of cerebromicrovascular health in safeguarding cognitive function over the long term. This enhanced understanding highlights the importance of prioritizing cerebromicrovascular health in the context of preserving cognitive abilities.
Keywords: Senescence, WBI, WBRT, whole brain radiation therapy, aging, vascular cognitive impairment, VCI, dementia
Introduction
Brain metastases rank as the second most frequent occurrence in cancer patients, with an incidence ranging from 10 to 30% among individuals with systemic malignancies [1, 2]. Whole brain irradiation (WBI) represents a widely employed therapeutic approach for patients afflicted with multiple brain metastases. While WBI effectively prolongs overall survival in individuals with cancer, it is distressing to note that more than half of the long-term survivors experience a gradual deterioration in cognitive function following WBI [3–9]. This cognitive decline significantly diminishes their quality of life and contributes to escalated healthcare expenses. Intriguingly, laboratory studies on animals have corroborated these clinical findings, as they demonstrate a progressive impairment of cognitive performance in response to WBI, thereby simulating the cognitive side effects observed in patients subjected to WBI treatment [3, 8, 10–14].
Recent advancements in preclinical investigations have provided valuable insights into the underlying mechanisms behind these deleterious effects, unveiling a complex injury within the cerebrovascular system [14–17]. This injury entails the activation of cellular senescence in diverse constituents of the neurovascular unit, including endothelial cells [15]. The emergence of cellular senescence subsequent to WBI has been linked to disruptions in the blood-brain barrier and impairments in neurovascular coupling responses post-irradiation [15].
It is worth noting that post-mitotic cells, such as mature neurons, are generally regarded as radioresistant. In contrast, proliferating cells within the neurovascular unit, specifically microvascular endothelial cells, demonstrate sensitivity to the damaging effects of ionizing radiation [15, 18]. Normal endothelial function in the cerebral microcirculation plays a multifaceted role in the preservation of cognitive health. An accumulating body of preclinical and clinical evidence highlights the critical, complex role of radiation-induced neurovascular and cerebromicrovascular injury in the manifestation of cognitive decline subsequent to WBI [13, 19–22]. Importantly, WBI-induced neurovascular injury associates with impaired neurovascular coupling responses [14, 15] and a decline in capillary density within the hippocampus, a phenomenon referred to as “cerebromicrovascular rarefaction” [19–21]. Maintaining microvascular health is also crucial for preserving the integrity of the blood-brain barrier (BBB) [23, 24]. Strong evidence suggests that WBI and γ-irradiation-induced microvascular injury contribute to BBB disruption [25–33], leading to neuroinflammation [34, 35].
One key aspect of endothelial function is the crucial role of endothelial NO-mediated vasodilation in regulating and maintaining cerebral blood flow (CBF). Endothelial cells release NO in response to various stimuli, including neurotransmitters and gliotransmitters (e.g., acetylcholine, ATP) and shear stress. NO acts as a potent vasodilator, relaxing the vascular smooth muscle cells and pericytes surrounding cerebral blood vessels, thereby decreasing resistance and increasing blood flow to active brain regions. This dynamic regulation of CBF is essential for supplying oxygen and nutrients to the brain and for effective wash-out of toxic metabolic by-products, ensuring optimal neuronal activity and synaptic plasticity. Furthermore, endothelial NO-mediated vasodilation is integral to the preservation of neurovascular coupling response, a critical hoeostatic process that ensures that increases in neuronal activity are matched with enhanced CBF to sustain neuronal function [36–51]. Importantly, intact endothelial function and NO-mediated vasodilation serve as a protective mechanism against ischemic events in the brain. The ability of endothelial cells to respond and dilate blood vessels helps maintain optimal blood flow even under conditions of reduced oxygen supply. Inadequate endothelial function compromises this protective response, rendering the brain more susceptible to ischemic damage and impairments in cognitive function. Taken together, normal endothelial function in the cerebral microcirculation is critical for the preservation of cognitive health. Despite the importance of endothelial NO-mediated vasodilation in regulation and maintenance of adequate CBF, the effects of WBI on it have not been well characterized.
Building upon the aforementioned findings, the present study aims to test the hypothesis that WBI-induced endothelial injury promotes endothelial dysfunction, which mimics the aging phenotype. To investigate this hypothesis, we employed a clinically relevant fractionated WBI protocol (5 Gy twice weekly for 4 weeks) on young mice. Both the WBI-treated and control mice were fitted with a cranial window, enabling the assessment of microvascular endothelial function. In order to evaluate the endothelium-dependent, NO-mediated cerebral blood flow (CBF) responses, we topically administered acetylcholine and ATP, and measured the resulting changes using laser Doppler flowmetry.
Materials and methods
Experimental animals and experimental design
C57BL/6J (3 months old, n = 20) male mice were purchased from the Jackson Laboratories (Bar Harbor, ME) and housed three per cage in the specific pathogen free animal facility at the University of Oklahoma Health Sciences Center (OUHSC). Animals were kept on a 12-h light/dark cycle and fed standard rodent chow and water ad libitum, following standard husbandry techniques. One week before radiation treatment, mice were transferred to the conventional facility (OUHSC) and housed under similar conditions. Mice were anesthetized and subjected to clinical series of WBI (n = 8, 5 Gy twice weekly for a total cumulative dose of 40 Gy) or used as a control group (n = 8). Mice were left to recover for 3 months in the original environment. At the end of the recovery period, mice were experimentally tested for cerebromicrovascular endothelial function. All animal protocols were approved by the Institutional Animal Care and Use Committee of OUHSC.
Fractionated whole brain irradiation protocol
After acclimating to the conventional facility for 1 week, mice were randomly assigned to either control or irradiated groups. Animals were weighed and anesthetized via i.p. injection of ketamine and xylazine (100/15 mg per kg). Mice in the irradiated group were subjected to clinically relevant WBI (5 Gy twice weekly for a total cumulative dose of 40 Gy) [17]. Radiation was administered using a 137Cesium gamma irradiator (GammaCell 40, Nordion International). A Cerrobend® shield was utilized to minimize exposure outside the brain. The radiation dose received by the mice was verified using film dosimetry, as described [13, 14, 16, 21].
Assessment of cerebromicrovascular endothelial function
To determine how accelerated brain senescence induced by WBI affects cerebromicrovascular function, CBF responses elicited by the endothelium-dependent vasodilator agents acetylcholine and ATP were assessed at 3 months post WBI treatment. Mice in both groups were anesthetized with isoflurane (4% induction and 1% maintenance), endotracheally intubated, and ventilated (MousVent G500; Kent Scientific Co, Torrington, CT). A thermostatic heating pad (Kent Scientific Co, Torrington, CT) was used to maintain rectal temperature at 37 °C [52]. The right femoral artery was canulated for arterial blood pressure measurement (Living Systems Instrumentations, Burlington, VT). The blood pressure was within the physiological range throughout the experiments (90–110 mmHg). Mice were immobilized and placed on a stereotaxic frame (Leica Microsystems, Buffalo Grove, IL), and the scalp and periosteum were pulled aside. Mice were equipped with an open cranial window, and changes in CBF were assessed above the left somatosensory cortex using a laser Doppler probe (Transonic Systems Inc., Ithaca, NY), as described [39, 52, 53]. The cranial window was superfused with artificial cerebrospinal fluid (ACSF, composition: NaCl 119 mM, NaHCO3 26.2 mM, KCl 2.5 mM, NaH2PO4 1 mM, MgCl2 1.3 mM, glucose 10 mM, CaCl2 2.5 mM, pH = 7.3, 37 °C). To assess microvascular endothelial function, endothelium-dependent, NO mediated CBF responses to topical administration of acetylcholine (ACh; 10−5 mol/L)), and ATP (10−6 mol/L)) were obtained following established protocols [45]. In each study, the experimenter was blinded to the treatment of the animals. At the end of the experiments, the animals were killed by decapitation. All reagents used in this study were purchased from Sigma-Aldrich (St Louis, MO) unless otherwise indicated.
Statistical analysis
The data are presented as means ± standard error of the mean (SEM). Statistical analysis was conducted using the GraphPad Prism 8 software (La Jolla, CA, USA) [50, 54]. Student’s two-sample T-test was employed to compare the experimental results, and differences were deemed significant at p < 0.05.
Results
WBI induces persisting endothelial dysfunction
In order to determine whether WBI results in persisting cerebromicrovascular endothelial dysfunction, we assessed endothelium-dependent vasodilation in the cerebral cortex in mice at 3 months post-WBI. We found that CBF responses in the somatosensory cortex elicited by acetylcholine were significantly decreased in WBI-treated mice compared to control animals indicating impaired endothelial function at 3 months post-irradiation (Fig. 1A). In control mice, topical administration of ATP also resulted in significant CBF increases in the somatosensory cortex, whereas these responses were significantly attenuated in WBI-treated mice (Fig. 1B).
Fig. 1.
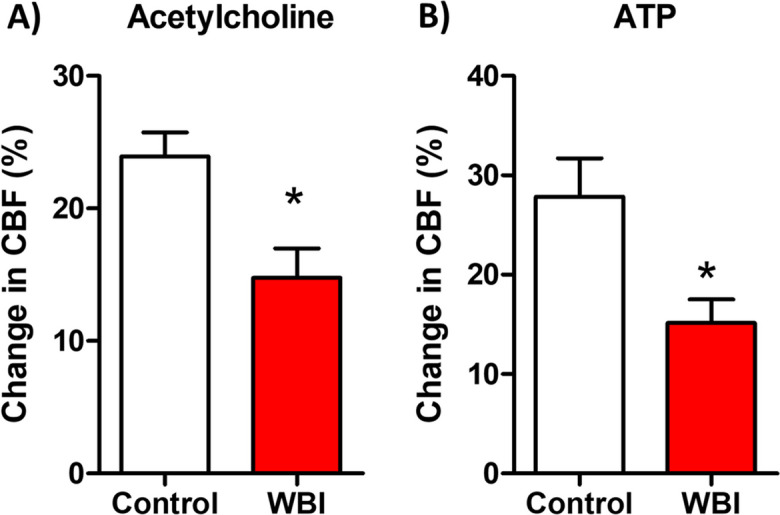
Whole brain irradiation (WBI) induces persistent endothelial dysfunction in the cerebral circulation. Laser Doppler probe measurements were utilized to assess changes in cerebral blood flow (CBF) in the cerebral cortex of mice following topical perfusion of the endothelium-dependent vasodilator agents acetylcholine (A) and ATP (B) at 3 months post-WBI. Bar graphs depict summary data. Importantly, both acetylcholine and ATP-induced CBF responses in the somatosensory cortex were significantly reduced in WBI-treated mice compared to control animals, indicative of impaired endothelial function persisting at 3 months post-irradiation. Data presented are mean ± S.E.M. (n = 8 for each data point). *P < 0.05 vs. control
Discussion
Our study aimed to investigate the hypothesis that WBI-induced endothelial injury promotes endothelial dysfunction, mimicking the aging phenotype. The results of our study provide compelling evidence of persisting cerebromicrovascular endothelial dysfunction following WBI. At 3 months post-irradiation, we observed a significant decrease in endothelium-dependent vasodilation in the cerebral cortex of WBI-treated mice compared to control animals. Specifically, the CBF responses elicited by acetylcholine were markedly impaired in WBI-treated mice, indicating compromised endothelial function. Additionally, the CBF responses induced by ATP, another endothelium-dependent vasodilator, were significantly attenuated in WBI-treated mice, further confirming the presence of endothelial dysfunction.
These findings align with previous studies demonstrating the detrimental effects of WBI on cerebromicrovascular health (Fig. 2). Endothelial cells play a crucial role in regulating CBF flow through the release of NO, a potent vasodilator, which relaxes vascular smooth muscle cells and pericytes. Impaired endothelial NO-mediated vasodilation decreases basal CBF and compromises the dynamic regulation of regional CBF, impeding the delivery of oxygen, nutrients to active brain regions, and the effective clearance of metabolic by-products. Consequently, this disruption in CBF can adversely affect neuronal activity and synaptic plasticity, contributing to cognitive impairment [41].
Fig. 2.
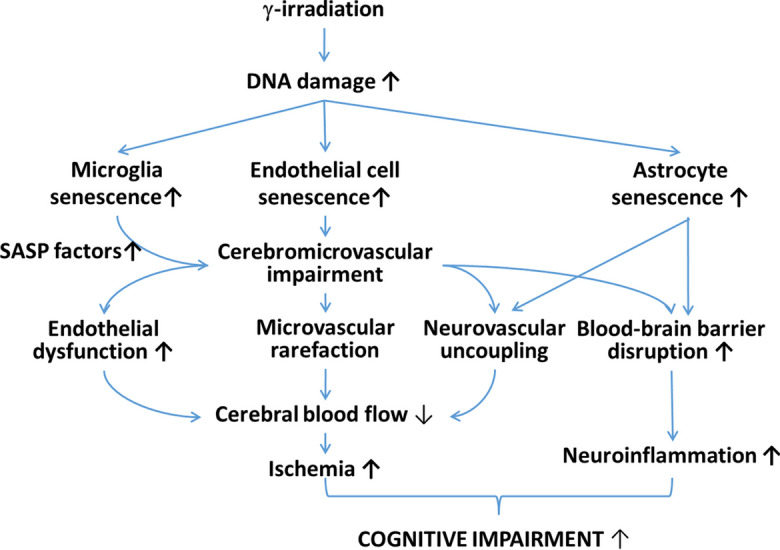
Proposed scheme for the contribution of neurovascular senescence and endothelial dysfunction to WBI-induced cognitive decline. γ-irradiation induced DNA damage and cellular senescence in cells of the neurovascular unit, including endothelial cells, perivascular microglia, and astrocytes. Endothelial senescence results in functional and structural impairment of the cerebral microcirculation, including endothelial dysfunction, impairment of neurovascular coupling responses, and microvascular rarefaction, all of which contribute to a significant decline in cerebral blood flow (CBF). γ-irradiation-induced neurovascular senescence disrupts the blood brain barrier, exacerbating neuroinflammation. Heightened inflammatory status of the neurovascular unit, due to endothelial senescence and the increased secretion of pro-inflammatory SASP factors from senescent microglia and astrocytes exacerbates cerebromicrovascular dysfunction and neuroinflammation. The resulting ischemic and inflammatory foci play a role in the pathogenesis of cognitive impairment. The model predicts that the aforementioned senescence-related structural and functional cerebromicrovascular alterations synergize to promote cognitive impairment in patients treated with WBI
The observed persisting endothelial dysfunction in WBI-treated mice adds to the growing body of evidence implicating cerebromicrovascular and neurovascular injury in the development of cognitive decline following WBI (Fig. 2). Our findings extend the results of previous studies demonstrating a decline in capillary density within the hippocampus, the cerebral cortex, and/or the white matter in response to WBI [13, 17, 19–21, 55]. Irradiation-induced microvascular rarefaction likely exacerbates the negative effects of impaired endothelium-mediated microvascular dilation and neurovascular coupling responses, compromising cerebral blood supply and, subsequently, cognition (Fig. 2). Furthermore, intact endothelial function is crucial for maintaining the integrity of the blood-brain barrier (BBB). Disruption of the BBB, as observed in WBI and γ-irradiation-induced microvascular injury [25–33], can lead to neuroinflammation and contribute to the genesis of cognitive impairment [34, 35] (Fig. 2).
One crucial mechanism underlying the detrimental effects of γ-irradiation on endothelial cells involves the generation of persistent DNA damage, triggering a chronic stress response known as cellular senescence [15, 18, 56, 57] (Fig. 2). Compelling evidence indicates that cerebromicrovascular endothelial cells (CMVECs) are particularly vulnerable to DNA damage induced by γ-irradiation, leading to the acquisition of senescent phenotypes in cultured CMVECs [18]. When cellular senescence is induced in culture, irradiated CMVECs undergo cell cycle arrest, display significant morphological changes, and develop a senescence-associated secretory phenotype (SASP), characterized by an increased secretion of pro-inflammatory cytokines [18]. The growing preclinical evidence highlights the contribution of senescent endothelial cells and their SASPs to the pathogenesis of microvascular disorders and cognitive decline associated with aging [49]. Moreover, studies in genetically modified mice, where cells expressing the senescence marker p16INK4A were depleted, have shown promising outcomes. These mice exhibit prolonged median lifespan, improved overall health, restored microvascular function, and enhanced cognition [58–67], providing further support for the pivotal role of cellular senescence in brain and cerebromicrovascular aging [49, 68–70].
Building upon these findings, our recent studies have demonstrated that eliminating senescent cells in WBI-treated mice yields protective effects on the regulation of cerebral blood flow and preserves cognitive performance [15]. These observations highlight the significance of targeting cellular senescence as a potential therapeutic strategy to mitigate the adverse consequences of WBI on cerebromicrovascular health and cognitive function (Fig. 2). Expanding upon these significant findings, our recent studies have provided compelling evidence that the elimination of senescent cells in WBI-treated mice confers remarkable protective effects on multiple aspects of cerebromicrovascular health and cognitive function [15]. Notably, the elimination of senescent cells was found to rescue neurovascular coupling responses [15]. The impaired neurovascular coupling observed in WBI-treated mice was ameliorated when senescent cells were targeted, suggesting a restoration of the intricate interplay between neurons, astrocytes, and endothelial cells within the neurovascular unit. This restoration of neurovascular coupling responses is of paramount importance as it ensures an adequate supply of oxygen and nutrients to active brain regions, thereby supporting optimal neuronal function. Furthermore, the elimination of senescent cells also exhibited a profound impact on preserving the integrity of the blood-brain barrier (BBB) in WBI-treated mice (Gulej et al. 2023 in press). Targeting senescent cells not only improves vasodilator function of the endothelial cells and restores BBB integrity but also likely mitigates the associated neuroinflammatory responses, thus providing a holistic therapeutic avenue to alleviate the genesis of cognitive decline following WBI treatment. These observations hold immense potential for the development of novel translational therapeutic strategies that can counteract the adverse consequences of WBI on cerebromicrovascular function and cognitive performance, ultimately improving the quality of life for individuals undergoing brain irradiation treatments. In the cerebral microcirculation, CMVECs are interconnected via specialized channels called gap junctions, facilitating the transfer of solutes and cytoplasmic signals [71]. This arrangement enables the formation of a functional syncytium [71], where a single senescent endothelial cell can directly influence the function and characteristics of neighboring CMVECs. Moreover, senescence can propagate within the microcirculation through the exposure of neighboring cells to paracrine factors released by senescent CMVECs, known as the senescence-associated secretory phenotype (SASP). This phenomenon, referred to as paracrine senescence or senescence-induced senescence, can result in the spread of senescence to adjacent cells. As a consequence, the increased presence of senescent endothelial and astroglial cells in the brains of WBI-treated mice is anticipated to impact a substantial portion of the cerebral microcirculatory network. Conversely, the elimination of senescent cells through treatment with senolytics is likely to confer protective effects throughout the entire cerebral microcirculation.
In this study, we have endeavored to provide valuable insights into the effects of WBI on cerebromicrovascular endothelial function. However, it is imperative to acknowledge certain limitations that impact the interpretation and generalizability of our findings, including the lack of comprehensive data on systemic cardiovascular function. Future research could also explore WBI-induced transcriptomic changes in endothelial cells, including changes in mRNA and protein expression of NOS isoforms and potential alterations of the local renin-angiotensin II system [72, 73].
In conclusion, our results support the hypothesis that WBI-induced endothelial injury promotes endothelial dysfunction, resembling the aging phenotype. The observed persisting endothelial dysfunction in WBI-treated mice contributes to our understanding of the long-term consequences of WBI and the role of cerebromicrovascular health in preserving cognitive function. Further research is warranted to elucidate the precise mechanisms underlying WBI-induced, senescence-related endothelial dysfunction, and its impact on cognitive decline, with the ultimate goal of developing targeted therapeutic strategies to mitigate these adverse effects.
Acknowledgements
We would like to express our sincere gratitude to Prof. William E. Sonntag (University of Oklahoma HSC) for his invaluable advice and assistance throughout the course of our research projects related to the pathophysiology of WBI-induced cognitive impairment. His expertise and guidance have been instrumental in shaping the design and execution of our experimental cohort. We are truly thankful for his unwavering support and dedication. The 3.5 version of ChatGPT, developed by OpenAI, was used as a language tool to refine our writing, enhancing the clarity of our work.
Funding
Open access funding provided by Semmelweis University. This work was supported by grants from the American Heart Association (ANT: AHA834339) and (ST: AHA CDA941290), the Oklahoma Center for the Advancement of Science and Technology, the National Institute on Aging (RF1AG072295, R01AG055395, R01AG068295; R01AG070915, K01AG073614, K01AG073613, R03AG070479), the National Institute of Neurological Disorders and Stroke (R01NS100782), the National Cancer Institute (R01CA255840), the Oklahoma Shared Clinical and Translational Resources (U54GM104938) with an Institutional Development Award (IDeA) from NIGMS, the Presbyterian Health Foundation, the Reynolds Foundation, the Oklahoma Nathan Shock Center (P30AG050911), and the Cellular and Molecular GeroScience CoBRE (P20GM125528), the NCI Cancer Center Support Grant (P30 CA225520) and the Oklahoma Tobacco Settlement Endowment Trust. TK was supported by project no. TKP2021-NKTA-47, implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-NKTA funding scheme; by funding through the National Cardiovascular Laboratory Program (RRF-2.3.1-21-2022-00003) provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund; project no. 135784 implemented with the support provided from the National Research, Development and Innovation Fund of Hungary, financed under the K_20 funding scheme and the European University for Well-Being (EUniWell) program (grant agreement number: 101004093/ EUniWell/EAC-A02-2019 / EAC-A02-2019-1).
Data Availability
All data generated or analyzed during this study are included in the manuscript and/or in its supplementary information files. The raw datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Disclaimer
The funding sources had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the American Heart Association, or the Presbyterian Health Foundation.
Competing interests
Dr. Anna Csiszar serves as Associate Editor for The Journal of Gerontology, Series A: Biological Sciences and Medical Sciences and GeroScience. Dr. Zoltan Ungvari serves as Editor-in-Chief for GeroScience. Dr. Stefano Tarantini and Dr. Andriy Yabluchanskiy serve as Associate Editors for GeroScience.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tamas Kiss, Anna Ungvari, Rafal Gulej and Ádám Nyúl-Tóth contributed equally to this work.
References
- 1.Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14:48–54. doi: 10.1007/s11912-011-0203-y. [DOI] [PubMed] [Google Scholar]
- 2.Jung EW, Rakowski JT, Delly F, Jagannathan J, Konski AA, Guthikonda M, Kim H, Mittal S. Gamma Knife radiosurgery in the management of brainstem metastases. Clin Neurol Neurosurg. 2013;115:2023–2028. doi: 10.1016/j.clineuro.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Welzel G, Fleckenstein K, Schaefer J, Hermann B, Kraus-Tiefenbacher U, Mai SK, Wenz F. Memory function before and after whole brain radiotherapy in patients with and without brain metastases. Int J Radiat Oncol Biol Phys. 2008;72:1311–1318. doi: 10.1016/j.ijrobp.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Bentzen SM, Li J, Renschler M, Mehta MP. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys. 2008;71:64–70. doi: 10.1016/j.ijrobp.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 5.Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994;12:627–642. doi: 10.1200/JCO.1994.12.3.627. [DOI] [PubMed] [Google Scholar]
- 6.Imperato JP, Paleologos NA, Vick NA. Effects of treatment on long-term survivors with malignant astrocytomas. Ann Neurol. 1990;28:818–822. doi: 10.1002/ana.410280614. [DOI] [PubMed] [Google Scholar]
- 7.Johannesen TB, Lien HH, Hole KH, Lote K. Radiological and clinical assessment of long-term brain tumour survivors after radiotherapy. Radiother Oncol. 2003;69:169–176. doi: 10.1016/S0167-8140(03)00192-0. [DOI] [PubMed] [Google Scholar]
- 8.Welzel G, Fleckenstein K, Mai SK, Hermann B, Kraus-Tiefenbacher U, Wenz F. Acute neurocognitive impairment during cranial radiation therapy in patients with intracranial tumors. Strahlenther Onkol. 2008;184:647–654. doi: 10.1007/s00066-008-1830-6. [DOI] [PubMed] [Google Scholar]
- 9.Silber JH, Radcliffe J, Peckham V, Perilongo G, Kishnani P, Fridman M, Goldwein JW, Meadows AT. Whole-brain irradiation and decline in intelligence: the influence of dose and age on IQ score. J Clin Oncol. 1992;10:1390–1396. doi: 10.1200/JCO.1992.10.9.1390. [DOI] [PubMed] [Google Scholar]
- 10.Lamproglou I, Martin S, Diserbo M, Multon E, Petiet A, Colas-Linhart N, Bok B, Martin C. Total body 4.5 Gy gamma irradiation-induced early delayed learning and memory dysfunction in the rat. Cell Mol Biol (Noisy-le-grand). 2001;47:453–457. [PubMed] [Google Scholar]
- 11.Shi L, Adams MM, Long A, Carter CC, Bennett C, Sonntag WE, Nicolle MM, Robbins M, D'Agostino R, Brunso-Bechtold JK. Spatial learning and memory deficits after whole-brain irradiation are associated with changes in NMDA receptor subunits in the hippocampus. Radiat Res. 2006;166:892–899. doi: 10.1667/RR0588.1. [DOI] [PubMed] [Google Scholar]
- 12.Soussain C, Ricard D, Fike JR, Mazeron JJ, Psimaras D, Delattre JY. CNS complications of radiotherapy and chemotherapy. Lancet. 2009;374:1639–1651. doi: 10.1016/S0140-6736(09)61299-X. [DOI] [PubMed] [Google Scholar]
- 13.Warrington JP, Csiszar A, Mitschelen M, Lee YW, Sonntag WE. Whole brain radiation-induced impairments in learning and memory are time-sensitive and reversible by systemic hypoxia. PLoS One. 2012;7:e30444. doi: 10.1371/journal.pone.0030444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ungvari Z, Tarantini S, Hertelendy P, Valcarcel-Ares MN, Fulop GA, Logan S, Kiss T, Farkas E, Csiszar A, Yabluchanskiy A. Cerebromicrovascular dysfunction predicts cognitive decline and gait abnormalities in a mouse model of whole brain irradiation-induced accelerated brain senescence. Geroscience. 2017;39:33–42. doi: 10.1007/s11357-017-9964-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yabluchanskiy A, Tarantini S, Balasubramanian P, Kiss T, Csipo T, Fulop GA, Lipecz A, Ahire C, DelFavero J, Nyul-Toth A, Sonntag WE, Schwartzman ML, Campisi J, Csiszar A, Ungvari Z. Pharmacological or genetic depletion of senescent astrocytes prevents whole brain irradiation-induced impairment of neurovascular coupling responses protecting cognitive function in mice. Geroscience. 2020;42:409–428. doi: 10.1007/s11357-020-00154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Institoris A, Murphy-Royal C, Tarantini S, Yabluchanskiy A, Haidey JN, Csiszar A, Ungvari Z, Gordon GR. Whole brain irradiation in mice causes long-term impairment in astrocytic calcium signaling but preserves astrocyte-astrocyte coupling. Geroscience. 2021;43:197–212. doi: 10.1007/s11357-020-00289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li B, Yabluchanskiy A, Tarantini S, Allu SR, Şencan-Eğilmez I, Leng J, Alfadhel MAH, Porter JE, Fu B, Ran C, Erdener SE, Boas DA, Vinogradov SA, Sonntag WE, Csiszar A, Ungvari Z, Sakadžić S. Measurements of cerebral microvascular blood flow, oxygenation, and morphology in a mouse model of whole-brain irradiation-induced cognitive impairment by two-photon microscopy and optical coherence tomography: evidence for microvascular injury in the cerebral white matter. Geroscience. 2023;45(3):1491–510. 10.1007/s11357-023-00735-3. [DOI] [PMC free article] [PubMed]
- 18.Ungvari Z, Podlutsky A, Sosnowska D, Tucsek Z, Toth P, Deak F, Gautam T, Csiszar A, Sonntag WE. Ionizing radiation promotes the acquisition of a senescence-associated secretory phenotype and impairs angiogenic capacity in cerebromicrovascular endothelial cells: role of increased DNA damage and decreased DNA repair capacity in microvascular radiosensitivity. J Gerontol A Biol Sci Med Sci. 2013;68:1443–1457. doi: 10.1093/gerona/glt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warrington JP, Ashpole N, Csiszar A, Lee YW, Ungvari Z, Sonntag WE. Whole brain radiation-induced vascular cognitive impairment: mechanisms and implications. J Vasc Res. 2013;50:445–457. doi: 10.1159/000354227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashpole NM, Warrington JP, Mitschelen MC, Yan H, Sosnowska D, Gautam T, Farley JA, Csiszar A, Ungvari Z, Sonntag WE. Systemic influences contribute to prolonged microvascular rarefaction after brain irradiation: a role for endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2014;307:H858–H868. doi: 10.1152/ajpheart.00308.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warrington JP, Csiszar A, Johnson DA, Herman TS, Ahmad S, Lee YW, Sonntag WE. Cerebral microvascular rarefaction induced by whole brain radiation is reversible by systemic hypoxia in mice. Am J Physiol Heart Circ Physiol. 2011;300:H736–H744. doi: 10.1152/ajpheart.01024.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lo EH, Frankel KA, Steinberg GK, DeLaPaz RL, Fabrikant JI. High-dose single-fraction brain irradiation: MRI, cerebral blood flow, electrophysiological, and histological studies. Int J Radiat Oncol Biol Phys. 1992;22:47–55. doi: 10.1016/0360-3016(92)90981-M. [DOI] [PubMed] [Google Scholar]
- 23.Wilhelm I, Nyul-Toth A, Kozma M, Farkas AE, Krizbai IA. Role of pattern recognition receptors of the neurovascular unit in inflamm-aging. Am J Physiol Heart Circ Physiol. 2017;313:H1000–H1012. doi: 10.1152/ajpheart.00106.2017. [DOI] [PubMed] [Google Scholar]
- 24.Costea L, Mészáros Á, Bauer H, Bauer HC, Traweger A, Wilhelm I, Farkas AE, Krizbai IA. The blood-brain barrier and its intercellular junctions in age-related brain disorders. Int J Mol Sci. 2019;20(21):5472. 10.3390/ijms20215472. [DOI] [PMC free article] [PubMed]
- 25.Bezek S, Trnovec T, Scasnar V, Durisova M, Kukan M, Kallay Z, Laginova V, Svoboda V. Irradiation of the head by 60Co opens the blood-brain barrier for drugs in rats. Experientia. 1990;46:1017–1020. doi: 10.1007/BF01940660. [DOI] [PubMed] [Google Scholar]
- 26.Fauquette W, Amourette C, Dehouck MP, Diserbo M. Radiation-induced blood-brain barrier damages: an in vitro study. Brain Res. 2012;1433:114–126. doi: 10.1016/j.brainres.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida Y, Sejimo Y, Kurachi M, Ishizaki Y, Nakano T, Takahashi A. X-ray irradiation induces disruption of the blood-brain barrier with localized changes in claudin-5 and activation of microglia in the mouse brain. Neurochem Int. 2018;119:199–206. doi: 10.1016/j.neuint.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Yuan H, Gaber MW, Boyd K, Wilson CM, Kiani MF, Merchant TE. Effects of fractionated radiation on the brain vasculature in a murine model: blood-brain barrier permeability, astrocyte proliferation, and ultrastructural changes. Int J Radiat Oncol Biol Phys. 2006;66:860–866. doi: 10.1016/j.ijrobp.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 29.Yuan H, Gaber MW, McColgan T, Naimark MD, Kiani MF, Merchant TE. Radiation-induced permeability and leukocyte adhesion in the rat blood-brain barrier: modulation with anti-ICAM-1 antibodies. Brain Res. 2003;969:59–69. doi: 10.1016/S0006-8993(03)02278-9. [DOI] [PubMed] [Google Scholar]
- 30.Allen BD, Limoli CL. Breaking barriers: neurodegenerative repercussions of radiotherapy induced damage on the blood-brain and blood-tumor barrier. Free Radic Biol Med. 2022;178:189–201. doi: 10.1016/j.freeradbiomed.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moretti R, Caruso P. An iatrogenic model of brain small-vessel disease: post-radiation encephalopathy. Int J Mol Sci. 2020;21(18):6506. 10.3390/ijms21186506. [DOI] [PMC free article] [PubMed]
- 32.Nair V, Roth LJ. Effect of X-irradiation and certain other treatments on blood brain barrier permeability. Radiat Res. 1964;23:249–264. doi: 10.2307/3571606. [DOI] [PubMed] [Google Scholar]
- 33.Remler MP, Marcussen WH, Tiller-Borsich J. The late effects of radiation on the blood brain barrier. Int J Radiat Oncol Biol Phys. 1986;12:1965–1969. doi: 10.1016/0360-3016(86)90133-1. [DOI] [PubMed] [Google Scholar]
- 34.Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG, Benzinger TLS, Fagan AM, Ringman JM, Schneider LS, Morris JC, Chui HC, Law M, Toga AW, Zlokovic BV. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25:270–276. doi: 10.1038/s41591-018-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-brain barrier: from physiology to disease and back. Physiol Rev. 2019;99:21–78. doi: 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EM. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc. 2014;3:e000787. doi: 10.1161/JAHA.114.000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am J Physiol Heart Circ Physiol. 2014;306:H299–H308. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci. 2014;69:1339–1352. doi: 10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell. 2015;14:1034–1044. doi: 10.1111/acel.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toth P, Tarantini S, Davila A, Valcarcel-Ares MN, Tucsek Z, Varamini B, Ballabh P, Sonntag WE, Baur JA, Csiszar A, Ungvari Z. Purinergic glio-endothelial coupling during neuronal activity: role of P2Y1 receptors and eNOS in functional hyperemia in the mouse somatosensory cortex. Am J Physiol Heart Circ Physiol. 2015;309:H1837–H1845. doi: 10.1152/ajpheart.00463.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol. 2017;312:H1–H20. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, Farkas E, Perz A, Rabinovitch PS, Sonntag WE, Csiszar A, Ungvari Z. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell. 2018;17(2):e12731. 10.1111/acel.12731. [DOI] [PMC free article] [PubMed]
- 43.Csiszar A, Yabluchanskiy A, Ungvari A, Ungvari Z, Tarantini S. Overexpression of catalase targeted to mitochondria improves neurovascular coupling responses in aged mice. Geroscience. 2019;41:609–617. doi: 10.1007/s11357-019-00111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lipecz A, Csipo T, Tarantini S, Hand RA, Ngo BN, Conley S, Nemeth G, Tsorbatzoglou A, Courtney DL, Yabluchanska V, Csiszar A, Ungvari ZI, Yabluchanskiy A. Age-related impairment of neurovascular coupling responses: a dynamic vessel analysis (DVA)-based approach to measure decreased flicker light stimulus-induced retinal arteriolar dilation in healthy older adults. Geroscience. 2019;41(3):341–9. 10.1007/s11357-019-00078-y. [DOI] [PMC free article] [PubMed]
- 45.Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, Hertelendy P, Kinter M, Ballabh P, Sule Z, Farkas E, Baur JA, Sinclair DA, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019;24:101192. doi: 10.1016/j.redox.2019.101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tarantini S, Yabluchanskiy A, Csipo T, Fulop G, Kiss T, Balasubramanian P, DelFavero J, Ahire C, Ungvari A, Nyul-Toth A, Farkas E, Benyo Z, Toth A, Csiszar A, Ungvari Z. Treatment with the poly(ADP-ribose) polymerase inhibitor PJ-34 improves cerebromicrovascular endothelial function, neurovascular coupling responses and cognitive performance in aged mice, supporting the NAD+ depletion hypothesis of neurovascular aging. Geroscience. 2019;41:533–542. doi: 10.1007/s11357-019-00101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiedenhoeft T, Tarantini S, Nyul-Toth A, Yabluchanskiy A, Csipo T, Balasubramanian P, Lipecz A, Kiss T, Csiszar A, Csiszar A, Ungvari Z. Fusogenic liposomes effectively deliver resveratrol to the cerebral microcirculation and improve endothelium-dependent neurovascular coupling responses in aged mice. Geroscience. 2019;41:711–725. doi: 10.1007/s11357-019-00102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Csipo T, Cassidy BR, Balasubramanian P, Drevets DA, Ungvari ZI, Yabluchanskiy A. Endothelial dysfunction and impaired neurovascular coupling responses precede cognitive impairment in a mouse model of geriatric sepsis. Front Aging Neurosci. 2021;13:644733. doi: 10.3389/fnagi.2021.644733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tarantini S, Balasubramanian P, Delfavero J, Csipo T, Yabluchanskiy A, Kiss T, Nyul-Toth A, Mukli P, Toth P, Ahire C, Ungvari A, Benyo Z, Csiszar A, Ungvari Z. Treatment with the BCL-2/BCL-xL inhibitor senolytic drug ABT263/Navitoclax improves functional hyperemia in aged mice. Geroscience. 2021;43:2427–2440. doi: 10.1007/s11357-021-00440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tarantini S, Nyul-Toth A, Yabluchanskiy A, Csipo T, Mukli P, Balasubramanian P, Ungvari A, Toth P, Benyo Z, Sonntag WE, Ungvari Z, Csiszar A. Endothelial deficiency of insulin-like growth factor-1 receptor (IGF1R) impairs neurovascular coupling responses in mice, mimicking aspects of the brain aging phenotype. Geroscience. 2021;43:2387–2394. doi: 10.1007/s11357-021-00405-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahire C, Nyul-Toth A, DelFavero J, Gulej R, Faakye JA, Tarantini S, Kiss T, Kuan-Celarier A, Balasubramanian P, Ungvari A, Tarantini A, Nagaraja R, Yan F, Tang Q, Mukli P, Csipo T, Yabluchanskiy A, Campisi J, Ungvari Z, Csiszar A. Accelerated cerebromicrovascular senescence contributes to cognitive decline in a mouse model of paclitaxel (Taxol)-induced chemobrain. Aging Cell. 2023;22(7):e13832. 10.1111/acel.13832. [DOI] [PMC free article] [PubMed]
- 52.Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari ZI. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and down-regulation of NADPH oxidas. Am J Physiol Heart Circ Physiol. 2014;306:H299–H308. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tarantini S, Hertelendy P, Tucsek Z, Valcarcel-Ares MN, Smith N, Menyhart A, Farkas E, Hodges E, Towner R, Deak F, Sonntag WE, Csiszar A, Ungvari Z, Toth P. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J Cereb Blood Flow Metab. 2015;35:1871–1881. doi: 10.1038/jcbfm.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tarantini S, Balasubramanian P, Yabluchanskiy A, Ashpole NM, Logan S, Kiss T, Ungvari A, Nyul-Toth A, Schwartzman ML, Benyo Z, Sonntag WE, Csiszar A, Ungvari Z. IGF1R signaling regulates astrocyte-mediated neurovascular coupling in mice: implications for brain aging. Geroscience. 2021;43:901–911. doi: 10.1007/s11357-021-00350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee WH, Cho HJ, Sonntag WE, Lee YW. Radiation attenuates physiological angiogenesis by differential expression of VEGF, Ang-1, tie-2 and Ang-2 in rat brain. Radiat Res. 2011;176:753–760. doi: 10.1667/RR2647.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coppe JP, Rodier F, Patil CK, Freund A, Desprez PY, Campisi J. Tumor suppressor and aging biomarker p16(INK4a) induces cellular senescence without the associated inflammatory secretory phenotype. J Biol Chem. 2011;286:36396–36403. doi: 10.1074/jbc.M111.257071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, Chung JW, Kim DH, Poon Y, David N, Baker DJ, van Deursen JM, Campisi J, Elisseeff JH. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23:775–781. doi: 10.1038/nm.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abdul-Aziz AM, Sun Y, Hellmich C, Marlein CR, Mistry J, Forde E, Piddock RE, Shafat MS, Morfakis A, Mehta T, Di Palma F, Macaulay I, Ingham CJ, Haestier A, Collins A, Campisi J, Bowles KM, Rushworth SA. Acute myeloid leukemia induces protumoral p16INK4a-driven senescence in the bone marrow microenvironment. Blood. 2019;133:446–456. doi: 10.1182/blood-2018-04-845420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim HN, Chang J, Iyer S, Han L, Campisi J, Manolagas SC, Zhou D, Almeida M. Elimination of senescent osteoclast progenitors has no effect on the age-associated loss of bone mass in mice. Aging Cell. 2019;18:e12923. doi: 10.1111/acel.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patil P, Dong Q, Wang D, Chang J, Wiley C, Demaria M, Lee J, Kang J, Niedernhofer LJ, Robbins PD, Sowa G, Campisi J, Zhou D, Vo N. Systemic clearance of p16(INK4a) -positive senescent cells mitigates age-associated intervertebral disc degeneration. Aging Cell. 2019;18:e12927. doi: 10.1111/acel.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, Negley BA, Sfeir JG, Ogrodnik MB, Hachfeld CM, LeBrasseur NK, Drake MT, Pignolo RJ, Pirtskhalava T, Tchkonia T, Oursler MJ, Kirkland JL, Khosla S. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med. 2017;23:1072–1079. doi: 10.1038/nm.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA, Sepe A, Johnson KO, Stout MB, Giorgadze N, Jensen MD, LeBrasseur NK, Tchkonia T, Kirkland JL. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife. 2015:4. [DOI] [PMC free article] [PubMed]
- 66.Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang-Verzosa G, Zhu Y, Schafer MJ, Tchkonia T, Kirkland JL, Miller JD. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15:973–977. doi: 10.1111/acel.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baar MP, Brandt RMC, Putavet DA, Klein JDD, Derks KWJ, Bourgeois BRM, Stryeck S, Rijksen Y, van Willigenburg H, Feijtel DA, van der Pluijm I, Essers J, van Cappellen WA, van IJcken WF, Houtsmuller AB, Pothof J, de Bruin RWF, Madl T, Hoeijmakers JHJ, Campisi J, de Keizer PLJ. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell. 2017;169(1):132–47.e16. 10.1016/j.cell.2017.02.031. [DOI] [PMC free article] [PubMed]
- 68.Kiss T, Nyul-Toth A, DelFavero J, Balasubramanian P, Tarantini S, Faakye J, Gulej R, Ahire C, Ungvari A, Yabluchanskiy A, Wiley G, Garman L, Ungvari Z, Csiszar A. Spatial transcriptomic analysis reveals inflammatory foci defined by senescent cells in the white matter, hippocampi and cortical grey matter in the aged mouse brain. Geroscience. 2022;44:661–681. doi: 10.1007/s11357-022-00521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kiss T, Nyul-Toth A, Balasubramanian P, Tarantini S, Ahire C, DelFavero J, Yabluchanskiy A, Csipo T, Farkas E, Wiley G, Garman L, Csiszar A, Ungvari Z. Single-cell RNA sequencing identifies senescent cerebromicrovascular endothelial cells in the aged mouse brain. Geroscience. 2020;42:429–444. doi: 10.1007/s11357-020-00177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fulop GA, Kiss T, Tarantini S, Balasubramanian P, Yabluchanskiy A, Farkas E, Bari F, Ungvari Z, Csiszar A. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience. 2018;40:513–521. doi: 10.1007/s11357-018-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ungvari Z, Tarantini S, Kiss T, Wren JD, Giles CB, Griffin CT, Murfee WL, Pacher P, Csiszar A. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol. 2018;15:555–565. doi: 10.1038/s41569-018-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dushpanova A, Agostini S, Ciofini E, Cabiati M, Casieri V, Matteucci M, Del Ry S, Clerico A, Berti S, Lionetti V. Gene silencing of endothelial von Willebrand Factor attenuates angiotensin II-induced endothelin-1 expression in porcine aortic endothelial cells. Sci Rep. 2016;6:30048. doi: 10.1038/srep30048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee TC, Greene-Schloesser D, Payne V, Diz DI, Hsu FC, Kooshki M, Mustafa R, Riddle DR, Zhao W, Chan MD, Robbins ME. Chronic administration of the angiotensin-converting enzyme inhibitor, ramipril, prevents fractionated whole-brain irradiation-induced perirhinal cortex-dependent cognitive impairment. Radiat Res. 2012;178:46–56. doi: 10.1667/RR2731.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in the manuscript and/or in its supplementary information files. The raw datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


