Abstract
Moraxella catarrhalis isolates express lipooligosaccharide (LOS) molecules on their surface, which share epitopes similar to that of the Neisseria and Haemophilus species. These common LOS epitopes have been implicated in various steps of pathogenesis for the different organisms. In this study, a cluster of three LOS glycosyltransferase genes (lgt) were identified in M. catarrhalis 7169, a strain that produces a serotype B LOS. Mutants in these glycosyltransferase genes were constructed, and the resulting LOS phenotypes were consistent with varying degrees of truncation compared to wild-type LOS. The LOS structures of each lgt mutant were no longer detected by a monoclonal antibody (MAb 4G5) specific to a highly conserved terminal epitope nor by a monoclonal antibody (MAb 3F7) specific to the serotype B LOS side chain. Mass spectrometry of the LOS glycoforms assembled by two of these lgt mutants indicated that lgt1 encodes an α(1-2) glucosyltransferase and the lgt2 encodes a β(1-4) galactosyltransferase. However, these structural studies could not delineate the function for lgt3. Therefore, M. catarrhalis lgt3 was introduced into a defined β(1-4) glucosyltransferase Haemophilus ducreyi 35000glu− mutant in trans, and monoclonal antibody analysis confirmed that Lgt3 complemented the LOS defect. These data suggest that lgt3 encodes a glucosyltransferase involved in the addition of a β(1-4)-linked glucose to the inner core. Furthermore, we conclude that this enzymatic step is essential for the assembly of the complete LOS glycoform expressed by M. catarrhalis 7169.
Moraxella catarrhalis is a gram-negative human respiratory pathogen that causes 15 to 20% of acute otitis media in children (56). In addition, this bacterium is responsible for 10 to 35% of lower respiratory infections in adults with chronic obstructive pulmonary disease, the fourth leading cause of death in the United States (20). The emergence of M. catarrhalis as an important human pathogen has occurred in the past 15 years due to the increasing prevalence of β-lactamase-positive strains, the high incidence of recurrent infections despite successful antibiotic treatment, and the lack of an effective vaccine (10, 39, 50, 56). Another factor that likely contributes to the persistence of M. catarrhalis disease is the lack of understanding of the basic bacterial factors and mechanisms that promote colonization and survival in the host.
Although there have been a number of putative virulence factors described for M. catarrhalis, the actual role of these components in pathogenesis remains largely undefined (13, 19, 20, 31, 53). One of the most prominent surface components expressed in the outer membrane of this bacterium is the lipooligosaccharide (LOS) (12). The LOS of M. catarrhalis is similar to the lipopolysaccharide (LPS) of other gram-negative organisms, but it lacks a repeating O antigen (21). Previous studies have suggested that LOS is important for the pathogenesis of other respiratory pathogens, such as Neisseria meningitidis and Haemophilus influenzae, aiding in adherence of the bacterium to the mucosal epithelium and in the destruction of the mucosal barrier (16, 30, 51, 52). Some studies have demonstrated that the LOS of M. catarrhalis may also aid in adherence of this bacterium to epithelial cells, while other studies have indicated that LOS may elicit antibody production, suggesting that this major glycolipid has potential as a vaccine candidate (2, 23, 24, 27).
Previous studies using polyclonal antisera and structural analyses have identified three major LOS serotypes for M. catarrhalis (7-9, 21, 55). Clinical isolates were grouped into LOS serotypes A (60%), B (30%), and C (5%), with 5% of the strains unidentified (21, 26, 55). However, some cross-reactivity exists between serotypes A and C, and there has yet to be a correlation established between LOS serotype as it relates to colonization and infection (21, 43-45). Despite these data, the genes and gene products responsible for the biosynthesis and assembly of M. catarrhalis LOS and the role of this molecule in pathogenesis remain only partially defined. Previous studies have identified two genes involved in the biosynthesis of M. catarrhalis LOS: the UDP-glucose-4-epimerase (galE) gene in a serotype A strain (59) and the 2-keto-3-deoxyoctulosonic acid (KDO)-8-phosphate synthase (kdsA) gene in a serotype B strain (33).
In this study, we have identified and characterized a cluster of three glycosyltransferase genes involved in the biosynthesis of LOS from a serotype B strain, M. catarrhalis 7169. The gene cluster was identified in the National Center for Biotechnology Information (NCBI) patented database of M. catarrhalis using nucleotide sequence homology to glycosyltransferase genes from other gram-negative respiratory pathogens. Construction of isogenic mutants for the first two genes in the cluster and analysis of corresponding LOS structures has determined that these lipooligosaccharide glycosyltransferase genes (lgt) encode an α(1-2) glucosyltransferase (Lgt1) and a β(1-4) galactosyltransferase (Lgt2). Deletion of the third putative glycosyltransferase (Lgt3) resulted in the production of a deeply truncated LOS molecule. Our studies suggest that Lgt3 functions by the addition of a β(1-4)-linked glucose to the inner core of the LOS, and this step plays a critical role that allows for the continuation of the biosynthesis of the major oligosaccharide glycoform assembled by M. catarrhalis. This report describes the isolation and characterization of glycosyltransferase genes involved in the biosynthesis of M. catarrhalis LOS, and these studies provide insight into the steps involved in the assembly of this important glycolipid.
MATERIALS AND METHODS
Strains, plasmids, and culture conditions.
The bacterial strains and plasmids used in this study are described in Table 1. M. catarrhalis 7169 was cultured on standard brain heart infusion (BHI) agar plates at 35.5°C in 5% CO2. Escherichia coli XL1-Blue was used as the host strain for plasmid DNA manipulations. E. coli was cultured in standard Luria-Bertani (LB) medium at 35.5°C in 5% CO2. Haemophilus ducreyi strains were cultured on chocolate agar plates or in BHI broth at 35.5°C in 5% CO2, as previously described (5). Antibiotics were supplemented as necessary as 20-μg/ml kanamycin, 100-μg/ml ampicillin, or chloramphenicol (1 μg/ml).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source and/or reference |
|---|---|---|
| Strains | ||
| E. coli XL1-Blue | Host strain used for cloning | Stratagene |
| M. catarrhalis | ||
| 7169 | Wild-type strain, pediatric middle ear isolate | Howard Faden (Children's Hospital, Buffalo, N.Y.); 34, 35 |
| 7169::lgt1KB | lgt1 kanamycin-resistant isogenic mutant of strain 7169 | This study |
| 7169::lgt2KM | lgt2 kanamycin-resistant isogenic mutant of strain 7169 | This study |
| 7169::lgt3KH | lgt3 kanamycin-resistant isogenic mutant of strain 7169 | This study |
| 7169::lgt1KB(REV) | 7169::lgt1KB mutant complemented to create a wild-type lgt1 revertant | This study |
| 7169::lgt1KM(REV) | 7169::lgt2KM mutant complemented to create a wild-type lgt2 revertant | This study |
| 7169::lgt3KH(REV) | 7169::lgt3KH mutant complemented to create a wild-type lgt3 revertant | This study |
| H. ducreyi | ||
| 35000 | Wild-type strain isolated in Winnipeg, Canada, binds MAb 1B2-1B7 | 17 |
| 35000glu− | Isogenic LOS mutant reported previously to be derived from strain 35000 by disruption of lgtF gene and insertion of a cat cassette, does not bind MAb 1B2-1B7 | 11 |
| 35000glu−(pLS3PR-E) | Strain derived from the electroporation of pLS3PR-E into strain 35000glu− to complement the inactivated lgtF gene, binds MAb 1B2-1B7 | This study |
| 35000glu−(pLS3IN-1) | Strain derived from the electroporation of pLS3IN-1 into strain 35000glu− to complement the inactivated lgtF genes, does not bind MAb 1B2-1B7 | This study |
| Plasmids | ||
| pGEM-T easy | Commercially available vector used for TA cloning and mutagenesis | Promega |
| pUC18K | Construct containing aphA-3 kanamycin resistance cassette | 37 |
| pLGT-1KE | lgt1 TA cloned into pGEM-T easy | This study |
| pLGT-2KE | lgt2 TA cloned into pGEM-T easy | This study |
| pLGT-3KE | lgt3 TA cloned into pGEM-T easy | This study |
| pLGT-1kanB | lgt1 containing an internal deletion and insertion of aphA-3 cassette | This study |
| pLGT-2kanM | lgt2 containing an internal deletion and insertion of aphA-3 cassette | This study |
| pLGT-3kanH | lgt3 containing an internal deletion and insertion of aphA-3 cassette | This study |
| pLS88 | E. coli-H. ducreyi shuttle vector, Kanr, Smr, Sulr | 6 |
| pLS3PR-E | pLS88 vector with a 2.2-kb insert of M. catarrhalis 7169 lgt3 gene (includes 265 bp of 5′ flanking DNA) | This study |
| pLS3IN-1 | pLS88 vector with a 1.3-kb insert of M. catarrhalis 7169 lgt3 gene (internal portion) | This study |
Standard recombinant DNA techniques.
Standard molecular biological techniques were used in this study as previously described (49). Plasmids were obtained from E. coli using QIAprep spin miniprep kits (QIAGEN, Chatsworth, Calif.). Chromosomal DNA was obtained from M. catarrhalis using standard methods, as previously described (48), and DNA was purified using QIAGEN purification kits. PCR was performed using Taq DNA polymerase (Invitrogen, Carlsbad, Calif.), and all other enzymes were obtained from New England Biolabs (Beverly, Mass.) and used according to company protocols. XL1-Blue E. coli was made electrocompetent, and plasmid DNA was introduced by electroporation, as previously described (49). DNA nucleotide sequences were obtained for all constructs via automated DNA sequencing at the RPCI Biopolymer Facility (Roswell Park Cancer Institute, Buffalo, N.Y.), and all sequences were analyzed using MacVector, version 7.2, software (Accelrys, San Diego, Calif.).
Construction of M. catarrhalis 7169 isogenic mutants.
The PCR primers used to clone and sequence lgt1, lgt2, and lgt3 from strain 7169 are listed in Table 2. The primers were designed based on the sequence homologies between the M. catarrhalis genome in the NCBI patent database and the reported sequence of other known glycosyltransferase genes. Each individual gene was ligated into the TA cloning vector pGEM-T Easy (Promega, Madison, Wis.) to create plasmids pLGT-1KE, pLGT-2KE, and pLGT-3KE (Table 1), and isogenic mutants were constructed using an inverse PCR strategy as previously described (14, 32, 33). Briefly, inverse PCR primers (Table 2) were designed to amplify a portion of the 5′ end and 3′ end of each gene using pLGT-1KE, pLGT-2KE, and pLGT-3KE as templates. This created an internal deletion with engineered restriction sites (Table 2) in each individual gene that allowed for the nonpolar insertion of the aphA-3 cassette (37) (kanamycin resistance) amplified from pUC18K (Table 1) as has been described previously by this laboratory (14, 33). The mutant constructs pLGT-1kanB, pLGT-2kanM, and pLGT-3kanH (Table 1) were linearized and introduced into M. catarrhalis 7169 by natural transformation using our standard techniques (14, 33). Chromosomal DNA was isolated from selected kanamycin-resistant transformants and subjected to PCR and sequence analysis to confirm that each inactivated gene had properly integrated into the genome.
TABLE 2.
Nucleotide sequence of oligonucleotide primers used for PCR-based cloning procedures in this study
| Primer | Sequencea | Brief description |
|---|---|---|
| Pr 406 | CAAAAGAAGACAAACAAGCAGC | Primer (sense) designed for sequencing and cloning of lgt1 |
| Pr 407 | CCCATTTAGTATCAGAAGATGACAC | Primer (antisense) designed for sequencing and cloning of lgt1 |
| Pr 422 | tataAGATCTTCAACTTCACTGTTACTGTGTTC | Inverse PCR primer (sense) with BamHI sites engineered for lgt1 mutagenesis |
| Pr 423 | gcgGGATCCAACCAAAAGCAAAGCCTG | Inverse PCR primer (antisense) with BglII sites engineered for lgt1 mutagenesis |
| Pr 508 | AAGAAGTGGGGCTTTTGTCAGAG | Primer (sense) designed for sequencing and cloning of lgt2 |
| Pr 509 | GAGAGTATGTCATTCGTGGCGAC | Primer (antisense) designed for sequencing and cloning of lgt2 |
| Pr 512 | gcgcCTCGAGGAATCGCTTTTCAGTAACCAAG | Inverse PCR primer (sense) with XhoI sites engineered for lgt2 mutagenesis |
| Pr 513 | tataAGATCTCAAGTTTTCATCAATCATCTGTTGC | Inverse PCR primer (antisense) with BglII sites engineered for lgt2 mutagenesis |
| Pr 698 | ATGAATACAGCCAAGCGG | Primer (sense) designed for sequencing and cloning of lgt3 |
| Pr 699 | AAGTCGTGATAGTTTGTCGG | Primer (antisense) designed for sequencing and cloning of lgt3 |
| Pr 681 | gcgcCTCGACCTTGTGCTTGATGCGGATGAAC | Inverse PCR primer (sense) with XhoI sites engineered for lgt3 mutagenesis |
| Pr 682 | tataAGATCTTCAGCCCATAACGACCTTGTAAG | Inverse PCR primer (antisense) with BglII sites engineered for lgt3 mutagenesis |
| Pr 342 | gcgGGATCCGTCGACTCTAGAGGATCCCCGGGTCATTA | Primer (antisense) designed to amplify the aphA-3 cassette with BamHI sites engineered for directional cloning and lgt1 mutagenesis |
| Pr 417 | tataAGATCTGGGTGACTAACTAGGAGGAATAAATGGCTA | Primer (sense) designed to amplify the aphA-3 with BglII sites engineered for directional cloning and mutagenesis |
| Pr 491 | tataCTCGAGGTCGACTCTAGAGGATCCCCGGGTCATTA | Primer (antisense) designed to amplify the aphA-3 cassette with XhoI sites engineered for directional cloning and lgt2 and lgt3 mutagenesis |
| Pr 836 | tataCCGCGGTTGTTGAGAGTCATTCCCC | Primer (sense) designed to amplify the lgt3 gene to include 5′ flanking DNA with SacII sites engineered for cloning into pLS88 |
| Pr 837 | taccCTGCAGTGATGTTGATACAGCAGGTTC | Primer (antisense) designed to amplify the lgt3 gene to include 5′ flanking DNA with PstI sites engineered for cloning into pLS88 |
| Pr 838 | taccCTGCAGATGAATACAGCCAAGCGG | Primer (sense) designed to amplify an internal portion of the lgt3 gene with SacII sites engineered for cloning into pLS88 |
| Pr 839 | tataCCGCGGCCATTTTGCCCCATAACTTTCG | Primer (antisense) designed to amplify an internal portion of the lgt3 gene with PstI sites engineered for cloning into pLS88 |
All primers are listed in the 5′ to 3′ direction. Any 5′ caps are shown in lowercase letters, and any restriction sites are shown in boldface type.
Restoration of native LOS in 7169 lgt mutants through reversion to wild type.
Primer sets 406-407, 508-509, and 698-699 (Table 2) were used to amplify the native lgt1, lgt2, and lgt3 genes, respectively, from M. catarrhalis 7169 chromosomal DNA. The resulting PCR products were purified using the MinElute kit (QIAGEN) and used to naturally transform 7169::lgt1KB, 7169::lgt2KM, and 7169::lgt3KH, respectively, by a method previously described (14). Potential revertant clones were tested for the loss of kanamycin resistance through replicate plating on both BHI and BHI plus kanamycin (20 μg/ml) agar plates. One clone for each lgt mutant revertant that demonstrated the loss of kanamycin resistance was chosen for further analyses (Table 1). Chromosomal DNA was isolated from each revertant strain (Table 1) and subjected to PCR and sequence analysis to confirm that each wild-type gene had properly integrated into the genome.
Preparation and analysis of M. catarrhalis LOS.
LOS was extracted from M. catarrhalis 7169 and all derivative strains using a modified proteinase K water-phenol method, as previously described (1, 25, 33). Briefly, M. catarrhalis was harvested from BHI plates, resuspended in a sodium dodecyl sulfate (SDS) buffer (0.06 M Tris base, 10 mM EDTA, 2% SDS), and heated at 100°C for 5 min. After addition of proteinase K at 50 μg/ml, the solution was incubated at 65°C for 1 h and then at 37°C overnight. The samples were sonicated for 15 min, and the solution was precipitated and washed three times with 100% ethyl alcohol and 3 M sodium acetate (pH 5.2) overnight at −20°C to remove the SDS. After the final precipitation, the pellets were resuspended in 20 mM Tris and incubated with 10 μg/ml of DNAseI and RNase for 2 h at 37°C. The LOS was extracted (two times) with equal volumes of phenol, heated at 65°C, and then placed on ice for 1 h. The aqueous layer was removed, and the LOS was precipitated and washed with ethyl alcohol and sodium acetate overnight (three times). After the final precipitation, the LOS molecules were resuspended in 200 μl of distilled water and resolved by SDS-polyacrylamide gel electrophoresis on a 17.5% acrylamide gel using a bilayer stacker as previously described (25) and then visualized by silver staining (54).
Western blot and colony lift analysis.
Purified LOS isolated from M. catarrhalis were transferred to 0.2-μm-pore-size nitrocellulose membranes using standard methods (28). Western blots were blocked for 1 h at room temperature with 3% (wt/vol) nonfat milk-phosphate-buffered saline and incubated overnight at 4°C with monoclonal antibodies (MAb) (34, 35). Colony lift assays were performed with H. ducreyi by a method previously described (35). MAb 4G5 (an immunoglobulin G2a [IgG2a]) and 3F7 (an IgM) were developed from a previous fusion (35). MAb 1B2-1B7 (an IgM) was obtained from the American Type Culture Collection (Manassas, Va.) and used as the probe in H. ducreyi colony lifts and Western blots. This antibody recognizes the surface-exposed, terminal lactosamine moiety on the LOS of wild-type H. ducreyi 35000 (11, 36, 58). Horseradish peroxidase-labeled protein A was used to detect MAb 4G5 and horseradish peroxidase-labeled goat anti-mouse IgM (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) was used to detect MAb 3F7 and MAb 1B2-1B7 reactivity, and then the blots were developed as previously described (4, 34, 35).
Growth curve and outer membrane protein (OMP) profile analyses.
Growth curves were performed for the M. catarrhalis strains as previously described (4, 14). Static-phase bacteria were harvested, and outer membranes were prepared and analyzed as previously described (4, 34, 35).
Mass spectrometric analysis of M. catarrhalis LOS.
LOS from M. catarrhalis 7169 and the lgt mutants were analyzed by mass spectrometry (MS) to determine molecular masses, generic compositions of the oligosaccharide, and the phosphorylation states of the lipid A moiety. LOS was O-deacylated by treatment with anhydrous hydrazine for 35 min at 37°C (18). The conversion of LOS to di-N-acyl LOS makes it more water soluble and amenable to mass spectrometric analysis (15). The O-deacylated LOS was dissolved in water and desalted by drop dialysis using a 0.025-μm-pore-size nitrocellulose membrane (Millipore, Bedford, Mass.). Approximately 25% of the sample was dried in a Speed-Vac (Savant Instruments, Inc., Holbrook, N.Y.), reconstituted in 1 μl of milli-Q water, and desalted with cation-exchange resin beads (DOWEX, 50×, NH4+) (40). Prior to spotting onto the stainless steel target, the sample was mixed with a matrix-assisted laser desorption ionization (MALDI) matrix consisting of 1 μl of 160 mM 2,5-dihydroxybenzoic acid, 87.5 mM 1-hydroxyisoquinoline solution in 4:1 acetone-water and air dried on the target (38). These O-deacylated LOS samples were then analyzed by MALDI-MS using an Applied Biosystems Voyager DESTR+ plus (Framingham, Mass.) with an N laser (337 nm) in negative-ion mode with linear optics. The delay time was 165 ns, and the grid voltage was 94% of the full acceleration voltage (20 kV). Spectra were acquired, averaged, and mass calibrated with an external calibrator consisting of an equimolar mixture of angiotensin I, ACTH 18-39, and ACTH 7-38 (Bachem, Torrance, Calif.).
Complementation of Haemophilus ducreyi 35000glu−.
pLS88 was used as an E. coli-H. ducreyi shuttle vector (Kanr, Smr, Sulr) for cloning and complementation in these studies (6, 11). PCR primers 836 and 837 (Table 2), engineered with SacII and PstI restriction sites, were used to amplify the M. catarrhalis lgt3 gene with 265 bp of 5′ flanking DNA, which was then ligated to SacII/PstI-digested pLS88, to construct plasmid pLS3PR-E (Table 1). Similarly, PCR primers 838 and 839 (Table 2) were used to amplify an internal portion of the M. catarrhalis lgt3 gene, which was then ligated to a SacII/PstI-digested pLS88, to construct plasmid pLS3IN-1 (Table 1). These plasmids were electroporated into H. ducreyi 35000glu− (Catr) as previously described (3, 11). Two kanamycin- and chloramphenicol-resistant transformants were selected, termed 35000glu−(pLS3PR-E) and 35000glu−(pLS3IN-1) as described in Table 1, for further analyses.
Nucleotide sequence accession numbers.
All primers were originally designed using sequence 31 of M. catarrhalis patent number WO0078968 (patent located at NCBI database under Incyte Genomics accession number AX067456). The sequence of the cluster of genes reported in this paper were deposited with GenBank and assigned the accession number AY789049.
RESULTS AND DISCUSSION
Identification, cloning, and analysis of glycosyltransferase genes of M. catarrhalis 7169.
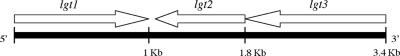
An NCBI BLAST search, with nucleotide sequences of known glycosyltransferase genes as queries, identified a cluster of three putative LOS genes in sequence 31 of the M. catarrhalis patented genome (NCBI patent number WO0078968). PCR primers, designed to each gene (Table 2), were used to amplify products from the chromosome of M. catarrhalis 7169, an LOS serotype B strain (33). Three amplicons corresponding to open reading frames (ORFs) of 984 bp, 765 bp, and 1,605 bp were obtained, and their nucleotide sequences were determined. Figure 1 illustrates the arrangement of the cluster with predicted gene products of 328, 255, and 535 amino acids for lgt1, lgt2, and lgt3, respectively. A database search with the translated sequences revealed homology with three glycosyltransferases from other gram-negative bacteria. Lgt1 has 26% similarity to the WbpZ of Pseudomonas aeruginosa and an uncharacterized glycosyltransferase from Methanosarcina mazei. WbpZ is a glycosyltransferase that adds the first rhamnose residue onto the A-band LPS molecule O-antigen region synthesized by P. aeruginosa (47). Lgt2 has 55% similarity to the glycosyltransferases LgtB of Neisseria meningitidis and LpsA of Mannheimia haemolytica. These genes encode a β(1-4) galactosyltransferase involved in the addition of galactose to the α-chain of the N. meningitidis LOS molecule (41, 57, 60) and a glycosyltransferase involved in LPS core biosynthesis for M. haemolytica (42), respectively. Lgt3 has 28% similarity to LgtF of Haemophilus ducreyi and WaaE of Klebsiella pneumoniae. These genes both encode β(1-4) glucosyltransferases involved in the addition of glucose to the LOS inner core (11, 46). Sequences upstream and downstream of the lgt cluster did not reveal significant homology to other LOS-associated genes.
FIG. 1.
Organization of the lgt cluster in the M. catarrhalis 7169 genome. The ORFs lgt1, lgt2, and lgt3 are 984 bp, 765 bp, and 1,605 bp in size, respectively.
Construction of M. catarrhalis LOS mutants.
To determine if the genes identified above code for specific glycosyltransferase enzymes, mutants were constructed by disrupting each lgt as described in Materials and Methods. Chromosomal DNA from selected kanamycin-resistant transformants were analyzed by PCR and sequence analysis to confirm that proper mutagenesis occurred in M. catarrhalis 7169, yielding the mutant strains 7169::lgt1KB and 7169::lgt2KM (Table 1). The arrangement of lgt3 and lgt2 (Fig. 1) suggested that these genes were cotranscribed, and reverse transcription-PCR confirmed this hypothesis (data not shown). Based on these data, a nonpolar mutation was constructed in lgt3 (as described in Materials and Methods), and the resulting transformant was named 7169::lgt3KH (Table 1).
To demonstrate that the loss of each Lgt expression was due to the internal deletion in each individual lgt as described above, the wild-type lgt1, lgt2, and lgt3 were reintroduced into the chromosome of each respective lgt mutant by natural transformation. Homologous recombination was confirmed by PCR and sequencing, and the LOS of each revertant was characterized compared to the corresponding mutant LOS as described below.
Comparative growth and OMP analyses.
There were no significant differences in growth rate or OMP content between the wild-type and the three lgt mutants.
Characterization of LOS profiles from lgt mutants and revertants.
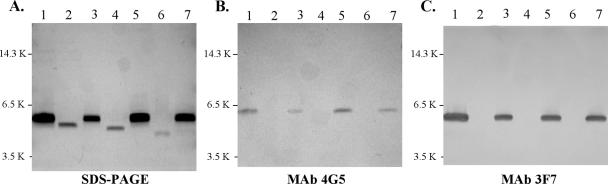
LOS isolated from wild-type 7169, the lgt mutants, and the lgt mutant revertants were analyzed by SDS-polyacrylamide gel electrophoresis (Fig. 2A). All three mutants synthesized truncated LOS molecules that migrated more rapidly than the wild-type 7169 glycoform (Fig. 2A, lane 1), indicating loss of carbohydrate moieties. The LOS synthesized by 7169::lgt2KM (Fig. 2A, lane 2) migrated slower than the LOS glycoforms from 7169::lgt1KB (Fig. 2A, lane 4) and 7169::lgt3KH (Fig. 2A, lane 6), suggesting a loss of only one or two sugar moieties compared to the wild-type LOS glycoform. The LOS glycoform synthesized by 7169::lgt3KH (Fig. 2A, lane 6) migrated the fastest relative to the wild-type LOS and the LOS from the other two mutants, suggesting a deeper truncation of the major glycoform. The LOS molecule synthesized by each lgt mutant revertant migrated the same as the wild-type LOS molecule (Fig. 2A, lanes 3, 5, and 7).
FIG. 2.
Composite of a silver-stained SDS-17.5% acrylamide gel (A) and the corresponding Western blots with monoclonal antibodies 4G5 (B) and 3F7 (C). The gel contains LOS samples from M. catarrhalis wild-type 7169 (lanes 1), 7169::lgt2KM (lanes 2), 7169::lgt2KM(REV) (lanes 3), 7169::lgt1KB (lanes 4), 7169::lgt1KB(REV) (lanes 5), 7169::lgt3KH (lanes 6), and 7169::lgt3KH(REV) (lanes 7). PAGE, polyacrylamide gel electrophoresis.
Monoclonal antibody analyses of M. catarrhalis lgt mutants and revertants.
Previous studies have shown that MAb 4G5 reacts to a conserved LOS epitope, containing the terminal portion of the main branch, expressed by all M. catarrhalis isolates tested (33, 59). Western blot analysis demonstrated that MAb 3F7 reacts to an epitope that is specific to M. catarrhalis strains expressing serotype B LOS, suggesting that the side chain is important for antibody reactivity (data not shown). These antibodies were used to characterize the phenotypic changes that resulted from the LOS truncations seen in each mutant and to confirm the restoration of the wild-type LOS. Figures 2B and C demonstrate that all three mutants lost reactivity to both MAb 4G5 (Fig. 2B, lanes 2, 4, and 6) and MAb 3F7 (Fig. 2C, lanes 2, 4, and 6). The loss of these LOS epitopes further supports the conclusion that this gene cluster is involved in the assembly of both the full-length chain and the serotype B-specific side chain. In addition, the LOS isolated from the corresponding revertants regained reactivity to both MAb 4G5 (Fig. 2B, lanes 3, 5, and 7) and MAb 3F7 (Fig. 2C, lanes 3, 5, and 7), confirming that the internal deletion of each gene alone was responsible for the resulting LOS phenotypes.
Structural analysis.
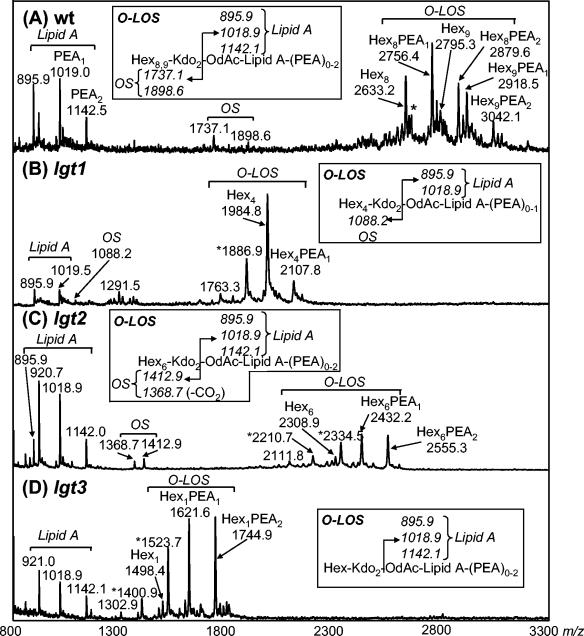
Mutant and wild-type LOS were O-deacylated (O-LOS) and analyzed by negative-ion linear MALDI-time of flight (TOF) mass spectrometry. The mass spectrum of the strain 7169 wild-type O-LOS corresponds with the previously published structure of serotype B LOS (9, 21) (Fig. 3A), yielding six deprotonated (M-H)− molecular ions. The ions with m/z of 2,633.2, 2,756.4, 2,795.3, 2,879.6, 2,918.5, and 3,042.1 are consistent with an O-LOS containing eight to nine hexoses, two KDO residues, and up to two phosphoethanolamine (PEA) groups but lacking any N-acetylhexosamine within the oligosaccharide. The lack of N-acetylhexosamine further confirms the LOS as B serotype, since both A- and C-serotype LOS contain a GlcNAc (7, 8, 21).
FIG. 3.
Linear negative-ion MALDI-TOF spectra of O-LOS from M. catarrhalis strains. (A) Wild-type 7169; (B) lgt1 mutant-7169::lgt1KB; (C) lgt2 mutant-7169::lgt1KM; (D) lgt3 mutant-7169::lgt3KH. All ions are represented as average (M-H)− masses. Intact O-LOS molecules show numerous molecular ions due to heterogeneity in hexose and phosphoethanolamine content. Molecular ions undergo in-source fragmentation to give lipid A and oligosaccharide prompt fragments as shown. *, ions occurring due to β-elimination of phosphoric acid (H3PO4) from the O-LOS; OS, oligosaccharide; lipid A, O-deacylated lipid A; Hex, Hexose; Kdo, 2-keto-3-deoxyoctonate.
O-LOS isolated from the lgt1 mutant showed two (M-H)− molecular ions with m/z of 2,107.8 and 1,984.8, corresponding to a truncated oligosaccharide consisting of four hexoses and two KDO moieties with zero to one PEA moieties, respectively (Fig. 3B). Also present in the mass spectrum is an ion with a m/z of 1,886.9, which results from the loss of phosphoric acid (H3PO4) from the ion with a four-hexose structure at a m/z of 1,984.8. Composition analysis showed the presence of glucose and KDO but no galactose in the LOS. Linkage analysis confirmed the presence of a 3-, 4-, 6-substituted glucose and terminal glucose only, whereas for the wild type, 2- and 4-substituted hexoses were also observed (data not shown).
This mutant is consistent with the loss of an α(1-2) glucosyltransferase, which would leave an oligosaccharide containing two KDO, one 3-substituted glucose, and three terminal glucose residues, thus:
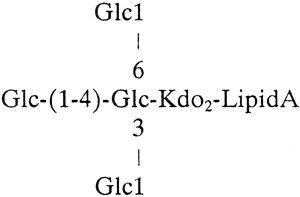 |
MALDI-TOF-MS of the lgt2 mutant O-LOS showed the presence of (M-H)− molecular ions smaller than the wild-type O-LOS but larger than the lgt1 mutant O-LOS (Fig. 3C). The ions with m/z of 2,308.9, 2,432.2, and 2,555.5 correlate to an O-LOS containing six hexoses, two KDO moieties, and zero to two PEA moieties. Also present were ions with m/z of 2,334.5 and 2,210.7, which are thought to arise from the loss of H3PO4 from ions with m/z of 2,434.2 and 2,308.9, respectively. Composition analysis of the O-LOS suggested that the major component of the oligosaccharide was glucose and that the mutant LOS lacks galactose. Linkage analysis of oligosaccharide derived from the LOS shows the presence of a 3-, 4-, 6-substituted glucose and terminal glucose, as seen for the lgt1 mutant; in addition, a partially methylated alditol acetate representing 2-substituted glucose was observed. Taken together, the data for this lgt2 mutant are consistent with a structure containing 2-substituted glucose but lacking galactose:
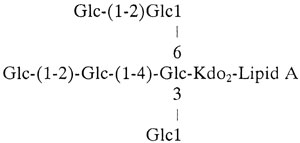 |
This structure, truncated at the site of galactose addition, would correlate with Lgt2 being a β(1-4) galactosyltransferase.
Finally, MALDI-TOF MS analysis of O-LOS isolated from the lgt3 mutant yielded ions with m/z of 2,308.9, 2,432.2, and 2,553.3 which correspond to an oligosaccharide containing one hexose, two KDO moieties, and zero, one, or two PEA moieties, respectively (Fig. 3D). Also present were ions with m/z of 1,400.9 and 1,523.7 corresponding to the loss of H3PO4 from the ions with m/z of 1,498.4 and 1,621.6, respectively. This mutant produces the most deeply truncated LOS molecules of the mutants studied and can only correspond to the structure:
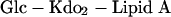 |
where linkage between the glucose and KDO is likely to be α(1-5), as previously shown by Edebrink et al. (9). It is unlikely that the Lgt3 enzyme is trifunctional; thus, it seems that if this enzyme is a glycosyltransferase, it adds either 3-, 4-, or 6-linked glucose to the growing LOS molecule and that this addition of a single glucose molecule is required before further elongation of the OS can occur.
As previously mentioned, Lgt3 has homology to LgtF of H. ducreyi, a known β(1-4) glucosyltransferase (11); thus, the following studies were performed to test this hypothesis.
Complementation of the glucosyltransferase (lgtF)-deficient H. ducreyi 35000glu−.
To determine whether the 7169 lgt3 functions as a β(1-4) glucosyltransferase, complementation studies were performed using the H. ducreyi 35000glu− mutant, previously developed in this laboratory (11). This isogenic mutant has a single defect in the β(1-4) glucosyltransferase, LgtF, and assembles a truncated LOS lacking the terminal, surface-exposed lactosamine epitope, expressed by the parent strain 35000 and detected by MAb 1B2-1B7 (36, 58). Two plasmids were constructed in the pLS88 shuttle vector using standard cloning techniques (see Materials and Methods). One plasmid, pLS3PR-E (Table 1), contained a 2.2-kb insert consisting of the entire M. catarrhalis 7169 lgt3 ORF as well as its 5′ flanking DNA of ∼265 bp. The other plasmid, pLS3IN-1 (Table 1), contained a 1.3-kb insert consisting of an internal portion of the 7169 lgt3 gene without any 5′ flanking DNA.
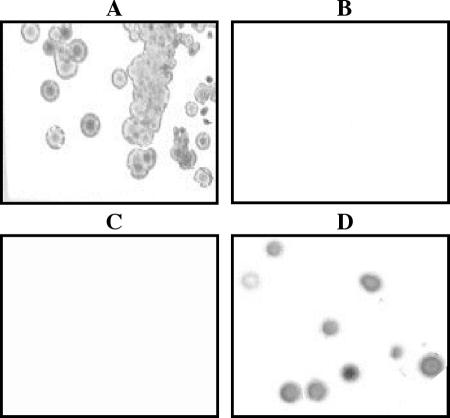
After electroporation of these constructs into H. ducreyi 35000glu−, kanamycin-chloramphenicol-resistant transformants were screened for the restoration of reactivity to MAb 1B2-1B7. Figure 4 is a composite showing colony lift assays probed with MAb 1B2-1B7, denoting the presence of the terminal lactosamine epitope on the LOS of the wild-type 35000 (Fig. 4A) and the loss of this epitope in 35000glu− (Fig. 4B) as expected (11, 36, 58). More importantly, these data demonstrate that MAb 1B2-1B7 reacts to the complemented 35000glu−(pLS3PR-E) (Fig. 4D), indicating that expression of lgt3 in trans corrected the defect, resulting in assembly of the full-length LOS chain. In addition, the mutant complemented with the internal portion of lgt3, 35000glu−(pLS3IN-1) did not restore the epitope recognized by MAb 1B2-1B7 (Fig. 4C). These results indicate that the 7169 Lgt3 was expressed in the LgtF-deficient H. ducreyi mutant, resulting in complementation of the defect, providing evidence that the Lgt3 can function as a β(1-4) glucosyltransferase in M. catarrhalis 7169.
FIG. 4.
A composite of colony lift assays of H. ducreyi 35000 (A), 35000glu− (B), 35000glu−(pLS3IN-1) (C), and 35000glu−(pLS3PR-E) (D) probed with MAb 1B2-1B7.
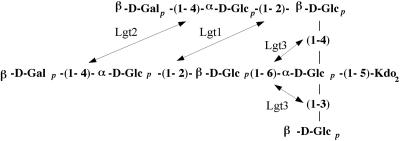
While the data presented in these studies conclude that Lgt1 and Lgt2 function as an α(1-2) glucosyltransferase and a β(1-4) galactosyltransferase, respectively, these data do not conclusively define the function of Lgt3. In addition, our data also suggests that the Lgt1 may be able to add a α(1-2)-linked glucose onto similar but not identical acceptor molecules in the two different chains (Fig. 5). It is interesting that both Lgt1 and Lgt2 likely function in the addition of carbohydrates to both the main branch and the serotype-specific branch of the LOS molecule; however, it is possible that the addition of only one sugar is occurring before another very similar enzyme is able to add the same sugar to the other branch.
FIG. 5.
Biochemical structure of the wild-type 7169 LOS molecule and the positions of the glycosyltransferase enzymes Lgt1, Lgt2, and Lgt3 as they may function in LOS biosynthesis for this organism as determined in these studies.
Perhaps the most interesting results from our studies involved the analysis of the lgt3. Based on the homology data, we were initially surprised by the structural studies that confirmed that the lgt3 mutant contained a single glucose attached to the KDO. This was not the result of pleiotropic effects, as our molecular analysis of the mutant and the restoration of the wild-type phenotype demonstrated that the LOS defect was the result of disrupting lgt3 alone. However, these data did not provide insight into a possible function for Lgt3. The LOS structural data suggested three possible functions for Lgt3 as either a β(1-3), a β(1-4), or β(1-6) glucosyltransferase. The other possible explanation was that Lgt3 is a multifunctional enzyme; however, while this would be a novel observation, more studies are needed to address this issue.
The heterologous complementation studies, using the H. ducreyi β(1-4) glucosyltransferase mutant, provided important insight into a probable function for Lgt3. Using the M. catarrhalis lgt3 to complement the isogenic 35000glu− mutant in trans indicated that Lgt3 can function as a β(1-4) glucosyltransferase. Furthermore, these data also show that the M. catarrhalis Lgt3 is capable of adding a glucose onto a heptose substrate in the H. ducreyi 35000glu− background. This is a somewhat surprising observation, as there is no heptose in M. catarrhalis LOS and Lgt3 would be predicted to add a glucose onto a glucose substrate in this bacterium. Nevertheless, based on the previously published characterization of the H. ducreyi 35000glu− mutant, a β(1-4)-linked glucose had to be added to the heptose to allow for the completion of the full-length H. ducreyi LOS chain and the restoration of the MAb 1B2-1B7 epitope (11, 36, 58). In addition, this is the first study describing the expression of a M. catarrhalis gene product in H. ducreyi, which is an important observation, as currently there are no reliable vectors that allow for complementation in trans in M. catarrhalis.
These studies further suggested that the function of the Lgt3 enzyme is essential for the continuation of both the main oligosaccharide chain and the serotype-specific side chain. Thus, in the absence of the β(1-4) linked glucose addition to the core glucose, the biosynthesis and assembly of the main LOS chain are disrupted. Previous studies with N. meningitidis and H. influenzae have reported that the order of addition of specific sugar residues onto the growing LOS molecule is critical to the function of enzymes that act further downstream in the biosynthesis of the full-length LOS molecule (22, 29). One study involving rfaK of N. meningitidis concluded that the absence of this transferase, responsible for the addition of N-acetylglucosamine to HepII, causes a lack of α-chain extension from HepI in the LOS rfaK mutant glycoform due to structural constraints imposed by the incomplete biosynthesis of the LOS inner core (29). Another study with glycosyltransferase enzymes of H. influenzae concluded that lpsA, lic2A, lgtC, and lgtD were all required for the sequential addition of the glycoses to the terminal inner core heptose to give the globotetraose structure of its LOS molecule (22). Based on our data and these previous studies, we hypothesize that Lgt3 functions at a critical point in biosynthesis and assembly of M. catarrhalis LOS, and in the absence of this enzymatic activity, the acceptor molecule will not be present for the proper function of glycosyltransferase enzymes that continue the elongation of either the main branch or the side chain, preventing the completion of the principal glycoform. However, further biochemical analyses of Lgt3 with regard to specific substrates and acceptor molecules are needed to delineate the precise action of this critical enzyme in M. catarrhalis LOS biosynthesis. Together, these data provide insight into the assembly of M. catarrhalis LOS, and the specific mutants provide valuable tools that will be essential in determining the role of this glycolipid in pathogenesis.
Acknowledgments
This research was supported by Public Health Service Research grants AI46422, DC005837 (A.A.C.), and AI31254 (B.W.G.). K.J.E. is also supported as a graduate student fellow by NIH training grant AI07614. This work was supported by the Department of Energy-funded (DE-FGOZ-93ER-20097) Center for Plant and Microbial Complex Carbohydrates.
We thank Melanie J. Filiatrault for the construction of the H. ducreyi 35000glu− (LgtF) mutant strain. We thank the Center for Complex Carbohydrate Research at the University of Georgia, in particular Trina Abney and Parastoo Azadi, for the preparation and analysis of the data involving the LOS isolated from the M. catarrhalis 7169 and lgt mutants. We also thank Alan Lesse for assistance in sequence analysis.
REFERENCES
- 1.Apicella, M. A., J. M. Griffiss, and H. Schneider. 1994. Isolation and characterization of lipopolysaccharides, lipooligosaccharides, and lipid A. Methods Enzymol. 235:242-252. [DOI] [PubMed] [Google Scholar]
- 2.Bakri, F., A. L. Brauer, S. Sethi, and T. F. Murphy. 2002. Systemic and mucosal antibody response to Moraxella catarrhalis after exacerbations of chronic obstructive pulmonary disease. J. Infect. Dis. 185:632-640. [DOI] [PubMed] [Google Scholar]
- 3.Bozue, J. A., L. Tarantino, and R. S. Munson, Jr. 1998. Facile construction of mutations in Haemophilus ducreyi using lacZ as a counter-selectable marker. FEMS Microbiol. Lett. 164:269-273. [DOI] [PubMed] [Google Scholar]
- 4.Campagnari, A. A., T. F. Ducey, and C. A. Rebmann. 1996. Outer membrane protein B1, an iron-repressible protein conserved in the outer membrane of Moraxella (Branhamella) catarrhalis, binds human transferrin. Infect. Immun. 64:3920-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campagnari, A. A., L. M. Wild, G. E. Griffiths, R. J. Karalus, M. A. Wirth, and S. M. Spinola. 1991. Role of lipooligosaccharides in experimental dermal lesions caused by Haemophilus ducreyi. Infect. Immun. 59:2601-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon, L. G., W. L. Albritton, and P. J. Willson. 1994. An analysis of the complete nucleotide sequence of the Haemophilus ducreyi broad-host-range plasmid pLS88. Plasmid 32:228-232. [DOI] [PubMed] [Google Scholar]
- 7.Edebrink, P., P. E. Jansson, M. M. Rahman, G. Widmalm, T. Holme, and M. Rahman. 1995. Structural studies of the O-antigen oligosaccharides from two strains of Moraxella catarrhalis serotype C. Carbohydr. Res. 266:237-261. [DOI] [PubMed] [Google Scholar]
- 8.Edebrink, P., P. E. Jansson, M. M. Rahman, G. Widmalm, T. Holme, M. Rahman, and A. Weintraub. 1994. Structural studies of the O-polysaccharide from the lipopolysaccharide of Moraxella (Branhamella) catarrhalis serotype A (strain ATCC 25238). Carbohydr. Res. 257:269-284. [DOI] [PubMed] [Google Scholar]
- 9.Edebrink, P., P. E. Jansson, G. Widmalm, T. Holme, and M. Rahman. 1996. The structures of oligosaccharides isolated from the lipopolysaccharide of Moraxella catarrhalis serotype B, strain CCUG 3292. Carbohydr. Res. 295:127-146. [DOI] [PubMed] [Google Scholar]
- 10.Enright, M. C., and H. McKenzie. 1997. Moraxella (Branhamella) catarrhalis-clinical and molecular aspects of a rediscovered pathogen. J. Med. Microbiol. 46:360-371. [DOI] [PubMed] [Google Scholar]
- 11.Filiatrault, M. J., B. W. Gibson, B. Schilling, S. Sun, R. S. Munson, Jr., and A. A. Campagnari. 2000. Construction and characterization of Haemophilus ducreyi lipooligosaccharide (LOS) mutants defective in expression of heptosyltransferase III and beta-1,4-glucosyltransferase: identification of LOS glycoforms containing lactosamine repeats. Infect. Immun. 68:3352-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fomsgaard, J. S., A. Fomsgaard, N. Høiby, B. Bruun, and C. Galanos. 1991. Comparative immunochemistry of lipopolysaccharides from Branhamella catarrhalis strains. Infect. Immun. 59:3346-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forsgren, A., M. Brant, M. Karamehmedovic, and K. Riesbeck. 2003. The immunoglobulin D-binding protein MID from Moraxella catarrhalis is also an adhesin. Infect. Immun. 71:3302-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furano, K., and A. A. Campagnari. 2003. Inactivation of the Moraxella catarrhalis 7169 ferric uptake regulator increases susceptibility to the bactericidal activity of normal human sera. Infect. Immun. 71:1843-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibson, B. W., A. A. Campagnari, W. Melaugh, N. J. Phillips, M. A. Apicella, S. Grass, J. Wang, K. L. Palmer, and R. S. Munson, Jr. 1997. Characterization of a transposon Tn916-generated mutant of Haemophilus ducreyi 35000 defective in lipooligosaccharide biosynthesis. J. Bacteriol. 179:5062-5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorter, A. D., J. Oostrik, P. van der Ley, P. S. Hiemstra, J. Dankert, and L. van Alphen. 2003. Involvement of lipooligosaccharides of Haemophilus influenzae and Neisseria meningitidis in defensin-enhanced bacterial adherence to epithelial cells. Microb. Pathog. 34:121-130. [DOI] [PubMed] [Google Scholar]
- 17.Hammond, G. W., C. J. Lian, J. C. Wilt, and A. R. Ronald. 1978. Antimicrobial susceptibility of Haemophilus ducreyi. Antimicrob. Agents Chemother. 13:608-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helander, I. M., K. Nummila, I. Kilpelèainen, and M. Vaara. 1995. Increased substitution of phosphate groups in lipopolysaccharides and lipid A of polymyxin-resistant mutants of Salmonella typhimurium and Escherichia coli. Prog. Clin. Biol. Res. 392:15-23. [PubMed] [Google Scholar]
- 19.Holm, M. M., S. L. Vanlerberg, I. M. Foley, D. D. Sledjeski, and E. R. Lafontaine. 2004. The Moraxella catarrhalis porin-like outer membrane protein CD is an adhesin for human lung cells. Infect. Immun. 72:1906-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holm, M. M., S. L. Vanlerberg, D. D. Sledjeski, and E. R. Lafontaine. 2003. The Hag protein of Moraxella catarrhalis strain O35E is associated with adherence to human lung and middle ear cells. Infect. Immun. 71:4977-4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holme, T., M. Rahman, P. E. Jansson, and G. Widmalm. 1999. The lipopolysaccharide of Moraxella catarrhalis structural relationships and antigenic properties. Eur. J. Biochem. 265:524-529. [DOI] [PubMed] [Google Scholar]
- 22.Hood, D. W., A. D. Cox, W. W. Wakarchuk, M. Schur, E. K. Schweda, S. L. Walsh, M. E. Deadman, A. Martin, E. R. Moxon, and J. C. Richards. 2001. Genetic basis for expression of the major globotetraose-containing lipopolysaccharide from H. influenzae strain Rd (RM118). Glycobiology 11:957-967. [DOI] [PubMed] [Google Scholar]
- 23.Hu, W. G., J. Chen, J. F. Battey, and X. X. Gu. 2000. Enhancement of clearance of bacteria from murine lungs by immunization with detoxified lipooligosaccharide from Moraxella catarrhalis conjugated to proteins. Infect. Immun. 68:4980-4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu, W. G., J. Chen, J. C. McMichael, and X. X. Gu. 2001. Functional characteristics of a protective monoclonal antibody against serotype A and C lipooligosaccharides from Moraxella catarrhalis. Infect. Immun. 69:1358-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inzana, T. J., and M. A. Apicella. 1999. Use of a bilayer stacking gel to improve resolution of lipopolysaccharides and lipooligosaccharides in polyacrylamide gels. Electrophoresis 20:462-465. [DOI] [PubMed] [Google Scholar]
- 26.Jèonsson, I., T. Holme, A. Krook, M. Rahman, and M. Thorāen. 1992. Variability of surface-exposed antigens of different strains of Moraxella catarrhalis. Eur. J. Clin. Microbiol. Infect. Dis. 11:919-922. [DOI] [PubMed] [Google Scholar]
- 27.Jiao, X., T. Hirano, Y. Hou, and X. X. Gu. 2002. Specific immune responses and enhancement of murine pulmonary clearance of Moraxella catarrhalis by intranasal immunization with a detoxified lipooligosaccharide conjugate vaccine. Infect. Immun. 70:5982-5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson, K. G., and M. B. Perry. 1976. Improved techniques for the preparation of bacterial lipopolysaccharides. Can. J. Microbiol. 22:29-34. [DOI] [PubMed] [Google Scholar]
- 29.Kahler, C. M., R. W. Carlson, M. M. Rahman, L. E. Martin, and D. S. Stephens. 1996. Inner core biosynthesis of lipooligosaccharide (LOS) in Neisseria meningitidis serogroup B: identification and role in LOS assembly of the α-1,2-N-acetylglucosamine transferase (RfaK). J. Bacteriol. 178:1265-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein, N. J., C. A. Ison, M. Peakman, M. Levin, S. Hammerschmidt, M. Frosch, and R. S. Heyderman. 1996. The influence of capsulation and lipooligosaccharide structure on neutrophil adhesion molecule expression and endothelial injury by Neisseria meningitidis. J. Infect. Dis. 173:172-179. [DOI] [PubMed] [Google Scholar]
- 31.Lafontaine, E. R., L. D. Cope, C. Aebi, J. L. Latimer, G. H. McCracken, Jr., and E. J. Hansen. 2000. The UspA1 protein and a second type of UspA2 protein mediate adherence of Moraxella catarrhalis to human epithelial cells in vitro. J. Bacteriol. 182:1364-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ling, M. M., and B. H. Robinson. 1997. Approaches to DNA mutagenesis: an overview. Anal. Biochem. 254:157-178. [DOI] [PubMed] [Google Scholar]
- 33.Luke, N. R., S. Allen, B. W. Gibson, and A. A. Campagnari. 2003. Identification of a 3-deoxy-d-manno-octulosonic acid biosynthetic operon in Moraxella catarrhalis and analysis of a KdsA-deficient isogenic mutant. Infect. Immun. 71:6426-6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luke, N. R., and A. A. Campagnari. 1999. Construction and characterization of Moraxella catarrhalis mutants defective in expression of transferrin receptors. Infect. Immun. 67:5815-5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luke, N. R., T. A. Russo, N. Luther, and A. A. Campagnari. 1999. Use of an isogenic mutant constructed in Moraxella catarrhalis to identify a protective epitope of outer membrane protein B1 defined by monoclonal antibody 11C6. Infect. Immun. 67:681-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melaugh, W., N. J. Phillips, A. A. Campagnari, R. Karalus, and B. W. Gibson. 1992. Partial characterization of the major lipooligosaccharide from a strain of Haemophilus ducreyi, the causative agent of chancroid, a genital ulcer disease. J. Biol. Chem. 267:13434-13439. [PubMed] [Google Scholar]
- 37.Menard, R., P. J. Sansonetti, and C. Parsot. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175:5899-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohr, M. D., K. O. Bèornsen, and H. M. Widmer. 1995. Matrix-assisted laser desorption/ionization mass spectrometry: improved matrix for oligosaccharides. Rapid Commun. Mass Spectrom. 9:809-814. [DOI] [PubMed] [Google Scholar]
- 39.Murphy, T. F. 1996. Branhamella catarrhalis: epidemiology, surface antigenic structure, and immune response. Microbiol. Rev. 60:267-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nordhoff, E., A. Ingendoh, R. Cramer, A. Overberg, B. Stahl, M. Karas, F. Hillenkamp, and P. F. Crain. 1992. Matrix-assisted laser desorption/ionization mass spectrometry of nucleic acids with wavelengths in the ultraviolet and infrared. Rapid Commun. Mass Spectrom. 6:771-776. [DOI] [PubMed] [Google Scholar]
- 41.Park, J. E., K. Y. Lee, S. I. Do, and S. S. Lee. 2002. Expression and characterization of beta-1,4-galactosyltransferase from Neisseria meningitidis and Neisseria gonorrhoeae. J. Biochem. Mol. Biol. 35:330-336. [DOI] [PubMed] [Google Scholar]
- 42.Potter, M. D., and R. Y. Lo. 1995. Cloning and characterization of a gene from Pasteurella haemolytica A1 involved in lipopolysaccharide biosynthesis. FEMS Microbiol. Lett. 129:75-81. [DOI] [PubMed] [Google Scholar]
- 43.Rahman, M., T. Holme, I. Jèonsson, and A. Krook. 1995. Lack of serotype-specific antibody response to lipopolysaccharide antigens of Moraxella catarrhalis during lower respiratory tract infection. Eur. J. Clin. Microbiol. Infect. Dis. 14:297-304. [DOI] [PubMed] [Google Scholar]
- 44.Rahman, M., and T. Holme. 1996. Antibody response in rabbits to serotype-specific determinants in lipopolysaccharides from Moraxella catarrhalis. J. Med. Microbiol. 44:348-354. [DOI] [PubMed] [Google Scholar]
- 45.Rahman, M., A. B. Jonsson, and T. Holme. 1998. Monoclonal antibodies to the epitope α-Gal-(1-4)-β-Gal-(1- of Moraxella catarrhalis LPS react with a similar epitope in type IV pili of Neisseria meningitidis. Microb. Pathog. 24:299-308. [DOI] [PubMed] [Google Scholar]
- 46.Reguāe, M., N. Climent, N. Abitiu, N. Coderch, S. Merino, L. Izquierdo, M. Altarriba, and J. M. Tomāas. 2001. Genetic characterization of the Klebsiella pneumoniae waa gene cluster, involved in core lipopolysaccharide biosynthesis. J. Bacteriol. 183:3564-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rocchetta, H. L., L. L. Burrows, J. C. Pacan, and J. S. Lam. 1998. Three rhamnosyltransferases responsible for assembly of the A-band D-rhamnan polysaccharide in Pseudomonas aeruginosa: a fourth transferase, WbpL, is required for the initiation of both A-band and B-band lipopolysaccharide synthesis. Mol. Microbiol. 28:1103-1119. [DOI] [PubMed] [Google Scholar]
- 48.Russo, T. A., J. E. Guenther, S. Wenderoth, and M. M. Frank. 1993. Generation of isogenic K54 capsule-deficient Escherichia coli strains through TnphoA-mediated gene disruption. Mol. Microbiol. 9:357-364. [DOI] [PubMed] [Google Scholar]
- 49.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 50.Sethi, S., N. Evans, B. J. Grant, and T. F. Murphy. 2002. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N. Engl. J. Med. 347:465-471. [DOI] [PubMed] [Google Scholar]
- 51.Swords, W. E., B. A. Buscher, K. Ver Steeg Ii, A. Preston, W. A. Nichols, J. N. Weiser, B. W. Gibson, and M. A. Apicella. 2000. Non-typeable Haemophilus influenzae adhere to and invade human bronchial epithelial cells via an interaction of lipooligosaccharide with the PAF receptor. Mol. Microbiol. 37:13-27. [DOI] [PubMed] [Google Scholar]
- 52.Swords, W. E., D. L. Chance, L. A. Cohn, J. Shao, M. A. Apicella, and A. L. Smith. 2002. Acylation of the lipooligosaccharide of Haemophilus influenzae and colonization: an htrB mutation diminishes the colonization of human airway epithelial cells. Infect. Immun. 70:4661-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Timpe, J. M., M. M. Holm, S. L. Vanlerberg, V. Basrur, and E. R. Lafontaine. 2003. Identification of a Moraxella catarrhalis outer membrane protein exhibiting both adhesin and lipolytic activities. Infect. Immun. 71:4341-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 55.Vaneechoutte, M., G. Verschraegen, G. Claeys, and A. M. Van Den Abeele. 1990. Serological typing of Branhamella catarrhalis strains on the basis of lipopolysaccharide antigens. J. Clin. Mcrobiol. 28:182-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verduin, C. M., C. Hol, A. Fleer, H. van Dijk, and A. van Belkum. 2002. Moraxella catarrhalis: from emerging to established pathogen. Clin. Microbiol. Rev. 15:125-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wakarchuk, W., A. Martin, M. P. Jennings, E. R. Moxon, and J. C. Richards. 1996. Functional relationships of the genetic locus encoding the glycosyltransferase enzymes involved in expression of the lacto-N-neotetraose terminal lipopolysaccharide structure in Neisseria meningitidis. J. Biol. Chem. 271:19166-19173. [DOI] [PubMed] [Google Scholar]
- 58.Young, W. W., Jr., J. Portoukalian, and S. Hakomori. 1981. Two monoclonal anticarbohydrate antibodies directed to glycosphingolipids with a lacto-N-glycosyl type II chain. J. Biol. Chem. 256:10967-10972. [PubMed] [Google Scholar]
- 59.Zaleski, A., N. K. Scheffler, P. Densen, F. K. Lee, A. A. Campagnari, B. W. Gibson, and M. A. Apicella. 2000. Lipooligosaccharide P(k) (Galα1-4Galβ1-4Glc) epitope of Moraxella catarrhalis is a factor in resistance to bactericidal activity mediated by normal human serum. Infect. Immun. 68:5261-5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu, P., C. M. Tsai, and C. E. Frasch. 2002. Immunologic and genetic characterization of lipooligosaccharide variants in a Neisseria meningitidis serogroup C strain. FEMS Immunol. Med. Microbiol. 34:193-200. [DOI] [PubMed] [Google Scholar]