Abstract
Cardiorespiratory fitness (CRF) mitigates age-related decline in cognition and brain volume. Little is known, however, about the effects of high-intensity interval training (HIIT) on cognitive aging and the relationship between HIIT, cognition, hippocampal subfield volumes, and cerebral oxygen extraction fraction (OEF). Older sedentary women participated in an 8-week HIIT intervention. We conducted cognitive assessments, fitness assessments (VO2max), MRI scans: asymmetric spin echo oxygen extraction fraction (ASE-OEF), high-resolution multiple image co-registration and averaging (HR-MICRA) imaging, and transcranial Doppler ultrasonography before and after the intervention. VO2max increased from baseline (M = 19.36, SD = 2.84) to follow-up (M = 23.25, SD = 3.61), Z = − 2.93, p < .001, r = 0.63. Composite cognitive (Z = − 2.05, p = 0.041), language (Z = − 2.19, p = 0.028), and visuospatial memory (Z = − 2.22, p = 0.026), z-scores increased significantly. Hippocampal subfield volumes CA1 and CA3 dentate gyrus and subiculum decreased non-significantly (all p > 0.05); whereas a significant decrease in CA2 (Z = − 2.045, p = 0.041, r = 0.436) from baseline (M = 29.51; SD = 24.50) to follow-up (M = 24.50; SD = 13.38) was observed. Right hemisphere gray matter was correlated with language z-scores (p = 0.025; r = 0.679). The subiculum was correlated with attention (p = 0.047; r = 0.618) and verbal memory (p = 0.020; r = 0.700). The OEF and CBF were unchanged at follow-up (all p > .05). Although we observed cognitive improvements following 8 weeks of our HIIT intervention, they were not explained by hippocampal, OEF, or CBF changes.
Keywords: Exercise, VO2max, Cognition, Brain, Oxygen extraction, Hippocampal subfields
Introduction
Even in the absence of brain pathology, aging is associated with a decline in cognitive function that begins in the 4th decade of life and progressively decreases with age [1–3]. Age-related cognitive changes are largely attributable to atrophy in the frontal and temporal lobes [4], which underlie cognitive and memory processes [5]. Other brain areas also experience age-related atrophy, including the basal ganglia, thalamic and diencephalic structures, accumbens, and cerebellum [4]. Studies show that age-related morphometric and cellular changes in the brain generally occur many years before onset of cognitive decline [6, 7]. How and why morphometric and functional changes occur are not completely understood. However, studies suggest an underlying vascular etiology [8–11], and that a 28–50% progressive reduction in cerebral blood flow (CBF) [9, 12] could be responsible. For instance, reductions in CBF are correlated with lower global cognitive scores and executive function [13] and may be a risk factor that precedes atrophy of amygdala and hippocampal structures [8].
In recent years, exercise interventions became a major focus when studies began reporting an effect of cardiorespiratory fitness (CRF) on age-related declines in cognition, brain structure, and CBF [12, 14–20]. For instance, exercise-induced increases in basal ganglia volume [21], caudate nucleus [22], gray matter [17, 23], hippocampus [14, 24–26], and the prefrontal cortex [27] were reported in adults. Erickson and Voss [14] examined the effects of a 1-year exercise intervention on hippocampal volume and memory functions in older participants (N = 120) between 55 and 80 years of age. After 1 year, performance on a spatial memory task improved in both groups, but the exercise group showed a 2% increase in hippocampal volume, whereas the stretching group showed a 1.4% decrease in the same regions [14]. Similarly, endurance-trained athletes had higher CBF than sedentary individuals [12, 19].
Cumulative evidence suggests that exercise-induced improvements in brain volume impact cognition in older adults. Zhao and Tranovich [28] compared cognitive function of elite master athletes (age > 55) and sedentary controls (N = 102) and observed faster reaction times and better verbal memory in athletes compared to the sedentary group. In addition, a number of randomized controlled trials showed that exercise interventions ranging from 12 to 52 weeks, resulting in improvements in maximal oxygen uptake (VO2max), were correlated with enhanced cognitive function, including attention, executive function, and processing speed in older healthy adults [5]; albeit, the exact exercise dosage and intensity needed to improve cognitive function remained unclear. Nevertheless, the positive benefits of exercise training on cognitive function were supported by later meta-analytic studies [5, 15, 29–34].
These studies support the role of CRF and increased fitness on CBF, brain volume, and cognition. However, blood flow represents only one aspect of cerebral hemodynamics: the availability of blood and oxygen in the cerebrovasculature in response to cerebral autoregulation [35, 36]. Yet, the efficiency of the brain tissues to extract the available oxygen from the circulation to satisfy the brain’s metabolic needs, or the oxygen extraction fraction (OEF), remains relatively unexplored. Findings about OEF suggest that OEF increases with age and in individuals with vascular pathologies, whereas it decreases in patients with Alzheimer’s pathology [37, 38], and recently, elevated watershed OEF was found to be associated with greater white matter hyperintensity burden and microstructural impairments in older participants with cerebral small vessel disease [39].
Based on previous studies that highlighted the role of exercise on brain volumes and cognition in older adults, we conducted a brief high-intensity interval training (HIIT) intervention in sedentary older women. Our primary aim was to assess the efficacy of our 8-week HIIT protocol to initiate cognitive improvements. In addition, we conducted exploratory analyses to examine exercise effects on hemispheric OEF and hippocampal subfield volumes and examined possible relationships with cognitive improvements. We hypothesized that improvements in cognition will be correlated with OEF and hippocampal subfield volumes.
Materials and methods
Participants
In light of the small sample of this study and the possible influence of sex, only women were recruited. Potential participants were recruited from the local community of Birmingham, Alabama, and underwent a phone screening interview which included a personal history questionnaire, the 6-item cognitive screen [40], and the CHAMPS physical activity questionnaire for older adults [41]. All participants were screened for exclusion/inclusion criteria prior to enrollment. Inclusion criteria were sedentary (< 120 min of moderate intensity exercise/week) older women between 60 and 75 years of age. Exclusion criteria included significant extracranial carotid disease on duplex Doppler, score < 5 on the 6-item cognitive screener [40], history of head trauma with loss of consciousness ≥ 30 min or seizures, major depression or anxiety disorder, diagnosis of dementia, recent substance abuse, uncorrected hearing or vision disorders, chronic obstructive pulmonary disease, resting systolic blood pressure ≥ 160 mmHg, uncontrolled diabetes, BMI ≥ 32, inadequate sonographic windows for transcranial Doppler, disease or condition that would preclude physical activity or exercise, and inability to safely undergo MRI (e.g., incompatible implant and claustrophobia). Those enrolled in the study underwent further assessment, which included a graded exercise test with a 12-lead electrocardiogram (ECG); cardiac enzyme levels to exclude for underlying major cardiovascular abnormalities and to determine whether participants were physically able to exercise, had no depression or cognitive impairments, and were able to undergo magnetic resonance imaging. Due to its efficiency at initiating rapid cardiometabolic changes across short durations [42], a HIIT protocol was developed (Table 1). The study was approved by the University of Alabama at Birmingham Institutional Review Board and all participants provided written informed consent and received monetary compensation for participation.
Table 1.
High-intensity interval protocol
| Week | ||||
|---|---|---|---|---|
| 1 and 2 | 3 and 4 | 5 and 6 | 7 and 8 | |
| Work/rest ratio (minutes) | 1:2 | 1:1 | 1:2 | 1:1 |
| Number of intervals | 10 | 10 | 10 | 10 |
| Interval intensity (%max HR) | 70–80% | 70–80% | 80–90% | 80–90% |
| Warm up/cool down (minutes) | 5/5 | 5/5 | 5/5 | 5/5 |
| Total session time in minutes | 40 | 30 | 40 | 30 |
Baseline assessments were completed within 1 week of the start of the intervention. Assessments of maximal oxygen consumption (VO2max) were carried out at baseline and follow-up via a graded maximal exercise test on a cycle ergometer at the UAB Center for Exercise Medicine’s (UCEM) clinical exercise facility. Blood was collected immediately prior to the graded exercise test. Cardiac specific troponin levels were measured to screen for underlying heart injury at baseline only. Hematocrit levels were collected at both time points for use in OEF calculations.
Maximal oxygen consumption-VO2max
During the exercise test, a 12-lead ECG and blood pressure (BP) were monitored continuously by a nurse practitioner, an exercise physiologist, and the researcher. The exercise test continued to maximum, which we defined as achievement of at least two of three criteria: (1) heart rate = 220 − age, (2) respiratory exchange ratio > 1.15; (3) leveling off of oxygen consumption with increasing workload. Expired gases were collected throughout the test to monitor O2 consumption and CO2 expiration, and the participant’s perceived effort was monitored at each test stage via the standard 6–20 rating of perceived exertion (RPE) scale [43]. The graded test was terminated prior to reaching physiologic criteria if the participant voluntarily stopped the test, the diastolic pressure increased drastically (≥ 115 mmHg), or an ECG abnormality was noted.
Intervention
Participants engaged in training sessions on stationary cycle 95 CS LifeFitness™ ergometers four times per week for 8 weeks. All participants wore a Polar™ heart rate monitor during each exercise session. Each session included 5-min warm-ups and cool downs, and 10 1-minute high-intensity intervals alternating with 1 or 2 minutes of active recovery. Training intensity was determined using maximum heart rate (MHR) (220 − age), starting at 70% of MHR (Table 1). All sessions were supervised by the researcher and a UCEM staff member.
Neuropsychological tests
Standardized neuropsychological tests designed to assess processing speed, memory, attention, and executive function, which have been shown sensitive to diffuse effects of cardiogenic and cerebral hypoperfusion, were administered. All tests (Table 2) have published age-adjusted norms and included Center for Epidemiologic Studies Depression Scale, Hopkins verbal learning test–revised, brief visual memory test–revised, controlled oral word association (verbal fluency, F, A, S), Boston naming test, Rey-Osterrieth complex figure, WAIS-IV digit span and digit symbol, and trail making A and B [25, 44, 45]. Our cognitive testing format was modified to prevent direct contact with eight participants who began the study after the COVID-19 lockdown. Participants were seated comfortably at a desk in front of a protective plexiglass shield behind which an iPad tablet displayed the test material. A baby monitor with a camera view allowed for communication between the psychometric technician and the participant.
Table 2.
Cognitive tests and domains
| Domains | Cognitive tests | Outcome variable |
|---|---|---|
| Visuospatial memory | Brief visuospatial memory test | Recognition |
| Executive | Digit symbol | Time/errors |
| Trail making-B | Time/errors | |
| Language | Controlled oral word association test | Time |
| Boston naming test | Number correct | |
| Attention | Digit span | Number correct |
| Trail making-A | Time/errors | |
| Visuospatial ability | Rey-Osterrieth complex figure test (copy) | Items correct |
| Verbal memory | Hopkins verbal learning test | Number correct |
| Domain composite | Average of all tests | –– |
For each domain, z-scores from the cognitive tests were summed and divided by the number of tests
Center for Epidemiologic Studies Depression Scale
The CES-D (4-item) is a short self-report scale designed to screen for depressive symptoms [46]. This measure was used for screening purposes only.
Hopkins Verbal Learning Test-revised
The HVLT-R measures verbal learning and memory [47]. The HVLT-R is available in six different forms, reducing practice effects on repeat administration. The HVLT-R includes three learning trials, a delayed recall trial (20–25 min), and a “yes”, “no” delayed recognition trial.
Brief Visuospatial Memory Test-revised
The BVMT-R is used to assess visual memory and consists of six alternate geometric figures presented in a matrix. The test includes three learning trials, a delayed recall trial (20–25 min), and a recognition trial. In each of the learning trials, each figure matrix is presented for 10 seconds, after which the participant is asked to reproduce the figures on a separate sheet in the same location as they had been presented in the original matrix display. The participant is asked to reproduce the figures after a time delay, which is followed by a recognition trial [48]
Controlled Oral Word Association
The purpose of this measure is to assess rapid, oral word list generation. The participant is asked to produce spontaneous words beginning with a given letter [49] (most commonly F, A, S) as quickly as possible within 60 seconds.
Boston Naming Test
The BNT consists of 30 drawings of items ranging from simple common words (“bed”) to uncommon words (“protractor”) to test oral naming abilities. Drawings are presented successively and two prompting cues (phonemic or stimulus) are provided if spontaneous object naming is not produced [50].
Rey-Osterrieth Complex Figure Test
Commonly, the RCFT is used to assess visuospatial construction ability and visual memory using a complex geometric figure and consists of a copy trial and recall trial [51].
Digit Span
The Digit Span is a measure of attention and working memory. The test is divided into forward span and backward span sections with varying difficulty levels from two digits forward to six digits backward. In the forward span, a sequence of numbers is read aloud to participants, who are asked to repeat the same sequence in the same order it was presented. Backward span requires that participants say the sequence of numbers in backward order [52]. WAIS–IV sequencing was not administered.
Digit Symbol
A measure of visual attention, scanning and tracking, which consists of one row of nine numbered squares with equivalent nonsense symbols that serve as a key, below which seven rows, each containing 20 blank squares are paired with a number from 1 to 9. Following a practice trial, the participant is asked to fill in each blank square with the equivalent nonsense symbol that is paired to the number as quickly as possible for 120 seconds [52].
Trail Making Test A & B
The Trail Making tests [53] consist of 25 circles randomly distributed on a sheet of paper. In trails-A, the circles are numbered 1–25. Participants are asked to draw lines connecting the circles in the correct ascending order (e.g., 1, 2, 3). In trails-B, the 25 circles include encircled letter circles (A–L) and number circles (1–13). The participant is asked to connect the circles beginning with 1 and alternating between a number and a letter in ascending order (e.g., 1-A, 2-B, 3-C).
For each test, a z-score was derived by calculating the differences between raw scores and the means from a normative population then dividing that number by the standard deviation of the normative population. Z-scores were corrected for age, education, and sex when available. Higher z-scores represent better function, and 0.5 SD change was considered clinically significant [54]. From these measures, domain composites were calculated (Table 2). For each composite cognitive domain, the z-scores from individual tests were averaged to calculate a composite z-score.
Transcranial Doppler
Transcranial Doppler (TCD) ultrasonography was completed at baseline and follow-up. The TCD assesses mean blood flow velocity in the anterior intracerebral circulation. Right and left middle cerebral arteries were insonated through the temporal acoustic windows at a depth of 50–58 mm using a 2-MHz probe to perform a continuous recording of the cerebral blood flow volume waveforms. After the stability of waveforms, mean flow velocity (MFV) was recorded.
Imaging
Imaging scans were conducted on a Siemens MAGNETOM Prisma 3.0-T whole-body MRI system with a 20-channel head coil for 11 subjects at baseline and after an exercise intervention. The parameters for each of the sequences were maintained between the two time points and were as follows: a T1-weighted magnetization prepared rapid acquisition with gradient echo (MPRAGE) sequence with an isotopic voxel resolution of 1.0 mm, repetition time (TR) and an echo time (TE) of 2300 ms and 2.98 ms respectively, a flip angle (FA) of 9, an echo spacing of 7.1 ms, a field of view (FOV) of 256 mm, and a turbo spin-echo (TSE) factor of 208; a three-dimensional fluid-attenuated inversion recovery (FLAIR) sequence with an in-plane resolution of 1 mm × 1 mm, an effective slice thickness of 1.2 mm, TR and TE of 4800 ms and 441 ms respectively, an echo spacing of 3.4 ms, an FOV of 256 mm, and a TSE factor of 243; and a variable flip-angle turbo spin echo sequence with an isotropic voxel resolution of 0.5 mm, a TR and echo time TE of 3200 ms and 561 ms respectively, an echo spacing of 5.34 ms, an echo train duration of 1319 ms, an FOV of 160 mm, and a TSE of 282. Additionally, an axial diffusion tensor imaging (DTI) sequence had isotropic voxel resolution of 2.0 mm, maximal b value of 1000 s/mm2, TR and TE of 7200 ms and 56 ms respectively, and monopolar diffusion scheme with b values of [0, 500, 1000, and 2000]. The variable flip-angle turbo spin echo sequence was repeated 4 times per session and the scans were resampled to 0.5 × 0.5 mm resolution in-plane resolution with 0.75-mm slice thickness (A-P) using FSL-FLIRT [55], with 3 high-resolution multi-contrast acquisition (HR-MICRA) scans created with scan resolutions of 2 × , 3 × , and 4 × respectively [56].
Asymmetric spin echo-oxygen extraction fraction process
The OEF was obtained using a dual-echo asymmetric spin echo sequence with the following imaging parameters: TE1/TE2/TR = 61.2/93.2/4100 ms, the time intervals (τ)between the center of π pulse and TE/2 are 0 to 22 ms with an increment of 0.5 ms, repetition = 2, in-plane voxel resolution = 1.72 × 1.72 mm after in-plane interpolation and slice thickness = 3 mm, and acquisition time = 6 min and 40 s. The shift of π pulse provided R2′ decay times of 2τ for the first echo (0 to 44 ms with an increment of 1 ms) and R2′ decay times of 2τ + TE2 − TE1 for the second echo (32 to 76 ms).To summarize, the OEF is calculated using the reversable transverse relaxation rate (R2′) measured by the ASE sequence [57, 58],
| 1 |
where γ is the gyromagnetic ratio; Hct is the fractional hematocrit; is the fractional venous cerebral blood volume; B0 is the magnetic field strength; is the susceptibility difference between the fully oxygenated and fully deoxygenated blood, which has been measured to be 0.19 ppm per unit Hct [59] and OEF is the oxygen extraction fraction. In this study, Hct was measured for each participant of the study and an Hct ratio of 0.85 [60] was employed to convert the large vessel Hct to a small vessel Hct.
Gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) probability maps were created using the Statistical Parametric Mapping toolbox (SPM12). Voxels with probability less than 0.9 of being classified as CSF, WM, or GM were removed from the mask. Left and right hemisphere subcortical segmentations were created using the FreeSurfer software ver. 7.0. Joint ROIs were created to review both the left and right hemispheres for the identified probability mappings of CSF, GM, and WM. The mean and standard deviation of the estimated OEF derived from these ROIs were obtained for each subject. To account for intrasubject variation, each subject’s estimated mean OEF were normalized as a ratio with respect to the mean OEF for the entire brain for their respective subject[61].
Automated segmentation of hippocampal subfields (ASHS) volumetry of hippocampal subfields
Hippocampal subregions were found using the ASHS software package [61] using the same combinations of T1 mprage file and a HR-MICRA. The atlas used was the Magdeburg Young Adult 7 T Atlas for sub-hippocampal structures [62] and Penn Temporal Lobe Epilepsy T1-MRI Whole Hippocampus ASHS Atlas [63] for general hippocampal structure and segmentation. The ASHS software calculates the volumes of each subfield automatically with a combination of multi-atlas segmentation and voting (MASV) and a learning-based bias correction/detection scheme [64]. The subregions produced by the Magdeburg Young Adult 7 T Atlas were both the left and right hemispheres; ErC, Area 35, Area 36, PhC, CA1, CA2, CA3, DG, Tail, Sub, and Cysts. However, in the current study only CA-1, 2, and 3; the dentate gyrus and subiculum, which constitute the hippocampus, are considered. Assessment of the labels produced in this study was manually reviewed for consistency and quality. While the editing of hippocampal anatomy is possible, it requires advanced knowledge of the regions of the hippocampus and multiple reviewers to account for interobserver variability. As such, the editing of segmentations was not considered for this project.
Statistical analyses
A Shapiro–Wilk normality test revealed non-normally distributed data. Given our small sample size, the effects of the intervention were examined using non-parametric, paired two–tailed Wilcoxon rank tests, and statistical significance was 0.05. Means, standard deviations, p-values, and effect sizes are presented for all variables. Two-tailed correlations using absolute difference scores were calculated to assess the strength of relationships between our variables of interest. Pearson’s correlation coefficients were calculated for normally distributed change scores, whereas Spearman’s rank correlation coefficients were used for non-normally distributed change scores. Statistical analyses were completed using SPSS Statistics 27 (IBM) software package and GraphPad Prism 9.4.
Results
Participants
Of the 128 women recruited for the study, 72 did not meet age requirements, declined due to time restrictions, or concerns over possible COVID-19 exposure. Another 38 were excluded for contraindicated conditions (COPD, asthma, vasculopathies, anxiety or depression, high BMI, diabetes, claustrophobia, sarcoidosis, or medications known to interact with brain function). The remaining 18 sedentary older women were enrolled in the study and underwent further assessment. The additional screening led to the exclusion of three women (2 could not complete the graded exercise test, one had a claustrophobic response to the MRI). Of the remaining 15 women, one participant dropped out of the study due to personal reasons, and follow-up data from three participants could not be collected due to closure of the university research facilities during the COVID-19 pandemic. Thus, eleven women between the ages of 60 and 75 (M = 67; SD = 3.99) participated in an 8-week exercise intervention. Adherence to the exercise sessions was 97% (31 out of 32 sessions completed) (Table 3).
Table 3.
Participant demographics
| (N = 11; Black 18%) | Mean | SD |
|---|---|---|
| Age | 67.09 | 3.99 |
| BMI | 28.93 | 3.90 |
| Education | 16.09 | 3.02 |
| Session adherence | 31.09 | 0.83 |
Maximum number of sessions = 32
VO2max
At baseline, the mean VO2max was 19.36 ml/kg/min (SD = 2.84). After the exercise intervention (Fig. 1), mean VO2max rose to 23.25 ml/kg/min (SD = 3.61), which was a statistically significant mean increase of 3.89 ml/kg/min, Z = − 2.93, p < 0.001, with a large effect size (r = 0.63).
Fig. 1.
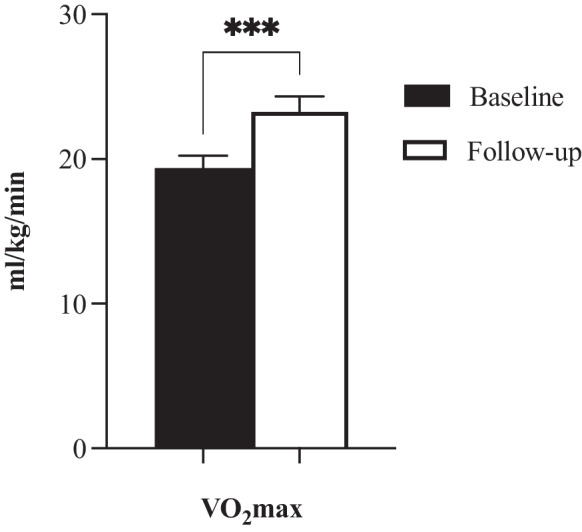
VO2max baseline and follow-up. Asterisks (***) indicate sig. < 0.001
Cognition
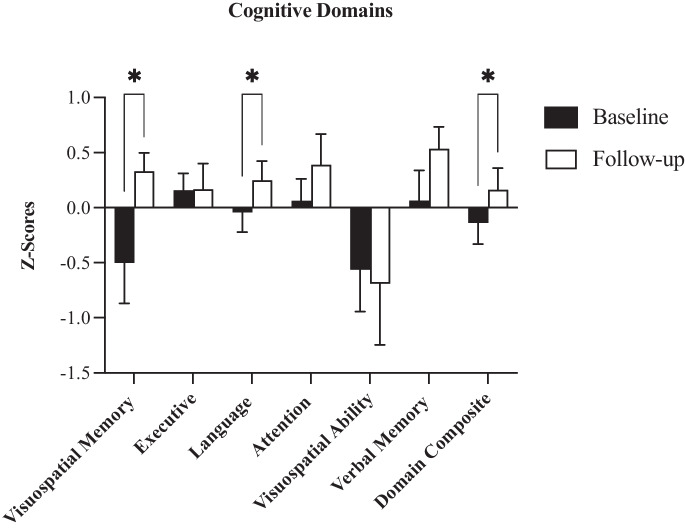
Baseline cognitive z-scores were within the normal range (M = 0.14; SD = 0.64). Eight weeks of HIIT had a positive effect on cognition in older women (Table 4; Fig. 2). Global cognition improved (Z = –2.05; p = 0.041) with a large effect size (r = 0.44). Visuospatial memory (Z = − 2.22; p = 0.026) and language domains (Z = − 2.19; p = 0.028), both improved significantly with large effect sizes (r = 0.47). There were no statistical differences in executive function, attention, verbal memory, and visuospatial ability (all p > 0.05).
Table 4.
Cognitive domains
| Baseline | Follow-up | Paired statistics | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Z | P | Effect size | |
| Visuospatial memory | − 0.37 | 0.51 | 0.27 | 0.83 | − 2.22 | 0.026 | − 0.47 |
| Executive | 0.16 | 0.51 | 0.17 | 0.78 | − 0.18 | 0.859 | − 0.04 |
| Language | − 0.04 | 0.60 | 0.25 | 0.59 | − 2.19 | 0.028 | − 0.47 |
| Attention | 0.06 | 0.67 | 0.39 | 0.92 | − 1.65 | 0.1 | − 0.35 |
| Verbal memory | − 0.06 | 0.91 | 0.53 | 0.67 | − 1.78 | 0.075 | − 0.38 |
| Visuospatial ability | − 0.56 | 1.27 | − 0.69 | 1.84 | − 0.87 | 0.386 | − 0.18 |
| Domain composite | − 0.14 | 0.64 | 0.16 | 0.66 | − 2.05 | 0.041 | − 0.44 |
Bold represents sig. < 0.05
Fig. 2.
Cognitive domains, baseline, and follow-up domain z-scores. Asterisk (*) indicates sig. < 0.05. Bars indicate SE
Hippocampal subfields
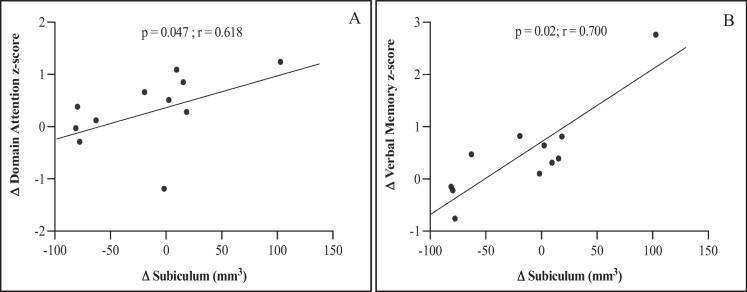
Subfield segmentations (mm3) showed a significant decrease from baseline (M = 29.51; SD = 10.84) to follow-up (M = 24.5; SD = 13.38) in the CA-2 hippocampal region, Z = − 2.045; p = 0.04, with a moderate effect size (r = 0.44). No significant changes were observed in any of the remaining subfields (Table 5). To test possible relationships between cognition and hippocampal subfields, correlations were calculated using change scores from cognitive domain z-scores and hippocampal subfields (Table 6). Results showed positive correlations between the subiculum and attention (p = 0.047; r = 0.618) and verbal memory (p = 0.020; r = 0.700) only (Fig. 3).
Table 5.
Hippocampal subfields in mm3
| Baseline | Follow-up | Paired differences | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Z | P | Effect size | |
| CA-1 | 702.91 | 102.76 | 694.08 | 95.99 | − 0.889 | 0.374 | − 0.190 |
| CA-2 | 29.51 | 10.84 | 24.50 | 13.38 | − 2.045 | 0.041 | − 0.436 |
| CA-3 | 120.24 | 19.68 | 110.92 | 22.42 | − 1.067 | 0.286 | − 0.227 |
| DG | 529.94 | 51.58 | 526.07 | 46.44 | − 0.489 | 0.624 | − 0.104 |
| Sub | 810.56 | 65.52 | 794.71 | 85.56 | − 0.711 | 0.477 | − 0.152 |
Bold represents sig. < 0.05
CA 1–3 cornu ammonis, DG dentate gyrus, Sub subiculum
Table 6.
Correlations between hippocampal subfields and cognitive domains
| Hippocampal subfields | |||||
|---|---|---|---|---|---|
| CA1 | CA2 | CA3 | DG | Sub | |
| Visuospatial memory | − 0.064 | − 0.100 | − 0.164 | − 0.009 | − 0.164 |
| Executive | − 0.310 | 0.087 | 0.237 | − 0.365 | 0.442 |
| Language | − 0.487 | − 0.301 | − 0.264 | 0.347 | 0.173 |
| Attention | − 0.227 | − 0.145 | 0.527 | − 0.415 | 0.618 |
| Visuospatial ability | − 0.410 | − 0.487 | − 0.178 | 0.249 | − 0.251 |
| Verbal memory | − 0.027 | 0.227 | 0.355 | − 0.123 | 0.700 |
| Domain composite | − 0.518 | − 0.200 | 0.264 | − 0.287 | 0.500 |
Bold represents sig. < 0.05
CA cornu ammonis, DG dentate gyrus, Sub subiculum
Fig. 3.
Cognition and hippocampal subfields. A As change scores in attention and B verbal memory composites z-scores increased, change score in the subiculum increased
Oxygen extraction fraction
From baseline to follow-up, we observed an increase of 5.8% in left hemisphere gray matter and 5.2% in white matter OEF. Right hemisphere gray matter decreased 1%, and white matter OEF increased 14%. All were statistically non-significant (all p > 0.05) with negligible effect sizes (Table 7). To assess whether improvements in cognition were associated with OEF, correlations were calculated using change scores from domain and composite z-scores, and OEF (Table 8). Only language correlated with right hemisphere gray matter OEF (p = 0.025; r = 0.679) (Fig. 4).
Table 7.
Oxygen extraction fraction
| Baseline | Follow-up | Paired statistics | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Z | P | Effect size | |
| Left hemisphere | |||||||
| Gray matter | 1.04 | 0.04 | 1.10 | 0.20 | − 0.356 | 0.722 | − 0.076 |
| White matter | 0.97 | 0.02 | 1.02 | 0.17 | − 0.267 | 0.790 | − 0.057 |
| Right hemisphere | |||||||
| Gray matter | 1.05 | 0.04 | 1.04 | 0.03 | − 1.511 | 0.131 | − 0.322 |
| White matter | 0.85 | 0.28 | 0.97 | 0.30 | − 0.533 | 0.594 | − 0.114 |
p < 0.05
Table 8.
Correlations between OEF and cognitive domains
| Oxygen extraction fraction | ||||
|---|---|---|---|---|
| Left hemisphere | Right hemisphere | |||
| Gray | White | Gray | White | |
| Visuospatial memory | − 0.009 | 0.191 | 0.055 | − 0.282 |
| Executive | 0.319 | 0.282 | 0.205 | 0.187 |
| Language | 0.269 | − 0.251 | 0.679 | − 0.132 |
| Attention | 0.218 | 0.336 | − 0.400 | 0.000 |
| Visuospatial ability | − 0.333 | − 0.715 | − 0.059 | − 0.337 |
| Verbal memory | 0.627 | 0.491 | − 0.118 | 0.582 |
| Domain composite | 0.236 | 0.082 | 0.082 | 0.045 |
Bold indicates sig. < 0.05
Fig. 4.
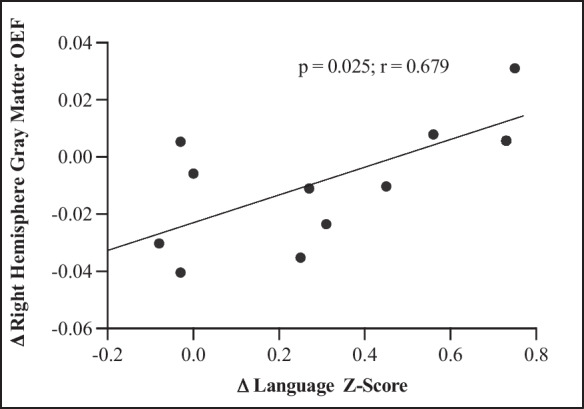
Language and OEF. As change scores in the language composite z-scores increased, change score in OEF increased in right hemisphere gray matter
CBF
Follow-up data for three participants were not collected due to COVID-19 restrictions. Therefore, data for only 8 participants were analyzed. Baseline MFV was 50.77 cc/s (SD = 6.46), which was in the normal range. At follow-up (M = 51.83; SD 6.81), no change in global hemispheric mean flow velocity (MFV) was observed (all p > 0.05).
Discussion
In this study, we showed the feasibility of an 8-week HIIT intervention in older sedentary women. Our high session completion rate (31/32 sessions; 97%) was robust. After 8 weeks, VO2max increased by 20%, consistent with previous studies. Østerås and Hoff [65] reported a significant increase (13.2%) in VO2max following 10 weeks of HIIT, while a later study reported a 9–13% increase after 8 weeks [42]. A strength of the present study was the response to our HIIT protocol in comparison to other short-duration HIIT studies, and moderate intensity programs ranging from 24 to 52 weeks [14, 66]. The increase in VO2max in our study demonstrates the effectiveness of our protocol, the success of which may be ascribed to various factors including (1) duration of each session, (2) frequency of training sessions, (3) time at intensity, and (4) supervised training.
In line with previous studies [14, 29, 33, 67, 68], our HIIT intervention improved some cognitive domains in older women. At follow-up, the overall cognitive composite, visuospatial memory, and language domains were significantly higher with no impact on executive, attention, verbal memory, and visuospatial ability. Importantly, it is possible that the improvements in cognition were a result of practice effects. Given the absence of a comparison control group, practice effects are difficult to establish. However, to help mitigate the potential for practice effects, form 1 was used at baseline, whereas form 3 was used at follow-up for the BVMT-R and HVLT-R.
Our exploratory aims found a global decrease in hippocampal subfield volumes after the intervention; however, only CA2 significantly decreased at follow-up. Nevertheless, our findings correspond with studies that showed significant reductions [69, 70] or no changes in hippocampal volumes [17, 71] following exercise training. By contrast, other studies reported an increase in hippocampal volumes following exercise [14, 26]. In older adults, the increase in the anterior hippocampal regions was correlated with spatial memory [14]; however, the longer duration, lower intensity of the intervention, and larger sample size prevent direct comparison with our study.
All domain mean z-scores except visuospatial ability improved following the intervention; however, only global cognition, visuospatial memory, and language domains were significantly different at follow-up. We assessed the relationship between cognitive domains and hippocampal subfields and found positive relationships between the subiculum and attention and verbal memory domains only. Given the role of the subiculum in working memory [72], our results are not surprising and suggest that larger volumes in the subiculum may be linked to functional performance.
Although our HIIT protocol significantly increased fitness levels in older women, we did not observe a change in global CBF or hemispheric CBF. Despite the small number of datasets (8) we were able to analyze, our findings correspond with previous research indicating no effect of an exercise program on CBF in otherwise healthy older individuals. Following a 28-week moderate-intensity exercise intervention in older men and women, resting middle cerebral artery blood flow velocity (MCAv) was relatively unchanged, whereas cerebrovascular reactivity in response to hypercapnia increased [73]. Similarly, a 12-week exercise intervention showed no change in MCAv in young and older adults [74]. Lastly, an interventional study by Kleinloog and Mensink [75] reported regional changes in CBF following a 12-week moderate intensity exercise intervention in older men. Using pseudo-continuous arterial spin labeling, CBF increased 27% in the frontal lobe and a 19% decrease in the right medial temporal lobe was reported, whereas global CBF, the gray matter CBF, and the right and left CBF were not different from baseline [75].
Our results did not show an effect of our exercise intervention on OEF. Although research examining the effects of a longitudinal HIIT intervention on OEF and cognition are scarce, our results are supported by one study that showed no change in OEF following an acute bout of exercise in young elite male athletes [76]. Our correlational analyses identified a positive association between the right hemisphere gray matter OEF and language, which was surprising given the presumed dominance of the left hemisphere [77] over the right hemisphere in verbal abilities. Recent findings, however, provided evidence for bilateral activations of both hemispheres with increasing word difficulty on verbal tasks, thereby supporting a potential contribution from the right hemisphere on tasks of word retrieval [78]. Our findings are supported by a recent study that examined OEF and cognition and found a positive correlation between OEF and cognitive performance in older adults [38, 79]. In the setting of vascular disease (e.g., hypertension, hypercholesterolemia, diabetes), however, higher OEF was associated with cognitive impairment [38]. Taking this into account, we considered vascular risk factors in the current study and found that although eight of our participants had risk factors, all eight were receiving antihypertensives and/or statins, and none was cognitively impaired at baseline or follow-up, which supports a protective effect of medications on cerebral hemodynamics and cognitive function [80, 81].
Limitations
Our results may be limited by several factors. First, our small study used a HIIT exercise modality. It is unclear if HIIT vs. moderate intensity confers different effects on the brain. Our small sample is underpowered to detect any changes in CBF or brain structures and we did not include a control group. The necessary sensitivity to measure changes in CBF, for instance, might have been improved with arterial spin labeling MRI. Furthermore, it is not clear if the absence of volumetric and OEF changes were due to the short duration of the study. This study represents a first application of the imaging sequences to assess changes following an exercise intervention. We collected 4 scans to assess hippocampal subfield volumes, which may have reduced the sensitivity of an otherwise powerful imaging tool to detect small variations. Additionally, to increase participant comfort during image acquisition, we used a 20-channel head coil instead of the 64-channel head coil. More scans (~ 8 +) and the use of the 64-channel head coil could have yielded better clarity providing more detailed measures of changes [56]. Similarly, our OEF sequence excludes subcortical and cerebellar structures. Considering that volumetric changes are known to occur selectively across the brain [14, 82], any potential changes in OEF in the subcortical regions including the hippocampus were not captured by our OEF imaging protocol. Therefore, it remains to be determined if our imaging sequences can be used to provide accurate estimates of the effects of exercise on the whole brain. Lastly, taking into account the brief interval between baseline and follow-up, it is possible our cognitive improvements were the result of practice effects rather than functional improvements.
In summary, our data suggest that a short-term HIIT exercise intervention is feasible and provides sufficient stimulus to increase VO2max and cognitive function significantly in older sedentary women. Furthermore, our intervention provided exploratory data on the effects of the 8 weeks of exercise on OEF, hippocampal subfield volumes, and CBF. Future studies would be well-served by a larger sample, a control group, a longer exercise intervention, and additional subfield scans to increase image clarity.
Funding
This manuscript was funded in part by the National Institute of General Medical Sciences (NIGMS) 5 T32 GM109780-4 (AMN), NIHR01NS082561 (HA), NIHRF1NS116565 (HA), and The Evelyn F. McKnight Brain Institute at the University of Alabama at Birmingham (RML).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
We certify that the submission is original work and is not under review at any other publication.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Salthouse TA. Independence of age-related influences on cognitive abilities across the life span. Dev Psychol. 1998;34(5):851. doi: 10.1037/0012-1649.34.5.851. [DOI] [PubMed] [Google Scholar]
- 2.Salthouse TA. Selective review of cognitive aging. J Int Neuropsychol Soc. 2010;16(5):754–760. doi: 10.1017/S1355617710000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon RA, et al. Episodic memory change in late adulthood: generalizability across samples and performance indices. Mem Cognit. 2004;32(5):768–778. doi: 10.3758/BF03195867. [DOI] [PubMed] [Google Scholar]
- 4.Fjell AM, Walhovd KB. Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci. 2010;21(3):187–222. doi: 10.1515/REVNEURO.2010.21.3.187. [DOI] [PubMed] [Google Scholar]
- 5.Colcombe SJ, Kramer AF. Fitness effects on the cognitive function of older adults- a meta-analytic study. Psychol Sci. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 6.Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5(2):87. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- 7.Downey A, et al. Health and medicine division; board on health sciences policy; committee on preventing dementia and cognitive impairment. Preventing cognitive decline and dementia: A way forward. Washington. DC: The National Academies Press; 2017. [PubMed]
- 8.Ruitenberg A, et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam study. Ann Neurol. 2005;57(6):789–794. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- 9.Beason-Held LL, et al. Longitudinal changes in cerebral blood flow in the older hypertensive brain. Stroke. 2007;38(6):1766–1773. doi: 10.1161/STROKEAHA.106.477109. [DOI] [PubMed] [Google Scholar]
- 10.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12(12):723. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson KI, et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci. 2010;30(15):5368–5375. doi: 10.1523/JNEUROSCI.6251-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ainslie PN, et al. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol. 2008;586(16):4005–4010. doi: 10.1113/jphysiol.2008.158279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poels MM, et al. Total cerebral blood flow in relation to cognitive function: the Rotterdam Scan Study. J Cereb Blood Flow Metab. 2008;28(10):1652–5. 10.1038/jcbfm.2008.62. [DOI] [PubMed]
- 14.Erickson KI, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bherer L, Erickson KI, Liu-Ambrose T. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J Aging Res. 2013;2013:657508. doi: 10.1155/2013/657508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erickson KI, et al. The brain-derived neurotrophic factor Val66Met polymorphism moderates an effect of physical activity on working memory performance. Psychol Sci. 2013;24(9):1770–1779. doi: 10.1177/0956797613480367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruscheweyh R, et al. Physical activity and memory functions: an interventional study. Neurobiol Aging. 2011;32(7):1304–1319. doi: 10.1016/j.neurobiolaging.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Floel A, et al. Physical activity and memory functions: are neurotrophins and cerebral gray matter volume the missing link? Neuroimage. 2010;49(3):2756–2763. doi: 10.1016/j.neuroimage.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 19.Bailey DM, et al. Elevated aerobic fitness sustained throughout the adult lifespan is associated with improved cerebral hemodynamics. Stroke. 2013;44(11):3235–3238. doi: 10.1161/STROKEAHA.113.002589. [DOI] [PubMed] [Google Scholar]
- 20.Lucas SJ, et al. Effect of age on exercise-induced alterations in cognitive executive function: relationship to cerebral perfusion. Exp Gerontol. 2012;47(8):541–551. doi: 10.1016/j.exger.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Becker L, Kutz DF, Voelcker-Rehage C. Exercise-induced changes in basal ganglia volume and their relation to cognitive performance. J Neurol Neuromed. 2016;1(5):19–24. doi: 10.29245/2572.942X/2016/5.1044. [DOI] [Google Scholar]
- 22.Verstynen TD, et al. Caudate nucleus volume mediates the link between cardiorespiratory fitness and cognitive flexibility in older adults. J Aging Res. 2012;2012:939285. doi: 10.1155/2012/939285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boraxbekk CJ, et al. Physical activity over a decade modifies age-related decline in perfusion, gray matter volume, and functional connectivity of the posterior default-mode network-a multimodal approach. Neuroimage. 2016;131:133–141. doi: 10.1016/j.neuroimage.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Killgore WD, Olson EA, Weber M. Physical exercise habits correlate with gray matter volume of the hippocampus in healthy adult humans. Sci Rep. 2013;3:3457. doi: 10.1038/srep03457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maass A, et al. Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. Neuroimage. 2016;131:142–154. doi: 10.1016/j.neuroimage.2015.10.084. [DOI] [PubMed] [Google Scholar]
- 26.Thomas AG, et al. Multi-modal characterization of rapid anterior hippocampal volume increase associated with aerobic exercise. Neuroimage. 2016;131:162–170. doi: 10.1016/j.neuroimage.2015.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinstein AM, et al. The association between aerobic fitness and executive function is mediated by prefrontal cortex volume. Brain Behav Immun. 2012;26(5):811–819. doi: 10.1016/j.bbi.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao E, et al. Chronic exercise preserves brain function in masters athletes when compared to sedentary counterparts. Phys Sportsmed. 2016;44(1):8–13. doi: 10.1080/00913847.2016.1103641. [DOI] [PubMed] [Google Scholar]
- 29.Angevaren M, et al. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane database of systematic reviews, 2008;(2). [DOI] [PubMed]
- 30.Barha CK, et al. Sex differences in exercise efficacy to improve cognition: a systematic review and meta-analysis of randomized controlled trials in older humans. Front Neuroendocrinol. 2017;46:71–85. doi: 10.1016/j.yfrne.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Gates N, et al. The effect of exercise training on cognitive function in older adults with mild cognitive impairment: a meta-analysis of randomized controlled trials. Am J Geriatr Psychiatry. 2013;21(11):1086–1097. doi: 10.1016/j.jagp.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 32.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil. 2004;85(10):1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 33.Kramer AF, et al. Fitness, aging and neurocognitive function. Neurobiol Aging. 2005;26(Suppl 1):124–127. doi: 10.1016/j.neurobiolaging.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Kramer AF, et al. Ageing, fitness and neurocognitive function. Nature. 1999;400:418. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- 35.Derdeyn CP. Hemodynamics and oxygen extraction in chronic large artery steno-occlusive disease: clinical applications for predicting stroke risk. J Cereb Blood Flow Metab. 2017; 271678X17732884. 10.1177/0271678X17732884. [DOI] [PMC free article] [PubMed]
- 36.Derdeyn CP, et al. Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodynamic impairment revisited. Brain. 2002;125(3):595–607. doi: 10.1093/brain/awf047. [DOI] [PubMed] [Google Scholar]
- 37.Catchlove SJ, et al. An investigation of cerebral oxygen utilization, blood flow and cognition in healthy aging. PLoS One. 2018;13(5):e0197055. doi: 10.1371/journal.pone.0197055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang D, et al. Brain oxygen extraction is differentially altered by Alzheimer’s and vascular diseases. J Magn Reson Imaging. 2020;52(6):1829–1837. doi: 10.1002/jmri.27264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang P, et al. Oxygen metabolic stress and white matter injury in patients with cerebral small vessel disease. Stroke. 2022;53(5):1570–1579. doi: 10.1161/STROKEAHA.121.035674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Callahan CM, et al. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;771–781. [DOI] [PubMed]
- 41.Stewart AL, et al. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Storen O, et al. The effect of age on the VO2max response to high-intensity interval training. Med Sci Sports Exerc. 2017;49(1):78–85. doi: 10.1249/MSS.0000000000001070. [DOI] [PubMed] [Google Scholar]
- 43.Borg G. Borg's perceived exertion and pain scales. Human kinetics; 1998.
- 44.Lezak MD. Neuropsychological assessment. Oxford University Press USA; 2004.
- 45.Maass A, et al. Vascular hippocampal plasticity after aerobic exercise in older adults. Mol Psychiatry. 2015;20(5):585–593. doi: 10.1038/mp.2014.114. [DOI] [PubMed] [Google Scholar]
- 46.Lewinsohn PM, et al. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997;12(2):277. doi: 10.1037/0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- 47.Benedict RH, et al. Hopkins verbal learning test–revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12(1):43–55. doi: 10.1076/clin.12.1.43.1726. [DOI] [Google Scholar]
- 48.Benedict RH. Brief visuospatial memory test--revised. PAR; 1997.
- 49.Benton A. Development of a multilingual aphasia battery: progress and problems. J Neurol Sci. 1969;9(1):39–48. doi: 10.1016/0022-510X(69)90057-4. [DOI] [PubMed] [Google Scholar]
- 50.Williams BW, Mack W, Henderson VW. Boston naming test in Alzheimer’s disease. Neuropsychologia. 1989;27(8):1073–1079. doi: 10.1016/0028-3932(89)90186-3. [DOI] [PubMed] [Google Scholar]
- 51.Meyers JE, Meyers KR. Rey complex figure test under four different administration procedures. Clin Neuropsychol. 1995;9(1):63–67. doi: 10.1080/13854049508402059. [DOI] [Google Scholar]
- 52.Wechsler D. WAIS-3., WMS-3: Wechsler adult intelligence scale, Wechsler memory scale: Technical manual. Psychol Corp; 1997.
- 53.Army U. Army individual test battery. Manual Dir Scoring; 1944.
- 54.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003:582–592. [DOI] [PubMed]
- 55.Jenkinson M, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1006/nimg.2002.1132. [DOI] [PubMed] [Google Scholar]
- 56.Ver Hoef L, et al. Clear and consistent imaging of hippocampal internal architecture with high resolution multiple image co-registration and averaging (HR-MICRA) Front Neurosci. 2021;15:546312. doi: 10.3389/fnins.2021.546312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.An H, Lin W. Impact of intravascular signal on quantitative measures of cerebral oxygen extraction and blood volume under normo-and hypercapnic conditions using an asymmetric spin echo approach. Magn Reson Med: Off J Int Soc Magn Reson Med. 2003;50(4):708–716. doi: 10.1002/mrm.10576. [DOI] [PubMed] [Google Scholar]
- 58.Guilliams KP, et al. Red cell exchange transfusions lower cerebral blood flow and oxygen extraction fraction in pediatric sickle cell anemia. Blood, J Am Soc Hematol. 2018;131(9):1012–1021. doi: 10.1182/blood-2017-06-789842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weisskoff RM, Kiihne S. MRI susceptometry: image-based measurement of absolute susceptibility of MR contrast agents and human blood. Magn Reson Med. 1992;24(2):375–383. doi: 10.1002/mrm.1910240219. [DOI] [PubMed] [Google Scholar]
- 60.Eichling JO, et al. In vivo determination of cerebral blood volume with radioactive oxygen-15 in the monkey. Circ Res. 1975;37(6):707–714. doi: 10.1161/01.RES.37.6.707. [DOI] [PubMed] [Google Scholar]
- 61.Yushkevich PA, et al. Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment. Hum Brain Mapp. 2015;36(1):258–287. doi: 10.1002/hbm.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berron D, et al. A protocol for manual segmentation of medial temporal lobe subregions in 7 Tesla MRI. NeuroImage: Clin. 2017;15:466–482. doi: 10.1016/j.nicl.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hadar PN, et al. Novel Multi-Slice Glutamate Imaging (GluCEST) of the Hippocampus in MRI-Negative Temporal Lobe Epilepsy. In ANNALS OF NEUROLOGY 2017 Oct 1 (Vol. 82, pp. S69-S69). 111 RIVER ST, HOBOKEN 07030-5774, NJ USA: WILEY.
- 64.Yushkevich PA, et al. Nearly automatic segmentation of hippocampal subfields in in vivo focal T2-weighted MRI. Neuroimage. 2010;53(4):1208–1224. doi: 10.1016/j.neuroimage.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Østerås H, Hoff J, Helgerud J. Effects of high-intensity endurance training on maximal oxygen consumption in healthy elderly people. J Appl Gerontol. 2016;24(5):377–387. doi: 10.1177/0733464804273185. [DOI] [Google Scholar]
- 66.Baker LD, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67(1):71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9(1):58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 68.Langlois F, et al. Benefits of physical exercise training on cognition and quality of life in frail older adults. J Gerontol B Psychol Sci Soc Sci. 2013;68(3):400–404. doi: 10.1093/geronb/gbs069. [DOI] [PubMed] [Google Scholar]
- 69.Wagner G, et al. Hippocampal structure, metabolism, and inflammatory response after a 6-week intense aerobic exercise in healthy young adults: a controlled trial. J Cereb Blood Flow Metab. 2015;35(10):1570–1578. doi: 10.1038/jcbfm.2015.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaiser A, et al. A randomized controlled trial on the effects of a 12-week high-vs. low-intensity exercise intervention on hippocampal structure and function in healthy, young adults. Front Psychiatry. 2022;12:780095. doi: 10.3389/fpsyt.2021.780095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jonasson LS, et al. Aerobic exercise intervention, cognitive performance, and brain structure: results from the physical influences on brain in aging (PHIBRA) study. Front Aging Neurosci. 2017;8:336. doi: 10.3389/fnagi.2016.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Böhm C, et al. Routes to, from and within the subiculum. Cell Tissue Res. 2018;373(3):557–563. doi: 10.1007/s00441-018-2848-4. [DOI] [PubMed] [Google Scholar]
- 73.Vicente-Campos D, et al. Impact of a physical activity program on cerebral vasoreactivity in sedentary elderly people. J Sports Med Phys Fitness. 2012;52(5):537. [PubMed] [Google Scholar]
- 74.Murrell CJ, et al. Cerebral blood flow and cerebrovascular reactivity at rest and during sub-maximal exercise: effect of age and 12-week exercise training. Age. 2013;35(3):905–920. doi: 10.1007/s11357-012-9414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kleinloog JP, et al. Aerobic exercise training improves cerebral blood flow and executive function: a randomized, controlled cross-over trial in sedentary older men. Front Aging Neurosci. 2019;11:333. doi: 10.3389/fnagi.2019.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bao D, et al. The effects of fatiguing aerobic exercise on the cerebral blood flow and oxygen extraction in the brain: a piloting neuroimaging study. Front Neurol. 2019;10:654. doi: 10.3389/fneur.2019.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kelley WM, et al. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron. 1998;20(5):927–936. doi: 10.1016/S0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- 78.Riès SK, Dronkers NF, Knight RT. Choosing words: left hemisphere, right hemisphere, or both? Perspective on the lateralization of word retrieval. Ann N Y Acad Sci. 2016;1369(1):111–131. doi: 10.1111/nyas.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lazar RM, et al. Baseline cognitive impairment in patients with asymptomatic carotid stenosis in the CREST-2 trial. Stroke. 2021;52(12):3855–3863. doi: 10.1161/STROKEAHA.120.032972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alosco ML, et al. The impact of hypertension on cerebral perfusion and cortical thickness in older adults. J Am Soc Hypertens. 2014;8(8):561–570. doi: 10.1016/j.jash.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Novak V, Hajjar I. The relationship between blood pressure and cognitive function. Nat Rev Cardiol. 2010;7(12):686–698. doi: 10.1038/nrcardio.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Becker L, Kutz DF, Voelcker-Rehage C. Exercise-induced changes in basal ganglia volume and their relation to cognitive performance. J Neurol Neuromed. 2016;1(5):19–24. doi: 10.29245/2572.942X/2016/5.1044. [DOI] [Google Scholar]