Abstract
The Arc two-component system, comprising the ArcB sensor kinase and the ArcA response regulator, modulates the expression of numerous genes in response to the respiratory conditions of growth. Under anoxic growth conditions, ArcB autophosphorylates and transphosphorylates ArcA, which in turn represses or activates its target operons. Under aerobic growth conditions, phosphorylated ArcA (ArcA-P) dephosphorylates and its transcriptional regulation is released. The dephosphorylation of ArcA-P has been shown to occur, at least in vitro, via an ArcAAsp54-P → ArcBHis717-P → ArcBAsp576-P → Pi reverse phosphorelay. In this study, the physiological significance of this pathway was assessed. The results demonstrate that the receiver and phosphotransfer domains of the tripartite sensor kinase ArcB are necessary and sufficient for efficient ArcA-P dephosphorylation in vivo.
The ability to respond to a vast array of environmental signals is vital for the growth and survival of microorganisms. The sensing and processing of these signals are carried out by molecular circuits within the cell, which detect, amplify, and integrate these signals into a specific response. In prokaryotes, these molecular circuits are typically organized by protein pairs, “sensory kinase” proteins and “response regulator” proteins, that belong to the large family of two-component systems. Two-component signal transduction systems that depend on histidine and aspartyl residues as phosphoryl group donors and acceptors have been reported to regulate diverse processes that include energy metabolism, symbiotic nitrogen fixation, chemotaxis, cell division, sporulation, and pathogenic interactions with both plant and animal hosts (1, 13, 29).
The prototypical system of this kind comprises a membrane-bound sensor kinase and a cytosol-located cognate response regulator. Upon signal reception, the sensor kinase undergoes an ATP-dependent autophosphorylation at a conserved His residue and subsequently transphosphorylates a conserved Asp residue in the cognate response regulator rendering it functional, in general as a transcriptional regulator. Upon cessation of signaling, both the cognate response regulator and the sensor kinase undergo dephosphorylation that results in silencing of the system.
The Arc (anoxic redox control) two-component system is a complex signal transduction system that plays a key role in regulating energy metabolism at the level of transcription in bacteria (23), including the pathogens Vibrio cholerae, Salmonella enterica serovar Typhimurium, Yersinia pestis, and Haemophilus influenzae (2, 3, 12, 25). This system comprises the ArcB protein as the sensor kinase and the ArcA protein as the response regulator (15, 17). ArcA is a typical response regulator comprising an N-terminal receiver domain with a conserved Asp residue (Asp54) and a C-terminal helix-turn-helix domain for DNA binding. By contrast, ArcB is unusually elaborate. First, there is no evident periplasmic domain (17, 20). Second, the cytosolic portion of the protein contains a putative leucine zipper (9) and a PAS (Per-Amt-Sim) domain (31). Third, ArcB possesses three cytosolic catalytic domains: an N-terminal transmitter domain (H1) with a conserved His residue at position 292, a central receiver domain (D1) with a conserved Asp residue at position 576, and a C-terminal transmitter domain (H2) with a conserved His residue at position 717 (14, 16).
Under reducing conditions, ArcB undergoes ATP-dependent autophosphorylation (10, 16), a process that is enhanced by certain anaerobic metabolites such as d-lactate, acetate, and pyruvate (7, 30), and transphosphorylates ArcA via a His292 → Asp576 → His717 → Asp54 phosphorelay (10, 18). Phosphorylated ArcA (ArcA-P), in turn, represses the expression of many operons involved in respiratory metabolism and activates a few operons encoding proteins involved in fermentative metabolism (23). Under aerobic conditions, the kinase activity of ArcB is inhibited by the quinone electron carriers that act as direct negative signals (8). In a recent study, it was demonstrated that the molecular mechanism of kinase silencing involves the oxidation of two cytosol-located redox-active cysteine residues (Cys180 and Cys241) that participate in intermolecular disulfide bond formation (24). Furthermore, dephosphorylation of ArcA-P, a reaction needed to curtail its regulatory activity, was previously shown to proceed, at least in vitro, via an ArcAAsp54-P → ArcBHis717-P → ArcBAsp576-P → Pi reverse pathway (9). However, no in vivo evidence was provided to support the physiological significance of such a pathway.
In this study, we address the question of whether the receiver (D1) and secondary transmitter (H2) of ArcB are involved in the in vivo dephosphorylation of ArcA-P.
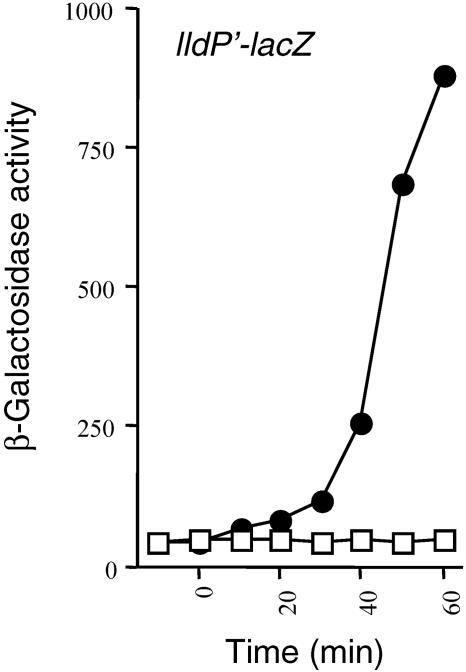
A shift to nonstimulating conditions leads to immediate release of the Arc-dependent transcriptional control. During anaerobic growth, the ArcA response regulator is activated upon phosphorylation by ArcB and regulates the transcription of some 40 operons. When oxygen becomes available, rapid dephosphorylation of both ArcB-P and ArcA-P is probably necessary for release of the Arc-dependent transcriptional control. To gain some insight into the mechanism of dephosphorylation, we examined the time lag for the response of the Arc system after a shift from anaerobic to aerobic conditions of growth. To this end, strain ECL5002 carrying the ArcA-P-repressible λΦ(lldP′-lacZ) reporter (20) was grown anaerobically in buffered Luria broth (LB) supplemented with 20 mM l-lactate to induce expression of the reporter (5). At an optical density at 600 nm (OD600) of ∼0.25, a sample was withdrawn and the expression level of the reporter, expressed in β-galactosidase units, was determined (depicted as −10 min). As expected, the expression of the reporter was very low, indicative of ArcA-P repression (Fig. 1). After an additional 10 min of growth, the culture was shifted to aerobiosis and the time course of the β-galactosidase activity was followed. As seen in Fig. 1, shifting the anaerobic culture to aerobiosis caused a rapid increase in reporter expression, suggesting that the Arc system responds quickly to changes in respiratory conditions. In this respect, it has to be mentioned that the presence of molecular oxygen leads to the oxidation of the quinone electron carriers that in turn inhibit ArcB phosphorylation (8) and thereby prevent further transphosphorylation of ArcA. The already formed ArcB-P dephosphorylates, most likely, by the intrinsic lability of the phospho-aspartyl bond in D1 (10). Also, the phosphohistidine phosphatase, SixA, which was previously reported to specifically dephosphorylate His717 of ArcB (28), may contribute to the dephosphorylation of ArcB-P. However, rapid dephosphorylation of ArcA-P is also necessary for reporter expression to resume after the shift to aerobiosis. Generally, the rate of dephosphorylation of response regulators appears to be controlled by the inherent lability of the mixed anhydride phospho-aspartyl bond and/or a phosphatase-like activity embodied in the cognate sensor kinase or another protein. Considering the fact that the in vitro half-life of the phospho-aspartyl bond of ArcA-P exceeds 60 min (9), we postulated that an ArcA-P-specific phosphatase might be needed for its dephosphorylation during oxidizing conditions of growth.
FIG. 1.
A shift to nonstimulating conditions leads to immediate release of the Arc-dependent transcriptional control. The ArcA/B two-component system responds very rapidly to changes in redox conditions of growth. Strain ECL5002 carrying the λΦ(lldP′-lacZ) reporter was grown anaerobically in LB containing 0.1 M MOPS (morpholinepropanesulfonic acid) (pH 7.4), 20 mM d-xylose, and 20 mM l-lactate as inducer. At an OD600 of 0.2, one aliquot was withdrawn for measuring the β-galactosidase activity (depicted as −10 min). At time 0 min, the cultures were divided in two: one of the subcultures continued growth under anaerobiosis, serving as control, while the other was shifted to aerobiosis. The β-galactosidase activity was monitored for 1 h with 10-min intervals. Open squares, anaerobic growth; closed circles, shift to aerobiosis.
ArcA-P-dependent repression is released upon expression of D1-H2 under nonstimulating conditions.
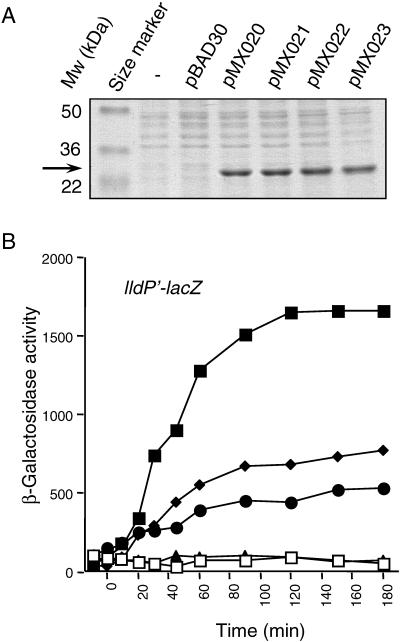
It has been previously reported that, at least in vitro, ArcA-P dephosphorylates via an ArcAAsp54-P → ArcBHis717-P → ArcBAsp576-P → Pi reverse pathway (9). Since the receiver (D1) and secondary transmitter (H2) of ArcB are involved in the in vitro dephosphorylation of ArcA-P, we argued that in vivo overexpression of these two domains might release the ArcA-P-dependent transcriptional control in the absence of stimuli. To test this, we constructed a series of plasmids expressing wild-type and mutant D1-H2 (ArcB521-778) proteins. The EcoRI-HindIII fragments of plasmids pQE30ArcB521-778 (10), pQE30ArcB521-778, D576A (9), pQE30ArcB521-778, H717Q (9), and pQE30ArcB521-778, D576A, H717Q (this study), containing the ribosomal binding site of the pQE30 vector and the arcB sequence that encodes the ArcB521-778 fragment, were cloned downstream from the ara promoter of plasmid pBAD30 (11) to generate pMX020, pMX021, pMX022, and pMX023 encoding ArcB521-778 (D1-H2), ArcB521-778, D576A (D1*-H2), ArcB521-778, H717Q (D1-H2*), and ArcB521-778, D576A, H717Q (D1*-H2*), respectively. Induction by arabinose revealed that all resultant D1-H2 constructs overexpress a protein of the expected size at similar steady-state levels (Fig. 2A).
FIG. 2.
ArcA-P-dependent repression is released upon expression of D1-H2 under nonstimulating conditions. (A) Strain ECL5003 carrying pBAD30, pMX020 (encodes D1-H2), pMX021 (encodes D1*-H2), pMX022 (encodes D1-H2*), or pMX023 (encodes D1*-H2*) was grown aerobically in LB containing 1.3 mM arabinose. At an OD600 of ∼0.4, a 1-ml sample was pelleted, washed with 1 ml of 10 mM Tris-HCl (pH 8.0), and solubilized by incubation at 95°C for 5 min in 100 μl of 2× sodium dodecyl sulfate sample buffer. Samples of 10 μl were subjected to electrophoresis in a sodium dodecyl sulfate-12% polyacrylamide gel, and the resolved proteins were visualized by Coomassie blue staining. (B) Strain ECL5047 (19) carrying a chromosomal tar-arcB hybrid (tab1), which encodes a protein with constitutive ArcB kinase activity, the λΦ(lldP′-lacZ) reporter fusion, and pBAD30, pMX020, pMX021, pMX022, or pMX023, was grown aerobically in LB containing 0.1 M MOPS (pH 7.4), 20 mM d-xylose, and 20 mM l-lactate. At an OD600 of 0.2, one aliquot was withdrawn for measuring the β-galactosidase activity (depicted as −10 min). At time 0 min, 1.3 mM arabinose was added in each culture and the β-galactosidase activity was followed. Open squares, ECL5047 transformed with pBAD30; closed squares, ECL5047 transformed with pMX020; closed diamonds, ECL5047 transformed with pMX021; closed circles, ECL5047 transformed with pMX022; closed triangles, ECL5047 transformed with pMX023.
The generated plasmids were transformed into strain ECL5047 (19), in which the wild-type arcB has been replaced with a tar-arcB hybrid (tab1) that encodes a protein with constitutive ArcB kinase activity and also carries the λΦ(lldP′-lacZ) reporter fusion. In this strain, ArcB autophosphorylates and transphosphorylates ArcA even during aerobic conditions of growth, and as a result, the β-galactosidase activity is always very low. Therefore, production of an ArcA-P phosphatase should lower the amount of ArcA-P and augment expression of the reporter. The transformants were grown aerobically in buffered LB to an OD600 of ∼0.3, and a sample for the β-galactosidase assay was withdrawn (depicted as −10 min). In agreement with previous results (19), the expression of the reporter was very low (Fig. 2B) in all tested strains. After an additional 10 min of growth, 1.3 mM arabinose was added in the cultures to induce transcription of the plasmid-encoded peptides, and the time course of β-galactosidase activity was monitored (Fig. 2B). It was found that addition of arabinose in the growth medium of the strain carrying either pBAD30 or the D1*-H2*-expressing plasmid (pMX023) did not affect the expression of the reporter. In contrast, addition of arabinose in the growth medium of the strain carrying the D1-H2-expressing plasmid (pMX020) resulted in an immediate increase of reporter expression, which reached a steady-state level 25 times higher than in the strains carrying either pBAD30 or pMX023. Finally, addition of inducer in the growth medium of the strains carrying either the D1*-H2- or the D1-H2*-expressing plasmid (pMX021 or pMX022) resulted in a moderate release of reporter repression, with D1*-H2 showing a stronger effect than D1-H2*. The effect of D1*-H2 is to be expected, if the reverse phosphorelay is the actual in vivo pathway for ArcA-P dephosphorylation, because the phosphoryl group will be transferred from ArcA-P to His717 of H2. An alternative should be that the mutant peptides attenuate the communication between ArcB and ArcA, by interfering with the phosphorelay in ArcB and/or by sequestrating ArcA and thereby blocking its transphosphorylation. Also, the possibility of intermolecular phosphoryl group transfer from H1 or H2 of ArcB to D1-H2* with concomitant hydrolysis of the Asp576-P cannot be excluded. Nonetheless, the above results provide strong evidence that the presence of D1-H2 abolishes the ArcA-P-dependent transcriptional regulation under nonstimulating conditions.
Expression of D1-H2 abrogates the ArcA-P transcriptional regulation under stimulating conditions.
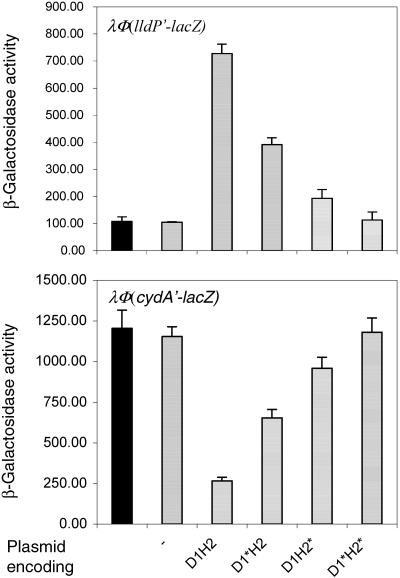
It is of interest that in the cases where a sensor kinase facilitates dephosphorylation of its cognate response regulator, it is not known whether the same conformation of the sensor allows both the phosphorylation and dephosphorylation reactions to be catalyzed. If distinct conformations of the sensor are needed to specify whether the kinase or the phosphatase activity will be allowed, it can be assumed that under stimulating conditions activation of the kinase will be followed by concomitant inhibition of the phosphatase, while nonstimulating conditions will have the opposite effect. To explore the possibility that distinct conformations might specify the reaction catalyzed by D1-H2, that is either the forward phosphorylation cascade or the reverse phosphorelay, we tested whether expression of D1-H2 under stimulating (anaerobic) conditions also abolishes the ArcA-P-dependent transcriptional control. To this end, the plasmids that express the wild-type or mutant D1-H2 proteins were transformed into strains ECL5002 and ECL5003 carrying, respectively, the ArcA-P-repressed λΦ(lldP′-lacZ) and the ArcA-P-activated λΦ(cydA′-lacZ) reporter in the lldPRD+ and cydAB+ background. The transformants were grown anaerobically in the presence of arabinose to an OD600 of ∼0.4, and the β-galactosidase activity was determined (Fig. 3). It was found that while the presence of D1-H2 abolished the activation of λΦ(cydA′-lacZ) and the repression of λΦ(lldP′-lacZ) during anaerobiosis, the presence of D1*-H2* did not affect the expression of either reporter. Also, in agreement with the previous result, expression of either D1*-H2 or D1-H2* reduced the ArcA-P-dependent transcriptional control, although at a considerably lower degree than the wild-type peptide. Thus, a conformational change of the D1-H2 peptide per se does not seem to be a determinant favoring any of the two opposing reactions of phosphorylation and dephosphorylation. However, this does not exclude the possibility that the full-length wild-type ArcB maintains different folds dependent on the redox state of the cell that could play a decisive role in dictating the direction of the phosphoryl group transfer.
FIG. 3.
Expression of D1-H2 abrogates the ArcA-P transcriptional regulation under stimulating conditions. The λΦ(lldP′-lacZ) and the λΦ(cydA′-lacZ) reporter strains ECL5002 and ECL5003 were transformed with either the wild-type or mutant D1-H2-expressing plasmid. The λΦ(cydA′-lacZ)-bearing strains were cultured anaerobically in buffered LB containing 0.1 M MOPS (pH 7.4), 20 mM d-xylose, and 1.3 mM arabinose. For the growth of λΦ(lldP′-lacZ)-bearing strains, the above medium was supplemented with 20 mM l-lactate. At an OD600 of 0.4, the cultures were harvested and the β-galactosidase activity was assayed and expressed in Miller units. The data are the average of four experiments, and the standard deviation values are indicated. Solid bars, untransformed strains; hatched bars, strains transformed with plasmids pBAD30, pMX020 (D1-H2), pMX021 (D1*-H2), pMX022 (D1-H2*), or pMX023 (D1*-H2*).
D1-H2 acts directly on ArcA-P.
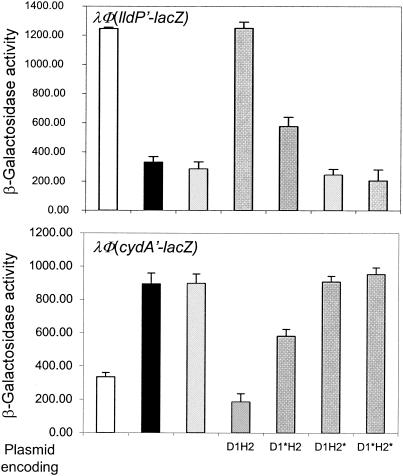
As mentioned earlier, the effect of D1-H2 on reporter expression is most likely the result of ArcA-P dephosphorylation. However, interception of the phosphoryl group transfer from ArcB to ArcA by D1-H2 should cause the same effect. Therefore, we attempted to generate ArcB-independent ArcA-P in vivo and examine whether D1-H2 is able to directly dephosphorylate ArcA-P. In doing this, we took advantage of the facts that in the absence of their cognate kinases many response regulators undergo in vivo autophosphorylation at the expense of acetyl-phosphate (4, 6, 21, 32) and that the intracellular concentration of acetyl-phosphate is an order of magnitude higher in cells grown aerobically on pyruvate than on glycerol as the sole carbon and energy source (26). The wild-type strains ECL5002 and ECL5003 and their isogenic ΔarcB strains ECL5004 and ECL5012, carrying either the λΦ(lldP′-lacZ) or the λΦ(cydA′-lacZ) reporters, were grown aerobically in defined medium supplemented with 20 mM pyruvate. At an OD600 of ∼0.4, the cultures were harvested and the β-galactosidase activity was determined (Fig. 4). It was found that the λΦ(cydA′-lacZ) expression was 3.5-fold higher and the expression of λΦ(lldP′-lacZ) was 3-fold lower in the arcB-null background than in the wild-type arcB+ background (Fig. 4). Thus, in the absence of its cognate sensor kinase, ArcA is able to autophosphorylate, most probably at the expense of acetyl phosphate, thus providing the means to examine the direct effect of the wild-type and mutant D1-H2 peptides on the dephosphorylation of ArcA-P. To this end, the ΔarcB strains ECL5004 and ECL5012 were transformed with plasmids pMX020 to -023, the transformants were grown aerobically in defined medium supplemented with pyruvate and arabinose, and at an OD600 of ∼0.4 the β-galactosidase activity was determined (Fig. 4). It was found that while the expression of D1-H2 abolished the activation of λΦ(cydA′-lacZ) and the repression of λΦ(lldP′-lacZ), D1*-H2* did not affect the expression level of either reporter. Furthermore, the expression of D1*-H2 reduced the ArcA-P-dependent transcriptional control, in a similar fashion to that observed in Fig. 1 and 2, suggesting that the effect of D1*-H2 is the result of phosphoryl group transfer from ArcA-P to His717 of D1*-H2. In contrast, the presence of D1-H2* in this setting failed to affect the expression of either reporter, indicating that D1-H2* interferes with the phosphoryl group transfer from ArcB to ArcA. However, more detailed studies are required to pinpoint the significance of such interference. Nonetheless, from the results presented here it can be concluded that the receiver and phosphotransfer domains of the ArcB sensor kinase are necessary and sufficient for efficient ArcA-P dephosphorylation in vivo. This result also has a bearing on the previously proposed hypothesis that the pivotal intermediate acetyl-P, containing carbon, phosphorus, and a high-energy bond, may be involved in maintaining and/or augmenting the basal level of many response regulators in the phosphorylated state (27). Such a hypothesis is attractive in view of the finding that acetyl-P levels in the cell can vary from <0.04 mM to 1.2 mM (26). However, this route of ArcA phosphorylation does not seem to be significant, as it is clear from our results that under physiological conditions the phosphatase activity of ArcB will nullify the acetyl-phosphate-dependent phosphorylation of ArcA.
FIG. 4.
Expression of D1-H2 annuls the acetyl-phosphate-dependent phosphorylation of ArcA in vivo. The λΦ(lldP′-lacZ) and the λΦ(cydA′-lacZ) reporter strains ECL5002 and ECL5003 and their ΔarcB isogenic strains ECL5012 and ECL5004 transformed with either the wild-type or mutant D1-H2-expressing plasmid were grown aerobically in defined medium [1 mM KH2PO4, 40 mM KCl, 34 mM NaCl, 20 mM (NH4)2SO4, 1 μM FeSO4, 0.3 mM MgSO4, 1 μM ZnCl2, 10 μM CaCl2, and 0.1 M MOPS, at a final pH of 7.6] supplemented with 20 mM pyruvate and 1.3 mM arabinose. For the growth of λΦ(lldP′-lacZ)-bearing strains, the above medium was supplemented with 20 mM l-lactate. At an OD600 of 0.4, the cultures were harvested and the β-galactosidase activity was assayed. The data are the average of four experiments, and the standard deviation values are indicated. Empty bars, ECL5002 and ECL5003, carrying the wild-type ArcB; solid bars, untransformed ECL5004 (ΔarcB) and ECL5012 (ΔarcB); hatched bars, ECL5004 and ECL5012 transformed with plasmids pBAD30, pMX020 (D1-H2), pMX021 (D1*-H2), pMX022 (D1-H2*), or pMX023 (D1*-H2*).
Concluding remarks.
Since the amplitude and duration of an adaptive response depend on the balance between the rates of phosphorylation and dephosphorylation of the response regulator, it will be important to characterize the factors that control the reaction rates in both directions. The FixL/FixJ system for the regulation of nitrogen fixation by Rhizobium meliloti provides an interesting illustration. O2 deprivation stimulates autophosphorylation of the heme-binding sensor kinase but inhibits the FixJ phosphatase activity, thereby promoting the anaerobic expression of nitrogen fixation genes with great effectiveness (22). For His-Asp-His-Asp phosphorelay systems, it would be useful to determine the equilibrium constants of the various partial reactions. This may facilitate the pinpointing of kinetic controls, since they are likely to influence reactions proceeding in the thermodynamically favorable direction. Another goal would be to identify additional physiological factors that may influence the rates of signal transmission and decay.
Acknowledgments
We thank E.C.C. Lin for advice and B. Michel for critically reading the manuscript.
This work was supported by grant 37342-N from the Consejo Nacional de Ciencia y Tecnología (CONACyT), by NIH research grant no. R03 TW06003, and by the 21st Century Frontier R&D Program grant from the Korean Ministry of Science and Technology.
REFERENCES
- 1.Barrett, J. F., and J. A. Hoch. 1998. Two-component signal transduction as a target for microbial anti-infective therapy. Antimicrob. Agents Chemother. 42:1529-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benson, D. A., I. Karsch-Mizrachi, D. J. Lipman, J. Ostell, B. A. Rapp, and D. L. Wheeler. 2000. GenBank. Nucleic Acids Res. 28:15-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blattner, F. R., G. Plunkett, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 4.Dailey, F. E., and H. C. Berg. 1993. Change in direction of flagellar rotation in Escherichia coli mediated by acetate kinase. J. Bacteriol. 175:3236-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong, J. M., J. S. Taylor, D. J. Latour, S. Iuchi, and E. C. Lin. 1993. Three overlapping lct genes involved in l-lactate utilization by Escherichia coli. J. Bacteriol. 175:6671-6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng, J., M. R. Atkinson, W. McCleary, J. B. Stock, B. L. Wanner, and A. J. Ninfa. 1992. Role of phosphorylated metabolic intermediates in the regulation of glutamine synthetase synthesis in Escherichia coli. J. Bacteriol. 174:6061-6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Georgellis, D., O. Kwon, and E. C. Lin. 1999. Amplification of signaling activity of the Arc two-component system of Escherichia coli by anaerobic metabolites. An in vitro study with different protein modules. J. Biol. Chem. 274:35950-35954. [DOI] [PubMed] [Google Scholar]
- 8.Georgellis, D., O. Kwon, and E. C. Lin. 2001. Quinones as the redox signal for the Arc two-component system of bacteria. Science 292:2314-2316. [DOI] [PubMed] [Google Scholar]
- 9.Georgellis, D., O. Kwon, P. De Wulf, and E. C. Lin. 1998. Signal decay through a reverse phosphorelay in the Arc two-component signal transduction system. J. Biol. Chem. 273:32864-32869. [DOI] [PubMed] [Google Scholar]
- 10.Georgellis, D., A. S. Lynch, and E. C. Lin. 1997. In vitro phosphorylation study of the Arc two-component signal transduction system of Escherichia coli. J. Bacteriol. 179:5429-5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guzman, L.-M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing arabinose pBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heidelberg, J. F., J. A. Eisen, W. C. Nelson, R. A. Clayton, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, L. Umayam, S. R. Gill, K. E. Nelson, T. D. Read, H. Tettelin, D. Richardson, M. D. Ermolaeva, J. Vamathevan, S. Bass, H. Qin, I. Dragoi, P. Sellers, L. McDonald, T. Utterback, R. D. Fleishmann, W. C. Nierman, and O. White. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoch, J. A. 2000. Two-component and phosphorelay signal transduction. Curr. Opin. Microbiol. 3:165-170. [DOI] [PubMed] [Google Scholar]
- 14.Ishige, K., S. Nagasawa, S. Tokishita, and T. Mizuno. 1994. A novel device of bacterial signal transducers. EMBO J. 13:5195-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iuchi, S., and E. C. Lin. 1988. arcA (dye), a global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc. Natl. Acad. Sci. USA 85:1888-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iuchi, S., and E. C. Lin. 1992. Mutational analysis of signal transduction by ArcB, a membrane sensor protein responsible for anaerobic repression of operons involved in the central aerobic pathways in Escherichia coli. J. Bacteriol. 174:3972-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iuchi, S., Z. Matzuda, T. Fujiwara, and E. C. Lin. 1990. The arcB gene of Escherichia coli encodes a sensor-regulator protein for anaerobic repression of the arc modulon. Mol. Microbiol. 4:715-727. [DOI] [PubMed] [Google Scholar]
- 18.Kwon, O., D. Georgellis, and E. C. Lin. 2000. Phosphorelay as the sole physiological route of signal transmission by the Arc two-component system of Escherichia coli. J. Bacteriol. 182:3858-3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon, O., D. Georgellis, and E. C. Lin. 2003. Rotational on-off switching of a hybrid membrane sensor kinase Tar-ArcB in Escherichia coli. J. Biol. Chem. 278:13192-13195. [DOI] [PubMed] [Google Scholar]
- 20.Kwon, O., D. Georgellis, A. S. Lynch, D. Boyd, and E. C. Lin. 2000. The ArcB sensor kinase of Escherichia coli: genetic exploration of the transmembrane region. J. Bacteriol. 182:2960-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, T. Y., K. Makino, H. Shinagawa, and A. Nakata. 1990. Overproduction of acetate kinase activates the phosphate regulon in the absence of the phoR and phoM functions in Escherichia coli. J. Bacteriol. 172:2245-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lois, A. F., M. Weinstein, G. S. Ditta, and D. R. Helinski. 1993. Autophosphorylation and phosphatase activities of the oxygen-sensing protein FixL of Rhizobium meliloti are coordinately regulated by oxygen. J. Biol. Chem. 268:4370-4375. [PubMed] [Google Scholar]
- 23.Lynch, A. S., and E. C. Lin. 1996. Responses to molecular oxygen, p. 1526-1538. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 24.Malpica, R., B. Franco, C. Rodriquez, and D. Georgellis. 2004. Identification of a quinone-sensitive redox switch in the ArcB sensor kinase. Proc. Natl. Acad. Sci. USA 101:13318-13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manukhov, I. V., Y. V. Bertsova, D. Y. Trofimov, A. V. Bogachev, and V. P. Skulachev. 2000. Analysis of HI0220 protein from Haemophilus influenzae, a novel structural and functional analog of ArcB protein from Escherichia coli. Biochemistry (Moscow) 65:1321-1326. [PubMed] [Google Scholar]
- 26.McCleary, W. R., and J. B. Stock. 1994. Acetyl phosphate and the activation of two-component response regulators. J. Biol. Chem. 269:31567-31572. [PubMed] [Google Scholar]
- 27.McCleary, W. R., J. B. Stock, and A. J. Ninfa. 1993. Is acetyl phosphate a global signal in Escherichia coli? J. Bacteriol. 175:2793-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogino, T., M. Matsubara, N. Kato, Y. Nakamura, and T. Mizuno. 1998. An Escherichia coli protein that exhibits phosphohistidine phosphatase activity towards the HPt domain of the ArcB sensor involved in the multistep His-Asp phosphorelay. Mol. Microbiol. 27:573-585. [DOI] [PubMed] [Google Scholar]
- 29.Parkinson, J. S., and E. C. Kofoid. 1992. Communication modules in bacterial signaling proteins. Annu. Rev. Genet. 26:71-112. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez, C., O. Kwon, and D. Georgellis. 2004. Effect of d-lactate on the physiological activity of the ArcB sensor kinase in Escherichia coli. J. Bacteriol. 186:2085-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor, B. L., and I. B. Zhulin. 1999. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol. Mol. Biol. Rev. 63:479-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wanner, B. L., and M. R. Wilmes-Riesenberg. 1992. Involvement of phosphotransacetylase, acetate kinase, and acetyl phosphate synthesis in the control of the phosphate regulon in Escherichia coli. J. Bacteriol. 174:2124-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]