Abstract
Age-related impairment of neurovascular coupling (NVC; “functional hyperemia”) is a critical factor in the development of vascular cognitive impairment (VCI). Recent geroscience research indicates that cell-autonomous mechanisms alone cannot explain all aspects of neurovascular aging. Circulating factors derived from other organs, including pro-geronic factors (increased with age and detrimental to vascular homeostasis) and anti-geronic factors (preventing cellular aging phenotypes and declining with age), are thought to orchestrate cellular aging processes. This study aimed to investigate the influence of age-related changes in circulating factors on neurovascular aging. Heterochronic parabiosis was utilized to assess how exposure to young or old systemic environments could modulate neurovascular aging. Results demonstrated a significant decline in NVC responses in aged mice subjected to isochronic parabiosis (20-month-old C57BL/6 mice [A-(A)]; 6 weeks of parabiosis) when compared to young isochronic parabionts (6-month-old, [Y-(Y)]). However, exposure to young blood from parabionts significantly improved NVC in aged heterochronic parabionts [A-(Y)]. Conversely, young mice exposed to old blood from aged parabionts exhibited impaired NVC responses [Y-(A)]. In conclusion, even a brief exposure to a youthful humoral environment can mitigate neurovascular aging phenotypes, rejuvenating NVC responses. Conversely, short-term exposure to an aged humoral milieu in young mice accelerates the acquisition of neurovascular aging traits. These findings highlight the plasticity of neurovascular aging and suggest the presence of circulating anti-geronic factors capable of rejuvenating the aging cerebral microcirculation. Further research is needed to explore whether young blood factors can extend their rejuvenating effects to address other age-related cerebromicrovascular pathologies, such as blood–brain barrier integrity.
Keywords: Ageing, Heterochronic parabiosis, Cerebral microcirculation, Neurovascular coupling, Reactive hyperemia, Endothelial dysfunction, Mouse brain, Cognitive health, Rejuvenation
Introduction
Age-related vascular cognitive impairment (VCI), characterized by cognitive deficits resulting from a spectrum of cerebrovascular pathologies, is a growing concern in many developed countries [1–3]. This concern is heightened by the increasing proportion of elderly individuals within their populations [4–8]. The demographic shift toward an older population is particularly evident in countries like Japan and several European Union nations, including Germany, Hungary, and Italy, where more than 20% of the populace is aged 65 or older [9,10]. Projections suggest that by 2050, 27% of the European Union’s population and 22% of the US population will have reached the age of 65 or older [9,11,12]. This demographic shift has profound implications, as a significant portion of these older individuals will experience VCI, leading to a gradual reduction in their functional independence and social engagement, and imposing substantial financial burdens on society [6,7,13–15]. Consequently, there is a pressing need to identify potentially reversible age-related pathophysiological mechanisms contributing to VCI and vascular dementia, as this knowledge could pave the way for the development of clinically relevant preventative treatments.
A complex landscape of age-related microvascular changes contributes to the genesis of VCI, encompassing disruptions in the blood–brain barrier, the accumulation of amyloid pathologies, microvascular rarefaction, microvascular inflammatory phenotypic changes, microhemorrhages, and other interconnected factors [3,16–25]. Among these multifaceted influences, emerging evidence underscores the critical role of aging-induced dysregulation of cerebral blood flow (CBF) in the genesis of cognitive impairment among older adults [26,27]. Despite constituting only 2% of the body’s weight, the brain consumes a remarkable 20% of its oxygen supply, necessitating a constant, tightly regulated supply of oxygen and glucose to maintain its metabolic demands. This requirement for metabolic homeostasis is further underscored during periods of heightened neuronal activity, where rapid adjustments in oxygen and glucose delivery, as well as efficient removal of harmful metabolites, are essential for sustaining the brain’s microenvironment. Achieving this delicate balance relies on a fundamental homeostatic mechanism known as neurovascular coupling (NVC) or functional hyperemia [3,28–40]. Mounting evidence has implicated aging-induced impairment of NVC responses as a contributor to age-related cognitive decline [3,35–37]. First, NVC responses are diminished in older adults, a finding consistently associated with impaired cognitive performance [27,30,32,41–44]. Second, pharmacological disruption of NVC in young mice can replicate aspects of the cognitive aging phenotype, further underscoring the link between NVC and cognitive function [45,46]. Third, interventions targeting cellular and molecular mechanisms of aging have demonstrated the potential to rescue NVC responses and improve cognitive performance in experimental rodent models [35–37,47–49].
The cellular underpinnings of NVC responses involve the release of vasodilator nitric oxide (NO) by cerebromicrovascular endothelial cells in response to gliotransmitters and mediators released from activated neurons [50,51]. Endothelium-derived NO operates by inducing relaxation in smooth muscle cells and is presumed to affect contractile pericytes within resistance arterioles and precapillary sphincters. Furthermore, endothelial cells play a pivotal role in promoting the upstream propagation of conducted vasodilation that initiates within the microcirculation, thus amplifying functional hyperemia [52]. Translational studies have highlighted compromised endothelial function in older adults [53] and aged laboratory animals [35,36,54], contributing significantly to the age-related decline in NVC responses. Understanding these cellular mechanisms affecting cerebromicrovascular aging holds immense promise for developing clinically translatable interventions aimed at neurovascular rejuvenation and enhancing cerebral blood flow, with the ultimate goal of preventing and delaying the onset of vascular cognitive impairment.
In the past, investigations into vascular aging predominantly concentrated on cell-autonomous mechanisms responsible for instigating functional decline [55]. These studies have contributed significantly to our grasp of essential factors, including mitochondrial dysfunction [36,56], heightened production of reactive oxygen species (ROS) [24,55,57–66], compromised nitric oxide availability [10,17–19], cellular NAD + depletion [35,67–69], epigenetic alterations (including sirtuin dysregulation) [58,70], and energetic dysfunctions [36] implicated in the etiology of vascular aging [55,71]. Nonetheless, in recent years, there has been an increasing awareness of the significance of non-cell autonomous mechanisms in instigating aging processes and contributing to the pathogenesis of age-related diseases [55]. Geroscience research has accumulated compelling evidence suggesting that circulating pro-geronic factors (increased with age and detrimental to vascular homeostasis, e.g., inflammatory cytokines) and/or the presence of anti-geronic factors (preventing cellular aging phenotypes and declining with age), derived from various organs like adipose tissue, the brain, the endocrine system, and the immune system, orchestrate cellular aging processes within the vascular wall [55,71]. Yet, further experimental investigation is warranted to elucidate the relative contributions of circulating factors versus cell-autonomous mechanisms to the development of neurovascular aging phenotypes.
To address this critical gap in our understanding, we employed heterochronic parabiosis [72–75], a surgical technique enabling the fusion of the circulatory systems of two animals, as a means to investigate the role of circulating factors in neurovascular aging. Building upon our previous research demonstrating the roles of circulating factors in driving vascular aging within the aorta [76,77], our study sought to experimentally test the hypothesis that age-related changes in circulating pro-geronic and/or anti-geronic factors contribute to the regulation of neurovascular aging processes in a non-cell autonomous manner. Leveraging heterochronic parabiosis, combined with the assessment of NVC responses with laser speckle contrast imaging (LSCI), we aimed to determine the extent to which exposure to an old or young environment could transpose neurovascular aging phenotypes within the young and old brain, respectively.
Materials and methods
Animal procurement
Young (4- to 5-month-old, C57BL/6, n = 16) and aged (18- to 19-month-old, C57BL/6, n = 16) male mice (Mus musculus) were procured from the aging colony maintained by the National Institute on Aging at Charles River Laboratories (Wilmington, MA). The animals were housed under specific pathogen-free barrier conditions in the Rodent Barrier Facility at the University of Oklahoma Health Sciences Center (OUHSC). They were maintained under a controlled photoperiod (12 h of light and 12 h of darkness), provided unlimited water access, and fed a standard AIN-93G diet ad libitum.
Parabiosis surgery
Parabiosis, a surgical technique that involves the fusion of the circulatory systems of two animals, has gained wide prominence for investigating the roles of circulating factors in the pathogenesis of various diseases [78–81], as well as for studying the regulation of aging processes [74,75,82–102]. This experimental tool, particularly heterochronic parabiosis, enables the exploration of the intricate interplay between cell-autonomous and non-cell autonomous mechanisms of aging [74,75,82–100].
The parabiosis procedure comprised a series of steps, including a 5-day pair acclimation period, the surgical intervention itself, 2 weeks of postoperative care, and an additional 4 weeks of parabiosis to allow for the effects of blood exchange (Fig. 1B). Subsequently, after 6 weeks of parabiosis, neurovascular coupling responses were assessed in each mouse. Throughout the pairwise habituation and post-intervention periods, animals were housed in the conventional rodent colony at OUHSC, with a controlled photoperiod (12 h of light and 12 h of darkness), unrestricted access to water, and ad libitum consumption of a standard AIN-93G diet.
Fig. 1.
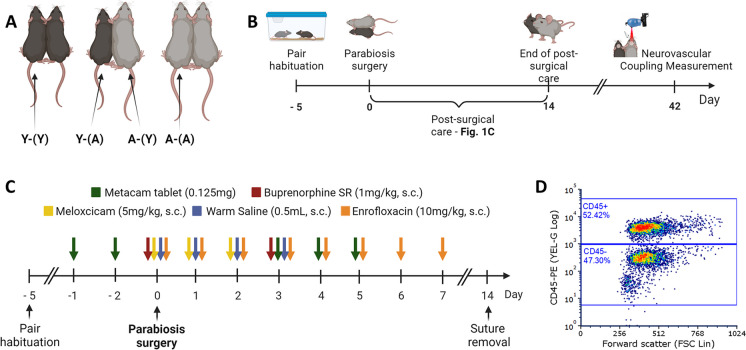
Parabiosis types and timelines. A This figure illustrates the four distinct experimental groups in our study, each represented by a unique combination of young (Y) and aged (A) parabionts, designated by the co-parabiont in parentheses. These groups include isochronic young (Y-(Y)), heterochronic young (Y-(A)), heterochronic aged (A-(Y)), and isochronic aged (A-(A)) parabionts. B The timeline for the parabiosis surgery procedure is depicted. Five days prior to surgery, mice are acclimated and assessed for potential aggression. Following surgery, animals are subjected to a meticulous 14-day monitoring period. Neurovascular coupling responses are evaluated in parabionts 6 weeks post-surgery. C This graph provides an overview of the comprehensive pre- and postsurgical care regimen administered to parabionts. To effectively manage pain and inflammation, parabionts receive treatment with buprenorphine and meloxicam. To prevent infection, surgery is performed in aseptic conditions and parabionts receive daily enrofloxacin injections for seven consecutive days. To prevent dehydration, parabionts are injected with warm saline. D Representative flow cytometry graph displaying mononuclear cells isolated from the blood of the non-injected parabiont. The graph emphasizes the presence of phycoerythrin-labeled leukocytes within the top gate, which originate from the injected co-parabiont. This observation serves as conclusive evidence confirming the successful exchange of blood between the parabionts
Presurgical procedures
In preparation for the surgery, animals were paired for 5 days to facilitate their acclimation. During this period, close monitoring ensured that aggressive behaviors between pairs were minimal, and any animals displaying aggression towards each other were excluded from undergoing parabiosis surgery. Furthermore, two days prior to the surgery, animals received bacon-flavored Metacam tablets (one per animal, 0.125 mg, MD275-0125, Bio-Serv, NJ, USA) to acquaint them with oral meloxicam administration and reduce the number of injections after the parabiosis surgery.
Parabiosis surgery
Parabiosis surgery adhered to established protocols by the Einstein Health Span Core, as previously reported [76–79,101,103]. Surgical unions were established between young animals (isochronic; young Y–(Y); n = 4 pairs), aged animals (isochronic old; A–(A); n = 4 pairs), and young and aged mice (heterochronic Y–(A) and A-(Y); n = 8 pairs) at the Oklahoma Center for Geroscience, resulting in four experimental groups: young mice exposed to young blood (Y-(Y)), young mice exposed to aged blood (Y-(A)), aged mice exposed to young blood (A-(Y)), and aged mice exposed to aged blood (A-(A)) (Fig. 1A).
During the parabiosis procedures, strict adherence to aseptic surgical protocols was maintained according to OUHSC guidelines. Mice were sedated using isoflurane anesthesia (3–4% induction, 2–3% maintenance, [Isoflurane USP, ref#: 029405, Covetrus, UK], 0.6–0.8 L/min O2) and subsequently transferred to the surgical preparation site. In isochronic young (Y-Y) and aged (A-A) pairs, anesthesia was maintained using a single vaporizer with a Y bifurcation splitter. In contrast, heterochronic pairs required two separate vaporizers to adjust isoflurane levels independently in the young and aged co-parabionts. The level of animal sedation was routinely assessed by monitoring paw and tail reflexes. The surgical procedure involved several steps, including preparation, incision, bone exposure, and connecting the two mice.
Preparation
The corresponding body sides of both animals were carefully shaved using clippers (Mini Arco®, Wahl, IL, USA), and any remaining hair was meticulously removed using a specialized hair removal lotion. The shaved areas were thoroughly cleaned with 70% ethanol, followed by the application of povidone-iodine surgical scrub (Betadine, Avrio Health, CT, USA), with this disinfection process repeated up to three times to ensure optimal sterility. Subsequently, an incision line was marked using a surgical marker, and the animals were then transferred to a surgically sterile environment.
Incision and bone exposure
A longitudinal incision was made on the side of the first animal, followed by a gentle blunt dissection of the superficial fascia and surrounding muscles to expose the femur bone. The periosteum of the femur was carefully scraped using a disposable scalpel. The shoulder blade was localized, and the supraspinatus and infraspinatus muscles were gently repositioned to partially expose the shoulder blade. To prevent tissue drying, a sterile saline solution was generously applied to the exposed bones and surrounding fascia. These steps were subsequently replicated for the co-parabiont.
Connecting the two mice
Two strands of 2–0 silk suture (SP118, Surgical Specialties, MA, USA) were passed underneath the femur of the first parabiont. The longer ends of the sutures were threaded beneath the femur of the co-parabiont, and the ends were securely fastened with square knots. The shoulder blades of the two mice were connected using a combination of one primary horizontal mattress suture and two additional surgical square knots, employing 4–0 silk suture (683G, Ethicon, OH, USA). The skin flaps on the dorsal and ventral surfaces were meticulously sutured together using a continuous interlocking suture technique. Additionally, two ends of continuous sutures were tied together using a single surgical knot between the necks of two parabionts. Three to four additional interrupted sutures (square surgical knots) were added to ensure the stability of the sutured connection. Subsequently, the animals were closely monitored until fully awake and then placed in a clean cage on a heating pad for recovery.
Postsurgical care
Following surgery, animals were placed on a partial heating pad overnight, and a multifaceted pharmacological treatment plan was initiated to ensure their well-being (Fig. 1C). To alleviate pain during and post-surgery, animals received two subcutaneous injections of buprenorphine (1 mg/kg, s.c., Buprenorphine E.R., 1 mg/ml, ZooPharm, WY, USA). The first dose was administered briefly before the surgery, followed by a second injection on day three post-surgery. Additionally, animals received meloxicam during and after surgery (5 mg/kg, s.c. Meloxicam injectable solution, Covetrus, UK; and Metacam, 0.125 mg/day, oral, MD275-0125, Bio-Serv, NJ, USA; on days 0–2 and 3–5, respectively). To prevent infections, parabionts were subcutaneously injected with enrofloxacin (10 mg/kg, Baytril, 2.27%, IN, USA) once daily for 7 days, starting on the day of the surgery. To prevent dehydration caused by reduced water intake post-surgery, animals were subcutaneously injected with 0.5 mL warmed saline during the surgery and on postsurgical days 1, 2, and 3. Additionally, animals received fresh DietGel Recovery (72–06-5022, ClearH2O, ME, USA) for the first 5 days. After the surgery, the parabionts’ condition, weight, coordination, and sutures were monitored twice a day for the first 2 weeks. Skin sutures were removed 2 weeks after the surgery, and animals were monitored daily until the experimental end point. Any concerning symptoms were promptly assessed and treated in consultation with OUHSC veterinarians. Animals remained joined for 6 weeks before being subjected to terminal experimentation and euthanasia.
Ethical considerations.
All experiments were conducted in strict adherence to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978) and were approved by the Institutional Animal Use and Care Committee of OUHSC. Rigorous measures were taken to minimize animal suffering and distress, and a highly skilled veterinarian affiliated with the Department of Comparative Medicine provided expert oversight at every stage of the surgical procedure, ensuring the highest standards of animal care and welfare throughout the study.
Measurement of neurovascular coupling responses
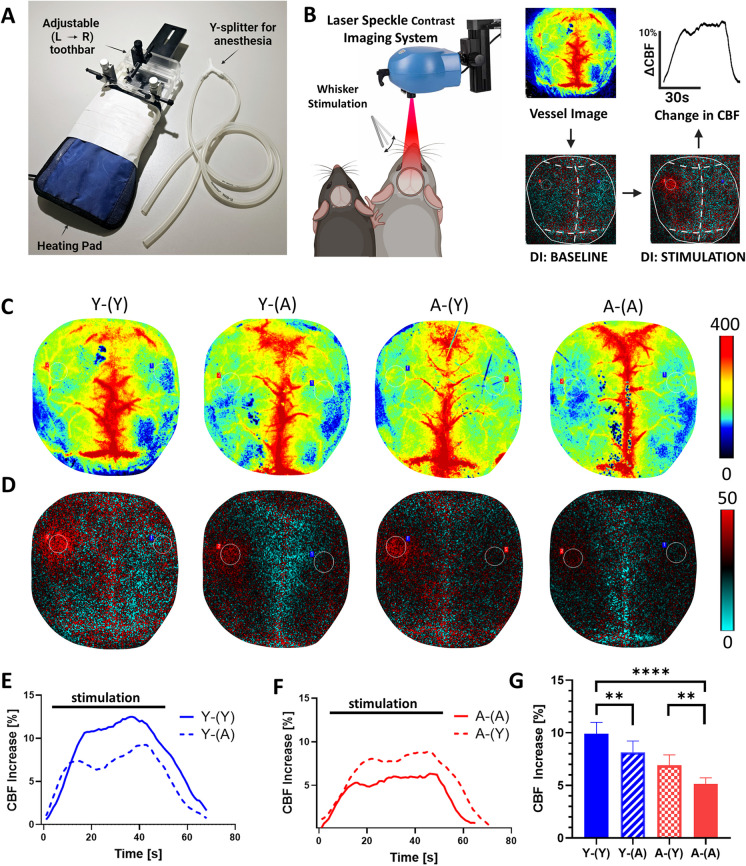
Parabiont pairs of mice in each group were anesthetized with isoflurane (induction at 3% and maintenance at 1%), facilitated by a Y bifurcation splitter. Endotracheal intubation was achieved using a 20Gx1 catheter (Ref: 266,741, Exel, CA, USA), followed by mechanical ventilation using a MousVent G500 system (Kent Scientific Co, Torrington, CT). To maintain a consistent body temperature of 37 °C, a thermostatic heating pad (Kent Scientific Co, Torrington, CT) was utilized [51]. End-tidal CO2 levels were controlled within the range of 3.2% and 3.7% to keep blood gas values within the physiological range, as described [39,104]. The parabiont mice were securely immobilized and affixed to a specially adapted stereotaxic frame designed for this study (Fig. 2A). This customized frame comprised a 3D-printed base featuring a lateral movable adaptor equipped with a mouse anesthesia mask (51,625 and 514609 M, Stoelting, USA). This configuration enabled sequential measurements on the left and right parabionts. Subsequently, the scalp and periosteum were retracted and secured aside, and a thin layer of nail polish was applied to the cranial surface to provide a smooth optical interface. Dual vaporizers were employed for anesthesia administration, affording independent control of sedation levels in each parabiont (1% and 2%, isoflurane, in measured and co-measured parabiont, respectively) A laser speckle contrast imager (Perimed, Järfälla, Sweden) was positioned 10 cm above the cranial surface, and to achieve the highest CBF responses, the left and right whiskers were stimulated for approximately 45 s at 5 Hz from side to side [36]. Differential perfusion maps of the brain surface were acquired, and changes in CBF were evaluated above the barrel cortex. This procedure was repeated in eight trials with 5-–10-min intervals between each trial. Changes in CBF were subsequently averaged and expressed as a percentage (%) increase relative to the baseline value [105].
Fig. 2.
Young blood rejuvenates NVC responses in aged heterochronic parabionts, whereas old blood impairs NVC responses in young heterochronic parabionts. A The parabiont-adjusted stage is illustrated, allowing for consecutive measurements of NVC responses in both parabionts. This specialized stage provides stabilization for the head of the measured animal while enabling unrestricted movement of the co-parabiont’s head. Furthermore, it facilitates separate anesthesia for both parabionts, with the measured parabiont receiving ventilation and the co-parabiont undergoing anesthesia via a face mask. B A schematic representation portrays both parabionts positioned beneath the laser speckle contrast imaging (LSCI) system for data acquisition. The difference image reveals alterations in cerebral blood flow during baseline and stimulation (highlighted in red within the somatosensory cortex). The percentage change in cerebral blood flow within this cortex region is quantified, representing the neurovascular coupling response. This quantification is depicted graphically as the change in cerebral blood flow over time. C Representative pseudocolor laser speckle flowmetry maps of baseline CBF in Y-(Y), Y-(A), A-(Y) and A-(A) parabionts, shown for orientation purposes. D Representative pseudocolor laser speckle flowmetry differential images showing CBF changes in the whisker barrel field relative to baseline during contralateral whisker stimulation (left oval, 30 s, 5 Hz) in Y-(Y), Y-(A), A-(Y) and A-(A) parabionts. Color bar represents CBF as a percent change from baseline. E, F The time course of CBF changes after the start of contralateral whisker stimulation (horizontal bars) in Y-(Y) and Y-(A) (E) and A-(Y) and A-(A) parabionts (F). Summary data are shown in G. Note that young blood rejuvenates NVC responses in aged heterochronic parabionts, whereas old blood impairs NVC responses in young heterochronic parabionts, mimicking the aging phenotype. Data are mean ± S.D. (one-way ANOVA with post-hoc Tukey’s tests, n = 8 in each group, **p < 0.01, ****p < 0.0001)
Blood exchange confirmation using flow cytometry
The primary objective of parabiosis surgery is to establish a shared circulatory system between two animals, thereby enabling the evaluation of the impact of circulating factors between the paired animals. To validate a blood exchange between parabionts, we employed an antibody-based circulating leukocyte labelling approach [106]. Briefly, 3 h prior to planned euthanasia, 50 µL of blood was collected from facial vein of both parabionts into EDTA-coated tubes (MiniCollect Tube, ref: 450,476, Greiner Bio-One, Austria). Subsequently, one of the parabionts in each pair received a retro-orbital injection of phycoerythrin-labeled anti-CD45 antibody (10 µg, 0.2 mg/mL, cat#: 103,106, BioLegend, CA, USA). After a 3-h interval, blood samples were once again collected from both parabionts following the same procedure. All blood samples were promptly processed, and peripheral blood mononuclear cells were isolated using the density gradient method with Ficoll-Paque (ref: 17,144,002, Cytiva, Sweden) as per the manufacturer’s instructions. The middle layer containing mononuclear cells was transferred into a sterile 1.5-mL centrifuge tube and washed once with PBS (500 g, 10 min, 20 °C). Subsequently, cells were resuspended in 150 µL of PBS, and the ratio of labeled to non-labeled leukocytes was determined for each blood sample (both pre- and post-injection in both parabionts) using flow cytometry. Ratios of CD45-positive and negative cells were acquired using the easyCyte flow cytometer (Guava easyCyte BGR HT System, Cytek, CA, USA), and data analysis and graph preparation were conducted using FCS ExpressPro (version 7.18, De Novo Software, CA, USA).
Statistical analysis
Graphs and statistical analyses were conducted using GraphPad Prism software (version 8.0.1, GraphPad Software, MA, USA). To assess group differences, one-way ANOVA followed by Sidak’s post hoc test was employed, as deemed appropriate for the analysis. Statistical significance was defined at p < 0.05 (**p < 0.01, ****p < 0.0001). Data are presented as the mean ± standard deviation. In the neurovascular coupling experiments, we conducted at least eight independent measurements for each experimental group.
Results
Successful parabiosis surgery rate, post-surgical complications, and blood exchange confirmation
Heterochronic parabiosis is a complex surgical procedure characterized by its extended duration, intricate surgical maneuvers, comprehensive pre- and post-operative care and protracted recovery phase. Our surgical team underwent rigorous training under the guidance of the small animal surgery experts at the Einstein Chronobiosis and Energetics/Metabolism of Aging Core (Bronx, NY, USA), who bring years of expertise in performing this intricate surgical procedure. Within our laboratory, we have consistently achieved a parabiosis surgical success rate of 95%. The overall post-surgical recovery rate stands at 80%, with the lowest recovery rate observed in the case of isochronic aged parabionts (67%, Table 1). This reduced recovery rate among aged parabionts can be attributed to age-related declines in resilience, heightened vulnerability to anesthesia, and a heightened incidence of post-surgical complications. These complications encompass dehydration, compromised motor coordination, limb irritation, wound dehiscence, and weight loss [101]. A noteworthy observation, both in our investigations and in related studies [101], is the substantial weight loss experienced by parabionts following the surgical procedure, with aged parabionts being particularly susceptible (Table 1). To address this challenge, we implemented a range of interventions aimed at ameliorating weight loss. These strategies encompassed dietary supplementation through gels, placement of food pellets on the cage floor, and acquainting the animals with these dietary adjustments prior to the parabiosis surgery. The ultimate objective of parabiosis is to establish a shared circulatory system between parabionts, primarily through wound healing during the initial days post-surgery [101]. To derive meaningful conclusions from parabiosis experiments, it is imperative to validate blood exchange between parabionts. To achieve this, we administered a retro-orbital injection of a fluorescently labelled anti-CD45 antibody to label circulating leukocytes in one of the parabionts. Subsequently, we employed flow cytometry to assess the presence of CD45+ cells in the bloodstream of both the injected and non-injected parabionts. This approach afforded us definitive confirmation of blood exchange in each category of parabiosis pairing (Table 1, Fig. 1D).
Table 1.
Post-surgery outcomes and recovery in heterochronic parabiosis models. This table presents key post-surgery outcomes and recovery statistics for different types of parabiosis models, including information on post-surgery survival rates, recovery rates, weight changes at various time points after surgery, and confirmation of successful blood exchange (flow cytometry) between parabionts. Y-A: Heterochronic parabiont pairs; Y-Y and A-A: Isochronic parabiont pairs
| Type of Parabiosis | Post-surgery survival (animals/total) | Post-surgery Recovery (animals/total) | Weight change at day 3 [%] | Weight change at day 14 [%] | Weight change at day 42 [%] | Confirmation of blood exchange (animals/total) |
|---|---|---|---|---|---|---|
| Y-Y | 100% (4/4) | 100% (4/4) | − 15 ± 2 | − 16 ± 4 | − 7 ± 3 | Yes (2/2) |
| Y-A | 100% (10/10) | 80% (8/10) | − 14 ± 2 | − 15 ± 3 | − 11 ± 1 | Yes (2/2) |
| A-A | 80% (5/6) | 67% (4/6) | − 17 ± 1 | − 16 ± 2 | − 14 ± 3 | Yes (2/2) |
Young blood rejuvenates NVC responses in aged heterochronic parabionts
Substantial experimental evidence underscores the pivotal role of endothelial dysfunction in age-related neurovascular impairments in the murine brain. Additionally, exposure to young blood has been shown to effectively restore endothelial function in the aorta of aged heterochronic parabionts [77]. To investigate the impact of systemic young blood factors on NVC responses within the aging mouse brain, we harnessed the heterochronic parabiosis model (Fig. 1A). This experimental framework enabled us to explore the effects of systemic young blood factors on aged heterochronic parabionts (A-(Y)), as well as the consequences of systemic factors enriched in aged blood on young heterochronic parabionts (Y-(A)). To mitigate potential confounding influences stemming from surgical stress associated with parabiosis, we incorporated isochronic young (Y-(Y)) and isochronic aged (A-(A)) parabionts as control groups.
To assess NVC responses within parabionts, we employed a stereotactic frame equipped with a heating pad, specifically designed to accommodate two animals simultaneously (Fig. 2A). This apparatus featured a tooth-bar holder with adjustable left/right positioning during measurements, facilitating the sequential assessment of NVC responses in both parabionts. Utilizing the LSCI system, we quantified the average change in cerebral blood flow within the somatosensory cortex in response to whisker stimulation (Fig. 2B).
Pseudocolor baseline CBF maps, generated via LSCI, were instrumental in offering enhanced anatomical and orientation details (Fig. 2C). Our results revealed a significant reduction in NVC responses within the somatosensory whisker barrel cortex of aged mice compared to their youthful counterparts, indicative of compromised functional hyperemia in advanced age (p < 0.0001, Fig. 2G) [31,35–37,40,107–109]. Notably, our study demonstrated a substantial increase in NVC responses among aged heterochronic parabionts following a 6-week exposure to a youthful systemic milieu when compared to their aged isochronic parabiont counterparts (p < 0.01, Fig. 2D–G). Although this exposure to young blood partially rejuvenated neurovascular function, NVC responses in aged heterochronic parabionts remained significantly lower than those observed in young isochronic parabionts.
Old blood impairs NVC responses in young heterochronic parabionts, recapitulating the aging phenotype
Remarkably, our investigations unveiled that young heterochronic parabionts exposed to the aged systemic milieu for 6 weeks exhibited a significant attenuation in NVC responses when compared to their young isochronic parabiont counterparts (p < 0.01, Fig. 2D–G), effectively mirroring the aging phenotype. Notably, the accelerated neurovascular aging triggered by exposure to aged blood was only partial, as NVC responses in young heterochronic parabionts remained significantly more robust than those observed in aged isochronic parabionts.
Discussion
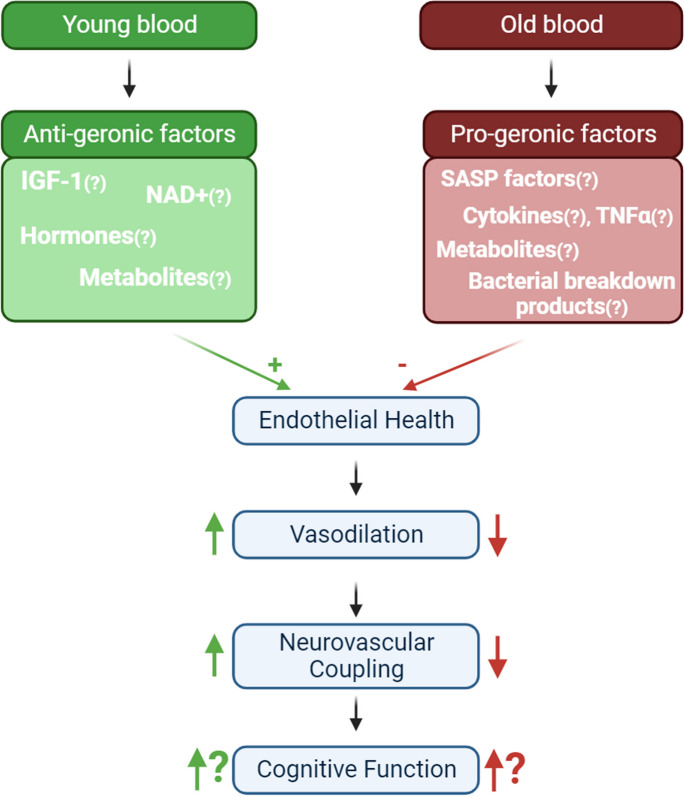
The pivotal finding of this study lies in the demonstration that even a relatively brief exposure to a youthful humoral environment can mitigate neurovascular aging phenotypes, leading to the rejuvenation of NVC responses. Conversely, a short-term encounter with an aged humoral milieu in young mice accelerates the acquisition of neurovascular aging traits characterized by impaired NVC responses (Fig. 3).
Fig. 3.
The impact of circulating factors on neurovascular aging. This schematic representation illustrates the potential presence of anti-geronic factors in young blood (depicted in green) and pro-geronic factors in old blood (depicted in red). These circulating factors act upon endothelial cells, influencing pathways responsible for endothelium-dependent vasodilation. Neurovascular coupling (NVC) responses are partially reliant on endothelial function; therefore, exposure to young blood can partially rejuvenate NVC responses in old parabionts. Conversely, exposure to old blood induces aging-like changes in endothelial cells, resulting in impaired NVC responses. These alterations in NVC are anticipated to have a direct impact on cognitive function
NVC stands as a critical homeostatic mechanism that ensures adequate blood supply to active brain regions. Neurons, devoid of energy or oxygen storage, heavily rely on the closely regulated delivery of nutrients and oxygen by the surrounding microvasculature to sustain their functionality. During neuronal activation, NVC orchestrates the adjustment of regional cerebral blood flow to increased demand. This intricate coupling between neuronal activity and the vascular response guarantees an uninterrupted supply of oxygen and nutrients, preventing any spatiotemporal functional impairment within the brain. Upon neuronal activation neurotransmitters are released and astrocytes undergo activation, releasing gliotransmitters (e.g., ATP). These signaling molecules act on endothelial cells, subsequently triggering endothelial NO synthase (eNOS) activation, NO release, and the relaxation of smooth muscle cells in precapillary arterioles and pericytes around capillaries, increasing regional CBF [110,111]. Furthermore, the microvascular endothelium plays a vital role in the retrograde transduction of vasorelaxation from capillary beds to pial arterioles, ensuring precisely localized vasodilation and safeguarding against “flow steal” from neighboring vascular networks [110].
With age, there is a progressive decline in endothelial function within the cerebral circulation, leading to diminished vasodilatory response and disrupted regulation of cerebral blood flow. The mechanisms of vascular aging [55,71] contributing to age-related endothelial dysfunction are multifaceted and include mitochondrial dysfunction [36,56,109], heightened production of reactive oxygen species (ROS) [24,55,57–66], compromised nitric oxide availability [10,17–19], cellular NAD + depletion [35,67–69], epigenetic alterations (including sirtuin dysregulation) [58,70], and energetic dysfunctions [36]. Impaired endothelium-mediated vasodilation compromises the aged brain’s ability to meet neuronal metabolic demands through NVC, ultimately contributing to cognitive deficits [112–114]. Our findings are of substantial importance, providing conclusive evidence that circulating factors play a central role in neurovascular aging and the age-associated decline in NVC responses.
The mechanisms underlying the promotion of neurovascular rejuvenation by young blood factors likely involve the rejuvenation of cerebromicrovascular endothelial cells. Several lines of evidence support this hypothesis. Firstly, our previous studies have established that aging-induced endothelial dysfunction is a pivotal contributor to neurovascular dysfunction in aged mice [31,35–37,40]. Crucially, we have demonstrated that heightened mitochondrial oxidative stress in microvascular endothelial cells is a critical mechanism through which aging impairs cerebromicrovascular endothelial dysfunction [31,35,36]. Notably, the administration of agents that mitigate mitochondrial oxidative stress, such as mitochondria-targeted antioxidative peptide SS-31 and resveratrol, has been shown to ameliorate NVC in mice, as well as improve cognitive function [36,109]. Secondly, our previous investigations have established that exposure to young blood rejuvenates endothelial vasodilation in the aorta using the same mouse parabiosis model employed in this study [76,77]. Importantly, this functional endothelial rejuvenation was accompanied by the rejuvenation of the vascular mitochondrial transcriptome and the mitigation of vascular oxidative stress [76,77]. Thirdly, recent research by Ximerakis et al. has demonstrated that, within the brain, endothelial cells are the most susceptible to age-related mitochondrial transcriptomic changes, while exhibiting the highest degree of responsiveness to rejuvenation induced by young systemic factors in a mouse model of heterochronic parabiosis [115]. Taken together, we propose that young blood-mediated neurovascular rejuvenation primarily arises from the rejuvenation of mitochondria in cerebromicrovascular endothelial cells. Further research is necessary to evaluate the roles of other related mechanisms, such as the reduction of DNA damage-induced cellular senescence, which may be attributed to the alleviation of oxidative stress, in the overall rejuvenation of cerebromicrovascular endothelial function.
Our observations regarding the rejuvenation of NVC responses by young blood factors align with prior findings from heterochronic parabiosis, plasma transfer, and plasma dilution experiments, all highlighting the beneficial impact of a youthful systemic milieu on brain aging. Exposure to factors enriched in young plasma has also been shown to reduce neuroinflammation, enhance hippocampal neurogenesis, improve cognitive function, and rejuvenate the transcriptome of various brain cell types [88,92,115]. Indeed, considering the direct exposure of endothelial cells to blood factors, it is tempting to envision them as the central hub for young blood-mediated rejuvenation. This scenario suggests that other cells might undergo a form of rejuvenation, albeit indirectly, facilitated by factors released from the rejuvenated endothelium, potentially encompassing cytokines, growth factors, and diverse signaling molecules. Furthermore, the enhancement of endothelial transport and barrier functions could potentially exert an indirect rejuvenating influence on parenchymal cells within the organ.
The precise nature of the anti-geronic factors present in the circulation of young animals remains a subject of active investigation (Fig. 3). Our recent bioinformatics analysis has identified a spectrum of potential pathways likely contributing to the rejuvenating effects of young blood. These pathways and factors include the inhibition of rapamycin-insensitive companion of mTOR (RICTOR) and the activation of insulin-like growth factor 1 receptor (IGF1R), VEGF receptors, and sirtuins. Notably, insulin-like growth factor 1 (IGF-1), a hormone responsible for mediating the anabolic effects of growth hormone and essential for normal growth and development, has been shown to significantly decrease in serum levels during aging [116] both in humans and laboratory animals. Strong evidence links circulating IGF-1 deficiency to cardiovascular aging and, in particular, neurovascular and brain aging [21,23,33,34,39,116–132]. Age-related IGF-1 deficiency has been causally associated with endothelial dysfunction, impaired neurovascular coupling responses, and cognitive deficits in both rodent models and humans [33,34,38,39]. In a significant demonstration of the importance of endothelial IGF-1 signaling, cell-specific IGFR1 knockdown in cerebrovascular endothelial cells resulted in an accelerated neurovascular aging phenotype and impaired NVC responses. Circulating IGF-1 levels have also been observed to correlate with NVC responses in older adults [38]. Consequently, further investigation is warranted to delineate the role of IGF-1 in young blood-mediated neurovascular rejuvenation. Another putative mechanism underlying the young blood-mediatedimprovement of NVC responses is the restoration of cellular NAD+ levels [35,133]. NAD+ serves as a co-factor for over four hundred enzymes, including vasoprotective and lifespan-extending sirtuins. With age, circulating and cellular NAD+ levels decline, including a decrease within cerebromicrovascular endothelial cells [35]. The replenishment of NAD+ through the administration of NAD precursors has been shown to rescue endothelial function, reverse age-related endothelial transcriptomic changes, and rejuvenate NVC responses [35,133]. Although initial evidence suggests that sirtuin activation may contribute to endothelial rejuvenation in the aortas of aged heterochronic parabionts, comprehensive mechanistic studies are needed to assess the role of sirtuin activation in the rejuvenation of cerebrovascular endothelial function and NVC responses. Other systemic factors (e.g., tissue plasminogen activator [tPA] [134–136], growth differentiation factor 11 [GDF-11] [92,137,138], tetrahydrobiopterin [BH4] [139,140]) may also contribute to the beneficial effects of young blood on NVC. However, the exact contributions of the aforementioned circulating factors to NVC rejuvenation are currently theoretical and warrant further exploration. Future studies, particularly those employing transgenic mice lacking specific circulating factors combined with heterochronic parabiosis models, are essential to pinpoint the precise factors and pathways involved in the rejuvenation effects of young blood on NVC. In addition, the observed enhancement of NVC responses in aged heterochronic parabionts could be linked to the dilution of pro-geronic factors present in aged plasma. This hypothesis aligns with the increasing evidence from both preclinical and clinical research, indicating beneficial outcomes from diluting aged plasma, such as improvements in neuroinflammation, cognitive function, and biological age [141–143]. Therefore, it is essential to explore further whether the dilution of pro-geronic factors by young blood significantly contributes to the improved NVC seen in aged heterochronic parabionts.
This study also highlights a crucial discovery that a relatively brief exposure of young mice to an aged humoral environment can accelerate the onset of neurovascular aging phenotypes. Notably, we demonstrate, for the first time, that mediators circulating in the blood of aged mice can expedite neurovascular aging. These findings build upon our prior research, which elucidated pro-geronic transcriptional alterations induced by aged blood in the aorta [76]. Moreover, our results further substantiate the notion that non-cell-autonomous mechanisms are pivotal in driving neurovascular aging processes, likely contributing to the pathogenesis of VCI. Earlier investigations have revealed that the presence of aged blood in the circulation of young heterochronic parabionts also prompts age-like phenotypic transformations in the liver, heart, and brain [31,47,49,52,55,98]. Interestingly, it has been demonstrated that old blood factors exert pro-geronic effects on the central nervous system [143,144]. This suggests that specific circulating anti-geronic factor(s) may traverse the blood–brain barrier or induce their detrimental impact on the brain by fostering accelerated neurovascular aging. The remarkable plasticity of neurovascular aging in response to both pro-geronic and anti-geronic circulating factors underscores the potential for therapeutic interventions aimed at counteracting the age-related dysfunction of cerebral microcirculation, either by directly or indirectly targeting the systemic milieu. Despite these findings, the precise nature and cellular origins of circulating pro-geronic factors, responsible for inducing accelerated neurovascular aging remain enigmatic. In a recent transcriptomic investigation, we identified potential cellular pathways induced by pro-geronic factors present in aged blood, including the possible involvement of TGFβ signaling, inhibition of the p53 pathway, serum response factor (SRF)-driven pathways, and inhibition of VEGF-A and IGF-1 signaling [76]. Furthermore, other circulating factors whose levels are modulated in young mice through heterochronic parabiosis or systemic administration of aged plasma or blood and which may bestow aging-like effects in the vasculature, encompass fibroblast growth factor 23 (FGF23) [145,146], β2-microglobulin, TGFβ family cytokines [93,100], TNFα [147], and other pro-inflammatory mediators secreted by senescent cells, collectively known as the senescence-associated secretory phenotype (SASP) [71,148,149]. Future studies should aim to identify pro-geronic factors responsible for accelerated endothelial aging. Uncovering these factors could be achieved through heterochronic parabiosis experiments using genetically engineered animals lacking certain pro-geronic factors134,150. Furthermore, the adverse effects on NVC noted in our study may partly stem from the dilution of protective young blood factors.
Recognizing the limitations and confounding factors of the heterochronic parabiosis model is crucial. This model’s primary strength lies in its ability to continuously expose both young and aged animals to their co-parabiont’s circulating factors, making it highly effective for detecting subtle pro- and anti-aging effects. However, this same strength poses a challenge in isolating and identifying specific factors and pathways responsible for the observed effects from heterochronic blood exchange. Additionally, the model involves not only exposure to the systemic factors of the co-parabiont but also indirect exposure to their organs, such as the liver and kidneys. This aspect could potentially enhance metabolic clearance and overall health, especially in the case of aged heterochronic parabionts. The surgery and subsequent shared circulation can induce stress in the animals, potentially influencing physiological responses. The altered physical activity levels in young and aged heterochronic parabionts add another layer of complexity, potentially influencing their health outcomes. Nutritional status can significantly impact aging processes and vascular health. In parabiosis experiments, the nutritional intake and metabolic status of one parabiont can indirectly influence the other, potentially skewing results related to circulating factors. It is pertinent to note that post-parabiosis surgery, parabionts often exhibit a significant weight reduction, which could impact various measured outcomes, similar to observations in caloric restriction studies. Despite controls like isochronic pairings, reduced mobility in heterochronic parabiosis poses challenges in specific physiological assessments, such as cognitive function evaluation or procedures requiring animal imaging and anesthesia. These challenges necessitate additional methodological adjustments, including the use of equipment and adaptation of protocols specifically designed for parabionts.
In summary, our study highlights the promising potential for reversing neurovascular aging by leveraging the systemic influences of both anti-geronic and pro-geronic circulating factors. While this study focused primarily on the rejuvenation of NVC responses, it remains to be explored whether young systemic factors can extend their rejuvenating effects to address other age-related cerebromicrovascular pathologies, such as blood–brain barrier disruption or microvascular rarefaction. Investigating these facets promises valuable insights into the broader implications of young blood-mediated brain rejuvenation. The circulation exposes each plasma constituent in blood, encompassing circulating hormones, cytokines, proteins, peptides, lipid mediators, micropeptides, metabolites, and circulating exosomes, to the vasculature. Each of these constituents may exert either anti-geronic or pro-geronic effects, warranting further investigation into the precise nature and cellular origins of circulating factors that mediate neurovascular rejuvenation and the induction of accelerated vascular aging, as observed in our study.
Utilizing transgenic animals in parabiosis experiments is a promising approach to uncover the factors that drive the rejuvenating effects of young blood and the detrimental impacts of aged blood, thereby enhancing the potential for translational applications in future research. Additionally, methods like plasma transfer, heterochronic blood exchange, and plasma dilution experiments provide valuable alternative models. These approaches can augment the findings from heterochronic parabiosis and contribute to a more comprehensive understanding of the mechanisms involved. The versatility of these models offers an avenue to extend our observations beyond the confines of parabiosis studies, shedding light on the broader implications of young blood factors in the context of age-related VCI and rejuvenation strategies.
Acknowledgements
We sincerely thank the Division of Comparative Medicine team at the University of Oklahoma Health Sciences Center for their invaluable support in supervising animal care and sharing their extensive expertise. Special recognition is extended to Dr. Shawn Lane, DVM, for his invaluable guidance and expertise in surgical and postsurgical care. We are grateful to Dr. Wendy Williams, DVM, for her instrumental role in designing appropriate pre- and postsurgical treatments. We also acknowledge Ms. Carlye Yancey, BS, for her exceptional animal husbandry knowledge and contributions to parabiosis housing. Furthermore, we wish to express our gratitude to Mr. Chad Cunningham, Electronic & Instrument Shop Supervisor Building Manager of the University of Oklahoma’s Department of Physics and Engineering for his essential assistance in fabricating the parabiont-adjusted components of the stereotactic frame, which was instrumental in facilitating simultaneous measurements of neurovascular coupling in parabionts.
Author contribution
The study’s conception, design, and data interpretation involved contributions from all authors. Parabiosis surgeries were executed by R.G. and B.Cs., while the post-surgical monitoring of animals was carried out by R.G., B.C., B.P., J.F., and S.S. The assessment of neurovascular coupling responses and subsequent data analysis were undertaken by R.G., ANT, S.N., and P.T. The initial draft of the manuscript was jointly composed by R.G., ANT, S.T., and Z.U. Subsequent revisions to the manuscript were conducted by all authors, who also collectively reviewed and provided their approval for the final version of the manuscript.
Funding
This work was supported by grants from the American Heart Association (R.G.: 916225, ANT: AHA834339, and S.T.: AHA CDA941290), the Oklahoma Center for the Advancement of Science and Technology, the National Institute on Aging (RF1AG072295, R01AG055395, R01AG068295; R01AG070915, K01AG073614, K01AG073613, R03AG070479), the National Institute of Neurological Disorders and Stroke (R01NS100782), the National Cancer Institute (R01CA255840), the Oklahoma Shared Clinical and Translational Resources (U54GM104938) with an Institutional Development Award (IDeA) from NIGMS, the Presbyterian Health Foundation, the Reynolds Foundation, the Oklahoma Nathan Shock Center (P30AG050911), and the Cellular and Molecular GeroScience CoBRE (P20GM125528), the NCI Cancer Center Support Grant (P30 CA225520) and the Oklahoma Tobacco Settlement Endowment Trust. ANT was supported by Project no. DMH is supported by P30AG038072. TKP2021-NKTA-47, implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-NKTA funding scheme; by funding through the National Cardiovascular Laboratory Program (RRF-2.3.1–21-2022–00003) provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund; Project no. 135784 implemented with the support provided from the National Research, Development and Innovation Fund of Hungary, financed under the K_20 funding scheme and the European University for Well-Being (EUniWell) program (grant agreement number: 101004093/ EUniWell/EAC-A02-2019 / EAC-A02-2019–1). The funding sources had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the American Heart Association, or the Presbyterian Health Foundation. The 3.5 version of ChatGPT, developed by OpenAI, was used as a language tool to refine our writing, enhancing the clarity of our work.
Declarations
Competing interests
Dr. Anna Csiszar serves as Associate Editor for The Journal of Gerontology, Series A: Biological Sciences and Medical Sciences and GeroScience. Dr. Zoltan Ungvari serves as Editor-in-Chief for GeroScience. Dr. Stefano Tarantini, Dr. Derek M. Huffman, and Dr. Andriy Yabluchanskiy serve as Associate Editors for GeroScience.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rafal Gulej and Ádám Nyúl-Tóth equal contribution.
References
- 1.Johnson AC. Hippocampal vascular supply and its role in vascular cognitive impairment. Stroke. 2023;54:673–685. doi: 10.1161/STROKEAHA.122.038263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iadecola C, Duering M, Hachinski V, Joutel A, Pendlebury ST, Schneider JA, Dichgans M. Vascular cognitive impairment and dementia: JACC Scientific Expert Panel. J Am Coll Cardiol. 2019;73:3326–3344. doi: 10.1016/j.jacc.2019.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol. 2017;312:H1–h20. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolters FJ, Ikram MA. Epidemiology of vascular dementia. Arterioscler Thromb Vasc Biol. 2019;39:1542–1549. doi: 10.1161/ATVBAHA.119.311908. [DOI] [PubMed] [Google Scholar]
- 5.Rizzi L, Rosset I, Roriz-Cruz M. Global epidemiology of dementia: Alzheimer’s and vascular types. Biomed Res Int. 2014;2014:908915. doi: 10.1155/2014/908915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rocca WA, Hofman A, Brayne C, Breteler MMB, Clarke M, Copeland JRM, Dartiques J-F, Engedal K, Hagnell O, Heeren TJ, Jonker C, Lindesay J, Lobo A, Mann AH, Mölsä PK, Morgan K, O’Connor DW, Droux AdS, Sulkava R, Kay DWK, Amaducci L, Group E-PR The prevalence of vascular dementia in europe: Facts and fragments from 1980–1990 studies. Annals of Neurology. 1991;30:817–824. doi: 10.1002/ana.410300611. [DOI] [PubMed] [Google Scholar]
- 7.Hébert R, Brayne C. Epidemiology of vascular dementia. Neuroepidemiology. 1995;14:240–257. doi: 10.1159/000109800. [DOI] [PubMed] [Google Scholar]
- 8.Tong X, Yang Q, Ritchey MD, George MG, Jackson SL, Gillespie C, Merritt RK. The Burden of Cerebrovascular Disease in the United States. Prev Chronic Dis. 2019;16:E52. doi: 10.5888/pcd16.180411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eurostat: Aging Europe. https://ec.europa.eu/eurostat/cache/digpub/ageing/ Accessed on 11/04/2022.
- 10.World Health Organization. Health data overview for Japan. https://data.who.int/countries/392 Accessed on 10/02/2023.
- 11.“Aging in the United States.” Population Reference Bureau, 2021, https://www.prb.org/aging-unitedstates-fact-sheet/. Accessed on 05/09/2023.
- 12.United States Census Bureau. 2020 Census: 1 in 6 People in the United States Were 65 and Over. https://www.census.gov/library/stories/2023/05/2020-census-united-states-older-population-grew.html Accessed on 10/02/2023.
- 13.Nichols E, Steinmetz JD, Vollset SE, Fukutaki K, Chalek J, Abd-Allah F, Abdoli A, Abualhasan A, Abu-Gharbieh E, Akram TT, Al Hamad H, Alahdab F, Alanezi FM, Alipour V, Almustanyir S, Amu H, Ansari I, Arabloo J, Ashraf T, Astell-Burt T, Ayano G, Ayuso-Mateos JL, Baig AA, Barnett A, Barrow A, Baune BT, Béjot Y, Bezabhe WMM, Bezabih YM, Bhagavathula AS, Bhaskar S, Bhattacharyya K, Bijani A, Biswas A, Bolla SR, Boloor A, Brayne C, Brenner H, Burkart K, Burns RA, Cámera LA, Cao C, Carvalho F, Castro-de-Araujo LFS, Catalá-López F, Cerin E, Chavan PP, Cherbuin N, Chu D-T, Costa VM, Couto RAS, Dadras O, Dai X, Dandona L, Dandona R, De la Cruz-Góngora V, Dhamnetiya D, Dias da Silva D, Diaz D, Douiri A, Edvardsson D, Ekholuenetale M, El Sayed I, El-Jaafary SI, Eskandari K, Eskandarieh S, Esmaeilnejad S, Fares J, Faro A, Farooque U, Feigin VL, Feng X, Fereshtehnejad S-M, Fernandes E, Ferrara P, Filip I, Fillit H, Fischer F, Gaidhane S, Galluzzo L, Ghashghaee A, Ghith N, Gialluisi A, Gilani SA, Glavan I-R, Gnedovskaya EV, Golechha M, Gupta R, Gupta VB, Gupta VK, Haider MR, Hall BJ, Hamidi S, Hanif A, Hankey GJ, Haque S, Hartono RK, Hasaballah AI, Hasan MT, Hassan A, Hay SI, Hayat K, Hegazy MI, Heidari G, Heidari-Soureshjani R, Herteliu C, Househ M, Hussain R, Hwang B-F, Iacoviello L, Iavicoli I, Ilesanmi OS, Ilic IM, Ilic MD, Irvani SSN, Iso H, Iwagami M, Jabbarinejad R, Jacob L, Jain V, Jayapal SK, Jayawardena R, Jha RP, Jonas JB, Joseph N, Kalani R, Kandel A, Kandel H, Karch A, Kasa AS, Kassie GM, Keshavarz P, Khan MAB, Khatib MN, Khoja TAM, Khubchandani J, Kim MS, Kim YJ, Kisa A, Kisa S, Kivimäki M, Koroshetz WJ, Koyanagi A, Kumar GA, Kumar M, Lak HM, Leonardi M, Li B, Lim SS, Liu X, Liu Y, Logroscino G, Lorkowski S, Lucchetti G, Lutzky Saute R, Magnani FG, Malik AA, Massano J, Mehndiratta MM, Menezes RG, Meretoja A, Mohajer B, Mohamed Ibrahim N, Mohammad Y, Mohammed A, Mokdad AH, Mondello S, Moni MAA, Moniruzzaman M, Mossie TB, Nagel G, Naveed M, Nayak VC, Neupane Kandel S, Nguyen TH, Oancea B, Otstavnov N, Otstavnov SS, Owolabi MO, Panda-Jonas S, Pashazadeh Kan F, Pasovic M, Patel UK, Pathak M, Peres MFP, Perianayagam A, Peterson CB, Phillips MR, Pinheiro M, Piradov MA, Pond CD, Potashman MH, Pottoo FH, Prada SI, Radfar A, Raggi A, Rahim F, Rahman M, Ram P, Ranasinghe P, Rawaf DL, Rawaf S, Rezaei N, Rezapour A, Robinson SR, Romoli M, Roshandel G, Sahathevan R, Sahebkar A, Sahraian MA, Sathian B, Sattin D, Sawhney M, Saylan M, Schiavolin S, Seylani A, Sha F, Shaikh MA, Shaji KS, Shannawaz M, Shetty JK, Shigematsu M, Shin JI, Shiri R, Silva DAS, Silva JP, Silva R, Singh JA, Skryabin VY, Skryabina AA, Smith AE, Soshnikov S, Spurlock EE, Stein DJ, Sun J, Tabarés-Seisdedos R, Thakur B, Timalsina B, Tovani-Palone MR, Tran BX, Tsegaye GW, Valadan Tahbaz S, Valdez PR, Venketasubramanian N, Vlassov V, Vu GT, Vu LG, Wang Y-P, Wimo A, Winkler AS, Yadav L, Yahyazadeh Jabbari SH, Yamagishi K, Yang L, Yano Y, Yonemoto N, Yu C, Yunusa I, Zadey S, Zastrozhin MS, Zastrozhina A, Zhang Z-J, Murray CJL and Vos T. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. The Lancet Public Health. 2022;7:e105-e125. [DOI] [PMC free article] [PubMed]
- 14.Nandi A, Counts N, Chen S, Seligman B, Tortorice D, Vigo D, Bloom DE. Global and regional projections of the economic burden of Alzheimer’s disease and related dementias from 2019 to 2050: A value of statistical life approach. EClinicalMedicine. 2022;51:101580. doi: 10.1016/j.eclinm.2022.101580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han EJ, Lee J, Cho E and Kim H. Socioeconomic costs of dementia based on utilization of health care and long-term-care services: a retrospective cohort study. Int J Environ Res Public Health. 2021;18. [DOI] [PMC free article] [PubMed]
- 16.Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol Rev. 2019;99:21–78. doi: 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nyul-Toth A, Tarantini S, DelFavero J, Yan F, Balasubramanian P, Yabluchanskiy A, Ahire C, Kiss T, Csipo T, Lipecz A, Farkas AE, Wilhelm I, Krizbai IA, Tang Q, Csiszar A, Ungvari Z. Demonstration of age-related blood-brain barrier disruption and cerebromicrovascular rarefaction in mice by longitudinal intravital two-photon microscopy and optical coherence tomography. Am J Physiol Heart Circ Physiol. 2021;320:H1370–H1392. doi: 10.1152/ajpheart.00709.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fulop GA, Ahire C, Csipo T, Tarantini S, Kiss T, Balasubramanian P, Yabluchanskiy A, Farkas E, Toth A, Nyul-Toth A, Toth P, Csiszar A, Ungvari Z. Cerebral venous congestion promotes blood-brain barrier disruption and neuroinflammation, impairing cognitive function in mice. Geroscience. 2019;41:575–589. doi: 10.1007/s11357-019-00110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tucsek Z, Toth P, Sosnowsk D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE, Ungvari Z, Csiszar A. Obesity in aging exacerbates blood brain barrier disruption, neuroinflammation and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2014;69:1212–26. doi: 10.1093/gerona/glt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Skike CE, Jahrling JB, Olson AB, Sayre NL, Hussong SA, Ungvari Z, Lechleiter JD, Galvan V. Inhibition of mTOR protects the blood-brain barrier in models of Alzheimer’s disease and vascular cognitive impairment. Am J Physiol Heart Circ Physiol. 2018;314:H693–H703. doi: 10.1152/ajpheart.00570.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller LR, Tarantini S, Nyul-Toth A, Johnston MP, Martin T, Bullen EC, Bickel MA, Sonntag WE, Yabluchanskiy A, Csiszar A, Ungvari ZI, Elliott MH, Conley SM. Increased susceptibility to cerebral microhemorrhages is associated with imaging signs of microvascular degeneration in the retina in an insulin-like growth factor 1 deficient mouse model of accelerated aging. Front Aging Neurosci. 2022;14:788296. doi: 10.3389/fnagi.2022.788296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nyul-Toth A, Fulop GA, Tarantini S, Kiss T, Ahire C, Faakye JA, Ungvari A, Toth P, Toth A, Csiszar A, Ungvari Z. Cerebral venous congestion exacerbates cerebral microhemorrhages in mice. Geroscience. 2022;44:805–816. doi: 10.1007/s11357-021-00504-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Springo Z, Fulop GA, Ashpole N, Gautam T, Giles CB, Wren JD, Sonntag WE, Csiszar A, Ungvari Z. Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell. 2017;16:469–479. doi: 10.1111/acel.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, Wren JD, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell. 2015;14:400–408. doi: 10.1111/acel.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ungvari Z, Tarantini S, Kirkpatrick AC, Csiszar A, Prodan CI. Cerebral microhemorrhages: mechanisms, consequences, and prevention. Am J Physiol Heart Circ Physiol. 2017;312:H1128–H1143. doi: 10.1152/ajpheart.00780.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarantini S, Tran CHT, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol. 2017;94:52–58. doi: 10.1016/j.exger.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorond FA, Hurwitz S, Salat DH, Greve DN, Fisher ND. Neurovascular coupling, cerebral white matter integrity, and response to cocoa in older people. Neurology. 2013;81:904–909. doi: 10.1212/WNL.0b013e3182a351aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarantini S, Tran CH, Gordon GR, Ungvari Z and Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol. 2016;94. 10.1016/j.exger.2016.11.004. [DOI] [PMC free article] [PubMed]
- 29.Csipo T, Lipecz A, Mukli P, Bahadli D, Abdulhussein O, Owens CD, Tarantini S, Hand RA, Yabluchanska V, Kellawan JM, Sorond F, James JA, Csiszar A, Ungvari ZI, Yabluchanskiy A. Increased cognitive workload evokes greater neurovascular coupling responses in healthy young adults. PLoS ONE. 2021;16:e0250043. doi: 10.1371/journal.pone.0250043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Csipo T, Mukli P, Lipecz A, Tarantini S, Bahadli D, Abdulhussein O, Owens C, Kiss T, Balasubramanian P, Nyul-Toth A, Hand RA, Yabluchanska V, Sorond FA, Csiszar A, Ungvari Z, Yabluchanskiy A. Assessment of age-related decline of neurovascular coupling responses by functional near-infrared spectroscopy (fNIRS) in humans. Geroscience. 2019;41:495–509. doi: 10.1007/s11357-019-00122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Csiszar A, Yabluchanskiy A, Ungvari A, Ungvari Z, Tarantini S. Overexpression of catalase targeted to mitochondria improves neurovascular coupling responses in aged mice. Geroscience. 2019;41:609–617. doi: 10.1007/s11357-019-00111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipecz A, Csipo T, Tarantini S, Hand RA, Ngo BN, Conley S, Nemeth G, Tsorbatzoglou A, Courtney DL, Yabluchanska V, Csiszar A, Ungvari ZI and Yabluchanskiy A. Age-related impairment of neurovascular coupling responses: a dynamic vessel analysis (DVA)-based approach to measure decreased flicker light stimulus-induced retinal arteriolar dilation in healthy older adults. Geroscience. 2019. [DOI] [PMC free article] [PubMed]
- 33.Tarantini S, Balasubramanian P, Yabluchanskiy A, Ashpole NM, Logan S, Kiss T, Ungvari A, Nyul-Toth A, Schwartzman ML, Benyo Z, Sonntag WE, Csiszar A, Ungvari Z. IGF1R signaling regulates astrocyte-mediated neurovascular coupling in mice: implications for brain aging. Geroscience. 2021;43:901–911. doi: 10.1007/s11357-021-00350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarantini S, Nyul-Toth A, Yabluchanskiy A, Csipo T, Mukli P, Balasubramanian P, Ungvari A, Toth P, Benyo Z, Sonntag WE, Ungvari Z, Csiszar A. Endothelial deficiency of insulin-like growth factor-1 receptor (IGF1R) impairs neurovascular coupling responses in mice, mimicking aspects of the brain aging phenotype. Geroscience. 2021;43:2387–2394. doi: 10.1007/s11357-021-00405-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, Hertelendy P, Kinter M, Ballabh P, Sule Z, Farkas E, Baur JA, Sinclair DA, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019;24:101192. doi: 10.1016/j.redox.2019.101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, Farkas E, Perz A, Rabinovitch PS, Sonntag WE, Csiszar A and Ungvari Z. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell. 2018;17. [DOI] [PMC free article] [PubMed]
- 37.Tarantini S, Yabluchanskiy A, Csipo T, Fulop G, Kiss T, Balasubramanian P, DelFavero J, Ahire C, Ungvari A, Nyul-Toth A, Farkas E, Benyo Z, Toth A, Csiszar A, Ungvari Z. Treatment with the poly(ADP-ribose) polymerase inhibitor PJ-34 improves cerebromicrovascular endothelial function, neurovascular coupling responses and cognitive performance in aged mice, supporting the NAD+ depletion hypothesis of neurovascular aging. Geroscience. 2019;41:533–542. doi: 10.1007/s11357-019-00101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toth L, Czigler A, Hegedus E, Komaromy H, Amrein K, Czeiter E, Yabluchanskiy A, Koller A, Orsi G, Perlaki G, Schwarcz A, Buki A, Ungvari Z, Toth PJ. Age-related decline in circulating IGF-1 associates with impaired neurovascular coupling responses in older adults. Geroscience. 2022;44:2771–2783. doi: 10.1007/s11357-022-00623-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell. 2015;14:1034–1044. doi: 10.1111/acel.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiedenhoeft T, Tarantini S, Nyul-Toth A, Yabluchanskiy A, Csipo T, Balasubramanian P, Lipecz A, Kiss T, Csiszar A, Csiszar A, Ungvari Z. Fusogenic liposomes effectively deliver resveratrol to the cerebral microcirculation and improve endothelium-dependent neurovascular coupling responses in aged mice. Geroscience. 2019;41:711–725. doi: 10.1007/s11357-019-00102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sorond FA, Kiely DK, Galica A, Moscufo N, Serrador JM, Iloputaife I, Egorova S, Dell’Oglio E, Meier DS, Newton E, Milberg WP, Guttmann CR, Lipsitz LA. Neurovascular coupling is impaired in slow walkers: the MOBILIZE Boston Study. Ann Neurol. 2011;70:213–220. doi: 10.1002/ana.22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zaletel M, Strucl M, Pretnar-Oblak J, Zvan B. Age-related changes in the relationship between visual evoked potentials and visually evoked cerebral blood flow velocity response. Funct Neurol. 2005;20:115–120. [PubMed] [Google Scholar]
- 43.Topcuoglu MA, Aydin H, Saka E. Occipital cortex activation studied with simultaneous recordings of functional transcranial Doppler ultrasound (fTCD) and visual evoked potential (VEP) in cognitively normal human subjects: effect of healthy aging. Neurosci Lett. 2009;452:17–22. doi: 10.1016/j.neulet.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 44.Stefanova I, Stephan T, Becker-Bense S, Dera T, Brandt T, Dieterich M. Age-related changes of blood-oxygen-level-dependent signal dynamics during optokinetic stimulation. Neurobiol Aging. 2013;34:2277–2286. doi: 10.1016/j.neurobiolaging.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 45.Tarantini S, Yabluchanksiy A, Fulop GA, Hertelendy P, Valcarcel-Ares MN, Kiss T, Bagwell JM, O’Connor D, Farkas E, Sorond F, Csiszar A, Ungvari Z. Pharmacologically induced impairment of neurovascular coupling responses alters gait coordination in mice. Geroscience. 2017;39:601–614. doi: 10.1007/s11357-017-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tarantini S, Hertelendy P, Tucsek Z, Valcarcel-Ares MN, Smith N, Menyhart A, Farkas E, Hodges EL, Towner R, Deak F, Sonntag WE, Csiszar A, Ungvari Z, Toth P. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J Cereb Blood Flow Metab. 2015;35:1871–1881. doi: 10.1038/jcbfm.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parikh I, Guo J, Chuang KH, Zhong Y, Rempe RG, Hoffman JD, Armstrong R, Bauer B, Hartz AM, Lin AL. Caloric restriction preserves memory and reduces anxiety of aging mice with early enhancement of neurovascular functions. Aging (Albany NY) 2016;8:2814–2826. doi: 10.18632/aging.101094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balasubramanian P, DelFavero J, Ungvari A, Papp M, Tarantini A, Price N, de Cabo R, Tarantini S. Time-restricted feeding (TRF) for prevention of age-related vascular cognitive impairment and dementia. Ageing Res Rev. 2020;64:101189. doi: 10.1016/j.arr.2020.101189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balasubramanian P, DelFavero J, Ungvari A, Papp M, Tarantini A, Price N, de Cabo R and Tarantini S. Time-restricted feeding (TRF) for prevention of age-related vascular cognitive impairment and dementia. Ageing Res Rev. 2020:101189. [DOI] [PMC free article] [PubMed]
- 50.Toth P, Tarantini S, Davila A, Valcarcel-Ares MN, Tucsek Z, Varamini B, Ballabh P, Sonntag WE, Baur JA, Csiszar A, Ungvari Z. Purinergic glio-endothelial coupling during neuronal activity: role of P2Y1 receptors and eNOS in functional hyperemia in the mouse somatosensory cortex. Am J Physiol Heart Circ Physiol. 2015;309:H1837–H1845. doi: 10.1152/ajpheart.00463.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari ZI. Resveratrol treatment rescues neurovascular coupling in aged mice:role of improved cerebromicrovascular endothelial function and down-regulation of NADPH oxidas. Am J Physiol Heart Circ Physiol. 2014;306:H299–308. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EM. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc. 2014;3:e000787. doi: 10.1161/JAHA.114.000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Csipo T, Lipecz A, Fulop GA, Hand RA, Ngo BN, Dzialendzik M, Tarantini S, Balasubramanian P, Kiss T, Yabluchanska V, Silva-Palacios F, Courtney DL, Dasari TW, Sorond F, Sonntag WE, Csiszar A, Ungvari Z, Yabluchanskiy A. Age-related decline in peripheral vascular health predicts cognitive impairment. Geroscience. 2019;41:125–136. doi: 10.1007/s11357-019-00063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab. 2007;27:1908–1918. doi: 10.1038/sj.jcbfm.9600491. [DOI] [PubMed] [Google Scholar]
- 55.Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of Vascular Aging. Circ Res. 2018;123:849–867. doi: 10.1161/CIRCRESAHA.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, Seals DR. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol. 2014;592:2549–2561. doi: 10.1113/jphysiol.2013.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Csiszar A, Gautam T, Sosnowska D, Tarantini S, Banki E, Tucsek Z, Toth P, Losonczy G, Koller A, Reglodi D, Giles CB, Wren JD, Sonntag WE, Ungvari Z. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am J Physiol Heart Circ Physiol. 2014;307:H292–306. doi: 10.1152/ajpheart.00307.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130:518–527. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 60.Csiszar A, Ungvari Z, Edwards JG, Kaminski PM, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.RES.0000020401.61826.EA. [DOI] [PubMed] [Google Scholar]
- 61.Labinskyy N, Csiszar A, Orosz Z, Smith K, Rivera A, Buffenstein R and Ungvari Z. Comparison of endothelial function, O2.- and H2O2 production, and vascular oxidative stress resistance between the longest-living rodent, the naked mole rat, and mice. Am J Physiol Lung Cell Mol Physiol. 2006;291:H2698–704. [DOI] [PubMed]
- 62.Csiszar A, Labinskyy N, Orosz Z, Xiangmin Z, Buffenstein R, Ungvari Z. Vascular aging in the longest-living rodent, the naked mole rat. Am J Physiol Lung Cell Mol Physiol. 2007;293:H919–H927. doi: 10.1152/ajpheart.01287.2006. [DOI] [PubMed] [Google Scholar]
- 63.Csiszar A, Labinskyy N, Zhao X, Hu F, Serpillon S, Huang Z, Ballabh P, Levy RJ, Hintze TH, Wolin MS, Austad SN, Podlutsky A, Ungvari Z. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell. 2007;6:783–797. doi: 10.1111/j.1474-9726.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- 64.Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci. 2012;67:811–820. doi: 10.1093/gerona/glr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fulop GA, Kiss T, Tarantini S, Balasubramanian P, Yabluchanskiy A, Farkas E, Bari F, Ungvari Z, Csiszar A. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience. 2018;40:513–521. doi: 10.1007/s11357-018-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, Ballabh P, de Cabo R, Sonntag WE, Csiszar A. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of Nrf2-mediated antioxidant response. Am J Physiol Heart Circ Physiol. 2011;301:H363–72. doi: 10.1152/ajpheart.01134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kiss T, Balasubramanian P, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Csipo T, Lipecz A, Reglodi D, Zhang XA, Bari F, Farkas E, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: a potential mechanism for prevention of vascular cognitive impairment. GeroScience. 2019;41:619–630. doi: 10.1007/s11357-019-00074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kiss T, Giles CB, Tarantini S, Yabluchanskiy A, Balasubramanian P, Gautam T, Csipo T, Nyul-Toth A, Lipecz A, Szabo C, Farkas E, Wren JD, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation promotes anti-aging miRNA expression profile in the aorta of aged mice, predicting epigenetic rejuvenation and anti-atherogenic effects. Geroscience. 2019;41:419–439. doi: 10.1007/s11357-019-00095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, Kim LJ, Osborne B, Joshi S, Lu Y, Trevino-Villarreal JH, Kang MJ, Hung TT, Lee B, Williams EO, Igarashi M, Mitchell JR, Wu LE, Turner N, Arany Z, Guarente L, Sinclair DA. Impairment oF AN ENDOTHELIAL NAD(+)-H2S signaling network is a reversible cause of vascular aging. Cell. 2018;173(74–89):e20. doi: 10.1016/j.cell.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mattison JA, Wang M, Bernier M, Zhang J, Park SS, Maudsley S, An SS, Santhanam L, Martin B, Faulkner S, Morrell C, Baur JA, Peshkin L, Sosnowska D, Csiszar A, Herbert RL, Tilmont EM, Ungvari Z, Pearson KJ, Lakatta EG, de Cabo R. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab. 2014;20:183–190. doi: 10.1016/j.cmet.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ungvari Z, Tarantini S, Sorond F, Merkely B, Csiszar A. Mechanisms of Vascular Aging, A Geroscience Perspective: JACC Focus Seminar. J Am Coll Cardiol. 2020;75:931–941. doi: 10.1016/j.jacc.2019.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ludwig FC, Elashoff RM. Mortality in syngeneic rat parabionts of different chronological age. Trans N Y Acad Sci. 1972;34:582–587. doi: 10.1111/j.2164-0947.1972.tb02712.x. [DOI] [PubMed] [Google Scholar]
- 73.Horrington EM, Pope F, Lunsford W, Mc CC. Age changes in the bones, blood pressure, and diseases of rats in parabiosis. Gerontologia. 1960;4:21–31. doi: 10.1159/000210970. [DOI] [PubMed] [Google Scholar]
- 74.Lunsford WR, Mc CC, Lupien PJ, Pope FE, Sperling G. Parabiosis as a method for studying factors which affect aging in rats. Gerontologia. 1963;7:1–8. doi: 10.1159/000211170. [DOI] [PubMed] [Google Scholar]
- 75.McCay CM, Pope F, Lunsford W, Sperling G, Sambhavaphol P. Parabiosis between old and young rats. Gerontologia. 1957;1:7–17. doi: 10.1159/000210677. [DOI] [PubMed] [Google Scholar]
- 76.Kiss T, Nyul-Toth A, Gulej R, Tarantini S, Csipo T, Mukli P, Ungvari A, Balasubramanian P, Yabluchanskiy A, Benyo Z, Conley SM, Wren JD, Garman L, Huffman DM, Csiszar A, Ungvari Z. Old blood from heterochronic parabionts accelerates vascular aging in young mice: transcriptomic signature of pathologic smooth muscle remodeling. Geroscience. 2022;44:953–981. doi: 10.1007/s11357-022-00519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kiss T, Tarantini S, Csipo T, Balasubramanian P, Nyul-Toth A, Yabluchanskiy A, Wren JD, Garman L, Huffman DM, Csiszar A, Ungvari Z. Circulating anti-geronic factors from heterochonic parabionts promote vascular rejuvenation in aged mice: transcriptional footprint of mitochondrial protection, attenuation of oxidative stress, and rescue of endothelial function by young blood. Geroscience. 2020;42:727–748. doi: 10.1007/s11357-020-00180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harris RB. Loss of body fat in lean parabiotic partners of ob/ob mice. Am J Physiol. 1997;272:R1809–R1815. doi: 10.1152/ajpregu.1997.272.6.R1809. [DOI] [PubMed] [Google Scholar]
- 79.Harris RB. Parabiosis between db/db and ob/ob or db/+ mice. Endocrinology. 1999;140:138–145. doi: 10.1210/endo.140.1.6449. [DOI] [PubMed] [Google Scholar]
- 80.Coleman DL. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia. 1973;9:294–298. doi: 10.1007/BF01221857. [DOI] [PubMed] [Google Scholar]
- 81.Coleman DL, Hummel KP. Effects of parabiosis of normal with genetically diabetic mice. Am J Physiol. 1969;217:1298–1304. doi: 10.1152/ajplegacy.1969.217.5.1298. [DOI] [PubMed] [Google Scholar]
- 82.Bitto A, Kaeberlein M. Rejuvenation: it’s in our blood. Cell Metab. 2014;20:2–4. doi: 10.1016/j.cmet.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cannata A, Marcon G, Cimmino G, Camparini L, Ciucci G, Sinagra G, Loffredo FS. Role of circulating factors in cardiac aging. J Thorac Dis. 2017;9:S17–S29. doi: 10.21037/jtd.2017.03.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Conboy MJ, Conboy IM, Rando TA. Heterochronic parabiosis: historical perspective and methodological considerations for studies of aging and longevity. Aging Cell. 2013;12:525–530. doi: 10.1111/acel.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fan X, Wheatley EG, Villeda SA. Mechanisms of hippocampal aging and the potential for rejuvenation. Annu Rev Neurosci. 2017;40:251–272. doi: 10.1146/annurev-neuro-072116-031357. [DOI] [PubMed] [Google Scholar]
- 86.Flemming A. Cardiovascular disease: Rejuvenating the ageing heart. Nat Rev Drug Discov. 2013;12:503. doi: 10.1038/nrd4064. [DOI] [PubMed] [Google Scholar]
- 87.Ghosh AK, O’Brien M, Mau T, Qi N, Yung R. Adipose tissue senescence and inflammation in aging is reversed by the young milieu. J Gerontol A Biol Sci Med Sci. 2019;74:1709–1715. doi: 10.1093/gerona/gly290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gontier G, Iyer M, Shea JM, Bieri G, Wheatley EG, Ramalho-Santos M, Villeda SA. Tet2 Rescues age-related regenerative decline and enhances cognitive function in the adult mouse brain. Cell Rep. 2018;22:1974–1981. doi: 10.1016/j.celrep.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Harrison DE, Astle CM. Loss of stem cell repopulating ability upon transplantation. Effects of donor age, cell number, and transplantation procedure. J Exp Med. 1982;156:1767–79. doi: 10.1084/jem.156.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hirayama R, Takemura K, Nihei Z, Ichikawa W, Takagi Y, Mishima Y, Utsuyama M, Hirokawa K. Differential effect of host microenvironment and systemic humoral factors on the implantation and the growth rate of metastatic tumor in parabiotic mice constructed between young and old mice. Mech Ageing Dev. 1993;71:213–221. doi: 10.1016/0047-6374(93)90085-6. [DOI] [PubMed] [Google Scholar]
- 91.Katsimpardi L, Kuperwasser N, Camus C, Moigneu C, Chiche A, Tolle V, Li H, Kokovay E and Lledo PM. Systemic GDF11 stimulates the secretion of adiponectin and induces a calorie restriction-like phenotype in aged mice. Aging Cell. 2019:e13038. [DOI] [PMC free article] [PubMed]
- 92.Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–634. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rebo J, Mehdipour M, Gathwala R, Causey K, Liu Y, Conboy MJ, Conboy IM. A single heterochronic blood exchange reveals rapid inhibition of multiple tissues by old blood. Nat Commun. 2016;7:13363. doi: 10.1038/ncomms13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sousa-Victor P, Neves J, Cedron-Craft W, Ventura PB, Liao CY, Riley RR, Soifer I, van Bruggen N, Kolumam GA, Villeda SA, Lamba DA, Jasper H. MANF regulates metabolic and immune homeostasis in ageing and protects against liver damage. Nat Metab. 2019;1:276–290. doi: 10.1038/s42255-018-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Despres S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, Smith LK, Bieri G, Lin K, Berdnik D, Wabl R, Udeochu J, Wheatley EG, Zou B, Simmons DA, Xie XS, Longo FM, Wyss-Coray T. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20:659–663. doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang H, Cherian R, Jin K. Systemic milieu and age-related deterioration. Geroscience. 2019;41:275–284. doi: 10.1007/s11357-019-00075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.DeCarolis NA, Kirby ED, Wyss-Coray T and Palmer TD. The role of the microenvironmental niche in declining stem-cell functions associated with biological aging. Cold Spring Harb Perspect Med. 2015;5. [DOI] [PMC free article] [PubMed]
- 99.Middeldorp J, Lehallier B, Villeda SA, Miedema SS, Evans E, Czirr E, Zhang H, Luo J, Stan T, Mosher KI, Masliah E, Wyss-Coray T. Preclinical Assessment of Young Blood Plasma for Alzheimer Disease. JAMA Neurol. 2016;73:1325–1333. doi: 10.1001/jamaneurol.2016.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smith LK, He Y, Park JS, Bieri G, Snethlage CE, Lin K, Gontier G, Wabl R, Plambeck KE, Udeochu J, Wheatley EG, Bouchard J, Eggel A, Narasimha R, Grant JL, Luo J, Wyss-Coray T, Villeda SA. beta2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat Med. 2015;21:932–937. doi: 10.1038/nm.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rodriguez SL, Carver CM, Dosch AJ, Huffman DM, Duke Boynton FD, Ayasoufi K, Schafer MJ. An optimized mouse parabiosis protocol for investigation of aging and rejuvenative mechanisms. Front Aging. 2022;3:993658. doi: 10.3389/fragi.2022.993658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yousefzadeh MJ, Robbins PD, Huffman DM. Heterochronic parabiosis: a valuable tool to investigate cellular senescence and other hallmarks of aging. Aging (Albany NY) 2022;14:3325–3328. doi: 10.18632/aging.204015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Morrison EJ, Champagne DP, Dzieciatkowska M, Nemkov T, Zimring JC, Hansen KC, Guan F, Huffman DM, Santambrogio L and D’Alessandro A. Parabiosis incompletely reverses aging-induced metabolic changes and oxidant stress in mouse red blood cells. Nutrients. 2019;11. [DOI] [PMC free article] [PubMed]
- 104.Tarantini S, Hertelendy P, Tucsek Z, Valcarcel-Ares MN, Smith N, Menyhart A, Farkas E, Hodges E, Towner R, Deak F, Sonntag WE, Csiszar A, Ungvari Z, Toth P. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J Cereb Blood Flow Metab. 2015;35:1871–1881. doi: 10.1038/jcbfm.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kazama K, Anrather J, Zhou P, Girouard H, Frys K, Milner TA, Iadecola C. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ Res. 2004;95:1019–1026. doi: 10.1161/01.RES.0000148637.85595.c5. [DOI] [PubMed] [Google Scholar]
- 106.Faulhaber LD, D’Costa O, Shih AY, Gust J. Antibody-based in vivo leukocyte label for two-photon brain imaging in mice. Neurophotonics. 2022;9:031917. doi: 10.1117/1.NPh.9.3.031917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tarantini S, Balasubramanian P, Delfavero J, Csipo T, Yabluchanskiy A, Kiss T, Nyul-Toth A, Mukli P, Toth P, Ahire C, Ungvari A, Benyo Z, Csiszar A, Ungvari Z. Treatment with the BCL-2/BCL-xL inhibitor senolytic drug ABT263/Navitoclax improves functional hyperemia in aged mice. Geroscience. 2021;43:2427–2440. doi: 10.1007/s11357-021-00440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci. 2014;69:1339–1352. doi: 10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am J Physiol Heart Circ Physiol. 2014;306:H299–308. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guerra G, Lucariello A, Perna A, Botta L, De Luca A and Moccia F. The Role of Endothelial Ca(2+) Signaling in neurovascular coupling: a view from the lumen. Int J Mol Sci. 2018;19. [DOI] [PMC free article] [PubMed]
- 111.Negri S, Faris P, Maniezzi C, Pellavio G, Spaiardi P, Botta L, Laforenza U, Biella G, Moccia DF. NMDA receptors elicit flux-independent intracellular Ca2+ signals via metabotropic glutamate receptors and flux-dependent nitric oxide release in human brain microvascular endothelial cells. Cell Calcium. 2021;99:102454. doi: 10.1016/j.ceca.2021.102454. [DOI] [PubMed] [Google Scholar]
- 112.Donato AJ, Machin DR, Lesniewski LA. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ Res. 2018;123:825–848. doi: 10.1161/CIRCRESAHA.118.312563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Toda N. Age-related changes in endothelial function and blood flow regulation. Pharmacol Ther. 2012;133:159–176. doi: 10.1016/j.pharmthera.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 114.Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Ame J Physiology-Heart Circulatory Physio. 2017;312:H1–H20. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ximerakis M, Holton KM, Giadone RM, Ozek C, Saxena M, Santiago S, Adiconis X, Dionne D, Nguyen L, Shah KM, Goldstein JM, Gasperini C, Gampierakis IA, Lipnick SL, Simmons SK, Buchanan SM, Wagers AJ, Regev A, Levin JZ, Rubin LL. Heterochronic parabiosis reprograms the mouse brain transcriptome by shifting aging signatures in multiple cell types. Nat Aging. 2023;3:327–345. doi: 10.1038/s43587-023-00373-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ashpole NM, Logan S, Yabluchanskiy A, Mitschelen MC, Yan H, Farley JA, Hodges EL, Ungvari Z, Csiszar A, Chen S, Georgescu C, Hubbard GB, Ikeno Y, Sonntag WE. IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience. 2017;39:129–145. doi: 10.1007/s11357-017-9971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, Bass C, Sonntag WE, Ungvari Z, Csiszar A. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:313–329. doi: 10.1093/gerona/glr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bailey-Downs LC, Sosnowska D, Toth P, Mitschelen M, Gautam T, Henthorn JC, Ballabh P, Koller A, Farley JA, Sonntag WE, Csiszar A, Ungvari Z. Growth Hormone and IGF-1 Deficiency Exacerbate High-fat diet-induced endothelial impairment in obese lewis dwarf rats: implications for vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:553–564. doi: 10.1093/gerona/glr197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Breese CR, Ingram RL, Sonntag WE. Influence of age and long-term dietary restriction on plasma insulin-like growth factor-1 (IGF-1), IGF-1 gene expression, and IGF-1 binding proteins. J Gerontol. 1991;46:B180–B187. doi: 10.1093/geronj/46.5.B180. [DOI] [PubMed] [Google Scholar]
- 120.D’Costa AP, Xu X, Ingram RL, Sonntag WE. Insulin-like growth factor-1 stimulation of protein synthesis is attenuated in cerebral cortex of aging rats. Neuroscience. 1995;65:805–813. doi: 10.1016/0306-4522(94)00495-Q. [DOI] [PubMed] [Google Scholar]
- 121.Deak F, Sonntag WE. Aging, synaptic dysfunction, and insulin-like growth factor (IGF)-1. J Gerontol A Biol Sci Med Sci. 2012;67:611–625. doi: 10.1093/gerona/gls118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Farias Quipildor GE, Mao K, Hu Z, Novaj A, Cui MH, Gulinello M, Branch CA, Gubbi S, Patel K, Moellering DR, Tarantini S, Kiss T, Yabluchanskiy A, Ungvari Z, Sonntag WE, Huffman DM. Central IGF-1 protects against features of cognitive and sensorimotor decline with aging in male mice. Geroscience. 2019;41:185–208. doi: 10.1007/s11357-019-00065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Logan S, Pharaoh GA, Marlin MC, Masser DR, Matsuzaki S, Wronowski B, Yeganeh A, Parks EE, Premkumar P, Farley JA, Owen DB, Humphries KM, Kinter M, Freeman WM, Szweda LI, Van Remmen H, Sonntag WE. Insulin-like growth factor receptor signaling regulates working memory, mitochondrial metabolism, and amyloid-beta uptake in astrocytes. Mol Metab. 2018;9:141–155. doi: 10.1016/j.molmet.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mitschelen M, Yan H, Farley JA, Warrington JP, Han S, Herenu CB, Csiszar A, Ungvari Z, Bailey-Downs LC, Bass CE, Sonntag WE. Long-term deficiency of circulating and hippocampal insulin-like growth factor I induces depressive behavior in adult mice: a potential model of geriatric depression. Neuroscience. 2011;185:50–60. doi: 10.1016/j.neuroscience.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Poe BH, Linville C, Riddle DR, Sonntag WE, Brunso-Bechtold JK. Effects of age and insulin-like growth factor-1 on neuron and synapse numbers in area CA3 of hippocampus. Neuroscience. 2001;107:231–238. doi: 10.1016/S0306-4522(01)00341-4. [DOI] [PubMed] [Google Scholar]
- 126.Ramsey MM, Weiner JL, Moore TP, Carter CS, Sonntag WE. Growth hormone treatment attenuates age-related changes in hippocampal short-term plasticity and spatial learning. Neuroscience. 2004;129:119–127. doi: 10.1016/j.neuroscience.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 127.Shi L, Linville MC, Tucker EW, Sonntag WE, Brunso-Bechtold JK. Differential effects of aging and insulin-like growth factor-1 on synapses in CA1 of rat hippocampus. Cereb Cortex. 2005;15:571–577. doi: 10.1093/cercor/bhh158. [DOI] [PubMed] [Google Scholar]
- 128.Sonntag WE, Deak F, Ashpole N, Toth P, Csiszar A, Freeman W, Ungvari Z. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front Aging Neurosci. 2013;5:27. doi: 10.3389/fnagi.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tarantini S, Giles CB, Wren JD, Ashpole NM, Valcarcel-Ares MN, Wei JY, Sonntag WE, Ungvari Z, Csiszar A. IGF-1 deficiency in a critical period early in life influences the vascular aging phenotype in mice by altering miRNA-mediated post-transcriptional gene regulation: implications for the developmental origins of health and disease hypothesis. Age (Dordr) 2016;38:239–258. doi: 10.1007/s11357-016-9943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tarantini S, Tucsek Z, Valcarcel-Ares MN, Toth P, Gautam T, Giles CB, Ballabh P, Wei JY, Wren JD, Ashpole NM, Sonntag WE, Ungvari Z, Csiszar A. Circulating IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: implications for cerebromicrovascular and brain aging. Age (Dordr) 2016;38:273–289. doi: 10.1007/s11357-016-9931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab. 2014;34:1887–1897. doi: 10.1038/jcbfm.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bickel MA, Csik B, Gulej R, Ungvari A, Nyul-Toth A, Conley SM. Cell non-autonomous regulation of cerebrovascular aging processes by the somatotropic axis. Front Endocrinol (Lausanne) 2023;14:1087053. doi: 10.3389/fendo.2023.1087053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kiss T, Nyul-Toth A, Balasubramanian P, Tarantini S, Ahire C, Yabluchanskiy A, Csipo T, Farkas E, Wren JD, Garman L, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation promotes neurovascular rejuvenation in aged mice: transcriptional footprint of SIRT1 activation, mitochondrial protection, anti-inflammatory, and anti-apoptotic effects. Geroscience. 2020;42:527–546. doi: 10.1007/s11357-020-00165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Furon J, Yetim M, Pouettre E, Martinez de Lizarrondo S, Maubert E, Hommet Y, Lebouvier L, Zheng Z, Ali C and Vivien D. Blood tissue Plasminogen Activator (tPA) of liver origin contributes to neurovascular coupling involving brain endothelial N-Methyl-D-Aspartate (NMDA) receptors. Fluids Barriers CNS. 2023;20:11. [DOI] [PMC free article] [PubMed]
- 135.Anfray A, Drieu A, Hingot V, Hommet Y, Yetim M, Rubio M, Deffieux T, Tanter M, Orset C, Vivien D. Circulating tPA contributes to neurovascular coupling by a mechanism involving the endothelial NMDA receptors. J Cereb Blood Flow Metab. 2020;40:2038–2054. doi: 10.1177/0271678X19883599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Park L, Zhou J, Koizumi K, Wang G, Anfray A, Ahn SJ, Seo J, Zhou P, Zhao L, Paul S, Anrather J, Iadecola C. tPA deficiency underlies neurovascular coupling dysfunction by amyloid-β. J Neurosci. 2020;40:8160–8173. doi: 10.1523/JNEUROSCI.1140-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mei W, Xiang G, Li Y, Li H, Xiang L, Lu J, Xiang L, Dong J, Liu M. GDF11 protects against endothelial injury and reduces atherosclerotic lesion formation in apolipoprotein E-null mice. Mol Ther. 2016;24:1926–1938. doi: 10.1038/mt.2016.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall’Osso C, Khong D, Shadrach JL, Miller CM, Singer BS, Stewart A, Psychogios N, Gerszten RE, Hartigan AJ, Kim MJ, Serwold T, Wagers AJ and Lee RT. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–39. [DOI] [PMC free article] [PubMed]
- 139.Yoshida Y-I, Eda S, Masada M. Alterations of tetrahydrobiopterin biosynthesis and pteridine levels in mouse tissues during growth and aging. Brain Develop. 2000;22:45–49. doi: 10.1016/S0387-7604(00)00144-3. [DOI] [PubMed] [Google Scholar]
- 140.Higashi Y, Sasaki S, Nakagawa K, Kimura M, Noma K, Hara K, Jitsuiki D, Goto C, Oshima T, Chayama K, Yoshizumi M. Tetrahydrobiopterin improves aging-related impairment of endothelium-dependent vasodilation through increase in nitric oxide production. Atherosclerosis. 2006;186:390–395. doi: 10.1016/j.atherosclerosis.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 141.Kim D, Kiprov DD, Luellen C, Lieb M, Liu C, Watanabe E, Mei X, Cassaleto K, Kramer J, Conboy MJ, Conboy IM. Old plasma dilution reduces human biological age: a clinical study. Geroscience. 2022;44:2701–2720. doi: 10.1007/s11357-022-00645-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mehdipour M, Mehdipour T, Skinner CM, Wong N, Liu C, Chen C-C, Jeon OH, Zuo Y, Conboy MJ, Conboy IM. Plasma dilution improves cognition and attenuates neuroinflammation in old mice. GeroScience. 2021;43:1–18. doi: 10.1007/s11357-020-00297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mehdipour M, Skinner C, Wong N, Lieb M, Liu C, Etienne J, Kato C, Kiprov D, Conboy MJ, Conboy IM. Rejuvenation of three germ layers tissues by exchanging old blood plasma with saline-albumin. Aging (Albany NY) 2020;12:8790–8819. doi: 10.18632/aging.103418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mehdipour M, Mehdipour T, Skinner CM, Wong N, Liu C, Chen CC, Jeon OH, Zuo Y, Conboy MJ and Conboy IM. Plasma dilution improves cognition and attenuates neuroinflammation in old mice. Geroscience. 2021. [DOI] [PMC free article] [PubMed]
- 145.McGrath ER, Himali JJ, Levy D, Conner SC, Pase MP, Abraham CR, Courchesne P, Satizabal CL, Vasan RS, Beiser AS, Seshadri S. Circulating fibroblast growth factor 23 levels and incident dementia: The Framingham heart study. PLoS ONE. 2019;14:e0213321. doi: 10.1371/journal.pone.0213321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mirza MA, Larsson A, Lind L, Larsson TE. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis. 2009;205:385–390. doi: 10.1016/j.atherosclerosis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 147.Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-TNFalfa treatment in aging. Am J Pathol. 2007;170:388–698. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schafer MJ, Zhang X, Kumar A, Atkinson EJ, Zhu Y, Jachim S, Mazula DL, Brown AK, Berning M, Aversa Z, Kotajarvi B, Bruce CJ, Greason KL, Suri RM, Tracy RP, Cummings SR, White TA and LeBrasseur NK. The senescence-associated secretome as an indicator of age and medical risk. JCI Insight. 2020;5. [DOI] [PMC free article] [PubMed]
- 149.Ogrodnik M, Evans SA, Fielder E, Victorelli S, Kruger P, Salmonowicz H, Weigand BM, Patel AD, Pirtskhalava T, Inman CL, Johnson KO, Dickinson SL, Rocha A, Schafer MJ, Zhu Y, Allison DB, von Zglinicki T, LeBrasseur NK, Tchkonia T, Neretti N, Passos JF, Kirkland JL, Jurk D. Whole-body senescent cell clearance alleviates age-related brain inflammation and cognitive impairment in mice. Aging Cell. 2021;20:e13296. doi: 10.1111/acel.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yousef H, Czupalla CJ, Lee D, Chen MB, Burke AN, Zera KA, Zandstra J, Berber E, Lehallier B, Mathur V, Nair RV, Bonanno LN, Yang AC, Peterson T, Hadeiba H, Merkel T, Korbelin J, Schwaninger M, Buckwalter MS, Quake SR, Butcher EC, Wyss-Coray T. Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1. Nat Med. 2019;25:988–1000. doi: 10.1038/s41591-019-0440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]