Abstract
Functional decline of physiological systems during ageing leads to age-related diseases. Dietary glycine increases healthy lifespan in model organisms and might decrease inflammation in humans, suggesting its geroprotective potential. This review summarises the evidence of glycine administration on the characteristics of eleven physiological systems in adult humans. Databases were searched using key search terms: ‘glycine’, ‘adult’, ‘supplementation’/ ‘administration’/ ‘ingestion’/ ‘treatment’. Glycine was administered to healthy and diseased populations (18 and 34 studies) for up to 14 days and 4 months, respectively. The nervous system demonstrated the most positive effects, including improved psychiatric symptoms from longer-term glycine administration in psychiatric populations. While longer-term glycine administration improved sleep in healthy populations, these studies had small sample sizes with a high risk of bias. Larger and long-term studies with more robust study designs in healthy populations to examine the effects of glycine administration on preventing, delaying or reversing the ageing process are warranted.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-023-00970-8.
Keywords: Ageing, Geroprotector, Glycine, Healthspan, Lifespan, Physiological systems
Introduction
Ageing is a complex biological phenomenon that occurs continuously in an organism with the passage of time; this results in a cumulative molecular damage and manifests as a progressive decline in organ functions of multiple physiological systems [1–4] leading to chronic diseases and disability [5, 6]. Therewith, optimising physiological function throughout the life course is critical to maximise an individual’s healthspan.
“Geroprotectors” are agents that enhance lifespan and healthspan in organisms by addressing the underlying cause of ageing and age-related diseases, thereby preventing, delaying, and/or reversing ageing processes [7]. The anti-cancer and anti-inflammatory effects of glycine have been observed in rodents [8–10]; and studies in humans suggest the potential of glycine supplementation to protect against metabolic diseases [8, 11], particularly by counteracting oxidative stress and inflammation [12, 13]. On the other hand, a chronic lack of glycine may impede growth, immune responses, and nutrient metabolism [14–16]. In animal models, glycine administration has been reported to extend the lifespan of C. elegans by up to 33% [17, 18], of rats by approximately 20% [19], and mice by 6% [20]. Given that glycine is inexpensive and likely safe for administration through oral supplementation, it is important to study its potential lifespan and healthspan enhancing properties as a geroprotector.
The physiological implications of glycine administration at the organ system level in human adults have not been comprehensively assessed. Hence, the aim of this systematic review is to summarise the effects of glycine administration on characteristics of physiological systems in adult humans.
Methods
Search strategy
This systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO; CRD42022312730) and conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Four electronic databases were searched from date of inception to 29 April 2022: Embase, PubMed, Web of Science, and Cochrane Central Register of Controlled Trials. The search strategy was developed with the assistance of a senior tertiary librarian from the National University of Singapore, with expertise in research and search strategies. Search terms included but were not limited to: ‘glycine’, ‘adult’, ‘supplementation’/ ‘administration’/ ‘ingestion’/ ‘treatment’. Snowballing was used to search references within identified articles.
Eligibility criteria
All study designs were considered. The inclusion criteria constituted the following: 1) population – adults (males and/or females) with a mean and/or median age of 18 years old and above; 2) intervention – administration of glycine in any combination of dose and medium through all reported routes (except topical administration), and independent of a placebo or control group were considered; 3) comparator(s)/control – glycine administration as the intervention compared with a placebo or no intervention, where applicable; 4) outcomes – characteristics of eleven physiological systems, which include the following systems: i) endocrine and metabolic, ii) nervous, iii) cardiovascular, iv) immune, v) digestive, vi) muscular, vii) renal, viii) reproductive, ix) integumentary, x) skeletal, and xi) respiratory.
Articles were excluded according to the following criteria: 1) animal and/or in vitro studies; 2) conference abstract, review, editorial, case reports, or letter to the editor; 3) studies investigating the following compounds: i) combined administration of glycine and another compound as an intervention, ii) precursors of glycine; glycine analogues; glycine derivatives; glycine (by-) products and intermediates; 4) studies investigating glycine administered topically, as a tracer compound, as an irrigation solution/fluid, and to solely measure pharmacokinetics and/or pharmacodynamics; 5) studies published in a non-English language; and 6) where full-text articles cannot be obtained.
Article selection and data extraction
Two reviewers (JS, SR or JL) independently screened titles, abstracts, and full text articles for inclusion. Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia) was used to screen the articles. Disagreements between reviewers were resolved by a third reviewer (JG). Data extraction was conducted independently by two authors (JS, SR or JL) and the following variables were extracted: author; year of publication; study design (e.g. randomised controlled trial, open label clinical trial, observational study, number of individuals per study arm, total sample size, dose and medium of glycine administration, intervention duration, and baseline conditions), population characteristics (age and sex); health status (e.g. healthy or diseased) and any reported changes in the characteristics of the aforementioned physiological systems.
Data analysis
The outcomes of glycine administration were presented in a descriptive fashion in which a significant change was considered for outcomes reported with p < 0.05 in the following instances: i) pre-post outcomes of glycine administration, and ii) glycine administered as the intervention compound vs. placebo/control comparator(s) at the end of the intervention period. Extracted outcomes for each reported population was then stratified according to the eleven physiological systems and health condition (healthy or diseased). Whether these changes implied an overall positive or negative effect on the respective physiological system(s) was based on the significant change (p < 0.05) in at least one of the measured characteristics of a physiological system. Where both positive and negative effects had been reported for a physiological system, the overall effect of glycine administration on the physiological system was considered to have mixed effects. Where these changes did not imply (a) positive or negative effect(s) on a physiological system, these outcomes were considered inconclusive to the overall effect on the physiological system. Studies determining the short-term effect of a single bolus of glycine within a day and the longer-term effect over a period longer than a day were separated.
Risk of bias
The risk of bias was assessed by two reviewers (JS, SR or JL). The revised Cochrane risk of bias tool for randomised trials [Cochrane risk of bias tool 2.0 (ROB 2)] was used to assess the risk of bias for randomised parallel-group [21] and crossover trials [22]. The tool used for randomised parallel group trials is based on five key sources of bias, namely: 1) the randomisation process, 2) deviations from intended intervention, 3) missing outcome data, 4) measurement of the outcome, and 5) selection of the reported result. For randomised crossover trials, the tool additionally includes bias arising from period and carryover effects. The risk of bias was categorised into “low risk”, “some concerns” and “high risk”. For non-randomised studies the Risk of Bias In Non-Randomised Studies – of Interventions (ROBINS-I) was employed [23]. This tool comprises seven key domains of bias: 1) confounding; 2) selection of participants; 3) classification of intervention; 4) deviation from interventions; 5) missing outcome data; 6) measurement of outcomes; and 7) selection of reported result overall. The risk of bias using ROBINS-I was rated accordingly: 0 – no information; 1 – low risk; 2 – moderate risk; 3 – serious risk; and 4 – critical risk.
Results
Study selection and characteristics of included studies
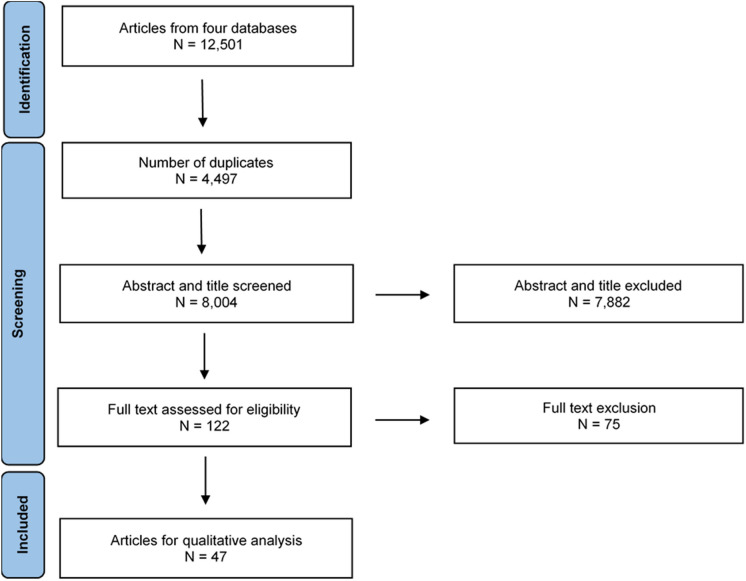
The article selection process is presented in Fig. 1. After excluding 4,497 duplicates, 8,004 articles underwent title and abstract screening, of which 122 progressed to full-text screening and 47 articles describing 50 studies were included. Table 1 shows a comprehensive overview of the included articles. Most studies (42/50) were randomised controlled trials (RCT), of which half were parallel-group trials. The majority of studies (41/50) reported oral glycine ingestion as the mode of delivery. Eighteen out of 50 studies were in healthy populations, 34/50 in diseased populations, and 2/50 contained both healthy and diseased populations. The mean or median age ranged from 21.5 to 41.4 years for healthy populations and 29.5 to 67 years for diseased populations. Glycine was administered for a period of one day (single bolus) to 14 days in healthy populations and up to 4 months in diseased populations. Figure 2 and Supplementary Table A provide a summary of the effects of glycine administration in healthy and diseased populations stratified by physiological systems and study types.
Fig. 1.
Schematic overview of article selection process
Table 1.
Articles describing glycine administration in humans stratified by characteristics of physiological systems measured
| First author (year) | Population | Study type | Age (y) | Sex (%F) | Sample size (n) | Glycine duration, dose, delivery, medium | Comparator(s) | |
|---|---|---|---|---|---|---|---|---|
| Glycine Comparator(s) | ||||||||
| RCT (//) | ||||||||
| Endocrine & Metabolic system | ||||||||
| González-Ortiz (2001) [24] | Healthy first-degree relatives of T2DM patients | RCT (//) | Gly: 23.7(4.1) Comparator: 24.7(8.0) | 67 | 6 | 6 | Single bolus (short-term), 5 g (30 min before test), O, NR | Placebo (Magnesium oxide) |
| Endocrine & Metabolic + Cardiovascular systems | ||||||||
| Díaz-Flores (2013) [12] | Metabolic Syndrome | RCT (//) | Glycine*: 47.5 (8.2) Comparator*: 46.9 (7.8) | 56* | 30 | 30 | 3 m, 5 g X 3/d (15 g/d), O, glycine powder dissolved in water | Placebo (starch) dissolved in water |
| Endocrine & Metabolic + Cardiovascular + Immune systems | ||||||||
| Cruz (2008) [13] | T2DM | RCT(//) | 58.5 (10) | 54 | 38 | 36 | 3 m, 5 g X 3/d (15 g/d), O, powder dissolved in water | Placebo (starch powder dissolved in water) |
| Endocrine & Metabolic + Immune + Renal systems | ||||||||
| Daly (1988) [25] | GI malignancies | RCT (//) | Glycine: 62 (10) Comparator: 66 (8) | 20 | 14 | 16 | 7d, 43 g X 1/d (43 g/d), E, L-glycine-supplemented enteral diet (solution) | L-arginine supplemented enteral diet (solution) |
| Nervous system | ||||||||
| Aliyev (2005) [26] | Alcohol hallucinosis | RCT (//) | Glycine: 42 (6.0) Comparator: 41.0 (5.0) | 0 | 20 | 20 | 7d, 700 mg/d, S, glycine tablets | Placebo (NR) |
| Greenberg (2009) [27] | Obsessive compulsive disorder | RCT (//) | Glycine: 44.2 (14.3) Comparator: 36.1 (12.2) | 63 | 12 | 12 | 12w, 30 g X 2/d (60 g/d), O, glycine powder dissolved in water or juice | Placebo (dextrose, fructose, fine granular citric acid, orange flavouring and ProSweet™ flavour enhancer dissolved in water or juice) |
| Greenwood (2018) [28] |
Schizophrenia/ Schizoaffective disorder; |
RCT (//) |
37.8 (8.4) | 57 | 12 | 10 | 6w, start @ 0.2 g/kg/d (0.2 g/kg X 2/d after 7d; 0.2 g/kg X 3/d after 14d onwards), O, NR | Placebo (NR) |
| Potkin (1999) [29] | Schizophrenia | RCT (//) |
Glycine: 35.3 (5.26) Comparator: 34.4 (4.75) |
12.5 | 12 | 12 | 12w, 10 g X 3/d after meals (30 g/d), O, solution of glycine dissolved in 1 oz water | Placebo (similar-tasting solution) |
| Javitt (1994) [30] | Schizophrenia |
i) RCT (//) ii) Open label trial |
i) Glycine: 36 (9.7) ii) Comparator: 38.1 (7.2) |
i) 0 ii) NR |
i) 7 ii) 15 |
i) 7 ii) NA |
i) 8w, start at 2 g/d to maximum dose (0.4 g/kg bw – approx. 30 g/d) during first 2w, O, glycine powder dissolved in juice ii) 8w, 0.4 g/kg bw (approx. 30 g/d), O, glycine powder dissolved in juice |
i) Placebo (taste-matched) ii) NA |
| Serrita (2019) [31] | Schizophrenia and alcohol dependence |
RCT (//) |
Glycine: 49.2 (4.84) Comparator: 48.6 (5.01) |
0 | 10 | 10 | 12w, 0.8 g/kg, O, glycine powder mixed in solution | Placebo (powder mixed in solution) |
| Nervous + Cardiovascular systems | ||||||||
| Gusev (2000) [32] | Acute ischaemic stroke |
RCT (//) |
63.7 (10.1)* | 45* |
0.5 g/d: 53 1.0 g/d: 53 2.0 g/d: 53 |
53 | 5d, 0.5 or 1.0 or 2.0 g/d, S, tablet | Placebo tablet (similar in appearance & taste) |
| Cardiovascular system | ||||||||
| Khan (2006) [33] | Obstructive CAD | RCT (//) | 61.1 | 21 | 111 | 112 | 6 m, 297.9 g X 2/d (595.8 g/d), O, glycine dissolved in water | Starch powder dissolved in water |
| Immune + Integumentary systems | ||||||||
| Peng (2006) [34] | Severe burn | RCT (//) |
Patients: 36.5 (11.4); 18 – 60 |
40 | 23 | 25 | 14d, 0.5 g/kg/d, oral or tube feeding, granules | Glutamine granules (oral or tube feeding) |
| Immune + Renal systems | ||||||||
| D’Angelo (2016) [35] | Early preeclampsia |
RCT (//) |
Glycine*: 32.7 (4.8) Comparator: 31.1 (4.3) |
100 | 20 | 20 | Up to 7d, daily bolus of 60 ml injectable water containing 1% glycine, NR, solution | Antithrombin dissolved in 60 ml injectable water |
| Immune + Digestive systems | ||||||||
| Den Hond (1999) [36] | Crohn’s disease | RCT (//) |
Glycine: 38.2 (13.4) Comparator: 25.0 (7.9) |
All: 71 Glycine: 57 Comparator: 86 |
7 | 7 |
4w, 7 g X 3/d (21 g/d), O, glycine powder dissolved in water |
Glutamine powder dissolved in water |
| Leite (2013) [37] | HIV/AIDS | RCT (//) |
All: 37.3 (3.0) Glycine: 40.1 (1.9) Comparator: 34.2 (1.7) |
22 | 24 | 22 | 10d, 25 g/d, O, 50 ml solution of orange juice enriched with glycine | Alanyl-glutamine in 50 ml solution of orange juice |
| Immune system | ||||||||
| Shabert (1999) [38] | HIV/AIDS |
RCT (//) |
Glycine*: 42; 33 – 53 Comparator*: 40; 30–50 |
All*: 10 Glycine*: 11 Compar-ator*: 8 |
9* | 12* | 12w, 40 g/d in 4 divided doses, NR, NR | L-glutamine + antioxidants (ascorbic acid, α-tocopherol, β-carotene, selenium, N-acetyl cysteine) |
| Digestive + Muscular systems | ||||||||
| Buchman (1999) [39] | Marathon runners | RCT (//) | 25 – 49 |
All: 39 Gly: 20 Arg: 38 |
17 | 17 | 14d, 10 g X 3/d (30 g/d), O, glycine dissolved in water or orange juice | L-arginine dissolved in water or orange juice |
| Digestive system | ||||||||
| Manir (2014) [40] | Nonmetastatic pelvic malignancy | RCT (//) |
Glycine*: 56.2 (9.6) Comparator*: 57.2 (8.1) |
66 | 43* | 42* | NR, NR (given 1 h prior radiation), O, NR | Glutamine granules dissolved in 100 ml fruit juice |
| Bushen (2004) [41] | HIV/AIDS | RCT (//) |
All: 36 (6); Median: 36; 23 – 52 |
29 | 9 |
Glutamine: 11 Lo Ala-Gln: 11 Hi Ala-Gln: 10 |
7d, 46 g/d (spectrum), O, NR |
i) Gln (30 g Gln + 15 g glycine/d) or, ii) Lo Ala-Gln (4 g Ala-Gln + 42 g glycine/d) or, iii) Hi Ala-Gln (44 g Ala-Gln/d) |
| Integumentary system | ||||||||
| Peng (2005) [42] | Severe burn | RCT (//) |
36.5 (11.4); 18 – 60 |
40 | 23 | 25 | 14d, 0.5 g/kg/d, oral or tube feeding, granules | Glutamine granules (oral or tube feeding) |
| RCT (X) | ||||||||
| Endocrine & Metabolic system | ||||||||
| Gannon (2002) [43] | Healthy | RCT (X) | 21 – 52 | 44 | 9 | 9 |
Single bolus (short-term) over 120 min, 1 mmol/kg lean bm, O |
i) Water, or ii) 25 g glucose, or, iii) 1 mmol glycine/kg lean bm + 25 g glucose |
| Endocrine & Metabolic + Immune + Digestive systems | ||||||||
| Genton (2021b) [44] | Chronic haemodialysis with PEW | RCT (X) |
Total*: 61.2 (13.7) |
36* | 37 | 37 | 4 m, 7 g X 2/d (14 g/d), O, granules | BCAA granules |
| Endocrine & Metabolic + Immune + Digestive + Muscular + Renal + Skeletal systems | ||||||||
| Genton (2021a) [45] | Chronic haemodialysis with PEW | RCT (X) |
Total*: 61.2 (13.7) BCAA-Glycine*: 63.3 (13.4) Glycine-BCAA*: 58.6 (14.2) |
36* | 37 | 37 | 4 m, 7 g X 2/d (14 g/d), O, granules | BCAA granules |
| Endocrine & Metabolic + Nervous systems | ||||||||
| Munts (2009) [46] | CRPS with dystonia | RCT (X) | 41{34 – 51} | 95 | 19 | 19 | 4w, 21 mg/ml, IT, solution | Placebo (0.9% sodium chloride IT solution) |
| Nervous system | ||||||||
| Bannai (2012) [47] | Healthy | RCT (X) |
41.4; 30 – 61 |
0 | 10 | 10 | 3 consecutive nights, 3 g/d (30 min before bedtime), O, flavoured glycine | Flavoured placebo (reduced form of malt sugar) |
| O’Neill (2011) [48] | Healthy | RCT (X) |
23 (4.1); 18 – 45 |
0 | 16 | 16 | Single bolus (short-term), 0.8 g/kg, O, glycine mixed with 200 ml orange juice | Placebo (flour powder mixed with 200 ml orange juice to mimic texture of glycine treatment) |
| Palmer (2008) [49] | Healthy | RCT (X) | 23.15 (4.26); 19 – 36 | 0 | 13 | 13 | Single bolus (short-term), 0.8 g/kg, O, glycine powder dissolved in 200 ml orange juice | Placebo (flour powder mixed with 200 ml orange juice) |
| Leung (2007) [50] | Healthy | RCT (X) |
23 (4.1); 19 – 36 |
0 | 16 | 16 | Single bolus (short-term), 0.8 g/kg bw, O, mixed with 200 ml orange juice | Flour mixed with 200 ml orange juice |
| O’Neill (2007) [27] | Healthy | RCT (X) |
23 (4.1); 19 – 36 |
0 | 16 | 16 | Single bolus (short-term) over 90 min, 0.8 g/kg bw, O, glycine powder mixed with 200 ml orange juice | Placebo (flour powder mixed with 200 ml orange juice) |
| Yamadera (2007) [51] | Healthy | RCT (X) |
40.5 (10.1); 30 – 57 |
73 | 11 | 11 | 2 consecutive nights, 3 g/d (1 h before bedtime), O, flavoured glycine | Flavoured placebo (reduced form of malt sugar) |
| Neumeister (2006) [52] | Healthy | RCT (X) | 28.5 (10.5) | 33* | 13 | 13 | Single bolus (short-term), 200 mg/kg bw over 45 min, IV infusion | Placebo (saline solution) |
| Inagawa (2006) [53] | Dissatisfaction with sleep | RCT (X) |
31.1; 24 – 53 |
100 | 19 | 0 | 4d, 3 g/d (1 h before bedtime), O, flavoured glycine (medium NR) | Placebo (flavoured) |
| Heresco-Levy (2004b) [54] | Schizophrenia | RCT (X) | 44.7 (10.8) | 24 | 17 | 17 | 6w, initiated at 4 g/d (↑ 4 g/d until 0.8 g/kg bw/d after 10d – 17d) in 3 divided doses, O, glycine powder dissolved in water (20% solution) | Placebo (glucose solution) |
| Heresco-Levy (2004a) [55] | Schizophrenia | RCT (X) | 42.4 | 41 | 17 | 17 | 6w, start at 4 g/d (↑ 4 g/d until 0.8 g/kg bw/d) in 3 divided doses, O, glycine powder dissolved in water | Placebo (glucose solution) |
| Javitt (2001) [56] | Schizophrenia | RCT (X) | 39.6 (5.5) | 33 | 12 | 12 | 6w, 0.8 g/kg/d, O, glycine powder dissolved in orange juice | Placebo (glucose) dissolved in orange juice |
| Heresco-Levy (1999) [57] | Schizophrenia | RCT (X) | 38.8 (11.0) | 45 | 22 | 22 | 6w, initiated at 4 g/d (↑ 4 g/d until 0.8 g/kg bw/d after 9d – 19d) in 3 divided doses, O, glycine powder dissolved in water (20% solution) | Placebo (glucose solution) |
| Heresco-Levy (1996) [58] | Schizophrenia | RCT (X) |
41.4* 22 – 60* |
55* | 12 | 12 | 6w, start at 4 g/d (↑ 4 g/d until 0.8 g/kg bw/d) in 3 divided doses, O, glycine powder dissolved in water | Placebo (glucose solution) |
| Digestive system | ||||||||
| Amin (2018) [59] |
i) Healthy ii) Healthy |
i) RCT (X) ii) RCT (X) |
i) 39.4 (11.4) ii) 36.0 (10.8) |
i) 86 ii) 89 |
i) 7 ii) 9 |
i) 7 ii) 9 |
For both studies: Single bolus (short-term), 17.1 mmol, O, hypromellose capsules |
For both studies: i) L-arginine hydrocholoride in hypromellose capsules or, ii) empty hypromellose capsules (vehicle) |
| Luiking (1998) [60] | Healthy | RCT (X) | 24.2 (4.1) | 0 | 10 | 10 | 8d, 13 g/d over 4 doses, O, solution | Placebo (glucose + chloride) solution |
| Muscular system | ||||||||
| Antonio (2002) [61] | Resistance-trained | RCT (X) | 21.5 (0.3) | NR | 6 | 6 | Single bolus (short-term), 0.3 g/kg, O, glycine mixed with 250 ml calorie-free fruit juice |
i) Glutamine mixed with calorie-free fruit juice, or ii) Placebo (calorie-free fruit juice only) |
| Non-randomised trials | ||||||||
| Endocrine & Metabolic systems | ||||||||
| Kasai (1980) [62] | Non-obese normal | Open-label trial (//) | 18 – 46 | 48 | 25 | NA | Single bolus (short-term) over 150 min, 4 g or 8 g, IV; single bolus (short-term) over 180 min, 12 g, IV | NA |
| Kasai (1978) [63] |
i) Non-obese normal; gastroduodenal anastomosis (partially gastrectomied) ii) Non-obese normal; non-obese diabetics |
i) Open-label trial ii) Open-label trial |
i) 20 – 70 ii) 20 – 70 |
i) 39 ii) 47 |
i) 31 ii) 15 |
i) NA ii) NA |
i) Single bolus (short-term) over 180 min, 250 ml 0.3 M, O ii) Single bolus (short-term) over 180 min, 250 ml 0.3 M, ID |
i) NA ii) NA |
| Nervous + Cardiovascular + Renal + Reproductive systems | ||||||||
| Sugaya (2021) [64] | Overactive bladder | Pilot (//) | 67 (16) | 20 | 20 | 20 | 4w, 3 g X 2/d (6 g/d), O, NR | Placebo (glucose) |
| Nervous system | ||||||||
| Truong (1988) [65] | Myoclonus | Open-label trial; crossov-er trial |
38; 18 – 58 |
NR | 7 | NA | Up to 9w, start @ 200 mg X 3/d (600 mg/d) then ↑ daily by 300 mg until therapeutic effect achieved or up to max dose 6 g/d in 3 divided doses over 6w, O, capsule | NA |
| Strzelecki (2011) [66] | Schizophrenia | Open-label trial |
32.3 (8.8)*; Median: 29.5; 20 – 50* |
45* | 32 | NA | 6w, 0.8 g/kg bm/24 h/3 doses, O, glycine crystillizate dissolution in approx. ½ glass of water or orange juice | NA |
| Javitt (1994) [30] | Schizophrenia |
i) RCT (//) ii) Open label trial |
i) Glycine: 36 (9.7) ii) Comparator: 38.1 (7.2) |
i) 0 ii) NR |
i) 7 ii) 15 |
i) 7 ii) NA |
i) 8w, start at 2 g/d to maximum dose (0.4 g/kg bw – approx. 30 g/d) during first 2w, O, glycine powder dissolved in juice ii) 8w, 0.4 g/kg bw (approx. 30 g/d), O, glycine powder dissolved in juice |
i) Placebo (taste-matched) ii) NA |
| Rosse (1989) [67] | Chronic psychotic disorder | Open-label trial |
38; 30 – 68 |
0 | 6 | NA | 4d – 8w, 10.8 g in 3 divided doses daily, O, glycine capsule | NA |
Age data are presented as: mean (SD); or median {IQR}
(X) crossover trial, (//) parallel trial, (*) value(s) given only for participants who completed trial and/or included in analysis, AIDS acquired immune deficiency syndrome, Arg arginine, BCAA branched-chain amino acid, bm body mass, bw body weight, d day, CAD coronary artery disease, DCS D-cycloserine, E enteral, F female, g gram, GI gastrointestinal, Gly glycine, Hi high dose, HIV human immunodeficiency virus, I inhalation, ID intraduodenal, IT intrathecal, IV intravenous, Lo Low dose, O oral ingestion, PEW protein energy wasting, NA not applicable, NR not reported, m month, RCT randomised controlled trial, T2DM type 2 diabetes mellitus, w week, y years
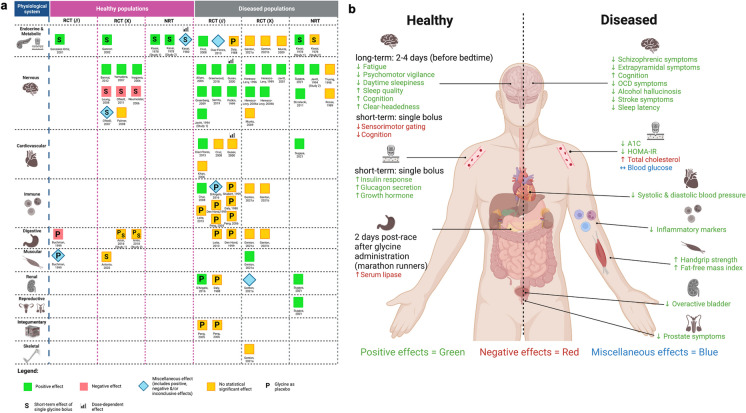
Fig. 2.
Summary of the effects of glycine administration in healthy and diseased populations. A Overall effects of administering glycine in healthy and diseased populations stratified by physiological systems and study types. B Summary of changes in characteristics on each physiological system in healthy and diseased populations with glycine administration. NRT, non-randomised trial; RCT (X), crossover randomised controlled trial; RCT (//), parallel-designed randomised controlled trial. Created with BioRender.com
Endocrine & metabolic systems
In healthy populations, 5/5 studies reported changes in endocrine and metabolic system where a single oral glycine bolus improved insulin responses [24, 43], and increased circulating concentrations of glucagon [43] and growth hormone [63]. In a non-obese healthy population, an inconclusive dose-dependent outcome was observed when a single intravenous (IV) administration of 4 g glycine increased serum growth hormone concentrations, while 12 g glycine increased serum blood sugar levels [62]. In diseased populations, oral glycine administration of 5 g X 3/day over 3 months showed positive effects in type 2 diabetes mellitus (T2DM) patients, including decreased glycosylated haemoglobin (A1C) (%), Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) and fasting blood glucose [13]; while the same dose over 3 months decreased A1C (%), but increased fasting blood glucose and total cholesterol levels when compared to baseline in metabolic syndrome (MetS) patients [12]. The latter study also reported miscellaneous effects that differed between males and females when glycine was compared to placebo administration instead, including: i) increased blood levels of glucose and high-density lipoprotein (HDL) in females; and ii) increased blood levels of total cholesterol, HDL, and systolic blood pressure; and decreased blood levels low-density lipoprotein and A1C (%) [12]. In gastroduodenal anastomosis patients, 0.3 M of a single oral or intraduodenal glycine bolus administration also increased circulating growth hormone concentrations [63] (Table 1, Fig. 2, Supplementary Table A).
Nervous system
The nervous system was examined in healthy populations in eight studies [47–53, 68]. Improved sleep quality, alertness and cognition, and decreased fatigue and sleepiness was observed in three populations receiving 3 g/day oral administration of glycine 30 min – 1 h before bedtime over 2 – 4 days [47, 51, 53]. Higher single bolus of 0.8 g/kg body weight orally or 200 mg/kg body weight intravenously showed negative effects on sensorimotor gating and cognitive performance in healthy populations [48, 50, 52]. Of the eight studies in healthy populations, one reported inconclusive effects with glycine administration [68], while another reported a statistically insignificant effect [49]. In diseased populations, 15/18 studies reported significant positive effects with glycine administration [26–32, 54–58, 64, 66], especially in psychiatric populations where oral glycine administration of 0.2 – 0.8 g/kg body weight daily over 6 – 12 weeks improved schizophrenic [28–31, 54–58, 66]/psychiatric symptoms, extrapyramidal symptoms and cognition. In overactive bladder patients, sleep latency decreased with 3 g X 2/day of oral glycine over 4 weeks [64] (Table 1, Fig. 2, Supplementary Table A).
Cardiovascular system
The cardiovascular system was not assessed in healthy populations. In diseased populations, positive effects included decreased systolic blood pressure with an oral glycine dose of 5 g X 3/day over 3 months in MetS patients [12], and with an oral glycine dose of 3 g X 2/day over 4 weeks in overactive bladder patients [64] (Table 1, Fig. 2, Supplementary Table A).
Immune system
The immune system was not assessed in healthy populations. In T2DM patients, significant positive immune system effects were observed after 3 months of 5 g X 3/day oral glycine ingestion, including decreased proinflammatory cytokines such as interleukin-6 (IL-6), interferon-gamma (IFN-γ), tumour necrosis factor- receptor 1 (TNF-RI), resistin, and interleukin-1 beta (IL-1β) [13] (Table 1, Fig. 2, Supplementary Table A).
Digestive system
In a healthy population of marathon runners, 10 g X 3/day oral glycine over 14 days prior to a marathon run showed significantly higher post-race serum lipase concentrations compared to baseline, suggesting mildly ameliorated pancreatic injury [39] (Table 1, Fig. 2, Supplementary Table A).
Muscular system
In a healthy population of marathon runners, 10 g X 3/day oral glycine over 14 days prior to a marathon run showed significant pre-post effects with increased 2 days post-race serum creatinine phosphokinase (CPK) concentrations (used as a surrogate marker for muscle injury) [39]. However, this rise in serum CPK concentrations was attributed to skeletal muscle trauma induced from marathon running; and it was concluded that glycine administration was not useful in preventing skeletal muscle injury [39]. No significant effect on upper and lower body strength was reported in resistance-trained adults with a single oral glycine bolus of 0.3 g/kg body weight 1 h prior to assessment [61]. Patients undergoing chronic haemodialysis with protein energy wasting (PEW) showed positive effects, including improvements in handgrip strength and fat-free mass index following oral glycine administration of 7 g X 2/day over 4 months [45] (Table 1, Fig. 2, Supplementary Table A).
Renal system
The renal system was not assessed in healthy populations. In diseased populations, positive effects included mitigation of symptoms in patients with overactive bladder administered with 3 g X 2/day of oral glycine over 4 weeks; and decreased daily proteinuria in early preeclampsia patients administered with placebo 1% glycine solution for up to 7 days [35]. Although increased levels of pre-dialysis urea and normalised protein catabolic rate (nPCR) were reported in chronic haemodialysis patients with PEW following oral glycine administration of 7 g X 2/day over 4 months, these results were attributed to the patients’ compliance to glycine treatment [45] (Table 1, Fig. 2, Supplementary Table A).
Reproductive system
The reproductive system was not assessed in healthy populations. In a diseased population with overactive bladder [64], positive effects on prostate symptoms, including nocturia and urinary urgency, were reported at a dose of 3 g X 2/day over 4 weeks [64] (Table 1, Fig. 2, Supplementary Table A).
Integumentary system
The integumentary system was not assessed in healthy populations. In diseased populations, both studies assessed the integumentary system in the same population with severe burn [34, 42]. No significant on the area and depth of burns was observed with oral glycine of 0.5 g/kg/day over 14 days [34, 42] (Table 1, Fig. 2, Supplementary Table A).
Skeletal system
The skeletal system was not assessed in healthy populations. Bone mineral density did not change in chronic haemodialysis patients with PEW given 7 g × 2/day of oral glycine administration over 4 months compared to baseline [45] (Table 1, Fig. 2, Supplementary Table A).
Respiratory system
The respiratory system was not assessed in healthy or diseased populations.
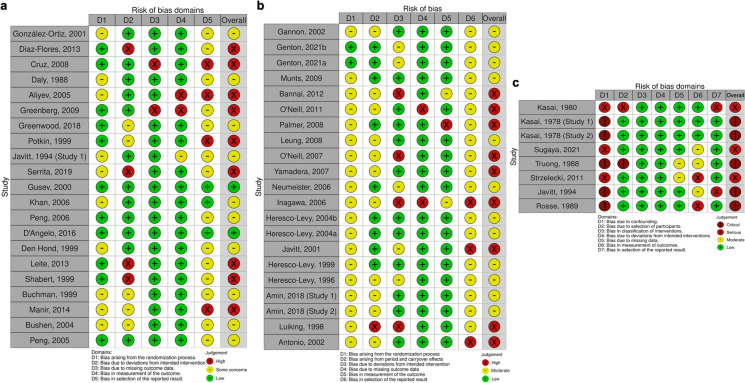
Risk of bias across studies
Figure 3a presents the Cochrane risk of bias ratings for parallel-designed RCTs. The majority of the studies were classified as either some concerns or high for overall risk of bias; with 10/21 and 9/21 of parallel-group RCTs, respectively; with the remaining 2/21 studies classified as having a “low” overall risk of bias [32, 35]. Figure 3b shows the Cochrane risk of bias ratings for crossover-designed RCTs. These studies were classified as either “some concerns” or “high” overall risk, by comprising of 12/21 and 9/21 of crossover-designed RCTs, respectively. Most of these studies were rated as “some concerns” for the domains “bias arising from the randomisation process” and “bias in selection of the reported result”; hence, none of the crossover studies was classified as having an overall “low” risk of bias. Figure 3c shows the Cochrane risk of bias ratings for non-randomised studies of interventions. The majority of these studies were open-label studies, and were either classified as “critical” (5/8) or “serious” (3/8) overall of bias. This result was attributed to all of these studies being rated as “critical” or “serious” risk for the domain “bias due to confounding”.
Fig. 3.
Overview of risk of bias based on the Cochrane risk of bias for included human studies. a ROB2 for RCT parallel group trials. b ROB2 for RCT crossover trials. c ROBINS-I for non-randomised trials
A meta-analysis combining the extracted data to ascertain the overall effect of glycine administration on the characteristics for each physiological system could not be performed due to the large heterogeneity and nature of reported outcomes and statistical presentation of the data.
Discussion
Glycine administration may improve the characteristics of multiple physiological systems, but there is limited evidence supporting their preventative effect for healthy populations. The majority of the physiological systems demonstrated significant positive effects on glycine that were mostly related to the nervous system with longer-term glycine administration, especially in diseased populations afflicted with psychiatric illnesses such as Schizophrenia. The positive effects reported on healthy populations included improved sleep and decreased daytime fatigue [47, 51, 53] and improved insulin responses [24, 43]. On the other hand, negative effects were mainly reported in studies giving a higher glycine dose in a single bolus [48, 50, 52]. This disparity in outcomes in studies on healthy populations may be attributed to variation in dosages and intervention periods.
Nutritional studies have highlighted that the amount of glycine available in humans and animals is inadequate to satisfy metabolic requirements, suggesting the need for dietary glycine supplementation [8, 14, 69, 70]. Several lines of evidence support the hypothesis of accelerated ageing in this psychiatric disorder which reduces the average lifespan of patients by 15 to 20 years compared with the general population [71, 72]. Besides increased mortality risk, Schizophrenia shares risk factors with other age-related conditions such as cognitive decline, metabolic abnormalities, and cardiovascular ageing [73]. However, the included studies on Schizophrenic populations in the present review have thus far only measured the effects of glycine administration on the nervous system, raising the issue of whether benefits may be observable in other physiological systems in this context.
Improved psychiatric symptoms in populations afflicted with psychiatric diseases, were accompanied by improved cognition [57, 58, 66] and extrapyramidal symptoms [54]. Moreover, in chronic haemodialysis patients with PEW, improvements were observed in handgrip strength and fat-free mass index [45]. Considering the involvement of the skeletal muscle in movement, together, it is plausible that these effects may, in turn, positively influence a host of functions associated with multiple physiological systems. Patients with sarcopenia, often have cognitive impairment associated with a decline in muscle strength, mass and function [74], along with other metabolic conditions such as diabetes mellitus [75] and metabolic syndrome [76].
Schizophrenia is hypothesised to result from the hypofunctioning of NMDA receptors [77]. Several reports cited herein have particularly underscored the potential effect of glycine on the N-methyl-D-aspartate (NMDA) receptor in eliciting positive neurological outcomes. Consistent with this notion, brain ageing is associated with the reduced NMDA receptor function, in turn leading to declined memory and learning performance [78]. Stimulation of glycine binding to NMDA receptors have been shown to ameliorate extrapyramidal symptoms of neuromuscular function [79] and a study has shown that glycine administration could improve extrapyramidal and cognitive symptoms in Schizophrenic patients [54]. NMDA receptors have also been implicated in other age-related diseases such as diabetes [80] and hypertension [81]. In healthy populations, oral glycine administration before bedtime has been shown to improve sleep quality through the action of glycine on NMDA receptors in the suprachiasmatic nucleus (SCN), the master circadian pacemaker, by promoting hypothermia and vasodilation [47]. Therefore, one possible mechanism by which glycine may confer its geroprotective effects may be through its action on NMDA receptors; although further research is required to understand the chronotherapeutic and tissue-specific effects of such an interaction and the interplay between multiple physiological systems in healthy and diseased populations.
Strength and limitations
This systematic review is the first to evaluate the effects of glycine on multiple physiological systems in adult humans; and is key in informing and substantiating health claims related to glycine.
In assessing the risk of bias across studies, the two studies classified as having a low overall risk of bias were parallel-group RCTs on diseased populations [32, 35], with none on healthy populations. Thus, conclusions drawn from the effect of glycine administration on the physiological systems should generally be treated judiciously, particularly for studies on healthy populations in improving in improving sleep quality, fatigue and alertness [47, 51, 53] where the evidence stem from studies of small sample sizes with overall high risk of bias. The search strategy was designed to be broad and inclusive, since articles on the topical administration of glycine such as its application on the skin have been excluded, this may account for the low number of studies on the integumentary system. Publication bias may have skewed analysis toward positive findings. Hence, the conclusions of this systematic review warrant judicious consideration. Formal statistical analysis was not conducted and results are interpreted on reported p-values, which is dependent on the sample size of the studies.
Conclusions
Glycine administration is most effective in improving characteristics of the nervous system, especially in ameliorating neurological symptoms in populations with psychiatric illnesses, most notably in Schizophrenia. Ageing is associated with the decline in function of various physiological systems and elucidating the molecular underpinnings and mechanisms of these disease states are critical in determining strategies to prevent ageing and age-related diseases. Although the administration of glycine may improve the characteristics of multiple physiological systems, there is currently limited evidence supporting their preventative effect for healthy populations, which warrants the need for future research. Importantly, larger and more robustly-designed RCTs are necessary to strengthen the current evidence on the potential of glycine administration in conferring benefits in adult humans. It would be prudent to conduct more studies on healthy populations to particularly establish the optimum dosage, route and medium of delivery, intervention duration, and timing of glycine administration for optimal organ function over multiple physiological systems to prevent the onset of age-related diseases, or to delay and potentially reverse the ageing process. Notwithstanding, the evidence to-date may suggest a simple and effective preventative strategy to enhance healthspan through oral glycine supplementation. Considering the pleiotrophic effect of glycine on multiple physiological systems demonstrated in this review, future studies should assess the effects of glycine administration on a diverse range of physiological systems in both healthy and diseased populations; and potentially, the differences in these outcomes between males and females.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful for the support of Teo Kim Kee, senior librarian of the National University of Singapore, for her assistance with the search strategy.
Author contributions
JS, ZXL, and AM were responsible for the concept and design. JS, SR, and JL did the study selection, data extraction, and critical appraisal. All authors contributed to data analysis and data interpretation. JS drafted the manuscript. All authors contributed to the reviewing and editing of the final manuscript. Supervision was provided by AM, BK, and JG.
Declarations
Conflict of interest
No conflict of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhavoronkov A, Cantor CR. "Methods for structuring scientific knowledge from many areas related to aging research," (in eng) PLoS One. 2011;6(7):e22597. doi: 10.1371/journal.pone.0022597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Correia-Melo C, Passos JF. "Mitochondria: Are they causal players in cellular senescence?," (in eng) Biochim Biophys Acta. 2015;1847(11):1373–1379. doi: 10.1016/j.bbabio.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Riera CE, Dillin A. "Tipping the metabolic scales towards increased longevity in mammals," (in eng) Nat Cell Biol. 2015;17(3):196–203. doi: 10.1038/ncb3107. [DOI] [PubMed] [Google Scholar]
- 4.Moskalev AA, Aliper AM, Smit-McBride Z, Buzdin A, Zhavoronkov A. "Genetics and epigenetics of aging and longevity," (in eng) Cell Cycle. 2014;13(7):1063–1077. doi: 10.4161/cc.28433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beard JR, Bloom DE. "Towards a comprehensive public health response to population ageing," (in eng) Lancet. 2015;385(9968):658–661. doi: 10.1016/s0140-6736(14)61461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried LP, et al. "Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment," (in eng) J Gerontol A Biol Sci Med Sci. 2009;64(10):1049–1057. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moskalev A, et al. Geroprotectors.org: a new, structured and curated database of current therapeutic interventions in aging and age-related disease, (in eng) Aging (Albany NY) 2015;7(9):616–628. doi: 10.18632/aging.100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W, Wu Z, Dai Z, Yang Y, Wang J, Wu G. "Glycine metabolism in animals and humans: implications for nutrition and health," (in eng) Amino Acids. 2013;45(3):463–477. doi: 10.1007/s00726-013-1493-1. [DOI] [PubMed] [Google Scholar]
- 9.Alarcon-Aguilar FJ, et al. "Glycine regulates the production of pro-inflammatory cytokines in lean and monosodium glutamate-obese mice," (in eng) Eur J Pharmacol. 2008;599(1–3):152–158. doi: 10.1016/j.ejphar.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 10.Zhong Z, et al. "L-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent," (in eng) Curr Opin Clin Nutr Metab Care. 2003;6(2):229–240. doi: 10.1097/00075197-200303000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Alves A, Bassot A, Bulteau AL, Pirola L, Morio B. "Glycine metabolism and its alterations in obesity and metabolic diseases," (in eng) Nutrients. 2019;11(6):1356. doi: 10.3390/nu11061356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Díaz-Flores M, et al. "Oral supplementation with glycine reduces oxidative stress in patients with metabolic syndrome, improving their systolic blood pressure," (in eng) Can J Physiol Pharmacol. 2013;91(10):855–860. doi: 10.1139/cjpp-2012-0341. [DOI] [PubMed] [Google Scholar]
- 13.Cruz M, et al. "Glycine treatment decreases proinflammatory cytokines and increases interferon-gamma in patients with type 2 diabetes," (in eng) J Endocrinol Invest. 2008;31(8):694–699. doi: 10.1007/bf03346417. [DOI] [PubMed] [Google Scholar]
- 14.Matilla B, Mauriz JL, Culebras JM, González-Gallego J, González P. "[Glycine: a cell-protecting anti-oxidant nutrient]," (in spa) Nutr Hosp. 2002;17(1):2–9. [PubMed] [Google Scholar]
- 15.de Koning TJ, Snell K, Duran M, Berger R, Poll-The BT, Surtees R. "L-serine in disease and development," (in eng) Biochem J. 2003;371(Pt 3):653–661. doi: 10.1042/bj20021785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis RM, Godfrey KM, Jackson AA, Cameron IT, Hanson MA. "Low serine hydroxymethyltransferase activity in the human placenta has important implications for fetal glycine supply," (in eng) J Clin Endocrinol Metab. 2005;90(3):1594–1598. doi: 10.1210/jc.2004-0317. [DOI] [PubMed] [Google Scholar]
- 17.Liu YJ, et al. "Glycine promotes longevity in Caenorhabditis elegans in a methionine cycle-dependent fashion," (in eng) PLoS Genet. 2019;15(3):e1007633. doi: 10.1371/journal.pgen.1007633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards C, et al. "Mechanisms of amino acid-mediated lifespan extension in Caenorhabditis elegans," (in eng) BMC Genet. 2015;16(1):8. doi: 10.1186/s12863-015-0167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brind J, et al. Dietary glycine supplementation mimics lifespan extension by dietary methionine restriction in Fisher 344 rats. FASEB J. 2011;25(S1):528.2–528.2. doi: 10.1096/fasebj.25.1_supplement.528.2. [DOI] [Google Scholar]
- 20.Miller RA, et al. "Glycine supplementation extends lifespan of male and female mice," (in eng) Aging Cell. 2019;18(3):e12953. doi: 10.1111/acel.12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne JAC, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPT, Sterne J, et al. (eds) "Revised Cochrane risk of bias tool for randomized trials (RoB 2) additional considerations for crossover trials." https://www.riskofbias.info/welcome/rob-2-0-tool/rob-2-for-crossover-trials. (Accessed 29 April 2022).
- 23.Sterne JA, et al. "ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions," (in eng) BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.González-Ortiz M, Medina-Santillán R, Martínez-Abundis E, von Drateln CR. "Effect of glycine on insulin secretion and action in healthy first-degree relatives of type 2 diabetes mellitus patients," (in eng) Horm Metab Res. 2001;33(6):358–360. doi: 10.1055/s-2001-15421. [DOI] [PubMed] [Google Scholar]
- 25.Daly JM, et al. "Immune and metabolic effects of arginine in the surgical patient," (in eng) Ann Surg. 1988;208(4):512–523. doi: 10.1097/00000658-198810000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aliyev NA, Aliyev ZN. "Application of glycine in acute alcohol hallucinosis," (in eng) Hum Psychopharmacol. 2005;20(8):591–594. doi: 10.1002/hup.735. [DOI] [PubMed] [Google Scholar]
- 27.Greenberg WM, et al. "Adjunctive glycine in the treatment of obsessive-compulsive disorder in adults," (in eng) J Psychiatr Res. 2009;43(6):664–670. doi: 10.1016/j.jpsychires.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Greenwood LM, et al. "The effects of glycine on auditory mismatch negativity in schizophrenia," (in eng) Schizophr Res. 2018;191:61–69. doi: 10.1016/j.schres.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 29.Potkin SG, Jin Y, Bunney BG, Costa J, Gulasekaram B. "Effect of clozapine and adjunctive high-dose glycine in treatment-resistant schizophrenia," (in eng) Am J Psychiatry. 1999;156(1):145–147. doi: 10.1176/ajp.156.1.145. [DOI] [PubMed] [Google Scholar]
- 30.Javitt DC, Zylberman I, Zukin SR, Heresco-Levy U, Lindenmayer JP. "Amelioration of negative symptoms in schizophrenia by glycine," (in eng) Am J Psychiatry. 1994;151(8):1234–1236. doi: 10.1176/ajp.151.8.1234. [DOI] [PubMed] [Google Scholar]
- 31.Serrita J, Ralevski E, Yoon G, Petrakis I. "A Pilot Randomized, Placebo-Controlled Trial of Glycine for Treatment of Schizophrenia and Alcohol Dependence," (in eng) J Dual Diagn. 2019;15(1):46–55. doi: 10.1080/15504263.2018.1549764. [DOI] [PubMed] [Google Scholar]
- 32.Gusev EI, et al. "Neuroprotective effects of glycine for therapy of acute ischaemic stroke," (in eng) Cerebrovasc Dis. 2000;10(1):49–60. doi: 10.1159/000016025. [DOI] [PubMed] [Google Scholar]
- 33.Khan M, et al. "Oral administration of glycine in the prevention of restenosis after coronary angioplasty. A double blind placebo controlled randomized feasibility trial evaluating safety and efficacy of glycine in the prevention of restenosis after angioplasty," (in eng) Acute Card Care. 2006;8(1):58–64. doi: 10.1080/14628840600643383. [DOI] [PubMed] [Google Scholar]
- 34.Peng X, Yan H, You Z, Wang P, Wang S. "Glutamine granule-supplemented enteral nutrition maintains immunological function in severely burned patients," (in eng) Burns. 2006;32(5):589–593. doi: 10.1016/j.burns.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 35.D'Angelo A, Valsecchi L. "High dose antithrombin supplementation in early preeclampsia: A randomized, double blind, placebo-controlled study," (in eng) Thromb Res. 2016;140:7–13. doi: 10.1016/j.thromres.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 36.Den Hond E, Hiele M, Peeters M, Ghoos Y, Rutgeerts P. "Effect of long-term oral glutamine supplements on small intestinal permeability in patients with Crohn's disease," (in eng) JPEN J Parenter Enteral Nutr. 1999;23(1):7–11. doi: 10.1177/014860719902300107. [DOI] [PubMed] [Google Scholar]
- 37.Leite RD, Lima NL, Leite CA, Farhat CK, Guerrant RL, Lima AA. "Improvement of intestinal permeability with alanyl-glutamine in HIV patients: a randomized, double blinded, placebo-controlled clinical trial," (in eng) Arq Gastroenterol. 2013;50(1):56–63. doi: 10.1590/s0004-28032013000100011. [DOI] [PubMed] [Google Scholar]
- 38.Shabert JK, Winslow C, Lacey JM, Wilmore DW. "Glutamine-antioxidant supplementation increases body cell mass in AIDS patients with weight loss: a randomized, double-blind controlled trial," (in eng) Nutrition. 1999;15(11–12):860–864. doi: 10.1016/s0899-9007(99)00213-0. [DOI] [PubMed] [Google Scholar]
- 39.Buchman AL, et al. "The effect of arginine or glycine supplementation on gastrointestinal function, muscle injury, serum amino acid concentrations and performance during a marathon run," (in eng) Int J Sports Med. 1999;20(5):315–321. doi: 10.1055/s-2007-971137. [DOI] [PubMed] [Google Scholar]
- 40.Manir K, Kallol B, Gaurav K, Arnab A, Amitabha M, Shaymal S. Role of glutamine versus placebo in prevention of acute gastrointestinal toxicity in pelvic radiotherapy: A randomized control study. Clin Cancer Investig J. 2014;3(6):508–513. doi: 10.4103/2278-0513.142637. [DOI] [Google Scholar]
- 41.Bushen OY, et al. "Diarrhea and reduced levels of antiretroviral drugs: improvement with glutamine or alanyl-glutamine in a randomized controlled trial in northeast Brazil," (in eng) Clin Infect Dis. 2004;38(12):1764–1770. doi: 10.1086/421394. [DOI] [PubMed] [Google Scholar]
- 42.Peng X, Yan H, You Z, Wang P, Wang S. "Clinical and protein metabolic efficacy of glutamine granules-supplemented enteral nutrition in severely burned patients," (in eng) Burns. 2005;31(3):342–346. doi: 10.1016/j.burns.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 43.Gannon MC, Nuttall JA, Nuttall FQ. "The metabolic response to ingested glycine," (in eng) Am J Clin Nutr. 2002;76(6):1302–1307. doi: 10.1093/ajcn/76.6.1302. [DOI] [PubMed] [Google Scholar]
- 44.Genton L, et al. Gut barrier and microbiota changes with glycine and branched-chain amino acid supplementation in chronic haemodialysis patients. J Cachexia Sarcopenia Muscle. 2021;12(6):1527–1539. doi: 10.1002/jcsm.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Genton L, et al. "Glycine increases fat-free mass in malnourished haemodialysis patients: a randomized double-blind crossover trial," (in eng) J Cachexia Sarcopenia Muscle. 2021;12(6):1540–1552. doi: 10.1002/jcsm.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munts AG, et al. "Intrathecal glycine for pain and dystonia in complex regional pain syndrome," (in eng) Pain. 2009;146(1–2):199–204. doi: 10.1016/j.pain.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 47.Bannai M, Kawai N. "New therapeutic strategy for amino acid medicine: glycine improves the quality of sleep," (in eng) J Pharmacol Sci. 2012;118(2):145–148. doi: 10.1254/jphs.11r04fm. [DOI] [PubMed] [Google Scholar]
- 48.O'Neill BV, et al. "High-dose glycine impairs the prepulse inhibition measure of sensorimotor gating in humans," (in eng) J Psychopharmacol. 2011;25(12):1632–1638. doi: 10.1177/0269881110372546. [DOI] [PubMed] [Google Scholar]
- 49.Palmer C, et al. The cognitive effects of modulating the glycine site of the NMDA receptor with high-dose glycine in healthy controls. Hum Psychopharmacol Clin Exp. 2008;23(2):151–159. doi: 10.1002/hup.904. [DOI] [PubMed] [Google Scholar]
- 50.Leung S, Croft RJ, O'Neill BV, Nathan PJ. "Acute high-dose glycine attenuates mismatch negativity (MMN) in healthy human controls," (in eng) Psychopharmacology. 2008;196(3):451–460. doi: 10.1007/s00213-007-0976-8. [DOI] [PubMed] [Google Scholar]
- 51.Yamadera W, Inagawa K, Chiba S, Bannai M, Takahashi M, Nakayama K. Glycine ingestion improves subjective sleep quality in human volunteers, correlating with polysomnographic changes. Sleep Biol Rhythms. 2007;5(2):126–131. doi: 10.1111/j.1479-8425.2007.00262.x. [DOI] [Google Scholar]
- 52.Neumeister A, et al. "Cerebral metabolic effects of intravenous glycine in healthy human subjects," (in eng) J Clin Psychopharmacol. 2006;26(6):595–599. doi: 10.1097/01.jcp.0000245558.14284.aa. [DOI] [PubMed] [Google Scholar]
- 53.Inagawa K, Hiraoka T, Kohda T, Yamadera W, Takahashi M. Subjective effects of glycine ingestion before bedtime on sleep quality. Sleep Biol Rhythms. 2006;4(1):75–77. doi: 10.1111/j.1479-8425.2006.00193.x. [DOI] [Google Scholar]
- 54.Heresco-Levy U, Ermilov M, Lichtenberg P, Bar G, Javitt DC. "High-dose glycine added to olanzapine and risperidone for the treatment of schizophrenia," (in eng) Biol Psychiatry. 2004;55(2):165–171. doi: 10.1016/s0006-3223(03)00707-8. [DOI] [PubMed] [Google Scholar]
- 55.Heresco-Levy U, Javitt DC. "Comparative effects of glycine and D-cycloserine on persistent negative symptoms in schizophrenia: a retrospective analysis," (in eng) Schizophr Res. 2004;66(2–3):89–96. doi: 10.1016/s0920-9964(03)00129-4. [DOI] [PubMed] [Google Scholar]
- 56.Javitt DC, et al. "Adjunctive high-dose glycine in the treatment of schizophrenia," (in eng) Int J Neuropsychopharmacol. 2001;4(4):385–391. doi: 10.1017/s1461145701002590. [DOI] [PubMed] [Google Scholar]
- 57.Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Silipo G, Lichtenstein M. "Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia," (in eng) Arch Gen Psychiatry. 1999;56(1):29–36. doi: 10.1001/archpsyc.56.1.29. [DOI] [PubMed] [Google Scholar]
- 58.Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Horowitz A, Kelly D. "Double-blind, placebo-controlled, crossover trial of glycine adjuvant therapy for treatment-resistant schizophrenia," (in eng) Br J Psychiatry. 1996;169(5):610–617. doi: 10.1192/bjp.169.5.610. [DOI] [PubMed] [Google Scholar]
- 59.Amin A, et al. "L-Arginine Increases Postprandial Circulating GLP-1 and PYY Levels in Humans," (in eng) Obesity (Silver Spring) 2018;26(11):1721–1726. doi: 10.1002/oby.22323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luiking YC, Weusten BL, Portincasa P, Van Der Meer R, Smout AJ, Akkermans LM. "Effects of long-term oral L-arginine on esophageal motility and gallbladder dynamics in healthy humans," (in eng) Am J Physiol. 1998;274(6):G984–G991. doi: 10.1152/ajpgi.1998.274.6.G984. [DOI] [PubMed] [Google Scholar]
- 61.Antonio J, Sanders MS, Kalman D, Woodgate D, Street C. "The effects of high-dose glutamine ingestion on weightlifting performance," (in eng) J Strength Cond Res. 2002;16(1):157–160. [PubMed] [Google Scholar]
- 62.Kasai K, Suzuki H, Nakamura T, Shiina H, Shimoda SI. "Glycine stimulated growth hormone release in man," (in eng) Acta Endocrinol (Copenh) 1980;93(3):283–286. doi: 10.1530/acta.0.0930283. [DOI] [PubMed] [Google Scholar]
- 63.Kasai K, Kobayashi M, Shimoda SI. "Stimulatory effect of glycine on human growth hormone secretion," (in eng) Metabolism. 1978;27(2):201–208. doi: 10.1016/0026-0495(78)90165-8. [DOI] [PubMed] [Google Scholar]
- 64.Sugaya K, et al. "Dietary glycine improves urine storage symptoms in urology outpatients," (in eng) J Complement Integr Med. 2021;18(3):617–620. doi: 10.1515/jcim-2020-0282. [DOI] [PubMed] [Google Scholar]
- 65.Truong DD, Fahn S. "Therapeutic trial with glycine in myoclonus," (in eng) Mov Disord. 1988;3(3):222–232. doi: 10.1002/mds.870030306. [DOI] [PubMed] [Google Scholar]
- 66.Strzelecki D, Kaluzynska O, Jozefowicz O. Initial glycine serum level is not a predictor of the recovery resulting from glycine augmentation of antipsychotic treatment. Arch Psychiatr Psychother, J Article. 2011;13(2):5–11. [Google Scholar]
- 67.Rosse RB, et al. "Glycine adjuvant therapy to conventional neuroleptic treatment in schizophrenia: an open-label, pilot study," (in eng) Clin Neuropharmacol. 1989;12(5):416–424. doi: 10.1097/00002826-198910000-00006. [DOI] [PubMed] [Google Scholar]
- 68.O'Neill BV, Croft RJ, Leung S, Oliver C, Phan KL, Nathan PJ. "High-dose glycine inhibits the loudness dependence of the auditory evoked potential (LDAEP) in healthy humans," (in eng) Psychopharmacology. 2007;195(1):85–93. doi: 10.1007/s00213-007-0870-4. [DOI] [PubMed] [Google Scholar]
- 69.Wu G. Recent advances in swine amino acid nutrition. Journal of Animal Science and Biotechnology. 2010;1(2):118–130. [Google Scholar]
- 70.Wu G. "Functional amino acids in growth, reproduction, and health," (in eng) Adv Nutr. 2010;1(1):31–37. doi: 10.3945/an.110.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Laursen TM, Nordentoft M, Mortensen PB. "Excess early mortality in schizophrenia," (in eng) Annu Rev Clin Psychol. 2014;10:425–448. doi: 10.1146/annurev-clinpsy-032813-153657. [DOI] [PubMed] [Google Scholar]
- 72.Hjorthøj C, Stürup AE, McGrath JJ, Nordentoft M. "Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis," (in eng) Lancet Psychiatry. 2017;4(4):295–301. doi: 10.1016/s2215-0366(17)30078-0. [DOI] [PubMed] [Google Scholar]
- 73.Kirkpatrick B, Messias E, Harvey PD, Fernandez-Egea E, Bowie CR. "Is schizophrenia a syndrome of accelerated aging?," (in eng) Schizophr Bull. 2008;34(6):1024–1032. doi: 10.1093/schbul/sbm140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peng TC, Chen WL, Wu LW, Chang YW, Kao TW. "Sarcopenia and cognitive impairment: A systematic review and meta-analysis," (in eng) Clin Nutr. 2020;39(9):2695–2701. doi: 10.1016/j.clnu.2019.12.014. [DOI] [PubMed] [Google Scholar]
- 75.Qiao Y-S et al. "The association between diabetes mellitus and risk of sarcopenia: accumulated evidences from observational studies," (in English), Front Endocrinol, Syst Rev. 2021;12. 10.3389/fendo.2021.782391. [DOI] [PMC free article] [PubMed]
- 76.Zhang H et al. "Association between Sarcopenia and Metabolic Syndrome in Middle-Aged and Older Non-Obese Adults: A Systematic Review and Meta-Analysis," (in eng), Nutrients. 2018;10(3). 10.3390/nu10030364 [DOI] [PMC free article] [PubMed]
- 77.Tsai GE, Lin PY. "Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis," (in eng) Curr Pharm Des. 2010;16(5):522–537. doi: 10.2174/138161210790361452. [DOI] [PubMed] [Google Scholar]
- 78.Newcomer JW, Farber NB, Olney JW. "NMDA receptor function, memory, and brain aging," (in eng) Dialogues Clin Neurosci. 2000;2(3):219–232. doi: 10.31887/DCNS.2000.2.3/jnewcomer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shimizu S et al. "Glycine-binding site stimulants of NMDA Receptors alleviate extrapyramidal motor disorders by activating the nigrostriatal dopaminergic pathway," (in eng), Int J Mol Sci. 2017;18(7). 10.3390/ijms18071416. [DOI] [PMC free article] [PubMed]
- 80.Marquard J, et al. "Characterization of pancreatic NMDA receptors as possible drug targets for diabetes treatment," (in eng) Nat Med. 2015;21(4):363–372. doi: 10.1038/nm.3822. [DOI] [PubMed] [Google Scholar]
- 81.Dumas SJ, et al. "NMDA-Type glutamate receptor activation promotes vascular remodeling and pulmonary arterial hypertension," (in eng) Circulation. 2018;137(22):2371–2389. doi: 10.1161/circulationaha.117.029930. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.