Abstract
Chronic inflammatory pathway activation, commonly referred to as “Inflammaging” or chronic inflammation (CI), is associated with frailty, cognitive and functional decline, and other causes of health span decline in older adults. We investigated the variability of candidate serum measures of CI among community-dwelling older adults selected for mild low-grade inflammation. We focused on serum cytokines known to be highly predictive of adverse health outcomes in older adults (sTNFR1, IL-6) during a short-term (weeks) and medium-term (months) follow-up, as well as immune markers that are less studied in aging but reflect other potentially relevant domains such as adaptive immune activation (sCD25), innate immune activation (sCD14 and sCD163), and the inflammation-metabolism interface (adiponectin/Acrp30) during short-term (weeks) follow up. We found that sTNFR1 was more reproducible than IL-6 over a period of weeks and months short-term and medium-term. The intra-class correlation coefficient (ICC) for sTNFR1 was 0.95 on repeated measures over 6 weeks, and 0.79 on repeated measures with mean interval of 14 weeks, while the ICC for IL-6 was 0.52 over corresponding short-term and 0.67 over corresponding medium-term follow-up. This suggests that sTNFR1 is a more reliable marker of CI than IL-6. This study provides new insights into the reproducibility of serum markers of CI in older adults. The findings suggest that sTNFR1 may be a better marker of CI than IL-6 in this population. Further studies are needed to confirm these findings and to investigate the clinical utility of sTNFR1 in older adults.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-023-01006-x.
Keywords: Chronic inflammation, TNFR1, IL-6, Biomarkers
Introduction
Chronic inflammatory pathway activation, commonly referred to as “Inflammaging” or chronic inflammation (CI), has gained increasing recognition as a significant contributor to frailty, cognitive and functional decline, sarcopenia, and worsening chronic disease states like cancer and congestive heart failure in older adults [1–8]. In addition, serum measures of inflammation have emerged as potential indicators of age-related biological changes such as mitochondrial decline and senescent cell burden [9, 10]. Therefore, the identification and selection of reliable and biologically relevant inflammatory markers are crucial for the identification of older adults at high risk of adverse health outcomes, for the development of CI-related clinical trial design, and for the identification of markers secreted by senescent cells as described within the emerging field of geroscience [3, 11].
Over the past decades, investigators have identified some clinically relevant markers of CI in human population studies. For example, interleukin (IL) 6 has been touted as the “cytokine for geriatricians” because of its strong associations with adverse health outcomes in older adults and because of its known pathological impact on stem cells and other biological processes when chronically expressed [12, 13]. Other studies identified C-reactive protein (CRP) and Tumor necrosis factor alpha (TNF-α) as important correlates of a range of adverse health outcomes in older adults [14–22]. In a more recent study, 15 inflammatory mediators were measured in the InCHIANTI study and identified TNF receptor 1 (TNFR1), IL1-RA, CRP, IL-18, and IL-6 as significantly correlated with mortality risk over 5 years [23]. These measures were validated in another population study as highly predictive of mortality over 10 years, with TNFR1 and IL-6 performing substantially better than the other three measures and were therefore combined as an inflammatory index score (IIS) [23]. Additional studies have demonstrated that these two markers of CI, and others, are also powerful predictors of adverse health outcomes [16, 24–33]. Though cytokines and chemokines have been a major focus of CI measurement in aging, other soluble factors including soluble receptors and adipokines have also been evaluated as markers of immune activation in aging and age-related diseases including soluble CD14 (sCD14), soluble CD163 (sCD163), soluble CD25 (sCD25/ soluble IL-2Rα), triggering receptor expressed on myeloid cells 2 (TREM2), soluble urokinase plasminogen activator receptor (suPAR), leptin, and adiponectin (Acrp30) [34–40]. These markers of CI also hold potential as measures of specific biological subdomains such as innate immune cell activation, adaptive immune cell activation, and metabolism.
Despite efforts to identify blood markers of CI, no single measure has become the gold standard for CI measurement and risk prediction [41]. This lack of consensus arises partially due to a limited understanding of the tissue origin of serum-measured cytokines and partly due to their variability over time. Particularly, short-term variability, i.e., intra-person variation over a period of days to a few weeks, introduces measurement errors that can affect the magnitude of the association of inflammation with aging outcomes. Stability over time is crucial for the discovery of highly predictive biomarkers that can help identify older adults at risk of developing long-term consequences associated with CI: for monitoring the efficacy of CI treatments and for investigating the underlying biology and consequences of cytokine production [6]. As short-term variability reflects measurement error, and long-term variability signifies biological variation, it becomes imperative to examine both shorter- and longer-term variation at the subject and population levels. Additionally, given that future trial designs may entail the specific enrollment of older adults with CI, it is important to understand the variability of established CI-associated cytokines among the specific subpopulation of older adults with elevated levels of a cytokine of interest during initial screening.
In order to identify a pool of stable markers of chronic inflammation that are predictive of adverse outcomes or that represent specific components of inflammatory pathway activation, we investigated the variability of candidate measures over a period of weeks and months. To capture a comprehensive range of measures representing different facets of immune system activation, we focused on serum cytokines known to be highly predictive of adverse health outcomes in older adults (sTNFR1, IL-6) during a short-term (weeks) and medium-term (months) follow-up, as well as immune markers that are less studied in aging and reflect other potentially relevant domains such as adaptive immune activation (sCD25), innate immune activation (sCD14 and sCD163), and the inflammation-metabolism interface (adiponectin/Acrp30) during short-term (weeks) follow-up.
Methods
Populations and study design
We utilized samples from two separate groups of older adults with known CI as measured by IL-6 or TNFR1. The first population, termed “main cytokine variability study” or CV, was recruited specifically for the purpose of determining the short-term variability of CI measures in very well-controlled circumstances. The second group, the ELCIE cohort, were adults over age 65 who were enrolled in a pilot clinical trial that evaluated the impact of lactoferrin on serum measures of CI with serum obtained during pre-intervention screening to identify evidence of CI (clinicaltrials.gov; NCT02968992) 42. Both studies were reviewed and approved by the Johns Hopkins Medical Institutional Review Board (IRB) and all participants provided written informed consent. Individuals were recruited from the JHU OAIC Registry of community-dwelling older adults who consented to be contacted for research for all studies. Consistent with NIH guidelines, all genders and all racial identities were eligible for inclusion.
In order to capture short-term variability data from the CV study, we collected early morning fasting blood samples every 2 weeks over a period of 6 weeks from 15 adults over age 65 (four fasting samples per subject). The inclusion criteria were the ability to complete 4-m timed walk, a walking speed ≤ 1.0 m/s, or a serum IL-6 level ≥ 2.5 pg/ml. Exclusion criteria included a Mini-Mental Status Exam score < 24, anti-inflammatory medication use (such as prednisone, infliximab, etanercept, methotrexate, daily non-steroidal anti-inflammatory drug (NSAID), aspirin greater than 325 mg per day), lower extremity mobility disability caused by Parkinson’s disease, history of stroke with residual motor deficit, severe osteoarthritis or rheumatoid arthritis, symptomatic claudication, hospitalization within 3 months for MI, angina, infection requiring antibiotics, or joint replacement, or a viral respiratory infection within the last 2 weeks, having received any vaccination within 2 weeks of first blood draw, or a diagnosis of any malignancy requiring treatment within the past 1 year. Participants were asked about these study exclusion criteria at each blood draw.
Longer term variability in CI measures was studied from samples taken and stored from 35 individuals who had participated in the ELCIE study [42]. All cytokine measures used in this analysis were obtained prior to the randomization and treatment with lactoferrin or placebo, during a peri-intervention period intended to establish the presence of CI. Blood was drawn from each participant three times over a varied period of weeks to months (mean time between visits 7 weeks, range 2 to 31 weeks). Subjects were not required to be fasted and blood was drawn at different times during the day. Inclusion criteria included age 70 years or older, able to complete 4-m timed walk, walking speed < 1.0 m/s, serum IL-6 level ≥ 2.5 pg/ml, or TNFR1 level ≥ 1500 pg/ml. Exclusion criteria for this study were a Mini-Mental Status Exam score < 21, IL-6 levels above 30.0 pg /ml, as well as the same exclusions for anti-inflammatory medication use, restricted mobility, past medical conditions, recent vaccinations, infections, or malignancies as outlined for the main cytokine variability study.
Laboratory measures
Blood was drawn and serum was extracted and frozen at − 80 °C within 2 h of each blood draw. Biomarkers were all measured in plasma serum after thawing using ELISAs according to manufacturers’ protocols. ELISAs for Human IL-6 (Cat# HS600B), Human sTNFR1 (Cat# DRT100), Human CD25 (Cat# DR2A00), Human CD14(Cat# DC140), and Human CD163 (Cat# DC1630) were purchased from R&D Systems, (Minneapolis, MN, USA). The Human Adiponectin (ACRP-30) ELISA was purchased from BioLegend (San Diego, CA, USA) (Cat# 558,409). An internal control was used for each assay and the results were standardized by the internal control.
Statistical analysis
In both the CV and ELCIE study, exploratory analysis was first performed, where the distributions of each cytokine were plotted at different times using violin plots. A violin plot is a combination of a box plot and density plot. It adds the information available from local density estimates to the basic summary statistics inherent in box plots. Thus, it provides a good visual representation of the distribution of the actual data points and is a useful tool for exploratory data analysis [43]. Each participant’s cytokine values at 4 time points were also plotted as trajectories. Reproducibility of cytokine levels over a relatively short time period (e.g., 1 month) was assessed using an intra-class correlation coefficient (ICC). ICC is formally defined as the ratio of inter-person variance to the total variance, where total variance is the sum of intra-person and inter-person variances. Larger ICC corresponds to greater reproducibility. Because the cytokines had skewed distributions, the values were log-transformed. ICC was calculated by fitting a one-way ANOVA model. The 95% confidence intervals for ICC were estimated using the method of Searle, as implemented in the R package called ICC [44, 45]. ICC was calculated in the overall sample, as well as stratified by sex (in men and women) and by BMI (< 30 and > = 30 kg/m2). For two participants whose IL-6 levels were above the upper limit of detection, the maximum value for the assay was imputed for the calculation of ICC.
Results
The mean age in both studies was 81 years, and the age distribution was similar in CV and ELICIE (73–95 and 72–92, respectively). There was a slightly higher proportion of female participants in both groups (60% and 63%, respectively). The majority of participants in both the CV and ELCIE groups were white (80% in both), and only one participant identified as Asian across both study cohorts (in ELCIE). There was a higher proportion of participants with a BMI greater than or equal to 30 kg/m2 in the CV group (53%) than in the ELCIE group (34%) (Table 1).
Table 1.
Characteristics of the participants by study
| Characteristics | Cytokine variability (CV) | ELCIE |
|---|---|---|
| Age—mean years (range) | 81 (73–95) | 81 (72–97) |
| Gender—female no./total no. (%) | 9/15 (60%) | 22/35 (63%) |
| Race—no./total no. (%) | ||
| Asian | 0/15 (0%) | 1/35 (3%) |
| Black or African–American | 3/15 (20%) | 6/35 (17%) |
| White | 12/15 (80%) | 28/35(80%) |
| BMI ≥ 30 kg/m2—no./total no. (%) | 8/15 (53%) | 12/35 (34%) |
Cytokine variability study
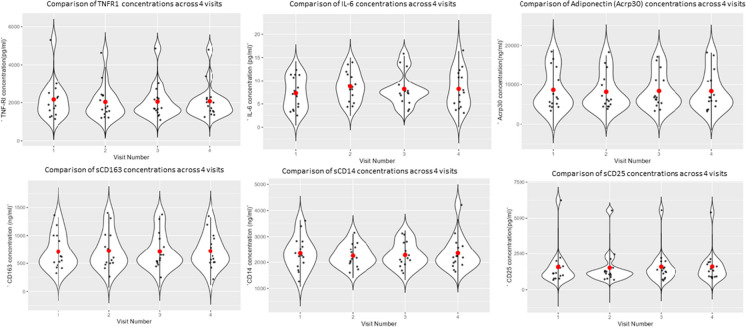
See Fig. 1.
Fig. 1.
Violin plots depicting the distribution of TNFR1 (pg/mL), IL-6 levels (pg/mL), adiponectin/Acrp30 (ng/ml), sCD63 (ng/ml), sCD14 (ng/ml), sCD25 (pg/ml) in patients at 4 time points. The x-axis indicates the visit number (2 weeks between each visit), y-axis indicates the relevant inflammatory marker serum concentration. The width of the violin represents the density of observations along the y-axis, the red dot represents the mean, and the error bars represent the standard deviation (Supplemental Fig. 1 provides individual participants between visit trajectories for each marker)
The stability of the markers in the cytokine variability (CV) study (Table 2) was determined using serum obtained under fasting conditions. Phlebotomy was performed in the morning at a baseline visit, and then every 2 weeks for a total of four visits in a 6-week-period of time. All the measures except IIS, IL6, and CD14 had an interclass coefficient (ICC) larger than 0.93, which indicates stability between visits. IL-6 and CD14 had the lowest ICCs of 0.52 and 0.74, respectively. Inflammatory index (IIS), which is a combination of IL-6 and TNFR1, had an ICC of 0.87.
Table 2.
Intra-class correlations in the CV study
| Cytokine | Between variability | Within variability | ICC (95% CI) |
|---|---|---|---|
| IL-6 | 0.117 | 0.108 | 0.52 (0.27, 0.77) |
| sTNFR1 | 0.138 | 0.008 | 0.95 (0.89, 0.98) |
| IIS | 0.100 | 0.015 | 0.87 (0.75, 0.95) |
| CD-14 | 0.040 | 0.014 | 0.74 (0.54, 0.89) |
| CD-25 | 0.27 | 0.02 | 0.93 (0.86, 0.97) |
| CD-163 | 0.19 | 0.0066 | 0.97 (0.93, 0.99) |
| Adiponectin | 0.28 | 0.01 | 0.96 (0.93, 0.99) |
Further investigation examining the biomarker stability between genders revealed that the variability in IL-6 was greater among the male participants (Table 3). The serum IL-6 and sTNFR1 levels at each time point for each participant are shown in Supplemental Tables 1 and 2. Of note, the ICC of sTNFR1, sCD14, sCD25, sCD163, and adiponectin were similar between male and female (Supplemental Table 3). Furthermore, obesity did not impact the ICC for any of the measures (Supplemental Table 3).
Table 3.
Intra-class correlation by gender in the CV study
| Cytokine | ICC (95% CI) Males |
ICC (95% CI) Females |
|---|---|---|
| IL-6 | 0.35 (0.0, 0.83) | 0.61 (0.29, 0.87) |
| sTNFR1 | 0.81 (0.52, 0.80) | 0.95 (0.88, 0.99) |
| IIS | 0.51 (0.12, 0.89) | 0.92 (0.80, 0.98) |
We also sought to determine if the ICC for both IL-6 and TNFR1 in the ELCIE study was similar to that from the CV study, as ELCIE measurements were obtained over a moderately longer average timeframe and the criteria for CI included either elevated IL-6 or elevated TNFR1 (in contrast to ELCIE, which used IL-6 alone for CI screening). The demographics of the ELCIE study were comparable to those of the CV study (Table 1). These samples were part of a collection of blood needed to screen and enroll participants, and all samples were collected prior to the study intervention. Samples were obtained at variable time points during the day over a period of months, without the requirement to fast. The ICC for IL-6 was higher in the ELCIE study under these conditions than those in the CV study, while the ICCs for TNFR1 and IIS were lower (Table 4). The serum IL-6 and sTNFR1 levels at each time point for each ELCIE participant are shown in Supplemental Tables 4 and 5. The ELCIE study revealed a difference in cytokine variability based on gender, like the CV study. The ICC for IL-6 was 0.67 for all ELCIE participants, while the ICC for males and females were 0.50 and 0.77, respectively. The ICC for sTNFR1 was 0.79 for all ELCIE participants, while the ICC for males and females were 0.85 and 0.76, respectively.
Table 4.
Intra-class correlation for ELCIE study
| Cytokine | Between variability | Within variability | ICC (95% CI) |
|---|---|---|---|
| IL-6 | 0.33 | 0.16 | 0.67 (0.51, 0.80) |
| sTNFR1 | 0.050 | 0.013 | 0.79 (0.68, 0.88) |
| IIS | 0.063 | 0.034 | 0.65 (0.48, 0.79) |
Discussion
Serum biomarkers are a viable low-risk target to identify those older adults with CI, which puts individuals at higher risk for the development of conditions that impact function, cognition, and overall health span. In addition, these biomarkers may be important in monitoring the impact of interventions under development for CI. In order for these markers to be clinically useful, they must lack variability between measures within the population under study and be stable after storage, in addition to possessing biological validity as predictors of adverse outcomes. The current study was therefore designed to measure the variability of a panel of candidate biomarkers that either have existing literature to support their relationship to age-associated adverse outcomes or are associated with an aspect of immunity that is biologically relevant to aging. We found that TNFR1, sCD25, sCD163, and adiponectin were highly stable over four fasting blood draws done over a period of 6 weeks. We also found that IL-6 and sCD14 were more variable, with ICC of 0.52 and 0.74, respectively. ICCs for TNFR1 and IL-6 were measured under less stringently controlled conditions in a separate study and were found to have somewhat different variability characteristics to those measured from fasting and morning blood draws, suggesting some impact on stability in proposed measures for CI. Notably, the ELCIE study included both IL-6 and TNFR1 as criteria for CI compared to IL-6 alone in the CV study. Gender appeared to play a role in this variability, with men having a much broader range than that demonstrated by women (Supplemental Table 3).
IL-6, a widely utilized marker for CI, was the most variable of the markers studied and more variable than TNFR1 in both sample populations (ICC 0.52 vs 0.95 in the main cytokine variability study). The findings for IL-6 differ from data reported in a prior study that reported an ICC of 0.87 for IL-6 compared to the ICC in the main cytokine variability study population (0.52) and in the ELCIE population (0.67) [46]. The discrepancy between our two studies and the study by Rao et al. may be due to the populations measured [46]. The prior included healthy individuals, 65 years and older, while the CV and ELCIE populations had elevated cytokine levels at baseline compared to those in the study by Rao et al. The small sample sizes of the studies may also impact the variation in IL-6 levels. For example, the sample size (four per group) in the prior study may have impacted the observation that there was no variance in IL-6 due to sex, which is in contrast to our findings [46]. Our findings for IL-6 variability observed in the CV study are closer to those seen in other recent studies including IL-6 ICC of 0.55 in a cohort of HIV seronegative men participating in the Multicenter AIDS Cohort Study (MACS cohort) [47]. The observed gender differences in IL-6 levels were not totally unexpected as there has been a precedent that sex influences inflammatory processes [48]. Notably, several genes on the X chromosome are involved with the inflammatory cascade [49]. Females have the advantage of silencing these rare genes with their second X chromosome. These observed sex differences in the stability of IL-6 measurement may impact study design and interventions that target IL-6. The IIS demonstrated intermediate variability between IL-6 and sTNFR1 and demonstrated a similar trend of slightly increased variability within the ELCIE study compared to CV(0.65 in ELCIE vs 0.87 in CV). Maximum flexibility with sustained stability will positively impact study design and logistics.
For the innate immune markers, sCD163 was quite stable (ICC 0.97), while sCD14 demonstrated somewhat greater variability (ICC 0.74). The ICC in prior studies for sCD14 was 0.56 in the MACS cohort [47]. The lower ICC for sCD14 in this prior study may reflect differences in exclusion criteria, differing intervals between sample collections, sample processing, and the specific assay used (Luminex platform multiplex vs single analyte ELISA). A literature review did not identify prior studies reporting ICC for sCD163, making the ICC reported here a useful contribution to the study of CI. sCD14 and sCD163 are both cell surface markers found on monocyte/macrophage cells that play an important role in moderating and sustaining inflammatory responses and could also help guide etiological studies of CI. Higher soluble sCD14 levels have been found in the cerebrospinal fluid of individuals with dementia compared to cognitively normal individuals, and sCD14 is also elevated in the plasma of individuals with incident dementia [34, 50]. In addition, sCD14 has been associated with an increased risk for hip fracture among community-dwelling older adults [51]. The inflammatory marker sCD163 increases with age [52]. In people with cancer, a recent meta-analysis demonstrated an age-associated mortality risk with elevated sCD163 [36]. In the context of diabetes mellitus, sCD163 is associated with diabetic complications [53]. Prior studies have also linked sCD163 to cardiovascular disease and atherosclerosis, including a recent study demonstrating an association between elevated sCD163 and all-cause and cardiovascular-specific mortality among older adults [54].
The adaptive immune marker sCD25 also demonstrated low variability (ICC 0.93), which is again somewhat higher than that observed in the MACS cohort (ICC 0.79) [47]. sCD25 is the soluble form of a transmembrane protein that is found in T cells. Alternatively known as soluble IL-2Rα receptor, it is one component of the trimeric IL-2 receptor and is required for mediating IL-2-induced effects including T cell proliferation, activation, and homeostasis. The protein is cleaved by a membrane metalloproteinase leading to ectodomain shedding. sCD25 is elevated in children less than 2 years of age, markedly declines and remains generally lower into middle age, then trends upward with advanced age [55–57]. sCD25 has been associated with an array of inflammatory processes (including infections, cardiovascular disease, malignancy, and autoimmune disorders) [55, 58–62]. The role of this marker has been less well studied in the setting of age-associated functional decline, frailty, or disability, though Rosenthal et al. demonstrated an association with 1-year mortality in hospitalized older men and Gao et al. found an association between elevated sCD25 and decreased cognitive performance among community-dwelling older adults [35, 63].
Finally, adiponectin also demonstrated a high ICC (0.96), making it a potentially useful marker at the immunity-metabolic interface. This finding is consistent with prior studies demonstrating a high ICC for adiponectin [64, 65]. Many aging-related conditions and syndromes linked to CI have also been associated with markers of metabolic regulation, including adiponectin [66–69]. This interaction is sometimes denoted by the term metaflammation [70]. Adiponectin levels have been demonstrated to increase with age across the life span, with a more prominent increase among women than men [71]. Early studies emphasized the positive associations of adiponectin with insulin-sensitizing, anti-apoptotic, and anti-inflammatory effects; more recent studies have added complexity to the understanding of how adiponectin affects various age-related diseases including diabetes mellitus type 2, coronary heart disease, and age-associated cognitive decline. In the cardiovascular context, both very low and very high levels of adiponectin have been associated with adverse outcomes including increased mortality, while moderately elevated adiponectin has been associated with better cardiovascular health. With increasing age, elevated adiponectin appears to shift from a cardioprotective role toward an association with increased cardiovascular disease in those older than 60 years [40]. Concerning age-associated decline, elevated adiponectin is associated with an increased risk for sarcopenia, according to a recent meta-analysis [72]. Such findings of adverse outcomes with low and high levels of adiponectin have been termed the “adiponectin paradox.” Further elucidation of the biology and epidemiology of adiponectin is required using prospective, longitudinal studies to clarify the clinical and prognostic significance of adiponectin levels in older adults.
As an exploratory analysis in the ELCIE study, the individual participant coefficient of variation (CV) was calculated for IL-6 and sTNFR1 as a proxy for “within-person variability.” There was no association between CV and age (Supplemental Fig. 2). Given the small sample size and the relatively older population of this study (mean = 81 years, minimum = 72 years), it is not possible to adequately address the possibility that “within-person variability” (or alternatively ICC within different age ranges) might vary across a wider age range. This might be relevant to study further in future research with larger numbers of human subjects.
Limitations
The studies included in this analysis included only one individual who did not identify their race as either White or Black/African–American. The study population was restricted to those who met multiple exclusion criteria to limit the likelihood of specific triggers of pro- or anti-inflammation states in order to measure marker variability in the absence of other perturbations. This restricted sampling limits the external validity when applied to populations with a less stringent design. In order to better understand measurement characteristics through intensive sampling, this study focused on a few inflammatory markers linked to age-associated chronic conditions. In the age of high-dimensional measures, it is clear that one or a few CI measures may not fully reflect an individual’s inflammatory state. The current findings nevertheless have value for the understanding of specific biomarkers as outcome measures for studies designed to mitigate age-associated CI.
Conclusions
A panel of stable serum biomarkers with known associations to adverse health outcomes will help to identify those individuals with clinically relevant chronic inflammation and facilitate the ability to detect the impact of future treatments and the identification of specific biological pathways that drive CI and its consequences to the health and well-being of older adults. TNFR1 is among the best predictors of adverse health outcomes related to CI and is stable across repeated measures, making it an attractive marker for future clinical translation and use as an outcome in studies of interventions for age-associated CI.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
National Institute on Aging at the National Institutes of Health, Grant/Award Number: R01 AG048272, R21 AG053681, T32 AG058527, and the Johns Hopkins Older Americans Independence Center P30 AG021334.
Declarations
Competing interests
TL, JL, and JW receive grant funding from MyMD Pharmaceuticals. JW has provided consultation services to MyMD for study design.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Langmann GA, Perera S, Ferchak MA, Nace DA, Resnick NM, Greenspan SL. Inflammatory markers and frailty in long-term care residents. J Am Geriatr Soc. 2017;65(8):1777–1783. doi: 10.1111/jgs.14876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang Z, Zhang T, Liu H, et al. Inflammaging: the ground for sarcopenia? Exp Gerontol. 2022;168:111931. doi: 10.1016/j.exger.2022.111931. [DOI] [PubMed] [Google Scholar]
- 3.Walker KA, Basisty N, Wilson DM, Ferrucci L. Connecting aging biology and inflammation in the omics era. J Clin Invest. 2022;132(14). 10.1172/JCI158448. [DOI] [PMC free article] [PubMed]
- 4.Cesari M, Kritchevsky SB, Nicklas B, et al. Oxidative damage, platelet activation, and inflammation to predict mobility disability and mortality in older persons: results from the health aging and body composition study. J Gerontol Ser A. 2012;67A(6):671–676. doi: 10.1093/gerona/glr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capri M, Yani SL, Chattat R, et al. Pre-operative, high-IL-6 blood level is a risk factor of post-operative delirium onset in old patients. Front Endocrinol. 2014;5. Accessed May 15, 2023. https://www.frontiersin.org/articles/10.3389/fendo.2014.00173. [DOI] [PMC free article] [PubMed]
- 6.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dick SA, Epelman S. Chronic heart failure and inflammation. Circ Res. 2016;119(1):159–176. doi: 10.1161/CIRCRESAHA.116.308030. [DOI] [PubMed] [Google Scholar]
- 9.Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krysko DV, Agostinis P, Krysko O, et al. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011;32(4):157–164. doi: 10.1016/j.it.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Justice JN, Ferrucci L, Newman AB, et al. A framework for selection of blood-based biomarkers for geroscience-guided clinical trials: report from the TAME biomarkers workgroup. GeroScience. 2018;40(5):419–436. doi: 10.1007/s11357-018-0042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ershler WB. Interleukin-6: a cytokine for gerontologists. J Am Geriatr Soc. 1993;41(2):176–181. doi: 10.1111/j.1532-5415.1993.tb02054.x. [DOI] [PubMed] [Google Scholar]
- 13.Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol Ser A. 2006;61(6):575–584. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162(20):2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 15.Aryan Z, Ghajar A, Faghihi-Kashani S, Afarideh M, Nakhjavani M, Esteghamati A. Baseline high-sensitivity C-reactive protein predicts macrovascular and microvascular complications of type 2 diabetes: a population-based study. Ann Nutr Metab. 2018;72(4):287–295. doi: 10.1159/000488537. [DOI] [PubMed] [Google Scholar]
- 16.Cushman M, Arnold AM, Psaty BM, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women. Circulation. 2005;112(1):25–31. doi: 10.1161/CIRCULATIONAHA.104.504159. [DOI] [PubMed] [Google Scholar]
- 17.Song IU, Chung SW, Kim YD, Maeng LS. Relationship between the hs-CRP as non-specific biomarker and Alzheimer’s disease according to aging process. Int J Med Sci. 2015;12(8):613–617. doi: 10.7150/ijms.12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tracy RP, Lemaitre RN, Psaty BM, et al. Relationship of C-reactive protein to risk of cardiovascular disease in the elderly. Results from the Cardiovascular Health Study and the Rural Health Promotion Project. Arterioscler Thromb Vasc Biol. 1997;17(6):1121–1127. doi: 10.1161/01.atv.17.6.1121. [DOI] [PubMed] [Google Scholar]
- 19.Davizon-Castillo P, McMahon B, Aguila S, et al. TNF-α–driven inflammation and mitochondrial dysfunction define the platelet hyperreactivity of aging. Blood. 2019;134(9):727–740. doi: 10.1182/blood.2019000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olson NC, Callas PW, Hanley AJG, et al. Circulating levels of TNF-α are associated with impaired glucose tolerance, increased insulin resistance, and ethnicity: the insulin resistance atherosclerosis study. J Clin Endocrinol Metab. 2012;97(3):1032–1040. doi: 10.1210/jc.2011-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puchta A, Naidoo A, Verschoor CP, et al. TNF drives monocyte dysfunction with age and results in impaired anti-pneumococcal immunity. PLoS Pathog. 2016;12(1):e1005368. doi: 10.1371/journal.ppat.1005368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giovannini S, Onder G, Liperoti R, et al. Interleukin-6, C-reactive protein, and tumor necrosis factor-alpha as predictors of mortality in frail, community-living elderly individuals. J Am Geriatr Soc. 2011;59(9):1679–1685. doi: 10.1111/j.1532-5415.2011.03570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varadhan R, Yao W, Matteini A, et al. Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J Gerontol A Biol Sci Med Sci. 2014;69A(2):165–173. doi: 10.1093/gerona/glt023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker KA, Gross AL, Moghekar AR, et al. Association of peripheral inflammatory markers with connectivity in large-scale functional brain networks of non-demented older adults. Brain Behav Immun. 2020;87:388–396. doi: 10.1016/j.bbi.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gross AL, Walker KA, Moghekar AR, et al. Plasma markers of inflammation linked to clinical progression and decline during preclinical AD. Front Aging Neurosci. 2019;11. https://www.frontiersin.org/articles/10.3389/fnagi.2019.00229. Accessed 18 May 2023. [DOI] [PMC free article] [PubMed]
- 27.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrucci L, Penninx BWJH, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50(12):1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 29.Sayed N, Huang Y, Nguyen K, et al. An inflammatory aging clock (iAge) based on deep learning tracks multimorbidity, immunosenescence, frailty and cardiovascular aging. Nat Aging. 2021;1:598–615. doi: 10.1038/s43587-021-00082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassan L, Medenwald D, Tiller D, et al. The association between change of soluble tumor necrosis factor receptor R1 (sTNF-R1) measurements and cardiovascular and all-cause mortality—results from the population-based (Cardiovascular Disease, Living and Ageing in Halle) CARLA study 2002–2016. PLoS ONE. 2020;15(10):e0241213. doi: 10.1371/journal.pone.0241213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gohda T, Maruyama S, Kamei N, et al. Circulating TNF receptors 1 and 2 predict mortality in patients with end-stage renal disease undergoing dialysis. Sci Rep. 2017;7(1):43520. doi: 10.1038/srep43520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puzianowska-Kuźnicka M, Owczarz M, Wieczorowska-Tobis K, et al. Interleukin-6 and C-reactive protein, successful aging, and mortality: the PolSenior study. Immun Ageing A. 2016;13:21. doi: 10.1186/s12979-016-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nidadavolu LS, Feger D, Chen D, et al. Associations between circulating cell-free mitochondrial DNA, inflammatory markers, and cognitive and physical outcomes in community dwelling older adults. Immun Ageing A. 2023;20:24. doi: 10.1186/s12979-023-00342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pase MP, Himali JJ, Beiser AS, et al. Association of CD14 with incident dementia and markers of brain aging and injury. Neurology. 2020;94(3):e254–e266. doi: 10.1212/WNL.0000000000008682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenthal AJ, McMurtry CT, Sanders KM, Jacobs M, Thompson D, Adler RA. The soluble interleukin-2 receptor predicts mortality in older hospitalized men. J Am Geriatr Soc. 1997;45(11):1362–1364. doi: 10.1111/j.1532-5415.1997.tb02937.x. [DOI] [PubMed] [Google Scholar]
- 36.Qian S, Zhang H, Dai H, et al. Is sCD163 a clinical significant prognostic value in cancers? A systematic review and meta-analysis. Front Oncol. 2020;10:585297. doi: 10.3389/fonc.2020.585297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber GE, Khrestian M, Tuason ED, et al. Peripheral sTREM2-related inflammatory activity alterations in early-stage Alzheimer’s disease. J Immunol. 2022;208(10):2283–2299. doi: 10.4049/jimmunol.2100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eugen-Olsen J, Andersen O, Linneberg A, et al. Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J Intern Med. 2010;268(3):296–308. doi: 10.1111/j.1365-2796.2010.02252.x. [DOI] [PubMed] [Google Scholar]
- 39.Filippi BM, Lam TKT. Leptin and aging. Aging. 2014;6(2):82–83. doi: 10.18632/aging.100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen KE, Katunaric B, Senthil Kumar G, McIntosh JJ, Freed JK. Vascular endothelial adiponectin signaling across the life span. Am J Physiol Heart Circ Physiol. 2022;322(1):H57–H65. doi: 10.1152/ajpheart.00533.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laskow T, Langdon J, Abadir P, Xue QL, Walston J. Lactoferrin for the treatment of age-associated inflammation-a pilot study. Physiol Int. Published online April 9, 2021. 10.1556/2060.2021.00010. [DOI] [PMC free article] [PubMed]
- 43.Hintze JL, Nelson RD. Violin plots: a box plot-density trace synergism. Am Stat. 1998;52(2):181–184. doi: 10.1080/00031305.1998.10480559. [DOI] [Google Scholar]
- 44.Searle SR. Linear Models. Wiley; 1971.
- 45.Wolak ME, Fairbairn DJ, Paulsen YR. Guidelines for estimating repeatability: guidelines for estimating repeatability. Methods Ecol Evol. 2012;3(1):129–137. doi: 10.1111/j.2041-210X.2011.00125.x. [DOI] [Google Scholar]
- 46.Murali Krishna Rao K, Pieper CS, Currie MS, Cohen HJ. Variability of plasma IL-6 and crosslinked fibrin dimers over time in community dwelling elderly subjects. Am J Clin Pathol. 1994;102(6):802–805. doi: 10.1093/ajcp/102.6.802. [DOI] [PubMed] [Google Scholar]
- 47.Epstein MM, Breen EC, Magpantay L, et al. Temporal stability of serum concentrations of cytokines and soluble receptors measured across two years in low-risk HIV seronegative men. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2013;22(11):10.1158/1055-9965.EPI-13-0379. [DOI] [PMC free article] [PubMed]
- 48.Lefèvre N, Corazza F, Valsamis J, et al. The number of X chromosomes influences inflammatory cytokine production following toll-like receptor stimulation. Front Immunol. 2019;10. https://www.frontiersin.org/articles/10.3389/fimmu.2019.01052. Accessed 7 Jun 2023. [DOI] [PMC free article] [PubMed]
- 49.Spolarics Z. The X-files of inflammation: cellular mosaicism of X-linked polymorphic genes and the female advantage in the host response to injury and infection. Shock Augusta Ga. 2007;27(6):597–604. doi: 10.1097/SHK.0b013e31802e40bd. [DOI] [PubMed] [Google Scholar]
- 50.Yin GN, Jeon H, Lee S, Lee HW, Cho JY, Suk K. Role of soluble CD14 in cerebrospinal fluid as a regulator of glial functions. J Neurosci Res. 2009;87(11):2578–2590. doi: 10.1002/jnr.22081. [DOI] [PubMed] [Google Scholar]
- 51.Bethel M, Bůžková P, Fink HA, et al. Soluble CD14 and fracture risk. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2016;27(5):1755–1763. doi: 10.1007/s00198-015-3439-9. [DOI] [PubMed] [Google Scholar]
- 52.Møller HJ. Soluble CD163. Scand J Clin Lab Invest. 2012;72(1):1–13. doi: 10.3109/00365513.2011.626868. [DOI] [PubMed] [Google Scholar]
- 53.Siwan E, Twigg SM, Min D. Alterations of CD163 expression in the complications of diabetes: a systematic review. J Diabetes Complications. 2022;36(4):108150. doi: 10.1016/j.jdiacomp.2022.108150. [DOI] [PubMed] [Google Scholar]
- 54.Durda P, Raffield LM, Lange EM, et al. Circulating soluble CD163, associations with cardiovascular outcomes and mortality, and identification of genetic variants in older individuals: the cardiovascular health study. J Am Heart Assoc. 2022;11(21):e024374. doi: 10.1161/JAHA.121.024374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Durda P, Sabourin J, Lange EM, et al. Plasma levels of soluble interleukin-2 receptor α. Arterioscler Thromb Vasc Biol. 2015;35(10):2246–2253. doi: 10.1161/ATVBAHA.115.305289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujita N, Okamoto Y, Gotoh Y, et al. Serum evaluation of the balance between soluble interleukin-2 and interleukin-4 receptors. Cytokine. 2005;32(3):143–148. doi: 10.1016/j.cyto.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 57.Rea IM, Stewart M, Campbell P, Alexander HD, Crockard AD, Morris TCM. Changes in lymphocyte subsets, interleukin 2, and soluble interleukin 2 receptor in old and very old age. Gerontology. 2009;42(2):69–78. doi: 10.1159/000213775. [DOI] [PubMed] [Google Scholar]
- 58.Xie M, Yunis J, Yao Y, et al. High levels of soluble CD25 in COVID‐19 severity suggest a divergence between anti‐viral and pro‐inflammatory T‐cell responses. Clin Transl Immunol. 2021;10(2). 10.1002/cti2.1251. [DOI] [PMC free article] [PubMed]
- 59.Huang CM, Xu XJ, Qi WQ, Ge QM. Prognostic significance of soluble CD25 in patients with sepsis: a prospective observational study. Clin Chem Lab Med CCLM. 2022;60(6):952–958. doi: 10.1515/cclm-2022-0068. [DOI] [PubMed] [Google Scholar]
- 60.Bakhshi H, Varadarajan V, Ambale-Venkatesh B, et al. Association of soluble interleukin-2 receptor α and tumour necrosis factor receptor 1 with heart failure: the multi-ethnic study of atherosclerosis. ESC Heart Fail. 2020;7(2):639–644. doi: 10.1002/ehf2.12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bien E, Balcerska A. Serum soluble interleukin 2 receptor α in human cancer of adults and children: a review. Biomarkers. 2008;13(1):1–26. doi: 10.1080/13547500701674063. [DOI] [PubMed] [Google Scholar]
- 62.Zhang RJ, Zhang X, Chen J, et al. Serum soluble CD25 as a risk factor of renal impairment in systemic lupus erythematosus—a prospective cohort study. Lupus. 2018;27(7):1100–1106. doi: 10.1177/0961203318760993. [DOI] [PubMed] [Google Scholar]
- 63.Gao Q, Camous X, Lu YX, Lim ML, Larbi A, Ng TP. Novel inflammatory markers associated with cognitive performance: Singapore longitudinal ageing studies. Neurobiol Aging. 2016;39:140–146. doi: 10.1016/j.neurobiolaging.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 64.Pischon T, Hotamisligil GS, Rimm EB. Adiponectin: stability in plasma over 36 hours and within-person variation over 1 year. Clin Chem. 2003;49(4):650–652. doi: 10.1373/49.4.650. [DOI] [PubMed] [Google Scholar]
- 65.Lee SA, Kallianpur A, Xiang YB, et al. Intra-individual variation of plasma adipokine levels and utility of single measurement of these biomarkers in population-based studies. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2464–2470. doi: 10.1158/1055-9965.EPI-07-0374. [DOI] [PubMed] [Google Scholar]
- 66.Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003;14(6):561. doi: 10.1097/00041433-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 67.Shibata R, Sato K, Pimentel DR, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11(10):1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li N, Zhao S, Zhang Z, et al. Adiponectin preserves metabolic fitness during aging. Isales C, Zaidi M, Isales C, eds. eLife. 2021;10:e65108. 10.7554/eLife.65108. [DOI] [PMC free article] [PubMed]
- 69.Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14(10):576–590. doi: 10.1038/s41574-018-0059-4. [DOI] [PubMed] [Google Scholar]
- 70.Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542(7640):177–185. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- 71.Obata Y, Yamada Y, Takahi Y, et al. Relationship between serum adiponectin levels and age in healthy subjects and patients with type 2 diabetes. Clin Endocrinol (Oxf) 2013;79(2):204–210. doi: 10.1111/cen.12041. [DOI] [PubMed] [Google Scholar]
- 72.Komici K, Dello Iacono A, De Luca A, et al. Adiponectin and sarcopenia: a systematic review with meta-analysis. Front Endocrinol. 2021;12:576619. doi: 10.3389/fendo.2021.576619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.