Abstract
Introduction
COVID-19 remains a significant risk for the immunocompromised given their lower responsiveness to vaccination or infection. Therefore, passive immunity through long-acting monoclonal antibodies (mAbs) offers a needed approach for pre-exposure prophylaxis (PrEP). Our study evaluated safety, anti-SARS-CoV-2 neutralizing activity, nasal penetration, and pharmacokinetics (PK) of two half-life-extended investigational mAbs, AER001 and AER002, providing the first demonstration of upper airway penetration of mAbs with the LS-modification.
Methods
This randomized, double-blind, placebo-controlled phase I study enrolled healthy adults (n = 80) who received two long-acting COVID mAbs (AER001 and AER002), AER002 alone, or placebo. The dose ranged from 100 mg (mg) to 1200 mg per mAb component. The primary objective was to describe the safety and tolerability following intravenous (IV) administration. Secondary objectives were to describe PK, anti-drug antibodies (ADA), neutralization activity levels, and safety evaluation through 6 months of follow-up.
Results
The majority (97.6%) of the reported adverse events (AE) post administration were of grade 1 severity. There were no serious adverse events (SAE) or ADAs. AER001 and AER002 successfully achieved an extended half-life of 105 days and 97.5 days, respectively. Participants receiving AER001 and AER002 (300 mg each) or AER002 (300 mg) alone showed 15- and 26-fold higher neutralization levels against D614G and omicron BA.1 than the placebo group 24 h post-administration. Single 300 or 1200 mg IV dose of AER001 and AER002 resulted in nasal mucosa transudation of approximately 2.5% and 2.7%, respectively.
Conclusion
AER001 and AER002 showed an acceptable safety profile and extended half-life. High serum neutralization activity was observed against D614G and Omicron BA.1 compared to the placebo group. These data support that LS-modified mAbs can achieve durability, safety, potency, and upper airway tissue penetration and will guide the development of the next generation of mAbs for COVID-19 prevention and treatment.
Trial Registration
EudraCT Number 2022-001709-35 (COV-2022-001).
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-023-00908-9.
Keywords: COVID-19, Monoclonal antibodies, Long acting, Neutralization, Immunocompromised, Transudation, Nasal mucosa, Pre-exposure prophylaxis
Key Summary Points
| Why carry out the study? |
| This placebo-controlled phase I study was carried out to evaluate safety, tolerability, pharmacokinetics (PK), immunogenicity and upper airway penetration of a single intravenous (IV) dose of AER001 and AER002 in healthy participants. |
| What was learned from this study? |
| AER001 and AER002 were safe and well tolerated in healthy adults with no serious adverse events (SAE) or ADAs. |
| The observed half-life supported that these monoclonal antibodies (mAbs) would be long-acting. |
| AER001 and AER002 achieve nasal mucosa transudation which is desirable for pre-exposure prophylaxis. |
Introduction
Although the COVID emergency has been declared over for the general population, worldwide, the risk of COVID remains a serious health threat to populations that cannot develop durable protective immunity. AER001 (P5C3) and AER002 (P2G3) are two long-acting fully human immunoglobulin G1 (IgG1) monoclonal antibodies (mAbs) selected from the plasma of convalescent and vaccinated patients with COVID-19 for potential treatment and prophylaxis of COVID-19 [1, 2]. AER001 and AER002 bind to the receptor binding domain (RBD) of the Spike protein (S-protein) on non-overlapping epitopes and compete with angiotensin-converting enzyme 2 (ACE2) to block viral attachment and subsequent entry into the human cell. Structural models based on cryogenic electron microscopy (Cryo-EM) structures show the simultaneous binding of both mAbs to the RBD of the spike protein. Each mAb carries the LS mutations (Met428Leu/Asn434Ser) in Fc to extend in vivo serum half-life [3]. AER001 and AER002 are produced by recombinant DNA technology in a Chinese hamster ovary (CHO) mammalian cell expression system. For AER001, the N-glycosylation site in hCDR3 has been removed. For AER002, asparagine deamidation and asparagine isomerization consensus motifs have been removed for manufacturing purposes.
Antibodies are classified on the basis of their binding mode to the S-protein. Class 1 antibodies are commonly derived from VH3-53/VH3-66 germlines and compete with ACE2 for binding site and only recognize “up” RBD. Class 3 antibodies bind to the RBD on the opposite side of class 1 binding epitope and can generally bind to both up and down RBD conformations [4, 5].
AER001 is a class 1 mAb that displays high levels of in vitro neutralizing activity against all early SARS-CoV-2 variants including Alpha, Beta, Delta, and Omicron BA.1 and BA.2 but not BA.4, BA.5, BA.4.6, BQ.1, or BQ.1.1.
AER002 is a class 3 mAb that potently neutralizes Alpha, Beta, Delta (B.1), and Omicron (BA.1, BA.1.1, BA.2, BA.4, BA.4.6, and BA.5). AER002 also retains some potential neutralization activity against the K444T-containing BQ.1 variant, albeit at a lower level than with earlier Omicron variants. Although AER002 is not effective against Omicron subvariants BQ.1.1, XBB.1, and XBB.1.5, it can potentially neutralize non-replicating S-protein of SARS-CoV-2 and subgenomic RNA from older variants that exist on the cell surface (S-protein) and can sometimes be detected in plasma of those with post-acute sequelae of COVID (i.e., long Covid) [6].
This study evaluated the safety profile of AER001 and AER002 as well as the pharmacokinetics (PKs), anti-SARS-CoV-2 neutralization activity, and nasal mucosal transudation in healthy adults.
Methods
Clinical Study Design
This randomized, double-blind, placebo-controlled, dose-escalation study enrolled 80 healthy adults to assess the safety, tolerability, PKs, and nasal mucosal penetration of a single mAb (AER002) and two tandemly administered mAbs (AER001 and AER002) in healthy adults. Placebo was a formulation buffer (solution) of 20 mM histidine, 8% (w/v) sucrose, 0.04% (w/v) Polysorbate 80, pH 6.0, same as that used for AER001 or AER002. Subject numbers were generated according to the randomization code generated by the biostatistician. A final randomization list was created, peer-reviewed, and approved by biostatisticians who were not members of the study team. The approved randomization list was transferred to the pharmacy and kept in a restricted area to which only the unblinded pharmacist had access. Study drug or placebo assignments were blinded to the investigator, subjects, contract research organization (CRO), and all clinical and research staff through day 85 post procedure, except for designated pharmacy staff who were unblinded to prepare the study drug or placebo. Good Clinical Practice (GCP) was followed as defined by the International Council for Harmonization (ICH) and all applicable federal and local regulations. The study protocol and informed consent documentation were reviewed and approved by Stichting Beoordeling Ethiek Biomedisch Onderzoek, an independent medical research ethics committee (MREC). Written informed consent was obtained from all study participants included in the study. The study protocol and informed consent form were reviewed and approved by the Netherlands National Ethics Committee (EC), known as the Central Committee on Research involving Human Subjects. The study was conducted at a single site, ICON-EDS in Groningen, Netherlands. The key inclusion criteria were adults at least 18 years old and less than 50 years, negative reverse transcription-polymerase chain reaction (RT-PCR) SARS-CoV-2 test less than 72 h before randomization and administration, body mass index (BMI) of 18 to 32 kg/m2. The key exclusion criteria were history of any hospitalization (> 24 h) within 30 days of first screening visit, history of any clinically significant medical conditions, and subjects who receive COVID-19 vaccine or booster at least 2 weeks prior to screening.
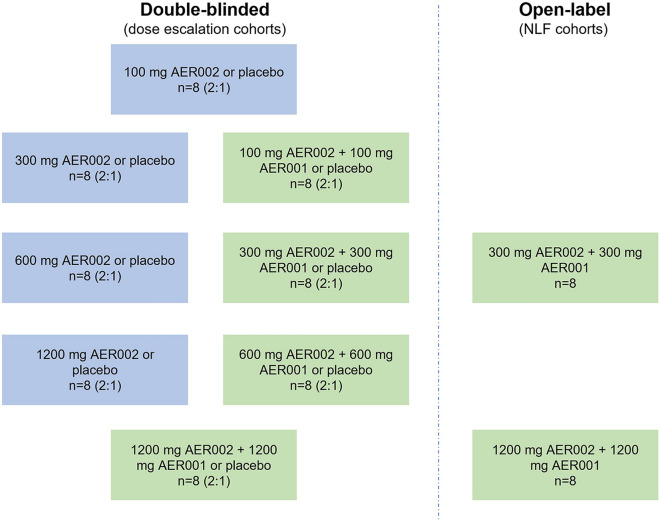
The study consisted of three cohorts of eight dosing regimens, 10 groups in total (Fig. 1). Different cohorts were designed to evaluate individual mAb doses ranging from 100 to 1200 mg. Participants in the active arms received an IV infusion of AER002 only, or a combination of AER001 and AER002 administered sequentially. There were four dose escalations in the study. For each dose escalation, the safety and tolerability data were reviewed by the principal investigator (PI), dose escalation review team (DRT), and the EC with agreement for the protocol-specified dosing to continue. Owing to AER001 and AER002 inactivity against the new Omicron subvariants BQ.1.1 and XBB.1.5, the study was terminated early.
Fig. 1.
Clinical trial design. AER002 dose escalation cohorts randomized 32 participants (4 groups), each received a single dose of AER002 (in blue; 100 mg, 300 mg, 600 mg, or 1200 mg). AER001 and AER002 dose escalation cohorts (in green) randomized 32 participants (4 groups), each received 2 sequential doses of AER002 and then AER001 (100 mg of each mAb, 300 mg of each mAb, 600 mg of each mAb, or 1200 mg of each mAb). NLF cohorts randomized 16 participants (2 groups), each received 2 sequential doses of AER002 and then AER001 (300 mg of each mAb or 1200 mg of each mAb)
Live Virus Neutralization Assay
Clinical trial serum samples were evaluated against the D614G (BavPat1/2020) and Omicron BA.1 (hCoV-19/Netherlands/NH-RIVM-72291/2021) VoC using the validated SARS-CoV-2 microneutralization assay (MN) at Viroclinics Bioscience. A standard number of SARS-CoV-2 infectious units were incubated at 37 °C, 5% CO2 with serial dilutions of serum. After a 60 ± 15 min pre-incubation period of the serum/virus mixtures, 100 µL of the mixture was added to Vero E6 cells for 60 ± 15 min and then removed and replaced by 100 µL of infection medium (Minimal Essential Medium (MEM), l-glutamine, non-essential amino acids (NEAA), (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES), fetal bovine serum-heat inactivated (FBS-HI) and antibiotic—antimycotic) with 1.6% carboxymetycellulose (CMC). After 22 ± 2 h incubation, cells were formalin-fixed followed by incubation with a mAb targeting the viral nucleocapsid protein, followed by a secondary anti-human IgG peroxidase conjugate (Thermo Fisher Scientific) and TrueBlue™ substrate. The formation of the blue precipitate on the nucleocapsid-positive cells in each well was acquired by a CTL ImmunoSpot™ analyzer, equipped with software to quantitate the nucleocapsid-positive cells (virus signal). The 80% neutralization titer (MN80) was calculated as described previously [7].
Upper Airway mAb Penetration PK Assay
Nasal lining fluid (NLF) samples were collected by Nasosorption™ FX.i device (Hunt Developments) from the surface of the inferior turbinate. NLFs were extracted with 1 mL of phosphate-buffered saline (PBS) containing 0.5% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate hydrate (CHAPS). NLF urea concentration was measured with a fluorometric assay kit (Cayman Chemicals, USA). The target mAbs, AER001 and AER002, were precipitated from the NLF with ammonium sulfate and digested using trypsin according to the procedure below.
An aliquot of 100 µL NLF extract was transferred to 1.5-mL tubes and mixed with 100 µL water saturated with ammonium sulfate. After vortex mixing, the tubes were incubated at room temperature for at least 15 min and centrifuged for 15 min at a minimum of 30,000 × g at 8 °C. After centrifugation, the tubes were incubated at room temperature for at least 15 min. The supernatant was removed and 20 µL of a mixture of 8 M urea in 50 mM ammonium bicarbonate/0.2 M dithiothreitol (DTT) in water (20:1, volume concentration of the solute in solution, v/v) was added to each tube. After vortex mixing, the tubes were incubated at 37 °C for 30 min. The digestion buffer was prepared immediately before use by adding 3 µg sequencing-grade trypsin per sample to 86 µL internal standard solution per well. Subsequently, 90–100 µL digestion buffer was added to each sample followed by an incubation at 37 °C for 2 h. Finally, 10 µL water/trifluoroacetic acid (9:1, v/v) was added and the samples were centrifuged for 10 min at a minimum of 30,000 × g at 8 °C. A 10-µL aliquot was injected into the high-performance liquid chromatography (HPLC) tandem mass spectrometry (MS/MS system. The analysis was done by separation using reversed-phase liquid chromatography followed by detection with triple-stage quadrupole MS/MS in the selected reaction monitoring mode. The ratio of urea concentrations in NLF eluant and in serum was used for normalization of AER001 and AER002 concentrations in the NLF to correct for differences in NLF sample volumes collected, and extraction efficiency.
Bioanalytical Assay of mAb Serum Concentrations
A 12-μL aliquot of serum sample was mixed with 180 μL water/water saturated with ammonium sulfate (1:1, v/v). After vortex mixing, the tubes were incubated at room temperature for at least 15 min and centrifuged for 15 min at a minimum of 30,000 × g at 8 °C. After centrifugation, the tubes were incubated at room temperature for at least 15 min. The supernatant was removed and 20 μL of a mixture of 8 M urea in 50 mM ammonium bicarbonate/0.2 M DTT in water (20:1, v/v) was added to each tube. After vortex mixing, the tubes were incubated at 37 °C for 30 min. The digestion buffer was prepared immediately before use by adding 3 μg sequencing-grade trypsin per sample to 86 μL internal standard solution per well. Subsequently, 90–100 μL digestion buffer was added to each sample followed by an incubation at 37 °C for 3 h. Finally, 10 μL water/trifluoroacetic acid (9:1, v/v) was added and the samples were centrifuged for 10 min at a minimum of 30,000 × g at 8 °C.
Each antibody was quantified by one unique signature peptide from its CDR1 region using an isotope-labelled internal standard peptide and LC–MS/MS. For this purpose, an aliquot of 10 μL of the tryptic digest was injected into the LC MS/MS system. The atmospheric pressure ionization interface was used as an ion source in positive ion mode. AER001 and AER002, and the internal standards AER001[13C6] SSYLGWYQQKPGQAPR and AER002[13C6,15N4] FDDYALHWVR were measured in selected reaction monitoring mode.
Safety
Throughout the study the subjects were closely monitored. AEs were evaluated by the principal investigator from the moment informed consent was signed by the subject through a minimum of 6 months follow-up. The safety assessment conducted by the principal investigator included serum chemistries, urinalysis, and hematologic testing pre-dose (day 0) and post-dose (days 2, 8, and 85).
Statistical Methods
Sample size (n = 80) was selected to provide information on safety, tolerability, PK, and pharmacodynamics (PD) following single/multiple doses of AER001 and AER002. Prospective calculations of statistical power were not done. The safety analysis set consisted of all the participants who had undergone randomization and received at least one infusion of AER002, AER001 + AER002, or placebo. AER001 and AER002 concentrations following IV administration are shown as mean with standard deviation (SD) and predicted mean. Neutralizing antibody titers are reported as geometric mean MN80 titer (SD). AER001 and AER002 concentrations in the NLF are shown as individual values and as means. The NLF serum partition ratio refers to the relative levels of AER001 or AER002 found in the NLF compared with their serum levels, expressed as a percentage. AER001 and AER002 NLF serum partition % are shown as individual values. GraphPad Prism software (versions 8.4.3 or higher) was used for data analysis and graph production. PK parameters were calculated using non-compartmental analysis with Phoenix WinNonLin™ version 6.3 (Pharsight Corp., St. Louis, Mo, USA).
Results
Enrollment and Demographic Information
Eighty participants were enrolled in the study between 29 August 2022 and 29 December 2022; 55.0% (44/80) were female and 45.0% (36/80) were male. Most subjects were white, 75/80 (93.8%). The mean age of the subjects was 26 years with a range from 18 to 50 years (Table 1). The proportion of individuals reporting prior vaccination or infection was 97.5% for the group that received AER001 and AER002, 95.8% for the group that received AER002, and 100% for the group that received placebo.
Table 1.
Demographics information
| Category or statistic | 100 mg AER002 (N = 6) |
300 mg AER002 (N = 6) |
600 mg AER002 (N = 6) |
1200 mg AER002 (N = 6) |
Placebo (N = 8) |
Total (N = 32) |
|
|---|---|---|---|---|---|---|---|
| Gender n (%) | Male | 2 (33.3) | 1 (16.7) | 5 (83.3) | 1 (16.7) | 6 (75.0) | 15 (46.9) |
| Female | 4 (66.7) | 5 (83.3) | 1 (16.7) | 5 (83.3) | 2 (25.0) | 17 (53.1) | |
| Race n (%) | White | 6 (100.0) | 5 (83.3) | 6 (100.0) | 6 (100.0) | 6 (75.0) | 29 (90.6) |
| American Indian or Alaska Native | 0 | 1 (16.7) | 0 | 0 | 0 | 1 (3.1) | |
| White + Black or African American | 0 | 0 | 0 | 0 | 2 (25.0) | 2 (6.3) | |
| Ethnicity n (%) | Hispanic or Latino | 1 (16.7) | 1 (16.7) | 0 | 0 | 1 (12.5) | 3 (9.4) |
| Not Hispanic or Latino | 5 (83.3) | 5 (83.3) | 6 (100.0) | 6 (100.0) | 7 (87.5) | 29 (90.6) | |
| Age (years) | n | 6 | 6 | 6 | 6 | 8 | 32 |
| Mean | 28.7 | 25.3 | 24.7 | 25.7 | 24.5 | 25.7 | |
| SD | 11.72 | 11.25 | 6.62 | 4.18 | 5.24 | 7.81 | |
| Median | 24.5 | 21.0 | 21.5 | 25.0 | 23.5 | 23.5 | |
| Min | 19 | 19 | 18 | 21 | 18 | 18 | |
| Max | 50 | 48 | 34 | 33 | 36 | 50 | |
| Height (cm)a | n | 6 | 6 | 6 | 6 | 8 | 32 |
| Mean | 170.5 | 165.7 | 185.0 | 177.0 | 176.0 | 174.9 | |
| SD | 6.80 | 9.46 | 5.18 | 4.69 | 4.24 | 8.66 | |
| Median | 170.0 | 166.5 | 185.5 | 178.0 | 176.5 | 177.0 | |
| Min | 161 | 153 | 179 | 168 | 169 | 153 | |
| Max | 182 | 181 | 191 | 181 | 183 | 191 | |
| Weight (kg)a | n | 6 | 6 | 6 | 6 | 8 | 32 |
| Mean | 70.73 | 64.62 | 73.87 | 77.85 | 76.66 | 72.99 | |
| SD | 9.111 | 7.479 | 8.279 | 10.134 | 7.427 | 9.232 | |
| Median | 72.30 | 63.15 | 71.80 | 77.95 | 77.05 | 72.50 | |
| Min | 54.9 | 56.0 | 64.7 | 64.5 | 63.2 | 54.9 | |
| Max | 79.8 | 75.4 | 87.3 | 92.1 | 86.8 | 92.1 | |
| BMI (kg/m2)a | n | 6 | 6 | 6 | 6 | 8 | 32 |
| Mean | 24.40 | 23.57 | 21.55 | 24.78 | 24.71 | 23.86 | |
| SD | 3.563 | 2.314 | 1.899 | 2.419 | 1.917 | 2.593 | |
| Median | 23.60 | 22.95 | 21.70 | 24.10 | 25.05 | 23.20 | |
| Min | 19.5 | 20.7 | 18.8 | 22.6 | 22.1 | 18.8 | |
| Max | 29.8 | 27.0 | 23.9 | 28.4 | 28.0 | 29.8 |
| Category or statistic | AER002/AER001 (100 mg each) (N = 6) | AER002/AER001 (300 mg each) (N = 6) | AER002/AER001 (600 mg each) (N = 6) | AER002/AER001 (1200 mg each) (N = 6) | Placebo (N = 8) |
Total (N = 32) |
|
|---|---|---|---|---|---|---|---|
| Gender n (%) | Male | 4 (66.7) | 2 (33.3) | 3 (50.0) | 3 (50.0) | 4 (50.0) | 16 (50.0) |
| Female | 2 (33.3) | 4 (66.7) | 3 (50.0) | 3 (50.0) | 4 (50.0) | 16 (50.0) | |
| Race n (%) | White | 5 (83.3) | 5 (83.3) | 6 (100.0) | 6 (100.0) | 8 (100.0) | 30 (93.8) |
| American Indian or Alaska Native | 0 | 1 (16.7) | 0 | 0 | 0 | 1 (3.1) | |
| Asian | 1 (16.7) | 0 | 0 | 0 | 0 | 1 (3.1) | |
| Ethnicity n (%) | Hispanic or Latino | 0 | 1 (16.7) | 0 | 0 | 0 | 1 (3.1) |
| Not Hispanic or Latino | 6 (100.0) | 5 (83.3) | 6 (100.0) | 6 (100.0) | 8 (100.0) | 31 (96.9) | |
| Age (years) | n | 6 | 6 | 6 | 6 | 8 | 32 |
| Mean | 29.0 | 23.8 | 25.5 | 28.8 | 30.8 | 27.8 | |
| SD | 10.77 | 3.82 | 3.99 | 7.57 | 8.55 | 7.50 | |
| Median | 25.0 | 22.5 | 26.0 | 28.5 | 30.0 | 26.0 | |
| Min | 19 | 21 | 20 | 21 | 20 | 19 | |
| Max | 48 | 31 | 31 | 42 | 46 | 48 | |
| Height (cm)a | n | 6 | 6 | 6 | 6 | 8 | 32 |
| Mean | 179.8 | 176.0 | 174.8 | 175.5 | 184.5 | 178.5 | |
| SD | 10.98 | 9.14 | 9.54 | 9.83 | 10.38 | 10.13 | |
| Median | 176.0 | 175.5 | 175.0 | 174.5 | 184.5 | 176.0 | |
| Min | 169 | 164 | 162 | 164 | 173 | 162 | |
| Max | 195 | 192 | 188 | 190 | 202 | 202 | |
| Weight (kg)a | n | 6 | 6 | 6 | 6 | 8 | 32 |
| Mean | 80.40 | 72.55 | 68.62 | 74.35 | 78.63 | 75.14 | |
| SD | 10.626 | 15.990 | 14.826 | 9.540 | 13.015 | 12.883 | |
| Median | 80.20 | 69.80 | 69.25 | 76.50 | 79.80 | 77.55 | |
| Min | 68.3 | 54.8 | 50.3 | 61.7 | 55.1 | 50.3 | |
| Max | 95.7 | 93.5 | 85.1 | 87.6 | 95.3 | 95.7 | |
| BMI (kg/m2)a | n | 6 | 6 | 6 | 6 | 8 | 32 |
| Mean | 24.83 | 23.25 | 22.20 | 24.27 | 23.06 | 23.49 | |
| SD | 1.844 | 3.731 | 2.764 | 3.575 | 3.427 | 3.096 | |
| Median | 24.60 | 23.60 | 21.60 | 24.15 | 21.95 | 23.40 | |
| Min | 22.5 | 18.5 | 19.2 | 20.3 | 18.4 | 18.4 | |
| Max | 27.9 | 28.5 | 26.0 | 28.6 | 28.8 | 28.8 |
BMI body mass index, Max maximum, Min minimum, SD standard deviation
aHeight, weight and BMI determined at screening
AER001 and AER002 Demonstrated an Acceptable Safety Profile
A total of 164 adverse events (AEs) were reported post administration in 77.5% (62/80) of subjects. The majority, 97.6% (160/164), of the reported AEs were of grade 1 (mild) severity and 2.5% (4/164) were of grade 2 (moderate) severity. No grade 3 (severe or medically significant but not immediately life; hospitalization or prolongation of hospitalization or disabling), grade 4 (life-threatening consequences; urgent intervention), or grade 5 (death related AEs) were reported. The most common reported AEs were infusion-related reactions 19.5% (32/164), headache 17.1% (28/164), common cold 13.4% (22/164), and blood sampling site reactions 5.5% (9/164). There were no SAEs or ADAs. Only 1.2% (2/164) AEs (infusion-related site reactions (IRSR), erythema, and tenderness) were rated as possibly related to the investigational products by the investigator. One subject discontinued from the study (withdrawal by subject because of relocation to another country, no AE reported) 14 days post administration (placebo recipient). There were four breakthrough COVID-19 cases post administration. Subject 1012 received 300 mg AER002 and tested positive at day 169 (March 2023), subject 1032 received 1200 mg AER002 and tested positive at day 120 (March 2023), subject 2004 received 100 mg each of AER001 and AER002 and tested positive at day 57 (November 2022), and subject 2017 received a placebo and tested positive at day 29 (November 2022). Subjects 1012, 1032, 2004, and 2017 had a history of receiving at least one dose of COVID-19 vaccine (1, 3, 2, and 2 doses, respectively). All the breakthrough COVID-19 cases were mild with no treatment required and the ones occurring after mAb administration occurred between November 2022 and March 2023, when more resistant subvariants were in dominant circulation.
AER001 and AER002 Exhibited a Prolonged Half-Life in Healthy Adults
The pharmacokinetic characteristics of AER001 and AER002 were evaluated in a randomized, double-blind, placebo-controlled study of 80 healthy adults (EUDRACT Number 2022-001709-35). The study is completed, and the data presented here are the 6-month AER002 and 4-month AER001 + AER002 data.
Participants in the active arm received an IV infusion dose of 100 mg, 300 mg, 600 mg, or 1200 mg of AER002 only, or a combination of AER001 and AER002. In the combination treatment, AER001 and AER002 were administered sequentially (AER002 followed by AER001, each at a 1:1 ratio), resulting in total combined doses of 200 mg, 600 mg, 1200 mg, or 2400 mg. Serum concentrations confirmed the extended half-life of 105 days for AER001 and 97.5 days for AER002 when administered at a dose of 300 mg (Fig. 2, Table 2). After a single IV dose of 300 mg of AER001 and AER002, the Cmax values were respectively 85.5 and 111 µg/mL for a Tmax at 0.50 h for AER001 and 0.49 h for AER002 (Fig. 2, Table 2).
Fig. 2.
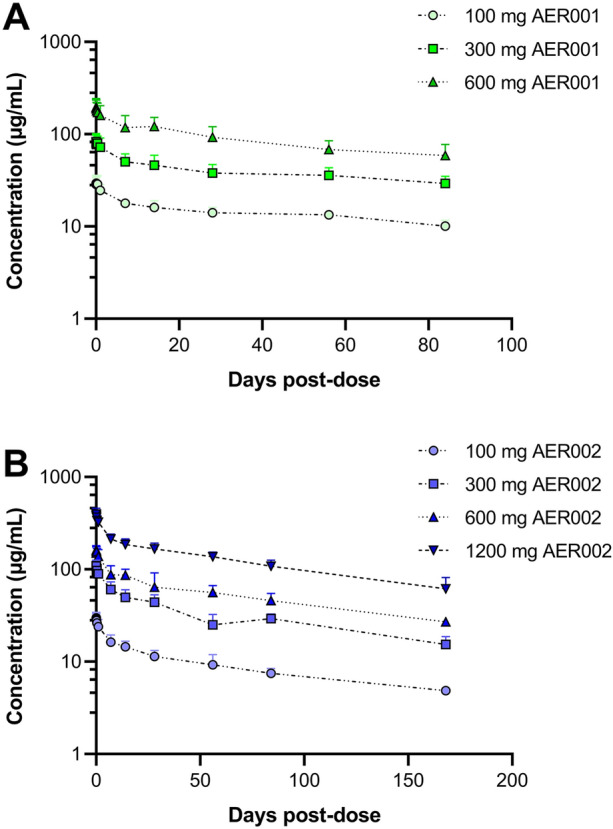
AER001 and AER002 exhibit extended half-life. Serum concentrations of AER001 and AER002 were measured over 3 months for AER001 (a) and 6 months for AER002 (b) following single intravenous (IV) administration of AER002 or AER001 and AER002 in healthy participants. Symbols represent the observed mean + SD and lines represent the predicted mean
Table 2.
Summary of pharmacokinetic parameters following a single intravenous administration of AER002 or AER001 and AER002
| PK Parameters | AER001 (300 mg)a | AER002 (300 mg)b |
|---|---|---|
| Cmax (µg/mL)c | 85.5 (19.8) | 111 (12.4) |
| Tmax (h)d | 0.50 (0.01–8.00) | 0.49 (0.03–1.00) |
| AUC0–28 (h·µg/mL)c | 32,408 (26.3) | 37,635 (15.5) |
| T1/2 (days)c | 105 (26.3) | 97.5 (29.3) |
| Kel (h−1)c | 0.000275 (26.3) | 0.000296 (29.3) |
aPharmacokinetic parameters from the 300 mg AER002 + 300 mg AER001 cohort
bPharmacokinetic parameters from the 300 mg AER002 cohort
cGeometric mean (geometric % CV)
dMedian (range)
AER001 and AER002 Transudate to the Mucosal Surface
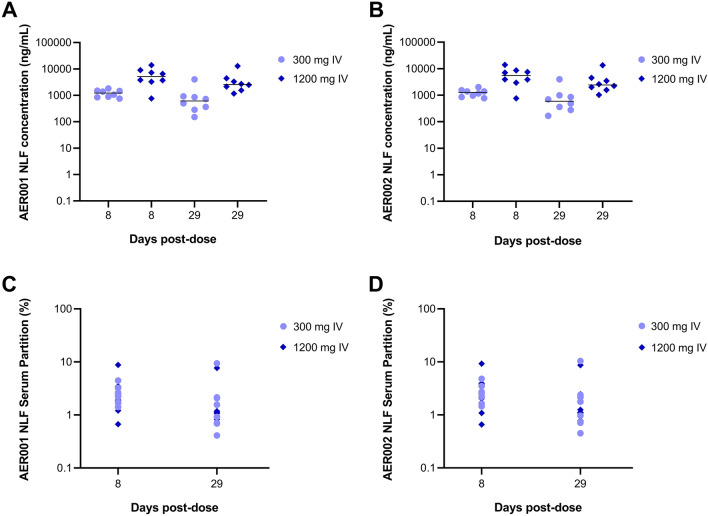
To evaluate the transudation of AER001 and AER002 to the nasal mucosa, we measured the concentration of AER001 and AER002 in the NLF at days 8 and 29 and we compared these concentrations to the level present in the serum. Following a single IV administration of AER001 and AER002 at 300 mg or 1200 mg each we measured an average ratio of 2.5% for AER001 concentration and 2.7% for AER002 concentration in the NLF compared to the serum which demonstrates the effective transcytosis of AER001 and AER002 carrying an LS-extended half-life mutation (Fig. 3).
Fig. 3.
AER001 and AER002 transudates to the nasal mucosal epithelium. a, b Concentrations of AER001 and AER002 were measured in the nasal lining fluid (NLF) after a single administration of 300 mg or 1200 mg intravenous (IV) doses of AER001 and AER002. c, d Serum partition of AER001 and AER002 present in the NLF after a single administration of 300 mg or 1200 mg IV doses of AER001 and AER002. Graph shows individual and median concentrations
AER001 and AER002 Exhibit a Level of Neutralizing Antibodies Against SARS-CoV-2 Superior to the Vaccination in Healthy Adults
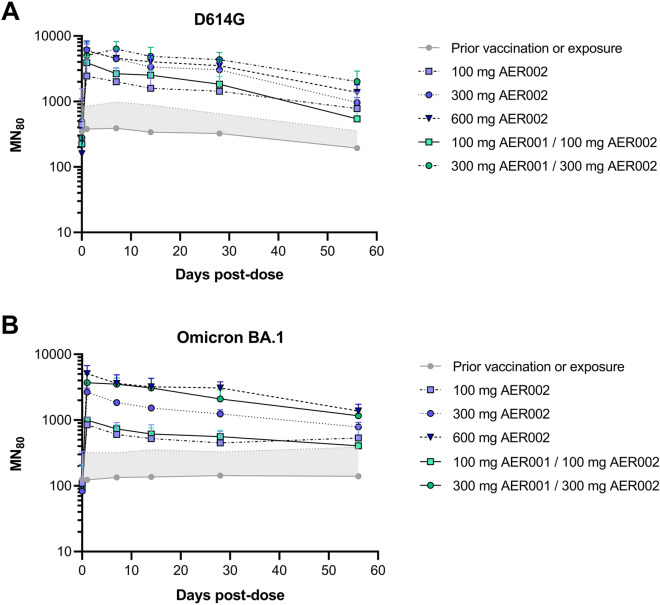
An interim neutralizing antibody analysis was conducted up to day 57 for the cohorts administered with 100 mg, 300 mg, 600 mg AER002 and 200 mg, 600 mg AER001 + AER002. Three participants (two in treatment groups, one in placebo) in the study were not previously vaccinated. A modified intention to treat analysis (ITT) analysis was conducted removing participants not vaccinated (n = 3), and participants infected during the trial (n = 2) and did not impact the results compared to the ITT. The geometric mean titers against D614G and Omicron BA.1 are presented in Fig. 4 for the modified ITT population. Figure 4a shows the results for D614G and Fig. 4b for Omicron BA.1. Overall, neutralizing antibodies following the 100 mg through 600 mg doses were observed at levels higher than those of the placebo/vaccinated control through the last measured time point of 57 days.
Fig. 4.
AER001 and AER002 confer higher anti-SARS-CoV-2 neutralizing antibody concentrations than the placebo, previously vaccinated group. Geometric mean neutralizing antibody titers (GMT) against SARS-CoV-2 (a D614G variant; b Omicron BA.1 variant) are shown over 2 months following single intravenous (IV) doses in healthy adult participants. Data represents the modified analysis of the geometric mean MN80 titer + SD for the vaccinated group, AER002 and AER001/AER002. Gray zone represents the upper bound of the GMT SD of the prior vaccination or exposure group at the different time points
Discussion
At this stage of the pandemic, healthy individuals are at much less risk of severe COVID owing to the availability of vaccines and antivirals. However, not everyone has been able to benefit from these advances. It is estimated that approximately 30 million people in the USA and EU [8, 9] have diagnosed immunodeficiencies which make them unable to mount adequate, durable immune responses following vaccination or infection. This number goes up substantially we include the elderly population, who not only are less likely to mount adequate immune responses but are also more likely to develop severe COVID [10]. Therefore, passive immunity using monoclonal antibodies presents a potentially optimal approach for the immunocompromised toward the prevention of SARS-CoV-2 infection. Monoclonal antibodies have been safely and effectively used for many years as targeted therapies for multiple diseases like malignancies, autoimmune or other infectious diseases [11]. The effectiveness of passive immunization using mAbs was well established by the studies of tixagevimab-cilgavimab (EVUSHELD™) and casirivimab and imdevimab (REGEN-COV™) early in the pandemic [12]. In fact, numerous independent lines of evidence now demonstrate that the neutralizing activity conferred by COVID mAbs correlates with clinical efficacy against COVID and even SARS-CoV-2 infection [13–18]. The level of neutralizing antibody-mediated activity (nAb) elicited by SARS-CoV-2 infection has also been associated with favorable disease outcome. Patients with COVID and low nAb levels had significant risk of death, while there were no deaths in a group of patients with high nAb levels [17]. In our trial, the enrolled population was non-naive, meaning they were previously vaccinated or infected, which is as expected for this stage of the pandemic. This resulted in a high level of the nAb titer in this group compared to some trials performed early in the pandemic [13]. With this context in mind, the lowest dose of AER002 (100 mg) at approximately 2 months following administration conferred a geometric mean neutralizing antibody titer (GMT) against the ancestral D614G and the omicron BA.1 strains that was still around fourfold greater than those detected in the vaccinated healthy population, which supports that COVID mAbs can provide better protection in the post-administration period than vaccines or natural exposure.
These results also support the extended duration of the protection level provided by LS-based COVID mAbs, which is an essential property for pre-exposure prophylaxis. These data provide proof of concept that the LS-based modifications (i.e., Met428Leu and Asn434Ser introduced in the Fc) can effectively extend serum half-life.
It has been previously demonstrated that LS mutations cause a decrease in the dissociation rate and an 11-fold improvement in binding activity between the Fc and human FcRn at pH 6. LS mutations conferred a threefold increase in antibody half-life in cynomolgus macaques and FcRn transgenic mice [3]. AER001 and AER002 LS-modified mAbs confirmed the extended half-life for all the different dosages ranging from 100 to 1200 mg that were administered in this study. The triple mutation Met252Tyr/Ser254Thr/Thr256Glu (YTE) is also used to reduce the antibody’s interaction with the FcRn and to slow down its degradation and clearance from the body [19]. For COVID mAbs the extended half-lives observed with YTE or LS mutations are very similar (Table 3).
Table 3.
Human half-life of COVID monoclonal antibodies with LS or YTE mutations
| Name | Extended half-life mutations | Plasma or serum half-life (days)a | References |
|---|---|---|---|
| AER001 | LS | 105 | na |
| AER002 | LS | 97.5 | na |
| S309 (sotrovimab) | LS | 56.5 | [23] |
| MabCo 19 (MAD0004J08) | LS | NA | NA |
| AZD8895 (tixagevimab) | YTE | 89 | [24] |
| AZD1061 (cilgavimab) | YTE | 84 | [24] |
| BRII-196 (amubarvimab) | YTE | 44.6–48.6 | [25] |
| BRII-198 (romlusevimab) | YTE | 72.2–83.0 | [25] |
na not applicable, NA not available, LS Met428Leu/Asn434Ser mutations in Fc to extend in vivo serum half-life, YTE Met252Tyr/Ser254Thr/Thr256Glu mutations in Fc to extend in vivo serum half-life
aVarious bioanalytical assays have been used
In our study, we reported three breakthrough COVID-19 cases post-administration. These were not considered unexpected as they occurred between November 2022 and March 2023, a period when subvariants resistant to AER001 and AER002 dominated circulation in the Netherlands [20].
The safety analysis shows that the investigational mAb infusions were safe and well tolerated. Most of the AEs were mild and self-limited and related to the actual insertion of the venous cannula for the infusion. Only two AEs were felt by the investigator to be possibly related to the mAbs. The AEs were localized IRSRs which is consistent with the most common AEs seen with mAbs. Future work will include development of additional routes of administration to provide alternatives for medical practice settings.
There were certain expected study limitations. As a phase 1 trial, the number of subjects recruited was accordingly small which makes the ability to observe rare AEs unlikely. Also, access to detailed medical records regarding COVID-19 testing methodology was limited, but all positive reports were included. Genotypic and phenotypic characterization of variants in the breakthrough cases was not conducted in the context of this trial. From a methodologic standpoint, any cross comparison to serum partition NLF can be impacted by laboratory-to-laboratory variation and should be considered for any comparisons to historical data. This trial only evaluated IV dosing, and future trials would evaluate additional routes of administration.
To our knowledge, this clinical study is also the first to demonstrate conserved transcytosis mucosal activity for a mAb carrying an LS-extended half-life mutation. The 2.5% to 2.7% average transudation from serum to the NLF at day 8 and day 29 is slightly higher than described for other mAbs carrying a YTE mutation that targets SARS-CoV-2 [21, 22]. This attribute makes the LS-modified COVID mAb approach reasonable for the prevention of upper airway infection.
Conclusion
AER001 and AER002 demonstrated an excellent safety profile with no SAEs or ADAs detected. Extended half-life was confirmed with an average T1/2 of 105 days for AER001 and 97.5 days for AER002 when administered at a dose of 300 mg each. Mucosal transcytosis was also confirmed, which is important for the prevention of upper airway infection. The level of nAbs 2 months post-administration was also higher than the vaccinated group at even the lowest tested dose of the COVID mAbs, which supports that immunocompromised individuals could realize the protection that the vaccination provides to healthy individuals using a mAb PrEP approach. Taken together, the results of this trial support the further development of LS-modified COVID mAbs to protect the immunocompromised.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to express our deep gratitude to the study staff and participants, ICON, Viroclinics, Swiss BioQuant AG and all Aerium Therapeutics employees for their expertise and support of this clinical trial.
Author Contribution
All authors contributed to data interpretation, writing, and editing of the manuscript, and all reviewed and approved the manuscript for submission. We confirm that there are no other persons who satisfied the criteria for authorship but are not listed and that order of authors listed in the manuscript has been approved by all of us.
Funding
This study and the journal’s Rapid Service fee were supported by Aerium Therapeutics.
Data Availability
Data underlying the findings described in this manuscript and the datasets analyzed during the current study may be requested from the corresponding author in accordance with Aerium Therapeutics data sharing policy.
Declarations
Conflict of Interest
Norman Moullan, Josephat Asiago, Kathryn Stecco, Moetaz Albizem, Holly Tieu, Kai Lin, Thomas Lengsfeld, Lauren Poffenbarger, David Liu, Rajeev Venkayya, Björn Hock and Prakash Bhuyan are employees of or former employees and hold or may hold stock in Aerium Therapeutics. Craig Fenwick, Didier Trono, and Giuseppe Pantaleo are inventors on patent applications PCT/IB2021/050621 that covers AER001/AER002 (P5C3/P2G3) and is entitled “Neutralizing antibodies and use thereof in the treatment of SARS-CoV-2 infection”. Salah Hadi has no conflicts of interest to disclose.
Ethical Approval
The study protocol and informed consent documentation were reviewed and approved by Stichting Beoordeling Ethiek Biomedisch Onderzoek, an independent medical research ethics committee (MREC). Informed consent was obtained from all study participants included in the study. The study protocol and informed consent form were reviewed and approved by the Netherlands National Ethics Committee (EC), known as the Central Committee on Research involving Human Subjects. We further confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fenwick C, Turelli P, Ni D, et al. Patient-derived monoclonal antibody neutralizes SARS-CoV-2 Omicron variants and confers full protection in monkeys. Nat Microbiol. 2022;7(9):1376–1389. doi: 10.1038/s41564-022-01198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fenwick C, Turelli P, Perez L, et al. A highly potent antibody effective against SARS-CoV-2 variants of concern. Cell Rep. 2021;37(2):109814. doi: 10.1016/j.celrep.2021.109814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zalevsky J, Chamberlain AK, Horton HM, et al. Enhanced antibody half-life improves in vivo activity. Nat Biotechnol. 2010;28(2):157–159. doi: 10.1038/nbt.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shrestha LB, Tedla N, Bull RA. Broadly-neutralizing antibodies against emerging SARS-CoV-2 variants. Front Immunol. 2021;12:752003. doi: 10.3389/fimmu.2021.752003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes CO, Jette CA, Abernathy ME, et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020;588(7839):682–687. doi: 10.1038/s41586-020-2852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor PC, Adams AC, Hufford MM, De la Torre I, Winthrop K, Gottlieb RL. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat Rev Immunol. 2021;21(6):382–393. doi: 10.1038/s41577-021-00542-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zielinska E, Liu D, Wu HY, Quiroz J, Rappaport R, Yang DP, et al. Development of an improved microneutralization assay for respiratory syncytial virus by automated plaque counting using imaging analysis. Virol J. 2005;2:84. doi: 10.1186/1743-422X-2-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel M, Chen J, Kim S. Analysis of MarketScan data for immunosuppressive conditions and hospitalizations for acute respiratory illness United States. Emerg Infect Dis. 2020;26(7):1720–1730. doi: 10.3201/eid2608.191493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ECPC . Joint statement on the protection of immunocompromised patients during the COVID-19 pandemic. Brussels: European Cancer Patient Coalition; 2022. [Google Scholar]
- 10.Quinti I, Locatelli F, Carsetti R. The immune response to SARS-CoV-2 vaccination: insights learned from adult patients with common variable immune deficiency. Front Immunol. 2021;12:815404. doi: 10.3389/fimmu.2021.815404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJT, et al. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9(4):325–338. doi: 10.1038/nrd3003. [DOI] [PubMed] [Google Scholar]
- 12.Al-Obaidi MM, Gungor AB, Kurtin SE, Mathias AE, Tanriover B, Zangeneh TT. The prevention of COVID-19 in high-risk patients using tixagevimab-cilgavimab (Evusheld): real-world experience at a large academic center. Am J Med. 2023;136(1):96–99. doi: 10.1016/j.amjmed.2022.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Earle KA, Ambrosino DM, Fiore-Gartland A, et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39(32):4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng S, Phillips DJ, White T, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27(11):2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Beltran WF, Lam EC, Astudillo MG, et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021;184(2):476–488.e11. doi: 10.1016/j.cell.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert PB, Donis RO, Koup RA, Fong Y, Plotkin SA, Follmann D. A Covid-19 milestone attained—a correlate of protection for vaccines. N Engl J Med. 2022;387(24):2203–2206. doi: 10.1056/NEJMp2211314. [DOI] [PubMed] [Google Scholar]
- 17.Goldblatt D, Alter G, Crotty S, Plotkin SA, et al. Correlates of protection against SARS-CoV-2 infection and COVID-19 disease. Immunol Rev. 2022;310(1):6–26. doi: 10.1111/imr.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 19.Dall’Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn) J Biol Chem. 2006;281(33):23514–23524. doi: 10.1074/jbc.M604292200. [DOI] [PubMed] [Google Scholar]
- 20.The Dutch Government. Coronavirus variants over time. https://coronadashboard.government.nl/landelijk/varianten. Accessed 20 Sept 2023.
- 21.Loo YM, McTamney PM, Arends RH, et al. The SARS-CoV-2 monoclonal antibody combination, AZD7442, is protective in nonhuman primates and has an extended half-life in humans. Sci Transl Med. 2022;14(635):eabl8124. doi: 10.1126/scitranslmed.abl8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levin MJ, et al. Intramuscular AZD7442 (tixagevimab-cilgavimab) for prevention of Covid-19. N Engl J Med. 2022;386(23):2188–2200. doi: 10.1056/NEJMoa2116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.European Medicines Agency . Xevudy product information. Winter Garden: EMA; 2021. [Google Scholar]
- 24.European Medicines Agency. Evusheld: EPAR - Product information. European Medicines Agency; 2023.
- 25.Hoy SM. Amubarvimab/romlusevimab: first approval. Drugs. 2022;82(12):1327–1331. doi: 10.1007/s40265-022-01759-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying the findings described in this manuscript and the datasets analyzed during the current study may be requested from the corresponding author in accordance with Aerium Therapeutics data sharing policy.