Abstract
Degenerative cervical myelopathy (DCM) is a leading cause of age-related non-traumatic spinal cord disorders resulting from chronic degeneration of the cervical spine. While traditional clinical assessments rely on patient-reported measures, this study used the NIH Toolbox Motor Battery (NIHTBm) as an objective, quantitative measure to determine DCM severity. The objective is to define NIHTBm cutoff values that can accurately classify the severity of DCM neuromotor dysfunction. A case-controlled pilot study of patients with DCM and age-matched controls. The focus was an in-depth quantitative motor assessment using the NIHTBm to understand the severity of neuromotor deficits due to degenerative spine disease. Motor assessments, dexterity, grip strength, balance, and gait speed were measured in 45 DCM patients and 37 age-matched healthy subjects (HC). Receiver operating curve (ROC) analysis determined cutoff values for mild and moderate-to-severe myelopathy which were validated by comparing motor assessment scores with disability scores. The ROC curves identified thresholds for mild dexterity impairment (T-score range 38.4 − 33.5, AUC 0.77), moderate-to-severe dexterity impairment (< 33.5, AUC 0.70), mild grip strength impairment (47.4 − 32.0, AUC 0.80), moderate-to-severe grip strength impairment (< 32.0, AUC 0.75), mild balance impairment (36.4 − 33.0, AUC 0.61), and moderate-to-severe balance impairment (< 33.0, AUC 0.78). Mild gait speed impairment was defined as 0.78–0.6 m/sec (AUC 0.65), while moderate-to-severe gait speed impairment was < 0.6 m/sec (AUC 0.65). The NIHTB motor score cutoff points correlated negatively with the DCM neck disability index (NDI) and showed balance and dexterity measures as independent indicators of DCM dysfunction. The use of NIHTB allows for precise delineation of DCM severity by establishing cutoff values corresponding to mild and moderate-to-severe myelopathy. The use of NIHTB in DCM allows enhanced clinical precision, enabling clinicians to better pinpoint specific motor deficits in DCM and other neurological disorders with motor deficits, including stroke and traumatic brain injury (TBI). Furthermore, the utility of objective assessment, NIHTB, allows us to gain a better understanding of the heterogeneity of DCM, which will enhance treatment strategies. This study serves as a foundation for future research to facilitate the discovery of innovative treatment strategies for DCM and other neurological conditions.
Keywords: Degenerative cervical myelopathy, NIH Toolbox, Dexterity, Grip strength, Balance, mJOA, Neck disability index
Introduction
Degenerative cervical myelopathy (DCM) is the leading cause of chronic progressive spinal cord impairment, predominantly affecting individuals aged 55 or older [1, 2]. This age-related condition results in significant motor dysfunction, such as progressive weakness in the upper and lower extremities, loss of dexterity, poor balance, impaired gait, and bladder dysfunction [2]. The primary treatment for DCM is surgical decompression of the spinal cord [3–5]. Clinical assessment of DCM, including the Nurick grade and modified Japanese Association (mJOA) score, augments treatment decisions and understanding of treatment efficacy [6–8]. However, both Nurick and mJOA scales are subjective, restricted, and have low interrater reliability [9–11]. This highlights the need for a more objective, repeatable, and reliable measure of DCM dysfunctions.
The NIH Toolbox Motor Assessment (NIHTBm) is a possible tool for evaluating the severity of DCM [12]. This broad scale has been applied in the motor assessment of traumatic brain injury, stroke, spinal cord injury (SCI), and DCM [12–16]. The NIHTBm battery measures hand coordination, upper and lower extremity strength, balance, and gait quality, with quantitative scores corrected for age, gender, handedness, BMI, and ethnicity, among other demographic parameters [17]. According to the Neuroscience Blueprint for Research, the mean T scores for dexterity, grip strength, and balance are set at 50, with a standard deviation (SD) of 10 [17–19]. However, these values do not directly translate to determining disease severity [12].
Despite its objective design and proven reliability in neurological diseases, the NIHTBm lacks agreed-upon cutoff values for defining the severity of neurological deficits and guiding treatment decisions [16]. This paper aims to determine the NIHTBm cutoff points for classifying DCM severity, facilitating a better understanding of DCM’s functional heterogeneity and informing appropriate treatment choices. Establishing these objective criteria is crucial for clinical management and research.
By utilizing the diverse motor assessment scales provided by NIHTBm [12, 19], we can elucidate the granularity of DCM symptoms, in contrast to the mJOA scale’s coarse classification into three severity groups [6]. This study seeks to establish clear thresholds for separating mild DCM cases, which can be managed conservatively, from moderate-to-severe cases that typically require surgical intervention [20, 21]. This approach will enable a more nuanced understanding of DCM's heterogeneity based on the presence, absence, and degree of specific motor deficits, beyond the limitations of the mean T scores specified on the NIHTB by the Neuroscience Blueprint for Research.
Methods
Participants: standard protocol approvals, registrations, and patient consents
This study received institutional approval from BLINDED institutional review board (IRB). This study presents a secondary analysis of previously collected data from patients with degenerative cervical myelopathy (DCM) that were included in the research conducted by Muhammad et al. [12] The prospective data collection took place between March 2021 and April 2023. The inclusion criteria for the patients were a confirmed diagnosis by at least one attending neurosurgeon and an age range of 20–80 years. Table 1 provides demographic information for healthy control (HC) and DCM patients. Informed consent was obtained from all participants. Clinical assessment measures included the Neck Disability Index (NDI), modified Japanese Orthopaedic Association (mJOA) scale, and NIH Toolbox motor assessment tests. These tests encompassed the Nine Hole Peg Test (9HPT), handgrip strength measured with the hand-held dynamometer, standing balance test, and walking gait test, as previously described in the study by Muhammad et al [12].
Table 1.
Demographic and clinical characteristics of participants
| Characteristics | DCM patients (n = 45) | HC (n = 37) | p-value |
|---|---|---|---|
| Age (years) | 56.53 (9.5) | 53.41 (6.9) | 0.1 |
| Sex: female | 55.60% | 81.08% | |
| mJOA | 13.0 (3.30) | 18.0 (0.00) | < 0.0001 |
| Mild (mJOA 15–17) | 38% | ||
| Moderate (mJOA 14–12) | 33.33% | ||
| Severe (mJOA < 12) | 28.89% | ||
| NDI | 22.00 (11.00) | 0.00 (0.00) | < 0.0001 |
| Dexterity (T-score) | 32.33 (12.88) | 50.54 (9.83) | < 0.0001 |
| Grip strength (T-score) | 32.82 (17.58) | 55.00 (9.56) | < 0.0001 |
| Balance (T-score) | 28.42 (15.59) | 41.89 (6.61) | 0.0027 |
| Gait speed (m/sec) | 0.69 (0.29) | 1.02 (0.22) | < 0.0001 |
Statistical analysis
All statistical analyses conducted for this study were performed in GraphPad Prism version 9.5, Boston MA [22]. The Shapiro–Wilk test was used to determine the normality of the demographic and clinical assessment data. A two-sample t-test was used to compare the NDI measures between DCM categories. Pearson’s analysis was used to examine the correlation between the NIH toolbox measures. Statistical significance was set at a p-value < 0.05.
Establishing cut-off values for mild and moderate-to-severe DCM
Receiver operating curve (ROC) analysis was performed to visualize and determine the cut-off values of the NIHTBm measures at which the highest sensitivity and specificity are reached [23]. The mJOA scoring system was used as the anchor to determine cutoff points between healthy controls (HC) and mild myelopathy and between mild and moderate-to-severe myelopathy. The mJOA scale classifies the severity of DCM, with scores of 15–17 indicating mild myelopathy, 14–12 representing moderate myelopathy, and ≤ 11 signifying severe myelopathy. In this study, we grouped DCM patients based on treatment recommendations into mild (conservative or surgical treatment) and moderate-to-severe myelopathy (surgical spinal cord decompression) categories. To find the optimal thresholds where the sensitivity and specificity of the measures are balanced, we employed Youden’s index, defined as J = sensitivity + specificity − 1 [24, 25].
Internal validation of the severity criteria
The validity of the NIHTBm cut-off values were tested by comparing them with the disability index scores (NDI). We hypothesize that patients in moderate-to-severe would have a higher disability index exhibiting functional disability more elevated than the DCM patients in the mild group do.
Sample size determination
A power analysis was performed with G*Power software for minimum sample size determination [26]. Based on the effect sizes obtained from data from Muhammad et al. 2023 which compared NIHTB measures between HC and patients with DCM, we determine the minimum sample size required to achieve 80% power at a significance criterion of a = 0.05, was 8 in each group for dexterity measure, 6 in each group for grip strength test, 17 in each group for balance test, and 7 in each group for gait speed test. Therefore, sample sizes of 45 and 37 for DCM and HC, respectively, are adequate to test this study hypothesis.
Results
Participant characteristics—distribution of symptoms based on mJOA and NIHTB
Our sample consisted of 45 DCM (25 females; 20 males) patients and 37 (30 females;7 males) age-matched healthy controls (HC). The mean ± SD age of the DCM 57 ± 9.5 years (range 34–81) was not significantly different from the mean ± SD age of the HCs at 53 ± 6.9 years (range 39–66); p = 0.10. The mean ± SD mJOA score of DCM patients was was 13 ± 3.3 compared to HC mean ± SD mJOA score of 18 ± 0.0 Thirty-eight percent (38%) of the DCM group had mild myelopathy (with mJOA score of 15–17), and 62% had moderate-to-severe myelopathy (with mJOA score of ≤ 14). In addition, the mean ± SD NDI of DCM patients was 22 ± 11. The mean ± SD dexterity T-score of DCM patients was 32.33 ± 12.88 compared to 50.54 ± 9.83 for HCs, p < 0.0001. The mean ± SD grip strength T-score of DCM patients was 32.82 ± 17.58 vs. 55.00 ± 9.56 for HCs, p < 0.0001. The mean ± SD standing balance T-score of DCM patients was 28.42 ± 15.59 vs. 41.89 ± 6.61 for HCs, p < 0.0001. Lastly, the mean ± SD gait speed of DCM patients was 0.69 ± 0.29 m/sec vs. 1.02 ± 0.22 m/sec for HCs, p < 0.0001 Table 1.
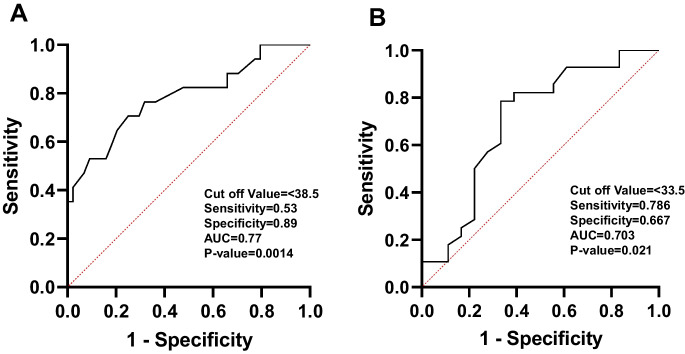
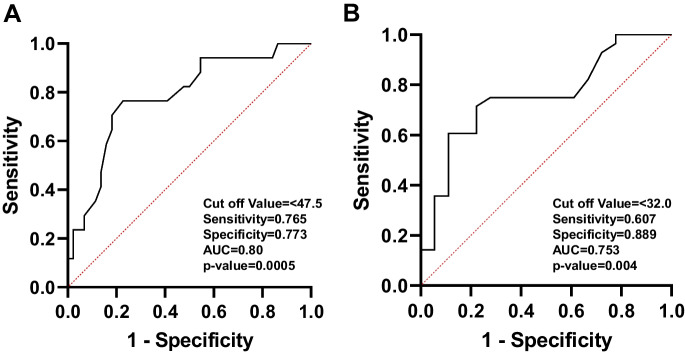
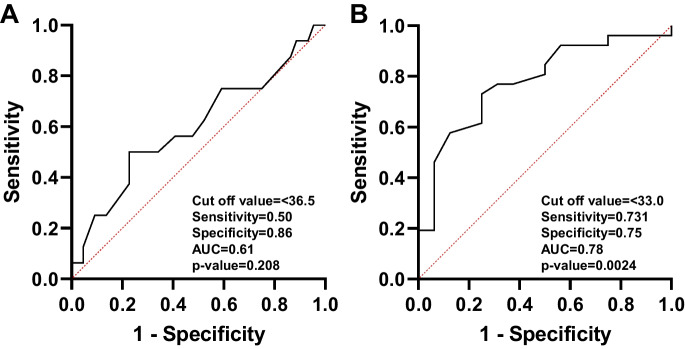
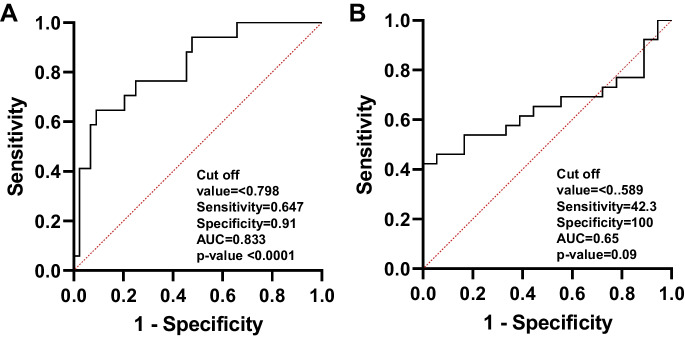
Figure 1A displays the ROC curves for determining the optimal cut-off values for dexterity measures in the NIHTB. The comparison between healthy and mild dexterity categories yielded an AUC of 0.77. The ideal balance between sensitivity and specificity for mild dexterity impairment was found with a T-score range of 38.4 to 33.5. In Fig. 1B, the ROC curve compares the mild and moderate-to-severe dexterity categories, with an AUC of 0.70. The optimal cut-off value for moderate-to-severe myelopathy was determined to be a T-score below 33.5. Figure 2 demonstrates an ROC curve with an AUC of 0.80, indicating that the threshold T-score for mild grip strength impairment ranges from 47.4 to 32.0. In contrast, the moderate-to-severe grip strength impairment threshold was identified as a T-score below 32.0, with an AUC of 0.75. Figure 3 presents the ROC curves for standing balance cut-off values. An AUC of 0.61 was found, with the optimal cut-off value for mild balance impairment being a T-score between 36.4 and 33.0. A T-score below 33.0 was determined for moderate-to-severe balance impairment, accompanied by an AUC of 0.78. The AUC for mild gait speed impairment was 0.65, with a threshold range of 0.78 to 0.6 m/sec. Similarly, an AUC of 0.65 was observed for moderate-to-severe gait speed impairment, with a threshold below 0.6 m/sec Fig. 4.
Fig. 1.
The ROC curve analyses for healthy to mild (A) and for mild and moderate-to-severe (B) dexterity categories
Fig. 2.
The ROC curve analyses for healthy to mild (A) and for mild and moderate-to-severe (B) grip strength categories
Fig. 3.
The ROC curve analyses for healthy to mild (A) and for mild and moderate-to-severe (B) balance categories
Fig. 4.
The ROC curve analyses for healthy to mild (A) and for mild and moderate-to-severe (B) gait speed categories
Validation of severity criteria
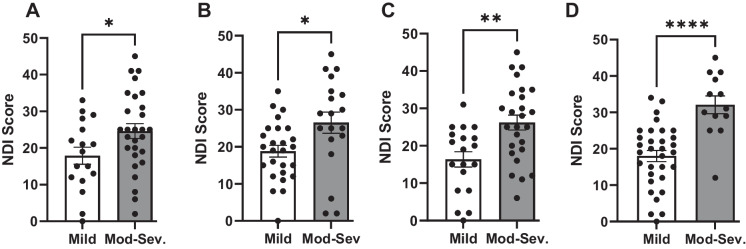
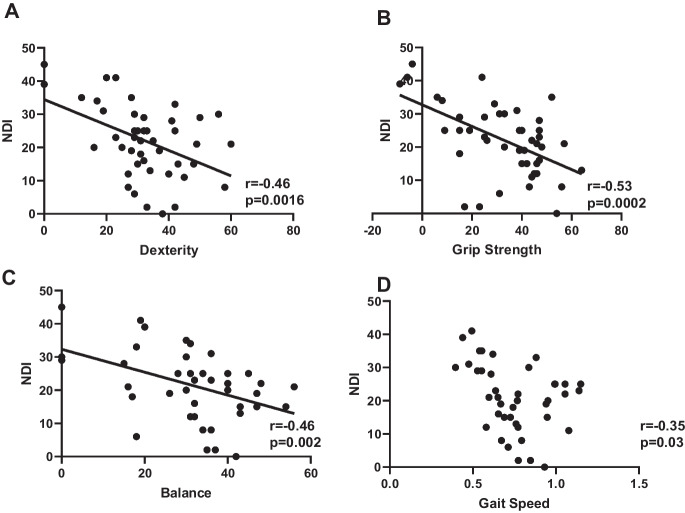
Based on the threshold criteria for mild and moderate-to-severe dysfunction, across all the NIHTBm measures, patients with moderate-to-severe myelopathy displayed significantly higher disability index (NDI); p < 0.05 compared to patients with mild myelopathy Fig. 5. We evaluated the correlation between each of the NIHTBm measures with the disability index (NDI) and we show that NDI showed a significant negative correlation with dexterity scores (r = 0.46, p = 0.0016), grip strength (r = 0.53, p = 0.0002), balance (r = 0.46, p = 0.002), and gait speed (r = 0.35, p = 0.03) Fig. 6.
Fig. 5.
Bar graphs showing patients with DCM with moderate-to-severe myelopathy have significantly higher myelopathy disability scores compared to DCM with mild myelopathy across the NIHTBm measures: dexterity (A), grip strength (B), balance(C), and gait speed (D). *p < 0.05, **p < 0.005, and **** p < 0.0001
Fig. 6.
Distribution of NIHTBm scores showing linear correlation with NDI scores: dexterity (A), grip strength (B), balance (C), and (D) gait speed. p < 0.05
Heterogeneity of DCM with NIHTBm measures
Based on the NIHTBm cutoff values, we observed that among DCM patients classified according to their mJOA scores, 26.7% (n = 12) of DCM patients had normal dexterity, 13.3% (n = 6) DCM patients had normal grip strength scores, 28.9% (n = 13) had normal standing balance scores, and 31.1% (n = 14) displayed normal gait speed scores. We utilized a correlation matrix to examine the relationships between the NIHTBm measures in DCM patients. We observed that dexterity scores were significantly correlated with both grip strength (ρ = 0.40; p = 0.007) and gait speed (ρ = 0.40; p = 0.007), but weak correlation with balance scores (ρ = 0.14; p = 0.372). Grip strength was significantly correlated with balance (ρ = 0.35; p = 0.017) but not gait speed (ρ = 0.26; p = 0.061). In addition, a significant correlation was observed between balance and gait speed (ρ = 0.36, p = 0.014; Fig. 7).
Fig. 7.
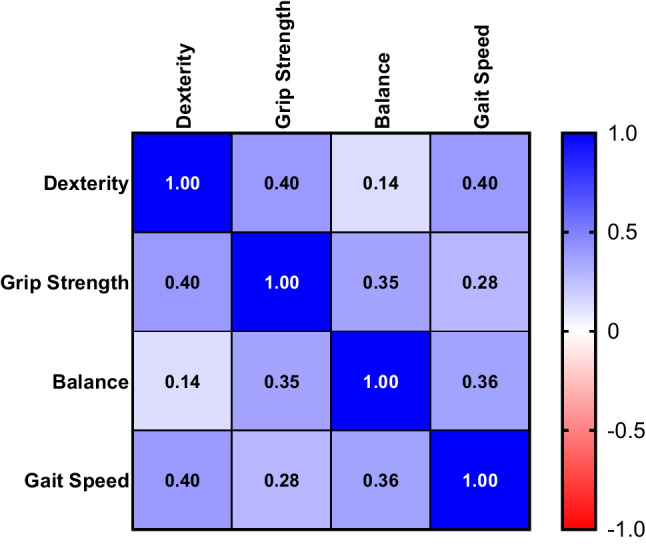
Correlation matrix for NIHTBm measures. Spearman’s correlation coefficient calculated between dexterity, grip strength, balance, and gait speed scores in patients with DCM. The degree of cross-correlation are color coded
Discussion
There are several gaps in the understanding of DCM severity and heterogeneity within the literature, primarily due to the lack of objective measures for neurological deficits. Current assessment tools, such as the mJOA, Nurick, and myelopathy index, are subjective and narrow in scope, limiting our comprehension of DCM [27]. Although the mJOA is a widely accepted measure for DCM, recent studies have suggested that it does not correlate well with DCM disability measures [28, 29]. Implementing an objective measure, like the NIHTBm, for evaluating the severity of DCM could lead to more accurate treatment stratification for patients, ultimately improving treatment outcomes and enhancing our understanding of the disease [12].
The NIHTBm assessment scale objectively evaluates motor dysfunction in both upper and lower extremities for neurological disorders such as stroke, TBI, DCM, and SCI [12, 16]. It offers quantitative measures of motor dysfunction in DCM and was developed by the NIH Blueprint for Neuroscience Research [17, 30], demonstrating proven internal consistency and good sensitivity in assessing motor, sensory, and cognitive dysfunction [17].
This study is the first to propose categorizing and defining cutoff values for motor dysfunction of myelopathy based on preferred treatment groups, distinguishing between mild and moderate-to-severe myelopathy. While the NIHTB platform establishes a mean score threshold for healthy individuals with a T-score of 50 and an SD of 10, it does not provide specific cut points to indicate the severity of motor dysfunction [31, 32]. This study assessed the validity of patient classification based on the degree of motor impairment with the NIHTBm. The main findings in this study suggest that the NIHTBm can effectively classify patients based on the degree of motor impairment and distinguish between mild and moderate-to-severe myelopathy. Further, this study demonstrated that patients with moderate-to-severe myelopathy exhibited worse disability scores. Ultimately enhancing our understanding of DCM could improve treatment outcomes.
The NIHTB was originally designed to evaluate and set a standardized motor assessment that is corrected for age, gender, BMI, ethnicity, etc. [17, 32] It is use as a reliable and repeatable measure with standardized value to assess neurological dysfunction [12, 14, 16]. NIHTB measures have been established and widely applied in the assessment of neurological disease including stroke and traumatic brain [16]. However, the established cutoffs for severity of motor deficits have not been established. We included these measures in DCM and elaborated that it is a reliable method of understanding DCM motor deficits [12]. In our earlier evaluations in DCM, including comparison with the mJOA, Nurick, and NDI, we observed that NIHTBm is a sensitive test that identifies myelopathy [12].
According to the findings in this study, DCM patients exhibit moderate-to-severe dexterity impairment when they present with hand dexterity scores of less than 33.5. Similarly, the moderate-to-severe imbalance is indicated by a standing balance score below 33.0, moderate-to-severe gait problems by a gait speed score below 0.6 m/sec, and moderate-to-severe grip strength impairment by a grip strength score less than 32.0. Establishing and understanding the extent of the cut-off points for motor dysfunctions in DCM highlights that not all DCM patients present with the same degree of motor dysfunction. It also demonstrates that DCM patients may manifest problems with dexterity independently of balance problems.
Our findings in this study significantly extend and refine the understanding of DCM severity by classifying motor measures obtained from the NIHTB. This classification can assist clinicians in more objectively categorizing DCM patients based on their motor dysfunction [12, 33, 34]. This categorization provides an objective, quantitative method that could help surgeons identify surgical candidates or design rehabilitative treatment strategies based on the specific dysfunction DCM patients present with. Using the quantitative measure, clinicians and patients are more likely to comprehend a quantitative description of their disease severity than relying solely on the mJOA classification [34].
Currently, mJOA is the accepted and preferred assessment measure for dexterity severity; however, it is subjective, provides a narrow band of score categories, and lacks the granularity to evaluate specific dysfunction [9, 27]. It is also prone to differences in surgeons' administration bias, leading to outcomes variations depending on who administers the survey. The mJOA scale validates the importance of using the NIHTB [12].
Limitations of this study
It is important to acknowledge the limitations of this study. The small sample size impacts the generalizability of the findings. DCM patients categorize into mild and moderate-to-severe given that most severe DCM patients have an urgent need for surgery. Large multisite studies with all spectra of DCM severity could enhance the interpretation of these defined cut-off values and their impact on treatment outcomes. The subjective scale mJOA was used as the anchor to define mild and moderate-to-severe myelopathy. It is challenging to determine how the predominant dysfunction might impact treatment outcomes, given that only baseline measures are presented in this pilot study. Future studies will include post-surgical evaluation and disability measures. The NIHTBm demonstrates that DCM patients could present differently without global dysfunction; however, this study is limited to motor dysfunction [12]. The inclusion of sensory dysfunction could complicate the understanding of heterogeneity with multiple subtypes of DCM.
Despite the limitations outlined above, this study serves as a starting point that could inform future analyses. In contrast to other studies of objective measures of dysfunction and subjective DCM assessments, our recent findings on the NIHTB highlight its potential to provide a more comprehensive and objective evaluation of DCM severity. This has broader application to other neurological disorders characterized by motor deficits. The standardized nature of NIHTB can facilitate and improve clinical assessment and allow for comparison of motor deficits across diseases. Furthermore, it can enhance our understanding of shared and distinct motor impairments in aging population that may manifest with multitude of neurological conditions.
In conclusion, this pilot study establishes a classification system to define DCM into treatment groups as mild or moderate-to-severe myelopathy based on motor assessments with the NIHTBm. These cut-off points and classifications are validated with the functional disability index, demonstrating effective differences in motor dysfunction severity in DCM. Our findings contribute to a growing body of evidence supporting the use of NIHTB as a more comprehensive and objective measure for DCM assessment and treatment planning.
Acknowledgements
Authors would like to thank the patients and participants of the study, neurosurgery clinic staff, and other researchers at the University of Oklahoma Health Sciences Center.
Funding
This work was supported by the NIH NINDS K23 grant (K23NS091430), Presbyterian Health Foundation Team Science Research, Presbyterian Health Foundation Bridge grant, and Oklahoma Shared Clinical and Translational Research (OSCTR) NIH-IDeA grant.
Data availability
Data used in this study are available upon request sent to the corresponding author.
Declarations
Conflict of interest
The authors declare are no competing interests.
Disclaimer
Funders had no role in the conduction of study and its results are not in any way influenced by them.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hejrati N, Moghaddamjou A, Marathe N, Fehlings MG. Degenerative cervical myelopathy: towards a personalized approach. Can J Neurol Sci. 2022;49(6):729–740. doi: 10.1017/cjn.2021.214. [DOI] [PubMed] [Google Scholar]
- 2.Hejrati N, Pedro K, Alvi MA, Quddusi A, Fehlings MG. Degenerative cervical myelopathy: where have we been? Where are we now? Where are we going? Acta Neurochir (Wien) 2023;165(5):1105–1119. doi: 10.1007/s00701-023-05558-x. [DOI] [PubMed] [Google Scholar]
- 3.Akter F, Yu X, Qin X, et al. The Pathophysiology of degenerative cervical myelopathy and the physiology of recovery following decompression. Front Neurosci. 2020;14:138. doi: 10.3389/fnins.2020.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vidal PM, Ulndreaj A, Badner A, Hong J, Fehlings MG. Methylprednisolone treatment enhances early recovery following surgical decompression for degenerative cervical myelopathy without compromise to the systemic immune system. J Neuroinflammation. 2018;15(1):222. doi: 10.1186/s12974-018-1257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shamji MF, Massicotte EM, Traynelis VC, Norvell DC, Hermsmeyer JT, Fehlings MG. Comparison of anterior surgical options for the treatment of multilevel cervical spondylotic myelopathy: a systematic review. Spine (Phila Pa 1976) 2013;38(22 Suppl 1):S195–209. doi: 10.1097/BRS.0b013e3182a7eb27. [DOI] [PubMed] [Google Scholar]
- 6.Tetreault L, Kopjar B, Nouri A, et al. The modified Japanese Orthopaedic Association scale: establishing criteria for mild, moderate and severe impairment in patients with degenerative cervical myelopathy. Eur Spine J. 2017;26(1):78–84. doi: 10.1007/s00586-016-4660-8. [DOI] [PubMed] [Google Scholar]
- 7.Gibson J, Nouri A, Krueger B, et al. Degenerative cervical myelopathy: a clinical review. Yale J Biol Med. 2018;91(1):43–48. [PMC free article] [PubMed] [Google Scholar]
- 8.Nurick S. The pathogenesis of the spinal cord disorder associated with cervical of cervical radiculopathy. A prospective outcome study. Acta Neurochir. 2005;147:1065–1070. doi: 10.1007/s00701-005-0542-2. [DOI] [PubMed] [Google Scholar]
- 9.Revanappa KK, Rajshekhar V. Comparison of Nurick grading system and modified Japanese Orthopaedic Association scoring system in evaluation of patients with cervical spondylotic myelopathy. Eur Spine J. 2011;20(9):1545–1551. doi: 10.1007/s00586-011-1773-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin AR, Jentzsch T, Wilson JRF, et al. Inter-rater reliability of the modified Japanese Orthopedic Association score in degenerative cervical myelopathy: a cross-sectional study. Spine (Phila Pa 1976) 2021;46(16):1063–1069. doi: 10.1097/brs.0000000000003956. [DOI] [PubMed] [Google Scholar]
- 11.Soufi KH, Perez TM, Umoye AO, Yang J, Burgos M, Martin AR. How is spinal cord function measured in degenerative cervical myelopathy? A systematic review. J Clin Med. 2022;11(5):1441. doi: 10.3390/jcm11051441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muhammad F, Baha A, Haynes G, et al. Isolating neurologic deficits in cervical spondylotic myelopathy: a case-controlled study, using the NIH Toolbox Motor Battery. Neurol Clin Pract. 2023;13(2):e200126. doi: 10.1212/cpj.0000000000200126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holdnack JA, Iverson GL, Silverberg ND, Tulsky DS, Heinemann AW. NIH toolbox cognition tests following traumatic brain injury: frequency of low scores. Rehabil Psychol. 2017;62(4):474–484. doi: 10.1037/rep0000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tulsky DS, Carlozzi NE, Holdnack J, et al. Using the NIH Toolbox Cognition Battery (NIHTB-CB) in individuals with traumatic brain injury. Rehabil Psychol. 2017;62(4):413–424. doi: 10.1037/rep0000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babakhanyan I, Carlozzi NE, McKenna BS, Casaletto KB, Heinemann AW, Heaton RK. National institutes of health toolbox emotion battery: application of summary scores to adults with spinal cord injury, traumatic brain injury, and stroke. Arch Phys Med Rehabil. 2019;100(10):1863–1871. doi: 10.1016/j.apmr.2019.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Carlozzi NE, Goodnight S, Casaletto KB, et al. Validation of the NIH Toolbox in individuals with neurologic disorders. Arch Clin Neuropsychol. 2017;32(5):555–573. doi: 10.1093/arclin/acx020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reuben DB, Magasi S, McCreath HE, et al. Motor assessment using the NIH Toolbox. Neurology. 2013;80(11 Suppl 3):S65–75. doi: 10.1212/WNL.0b013e3182872e01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodes RJ, Insel TR, Landis SC. The NIH toolbox: setting a standard for biomedical research. Neurology. 2013;80(11 Suppl 3):S1. doi: 10.1212/WNL.0b013e3182872e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ. NIH toolbox for assessment of neurological and behavioral function. Neurology. 2013;80(11 Suppl 3):S2–6. doi: 10.1212/WNL.0b013e3182872e5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fehlings MG, Ibrahim A, Tetreault L, et al. A global perspective on the outcomes of surgical decompression in patients with cervical spondylotic myelopathy: results from the prospective multicenter AOSpine international study on 479 patients. (Phila Pa 1976) 2015;40(17):1322–8. doi: 10.1097/brs.0000000000000988. [DOI] [PubMed] [Google Scholar]
- 21.Kadaňka Z, Bednařík J, Novotný O, Urbánek I, Dušek L. Cervical spondylotic myelopathy: conservative versus surgical treatment after 10 years. Eur Spine J. 2011;20(9):1533–1538. doi: 10.1007/s00586-011-1811-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitteer DR, Greer BD. Using GraphPad prism's heat maps for efficient, fine-grained analyses of single-case data. Behav Anal Pract 2022;15(2):505–14. 10.1007/s40617-021-00664-7 [DOI] [PMC free article] [PubMed]
- 23.Davies BM, Nourallah B, Venkatesh A, et al. Establishing mild, moderate and severe criteria for the myelopathy disability index in cervical spondylotic myelopathy. Br J Neurosurg. 2023;37(5):1018–22. 10.1080/02688697.2020.1839741 [DOI] [PubMed]
- 24.Hughes G. Youden's index and the weight of evidence. Methods Inf Med. 2015;54(2):198–199. doi: 10.3414/me14-04-0003. [DOI] [PubMed] [Google Scholar]
- 25.Ruopp MD, Perkins NJ, Whitcomb BW, Schisterman EF. Youden Index and optimal cut-point estimated from observations affected by a lower limit of detection. Biom J. 2008;50(3):419–430. doi: 10.1002/bimj.200710415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang H. Sample size determination and power analysis using the G*Power software. J Educ Eval Health Prof. 2021;18:17. doi: 10.3352/jeehp.2021.18.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pons Carreto A, Ramírez Valencia M, de García FA, et al. Myelopathy disability index: establishing criteria for mild, moderate and severe impairment in patients with degenerative cervical myelopathy. Eur Spine J. 2023;32(2):584–589. doi: 10.1007/s00586-022-07506-2. [DOI] [PubMed] [Google Scholar]
- 28.Yee TJ, Upadhyaya C, Coric D, et al. Correlation of the modified japanese orthopedic association with functional and quality-of-life outcomes after surgery for degenerative cervical myelopathy: a quality outcomes database study. Neurosurgery. 2022;91(6):952–960. doi: 10.1227/neu.0000000000002161. [DOI] [PubMed] [Google Scholar]
- 29.Owen RJ, Zebala LP, Peters C, McAnany S. PROMIS physical function correlation with NDI and mJOA in the surgical cervical myelopathy patient population. Spine (Phila Pa 1976) 2018;43(8):550–555. doi: 10.1097/brs.0000000000002373. [DOI] [PubMed] [Google Scholar]
- 30.Weintraub S, Bauer PJ, Zelazo PD, et al. I. NIH Toolbox Cognition Battery (CB): introduction and pediatric data. Monogr Soc Res Child Dev. 2013;78(4):1–15. doi: 10.1111/mono.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holdnack JA, Tulsky DS, Brooks BL, et al. Interpreting patterns of low scores on the NIH Toolbox Cognition Battery. Arch Clin Neuropsychol. 2017;32(5):574–584. doi: 10.1093/arclin/acx032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casaletto KB, Umlauf A, Beaumont J, et al. Demographically corrected normative standards for the english version of the NIH Toolbox Cognition Battery. J Int Neuropsychol Soc. 2015;21(5):378–391. doi: 10.1017/s1355617715000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh A, Crockard HA. Quantitative assessment of cervical spondylotic myelopathy by a simple walking test. Lancet. 1999;354(9176):370–3. doi: 10.1016/s0140-6736(98)10199-x. [DOI] [PubMed] [Google Scholar]
- 34.Singh A, Gnanalingham KK, Casey AT, Crockard A. Use of quantitative assessment scales in cervical spondylotic myelopathy–survey of clinician’s attitudes. Acta Neurochir (Wien) 2005;147(12):1235–8. doi: 10.1007/s00701-005-0639-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in this study are available upon request sent to the corresponding author.