Abstract
The human pathogen Vibrio cholerae is a highly motile organism by virtue of a polar flagellum, and motility has been inferred to be an important aspect of virulence. It has previously been demonstrated that the σ54-dependent activator FlrC is necessary for both flagellar synthesis and for enhanced intestinal colonization. In order to characterize FlrC binding, we analyzed two FlrC-dependent promoters, the highly transcribed flaA promoter and the weakly transcribed flgK promoter, utilizing transcriptional lacZ fusions, mobility shift assays, and DNase I footprinting. Promoter fusion studies showed that the smallest fragment with wild-type transcriptional activity for flaAp was from positions −54 to +137 with respect to the start site, and from −63 to +144 for flgKp. Gel mobility shift assays indicated that FlrC binds to a fragment containing the region from positions +24 to +95 in the flaAp, and DNase I footprinting identified a protected region between positions +24 and +85. Mobility shift and DNase I footprinting indicated weak binding of FlrC to a region downstream of the flgKp transcription start site. These results demonstrate a relatively novel σ54-dependent promoter architecture, with the activator FlrC binding downstream of the σ54-dependent transcription start sites. When the FlrC binding site(s) in the flaA promoter was moved a large distance (285 bp) upstream of the transcription start site of either flaAp or flgKp, high levels of FlrC-dependent transcription resulted, indicating that this binding region functions as an enhancer element. In contrast, the relatively weak FlrC binding site(s) in the flgK promoter failed to function as an enhancer element at either promoter, suggesting that FlrC binding strength contributes to enhancer activity. Our results suggest that the differences in FlrC binding to various flagellar promoters results in the differences in transcription levels that mirror the relative requirement for the flagellar components within the flagellum.
The life-threatening diarrheal disease cholera is caused by the gram-negative bacterium Vibrio cholerae. This bacterium is highly motile by means of a single, polar sheathed flagellum and enters the human host through the ingestion of contaminated food or water. Within the intestine, V. cholerae expresses a number of virulence factors including cholera toxin, which causes the profuse watery diarrhea (23, 27), and the toxin-coregulated pilus, which is essential for intestinal colonization (38).
Motility is also a virulence determinant for V. cholerae. Nonmotile mutants have been shown to produce less disease in rabbit models (8, 34). Some studies have seen minimal colonization defects of nonmotile mutants in the infant mouse model (11, 21, 34), whereas other studies have noted significant colonization defects of nonmotile mutants in infant mice (21, 22); these discrepancies are likely due to differences in bacterial strains and flagellar mutations. Importantly, nonmotile mutants of live attenuated V. cholerae vaccine strains show reduced reactogenicity (i.e., disease symptoms) in humans while maintaining their ability to colonize the intestine (6, 19), providing support for a role for motility in human cholera. Chemotaxis, which requires a functional flagellum, also contributes to virulence factor expression and intestinal colonization (2, 8, 9, 22).
V. cholerae utilizes more than 40 genes for the production of its flagellum. Transcription of these genes is organized into a hierarchy of four classes, (32). The sole gene in class I is flrA, which encodes a σ54-dependent transcriptional activator. Class II genes, which are FlrA and σ54 dependent, primarily encode the switch-export apparatus and MS ring (FliF), as well as the regulatory genes flrBC and fliA. FlrC is also a σ54-dependent activator and, along with σ54, is responsible for transcription of class III genes, which encode the rest of the basal body structure, the hook, and the “core” flagellin, FlaA (20). Finally, class IV genes are FliA (σ28) dependent and encode the four additional flagellins, as well as the other motor components. The expression of class IV genes is controlled through modulation of the activity of σ28 by an anti-σ28 factor, FlgM, which is secreted through the sheathed flagellum (4).
Expression of class III genes is also controlled by modulation of the activity of FlrC. Phosphorylation of the conserved aspartate residue (D54) in the response regulatory N-terminal domain by the histidine kinase FlrB is required for FlrC-dependent transcriptional activation, as well as enhanced intestinal colonization (5, 21). FlrC shares homology over its entire length with the well-characterized σ54-dependent response regulator NtrC, and thus its mechanism of transcription activation can be inferred from studies on NtrC (29). The central domain of NtrC is shared among all σ54-dependent activators and contains an ATPase activity that is essential for isomerization of closed complexes to open complexes; phosphorylation modulates this enzymatic activity (40). The C-terminal region contains the DNA-binding domain that binds to an enhancer element within the promoter region (3, 29). The enhancer is composed of two NtrC binding sites located on the same side of the DNA helix and serves two main functions. First, the enhancer serves to tether NtrC in close proximity to the σ54-holoenzyme binding site to facilitate contact between NtrC and RNAP (39). Second, the two binding sites facilitate the cooperative interactions between NtrC that stimulate the ATPase activity necessary for transcriptional activation (31). Although the NtrC binding sites constitute a true enhancer, i.e., they can function to stimulate transcription both upstream and downstream and at a large distance from the transcription start site (33), the enhancers bound by NtrC and virtually all characterized σ54-dependent activators are normally found upstream of the binding site of σ54-holoenzyme.
Homologues of FlrC that regulate a similar class of flagellar genes are found in all other Vibrio spp. studied (26), as well as in Pseudomonas aeruginosa (7) and Campylobacter jejuni (14, 17), suggesting that similar mechanisms underlie the regulation of class III genes in polar flagellates. Our previous results showed that strains containing FlrC locked into an inactive (D54A) or constitutively active (M114I) state demonstrated more severe defects in intestinal colonization than strains lacking FlrC entirely (5). We reasoned that this observation could be due to a repressive effect of FlrC binding upon some gene(s) necessary for V. cholerae virulence, and undertook the characterization of FlrC DNA binding.
In the present study, we characterized FlrC binding at two flagellar promoters, the highly transcribed flaA promoter and the weakly transcribed flgK promoter. Our results demonstrate that (i) FlrC binds downstream of the transcription start site in both promoters, (ii) FlrC binds more strongly to the flaAp than flgKp, (iii) the FlrC binding site(s) within the flaAp function as an enhancer, and thus (iv) the strength of FlrC binding dictates promoter strength and the ability of the binding site to behave as an enhancer.
MATERIALS AND METHODS
Bacterial strains and media.
Escherichia coli DH5α (12) was used for cloning manipulations. V. cholerae strains used in the present study are listed in Table 1. Luria broth (LB) was used for both liquid and agar, and ampicillin at 50 μg/ml was added when appropriate.
TABLE 1.
Bacterial strains, plasmids, and oligonucleotide primers used in this study
| V. cholerae strain, plasmid, or oligonucleotide | Relevant genotype or sequence (5′-3′)a | Source or reference or oligonucleotide used |
|---|---|---|
| Strains | ||
| KKV598 | 0395; ΔlacZ | 5 |
| KKV56 | 0395; ΔrpoN ΔlacZ | 21 |
| KKV98 | 0395; ΔflrC ΔlacZ | 21 |
| Plasmids | ||
| pRS551 | Transcriptional lacZ fusion vector; Ampr Kanr | 37 |
| pKEK75 | pRS551 with BglII and XbaI sites added | 21 |
| pKEK80 | −569 to +581 of flaAp in pRS551 | FLGLD1/FLAAP1 |
| pKEK147 | −54 to +581 of flaAp in pRS551 | FLAAPG2/FLAAP1 |
| pKEK158 | −285 to +347 of flaAp in pRS551 | FLAAPG4/FLAAPG3 |
| pKEK184 | −285 to +66 of flaAp in pRS551 | FLAAPG4/FLAAPG1 |
| pKEK445 | −54 to +206 of flaAp in pRS551 | FLAAPG2/FLAAPG5 |
| pKEK446 | −569 to +66 of flaAp in pRS551 | FLGLD1/FLAAPG1 |
| pKEK447 | −54 to +66 of flaAp in pRS551 | FLAAPG2/FLAAPG1 |
| pKEK594 | −54 to +137 of flaAp in pRS551 | FLAAPG2/FLAAPG7 |
| pKEK617 | −285 to +10 of flaAp in pRS551 | FLAAP10down, FLAAPG4like, FLAAP10up, FLAAPG5like |
| pKEK687 | −54 to +85 of flaAp in pRS551 | FLAAPG2/FLAAPG8 |
| pKEK691 | −54 to +95 of flaAp in pRS551 | FLAAPG2/FLAAP95 |
| pKEK692 | −54 to +110 of flaAp in pRS551 | FLAAPG2/FLAAPG9 |
| pKEK331 | −220 to +255 of flgKp in pRS551 | FLGKP1/FLGKP2 |
| pKEK580 | −220 to +27 of flgKp in pRS551 | FLGKP1/FLGKG2 |
| pKEK581 | −63 to +255 of flgKp in pRS551 | FLGKG1/FLGKP2 |
| pKEK657 | −63 to +207 of flgKp in pRS551 | FLGKG1/FLGKG4 |
| pKEK658 | −63 to +144 of flgKp in pRS551 | FLGKG1/FLGKG3 |
| pKEK667 | +11 to +144 flgKp fused to −220 to +10 flgKp in pRS551 | FLGKP220, FLGKP144, FLGKP10up, FLGKP10down |
| pKEK670 | −220 to +10 of flgKp in pRS551 | Same as pKEK667, digested with MfeI and BamHI to get rid of downstream region |
| pKEK695 | +24 to +110 flaAp fused upstream of −285 to +10 flaAp in pRS551 | FLAAP24, FLAAPG11, FLAAPG10, FLAAP10up |
| pKEK696 | +24 to +110 flaAp fused upstream of −220 to +10 flgKp in pRS551 | FLAAP24, FLAAK4, FLAAK3, FLGKP10up |
| pKEK697 | +11 to +144 flgKp fused upstream of −285 to +10 flaAp in pRS551 | FLGK10down, FLAAK2, FLAAK1, FLAAP10up |
| pKEK707 | +24 to +137 flaAp fused upstream of −285 to +10 flaAp in pRS551 | FLAAP24, FLAAPG13, FLAAPG12, FLAAP10up |
| pKEK714 | Same as pKEK707 with G+38→T in pRS551 | FLAAPG38T2/FLAAP10up |
| pKEK715 | Same as pKEK707 with G+78→T in pRS551 | FLAAP24, FLAAP88, FLAAP68, FLAAP10up |
| pKEK716 | Same as pKEK707 with G+38→T and G+78→T in pRS551 | FLAAP38T, FLAAP88, FLAAP68, FLAAP10up |
| Oligonucleotide | ||
| FLGLD1 | GCTCTAGAGGCTATCAGTTAGAGCGTAA | |
| FLAAP1 | GCAGATCTCGATTCATTCATCGCACCT | |
| FLAAPG1 | GCGGATCCAGCGGCAATCCATTTTGCCGC | |
| FLAAPG2 | GCTCTAGATATGAGCGAAGTGAGTTGAG | |
| FLAAPG3 | GCGGATCCGGTATTTACGTTAATGGTCATA | |
| FLAAPG4 | GCTCTAGAATTGGTGCGCGTTTGAGTACGC | |
| FLAAPG5 | GCGGATCCTACAACAACTCTGATGTACTTTA | |
| FLAAPG6 | GCGAATTCCACCGAAATCGGCAACTTTGAG | |
| FLAAPG7 | GCGGATCCCATCGCTTATTTTCAATGAGT | |
| FLAAPG8 | GCGGATCCAAAGTTGCCGATTTCGGTGAGC | |
| FLAAPG9 | GCGAATTCGGATCCGTCTATCTAATTCAATCAGC | |
| FLAAPG10 | TTAGATAGACATTGGTGCGCGTTTGAGTACG | |
| FLAAPG11 | GCGCACCAATGTCTATCTAATTCAATCAGC | |
| FLAAPG12 | ATAAGCGATGATTGGTGCGCGTTTGAGTACG | |
| FLAAPG13 | GCGCACCAATCATCGCTTATTTTCAATGAG | |
| FLAAP10down | GCGAATTCGAAAAGTTGTACAGGGTAACCA | |
| FLAAP10up | GCGGATCCACCAAGGCTCAATGTTGTATTAG | |
| FLAAPG4like | AGTTGTTGTACAATTGATTGGTGCGCGTTTGAGTAC | |
| FLAAPG5like | GCGCACCAATCAATTGTACAACAACTCTGATGTACT | |
| FLAAP24 | GCGAATTCGGGTAACCAAAGCGGCAAG | |
| FLAAP38T2 | GCGAATTCGGGTAACCAAAGCGTCAAGTCAGCG | |
| FLAAP68 | ACCGAAATCGTCAACTTTGAG | |
| FLAAP85 | GCGAATTCAAAGTTGCCGATTTCGGTGAGC | |
| FLAAP88 | CTCAAAGTTGACGATTTCGGT | |
| FLAAP95 | GCGAATTCGGATCCTCAGCCACTCAAAGTTGC | |
| FLAAP95GT | GCGAATTCGGATCCTCAGCCACTCAAAGTTGACGATTTC | |
| FLAAK1 | TAATACAGAGATTGGTGCGCGTTTGAGTACG | |
| FLAAK2 | GCGCACCAATCTCTGTATTAACATTGGAAAT | |
| FLAAK3 | TTAGATAGACCACCGTGCTGGATATGCGAC | |
| FLAAK4 | CAGCACGGTGGTCTATCTAATTCAATCAGC | |
| FLGKP1 | GCGAATTCCACCGTGCTGGATATGCGACC | |
| FLGKP2 | GCGGATCCTTGATCCCACGAGCGGCGAAC | |
| FLGKG1 | GCGAATTCTGCTATTTTTATTAGAGTAAATC | |
| FLGKG2 | GCGGATCCATTAATCAGTAAAATTCACTGAA | |
| FLGKG3 | GCGGATCCCTCTGTATTAACATTGGAAAT | |
| FLGKG4 | GCGGATCCGGTTTCACCGCCGTATTGGCG | |
| FLGKP10down | GCGAATTCGAATTTTACTGATTAATATCGG | |
| FLGKP10up | GCGGATCCACTGAATCTAAACAGTAGATAG | |
| FLGKP144 | CAGCACGGTGCAATTGCTCTGTATTAACATTGGAAATG | |
| FLGKP220 | TAATACAGAGCAATTGCACCGTGCTGGATATGCGACC |
Ampr, ampicillin resistance; Kanr, kanamycin resistance.
Construction of flaAp and flgKp-lacZ fusions.
Different size fragments containing flaA or flgK promoter regions were PCR amplified by using the corresponding oligonucleotides listed in Table 1. V. cholerae classical O1 strain O395 chromosomal DNA was used as a template for PCR. Restriction sites for XbaI or EcoRI and BamHI or BglII were added to the upstream and downstream primers, respectively, for cloning into pRS551 or pKEK75 (pRS551 with XbaI and BglII sites added) (21) that had been digested similarly. Plasmids pKEK667, pKEK695, pKEK696, pKEK707, pKEK715, and pKEK716 (Table 1) were constructed by a two-step PCR technique in which overlapping PCR fragments containing the mutation of interest were generated and then used as templates in a second PCR with the outermost primers. Then, the PCR products from the second reaction were digested with EcoRI and BamHI and ligated into pRS551 (37) that had been digested similarly. All primer sequences were designed based on the complete V. cholerae genome sequence (13). The correct construction of the plasmids was verified by DNA sequencing.
β-Galactosidase assays.
ΔlacZ wild-type, ΔrpoN, and ΔflrC V. cholerae strains KKV598, KKV56, and KKV98, respectively, were transformed with the promoter-lacZ fusion-containing plasmids, grown at 37°C in LB supplemented with 2 mM glutamine (this allows for wild-type growth of ΔrpoN strains) (21) and 50 μg of ampicillin/ml, and harvested at an optical density at 600 nm of ca. 0.4 to 0.6. Bacterial cells were permeabilized with chloroform and sodium dodecyl sulfate (SDS) and assayed for β-galactosidase activity according to the method of Miller (28).
Protein purification.
Expression and purification of MBP-FlrC and MBP-FlrB proteins has been previously described (5).
Primer extension.
Total RNA was isolated from mid-log-phase wild-type, ΔrpoN, and ΔflrC V. cholerae strains by using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Oligonucleotides (FLAAPG5 for flaAp and FLGKG4 for flgKp) were end labeled with [γ-32P]ATP (Amersham Pharmacia) and T4 polynucleotide kinase (New England Biolabs) as described by Sambrook and Russell (36). For primer extension, the SuperScript First Strand Synthesis system for reverse transcription-PCR (Invitrogen) was used, and the manufacturer's instructions were followed with 30 μg of RNA. The primer extension products were run in parallel with a sequencing reaction (Sequenase version 2.0; USB) performed with the same primer used for primer extension but labeled with [α-32P]dATP (Amersham Pharmacia), loaded onto a 6% polyacrylamide-7 M urea sequencing gel, and then visualized by autoradiography.
DNase I footprinting.
A 191-bp fragment containing the region from positions −54 to +137 of flaAp was PCR amplified by using oligonucleotides FLAAPG2 and FLAAPG7. After gel purification, this PCR product was end labeled with [γ-32P]ATP (Amersham Pharmacia) and T4 polynucleotide kinase (New England Biolabs) and digested with either BamHI (for labeling of the upper strand) or XbaI (for labeling of the lower strand). After enzymatic digestion, the mixture was passed through a Nuc-Trap column (Stratagene), and the eluate was then precipitated with ethanol and resuspended in 10 mM Tris-HCl (pH 7.5)-1 mM EDTA (pH 8.0) (TE) buffer. The labeled fragment (∼150,000 cpm) was incubated with various concentrations of FlrC protein (0.18-5.76 μM) in a final volume of 50 μl with binding buffer (50 mM Tris-HCl [pH 8.0], 100 mM KCl, 1 mM MgCl2, 1 mM EDTA, 15% glycerol, 1 mM dithiothreitol) for 10 min at 37°C. Then, 50 μl of Ca+2/Mg+2 solution (5 mM CaCl2, 10 mM MgCl2) was added, followed by incubation for 1 min at room temperature. DNase I (Gibco/BRL) was added to each reaction mixture for 2 min at room temperature, and the reactions were stopped by adding 90 μl of stop solution (200 mM NaCl, 2 mM EDTA, 1% SDS, 100 μg of yeast tRNA/ml). The digestion products were extracted with phenol, precipitated with ethanol, and resuspended in 10 μl of loading solution (from Sequenase version 2.0) for subsequent loading onto a 6% polyacrylamide-7 M urea gel. A sequencing reaction was run in parallel to the DNase I digestion products and then visualized by autoradiography. The 5′ end of the primer used for sequencing reactions corresponded to the 5′ end of the footprinting probe.
Mobility shift assays.
flaA and flgK promoter fragments were amplified by PCR by using a γ-32P-labeled oligonucleotide (labeling procedure described above in primer extension). The flaA and flgK promoter fragments were PCR amplified by using the primers listed in Table 1 to give the appropriate size fragments. The PCR products were passed through a Nuc-Trap Column (Stratagene), and they were further purified by 5% polyacrylamide nondenaturing gel electrophoresis and then resuspended in TE. The binding reaction (10-μl final volume) contained 20,000 cpm of labeled probe, 1 μg of poly(dI-dC), and various concentrations of MBP-FlrC in low-salt buffer (10 mM sodium phosphate, 30 mM NaCl, 5% glycerol). The reactions were incubated at 37°C for 10 min, loaded onto a 5% polyacrylamide nondenaturing gel (Tris base, 6.0 g; glycine, 28.54 g/liter; pH ∼8.5), and visualized by autoradiography. In experiments involving phosphorylation of FlrC, either MBP-FlrB (with or without 2.5 mM ATP) (5) or acetyl phosphate (50 mM final concentration) was added to the binding reaction. Competition experiments were performed by using an excess of unlabeled PCR products as specific and nonspecific competitors; the chloramphenicol resistance gene from pACYC184 was used as the nonspecific competitor.
For quantitation of mobility shift experiments, gels were quantitated utilizing a Typhoon Imager (Amersham Biosciences) and the program ImageQuant (Molecular Dynamics). The percent shifted species was calculated by subtracting the unshifted species from the total loaded per lane divided by the total loaded per lane; this corrects for loading differences and any instability of complexes during separation.
RESULTS
Determination of the transcription start site for flaA and flgK promoters.
The V. cholerae polar flagellar filament is composed of five distinct flagellins, but only one of these, FlaA, is essential for motility (20). In Salmonella enterica serovar Typhimurium as many as 20,000 flagellin subunits are typically assembled into a functional flagellum (25), and it is anticipated that similar large amounts of FlaA are present in the V. cholerae flagellum. The products of the flgKL operon are hook-associated proteins (HAP1 and HAP3; encoded by VC2191 and VC2190, respectively) that function to connect the hook to the filament (15); normally, only very few HAP subunits are found in a flagellum (ca. 10 to 20 subunits of FlgK/flagellum and 10 to 40 subunits of FlgL) (16). Both the flaA and the flgKL promoters are σ54 and FlrC dependent, and yet transcription from flaAp is significantly higher (∼10-fold) than from flgKp, apparently reflecting the relative requirements for these subunits within the flagellum (32). This led us to examine FlrC binding at these two promoters in greater detail to determine whether differences in FlrC binding dictated promoter strength.
We performed primer extension of the flaA and flgK promoters by using RNA isolated from wild-type, ΔflrC, and ΔrpoN (i.e., lacking σ54) isogenic V. cholerae strains. A single σ54- and FlrC-dependent transcript was detected at both promoters. The transcription start sites were immediately downstream of consensus σ54-binding sites, which we had previously predicted to be the relevant σ54 recognition sequences in these two promoters (32). The start sites mapped to a G residue in the flaAp located 326 bp upstream from the FlaA start codon and to an A residue in the flgKp located 45 bp upstream from the FlgK start codon (Fig. 1).
FIG. 1.
Identification of the flaAp and flgKp transcription start sites. Primer extension of the flaA (A) and flgK promoters (B) was performed in wild-type, ΔrpoN, and ΔflrC V. cholerae strains. The σ54- and FlrC-dependent transcription start sites correspond to the G and A residues for flaAp and flgKp, respectively (denoted by asterisks). These transcription start sites are 326 and 45 bp upstream from the translational start codons of FlaA and FlgK, respectively.
FlrC-dependent activation requires downstream elements in the flaAp and flgKp.
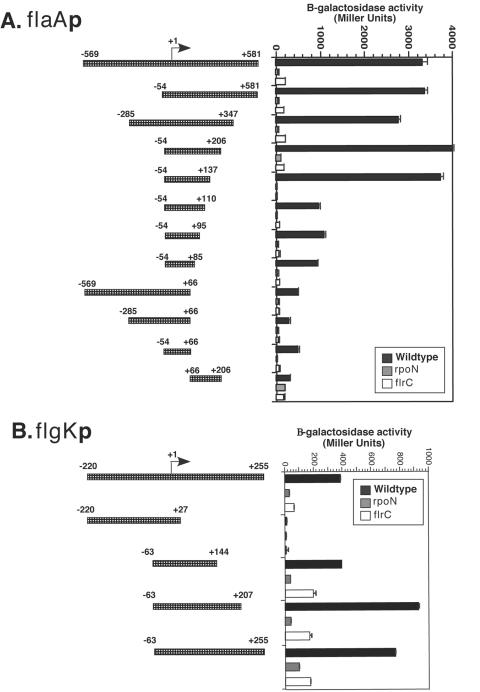
To define the flaAp elements necessary for FlrC-dependent activation, we used truncated fragments of the flaAp, from positions −569 to +581 with respect to the transcription start site, fused to a transcriptional lacZ reporter. β-Galactosidase activity was then measured in wild-type, ΔflrC, and ΔrpoN V. cholerae strains carrying these flaAp-lacZ fusions (Fig. 2A). σ54-Holoenzyme has been shown to bind to regions as far as position −39 with respect to the transcription start site (30) so, to ensure efficient σ54-holoenzyme binding, truncations that removed the upstream regions retained flaAp sequence up to position −54. Truncations of the flaAp that removed the region upstream (positions −569 to −54) of the σ54-holoenzyme binding site retained levels of transcriptional activity equivalent to the full-length promoter fragment, indicating that the upstream region is not required for full FlrC-dependent transcriptional activity. In contrast, truncation of the downstream region to less than +137 reduced transcription approximately three- to fourfold, and truncation of the downstream region to less than +85 reduced transcription by an additional two- to threefold. These results suggest that element(s) located downstream of the transcription start site, between positions +66 and + 137, are important for FlrC-dependent transcription. The smallest fragment tested, from positions −54 to +66, still retained low levels of FlrC- and σ54-dependent transcription, suggesting that some elements recognized by FlrC remain in this fragment.
FIG. 2.
flaA and flgK promoter lacZ fusions identify downstream elements important for σ54- and FlrC-dependent transcription. Various truncated promoter fragments from flaAp (A) and flgKp (B) were fused to lacZ in the reporter plasmid pRS551. On the left, the fragments are depicted with numbering in relation to the transcription start site. The β-galactosidase activity of these promoter constructs was measured in wild-type, ΔrpoN, and ΔflrC V. cholerae strains, as shown on the right. The results represent assays performed in triplicate with the standard deviations shown. Note that all promoter fragments (with the exception of flaAP +67/+206) contain the predicted σ54-holoenzyme binding site.
We analyzed transcription at the FlrC-dependent flgK promoter similarly by creating truncations of the flgK promoter fused to the lacZ reporter and measuring β-galactosidase activity in wild-type, ΔflrC, and ΔrpoN V. cholerae strains (Fig. 2B). Note that transcription from the full-length flgK promoter was almost 10-fold lower than transcription from the full-length flaA promoter. Although relatively few fragments were tested, it was clear that downstream elements are important for FlrC-dependent transcription at this promoter, since a fragment lacking sequence downstream of position +27 showed essentially no transcriptional activity, while fragments containing the downstream region up to position +144 exhibited wild-type levels of FlrC-dependent transcription. These results indicate the presence of element(s) between positions +27 and +144 that are important for FlrC-dependent activation of flgKp. Fragments that lacked sequence upstream of −63 and contained downstream sequence past +144 showed transcriptional activity even greater than the full-length promoter, again emphasizing the lack of sequences important for FlrC-dependent activation upstream of position −63.
FlrC binding to flaAp and flgKp in vitro.
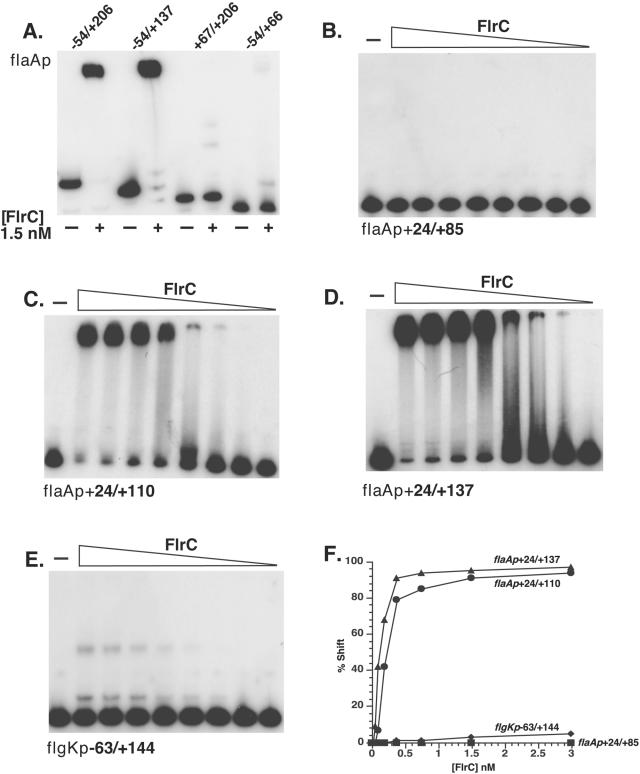
To determine whether FlrC binds directly to flaAp and/or flgKp, gel mobility shift assays were performed utilizing purified MBP-FlrC (5) and 32P-radiolabeled forms of the various truncated promoter fragments (Fig. 3). As seen in Fig. 3A, FlrC causes a mobility shift of the flaAp fragments containing primarily the downstream region (positions −54 to +206 and −54 to +137). The fragment spanning positions −54 to +206 was split in half, forming fragments from positions −54 to +66 and from positions +67 to +206, and then each fragment was subjected separately to mobility shift assay with FlrC. Only an extremely weak mobility shift of each fragment could be detected, suggesting that the FlrC-binding site(s) likely span the region surrounding position +66. The binding of FlrC to the −54-to-+206 and −54-to-+137 fragments was determined to be specific by experiments with an excess of cold specific competitor DNA (flaAp), which was able to eliminate the shifted species, whereas an equivalent amount of cold nonspecific DNA (cat fragment PCR amplified from pACYC184) could not (data not shown).
FIG. 3.
Mobility shift assays indicate stronger binding of FlrC to flaAp than flgKp. Mobility shift assays were performed as described in Materials and Methods with MBP-FlrC and 32P-labeled DNA fragments. The 5′ and 3′ ends of the fragments are noted in relation to the transcription start site. (A) flaAp −54/+206, −54/+137, +67/+206, and −54/+66 fragments were used in mobility shift assays either in the absence (−) or presence (+) of 1.5 nM MBP-FlrC. Fragments corresponding to flaAp (+24/+85) (B), flaAp (+24/+110) (C), flaAp (+24/+137) (D), and flgKp (−63/+144) (E) were incubated either in the absence (−) or presence (+) of MBP-FlrC at final concentrations of 3.0, 1.5, 0.75, 0.375, 0.187, 0.09, 0.046, and 0.023 nM. (F) The percent shifted species in panels B to E were calculated as described in Materials and Methods.
The region of FlrC binding within the flaAp was further delineated by examining the mobility shift patterns of truncated flaAp fragments with increasing concentrations of purified FlrC. Identical gel mobility shift conditions with an identical preparation of purified FlrC were utilized for the experiments shown in Fig. 3B to E, allowing the relative binding of FlrC obtained by densitometric analysis to be directly compared as a function of the percentage of shifted species (Fig. 3F). FlrC specifically binds the downstream fragment from +24 to +137 (+24/+137 fragment) (Fig. 3D) with the same apparent affinity as the −54/+137 fragment (not shown), indicating that the FlrC binding site(s) lie downstream of position +24. Moreover, FlrC binds to the +24/+110 fragment (Fig. 3C) with the same apparent affinity as to the +24/+137 fragment (Fig. 3F), indicating that the FlrC binding site(s) lie upstream of position +110. However, FlrC failed to bind to the +24/+85 fragment (Fig. 3B, 3F), demonstrating the existence of critical elements required for FlrC binding within the +85/+110 region. These results identify the +24/+110 region as containing the FlrC binding site(s) for this promoter. Interestingly, the region between positions +110 and +137, while not required for FlrC binding, is required for wild-type levels of transcriptional activation from this promoter (Fig. 2A).
Gel mobility shift assays were also performed with purified MBP-FlrC and 32P-labeled flgK promoter fragments. In preliminary experiments, two fragments spanning the −220/+27 and −63/+255 regions (both thus contain the σ54-holoenzyme binding site and the transcription start site) were tested for their ability to be bound by FlrC. The upstream fragment (−220/+27) was not shifted by FlrC, whereas the downstream fragment (−63/+255) demonstrated weak binding to FlrC (data not shown). A fragment spanning the −63/+144 flgKp region (Fig. 3E) was bound by FlrC similarly to the −63/+255 region (not shown), indicating that the FlrC binding site(s) within this promoter was localized to the region upstream of position +144. The binding of FlrC to the −63/+144 flgKp fragment was determined to be specific by experiments with an excess of cold specific competitor DNA (flgKp), which was able to eliminate the shifted species, whereas an equivalent amount of cold nonspecific DNA (cat fragment PCR amplified from pACYC184) could not (data not shown).
Because the gel mobility shift of the −63/+144 flgKp fragment (Fig. 3E) was performed under identical conditions as those used for the flaAp fragments (Fig. 3B to D), the relative affinity of FlrC for these two promoters can be directly compared (Fig. 3F). The percentage of shift for flgK promoter −63/+144 fragment was ∼10-fold less than that for the flaA promoter +24/+137 and + 24/+110 fragments, which approached 100% at relatively low FlrC concentrations. It is clear that FlrC has lower affinity for the binding site(s) within the flgKp than for those within the flaAp.
It has been shown for NtrC that phosphorylation increases cooperative DNA binding by enhancing protein-protein interactions (31). To determine whether phosphorylation of FlrC alters DNA binding, we performed gel mobility shift assays with increasing concentrations of FlrC and the flaAp fragment (−54/+206) or the flgKp fragment (−63/+255) under two different conditions that promote phosphorylation of FlrC: (i) in the presence of the cognate histidine kinase FlrB and ATP (5) and (ii) in the presence of the phosphodonor acetylphosphate (24). We compared the binding patterns of the wild-type FlrC protein under phosphorylated and unphosphorylated conditions to the binding patterns of a mutant form of FlrC (FlrCD54A), which cannot be phosphorylated (5). We detected no difference in the binding patterns of FlrC to the flaA or flgK promoter fragments (data not shown). Our results suggest that phosphorylation of FlrC does not perceptibly alter its DNA-binding capability to the flaA or flgK promoters.
DNase I footprinting of FlrC binding site(s):.
To define the exact region of DNA that FlrC binds in the flaA promoter, we performed DNase I footprinting with the −54/+137 fragment, which retained wild-type transcriptional activity (Fig. 2A) and was bound by FlrC (Fig. 3). This 191-bp fragment was labeled at either end with [γ32P]ATP, incubated with different concentrations of FlrC, and then subjected to DNase I digestion, and the products were separated by gel electrophoresis. FlrC protected a region from positions +32 to +85 on one strand and from positions +24 to +75 on the other strand at the lowest concentrations of FlrC (Fig. 4 and data not shown). Additional partial protection extending from positions +24 to −42 could be detected at higher concentrations of FlrC; this region overlaps with the σ54-holoenzyme binding site and is thus likely to be occupied by E-σ54 during active transcription. The asterisk in Fig. 4 indicates an area of enhanced cleavage which corresponds to the A residue at position +58.
FIG. 4.
DNase I footprint of FlrC at the flaA promoter. A flaAp fragment (−54/+137) was radiolabeled and subjected to DNase I digestion as described in Materials and Methods; the bottom strand is shown. The start site of transcription is indicated by an arrow, the downstream area protected at the lowest FlrC concentrations is delineated by a thick line at right (+32/+85), the region protected at higher FlrC concentrations is delineated by a thin line at right (−42/+24), and the enhanced cleavage site is marked with an asterisk. FlrC was added to final concentrations of 1.5, 0.75, 0.375, 0.187, 0.09, and 0.046 nM.
We performed similar DNase I footprinting of FlrC on the flgK promoter but were unable to detect specific protection despite several attempts, thus suggesting weaker FlrC binding at this promoter.
The FlrC binding site(s) at the flaA promoter act as an enhancer element.
The FlrC homologue NtrC is known to be an enhancer-binding protein, i.e., the NtrC binding sites that constitute the enhancer function to stimulate transcription at large distances either upstream or downstream of the transcription start site. To determine whether the FlrC binding site(s) located downstream of the transcription start sites in flaAp and flgKp can function as enhancer elements (i.e., function upstream of the transcription start site), we constructed promoters in which the downstream FlrC binding regions of the flaA promoter from positions +11 to +110 and the flgK promoter from positions +11 to +144 were moved upstream of the corresponding transcription start site at position −285 for flaAp and at position −220 for flgKp. The transcriptional activity of these constructs was measured in wild-type, ΔrpoN, and ΔflrC V. cholerae strains (Fig. 5).
FIG. 5.
The flaAp FlrC binding site(s) act as an enhancer element. Various native and engineered promoter fragments from flaAp and flgKp were fused to lacZ in the reporter plasmid pRS551. On the left, the fragments are depicted with numbering in relation to the transcription start site. The first and fourth promoters from the top, respectively, depict the native flaAp and flgKp. The FlrC binding regions of flaAp (positions +11 to +110; hatched areas) were moved upstream of the transcription start sites to −285 for flaAp and to −220 for flgKp (the third and eighth promoters from the top, respectively). The FlrC binding regions of flgKp (positions +11 to +144; shaded areas) were moved upstream of the transcription start sites, to −285 for flaAp and to −220 for flgKp (the sixth and seventh promoters from the top, respectively). The β-galactosidase activity of these promoter constructs was measured in wild-type, ΔrpoN, and ΔflrC V. cholerae strains as shown on the right. Results represent assays performed in triplicate, with the standard deviations shown.
The flaAp fragment lacking the FlrC binding site(s) between positions +11 and +110 (−285/+10) has approximately one-fourth the activity of the flaAp with these sites located at their native position downstream of the start site (−54/+110). Likewise, when the FlrC binding site(s) between positions +11 and +144 was removed from the flgKp (−220/+10), the resulting truncated flgKp has approximately one-fifth the transcriptional activity of the flgKp with these sites located at their native position downstream of the start site (−63/+144). These results again confirm that the FlrC binding sites downstream of the respective transcription start sites contribute to FlrC-dependent transcription of these two promoters.
When the flaAp FlrC binding site(s) normally located at positions +11 to +110 are moved 285 bp upstream of the flaAp start site, transcription is increased approximately fivefold compared to the flaAp lacking these site(s) (−285/+10), demonstrating that these FlrC binding site(s) function as an enhancer element. Also, when the flaAp FlrC binding sites located at positions +11 to +110 are moved 220 bp upstream of the flgKp startsite, transcription is increased approximately sevenfold compared to the flgKp lacking the native FlrC binding site(s) (−220/+10). These results clearly demonstrate that the FlrC binding site(s) within the flaAp (located between +11 and +110) can function as an enhancer, at both the flaA and flgK promoters.
In contrast, when the flgKp FlrC binding site(s) normally located at +11 to +144 are moved 220 bp upstream of the flgKp startsite, no significant increase in transcription was seen compared to the flgKp lacking these site(s) (−220/+10), demonstrating that these FlrC binding site(s) do not function as an enhancer element. Also, when the flgKp FlrC binding sites located at positions +11 to +144 are moved 285 bp upstream of the flaAp start site, there was no increase in transcription, compared to the flaAp lacking the native FlrC binding site(s) (−285/+10). Given the weaker binding of FlrC to the flgKp binding site(s) compared to the flaAp binding site(s) (Fig. 3), our results suggest that the strength of FlrC binding contributes to enhancer function.
Identification of specific nucleotides important for FlrC DNA binding.
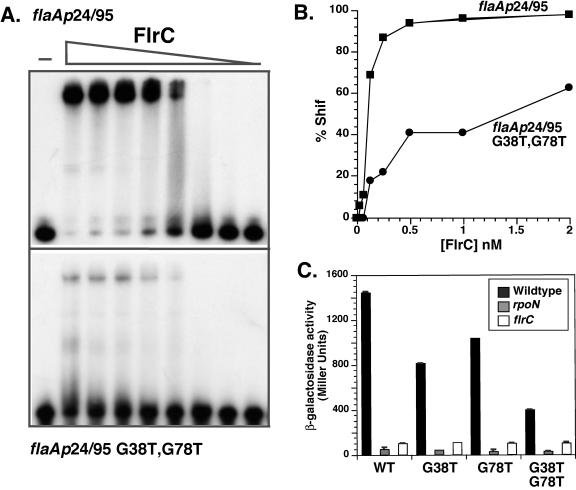
Within the FlrC binding region of the flaA promoter, there is a 6-bp sequence motif, CGGCAA, that is repeated four times. To determine whether this sequence was important for FlrC binding, we changed the second G residue in the motif to T in the first and last sequence motifs (G38T and G78T) and then tested the resultant promoter fragments for both FlrC-dependent transcriptional activation and DNA binding. Due to the relative ease of construction, for these studies the reengineered flaA promoter with the FlrC enhancer region (+11/+110) placed 285 bp upstream (described above) was used for lacZ fusion transcriptional analysis.
Promoters carrying either individual mutation (G38T or G78T) were less transcriptionally active than the parent promoter when measured by lacZ transcriptional fusions, but the decrease in transcription was less than twofold (Fig. 6C). However, a promoter carrying both mutations (G38T G78T) was reduced almost fourfold for transcription. When both mutations were introduced into the flaAp FlrC binding fragment (+24/+95), a significant decrease in FlrC binding could be detected by gel mobility shift assay in comparison with the native fragment (Fig. 6A) corresponding to an approximate fourfold decrease in binding at lower concentrations of FlrC (Fig. 6B). These results demonstrate that the G residues at positions +38 and +78, which are located within the CGGCAA motifs in the flaA promoter, are important for FlrC binding. Moreover, the close agreement between FlrC binding and FlrC-dependent transcriptional activation demonstrates that the strength of FlrC binding dictates the transcriptional strength of FlrC-dependent promoters.
FIG. 6.
The G residues in flaAp at positions +38 and +78 are important for FlrC binding and transcription. (A) Mobility shift assays of MBP-FlrC (0.016 to 2.0 nM) incubated with the native flaAp fragment (+24/+95) (upper panel) or the same fragment containing the G+38→T and G+78→T changes (lower panel). MBP-FlrC was added to final concentrations of 2.0, 1.0, 0.5, 0.25, 0.125, 0.062, 0.031, and 0.016 nM. (B) The percent shifted species in panel A was calculated as described in Materials and Methods. (C) The reengineered flaAp containing the FlrC binding sites 285 bp upstream of the start site (+11/+110 fused to −285/+10) and the same promoter fragment with the G + 38→T and/or G + 78→T mutations were fused to lacZ in the reporter plasmid pRS551. The β-galactosidase activity of these promoter constructs was measured in wild-type, ΔrpoN, and ΔflrC V. cholerae strains. The results represent assays performed in triplicate, with the standard deviations shown.
DISCUSSION
Motility has been identified as a virulence factor for V. cholerae (9-11, 21, 22, 34), but the molecular mechanism(s) by which flagellar function affects pathogenesis has not been determined. It has been suggested that expression of motility may repress expression of virulence factors and vice versa (11). We previously discovered a link between motility and virulence that involves the σ54-dependent activator, FlrC (5). FlrC activates the transcription of class III flagellar genes (32), and locking FlrC into an inactive (D54A) state prevents flagellar synthesis and also inhibits intestinal colonization (5). Interestingly, locking FlrC into a constitutively active (M114I) state also inhibits intestinal colonization, suggesting that modulation of the transcriptional activity of FlrC is important for the colonization process. One possible mechanism by which both inactive and constitutively active FlrC could inhibit intestinal colonization would be through a DNA-binding-mediated repression of virulence genes. However, FlrC DNA binding has not previously been characterized; thus, the present study sought to analyze FlrC binding at two flagellar promoters, flaA and flgK.
Our studies demonstrate that FlrC binds to elements located downstream of the σ54- and FlrC-dependent transcription start sites of both the flaA and the flgK promoters. The FlrC binding site(s) in the flaA promoter lie in the region located at positions +24 to +95 with respect to the transcription start site (as determined by gel mobility shift in Fig. 3 and 6) within a large (326-bp) untranslated region upstream of the flaA coding sequence (Fig. 7A). Although the annotation of the V. cholerae genome (www.tigr.org/tigr-scripts/CMR2/GenomePage3.spl?database=gvc) indicates the presence of an open reading frame (VC2189) encoding a small 59-amino-acid hypothetical protein within the region upstream of the flaA coding sequence, this region is unlikely to contain an open reading frame because (i) the transcription start site mapped to a nucleotide downstream of the putative start codon of VC2189, which corresponded to a TTG rather than the typical ATG, and (ii) VC2189 shares no homology with any gene within any database, including closely related Vibrio spp. The long untranslated leader sequence of the flaA transcript may play a role in the regulation of FlaA translation, as has been demonstrated for the S. enterica flagellin, FliC (1). FlrC also binds downstream of the σ54- and FlrC-dependent transcription start site of the flgK promoter, in a region from positions +11 to +144 with respect to the transcription start site; this region contains the start codon for FlgK.
FIG. 7.
Schematic representation of the flaA and flgK promoter regions. The transcriptional start site (position +1) and consensus sequence for E-σ54 binding are shown for flaAp (A) and flgKp (B). The region of flaAp protected by FlrC, as deduced by DNase I footprinting, is indicated with a thin line for the top strand and thick line for the bottom strand. Horizontal arrows in flaAp indicate the CGGCAA repeats. Mutagenized bases in flaAp (G+38 and G+78) are indicated by small vertical arrows. The asterisk indicates the flaAp base pair demonstrating enhanced cleavage when bound by FlrC during DNase I digestion. Dashed lines in flgKp indicate the regions of homology between flaAp and flgKp, which are also shown in panel C. The initiating translational start codon for FlgK is shown by a small square. (D) Homology between flaAp and the hemolysin genes VC1888 and VCA0219.
σ54-dependent activators typically bind to sites located upstream of the σ54-holoenzyme binding site and contact RNAP by a DNA looping mechanism (43). The σ54-dependent activator binding site(s) serve to tether the activating protein in close proximity to σ54-holoenzyme to facilitate productive interactions (39). The presence of FlrC binding sites downstream of the transcriptional start sites not only suggests that these sites tether FlrC in close proximity to σ54-holoenzyme but that FlrC binding itself contributes to transcription regulation, perhaps by blocking the progression of transcription elongation complexes. However, FlrC binds with highest affinity to the site(s) within flaAp, and yet this promoter is also the most transcriptionally active, indicating that FlrC bound within the DNA being actively transcribed does not prevent progression of elongation complexes. σ54-dependent transcription activation has only been studied in mechanistic detail at promoters where the activator binding sites lie outside the region being transcribed, so the effect of binding site placement within the transcribed region on σ54-dependent transcription awaits future experimentation.
Although the downstream location of FlrC binding sites is unusual, there are a few other examples of σ54-dependent activators binding downstream of the transcription start site. Notably, FleQ of P. aeruginosa (18), a homologue of V. cholerae FlrA and activator of class II flagellar genes, binds in the downstream region of the transcription start site of the flagellar promoters flhA, fliE and fliL. Ramphal and coworkers suggested that the close proximity of FleQ binding sites to the σ54-dependent transcription start sites is incompatible with a DNA looping mechanism and argues for a direct interaction between activator and RNAP without looping. The DNA-binding site(s) for V. cholerae FlrA has not yet been determined, but if it is similarly located downstream, this σ54-dependent promoter architecture may be common in V. cholerae.
The FlrC binding region in the flaAp probably corresponds to sites for at least two FlrC dimers based on the NtrC paradigm. Two dimers of NtrC are necessary for stimulation of the ATPase activity required for transcriptional activation. The two NtrC sites in glnAp that compose the enhancer lie on the same face of the DNA helix, which facilitates cooperative binding that is enhanced by NtrC phosphorylation (31). The cooperative interactions facilitated by the NtrC enhancer are so strong that only a single shifted species that contains two NtrC dimers bound to DNA is seen in a gel mobility shift assay; a single NtrC dimer binds poorly to each individual site. This architecture essentially assures constant occupancy at the glnAp enhancer by two dimers. We believe a similar architecture exists for the FlrC binding site(s) in the flaAp, because (i) the motif CGGCAA is found four times in this region, which suggests recognition by two dimers, and mutation of the second G in the first and last motif diminishes both FlrC binding and FlrC-dependent transcriptional activation; (ii) a single shifted species of the entire region is seen in gel mobility shift assays, and when this region is split (at position +66), FlrC binds to each individual fragment very weakly; (iii) enhanced DNase I cleavage is seen at position +58, which would be expected if two interacting dimers bind on either side and cause bending of the intervening DNA; and (iv) the FlrC binding region in flaAp acts as an enhancer, since it can be moved a large distance upstream and still function; because there is likely to be a requirement for two dimers to activate σ54-dependent transcription, and the flaAp binding region can function upstream as well as downstream. This implies that the binding region contains binding sites for at least two FlrC dimers.
Our results from gel shift assays with purified FlrC and the flaAp fragment under conditions that promote phosphorylation of FlrC, i.e., the presence of histidine kinase FlrB and ATP or using acetyl phosphate as a phospho-donor, failed to detect differences in DNA binding by phosphorylated FlrC to these promoter fragments. Phosphorylation of NtrC increases the cooperative interactions between NtrC dimers (31). However, in order to visualize the effect of phosphorylation on DNA binding, an artificial glnAp enhancer needed to be created with two “strong” NtrC binding sites, since a single dimer bound to one of the natural “weak” NtrC sites was unable to be visualized. In other words, NtrC cooperative interactions could only be measured if a singly bound shifted species could also be visualized, and gel shifts with the native enhancer failed to produce single dimer-DNA interactions. A similar situation likely exists at the FlrC binding sites in the flaAp, where two relatively weak individual FlrC binding sites are situated to promote strong cooperative interactions.
Comparison of the flaAp FlrC binding region to flgKp revealed a 14-bp stretch of homology (Fig. 7C), GGGTAACCAAAGCG, that lies within the coding sequence for FlgK. However, the CGGCAA motifs are not found within the flgKp. This is perhaps not surprising; since FlrC binds the flaAp binding sites more strongly than the flgKp binding sites, more degeneracy is likely to be present within the flgKp binding sites. When the 72 bp contained in the flaAp fragment from positions +25 to +95 (minimum size fragment bound by FlrC) is used to search the entire V. cholerae genome, a number of matches are found. A total of 55 matches were found in chromosome 1 and 22 matches were found in chromosome 2 that were at least 12 bp long; 73% of these contained the CGGCAA motif. Interestingly, two of the matches were within the coding sequences for the hemolysin HlyA (VCA0219) and the hemolysin-related protein VC1888 (Fig. 7D). We previously reported increased hemolytic activity in a V. cholerae ΔflrC strain (5), so it will be interesting to determine whether FlrC binding at either of these sites negatively regulates hemolytic activity.
Surprisingly, none of the known FlrC-regulated promoters (32) were identified in the search of the genome with the flaAp binding region (+24/+95). Our results demonstrate that FlrC binds flaAp stronger than flgKp, and we suggest that the flaAp sites are likely the strongest FlrC binding sites within the genome. The other FlrC binding sites within the genome are probably more degenerate from the flaAp binding site sequence, reflecting the lower levels of transcription necessary for the other FlrC-dependent genes. Thus, although FlrC activates multiple promoters, different levels of FlrC-dependent transcription are achieved in part by the strength of FlrC binding to the individual promoters, as has also been suggested for the FlrC homologue from Caulobacter crescentus, the flagellar regulator FlbD (42). It follows from this logic that enhancer strength also depends on strength of FlrC binding; the flaAp sites constitute an enhancer because FlrC binds more strongly to these sites, and the higher occupancy of FlrC at these sites facilitates FlrC-RNAP contacts, even from a distance. In contrast, the weak binding of FlrC to flgKp not only leads to lower levels of transcription at this promoter but also to an inability of these binding site(s) to act as an enhancer, due to lower occupancy of FlrC, which leads to an inability to facilitate FlrC-RNAP contacts from a distance.
Additional elements also appear to contribute to the high levels of flaAp transcription. We were unable to detect any FlrC binding downstream of position +95, yet the region from positions +110 to +137 is necessary for full transcriptional activity of this promoter. The nature of this enhancing activity is unclear, but this region shares homology with the sequence surrounding the flgKp transcription start site (Fig. 7C). It will be interesting to determine whether the FlrC homologues found in other polar flagellates, i.e., V. parahaemolyticus FlaM (26), C. jejuni FlgR (14, 17, 41), and P. aeruginosa FleR (7, 35), exhibit similar enhancer binding characteristics.
Acknowledgments
We thank Sinem Beyhan and Fitnat Yildiz for assistance with the V. cholerae genome searches.
This research was supported by NIH AI43486 to K.E.K.
REFERENCES
- 1.Bonifield, H. R., and K. T. Hughes. 2003. Flagellar phase variation in Salmonella enterica is mediated by a posttranscriptional control mechanism. J. Bacteriol. 185:3567-3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler, S. M., and A. Camilli. 2004. Both chemotaxis and net motility greatly influence the infectivity of Vibrio cholerae. Proc. Natl. Acad. Sci. USA 101:5018-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Contreras, A., and M. Drummond. 1988. The effect on the function of the transcriptional activator NtrC from Klebsiella pneumoniae of mutations in the DNA-recognition helix. Nucleic Acids Res. 16:4025-4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Correa, N. E., J. R. Barker, and K. E. Klose. 2004. The Vibrio cholerae FlgM homologue is an anti-sigma28 factor that is secreted through the sheathed polar flagellum. J. Bacteriol. 186:4613-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Correa, N. E., C. M. Lauriano, R. McGee, and K. E. Klose. 2000. Phosphorylation of the flagellar regulatory protein FlrC is necessary for Vibrio cholerae motility and enhanced colonization. Mol. Microbiol. 35:743-755. [DOI] [PubMed] [Google Scholar]
- 6.Coster, T. S., K. P. Killeen, M. K. Waldor, D. T. Beattie, D. R. Spriggs, J. R. Kenner, A. Trofa, J. C. Sadoff, J. J. Mekalanos, and D. N. Taylor. 1995. Safety, immunogenicity, and efficacy of live attenuated Vibrio cholerae O139 vaccine prototype. Lancet 345:949-952. [DOI] [PubMed] [Google Scholar]
- 7.Dasgupta, N., M. C. Wolfgang, A. L. Goodman, S. K. Arora, J. Jyot, S. Lory, and R. Ramphal. 2003. A four-tiered transcriptional regulatory circuit controls flagellar biogenesis in Pseudomonas aeruginosa. Mol. Microbiol. 50:809-824. [DOI] [PubMed] [Google Scholar]
- 8.Freter, R., and P. C. O'Brien. 1981. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: chemotactic responses of Vibrio cholerae and description of motile nonchemotactic mutants. Infect. Immun. 34:215-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freter, R., and P. C. O'Brien. 1981. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: fitness and virulence of nonchemotactic Vibrio cholerae mutants in infant mice. Infect. Immun. 34:222-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freter, R., P. C. O'Brien, and M. S. Macsai. 1981. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vivo studies. Infect. Immun. 34:234-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardel, C. L., and J. J. Mekalanos. 1996. Alterations in Vibrio cholerae motility phenotypes correlate with changes in virulence factor expression. Infect. Immun. 64:2246-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 13.Heidelberg, J. F., J. A. Eisen, W. C. Nelson, R. A. Clayton, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, L. Umayam, S. R. Gill, K. E. Nelson, T. D. Read, H. Tettelin, D. Richardson, M. D. Ermolaeva, J. Vamathevan, S. Bass, H. Qin, I. Dragoi, P. Sellers, L. McDonald, T. Utterback, R. D. Fleishmann, W. C. Nierman, and O. White. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hendrixson, D. R., and V. J. DiRita. 2003. Transcription of sigma54-dependent but not sigma28-dependent flagellar genes in Campylobacter jejuni is associated with formation of the flagellar secretory apparatus. Mol. Microbiol. 50:687-702. [DOI] [PubMed] [Google Scholar]
- 15.Homma, M., and T. Iino. 1985. Locations of hook-associated proteins in flagellar structures of Salmonella typhimurium. J. Bacteriol. 162:183-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikeda, T., M. Homma, T. Iino, S. Asakura, and R. Kamiya. 1987. Localization and stoichiometry of hook-associated proteins within Salmonella typhimurium flagella. J. Bacteriol. 169:1168-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jagannathan, A., C. Constantinidou, and C. W. Penn. 2001. Roles of rpoN, fliA, and flgR in expression of flagella in Campylobacter jejuni. J. Bacteriol. 183:2937-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jyot, J., N. Dasgupta, and R. Ramphal. 2002. FleQ, the major flagellar gene regulator in Pseudomonas aeruginosa, binds to enhancer sites located either upstream or atypically downstream of the RpoN binding site. J. Bacteriol. 184:5251-5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenner, J. R., T. S. Coster, D. N. Taylor, A. F. Trofa, M. Barrera-Oro, T. Hyman, J. M. Adams, D. T. Beattie, K. P. Killeen, D. R. Spriggs, et al. 1995. Peru-15, an improved live attenuated oral vaccine candidate for Vibrio cholerae O1. J. Infect. Dis. 172:1126-1129. [DOI] [PubMed] [Google Scholar]
- 20.Klose, K. E., and J. J. Mekalanos. 1998. Differential regulation of multiple flagellins in Vibrio cholerae. J. Bacteriol. 180:303-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klose, K. E., and J. J. Mekalanos. 1998. Distinct roles of an alternative sigma factor during both free-swimming and colonizing phases of the Vibrio cholerae pathogenic cycle. Mol. Microbiol. 28:501-520. [DOI] [PubMed] [Google Scholar]
- 22.Lee, S. H., S. M. Butler, and A. Camilli. 2001. Selection for in vivo regulators of bacterial virulence. Proc. Natl. Acad. Sci. USA 98:6889-6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lospalluto, J. J., and R. A. Finkelstein. 1972. Chemical and physical properties of cholera exo-enterotoxin (choleragen) and its spontaneously formed toxoid (choleragenoid). Biochim. Biophys. Acta 257:158-166. [DOI] [PubMed] [Google Scholar]
- 24.Lukat, G. S., W. R. McCleary, A. M. Stock, and J. B. Stock. 1992. Phosphorylation of bacterial response regulator proteins by low molecular weight phospho-donors. Proc. Natl. Acad. Sci. USA 89:718-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macnab, R. M. 2003. How bacteria assemble flagella. Annu. Rev. Microbiol. 57:77-100. [DOI] [PubMed] [Google Scholar]
- 26.McCarter, L. L. 2001. Polar flagellar motility of the Vibrionaceae. Microbiol. Mol. Biol. Rev. 65:445-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mekalanos, J. J., D. J. Swartz, G. D. Pearson, N. Harford, F. Groyne, and M. de Wilde. 1983. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature 306:551-557. [DOI] [PubMed] [Google Scholar]
- 28.Miller, J. H. 1992. A Short Course in Bacterial Genetics. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 29.North, A. K., K. E. Klose, K. M. Stedman, and S. Kustu. 1993. Prokaryotic enhancer-binding proteins reflect eukaryote-like modularity: the puzzle of nitrogen regulatory protein C. J. Bacteriol. 175:4267-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popham, D. L., D. Szeto, J. Keener, and S. Kustu. 1989. Function of a bacterial activator protein that binds to transcriptional enhancers. Science 243:629-635. [DOI] [PubMed] [Google Scholar]
- 31.Porter, S. C., A. K. North, A. B. Wedel, and S. Kustu. 1993. Oligomerization of NTRC at the glnA enhancer is required for transcriptional activation. Genes Dev. 7:2258-2273. [DOI] [PubMed] [Google Scholar]
- 32.Prouty, M. G., N. E. Correa, and K. E. Klose. 2001. The novel sigma54- and sigma28-dependent flagellar gene transcription hierarchy of Vibrio cholerae. Mol. Microbiol. 39:1595-1609. [DOI] [PubMed] [Google Scholar]
- 33.Reitzer, L. J., and B. Magasanik. 1986. Transcription of glnA in Escherichia coli is stimulated by activator bound to sites far from the promoter. Cell 45:785-792. [DOI] [PubMed] [Google Scholar]
- 34.Richardson, K. 1991. Roles of motility and flagellar structure in pathogenicity of Vibrio cholerae: analysis of motility mutants in three animal models. Infect. Immun. 59:2727-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ritchings, B. W., E. C. Almira, S. Lory, and R. Ramphal. 1995. Cloning and phenotypic characterization of fleS and fleR, new response regulators of Pseudomonas aeruginosa which regulate motility and adhesion to mucin. Infect. Immun. 63:4868-4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 37.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 38.Taylor, R. K., V. L. Miller, D. B. Furlong, and J. J. Mekalanos. 1987. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. USA 84:2833-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wedel, A., D. S. Weiss, D. Popham, P. Droge, and S. Kustu. 1990. A bacterial enhancer functions to tether a transcriptional activator near a promoter. Science 248:486-490. [DOI] [PubMed] [Google Scholar]
- 40.Weiss, D. S., J. Batut, K. E. Klose, J. Keener, and S. Kustu. 1991. The phosphorylated form of the enhancer-binding protein NTRC has an ATPase activity that is essential for activation of transcription. Cell 67:155-167. [DOI] [PubMed] [Google Scholar]
- 41.Wosten, M. M., J. A. Wagenaar, and J. P. van Putten. 2004. The FlgS/FlgR two-component signal transduction system regulates the fla regulon in Campylobacter jejuni. J. Biol. Chem. 279:16214-16222. [DOI] [PubMed] [Google Scholar]
- 42.Wu, J., A. K. Benson, and A. Newton. 1995. Global regulation of a sigma 54-dependent flagellar gene family in Caulobacter crescentus by the transcriptional activator FlbD. J. Bacteriol. 177:3241-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu, H., and T. R. Hoover. 2001. Transcriptional regulation at a distance in bacteria. Curr. Opin. Microbiol. 4:138-144. [DOI] [PubMed] [Google Scholar]