Abstract
In the ageing process, the vascular system undergoes morphological and functional changes that may condition brain functioning; for this reason, the aims of this study were to assess the effect of vascular function indirectly measured by ankle-brachial index (ABI) on both cognitive performance at baseline and change in cognitive performance at end of follow-up. We developed a prospective, population-based, cohort study with 1147 participants aged > 65 years obtained from the Toledo Study for Healthy Ageing who had cognitive assessment and measured ABI in the first wave (2006–2009) were selected for the cross-sectional analysis. Those participants who also performed the cognitive assessment in the second wave (2011–2013) were selected for the prospective analysis. Cognitive impairment diagnosis and symptoms and/or history of cardio/neurovascular disease were used as exclusion criteria. Multivariate segmented regression model was used to assess the associations between ABI and cognitive performance in both the cross-sectional and prospective analyses. As ABI score decreased from 1.4, the cross-sectional analysis showed a higher decrease in cognitive performance and the prospective analysis showed a higher degree of worsening in cognitive performance. Our findings suggest that the ABI, a widespread measure of vascular health in primary care, may be a useful tool for predicting cognitive performance and its evolution.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-023-00966-4.
Keywords: Vascular function (VF), Ankle-brachial index (ABI), Cognitive performance, Cognitive test, Ageing
Introduction
The ageing process is a natural and multi-factorial phenomenon characterised by the accumulation of degenerative processes [1]. This process compromises the capabilities of the physiological processes that allow us to maintain the correct structure and function of the various molecules, cells, tissues, organs, and systems, to adapt to variations in the internal and external environment [2]. Cardiovascular system (CVS) and the central nervous system (CNS) are involved in the ageing process. Both systems undergo morphological and functional changes that may be compromised sometimes directly by ageing and sometimes as a consequence of the involvement of other systems [3]. These two systems are interrelated; an adequate blood flow in the brain structures ensures a normal brain function [4]. Furthermore, the CNS plays an important role in maintaining homeostasis of the cardiovascular system [5], through its modulation of autonomic activity [6, 7].
The blood flow regulation, blood pressure, capillary recruitment and filtration, and central venous pressure all fall under a generalised term used to describe them: vascular function (VF) [8]. An alteration of the VF can lead to an abnormal brain function and consequently the development of future cognitive decline and neurodegenerative disorders [9], with enormous social repercussions in terms of loss of quality of life and economic burden [10].
The ankle-brachial index (ABI) is a non-invasive test used in routine clinical practice to indirectly assess VF. Abnormal ABI score represents different changes in the vascular structure (ABI < 0.9 correlates with vascular stenosis and ABI > 1.4 with vascular stiffness) and both are associated with risk of cardiovascular events [11].
The ABI score ≤ 0.9 is the most studied, and this threshold has been shown to have a sensitivity and specificity > 90% for detecting peripheral artery disease compared to angiography [11]. However, other studies confirmed that the cardiovascular risk increases as ABI score decreases below a threshold of 1.10 [12, 13]. This value is more likely to capture a larger number of asymptomatic participants in whom early intervention is possible to avoid consequences in other organs.
Studies have shown the relationship between ABI and cognitive decline [14–16] and they have demonstrated that low ABI score may be an early predictor with potential value in identifying individuals at increased risk of cognitive decline [17, 18].
Some cognitive domains are damaged prematurely during the ageing process [19] and the degree of damage is related to the presence of subclinical cerebrovascular disorder [20], so it is interesting to analyse cognitive performance according to the ABI score, even within the normal range.
Gender is another factor to consider in the analysis between ABI score and cognitive decline. Females are more likely to suffer cognitive decline, probably related to cardiovascular impairment [21], but other factors may also play a role.
Identification of clinical predictors cognitive decline in elderly people is often considered useful to intervene early and to alleviate the public health burden of cognitive decline, and for this reason, the aims of the present study were: (1) to find out how VF indirectly measured by ABI score modulates the cognitive performance and (2) to assess the effect of ABI score on change in cognitive performance along follow-up in a sample of the Toledo Study for Healthy Ageing (TSHA).
Methods
Study design and sample
Data was obtained from the TSHA. This study is a population-based, prospective, cohort study, with waves performed every 3–4 years. The aim of TSHA was to evaluate the characteristics and consequences of frailty in participants ≥ 65 years old living in the province of Toledo (Spain), which is described elsewhere [22].
Basically, this study was carried out in three different phases: in the first one, a team of trained psychologists conducted an interview at the participant’s home and collected socio-demographic data, performed an extensive neuropsychological battery, and collected self-reported information on comorbidities and drug intake, cardio/neurological symptoms, and alcohol and tobacco consumption; in addition, in case of doubt, the participants’ medical record was checked. Second, a team of trained nurses carried out a physical battery test and collected weight, height, hip and waist circumference, ABI, and blood pressure at rest were measured according to measurement standards. Third, a nurse obtained fasting blood.
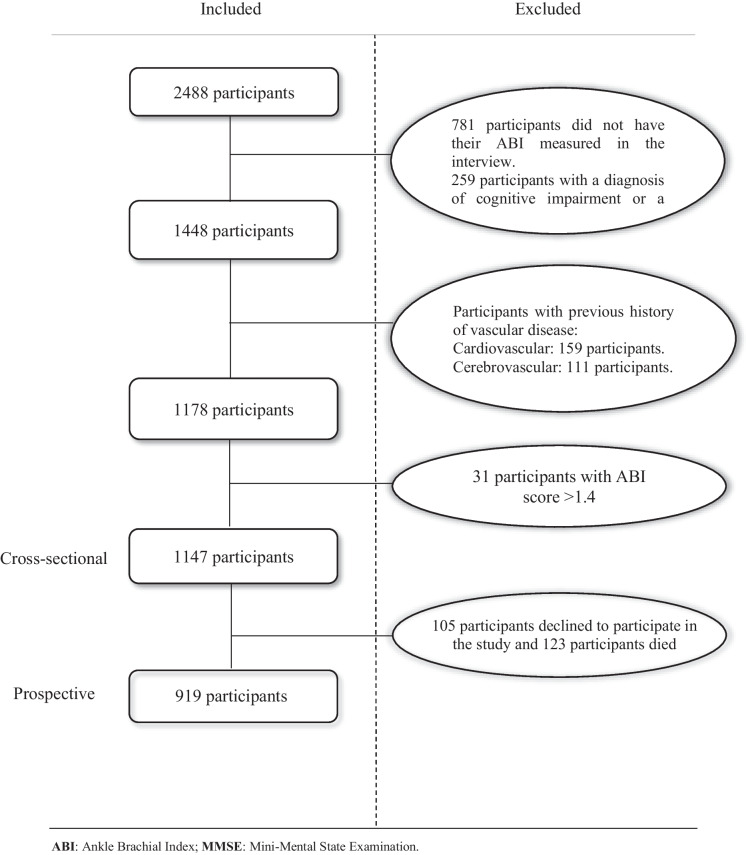
For our analysis, we obtained 2488 participants which were included in the first wave of the TSHA, of which we excluded 781 participants who did not have their ABI measured; 259 participants with cognitive impairment or with Mini-Mental State Examination (MMSE) score below the educational level cut-off point (< 18 for illiterate, < 21 for those with primary education, and < 24 for those with higher educational level) [23]; 159 participants with myocardial infarction, angina pectoris, and intermittent claudication; and 111 participants with transient ischemic attack and an active screening of stroke, so participants with unknown pathology or preclinical stage of the disease were analysed. In addition, due to their under-representation in the sample analysed, 31 participants with ABI > 1.4 were excluded. A total of 1147 participants who underwent cognitive assessment and the ABI measured in the first wave (between 2006 and 2009) remained for the cross-sectional analysis. For the prospective analysis, participants were selected from the first wave who also underwent cognitive assessment in the second wave and consented to follow-up (between 2011 and 2013), of whom 105 participants declined to participate in the study and 123 participants died, leaving a total of 919 participants for follow-up (Fig. 1).
Fig. 1.
Flow chart
The study complies with Spanish law on biomedical research and obtained approval by our Centre’s Clinical Research Ethic Committee [ref: 22/2005]. Before data acquisition, participants signed written informed consent.
Measurements
Neuropsychological evaluation
An extensive neuropsychological assessment was carried out; global cognitive performance was examined using the MMSE (Spanish Version) [23] (score range from 30 points [best] to 0 [worst]). We also measured other compound tests such as 7 Minute Neurocognitive Screening Battery (7-M test) (score range from 5.52 [best] to − 7.14 [worst]) [24, 25], and the Cumulative Executive Dysfunction Index (score range from 16 point [best] to 0 [worst]) [26]. The 7-M test consists of 4 tests representing 4 cognitive areas: (1) short-term memory, assessed by free and facilitated recalling, (2) verbal fluency, assessed by language test that consists of saying as many animals as possible in 1 min, (3) visuospatial and visuoconstruction, assessed by the Clock Drawing Test [27], and (4) orientation for time, assessed by the Benton test; and the Cumulative Executive Dysfunction Index included: (1) verbal fluency (7-M test), (2) digit span [28], (3) Luria’s Orders Tests [29], and (4) go/no-go task [30, 31].
Other individual’s neuropsychological tests assessed were short- and long-term memory by free and facilitated memory, being the sum of both total memory capacity [24]. Long-term memory was evaluated by free and facilitated memory 15 min after short-term memory. During this time, a distraction task was conducted. Hole Peg Test was used to assess manual dexterity and executive function [32, 33].
For each test, we used an internationally recognised scoring rule to assess the participant’s cognitive performance.
Vascular function
It was carried out by measuring the ABI, using eco-Doppler (HADECO mini-Doppler ES-100 ×) according to standards operation [34].
Cardiovascular and cerebrovascular disease
We considered that a participant had the disease based on self-reported information, medical records, and intake of drugs related to cardio/neurovascular diseases.
Cardiovascular risk factors
Body mass index (BMI) was calculated by dividing the weight in kilograms by the square of height in meters.
Hypertension, diabetes mellitus, and dyslipidemia: We considered that a participant had the disease based on self-reported information, medical records, and intake of drugs related to these diseases.
Tobacco: Participants were classified into three categories: non-smokers, active smokers, and former smokers based on their responses to the questions “Have you smoked every day for at least 1 year?” and “are you still smoking?”.
Statistical analysis
Descriptive data was presented as mean (std) and N (percentages). P for trend was used to analyse the relationship between ABI categories and variables.
We performed both a cross-sectional analysis and a prospective analysis using multivariate segmented regression models. In the cross-sectional analysis, we analysed the relationship between basal ABI scores and neuropsychological evaluation scores. And, in the prospective analysis, we analysed the relationship between basal ABI score and the change of neuropsychological evaluation scores between both visits. We used age, gender, BMI, DM, hypertension, educative level, dyslipidemia, and smoker as potential confounders. Additionally, we assessed the associations for each gender separately. Since cognitive function test measures different domains, we did not use the Bonferroni correction for multiple comparisons.
All analyses were performed with the Statistical Package R for windows (Vienna, Austria) (http://www.r-project.org), version 3.5.3. Statistical significance was set as p-value < 0.05.
Results
Table 1 shows the baseline characteristics of the 1147 participants analysed, the mean (SD) of age was 74.3 ± 5.6 years, and 486 participants (42.3%) were males; in terms of cardiovascular risk factors, 48.8% had hypertension, 16% had DM, and 35.8% had dyslipidemia, while 8% were active smokers and 21.4% were former smokers, in addition a mean BMI score of 29.2 ± 4.4 points was observed. In the global cognitive tests measured by MMSE, the mean score was 25.2 points, and the mean score in compound tests, the 7-M test was 1.01 points, while the score of the Cumulative Executive Dysfunction Index was 5.9 points.
Table 1.
Study sample characteristics
| Variable | Overall | ABI < 0.8 | ABI between 0.8– < 1.1 | ABI between > 1.1–1.4 | P for trend |
|---|---|---|---|---|---|
| N | 1147 | 62 | 739 | 346 | |
| Age (y) | 74.32 (5.61) | 75.60 (6.74) | 74.58 (5.52) | 73.55 (5.52) | 0.0006 |
| Gender, male (%) | 486 (42.37) | 30 (48.39) | 300 (40.60) | 156 (45.09) | 0.3776 |
| Charlson Index | 0.92 (1.53) | 0.95 (1.55) | 0.92 (1.51) | 0.93 (1.58) | 0.9503 |
| Educative level (%) | |||||
| - None | 760 (66.26) | 42 (67.74) | 489 (66.17) | 229 (66.18) | 0.8499 |
| - Primary incomplete | 206 (17.96) | 9 (14.52) | 131 (17.73) | 66 (19.08) | 0.4618 |
| - Primary complete | 79 (6.89) | 5 (8.06) | 54 (7.31) | 20 (5.78) | 0.3483 |
| - Secondary or higher | 102 (8.89) | 6 (9.68) | 65 (8.80) | 31 (8.96) | 0.8755 |
| Risk factors | |||||
| - BMI* | 29.26 (4.44) | 29.78 (4.71) | 29.18 (4.50) | 29.35 (4.26) | 0.9327 |
| - DM + | 184 (16.04) | 12 (19.35) | 116 (15.70) | 56 (16.18) | 0.8794 |
| - Hypertension + | 560 (48.82) | 30 (48.39) | 369 (49.93) | 161 (46.53) | 0.3711 |
| - Tobacco use + | |||||
| · Never | 810 (70.62) | 43 (69.35) | 525 (71.04) | 242 (69.94) | 0.7224 |
| · Active | 92 (8.02) | 6 (9.68) | 62 (8.39) | 24 (6.94) | 0.2610 |
| · Former | 245 (21.36) | 13 (20.97) | 152 (20.57) | 80 (23.12) | 0.2544 |
| - Dyslipidemia + | 411 (35.86) | 20 (32.26) | 277 (37.53) | 114 (32.95) | 0.2931 |
| Cognitive performance* | |||||
| - MMSE (total score) | 25.21 (3.30) | 25.31 (3.38) | 25.13 (3.29) | 25.39 (3.30) | 0.3202 |
| - Cumulative Executive Dysfunction Index | 5.91 (2.66) | 6.32 (3.03) | 5.91 (2.64) | 5.82 (2.63) | 0.2558 |
| - 7-MT (total score) | 1.01 (2.02) | 0.61 (2.42) | 0.99 (2.02) | 1.13 (1.93) | 0.1962 |
| 7-MT (Clock Drawing Test) | 0.14 (0.91) | 0.01 (1.10) | 0.13 (0.91) | 0.20 (0.88) | 0.1888 |
| 7-MT (free and facilitated memory) | 0.28 (0.94) | 0.08 (1.06) | 0.30 (0.89) | 0.27 (1.01) | 0.8262 |
| 7-MT (verbal fluency) | 0.09 (1.08) | − 0.15 (1.10) | 0.10 (1.05) | 0.11 (1.15) | 0.3414 |
| 7-MT (Benton’s Temporal Orientation Test) | 0.27 (0.38) | 0.32 (0.03) | 0.26 (0.44) | 0.29 (0.25) | 0.6019 |
| - Denomination | 15.80 (0.57) | 15.69 (0.63) | 15.82 (0.56) | 15.78 (0.58) | 0.9584 |
| - Immediate memory | 15.79 (0.79) | 15.73 (0.62) | 15.77 (0.90) | 15.86 (0.51) | 0.1357 |
| - Total short-term memory | 15.03 (1.67) | 14.67 (1.89) | 15.06 (1.59) | 15.02 (1.80) | 0.8256 |
| - Free short-term memory | 6.80 (2.56) | 6.73 (3.06) | 6.84 (2.54) | 6.75 (2.50) | 0.6728 |
| - Facilitated short-term memory | 8.25 (2.35) | 8.11 (2.49) | 8.26 (2.35) | 8.27 (2.34) | 0.8946 |
| - Total long-term memory | 15.06 (1.62) | 14.58 (1.97) | 15.06 (1.64) | 15.16 (1.50) | 0.1356 |
| - Free long-term memory | 7.52 (3.03) | 6.71 (3.30) | 7.50 (3.07) | 7.73 (2.84) | 0.0344 |
| - Facilitated long-term memory | 7.54 (2.78) | 7.87 (3.12) | 7.57 (2.77) | 7.42 (2.74) | 0.1515 |
| - Hole Peg Test (dominant hand) | 24.97 (7.13) | 25.90 (7.38) | 24.83 (7.15) | 25.10 (7.07) | 0.9627 |
*Mean values and standard deviation (SD); + : N (%). BMI, body mass index; DM, diabetes mellitus; MMSE, Mini-Mental State Examination; 7-MT, 7-min test
According to the ABI score, 346 participants had an ABI score between > 1.1 and 1.4; 739 participants had an ABI score between 0.8 and < 1.1, and 62 participants had an ABI < 0.8. When we compared the basal characteristics between ABI severity categories, we observed that as ABI category severity (lower ABI) was higher, participants were older (p < 0.05) and their performances in free long-term memory test were lower (p = 0.03).
Table 2 shows the cross-sectional analysis of the total effect of ABI score in cognitive performance, showing that for every tenth that the ABI score decreases, there is a more pronounced deterioration in the score of the different cognitive tests in the three ABI categories analysed (1.4 to > 1.1, < 1.1 to 0.8, and < 0.8), and in case of MMSE, the changes in the score were 0.148, 0.199, and 0.321, respectively, per tenth decrease in ABI, with the ABI category < 0.8 being statistically significant (p = 0.047). The evolution observed in free short-term memory showed a statistically significant association for the three ABI categories with a decrease in recall in the number of words of 0.185, 0.249, and 0.450 for each ABI category, respectively. The same evolution was observed in the clock drawing test which showed a significant deterioration in the score of 0.084, 0.122, and 0.159 for each ABI category, respectively. The rest of the tests analysed showed a non-significant trend of worsening in the score for each tenth that the ABI score decreased. To show the impact of low ABI on cognition, for each test, we plotted the difference in test score using a reference value of ABI = 1.4 (Supplementary Figs. 1–4).
Table 2.
Cross-sectional analysis of total effect of ABI score decrease (tenths*) in cognitive performance
| Variable | ABI < 0.8 | ABI between 0.8– < 1.1 | ABI between > 1.1–1.4 | All | ||||
|---|---|---|---|---|---|---|---|---|
| p-value | Beta (95% CI) | p-value | Beta (95% CI) | p-value | Beta (95% CI) | p-value | Beta (95% CI) | |
| MMSE (total score) | 0.0475 | 0.321 (0.004, 0.638) | 0.0659 | 0.199 (− 0.013, 0.411) | 0.1022 | 0.148 (− 0.029, 0.326) | 0.9732 | 0.002 (− 0.120, 0.124) |
| Cumulative Executive Dysfunction Index | 0.1683 | − 0.175 (− 0.423, 0.074) | 0.0941 | − 0.142 (− 0.308, 0.024) | 0.1360 | − 0.106 (− 0.245, 0.033) | 0.5253 | − 0.031 (− 0.127, 0.065) |
| 7-MT (total score) | 0.0634 | 0.210 (− 0.011, 0.410) | 0.0229 | 0.172 (0.024, 0.320) | 0.0547 | 0.122 (− 0.002, 0.246) | 0.6276 | 0.021 (− 0.065, 0.107) |
| 7-MT (Clock Drawing Test) | 0.0315 | 0.159 (0.014, 0.303) | 0.0123 | 0.122 (0.027, 0.216) | 0.0412 | 0.084 (0.004, 0.164) | 0.9510 | 0.002 (− 0.055, 0.059) |
| 7-MT (free and facilitated memory) | 0.0056 | 0.183 (0.054, 0.311) | 0.0046 | 0.124 (0.038, 0.210) | 0.0120 | 0.093 (0.021, 0.165) | 0.6838 | 0.011 (− 0.040, 0.061) |
| 7-MT (verbal fluency) | 0.1592 | 0.098 (− 0.038, 0.234) | 0.0236 | 0.104 (0.014, 0.195) | 0.0728 | 0.070 (− 0.008, 0.144) | 0.6197 | 0.014 (− 0.040, 0.067) |
| 7-MT (Benton’s Temporal Orientation Test) | 0.9357 | 0.002 (− 0.049, 0.053) | 0.8702 | − 0.003 (− 0.037, 0.031) | 0.8462 | − 0.003 (− 0.031, 0.026) | 0.6094 | − 0.005 (− 0.025, 0.015) |
| Denomination | 0.6155 | − 0.016 (− 0.076, 0.045) | 0.8227 | − 0.005 (− 0.045, 0.036) | 0.5771 | − 0.010 (− 0.044, 0.024) | 0.3150 | − 0.012 (− 0.035, 0.011) |
| Immediate memory | 0.2407 | 0.048 (− 0.032, 0.128) | 0.5283 | 0.017 (− 0.036, 0.071) | 0.5312 | 0.014 (− 0.030, 0.059) | 0.6799 | − 0.006 (− 0.037, 0.024) |
| Total short-term memory | 0.0589 | 0.164 (− 0.006, 0.333) | 0.1034 | 0.094 (− 0.019, 0.207) | 0.2360 | 0.057 (− 0.037, 0.152) | 0.2543 | − 0.038 (− 0.104, 0.027) |
| Free short-term memory | 0.0006 | 0.450 (0.193, 0.708) | 0.0046 | 0.249 (0.077, 0.421) | 0.0120 | 0.185 (0.041, 0.330) | 0.6443 | − 0.024 (− 0.123, 0.076) |
| Facilitated short-term memory | 0.0348 | − 0.269 (− 0.518, − 0.020) | 0.0719 | − 0.153 (− 0.320, 0.013) | 0.0691 | − 0.129 (− 0.269, 0.010) | 0.5246 | − 0.031 (− 0.127, 0.065) |
| Total long-term memory | 0.0689 | 0.161 (− 0.012, 0.335) | 0.0371 | 0.124 (0.008, 0.240) | 0.0573 | 0.095 (− 0.003, 0.192) | 0.4109 | 0.028 (− 0.039, 0.095) |
| Free long-term memory | 0.1293 | 0.240 (− 0.070, 0.551) | 0.0219 | 0.243 (0.035, 0.451) | 0.0212 | 0.205 (0.031, 0.379) | 0.0171 | 0.146 (0.026, 0.265) |
| Facilitated long-term memory | 0.5402 | − 0.093 (− 0.389, 0.204) | 0.1999 | − 0.130 (− 0.328, 0.069) | 0.1597 | − 0.119 (− 0.286, 0.047) | 0.0351 | − 0.123 (− 0.237, − 0.009) |
| Hole Peg Test (dominant hand) | 0.4540 | − 0.369 (− 1.335, 0.597) | 0.2746 | − 0.359 (− 1.001, 0.284) | 0.4085 | − 0.226 (− 0.763, 0.310) | 0.9289 | − 0.017 (− 0.387, 0.354) |
*tenths = 0.1. MMSE, Mini-Mental State Examination; 7-MT, 7-min test, Significant effect in bold (p < 0.05)
In the gender stratified analysis, both genders showed the same decrease in cognitive tests score as the ABI score decreased, but these were not significant in males, whereas in females, tests such as the MMSE, total short-term memory, free short-term memory, free long-term memory, 7-M test, and Cumulative Executive Dysfunction Index showed a pronounced deterioration in scores when analysing the three categories (p < 0.05) (Table 3 and Supplementary material 1).123456
Table 3.
Cross-sectional analysis of the relationship between ABI score decrease (tenths*) and worse cognitive performance, stratified by gender females
| Variable | ABI < 0.8 | ABI between 0.8– < 1.1 | ABI between > 1.1–1.4 | All | ||||
|---|---|---|---|---|---|---|---|---|
| p-value | Beta (95% CI) | p-value | Beta (95% CI) | p-value | Beta (95% CI) | p-value | Beta (95% CI) | |
| MMSE (total score) | 0.0100 | 0.603 (0.146, 1.060) | 0.0197 | 0.362 (0.059, 0.666) | 0.0311 | 0.282 (0.026, 0.538) | 0.7566 | 0.028 (− 0.151, 0.208) |
| Cumulative Executive Dysfunction Index | 0.0290 | − 0.367 (− 0.696, − 0.038) | 0.0223 | − 0.255 (− 0.473, − 0.037) | 0.0336 | − 0.199 (− 0.383, − 0.016) | 0.4279 | − 0.052 (− 0.181, 0.077) |
| 7-MT (total score) | 0.0036 | 0.429 (0.142, 0.717) | 0.0005 | 0.343 (0.152, 0.533) | 0.0023 | 0.252 (0.091, 0.413) | 0.3529 | 0.054 (− 0.060, 0.169) |
| 7-MT (Clock Drawing Test) | 0.0315 | 0.159 (0.014, 0.304) | 0.0123 | 0.122 (0.027, 0.217) | 0.0412 | 0.084 (0.004, 0.165) | 0.9510 | 0.002 (− 0.055, 0.059) |
| 7-MT (free and facilitated memory) | 0.0056 | 0.183 (0.054, 0.312) | 0.0046 | 0.124 (0.039, 0.210) | 0.0120 | 0.093 (0.021, 0.165) | 0.6838 | 0.011 (− 0.040, 0.061) |
| 7-MT (verbal fluency) | 0.1592 | 0.098 (− 0.038, 0.234) | 0.0236 | 0.104 (0.014, 0.195) | 0.0728 | 0.070 (− 0.006, 0.146) | 0.6197 | 0.014 (− 0.040, 0.067) |
| 7-MT (Benton’s Temporal Orientation Test) | 0.9397 | 0.002 (− 0.049, 0.053) | 0.8702 | − 0.003 (− 0.037, 0.031) | 0.8462 | − 0.003 (− 0.031, 0.026) | 0.6094 | − 0.005 (− 0.025, 0.015) |
| Denomination | 0.7134 | 0.017 (− 0.074, 0.108) | 0.5913 | 0.017 (− 0.044, 0.077) | 0.9137 | 0.003 (− 0.048, 0.054) | 0.2549 | − 0.021 (− 0.057, 0.015) |
| Immediate memory | 0.9456 | 0.004 (− 0.116, 0.125) | 0.7700 | − 0.012 (− 0.092, 0.068) | 0.9047 | − 0.004 (− 0.071, 0.063) | 0.9552 | 0.001 (− 0.046, 0.048) |
| Total short-term memory | 0.0056 | 0.327 (0.096, 0.558) | 0.0046 | 0.222 (0.069, 0.376) | 0.0121 | 0.166 (0.037, 0.295) | 0.6830 | 0.019 (− 0.072, 0.110) |
| Free short-term memory | 0.0004 | 0.653 (0.293, 1.014) | 0.0004 | 0.433 (0.194, 0.673) | 0.0012 | 0.334 (0.132, 0.536) | 0.4208 | 0.059 (− 0.084, 0.201) |
| Facilitated short-term memory | 0.0533 | − 0.342 (− 0.689, 0.004) | 0.0597 | − 0.222 (− 0.452, 0.009) | 0.0710 | − 0.179 (− 0.373, 0.015) | 0.4883 | − 0.048 (− 0.184, 0.088) |
| Total long-term memory | 0.0693 | 0.213 (− 0.016, 0.442) | 0.0107 | 0.199 (0.047, 0.352) | 0.0145 | 0.161 (0.032, 0.289) | 0.0515 | 0.089 (0.000, 0.179) |
| Free long-term memory | 0.0095 | 0.558 (0.137, 0.979) | 0.0013 | 0.460 (0.181, 0.740) | 0.0018 | 0.377 (0.141, 0.612) | 0.0249 | 0.189 (0.024, 0.354) |
| Facilitated long-term memory | 0.0874 | − 0.345 (− 0.740, 0.050) | 0.0517 | − 0.261 (− 0.524, 0.001) | 0.0558 | − 0.216 (− 0.437, 0.005) | 0.2057 | − 0.100 (− 0.254, 0.055) |
| Hole Peg Test (dominant hand) | 0.2781 | − 0.659 (− 1.848, 0.530) | 0.0667 | − 0.741 (− 1.530, 0.049) | 0.0893 | − 0.578 (− 1.244, 0.087) | 0.1473 | − 0.354 (− 0.831, 0.124) |
*tenths = 0.1. MMSE, Mini-Mental State Examination; 7-MT, 7-min test, Significant effect in bold (p < 0.05)
As observed in the cross-sectional analysis, our prospective analysis also showed that there was higher worsening in cognitive performance as the ABI score decreased from 1.4, with cognitive worsening being more pronounced at ABI score < 1.1. Therefore, two categories were established, ranging from an ABI score of 1.4 to > 1.1 and ABI score < 1.1 (Table 4).
Table 4.
Relationship between ABI score decrease (tenths*) and worse cognitive performance, prospective analysis after 5 years
| Variable | Overall | |||
|---|---|---|---|---|
| ABI < 1.1 | ABI between > 1.1–14 | |||
| p-value | Beta (95% CI) | p-value | Beta (95% CI) | |
| MMSE (total score) | 0.4613 | − 0.118 (− 0.433, 0.196) | 0.4723 | − 0.097 (− 0.361, 0.167) |
| Cumulative Executive Dysfunction Index | 0.0239 | 0.200 (0.027, 0.373) | 0.0126 | 0.185 (0.040, 0.330) |
| 7-MT (total score) | 0.3055 | − 0.079 (− 0.230, 0.072) | 0.1866 | − 0.086 (− 0.213, 0.041) |
| 7-MT (Clock Drawing Test) | 0.6033 | -0.018 (− 0.087, 0.050) | 0.3919 | − 0.025 (− 0.083, 0.032) |
| 7-MT (free and facilitated memory) | 0.0305 | − 0.102 (− 0.193, − 0.010) | 0.0257 | − 0.088 (− 0.164, − 0.011) |
| 7-MT (verbal fluency) | 0.1021 | − 0.056 (− 0.123, 0.011) | 0.1091 | − 0.046 (− 0.102, 0.010) |
| 7-MT (Benton’s Temporal Orientation Test) | 0.2761 | 0.030 (− 0.024, 0.084) | 0.1591 | 0.033 (− 0.013, 0.078) |
| Denomination | 0.4733 | 0.025 (− 0.043, 0.093) | 0.3472 | 0.027 (− 0.030, 0.085) |
| Immediate memory | 0.4847 | − 0.036 (− 0.136, 0.065) | 0.5774 | − 0.024 (− 0.108, 0.060) |
| Total short-term memory | 0.0310 | − 0.181 (− 0.345, − 0.017) | 0.0259 | − 0.157 (− 0.294, − 0.019) |
| Free short-term memory | 0.0327 | − 0.188 (− 0.360, − 0.016) | 0.0497 | − 0.144 (− 0.288, 0.000) |
| Facilitated short-term memory | 0.9870 | 0.002 (− 0.190, 0.194) | 0.8215 | − 0.019 (− 0.179, 0.142) |
| Total long-term memory | 0.0030 | − 0.200 (− 0.331, − 0.068) | 0.0054 | − 0.157 (− 0.267, − 0.047) |
| Free long-term memory | 0.0107 | − 0.281 (− 0.496, − 0.066) | 0.0159 | − 0.222 (− 0.402, − 0.042) |
| Facilitated long-term memory | 0.4846 | 0.076 (− 0.137, 0.288) | 0.5052 | 0.060 (− 0.117, 0.238) |
| Hole Peg Test (dominant hand) | 0.0595 | 0.781 (− 0.030, 1.592) | 0.0911 | 0.584 (− 0.092, 1.260) |
*tenths = 0.1. MMSE, Mini-Mental State Examination; 7-MT, 7-min test, Significant effect in bold (p < 0.05)
The statistically significant decreases in cognitive test score for every tenth decrease in ABI score within < 1.1 (1.1–1.4) range were 0.281 (0.222) for free long-term memory; 0.188 (0.144) for free short-term memory; 0.181 (0.157) for total short-term memory; and 0.200 (0.157) for total long-term memory; regarding motor asses the Cumulative Executive Dysfunction Index, the result was 0.200 (0.185). To show the impact of low ABI on cognition, for each test, we plotted the difference in change in test score along follow-up using a reference value of ABI = 1.4 (Supplementary Figs. 5–8).
The prospective analysis stratified by gender showed an association between ABI and cognitive performance; in males, free long-term memory showed a statistically significant worsening only for ABI score < 1.1 (B = 0.334, p < 0.05). In addition, worsening in free short-term memory was affected in the ABI category between 1.4 and > 1.1 (B = 0.255, p < 0.05); as for the ABI category < 1.1 (B = 0.330, p < 0.05) (Supplementary material 2). For females, animal naming, total short- and long-term memory, as well as the executive function test, and Hole Peg Test showed a significant association between lower ABI score and higher worsening cognitive performance (p < 0.05) (Table 5).
Table 5.
Relationship between ABI score decrease (tenths*) and worse cognitive performance stratified by gender females, prospective analysis after 5 years
| Variable | ABI < 1.1 | ABI between > 1.1–14 | ||
|---|---|---|---|---|
| p-value | Beta (95% CI) | p-value | Beta (95% CI) | |
| MMSE (total score) | 0.3762 | − 0.189 (− 0.607, 0.229) | 0.2853 | − 0.192 (− 0.544, 0.160) |
| Cumulative Executive Dysfunction Index | 0.0015 | 0.378 (0.146, 0.610) | 0.0009 | 0.333 (0.137, 0.528) |
| 7-MT (total score) | 0.2401 | − 0.128 (− 0.340, 0.085) | 0.1399 | − 0.136 (− 0.315, 0.044) |
| 7-MT (Clock Drawing Test) | 0.3762 | − 0.042 (− 0.134, 0.051) | 0.2514 | − 0.046 (− 0.124, 0.032) |
| 7-MT (free and facilitated memory) | 0.0383 | − 0.136 (− 0.264, − 0.008) | 0.0410 | − 0.113 (− 0.220, − 0.005) |
| 7-MT (verbal fluency) | 0.0131 | − 0.114 (− 0.204, − 0.024) | 0.0066 | − 0.106 (− 0.181, − 0.030) |
| 7-MT (Benton’s Temporal Orientation Test) | 0.5586 | 0.024 (− 0.057, 0.106) | 0.3915 | 0.030 (− 0.039, 0.099) |
| Denomination | 0.7758 | 0.015 (− 0.091, 0.122) | 0.8955 | 0.006 (− 0.084, 0.096) |
| Immediate memory | 0.1686 | − 0.114 (− 0.275, 0.048) | 0.2218 | − 0.085 (− 0.221, 0.051) |
| Total short-term memory | 0.0383 | − 0.243 (− 0.472, − 0.014) | 0.0411 | − 0.202 (− 0.394, − 0.009) |
| Free short-term memory | 0.5667 | − 0.071 (− 0.312, 0.171) | 0.6347 | − 0.049 (− 0.253, 0.154) |
| Facilitated short-term memory | 0.2429 | − 0.160 (− 0.428, 0.108) | 0.2163 | − 0.143 (− 0.369, 0.083) |
| Total long-term memory | 0.0215 | − 0.201 (− 0.372, − 0.030) | 0.0249 | − 0.165 (− 0.309, − 0.021) |
| Free long-term memory | 0.1139 | − 0.241 (− 0.539, 0.057) | 0.0822 | − 0.223 (− 0.474, 0.028) |
| Facilitated long-term memory | 0.7860 | 0.040 (− 0.248, 0.328) | 0.6400 | 0.058 (− 0.185, 0.300) |
| Hole Peg Test (dominant hand) | 0.0023 | 1.685 (0.609, 2.761) | 0.0058 | 1.288 (0.380, 2.196) |
*tenths = 0.1. MMSE, Mini-Mental State Examination; 7-MT, 7-min test, Significant effect in bold (p < 0.05)
Discussion
This study shows how VF measured indirectly by ABI modulates the cognitive performance and its evolution. In this sense, and from a global point of view, this study shows that as ABI score decreases, cross-sectionally, there is a decrease in cognitive performance, and prospectively, there is a higher degree of worsening in cognitive performance, this was observed mainly in women. Interestingly, this phenomenon occurs in both ABI score classically associated with vascular dysfunction (ABI score < 0.9) and those ABI scores identified as normal (ABI score between 1 and 1.4). This may indicate that the effect of vascular dysfunction on cognition does not have a clear threshold and has rather a continuous effect. To our knowledge, this is the first study to show this effect.
Atherosclerosis causes a chronic reduction of vascularisation with consequent impairment of the performance of organs, like the brain [35]. This inadequate cerebral blood flow can reduce the supply of oxygen and glucose, and other nutrients to neuronal cells, thus slowly initiating a pathway of progressive impairment of brain integrity, neuronal metabolism, and subsequent cognitive impairment [36].
Although representing only 2% of total body mass, the brain consumes ~ 20% of the body’s glucose and oxygen and can rapidly increase blood flow and oxygen delivery to its activated regions. This process is known as neurovascular coupling [37] which is compromised when vascular dysfunction is present in the brain. So, the maintenance of the microvascular network is crucial for adequate brain perfusion, the impairment of which is a source of cognitive impairment associated with ageing [38].
Our results are in line with previous studies [39, 40], showing that as ABI score decreased, cognitive test scores were lower in the cross-sectional analysis and the worsening in test scores was higher in the prospective analysis. But we have quantified in a dose–response manner the effect of ABI on cognitive test and its evolution, even for ABI scores included in the normal range [41]. Allowing for any two ABI scores to estimate the difference in test scores in the cross-sectional analysis and the difference in the change of test scores in the prospective analysis, from a clinical point of view, being able to assess these differences allows a more accurate evaluation of cognitive decline and the risk of developing cognitive impairment-related diseases, especially when this cognitive decline process is starting, where small changes in cognitive performance imply big changes in risk of these diseases’ development [42].
ABI is an important marker of generalised atherosclerosis and cardiovascular risk, but it is not a specific measure of cerebrovascular health. However, available data suggest that individuals with ABI < 0.9 experience more cognitive deficits [43]. It is known that atherosclerosis occurs an endothelial vascular dysfunction caused by the presence of circulating factors leading to pro-inflammatory and pro-oxidant changes in endothelial cells [44]. These changes lead to a brain-microvascular dysfunction and a microvascular damage induced by endothelial oxidative stress and inflammation which have been recognised as critical contributors to the genesis of vascular cognitive decline [45]. Additionally, it is known that aortic stiffness is associated with memory impairment and that this association is mediated, in part, by microvascular parenchymal damage [46]. So, it is intuitive to think that cognitive performance deficits secondary at lowering ABI score are related to cerebrovascular disorder.
Another important finding in the current study is a marked difference by gender observed. In the cross-sectional analysis, only women showed significant associations between ABI score and cognitive performance in different tests analysed, and in the prospective analysis, females also showed significant changes in cognitive tests as ABI score decreased, reinforcing that over follow-up, the change is even more marked.
The differences by gender can be explained by several factors, including the role of estrogen in the neural structures functions that control learning, memory, and executive functions, such as the prefrontal cortex, hippocampus, amygdala, and posterior cingulate cortex. Decreased estrogen levels after menopause induces a hypometabolic brain glucose state, reduced mitochondrial function, and consequent oxidative damage promotes neuronal dysfunction [47]. In addition, women have a greater longevity and prevalence of both subclinical and clinical cardiovascular disease triggering greater exposure to the deleterious effects of cardiovascular disease [21]. Also, women may be more likely than men to have subtle changes in vascular function (e.g., peripheral arterial stiffness) that lead to microvascular changes in the white matter [48].
Finally, this study has some strengths and weaknesses that should be commented on. Among the strengths we can highlight that this is a population-based, prospective, cohort study, whose combination of both cross-sectional and prospective data reinforces the causal relationship link between worsening vascular function, indirectly measured by the ABI and cognitive function, also features a large number of participants representative of the elderly population that has allowed us to perform a stratified analysis by gender, in participants without known cerebrovascular disease; furthermore, we have used a sufficiently broad battery of cognitive tests that has allowed us to assess different cognitive domains and their affectation from preclinical vascular stages, but certain weaknesses of our study must also be considered, like our study included a low number of participants with ABI score > 1.4. This fact provokes a lack of statistical power did not allow to estimate the association in this group and we decided to exclude them. In addition, the use of a strict exclusion criteria allowed to obtain associations without the effects of cardio- and cerebrovascular events and their dosage treatments on cognition. This resulted in a low number of participants with low ABI at baseline, constraining the validity of the results within this group. Another weakness of this study is that prospective analysis may be affected by loss of follow-up bias (due to death and drop out of the study). And finally, by not analysing neuroimaging evidence, silent brain infarcts are likely to be underestimated, but there are also silent cardiovascular diseases that are often under-diagnosed.
In conclusion, this study shows how the ABI, a widespread measure of vascular health in primary care for the detection of peripheral arterial disease, but not at the cerebrovascular level, can also be a predictor of cognitive performance. There is a dose–effect relationship between ABI score and cognitive performance that is demonstrated by a worse score on different cognitive tests as ABI score decreases. In addition, at the prospective analysis, we demonstrated that there is a greater cognitive worsening in relation to lower ABI scores.
This association showed marked differences by gender, with a higher prevalence in females, being independent of educative level and cardiovascular risk factors such as smoking, high blood pressure, diabetes mellitus, and dyslipidemia.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the support received by grants from the Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable (CIBERFES) and FEDER funds, from the Instituto de Salud Carlos III, from FISCAM (Junta de Comunidades de Castilla-La Mancha, Spain), and from FP7-Health-2012-Innovation (European Union).
Funding
This work was supported by grants [CB16/10/00456 and CB16/10/00464] from the European Union (the Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable (CIBERFES) and FEDER funds), [PI15/01305] from the Instituto de Salud Carlos III (Ministerio de Ciencia e Innovación, Spain), [DEP2015-69386-R and RD12/0043] from the Instituto de Salud Carlos III (Ministerio de Economía y Competitividad, Spain), [PI2010/020] from FISCAM (Junta de Comunidades de Castilla-La Mancha, Spain), and [FP7-305483–2] (“Frailomic Iniciative”) from FP7-Health-2012-Innovation (European Union).
Declarations
Ethical standards
This work was performed according to the ethical standards laid down in the 1964 Declaration of Helsinki and later amendments; complies with Spanish law on biomedical research and obtained approval by our centre’s Clinical Research Ethics Committee.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wagner KH, Cameron-Smith D, Wessner B, Franzke B. Biomarkers of aging: from function to molecular biology. Nutrients. 2016;8(6):E338. doi: 10.3390/nu8060338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libretti S, Puckett Y. Physiology, homeostasis. [Updated 2022 May 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559138/. [PubMed]
- 3.Felipe Salech M, Rafael Jara L, Luis MA. Cambios fisiológicos asociados al envejecimiento. Rev Médica Clínica Las Condes. 2012;23(1):19–29. doi: 10.1016/S0716-8640(12)70269-9. [DOI] [Google Scholar]
- 4.Fantini S, Sassaroli A, Tgavalekos KT, Kornbluth J. Cerebral blood flow and autoregulation: current measurement techniques and prospects for noninvasive optical methods. Neurophotonics. 2016;3(3):031411. doi: 10.1117/1.NPh.3.3.031411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talman WT, Kelkar P. Neural control of the heart. Central and peripheral. Neurol Clin. 1993;11(2):239–256. doi: 10.1016/S0733-8619(18)30151-8. [DOI] [PubMed] [Google Scholar]
- 6.Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci. 2018;21(10):1318–1331. doi: 10.1038/s41593-018-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thijssen DHJ, Carter SE, Green DJ. Arterial structure and function in vascular ageing: are you as old as your arteries?: arterial structure and function in vascular ageing. J Physiol. 2016;594(8):2275–2284. doi: 10.1113/JP270597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quiñónez-Bareiro F, Carnicero JA, Alfaro-Acha A, et al. Risk of frailty according to the values of the ankle-brachial index in the Toledo Study for Healthy Aging. J Frailty Aging. Published online 2022. 10.14283/jfa.2022.25 [DOI] [PubMed]
- 9.Ouellette J, Lacoste B. From neurodevelopmental to neurodegenerative disorders: the vascular continuum. Front Aging Neurosci. 2021;13:749026. doi: 10.3389/fnagi.2021.749026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bos D, Vernooij MW, de Bruijn RFAG, et al. Atherosclerotic calcification is related to a higher risk of dementia and cognitive decline. Alzheimers Dement. 2015;11:639–647. doi: 10.1016/j.jalz.2014.05.1758. [DOI] [PubMed] [Google Scholar]
- 11.Dachun Xu, Li J, Zou L, et al. Sensitivity and specificity of the ankle-brachial index to diagnose peripheral artery disease: a structured review. Vasc Med. 2010;15(5):361–369. doi: 10.1177/1358863X10378376. [DOI] [PubMed] [Google Scholar]
- 12.Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126(24):2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 13.Hicks CW, Al-Qunaibet A, Ding N, et al. Symptomatic and asymptomatic peripheral artery disease and the risk of abdominal aortic aneurysm: the Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis. 2021;333:32–38. doi: 10.1016/j.atherosclerosis.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarraf W, Criqui MH, Allison MA, et al. Ankle brachial index and cognitive function among Hispanics/Latinos: results from the Hispanic Community Health Study/Study of Latinos. Atherosclerosis. 2018;271:61–69. doi: 10.1016/j.atherosclerosis.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Espeland MA, Newman AB, Sink K, et al. Associations between ankle-brachial index and cognitive function: results from the lifestyle interventions and independence for elders trial. J Am Med Dir Assoc. 2015;16(8):682–689. doi: 10.1016/j.jamda.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.López M, Ríos A, Romaguera D, et al. Association between ankle-brachial index and cognitive function in participants in the PREDIMED-Plus study: cross-sectional assessment. Rev Esp Cardiol Engl Ed. 2021;74(10):846–853. doi: 10.1016/j.rec.2020.06.041. [DOI] [PubMed] [Google Scholar]
- 17.Laukka EJ, Starr JM, Deary IJ. Lower ankle-brachial index is related to worse cognitive performance in old age. Neuropsychology. 2014;28(2):281–289. doi: 10.1037/neu0000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desormais I, Aboyans V, Guerchet M, et al. Ankle–brachial index: an ubiquitous marker of cognitive impairment—the EPIDEMCA Study. Angiology. 2018;69(6):497–506. doi: 10.1177/0003319717736608. [DOI] [PubMed] [Google Scholar]
- 19.Harada CN, Natelson Love MC, Triebel KL. Normal cognitive aging. Clin Geriatr Med. 2013;29(4):737–52. doi: 10.1016/j.cger.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jokinen H, et al. Longitudinal cognitive decline in subcortical ischemic vascular disease—the LADIS Study. Cerebrovasc Dis (Basel Switzerland) 2009;27(4):384–91. doi: 10.1159/000207442. [DOI] [PubMed] [Google Scholar]
- 21.Volgman AS, Bairey Merz CN, Aggarwal NT, et al. Sex differences in cardiovascular disease and cognitive impairment: another health disparity for women? J Am Heart Assoc. 2019;8(19):e013154. doi: 10.1161/JAHA.119.013154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Garcia FJ, Gutierrez Avila G, Alfaro-Acha A, et al. The prevalence of frailty syndrome in an older population from Spain. The Toledo study for healthy aging. J Nutr Health Aging. 2011;15(10):852–856. doi: 10.1007/s12603-011-0075-8. [DOI] [PubMed] [Google Scholar]
- 23.Escribano-Aparicio MV, Pérez-Dively M, García-García FJ, et al. Validación del MMSE de Folstein en una población española de bajo nivel educativo1. Rev Esp Geriatría y Gerontol. 1999;34:319e326. [Google Scholar]
- 24.Solomon PR, Hirschoff A, Kelly B, et al. A 7-minute neurocognitive screening battery highly sensitive to Alzheimer’s disease. Arch Neurol. 1998;55:349e355. doi: 10.1001/archneur.55.3.349. [DOI] [PubMed] [Google Scholar]
- 25.del SerQuijano T, Sanchez Sanchez F, Garcia de Yebenes MJ, et al. Spanish version of the 7 Minute screening neurocognitive battery. Normative data of an elderly population sample over 70. Neurologia. 2004;19:344e358. [PubMed] [Google Scholar]
- 26.Rosado-Artalejo C, Carnicero JA, Losa-Reyna J, et al. Global performance of executive function is predictor of risk of frailty and disability in older adults. J Nutr Health Aging. 2017;21(9):980–987. doi: 10.1007/s12603-017-0895-2. [DOI] [PubMed] [Google Scholar]
- 27.Agrell B, Dehlin O. The clock-drawing test. 1998. Age Ageing. 2012;41 Suppl 3:iii41–45. doi: 10.1093/ageing/afs149. [DOI] [PubMed] [Google Scholar]
- 28.Petrides M, Milner B. Deficits on subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia. 1982;20(3):249–262. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- 29.Luria AR. The frontal lobes and the regulation of behavior. In: Pribram KH, Luria AR, editors. Psychophysiology of the Frontal Lobes. New York, NY: Aca- demic Press; 1973. p. 3e26.
- 30.Drewe EA. Go-no go learning after frontal lobe lesions in humans. Cortex. 1975;11:8e16. doi: 10.1016/S0010-9452(75)80015-3. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz-Sánchez de León JM, Llanero-Luque M, Montenegro-Peña M, Montejo-Carrasco P. Tarea de inhibición frontal (go/no go) para la evaluación del envejecimiento normal, el deterioro cognitivo ligero y demencia de tipo Alzheimer leve. Neurología. 2008;23:839. [Google Scholar]
- 32.Mathiowetz V, Weber K, Kashman N, Volland G. Adult norms for the nine Hole Peg Test of finger dexterity. Occup Ther J Res. 1985;5(1):24–38. doi: 10.1177/153944928500500102. [DOI] [PubMed] [Google Scholar]
- 33.Kellor M, Frost J, Silberberg N, Iversen I, Cummings R. Hand strength and dexterity. Am J Occup Ther Off Publ Am Occup Ther Assoc. 1971;25(2):77–83. [PubMed] [Google Scholar]
- 34.Fowkes FGR. Ankle-brachial index and extent of atherothrombosis in 8891 patients with or at risk of vascular disease: results of the international AGATHA study. Eur Heart J. 2006;27(15):1861–1867. doi: 10.1093/eurheartj/ehl114. [DOI] [PubMed] [Google Scholar]
- 35.Nelson AR, Sweeney MD, Sagare AP, Zlokovic BV. Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer’s disease. Biochim Biophys Acta BBA - Mol Basis Dis. 2016;1862(5):887–900. doi: 10.1016/j.bbadis.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017;18:419–434. doi: 10.1038/nrn.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lourenço CF, Ledo A, Caetano M, Barbosa RM, Laranjinha J. Age-dependent impairment of neurovascular and neurometabolic coupling in the hippocampus. Front Physiol. 2018;9:913. doi: 10.3389/fphys.2018.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Silva TM, Faraci FM. Microvascular dysfunction and cognitive impairment. Cell Mol Neurobiol. 2016;36(2):241–258. doi: 10.1007/s10571-015-0308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price JF, McDowell S, Whiteman MC, Deary IJ, Stewart MC, Fowkes FGR. Ankle brachial index as a predictor of cognitive impairment in the general population: ten-year follow-up of the Edinburgh Artery Study. J Am Geriatr Soc. 2006;54(5):763–769. doi: 10.1111/j.1532-5415.2006.00702.x. [DOI] [PubMed] [Google Scholar]
- 40.Johnson W, Price JF, Rafnsson SB, Deary IJ, Fowkes FGR. Ankle-brachial index predicts level of, but not change in, cognitive function: the Edinburgh Artery Study at the 15-year follow-up. Vasc Med. 2010;15(2):91–97. doi: 10.1177/1358863X09356321. [DOI] [PubMed] [Google Scholar]
- 41.Aboyans V, Ricco JB, Bartelink MLEL, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS) Eur Heart J. 2018;39(9):763–816. doi: 10.1093/eurheartj/ehx095. [DOI] [PubMed] [Google Scholar]
- 42.Meulen EFJ. The seven minute screen: a neurocognitive screening test highly sensitive to various types of dementia. J Neurol Neurosurg Psychiatry. 2004;75(5):700–705. doi: 10.1136/jnnp.2003.021055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guerchet M, Aboyans V, Nubukpo P, Lacroix P, Clément JP, Preux PM. Ankle-brachial index as a marker of cognitive impairment and dementia in general population. A systematic review. Atherosclerosis. 2011;216(2):251–257. doi: 10.1016/j.atherosclerosis.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 44.Gardner AW, Parker DE, Montgomery PS, Sosnowska D, Casanegra AI, Esponda OL, et al. Impaired vascular endothelial growth factor A and inflammation in patients with peripheral artery disease. Angiology. 2014;65:683–690. doi: 10.1177/0003319713501376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cortes-Canteli M, Iadecola C. Alzheimer’s disease and vascular aging. J Am Coll Cardiol. 2020;75(8):942–951. doi: 10.1016/j.jacc.2019.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooper LL, Woodard T, Sigurdsson S, et al. Cerebrovascular damage mediates relations between aortic stiffness and memory. Hypertension. 2016;67(1):176–182. doi: 10.1161/HYPERTENSIONAHA.115.06398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheyer O, Rahman A, Hristov H, et al. Female sex and Alzheimer’s risk: the menopause connection. J Prev Alzheimers Dis. Published online 2018:1–6. 10.14283/jpad.2018.34 [DOI] [PMC free article] [PubMed]
- 48.Reas ET, Laughlin GA, Hagler DJ, Lee RR, Dale AM, McEvoy LK. Age and sex differences in the associations of pulse pressure with white matter and subcortical microstructure. Hypertension. 2021;77(3):938–947. doi: 10.1161/HYPERTENSIONAHA.120.16446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.