Abstract
Effort toward reproduction is often thought to negatively influence health and survival. Reproduction has been shown to influence metabolism, but the pathways and mechanisms have yet to be thoroughly elucidated. In the current experiments, our aim was to dissect the role of young and old ovarian tissues in the response to oxidative stress, through changes in liver oxidative stress response proteins. Liver proteins were analyzed in control mice at 4, 13, and 27 months of age and compared to 23-month-old mice which received young ovarian tissue transplants (intact or follicle-depleted) at 13 months of age. In control mice, of the 29 oxidative stress response proteins measured, 31% of the proteins decreased, 52% increased, and 17% were unchanged from 13 to 27 months. The greatest changes were seen during the period of reproductive failure, from 4 to 13 months of age. In transplanted mice, far more proteins were decreased from 13 to 23 months (93% in follicle-containing young ovary recipients; 62% in follicle-depleted young ovary recipients). Neither transplant group reflected changes seen in control mice between 13 and 27 months. Estradiol levels in transplant recipient mice were not increased compared with age-matched control mice. The current results suggest the presence of a germ cell- and estradiol-independent ovarian influence on aging-associated changes in the response to oxidative stress, which is manifest differently in reproductive-aged adults and post-reproductive-aged mice. The results presented here separate chronological and ovarian aging and the influence of estradiol in the response to aging-associated oxidative stress and support a novel, estradiol-independent role for the ovary in female health and survival.
Keywords: Menopause, Oxidative stress, Estradiol, Follicle, Ovarian
Introduction
Aging is often associated with a loss of metabolic homeostasis and plasticity, which is causally linked to multiple age-onset pathologies [45]. Many established interventions that increase lifespan have been found to slow aging and protect against age-related disease. Many of these interventions target central metabolic pathways and/or act through insulin-like growth factor (IGF), the mechanistic target of rapamycin (mTOR), AMP-activated protein kinase (AMPK), nicotinamide adenine dinucleotide (NAD+), sirtuins, and other metabolic signaling pathways [5, 17, 67, 70]. Most of these pathways are highly conserved across species [3] and are crucial for balancing resource utilization between maintenance, survival, and reproductive effort.
Reproductive effort is often thought to negatively influence health and survival [63, 78]. Prior to the reproductive decline, females hold a significant health advantage over males of the same age [20]. However, after reproductive failure/menopause, the increase in disease risks for women significantly outpace those of men. This dependence on reproductive function for the maintenance of health is exemplified in surgically menopausal women and in women with premature ovarian failure, who suffer from a decline in health at a much younger age than in women with traditional menopausal timing [37, 68–71]. Previous experiments by our group have demonstrated significant health and longevity benefits when older females receive ovarian transplants from young mice [27, 28, 46–49, 60–62, 72]. Based on these observations, reproductive function can significantly influence non-reproductive biological functions and is exquisitely sensitive to aging-related changes. Reproduction has been shown to influence metabolism, but the pathways and mechanisms have yet to be thoroughly elucidated [61, 62, 72].
The free radical/oxidative stress theory of aging is based on the hypothesis that age-associated functional losses are due to an imbalance between the production of reactive oxygen species (ROS) and antioxidant defenses, resulting in the accumulation of ROS-induced damage to macromolecules. Several studies have provided a broad understanding of the roles of increased production of ROS in the etiology of mammalian aging [7–10, 40–42, 73–75].
Females that undergo dietary restriction display improved resistance to oxidative stress and improved metabolic parameters, and, as a general rule, they preserve reproductive function for later in life [6, 21, 79]. Therefore, there appears to be a link between improved metabolic function, resistance to oxidative stress, and reproductive function. However, the role of ROS in reproductive aging, and more specifically, ovarian aging, has not been well studied independent of the role of estradiol.
A common doctrine is that the depletion of estrogen at menopause causes increases in oxidative stress and metabolic perturbations. Estrogen at high concentrations can have a beneficial antioxidant effect, but at low concentrations, estrogen can have pro-oxidant-like effects [54]. Pro-oxidant biomarkers were found to be higher in post-menopausal women than in premenopausal women [69] suggesting that there is a high degree of oxidative stress in the post-menopausal state [52]. Increased levels of ROS can also lead to cellular senescence and an irreversible senescence-associated secretory phenotype. Cellular senescence increases with age in the ovary can be decreased/reversed by treatment with senolytics [1, 30].
A major roadblock in investigating this issue has been the inability to separate chronological and reproductive aging in mammals. In mammals, dietary restriction extends reproductive potential but changes the relationship between reproductive aging and chronological aging. Our approach allows the separation of chronological and reproductive aging surgically by utilizing heterochronic ovarian tissue transplantation procedures [50]. Transplantation of young ovarian tissue/cells to post-reproductive females improves health and extends longevity. These effects are independent of ovarian germ cells/follicles and are not dependent on changes in estradiol levels [28]. Whether these changes (which appear to be germ cell and hormone independent) influence metabolic function and resistance to oxidative stress remains to be seen. In the current experiments, changes in liver oxidative stress response proteins were examined to dissect the role of young ovarian tissue in the response to oxidative stress.
Materials and methods
Animals
The CBA/J and DBA mice are common laboratory strains used in aging studies because of the unique loss of ovarian follicles early in life [27, 47, 48]. The present study used the CBA/J mouse strain as an aging model for post-reproductive health. Female CBA/J mice were obtained from the National Institute of Health-National Institute of Aging (Bethesda, MD, USA) and Jackson Laboratory (Bar Harbor, ME, USA). Each mouse was individually housed in ventilated cages (Green Line IVC Sealsafe Plus, Techniplast, West Chester, PA, USA) containing corncob bedding (7097 Corncob, Harlan Teklad, Bartonville, IL, USA). Each home cage had deionized water, laboratory rodent diet ad libitum (2018 Teklad Global 18% Protein Rodent Diet, Harlan Teklad, Bartonville, IL, USA), and added enrichment (4 cm diameter × 10 cm L paper tubes, nestlets, Kimwipes). All females were exposed to male mice (no more than 2 cages away from a cage containing a male/males)/male bedding (a small amount of soiled bedding from a male’s cage was mixed with fresh bedding at cage changes) throughout the study. The colony environment had fresh filtered air (15 changes/hour), regulated temperatures of 21 ± 2 °C, humidity of 50 ± 20%, and an even light–dark cycle (12:12 h) and was located in the Utah Science, Technology and Research Center (USTAR) at Utah State University. USTAR is an American Association for Accreditation of Laboratory Animal Care (AAALAC) approved facility in accordance with the National Institutes of Health and Animal Use guidelines. Animal care protocols were developed under the National Research Council guidelines found in the Guide for the Care and Use of Laboratory Animals. Protocols were approved by the Utah State University Institutional Animal Care and Use Committee (IACUC-10222).
Surgical procedures
Surgical procedures included anesthetics for both donor and recipient mice and post-operative administration of analgesia with extended administration of analgesia if necessary. Euthanasia of donors and recipient mice occurred by cervical dislocation followed by thoracotomy with rapid exsanguination by cardiocentesis. Mice were monitored twice daily and more frequently if health concerns arose. Mice with acute weight loss were treated with moistened food and subcutaneous (SC) fluids. Individuals with acute urine staining or rectal/vaginal prolapse were manually cleaned and treated with Desitin®. Any moribund, aged mice who exhibited overt clinical signs of catatonia were euthanized. Criteria for potential euthanasia were determined in coordination with the attending veterinarian and included but were not limited to: mice found in poor condition with or without crusting around the perineum, diarrhea, urine staining, persistent vaginal prolapse, chronic vulva/rectal swelling, respiratory distress, anorexia, kyphosis, poor coat, and unusual weight loss or gain. An increased rate of weight loss was the most critical factor for determining a moribund state in aged mice. Unexpected deaths were uncommon but included euthanasia due to neoplastic growths, decubitus ulcers, or uncontrolled cataleptic seizures [46].
Experimental design
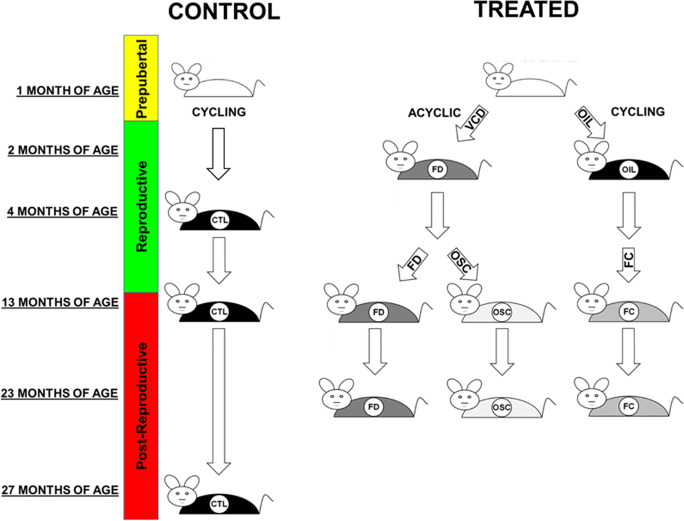
Animals were randomly assigned to control or experimental groups (Fig. 1). Control groups consisted of mice with their original ovaries intact (not sham-operated). In previous experiments, sham-operated mice were no different from unoperated mice in terms of lifespan, cardiomyopathy, or osteoarthritis [46–49]. Experimental groups consisted of CBA/J females who received ovarian tissue/cell transplants. CBA/J mice at 13 months of age, who were assessed as reproductively senescent (acyclic) via vaginal cytology, received germ cell/follicle-containing (FC) 60-day old ovaries, germ cell/follicle-depleted (FD) 60-day-old ovaries, or isolated ovarian somatic cells (OSC) from 60-day-old CBA/J mice.
Fig. 1.
Experimental design. Donor mice received treatment with 4-vinylcyclohexide diepoxide (VCD) or vehicle (OIL) for 20 days. Ovarian tissues from 60-day-old donor mice were collected, processed, and transplanted to 13-month recipient mice. Control mice were analyzed for proteins at 4 (n=6), 13 (n=3), and 27 (n=4) months of age. Ovarian tissue/cell recipients were analyzed for proteins at 23 months of age (see comments in Methods regarding collection ages). OIL, animal treated with sesame oil (vehicle) only; FD, follicle-depleted ovary (n=12); FC, follicle-containing ovary (n=9); OSC, isolated ovarian somatic cells (n=7); CTL, control; TX, young ovarian tissue/cell recipient
Ovarian transplantation
CBA/J mice at 13 months of age underwent a bilateral ovariectomy and bilateral ovarian transplantation with a pair of ovaries or ovarian cells from 60-day-old donor mice of the same strain. Bilateral ovarian transplantations were performed as previously described [46–50]. Briefly, recipient and donor mice were anesthetized using an intraperitoneal injection of an anesthetic cocktail (100 mg/kg ketamine, 20 mg/kg xylazine, and 10 mg/kg acepromazine). The ovaries of the donor mice were exposed starting with a paralumbar incision. Once through the skin and peritoneum, the ovarian fat pad, located distal to the kidney, was placed into the field of view and positioned with a clamp to expose the ovarian bursa containing the ovary. The ovary was then removed by incising the bursa opposite the ovarian hilum. The ovary was gently removed from the bursa, and the hilum was clamped to prevent bleeding. The excised ovaries were placed in cold saline or digested to a single-cell suspension prior to transfer.
The FC/FD recipient mice underwent the same process to remove their endogenous, acyclic ovaries. After the endogenous ovaries were removed, young ovaries or isolated somatic cells were placed into the recipient bursa through the original incision. The bursa was closed with sutures using 9–0 Ethicon monofilament (Ethicon, Inc.) and placed back into the body cavity. The abdominal wall was sutured with 6–0 Vicryl (Ethicon, Inc.), and the skin was closed using 9 mm wound clips (MikRon Precision, Inc.). The procedure was repeated on both ovaries in each recipient mouse. Mice were given analgesia and electrolytes and dextrose with continuous post-operative monitoring for any concerns until each mouse was bright, alert, and responsive. Post-operative mortality was less than 5%. The OSC recipient mice retained their endogenous, acyclic ovaries for subsequent OSC injection.
Follicle (germ cell) depletion
We used 4-vinylcyclohexene diepoxide (VCD) to deplete germ cell-containing follicles in the ovaries of young mice [28]. Treatment with VCD selectively destroys primordial and primary follicles in the ovaries of rats and mice (reviewed in [34]. In contrast to ovariectomy, VCD treatment induces gradual ovarian failure through depletion of the germ cell-containing follicles, while leaving the somatic ovary tissue intact. Germ cell-depleted CBA/J mice are currently produced in our laboratory following the method of Lohff et al. [44] with minor modifications.
At 28 days of age, CBA/J female mice received daily intraperitoneal (I.P.) injections of 160 mg/kg VCD in sesame oil for 20 days. FC ovary-donor mice were treated with sesame oil (vehicle) on the same schedule as the VCD-treated mice and stopped VCD and vehicle treatments at 48 days of age. At 60 days of age, ovaries were collected from VCD-treated and vehicle-treated mice for transplantation to 13-month-old, virgin CBA/J mice. Ovaries from 60 days of VCD-treated mice were also used for the isolation of young ovarian somatic cells for transplantation. CBA/J mice analyzed at only 37 days after the initiation of VCD treatment already displayed cessation of reproductive cyclicity (persistent vaginal cornification), reduced ovarian weights (1.7 mg in oil-only vs. 0.9 mg in VCD-treated, P = 0.030), and depleted primordial (P = 0.004) and primary (P = 0.029) ovarian follicles compared with controls. Primordial and primary follicle depletion is normally complete by day 46 after the initiation of VCD treatment, as reported previously in other strains of mice [65]. VCD-treated donor mice were superovulated prior to ovary collection to decrease any remaining secondary or later-stage follicles. The mice were superovulated using 5 IU PMSG injected SC and subsequently received 5 IU hCG 46 h later.
Somatic cell isolation
VCD-treated ovaries were used for the isolation of young OSCs for transplant. Somatic cells collected from one young ovary were injected into each recipient’s endogenous ovary. Somatic cells were isolated from young ovaries using mechanical and enzymatic tissue disruption/digestion. Ovaries were collected in PBS + (pH3.0, with Ca + 2 and Mg + 2) and placed in a depression slide that contained 50 µl of digestion media (Collagenase/Dispase/DNase) and minced with 19 ga needles for 5 min. After 5 min, the digestion media/ovaries solution was put into a 1.5 ml Eppendorf tube. The slide was then washed with an additional 450 µl digestion buffer, which was added to the 1.5 ml tube, and the solution was incubated at 37 °C for an additional 10 min with frequent pipetting (in a water bath). After a total of 15 min, 500 µl of serum was added to the 1.5 ml tube, mixed well, and the solution was centrifuged and washed 2 × with PBS − (no Ca + 2 or Mg + 2). The somatic cells were then isolated from the ovarian cell mixture using a 70-um filter to remove cellular debris.
We initially lose slightly more of the somatic cells using this procedure compared with differential attachment culture (the ovarian cell mixture is allowed to attach to a culture plate overnight, washed, detached, and reattached, and most of the attached cells are somatic in origin). However, attachment to a culture plate can significantly change the phenotype/behavior of the cells. Our procedure is very quick and subjects the cells to very few disruptive environments prior to transplantation. The isolated cells were resuspended in 0.1% F68 surfactant in saline and transplanted to the OSC recipient's endogenous ovary in a 10 µl volume or cryopreserved for future use. Transplantation with other vehicles (i.e., phytohemagglutinin, [14] decreases the viability of re-aggregated OSCs (personal communication, Dr. Suzannah Williams, University of Oxford). Preliminary cell transplantations with F68/saline have resulted in the recovery of live cells 7 days post-transplantation.
Oxidative stress response protein assay
Liver samples were collected from all groups at the time of euthanasia and flash frozen. Frozen liver samples were pulverized in a freezer mill. Pulverized liver samples (50 to 200 mg) were quantified and sent to Dr. Michael Kinter at the Aging and Metabolism Research Program, Oklahoma Medical Research Foundation, Oklahoma City, Oklahoma for analysis. Briefly, pulverized liver samples were reconstituted in 3.5 mL of Ripa buffer, mixed well, and heated at 70 °C for 15 min. The samples were frozen overnight, thawed, and cleared by centrifugation at 500 × g for 5 min. A total of 1 mL aliquots of the supernatant were collected and flash frozen in liquid nitrogen and stored at − 80 °C until further processing. An aliquot of each sample was thawed, and a volume containing 100 µg total protein was taken for analysis. BSA was added as a non-endogenous internal standard. The samples were mixed, heated at 70 °C, and precipitated with acetone overnight. The precipitate was reconstituted in Laemmli sample buffer at 1 µg/uL and run 1.5 cm into an SDS-PAGE gel. Each 1.5 cm lane was cut, chopped into smaller pieces, washed, reduced, alkylated, and digested with 1 µg trypsin overnight at room temperature. Peptides were extracted from the gel in 50% acetonitrile; the extracts were taken to dryness by Speedvac and reconstituted in 200 µL 1% acetic acid for analysis. For high-resolution accurate mass analysis (HRAM), a Thermo Scientific QEX plus instrument was used in the full MS mode, scanning from m/z 300 to 1100 with a resolution of 280,000 and scan times of 1 scan/second. The LC conditions were a linear gradient elution from 2%B to 45%B in 60 min. Total analysis time is approximately 1.5 h per sample. Data were analyzed using the program Skyline. The program finds and integrates the proper chromatographic peaks. Proper retention times are predicted based on retention time calibration using standard BSA and trypsin peptides. This process is edited by manually inspecting the data as needed. Peak areas are exported to excel. The assay for each protein is set to find and integrate 2 peptides per protein. All peptides in these assays have been validated in prior experiments. Calculations determined the total protein response from the geomean of the two monitored peptides. Results were normalized to the BSA internal standard and expressed as pmol/100 µg total protein.
Hormone assay
Serum for hormone measurements was collected after a 4–5 h fast from mice verified as acyclic or in the diestrus stage of the estrous cycle. Samples were collected in gel serum tubes with added protease inhibitor (in-house), allowed to clot for 15 min, and spun down, and the serum was collected, flash frozen, and stored at − 80 °C until analysis. Estradiol was measured using a Mouse/Rat Estradiol ELISA kit (Calbiotech Inc., cat# Es180S-100, El Cajon, CA; detection parameters for the estradiol assay are 3–300 pg/ml with a sensitivity of 3 pg/ml). AMH was measured using a mouse and rat AMH ELISA kit (ANSH LABS, cat#AL-113, Webster, TX; detection parameters for the AMH assay are 3.36–215.0 ng/ml with a sensitivity of 3.36 ng/ml).
Statistical analysis
Statistical analysis was performed with GraphPad Prism 7.04 (GraphPad Software, Inc., La Jolla, CA, USA). The D’Agostino-Pearson omnibus test was performed to determine normality. Data were analyzed with a two-factor ANOVA, and a Tukey–Kramer post hoc test was used to determine differences between the groups. Student’s two-tailed t-test was performed on individual treatments assuming the unequal distribution of variance. Test results were considered significant for P values P < 0.05. The average SE of the mean for percent change in pmol protein was 1.6%. Percent change values below 1.6% were reported as no change. Experimental mice received ovarian transplant at 13 months of age and were collected at 23 months of age.
Because of COVID-19-associated restricted facility access, there were no age-matched control mice. Therefore, the data was used to extrapolate where 23-month-old mice would fall on a standard curve specific for each protein. The values from the mice that received transplanted ovaries were reported as either increased, decreased, or unchanged compared to the predicted value of a 23-month-old mouse. These values were used to either confirm or refute predicted ovarian influence on aging-associated changes in oxidative stress response proteins.
Results
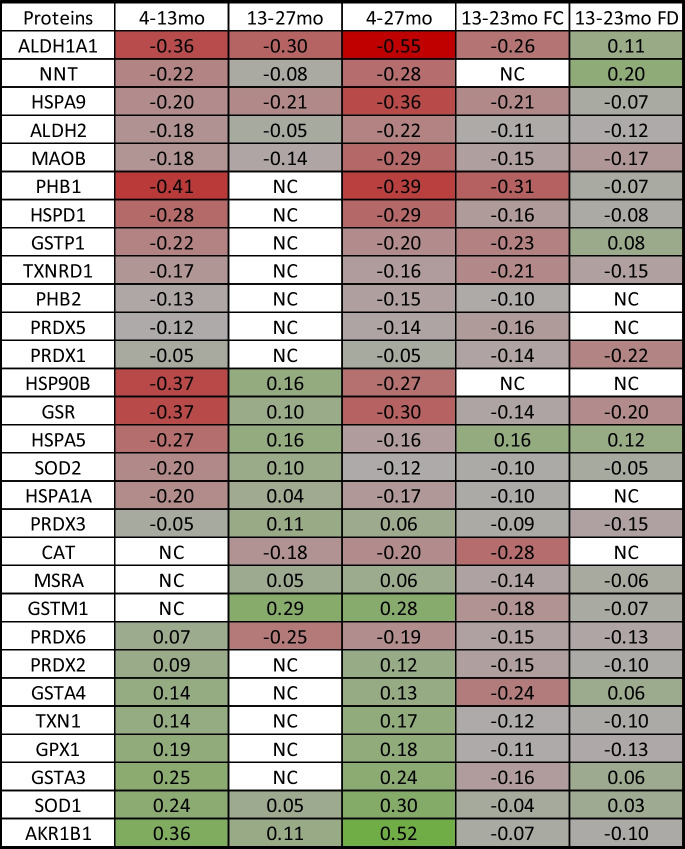
We measured oxidative stress response proteins at three different life stages in control mice, including young, healthy, and reproductively active mice at 4 months of age, mice that had recently undergone reproductive failure/complete cessation of reproductive cycling at 13 months of age (most mice experience reproductive failure/complete cessation of reproductive cycling by approximately 12 months of age in this strain), and old mice at 27 months of age. Proteins were also measured at 23 months of age in mice that had received ovarian transplantations at 13 months of age, just past reproductive failure (the average SE of the mean for percent change in pmol protein was 1.6%. Percent change values below 1.6% were reported as no change [NC], Table 1).
Table 1.
Changes in protein levels from young adult to reproductive failure (4–13 months), from post-reproductive to aged (13–27 months), over the lifespan from young adult to aged (4–27 months), and with exposure to young ovarian tissues (FC, follicle-containing ovary; FD, follicle-depleted ovary). NC, no change.
The measured proteins displayed four distinct patterns of change from 4 to 27 months of age:
In the first group, proteins were low at 4 months and increased at 13 months and continued to increase or did not change from 13 to 27 months. This group included AKR1B1, GSTA3, SOD1, GPX1, TXN1, GSTA4, PRDX2, and MSRA.
In the second group, proteins decreased from 4 to 13 months and continued to decrease or did not change from 13 to 27 months. This group included ALDH1A1, HSPD1, NNT, HSPA9, ALDH2, MAOB, PHB2, PRDX5, and PRDX1.
In the third group, one protein increased from 4 to 13 months but decreased from 13 to 27 months (only PRDX6).
In the fourth group, proteins were high at 4 months and decreased at 13 months but increased from 13 to 27 months. This group includes PHB1, HSP90B, GSR, HSPA5, GSTP1, SOD2, HSPA1A, TXNRD1, and PRDX3.
An additional pattern was displayed where proteins did not change with ovarian failure at 13 months but decreased (CAT) or increased (GSTM1) from 13 to 27 months.
Transplantation of young FC ovaries decreased oxidative stress response protein levels in 27 of 29 proteins observed. Protein HSPA5 was increased, NNT was unchanged, and HSP90B, SOD1, AKR1B1, PRDX3, HSPA1, SOD2, PHB2, ALDH2, GPX1, TXN1, GSR, PRDX1, MSRA, PRDX6, PRDX2, MAOB, GSTA3, PRDX5, HSPD1, GSTM1, HSPA9, TXNRD1, GSTP1, GSTA4, ALDH1A1, CAT, and PHB1 were all decreased, compared with predicted values for age-matched controls.
Transplantation of young FD ovaries influenced 27 of 29 proteins observed. Proteins NNT, HSPA5, ALDH1A1, GSTP1, GSTA4, GSTA3, SOD1, HSP90B, and PHB2 were increased; PRDX5 and HSPA1A were unchanged; and CAT, SOD2, MSRA, HSPA9, PHB1, GSTM1, HSPD1, AKR1B1, PRDX2, TXN1, ALDH2, GPX1, PRDX6, PRDX3, TXNRD1, MAOB, GSR, and PRDX1 were all decreased, compared with predicted values for age-matched controls.
In most proteins, the change in protein level (increase/decrease) was greatest during the period encompassing reproductive failure (4–13 months). The strongest argument for an ovarian influence is in the proteins that increased/decreased from 13 to 27 months and displayed an opposite or blunted response in mice that received new ovaries. Interestingly, the response was different for some proteins between FC and FD mice. Example graphs are presented to emphasize how proteins may differ in their reaction to changes in reproductive status and changes in chronological age (Fig. 2).
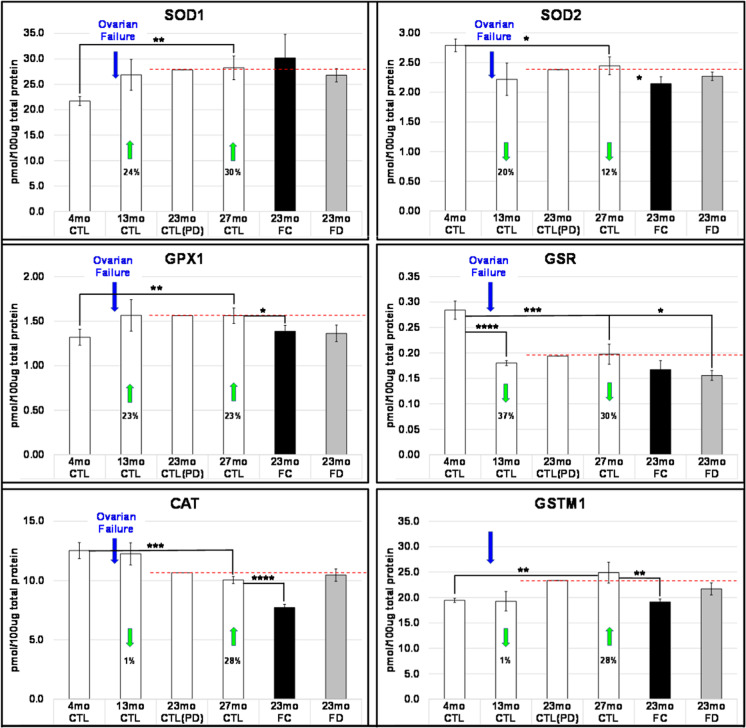
Fig. 2.
Examples of diverse responses of oxidative stress response proteins to reproductive failure and chronological aging. Cytoplasmic superoxide dismutase 1 (Sod1) and glutathione peroxidase 1 (Gpx1) both increased due to ovarian failure but showed little change to 27 months. Mitochondrial superoxide dismutase 2 (Sod2) and glutathione reductase (gsr) both decreased due to ovarian failure but also showed little change to 27 months. Both catalase (cat) and glutathione S-transferase Mu 1 (gstm1) did not appear to be sensitive to ovarian failure but did change with subsequent chronological aging. Responses to the restoration of young ovarian tissue/cells were not predictable based on response to ovarian failure at 13mo. *P < 0.200, **P < 0.100, ***P < 0.050, ****P < 0.010
Hormones
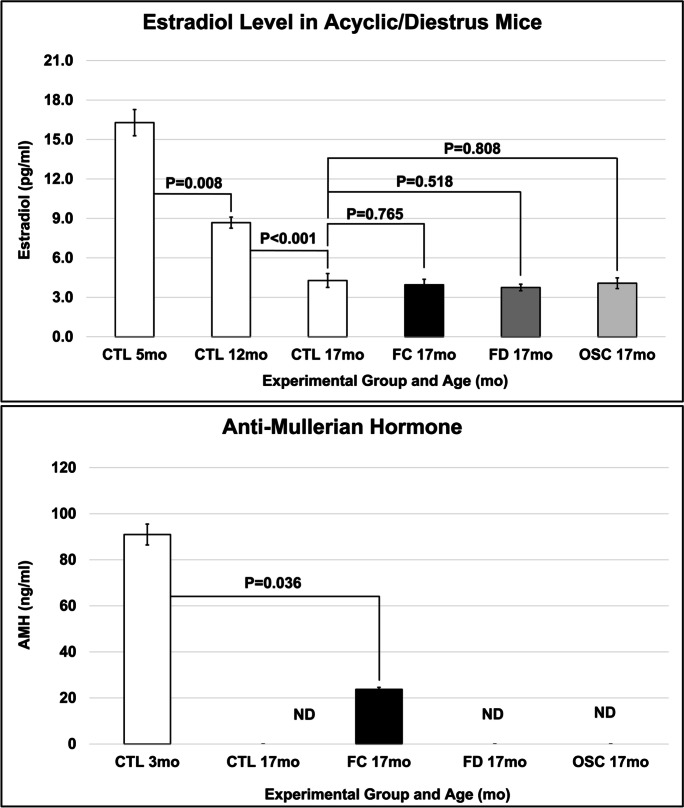
Estradiol levels for these groups of mice have been reported previously [28]. Briefly, estradiol levels decreased at the time of ovarian failure and decreased further with increased aging but did not increase with ovarian tissue transplantation in FC, FD, or OSC recipient mice. AMH decreased with reproductive failure in control mice. Transplantation of young, FC ovaries increased AMH levels, but AMH levels were not increased in FD or OSC recipient mice (Fig. 3).
Fig. 3.
Age and treatment changes in hormone levels. Estradiol levels decreased with age and were not changed from age-matched control mice with transplantation of young ovarian tissue. AMH levels also decreased with age and with the transplantation of FC, but not FD ovaries or OSC cells. FC, follicle-containing young ovary transplant; FD, follicle-depleted young ovary transplant; OSC, transplanted with young ovarian somatic cells; ND, not detected
Discussion
The CBA/J strain of mice is different from many other strains used for aging or reproductive research in that they prematurely lose their ovarian follicles, often becoming reproductively incompetent by 10–12 months of age [4, 16, 18, 39, 66, 82]. In addition, compared with the commonly-used C57BL/6 strain, female CBA/J mice have a very short constant estrus phase and move into a diestrus state much more quickly at the time of reproductive failure. Since a reduction of ovarian follicles in the human is normally associated with the onset of menopause, it has been suggested that CBA strain mice may serve as an appropriate experimental model to study age-related changes in the human reproductive system [2, 4, 22, 59, 66]. Similar transplantation experiments in C57BL/6 strain mice and CB6F1 hybrid mice have produced similar results in physiological/health span assays and molecular studies in these strains are currently underway. In the current experiments, all CBA/J female mice used were acyclic at 13 months of age. FC recipient mice initially resumed reproductive cycling post-operatively but were acyclic by 17 months. Immediately postoperatively, cycles in FC transplant recipient mice were irregular and extended. The observed increase in AMH was likely due to recruitment/progression from the primordial to primary follicle stage. However, progression past the secondary stage was not apparent due to the low levels of estradiol detected. This was likely due to the aged hypothalamus/pituitary tissues being unable to fully respond to inputs from the young ovaries. As most of the ovarian tissues from these mice were used for molecular studies, histological assessment was not possible. FD and OSC mice did not cycle post-operatively (Mean lifespan for control CBA/J female mice is ~ 644 days/21 months, 780 days/26 months for FC mice, and 880 days/29 months for FD mice).
In control mice, of the 29 oxidative stress response proteins measured, 66% of the proteins decreased from 4 to 27 months of age. The greatest changes were seen during the period of reproductive failure, from 4 to 13 months of age. This period included cessation of reproductive cycling, depletion of both the follicles and the associated/recruited ovarian somatic cells, and decreased cyclic estradiol. Changes in oxidative stress response protein levels during this period could, therefore, be due to changes in circulating levels of estradiol, to changes in the influence of ovarian somatic cells, to chronological changes in hypothalamic-pituitary–gonadal axis signaling, or a combination of multiple factors.
In FC-treated mice, of the 29 oxidative stress response proteins measured, from 13 to 23 months of age, 93% of the proteins decreased, and in FD-treated mice, 62% of the proteins decreased. Neither transplant group reflected changes reported in control mice between 13 and 27 months, where only 31% of the proteins decreased and 52% increased. The transplanted ovaries were acyclic for the majority of their time in recipient mice, suggesting a strong, non-hormonal influence of the young ovarian tissue in the old mice. The differences seen between FC and FD recipient mice could potentially be due to the brief period of cyclic activity in FC mice or to an extended duration of young, somatic cell survival influence in the FD mice. In mice that received young, FC ovaries, many of the ovarian somatic cells may have been tasked with supporting reproduction due to the presence of follicles/germ cells. In the FD ovaries, there were no follicles/germ cells present. Therefore, the ovarian somatic cells would potentially be free to focus their efforts on the survival/maintenance of the organism.
Systemic hormone levels change throughout chronological and reproductive life. In mammals, cyclic estradiol is produced by developing ovarian follicles during the reproductively-competent period of the lifespan [11, 12, 14–18, 20–30]. Total estrogen levels are reduced due to “ovarian failure” at menopause [29]. The group of CBA/J female control mice used in the current study displayed decreased levels of estradiol from 5 to 17 months of age (measured in the diestrus phase of the estrous cycle in cycling mice). In mice that received increased exposure to young ovarian tissue, either through tissue transplantation or cell injection, estradiol levels were not significantly different from the age-matched control group. Therefore, observed changes in the response to oxidative stress were not due to increased levels of estradiol upon ovarian tissue/cell transplantation, as might have been expected. The increase in AMH in FC transplant recipients confirmed the presence of young follicles in FC recipients.
The superoxide dismutase proteins explored here (SOD1/Cu–Zn SOD, functions mainly in the cytoplasm) and (SOD2/Mn-SOD, largely in the mitochondria) displayed opposing responses to reproductive failure (SOD1 increased; SOD2 decreased). Previously, SOD1-null mice exhibited increased DNA damage, increased senescent cells, and increased inflammation [81]. Overexpression of SOD1 results in neurodegenerative changes [36]. Reducing SOD2 activity in mice increases the levels of oxidative damage to DNA [76], while SOD2 overexpression in mice increases lifespan and provides a protective role against oxidative stress, fibrosis, and apoptosis [35]. Interestingly, the loss of SOD2 correlates with the overexpression of SOD1 [58], which may suggest that the increase in SOD1 in the current study may be a compensatory response to the decrease in SOD2.
Both SOD1 and SOD2 responded to the loss of estradiol from 4 to 13 months, but SOD1 did not appear to be influenced by the transplantation of young ovarian tissue, whereas SOD2 demonstrated a modest response to the transplanted tissue. Because the young, transplanted tissues did not restore young levels of estradiol, changes in SOD1 appeared to be sensitive to changes in estradiol, but not to other, non-estradiol signaling from the young ovarian tissue. Changes in SOD2 appeared to be sensitive to estradiol and non-estradiol ovarian signaling.
Glutathione peroxidase 1 (GPX1) is the most abundant glutathione peroxidase, is found in almost all mammalian tissues, and plays a major role in protecting cells from oxidative stress [53]. Mice without GPX1 are more susceptible to oxidative stress [12]. Mice that overexpress GPX1 develop insulin resistance and obesity [51]. Ovarian transplant recipient mice display improved insulin sensitivity and decreased fat mass [25, 72]. Glutathione-disulfide reductase (GSR) is a homodimeric flavoprotein that maintains the ratio of GSH/GSSG and prevents oxidative stress in red blood cells [31]. Overexpression of GSR in mice results in increased anxiety-like behavior, while inhibition decreases anxiety-like behavior [33]. In transplant recipients that displayed decreased GSR, sensory function and cognitive behavior were also improved [26, 60].
Catalase was reduced by 28% in FC transplant recipients but was not reduced in FD recipients. Mice with cat overexpression display extended longevity, increased resistance to age-related skeletal muscle dysfunction and exhibit improved voluntary exercise, increased muscle force, decreased intracellular Ca(2+) leak, and increased sarcoplasmic reticulum Ca(2+) load [73]. Catalase activity is significantly reduced in patients with Alzheimer-type dementia [24], suggesting that reactive oxygen species could contribute to the pathogenesis of Alzheimer’s disease. Muscle force was increased [28], and sarcopenia was decreased [25, 61] in FC and FD ovary transplant recipients, suggesting an indirect connection between cat function and skeletal muscle function.
Aging can induce the loss of the protective functions of FoxO transcription factors, which, in mammals, can exert their protective effects through the regulation of superoxide dismutases and which have roles in the regulation of glutathione-mediated detoxification by inducing the transcription of GSTM1 [23, 55]. Lack of GSTM1 is associated with greater susceptibility to genomic damage [15], while animals with GSTM1 overexpression demonstrate significantly lower blood pressure and reduced oxidative stress [57]. Our analysis has revealed that both GSTM1 and SOD1 are increased with aging, which may reflect oxidative stress in the local environment [32]. An increase in both proteins was also observed with aging in human ovaries (unpublished observations, Dr. Miguel Brieño-Enríquez, University of Pittsburgh). Preliminary work in our lab has revealed that FoxO1 protein was abundant in ovarian transudate from 60-day-old ovaries but was not detected in the ovaries of 18-month-old mice (unpublished observations, Mason lab).
We have demonstrated that transplantation of young ovarian somatic tissues/cells improves the health of postreproductive recipient mice, independent of follicles and cyclic estrogens [25, 26, 28, 72]. In addition, several age-related miRNA changes in serum are reversed in old recipients of young ovarian tissue (unpublished observations). We and others have identified specific, health-associated miRNAs [19, 56, 64, 77]. In addition, exogenous exosomes can improve health through alterations in miRNA pathways [13, 38, 43, 80]. Based on these observations, we hypothesize that ovarian somatic tissues confer anti-aging benefits to old mice by secreting putative pro-survival exosomal miRNAs that circulate systemically and which regulate distant tissues.
In summary, the current results suggest the presence of a germ cell- and estradiol-independent ovarian influence on aging-associated changes in the response to oxidative stress, which manifest differently in reproductive-aged and post-reproductive-aged mice. The results presented here help to separate chronological and ovarian aging and the influence of estradiol on the response to oxidative stress, bring into question the role of follicle-driven hormone production in the response to oxidative stress, and support a novel, estradiol-independent role for the ovary in female health and survival.
Acknowledgements
The authors thank Dr. Aaron Olsen, Mrs. Lisa DeSoi, and Nate Johnson for their help with the mice and Dr. Miguel Brieño-Enríquez and Dr. Suzannah Williams for the discussions and contributions regarding ovarian structure and function. Additionally, the authors thank the Utah Science Technology and Research Initiative (USTAR), the USDA ARS Poisonous Plant Research Laboratory, Utah State University, and the Utah State University, College of Veterinary Medicine and the Department of Animal, Dairy, and Veterinary Sciences.
Funding
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under award number R15AG061795 to J.B.M. and R56AG074499 to M.M.M and J.B.M. This work was also supported by the Nathan Shock Center Pilot Funding Program and the Oklahoma Nathan Shock Center core facility under award number P30 AG050911, the Utah Agricultural Experiment Station, grant number UTA01159, Utah State University, and by the College of Veterinary Medicine, Department of Animal, Dairy and Veterinary Sciences, Utah State University.
Data availability
The data presented in the work are available from the corresponding author upon request.
Declarations
Ethics approval
Animals were housed, and procedures were performed in an American Association for Accreditation of Laboratory Animal Care (AAALAC) approved facility in accordance with the National Institutes of Health and Animal Use guidelines. Animal care protocols were developed under the National Research Council guidelines found in the Guide for the Care and Use of Laboratory Animals. Protocols were approved by the Utah State University Institutional Animal Care and Use Committee (IACUC-10222).
Competing interests
The authors declare no competing interests.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ansere VA, Ali-Mondal S, Sathiaseelan R, Garcia DN, Isola JVV, Henseb JD, Saccon TD, Ocañas SR, Tooley KB, Stout MB, Schneider A, Freeman WM. Cellular hallmarks of aging emerge in the ovary prior to primordial follicle depletion. Mech Ageing Dev. 2021;94:111425. doi: 10.1016/j.mad.2020.111425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnett KR, Schilling C, Greenfeld CR, Tomic D, Flaws JA. Ovarian follicle development and transgenic mouse models. Hum Reprod Update. 2006;12(5):537–555. doi: 10.1093/humupd/dml022. [DOI] [PubMed] [Google Scholar]
- 3.Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61(6):1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boot L, Muhlbock O, Thung P. Senile changes in the oestrous cycle and in ovarian structure in some inbred strains of mice. Acta Endocrinol. 1956;23(1):8–32. doi: 10.1530/acta.0.0230008. [DOI] [PubMed] [Google Scholar]
- 5.Bridgeman SC, Ellison GC, Melton PE, Newsholme P, Mamotte CDS. Epigenetic effects of metformin: from molecular mechanisms to clinical implications. Diabetes Obes Metab. 2018;20(7):1553–1562. doi: 10.1111/dom.13262. [DOI] [PubMed] [Google Scholar]
- 6.Casalino SM, Linares JA, Goldraij A. Different effect of a restricted diet on isolated uteri of ovariectomized and non-ovariectomized rats. Influence of indomethacin and prostaglandins. Prostaglandins Leukot Essent Fatty Acids. 1994;51(1):41–5. doi: 10.1016/0952-3278(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 7.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90(11):1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 8.Csiszar A, Labinskyy N, Zhao X, Hu F, Serpillon S, Huang Z, Ballabh P, Levy RJ, Hintze TH, Wolin MS, Austad SN, Podlutsky A, Ungvari Z. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell. 2007;6(6):783–797. doi: 10.1111/j.1474-9726.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- 9.Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130(8):518–527. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Csiszar A, Gautam T, Sosnowska D, Tarantini S, Banki E, Tucsek Z, Toth P, Losonczy G, Koller A, Reglodi D, Giles CB, Wren JD, Sonntag WE, Ungvari Z. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am J Physiol Heart Circ Physiol. 2014;307(3):H292–306. doi: 10.1152/ajpheart.00307.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalal PK, Agarwal M. Postmenopausal syndrome. Indian J Psychiatry. 2015;57(2):S222–S232. doi: 10.4103/0019-5545.161483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Haan JB, Bladier C, Griffiths P, Kelner M, O'Shea RD, Cheung NS, Bronson RT, Silvestro MJ, Wild S, Zheng SS, Beart PM, Hertzog PJ, Kola I. Mice with a homozygous null mutation for the most abundant glutathione peroxidase, Gpx1, show increased susceptibility to the oxidative stress-inducing agents paraquat and hydrogen peroxide. J Biol Chem. 1998;273(35):22528–22536. doi: 10.1074/jbc.273.35.22528. [DOI] [PubMed] [Google Scholar]
- 13.Duan S, Wang F, Cao J, Wang C. Exosomes derived from MicroRNA-146a-5p-enriched bone marrow mesenchymal stem cells alleviate intracerebral hemorrhage by inhibiting neuronal apoptosis and microglial M1 polarization. Drug Des Devel Ther. 2020;5(14):3143–3158. doi: 10.2147/DDDT.S255828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eppig JJ, Wigglesworth K. Development of mouse and rat oocytes in chimeric reaggregated ovaries after interspecific exchange of somatic and germ cell components. Biol Reprod. 2000;63(4):1014–1023. doi: 10.1095/biolreprod63.4.1014. [DOI] [PubMed] [Google Scholar]
- 15.Eshkoor SA, Marashi SJ, Ismail P, Rahman SA, Mirinargesi M, Adon MY, Devan RV. Association of GSTM1 and GSTT1 with ageing in auto repair shop workers. Genet Mol Res. 2012;11(2):1486–1496. doi: 10.4238/2012.May.21.5. [DOI] [PubMed] [Google Scholar]
- 16.Faddy MJ, Telfer E, Gosden RG. The kinetics of pre-antral follicle development in ovaries of CBA/Ca mice during the first 14 weeks of life. Cell Tissue Kinet. 1987;20(6):551–560. doi: 10.1111/j.1365-2184.1987.tb01364.x. [DOI] [PubMed] [Google Scholar]
- 17.Fang EF, Lautrup S, Hou Y, Demarest TG, Croteau DL, Mattson MP, Bohr VA. NAD+ in aging: molecular mechanisms and translational implications. Trends Mol Med. 2017;23(10):899–916. doi: 10.1016/j.molmed.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finch CE. The menopause and aging, a comparative perspective. J Steroid Biochem Mol Biol. 2014;142:132–141. doi: 10.1016/j.jsbmb.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francisco S, Martinho V, Ferreira M, Reis A, Moura G, Soares AR, Santos MAS. The role of MicroRNAs in Proteostasis decline and protein aggregation during brain and skeletal muscle aging. Int J Mol Sci. 2022;23(6):3232. doi: 10.3390/ijms23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fryar CD, Chen TC, Li X. Prevalence of uncontrolled risk factors for cardiovascular disease: United States, 1999–2010. NCHS Data Brief. 2012;103:1–8. [PubMed] [Google Scholar]
- 21.Garcia DN, Saccon TD, Pradiee J, Rincón JAA, Andrade KRS, Rovani MT, Mondadori RG, Cruz LAX, Barros CC, Masternak MM, Bartke A, Mason JB, Schneider A. Effect of caloric restriction and rapamycin on ovarian aging in mice. Geroscience. 2019;41(4):395–408. doi: 10.1007/s11357-019-00087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gosden RG, Jones EC, Jacks F. Pituitary-ovarian relationships during the post-reproductive phase of inbred mice. Exp Gerontol. 1978;13(3–4):159–166. doi: 10.1016/0531-5565(78)90008-6. [DOI] [PubMed] [Google Scholar]
- 23.Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007;282(41):30107–30119. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- 24.Gsell W, Conrad R, Hickethier M, Sofic E, Frölich L, Wichart I, Jellinger K, Moll G, Ransmayr G, Beckmann H, et al. Decreased catalase activity but unchanged superoxide dismutase activity in brains of patients with dementia of Alzheimer type. J Neurochem. 1995;64(3):1216–1223. doi: 10.1046/j.1471-4159.1995.64031216.x. [DOI] [PubMed] [Google Scholar]
- 25.Habermehl TL, Mason JB. Decreased sarcopenia in aged females with young ovary transplants was preserved in mice that received germ cell-depleted young ovaries. J Clin Med. 2019;8(1):40. doi: 10.3390/jcm8010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Habermehl, T.L., Parkinson, K.C. and Mason, JB. (2019). Germ cell depletion influenced neuromuscular, sensory, renal and metabolic function in postreproductive female mice. Obstetrics and Gynecology: Open Access: OBOA-131. 10.29011/2577-2236/100031
- 27.Habermehl TL, Parkinson KC, Hubbard GB, Ikeno Y, Engelmeyer JI, Schumacher B, Mason JB. Extension of longevity and reduction of inflammation is ovarian-dependent, but germ cell-independent in post-reproductive female mice. Geroscience. 2019;41(1):25–38. doi: 10.1007/s11357-018-0049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Habermehl TL, Underwood KB, Welch KD, Gawrys SP, Parkinson KC, Schneider A, Masternak MM, Mason JB. Aging-associated changes in motor function are ovarian somatic tissue-dependent, but germ cell and estradiol independent in post-reproductive female mice exposed to young ovarian tissue. Geroscience. 2022;44(4):2157–2169. doi: 10.1007/s11357-022-00549-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henderson VW. Aging, estrogens, and episodic memory in women. Cogn Behav Neurol. 2009;22(4):205–214. doi: 10.1097/WNN.0b013e3181a74ce7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hense JD, Garcia DN, Isola JV, Alvarado-Rincón JA, Zanini BM, Prosczek JB, Stout MB, Mason JB, Walsh PT, Brieño-Enríquez MA, Schadock I, Barros CC, Masternak MM, Schneider A. Senolytic treatment reverses obesity-mediated senescent cell accumulation in the ovary. Geroscience. 2022;23. 10.1007/s11357-022-00573-9 [DOI] [PMC free article] [PubMed]
- 31.Ho HY, Cheng ML, Chiu DT. Glucose-6-phosphate dehydrogenase–from oxidative stress to cellular functions and degenerative diseases. Redox Rep. 2007;12(3):109–118. doi: 10.1179/135100007X200209. [DOI] [PubMed] [Google Scholar]
- 32.Ho E, Karimi Galougahi K, Liu CC, Bhindi R, Figtree GA. Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biol. 2013;1(1):483–491. doi: 10.1016/j.redox.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hovatta I, Tennant RS, Helton R, Marr RA, Singer O, Redwine JM, Ellison JA, Schadt EE, Verma IM, Lockhart DJ, Barlow C. Glyoxalase 1 and glutathione reductase 1 regulate anxiety in mice. Nature. 2005;438(7068):662–666. doi: 10.1038/nature04250. [DOI] [PubMed] [Google Scholar]
- 34.Hoyer PB, Sipes IG. Assessment of follicle destruction in chemical-induced ovarian toxicity. Annu Rev Pharmacol Toxicol. 1996;36:307–331. doi: 10.1146/annurev.pa.36.040196.001515. [DOI] [PubMed] [Google Scholar]
- 35.Hu D, Cao P, Thiels E, Chu CT, Wu GY, Oury TD, Klann E. Hippocampal long-term potentiation, memory, and longevity in mice that overexpress mitochondrial superoxide dismutase. Neurobiol Learn Mem. 2007;87(3):372–384. doi: 10.1016/j.nlm.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaarsma D, Haasdijk ED, Grashorn JA, Hawkins R, van Duijn W, Verspaget HW, London J, Holstege JC. Human Cu/Zn superoxide dismutase (SOD1) overexpression in mice causes mitochondrial vacuolization, axonal degeneration, and premature motoneuron death and accelerates motoneuron disease in mice expressing a familial amyotrophic lateral sclerosis mutant SOD1. Neurobiol Dis. 2000;7(6 Pt B):623–43. 10.1006/nbdi.2000.0299. [DOI] [PubMed]
- 37.Jacobsen BK, Knutsen SF, Fraser GE. Age at natural menopause and total mortality and mortality from ischemic heart disease: the Adventist Health Study. J Clin Epidemiol. 1999;52(4):303–307. doi: 10.1016/s0895-4356(98)00170-x. [DOI] [PubMed] [Google Scholar]
- 38.Jin Z, Ren J, Qi S. Human bone mesenchymal stem cells derived exosomes overexpressing microRNA-26a-5p alleviate osteoarthritis via down-regulation of PTGS2. Int Immunopharmacol. 2020;78. 10.1016/j.intimp.2019.105946 [DOI] [PubMed]
- 39.Jones EC, Krohn PL. The relationships between age, numbers of oocytes and fertility in virgin and multiparous mice. J Endocrinol. 1961;21:469–495. doi: 10.1677/joe.0.0210469. [DOI] [PubMed] [Google Scholar]
- 40.Keshari RS, Verma A, Barthwal MK, Dikshit M. Reactive oxygen species-induced activation of ERK and p38 MAPK mediates PMA-induced NETs release from human neutrophils. J Cell Biochem. 2013;114(3):532–540. doi: 10.1002/jcb.24391. [DOI] [PubMed] [Google Scholar]
- 41.Kruk J, Aboul-Enein HY, Kładna A, Bowser JE. Oxidative stress in biological systems and its relation with pathophysiological functions: the effect of physical activity on cellular redox homeostasis. Free Radic Res. 2019;53(5):497–521. doi: 10.1080/10715762.2019.1612059. [DOI] [PubMed] [Google Scholar]
- 42.Labinskyy N, Csiszar A, Orosz Z, Smith K, Rivera A, Buffenstein R, et al. Comparison of endothelial function, O2.- and H2O2 production, and vascular oxidative stress resistance between the longest-living rodent, the naked mole rat, and mice. Am J Physiol. 2006;291:2698–704. doi: 10.1152/ajpheart.00534.2006. [DOI] [PubMed] [Google Scholar]
- 43.Lee JY, Ryu D, Lim SW, Ryu KJ, Choi ME, Yoon SE, Kim K, Park C, Kim SJ. Exosomal miR-1305 in the oncogenic activity of hypoxic multiple myeloma cells: a biomarker for predicting prognosis. J Cancer. 2021;12(10):2825–2834. doi: 10.7150/jca.55553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lohff JC, Christian PJ, Marion SL, Arrandale A, Hoyer PB. Characterization of cyclicity and hormonal profile with impending ovarian failure in a novel chemical-induced mouse model of perimenopause. Comp Med. 2005;55(6):523–527. [PubMed] [Google Scholar]
- 45.Mason JB, Habermehl TL, Underwood KB, Schneider A, Brieño-Enriquez MA, Masternak MM, Parkinson KC. The interrelationship between female reproductive aging and survival. J Gerontol A Biol Sci Med Sci. 2021;. 10.1093/gerona/glab252 [DOI] [PMC free article] [PubMed]
- 46.Mason JB, Cargill SL, Anderson GB, Carey JR. Transplantation of young ovaries to old mice increased life span in transplant recipients. J Gerontol A Biol Sci Med Sci. 2009;64(12):1207–1211. doi: 10.1093/gerona/glp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mason JB, Cargill SL, Anderson GB, Carey JR. Ovarian status influenced the rate of body-weight change but not the total amount of body-weight gained or lost in female CBA/J mice. Exp Gerontol. 2010;45(6):435–441. doi: 10.1016/j.exger.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mason JB, Cargill SL, Griffey SM, Reader JR, Anderson GB, Carey JR. Transplantation of young ovaries restored cardioprotective influence in postreproductive-aged mice. Aging Cell. 2011;10(3):448–456. doi: 10.1111/j.1474-9726.2011.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mason JB, Terry BC, Merchant SS, Mason HM, Nazokkarmaher M. Manipulation of ovarian function significantly influenced trabecular and cortical bone volume, architecture and density in mice at death. PLoS One. 2015;10(12):e0145821. doi: 10.1371/journal.pone.0145821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mason JB, Parkinson KC, Habermehl TL. Orthotopic ovarian transplantation procedures to investigate the life- and health-span influence of ovarian senescence in female mice. J Vis Exp. 2018;132:56638. doi: 10.3791/56638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McClung JP, Roneker CA, Mu W, Lisk DJ, Langlais P, Liu F, Lei XG. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci U S A. 2004;101(24):8852–8857. doi: 10.1073/pnas.0308096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McLean RR. Proinflammatory cytokines and osteoporosis. Curr Osteoporos Rep. 2009;7:134–139. doi: 10.1007/s11914-009-0023-2. [DOI] [PubMed] [Google Scholar]
- 53.Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43(4):477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 54.Nilsen J. Estradiol and neurodegenerative oxidative stress. Front Neuroendocrinol. 2008;29(4):463–475. doi: 10.1016/j.yfrne.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 55.Nogueira V, et al. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008;14(6):458–470. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nunes ADC, Weigl M, Schneider A, Noureddine S, Yu L, Lahde C, Saccon TD, Mitra K, Beltran E, Grillari J, Kirkland JL, Tchkonia T, Robbins PD, Masternak MM. miR-146a-5p modulates cellular senescence and apoptosis in visceral adipose tissue of long-lived Ames dwarf mice and in cultured pre-adipocytes. Geroscience. 2022;44(1):503–518. doi: 10.1007/s11357-021-00490-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olson E, Pravenec M, Landa V, Koh-Tan HHC, Dominiczak AF, McBride MW, Graham D. Transgenic overexpression of glutathione S-transferase μ-type 1 reduces hypertension and oxidative stress in the stroke-prone spontaneously hypertensive rat. J Hypertens. 2019;37(5):985–996. doi: 10.1097/HJH.0000000000001960. [DOI] [PubMed] [Google Scholar]
- 58.Papa L, Hahn M, Marsh EL, Evans BS, Germain D. SOD2 to SOD1 switch in breast cancer. J Biol Chem. 2014;289(9):5412–5416. doi: 10.1074/jbc.C113.526475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parkening TA, Fabricant JD, Heussner JC, Collins TJ, Smith ER. Orthotopic ovarian transplantations in young and aged C57BL/6J mice. Biol Reprod. 1985;32(5):989–997. doi: 10.1095/biolreprod32.5.989. [DOI] [PubMed] [Google Scholar]
- 60.Parkinson KC, Peterson RL, Mason JB. Cognitive behavior and sensory function were significantly influenced by restoration of active ovarian function in postreproductive mice. Exp Gerontol. 2017;92:28–33. doi: 10.1016/j.exger.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 61.Peterson RL, Parkinson KC, Mason JB. Manipulation of ovarian function significantly influenced sarcopenia in postreproductive-age mice. J Transplant. 2016;2016:4570842. doi: 10.1155/2016/4570842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peterson RL, Parkinson KC, Mason JB. Restoration of immune and renal function in aged females by re-establishment of active ovarian function. Reprod Fertil Dev. 2017;29:2052–2059. doi: 10.1007/s11357-018-0049-4. [DOI] [PubMed] [Google Scholar]
- 63.Pinto YM, Paul M, Ganten D. Lessons from rat models of hypertension: from Goldblatt to genetic engineering. Cardiovasc Res. 1998;39(1):77–88. doi: 10.1016/S0008-6363(98)00077-7. [DOI] [PubMed] [Google Scholar]
- 64.Piotrowski I, Zhu X, Saccon TD, Ashiqueali S, Schneider A, de Carvalho Nunes AD, Noureddine S, Sobecka A, Barczak W, Szewczyk M, Golusiński W, Masternak MM, Golusiński P. miRNAs as biomarkers for diagnosing and predicting survival of head and neck squamous cell carcinoma patients. Cancers (Basel) 2021;13(16):3980. doi: 10.3390/cancers13163980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rivera Z, Christian PJ, Marion SL, Brooks HL, Hoyer PB. Steroidogenic capacity of residual ovarian tissue in 4-vinylcyclohexene diepoxide-treated mice. Biol Reprod. 2009;80(2):328–336. doi: 10.1095/biolreprod.108.070359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robert H, Ferguson L, Reins O, Greco T, Prins ML, Folkerts M. Rodent estrous cycle monitoring utilizing vaginal lavage: no such thing as a normal cycle. J Vis Exp. 2021;(174):10.3791/62884 [DOI] [PMC free article] [PubMed]
- 67.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65(2):161–166. doi: 10.1016/j.maturitas.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Signorelli SS, Neri S, Sciacchitano S, Pino LD, Costa MP, Marchese G, Celotta G, Cassibba N, Pennisi G, Caschetto S. Behaviour of some indicators of oxidative stress in postmenopausal and fertile women. Maturitas. 2006;53(1):77–82. doi: 10.1016/j.maturitas.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 70.Suh Y, Atzmon G, Cho MO, et al. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci USA. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thom T, Haase N, Rosamond W, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics 2006 update a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113(6):85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 72.Tyler KA, Habermehl TL, Mason JB. Manipulation of ovarian function influenced glucose metabolism in CBA/J mice. Exp Gerontol. 2019;126:110686. doi: 10.1016/j.exger.2019.110686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Umanskaya A, Santulli G, Xie W, Andersson DC, Reiken SR, Marks AR. Genetically enhancing mitochondrial antioxidant activity improves muscle function in aging. Proc Natl Acad Sci U S A. 2014;111(42):15250–15255. doi: 10.1073/pnas.1412754111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation. 2003;108:1253–1258. doi: 10.1161/01.CIR.0000079165.84309.4D. [DOI] [PubMed] [Google Scholar]
- 75.Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, et al. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of Nrf2-mediated antioxidant response. Am J Physiol Heart Circ Physiol. 2011;301:H363–H372. doi: 10.1152/ajpheart.01134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van Remmen H, Ikeno Y, Hamilton M, Pahlavani M, Wolf N, Thorpe SR, Alderson NL, Baynes JW, Epstein CJ, Huang TT, Nelson J, Strong R, Richardson A. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16(1):29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- 77.Victoria B, Nunez Lopez YO, Masternak MM. MicroRNAs and the metabolic hallmarks of aging. Mol Cell Endocrinol. 2017;5(455):131–147. doi: 10.1016/j.mce.2016.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wexler BC, Iams SG, Judd JT. Arterial lesions in repeatedly bred spontaneously hypertensive rats. Circ Res. 1976;38(6):494–501. doi: 10.1161/01.res.38.6.494. [DOI] [PubMed] [Google Scholar]
- 79.Yamaza H, Komatsu T, Wakita S, Kijogi C, Park S, Hayashi H, Chiba T, Mori R, Furuyama T, Mori N, Shimokawa I. FoxO1 is involved in the antineoplastic effect of calorie restriction. Aging Cell. 2010;9(3):372–382. doi: 10.1111/j.1474-9726.2010.00563.x. [DOI] [PubMed] [Google Scholar]
- 80.Yao S, Yin Y, Jin G, Li D, Li M, Hu Y, Feng Y, Liu Y, Bian Z, Wang X, Mao Y, Zhang J, Wu Z, Huang Z. Exosome-mediated delivery of miR-204-5p inhibits tumor growth and chemoresistance. Cancer Med. 2020;9(16):5989–5998. doi: 10.1002/cam4.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y, Unnikrishnan A, Deepa SS, Liu Y, Li Y, Ikeno Y, Sosnowska D, Van Remmen H, Richardson A. A new role for oxidative stress in aging: the accelerated aging phenotype in Sod1-/- mice is correlated to increased cellular senescence. Redox Biol. 2017;11:30–37. doi: 10.1016/j.redox.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou Y, Richard S, Batchelor NJ, Oorschot DE, Anderson GM, Pankhurst MW. Anti-Müllerian hormone-mediated preantral follicle atresia is a key determinant of antral follicle count in mice. Hum Reprod. 2022;37(11):2635–2645. doi: 10.1093/humrep/deac204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in the work are available from the corresponding author upon request.