Abstract
Microbiota composition has been linked to physical activity, health measures, and biological age, but a shared profile has yet to be shown. The aim of this study was to examine the associations between microbiota composition and measures of function, such as a composite measure of physical capacity, and biological age in midlife, prior to onset of age-related diseases. Seventy healthy midlife individuals (age 44.58 ± 0.18) were examined cross-sectionally, and their gut-microbiota profile was characterized from stool samples using 16SrRNA gene sequencing. Biological age was measured using the Klemera-Doubal method and a composition of blood and physiological biomarkers. Physical capacity was calculated based on sex-standardized functional tests. We demonstrate that the women had significantly richer microbiota, p = 0.025; however, microbiota diversity was not linked with chronological age, biological age, or physical capacity for either women or men. Men had slightly greater β-diversity; however, β-diversity was positively associated with biological age and with physical capacity for women only (p = 0.01 and p = 0.04; respectively). For women, an increase in abundance of Roseburia faecis and Collinsella aerofaciens, as well as genus Ruminococcus and Dorea, was significantly associated with higher biological age and lower physical capacity; an increase in abundance of Akkermansia muciniphila and genera Bacteroides and Alistipes was associated with younger biological age and increased physical capacity. Differentially abundant taxa were also associated with non-communicable diseases. These findings suggest that microbiota composition is a potential mechanism linking physical capacity and health status; personalized probiotics may serve as a new means to support health-promoting interventions in midlife. Investigating additional factors underlying this link may facilitate the development of a more accurate method to estimate the rate of aging.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-023-00905-3.
Keywords: Biological age, Exercise, Non-communicable disease, Microbiome
Introduction
Aging is a major risk factor for chronic diseases and decline in function; however, the aging process is highly diverse [1]. This diversity is seen both interpersonally and between sexes; women have longer lifespans but have a lower proportion of life expectancy in good health, identified as the health-survival paradox [2]. In this vein, geroscientists are searching for an accurate and feasible set of markers to represent or estimate the rate of aging, support predictions of health outcomes, and identify potential aging mechanisms [3].
The concept of biological age (BA), which captures aging diversity by estimating the overall state of the body, was found to be associated with health and mortality measures [4]. Accepted methods for BA assessment rely on physiologically-based analyses (e.g., level of DNA methylation [5], different cells’ telomere lengths [4], composition of physiological markers [6]), or behavioral ones (e.g., measures of function [7]). Each method sheds light on a unique representation of the aging process and offers a possible avenue to support interventions promoting healthier aging; thus, no single gold standard exists [8]. In recent years, the gut microbiota, which plays a role in a large variety of physiological pathways, has offered a new avenue to understanding aging processes [9, 10], as it makes a unique contribution to many physiological processes and is associated with both physiological and behavioral markers of aging, such as physical capacity [11].
Engaging with physical activity on a regular basis leads to improved physical fitness [12] or physical capacity, an umbrella term for the ability to perform physical function [13], and it has the potential to attenuate many negative age-related health changes (e.g., skeletal muscle changes, vascular system adaptation, metabolic processes) [14, 15]. Physical capacity can be used as a behavioral marker of aging, and as such, it is associated with BA based on physiological markers of aging [6, 16]. Additionally, physical activity is widely investigated in the context of microbiota, showing its contribution to changes in microbiota composition [11, 17].
Our microbiota is a collection of bacteria and other species living in our gastrointestinal tract that changes as we age; these changes can be studied in the context of health and longevity [9]. Described as “an organ inside another organ,” our microbiota plays a complex and intricate role in regulating human physiology [18], and accumulation of negative alterations in its composition is associated with impaired health and can be regarded as a hallmark of aging [19, 20]. In contrast, improving microbiota composition (e.g., increasing its diversity or changing abundance of specific taxa) may serve as a means to ameliorate age-related disease prevalence [21]. Moreover, microbiota composition is also associated with non-communicable disease development [22], here investigated as age-related diseases [23], further supporting the need to investigate microbiota composition metrics as promising markers to identify negative aging trajectories. One shared factor that has the potential to improve health and consequently both the microbiota composition and BA is performing regular physical activity [11, 15].
To the best of our knowledge, to date, the link between BA, physical capacity, and microbiota composition was not investigated; exploring this link in midlife, prior to the onset of age-related diseases, may help identify possible aging mechanisms and highlight feasible modifiable markers of aging applicable for population-wide studies [24]. Thus, the aim of this study was to identify common microbiota composition characteristics linking physical capacity and BA, measured using a combination of physiological biomarkers while considering possible sex-related differences. We hypothesized that specific microbiota characteristics, namely, diversity metrics and specific taxa, such as the genus Roseburia [25] and species Akkermansia muciniphila [26], will be associated with both younger BA and higher physical capacity as well as with non-communicable diseases [22], as non-communicable diseases may represent age-related negative health status. Identified characteristics may support the future development of a robust measure for BA and facilitate potential interventions for healthier aging.
Study sample
This study comprises a subset of adults from the Jerusalem Perinatal Study (JPS), which includes all births between 1974 to 1976 in West Jerusalem [27]. The current sub-set is of individuals who participated in the most recent follow-up examination at age 42–45 (between 2016 and 2021), were willing to take part in this extension of the study, and were inclined to provide a stool sample. The Ethics Committee of the Faculty of Social Welfare and Health Sciences at the University of Haifa (#407/20) and Hadassah Hospital Institutional Review Board (#10–01.04.05) provided ethical approval for the recent extension of the JPS follow-up. All study participants provided an informed consent prior to any study procedure. In short, included participants were healthy midlife adults with no physical or other health limitations prohibiting participation in leisure physical activity.
Biological age estimation and physical capacity measurements
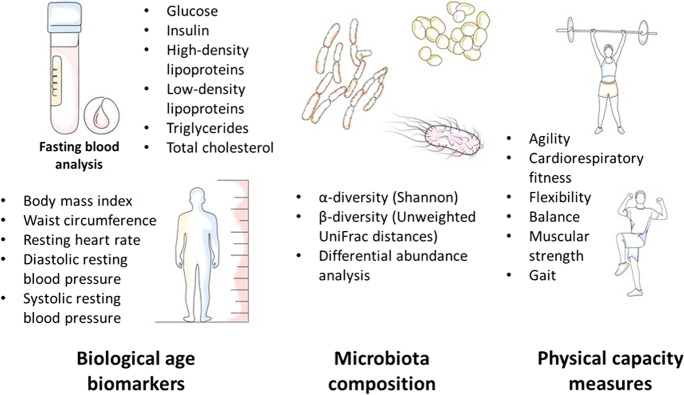
We estimated BA using the Klemera-Doubal method (KDM) [28] and a set of fasting blood markers with additional physiological measures collected during the most recent JPS follow-up at approximate chronological age 44 (Fig. 1). This collection of biomarkers focuses on cardio-metabolic health, here used as an estimated measure of biological aging. The BioAge R package [29] was used to establish BA; additional information on the used methods is given in the supplementary data, eTable 1.
Fig. 1.
Measured biomarkers, physical capacity domains, and microbiota variables
We focused on physical capacity metrics to estimate individuals’ engagement in physical activity, obtaining immediate quantity estimation for objective function across multiple physiological systems; lower physical capacity reflects lower participation in regular physical activity. Moreover, these measures provide a feasible behavioral goal for positive lifestyle modification. Measures of physical capacity were based on a comprehensive testing battery of functional performance, including five different physical fitness domains associated with health as well as gait analysis under normal and dual-task conditions [16] (Fig. 1). Each domain included several tests, the results of which were standardized using percentiles of total cohort measures to obtain a composite score ranging from 0 (minimum score, very low physical capacity) to 6 (maximum score). These composite scores were calculated seperately for each sex group, to avoid any sex-related differences in performance. A complete list of conducted tests is provided in the supplementary data, eTable 1.
Microbiome sample collection and analysis
Fixed protocols were followed for the collection and processing of stool samples [30] (supplementary data, eTable1). Briefly, following DNA extraction, the V4 region of the 16S rRNA gene was amplified, and amplicons were sequenced using the Illumina MiSeq platform. Sequences were processed in the QIIME2 pipeline [31] (version qiime2-2020.8), and then data was exported for further analyses in R software. Shannon diversity [32] was used as a measure of α-diversity (within-individuals diversity), and the unweighted UniFrac distances [33] were used for β-diversity (between-individuals diversity). α- and β-diversity were compared between males and females in the context of BA, chronological age, and physical capacity, and differential abundance analysis was performed. Associations between microbiota composition, BA, and physical capacity were assessed, and correlation between BA and physical capacity was examined using Pearson’s correlation to confirm the expected association between lower BA and higher physical capacity [16].
To further investigate the association between identified bacteria and aging, we focused on the link between specific microbial taxa and non-communicable diseases or risk factors associated with non-communicable diseases, as those are recognized as being linked with aging. We then conducted a focused literature search (https://disbiome.ugent.be/home and https://scholar.google.com) to highlight possible pathways linking these taxa to health.
Results
Seventy midlife individuals provided samples and were included in the analysis (mean age 44.58 ± 0.18, see Table 1). One participant did not provide a blood sample, and four participants did not complete the physical capacity testing phase. For included participants, physical capacity was negatively associated with BA adjusting for chronological age (partial Pearson correlation r = − 0.42, p = 0.001), demonstrating that higher physical capacity was linked with better health represented by lower BA.
Table 1.
Main variables
| Men | Women | |
|---|---|---|
| Chronological age1 (N = 70, 38 men) | 44.55 ± 0.25 (41.78–46.82) | 44.62 ± 0.26 (41.83–46.49) |
| BA1 (N = 69, 38 men) | 44.71 ± 1.37 (30.06–71.41) | 42.53 ± 1.19 (26.27–56.52) |
| Physical capacity1,2 (N = 66, 34 men) | 3.18 ± 0.22 (0.85–5.05) | 3.16 ± 0.19 (1–5.05) |
| BMI1,3 (N = 70, 38 men) | 27.82 ± 0.69 (21.33–41.83) | 26.03 ± 0.72 (17.93–35.19) |
1Differences between men and women were examined using T-test and found to be insignificant for all reported measures (p = 0.1 to 0.97)
2Composite score based on various tests, range 0–6; higher score is considered better physical capacity
3BMI was used as a biomarker for calculating BA and provided here as a measure for describing the cohort population
Within-person and between-person microbiome composition comparisons (α-diversity, β-diversity)
No significant links between α-diversity and chronological age, BA, or physical capacity were found. A comparison of α-diversity between men and women revealed that the women had significantly richer microbiota (Shannon α-diversity, Kruskal − Wallis test, p = 0.025, see supplementary eTable 2: Results—figures). However, no sex-specific links with chronological age, BA, or physical capacity were found.
Similarly, no associations between β-diversity (unweighted UniFrac distances) and chronological age, BA, or physical capacity were found when using PERMANOVA for the entire cohort. Again, there was a marginal effect of sex on microbiota diversity (PERMANOVA, p = 0.085) showing that men have slightly greater β-diversity. Examination of microbiota in the context of chronological age, BA, and physical capacity in men only revealed a trend toward a significant positive association between microbiota β-diversity and chronological age (PERMANOVA, p = 0.052). However, the associations between their β-diversity and BA and physical capacity were statistically insignificant. For women, no significant association was found for chronological age; there was a positive association of β-diversity with BA (PERMANOVA, p = 0.01) and with physical capacity (PERMANOVA, p = 0.04).
Differentially abundant taxa
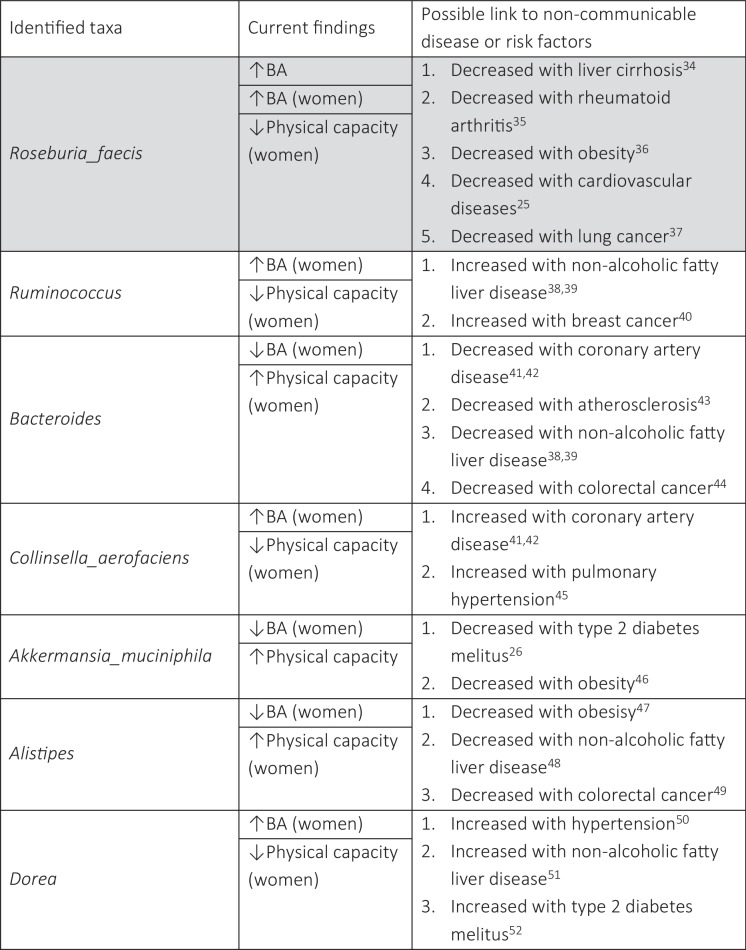
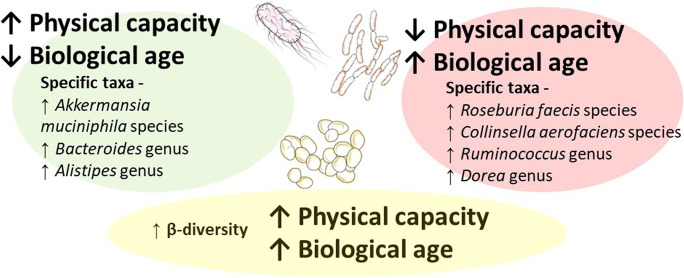
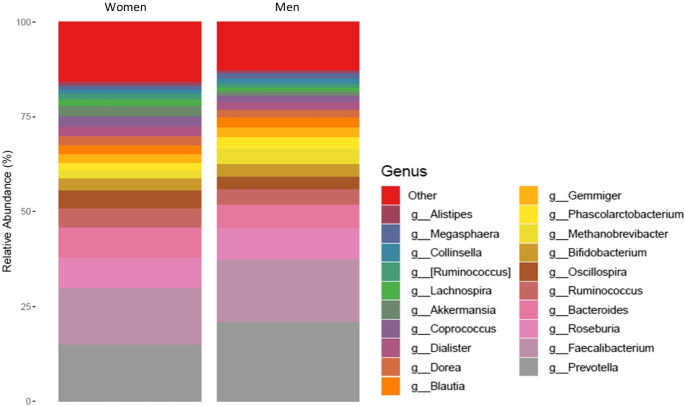
We identified specific taxa significantly associated with BA and physical capacity, linking microbiota composition to early manifestation of aging. Table 2 lists taxa that were significantly differentially abundant across BA, and physical capacity measures and presents possible associated non-communicable diseases or risk factors reported in previous publications. Figure 2 provides a graphic summary of the main findings; the full list of taxa significantly differentially abundant with the study variables (i.e., chronological age, BA, and physical capacity) is in the supplementary data, eTable 2. Figures 3 and 4 present the top 20 abundant genera and observed correlations with BA and physical capacity.
Table 2.
Taxa, linked variables, and non-communicable diseases
Fig. 2.
Significant microbiota composition variables associated with both physical capacity and BA
Fig. 3.
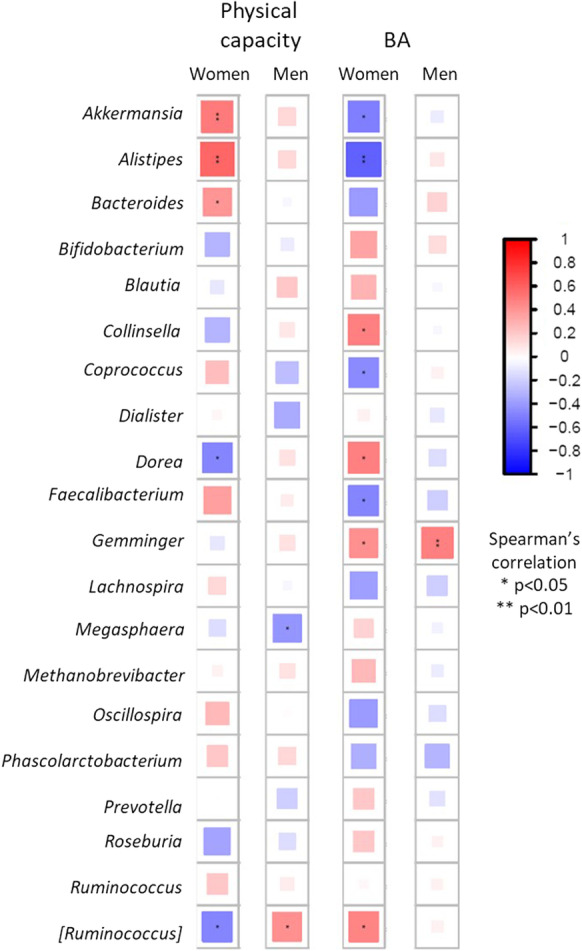
Top 20 abundant genera correlations with physical capacity and BA
Fig. 4.
Top 20 abundant genera by sex group
Discussion
In line with our hypothesis, the findings demonstrated that physical capacity and estimated BA share microbial characteristics; however, these results were mainly significant for women. In contrast to our expectation, α-diversity was not correlated with either BA or physical capacity; between-person diversity was positively correlated with physical capacity and BA for women only; thus, increased between-person diversity (β-diversity) was associated with accelerated aging status as well as with higher physical function levels. An increase in abundance of Roseburia faecis and Collinsella aerofaciens, as well as genus Ruminococcus and Dorea, was significantly associated with higher BA and lower physical capacity. An increase in abundance of Akkermansia muciniphila, as well as genus Bacteroides and Alistipes, was associated with lower BA and increased physical capacity (see Fig. 2). Additionally, a composite physical capacity score, as a quantitative measure of function, was negatively associated with BA in midlife healthy adults, supporting the link between increased physical capacity and a positive health status represented by younger BA.
Microbiota diversity in midlife
For the entire cohort of healthy midlife individuals, we did not find an increase in microbiota α- or β-diversity in participants evaluated as biologically younger or with better physical capacity. Considering previous reports [53], increased α-diversity was expected to be linked with higher physical capacity and lower BA, representing healthier, younger physiological status [9]. Microbial α-diversity measures the richness of our gut ecosystem [54]; in general, increased α-diversity contributes to higher resilience of the microbiota, supporting its ability to withstand dietary challenges, alcohol, or pathogens [55]. Higher microbial α-diversity may be linked with increased physical activity levels [56] and is widely reported for its association with better health metrics [55, 57]. Physical activity leads to widespread perturbations in numerous cells, tissues, and organs, resulting in multiple health-promoting benefits [58] as well as the mainly positive alteration in microbial α-diversity. Although differences in β-diversity between individuals with different lifestyles or chronological age may be observed [9], and a previous report linked β-diversity to higher levels of physical activity [59], we identified a significant, positive association between β-diversity and physical capacity as well as with BA in the women sub-cohort only. This finding opposes our hypothesis linking microbiota to both slower aging state and higher physical ability.
Although sex differences in BA and physical capacity were insignificant, we observed sex-related differences in microbiota diversity (women had significantly higher α-diversity and men had higher β-diversity), suggesting microbiota diversity may not be an accurate measure for assessing the health status of otherwise comparable men and women. Although relying on microbiota diversity as a health marker is plausible, it is a limited measure [60] as it may change rapidly. Lack of significant association between higher microbiota diversity and better BA or physical capacity could partially be explained by the relatively small sample size or by dietary differences that were not accounted for [61, 62]. Future studies should closely monitor dietary patterns to ensure the accuracy of microbiota diversity measures and offer a more robust assessment of its association with physical capacity and BA.
Contribution of specific taxon abundance to the link between physical capacity, biological age, and non-communicable diseases
We identified seven distinct taxa (four genera: Ruminococcus, Bacteroides, Dorea, and Alistipes; three species: Roseburia faecis, Collinsella aerofaciens, and Akkermansia muciniphila) associated with both BA and physical capacity (see Table 2 and Fig. 2). Identified taxa support the hypothesis that lower BA (being younger biologically) and higher physical capacity (being more physically fit), or vice versa, share a link with specific microbiota characteristics. Moreover, six taxa were also identified as being associated with non-communicable diseases. An increase in one of the identified species (Roseburia faecis) was significantly associated with being biologically older and having lower physical capacity as expected; however, available findings suggest that lower abundance of this species is associated with a variety of non-communicable diseases, such as cardiovascular disease [25] or health-related risk factors, such as obesity [36], while higher abundance is linked with beneficial production of short-chain fatty acids, specifically in older women [63] (therefore, colored differently in Table 2). Keeping in mind that there are many other short-chain fatty acid-producing bacteria at play and that here we focus only on bacteria associated with physical capacity and BA, Roseburia faecis could be associated with additional metabolic pathways that are not necessarily beneficial. All identified taxa represent one possible microbiota phenotype associated with an active lifestyle and a healthy younger physiological aging status.
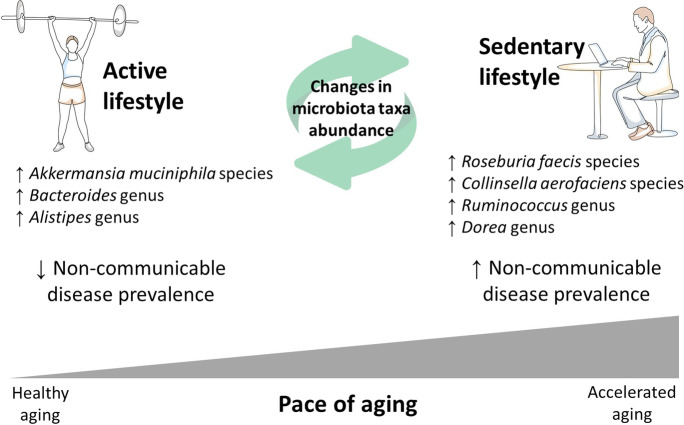
Previous publications suggest that an active lifestyle supports microbial changes that result in a health-supporting microbiota phenotype [64]; in contrast, a sedentary lifestyle promotes dysbiosis, loss of bacteria promoting positive metabolic activity, an increase in opportunistic species, and reduced diversity [65], thus contributing to accelerated aging state and non-communicable disease development [66] (see Fig. 5). As presented in the conceptual model in Fig. 5, microbiota composition changes present a possible mechanism linking lifestyle with pace of aging and non-communicable disease prevalence. Keeping in mind that bacteria work as communities, interacting with each other and with their host [67], these specific taxa represent only one possible microbiota-based phenotype linked with increased physical capacity and better health.
Fig. 5.
Conceptual model describing the link between active lifestyle, microbiota composition, and biological aging
The focused literature search on the identified taxa suggests they may also play a role in non-communicable diseases (e.g., non-alcoholic fatty liver disease, coronary artery disease, type 2 diabetes mellitus). Note that although all participants were in good health, non-communicable diseases develop slowly and are associated with lifestyle choices, such as physical inactivity [68]; thus, there may be microbial precursors to these disorders prior to diagnosis of such conditions in this study population.
Sex difference impact on microbiota composition and estimated BA
Comparisons of women’s and men’s physiological and physical traits are often significantly different [69], especially when studying aging [2]. In this cohort, studied variables (i.e., chronological age, BA, physical capacity) across sexes were comparable, as both BA and physical capacity were calculated separately for each sex and the cohort used was chronological age homogenous. However, we identified some differences in microbiota diversity measures, suggesting that sex-based microbiota composition differences could not be disregarded. Moreover, specific taxon abundance associations with study variables were mostly observed when investigating women separately from men, limiting our ability to generalize specific taxa roles in aging-related mechanisms. These findings are in-line with current literature on sex contributions to microbiota compositional changes [70] and can potentially explain these results. Sex hormones (e.g., testosterone, estradiol) are correlated with microbiota composition characteristics [71], such as diversity or specific taxon abundance. Presented results suggest that women could be more sensitive to microbiota compositional changes associated with aging mechanisms or physical capacity status, explaining the main findings being observed only in midlife women.
Strengths and limitations
This study utilizes physical capacity, based on an extended testing battery for measuring physical fitness as well as gait, to represent a robust estimation of participants’ physical status, thus providing a comprehensive estimation for the physical state. This method offers additional insight on the role behavioral measures play in the aging processes. Additionally, investigating a midlife cohort with relatively homogenous age enables us to capture possible variations in all investigated variables before the onset of any age-related illness. This study has several limitations: (1) our sample size, although acceptable for microbiota studies, is relatively small thus limiting our analysis, particularly when assessing the impact of body weight or additional lifestyle factors. (2) The cross-sectional design does not provide an in-depth understanding of the association between microbiota and physical capacity; although it can be inferred, having a longitudinal comparison demonstrating the impact of changes in physical capacity on microbiota composition would have added another important perspective on these complicated interactions. (3) 16S rRNA analysis mainly allowed us to characterize the microbiota composition. Whole genome shotgun sequencing and meta-transcriptomics may bridge this gap by identifying specific bacterial gene involvement and allowing for a comprehensive understanding of the role certain bacteria play [72], supporting the idea that better health represented by lower BA shares similar microbiota functions with higher physical capacity. To give context to our results, we characterized possible shared pathways using functional analysis of the 16S rRNA data and did not reveal any shared functional link between physical capacity and BA (see supplementary eTable 2: Results—figures).
Conclusions
We show that several taxa in the microbiota were associated with both BA and physical capacity, suggesting microbiota composition plays a role in the association between physical capacity and our health status, in community dwelling midlife individuals. As such, altered microbiota composition may be regarded as an aging mechanism and specific modifications to its composition could be established as a leverage point for interventions addressing accelerated aging status. Additional dimensions of microbiota composition, studied by microbiota diversity metrics (within-person and between-person diversity), were not significantly associated with study variables, possibly due to study participants being healthy or the limited sample size. The rising prevalence of sedentary lifestyle increases the risk of age-related disease and a negative aging trajectory; investigating additional factors underlying the link between physical capacity and BA may facilitate the development of a more accurate method to estimate the rate of aging. Moreover, these findings may assist in the development of new intervention strategies. Future studies should account for microbiota composition as a possible force multiplier, enhancing physical activity interventions’ positive contribution to health with additional microbiota alteration methods supporting the same aim.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are thankful to all the participants for their willingness to contribute to this study.
Data availability
The microbiota data used here is publicly available in the European Bioinformatics Institute (EBI) database, https://www.ebi.ac.uk/ena/browser/view/PRJEB63185.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Omry Koren and Maayan Agmon contributed equally to this work.
References
- 1.Jazwinski SM, Kim S. Examination of the dimensions of biological age. Front Genet. 2019;10:263. doi: 10.3389/fgene.2019.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oksuzyan A, Juel K, Vaupel JW, Christensen K. Men: good health and high mortality. Sex differences in health and aging. Aging Clin Exp Res. 2008;20(2):91–102. doi: 10.1007/bf03324754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cummings SR, Kritchevsky SB. Endpoints for geroscience clinical trials: health outcomes, biomarkers, and biologic age. GeroScience. 2022;44(6):2925–2931. doi: 10.1007/s11357-022-00671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jylhävä J, Pedersen NL, Hägg S. Biological age predictors. EBioMedicine. 2017;21:29–36. doi: 10.1016/j.ebiom.2017.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell CG, Lowe R, Adams PD, et al. DNA methylation aging clocks: challenges and recommendations. Genome Biol. 2019;20(1). https://link.springer.com/article/10.1186/s13059-019-1824-y. [DOI] [PMC free article] [PubMed]
- 6.Belsky DW, Moffitt TE, Cohen AA, et al. Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: do they measure the same thing? Am J Epidemiol. 2018;187(6):1220–1230. doi: 10.1093/aje/kwx346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finkel D, Sternäng O, Jylhävä J, Bai G, Pedersen NL. Functional aging index complements frailty in prediction of entry into care and mortality. J Gerontol Ser A. 2019;74(12):1980–1986. doi: 10.1093/gerona/glz155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Ploner A, Wang Y, et al. Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up. eLife. 2020;9. https://elifesciences.org/articles/51507. [DOI] [PMC free article] [PubMed]
- 9.Badal VD, Vaccariello ED, Murray ER, et al. The gut microbiome, aging, and longevity: a systematic review. Nutrients. 2020;12(12):3759. doi: 10.3390/nu12123759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galkin F, Mamoshina P, Aliper A, et al. Human gut microbiome aging clock based on taxonomic profiling and deep learning. iScience. 2020;23(6):101199. doi: 10.1016/j.isci.2020.101199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tzemah Shahar R, Koren O, Matarasso S, Shochat T, Magzal F, Agmon M. Attributes of physical activity and gut microbiome in adults: a systematic review. Int J Sports Med. 2020;41(12):801–814. doi: 10.1055/a-1157-9257. [DOI] [PubMed] [Google Scholar]
- 12.Corbin CBP, Robert P, Franks, B. Don. Definitions: health, fitness, and physical activity. President's Council on Physical Fitness and Sports, Washington, DC. 2000;(Series 3 n9).
- 13.Tzemah-Shahar R, Hochner H, Iktilat K, Agmon M. What can we learn from physical capacity about biological age? A systematic review. Ageing Res Rev. 2022;77:101609. doi: 10.1016/j.arr.2022.101609. [DOI] [PubMed] [Google Scholar]
- 14.McGee SL, Hargreaves M. Exercise adaptations: molecular mechanisms and potential targets for therapeutic benefit. Nat Rev Endocrinol. 2020;16(9):495–505. doi: 10.1038/s41574-020-0377-1. [DOI] [PubMed] [Google Scholar]
- 15.Duggal NA, Niemiro G, Harridge SDR, Simpson RJ, Lord JM. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat Rev Immunol. 2019;19(9):563–572. doi: 10.1038/s41577-019-0177-9. [DOI] [PubMed] [Google Scholar]
- 16.Tzemah-Shahar R, Shapiro I, Kodesh E, et al. Physical-capacity based evaluation battery for rate of aging at midlife: a preliminary study. Submitted to European Review of Aging and Physical Activity, 2023.
- 17.Ramos C, Gibson GR, Walton GE, Magistro D, Kinnear W, Hunter K. Systematic review of the effects of exercise and physical activity on the gut microbiome of older adults. Nutrients. 2022;14(3):674. doi: 10.3390/nu14030674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baquero F, Nombela C. The microbiome as a human organ. Clin Microbiol Infect. 2012;18:2–4. doi: 10.1111/j.1469-0691.2012.03916.x. [DOI] [PubMed] [Google Scholar]
- 19.Bana B, Cabreiro F. The microbiome and aging. Annu Rev Genet. 2019;53:239–261. doi: 10.1146/annurev-genet-112618-043650. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: an expanding universe. Cell. 2023;186(2):243–278. doi: 10.1016/j.cell.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Nagpal R, Mainali R, Ahmadi S, et al. Gut microbiome and aging: physiological and mechanistic insights. Nutr Healthy Aging. 2018;4(4):267–285. doi: 10.3233/NHA-170030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noce A, Marrone G, Di Daniele F, et al. Impact of gut microbiota composition on onset and progression of chronic non-communicable diseases. Nutrients. 2019;11(5):1073. doi: 10.3390/nu11051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennett JE, Stevens GA, Mathers CD, et al. NCD countdown 2030: worldwide trends in non-communicable disease mortality and progress towards sustainable development goal target 3.4. The Lancet. 2018;392(10152):1072–1088. doi: 10.1016/s0140-6736(18)31992-5. [DOI] [PubMed] [Google Scholar]
- 24.Belsky DW, Caspi A, Houts R, et al. Quantification of biological aging in young adults. Proc Natl Acad Sci. 2015;112(30):E4104–E4110. doi: 10.1073/pnas.1506264112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang R, Ju Z, Zhou PK. A gut dysbiotic microbiota-based hypothesis of human-to-human transmission of non-communicable diseases. Sci Total Environ. 2020;745:141030. doi: 10.1016/j.scitotenv.2020.141030. [DOI] [PubMed] [Google Scholar]
- 26.Zhong H, Ren H, Lu Y, et al. Distinct gut metagenomics and metaproteomics signatures in prediabetics and treatment-naïve type 2 diabetics. EBioMedicine. 2019;47:373–383. doi: 10.1016/j.ebiom.2019.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harlap S, Davies AM, Deutsch L, et al. The Jerusalem perinatal study cohort, 1964?2005: methods and a review of the main results. Paediatr Perinat Epidemiol. 2007;21(3):256–273. doi: 10.1111/j.1365-3016.2007.00799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klemera P, Doubal S. A new approach to the concept and computation of biological age. Mech Ageing Dev. 2006;127(3):240–248. doi: 10.1016/j.mad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Kwon D, Belsky DW. A toolkit for quantification of biological age from blood chemistry and organ function test data: BioAge. Geroscience. 2021;43(6):2795–2808. doi: 10.1007/s11357-021-00480-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uzan-Yulzari A, Morr M, Tareef-Nabwani H, et al. The intestinal microbiome, weight, and metabolic changes in women treated by adjuvant chemotherapy for breast and gynecological malignancies. BMC Med. 2020;18(1):281. doi: 10.1186/s12916-020-01751-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bolyen E, Rideout JR, Dillon MR, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(8):852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim BR, Shin J, Guevarra R, et al. Deciphering diversity indices for a better understanding of microbial communities. J Microbiol Biotechnol. 2017;27(12):2089–2093. doi: 10.4014/jmb.1709.09027. [DOI] [PubMed] [Google Scholar]
- 33.Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R. UniFrac: an effective distance metric for microbial community comparison. ISME J. 2011;5(2):169–172. doi: 10.1038/ismej.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santiago A, Pozuelo M, Poca M, et al. Alteration of the serum microbiome composition in cirrhotic patients with ascites. Sci Rep. 2016;6(1):25001. doi: 10.1038/srep25001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breban M, Tap J, Leboime A, et al. Faecal microbiota study reveals specific dysbiosis in spondyloarthritis. Ann Rheum Dis. 2017;76(9):1614–1622. doi: 10.1136/annrheumdis-2016-211064. [DOI] [PubMed] [Google Scholar]
- 36.Jolivet-Gougeon MB-MA. Roseburia spp.: a marker of health. Future Microbiol. 2017;12(2):157–170. doi: 10.2217/fmb-2016-0130. [DOI] [PubMed] [Google Scholar]
- 37.Zheng Y, Fang Z, Xue Y, et al. Specific gut microbiome signature predicts the early-stage lung cancer. Gut Microbes. 2020;11(4):1030–1042. doi: 10.1080/19490976.2020.1737487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boursier J, Mueller O, Barret M, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63(3):764–775. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Da Silva HE, Teterina A, Comelli EM, et al. Nonalcoholic fatty liver disease is associated with dysbiosis independent of body mass index and insulin resistance. Sci Rep. 2018;8(1). https://www.nature.com/articles/s41598-018-19753-9. [DOI] [PMC free article] [PubMed]
- 40.Kovács T, Mikó E, Ujlaki G, et al. The involvement of oncobiosis and bacterial metabolite signaling in metastasis formation in breast cancer. Cancer Metastasis Rev. 2021;40(4):1223–1249. doi: 10.1007/s10555-021-10013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emoto T, Yamashita T, Kobayashi T, et al. Characterization of gut microbiota profiles in coronary artery disease patients using data mining analysis of terminal restriction fragment length polymorphism: gut microbiota could be a diagnostic marker of coronary artery disease. Heart Vessels. 2017;32(1):39–46. doi: 10.1007/s00380-016-0841-y. [DOI] [PubMed] [Google Scholar]
- 42.Liu Z, Li J, Liu H, et al. The intestinal microbiota associated with cardiac valve calcification differs from that of coronary artery disease. Atherosclerosis. 2019;284:121–128. doi: 10.1016/j.atherosclerosis.2018.11.038. [DOI] [PubMed] [Google Scholar]
- 43.Jie Z, Xia H, Zhong S-L, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8(1). https://www.nature.com/articles/s41467-017-00900-1. [DOI] [PMC free article] [PubMed]
- 44.Nakatsu G, Li X, Zhou H, et al. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat Commun. 2015;6(1):8727. doi: 10.1038/ncomms9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim S, Rigatto K, Gazzana MB, et al. Altered gut microbiome profile in patients with pulmonary arterial hypertension. Hypertension. 2020;75(4):1063–1071. doi: 10.1161/hypertensionaha.119.14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ettehad Marvasti F, Moshiri A, Taghavi MS, et al. The first report of differences in gut microbiota composition between obese and normal weight Iranian subjects. Iran Biomed J. 2020;24(3):148–54. doi: 10.29252/ibj.24.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu L, Baker SS, Gill C, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57(2):601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 48.Jiang W, Wu N, Wang X, et al. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep. 2015;5(1):8096. doi: 10.1038/srep08096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loke MF, Chua EG, Gan HM, et al. Metabolomics and 16S rRNA sequencing of human colorectal cancers and adjacent mucosa. Plos One. 2018;13(12):e0208584. doi: 10.1371/journal.pone.0208584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim S, Goel R, Kumar A, et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci. 2018;132(6):701–718. doi: 10.1042/cs20180087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raman M, Ahmed I, Gillevet PM, et al. Fecal Microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2013;11(7):868–875.e3. doi: 10.1016/j.cgh.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 52.Li Q, Chang Y, Zhang K, Chen H, Tao S, Zhang Z. Implication of the gut microbiome composition of type 2 diabetic patients from northern China. Sci Rep. 2020;10(1). https://www.nature.com/articles/s41598-020-62224-3. [DOI] [PMC free article] [PubMed]
- 53.Ortiz-Alvarez L, Xu H, Martinez-Tellez B. Influence of exercise on the human gut microbiota of healthy adults: a systematic review. Clin Transl Gastroenterol. 2020;11(2):e00126. doi: 10.14309/ctg.0000000000000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Larsen OFA, Claassen E. The mechanistic link between health and gut microbiota diversity. Sci Rep. 2018;8(1). https://www.nature.com/articles/s41598-018-20141-6. [DOI] [PMC free article] [PubMed]
- 55.Dogra SK, Dore J, Damak S. Gut microbiota resilience: definition, link to health and strategies for intervention. Front Microbiol. 2020;11:572921. doi: 10.3389/fmicb.2020.572921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aya V, Flórez A, Perez L, Ramírez JD. Association between physical activity and changes in intestinal microbiota composition: a systematic review. Plos One. 2021;16(2):e0247039. doi: 10.1371/journal.pone.0247039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rinninella E, Raoul P, Cintoni M, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7(1):14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hawley JA, Joyner MJ, Green DJ. Mimicking exercise: what matters most and where to next? J Physiol. 2021;599(3):791–802. doi: 10.1113/jp278761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Langsetmo L, Johnson A, Demmer RT, et al. The association between objectively measured physical activity and the gut microbiome among older community dwelling men. J Nutr Health Aging. 2019;23(6):538–546. doi: 10.1007/s12603-019-1194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma Z, Li L, Gotelli NJ. Diversity-disease relationships and shared species analyses for human microbiome-associated diseases. ISME J. 2019;13(8):1911–1919. doi: 10.1038/s41396-019-0395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dziewiecka H, Buttar HS, Kasperska A, et al. Physical activity induced alterations of gut microbiota in humans: a systematic review. BMC Sports Sci Med Rehabil. 2022;14(1). doi:10.1186/s13102-022-00513-2. [DOI] [PMC free article] [PubMed]
- 62.Divella R, De Palma G, Tufaro A, et al. Diet, probiotics and physical activity: the right allies for a healthy microbiota. Anticancer Res. 2021;41(6):2759–2772. doi: 10.21873/anticanres.15057. [DOI] [PubMed] [Google Scholar]
- 63.Lv WQ, Lin X, Shen H, et al. Human gut microbiome impacts skeletal muscle mass via gut microbial synthesis of the short-chain fatty acid butyrate among healthy menopausal women. J Cachexia Sarcopenia Muscle. 2021;12(6):1860–1870. doi: 10.1002/jcsm.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barton W, Penney NC, Cronin O, et al. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut. 2017:gutjnl-2016–313. https://gut.bmj.com/content/67/4/625.abstract?casa_token=uG5E_mzSPdsAAAAA:IpoRgMfcqa5L6qynNLApYQjQWbj166O_ICGZOi1lhIuDc6PZdZZtlDZRHkNWxVApc7eM0WVNz7U. [DOI] [PubMed]
- 65.Allam-Ndoul B, Castonguay-Paradis S, Veilleux A. Gut microbiota and intestinal trans-epithelial permeability. Int J Mol Sci. 2020;21(17):6402. doi: 10.3390/ijms21176402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park JH, Moon JH, Kim HJ, Kong MH, Oh YH. Sedentary lifestyle: overview of updated evidence of potential health risks. Korean J Fam Med. 2020;41(6):365–373. doi: 10.4082/kjfm.20.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tett A, Pasolli E, Masetti G, Ercolini D, Segata N. Prevotella diversity, niches and interactions with the human host. Nat Rev Microbiol. 2021;19(9):585–599. doi: 10.1038/s41579-021-00559-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.OECD, Organization WH. Step up! Tackling the burden of insufficient physical activity in Europe. 2023.
- 69.Bhargava A, Arnold AP, Bangasser DA, et al. Considering sex as a biological variable in basic and clinical studies: an Endocrine Society scientific statement. Endocr Rev. 2021;42(3):219–258. doi: 10.1210/endrev/bnaa034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Valeri F, Endres K. How biological sex of the host shapes its gut microbiota. Front Neuroendocrinol. 2021;61:100912. doi: 10.1016/j.yfrne.2021.100912. [DOI] [PubMed] [Google Scholar]
- 71.Shin JH, Park YH, Sim M, Kim SA, Joung H, Shin DM. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res Microbiol Jun-Aug. 2019;170(4–5):192–201. doi: 10.1016/j.resmic.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 72.Hall CV, Lord A, Betzel R, et al. Co-existence of network architectures supporting the human gut microbiome. iScience. 2019;22:380–391. doi: 10.1016/j.isci.2019.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.(CDC) CfDCaP. Data from: National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services.
- 74.Shapiro I, Belsky DW, Israel S, Youssim I, Friedlander Y, Hochner H. Familial aggregation of the aging process: biological age measured in young adult offspring as a predictor of parental mortality. GeroScience. 2023;45(2):901–913. doi: 10.1007/s11357-022-00687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caporaso JG, Lauber CL, Walters WA, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6(8):1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13(7):581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72(7):5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8(4):e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12). https://genomebiology.biomedcentral.com/articles/10.1186/s13059-014-0550-8?ref=https://githubhelp.com. [DOI] [PMC free article] [PubMed]
- 80.Wickham H. Programming with ggplot2. Springer International Publishing; 2016:241–253. https://link.springer.com/chapter/10.1007/978-3-319-24277-4_12.
- 81.Douglas GM, Maffei VJ, Zaneveld JR, et al. PICRUSt2 for prediction of metagenome functions. Nat Biotechnol. 2020;38(6):685–688. doi: 10.1038/s41587-020-0548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Caspi R, Billington R, Keseler IM, et al. The MetaCyc database of metabolic pathways and enzymes - a 2019 update. Nucleic Acids Res. 2019;48(D1):D445–D453. doi: 10.1093/nar/gkz862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The microbiota data used here is publicly available in the European Bioinformatics Institute (EBI) database, https://www.ebi.ac.uk/ena/browser/view/PRJEB63185.