Abstract
To determine the optimal inductive sites for immunization against Helicobacter pylori infection, the protective efficacy of recombinant urease (rUre) was assessed for mice given the vaccine by either the oral (p.o.), intranasal (i.n.), or rectal route. When mice were immunized with rUre (25 μg p.o. or rectally or 10 μg i.n.) plus heat-labile toxin from Escherichia coli as the mucosal adjuvant, all routes afforded protection against challenge with H. pylori, as indicated by a significant reduction in gastric urease activity (P < 0.0005) compared to that of sham-immunized controls. Quantitative H. pylori culture of stomach tissue demonstrated a >97% reduction in bacterial burden in mice immunized by all routes (P < 0.05). Induction of antiurease immunoglobulin A (IgA) levels in gastric luminal secretions after p.o. immunization was greater than after i.n. administration (means, 6.0 and 1.02 ng/ml, respectively) and was dependent upon challenge with H. pylori. However, immunization by the rectal route resulted in the generation of the highest levels of gastric antiurease IgA (mean, 40.89 ng/ml), which was detectable prior to challenge with H. pylori. Immunohistochemical staining of stomach tissue for cells secreting urease-specific antibody and CD4+ T cells showed levels of recruitment to be dependent upon challenge with H. pylori and equivalent for all routes. These results identify both the rectum and nasal passages as suitable inductive sites for urease immunization.
Infection with the gastroduodenal pathogen Helicobacter pylori is now established as the etiologic agent of chronic gastritis and most cases of peptic ulcer disease worldwide (23). Mounting evidence has also linked this chronic infection with gastric adenocarcinoma of the distal stomach and with gastric lymphoma (7, 26), which has resulted in the World Health Organization’s classification of this bacterium as a class I carcinogen (27). The risk of developing gastric cancer has been estimated to be three- to sixfold greater for H. pylori-infected individuals than for uninfected age-matched controls (18, 22), and this bacterium is considered to be the second leading cause of cancer deaths in many developing countries. Prevention of this disease by means of an effective vaccine against H. pylori is thus a high priority. An additional indication of the need for a vaccine strategy is peptic ulcer disease. Treatment of patients presenting with peptic ulcer disease consists of acid suppression and antimicrobial therapy. Although current treatment regimens are effective and the incidence of reinfection (16) is low in industrialized countries, reinfection and recrudescence in the developing world are significant problems (20). The emergence of antibiotic-resistant strains (14), poor patient compliance, and the high cost of therapy and patient management represent additional problems for management of H. pylori-infected individuals, especially in the developing world, where antimicrobial treatment has not been very effective.
Prevention of H. pylori infection by active immunization has been demonstrated with several animal models (2, 3, 5, 11, 13, 15, 17, 21). These studies have evaluated both whole-cell Helicobacter sonicates and recombinant subunit vaccines, such as those whose active component is urease apoenzyme, heat shock protein A (HspA), vacuolating cytotoxin A (VacA), or catalase. Significant protection against challenge with different species of Helicobacter has been demonstrated when these antigenic compositions were coadministered by a mucosal route with a suitable adjuvant such as cholera toxin (CT) or heat-labile toxin (LT) from Escherichia coli. In those studies, vaccine antigens were administered by the intragastric (3) or oral (13) route, both of which were determined to induce protection against challenge. Although its role was not clearly defined, those studies suggested that antigen-specific secretory immunoglobulin A (sIgA) helps protect against infection (3, 13, 21). A role for sIgA in protection against infection with H. pylori in humans has been suggested in a study of passive immunity (24). In that study, human breast milk IgA titers were shown to correlate with a delay in the onset of H. pylori infection in infants.
More recently, we have demonstrated that administration of antigen with LT adjuvant by the intranasal (i.n.) route generated protective immunity against infection with Helicobacter felis (25). However, this study demonstrated that animals immunized by the i.n. route, in the absence of a mucosal adjuvant, were not protected from challenge, even in the presence of elevated levels of sIgA in saliva and fecal extracts. This result suggested that sIgA was not a mediator of protection or that i.n. immunization did not elicit local antibody production in the gastric compartment. The existence of a common mucosal immune system and the induction of mucosal antibody at distant sites is well documented, but there is also evidence for preferential specific sIgA induction at the site of administration (9). Rectal immunization with CT as the candidate antigen was determined to elicit higher levels of specific sIgA in duodenal, colonic-rectal, and vaginal secretions than in saliva (9). The rectum as an inductive site for the generation of specific sIgA in gastric secretions has not yet been evaluated in a model of Helicobacter infection.
In all immunization studies of infection with Helicobacter, protection has primarily been defined by using either a qualitative or a quantitative assessment of gastric urease activity in intact stomach tissue or by histology. The recent development of a murine model of H. pylori infection (10, 12, 15) has allowed efficacy studies of other antigens conserved in H. pylori (11, 15) and more-sensitive determinations of the protective efficacies of these candidate antigens after direct quantitation of the number of colonizing bacteria in the stomach by culture.
In the present study we used recombinant urease (rUre) with LT as the vaccine to compare and contrast results of inoculation by the oral (p.o.), i.n., and rectal routes separately to determine a suitable means for inducing protective immunity against infection with H. pylori. In the absence of complete protection (sterilizing immunity) by single-site immunization against H. pylori challenge, we also evaluated a role for priming at one mucosal site followed by three booster doses at one of the other mucosal sites investigated as part of a prime-boost strategy. The level of protection was then compared to that obtained by single-site immunization by either the p.o., i.n., or rectal route. Protection was evaluated by quantitative determination of H. pylori bacterial densities in gastric mucosae and by a quantitative gastric urease assay. A comprehensive analysis of gastric secretion and serum antiurease responses was performed after immunization and following challenge with H. pylori. Induction of immune cells in gastric tissue was examined by quantitating IgA+ B cells, recombinant-urease-specific-antibody-containing cells (rUre-ACC), and CD4+ and CD8+ T cells.
MATERIALS AND METHODS
Bacterial growth conditions.
H. pylori X47-2AL (ORV2001), originally isolated from a domestic cat, was kindly provided by J. Fox, Massachusetts Institute of Technology, and was adapted to Swiss-Webster mice by sequential in vivo passaging (10). A natural streptomycin-resistant mutant of H. pylori X47-2AL was isolated and used for all challenge experiments. A frozen vial was plated on Mueller-Hinton agar containing 10% whole sheep blood, 5 μg of amphotericin (Sigma Chemical Co., St. Louis, Mo.) per ml, TVP (5 μg of trimethoprim per ml, 10 μg of vancomycin per ml, 10 U of polymixin B sulfate per ml; Sigma), and 50 μg of streptomycin per ml and grown for 48 h at 37°C in a 7% CO2 atmosphere. Cells were harvested into phosphate-buffered saline (PBS; pH 7.0), washed once, resuspended in PBS to an optical density at 600 nm (OD600) of 1.0, and used as a starter inoculum. Cells were diluted to a final OD600 of 0.05 in 100 ml of brucella broth (Difco Laboratories, Detroit, Mich.) containing 5% fetal bovine serum (JRH Biosciences, Lenexa, Kans.) with helicobacter-selective antibiotics and grown statically overnight at 37°C in a 7% CO2 atmosphere. Bacteria were viewed by phase-contrast microscopy for viability and motility as wet mounts and then harvested by centrifugation at 3,000 rpm (Beckman centrifuge) for 5 min. Cells were resuspended in brucella broth to an OD600 of 1.0 (approximately 108 CFU/ml).
Immunizations, H. pylori challenge, and sampling procedures.
Groups of 5 to 10 outbred specific-pathogen-free, 6- to 8-week-old Swiss-Webster mice (Taconic Farms, Germantown, N.Y.) were immunized once each day on days 0, 7, 14, and 21, with H. pylori rUre (25) (25 μg p.o. or rectally or 10 μg i.n.) plus LT holotoxin (5 μg p.o. or i.n. or 25 μg rectally) suspended in PBS. H. pylori rUre was purified from E. coli (ORV214) fermentor-grown cultures to >99% purity as an assembled (hexameric) inactive enzyme and stored in 2% sucrose as a lyophilized powder until required (13). Ten micrograms of rUre antigen was administered by the i.n. route per dose, previously determined to elicit protection equivalent to that observed for administration of 25 μg given by the p.o. route. While previous dose-response studies had demonstrated that the optimal and most reproducible LT dose required to induce a protective immune response by the p.o. and i.n. routes was 5 μg, the dose of LT required to elicit protective immunity by the rectal route was not determined. A fivefold increase in LT dose over that routinely administered by the other routes was therefore investigated. The inocula were administered either p.o. (25 μg given in a 50-μl volume), i.n. (10 μg given in a 10-μl volume, 5 μl per nostril), or rectally (25 μg given in a 25-μl volume) without anesthesia. Animals immunized by a prime-boost strategy were primed by the p.o., i.n., or rectal route and then boosted three times by one of the other routes under investigation. Animals received concentrations of antigen in these prime-boost regimens identical to those administered by the single route. Control mice received LT alone by either the p.o., i.n., or rectal route according to the same schedule. Two weeks after immunization, animals were either euthanized to determine postimmunization local and serum antibody responses or challenged once intragastrically in the absence of sodium bicarbonate with 100 μl of a 108-CFU/ml suspension of H. pylori X47-2AL. Back titrations were performed to assess the viability of the culture at the end of the challenge procedure. Two weeks after challenge, mice were euthanized and infection with H. pylori was determined in a quantitative fashion.
Antibody responses were measured in sera, saliva, and gastric secretions pre- and postchallenge with H. pylori. Blood was collected from the retro-orbital sinus under isoflurane inhalation anesthesia. Saliva was collected with a micropipette after intraperitoneal injection of 100 μg of pilocarpine. Gastric secretions were collected by wrapping the mucosal side of an intact stomach around two cellulose wicks for 3 min (9). Wicks were frozen at −80°C until tested. Gastric antibody was eluted from the wicks into a protease inhibitor cocktail containing 5% nonfat dry milk, 0.2 μM aminoethyl-benzene sulfonyl fluoride (Calbiochem, La Jolla, Calif.), 1 μg of aprotinin (Sigma) per ml, and 10 μM leupeptin (Sigma). Samples were vortexed vigorously, and insoluble material was removed by centrifugation.
Determination of H. pylori infection in gastric tissue specimens.
Two longitudinal strips from the antral region of each stomach were taken for a quantitative analysis of H. pylori infection. One tissue strip was placed in 0.5 ml of urea broth containing phenol red indicator, and gastric urease activity was measured spectrophotometrically (OD550) after 4 h of incubation at room temperature. H. pylori infection was quantitated on the basis of the extent of urea hydrolysis. Animals were considered protected from infection when the absorbance at 550 nm (A550) was within 2 standard deviations of the mean value determined for uninfected control mice.
The other antral section was placed in 1 ml of brucella broth and homogenized in a 7-ml dounce homogenizer until the tissue was completely disrupted. Tenfold serial dilutions were then prepared and plated onto helicobacter-selective agar, as described above. Bacterial counts were determined after 3 to 5 days of growth in a 7% CO2 incubator.
Quantitative assessment of mucosal and systemic immune responses to rUre.
Sera, saliva, and gastric secretions were harvested from animals on the day of termination and were quantitated for urease-specific IgG1, IgG2a, and IgA by standard enzyme-linked immunosorbent assay. Specific antibody was measured from a standard curve with monoclonal IgA (3) (monoclonal antibody 70), IgG1, and IgG2a (710C3 and 701C2; kindly provided by Pasteur Merieux Connaught, Lyons, France) as outlined below, and specific antibody measurements were expressed in micrograms or nanograms per milliliter. Sera were assayed at dilutions of 1:100 through 1:200,000. Gastric secretions were assayed for specific IgA at a dilution of 1:5 and were assayed for IgG at dilutions of 1:10 through 1:100. Saliva samples were assayed at dilutions of 1:5 through 1:50.
Enzyme-linked immunosorbent assay was carried out with flat-bottom 96-well microtiter plates (Corning Glass, Corning, N.Y.) coated with 5 μg (0.5 μg/well) of purified native H. pylori urease per ml in 0.1 M carbonate buffer (pH 9.6) and incubated overnight at 4°C. After three washes with PBS-Tween 20 (0.05% Tween 20), plates were blocked with PBS-Tween 20 containing 2.5% nonfat dry milk. Blocking buffer was used as the diluent for all test and control reagents in all subsequent steps. All incubations steps were carried out at 37°C for 1 h. After being blocked, duplicate 100-μl aliquots of each test sample were added and the plates were reincubated. After being washed, the plates were incubated with either goat anti-mouse IgG or goat anti-mouse IgA conjugated to alkaline phosphatase (Southern Biotechnology Associates, Birmingham, Ala.) diluted 1:1,000 and reincubated. After being washed, the plates were developed with p-nitrophenyl phosphate (Sigma) substrate tablets dissolved to a final concentration of 1 mg/ml in 1 M diethanolamine buffer, pH 9.6, containing 5 mM MgCl2. The plates were incubated for 20 min at room temperature, and the A405 was read with a Vmax microplate reader (Molecular Devices, Menlo Park, Calif.). Absorbance values were converted to specific-antibody measurements with the standard curves outlined with SOFTmax 881 software (Molecular Devices).
Immunohistochemical analysis of gastric tissue.
Longitudinal sections of gastric tissue including the corpus and antrum were mounted in O.C.T. compound (Miles Scientific, Naperville, Ill.) and frozen in liquid-nitrogen-cooled Freon 22 (8).
Analysis of IgA-containing cells.
Frozen sections (thickness, 7 μm) were placed on precoated slides, air dried, and fixed in acetone. Sections were blocked with blocking reagent containing avidin-biotin (Vector Laboratories, Burlingame, Calif.) and incubated sequentially with biotinylated monoclonal antibody against IgA (Pharmingen, San Diego, Calif.) or biotinylated rabbit anti-IgA (Southern Biotechnology Associates) and then with avidin conjugated to biotinylated glucose oxidase (ABC-GO; Vector Laboratories). Labeled sites were visualized with TNBT tetrazolium salt (glucose oxidase kit II; Vector Laboratories) and counterstained with methyl green. Control sections were incubated without primary antibodies.
Analysis of rUre-ACC.
Sections were blocked with blocking reagents containing avidin-biotin and sequentially incubated with rUre (10 μg/ml), rabbit anti-rUre, biotinylated donkey anti-rabbit Ig (Amersham Corp., Arlington Heights, Ill.), ABC-GO, TNBT tetrazolium salt, and methyl green. Control sections were incubated without rUre, or without rUre and rabbit-anti-rUre.
Analysis of T-cell phenotype.
Cryosections were blocked with blocking reagents containing avidin-biotin (Vector Laboratories) and then incubated with rat monoclonal antibody against mouse CD4 (clone RM4-5) or CD8 (clone 53-6.7) (Pharmingen). Sections were then sequentially incubated with biotinylated rabbit anti-rat IgG (Vector Laboratories), horseradish peroxidase conjugated to an avidin-biotin complex (Vector Laboratories), diaminobenzidine, and methyl green.
Morphometric evaluation.
IgA+ B cells, rUre-ACC, CD4+ cells, and CD8+ cells were counted in cross sections of antral mucosae and expressed per square-millimeter field of gastric mucosa. All counts were made at the antrum-corpus junction in areas shown to be colonized by H. pylori in murine stomachs (12).
RESULTS
Protection against infection with H. pylori.
Mice immunized p.o., i.n., and rectally with rUre plus LT were significantly protected against challenge with H. pylori, as determined by a urea broth assay (P < 0.0005), in comparison to sham-immunized controls (Fig. 1). A statistically significant reduction in the bacterial burden was also observed by all three routes (P < 0.05), as assessed by quantitative H. pylori culture of gastric tissue (Fig. 2). No statistically significant differences in results were observed between groups immunized by the three different routes. The gastric bacterial density in each immunized mouse was reduced by >97%, compared to those of controls. These results confirm the suitability of all three inductive sites for the generation of protective immunity against H. pylori infection.
FIG. 1.
Urease activities of gastric tissues from mice immunized with rUre plus LT by either the p.o., i.n., or rectal (R) route. Mice were immunized with 25 μg of rUre by the p.o. and rectal routes and with 10 μg by the i.n. route. Animals were challenged with H. pylori 2 weeks postimmunization and euthanized 2 weeks postchallenge. Infection was determined by incubating one-fourth of the antrum from each animal in urea broth and measuring the gastric urease activity spectrophotometrically at an A550 after a 4-h incubation at room temperature. Mice were considered protected from infection if the urease activity was within 2 standard deviations of the mean value for unchallenged control mice. Mice were significantly protected by all routes compared to LT sham-immunized controls (P < 0.0005; Wilcoxon rank sum test). As no significant differences between results for control groups were apparent, all results for LT sham-immunized animals were pooled for this analysis. Solid lines represent the arithmetic mean for each group studied.
FIG. 2.
Quantitative H. pylori culture of gastric tissue, represented on a logarithmic scale, from mice immunized with rUre plus LT by either the p.o., i.n., or rectal (R) route. Gastric tissue from one-fourth of the antrum of each mouse was homogenized in a dounce homogenizer (7 ml), and serial dilutions from each homogenate were plated onto helicobacter-selective agar. Colony counts are presented as CFU per milliliter of the dilutions of the one-quarter antral tissue section of each animal. Only two animals from rUre-immunized groups were determined to have zero bacteria (indicated as 0.1 on a log10 scale). A significant reduction in bacterial burden (2 logs) was observed for all routes when burdens were compared to that of the pooled LT controls (P < 0.05; Wilcoxon rank sum test). Solid lines represent the geometric mean for each group studied.
In the same study, groups of mice were primed by either the p.o., i.n., or rectal route and boosted three times, according to the same weekly schedule, by either the p.o., i.n., or rectal route. Each combination regimen afforded protection, as was demonstrated by a significant reduction in the number of colonizing bacteria, but there was no increase in the level of protection when compared to that produced by single-site immunization (Table 1).
TABLE 1.
Protection against H. pylori challenge by a combination prime-boost strategy for mucosal immunization
| First, second route of immunizationa | Mean from quantitative H. pylori cultureb (CFU/ml) | P valuec |
|---|---|---|
| p.o., p.o. | 377 | 0.0017 |
| p.o., i.n. | 858 | 0.0065 |
| p.o., r. | 1,590 | 0.0049 |
| i.n., i.n. | 79 | 0.0003 |
| i.n., p.o. | 1,293 | 0.0017 |
| i.n., r. | 650 | 0.0007 |
| r., r. | 394 | 0.0014 |
| r., p.o. | 428 | 0.0027 |
| r., i.n. | 233 | 0.0037 |
| All | 14,217 |
Weekly immunizations were given on days 0, 7, 14, and 21 by either the p.o. (50 μl of soluble antigen delivered by pipette into the buccal cavity), the i.n. (5 μl of soluble antigen delivered to each external naris of unanesthetized mice), or the rectal (r.) (25 μl of soluble antigen delivered with an olive-tipped catheter directly into the rectum) route. The number of booster doses given by the second route specified was 3 (does not apply to sham-immunized controls). Sham-immunized control animals (n = 15) received LT alone by either the p.o., i.n., or rectal route (n = 5/route), and results were pooled for this analysis (“All”).
Quantitative H. pylori culture was performed on homogenized stomach tissue (one-fourth of the antral tissue specimen) harvested at the time of termination (2 weeks postchallenge). Results are expressed as geometric means of CFU per milliliter per biopsy section for each of 10 mice immunized with rUre plus LT and each of 15 control mice sham-immunized with LT.
By the Wilcoxon rank sum test. Values are relative to those for the LT sham-immunized control group.
Serum antibody responses to urease by route of immunization.
Primary immunization with rUre and LT by the p.o., i.n., or rectal route elicited elevated levels in sera of both IgG and IgA against native H. pylori urease (Table 2). Significantly higher levels of IgG1 and IgG2a antibodies were found in the sera of all immunized groups than in the sera of sham-immunized controls. Differences were observed in the IgG1/IgG2a ratios that were specific to the site of immunization and dependent upon challenge with H. pylori. Oral immunization resulted in a greater IgG2a response, as was indicated by an IgG1/IgG2a ratio of 0.16, and levels of IgG2a remained unaltered after challenge, indicative of a type 1 helper T-cell (Th1) response. Rectal immunization with the same formulation and antigen dose resulted in a markedly different serum IgG response than that induced by the p.o. route. IgG1 predominated, with an IgG1/IgG2a ratio of 2.5. Challenge again shifted the response towards Th1, resulting in a more balanced Th1/Th2 response (IgG1/IgG2a ratio of 0.88) after infection with H. pylori. Immunization by the i.n. route resulted in elevated levels of IgG2a (indicative of a Th1 response; P = 0.04 by the Wilcoxon rank sum test), with an IgG1/IgG2a ratio of 0.29. Challenge with H. pylori further increased the IgG2a response (IgG1/IgG2a ratio of 0.08; P = 0.04 by the Wilcoxon rank sum test, comparing ratios pre- and postchallenge). A shift towards a Th1 response after challenge with H. pylori was observed for all animals within each immunization group.
TABLE 2.
Serum antibody responses induced by rUre immunization
| Routea | Vaccineb | Challengec | Mean leveld (μg/ml) in serum of:
|
IgG1/IgG2a ratio | Mean level of IgAd (μg/ml) in serum | |
|---|---|---|---|---|---|---|
| IgG1 | IgG2a | |||||
| p.o. | rUre + LT | − | 30.57 | 183.70 | 0.16 | 1.86 |
| + | 20.72 | 83.51 | 0.25 | 1.69 | ||
| r. | rUre + LT | − | 220.60 | 88.04 | 2.51 | 18.34 |
| + | 121.92 | 139.09 | 0.88 | 38.48 | ||
| i.n. | rUre + LT | − | 120.40 | 416.50e | 0.29 | 0.64 |
| + | 85.06 | 1,017.87e | 0.08f | 3.17 | ||
| All | LT | + | 0.42 | 0.92 | 0.45 | 0.13 |
Weekly immunizations were given on days 0, 7, 14, and 21 by either the p.o. (50 μl of soluble antigen delivered by pipette into the buccal cavity), the i.n. (5 μl of soluble antigen delivered to each external naris of unanesthetized mice), or the rectal (r.) (25 μl of soluble antigen delivered with an olive-tipped catheter) route. Sham-immunized control animals (n = 15) received LT alone by either the p.o., i.n., or rectal route, and results were pooled for this analysis (“All”).
By the p.o. or rectal route, animals were administered 25-μg doses of rUre plus LT (5 μg p.o. and 25 μg rectally), and by the i.n. route, animals were administered 10-μg doses of rUre plus LT (5 μg) (n = 10/group).
Animals were challenged once with 100 μl of a 108-CFU/ml suspension of streptomycin-resistant H. pylori X47-2AL.
Serum antibody measurements are presented as geometric means.
Statistical significance (P < 0.05) was determined by the Wilcoxon rank sum test by comparing levels of IgG1 to those of IgG2a.
Statistical significance (P < 0.05) was determined by the Wilcoxon rank sum test by comparing results for challenged animals to those for similarly treated unchallenged animals.
Serum IgA responses to rUre were lower than those observed for IgG and were highest after rectal immunization. IgA levels increased only slightly after challenge with H. pylori (Table 2). Infection with H. pylori in control (sham-immunized) mice given LT induced only much lower levels of both IgG and IgA in sera compared to levels in animals receiving vaccine. Serum responses to infection alone were indicative of a Th1 cell response.
Urease-specific gastric and salivary antibody responses by route of immunization.
Mucosal immunization with rUre plus LT induced gastric IgA responses against the vaccine that appeared to be dependent upon both the route of administration and challenge with H. pylori (Table 3). p.o. immunization resulted in production of urease-specific gastric IgA, which was detectable only after challenge with H. pylori. Primary rectal immunization resulted in an elevated urease-specific gastric IgA response prior to challenge, and the response was boosted further after challenge. Levels of gastric IgA after rectal immunization were significantly higher than those observed in p.o. immunized, challenged animals (P = 0.014; Wilcoxon rank sum test) but might also reflect the fivefold increase in LT concentration given by this route. i.n. immunization gave rise to the lowest level of urease-specific gastric IgA, which was not significantly boosted after challenge with H. pylori.
TABLE 3.
Mucosal antibody responses induced by rUre immunization
| Routea | Vaccineb | Challengec | Mean level of IgAd (ng/ml) in:
|
|
|---|---|---|---|---|
| Gastric secretions | Saliva | |||
| p.o. | rUre + LT | − | 0.31 | 798.56 |
| + | 6.00e | 832.12 | ||
| r. | rUre + LT | − | 40.89e | 82.16 |
| + | 72.40e | 176.65 | ||
| i.n. | rUre + LT | − | 0.25 | 439.23 |
| + | 1.02e | 1,258.72f | ||
| All | LT | + | 0.12 | 0.10 |
Weekly immunizations were given on days 0, 7, 14, and 21 by either the p.o. (50 μl of soluble antigen delivered by pipette into the buccal cavity), the i.n. (5 μl of soluble antigen delivered to each external naris of unanesthetized mice), or the rectal (r.) (25 μl of soluble antigen delivered with an olive-tipped catheter directly into the rectum) route. Sham-immunized control animals (n = 15) received LT alone by either the p.o., i.n., or rectal route, and results were pooled for this analysis (“All”).
By the p.o. or rectal route, animals were administered 25-μg doses of rUre plus LT (5 μg p.o. and 25 μg rectally), and by the i.n. route, animals were administered 10-μg doses of rUre plus LT (5 μg) (n = 10/group).
Animals were challenged once with 100 μl of a 108-CFU/ml suspension of streptomycin-resistant H. pylori X472AL.
Gastric and salivary IgA antibody responses are presented as geometric means.
Statistical significance (P < 0.005) was determined by the Wilcoxon rank sum test, with results for immunized animals being compared to those for sham-immunized controls.
Statistical significance (P < 0.05) was determined by the Wilcoxon rank sum test, with results for challenged animals being compared to those for similarly treated unchallenged animals.
Urease-specific salivary IgA was induced by all routes, with levels after p.o. and i.n. immunization exceeding those after rectal immunization (Table 3). After challenge with H. pylori, antiurease salivary IgA was significantly boosted only in those animals immunized by the i.n. route (P = 0.007; Wilcoxon rank sum test). The presence of high levels of salivary IgA but low levels of gastric IgA in those animals immunized by the i.n. route indicated that gastric IgA measured after p.o. or rectal immunization was unlikely to be due to contaminating salivary IgA. This conclusion is in agreement with previous findings, where the level of salivary IgA directed against urease did not correlate with protection (19). The lower salivary IgA response in rectally immunized animals also suggests that IgA detected in gastric wicks is unlikely to come from saliva. Neither gastric nor salivary IgA against native urease was detected in sham-immunized, infected animals.
IgG against H. pylori urease was also detected in the gastric compartments of animals immunized by all routes, although at levels 1,000-fold lower than those found in sera (results not shown). Analysis of the IgG subclass response identified an IgG1/IgG2a ratio in stomachs similar to that observed in sera, with similar boosting and subclass switching after challenge with H. pylori (Table 4). This result suggested that the presence of IgG in gastric secretions may have been the result of transudation of serum into the gastric compartment. If serum IgG transudate is present in gastric secretions, then serum-derived (monomeric) IgA may also contribute to IgA found in gastric secretions. However, immunization by the p.o. route resulted in serum IgA levels that were unaffected by challenge, whereas gastric IgA levels were significantly increased. The minimal increase observed upon challenge in salivary IgA is also unlikely to account for the increase in IgA detected in gastric secretions. In addition, challenge of animals immunized by the rectal route showed a boost in serum IgA that was not reflected in gastric secretions. Thus, while serum cannot account entirely for the IgA response measured in gastric secretions, gastric IgA may be considered to result from both local production and transudation of serum IgA. Immunohistochemical evidence for local IgA production (data presented below) supports this hypothesis.
TABLE 4.
Urease-specific serum and gastric IgG subclass responses
| Route of immunizationa | Antiurease IgG1/IgG2a antibody ratio in indicated fluid
|
|||
|---|---|---|---|---|
| Prechallenge with H. pylori
|
Postchallenge with H. pylori
|
|||
| Serum | Gastric juice | Serum | Gastric juice | |
| p.o. | 0.16 | 0.35 | 0.25 | 0.19 |
| r. | 2.51 | 1.65 | 0.88 | 0.49 |
| i.n. | 0.29 | 0.18 | 0.08 | 0.09 |
Weekly immunizations with rUre plus LT were given on days 0, 7, 14, and 21 by either the p.o. (50 μl of soluble antigen delivered by pipette into the buccal cavity), the i.n. (5 μl of soluble antigen delivered to each external naris of unanesthetized mice), or the rectal (r.) (25 μl of soluble antigen delivered with an olive-tipped catheter directly into the rectum) route.
Recruitment of immune cells to the gastric mucosa.
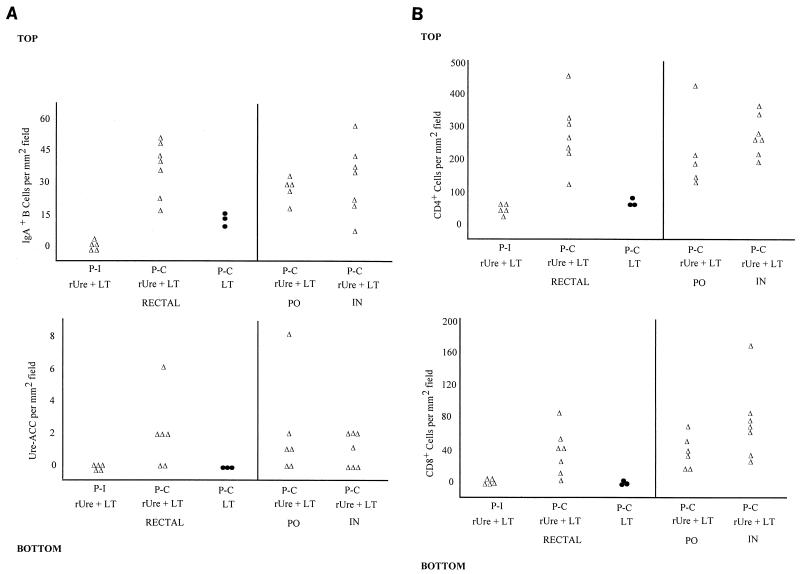
Gastric tissues from both rUre-LT-immunized and LT sham-immunized mice identified IgA+ B cells and rUre-ACC to be dependent upon immunization and challenge. IgA+ B cells were found at their highest frequency at the junction between the antrum and corpus, whereas rUre-ACC were found only in this region. Prior to challenge with H. pylori, there were few IgA+ cells and no rUre-ACC in the gastric mucosae of mice immunized with rUre plus LT by all routes of immunization (Fig. 3A). This result was in agreement with the low levels of urease-specific gastric IgA identified in wick-harvested secretions from animals immunized by the p.o. and i.n. routes, but it did not reflect the results for urease-specific gastric IgA in secretions from animals immunized by the rectal route (Table 3). After challenge with H. pylori, mice immunized by all routes with rUre plus LT showed an elevated B-cell response in gastric tissue (Fig. 3A). Of the IgA+ B cells recruited, between 5 and 10% could be attributed to rUre-ACC. This finding is in contrast to results with infected animals immunized with LT alone, where a reduced B-cell response and no urease-specific response was observed after challenge (Fig. 3A, bottom; only results for the rectal LT control group are shown).
FIG. 3.
Gastric immune responses induced to rUre immunization by route of immunization. (A and B) Recruitment of IgA+ B cells (top) and rUre-specific ACC (bottom) and of CD4+ (top) and CD8+ (bottom) T cells, respectivley, into the gastric mucosae of mice immunized with rUre plus LT is dependent upon challenge with H. pylori. Results for LT controls are shown for rectally immunized animals only. P-I, postimmunization (only results for the rectally immunized group are shown); P-C, postchallenge. Results for sham-immunized control mice are represented with closed circles.
An evaluation of T-cell subsets identified CD4+ and CD8+ cells in the gastric mucosae (Fig. 3B). Levels of T cells induced were comparable by each route of immunization and were clearly dependent upon challenge with H. pylori. CD4+ cells outnumbered both IgA+ B cells and CD8+ cells by 5- to 10-fold and were found throughout the antrum and corpus regions of the stomach. In contrast, CD8+ cells were predominantly found in regions adjacent to the junction of the antrum and corpus. The strongest CD4+ cell responses were observed after immunization by the rectal and i.n. routes, and the strongest CD8+ cell responses were observed after immunization by the i.n. route, although there were no statistically significant differences between groups. In the gastric mucosae of sham-immunized, infected animals, CD4+ cells were greatly reduced in number relative to levels in rUre-immunized, protected animals, and CD8+ cells appeared to be absent.
DISCUSSION
We have developed a murine model of H. pylori infection (10) that provides quantitative determinations of the levels of protection afforded by vaccine formulations and that permits us to evaluate novel H. pylori antigens and delivery systems. In this study, mucosal immunization by the p.o., i.n., and rectal routes with rUre plus LT elicited significant protection against colonization with H. pylori. In previous studies, the gastric urease assay has suggested complete protection against infection in the heterologous H. felis challenge model. We showed that the urease assay is relatively insensitive in detecting H. pylori in gastric tissue below approximately 103 CFU and thus does not indicate complete protection (sterilizing immunity). In the present study, a more sensitive quantitative culture approach was used to determine H. pylori colonization of gastric tissue. Immunization by all three mucosal routes gave significant protection, with >97% reduction in bacterial burden relative to that of unimmunized controls. Although immunization by the i.n. route appeared to result in the lowest level of colonization, we observed no significant differences between the levels of protection of the routes investigated. Complex immunization regimens wherein mice were immunized by one mucosal route and boosted (three times) by another route afforded no greater protection than immunization by a single mucosal route. No significant differences between single-site and prime-boost immunization strategies were observed. Complete protection, demonstrated by the absence of bacteria by culture, was achieved for only 10 to 20% of animals immunized mucosally with rUre plus LT adjuvant, all remaining animals showing significant decreases in their bacterial densities. Whether sterilizing immunity is indeed achievable through mucosal immunization is currently being addressed by evaluating synergy of novel protective antigens with rUre.
The residual infection (incomplete protection) in our model may have been due to one of the following: (i) the high challenge inoculum (1,000 90% infective doses) may have resulted in a breakthrough of the level of immunity achieved by vaccination; (ii) urease shed from the bacterial surface may have acted as a decoy for antibody; (iii) residual H. pylori organisms may have occupied an immunologically privileged site that is inaccessible to antibody or the effector function of T cells; (iv) there may have been a need for alternate antigens and/or adjuvants to stimulate a more effective response; or (v) there may have been reduced levels of urease on the surface of H. pylori cells as the bacterial density decreased. Regardless of the reason for residual infection, the increased sensitivity and reproducibility of the results of the H. pylori model and quantitative culture assay provide a means for investigating alternate methods for improving efficacy.
An evaluation of the immune responses induced by rUre immunization at different inductive sites revealed both qualitative and quantitative differences that appeared to shift upon challenge with H. pylori. Immunization by all routes elicited elevated levels of antiurease IgA and IgG in serum compared to levels in infected, unimmunized controls. Analysis of the serum IgG subclass responses revealed that each route of immunization induced a characteristic IgG1/IgG2a ratio. p.o. and i.n. immunization resulted predominantly in an IgG2a response, whereas rectal immunization resulted in a stronger IgG1 response. Regardless of the orientation of the primary immune response to p.o., i.n., or rectal immunization, challenge with H. pylori shifted the immune response to one that favors IgG2a (Th1), particularly with p.o. and i.n. immunization. This was consistent with the predominance of Th1 responses in sera from unimmunized animals infected with H. pylori. IgG subclass responses in the gastric compartments identified similar IgG1/IgG2a ratios prior to challenge with H. pylori and corresponding shifts in the direction of an IgG2a response after challenge. These findings suggest that the IgG detected in gastric secretions may result from serum, presumably through transudation into the gastric compartment. In a previous study, we had demonstrated elevated levels of both IgG1 and IgG2a in the sera of animals immunized with rUre, in the absence of adjuvant, by the i.n. route, with no detectable protection observed against H. felis challenge, which indicates that IgG in serum is unlikely to play a role in mediating protection (25). In contrast, Ferrero et al. have presented evidence for local generation of IgG antibodies and have suggested that IgG may play a role in protection (6). These discrepant results may be due to differences in mouse strains, immunization regimens, or analytical methods used to determine local immune responses.
Both p.o. and rectal immunization with rUre induced IgA antibody in gastric secretions. These findings are consistent with other reports that have identified IgA as a marker of protection against Helicobacter infection (3, 13, 21). In the present study, a clear difference between routes of administration was the induction of IgA in the gastric compartment prior to challenge after rectal but not p.o. immunization (Table 3). Even though the LT dose administered rectally was fivefold that administered p.o., the magnitude of the sIgA responses in the gastric compartment appears unlikely to be a result of the increase in LT concentration alone. Similar findings were reported from a study where elevated levels of IgA against CT were detected in colonic-rectal secretions harvested from mice immunized by the rectal route (9). It has previously been suggested that p.o. immunization primes the mucosal compartment and that upon challenge with H. felis, a boosting of sIgA in the gastric compartment, as measured in fecal extracts, occurs, possibly as a result of reexposure to urease presented by the challenge inoculum (21). In our study, challenge with H. pylori resulted in a modest increase in gastric IgA in animals immunized by both the p.o. and rectal routes. The lack of complete protection (sterilizing immunity) even in those animals with preexisting sIgA in the gastric compartment was an unexpected finding and may indicate that sIgA is not the sole effector molecule. We have recently completed a study demonstrating protective immunity against novel H. pylori challenge strains and demonstrated that complete protection (sterilizing immunity) is achievable through rectal immunization with rUre, but only against strains that infect at significantly lower levels (unpublished findings). In every case a 2- to 3-log reduction in bacterial density was observed regardless of the challenge strain, indicating that sterilizing immunity may be dependent upon host-pathogen interaction.
Evaluation of IgA+ B cells and urease-specific ACC in situ revealed that these cells were absent in the antral mucosae prior to challenge with H. pylori. These findings reflected the low specific-antibody determinations for mice immunized by the i.n. and p.o. routes but did not reflect data for the rectal route. After challenge, there was significant recruitment of IgA+ B cells and urease-specific ACC into the lamina propria of the stomach. The magnitudes of the B-cell responses when we compared the results of immunization by different routes appeared similar, although the actual levels of secretion of urease-specific IgA appeared to differ among the different routes investigated. It is not clear why these two measurements are not in agreement. However, the difference may be related to the limited surface area of the stomach sampled by immunohistochemistry, differences in tissue and surface antibody levels, or differences in levels of antibody secretion or in transepithelial transport rates following immunization by each route.
The immunological specificity and function of the T cells found in the gastric mucosae of vaccinated mice have not been well defined. However, the T cells induced in the gastric mucosae of urease-immunized mice in response to H. felis challenge expressed mucosal integrins (4), suggesting that their presence is a result of mucosal immunization.
A correlate of protection in mice immunized with rUre was the appearance of large numbers of CD4+ cells and low numbers of CD8+ cells in the gastric mucosae after H. pylori challenge. In a previous study with H. felis, primarily CD4+ cells were induced in the gastric mucosae, with significant levels of CD8+ cells observed only several weeks after challenge, indicating distinctly different kinetics for T-cell induction between H. pylori and H. felis (4). Current studies are focusing on (i) determining the antigen specificity of the T-cell population apparent after challenge with H. pylori and (ii) using reverse transcription-PCR as a means to monitor regulation of both Th1- and Th2-oriented cytokines and T-cell recruitment as a function of challenge.
In conclusion, we have shown that the rectal and i.n. routes, in addition to the p.o. route, are suitable mucosal routes for inducing protective immunity against infection with H. pylori. Each route appeared unique in the generation of both urease-specific serum and mucosal antibody, with no significant difference observed in the levels of protection afforded by the different routes, either by urease activity determinations or quantitative H. pylori culture. The immunological basis for protection observed in mice after active immunization with rUre remains uncertain, although our results suggest a role for sIgA and CD4+ T cells as a result of immunization by any of the routes examined.
Although sterilizing immunity was not achieved for all animals, a previous study of immunological longevity as a result of mucosal immunization demonstrated significant protection with rUre up to 1 year after immunization (19). The association of clinical symptoms with bacterial density (1) and the greatly reduced levels of infection observed in this study as a direct result of mucosal immunization may therefore be sufficient to significantly alter the kinetics of induction of gastroduodenal pathologies associated with this chronic infection.
ACKNOWLEDGMENTS
We thank Jacques Pappo for his valuable advice in the determination of specific-antibody measurements for this study.
This work was funded in part from an SBIR grant (R44-AI34679-02A1) from the National Institute of Allergy and Infectious Disease and by Pasteur-Merieux Connaught.
REFERENCES
- 1.Atherton J C, Tham K T, Peek R M, Jr, Cover T L, Blaser M J. Density of Helicobacter pylori infection as assessed by quantitative culture and histology. J Infect Dis. 1996;174:552–556. doi: 10.1093/infdis/174.3.552. [DOI] [PubMed] [Google Scholar]
- 2.Chen M, Lee A, Hazell S, Hu P, Li Y. Immunization against infection with Helicobacter species: first step in the prophylaxis of gastric cancer? Zentbl Bakteriol. 1993;280:155–165. doi: 10.1016/s0934-8840(11)80952-7. [DOI] [PubMed] [Google Scholar]
- 3.Czinn S J, Cai A, Nedrud J G. Protection of germ-free mice from infection by Helicobacter felis after oral or passive IgA immunization. Vaccine. 1993;11:637–642. doi: 10.1016/0264-410x(93)90309-l. [DOI] [PubMed] [Google Scholar]
- 4.Ermak T H, Ding R, Ekstein B, Myers G, Lee C K, Pappo J, Kleanthous H, Monath T P. Gastritis in urease-immunized mice after Helicobacter felis challenge may be due to residual bacteria. Gastroenterology. 1997;113:1118–1128. doi: 10.1053/gast.1997.v113.pm9322506. [DOI] [PubMed] [Google Scholar]
- 5.Ferrero R L, Thilberge J M, Kansau I, Wuchser N, Huerra M, Labigne A. The GroES homologue of Helicobacter pylori confers protective immunity against mucosal infection in mice. Proc Natl Acad Sci USA. 1995;92:6499–6503. doi: 10.1073/pnas.92.14.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrero, R. L., J. M. Thilberge, and A. Labigne. 1996. Local immunoglobulin G antibodies, and not IgA, contribute to protective immunity against gastric H. felis infection in mice. Gut 39(Suppl. 2):A44.
- 7.Forman D, Webb P. Geographic distribution and association with gastric cancer. In: Northfield T C, et al., editors. Helicobacter pylori infection. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1993. pp. 11–20. [Google Scholar]
- 8.Fox J G, Blanco M, Murphy J C, Taylor N S, Lee A, Kabok Z, Pappo J. Local and systemic immune responses in murine Helicobacter felis active chronic gastritis. Infect Immun. 1993;61:2309–2315. doi: 10.1128/iai.61.6.2309-2315.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haneberg B, Kendall D, Amerongen H M, Apter F M, Kraehenbuhl J-P, Neutra M R. Induction of specific immunoglobulin A in the small intestine, colon-rectum, and vagina measured by a new method for collection of secretions from local mucosal surfaces. Infect Immun. 1994;62:15–23. doi: 10.1128/iai.62.1.15-23.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleanthous H, Tibbitts T, Bakios T J, Georgokopoulos K, Myers G, Ermak T H, Fox J G, Monath T P. In-vivo selection of a highly adapted H. pylori isolate and the development of an H. pylori mouse model for studying vaccine efficacy. Gut. 1995;37:A94. [Google Scholar]
- 11.Kolesnikow, T., F. J. Radcliff, S. L. Hazell, C. Doidge, and A. Lee. 1996. Helicobacter pylori catalase: a novel antigen for vaccination. Gut 39(Suppl. 2):A46. [DOI] [PMC free article] [PubMed]
- 12.Lee A, O’Rourke J, Corazon de Ungria M, Robertson B, Daskalopoulos G, Dixon M F. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 13.Lee C K, Weltzin R, Thomas W D, Jr, Kleanthous H, Ermak T H, Gopalan S, Hill J E, Ackerman S K, Monath T P. Oral immunization with recombinant Helicobacter pylori urease induces secretory IgA antibodies and protects mice from challenge with Helicobacter felis. J Infect Dis. 1995;172:161–172. doi: 10.1093/infdis/172.1.161. [DOI] [PubMed] [Google Scholar]
- 14.Malfertheiner, P. 1993. Compliance, adverse events and antibiotic resistance in Helicobacter pylori treatment. Scand. J. Gastroenterol. 28(Suppl. 196):34–37. [DOI] [PubMed]
- 15.Marchetti M, Arico B, Burroni D, Figura N, Rappuoli R, Ghiara P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 1995;267:1655–1658. doi: 10.1126/science.7886456. [DOI] [PubMed] [Google Scholar]
- 16.Marshall J. Epidemiology of H. pylori in Western countries. In: Hunt R H, Tytgat G N J, editors. Helicobacter pylori. Basic mechanisms top clinical cure. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 75–84. [Google Scholar]
- 17.Michetti P, Corthesy-Theulaz I, Davin C, Haas R, Vaney A-C, Heitz M, Bille J, Kraehenbuhl J-P, Saraga E, Blum A L. Immunization of BALB/c mice against Helicobacter felis infection with Helicobacter pylori urease. Gastroenterology. 1994;107:1002–1011. doi: 10.1016/0016-5085(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell H M. The epidemiology of Helicobacter pylori infection and its relation to gastric cancer. In: Goodwin C S, Worsley B W, editors. Helicobacter pylori: biology and clinical practice. Boca Raton, Fla: CRC Press; 1993. pp. 95–114. [Google Scholar]
- 19.Myers, G., T. H. Ermak, K. Georgokopoulos, T. Tibbits, J. Bakios, H. Gray, J. Pappo, H. Kleanthous, C. K. Lee, and T. P. Monath. 1996. Oral immunization with recombinant urease confers long-lasting immunity. Gut 39(Suppl. 2):A44. [DOI] [PubMed]
- 20.Oderda G, Vaira D, Ainley C. Eighteen month follow-up of H. pylori positive children treated with amoxycillin and tinidazole. Dig Dis Sci. 1992;36:572–576. doi: 10.1136/gut.33.10.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pappo J, Thomas W D, Jr, Kabok Z, Taylor N S, Murphy J C, Fox J G. Effect of oral immunization with recombinant urease on murine Helicobacter felis gastritis. Infect Immun. 1995;63:1246–1252. doi: 10.1128/iai.63.4.1246-1252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parsonnet J, Samloff I M, Nelson L M, et al. Helicobacter pylori, pepsinogen and risk for gastric adenocarcinoma. Cancer Epidemiol Biomark Prev. 1993;2:1–7. [PubMed] [Google Scholar]
- 23.Solnick L V, Tompkins L S. Helicobacter pylori and gastroduodenal disease: pathogenesis and host-parasite interaction. Infect Agents Dis. 1993;1:294–309. [PubMed] [Google Scholar]
- 24.Thomas J E, Austin S, Dale A, et al. Protection by human milk IgA against Helicobacter pylori infection in infancy. Lancet. 1993;342:121. doi: 10.1016/0140-6736(93)91327-i. [DOI] [PubMed] [Google Scholar]
- 25.Weltzin R, Kleanthous H, Guirakhoo F, Monath T P, Lee C K. Novel intranasal immunization techniques for antibody induction and protection of mice against gastric Helicobacter felis infection. Vaccine. 1997;15:370–376. doi: 10.1016/s0264-410x(97)00203-x. [DOI] [PubMed] [Google Scholar]
- 26.Witherspoon A C, Doglioni C, Diss T C, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575–577. doi: 10.1016/0140-6736(93)91409-f. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. The state of world health. Geneva, Switzerland: World Health Organization; 1996. Infectious diseases and cancer; pp. 59–62. [Google Scholar]