Abstract
Over the past decades, global climate change has led to a significant increase in the average ambient temperature causing heat stress (HS) waves. This increase has resulted in more frequent heat waves during the summer periods. HS can have detrimental effects on poultry, including growth retardation, imbalance in immune/antioxidant pathways, inflammation, intestinal dysfunction, and economic losses in the poultry industry. Therefore, it is crucial to find an effective, safe, applicable, and economically efficient method for reducing these negative influences. Medicinal plants (MPs) contain various bioactive compounds with antioxidant, antimicrobial, anti-inflammatory, and immunomodulatory effects. Due to the biological activities of MPs, it could be used as promising thermotolerance agents in poultry diets during HS conditions. Nutritional supplementation with MPs has been shown to improve growth performance, antioxidant status, immunity, and intestinal health in heat-exposed chickens. As a result, several types of herbs have been supplemented to mitigate the harmful effects of heat stress in chickens. Therefore, several types of herbs have been supplemented to mitigate the harmful effects of heat stress in chickens. This review aims to discuss the negative consequences of HS in poultry and explore the use of different traditional MPs to enhance the health status of chickens.
Key words: medical plant, antioxidant, heat stress, chicken
INTRODUCTION
Since the 18th century, when modern commercial enterprise began, human activities have elevated the planet temperature in a phenomena called climate change (Biswal et al., 2022). The heat stress (HS) evoked by global warming lead to weaken the productivity, health and wellbeing of chickens (Khan et al., 2023). Poultry farmers in tropical and subtropical areas are facing significant issues since HS has a negative consequence on the poultry industry (Khan et al., 2023). HS phenomena occur when the amount of heat formed by the body of an animal is superior than the amount of heat dissipated to their direct environment (Brugaletta et al., 2023; Kuter et al., 2023). Chickens are sensitive to HS conditions for many reasons such as the high metabolic rate and the lack of sweat glands in their skin (Hidayat et al., 2023; Khan et al., 2023). Literatures (Chen et al., 2023b; Khan et al., 2023; Vandana et al., 2021) stated that HS generates high amounts of reactive oxygen species (ROS), resulting in oxidative stress (OS) in the cellular system of the broiler chickens. Elevation of OS is accompanying with impairment to vital proteins, lipids, DNA, and disrupts the balance of redox processes and immune function. This ultimately results in the buildup of tissue damage and a decline in the quality of meat (Saeed et al., 2019; Brugaletta et al., 2023; Hidayat et al., 2023).
Heat stress reduces the efficiency of feed, metabolism, hormones, and the immune system (Zhao et al., 2023), and intestinal tissue (Saracila et al., 2023). Moreover, HS triggers inflammation, and causes microbiota dysbiosis (Zwirzitz et al., 2023). Moreover, HS could increase the intestinal inflammation, oxidative stress, diminishing the antioxidant and immune markers in broiler. Reinforcing the defense system of chickens through the administration of immune-stimulants natural compounds in their diet has become a priority for ameliorating stress in the poultry sector. Amongst different immuno-stimulants, medicinal herbs incorporate chemical component that improve immunity, antioxidant capacity, enhance gut microbitoa and health as well as reduce the oxidative/inflammation pathways (Yang et al., 2021; Reith et al., 2022), making the animal more resistant to external stressors (Al-Garadi et al., 2023; Saracila et al., 2023). The mode of action of medicinal plants on heat-stressed broilers can be one of the following: 1) stimulating health state by improving the antioxidant system which can directly remove ROS produced as a result of stress (Abo Ghanima et al., 2023), and 2) MPs can also activate antioxidant enzymes and constrain pro-oxidant enzymes, supporting the health status of broiler exposed to HS.
Dietary inclusion of medicinal plants can maintain their efficacy in supporting health via promotion the antioxidant and immune system (Abo Ghanima et al., 2023; Hidayat et al., 2023; Wang et al., 2023; Zhao et al., 2023). MPs have the ability to target the production of ROS associated with environmental HS by inhibiting enzymes involved in cellular damages. They can also enhance mitochondrial function pathways (Wang et al., 2023) reducing the synthesis of OS and providing increased synthesis and provide more energy resources (Chen et al., 2023a). Based on its various biological activities, incorporation of poultry diets may be an effective, safe, applicable, and economically efficient technique for reducing these negative influences in poultry sector. The present review aims to provide an updated overview of the protective roles of medicinal plants in alleviating the negative effects of heat stress on poultry.
Effects of heat stress on performance
Growth performance reflects the managerial and ecological effects on an animal's productivity. In hot and subtropical regions, the growth characteristics of farm animals are weakened due to significant fluctuations in biological processes, such as variations in nutritional compounds like protein, minerals, water, and energy metabolism. Numerous previous trails have informed that HS negatively impacts growth characteristics in poultry (Song et al., 2018; Zhang et al., 2022; Abbas et al., 2022; AAbo Ghanima et al., 2023; Al-Garadi et al. 2023; Deng et al., 2023; Kim and Lee, 2023; Zwirzitz et al., 2023). The reduction in growth performance of chickens during HS may be related to a reduction in feed consumption and body weight gain, as well as an increase in feed conversion ratio (FCR) (Du et al., 2023; Kim and Lee, 2023; Sun et al., 2023). Based on the literature, exposure to climatic stress significantly decreased the daily body mass and feed intake of chickens (Abo Ghanima et al., 2023; Brugaletta et al., 2023; Zwirzitz et al., 2023).
Heat stress increases the heat load on chickens, leading to a decline in feed intake as a behavioral response to reduce the heat production (Mashaly et al., 2004; Rostagno, 2020; Vandana et al. 2021). Stressed chickens have struggle attaining a balance between body heats generated and body heat loss. Moreover, HS decreases feed efficiency, nutrients digestibility and its metabolism and absorption, thereby reducing nutrient availability to cells and physiological aspects (Mashaly et al., 2004; Amedy et al., 2011; Bilal et al., 2021; Ringseis and Eder, 2022). As a result, the reduction of feed intake induced by HS may lead to a decrease in the energy provided for tissues building and impair biological functions in the cellular systems of the body (Cheng et al., 2019; Chen et al. 2023a; Deng et al., 2023). Additionally, HS also causes intestinal dysfunction via distributing the intestinal structure, and decreasing the villus high, which play critical role in nutrients absorption (Karl et al., 2017; Raya-Sandino et al., 2021; Yang et al., 2021; Gieryńska et al., 2022).
Moreover, hormonal imbalances such as altered thyroid hormones have been detected in broiler chickens after exposure to climatic stress. Thyroid hormones are critical for the growth, metabolism and other critical biological events of animals; however, HS can disrupt their balance resulted in weakened in growth (Beckford et al., 2020). Moreover, the linkage between the intestinal morphology, microbiota changes and reducing growth indices in stressed chickens were also stated in many experiments (Karl et al., 2017; Raya-Sandino et al., 2021; Yang et al., 2021; Gieryńska et al., 2022). Some studies reported that the decrease in growth indices in broilers could be attributed to intestinal impairment, resulting in the translocation of intestinal pathogens and increased intestinal permeability to endotoxin induced by HS (Abd El-Hack et al., 2019; He et al., 2021; Gieryńska et al., 2022). Moreover, chronic HS increases the hepatic index (weight and liver enzymes), abdominal fat, lipid accumulation, thereby reducing the availability of nutrition for growth and development (Oladeinde et al., 2023). This feature was explained by (Li et al., 2023a), who found that HS induces up-regulation of fat synthesis genes and down regulated lipolysis-related genes in broiler.
Effects of heat stress on immunity
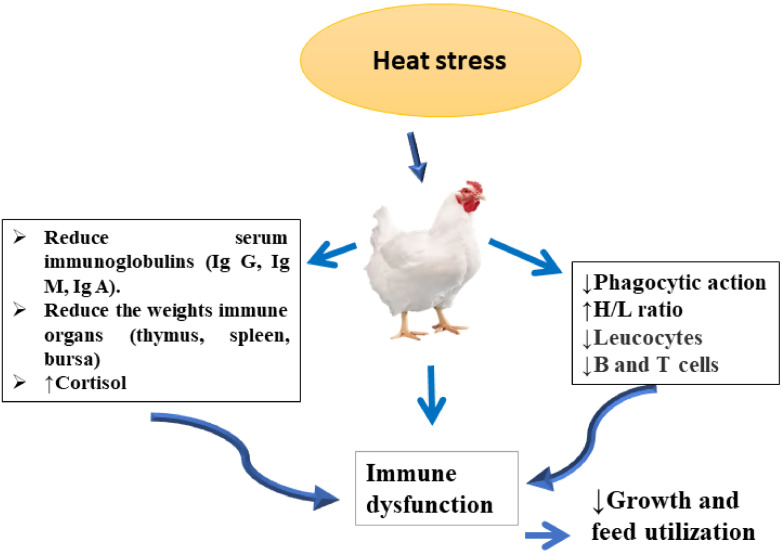
High temperature stress can affect hormonal stress markers such cortisol. Additionally, the sympathetic-adrenal–medullary (SAM) and hypothalamic-pituitary-adrenal (HPA) axis are activated to maintain redox homeostasis and immune functionality in response to stress (Sejian et al., 2021). During acute periods of HS, the production of cortisol hormone may serve as a trigger for the immune system. However, in case of chronic stress, the release of cortisol has been linked to immune suppression (Sun et al., 2023). Suppressed immune function makes animal more susceptible to diseases and immune challenges. Of the dual immune reactions, the adaptive and innate response, the adaptive reaction is more multifaceted and involves prolonged immunological challenge (Mashaly et al. 2004; Amedy et al., 2011; Bilal et al., 2021; Ringseis and Eder, 2022). Serum immunoglobulin G (IgG), and secretory immunoglobulin (SIgA) were significantly decreased in heat stressed broilers (Li et al., 2023b). SIgA and IgG are 2 main kinds of immunoglobulin in chickens that show a role in maintaining immunity. SIgA has a significant role in protecting and regulating intestinal mucosal health by unraveling the external environment from the inside of the body, limiting the entry of microbes and mucosal antigens into the delicate mucosal barriers. The IgG is formed by B cells, and directly part to an immune response including neutralization of viruses and toxins (Song et al., 2018). Numerous studies have reported the harmful effects of HS on immunological responses (Amedy et al., 2011; Farag and Alagawany, 2018; Sun et al., 2023). HS is responsible for inhibiting the phagocytic activity of leukocytes and suppressing the biosynthesis of B and T lymphocytes (Li et al., 2023b). The heterophil to lymphocyte ratio (H/L ratio) is a popular signal of stress in broilers (Al-Murrani et al., 2006). It has been observed that the H/L ratio was augmented in broiler exposed to HS, indicating immune dysfunction (Mcfarlane and Curtis, 1989). Likewise, Altan et al. (2003) and Nofal et al. (2015) discovered that high ambient temperature significantly increased the H/L and basophil ratios while decreasing hematocrit in birds. Inversely, Mashaly et al. (2004) recognized that high ambient temperature reduced the H/L ratio in table egg laying hens in addition to decay the actions and quantities of leukocytes. Figure 1 provides a summary of the potential effects of HS-induced immune dysfunction in poultry.
Figure 1.
Heat stress (HS) can trigger immune dysfunction in poultry. The reduction in feed intakes during HS promotes the decreasing of immune organs weights, thus reducing the immunoglobulins (IgA, IgG, and IgM) in the blood. HS can inhibit the phagocytic activity and suppressing the biosynthesis of B and T lymphocytes.
Heat stress induce inflammation
HS leads to reduced growth rates and a weakened immune system in the majority of the poultry population. However, in stressful circumstances, both pro- and anti-inflammatory cytokines are released from different excrete of various immune tissues and could play crucial roles in modulating the immune status of broiler. Generally, pro-inflammatory mediators facilitate inflammatory destruction, while anti-inflammatory mediators mitigate inflammation and stimulate the healing process during the environmental stimuli (Bamias et al., 2014). Interleukin-10 (IL-10) is a critical anti-inflammatory mediator involved in the inflammatory response. Some studies reported that IL-10 is one of the most significant cytokines associated with numerous pathophysiological circumstances, where it constrains the production of pro-inflammatory mediators (Hidayat et al., 2023). On the other hand, tumor necrosis factor alpha (TNF-α) is a pro-inflammatory mediator, that is widely considered in animal models (Yue et al., 2017; Abdelnour et al., 2019). Among inflammatory cytokines, TNF-α is an early and important mediator of hepatic damage (Hoek and Pastorino, 2002). It is well established that HS is a significant environmental factor responsible for liver damage. Therefore, the elevation of TNF-α levels in the liver or serum may contribute to liver dysfunction (Li et al., 2023a).
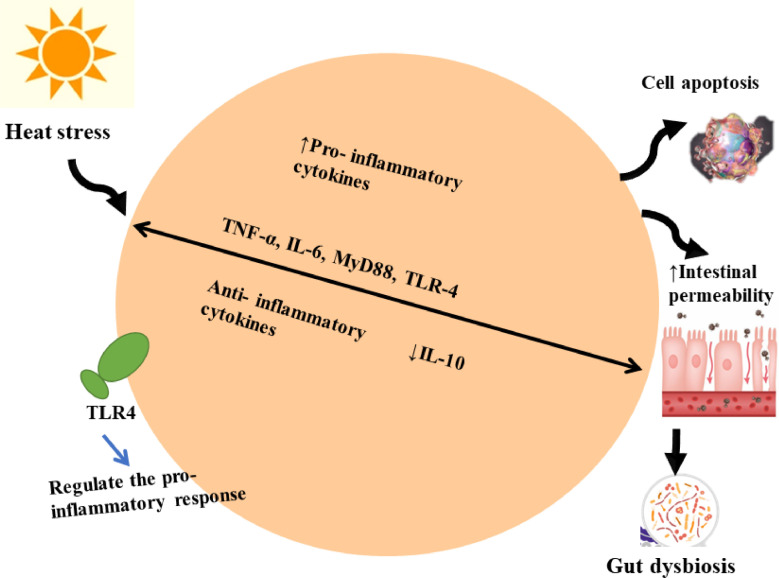
Remarkably, interleukin 6 (IL-6) has both pro- and anti-inflammatory actions; contributing to both metabolic and inflammatory pathways (Su et al., 2013). Furthermore, IL-6 affects the tight junctions of the gastrointestinal tract, where TNF-α increases intestinal permeability (Su et al., 2013). Inflammatory markers such as myeloid differentiation primary response 88 (MyD88), Nuclear factor-κB (NF-κB), and toll-like receptor 4 (TLR-4) were observed to be upregulated in intestinal tissues (Figure 2), leading to impaired gut health (Zhang et al., 2022). Thus, immunomodulatory mediators could potentially contribute to mitigating the harmful impacts of HS by improving antioxidant status, controlling cytokine responses, and adjusting gut microbiota and its function. Previous studies have reported that HS has detrimental effects on the relative weight of immune structures (thymus, liver, and spleen) and functions (leukocytes and immunoglobulins) in animals (Yue et al., 2017; Abdelnour et al., 2019; He et al., 2019). Moreover, HS can trigger inflammation via releasing inflammatory markers such as interleukin 2 (IL-2), TNF-α and IL-4 (He et al., 2019). As the primary participant recognized by the toll-like receptor (TLR) group is the chief stress-related biosensor. TLR4 can stimulate NF-κB, the main nuclear transcription factor of the inflammatory/immune response, thereby influencing the expression of a sequence of inflammatory-associated elements such as IL-6, TNF-α, and IL-1β (He et al., 2019). Earlier studies reported that HS upregulates the expression of NF-κB and TLR4 (Cheng et al., 2019). NF-κB is a main intracellular signaling protein that regulates the transcription of numerous genes associated with cell development, inflammatory reactions, and cell apoptosis (Liu et al., 2015). NF-κB has been found to have a distinct function in association with mitogen-activated protein kinases (MAPKs), ROS and heat shock protein 27 (HSP27), suggesting a protective effect against cell apoptosis induced by HS, as indicated by in vitro studies. Consequently, the activation of the inflammatory signaling pathway could be one of the primary reasons for the impairment of HS-induced innate immunity and the triggering of an inflammatory response during HS.
Figure 2.
Heat stress caused an increase in the levels of pro-inflammatory cytokines (TNF, MyD88, IL-4, and TRL4) and reduced the anti-inflammatory cytokines (IL-10). This imbalance could promote the cell apoptosis and increase the intestine permeability resulted in gut dysbiosis.
Heat stress induce oxidative stress
Heat stress is a well-known environmental issue that contributes to the induction of increased oxidative stress levels in the cellular system (Akbarian et al., 2016). This phenomenon can lead to an discrepancy between ROS and the body's defense structure. This imbalance can lead to disorders in cellular components such as lipids, DNA, and proteins (Altan et al., 2003; Salah et al., 2021; Reith et al., 2022; Zhao et al., 2023). Animal cells naturally produce ROS through the electron transport chain in the mitochondria during metabolism, particularly during heat production (Sun et al., 2023; Wang et al., 2023). The immune system also produces larger amounts of specific ROS, like superoxide radicals and nitric oxide, to combat harmful agents. ROS also play important roles in cytokine transcription and ion transport (Bilal et al., 2021).
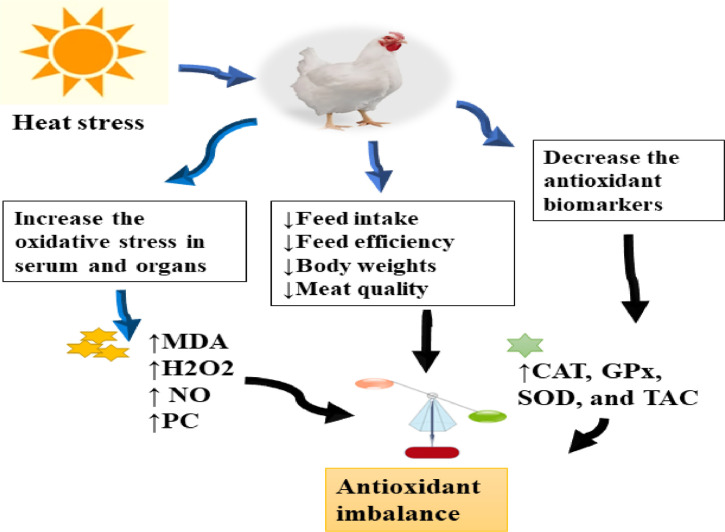
During heat stress, the demand for cellular energy increases, leading to higher production of mitochondrial ROS (Salah et al., 2021). To prevent oxidative damage, the body relies on its antioxidant system to neutralize ROS. The main antioxidant enzymes secreted by the body are catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx). However, prolonged heat stress can denature these enzymes, causing tissue damage and cell lesions (Calik et al., 2022). Heat stress elevates the cellular ROS levels in broiler chickens and impairs the effectiveness of the antioxidant system. As a result, the enzymatic antioxidant activities decrease (Yang et al., 2021; Sun et al., 2023). In broilers, HS leads to significant reductions in various antioxidant elements, such as GPx, SOD, and CAT, the total antioxidant capacity and nuclear muscle factor erythroid 2-related factor 2 (Nrf2), while instantaneously aggregate muscle Kelch-like ECH-associated protein 1 (Keap1) transcript and the levels of malondialdehyde (MDA) (Algothmi et al., 2023; Hidayat et al., 2023).
A key factor leading to the production of ROS is the final outcome of the respiratory chain, which occurs within the inner mitochondrial membrane. Here, the electron transport chain complexes within the mitochondria transfer electrons to oxygen (Deng et al., 2023). The Nrf2-mediated antioxidant response pathway maintains cellular redox homeostasis by inducing the transcription of a variety of cytoprotective genes (Lu et al., 2023). Additionally, Du et al. (2023) stated that HS displayed a less SOD level in jejunal tissues and a greater MDA aggregations in serum, hepatic and intestinal tissues in stressed broilers. When the level of ROS increases, cellular molecules such as enzymes, phospholipids, and side chains of polyunsaturated fatty acids (PUFAs), and nucleic acids will lead to modification in the permeability and fluidity of cellular membranes, and eventually in fluctuations in cell function and structure (Hashem et al., 2021).
Deng et al. (2023) reported that the levels of OS indicators (such as MDA and H2O2) were increased in serum under HS condition. Recently, multiple studies indicated that phytochemicals can alleviate OS in various syndrome and stress situations and reduce lipid peroxidation in the renal-hepatic tissues of heat-exposed broilers (Ali et al., 2023). Additionally, these phytochemicals may diminish the damage of antioxidant enzymes activity (SOD, GPX, and CAT) induced by HS via constraining NF-κB stimulation to reduce ROS production (Abd El-Hack et al., 2020). Moreover, they can restrict ROS via stimulating the phosphatidylinositol 3 kinase (PI3K)/protein kinase B (Akt) pathway (Amin et al., 2016). The PI3K/AKT/mTOR pathway is an intracellular signaling pathway that theaters a significant function in regulating the cell cycle. According to some trials, (Lee et al., 2013; Shehata et al., 2020) stated that the PI3K/Akt can mediate the HS conditions via promoting the production of heat shock proteins (HSPs), which represent protector mediator against any environmental issues. Collectively, HS induces redox dysfunction and hemostasis imbalance by reducing the activities of SOD, GPx, CAT, and total antioxidant capacity in serum and ileum tissues, and increasing the synthesis of oxidative markers such as MDA and H2O2 levels.
Heat stress induce gut dysfunction
The diverse population of microbes in the gut (gut microbiota) plays a critical role in breaking down food, extracting essential nutrients, and supporting the development and function of the immune system (Rowland et al., 2018). The gut acts as a barrier, removing toxins, pathogenic bacteria and infectious agents as shown in Figure 3. However, gut health is affected by exposure to heat conditions, which leads to reduced nutrient absorption, weakened immunity, and dysfunction in the intestinal system (Rostagno, 2020). Under normal conditions, the gut has the ability to fully digest and absorb feed, water, and electrolytes through trans and intracellular transport.
Figure 3.
Heat stress can increase the oxidative stress related biomarkers (MDA, NO, protein carbonyl, H2O2) and decreased the antioxidative related biomarkers (CAT, GPx, SOD, and total antioxidant capacity) in the blood serum and tissues. This imbalance in redox status may trigger diminishing the growth and feed efficiency in poultry.
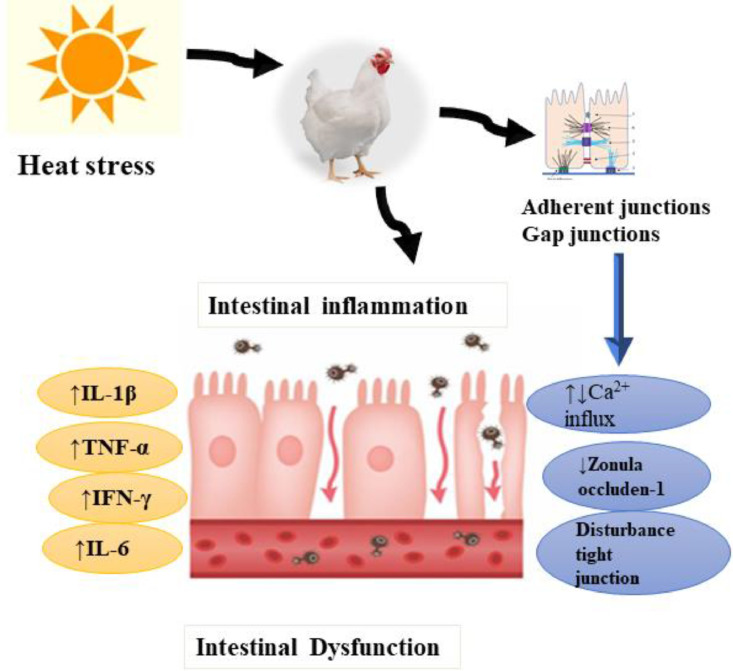
The epithelial cells in intestinal canal are strongly associated with the functionality of intercellular junctions. The multiplexes are an important constituent of the intestinal health and sustain the integrity of the epithelial barrier (Gieryńska et al., 2022). Conferring to previous reports (Garcia et al. 2018; Raya-Sandino et al., 2021), gut epithelia connection multiplexes contains Desmosomes, adherent junctions (AJ), gap junctions (GJ), and tight junction (TJ), are accompanying with keratin filaments where AJ are positioned underneath the TJ and are complicated by an intracellular communication. The TJ is a multifaceted protein assembly that forms trans-membrane protein channels through the epithelium, permits the transportation of various ingredients via signaling pathways, and interrelates with trans-membrane proteins. During HS situations, the TJ barrier was damaged and luminal substances arrive into the blood flow (Yang et al., 2021; Deng et al., 2023). Consequently, leaky gut persuades chronic systemic inflammation which decreases the disease-resistance capability of broilers (Zhang et al., 2022; Saracila et al., 2023). Recently, Xu et al. (2023) proposed that HS could increase the intestinal inflammation via increasing the circulating lipopolysaccharides (LPS) level and decrease DNA methylation level in the promoter region of TRPV4 (Transient Receptor Potential Cation Channel Subfamily V Member 4), which constrain TRPV4 expression, thus dropping Ca2+ influx, and lastly aggravating inflammation via disturbing NF-κB signaling pathway in broilers (Figure 4). Moreover, HS increased serum levels of interferon-γ (IFN-γ), IL-1β and jejunal mucosa of IL-1β level in broilers (Du et al., 2023). Moreover, it was found that HS elevated the transcripts of IFN-γ and IL-1β and downregulated levels of zonula occluden-1 (ZO-1) and occluding in jejunal mucosa of broilers (Figure 4) (Du et al., 2023). Collectively, HS induces intestinal dysfunction by altering the tight junction proteins, resulting in a reduction of the nutrients absorption and, consequently, causing growth retardation in broiler.
Figure 4.
Heat stress induced intestinal dysfunction via promoting the inflammation pathways. HS induces intestinal dysfunction by altering the tight junction proteins, resulting in a reduction of the nutrients absorption and, consequently, causing growth retardation in broiler chickens.
Effects of medical plants on alleviating the effects of heat stress on chickens
A number of studies have highlighted on the significant role of MPs as ordinary feed additives in poultry diets, aiming to increase the quantity and quality of meat and eggs (Vandana et al., 2021; Abbas et al., 2022; Kim and Lee, 2023). There has been considerable attention focused on how MPs can be applied to mitigate the adversative effects of HS, as shown in Table 1. This approach can help improve the production and performance of broiler chickens. Certain herbal plants have been employed the alleviate the adverse impacts of HS, including Artemisia spp., Olea europaea L., Silybum marianum, Foeniculum vulgare Mill, Thymus vulgari, Salvia rosmarinus.
Table 1.
Potential roles of some medicinal herbs used in this review to mitigate the negative effects of HS in broiler.
| Medical plants | Heat stress condition | Level | Main findings | References |
|---|---|---|---|---|
| Artemisia annua L. | 34 ± 1°C for 8 h from d 22 to d 42 of age | 0.75 g-1.25 g/kg diet |
|
Wan et al. (2017) |
| 34 ± 1°C for 8 h from d 22 to d 41 of age | 1 g/kg diet |
|
Song et al. (2017) | |
| 34 ± 1°C for 8 h from d 22 to d 41 of age |
|
|
Song et al. (2018) | |
| O. europaea L. | 33 ± 1°C for 5 h from 28 to 42 d | olive leaf extract (200 or 400) mg/kg |
|
Agah et al. (2019) |
| 34 ± 1°C from 28 to 42 d | Olive leaf extract (0, 5, 10 or 15 mL/L dirking water) |
|
Oke et al. (2017) | |
| 34°C for 12 h, 21 d of age | 6.7% olive oil |
|
Mujahid et al. (2009) | |
| S. marianum | Natural summer | 5, 10 15 g/kg diet |
|
Ahmad et al. (2020) |
| F. vulgare Mill | Cyclic heat stress cycle | 15, 20, 25 g/kg diet |
|
Fatima et al. (2022) |
| 30°C | 50, 100 or 200 mg/kg diet |
|
Mirzaei et al. (2023) | |
| S. rosmarinus | 42 ± 1°C for 1, 3, 5, and 10 h | 40 mg per d |
|
Tang et al. (2018) |
| T. vulgaris | 38 ± 1°C from d 22–42 | 100, 150 or 200 mg/kg |
|
Rafat Khafar et al. (2019) |
| 35°C for 8 h from d 26–42 | Mixture of thymol and carvacrol (60, 100, and 200 mg/kg) |
|
Saadat Shad et al. (2016) |
Artemisia spp
Artemisia spp. belongs to the Asteraceae family and it contains high levels of bioactive constituents, including b-camphene, essential oils, phenolics, flavonoids, steroids, and coumarins, as well as vitamins, amino acids, and minerals. Artemisia spp. has antibacterial, antioxidant, antihypertensive, anti-inflammatory and nutritional benefits (Sharifi-Rad et al., 2022). Studies have reported the antimicrobial effects of Artemisia spp. against some pathogenic bacteria such as Eimeria tenella and E. acervulina has been reported in broilers (Arab et al., 2006).
Supplementation of Artemisia annua L. (0.75–1.25 g/kg diet) to the diets of stressed broiler significantly enhanced the body weight, reduced the oxidative stress biomarkers (MDA, corticosterone), improved the hepatic function (aspartate transaminase [AST] and alanine aminotransferase [ALT]), and antioxidant capacity (Wan et al., 2017). Another study, Song et al. (2017) clarified that stressed broilers (34 ± 1°C for 8 h: 21-day-old male) fed with 1 gram of Artemisia annua L. decreased plasma diamine oxidase (DAO) activity, the mRNA expression of heat shock protein 70 (HSP70), TLR-4, IL-6, IL-1β, and IFN‐γ in intestinal tissues. In the intestinal mucosa, the mature upper villus cells exhibit high enzymatic activity for oxidation, facilitated by an enzyme called DAO.
Monitoring plasma DAO activities can be valuable for assessing intestinal tract impairments by environmental stressors. Furthermore, Song et al. (2018) reported that dietary inclusion of Artemisia annua had favorable impacts on growth indices, intestinal architecture, digestive enzymes, immune status, and redox status in broilers exposed to high temperature. Enhancing the intestinal integrity and function may be an effective attitude to partly reduce the unfavorable effects of HS on immunity, growth and health in chickens (Song et al., 2018). Taken together, these verdicts propose that Artemisia annua has promising properties to overcome the undesirable impacts of HS in broiler chickens.
Olea europaea L.
Olive trees are a characteristic plant found in the Mediterranean region. The olive oil market serves as a significant economic sector within the agricultural, pharmaceutical, and related industries (Solomou and Sfougaris, 2021). Olive (O. europaea) is a medicinal plant rich in polyphenols such as tyrosol, hydroxytyrosol, oleuropein, and pinoresinol (de Bock et al., 2013), which have strong antioxidant, antimicrobial, and anti-inflammatory properties. In earlier study, Oke et al. (2017) assessed the impacts of various gradual of olive leaf extract (5–15 mL/L) supplemented to the drinking water of broiler chickens on the growth, hematology profile, thyroid activity, and oxidative related indicators under hot climates. A significant enhancement in feed efficiency (higher feed intake, lower feed conversation ratio) and final body weight of broilers given high levels of the extract (10 or 15 mL/L) than those of the other experimental treatments (Oke et al., 2017). The improvement of growth in broiler might be accompanying with the capacity of olive leaves extract to boost the production of digestive enzymes, thus improving the digestibility of nutrients as reported by (Agah et al., 2019). Improving nutrients digestibility can compensate for the reduction in feed intakes triggered by HS. It is known that HS significantly reduces feed intake in broilers and other animal species. This reduction may be linked to the ability of O. europaea L. to stimulate digestive enzymes and improve nutrient digestibility, as stated by (Elbaz et al., 2022).
The dietary incorporation of olive oil was also found to improve the meat quality of broilers via boosting the antioxidant biomarkers and reducing OS (Tufarelli et al., 2016). The previous work suggested that antioxidant action of olive oil can protect the hepatic tissues of broilers from HS, which is responsible for the homeostasis of the entire organism and exposed to OS (Tufarelli et al., 2016). Another trial, Agah et al. (2019) found no significant effects of olive leaves extracts on body weight gains, and feed efficiency of heat stressed broilers (5 h, 33°C; day old). In contrast, olive leaves extracts were found to decrease lipid markers (cholesterol and triglyceride), and liver related functions (AST and ALT), MDA values and increase GPx (Agah et al., 2019; Sierzant et al., 2019). Moreover, it was reported that the addition of olive extracts (15 mL/L in dirking water) deceased the MDA values in broilers by 40.18% and increased SOD (18.13%) and T3 relative to the control group (Oke et al., 2017). Additionally, Agah et al. (2019) demonstrated that saturated fats, especially long-chain ones in olive oil, may reduce heat production in broilers (Oke et al., 2017). SOD is a main antioxidant enzyme that defends cells and organisms from the destructive impacts of superoxide anion. The antioxidant result of olive oil or its leaf extracts has been stated previously (Lee and Lee, 2010), by increasing plasma SOD levels and diminishing MDA integrity, thus enhancing the health and redox status balance of the chickens. This might be explained by the capability of OLE to confer suitable antioxidant shield alongside lipid peroxidation on the broiler during HS conditions (Oke et al., 2017; Agah et al., 2019). Many studies have reported that avian uncoupling protein (avUCP) was considerably downregulated (by ↓70%) when broilers were exposed to HS (34°C, 12 h; 35 d), thought provoking ROS production and oxidative damage (Oke et al., 2017; Agah et al., 2019). Thus, targeting avUCP by feeding broiler with olive oil (6.7%) in their diets can attenuate the ROS, and improve mitochondrial dysfunction induced by HS (Mujahid et al., 2009). The presence of oleic acid in olive oil may activate UCP3 mRNA expression, contributing to its unique properties (Jaburek et al., 2004) or be regulation by respiratory chain activity (Hosseindoust et al., 2022). Furthermore, this augmentation of the avUCP protein in mitochondria could participate in decreasing mitochondrial ROS creation in broilers exposed to HS, probably via an augmented inducible proton leakage that would accompany the decreased mitochondria function.
Silybum marianum
Milk thistle (S. marianum) contains high levels of bioactive constituents including silymarin, silychristine and silydianin (Aziz et al., 2021). Milk thistle extract is rich in silymarin (60%), a natural compound with health benefits. Administering an injection of S. marianum extract mixture (60 μL) at the time of hatching, monitored by the addition of 0.25 mL/L via drinking water throughout the raising stage could be a beneficial approach to improve the performance, blood markers and health status of broiler kept under hot climates (Parandoosh et al., 2023). Moreover, Ahmad et al. (2020) demonstrated that the growth rate, feed efficacy, and carcass traits were enhanced in stressed broilers fed a diet supplemented with milk thistle (15 g/kg). They suggested that milk thistle could considerably diminish the destructive effects of natural summer stress in broilers via boosting antioxidative and immune markers and reduce the oxidative pathways. More recent, Baradaran et al. (2019) described that silymarin may relieve the opposing effects of oxidative stress in poultry farms. Other studies have reported that milk thistle seeds possess antioxidant, anti-inflammatory, neutralize ROS and inhibiting lipid peroxidation (Yousefdoost et al., 2019; Aziz et al., 2021; Feshanghchi et al., 2022).
Foeniculum vulgare Mill
F. vulgare, ordinarily identified as Fennel, belongs to the Apiaceae family. Fennel has been found to possess antioxidant, antimicrobial, and strong hepatoprotective properties (Özbek et al., 2003).
Study of Ragab et al. (2013) reported that supplementing broilers raised under hot conditions and fed diets with 1 or 2% Fennel had greater feed utilization, meat breast yield, and leukocyte count than un-treated group. Similarly, Gharaghani et al. (2015) described that adding fennel fruits (10 or 20 g/kg) in the diet of laying hens reared under HS upgraded the quality of eggs, decreased MDA, protein carboxyl levels in eggs, and decreased the contents of triglyceride and cholesterol.
In a recent study, Fatima et al. (2022) explored the protective effects of fennel seed in improving the growth rate, carcass features, and blood components of stressed broiler. The study revealed that fennel seeds (20–25 g/kg) improved broiler growth, antioxidant status, carcass features, and immunological responses under HS conditions. Mirzaei et al. (2023) investigated the effects of fennel essential oil nanoemulsion (50, 100 or 200 mg/kg diet) under HS conditions in broilers. They found that nanoemulsion of fennel significantly improved antioxidant capacity, immunity, and promoted hepatic function in stressed broilers. Furthermore, it led to a reduction in Escherichia coli levels and an increase in beneficial bacteria such as lactobacillus and coliform. In another study, Al-Sagan et al. (2020) found that 3.2% fennel seeds powder into the diet of broilers had superior effects on growth indices and meat characteristics under chronic HS.
Thymus vulgaris
Thyme (T. vulgaris L.) is an indigenous herb that is mainly cultivated in the Mediterranean regions. It contains high levels of active constituents such as thymol, carvacrol, resin, tannins, steroids, saponins, flavonoids, alkaloids, PUFAs, vitamins, and glutamic acid (Khalil et al., 2020).
The favorable functions of thyme and its extracts have been itemized in variou in vitro and in vivo animal models (Puvača et al., 2022), including antioxidant, antimicrobial, and anti-inflammatory characteristics. In poultry, thyme has been found to have immunomodulating properties when used as a feed supplement or to mitigate the toxic effects of aflatoxins (Ahmadzadeh et al., 2022). Further, Rafat Khafar et al. (2019) found that thyme essential oil (150 or 200 mg/kg) in broiler diets improved growth indices, immunity and blood metabolites as well as decreased stress-related biomarkers (corticosterone and MDA) under HS condition. Moreover, Saadat Shad et al. (2016) reported that dietary inclusion of thyme (250 mg/kg) might positively attenuate the undesirable effects of heat exposed broilers, through enhancing feed and water intake, and antioxidative potential of blood in stressed broilers. Additionally, Nazar et al. (2019), found that the addition of thymol (≈80 mg/quail/d) increased blood protein levels and reduced inflammatory responses. In a recent study carried out by Señas-Cuesta et al. (2023), it was shown that a diet containing essential oils with a thymol chemotype could enhance the growth of broiler chickens during cyclic heat stress.
Salvia rosmarinus
S. rosmarinus commonly known as rosemary, belongs to the Labiatae family. The flowers of rosemary can come in various colors, including purple, pink, blue or white. Rosemary contains high levels of active constituents as caffeic acid, rosmarinic acid, ursolic acid, betulinic acid, and camphor. Among these, carnosol and carnosic acid are the most powerful antioxidants (Rostami et al., 2018; Liu et al., 2022).
According to Tang et al. (2018), rosemary extract has been shown to reduce cardiac stress markers (lactate dehydrogenase [LDH], creatine kinase [CK], and myocardial CK [CKMB]) and MDA. Additionally, it improves protective markers such as crystallin alpha B (CRYAB) and HSP70 in stressed broilers. In a study by Karami and Rahimian (2022), found that it was found that including rosemary (0.5%) in the diet improved the growth matrices, boosted antioxidant capacity, increased thyroid hormones in stressed broiler chicks. The positive effects of rosemary on productivity and broiler health under stressful conditions may be attributed to its ability to enhance antioxidant activity (Hosseinzadeh et al., 2023), modulate intestinal microbiota population (Liu et al., 2022), improve intestinal morphology, enhance immune activity (Rostami et al., 2018), and optimize plasma biochemistry parameters in broiler chickens (Torki et al., 2018). According to the current review, herbs or medicinal plants has been significantly investigated and utilized as an alternative to antibiotic growth promoters, while its potential roles in mitigating the negative effects of heat stress are still need further explorations. Moreover, the use of byproducts of these medicinal plants could offer effective and applicable methods for contributing to a more sustainable and efficient poultry industry.
CONCLUSION
Overall, heat stress can limit broiler productivity by causing growth retardation, impairing immune function, promoting intestinal inflammation and health issues, and increasing oxidative and inflammation pathways. Medicinal plants show promise approach to mitigate these unfavorable effects in chickens raised under heat stress conditions. They can stimulate gut health, sustain the microbiota community, reduce the inflammation/oxidative stress pathways, boost the immunity and antioxidative status and improve productivity in chickens under heat stress conditions. However, further investigation is needed to explore the molecular fluctuations caused by medicinal plants, as well as the interactions between active ingredients, intestinal microbiota, and intestinal barriers. These approaches can promote the welfare of broiler chickens and contribute to a more sustainable and efficient poultry industry.
ACKNOWLEDGMENTS
The authors would like to express their deepest gratitude to the deanship of scientific research at the University of Jordan and Higher Council for Science and Technology for thier financial support to conduct this research.
Ethical Approval: Not applicable.
Animal Welfare Statement: No ethical approval was required as this is a review article with no original research data.
Authors’ Contributions: All authors contributed equally to this review.
DISCLOSURES
The authors declare that they have no conflicts of interests.
REFERENCES
- Abbas G., Arshad M., Tanveer A.J., Jabbar M.A., Arshad M., AL-Taey D.K.A., Mahmood A., Khan M.A., Khan A.A., Konca Y., Sultan Z., Qureshi R.A.M., Iqbal A., Amad F., Ashraf M., Asif M., Mahmood R., Abbas H., Mohyuddin S.G., Jiang M.Y. Combating heat stress in laying hens a review. Pak. J. 2022;73:633–655. [Google Scholar]
- Abd El-Hack M.E, Alagawany M., Abdelnour S. Responses of growing rabbits to supplementing diet with a mixture of black and red pepper oils as a natural growth promoter. J. Anim. Physiol. Anim. Nutr. 2019;103:509–517. doi: 10.1111/jpn.13045. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., Abdelnour S.A., Taha A.E., Khafaga A.F., Arif M., Ayasan T., Swelum A.A., Abukhalil M.H., Alkahtani S., Aleya L. Herbs as thermoregulatory agents in poultry: an overview. Sci. Total. Environm. 2020;703 doi: 10.1016/j.scitotenv.2019.134399. [DOI] [PubMed] [Google Scholar]
- Abdelnour S.A., Abd El-Hack M.E., Khafaga A.F., Arif M., Taha A.E., Noreldin A.E. Stress biomarkers and proteomics alteration to thermal stress in ruminants: a review. J. Therm. Biol. 2019;79:120–134. doi: 10.1016/j.jtherbio.2018.12.013. [DOI] [PubMed] [Google Scholar]
- Abo Ghanima M.M., Aljahdali N., Abuljadayel D.A., Shafi M.E., Qadhi A., Abd El-Hack M.E., Mohamed L.A. Effects of dietary supplementation of Amla, Chicory and Leek extracts on growth performance, immunity and blood biochemical parameters of broilers. Ital. J. Anim. Sci. 2023;22:24–34. [Google Scholar]
- Agah M., Mirakzehi M., Saleh H. Effects of olive leaf extract (Olea europea L.) on growth performance, blood metabolites and antioxidant activities in broiler chickens under heat stress. J. Anim. Plant Sci. 2019;29 (3) [Google Scholar]
- Ahmad M., Chand N., Khan R.U., Ahmad N., Khattak I., Naz S. Dietary supplementation of milk thistle (Silybum marianum): growth performance, oxidative stress, and immune response in natural summer stressed broilers. Trop. Anim. Health Prod. 2020;52:711–715. doi: 10.1007/s11250-019-02060-4. [DOI] [PubMed] [Google Scholar]
- Ahmadzadeh A., Nobakht A., Mehmannavaz Y. Supplementary prebiotics, probiotics, and thyme (Thymus vulgaris) essential oil for broilers: performance, intestinal morphology, and fecal nutrient composition. Probiotics Antimicrob Proteins. 2022;15:903–911. doi: 10.1007/s12602-022-09927-3. [DOI] [PubMed] [Google Scholar]
- Akbarian A., Michiels J., Degroote J., Majdeddin M., Golian A., De Smet S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016;7:1–14. doi: 10.1186/s40104-016-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algothmi K.M., Mahasneh Z.M., Abdelnour S.A., Khalaf Q.A., Noreldin A.E., Barkat R.A., Khalifa N.E., Khafaga A.F., Tellez-Isaias G., Alqhtani A.H., Swelum A.A., Abd El-Hack M.E. Protective impacts of mitochondria enhancers against thermal stress in poultry. Poult. Sci. 2023 doi: 10.1016/j.psj.2023.103218. 103 (1), 103218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Garadi M.A., Suliman G.M., Hussein E.O., Al-Owaimer A.N., Swelum A.A., Almalamh N.A., Alhotan R.A., Qaid M.M. The effects of betaine and nano-emulsified plant-oil supplementation on growth performance and serum biochemistry indices of heat-stressed broiler chickens. Ital. J. Anim. Sci. 2023;22:398–406. [Google Scholar]
- Al-Murrani W.K., Al-Rawi A.J., Al-Hadithi M.F., Al-Tikriti B. Association between heterophil/lymphocyte ratio, a marker of “resistance” to stress, and some production and fitness traits in chickens. Br. Poult. Sci. 2006;47:443–448. doi: 10.1080/00071660600829118. [DOI] [PubMed] [Google Scholar]
- Al-Sagan A.A., Khalil S., Hussein E.O., Attia Y.A. Effects of fennel seed powder supplementation on growth performance, carcass characteristics, meat quality, and economic efficiency of broilers under thermoneutral and chronic heat stress conditions. Animals. 2020;10:206. doi: 10.3390/ani10020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N.M., Mohamed G.A., El-Demerdash A.S. Impact of oral administration of chitosan–nanoparticles on oxidative stress index and gut microbiota of heat stressed broilers. J. Adv. Vet. Res. 2023;13:997–1003. [Google Scholar]
- Altan Ö., Pabuçcuoğlu A., Altan A., Konyalioğlu S., Bayraktar H. Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. Br. Poult. Sci. 2003;44:545–550. doi: 10.1080/00071660310001618334. [DOI] [PubMed] [Google Scholar]
- Amedy V.J., Tayeb I.T., Yokhana J.S. Effects of supplemental ascorbic acid on humeral immune response in broilers reared under heat-stress condition. Res. Opin. Anim. Vet. Sci. 2011;1:459–462. [Google Scholar]
- Amin F.U., Shah S.A., Kim M.O. Glycine inhibits ethanol-induced oxidative stress, neuroinflammation and apoptotic neurodegeneration in postnatal rat brain. Neurochem. Int. 2016;96:1–12. doi: 10.1016/j.neuint.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Arab H.A., Rahbari S., Rassouli A., Moslemi M.H., Khosravirad F. Determination of artemisinin in Artemisia sieberi and anticoccidial effects of the plant extract in broiler chickens. Trop. Anim. Health Prod. 2006;38:497–503. doi: 10.1007/s11250-006-4390-8. [DOI] [PubMed] [Google Scholar]
- Aziz M., Saeed F., Ahmad N., Ahmad A., Afzaal M., Hussain S., Mohamed A.A., Alamri M.S., Anjum F.M. Biochemical profile of milk thistle (Silybum Marianum L.) with special reference to silymarin content. Food Sci. Nutr. 2021;9:244–250. doi: 10.1002/fsn3.1990. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bamias G., Arseneau K.O., Cominelli F. Cytokines and mucosal immunity. Curr. Opin. Gastroenterol. 2014;30:547. doi: 10.1097/MOG.0000000000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baradaran A., Samadi F., Ramezanpour S.S., Yousefdoust S. Hepatoprotective effects of silymarin on CCl4-induced hepatic damage in broiler chickens model. Toxicol. Rep. 2019;6:788–794. doi: 10.1016/j.toxrep.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckford R.C., Ellestad L.E., Proszkowiec-Weglarz M., Farley L., Brady K., Angel R., Liu H.C., Porter T.E. Effects of heat stress on performance, blood chemistry, and hypothalamic and pituitary mRNA expression in broiler chickens. Poult. Sci. 2020;99:6317–6325. doi: 10.1016/j.psj.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilal R.M., Hassan F., Farag M.R., Nasir T.A., Ragni M., Mahgoub H.A., Alagawany M. Thermal stress and high stocking densities in poultry farms: potential effects and mitigation strategies. J. Therm. Biol. 2021;99 doi: 10.1016/j.jtherbio.2021.102944. [DOI] [PubMed] [Google Scholar]
- Biswal J., Vijayalakshmy K., Rahman T.B. Impact of heat stress on poultry production. Worlds Poult. Sci. J. 2022;78:179–196. [Google Scholar]
- Brugaletta G., Laghi L., Zampiga M., Oliveri C., Indio V., Piscitelli R., Pignata S., Petracci M., De Cesare A., Sirri F. Metabolic and microbiota response to arginine supplementation and cyclic heat stress in broiler chickens. Front. Physiol. 2023:14. doi: 10.3389/fphys.2023.1155324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calik A., Emami N.K., Schyns G., White M.B., Walsh M.C., Romero L.F., Dalloul R.A. Influence of dietary vitamin E and selenium supplementation on broilers subjected to heat stress, Part II: oxidative stress, immune response, gut integrity, and intestinal microbiota. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Liu H., Zhang J., Zhou B., He X., Wang T., Wang C. Dietary rutin improves breast meat quality in heat-stressed broilers and protects mitochondria from oxidative attack via the AMPK/PINK1–Parkin pathway. J. Sci. Food Agric. 2023;103:2367–2377. doi: 10.1002/jsfa.12431. [DOI] [PubMed] [Google Scholar]
- Chen Z., Sun X., Li X., Liu N. Oleoylethanolamide alleviates hyperlipidaemia-mediated vascular calcification via attenuating mitochondrial DNA stress triggered autophagy-dependent ferroptosis by activating PPARα. Biochem. Pharmacol. 2023;208 doi: 10.1016/j.bcp.2022.115379. [DOI] [PubMed] [Google Scholar]
- Cheng K., Yan E., Song Z., Li S., Zhang H., Zhang L., Wang C., Wang T. Protective effect of resveratrol against hepatic damage induced by heat stress in a rat model is associated with the regulation of oxidative stress and inflammation. J. Therm. Biol. 2019;82:70–75. doi: 10.1016/j.jtherbio.2019.03.012. [DOI] [PubMed] [Google Scholar]
- de Bock M., Thorstensen E.B., Derraik J.G., Henderson H.V., Hofman P.L., Cutfield W.S. Human absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea europaea L.) leaf extract. Mol. Nutr. Food Res. 2013;57:2079–2085. doi: 10.1002/mnfr.201200795. [DOI] [PubMed] [Google Scholar]
- Deng C., Zheng J., Zhou H., You J., Li G. Dietary glycine supplementation prevents heat stress-induced impairment of antioxidant status and intestinal barrier function in broilers. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M., Cheng Y., Chen Y., Wang S., Zhao H., Wen C., Zhou Y. Dietary supplementation with synbiotics improves growth performance, antioxidant status, immune function, and intestinal barrier function in broilers subjected to cyclic heat stress. Environ. Sci. Pollut. Res. 2023;30:18026–18038. doi: 10.1007/s11356-022-23385-y. [DOI] [PubMed] [Google Scholar]
- Elbaz A.M., Ashmawy E.S., Salama A.A., Abdel-Moneim A.E., Badri F.B., Thabet H.A. Effects of garlic and lemon essential oils on performance, digestibility, plasma metabolite, and intestinal health in broilers under environmental heat stress. BMC Vet. Res. 2022;18:430. doi: 10.1186/s12917-022-03530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag M.R., Alagawany M. Physiological alterations of poultry to the high environmental temperature. J. Therm. Biol. 2018;76:101–106. doi: 10.1016/j.jtherbio.2018.07.012. [DOI] [PubMed] [Google Scholar]
- Fatima A., Chand N., Naz S., Saeed M., Khan N.U., Khan R.U. Coping heat stress by crushed fennel (Foeniculum vulgare) seeds in broilers: growth, redox balance and humoral immune response. Livest. Sci. 2022;265 [Google Scholar]
- Feshanghchi M., Baghban-Kanani P., Kashefi-Motlagh B., Adib F., Azimi-Youvalari S., Hosseintabar-Ghasemabad B., Slozhenkina M., Gorlov I., Zangeronimo M.G., Swelum A.A., Seidavi A., Khan R.U., Ragni M., Laudadio V., Tufarelli V. Milk Thistle (Silybum marianum), Marine Algae (Spirulina platensis) and Toxin binder powders in the diets of broiler chickens exposed to aflatoxin-b1: growth performance, humoral immune response and cecal microbiota. Agriculture. 2022;12:805. [Google Scholar]
- Garcia M.A., Nelson W.J., Chavez N. Cell-cell junctions organize structural and signaling networks. Cold Spring Harb Perspect Biol. 2018;10(4):a029181. doi: 10.1101/cshperspect.a029181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharaghani H., Shariatmadari F., Torshizi M. Effect of fennel (Foeniculum vulgare Mill.) used as a feed additive on the egg quality of laying hens under heat stress. Braz. J. Poult. Sci. 2015;17:199–207. [Google Scholar]
- Gieryńska M., Szulc-Dąbrowska L., Struzik J., Mielcarska M.B., Gregorczyk-Zboroch K.P. Integrity of the intestinal barrier: the involvement of epithelial cells and microbiota—a mutual relationship. Animals. 2022;12(2):145. doi: 10.3390/ani12020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashem N.M., Abdelnour S.A., Alhimaidi A.R., Swelum A.A. Potential impacts of COVID-19 on reproductive health: scientific findings and social dimension. Saudi J. Biol. Sci. 2021;28:1702–1712. doi: 10.1016/j.sjbs.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S., Yu Q., He Y., Hu R., Xia S., He J. Dietary resveratrol supplementation inhibits heat stress-induced high-activated innate immunity and inflammatory response in spleen of yellow-feather broilers. Poult. Scie. 2019;98:6378–6387. doi: 10.3382/ps/pez471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Maltecca C., Tiezzi F. Potential Use of gut microbiota composition as a biomarker of heat stress in monogastric species: a review. Animals. 2021;11:1833. doi: 10.3390/ani11061833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidayat D.F., Mahendra M.N., Kamaludeen J., Pertiwi H. Lycopene in feed as antioxidant and immuno-modulator improves broiler chicken's performance under heat-stress conditions. Vet. Med. Int. 2023;2023 doi: 10.1155/2023/5418081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek J.B., Pastorino J.G. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol. 2002;27:63–68. doi: 10.1016/s0741-8329(02)00215-x. [DOI] [PubMed] [Google Scholar]
- Hosseindoust A., Kang H., Kim J. Quantifying heat stress; the roles on metabolic status and intestinal integrity in poultry: a review. Domest. Anim. Endocrinol. 2022;81 doi: 10.1016/j.domaniend.2022.106745. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh S., Shariatmadari F., Karimi Torshizi M.A., Ahmadi H., Scholey D. Plectranthus amboinicus and rosemary (Rosmarinus officinalis L.) essential oils effects on performance, antioxidant activity, intestinal health, immune response, and plasma biochemistry in broiler chickens. Food Sci. Nutr. 2023;11:3939–3948. doi: 10.1002/fsn3.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaburek M., Miyamoto S., Mascio D.P., Garlid K.D., Jezek P. Hydroperoxy fatty acid cycling mediated by mitochondrial uncoupling protein UCP2. J. Biol. Chem. 2004;279:53097–53102. doi: 10.1074/jbc.M405339200. [DOI] [PubMed] [Google Scholar]
- Karami M., Rahimian Y. Performance, thyroid hormones activities and antioxidant status in broilers of using Eucalyptus and Rosemary leaf powders, reared under heat stress condition. J. Appl. Anim. Res. 2022;11:63–74. [Google Scholar]
- Karl J.P., Margolis L.M., Madslien E.H., Murphy N.E., Castellani J.W., Gundersen Y., Hoke A.V., Levangie M.W., Kumar R., Chakraborty N. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;312:G559–G571. doi: 10.1152/ajpgi.00066.2017. [DOI] [PubMed] [Google Scholar]
- Khalil S.R., Elhakim Y.A., Abd El-fattah A.H., Ragab F.M., Abd El-Hameed N.E., El-Murr A.E. Dual immunological and oxidative responses in Oreochromis niloticus fish exposed to lambda cyhalothrin and concurrently fed with Thyme powder (Thymus vulgaris L.): stress and immune encoding gene expression. Fish Shellfish Immunol. 2020;100:208–218. doi: 10.1016/j.fsi.2020.03.009. [DOI] [PubMed] [Google Scholar]
- Khan R.U., Naz S., Ullah H., Ullah Q., Laudadio V., Qudratullah G., Bozzo G., Tufarelli V. Physiological dynamics in broiler chickens under heat stress and possible mitigation strategies. Anim. Biotechnol. 2023;34:438–447. doi: 10.1080/10495398.2021.1972005. [DOI] [PubMed] [Google Scholar]
- Kim D.H. and Lee K.W., An update on heat stress in laying hens, World’s Poult.Sci. J., 2023, 1–24. 79(4), 689-712.
- Kuter E., Cengiz Ö., Köksal B.H., Sevim Ö., Tatlı O., Ahsan U., Güven G., Önol A.G., Bilgili S.F. Litter quality and incidence and severity of footpad dermatitis in heat stressed broiler chickens fed supplemental zinc. Livest. Sci. 2023;267 [Google Scholar]
- Lee K.H., Jeong J., Yoo C.G. Positive feedback regulation of heat shock protein 70 (Hsp70) is mediated through Toll-like receptor 4-PI3K/Akt-glycogen synthase kinase-3β pathway. Exp. Cell Res. 2013;319:88–95. doi: 10.1016/j.yexcr.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Lee O.H., Lee B.Y. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour. Technol. 2010;101:3751–3754. doi: 10.1016/j.biortech.2009.12.052. [DOI] [PubMed] [Google Scholar]
- Li L., Cui Z., Wang H., Huang B., Ma H. Dietary supplementation of dimethyl itaconate protects against chronic heat stress-induced growth performance impairment and lipid metabolism disorder in broiler chickens. J. Anim. Sci. 2023:skad120. doi: 10.1093/jas/skad120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Liu A., Xu J., Zhang C. Resveratrol attenuates heat-stress-impaired immune and inflammatory responses of broilers by modulating toll-like receptor-4 signaling pathway. Braz. J. Poult. Sci. 2023;25 eRBCA-2022-1668. [Google Scholar]
- Liu Y., Li C., Huang X., Zhang X., Deng P., Jiang G., Dai Q. Dietary rosemary extract modulated gut microbiota and influenced the growth, meat quality, serum biochemistry, antioxidant, and immune capacities of broilers. Front. Microbiol. 2022;13:1024682. doi: 10.3389/fmicb.2022.1024682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhou G., Wang Z., Guo X., Xu Q., Huang Q., Su L. NF-κB signaling is essential for resistance to heat stress-induced early stage apoptosis in human umbilical vein endothelial cells. Sci. Rep. 2015;5:13547. doi: 10.1038/srep13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M.H., Ding K.N., Liang S.S., Guo Y.N., He Y.M., Tang L.P. Resveratrol inhibits oxidative damage in lungs of heat-stressed broilers by activating Nrf2 signaling pathway and autophagy. Ecotoxicol. Environ. Saf. 2023;258 doi: 10.1016/j.ecoenv.2023.114949. [DOI] [PubMed] [Google Scholar]
- Mashaly M., Hendricks G., Kalama M., Gehad A., Abbas A., Patterson P. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult. Sci. 2004;83:889–894. doi: 10.1093/ps/83.6.889. [DOI] [PubMed] [Google Scholar]
- Mcfarlane J.M., Curtis S.E. Multiple concurrent stressors in chicks.: 3. Effects on plasma corticosterone and the heterophil: lymphocyte ratio. Poult. Sci. 1989;68:522–527. doi: 10.3382/ps.0680522. [DOI] [PubMed] [Google Scholar]
- Mirzaei H., Ghorbani M.R., Salari S., Mehrnia M.A. Antioxidant properties of the fennel essential oil nanoemulsion: effect on European production efficiency factor, blood metabolites, immune system and cecal microbial population of heat stressed broiler chickens. J. Livest. Sci. Technol. 2023;11:53–60. [Google Scholar]
- Mujahid A., Akiba Y., Toyomizu M. Olive oil-supplemented diet alleviates acute heat stress-induced mitochondrial ROS production in chicken skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:R690–R698. doi: 10.1152/ajpregu.90974.2008. [DOI] [PubMed] [Google Scholar]
- Nazar F.N., Videla E.A., Marin R.H. Thymol supplementation effects on adrenocortical, immune and biochemical variables recovery in Japanese quail after exposure to chronic heat stress. Animal. 2019;13:318–325. doi: 10.1017/S175173111800157X. [DOI] [PubMed] [Google Scholar]
- Nofal M., Magda A.G., Mousa S., Doaa M., Bealsh A. Effect of dietary betaine supplementation on productive, physiological and immunological performance and carcass characteristic of growing developed chicks uinder the condition of heat stress, Egypt. Poult. Sci. J. 2015:35.1. [Google Scholar]
- Oke O., Emeshili U., Iyasere O., Abioja M., Daramola J., Ladokun A., Abiona J., Williams T., Rahman S., Rotimi S. Physiological responses and performance of broiler chickens offered olive leaf extract under a hot humid tropical climate. J. Appl. Poult. Res. 2017;26:376–382. [Google Scholar]
- Oladeinde A., Awosile B., Woyda R., Abdo Z., Endale D., Strickland T., Lawrence J.P., Cudnik D., House S., Cook K. Management and environmental factors influence the prevalence and abundance of food-borne pathogens and commensal bacteria in peanut hull-based broiler litter. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özbek H., Uğraş S., Dülger H., Bayram I., Tuncer I., Öztürk G., Öztürk A. Hepatoprotective effect of Foeniculum vulgare essential oil. Fitoterapia. 2003;74:317–319. doi: 10.1016/s0367-326x(03)00028-5. [DOI] [PubMed] [Google Scholar]
- Parandoosh H., Khodaei-Motlagh M., Ghasemi H.A., Farahani A.H.K. Effects of day-of-hatch intramuscular administration of a herbal extract mixture and its re-supplementation in drinking water on growth performance, stress indicators, and antioxidant status of broiler chickens reared under hot summer conditions. Trop. Anim. Health Prod. 2023;55:196. doi: 10.1007/s11250-023-03597-1. [DOI] [PubMed] [Google Scholar]
- Puvača N., Tufarelli V., Giannenas I. Essential oils in broiler chicken production, immunity and meat quality: review of Thymus vulgaris, Origanum vulgare, and Rosmarinus officinalis. Agriculture. 2022;12:874. [Google Scholar]
- Rafat Khafar K., Mojtahedin A., Rastegar N., Kalvani Neytali M., Olfati A. Dietary inclusion of thyme essential oil alleviative effects of heat stress on growth performance and immune system of broiler chicks. Iran. J. Appl. Anim. Sci. 2019;9:509–517. [Google Scholar]
- Ragab M., Namra M., Aly M., Fathi M. Impact of inclusion fennel seeds and thyme dried leaves in broiler diets on some productive and physiological performance during summer season. Egypt. Poult. Sci. J. 2013;33:197–219. [Google Scholar]
- Raya-Sandino A., Luissint A.C., Kusters D.H.M., Narayanan V., Flemming S., Garcia-Hernandez V., Godsel L.M., Green K.J., Hagen S.J., Conway D.E., Parkos C.A., Nusrat A. Regulation of intestinal epithelial intercellular adhesion and barrier function by desmosomal cadherin desmocollin-2. Mol. Biol. Cell. 2021;32:753–768. doi: 10.1091/mbc.E20-12-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith R.R., Sieck R.L., Grijalva P.C., Swanson R.M., Fuller A.M., Diaz D.E., Schmidt T.B., Yates D.T., Petersen J.L. Transcriptome analyses indicate that heat stress-induced inflammation in white adipose tissue and oxidative stress in skeletal muscle is partially moderated by zilpaterol supplementation in beef cattle. J. Anim. Sci. 2022;100:skac019. doi: 10.1093/jas/skac019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringseis R., Eder K. Heat stress in pigs and broilers: role of gut dysbiosis in the impairment of the gut-liver axis and restoration of these effects by probiotics, prebiotics and synbiotics. J. Anim. Sci. Biotechnol. 2022;13:1–16. doi: 10.1186/s40104-022-00783-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostagno M.H. Effects of heat stress on the gut health of poultry. J. Anim. Sci. 2020 doi: 10.1093/jas/skaa090. 98(4), skaa090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami H., Seidavi A., Dadashbeiki M., Asadpour Y., Simões J., Shah A.A., Laudadio V., Losacco C., Perillo A., Tufarelli V. Supplementing dietary rosemary (Rosmarinus officinalis L.) powder and vitamin E in broiler chickens: evaluation of humoral immune response, lymphoid organs, and blood proteins. Environ. Sci. Pollut. Res. 2018;25:8836–8842. doi: 10.1007/s11356-018-1209-x. [DOI] [PubMed] [Google Scholar]
- Rowland I., Gibson G., Heinken A., Scott K., Swann J., Thiele I., Tuohy K. Gut microbiota functions: metabolism of nutrients and other food components. Eur. J. Nutr. 2018;57:1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadat Shad H., Mazhari M., Esmaeilipour O., Khosravinia H. Effects of thymol and carvacrol on productive performance, antioxidant enzyme activity and certain blood metabolites in heat stressed broilers. Iran. J. Appl. Anim. Sci. 2016;6:195–202. [Google Scholar]
- Saeed M., Abbas M., Alagawany A., Kamboh A., Abd El-Hack M.E., Khafaga A.F., Chao S. Heat stress management in poultry farms: a comprehensive overview. J. Therm. Biol. 2019;84:414–425. doi: 10.1016/j.jtherbio.2019.07.025. [DOI] [PubMed] [Google Scholar]
- Salah A.S., Ahmed-Farid O.A., Nassan M.A., El-Tarabany M.S. Dietary curcumin improves energy metabolism, brain monoamines, carcass traits, muscle oxidative stability and fatty acid profile in heat-stressed broiler chickens. Antioxidants. 2021;10:1265. doi: 10.3390/antiox10081265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saracila M., Panaite T.D., Predescu N.C., Untea A.E., Vlaicu P.A. Effect of dietary salicin standardized extract from salix alba bark on oxidative stress biomarkers and intestinal microflora of broiler chickens exposed to heat stress. Agriculture. 2023;13:698. [Google Scholar]
- Sejian V., Silpa M., Lees A.M., Krishnan G., Devaraj C., Bagath M., Anisha J., Reshma Nair M., Manimaran A., Bhatta R. Opportunities, challenges, and ecological footprint of sustaining small ruminant production in the changing climate scenario. Agroecol. Sustain. Food Syst. 2021:365–396. [Google Scholar]
- Señas-Cuesta R., Stein A., Latorre J.D., Maynard C.J., Hernandez-Velasco X., Petrone-Garcia V., Greene E.S., Coles M., Gray L., Laverty L., Martin K., Loeza I., Uribe A.J., Martínez B.C., Angel-Isaza J.A., Graham D., Owens C.M., Hargis B.M., Tellez-Isaias G. The effects of essential oil from Lippia origanoides and herbal betaine on performance, intestinal integrity, bone mineralization and meat quality in broiler chickens subjected to cyclic heat stress. Front. Physiol. 2023 doi: 10.3389/fphys.2023.1184636. 14, 1184636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi-Rad J., Herrera-Bravo J., Semwal P., Painuli S., Badoni H., Ezzat S.M., Farid M.M., Merghany R.M., Aborehab N.M., Salem M.A., Sen S., Acharya K., Lapava N., Martorell M., Tynybekov B., Calina D., Cho W.C. Artemisia spp.: an update on its chemical composition, pharmacological and toxicological profiles. Oxid. Med. Cell Longev. 2022;2022 doi: 10.1155/2022/5628601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata A.M., Saadeldin I.M., Tukur H.A., Habashy W.S. Modulation of heat-shock proteins mediates chicken cell survival against thermal stress. Animals. 2020;10:2407. doi: 10.3390/ani10122407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierzant K., Orda J., Korzeniowska M., Malicki A. Effect of dietary supplementation with extracts of rosemary, olive leaves, pine bark and quercetin on selected performance indices of broiler chickens and microbiological status of their ileum. Med. Weter. 2019;75:247–252. [Google Scholar]
- Solomou A.D., Sfougaris A. Contribution of agro-environmental factors to yield and plant diversity of olive grove ecosystems (Olea europaea L.) in the Mediterranean landscape. Agronomy. 2021;11:161. [Google Scholar]
- Song Z., Cheng K., Zhang L., Wang T. Dietary supplementation of enzymatically treated Artemisia annua could alleviate the intestinal inflammatory response in heat-stressed broilers. J. Therm. Biol. 2017;69:184–190. doi: 10.1016/j.jtherbio.2017.07.015. [DOI] [PubMed] [Google Scholar]
- Song Z.H., Cheng K., Zheng X.C., Ahmad H., Zhang L.L., Wang T. Effects of dietary supplementation with enzymatically treated Artemisia annua on growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poult. Sci. 2018;97:430–437. doi: 10.3382/ps/pex312. [DOI] [PubMed] [Google Scholar]
- Su L., Nalle S.C., Shen L., Turner E.S., Singh G., Breskin L.A., Khramtsova E.A., Khramtsova G., Tsai P.Y., Fu Y.X. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology. 2013;145:407–415. doi: 10.1053/j.gastro.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S., Li B., Wu M., Deng Y., Li J., Xiong Y., He S. Effect of dietary supplemental vitamin C and betaine on the growth performance, humoral immunity, immune organ index, and antioxidant status of broilers under heat stress. Trop. Anim. Health Prod. 2023;55:96. doi: 10.1007/s11250-023-03500-y. [DOI] [PubMed] [Google Scholar]
- Tang S., Yin B., Xu J., Bao E. Rosemary reduces heat stress by inducing CRYAB and HSP70 expression in broiler chickens. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/7014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torki M., Sedgh-Gooya S., Mohammadi H. Effects of adding essential oils of rosemary, dill and chicory extract to diets on performance, egg quality and some blood parameters of laying hens subjected to heat stress. J. Appl. Anim. Res. 2018;46:1118–1126. [Google Scholar]
- Tufarelli V., Laudadio V., Casalino E. An extra-virgin olive oil rich in polyphenolic compounds has antioxidant effects in meat-type broiler chickens. Environ. Sci. Pollut. Res. 2016;23:6197–6204. doi: 10.1007/s11356-015-5852-1. [DOI] [PubMed] [Google Scholar]
- Vandana G.D., Sejian V., Lees A.M., Pragna P., Silpa M.V., Maloney S.K. Heat stress and poultry production: impact and amelioration. Int. J. Biometeorol. 2021;65:163–179. doi: 10.1007/s00484-020-02023-7. [DOI] [PubMed] [Google Scholar]
- Wan X., Jiang L., Zhong H., Lu Y., Zhang L., Wang T. Effects of enzymatically treated Artemisia annua L. on growth performance and some blood parameters of broilers exposed to heat stress. Anim. Sci. J. 2017;88:1239–1246. doi: 10.1111/asj.12766. [DOI] [PubMed] [Google Scholar]
- Wang Y., Yang X., Li S., Wu Q., Guo H., Wang H., Su P., Wang J. Heat stress affects immune and oxidative stress indices of the immune organs of broilers by changing the expressions of adenosine triphosphate-binding cassette subfamily G member 2, sodium dependent vitamin C transporter-2, and mitochondrial calcium uniporter. Poult. Sci. 2023 doi: 10.1016/j.psj.2023.102814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., Lin H., Jiao H., Zhao J., Wang X. Chicken embryo thermal manipulation alleviates postnatal heat stress-induced jejunal inflammation by inhibiting transient receptor potential V4. Ecotoxicol. Environ. Saf. 2023;256 doi: 10.1016/j.ecoenv.2023.114851. [DOI] [PubMed] [Google Scholar]
- Yang C., Luo P., Chen S.J., Deng Z.C., Fu X.L., Xu D.N., Tian Y.B., Huang Y.M., Liu W.J. Resveratrol sustains intestinal barrier integrity, improves antioxidant capacity, and alleviates inflammation in the jejunum of ducks exposed to acute heat stress. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefdoost S., Samadi F., Jafari S.M., Ramezanpour S.S., Hassani S., Ganji F. Application of nanoencapsulated silymarin to improve its antioxidant and hepatoprotective activities against carbon tetrachloride-induced oxidative stress in broiler chickens. Livest. Sci. 2019;228:177–186. [Google Scholar]
- Yue Y., Guo Y., Yang Y. Effects of dietary L-tryptophan supplementation on intestinal response to chronic unpredictable stress in broilers. Amino Acids. 2017;49:1227–1236. doi: 10.1007/s00726-017-2424-3. [DOI] [PubMed] [Google Scholar]
- Zhang X., Akhtar M., Chen Y., Ma Z., Liang Y., Shi D., Cheng R., Cui L., Hu Y., Nafady A.A., Ansari A.R., Abdel-Kafy E.S.M., Liu H. Chicken jejunal microbiota improves growth performance by mitigating intestinal inflammation. Microbiome. 2022;10:107. doi: 10.1186/s40168-022-01299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Liu M., Sun H., Yang J.C., Huang Y.X., Huang J.Q., Lei X., Sun L.H. Selenium deficiency-induced multiple tissue damage with dysregulation of immune and redox homeostasis in broiler chicks under heat stress. Sci. China Life Sci. 2023:1–14. doi: 10.1007/s11427-022-2226-1. [DOI] [PubMed] [Google Scholar]
- Zwirzitz B., Oladeinde A., Johnson J., Zock G., Milfort M.C., Fuller A.L., Ghareeb A.A., Foutz J.C., Teran J.A., Woyda R., Abdo Z., Looft T., Lawrence J.P., Cudnik D., Aggrey S.E. Temporal dynamics of the cecal and litter microbiome of chickens raised in two separate broiler houses. Front. Physiol. 2023;14:326. doi: 10.3389/fphys.2023.1083192. [DOI] [PMC free article] [PubMed] [Google Scholar]