Abstract
Purpose
To describe a case of retinal vaso-occlusive vasculitis with associated lid edema and conjunctivitis following intravitreal pegcetacoplan administration in a patient with geographic atrophy (GA).
Observation
A 78 year old Caucasian woman presented with complaints of lid edema, conjunctival injection, loss of vision, and mild ocular discomfort eleven days after receiving an intravitreal pegcetacoplan injection in the left eye for geographic atrophy. Visual acuity on presentation was decreased to 20/400 from 20/200 previously in that eye. Eyelid edema and conjunctival injection were present with minimal anterior chamber reaction. Dilated fundus examination revealed hemorrhages throughout the retina and signs of retinal vasculitis. The patient subsequently developed hyphema and vitreous hemorrhage. Laboratory evaluations for common infectious and inflammatory causes including aqueous and vitreous cultures for bacteria and Herpes simplex PCR were normal or negative. A delayed hypersensitivity to pegcetacoplan was suspected and was treated with topical, oral subconjunctival and intravitreal steroids.
Conclusion
This index report illustrates a case of retinal vaso-occlusive vasculitis associated with intravitreal pegcetacoplan associated with lid edema and conjunctival injection and subsequent hyphema and vitreous hemorrhage. Therapy with steroids topically, systemically, periocularly and intravitreally were used to treat the inflammatory process and prevent further visual loss.
Keywords: Occlusive vasculitis, Retinal vasculitis, Pegcetacoplan, Geographic atrophy, Lid edema, Conjunctivitis, Hyphema, Vitreous hemorrhage
1. Introduction
Intravitreal pegcetacoplan providing local C3 inhibition has been shown to result in statistically significant reductions in the growth of GA compared to sham treatment.1 Associated adverse events have been reported to include culture positive endophthalmitis, culture-negative endophthalmitis, intraocular pressure increase, retinal detachment, and the development of exudative disease requiring treatment with anti-VEGF agents.1 We present a case of pegcetacoplan associated vaso-occlusive vasculitis atypically presenting with associated lid edema and conjunctivitis, and subsequently developing hyphema and vitreous hemorrhage.
2. Case report
A 78 year old Caucasian female with geographic atrophy threatening the fovea in both eyes had been followed for a number of years. The patient had an ocular history significant for cataract surgery in the left eye two years prior. Her medical history was significant for Hashimoto's thyroiditis and vertigo. Her systemic medications included levothyroxine. The patient had no history of any prior anti-VEGF treatments, no history of recent infections, specifically, CMV, Herpes simplex, toxoplasmosis and no history of autoimmune diseases like lupus or any malignancy. She did have a history of Hashimoto's thyroiditis and had COVID three months prior to presentation.
In March 2023, best-corrected visual acuity (VA) was 20/80 in both eyes. Autofluorescence photography revealed significant progression of the geographic atrophy over the past six years and it was elected to start treating both eyes with pegcetacoplan intravitreal injections. On the day of her first pegcetacoplan injection, visual acuity had dropped to 20/200 in the left eye. Pegcetacoplan was injected in that eye using aseptic technique, lid speculum, masking of the patient and doctor, use of betadine irrigation and antibiotic drops.
Eleven days after her injection, the patient presented to the ER with left eyelid edema, ocular discomfort and conjunctival injection. She also complained of headache and loss of vision. Topical treatment with antibiotic drops (Moxifloxacin) and ophthalmologic follow-up was recommended by the ER physician.
Two days after the ER visit (thirteen days after receiving the pegcetacoplan injection), the patient came for ophthalmologic evaluation. Visual acuity had decreased to 20/400 in the left eye. Anterior segment examination revealed 1+ conjunctival injection and 1+ lid edema of the left eye with minimal anterior chamber reaction. Intraocular pressure (IOP) was 33 mm Hg in her left eye. Funduscopic examination revealed diffuse intraretinal hemorrhages with vascular cuffing and macular edema. Fluorescein angiography showed macular staining of the geographic atrophy with cystoid changes. Blockage in the areas of hemorrhages was noted together with optic nerve and retinal vascular leakage and peripheral nonperfusion. No delay in filling time was noted (Fig. 1, Fig. 2, Fig. 3). The patient was advised to continue the topical antibiotic drops. Topical prednisolone acetate 1 % eyedrops four times daily were added to the regimen for mild inflammation and glaucoma therapy (Brimonidine/timolol, latanoprostene bunod, and netarsudil/latanoprost) was started to lower her intraocular pressure.
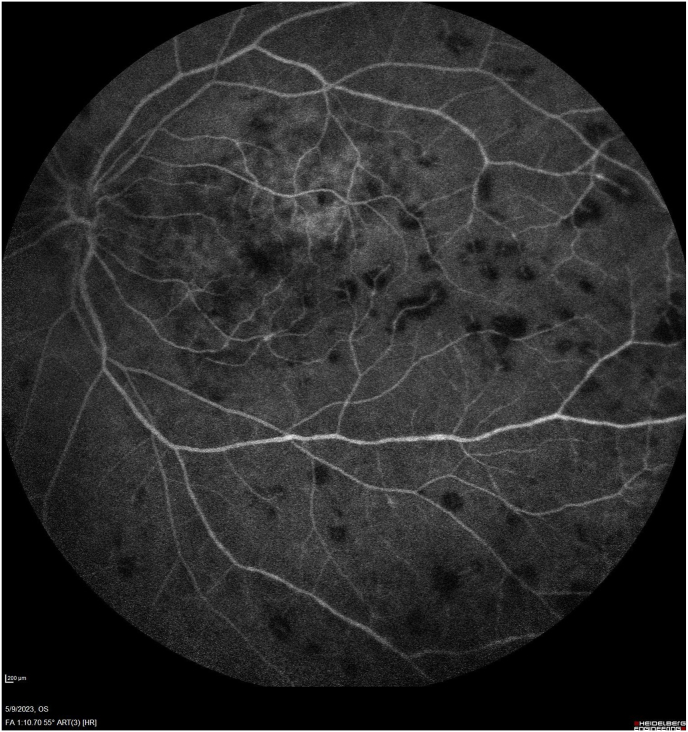
Fig. 1.
Fluorescein angiography at 1:10 displays blockage from intraretinal and perivascular hemorrhages scattered throughout the fundus and some central staining of the geographic atrophy. No vessel tortuosity is noted.
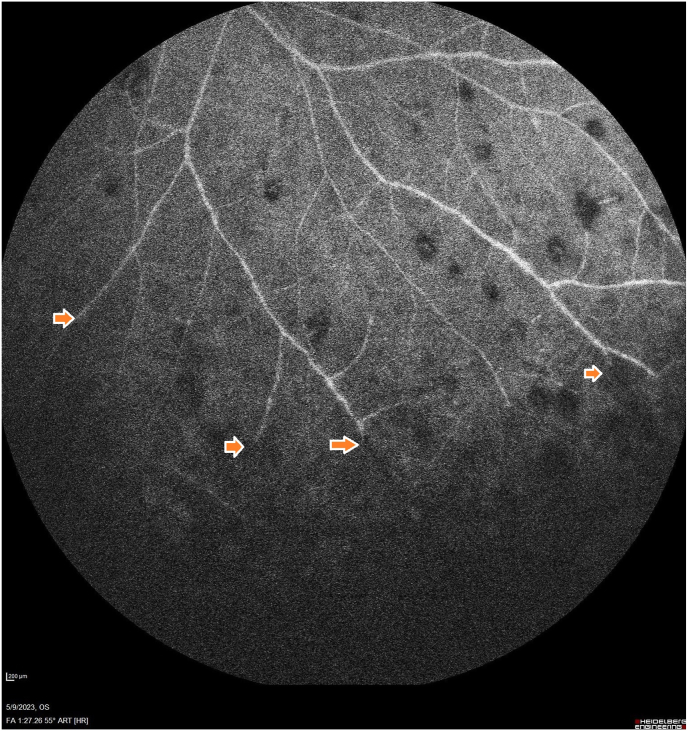
Fig. 2.
Fluorescein angiography at 1:27 peripherally displays nonperfusion and vascular leakage. The arrows indicate areas of vascular peripheral occlusion with nonperfusion.
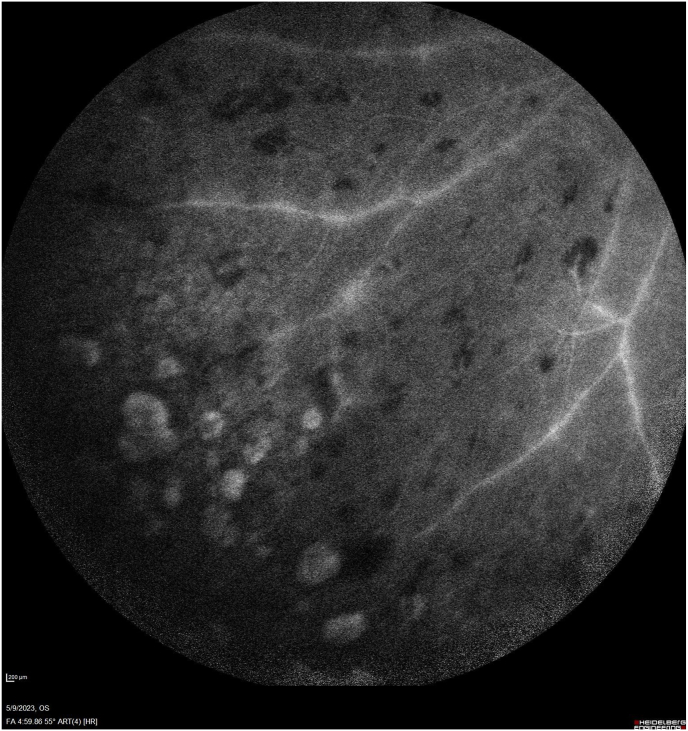
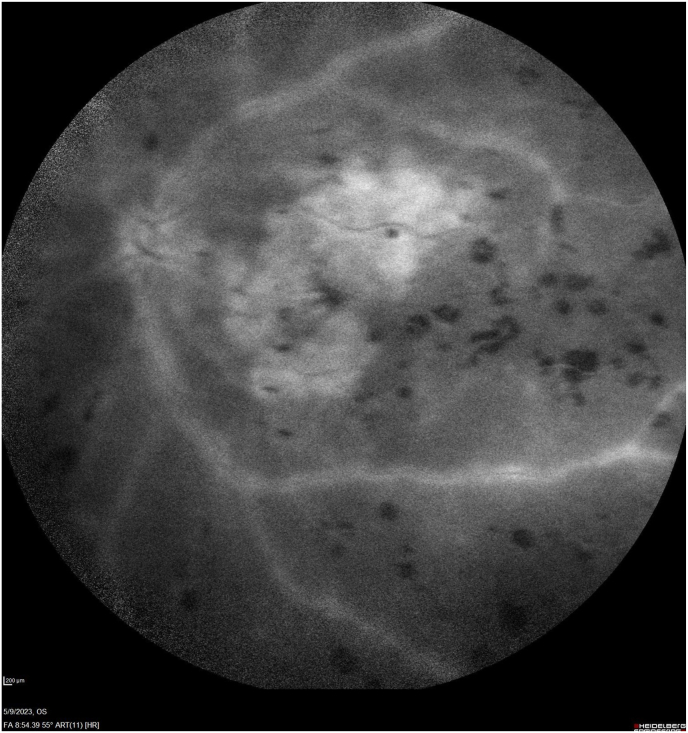
Fig. 3.
Fluorescein angiography late images at 4:59 and 8:54 display vascular leakage and optic nerve hyperfluorescence with central cystoid changes and staining of the geographic atrophy.
The next day (fourteen days post pegcetacoplan injection), VA remained 20/400 OS and IOP was 6 mm Hg OS. Examination revealed increased macular thickening and edema compared to the prior visit (Fig. 4). The possible need for anti-VEGF treatment was discussed and the latanoprostene bunod and netarsudil/latanoprost were discontinued, while steroid and antibiotic drops were continued.
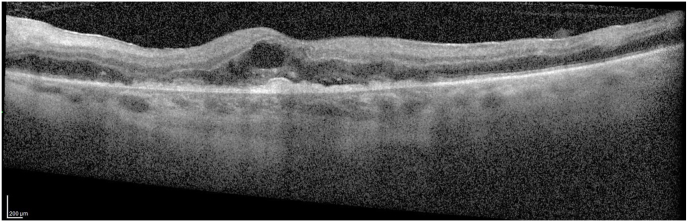
Fig. 4.
Thse optical coherence tomography lines (Spectralis: Heidelberg, Germany) portray the worsening in the macular edema in 24 hours just prior to the vitreous hemorrhage and hyphema presentation the following day.
The following day (fifteen days post pegcetacoplan injection), the VA in the left eye decreased to count fingers. By now, the lid edema had resolved but there still was conjunctival injection and there was an approximately 10 % hyphema (Fig. 5) and significant vitreous hemorrhage. No rubeosis iridis was present. IOP was 7 mm Hg in the left eye. B scan ultrasonography revealed a flat retina. An aflibercept intravitreal injection was administered and the patient was started on 50 mg Prednisone daily and was advised to continue the topical moxifloxacin and prednisolone acetate 1 % eye drops and to discontinue the brimonidine/timolol. Cyclopentolate 1 % drops were also added at bedtime because of the hyphema.
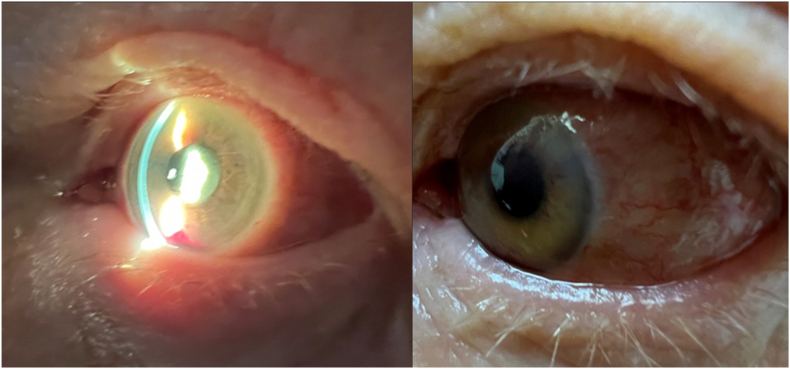
Fig. 5.
These photographs portray the hyphema and the conjunctival injection that was present on the day that the aflibercept injection was given and the patient was started on PO steroids.
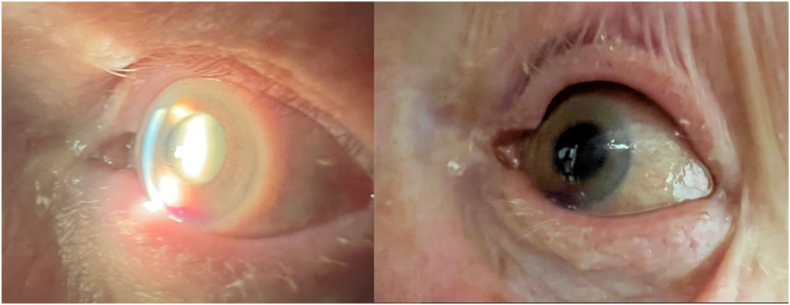
The following day (sixteen days post pegcetacoplan injection), VA remained at count fingers and IOP was 3 mm Hg. There was improvement in the conjunctival injection after one day of oral steroids, and the ocular discomfort was less (Fig. 6). Oral antibiotics were prescribed, and moxifloxacin eye drops were increased to q2 hours just in case this vasculitis was from an infectious etiology.
Fig. 6.
One day after the aflibercept injection and starting Prednisone 50 mg qd, there is improvement in the conjunctival injection of the left eye.
The following day (seventeen days post pegcetacoplan injection), the exam and vision was unchanged and IOP was 2 mm Hg with no further improvement. The IOP remained low and a small choroidal detachment was noted on B scan. It was decided to administer intravitreal ceftazidime and the patient was taken to the OR for a vitreous and aqueous tap and injection of intravitreal ceftazidime and subconjunctival dexamethasone. Vancomycin was not injected. Aqueous and vitreous specimens were sent for cultures and Herpes simplex PCR. The patient was placed on oral moxifloxacin in addition to the topical therapy.
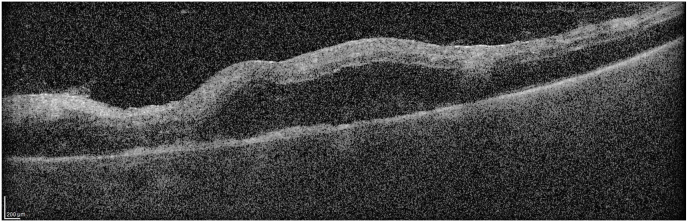
Postoperatively, the choroidal detachment resorbed. By Day 12 after the vitreous hemorrhage occurred (twenty six days post pegcetacoplan injection), visual acuity was count fingers with some improvement noted by the patient. The view of the retina was clearer and with the mild clearing of the vitreous hemorrhage and IOP had increased to 14 mm Hg in the left eye. The clearer view allowed imaging of the macula with OCT revealing that the edema had resolved.
Oral steroids were tapered slowly over a period of a month. Aqueous and vitreous cultures and Herpes simplex PCR of the aqueous and vitreous were negative. Inflammatory workup including complete blood count, C-reactive protein, erythrocyte sedimentation rate (ESR), antineutrophil cytoplasmic antibodies (c-ANCA), peripheral antineutrophil cytoplasmic antibodies (p-ANCA), angiotensin converting enzyme (ACE), lysozyme, Quantiferon Gold, syphilis screen, herpes simplex IgM and IgG titers were all negative. COVID titers were positive from her prior infection. Positive igG antibodies to Herpes Zoster were also detected.
When oral steroids were tapered to prednisone 5 mg every other day (six weeks post pegcetacoplan injection), ocular complaints recurred and a 1+ anterior reaction was noted. Visual acuity had decreased to count fingers. Topical steroid drops were increased, oral prednisone was increased, and an intravitreal dexamethasone implant was administered.
Four days following treatment, visual acuity recovered to 20/400 and remained stable from that point on. Another aflibercept injection was given five days after the dexamethasone implant placement to help clear the vitreous and retina hemorrhages. Retinal findings returned to baseline three months after the pegcetacoplan injection on continuing treatment with oral steroids. When the macular edema recurred as the prednisone was tapered down to 2.5 mg every other day another aflibercept injection was given. Oral steroids were discontinued five months post pegcetacoplan injection with no recurrence of ocular inflammation. Vision stabilized in the left eye at 20/400 but macular edema recurred which necessitated one additional aflibercept injection.
3. Discussion
During clinical trials of intravitreal pegcetacoplan injection, no events of occlusive vasculitis or retinitis were observed.2 However, a few cases of vasculitis were reported to the American Society of Retina Specialists after the medication was used in practice following FDA approval.3
The patient described in this report presented 11 days after an intravitreal pegcetacoplan injection with ocular signs of lid edema, conjunctival injection, ocular discomfort, mild anterior chamber reaction and elevated intraocular pressure. She was noted at that time to have diffuse intraretinal hemorrhages and signs of retinal vasculitis with peripheral occlusion and vascular leakage on fluorescein angiography. Although the external presentation resembled conjunctivitis with lid edema and conjunctival injection, the intraocular and angiographic findings were most consistent with a vaso-occlusive vasculitis.
Differential diagnosis included central retinal vein occlusion. However, the patient had no risk factors for central retinal vein occlusion such as diabetes or hypertension. In addition, there was no associated vascular tortuosity or engorgement of the veins while there was obvious vascular sheathing which is not consistent with central retinal vein occlusion. The patient did develop a vitreous hemorrhage, elevated intraocular pressure and hyphema which can also be seen in the setting of central retinal vein occlusion, but had no rubeosis and an open angle with no vessels in the angle. The vitreous hemorrhage may have occurred from retinal neovascularization with spillover of hemorrhage into the anterior chamber presenting with a hyphema.
Hemorrhagic occlusive retinal vasculitis (HORV) was also included in the differential diagnosis. HORV can also present with diffuse retinal hemorrhages and has been associated with intraocular use of Vancomycin. Our patient did not receive intraoperative or intracameral vancomycin or other agent given prior to her presentation. Similar to our patient's vaso-occlusive vasculitis, HORV is also a delayed onset hypersensitivity reaction and typically presents after 1–21 days4 after exposure to Vancomycin. This time frame is similar to the timing after injection of pegcetacoplan in our case. HORV may also progress to neovascular glaucoma and hemorrhage, but as noted before, our patient had no rubeosis to suggest this etiology for her ocular hypertension.
Retinal vasculitis has also been reported after injection of brolucizumab.5 In those cases, vasculitis was associated with intraocular inflammation, vascular sheathing and vascular leakage on fluorescein angiography similar to our case. Vasculitis from brolucizumab ranged from peripheral vasculitis to occlusion of large retinal arteries around the optic nerve and macula. Our case had peripheral occlusions, but no large arterial occlusions.
The patient presented here was initially treated with topical antibiotic drops for her external symptoms and signs. Topical steroids were added to help with the vasculitis and then with the onset of the hyphema, vitreous hemorrhage, retinal hemorrhages, and macular edema, an aflibercept intravitreal injection was given which was effective at resolving the macular edema. In addition, after repeat anti-VEGF treatment the hyphema, vitreous hemorrhage and retinal hemorrhages cleared. Recurrence of the macular edema after oral steroids were reduced to 2.5 mg every other day necessitated another aflibercept injection which was again effective. Treatment every 3 months with an anti-VEGF therapy may be needed in this patient going forward.
High dose (50 mg daily) oral prednisone was highly effective at treating the retinal vasculitis and had an immediate and marked effect on resolving the conjunctival signs and ocular discomfort as was seen in Fig. 6. When oral steroids were tapered because of side effects, symptoms and signs returned with a more prominent anterior chamber reaction. At that time an intravitreal dexamethasone implant was inserted which again resulted in suppression of inflammation.
Vitrectomy was performed mainly to obtain cultures and to administer intravitreal ceftazidime although the likelihood of an infectious etiology was remote. Cultures were as expected negative. It can of course be argued that the patient had also been given oral and topic antibiotics prior to the vitrectomy which may have affected the results. Clinically however, the presentation was very atypical for an infection-associated retinal vasculitis. In addition, the excellent response to systemic steroids strongly suggests an inflammatory etiology instead of an infectious one.
Like the brolucizumab and hemorrhagic occlusive retinal vasculitis cases, this presentation most likely represents a delayed hypersensitivity reaction. Hypersensitivity reactions have been reported after anti-VEGF therapy. These are mostly type I hypersensitivity reactions due to exogenous substances present in intravitreal injection6,7 and type IV or delayed-type hypersensitivity reactions to the anti-VEGF agent itself.8,9 Unlike most of the brolucizumab associated vasculitis,10 in our case, there had been no intraocular injections prior to the administration of pegcetacoplan and there was only a minimal anterior chamber reaction upon presentation.
4. Conclusion
In conclusion, medication-associated retinal vasculitis is a rare adverse effect of intravitreal pegcetacoplan administration and can be associated with a mild to severe level of inflammation and may lead to varying degrees of visual loss. Being vigilant after the administration of intraocular pegcetacoplan and encouraging prompt evaluation of patients who present with new symptoms post injection is critical. Prompt treatment with topical, local, systemic corticosteroids is critical to control the inflammatory response to prevent further visual loss. Anti-VEGF agents may also be helpful to treat any macular edema and associated vitreous hemorrhage.
Patient consent
Verbal and written consents have been obtained from the patient.
Authorship
All authors attest that they met the current ICMJE criteria.
CRediT authorship contribution statement
Stella Douros: Conceptualization, Data curation, Supervision, Writing – original draft, Writing – review & editing. David Mostafavi: Data curation. Mary Danias: Data curation, Software, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Liao D.S., Grossi F.V., El Mehdi D., et al. Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: a randomized phase 2 trial. Ophthalmology. 2020;127(2):186–195. doi: 10.1016/j.ophtha.2019.07.011. Feb. [DOI] [PubMed] [Google Scholar]
- 2.Apellis announces 24-month results showing increased effects over time with pegcetacoplan in phase 3 derby and oaks studies in geographic atrophy (GA) 2022. https://investors.apellis.com/news-releases/news-release-details/apellis-announces-24-month-results-showing-increased-effects
- 3.Kuriyan A.E., Witkin A.J. 2023. ReST Committee Update on Inflammation after Intravitreal Injections. [Google Scholar]
- 4.Witkin A.J., Chang D.F., Jumper J.M., et al. Vancomycin-associated hemorrhagic occlusive retinal vasculitis: clinical characteristics of 36 eyes. Ophthalmology. May 2017;124(5):583–595. doi: 10.1016/j.ophtha.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 5.Witkin A.J., Hahn P., Murray T.G., et al. Brolucizumab-associated intraocular inflammation in eyes without retinal vasculitis. Journal of vitreoretinal diseases. Jul 2021;5(4):326–332. doi: 10.1177/2474126420975303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleris R.S., Keswani A., Lugar P. The eyes have it: eyelid swelling and rash in a 79-year-old woman with macular degeneration. Allergy Rhinol (Providence) Jan-Dec 2018;9 doi: 10.1177/2152656718763385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veramme J., de Zaeytijd J., Lambert J., Lapeere H. Contact dermatitis in patients undergoing serial intravitreal injections. Contact Dermatitis. 2016;74(1):18–21. doi: 10.1111/cod.12478. Jan. [DOI] [PubMed] [Google Scholar]
- 8.Meredith G.G., Schkade P.A., Joondeph B.C. Allergic reaction upon intravitreal administration of anti-vascular endothelial growth factor agents. Retin Cases Brief Rep. Summer. 2019;13(3):287–289. doi: 10.1097/ICB.0000000000000581. [DOI] [PubMed] [Google Scholar]
- 9.Nagai N., Ibuki M., Shinoda H., Kameyama K., Tsubota K., Ozawa Y. Maculopapular rash after intravitreal injection of an antivascular endothelial growth factor, aflibercept, for treating age-related macular degeneration: a case report. Medicine (Baltim) May 2017;96(21) doi: 10.1097/MD.0000000000006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumal C.R., Spaide R.F., Vajzovic L., et al. Retinal vasculitis and intraocular inflammation after intravitreal injection of brolucizumab. Ophthalmology. 2020;127(10):1345–1359. doi: 10.1016/j.ophtha.2020.04.017. Oct. [DOI] [PubMed] [Google Scholar]