Abstract
Background
Magnetic resonance‐guided focused ultrasound (MRgFUS) thalamotomy is increasingly used to treat drug‐resistant essential tremor (ET). Data on MRgFUS thalamotomy in dystonic tremor (DT) are anecdotal.
Objectives
To investigate efficacy, safety, and differences in target coordinates of MRgFUS thalamotomy in DT versus ET.
Methods
Ten patients with DT and 35 with ET who consecutively underwent MRgFUS thalamotomy were followed for 12 months. Although in both groups the initial surgical planning coordinates corresponded to the ventralis intermediate (Vim), the final target could be modified intraoperatively based on clinical response.
Results
Tremor significantly improved in both groups. The thalamic lesion was significantly more anterior in DT than ET. Considering both ET and DT groups, the more anterior the lesion, the lower the odds ratio for adverse events.
Conclusions
MRgFUS thalamotomy is safe and effective in DT and ET. Compared to classical Vim coordinates used for ET, more anterior targeting should be considered for DT.
Keywords: MRgFUS, thalamotomy, essential tremor, dystonic tremor, dystonia
Magnetic resonance guided focused ultrasound surgery (MRgFUS) is an ablative technique, which allows to create precise thermal lesions without craniotomy. MRgFUS thalamotomy in the ventralis intermediate (Vim) nucleus has been shown to improve tremor in patients with essential tremor (ET) and Parkinson's disease. 1 , 2 A long‐lasting clinical benefit up to 5 years after surgery has been consistently demonstrated in ET. 3 , 4 , 5 , 6
Tremor is a common neurological symptom and causes disability and loss of independence in daily activities. 7 , 8 It has been recently suggested that ET should be considered a syndrome rather than a specific disease and that tremor may be an intrinsic part of dystonia (dystonic tremor [DT]). 9 , 10 Different tremor etiologies may underpin distinct pathophysiological mechanisms of tremor generation. Therefore, it is conceivable that different therapeutic approaches are needed to optimize clinical response in DT versus ET. 11
Although Vim is the main target for the surgical treatment of ET, 12 different nuclei have been proposed for DT: the globus pallidus, 13 , 14 the subthalamus, 15 and the motor thalamus. 16 , 17 To date, there is only one report about the effects of MRgFUS Vim thalamotomy on two patients with DT. 18 Other groups reported the effect of MRgFUS pallido‐thalamic tractotomy 19 or pallidotomy 20 on dystonic patients, but without mentioning tremor as dystonic feature. To the best of our knowledge, there is still no consensus on whether patients with DT who undergo MRgFUS thalamotomy require different targeting than ET. Considering the different pathophysiology, it is conceivable that the most effective target coordinates may differ between ET and DT. This has also been suggested in a recent study on patients with DT and ET treated with thalamic stimulation in which the authors found that the optimal stimulation region was more anterior in DT compared with ET. 11
In this study, we investigated efficacy, safety, and stereotactic targeting of MRgFUS thalamotomy in a consecutive cohort of patients with drug‐resistant DT or ET prospectively followed for 12 months.
Methods
We prospectively enrolled consecutive patients with DT or ET with disabling drug‐resistant tremor who underwent MRgFUS thalamotomy at our Institute from January 2019 to December 2021.
ET and DT were diagnosed accordingly to the Consensus Statement on the Classification of Tremors. 10 More specifically, patients with tremor in the same body region affected by dystonia were classified as DT, whereas patients with questionable dystonic signs were included in the ET group (clinical diagnosis of ET plus). 10 Patients with concomitant tremor and dystonia, but in different body regions (ie, tremor associated with dystonia) were excluded from this study. 10
Drug resistance was defined as <30% improvement in the Tremor Research Group Essential Tremor Rating scale (TETRAS21, 22 after adequate trial of at least two different medications. 23
Extensive inclusion and exclusion criteria for eligibility to surgery are detailed in the Data S1. 24
Patients were evaluated at baseline (3 ± 1 weeks before surgery) and at 12 months follow‐up by means of TETRAS scale. 21 , 22 TETRAS is divided in two parts, assessing the disability in the activities of daily living (ADL section) and evaluating the clinical severity of tremor (Performance section). Higher scores indicate more severe impairment. The total score, a sub‐score for the treated body side (sum of subitems 4a, 4b, 4c, 6, 7 [if the treated side was the dominant side], and 8 of the treated body side) and the subscore for axial tremor (sum of subitems 1, 2, 3, and 9) were computed.
Adverse events (AE) related to thalamotomy were collected at 1, 6, and 12 months.
MRgFUS Thalamotomy
Patients' preparation, targeting, and execution of MRgFUS thalamotomy followed standard workup previously described. 1 , 25 The procedure was performed using a 1.5 Tesla (T) magnetic resonance imaging (MRI) scanner with the ExAblate 4000 device (InSightec, Haifa, Israel). Initial stereotactic coordinates were calculated based on previous literature, 1 targeting the Vim for both DT and ET patients. During the procedure the target for the final sonication was refined accordingly to the intraoperative tremor improvement. More specifically, tremor was clinically evaluated at baseline (before the first sonication) and after sonications with moderate heating (temperature, 50°C–54°C), which produce transient clinical effects. Intraoperative clinical examination included writing a simple sentence, drawing spirals and lines, evaluation of rest, postural, and kinetic tremor. 26 During the procedure, the target was modified in case of adverse effects or poor tremor control by gradually moving 0.5 to 1 mm in the following directions: anterior > medial > superior > lateral > posterior. This pre‐defined plan was systematically used for all patients, with the same approach in both ET and DT patients. These directions were chosen based on previous reports indicating that adverse effects incidence and type depend on lesion location (ie, sensory and motor AE are associated with lesions extending, respectively, too posteriorly or laterally, whereas gait difficulties, dysmetria and speech, effect may occur when lesion are located infero‐laterally to the thalamus; speech effects may also occur when lesion are located medially to the Vim). 27
Lesion Volume and Coordinates
The thalamic lesion of each patient was delineated in the axial plane with MRIcro software (https://people.cas.sc.edu/rorden/mricro/mricro.html), using post‐contrast volumetric T1‐weighted sequences (isotropic voxel size, 0.9 mm) acquired 1 day after treatment. The lesion outline was delineated considering the cytotoxic edema as the margin, visible as an irregular perilesional hyperintensity. 28
Patient‐specific coordinates of the lesion center were calculated on T1‐weighed 3D‐MRI scans performed at 1 month follow‐up on 3 T MRI scanner (Achieva TX, Philips Healthcare BV, Best, The Netherlands). The lesion center was manually detected and the stereotactic coordinates (X, Y, and Z) were computed. X represents the distance between the lesion center and midline; this value has been acquired as a positive number, independently from the hemisphere of the lesion. Y indicates the distance from posterior commissure (PC) in anteroposterior axis. This coordinate was expressed as absolute length and as percentage of the (anterior commissure‐posterior commissure) ACPC distance. Z indicates the distance from an axial plane passing through ACPC; positive values indicate that the lesion center was above that plane.
Ethics
All patients gave their written informed consent to participate in this study. The study was conducted in accordance with the Declaration of Helsinki and approved by the local ethics committee (CE no. 59/2020).
Statistical Analysis
Comparison of baseline characteristics between ET and DT and differences in the incidence of AEs were computed with the Mann–Whitney U‐test or Fischer's exact test as appropriate. Longitudinal modifications of clinical scales were evaluated with repeated measures analysis of variance, with time and diagnosis as factors. A stepwise binary logistic regression model based on Akaike information criterion was used to verify the relationship between the lesion coordinates and AEs. Statistical significance was set at P < 0.05. Statistics were computed with R. 29 , 30 , 31 , 32 , 33
Results
A total of 58 patients (11 DT and 47 ET) underwent MRgFUS thalamotomy during the 3 years study period. One patient with DT and 12 patients with ET were excluded from analysis for various reasons (see Data S1). Data from 10 patients with DT and 35 with ET were analyzed.
Demographic, clinical features, lesion volume, and coordinates of the two groups are reported in the Table 1. No significant differences were found at baseline on TETRAS ADL or Performance section scores. We found no differences in sonication parameters between the two groups (Data S1). ADL, Performance total scores, and Performance treated side score significantly improved similarly in both groups. Axial score improved significantly in ET patients and trended toward improvement in DT (P = 0.033 and P = 0.075, respectively).
TABLE 1.
Demographic information, clinical variables, lesion volume, lesion coordinates, and AE in patients with DT and ET
| DT (n = 10) | ET (n = 35) | p‐value (DT vs. ET) | ||||
| Baseline | 12 months | Baseline | 12 months | |||
| Demographic information and clinical variables | ||||||
| Women, No. (%) | 3 (30) | – | 7 (20) | – | 0.668 | |
| Right handed, No. (%) | 9 (90) | – | 35 (100) | – | 0.222 | |
| Treated side left, No. (%) | 9 (90) | – | 32 (91.4) | – | 0.999 | |
| Disease duration, years | 35 [23, 41] | – | 26 [17, 55] | – | 0.801 | |
| Age at surgery, years | 56 [44, 66] | – | 73.0 [67, 76] | – | <0.001 | |
| SDR | 0.50 [0.47, 0.52] | – | 0.54 [0.48, 0.59] | – | 0.922 | |
| ADL a | 27 [24, 29] | 10 [6, 14]*** | 29 [26, 31] | 11 [8, 19]*** | 0.089 d | |
| Performance (tot) a | 22 [21, 27] | 17 [13, 21]** | 27 [22,33] | 20 [16, 23]*** | 0.142 d | |
| Performance (treated side) a , b | 13 [12, 14] | 5 [4, 7]*** | 15 [12, 17] | 7 [5, 9]*** | 0.071 d | |
| Performance (axial score) c | 1.5 [0, 4.5] | 0 [0, 1] | 2 [0, 3] | 1 [0, 2]* | 0.999 d | |
| Lesion volume and coordinates | ||||||
| Lesion volume (mm3) | 204 [172, 243] | 170 [101, 231] | 0.181 | |||
| ACPC, mm | 26.8 [26.0, 27.0] | 27.0 [25.8, 28.3] | 0.410 | |||
| X (latero‐lateral), mm e | 13.3 [12.6, 14.0] | 13.5 [13.0, 14.5] | 0.189 | |||
| Y (anteroposterior), mm f | 9.0 [8.0, 9.5] | 8.0 [7.0, 9.0] | 0.025 | |||
| Y (anteroposterior), % of ACPC f | 33 [31, 35] | 29 [27, 31] | 0.007 | |||
| Z (superoinferior), mm g | 1.3 [1.0, 1.7] | 1.5 [0.5, 2.5] | 0.752 | |||
| Adverse Events | ||||||
| Patients with 1 or more AE, No. (%) | ||||||
| 1 month | 1 (10) | 12 (34.3) | 0.238 | |||
| 6 months | – | 8 (22.9) | 0.168 | |||
| 12 months | – | 5 (14.3) | 0.571 | |||
| Total no. of AE | 2 | 21 | ||||
| Imbalance | Transient h | 1 | 6 | |||
| Persistent i | – | – | ||||
| Dysarthria | Transient | 1 | 4 | |||
| Persistent | – | 1 | ||||
| Limb ataxia | Transient | – | 2 | |||
| Persistent | – | – | ||||
| Oral paresthesias | Transient | – | – | |||
| Persistent | – | 2 | ||||
| Hand paresthesias | Transient | – | 2 | |||
| Persistent | – | 1 | ||||
| Inferior limb weakness | Transient | – | 2 | |||
| Persistent | – | – | ||||
| Ataxia‐hemiparesis | Transient | – | – | |||
| Persistent | – | 1 | ||||
Note: data are expressed as median [IQR] unless otherwise specified. Significant P‐values are highlighted in bold, trends towards significance in italics.
Abbreviations: AE, adverse events; DT, dystonic tremor; ET, essential tremor; SDR, skull density ratio; ADL, activities of daily living; ACPC, anterior commissure‐posterior commissure; IQR, interquartile range; TETRAS, Tremor Research Group Essential Tremor Rating scale; rmANOVA, repeated measures analysis of variance.
P < 0.05 compared to baseline values.
P < 0.01 compared to baseline values.
P < 0.001 compared to baseline values.
Evaluated with the TETRAS scale. 23
Sum of subitems of the Performance section 4a, 4b, 4c, 6, and 7 (if the treated side was the dominant side) and 8 relative only to the treated side.
Sum of subitems of the Performance section 1, 2, 3, and 9.
P‐value for the between‐group difference (DT vs. ET) in the rmANOVA.
Distance of the center of the lesion from midline.
Distance of the center of the lesion from the posterior commissure in anteroposterior axis.
Distance of the center of the lesion from the axial plane passing through ACPC line.
AE that resolved between 1 and 12 months after thalamotomy.
AE that were persistent 12 months after thalamotomy.
The analysis of the lesion coordinates revealed that in DT patients the thalamotomy was significantly more anterior than in ET patients (see Table 1).
In the DT group, only one patient had mild AE related to thalamotomy (imbalance and dysarthria without any substantial impairment in walking or speaking), which completely resolved 2 months after surgery. AEs were more common among ET, and at 12 months five patients (14.3%) had persistent mild AE. However, the frequency of AEs did not differ between the two groups (P = 0.238) (Table 1).
One ET patient reported a major AE (ataxic hemiparesis persistent at 12 months follow‐up).
Considering the whole study population, a multivariable logistic regression analysis with the dichotomic variable presence/absence of at least one AE as dependent variable and the lesion coordinates (X, Y, and Z), lesion volume, and age at surgery as independent variables revealed the association between a more anterior lesion and a reduced odds ratio (OR) for AE (OR = 0.751, 95% confidence interval [CI] = [0.589–0.957], P = 0.021). The lesion volume trended toward greater incidence of AE (OR = 1.010, 95% CI = [1.000–1.020], P = 0.057).
X and Y coordinates of the lesion in patients with or without AEs are shown in the Figure 1. The majority of patients with AEs have a more posterior (and slightly more lateral) lesion.
FIG. 1.
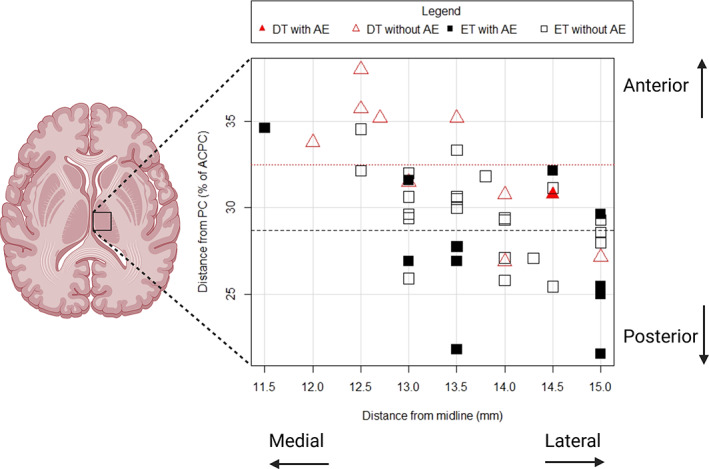
Graphical representation of the center of thalamic lesions in dystonic tremor (DT) and essential tremor (ET) patients. The position of the lesion is reported in two dimensions, with the coordinates X (latero‐lateral coordinate, ie, distance of the center of the lesion from midline) and Y (anteroposterior coordinate, expressed as a percentage of the length of anterior commissure‐posterior commissure [ACPC] from the posterior commissure). Filled symbols represent patients with one or more adverse events. Dotted and dashed lines represent the mean antero‐posterior position of the thalamic lesion in DT and ET, respectively. The mean anteroposterior position of the thalamic lesion is more anterior in DT compared to ET. Partially created with BioRender.com.
Discussion
Vim nucleus is the established surgical target for drug‐resistant ET. 12 Conversely, the surgical target for DT is still a matter of debate. 11 Although both lesional techniques and deep brain stimulation (DBS) in the motor thalamus have been adopted for DT, published literature is limited to small case series or case reports. 17 The motor thalamus includes the ventral anterior (which receives fibers from internal pallidum and substantia nigra pars reticulata and encompasses the ventralis oralis anterior [Voa] and posterior [Vop] nuclei) 34 and the ventral lateral nucleus (that receives cerebellar afferences and corresponds to the Vim). 16 , 34
Currently, MRgFUS thalamotomy may rely solely on indirect targeting, 1 , 25 because 1.5 T and 3 T MRI cannot precisely identify intrathalamic nuclei. MRgFUS thalamotomy allows target refinement before permanent lesioning using moderate heating (50°C–54°C). 1 Intraoperative assessment for tremor reduction and side effects guides the escalation of acoustic energy to achieve tissue ablation. 1 , 35 In our study, both the processes of initial targeting and the target adjustments to improve safety and efficacy during sublesional sonications were identical between DT and ET. However, the careful process of intraoperative patient assessment determined, in DT patients, a refinement of the target more anterior than initial surgical planning, with the placement of the thalamic lesion significantly more anterior in DT compared with ET, confirmed by the lesion coordinates 1 month after surgery (Table 1).
A similar finding has been reported in a recent connectivity study, where the optimal stimulation region for tremor control was more anterior in DT than in ET. 11 This may be because of the involvement of the pallidothalamic tract, which primarily projects to the Vop and plays a fundamental role in the pathophysiology of DT. 11
ADL, Performance total score and Performance treated‐side score significantly improved in both DT and ET groups. In ET patients, we also observed a significant improvement in axial‐score, confirming previous findings in a larger cohort of ET patients. 36 The improvement in axial‐score clearly trended toward significance in DT patients; studies in larger cohorts are needed to confirm our preliminary findings. Such an effect would be in line with the efficacy of bilateral DBS of ventrolateral thalamus on dystonic head tremor. 37 The relatively small sample size and the unilateral thalamic lesion in the present study may partly justify this discrepancy.
The AEs profile in our ET patients is comparable with previous reports. 1 , 38 A retrospective study found that the AEs related to stimulation (with dysarthria and gait imbalance being the most common) were not different between ET and DT after Vim DBS. 39 Our study overall confirms this finding. However, it should be noted that, despite the lack of statistically significant differences in AEs frequency between ET and DT, only one patient with DT had mild AEs, which completely resolved a few weeks after surgery.
Fasano et al 18 reported one DT patient who developed paresthesia after MRgFUS thalamotomy, which persisted at 6 months. We did not register sensory deficits among DT patients and this may be because of the more anterior lesion location respective to classical Vim coordinates, 1 with sparing of thalamic sensory nuclei. Sensory complaints are, in fact, a common AE after MRgFUS Vim thalamotomy in ET. 1 , 23 , 35 , 40 Here, the volume of the lesion trended toward greater incidence of AE despite not reaching statistical significance, supporting previous evidence suggesting that larger lesions increase the risk of AEs. 41
Vim targeting in MRgFUS differs in current literature, particularly for the anteroposterior coordinate. In fact, although some used the same targeting on every patient (eg, 7 mm anteriorly to PC), 35 others used a patient‐specific approach (that is the one adopted by our group). 1
Based on our results, we suggest that a more anteromedial initial targeting (one third of the ACPC length and 13–13.5 mm lateral to midline) could be considered for DT patients.
There are limitations to acknowledge. First, the limited sample size of the DT group; accordingly, this should be considered a pilot study, whose results need to be replicated in larger cohorts. Second, we provided data on the location of the center of the lesion in terms of stereotactic coordinates. We are currently analyzing the relationship between outcome, lesion volume, and the involvement of the dentato‐rubro‐thalamic tract. Third, the TETRAS scale was used in both groups to ensure uniformity in representing tremor severity. We acknowledge that this scale has been specifically validated only in ET and may not represent the best clinical tool for assessing DT. Last, our study was not designed to specifically identify which target to prefer in DT patients due to the lack of a control group of patients with DT in whom the actual lesion was made at classical Vim coordinates. Moreover, we did not systematically explore all the directions around the initial target, because we used the same pre‐defined scheme to modify the target during sublesional sonications for all patients.
Furthermore, it is worth emphasizing that this is the first study suggesting that the initial coordinates to be considered in the surgical planning for MRgFUS thalamotomy in DT may be more anterior than standard Vim coordinates commonly used in ET. We provided preliminary evidence that a differential targeting is probably associated with good clinical outcomes in terms of both efficacy and safety. The present data emphasize the importance of a correct differential diagnosis between ET and DT and its implications within the framework of “precision medicine” in the setting of MRgFUS treatment.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
N.G.A.: 1A, 1B, 1C, 2A, 2B, 3A, 3B
A.B.: 1C, 2C, 3A, 3B
V.L.: 1B, 1C, 2C, 3B
S.R.: 1B, 1C, 2C, 3B
F.G.: 1A, 1B, 2C, 3C
R.C.: 1A, 1B, 2A, 2C, 3A, 3B
L.M.R.: 1C, 2C, 3B
S.B.: 1B, 1C, 2C, 3B
A.E.E.: 1B, 2C, 3B
G.D.: 1C, 2C, 3B
R.T.: 1B, 2C, 3B
F.C.: 1C, 2C, 3B
M.G.B.: 1B, 1C, 2C, 3B
G.M.: 1C, 2C, 3B
M.C.: 1C, 2C, 3B
M.S.: 1B, 1C, 2C, 3B
V.C.: 1C, 2C, 3B
S.P.: 1C, 2C, 3B
P.A.: 1C, 2C, 3B
M.F.P.: 1C, 2C, 3B
S.H.M.P.: 1B, 1C, 2C, 3B
M.G.: 1C, 2C, 3B
E.F.M.C.: 1B, 1C, 2C, 3B
F.D.: 1B, 1C, 2C, 3B
R.E.: 1A, 1B, 1C, 2A, 2C, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. Ethics Committee of the Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano (reference number: CE no. 59/2020). The study was conducted in accordance with the declaration of Helsinki and written informed consent was obtained by study participants.
Funding Sources and Conflicts of Interest: All the authors report no conflict of interest related to this manuscript.
Financial Disclosures for the Previous 12 Months: N.G.A., A.B., S.R., F.G., L.M.R., S.B., A.E.E., G.D., R.T., F.C., M.G.B., G.M., M.C., M.S., V.C., S.P., P.A., M.F.P., S.H.M.P., M.G., E.F.M.C., F.D., and R.E. have no financial disclosures to report. R.C. has received speaking honoraria from Zambon Italia, Zambon SAU, and Bial Italia Srl; has received advisory board fees from Bial; has received research support from the Italian Ministry of Health; is the Editor‐in‐Chief of the neuromuscular and movement disorders section of Brain Sciences; and is the Associate Editor of Parkinsonism and Related Disorders, Frontiers in Neurology, and Frontiers in Aging Neuroscience. V.L. received honoraria from Medtronic SPA and Wise SRL.
Supporting information
Data S1. Supporting Information.
Acknowledgment
Open access funding provided by BIBLIOSAN.
Relevant disclosures and conflict of interest are listed at the end of this article.
Data Availability Statement
The data supporting the findings of this study will be available on request on https://zenodo.org.
References
- 1. Elias WJ, Lipsman N, Ondo WG, et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med 2016;375:730–739. 10.1056/nejmoa1600159. [DOI] [PubMed] [Google Scholar]
- 2. Bond AE, Shah BB, Huss DS, et al. Safety and efficacy of focused ultrasound thalamotomy for patients with medication‐refractory, tremor‐dominant Parkinson disease: a randomized clinical trial. JAMA Neurol 2017;74:1412–1418. 10.1001/jamaneurol.2017.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chang JW, Park CK, Lipsman N, et al. A prospective trial of magnetic resonance–guided focused ultrasound thalamotomy for essential tremor: results at the 2‐year follow‐up. Ann Neurol 2018;83:107–114. 10.1002/ana.25126. [DOI] [PubMed] [Google Scholar]
- 4. Park Y, Jung NY, Na YC, Chang JW. Four‐year follow‐up results of magnetic resonance‐guided focused ultrasound thalamotomy for essential tremor. Mov Disord 2019;34:727–734. 10.1002/mds.27637. [DOI] [PubMed] [Google Scholar]
- 5. Sinai A, Nassar M, Eran A, Constantinescu M, Zaaroor M, Sprecher E, Schlesinger I. Magnetic resonance–guided focused ultrasound thalamotomy for essential tremor: a 5‐year single‐center experience. J Neurosurg 2020;133:417–424. 10.3171/2019.3.jns19466. [DOI] [PubMed] [Google Scholar]
- 6. Cosgrove GR, Lipsman N, Lozano AM, et al. Magnetic resonance imaging–guided focused ultrasound thalamotomy for essential tremor: 5‐year follow‐up results. J Neurosurg 2022;138:1–6. 10.3171/2022.6.jns212483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schneider SA, Deuschl G. The treatment of tremor. Neurotherapeutics 2014;11:128–138. 10.1007/s13311-013-0230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zaaroor M, Sinai A, Goldsher D, Eran A, Nassar M, Schlesinger I. Magnetic resonance–guided focused ultrasound thalamotomy for tremor: a report of 30 Parkinson's disease and essential tremor cases. J Neurosurg 2018;128:202–210. 10.3171/2016.10.jns16758. [DOI] [PubMed] [Google Scholar]
- 9. Deuschl G, Bain P, Brin M, Committee AHS. Consensus statement of the Movement Disorder Society on tremor. Mov Disord 1998;13:2–23. 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 10. Bhatia KP, Bain P, Bajaj N, et al. Consensus statement on the classification of tremors. From the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord 2018;33:75–87. 10.1002/mds.27121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tsuboi T, Wong JK, Eisinger RS, et al. Comparative connectivity correlates of dystonic and essential tremor deep brain stimulation. Brain 2021;144:1774–1786. 10.1093/brain/awab074. [DOI] [PubMed] [Google Scholar]
- 12. Ferreira JJ, Mestre TA, Lyons KE, et al. MDS evidence‐based review of treatments for essential tremor. Mov Disord 2019;34:950–958. 10.1002/mds.27700. [DOI] [PubMed] [Google Scholar]
- 13. Tsuboi T, Au KLK, Deeb W, Almeida L, Foote KD, Okun MS, Ramirez‐Zamora A. Motor outcomes and adverse effects of deep brain stimulation for dystonic tremor: a systematic review. Parkinsonism Relat D 2020;76:32–41. 10.1016/j.parkreldis.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 14. Trompette C, Giordana C, Leplus A, Grabli D, Hubsch C, Marsé C, Fontaine D. Combined thalamic and pallidal deep brain stimulation for dystonic tremor. Parkinsonism Relat D 2022;103:29–33. 10.1016/j.parkreldis.2022.08.003. [DOI] [PubMed] [Google Scholar]
- 15. Kitagawa M, Murata J, Kikuchi S, Sawamura Y, Saito H, Sasaki H, Tashiro K. Deep brain stimulation of subthalamic area for severe proximal tremor. Neurology 2000;55:114–116. 10.1212/wnl.55.1.114. [DOI] [PubMed] [Google Scholar]
- 16. Mai JK, Majtanik M. Toward a common terminology for the thalamus. Front Neuroanat 2019;12:114. 10.3389/fnana.2018.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mongardi L, Rispoli V, Scerrati A, et al. Deep brain stimulation of the ventralis oralis anterior thalamic nucleus is effective for dystonic tremor. Parkinsonism Relat D 2020;81:8–11. 10.1016/j.parkreldis.2020.09.040. [DOI] [PubMed] [Google Scholar]
- 18. Fasano A, Llinas M, Munhoz RP, Hlasny E, Kucharczyk W, Lozano AM. MRI‐guided focused ultrasound thalamotomy in non‐ET tremor syndromes. Neurology 2017;89:771–775. 10.1212/wnl.0000000000004268. [DOI] [PubMed] [Google Scholar]
- 19. Jamora RDG, Chang W‐C, Taira T. Transcranial magnetic resonance‐guided focused ultrasound in X‐linked dystonia‐parkinsonism. Life 2021;11:392. 10.3390/life11050392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Horisawa S, Yamaguchi T, Abe K, et al. Magnetic resonance‐guided focused ultrasound thalamotomy for focal hand dystonia: a pilot study. Mov Disord 2021;36:1955–1959. 10.1002/mds.28613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elble R, Comella C, Fahn S, et al. Reliability of a new scale for essential tremor. Mov Disord 2012;27:1567–1569. 10.1002/mds.25162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ondo W, Hashem V, LeWitt PA, et al. Comparison of the Fahn‐Tolosa‐Marin clinical rating scale and the essential tremor rating assessment scale. Mov Disord Clin Pract 2018;5:60–65. 10.1002/mdc3.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elias WJ, Huss D, Voss T, et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N Engl J Med 2013;369:640–648. 10.1056/nejmoa1300962. [DOI] [PubMed] [Google Scholar]
- 24. Andreasi NG, Cilia R, Romito LM, et al. Magnetic resonance‐guided focused ultrasound thalamotomy may spare dopaminergic therapy in early‐stage tremor‐dominant Parkinson's disease: a pilot study. Mov Disord 2022;37:2289–2295. 10.1002/mds.29200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chang WS, Jung HH, Kweon EJ, Zadicario E, Rachmilevitch I, Chang JW. Unilateral magnetic resonance guided focused ultrasound thalamotomy for essential tremor: practices and clinicoradiological outcomes. J Neurol Neurosurg Psychiatry 2015;86:257–264. 10.1136/jnnp-2014-307642. [DOI] [PubMed] [Google Scholar]
- 26. Walters H, Shah BB. Focused ultrasound and other lesioning therapies in movement disorders. Curr Neurol Neurosci 2019;19:66. 10.1007/s11910-019-0975-2. [DOI] [PubMed] [Google Scholar]
- 27. Boutet A, Ranjan M, Zhong J, et al. Focused ultrasound thalamotomy location determines clinical benefits in patients with essential tremor. Brain 2018;141:3405–3414. 10.1093/brain/awy278. [DOI] [PubMed] [Google Scholar]
- 28. Wintermark M, Druzgal J, Huss DS, et al. Imaging findings in MR imaging‐guided focused ultrasound treatment for patients with essential tremor. Am J Neuroradiol 2014;35:891–896. 10.3174/ajnr.a3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2022. https://www.R-project.org/. [Google Scholar]
- 30. Fox J. The R commander: a basic statistics graphical user Interface to R. J Stat Softw 2005;14(9):1–42. [Google Scholar]
- 31. Fox J. Using the R Commander: A Point‐and‐Click Interface or R. Boca Raton FL: Chapman and Hall/CRC Press; 2017. [Google Scholar]
- 32. Fox J, Bouchet‐Valat M. Rcmdr: R commander; 2022. R Package Version 2.8‐0.
- 33. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transpl 2013;48:452–458. 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hassler R, Schaltenbrand G, Wahren W. Architectonic organization of the thalamic nuclei. Atlas for stereotaxy of the human brain: with an accompanying guide. 2nd ed. Stuttgart, Germany: Thieme; 1977. [Google Scholar]
- 35. Lipsman N, Schwartz ML, Huang Y, et al. MR‐guided focused ultrasound thalamotomy for essential tremor: a proof‐of‐concept study. Lancet Neurol 2013;12:462–468. 10.1016/s1474-4422(13)70048-6. [DOI] [PubMed] [Google Scholar]
- 36. Yamamoto K, Sarica C, Elias GJB, et al. Ipsilateral and axial tremor response to focused ultrasound thalamotomy for essential tremor: clinical outcomes and probabilistic mapping. J Neurol Neurosurg Psychiatry 2022;93:1049–1058. 10.1136/jnnp-2021-328459. [DOI] [PubMed] [Google Scholar]
- 37. Pauls AK, Hammesfahr S, Moro E, et al. Deep brain stimulation in the ventrolateral thalamus/subthalamic area in dystonia with head tremor. Mov Disord 2014;29:953–959. 10.1002/mds.25884. [DOI] [PubMed] [Google Scholar]
- 38. Segar DJ, Lak AM, Lee S, et al. Lesion location and lesion creation affect outcomes after focused ultrasound thalamotomy. Brain 2021;144:3089–3100. 10.1093/brain/awab176. [DOI] [PubMed] [Google Scholar]
- 39. Tsuboi T, Jabarkheel Z, Zeilman PR, Barabas MJ, Foote KD, Okun MS, Wagle Shukla A. Longitudinal follow‐up with VIM thalamic deep brain stimulation for dystonic or essential tremor. Neurology 2020;94:e1073–e1084. 10.1212/wnl.0000000000008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fishman PS, Elias WJ, Ghanouni P, et al. Neurological adverse event profile of magnetic resonance imaging–guided focused ultrasound thalamotomy for essential tremor. Mov Disord 2018;33:843–847. 10.1002/mds.27401. [DOI] [PubMed] [Google Scholar]
- 41. Kapadia AN, Elias GJB, Boutet A, et al. Multimodal MRI for MRgFUS in essential tremor: post‐treatment radiological markers of clinical outcome. J Neurol Neurosurg Psychiatry 2020;91:921–927. 10.1136/jnnp-2020-322745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.
Data Availability Statement
The data supporting the findings of this study will be available on request on https://zenodo.org.


