Abstract
In order to investigate the effects of a single dose of ketamine on duodenal motility, the present study focused on the electric impedance technique. Five pigs (32 to 40 kg, CVC group) were instrumented with a central venous catheter 1 d before measurements. The next day, general anesthesia was started and maintained via central venous catheter by propofol and fentanyl. In contrast, the pigs of the KETA group (n = 5) received ketamine intramuscularly prior to the induction of anesthesia by the injection of propofol-fentanyl via an ear vein. An intraluminal impedance catheter was manually introduced into the proximal duodenum. Measurements were recorded for 4 h. The KETA group showed a median duration of phase II that was shortened by 35%, while phase I was prolonged by 73% (P < 0.05). In conclusion, when gastrointestinal motility has to be investigated, the effects of a single dose of ketamine, even for premedication, should be taken into consideration.
Abstract
Résumé — Effets d’une dose unique de kétamine sur la motilité duodénale du porc. Cette expérience, basée sur une technique d’impédance électrique, a été réalisée dans le but d’étudier les effets d’une dose unique de kétamine sur la motilité duodénale. Un cathéter veineux central a été mis en place chez 5 porcs d’un poids de 32 à 40 kg (groupe CVC) un jour avant l’expérimentation. Le jour suivant, une anesthésie générale a été induite et maintenue via le cathéter veineux central à l’aide de propofol et de fentanyl. Par ailleurs, les 5 porcs du groupe KETA ont reçu de la kétamine par voie intramusculaire avant l’induction de l’anesthésie par injection de propofol-kétamine par une veine de l’oreille. Un cathéter intraluminal à impédance a été introduit manuellement dans le duodénum proximal. Les résultats ont été enregistrés pendant 4 heures. Le groupe KETA a montré une diminution de la durée médiane de la phase 11 de 35 % alors que la phase 1 était prolongée de 73 % (P < 0,05). En conclusion, lorsque la motilité gastro-intestinale doit être évaluée, les effets d’une dose unique de kétamine, même en prémédication, devraient être pris en considération.
(Traduit par Docteur André Blouin)
Introduction
Ketamine has been used extensively in pigs for minor surgical and diagnostic procedures, as well as for experimental research (1). The effects of ketamine on intestinal motility are still controversial: On one hand, anesthetic and subanesthetic doses of ketamine did not influence small intestine peristalsis, duodenal motility patterns, or iliac contractile forces in dogs (2–4); on the other hand, in an in vitro study in which guinea-pig ileum was used, an inhibitory effect of ketamine on intestinal motility was demonstrated in a concentrate-related manner (5). These findings could be supported by clinical data that showed moderate inhibitory effects of ketamine on gastrointestinal motility in humans (6). There is a lack of data concerning the effects of a single dose of ketamine on intestinal motility in the pig.
During the last decade, intraluminal impedancometry has been used as an alternative to manometry for measuring intestinal motility (7–9). The impedance technique allows the gastroduodenal motility patterns to be determined by the acquisition of the electric impedance in the surrounding body volume conductor from a number of annular electrodes (10). Passage of air or gas resulted in a temporary increase in intraluminal impedance, while the passage of hyperconductive fluid was followed by a decrease in impedance (11). The accuracy of impedancometry results was confirmed by comparing the results of gastroduodenal peristalsis obtained with video-fluoroscopy and manometry with those obtained by the impedance technique (12). Additionally, the impedance technique might describe gastroduodenal fluid transport independent of associated gastric pressure events and, therefore, be more precise than manometry (11).
The aim of the present study was to investigate the effects of a single dose of ketamine, IM, on duodenal motility in pigs, measured by the intraluminal impedance technique.
Material and methods
Subjects
This investigation was approved by the local Institutional Animal Ethics Committee and followed the Canadian Council on Animal Care Guidelines on Animal Use. To avoid introducing the possibility of gender bias on intestinal motility, 10 male castrated German Landrace, all obtained from the same breeder and with body weights (BW) between 32 and 40 kg, were used. All pigs were allowed to adjust to both local housing conditions and the investigators over a period of 9 d. The pigs had free access to water and were fed twice a day with a standard diet (Muskator; Muskator-Werk, Germany).
Instrumentation
The pigs were divided into 2 groups; namely, the CVC group (n = 5), which received a central venous catheter (CVC), and the KETA group (n = 5), which received ketamine. In both groups, each pig was fasted overnight but still had free access to water prior to instrumentation.
The pigs of the CVC group were instrumented as follows: After a premedication with 4 mg/kg bodyweight (BW) azaperone (Stresnil; Janssen-Cilag GmbH, Neuss, Germany) and 0.5 mg atropine (Atropin; B. Braun Melsungen, Melsungen, Germany), IM, 10 mg/kg BW ketamine (Ketamin; Sanofi-Cefa GmbH, Berlin, Germany) was injected, IM. Once IV access had been established via an ear vein, general anesthesia was induced with propofol (Propofol; Parke-Davis, Karlsruhe, Germany), 2 to 4 mg/kg BW, IV. Anesthesia was maintained with a continuous infusion of propofol at a rate of 25 to 35 mg/kg BW/h in combination with a continuous infusion of fentanyl at a rate of 1 to 3 μg/kg BW/h. The doses of propofol and fentanyl were guided by clinical signs to ensure appropriate depth of anesthesia. The pigs underwent orotracheal intubation and were mechanically ventilated. Oxygen saturation (SaO2) of between 94% and 99%, and expiratory carbon dioxide partial pressures (pETCO2) of between 35 and 38 mmHg were maintained. Fluid was replaced during surgery with a crystalloid solution at a rate of 2 mL/kg BW/h. Blood pressure was measured through noninvasive methods on the hind limb (Riva-Rocci; AS/3 Compact, Datex Ohmeda, Helsinki, Finnland and Dura Cuff, Infant Cuff; Criticon, Tampa, Florida, USA). Mean arterial blood pressures (MAP), heart rate (HR), SaO2, and pETCO2 were assessed at 15-min intervals during the surgical procedure. The animals were instrumented with a CVC inserted into the left jugular vein via venous cut down and tunnelled paravertebrally and subcutaneously towards the neck. Finally, the CVC was placed in a catheter bag, which was sutured on to the back (13). Body temperature (Temp) was measured and white blood cells (WBC) counted twice a day. Postoperative analgesia had already been started under general anesthesia with a first dose of the nonopioid analgesic metamizol-natrium (Novalgin; Hoechst), 20 mg/kg BW, IV, and carprofen (Rimadyl; Pfizer GmbH, Karlsruhe, Germany), 4 mg/kg BW, SC. The pigs were then fed a standard diet in the evening and had free access to water overnight.
In contrast to the CVC group, the pigs of the KETA group did not receive any instrumentation and were also fed a standard diet in the evening with free access to water overnight prior to the day of measurements.
The next morning (measurement day), after routine morning feeding, the pigs were instrumented as follows: The pigs of the CVC group were anesthetized via the CVC with a bolus of propofol, 2 to 4 mg/kg BW, followed by a continuous infusion of propofol, 20 to 35 mg/kg BW/h, and fentanyl, 1 to 3 μg/kg BW/h. As described above, doses of propofol and fentanyl were guided by clinical signs to ensure appropriate depth of anesthesia. Comparable with the previous day, pigs underwent orotracheal intubation for mechanical ventilation. Fluids were replaced with a crystalloid solution, 2 mL/kg BW/h, during the investigation period. A laparotomy of the dorsal part of the abdominal wall on the right side by using a 15-cm incision was followed by an antimesenteric incision of the proximal duodenum, 5 to 10 cm aboral to the pylorus. The catheter for assessing duodenal motility by impedancometry (2 mm diameter, 100 cm long, flexible, and with 16 electrodes distributed over the distal 32 cm; Femu-Research Institute, University Hospital Aachen, Germany) was introduced into the duodenum. Subsequently, the duodenum and abdominal wall were closed by manual sutures. While the duodenum was closed by performing a pursestring suture, the peritoneum, abdominal muscles, and the skin were closed in layers with interrupted sutures. Finally, the catheters were connected to the signal transducer for online measurements, which were recorded for the following 4 h. At the conclusion of the proceedings, the animals were euthanized by an overdose of barbiturate. Necropsy was subsequently performed.
The pigs of the KETA group received a premedication with ketamine, 10 mg/kg BW, IM. After 10 min, general anesthesia was started IV via an ear vein by using the same anesthetics and doses as in the CVC group. The procedures for anesthesia and duodenotomy were similar to those of the CVC group.
Impedancometry
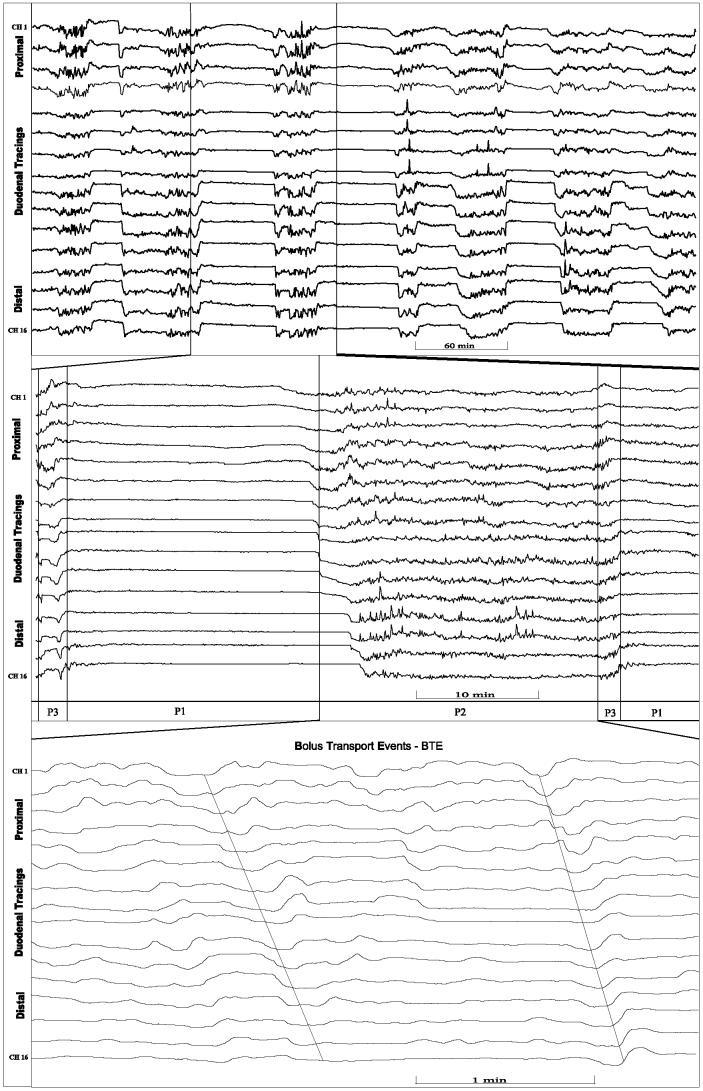
The basis of impedancometry is illustrated in Figure 1. Impedance signals were stored online with a personnel computer (PC). The impedance tracings were visually analyzed on a PC-screen. Locally developed software (Femu-Research Institute, University Hospital Aachen, Germany) was used for the data acquisition, analysis, and graphic presentation. Duodenal motility activity was defined as waves that demonstrated a drop from the baseline of more than 30%. These waves could be differentiated from those of respiration, which moved at a rate between 16 and 29 cycles/min, and from heart beats with a pulse frequency of greater than 90 beats/min. Fasting digestive motility is normally characterized by the periodic occurrence of the migrating motor complex (MMC). The MMC is conventionally divided into 3 phases: a period of quiescence (phase I) is followed by irregular contractile activity (phase II), which is replaced by a shorter period of regular contractions (phase III) or “activity front” of the MMC (14). According to established manometric criteria, the duration of the MMC is described as the time interval between 2 successive phase III patterns. The MMC is interrupted by feeding and replaced by continuous phase II-like activities. The following parameters were investigated: the duration of duodenal interdigestive phases I to III, MMC, and the quantity of bolus transport events (BTE), particular impedance tracings that are related to a bolus passage detected over 3 measuring channels (15).
Figure 1.
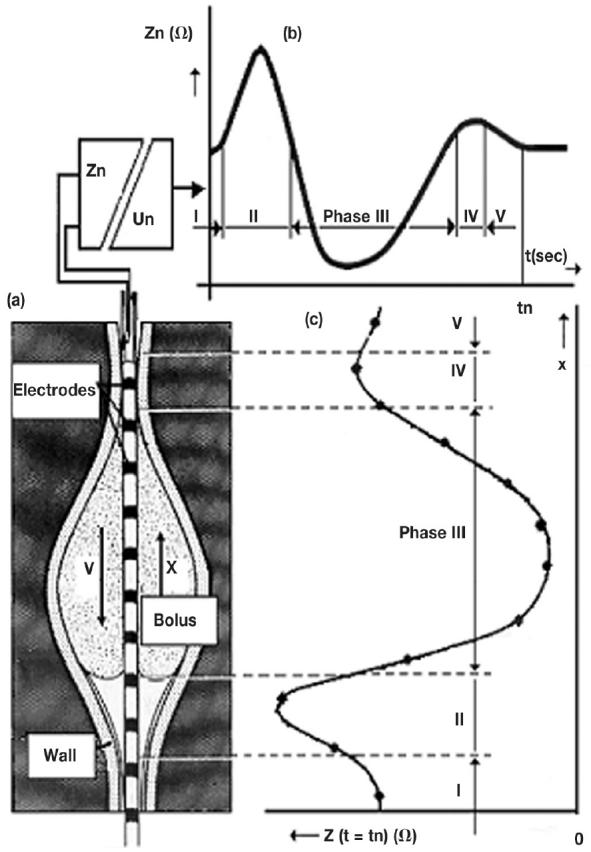
Cylinder shaped metallic electrodes are mounted on a thin plastic catheter. Each neighbouring electrode pair is connected to an impedance voltage transducer outside the body (Zn = measured impedance at the moment, which is the ratio between the applied voltage [Un] and the resulting current). The instantaneous output voltage of each transducer represents the average electrical impedance of the volume conductor around the catheter. Estimation of the impedance changes caused by a bolus (Bolus) and an air volume in front of it which move distally with a velocity (V) in the reverse x-direction. (a) Illustration of a bolus shape at the moment (tn). (b) Temporal impedance changes between two neighbouring electrodes which give a characteristic shape of the impedance phases I to V. (c) Spatial impedance distribution of the impedance in the measured segment along the catheter at the time tn.
Statistical analysis
Differences between the groups were analyzed by using the Mann-Whitney U test. Data are expressed as medians and 95% confidence interval (95% CI). Significance was defined as P < 0.05.
Results
The median duration of the interdigestive phases I to III, and MMC-cycles are demonstrated in Figure 3. The duration of phase I in the CVC group (n = 16) was 14:59 min (95% CI: 10:14 to 28:23), which was prolonged in the KETA group (n = 18) by 73% (P < 0.05). The duration of phase II in the CVC group (n = 16) was 40:55 min (95% CI: 37:37 to 71:11), which was shortened in the KETA group (n = 16) by 35% (P < 0.05). Phase III took 3:27 min (95% CI: 3:25 to 4:45) in the CVC group (n = 15), which was comparable with the phase III of the KETA group (n = 18) (5:49 min; 95% CI: 4:22 to 6:54; P = 0.11). The CVC group demonstrated a MMC cycle length of 60:08 min (95% CI: 53:04 to 76:43), which was comparable with that of the KETA group (69:19 min, 95% CI: 44:40 to 98:59; P = 0.96).
Figure 3.
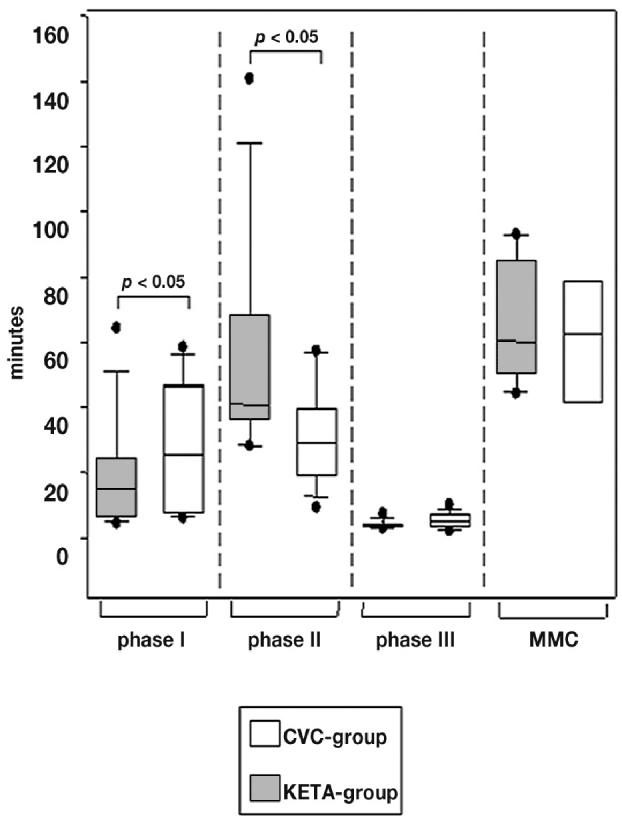
The duration of the different interdigestive phases of both groups. Data are shown as box blots indicating median, 25% and 75% quartiles, and range. Significance was defined as P < 0.05.
The number of the phases I to III during the measurements and the quantity of BTE during the different interdigestive phases I to III are demonstrated in Table 1. The median number of BTE during phase I was 0 (95% CI: 0 to 1.2) in the CVC group, compared with 2 in the KETA group (95% CI: 1.6 to 9.1; P < 0.01). During phase II, 74 BTE (95% CI: 60.2 to 129.7) were observed in the CVC group, compared with 29 in the KETA group, a reduction of 61% (P < 0.001). During phase III, 1 BTE was observed in the CVC group (95% CI: 0.6 to 3), which was comparable between the groups (P = 0.7)
Table 1.
Number of phases (I to III) during the measurements and quantity of bolus transport events (BTE) during the interdigestive phases I to III in median and 95% CI. The number of BTE/phase between the groups was significant when P < 0.05
| CVC Group
|
KETA Group
|
||||
|---|---|---|---|---|---|
| BTE/phase | BTE/phase | P | |||
| Phase I | n = 16 | 0 (0 to 1.2) | n = 18 | 2 (1.6 to 9.1) | <0.01 |
| Phase II | n = 16 | 74 (60.2 to 129.7) | n = 16 | 29 (15.6 to 42.9) | <0.001 |
| Phase III | n = 15 | 1 (0.6 to 3) | n = 18 | 1 (0.5 to 2.4) | 0.7 |
All animals could be included in the study and duodenal motility of each animal could be recorded continuously for 4 h. Biometrics and anesthetic doses are shown in Table 2. The pigs in the CVC group showed a lower expiratory pETCO2 (P < 0.01), which was associated with a higher respiratory rate (P < 0.05). Additionally, the CVC group demonstrated a slight increase in SaO2 (P < 0.05), while body temperature was slightly lower (P < 0.05) in the CVC group compared with the KETA group. Doses of propofol were comparable between the groups, while the dose of fentanyl was higher in the CVC group (P < 0.05).
Table 2.
Biometrics and anesthetic doses of each group are demonstrated as median and 95% CI. Significance was defined as P < 0.05
| CVC | Group | KETA | |
|---|---|---|---|
| BW (kg) | 34 (30.6 to 38) | 37 (34.8 to 38.8) | . 0.2 |
| HR (/min) | 106 (88.9 to 129.8) | 129 (106.2 to 151.1) | . 0.3 |
| MAP (mmHg) | 82 (70.1 to 103.3) | 70 (57 to 96.8) | . 0.1 |
| RR (/min) | 28 (23.4 to 31) | 24 (20.6 to 28) | < 0.05 |
| pETCO2 (mmHg) | 37 (35.8 to 37.5) | 43 (42.2 to 44.9) | < 0.01 |
| pH | 7.39 (7.23 to 7.6) | 7.4 (7.33 to 7.4) | . 0.8 |
| SaO2 (%) | 99 (98 to 100) | 97 (95.7 to 98.8) | < 0.05 |
| Temp (°C) | 39.2 (39.0 to 39.6) | 39.8 (39.3 to 40.2) | < 0.05 |
| Hkt | 0.27 (0.24 to 0.33) | 0.28 (0.26 to 0.29) | . 0.9 |
| WBC (. 109/L) | 19.1 (13.5 to 24.2) | 19.5 (11.8 to 26) | . 1.0 |
| Propofol (mg/kg BW/h) | 32 (24.2 to 36.8) | 26 (20.8 to 32.6) | . 0.3 |
| Fentanyl (μg/kg BW/min) | 0.06 (0.05 to 0.06) | 0.05 (0.03 to 0.06) | < 0.05 |
BW — body weight; HR — heart rate; MAP — mean arterial blood pressure; RR — respiratory rate; pETCO2 — carbon dioxide partial pressures; SaO2 — oxygen saturation; Temp — body temperature; Hkt — hematocrit; WBC — white blood cell
Necropsies showed full gastric contents in all pigs. Macroscopically, gastric contents seemed to be the remnants of the morning feeding.
Discussion
This use of the intraluminal impedance technique in the pig to demonstrate duodenal motility is the first to show that a single dose of ketamine shortened phase II and prolonged phase I of the MMC, while the duration of the MMC-cycle was unaffected.
While opiates inhibit gastrointestinal motility in a dose dependent manner, ketamine has been used as an alternative analgesic in order to minimize these gastrointestinal side effects (1,16). The results from this study suggest that ketamine influences the time periods of the interdigestive phases, so that the most important promotility phase (phase II) is shortened, while the phase of quiescence (phase I) is compensatorily prolonged. These results are supported by those of Savoye-Collet et al (17), who characterized phase II as being mainly involved in peristaltic mechanisms for the transpyloric gastroduodenal flow. A premature cessation of the phase II might explain, at least partially, the inhibitory effect of ketamine on intestinal motility, which has already been described (5,6), as would the finding in this study of a simultaneous reduction of BTE during phase II (15).
As described above, BTE were mainly found during the phase II (15). In contrast, phase I is described as a periodic period of quiescence that normally shows a lack of any pronounced motility activity (14). In both groups, the number of BTE during phase I was very low. However, there was a slight increase in BTE after ketamine during this phase I. This finding still remains unclear. Accordingly, it might be challenging to investigate the effects of continuous administration of ketamine on motility activity during this phase of quiescence (phase I).
All pigs were mechanically ventilated. The CVC group showed lower end-tidal pETCO2 that were due to higher respiration rates. Although distinct hypercarbia might influence gastric motility (18,19), the slightly increased end-tidal pETCO2 in the KETA group were close to normal and, therefore, might be neglected as an inhibitory source of duodenal motility.
Clinical and in vitro investigations have demonstrated a delay in gastric emptying associated with decreased contractile activity during propofol sedation (20,21). Propofol is often used in combination with opiates, which additionally delay gastric emptying in a dose-dependent manner (16). In all pigs, the doses of propofol and fentanyl were guided by clinical signs to ensure appropriate depth of anesthesia. While the propofol doses being used were comparable between both groups, the dose of fentanyl was slightly higher in the CVC group. In total, this was not followed by a more pronounced inhibition of duodenal motility, when compared with the KETA group.
The intraluminal impedancometry is a novel and validated approach that promotes measurements of motility. In contrast to manometry, the intraluminal electric impedance technique is independent of the surrounding pressures. As described by Savoye-Collet et al (17), peristalsis without luminal occlusion could be underestimated by manometry. When compared with manometry and real-time ultrasound, the intraluminal impedance technique might be an alternative, practicable, and more accurate approach to monitor gastroduodenal motility under clinical settings (11).
In order to introduce general anesthesia without any distress or agents usually used for premedication on the day of measurements, the CVC group was instrumented with a CVC 1 d before measurements were taken. For instrumentation, the pigs were premedicated with ketamine. Although the short half life of ketamine might contradict residual effects on motility measurements on the following day, this side effect can not be excluded. Nevertheless, the use of ketamine is a standard procedure during premedication, since ketamine sufficiently promotes analgesia during orotracheal intubation (1). Therefore, a single dose of ketamine was used in the CVC group. In contrast, the KETA group was instrumented without a CVC in order to imitate the experimental proceedure, including premedication, instrumentation, and measurements on the same day. Although the KETA group was instrumented less invasively, duodenal motility was affected by the single IM injection of ketamine.
In order to demonstrate a wide range of values more accurately we decided to use the median plus the confidence interval. In addition, the number of BTE is better characterized by the median than by the mean.
In conclusion, when gastrointestinal motility has to be investigated in pigs, the effects of a single dose of ketamine, even for premedication, should be taken into consideration. Further studies are necessary to link the effects of changed time period of the interdigestive phases with intestinal transit times. CVJ
Figure 2.
Duodenal impedance tracings of ketamine treated pig demonstrating 16 pairs of electrodes (Ch 1 = proximal channel 1, Ch 16 = distal channel 16). Top: time period of 8 h containing interdigestive phases. Middle: time period expanded. p1 = phase I, p2 = phase II, p3 = phase III. Bottom: phase II further expanded demonstrating bolus transport events (BTE).
Footnotes
This study was financially supported by START, RWTH Aachen, Germany, and B. Braun Melsungen AG, Melsungen, Germany.
References
- 1.Lin HC. Dissociative Anesthetics. In: Thurmon JC, Tranquilli WJ, Benson J, eds. Lumb & Jones’ Veterinary Anesthesia. Third Edition. Philadelphia: Lippincott Williams & Wilkins, 1993:241–296.
- 2.Dick W, Hofmann S. Effect of dehydrobenzperidol, fentanyl and ketamines on the intestinal motility in young dogs. (Article in German) Anaesthesist. 1970;19:205–208. [PubMed] [Google Scholar]
- 3.Fass J, Bares R, Hermsdorf V, Schumpelick V. Effects of intravenous ketamine on gastrointestinal motility in the dog. Intensive Care Med. 1995;21:584–589. doi: 10.1007/BF01700164. [DOI] [PubMed] [Google Scholar]
- 4.An YJ, Lee H, Chang D, et al. Application of pulsed Doppler ultrasound for the evaluation of small intestinal motility in dogs. J Vet Sci. 2001;2:71–74. [PubMed] [Google Scholar]
- 5.Herbert MK, Kuhn H, Roewer N. Effect of racemic and S(+)- ketamine on the peristaltic reflex in guinea-pig ileum in vitro. Comparison to fentanyl. (Article in German). Anästhesiol Intensivmed Notfallmed Schmerzther. 1998;33:613–614. [Google Scholar]
- 6.Freye E, Knufermann V. No inhibition of intestinal motility following ketamine-midazolam anaesthesia. A comparison of anaesthesia with enflurane and fentanyl/midazolam. (Article in German) Anaesthesist. 1994;43:87–91. doi: 10.1007/s001010050036. [DOI] [PubMed] [Google Scholar]
- 7.Frieling T, Hermann S, Kuhlbusch R, et al. Comparison between intraluminal multiple electric impedance measurement and manometry in the human oesophagus. Neurogastroenterol Motil. 1996;8:45–50. doi: 10.1111/j.1365-2982.1996.tb00241.x. [DOI] [PubMed] [Google Scholar]
- 8.Sifrim D, Holloway R, Silny J, Tack J, Lerut A, Janssens J. Composition of the postprandial refluxate in patients with gastroesophageal reflux disease. Am J Gastroenterol. 2001;96:647–655. doi: 10.1111/j.1572-0241.2001.03598.x. [DOI] [PubMed] [Google Scholar]
- 9.Peter CS, Wiechers C, Bohnhorst B, Silny J, Poets CF. Detection of small bolus volumes using multiple intraluminal impedance in preterm infants. J Pediatr Gastroenterol Nutr. 2003;36:381–384. doi: 10.1097/00005176-200303000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Silny J. Intraluminal multiple electric impedance procedure for measurement of gastrointestinal motility. J Gastrointest Mot. 1991;3:151–162. [Google Scholar]
- 11.Savoye G, Savoye-Collet C, Oors J, Smout AJ. Interdigestive transpyloric fluid transport assessed by intraluminal impedance recording. Am J Physiol Gastrointest Liver Physiol. 2003;284:G663–669. doi: 10.1152/ajpgi.00403.2002. [DOI] [PubMed] [Google Scholar]
- 12.Imam H, Sanmiguel C, Larive B, Bhat Y, Soffer E. Study of intestinal flow by combined video-fluoroscopy, manometry, and multiple intraluminal impedance. Am J Physiol Gastrointest Liver Physiol. 2004;286:G263–270. doi: 10.1152/ajpgi.00228.2003. [DOI] [PubMed] [Google Scholar]
- 13.Unger JK, Gerlach JC, Juhr NC, Rossaint R. Development of a special catheterbag to enable artificial organ evaluation in concious, unrestrained pigs: technical note. Int J Artif Organs. 2000;23:268–274. [PubMed] [Google Scholar]
- 14.Malagelada, J, Camilleri M, Stanghellini V. Physiologic basis of gastrointestinal motility disorders. In: Malagelada J, ed. Manometric diagnosis of gastrointestinal motility disorders. New York: Thieme, 1986:1–11.
- 15.Nguyen HN, Silny J, Wuller S, Marschall HU, Rau G, Matern S. Chyme transport patterns in human duodenum, determined by multiple intraluminal impedancometry. Am J Physiol. 1995;268:G700–708. doi: 10.1152/ajpgi.1995.268.4.G700. [DOI] [PubMed] [Google Scholar]
- 16.Kurz A, Sessler DI. Opioid-induced bowel dysfunction: pathophysiology and potential new therapies. Drugs. 2003;63:649–671. doi: 10.2165/00003495-200363070-00003. [DOI] [PubMed] [Google Scholar]
- 17.Savoye-Collet C, Savoye G, Smout A. Determinants of transpyloric fluid transport: a study using combined real-time ultrasound, manometry and impedance recording. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1147–1152. doi: 10.1152/ajpgi.00208.2003. [DOI] [PubMed] [Google Scholar]
- 18.Schulze-Delrieu K, Lepsien G. Depression of mechanical and electrical activity in muscle strips of opossum stomach and esophagus by acidosis. Gastroenterology. 1982;82:720–724. [PubMed] [Google Scholar]
- 19.Toumadre JP, Barclay M, Fraser R, et al. Small intestinal motor patterns in critically ill patients after major abdominal surgery. Am J Gastroenterol. 2001;96:2418–2426. doi: 10.1111/j.1572-0241.2001.03951.x. [DOI] [PubMed] [Google Scholar]
- 20.Mushambi MC, Rowbotham DJ, Bailey SM. Gastric emptying after minor gynaecological surgery. The effect of anaesthetic technique. Anaesthesia. 1992;47:297–299. doi: 10.1111/j.1365-2044.1992.tb02167.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee TL, Ang SB, Dambisya YM, Adaikan GP, Lau LC. The effect of propofol on human gastric and colonic muscle contractions. Anesth Analg. 1999;89:1246–1249. [PubMed] [Google Scholar]