Abstract
Neospora caninum is one of the most important causes of abortion in cows. The occurrence of N. caninum infection in beef and dairy cattle has been reported worldwide, and in most provinces in Canada. The objective of this review is to summarize our current understanding of N. caninum in dairy and beef cattle for Canadian bovine practitioners. The review covers the life cycle of the agent, its mechanisms of transmission, clinical signs, and tests for diagnosing the infection. Data on the prevalence of the infection in Canadian dairy and beef cattle are reviewed and briefly compared with estimates from other parts of the world. Most importantly for Canadian bovine practitioners, the impacts of the infection, risk factors for its occurrence, and methods of control are also discussed. By reviewing the scientific literature on N. caninum from a Canadian perspective, culling decisions based on the interpretation of diagnostic tests are more effectively made in the control of N. caninum-associated disease.
Abstract
Résumé — Neospora caninum chez les bovins laitiers et de boucherie : mise au point dans une perspective canadienne. Neospora caninum est l’une des causes les plus importantes d’avortements chez les vaches. La présence d’infections à N. caninum chez les bovins de boucherie et laitiers a été rapportée à la grandeur de la planète ainsi que dans la majorité des provinces canadiennes. L’objectif de cette mise au point est d’actualiser les connaissances sur N. caninum chez les bovins laitiers et de boucherie pour les praticiens bovins du Canada. Ce tour d’horizon comprend le cycle vital de l’agent, ses mécanismes de transmission, les signes cliniques et les tests destinés au diagnostic de l’infection. Les données sur la prévalence de l’infection chez les bovins laitiers et de boucherie du Canada sont revues et brièvement comparées avec les estimations provenant d’autres parties du monde. Le tout est suivi d’une discussion sur les conséquences de l’infection, sur les facteurs favorisant son apparition et sur les méthodes de contrôle à l’intention des praticiens bovins du Canada. En passant en revue la littérature scientifique sur N. caninum dans une perspective canadienne, les décisions concernant les animaux à éliminer en vertu de l’interprétation des tests de diagnostic conduisent à un contrôle plus efficace de la maladie causée par N. caninum.
(Traduit par Docteur André Blouin)
Introduction
Neospora caninum is an apicomplexan protozoan that was first recognized in dogs in Norway (1) in 1984. In 1988, a new protozoan species, N. caninum, was proposed under a new genus, Neospora (2). This parasite is now recognized as an important cause of reproductive problems and abortion in cows. It is found worldwide, with widespread occurrence of neosporosis in beef cattle, dairy cattle, or both, in most provinces in Canada, including the Maritime provinces, Ontario, Quebec, Manitoba, Saskatchewan, Alberta, and British Columbia (3–7). Since its discovery, there has been much research about this parasite, and several general reviews have been written (8–12). The aim of this paper is to summarize the current state of knowledge of neosporosis in dairy and beef cattle, with a particular focus on its relevance to the Canadian cattle industry.
Methods
Medline (accessed via PubMed from 1966 to present), The Commonwealth Animal Bureaux (CAB) (accessed via VetCD and ParasiteCD from 1973 to present), and Agricola, produced by the National Agricultural Library of the U.S. Department of Agriculture (accessed via National Agricultural Library from 1970 to present) were used to collect the majority of the references that were used in this paper. The keywords used in the search of the databases were neospora, neosporosis, Canada, Canadian, and cattle. In addition, a small number of papers were identified from the reference lists of other papers, or through personal knowledge of reports or conference proceedings.
History
Before Dubey et al (2) described N. caninum in 1988, many researchers already suspected that a new, different genus of protozoa was causing abortion in cows. In 1987, O’Toole and Jeffrey (13) described a sporozoan-associated disease in a weak newborn calf in England that was tested for toxoplasmosis and sarcocystis by an immunoperoxidase test with negative results. The cause of the disease would later be confirmed as N. caninum.
The characteristics of the oocyst of N. caninum are quite similar to those of oocysts of Hammondia heydorni from dog feces and of Toxoplasma gondii and Hammondia hammondi from cat feces (14). Furthermore, the agents’ tachyzoites and bradyzoites appear similar under a light microscope, but they can be distinguished under an electron microscope by the number, appearance, and location of their rhoptries (2,15,16), leading to the conclusion that they are different protozoa (17).
In Canada, the first report of N. caninum being associated with clinical disease was in 1994 (18), when a 3-day-old calf in Alberta presented with clinical neurological signs. Histopathologic examination revealed tissue cysts and lesions in the central nervous system (CNS). In the same journal issue, it was reported that a Santa Gertrudis cow in British Columbia had aborted in her 8th mo of pregnancy (19). The calf and the fetus were both confirmed as being N. caninum-positive by immunohistochemical (IHC) staining, while tests for Toxoplasma gondii and Sarcocystis spp. were negative.
The earliest known outbreak of abortions due to N. caninum in Canada was also reported in 1994 (20). On a dairy farm in eastern Ontario, 15 of 80 cows aborted in an 18-day period in January and February 1994. The cows were 3 to 7 y of age and aborted at 4 to 8 mo gestation. From the 15 abortions, 4 fetuses were submitted to a provincial veterinary diagnostic laboratory, where lesions typical of N. caninum were found and the diagnosis of N. caninum infection was confirmed by IHC (20). Comparative testing was carried out for infectious bovine rhinotracheitis (IBR), bovine viral diarrhea (BVD), and leptospirosis with negative results. From the same laboratory, lesions of neosporosis were subsequently identified in cattle from stored samples received from 24 other farms between February 1993 and July 1995, using histologic examination and IHC tests. Since that time, N. caninum has been a commonly diagnosed cause of abortion in cattle in many parts of Canada (4,20–22).
Biology and life cycle of N. caninum
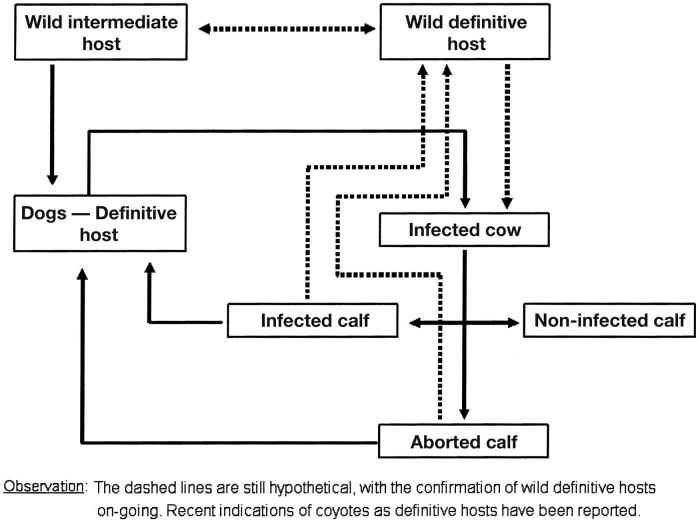
Figure 1 depicts the life cycle of N. caninum as it is understood today. The dog is a definitive host of N. caninum, although it is suspected that the dog may also serve as an intermediate host. As a definitive host, the dog sheds unsporulated oocysts in the environment for 5 to 17 d after the ingestion of tissue cysts (23,24). After 3 d in the environment, the oocysts (the sexual stage) sporulate to form 2 sporocysts, each containing 4 sporozoites. It is unclear how long oocysts will survive in the environment. Intermediate hosts (cattle) ingest oocysts that are found in contaminated food and water. Sporozoites are released in the intestinal tract where they penetrate cells and become tachyzoites (a rapidly dividing asexual phase). Tachyzoites divide and quickly spread to other host cells, which they invade and often destroy. Tachyzoites have been found in neural cells, macrophages, fibroblasts, vascular endothelial cells, hepatocytes, and muscular cells including those of myocardium (25), and the placenta in pregnant cows (26). The tachyzoites can be transmitted vertically from a dam through the placenta to the fetus. In neural cells, tachyzoites can transform into bradyzoites (a slowly dividing asexual phase) when a strong immune response is mounted against the protozoa elsewhere in the body. The bradyzoites form tissue cysts around themselves for protection; they remain latent until the immune system of the intermediate host is suppressed, allowing them to recrudesce. Cysts have been found in the brain, spinal cord, and retina (23). Tachyzoites in placental tissue (27) (and likely bradyzoites in tissue cysts), when consumed by a dog, implant in the gastrointestinal tract where they mature, begin to shed oocysts, and complete the horizontal transmission cycle (28).
Figure 1.
Diagram of the life cycle of Neospora caninum.
When calves were infected experimentally by oral inoculation with N. caninum oocysts collected from dog feces, the calves seroconverted within 2 to 4 wk (27). In another experiment, uninfected calves that were bottle-fed colostrum with added tachyzoites also seroconverted, introducing another possible mechanism of transmission, but this needs confirmation under commercial conditions. It is unknown whether colostrum with tachyzoites from naturally infected cows would infect calves (29).
Serological evidence of N. caninum infection or confirmation of its presence by using IHC or polymerase chain reaction (PCR) has been found in many mammals other than cattle and dogs; these include goats (30–32), sheep (33), horses (34), deer (35), foxes (36–38), dingoes (39), raccoons (40), and coyotes (41). A very recent article has confirmed that coyotes are a definitive host as well (42). The other canids listed above may also be definitive hosts, while the herbivores listed above may also be intermediate hosts. It also has been suggested that birds may act as reservoir hosts for N. caninum (43–45), but none of these suppositions has been proven yet, and further research on these potential hosts is required.
The principal route of infection in cattle is transplacental (vertical) transmission (46,47) and the same cow can pass the infection to multiple offspring (48). The probability of a seropositive dam producing a calf that is seropositive prior to consumption of colostrum has been widely reported as ranging between 81% and 100% (49–52). However, these reports are based on a small number of herds with a high prevalence of seropositive animals, including a large cow-calf herd in northern Alberta following a N. caninum-associated abortion epidemic (53). This high probability of vertical transmission may be appropriate for high prevalence herds, particularly if they are having abortion problems. However, lower probabilities of vertical transmission were found in a study of 23 dairy herds in Quebec with a range of seroprevalences from 4.3% to 61.8%; these farms may be more representative of the majority of infected dairy and beef herds in Canada. In this study in Quebec, a range of vertical transmission probabilities, from 0% to 86% were found (54), with the high probabilities occurring in the herds with high seroprevalences.
A number of factors may have contributed to the lower vertical transmission probabilities in the herds in Quebec compared with those reported elsewhere. First, the positive predictive value of tests are lower in low prevalence herds, so a higher proportion of test positive dams will, in fact, be false positives. False positive dams will not deliver a congenitally infected calf, so when they are mixed in with true positive dams, a lower perceived probability of vertical transmission compared with what would be observed if only truly positive dams were included will be obtained. Second, vertical transmission estimates could differ, depending on the sensitivities and specificities of the tests used to determine seropositivity. Third, selective culling of infected cattle without identification of their serological status prior to culling could also bias vertical transmission probabilities downward, since congenitally infected cows that abort are more likely to be culled for abortion-related reasons (55) (see details in impacts section below); consequently, they are lost to follow-up for vertical transmission assessments, leading to a possible downward bias (56). Fourth, the use of embryo transfer will prevent N. caninum infection in daughters of seropositive cows, provided that the embryos are implanted in seronegative recipients (57) and will also reduce the apparent probability of vertical transmission in these herds. Finally, in small herds with moderate seroprevalences or large herds with low seroprevalences, the estimates of vertical transmission will be dependent on only a few seropositive dams and their progeny, making these estimates highly unstable and susceptible to the biases mentioned above.
While the above reasons may partially explain the differences found in the results between the herds in Quebec and high prevalence herds, there are also possible biological reasons for the lower probabilities of vertical transmission in the herds in Quebec. First, in low and moderate prevalence herds where there is no horizontal transmission, infections occur in utero and turn into latent infections by the time female calves reach a reproductive age. Without circulating tachyzoites, daughters of latently infected dams are unlikely to become infected congenitally, unless there is sufficient immune system down-regulation in mid-gestation to allow recrudescence (58). Evidence for this effect was recently demonstrated in a study of dairy herds in The Netherlands with a history of abortion problems. Even with abortion problems evident in the herd, vertical transmission decreased with age, with 66% of seropositive cows in their 4th or higher parity having seropositive calves compared with > 80% in their 1st lactation (52).
Second, differences in herd factors (such as herd size, average age of cows, and production level) between the herds in Quebec and those large herds citing high vertical transmission risks may reflect lower stress levels in the herds in Quebec, thereby reducing the down-regulation of the immune system in mid-gestation (59) and recrudescence of predominantly latently infected cattle.
True vertical transmission probabilities for most Canadian dairy (and perhaps beef) herds not having abortion problems are unlikely to be 0%. A study in a population of randomly selected Canadian dairy herds would be useful to confirm whether the average vertical transmission level of 44% found in the case control study in Quebec (54) is representative of the Canadian dairy industry as a whole. Because N. caninum infection is thought to be maintained by vertical infection on most farms, the true level of vertical transmission would be important to determine the need for culling seropositive cattle.
Based on available reports in California and England, estimated incidence rates of horizontal transmission of infection appear to be generally quite low, 1 infection per 100 cow-years at risk in one study (60) and 1.9 infections per 100 heifer-years at risk in another study (49), respectively. Specific farms undergoing a horizontally transmitted abortion outbreak due to N. caninum could have a much higher incidence rate, but because outbreaks are not common, the overall rate is likely to be low.
Dogs have shed oocysts after ingesting tissue from a number of different infected species, including cattle, goats, sheep, guinea pigs, rats, and mice (52,61,62). One study showed that 2 dogs already infected with N. caninum did not shed oocysts again upon reexposure (28). However, confirmation of this finding is required in a larger number of dogs (and possibly wild canids) with different types of tissues having varying levels of N. caninum. Recrudescence of shedding in dogs (and possibly wild canids) also needs to be investigated further to determine the possibility of it occurring under varying circumstances and stress levels (pregnancy).
The relative proportion of vertical versus horizontal transmission in a farm or region is likely dependent on the current seroprevalence of infection in the cattle population, as well as the distribution of infected dogs and, maybe, other canids in the region and their access to cattle and their feeds.
Diagnosis
Clinical signs and lesions
When a naïve cow is infected with N. caninum, there are 2 main factors that determine which of 4 manifestations (early embryonic death, abortion, stillbirth or birth of a feeble abnormal calf, and birth of a normal calf with no obvious effect of N. caninum infection) occurs: whether the animal is pregnant or not at the time of infection, and the phase of gestation — early, mid, or late (59).
If a naïve cow is not pregnant when infected, the infection usually produces no clinical signs, but seroconversion occurs, along with the development of cell-mediated immunity (CMI) (involving cell proliferation and interferon [IFN]-gamma production). Infection leads to limited multiplication of intracellular parasites due to IFN-gamma produced by CD4 T-cells, with persistent infection (as bradyzoites) within tissue cysts in the central nervous system (CNS) (59).
If a naïve cow is pregnant and in early gestation (< 2 to 3 mo) when infected, the infection leads to early embryonic death (EED). It is likely that the EED is caused by the presence of pro-inflammatory T helper (Th) type-1 cytokines at the maternal-fetal interface (placenta) damaging the placental connection, because the maternal immune system develops a strong cell proliferation response with production of IFN-gamma in response to parasite antigen (59).
If a naïve cow is pregnant and in mid-gestation (3 to 7 mo) when infected, the infection leads to either abortion or birth of a weak, abnormal calf, depending on the month of gestation. At this stage of gestation, the fetus has an immature immune system and is unable to fully fight off the infection. With the down-regulation of the maternal type 1 T-cell response by the placental type 2 cytokine, the immunological defence of the cow is reduced at this stage, allowing for an increase in N. caninum population, with subsequent invasion of the placenta and calf by the N. caninum tachyzoites. If the N. caninum exposure completely overcomes the immune system of the calf, tachyzoites explode in population size, leading to extensive tissue damage and the abortion of an autolysed fetus. If the immune system of the calf is nearly completely developed, a weak abnormal calf is born with poor formation of the CNS or encephalomyelitis due to the mild or moderate tachyzoite-induced tissue damage, leading to neurological symptoms and low weight at birth (18) (59).
If the naïve cow is pregnant and in late gestation when infected, the infection leads to the birth of a weak or normal calf that is seropositive for N. caninum. During this stage of gestation, the immune system of the fetus is more mature than that of a younger fetus; therefore, it is more able to control the infection, leading to limited or no clinical signs in the newborn calf (59).
Diagnostic tests
Since the discovery of N. caninum, many diagnostic tests have been developed to help in diagnosing this parasitic infection. They include IHC staining (63), indirect fluorescent antibody tests (IFAT) (25), enzyme-linked immunosorbent assays (ELISA) (64,65), direct agglutination tests (DAT) (66,67), Western blot analysis (WB) (68), and polymerase chain reaction (PCR) (69). This paper will focus on the tests used most frequently and emphasize those that are commercially available in Canada
Immunohistochemical staining, in which an avidin-biotin-peroxidase complex was used, was the first test produced to identify the parasite and demonstrate that there was no cross-reaction with the closely related Toxoplasma gondii or other extra-intestinal coccidia (63). This test is still used to confirm N. caninum parasites in tissue where characteristic inflammatory lesions are observed on histologic examination; however, IHC staining can underestimate the true prevalence of infection due to low sensitivity in severely autolysed fetuses (70). From fetal material, brain provides the tissue of choice for the diagnosis of N. caninum when IHC staining or IFAT is used, although frequently tissue cysts or tachyzoites can also be found in lung, kidney, and skeletal muscle. From cows, IHC staining can be carried out in samples of brain, liver, or heart.
The main tests used for serologic examination for N. caninum infection are IFAT and ELISA. The IFAT is very specific and there are no cross-reactions between N. caninum and T. gondii, although they share several antigens (25). Comparisons of IFAT results from different laboratories are extremely difficult, given the different antigen preparations, reagents, and serum dilutions used and the diversity in cutpoints selected. Cutpoint dilutions of 1:200 (71) to 1:640 (70,72) have been suggested for infections in adult cattle. For bovine fetuses and precolostrum newborn calves, lower values, such as 1:80, have been suggested as being indicative of infection (73). For 1-week-old heifers that have been fed colostrum, a cutpoint of ≥ 1:5120 has been reported to be a good test for the early diagnosis of seropositive heifers (74).
However, the IFAT is time-consuming and expensive compared with ELISA; therefore, it is not used routinely for screening cattle populations for N. caninum infection. An indirect ELISA (i-ELISA), using a crude tachyzoite antigen derived from an aborted fetus, was first developed in 1994 and was reported to have a sensitivity and specificity of 88.6% and 96.5%, respectively (64). This test is the basis of both the IDEXX (IDEXX Laboratories, Westbrook, Maine, USA) and Biovet (BIOVET Laboratories, St. Hyacinthe, Quebec) ELISA test kits, 2 indirect N. caninum ELISAs available in Canada. Sample-to-positive control (S/P) ratios of 0.45 and 0.60 for the IDEXX and Biovet ELISAs, respectively, were determined as the optimal cutpoints in order to differentiate between infected and noninfected cattle. A recent independent validation study of the IDEXX and Biovet ELISAs compared them with immunoblotting (the gold standard) by using 150 field sera from an infected beef herd (75). The results showed that the 2 ELISAs worked equally well and there was no statistically significant difference between the performances of the 2 tests. Both tests showed high reproducibility, repeatability, and substantial agreement with results from 2 other laboratories. Sensitivities for the Biovet and IDEXX ELISAs on the field samples were 95.1% and 97.6%, respectively, while specificities were 100% and 98.5%, respectively (75).
However, there has been concern regarding cross-reaction of the indirect ELISA with antibodies to Sarcocystis spp. (25), leading to false positive test results. Use of higher, more specific cut-off values would reduce the number of false positive test results, but would lower the sensitivity of the test for identifying N. caninum infected cattle.
A competitive inhibition ELISA test (c-ELISA) (VMRD Laboratories, Pullman, Washington, USA) has been shown to be unreactive to antigens of 2 closely related apicomplexan protozoa, Toxoplasma gondii and Sarcocystis cruzi (65). An independent assay to validate the test used a “gold standard” set of 184 cow sera (42 positives and 142 negatives) defined by fetal histopathologic examination and N. caninum IHC staining and by maternal N. caninum IFAT at a 1:200 serum dilution. The sensitivity was 97.6% and specificity was 98.6% (76). This ELISA test has recently been adopted by many laboratories in Canada as the test of choice for detecting antibody against N. caninum.
All serological results for N. caninum should, however, be interpreted with caution, because the immune system is not static and antibody levels fluctuate, particularly for parasites that form cysts that wall themselves off from the host’s immune system and can recrudesce with immunosuppression (6). A single serum sample from an individual cow may not reflect her infection status accurately, particularly on farms without a history of N. caninum abortions. On these farms, only consistent results on multiple tests during different years or seasons of the year should be used to make culling decisions, particularly when S/P ratios are close to the cut-off values of the test. For example, congenitally infected heifers that have had a history of positive N. caninum titers have had negative titers at calving, while giving birth to a N. caninum-infected calf (74). Similarly, cows that abort a N. caninum-infected fetus may no longer have a significantly elevated titer at the time of abortion (77).
In a Dutch study of 21 dairy farms with a history of N. caninum abortion, blood was sampled multiple times to evaluate a single serological screening (52). Based on subsequent test results, the 1st test provided a relative sensitivity and specificity of 94.7% and 95.6%, respectively, and a positive predictive value (PPV) and negative predictive value (NPV) of 92.4% and 97%, respectively. However, there are 2 concerns related to applying the results of this study to Canadian dairy herds: First, no gold standard tests were used in this study to determine the true N. caninum status of the tested animals; therefore, cross-reactions or other misclassification problems may have occurred. Also, 36.8% of animals tested positive in this study, a prevalence that is considerably higher than most Canadian dairy and beef farms (as discussed in detail below). This difference is a concern, because the PPV and the NPV of test results are affected by the estimated true prevalence of infection in the population being tested. With lower seroprevalences, such as those found in Canada, PPV decreases, making confirmation of an initial positive test necessary, particularly if the positive titer is close to the cut-off value. Conversely, on the small number of Canadian farms with seroprevalences approaching 80% to 100%, NPV may decrease, making confirmation of an initial negative test necessary.
Accurate comparison of results between samplings or studies that use different tests is also challenging, unless duplicate samples, tested by the different tests, have produced similar results, something that is rarely done. Using estimated true prevalences, adjusted for test sensitivity and specificity, can reduce the bias in prevalence estimates (78). However, populations of cattle with a high average age could demonstrate a low N. caninum prevalence simply because most infected animals are in latent stages that are less likely to test positive.
One potentially useful test for N. caninum diagnosis in outbreak investigations is an immunoglobulin (Ig) G avidity ELISA, which can be used to differentiate acute and chronic infections. With this test, the binding strength (avidity) of the IgG antibodies to a N. caninum antigen is measured. The IgG avidity increases with time after infection; consequently, low (< 50) and high (> 50) avidities indicate recent and chronic infection, respectively. This test is not yet commercially available in Canada (79); further research is needed to validate it.
At the time of writing, the following Canadian laboratories offer the c-ELISA (VMRD) test: Diagnostic Services of the Atlantic Veterinary College (AVC/UPEI) in Charlottetown, Prince Edward Island; the Animal Health Centre in Abbotsford, British Columbia; Prairie Diagnostic Services in Regina, Saskatchewan; and Veterinary Laboratory Services in Guelph, Ontario. The Biovet ELISA is available at the source laboratory. The IDEXX ELISA is available at the International Bio Institute in Fergus, Ontario, and the Veterinary Services Laboratory in Winnipeg, Manitoba. The IFAT is available for diagnostic confirmation at the Animal Health Centre in Abbotsford. The PCR for aborted fetuses is available at the Veterinary Services Laboratory in Winnipeg. The IHC test is available at Prairie Diagnostic Services in Regina, Saskatchewan.
Prevalence of N. caninum infection
Prevalence in dairy cattle
Based on i-ELISA testing of 30 randomly selected dairy cattle in each of 181 randomly selected herds in Manitoba (7), Saskatchewan (7), and the 3 Maritime provinces (3), and from a representative population of 51 herds in Ontario (7), N. caninum infection can be found in a large number of herds in Canada. Cow-level prevalences ranged from 5.6% to 7.0% in western Canada, were 7.5% to 8.2% in Quebec and Ontario, and ranged from 10.4% to 25.5% in Atlantic Canada (Table 1) (3,80). Analysis of samples from PEI in 1979, 1989, and 1998 showed the same prevalence in 1998 and 1989, but a lower level in 1979, suggesting a possible expansion of the disease in the 1980s but a stable prevalence throughout the 1990s (3).
Table 1.
Summary of seroprevalence for Neospora caninum in dairy and beef cattle in Canada
| Number of herds | Number of cows | Herd level prevalence | Cow level prevalence | Reference | |
|---|---|---|---|---|---|
| Dairy prevalence | |||||
| New Brunswick | 30 | 900 | 90.0 | 25.5 | 3, 80 |
| Nova Scotia | 30 | 900 | 83.0 | 21.3 | 3, 80 |
| Prince Edward Island | 30 | 900 | 63.0 | 10.4 | 3, 80 |
| Quebeca | 22 | 1463b | 73.0 | 7.5 | 4 |
| Ontario | 31 | 930 | 71.0 | 8.2 | 3, 80 |
| Manitoba | 40 | 1200 | 38.0 | 7.0 | 3, 80 |
| Saskatchewan (Sask) | 51 | 2040 | 44.0 | 5.6 | 3, 80 |
| Beef prevalence | |||||
| Alberta (Alta) | 174 | 1806 | 36.0 | 9.0 | 5 |
| Manitoba (Man) | 49 | 1417 | 78.0 | 8.8 | Unpub. data |
| Man, Sask, Alta, British Columbia | — | 1976 | — | 6.5 | 81 |
aOnly control data were used from the case-control study to estimate prevalence
bEstimated because the exact number of control group cows was unavailable from the paper
In a case-control study in Quebec, 3059 dairy cows were i-ELISA tested from 24 case herds (presence of a N. caninum aborted fetus confirmed histologically and immunohistochemically) and 22 control herds (no presence of N. caninum suspected). All case herds and 73% of the control herds had at least 1 seropositive cow. Based on the within-herd prevalence in the control group, it was estimated that the provincial cow-level prevalence was at least 7.5% and that N. caninum exists in the majority of farms in the province (4). However, this estimate is likely to be an underestimate of the true prevalence in Quebec, because it is based only on herds that have never had N. caninum infections reported, rather than a random sample of herds that would likely include some herds with reported N. caninum infections. Therefore, the true seroprevalence in Quebec is likely to be closer to that of Ontario or the Maritime provinces, rather than that of western Canada.
Based on the data from dairy cattle, there appears to be some ecological (proximity of farms to domestic or wild canid populations) or management (pasture use or cattle density on pasture) differences that have lead to substantially higher N. caninum infection levels in eastern Canada compared with western Canada. The N. caninum prevalences found in eastern Canada are similar to those reported among dairy cattle in the United States (81) and elsewhere (70,82,83).
Prevalence in beef cattle
In a 2004 report on Canadian beef cattle, a random sample of 1976 steers and bull calves from 4 feedlots in northern Alberta were tested by i-ELISA, with 128 (6.5%) testing positive. The cattle came from British Columbia, Alberta, Saskatchewan, and Manitoba (84). Another study involved the random collection of blood samples from 1806 cows from 174 herds at auction in northern Alberta in 1998; 162 (9.0%) cows were positive and 62 (36%) herds had at least 1 positive cow by an i-ELISA. A total of 260 samples had been collected in the same region in 1980, when 35 (11.5%) were positive (5).
In contrast to these low seroprevalences in randomly selected cattle, in a study to determine associations between N. caninum infection and reproductive performance, the N. caninum seroprevalence in 419 cows from 8 progressive cow-calf herds in central Alberta was estimated to be 30% in beef cattle, based on IFAT results from blood samples taken between 1992 and 1995 (6). However, this study likely overestimated the true prevalence in the beef industry in Alberta, because the authors selected herds in order to achieve their primary objective, which was to determine the association between serologic status and rate of abortion, stillbirth, calf mortality, and reproductive failure, not to estimate the true prevalence of N. caninum infection in Alberta. Eight herds would be an inappropriate number of herds to give a valid representation of the estimated true prevalence in the whole province. Another reason for the likely overestimation is that horizontal transmission of N. caninum had occurred in at least some of this select group of herds. Indeed, in an outbreak investigation of a herd that suffered an abortion storm in northern Alberta, over 80% of cows, heifers, and calves were seropositive shortly after the outbreak, demonstrating how widespread the infection can become in some herds (22,53,85).
Based on the studies specifically designed to determine N. caninum seroprevalence in representative cattle, dairy and beef cattle in Saskatchewan and Manitoba appear to have similar N. caninum seroprevalences, albeit based on studies with some differences in methods and results. Currently, there are no data on the seroprevalence of N. caninum in beef cattle in eastern Canada to confirm that dairy and beef cattle have similar prevalences.
Prevalence in dogs
There is little reported information about the prevalence of N. caninum infection in dogs in Canada. One study that involved 1077 serum samples collected from dogs in 35 American states and 3 Canadian provinces determined that 75 dogs (7.0%) were IFAT positive, with no difference in prevalence between males and females. In PEI, all 3 samples were negative. In Alberta, 6 of 8 (75%) samples were positive, while in Ontario, 5 of 77 (6.5%) samples were positive (86). In one other North American study, a 2% prevalence was reported in dogs that were tested in Kansas (87). However, these estimates of seroprevalence should be interpreted with caution, because diagnostic tests for N. caninum infection in cattle have not been evaluated to determine their sensitivity and specificity in dogs. Furthermore, the small sample sizes in 2 of the 3 provinces certainly cannot be considered representative of the dog population in these provinces. Also, while dogs have been shown to develop a measurable antibody response to N. caninum, some dogs remain seronegative even after producing N. caninum oocysts (23,24). In studies from Japan, Korea, and Mexico, a higher level of infection was reported in rural dogs than in urban dogs (88–90), which is what would be expected where farm dogs are likely to have greater access to placental material from cows and tissues from dead stock.
Effects on productivity
The possible effects of neosporosis on productivity in cattle include reproductive losses, reduction in milk production, premature culling, and reduced weight gain.
Reproductive losses
Neosporosis can cause abortions at sporadic, endemic, and epidemic levels in herds. In herds with low seroprevalence of N. caninum (< 5%), abortions due to N. caninum infection may occur at a rate of 1 per 100 cows/year, or less, because of the low seroprevalence and unpredictability with which seropositive cattle recrudesce and abort the fetus. In herds with moderate (10% to 20%) or high (> 20%) seroprevalence of N. caninum infection and no evidence of horizontal transmission, abortion due to N. caninum infection may be frequent and distributed throughout the year (51). Abortion storms, involving 10% to 60% of cows (91), can occur either in herds with recently infected cows (horizontal transmission) (22,92) or in herds with moderate or high seroprevalence due to previous N. caninum infection that have been exposed to factors that have lead to recrudescence and abortion (91,93–95).
The risk of abortion is 2 to 3 times higher in seropositive than in seronegative dairy cows (82,91,96). However, this risk is age-dependent and can be 7 times higher, as was observed in congenitally infected heifers in their 1st pregnancy in a large dairy herd in California (51). On this same farm, seropositive cows were only 1.7 times more likely to abort in their 2nd pregnancy (during their first lactation).
A study of 8 beef herds in central Alberta with moderate to high levels of N. caninum infection demonstrated a similar increased risk of abortion (OR = 5.7) and even stillbirth (OR = 2.8) among seropositive cows compared with seronegative cows (6). In at least 2 of these herds, there was evidence for horizontal transmission, which perhaps explains the higher risk of abortion compared in this study with that in many reports in dairy cattle. From an investigation of an abortion storm on a beef farm in Alberta with evidence of N. caninum horizontal transmission, seropositive cows were also 6 times more likely to abort or be open at pregnancy check compared with seronegative cows (22). Therefore, the risk of abortion appears to be highest soon after the time of initial N. caninum infection. A possible explanation for this time dependency could be that, as time passes, the encysted bradyzoites are less likely to recrudesce. Support for this theory can be seen with the negative dose:response relationship between cow age and level of vertical transmission, as discussed earlier (52).
While it is clear that seropositive cows are more likely to abort than are seronegative cows, it is also clear that many seropositive cows do not abort. The factors that enhance the likelihood of any given seropositive cow aborting remain largely unknown, as are the factors that enhance the likelihood of any given seropositive cow aborting a second time. Dairy cows that are seropositive for N. caninum and have aborted are 2 to 3 times more likely to have a subsequent abortion than are seronegative cows (51). However, in the investigation of an outbreak of abortions on a beef farm in Alberta, seropositive cows that aborted during the initial abortion storm were not at increased risk of abortion compared with seronegative herdmates during the subsequent 2 y of observation (53). Perhaps the stress of high milk production in dairy cows, accompanied by the normal down-regulation of the immune system during mid-gestation, may explain the increased risk of multiple abortions in dairy cows but not in beef cows.
Since the discovery of N. caninum as a cause of cattle abortions in the late 1980s, N. caninum has become the most commonly diagnosed cause of abortions in many parts of Canada and elsewhere. Diagnosis of N. caninum-associated abortions in dairy cattle in Ontario increased from 1.6% of abortion submissions in 1993 to 1994 to 5.7%, 11.4%, 12.5%, and 14% to 15% in 1994 to 1995, 1995 to 1996, 1996 to 1997, and 1997 to 2000, respectively (97). Since 1994, N. caninum has been the most commonly diagnosed cause of abortion in dairy herds in Ontario. In Quebec, 11.4% of all aborted bovine fetuses submitted to diagnostic laboratories in 1996 were infected with N. caninum (4). Similar estimates of 15% to 20% have been found in California and The Netherlands, demonstrating the large impact of N. caninum in other dairy producing areas of the world (98–100).
Reduced milk production
The association between seropositive dairy cows and milk production depends on whether N. caninum is causing abortions. In 90 randomly selected herds in Maritime Canada, with seroprevalence levels ranging from 0% to 73%, N. caninum-seropositive cows did not have a significantly different 305-day milk production compared with N. caninum-seronegative cows (80). However, in a case control study of 83 herds in Ontario, 305-day milk production for seropositive cows was 250 to 300 kg less than for seronegative cows in herds with a history of N. caninum abortion problems, but it was not less in herds without a history of abortion problems (101). These results would explain previous studies in large herds with abortion problems in the United States that demonstrated similar reductions in milk production associated with seropositive cows compared with seronegative herdmates (102,103).
Premature culling
The association in dairy cows between seropositivity and premature culling also depends on whether N. caninum is causing abortions. In 56 dairy herds in Ontario, N. caninum seropositivity was not significantly associated with either time to culling or risk of culling (104). In 90 randomly selected dairy herds in Maritime Canada, N. caninum-seropositive cows also did not have an increased risk of culling compared with N. caninum-negative cows (105). There was no information on abortions in either of these Canadian studies. Conversely, in 1 large dairy herd with N. caninum abortion problems in California, seropositive cows were 1.7 times more likely to have been culled during the study period than were seronegative cows and were 6.3 mo younger at culling (55). An interaction was found between the number of abortions and the serological status of the animal, indicating that cows were more likely to be culled if they were seropositive and had aborted, than if they were seropositive but hadn’t aborted or if they had aborted but were not seropositive. For example, a seropositive cow that had aborted was 3 times more likely to be culled than a seronegative cow that had aborted (55). Seropositive cows were also twice as likely to have been culled for low milk production or mastitis compared with seronegative cows (55).
There is limited information on the risk of culling among seropositive beef cattle. In a study of 8 beef herds in central Alberta with moderate to high levels of N. caninum infection, seropositive cows had a 1.9 times higher risk of being culled for any reason, and 2.5 times higher risk for reproductive failure compared with seronegative herdmates (53). It is unlikely that these herds are representative of the majority of beef herds in Canada, because their seroprevalences, ranging from 20% to 50%, were considerably higher than those found on most beef farms. Additional research is required to determine if seropositive beef cows are at higher risk of premature culling in herds that do not have abortion problems.
Reduced weight gain/feed efficiency
Following the outbreak investigation of a N. caninum abortion storm in a beef farm in Alberta, fall calf weights for 75 calves with antibodies to N. caninum at birth were slightly lower (4.2 kg), but not significantly different, from those of 37 calves with no antibodies, after adjusting for calf sex, dam age, calf birth weight, calf age at weighing, and sire group (85).
In another recent, larger study of 1976 male calves from all 4 of the western Canadian provinces, 128 seropositive calves also had a slightly lower (0.04 kg/d) but not significantly different postweaning average daily gain (ADG) compared with the seronegative calves. These findings can be compared with results from a study of 1009 weaned steers from 92 herds in Texas. This latter study found a significantly (P < 0.05) lower postweaning ADG (0.05 kg/d) and slaughter weight (7.5 kg) in seropositive steers compared with seronegative steers. No significant difference was found in feed conversion. However, in a small, detailed longitudinal study of 34 feedlot steers in Texas, seropositive steers required 2.2 kg more feed (on a dry matter basis) for each 1.0 kg of live body weight gain than did seronegative steers. This extra feed requirement demonstrated a significant impairment in feed efficiency (106).
The 2 Canadian studies, neither of which examined feed efficiency, showed a trend toward lower ADG in seropositive cattle compared with seronegative cattle, a trend that may have developed into a statistically significant difference with a larger sample size. With the ADG and total weight gain findings being numerically similar in the studies from both Canada and the United States, it would appear that N. caninum infection has a negative impact on growth in beef cattle under North American production systems.
Economic impact
There are few firm data on the economic losses due to N. caninum in the dairy or beef cattle industries in Canada or elsewhere. As indicated in the previous section, direct productivity losses due to N. caninum include reproductive problems, such as stillbirths; abortions; early fetal death and resorption, manifested as return to service; increased time to conception; or infertility. Other direct costs include loss of milk yield in cows aborting due to N. caninum, increased culling of cows aborting due to N. caninum, and reduced growth and feed efficiency (107). A reduction in the value of breeding stock is also likely, although there is no documented evidence of this impact. Potential indirect costs include professional costs and costs associated with diagnoses, rebreeding, increased lactation time, and replacement of a positive cow that has been culled (107).
In a Canadian study, based on data from the Maritime provinces, the cost of neosporosis was estimated at the herd and regional level. At the herd level, direct costs (premature culling, reduced cull value, abortions, and reproductive losses) and treatment costs (the cost of veterinary services, medication, and extra labor) were estimated at $2304.98 per year per infected herd of 50 cows, based on a 20% within-herd prevalence of infection. At the regional level, annual losses were estimated to be $1 909 794.00 for the 3 provinces (108).
A California study, based only on the number of possible abortions, estimated the loss at US $35 million per year in California alone (9). By applying the assumptions used in the California study that 2% to 5% of all pregnancies end in abortion due to N. caninum infection, annual losses in Canada could be between 24 000 and 60 000 pregnancies/y (9).
There is one estimate of the economic losses associated with N. caninum infection in beef cattle, based on effects estimated in beef herds in Texas. The predicted loss, using a stochastic model that allows for ranges of estimated costs with likely distributions of these ranges, was between US $23.29 to US $35.21 per head or US $978.18 to US $1478.82 per 42-head infected herd with a 20% prevalence of infection (109). Estimated financial losses to the Texan beef industry ranged from US $15 to 24 million. However, this estimate may not be applicable to Canadian herds, due to differences in prices and market conditions between the 2 countries.
Risk factors
Identified risk factors for N. caninum transmission can be subdivided, based on the type of outcome that was investigated, into those associated with seroprevalence, confirmed postnatal infection, and abortion storms. However, it is unlikely that the risk factors associated with each of the types of investigated outcomes are independent. Documented abortion storms have frequently resulted from infection of previously uninfected pregnant animals (43,82,110), although they could result from group recrudescence among congenitally infected cattle (111). High seroprevalence herds could have reached that status through long-term accumulation of congenitally infected cattle, widespread postnatal infection, or both (52). It is likely that there are factors that contribute to horizontal transmission and factors that contribute to vertical transmission on farms with and without existing N. caninum infection on the farm. However, many exploratory risk factor studies are unable to specifically identify whether the factors are contributing to horizontal or vertical transmission, unless the factors are biologically associated with one or the other, as demonstrated below.
There has been only one published Canadian study to determine risk factors of N. caninum seroprevalence and N. caninum abortion on dairy farms. The presence and number of farm dogs on the dairy farm premises at the time of the study visit, as well as during the previous 3 y, were the only 2 factors significantly associated with a herd being a case herd (N. caninum confirmed abortion) and a herd having a high (≥ 10%) seroprevalence (4). These findings have been corroborated elsewhere (43,112) and with additional detail from a study on 8 dairy farms in The Netherlands with abortion storms and evidence of horizontal transmission. All 8 farms reported the introduction of a new farm dog within a period of 1.5 y before the first indication of N. caninum infection (either a N. caninum abortion or N. caninum infected calf). As there was evidence in all herds of vertical transmission of N. caninum for years, it was hypothesized that the newly introduced (probably naïve) dog was infected with N. caninum through placental (or other) material from already infected cattle and that it subsequently transmitted the infection to other cattle by shedding oocysts (113). However, there were no uninfected farms in this small study; therefore, it is unknown whether a randomly selected group of uninfected dairy farms would also have had new farm dogs introduced within the past 1.5 y, or whether the 8 farms are representative of typical management seen in the dairy industry in The Netherlands or Canada.
Another Dutch study found that female dogs were twice as likely to be seropositive as male dogs (112). Recrudescence of infection during pregnancy and a subsequent rise in antibodies may explain this finding, a phenomenon that is well known to exist in cows (58,96) and has been demonstrated to occur in a single dog (114). Confirmation of this theory is required in a larger population of dogs.
A variety of other risk factors have been reported to be related to N. caninum seroprevalence in cattle from studies done outside Canada, factors that may, in the future, prove to be relevant to the control of N. caninum in dairy and beef farms in Canada. In one study of 42 dairy farms in France, 27 seropositive farms were associated with having contact with rabbits, ducks, and poultry, as well as with tethered housing and pond water supply (45). A Dutch study of risk factors among 50 dairy farms with N. caninum abortion storms (43) also found a significant association with the number of poultry present on the farm. With a number of avian species having been shown to have antibodies against N. caninum, it is possible that poultry and other avian species may be another intermediate host, although this still requires confirmation. The Dutch study also found an association between the farms with abortion storms and the feeding of moldy corn-silage during the summer. This finding may be related to the possible immunosuppression that moldy feed can impart on cattle, leading to recrudescence of already infected cattle.
In the above mentioned French study (45), long calving intervals, high somatic cell counts, and the presence of cats were found to be negatively associated with the risk of N. caninum infection (45), although it is unclear by what mechanism these factors could protect against a positive antibody test for N. caninum.
In a Dutch study, 12 dairy farms with demonstrated horizontal N. caninum infection were compared with 21 control farms with no evidence of postnatal infection. The 12 farms were significantly more likely to have farm dogs that ingested colostrum, milk, uterine discharge, and placental material, and defecate in feed alleys and storage areas for grass and corn silage compared to control farms (93). However, multivariable regression analyses were not conducted on these data to determine which of these variables were most significantly associated with horizontal infection. For example, it is conceivable that most farm dogs that ingested placental material also ingested uterine discharge, and without this additional analysis, one could prematurely conclude that uterine discharge consumption can lead to horizontal transmission of N. caninum. Two dogs fed colostrum spiked with culture-derived tachyzoites did not develop a titer or shed oocysts; so, based on this small Dutch study, consumption of tachyzoite contaminated colostrum appears not to be a means of transmission to dogs (28). Confirmation of this finding from naturally contaminated colostrum from N. caninum infected cows under a commercial setting is required.
A number of authors have suspected that concurrent infections with other agents may lead to immunosuppression, allowing recrudescence in latently infected cattle. However, herd-level prevalence of antibodies to bovine herpesvirus 1, Leptospira hardjo, or Salmonella dublin were not associated with the risk of abortion storms on Dutch dairy farms (43). Similarly, an increased risk of abortion was not observed when cows were seropositive to both N. caninum and bovine viral diarrhea virus (BVDV) infections (43,110,115).
Interesting possible risk factors for transmission of infection were identified among 760 calves from 76 herds in Texas, where 99 (13%) calves were positive and 59% of the herds had at least 1 seropositive calf. In the final multivariable regression model, the following factors (possibly related to horizontal transmission) were associated with an increased risk among seropositive calves: calving in the spring, stocking density > 1 cow/calf unit/2.2 ha, use of a round-bale feeder, and wildlife access to the weaning supplement. The use of a self-contained feeder for cow supplements was associated with a reduced risk for seropositivity, potentially reducing the risk for horizontal transmission. Ranches that self-reared replacement heifers also had an increased risk for seropositivity, supporting the hypothesis that some of the calves were exposed to N. caninum through vertical transmission. A decrease in the risk for seropositivity was associated with the use of a cattle-working dog, which, the authors speculated, may have prevented contamination of feed and water sources by other canids (116).
A spatial analysis of seropositivity among 131 steers on 54 of 94 ranches tested in Texas showed associations between seroprevalence of N. caninum and cattle density and abundances of gray foxes, coyotes, or both, which seems to corroborate the association between cattle density and seroprevalence found elsewhere, and is also indicative of how coyotes and perhaps foxes may be responsible for sylvatic transmission of N. caninum (117). Additional studies are needed to confirm whether these possible risk and protective factors are representative of other cattle-rearing locations, including Canada.
In 5 north-western states in the United States, which have an ecology more representative of western Canada than Texas, a significant association between seroprevalence and higher winter stocking density was found (118).
Prevention and control
Uninfected herds
On uninfected farms, preventing the introduction of the parasite through normal biosecurity measures is the primary focus. With the waxing and waning of titers in infected animals, the best method to ensure that the parasite is not introduced by carrier cattle is to maintain a closed herd. If animals are purchased, they should be obtained only from herds that have been tested and are known to be test-negative. Alternatively, purchased animals should be test-negative, but false-negative identifications on single tests at the individual animal level are more likely to occur than false-negative herd tests, especially with repeated testing within the herd.
Even with careful purchasing of animals, N. caninum could be introduced into a herd; therefore, risk factors for horizontal transmission should be minimized. Access of dogs (both farm and feral) to ruminants and their feed and calving areas should be restricted as much as possible (4,23). On farms where cows are pastured, it is impossible to keep dogs out of pastures, so mangers and feed storage areas should be the focus of protection from contamination by dog feces. Any feeding equipment used on neighboring farms should be cleaned well before use on an uninfected farm.
An effective monitoring program to confirm that N. caninum is not in the herd is also recommended. This program should include the serological testing of all cows that aborted, their fetuses, and their placentas for N. caninum (using IHC). A cow with a negative test in a herd with no history of N. caninum is likely to be a true negative (have a very high negative predictive value of the test, due to a low prevalence and high test sensitivity). A positive antibody test from a cow that aborts should only implicate N. caninum as the cause of the abortion in the absence of negative tests for other abortifacients. A positive IHC test for N. caninum on fetal or placental tissue would confirm the cause of the abortion.
If herd status is important for genetic sales, periodic testing of the herd (every 1 or 2 y) to confirm that N. caninum has not been introduced into the herd may be cost-beneficial. The ability to detect N. caninum-infected herds through bulk tank milk ELISA for antibodies to N. caninum is available in other countries and is currently being evaluated in Canada, and may become a useful monitoring tool for early detection of N. caninum in uninfected herds before N. caninum associated abortions occur. The testing of a farm dog for N. caninum infection status is unlikely to yield useful results, due to the limited information on the interpretation of test results in dogs for tests intended for cattle use only. There are no tests commercially available specifically for N. caninum infection in dogs.
Infected herds
The primary management goals for infected herds include preventing abortions and reducing the risk of both vertical and horizontal transmission of N. caninum, so that the prevalence of infection in the herd is reduced in the longterm. Reducing the risk of introduction of the parasite into the herd, as discussed above, is also important, so that on-farm transmission control efforts are not offset by the reintroduction of N. caninum from outside the farm.
In order to determine the extent of N. caninum infection in the herd, systematic serological testing of old and young stock (precolostrally or after 6 mo of age to avoid maternal antibody false positives) should be instituted to identify infected and uninfected animals. Based on serological test results, selective rearing of seronegative youngstock should be the backbone for long-term prevalence reduction. In herds with a seroprevalence above 30%, a single positive ELISA is sufficient to consider an animal infected and a potential cull (52). In herds with a lower seroprevalence, all cattle with more than 1 positive antibody titer, or a strong positive antibody titer on a single test, should be classified as N. caninum positive and considered for culling. However, due to the limited impacts on production parameters that have been confirmed to date, other than when N. caninum abortions occur, N. caninum infected cattle should not be culled automatically. Even though a N. caninum infected cow is more likely to abort than an uninfected cow (6,96), and cows that abort due to N. caninum in the past are more likely to abort due to N. caninum again in the future (91), it is impossible to predict whether a specific N. caninum-infected cow will abort in the future. Furthermore, while many calves born to seropositive cows become congenitally infected, the probability of vertical transmission is not 100% and can vary considerably from farm to farm (28,54). Also, embryo transfer can be used on seropositive donor cows to harvest uninfected embryos for implantation into seronegative recipient cattle, a practice that has been shown to produce seronegative calves (54,57). Although various antimicrobial agents have been tested against N. caninum in vitro, there is currently no known effective treatment to clear a cow of N. caninum infection.
Farmers do, however, need to consider a N. caninum infected cow as a reservoir for N. caninum that could be spread to the rest of the herd, either slowly and insidiously through vertical transmission or rapidly and explosively through horizontal transmission, if risk factors for this are in place on the farm. A N. caninum-infected cow in a herd could lead to a N. caninum abortion outbreak if she has a N. caninum abortion and an uninfected dog has access to and consumes some of the N. caninum-infected placenta or fetus. A dog could also consume the parasite through N. caninum-infected deadstock, if it is not properly disposed of. If this same dog becomes N. caninum infected and defecates in areas where cattle feed or water is stored, prepared, or consumed, when it is shedding oocysts, and if uninfected cattle subsequently consume the contaminated feed or water before the oocysts have been destroyed by environmental conditions, the horizontal transmission cycle will be completed. An extraneous source of the parasite may also be other domestic or wildlife reservoirs on or off the farm. These factors should be carefully assessed prior to the culling decision, particularly if the infected animal is valuable for breeding. For some farms, particularly those with confined herds, it may be more cost-effective to mitigate these risk factors (control access of dogs to the barn and feed storage areas, and properly dispose of placenta, fetuses, and all deadstock) than to lose genetically valuable animals.
There is a killed vaccine on the market in the US, conditionally approved in December of 1998 by the US Department of Agriculture. It is recommended that the vaccine be used twice in early gestation. Preliminary evaluations of this vaccine suggest that it might be able to induce protection against abortion (119,120), although this protection may only be measurable on farms with on-going risks of abortion in the 10% or higher range, as demonstrated in a field trial in New Zealand (121).
However, there are still concerns about the use of this vaccine. Test-and-cull control strategies that use immunological (ELISA) tests can no longer be used in vaccinated herds (59). Additionally, in the vaccine efficacy trial in New Zealand, more vaccinates were found to be open at their expected calving date compared with non-vaccinates, possibly due to early embryonic death (EED) from the immune response to the vaccine and its subsequent effects on placental attachment in early gestation. (121). Vaccination prior to gestation may be preferred to avoid this EED. While exposure to tachyzoites prior to gestation has been shown to prevent congenital infection with N. caninum in a majority of the experimental animals (58), there is still no scientific evidence to indicate that the vaccine can prevent fetal infection in commercial herds. Therefore, the use of this vaccine as it is currently recommended is controversial and requires further evaluation.
Conclusions
Neospora caninum is a major cause of abortion on dairy farms worldwide and is widespread in Canada. In the past 15 y, great progress has been made in understanding the pathogenesis of neosporosis. However, there are still risk factors of transmission that must be better understood, and gaps remain in the knowledge about the impact and control of the disease. Vertical transmission is the major route of transmission, but elimination of vertical transmission may not be enough to eliminate the infection from a herd, because horizontal transmission may occur. Control of N. caninum involves the prevention of both vertical and horizontal transmission, including testing and culling seropositive cows, where appropriate; use of embryo transfer; and limiting access of dogs to cattle and their feed. The ELISA has sensitivity ≥ 95% and specificity ≥ 97%, making it a good test for the determination of infected animals and herds, particularly with multiple or strong positive results, or both. The economic impact of N. caninum on dairy production is substantial and losses from abortion alone are enough to warrant control of this disease.
Acknowledgments
The authors thank Drs. Camilla Bjorkman and Herman Barkema for their helpful suggestions. CVJ
Footnotes
Support was provided by the Production Limiting Diseases Committee (PLDC), CAPES — Brazilian government, Dairy Farmers of Canada, PEI Agricultural Research Investment Fund, and Atlantic Veterinary College.
CAPES — BRAZIL provided stipend support for Dr. Haddad.
References
- 1.Bjerkas I, Mohn SF, Presthus J. Unidentified cyst-forming sporozoon causing encephalomyelitis and myositis in dogs. Z Parasitenkd. 1984;70:271–274. doi: 10.1007/BF00942230. [DOI] [PubMed] [Google Scholar]
- 2.Dubey JP, Carpenter JL, Speer CA, Topper MJ, Uggla A. Newly recognized fatal protozoan disease of dogs. J Am Vet Med Assoc. 1988;192:1269–1285. [PubMed] [Google Scholar]
- 3.Keefe GP, VanLeeuwen JA. Neospora then and now: prevalence of Neospora caninum in maritime Canada in 1979, 1989, and 1998. Can Vet J. 2000;41:864–866. [PMC free article] [PubMed] [Google Scholar]
- 4.Pare J, Fecteau G, Fortin M, Marsolais G. Seroepidemiologic study of Neospora caninum in dairy herds. J Am Vet Med Assoc. 1998;213:1595–1598. [PubMed] [Google Scholar]
- 5.Waldner CL, Henderson J, Wu JTY, Coupland R, Chow EYW. Seroprevalence of Neospora caninum in beef cattle in northern Alberta. Can Vet J. 2001;42:130–132. [PMC free article] [PubMed] [Google Scholar]
- 6.Waldner CL, Janzen ED, Ribble CS. Determination of the association between Neospora caninum infection and reproductive performance in beef herds. J Am Vet Med Assoc. 1998;213:685–690. [PubMed] [Google Scholar]
- 7.VanLeeuwen JA. Infection prevalence of production limiting disease in Canada. Proc Atlantic Bovine Pract Assoc Conf — Moncton 2003, ABPA 2003.
- 8.Dubey JP, Lindsay DS. A review of Neospora caninum and neosporosis. Vet Parasitol. 1996;67:1–59. doi: 10.1016/s0304-4017(96)01035-7. [DOI] [PubMed] [Google Scholar]
- 9.Dubey JP. Neosporosis in cattle: biology and economic impact. J Am Vet Med Assoc. 1999;214:1160–1163. [PubMed] [Google Scholar]
- 10.Dubey JP. Recent advances in Neospora and neosporosis. Vet Parasitol. 1999;84:349–367. doi: 10.1016/s0304-4017(99)00044-8. [DOI] [PubMed] [Google Scholar]
- 11.Anderson ML, Andrianarivo AG, Conrad PA, et al. Neosporosis in cattle. Anim Reprod Sci 2000;60–61:417–431. [DOI] [PubMed]
- 12.Hemphill A, Gottstein B, Conraths FJ, et al. A European perspective on Neospora caninum. Int J Parasitol. 2000;30:877–924. doi: 10.1016/s0020-7519(00)00072-2. [DOI] [PubMed] [Google Scholar]
- 13.O’Toole D, Jeffrey M. Congenital sporozoan encephalomyelitis in a calf. Vet Rec. 1987;121:563–566. [PubMed] [Google Scholar]
- 14.Lindsay DS, Upton SJ, Dubey JP. A structural study of the Neospora caninum oocyst. Int J Parasitol. 1999;29:1521–1523. doi: 10.1016/s0020-7519(99)00121-6. [DOI] [PubMed] [Google Scholar]
- 15.Lindsay DS, Speer CA, Toivio-Kinnucan MA, Dubey JP, Blagburn BL. Use of infected cultured cells to compare ultrastructural features of Neospora caninum from dogs and Toxoplasma gondii. Am J Vet Res. 1993;54:103–106. [PubMed] [Google Scholar]
- 16.Speer CA, Dubey JP, McAllister MM, Blixt JA. Comparative ultrastructure of tachyzoites, bradyzoites, and tissue cysts of Neospora caninum and Toxoplasma gondii. Int J Parasitol. 1999;29:1509–1519. doi: 10.1016/s0020-7519(99)00132-0. [DOI] [PubMed] [Google Scholar]
- 17.Dubey JP, Barr BC, Barta JR, et al. Redescription of Neospora caninum and its differentiation from related coccidia. Int J Parasitol. 2002;32:929–946. doi: 10.1016/s0020-7519(02)00094-2. [DOI] [PubMed] [Google Scholar]
- 18.Bryan LA, Gajadhar AA, Dubey JP, Haines DM. Bovine neonatal encephalomyelitis associated with a Neospora sp. protozoan. Can Vet J. 1994;35:111–113. [PMC free article] [PubMed] [Google Scholar]
- 19.McIntosh DW, Haines DM. Neospora infection in an aborted fetus in British Columbia. Can Vet J. 1994;35:114–115. [PMC free article] [PubMed] [Google Scholar]
- 20.Duivenvoorden J. Neospora abortions in eastern Ontario dairy herds. Can Vet J. 1995;36:623. [PMC free article] [PubMed] [Google Scholar]
- 21.Bildfell R, Davidson J, Dubey JP. Neospora-induced protozoal bovine abortion in Prince Edward Island. Can Vet J. 1994;35:122. [PMC free article] [PubMed] [Google Scholar]
- 22.Waldner CL, Janzen ED, Henderson J, Haines DM. Outbreak of abortion associated with Neospora caninum infection in a beef herd. J Am Vet Med Assoc. 1999;215:1485–1490. [PubMed] [Google Scholar]
- 23.McAllister MM, Dubey JP, Lindsay DS, Jolley WR, Wills RA, McGuire AM. Dogs are definitive hosts of Neospora caninum. Int J Parasitol. 1998;28:1473–1478. [PubMed] [Google Scholar]
- 24.Lindsay DS, Dubey JP, Duncan RB. Confirmation that the dog is a definitive host for Neospora caninum. Vet Parasitol. 1999;82:327–333. doi: 10.1016/s0304-4017(99)00054-0. [DOI] [PubMed] [Google Scholar]
- 25.Dubey JP, Lindsay DS, Adams DS, et al. Serologic responses of cattle and other animals infected with Neospora caninum. Am J Vet Res. 1996;57:329–336. [PubMed] [Google Scholar]
- 26.Shivaprasad HL, Ely R, Dubey JP. A Neospora-like protozoan found in an aborted bovine placenta. Vet Parasitol. 1989;34:145–148. doi: 10.1016/0304-4017(89)90174-x. [DOI] [PubMed] [Google Scholar]
- 27.De Marez T, Liddell S, Dubey JP, Jenkins MC, Gasbarre L. Oral infection of calves with Neospora caninum oocysts from dogs: humoral and cellular immune responses. Int J Parasitol. 1999;29:1647–1657. doi: 10.1016/s0020-7519(99)00154-x. [DOI] [PubMed] [Google Scholar]
- 28.Dijkstra T, Eysker M, Schares G, Conraths FJ, Wouda W, Barkema HW. Dogs shed Neospora caninum oocysts after ingestion of naturally infected bovine placenta but not after ingestion of colostrum spiked with Neospora caninum tachyzoites. Int J Parasitol. 2001;31:747–752. doi: 10.1016/s0020-7519(01)00230-2. [DOI] [PubMed] [Google Scholar]
- 29.Uggla A, Stenlund S, Holmdahl OJ, et al. Oral Neospora caninum inoculation of neonatal calves. Int J Parasitol. 1998;28:1467–1472. doi: 10.1016/s0020-7519(98)00110-6. [DOI] [PubMed] [Google Scholar]
- 30.Barr BC, Anderson ML, Woods LW, Dubey JP, Conrad PA. Neospora-like protozoal infections associated with abortion in goats. J Vet Diagn Invest. 1992;4:365–367. doi: 10.1177/104063879200400331. [DOI] [PubMed] [Google Scholar]
- 31.Dubey JP, Acland HM, Hamir AN. Neospora caninum (Apicomplexa) in a stillborn goat. J Parasitol. 1992;78:532–534. [PubMed] [Google Scholar]
- 32.Dubey JP, Morales JA, Villalobos P, Lindsay DS, Blagburn BL, Topper MJ. Neosporosis-associated abortion in a dairy goat. J Am Vet Med Assoc. 1996;208:263–265. [PubMed] [Google Scholar]
- 33.Dubey JP, Hartley WJ, Lindsay DS, Topper MJ. Fatal congenital Neospora caninum infection in a lamb. J Parasitol. 1990;76:127–130. [PubMed] [Google Scholar]
- 34.Dubey JP, Porterfield ML. Neospora caninum (Apicomplexa) in an aborted equine fetus. J Parasitol. 1990;76:732–734. [PubMed] [Google Scholar]
- 35.Woods LW, Anderson ML, Swift PK, Sverlow KW. Systemic neosporosis in a California black-tailed deer (Odocoileus hemionus columbianus) J Vet Diagn Invest. 1994;6:508–510. doi: 10.1177/104063879400600425. [DOI] [PubMed] [Google Scholar]
- 36.Buxton D, Maley SW, Pastoret PP, Brochier B, Innes EA. Examination of red foxes (Vulpes vulpes) from Belgium for antibody to Neospora caninum and Toxoplasma gondii. Vet Rec. 1997;141:308–309. doi: 10.1136/vr.141.12.308. [DOI] [PubMed] [Google Scholar]
- 37.Simpson VR, Monies RJ, Riley P, Cromey DS. Foxes and neosporosis. Vet Rec. 1997;141:503. [PubMed] [Google Scholar]
- 38.Almeria S, Ferrer D, Pabon M, Castella J, Manas S. Red foxes (Vulpes vulpes) are a natural intermediate host of Neospora caninum. Vet Parasitol. 2002;107:287–294. doi: 10.1016/s0304-4017(02)00162-0. [DOI] [PubMed] [Google Scholar]
- 39.Barber JS, Gasser RB, Ellis J, Reichel MP, McMillan D, Trees AJ. Prevalence of antibodies to Neospora caninum in different canid populations. J Parasitol. 1997;83:1056–1058. [PubMed] [Google Scholar]
- 40.Lindsay DS, Spencer J, Rupprecht C, Blagburn BL. Prevalence of agglutinating antibodies to Neospora caninum in raccoons, Procyon lotor. J Parasitol. 2001;87:1197–1198. doi: 10.1645/0022-3395(2001)087[1197:POAATN]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 41.Lindsay DS, Kelly EJ, McKown RD, et al. Prevalence of Neospora caninum and Toxoplasma gondii antibodies in coyotes (Canis latrans) and experimental infections of coyotes with Neospora caninum. J Parasitol. 1996;82:657–659. [PubMed] [Google Scholar]
- 42.Gondim LF, McAllister MM, Pitt WC, Zemlicka DE. Coyotes (Canis latrans) are definitive hosts of Neospora caninum. Int J Parasitol. 2004;34:159–161. doi: 10.1016/j.ijpara.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Bartels CJ, Wouda W, Schukken YH. Risk factors for Neospora caninum-associated abortion storms in dairy herds in The Netherlands (1995 to 1997) Theriogenology. 1999;52:247–257. doi: 10.1016/S0093-691X(99)00126-0. [DOI] [PubMed] [Google Scholar]
- 44.McAllister M, Wills RA, McGuire AM, et al. Ingestion of Neospora caninum tissue cysts by Mustela species. Int J Parasitol. 1999;29:1531–1536. doi: 10.1016/s0020-7519(99)00098-3. [DOI] [PubMed] [Google Scholar]
- 45.Ould-Amrouche A, Klein F, Osdoit C, et al. Estimation of Neospora caninum seroprevalence in dairy cattle from Normandy, France. Vet Res. 1999;30:531–538. [PubMed] [Google Scholar]
- 46.Dubey JP, Lindsay DS, Anderson ML, Davis SW, Shen SK. Induced transplacental transmission of Neospora caninum in cattle. J Am Vet Med Assoc. 1992;201:709–713. [PubMed] [Google Scholar]
- 47.Barr BC, Rowe JD, Sverlow KW, et al. Experimental reproduction of bovine fetal Neospora infection and death with a bovine Neospora isolate. J Vet Diagn Invest. 1994;6:207–215. doi: 10.1177/104063879400600212. [DOI] [PubMed] [Google Scholar]
- 48.Barr BC, Conrad PA, Breitmeyer R, et al. Congenital Neospora infection in calves born from cows that had previously aborted Neospora-infected fetuses: four cases (1990–1992) J Am Vet Med Assoc. 1993;202:113–117. [PubMed] [Google Scholar]
- 49.Davison HC, Otter A, Trees AJ. Estimation of vertical and horizontal transmission parameters of Neospora caninum infections in dairy cattle. Int J Parasitol. 1999;29:1683–1689. doi: 10.1016/s0020-7519(99)00129-0. [DOI] [PubMed] [Google Scholar]
- 50.Pare J, Thurmond MC, Hietala SK. Congenital Neospora caninum infection in dairy cattle and associated calfhood mortality. Can J Vet Res. 1996;60:133–139. [PMC free article] [PubMed] [Google Scholar]
- 51.Thurmond MC, Hietala SK. Effect of congenitally acquired Neospora caninum infection on risk of abortion and subsequent abortions in dairy cattle. Am J Vet Res. 1997;58:1381–1385. [PubMed] [Google Scholar]
- 52.Dijkstra T, Barkema HW, Eysker M, Beiboer ML, Wouda W. Evaluation of a single serological screening of dairy herds for Neospora caninum antibodies. Vet Parasitol. 2003;110:161–169. doi: 10.1016/s0304-4017(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 53.Waldner CL, Henderson J, Wu JTY, Breker K, Chow EYW. Reproductive performance of a cow-calf herd following a Neospora caninum-associated abortion epidemic. Can Vet J. 2001;42:355–360. [PMC free article] [PubMed] [Google Scholar]
- 54.Bergeron N, Fecteau G, Pare J, Martineau R, Villeneuve A. Vertical and horizontal transmission of Neospora caninum in dairy herds in Quebec. Can Vet J. 2000;41:464–467. [PMC free article] [PubMed] [Google Scholar]
- 55.Thurmond MC, Hietala SK. Culling associated with Neospora caninum infection in dairy cows. Am J Vet Res. 1996;57:1559–1562. [PubMed] [Google Scholar]
- 56.Wouda W, Moen AR, Schukken YH. Abortion risk in progeny of cows after a Neospora caninum epidemic. Theriogenology. 1998;49:1311–1316. doi: 10.1016/S0093-691X(98)00078-8. [DOI] [PubMed] [Google Scholar]
- 57.Baillargeon P, Fecteau G, Pare J, Lamothe P, Sauve R. Evaluation of the embryo transfer procedure proposed by the International Embryo Transfer Society as a method of controlling vertical transmission of Neospora caninum in cattle. J Am Vet Med Assoc. 2001;218:1803–1806. doi: 10.2460/javma.2001.218.1803. [DOI] [PubMed] [Google Scholar]
- 58.Innes EA, Wright SE, Maley S, et al. Protection against vertical transmission in bovine neosporosis. Int J Parasitol. 2001;31:1523–1534. doi: 10.1016/s0020-7519(01)00284-3. [DOI] [PubMed] [Google Scholar]
- 59.Innes EA, Andrianarivo AG, Bjorkman C, Williams DJL, Conrad PA. Immune responses to Neospora caninum and prospects for vaccination. Trends Parasitol. 2002;18:497–504. doi: 10.1016/s1471-4922(02)02372-3. [DOI] [PubMed] [Google Scholar]
- 60.Hietala SK, Thurmond MC. Postnatal Neospora caninum transmission and transient serologic responses in two dairies. Int J Parasitol. 1999;29:1669–1676. doi: 10.1016/s0020-7519(99)00102-2. [DOI] [PubMed] [Google Scholar]
- 61.Schares G, Heydorn AO, Cuppers A, Conraths FJ, Mehlhorn H. Cyclic transmission of Neospora caninum: serological findings in dogs shedding oocysts. Parasitol Res. 2001;87:873–877. doi: 10.1007/s004360100459. [DOI] [PubMed] [Google Scholar]
- 62.Schares G, Heydorn AO, Cuppers A, Conraths FJ, Mehlhorn H. Hammondia heydorni-like oocysts shed by a naturally infected dog and Neospora caninum NC-1 cannot be distinguished. Parasitol Res. 2001;87:808–816. doi: 10.1007/s004360100445. [DOI] [PubMed] [Google Scholar]
- 63.Lindsay DS, Dubey JP. Immunohistochemical diagnosis of Neospora caninum in tissue sections. Am J Vet Res. 1989;50:1981–1983. [PubMed] [Google Scholar]
- 64.Pare J, Hietala SK, Thurmond MC. An enzyme-linked immunosorbent assay (ELISA) for serological diagnosis of Neospora sp. infection in cattle. J Vet Diagn Invest. 1995;7:352–359. doi: 10.1177/104063879500700310. [DOI] [PubMed] [Google Scholar]
- 65.Baszler TV, Knowles DP, Dubey JP, Gay JM, Mathison BA, McElwain TF. Serological diagnosis of bovine neosporosis by Neospora caninum monoclonal antibody-based competitive inhibition enzyme-linked immunosorbent assay. J Clin Microbiol. 1996;34:1423–1428. doi: 10.1128/jcm.34.6.1423-1428.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romand S, Thulliez P, Dubey JP. Direct agglutination test for serologic diagnosis of Neospora caninum infection. Parasitol Res. 1998;84:50–53. doi: 10.1007/s004360050355. [DOI] [PubMed] [Google Scholar]
- 67.Packham AE, Sverlow KW, Conrad PA, et al. A modified agglutination test for Neospora caninum: development, optimization, and comparison to the indirect fluorescent-antibody test and enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 1998;5:467–473. doi: 10.1128/cdli.5.4.467-473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harkins D, Clements DN, Maley S, et al. Western blot analysis of the IgG responses of ruminants infected with Neospora caninum and with Toxoplasma gondii. J Comp Pathol. 1998;119:45–55. doi: 10.1016/s0021-9975(98)80070-4. [DOI] [PubMed] [Google Scholar]
- 69.Lally NC, Jenkins MC, Dubey JP. Development of a polymerase chain reaction assay for the diagnosis of neosporosis using the Neospora caninum 14-3-3 gene. Mol Biochem Parasitol. 1996;75:169–178. doi: 10.1016/0166-6851(95)02530-8. [DOI] [PubMed] [Google Scholar]
- 70.McNamee PT, Trees AJ, Guy F, Moffett D, Kilpatrick D. Diagnosis and prevalence of neosporosis in cattle in Northern Ireland. Vet Rec. 1996;138:419–420. doi: 10.1136/vr.138.17.419. [DOI] [PubMed] [Google Scholar]
- 71.Dubey JP, Jenkins MC, Adams DS, et al. Antibody responses of cows during an outbreak of neosporosis evaluated by indirect fluorescent antibody test and different enzyme-linked immunosorbent assays. J Parasitol. 1997;83:1063–1069. [PubMed] [Google Scholar]
- 72.Conrad PA, Sverlow K, Anderson M, et al. Detection of serum antibody responses in cattle with natural or experimental Neospora infections. J Vet Diagn Invest. 1993;5:572–578. doi: 10.1177/104063879300500412. [DOI] [PubMed] [Google Scholar]
- 73.Barr BC, Anderson ML, Sverlow KW, Conrad PA. Diagnosis of bovine fetal Neospora infection with an indirect fluorescent antibody test. Vet Rec. 1995;137:611–613. [PubMed] [Google Scholar]
- 74.Anderson ML, Reynolds JP, Rowe JD, et al. Evidence of vertical transmission of Neospora sp infection in dairy cattle. J Am Vet Med Assoc. 1997;210:1169–1172. [PubMed] [Google Scholar]
- 75.Wu JT, Dreger S, Chow EY, Bowlby EE. Validation of 2 commercial Neospora caninum antibody enzyme linked immunosorbent assays. Can J Vet Res. 2002;66:264–271. [PMC free article] [PubMed] [Google Scholar]
- 76.Baszler TV, Adams S, Vander SJ, Mathison BA, Kostovic M. Validation of a commercially available monoclonal antibody-based competitive-inhibition enzyme-linked immunosorbent assay for detection of serum antibodies to Neospora caninum in cattle. J Clin Microbiol. 2001;39:3851–3857. doi: 10.1128/JCM.39.11.3851-3857.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Otter A. Neospora and bovine abortion. Vet Rec. 1997;140:239. [PubMed] [Google Scholar]
- 78.Dohoo IR, Martin SW, Stryhn H. Veterinary Epidemiology Research. Charlottetown, Prince Edward Island: AVC Inc., 2003.
- 79.Bjorkman C, Naslund K, Stenlund S, Maley SW, Buxton D, Uggla A. An IgG avidity ELISA to discriminate between recent and chronic Neospora caninum infection. J Vet Diagn Invest. 1999;11:41–44. doi: 10.1177/104063879901100106. [DOI] [PubMed] [Google Scholar]
- 80.VanLeeuwen JA, Keefe GP, Tiwari A. Seroprevalence and productivity effects of infection with bovine leukemia virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum in Maritime Canadian dairy cattle. Bovine Pract. 2002;36:86–91. [Google Scholar]
- 81.Rodriguez I, Choromanski L, Rodgers SJ, Weinstock D. Survey of Neospora caninum antibodies in dairy and beef cattle from five regions of the United States. Vet Ther. 2002;3:396–401. [PubMed] [Google Scholar]
- 82.Davison HC, French NP, Trees AJ. Herd-specific and age-specific seroprevalence of Neospora caninum in 14 British dairy herds. Vet Rec. 1999;144:547–550. doi: 10.1136/vr.144.20.547. [DOI] [PubMed] [Google Scholar]
- 83.Buxton D, Caldow GL, Maley SW, Marks J, Innes EA. Neosporosis and bovine abortion in Scotland. Vet Rec. 1997;141:649–651. [PubMed] [Google Scholar]
- 84.Waldner CL, Wild BK, Hill BW, et al. Determination of the seroprevalence of Neospora caninum in feedlot steers in Alberta. Can Vet J. 2004;45:218–224. [PMC free article] [PubMed] [Google Scholar]
- 85.Waldner CL. Pre-colostral antibodies to Neospora caninum in beef calves following an abortion outbreak and associated fall weaning weights. Bovine Pract. 2002;36:81–85. [Google Scholar]
- 86.Cheadle MA, Lindsay DS, Blagburn BL. Prevalence of antibodies to Neospora caninum in dogs. Vet Parasitol. 1999;85:325–330. doi: 10.1016/s0304-4017(99)00105-3. [DOI] [PubMed] [Google Scholar]
- 87.Lindsay DS, Dubey JP, Upton SJ, Ridley RK. Serological prevalence of Neospora caninum and Toxoplasma gondii in dogs from Kansas. J Helminthol Soc Washington. 1990;57:86–88. [Google Scholar]
- 88.Sawada M, Park CH, Kondo H, et al. Serological survey of antibody to Neospora caninum in Japanese dogs. J Vet Med Sci. 1998;60:853–854. doi: 10.1292/jvms.60.853. [DOI] [PubMed] [Google Scholar]
- 89.Kim JH, Kang MS, Lee BC, et al. Seroprevalence of antibodies to Neospora caninum in dogs and raccoon dogs in Korea. Korean J Parasitol. 2003;41:243–245. doi: 10.3347/kjp.2003.41.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sanchez GF, Morales SE, Martinez MJ, Trigo JF. Determination and correlation of anti-Neospora caninum antibodies in dogs and cattle from Mexico. Can J Vet Res. 2003;67:142–145. [PMC free article] [PubMed] [Google Scholar]
- 91.Moen AR, Wouda W, Mul MF, Graat EAM, Werven TV, Van Werven T. Increased risk of abortion following Neospora caninum abortion outbreaks: A retrospective and prospective cohort study in four dairy herds. Theriogenology. 1998;49:1301–1309. doi: 10.1016/S0093-691X(98)00077-6. [DOI] [PubMed] [Google Scholar]
- 92.Thilsted JP, Dubey JP. Neosporosis-like abortions in a herd of dairy cattle. J Vet Diagn Invest. 1989;1:205–209. doi: 10.1177/104063878900100301. [DOI] [PubMed] [Google Scholar]
- 93.Dijkstra T, Barkema HW, Eysker M, Hesselink JW, Wouda W. Natural transmission routes of Neospora caninum between farm dogs and cattle. Vet Parasitol. 2002;105:99–104. doi: 10.1016/s0304-4017(02)00010-9. [DOI] [PubMed] [Google Scholar]
- 94.McAllister MM, Huffman EM, Hietala SK, Conrad PA, Anderson ML, Salman MD. Evidence suggesting a point source exposure in an outbreak of bovine abortion due to neosporosis. J Vet Diagn Invest. 1996;8:355–357. doi: 10.1177/104063879600800313. [DOI] [PubMed] [Google Scholar]
- 95.Thornton RN, Thompson EJ, Dubey JP. Neospora abortion in New Zealand cattle. NZ Vet J. 1991;39:129–133. doi: 10.1080/00480169.1991.35679. [DOI] [PubMed] [Google Scholar]
- 96.Pare J, Thurmond MC, Hietala SK. Neospora caninum antibodies in cows during pregnancy as a predictor of congenital infection and abortion. J Parasitol. 1997;83:82–87. [PubMed] [Google Scholar]
- 97.Ontario Ministry of Agriculture, Food and Rural Affairs. Veterinary Laboratory Services Branch Annu Rep. 2001.
- 98.Wouda W, Moen AR, Visser IJ, van Knapen F. Bovine fetal neosporosis: a comparison of epizootic and sporadic abortion cases and different age classes with regard to lesion severity and immunohistochemical identification of organisms in brain, heart, and liver. J Vet Diagn Invest. 1997;9:180–185. doi: 10.1177/104063879700900212. [DOI] [PubMed] [Google Scholar]
- 99.Anderson ML, Blanchard PC, Barr BC, Dubey JP, Hoffman RL, Conrad PA. Neospora-like protozoan infection as a major cause of abortion in California dairy cattle. J Am Vet Med Assoc. 1991;198:241–244. [PubMed] [Google Scholar]
- 100.Anderson ML, Palmer CW, Thurmond MC, et al. Evaluation of abortions in cattle attributable to neosporosis in selected dairy herds in California. J Am Vet Med Assoc. 1995;207:1206–1210. [PubMed] [Google Scholar]
- 101.Hobson JC, Duffield TF, Kelton D, et al. Neospora caninum serostatus and milk production of Holstein cattle. J Am Vet Med Assoc. 2002;221:1160–1164. doi: 10.2460/javma.2002.221.1160. [DOI] [PubMed] [Google Scholar]
- 102.Thurmond MC, Hietala SK. Effect of Neospora caninum infection on milk production in first-lactation dairy cows. J Am Vet Med Assoc. 1997;210:672–674. [PubMed] [Google Scholar]
- 103.Hernandez J, Risco C, Donovan A. Association between exposure to Neospora caninum and milk production in dairy cows. J Am Vet Med Assoc. 2001;219:632–635. doi: 10.2460/javma.2001.219.632. [DOI] [PubMed] [Google Scholar]
- 104.Cramer G, Kelton D, Duffield TF, et al. Neospora caninum serostatus and culling of Holstein cattle. J Am Vet Med Assoc. 2002;221:1165–1168. doi: 10.2460/javma.2002.221.1165. [DOI] [PubMed] [Google Scholar]
- 105.Tiwari A, VanLeeuwen JA, Dohoo IR, Keefe GP. Effects of seropositivity with bovine leukosis virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum on risk of culling in dairy cattle. Annu Conv Am Assoc Bovine Pract, Columbus, Ohio. 2003. 2004:160.
- 106.Barling KS, Lunt DK, Snowden KF, Thompson JA. Association of serologic status for Neospora caninum and postweaning feed efficiency in beef steers. J Am Vet Med Assoc. 2001;219:1259–1262. doi: 10.2460/javma.2001.219.1259. [DOI] [PubMed] [Google Scholar]
- 107.Trees AJ, Davison HC, Innes EA, Wastling JM, Tenter AM, Shirley MW. Towards evaluating the economic impact of bovine neosporosis. Int J Parasitol. 1999;29:1195–1200. doi: 10.1016/s0020-7519(99)00093-4. [DOI] [PubMed] [Google Scholar]
- 108.Chi J, VanLeeuwen JA, Weersink A, Keefe GP, Chi J. Direct production losses and treatment costs from bovine viral diarrhoea virus, bovine leukosis virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum. Prev Vet Med. 2002;55:137–153. doi: 10.1016/s0167-5877(02)00094-6. [DOI] [PubMed] [Google Scholar]
- 109.Kasari TR, Barling K, McGrann JM. Estimated production and economic losses from Neospora caninum infection in Texas beef herds. Bovine Pract. 1999;33:113–120. [Google Scholar]
- 110.Bjorkman C, Alenius S, Emanuelsson U, Uggla A. Neospora caninum and bovine virus diarrhoea virus infections in Swedish dairy cows in relation to abortion. Vet J. 2000;159:201–206. doi: 10.1053/tvjl.1999.0446. [DOI] [PubMed] [Google Scholar]
- 111.Wouda W, Bartels CJM, Moen AR. Characteristics of Neospora caninum-associated abortion storms in dairy herds in the Netherlands (1995 to 1997) Theriogenology. 1999;52:233–245. doi: 10.1016/s0093-691x(99)00125-9. [DOI] [PubMed] [Google Scholar]
- 112.Wouda W, Dijkstra T, Kramer AM, van Maanen C, Brinkhof JM. Seroepidemiological evidence for a relationship between Neospora caninum infections in dogs and cattle. Int J Parasitol. 1999;29:1677–1682. doi: 10.1016/s0020-7519(99)00105-8. [DOI] [PubMed] [Google Scholar]
- 113.Dijkstra T, Barkema HW, Hesselink JW, Wouda W. Point source exposure of cattle to Neospora caninum consistent with periods of common housing and feeding and related to the introduction of a dog. Vet Parasitol. 2002;105:89–98. doi: 10.1016/s0304-4017(02)00009-2. [DOI] [PubMed] [Google Scholar]
- 114.Rasmussen K, Jensen AL. Some epidemiologic features of canine neosporosis in Denmark. Vet Parasitol. 1996;62:345–349. doi: 10.1016/0304-4017(95)00867-5. [DOI] [PubMed] [Google Scholar]
- 115.Mainar Jaime RC, Berzal HB, Arias P, Rojo Vazquez FA. Epidemiological pattern and risk factors associated with bovine viral-diarrhoea virus (BVDV) infection in a non-vaccinated dairycattle population from the Asturias region of Spain. Prev Vet Med. 2001;52:63–73. doi: 10.1016/s0167-5877(01)00239-2. [DOI] [PubMed] [Google Scholar]
- 116.Barling KS, McNeill JW, Paschal JC, et al. Ranch-management factors associated with antibody seropositivity for Neospora caninum in consignments of beef calves in Texas, USA. Prev Vet Med. 2001;52:53–61. doi: 10.1016/s0167-5877(01)00233-1. [DOI] [PubMed] [Google Scholar]
- 117.Barling KS, Sherman M, Peterson MJ, et al. Spatial associations among density of cattle, abundance of wild canids, and seroprevalence to Neospora caninum in a population of beef calves. J Am Vet Med Assoc. 2000;217:1361–1365. doi: 10.2460/javma.2000.217.1361. [DOI] [PubMed] [Google Scholar]
- 118.Sanderson MW, Gay JM, Baszler TV. Neospora caninum seroprevalence and associated risk factors in beef cattle in the northwestern United States. Vet Parasitol. 2000;90:15–24. doi: 10.1016/s0304-4017(00)00234-x. [DOI] [PubMed] [Google Scholar]
- 119.Choromanski L, Block W. Humoral immune responses and safety of experimental formulations of inactivated Neospora vaccines. Parasitol Res. 2000;86:851–853. doi: 10.1007/pl00008512. [DOI] [PubMed] [Google Scholar]
- 120.Williams DJ, Guy CS, Smith RF, et al. First demonstration of protective immunity against foetopathy in cattle with latent Neospora caninum infection. Int J Parasitol. 2003;33:1059–1065. doi: 10.1016/s0020-7519(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 121.Heuer C, Nicholson C, Bielsa JM, Weston JF. Efficacy of a vaccine against Neospora caninum related abortions in New Zeland dairy herds. Proc 10th Symp Int Soc Vet Epidemiol Econ, Vina del mar, Chile, 2003.