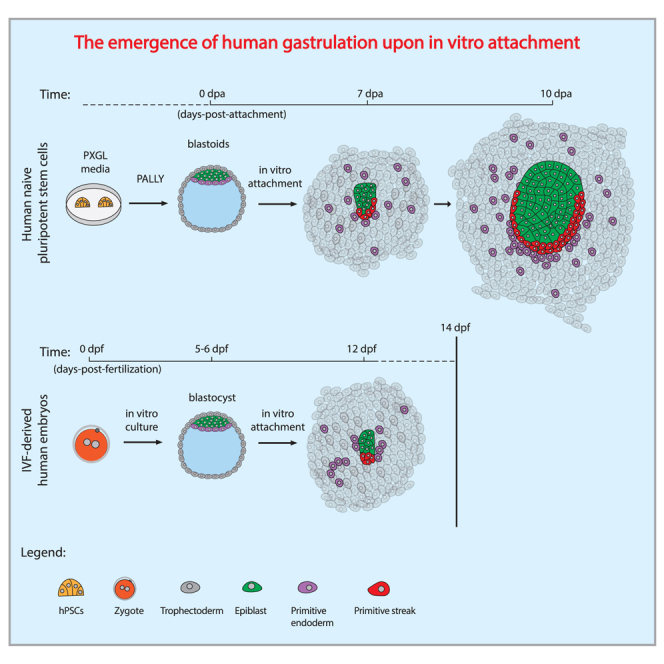
Summary
While studied extensively in model systems, human gastrulation remains obscure. The scarcity of fetal biological material as well as ethical considerations limit our understanding of this process. In vitro attachment of natural blastocysts shed light on aspects of the second week of human development in the absence of the morphological manifestation of gastrulation. Stem cell-derived blastocyst models, blastoids, provide the opportunity to reconstitute pre- to post-implantation development in vitro. Here we show that upon in vitro attachment, human blastoids self-organize a BRA+ population and undergo gastrulation. Single-cell RNA sequencing of these models replicates the transcriptomic signature of the human gastrula. Analysis of developmental timing reveals that in both blastoid models and natural human embryos, the onset of gastrulation as defined by molecular markers, can be traced to timescales equivalent to 12 days post fertilization. In all, natural human embryos and blastoid models self-organize primitive streak and mesoderm derivatives upon in vitro attachment.
Keywords: gastrulation, blastoids, human embryo, in vitro attachment, scRNA-seq
Graphical abstract
Highlights
-
•
In vitro attached human blastoids self-organize mesoderm populations in 7–10 days
-
•
scRNA-seq of in vitro attached blastoids recapitulates the human gastrula
-
•
In vitro attached human embryos self-organize a BRA+ axial population at 12 dpf
In this article, Brivanlou and colleagues show that stem cell-derived blastocyst models, blastoids, self-organize a BRA+ population and undergo gastrulation in 7–10 days upon in vitro attachment. A side-by-side comparison with natural human embryos revealed that the earliest sign of gastrulation, as defined by molecular markers, can be traced to timescales equivalent to 12 days post fertilization.
Introduction
Gastrulation is one of the most dramatic moments in our early life: a time when in the implanted embryo, a sheet of cells called the epiblast that gives rise to the entire embryo, breaks symmetry, undergoes morphogenetic movements, and establishes the primordia of the three germ layers and body axis. This process can be recognized by the emergence of morphological landmarks such as the formation of the organizer in teleosts and amphibians, or node and primitive streak in birds and mammals (Solnica-Krezel and Sepich, 2012; Ghimire et al., 2021). However, the molecular program initiating gastrulation occurs before the emergence of the morphological landmarks as demarcated by the expression of BRACHYURY (BRA) (Rivera-Pérez and Magnuson, 2005).
The establishment of culture systems that allowed in vitro attachment of natural human blastocysts (i.e., blastocysts obtained from fertilization) shed light on the second week of human development and unveiled an unexpected level of self-organization embedded in the pre-gastrulating embryo (Deglincerti et al., 2016; Shahbazi et al., 2016). These embryos, however, did not display the morphological signatures of gastrulation. We therefore asked whether the onset of human gastrulation can be recognized by the expression of molecular markers before the emergence of morphological features. It is self-evident that, regardless of rules and guidelines, the sheer number of human embryos required to achieve the same level of scrutiny as in model systems is unrealistic. To alleviate this inevitable limitation, in vitro models of the human gastrula derived from human pluripotent stem cells (hPSCs) were recently developed (Warmflash et al., 2014; Shao et al., 2017; Martyn et al., 2018; Simunovic et al., 2019; Zheng et al., 2019; Moris et al., 2020; Simunovic et al., 2022; reviewed in Shahbazi, 2020; van den Brink and van Oudenaarden, 2021). However, these systems do not self-organize from a blastocyst-like structure, lack pre-implantation extraembryonic cells, and rely on the exogenous presentation of morphogens such as BMP4, WNT, and Activin A.
Recently, naive human embryonic stem cells (hESCs) were shown to be able to self-organize into 3D blastula models (blastoids) that attach in vitro and recapitulate features of human post-implantation development until 13 days post fertilization (dpf) in the absence of cellular and morphological manifestations of primitive streak formation (Yu et al., 2021; Yanagida et al., 2021; Kagawa et al., 2022). Following these pioneering studies, we show that human blastoids generated from PXGL naive hESCs can gastrulate and self-organize to generate embryonic and extraembryonic germ layers upon in vitro attachment, reconstituting the molecular signature of the human primitive streak. Our study traces the origin of human gastrulation upon in vitro attachment and provides the in vitro-reconstituted transcriptomic signature of early human development.
Results
In vitro attached human blastoids gastrulate
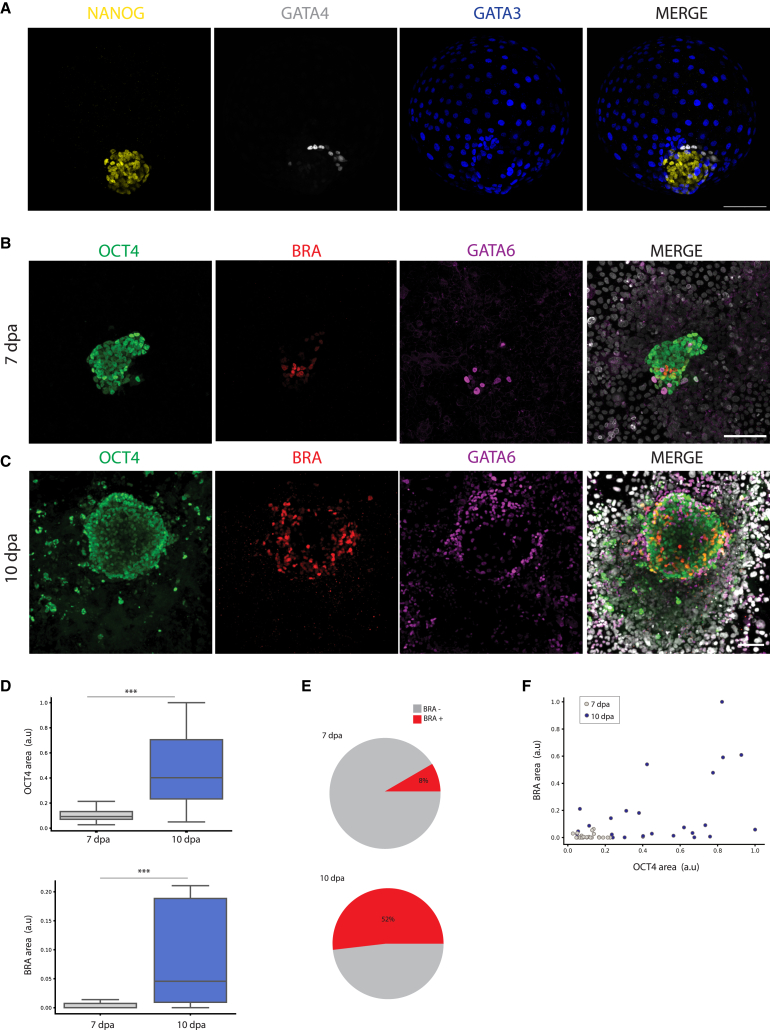
RUES2 (NIHhESC-09-0013) cells were reprogrammed from post-implantation “primed” to a pre-implantation “naive” state by chemical resetting (Guo et al., 2017). This was manifested by a change in colony morphology, from flat epithelial to dome-shaped, accompanied by the expression of pre-implantation epiblast markers, KLF17/SUDS2 (Figures S1A and S1B) and the absence of post-implantation epiblast and mesodermal markers, CD24/SSEA4 and BRA (Figures S1C–S1E) (Bredenkamp et al., 2019). Upon differentiation in microwells (Aggrewells) using PALLY-LY medium (Kagawa et al., 2022), naive RUES2 cells self-organized into 3D blastoids, harboring epiblast (NANOG+, OCT4+), primitive endoderm (GATA4+, SOX17+), and trophectoderm (GATA3+) populations (Figures 1A and S1F–S1H) (Kagawa et al., 2022). We modified our in vitro attachment protocol for natural human embryos (Deglincerti et al., 2016; Shahbazi et al., 2016) to include conditions developed for the maintenance of extended culture of human and non-human primate embryos (Ma et al., 2019; Niu et al., 2019; Xiang et al., 2020). RUES2 blastoids were attached on plastic substrates coated with laminin-521 with ROCK inhibitor (Y-27632) and extracellular matrix in the medium (5% Geltrex) (experimental procedures). Primitive streak and mesoderm formation were evaluated by expression of OCT4+ (epiblast/ectoderm), GATA6+ (endoderm), and OCT4+/BRA+ (primitive streak) at 7 days post attachment (dpa). We use dpa instead of dpf because hPSCs do not have a time zero as identified by fertilization upon which time is counted in natural human embryos. At 7 dpa, a subset of blastoids displayed a small clump of OCT4+/BRA+ cells, matching the molecular signature of an early primitive streak (Figure 1B). Immunostaining analysis of in vitro attached blastoids at 10 dpa resulted in a prominent BRA+ territory in a fraction of in vitro attached blastoids (Figure 1C). Both OCT4+ and OCT4+/BRA+ populations increased over time, suggesting an enlargement of the epiblast population as well as induction and expansion of primitive streak and mesoderm populations (Figure 1D, n = 51 from three independent biological replicates). The fraction of blastoids that initiated primitive streak and mesoderm differentiation expanded over time and was 8% at 7 dpa and 52% at 10 dpa (Figure 1E). A BRA+ population was induced across a wide range of OCT4+ epiblast areas, suggesting that the absolute size of the epiblast was not associated with primitive streak and mesoderm induction (Figures 1F and S2A). Self-organization of a gastrulating epiblast was also observed in blastoids derived from an additional human naive PSC line, niPSC75.2 (Figure S2B). Culture conditions and substrate coating had a marginal effect on the induction of the OCT4+/BRA+ population, since it was detected across several different culture conditions, including in the absence of KOSR or laminin-521 coating (Figure S2C). We conclude that in vitro attached human blastoids self-organize an OCT4+/BRA+ human primitive streak population in 7–10 dpa.
Figure 1.
In vitro attached human blastoids self-organize primitive streak and mesoderm populations
(A) Blastoid immunostaining displays NANOG (yellow), GATA4 (gray), and GATA3 (blue) marker genes distinguishing epiblast, primitive endoderm, and trophectoderm before in vitro attachment. Scale bar, 100 μm.
(B) In vitro attached human blastoids analyzed by immunostaining show OCT4+/BRA+ cells at 7 days post attachment (dpa). OCT4+ cells label the epiblast territory, GATA6+ the primitive endoderm, and BRA+ the primitive streak and mesoderm populations. Immunostaining pseudocolors: OCT4 (green), BRA (red), GATA6 (magenta), and merge with DAPI (gray). Scale bar, 100 μm.
(C) Immunostaining at 10 dpa displays OCT4+-epiblast, BRA+-primitive streak and mesoderm, and GATA6+-endoderm cells. Immunostaining pseudocolors: OCT4 (green), BRA (red), GATA6 (magenta), and merge with DAPI (gray). Scale bar, 100 μm.
(D) Scaled area quantification of OCT4+ and BRA+ cells at 7 and 10 dpa. Data are displayed as a box plot (center line displays the median, and box limits are the 25th and 75th percentiles) (n = 51 from three independent differentiations). Student’s t test, unpaired with two tails. ∗∗∗p < 0.001.
(E) Pie chart of in vitro attached blastoids containing at least five BRA-positive cells at 7 and 10 dpa.
(F) Area quantification is displayed as a scatterplot. x axis reports the scaled OCT4 area, while the y axis is the BRA scaled area. Each dot represents an individual attached blastoid that has been color coded according to the time of analysis. Gray dots represent blastoids at 7 dpa and blue dots blastoids at 10 dpa.
scRNA-seq of in vitro attached human blastoids reveals convergent features with the human gastrula
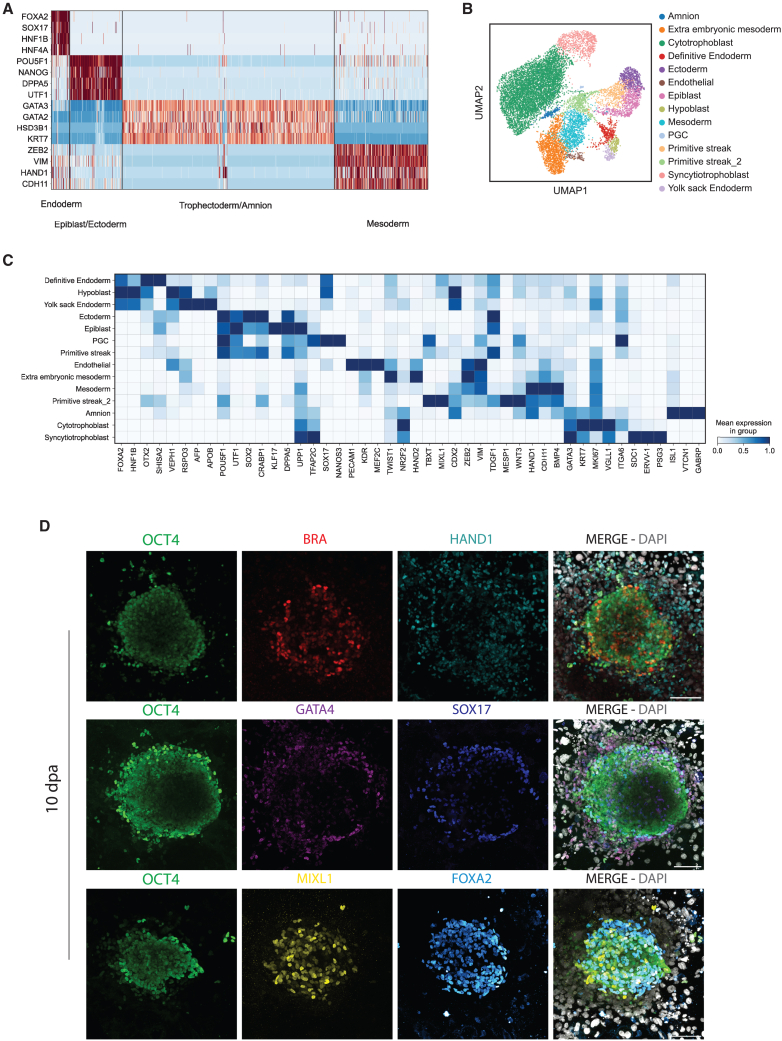
Two different time points (7 and 10 dpa) were analyzed by single-cell RNA sequencing (scRNA-seq). Sixteen distinct clusters comprising derivatives of the three germ layers and extraembryonic cells were identified (Figures 2A, 2B, and S2D). Cell-type annotation was based on the expression of marker genes and revealed the presence of epiblast, ectoderm, primordial germ cells, two distinct populations of the primitive streak, mesoderm, extraembryonic mesoderm, endothelial progenitors, definitive endoderm, hypoblast, yolk sac endoderm, cytotrophoblast, syncytiotrophoblast, and amniotic cells (Figures 2C and S2E–S2G; Table S1). Immunostaining analysis displayed MIXL1+, HAND1+, FOXA2+, SOX17+, and GATA4+ cells that were self-organized with proximal-distal coordinates from the central epiblast territory, distinguishing primitive streak, mesoderm, and endoderm populations in in vitro attached blastoids at 10 dpa (Figure 2D). Among the GATA4+ cells, we detected a small subpopulation also positive for NR2F2 and VIM, identifying the extraembryonic mesoderm population, that increased over time (Figure S2H) (Pham et al., 2022). In addition to derivatives of the three germ layers and the extraembryonic mesoderm, we identified trophoblast (GATA3+/KRT7+) and amnion cells (ISL1+/OCT4+/GATA3+), although no amniotic cavity was detected at 7–10 dpa (Figures S2I–S2K and S3A). Thus, single-cell transcriptomics of in vitro attached human blastoids revealed the presence of derivatives of the three germ layers and extraembryonic cellular populations at 7–10 dpa.
Figure 2.
Single-cell transcriptomics of in vitro attached human blastoids distinguish embryonic and extraembryonic populations
(A) Heatmap displays Z-score scaled expression values for marker genes that distinguish extraembryonic (GATA3, GATA2, HSD3B1, KRT7), epiblast/ectoderm (POU5F1, NANOG, DPP5, UTF1), mesoderm (ZEB2, VIM, HAND1, CDH11), and endoderm (FOXA2, SOX17, HNF1B, HNF4A) populations at 7 and 10 dpa (7 dpa, 7,354 cells; 10 dpa, 5,489 cells).
(B) UMAP plot shows the identified cell populations: cytotrophoblast, syncytiotrophoblast, amnion, epiblast, ectoderm, primordial germ cells, primitive streak 1–2, mesoderm, extraembryonic mesoderm, endothelial, definitive endoderm, hypoblast, and yolk sac endoderm.
(C) Heatmap of average scaled expression displaying marker genes that distinguish individual cell populations.
(D) Immunostaining of 10-dpa human blastoids. OCT4 (green) and DAPI (gray) are used to identify blastoids. MIXL1 (yellow), HAND1 (cyan), GATA4 (magenta), FOXA2 (hot cyan), and SOX17 (blue) identify mesoderm and endoderm populations. Scale bar, 100 μm.
To identify the gene trends occurring during primitive streak formation, pseudotime analysis was performed on the primitive streak clusters (Figure S3B and Table S2). Gene trend analysis found waves of gene expression that recapitulate the transition from early to late primitive streak, mirroring in vivo mesoderm development (Figures S3C and S3D) (Tyser et al., 2021). Gene ontology analysis enriched categories associated with “anterior/posterior axes formation” and “gastrulation” validating our findings (Figure S3E). Furthermore, scRNA-seq analysis distinguished the molecular signature of anterior and posterior primitive streak populations, with the anterior population resembling the Spemann organizer (GSC+/CER1+/FST+/NOG+) (Figures S3F and S3G).
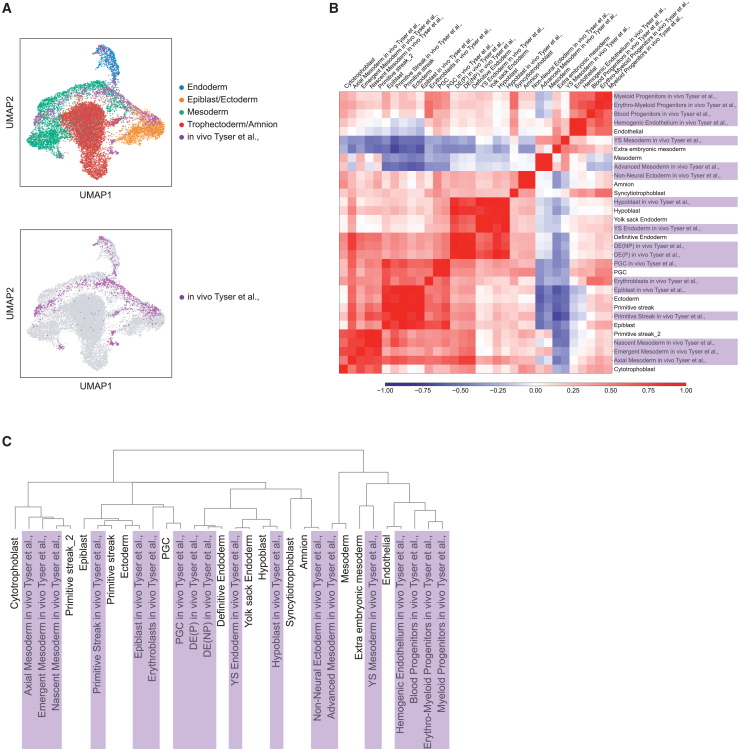
We then compared our dataset to the recently published scRNA-seq transcriptome profile of the in vivo gastrulating human embryo during the third week of development (16–19 dpf) (Tyser et al., 2021). Dataset integration displayed preferential alignment of the human gastrula within the epiblast/ectoderm, mesoderm, and endoderm clusters (Figure 3A). Cluster level correlation analysis showed several blastoid cell types displaying high correlation with distinct in vivo populations (Figure 3B). Hierarchical clustering identified multiple subgroups containing in vitro cell populations and their corresponding in vivo counterparts (Figure 3C), suggesting cell-type-specific convergent gene expression between in vitro attached blastoids and in vivo gastrulating human embryos. To further corroborate our classification, we harnessed an embryonic cell classifier to validate our dataset and cell-type classification (Zhao et al., 2021). The classifier predicted cell types that match our marker-based cell-type classification (Figure S3H and Table S3). To further support our analysis, we integrated our scRNA-seq data with published in vivo mouse and monkey datasets (Pijuan-Sala et al., 2019; Zhai et al., 2022), and with in vitro cultured human and monkey embryos (Ma et al., 2019; Xiang et al., 2020), which altogether displayed convergent gene expression matching cellular populations in embryonic and extraembryonic tissues of in vitro attached human blastoids (Figures S3I–S3L). In conclusion, single-cell transcriptomics of in vitro attached human blastoids displayed the molecular signatures of embryonic and extraembryonic populations that replicate in vivo populations, including those of the human gastrula in the third week of development.
Figure 3.
In vitro attached human blastoids recapitulate the cellular populations of the human gastrula
(A) UMAP shows the integration of 7–10 dpa blastoid scRNA-seq and the human gastrula dataset at 16–19 days post fertilization (dpf) (Tyser et al., 2021). In vitro attached blastoids are color coded according to their lineage. The human gastrula dataset preferentially integrates with the epiblast/ectoderm, mesoderm, and endoderm lineages, though not with the trophectoderm/amnion population.
(B) Correlation analysis using the commonly detected highly variable genes between the human gastrula dataset (Tyser et al., 2021) and the in vitro attached human blastoid datasets displayed as a Z-score heatmap. The clusters from the human gastrula dataset are shown with their original annotations followed by an “in vivo Tyser et al.” label and highlighted in light purple.
(C) Hierarchical clustering is displayed as a dendrogram. The human gastrula (Tyser et al., 2021) labels are highlighted in light purple.
The onset of gastrulation is regulated by the signaling cascade that starts with BMP4, which then induces WNT, that in combination with NODAL, controls mesoderm and endoderm differentiation (Arnold and Robertson, 2009; Warmflash et al., 2014; Morgani et al., 2018; Martyn et al., 2018). scRNA-seq analysis of in vitro attached human blastoids displayed endogenous expression of WNT3 and BMP4 in the amnion and mesodermal clusters, while NODAL expression was mostly confined to the epiblast, ectoderm, endoderm, and primitive streak populations (Figure S4A). In agreement with cell-type-specific ligand expression, we also observed induction of ID2 and LEF1 gene expression, which are immediate-early target genes of the BMP and WNT pathways, suggesting self-organized active signaling (Figure S4B) (Sasaki et al., 2016; Martyn et al., 2019). Treatment with small molecules inhibiting BMP (LDN-193189) or WNT (IWR1-endo) pathways starting from 5 dpa significantly reduced BRA expression, demonstrating the BMP- and WNT-dependent induction of the primitive streak population upon in vitro attachment (Figure S4C). BMP signaling regulates amnion specification controlling primitive streak and mesoderm induction (Shao et al., 2017; Zheng et al., 2019; Yang et al., 2021). Upon BMP inhibition, immunostaining displayed a reduction of ISL1+ cells, suggesting that the reduced BRA+ population might be the consequence of impaired ISL1+ amnion formation (Figure S4D). Collectively, these data show that in vitro attached blastoids self-organize BMP, WNT, and NODAL signaling pathways in a cell-type-specific manner, regulating the emergence of primitive streak and mesoderm populations upon in vitro attachment.
In vitro attached natural human embryos self-organize a BRA-positive population at 12 dpf
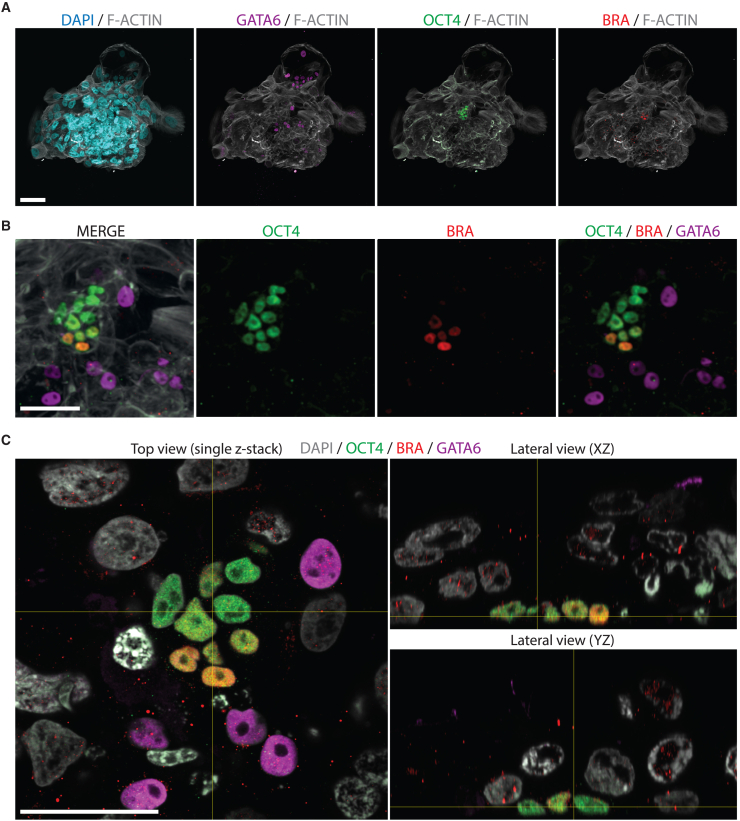
In vitro attached human blastoids self-organized a BRA+ population in the epiblast territory around 7 dpa (Figure 1C). We matched this early sign of primitive streak induction with in vitro attached natural human embryos for analysis at 12–13 dpf. As with the blastoids, in vitro attachment was performed on functionalized plastic surfaces with laminin-521, and immunostaining analysis of cell-type-specific markers was performed at 12 dpf. OCT4 staining was used to identify the epiblast population, GATA6 for primitive endoderm, BRA for primitive streak and mesoderm, and F-ACTIN and DAPI for the embryo structure (Figure 4A). Among the OCT4+ epiblast cells, we detected a subset of cells that was also positive for BRA and negative for GATA6, indicative of an early primitive streak and mesoderm population at 12 dpf (Figure 4B, n = 4 embryos). 3D rendering of the in vitro attached human embryo localized the OCT4+/BRA+ population on one side of the epiblast, suggesting the establishment of an anterior-posterior axis in a natural human embryo at 12 dpf (Figure 4C). We further investigated whether the use of laminin-521 functionalized plastic substrates for in vitro attachment was required for BRA induction. Human embryos were attached in vitro according to Deglincerti et al. (2016) in the absence of functionalized substrates, and consistently displayed self-organization of a BRA+ population at 12 dpf (Figure S4E, n = 2 embryos).
Figure 4.
In vitro attached human embryos self-organize a BRA-positive axial population at 12 dpf
(A) Immunostaining of the in vitro attached human embryo at 12 dpf. A low-magnification field of view shows DAPI (cyan), GATA6 (magenta), OCT4 (green), and BRA (red) signals, all overlaid on the F-ACTIN (gray) staining. OCT4 localizes the epiblast territory and GATA6 the primitive endoderm cells. BRA is used as a primitive streak and mesoderm marker. Scale bar, 100 μm.
(B) Immunostaining of the in vitro attached human embryo at 12 dpf. Zoomed-in field of view shows the merged image of OCT4 (green), BRA (red), GATA6 (magenta), and F-ACTIN (gray) signals. Scale bar, 50 μm.
(C) 3D orthogonal projections of the in vitro attached human embryo at 12 dpf. Top and lateral views show the merge of OCT4 (green), BRA (red), GATA6 (magenta), and DAPI (gray). Scale bar, 50 μm.
In all, in vitro attached human embryos displayed the earliest sign of primitive streak and mesoderm differentiation at 12 dpf, self-organizing an anterior-posterior axis marked by the expression of BRA in the epiblast territory.
Discussion
We have shown that upon in vitro attachment, natural human embryos and stem cell models of human blastocysts, blastoids, progress through stages of post-implantation development, including the onset of primitive streak and mesoderm formation, modeling key aspects of human gastrulation in vitro. Recently, another study showed similar findings using human blastoids derived from 5i/L/A naive cells, which self-organized primitive streak and mesoderm populations upon 3D in vitro culture (Karvas et al., 2023). Taking advantage of stem cell-derived human blastoids, we defined the timeline of primitive streak induction upon in vitro attachment, discovering the earliest molecular sign of primitive streak formation at 12 dpf in vitro attached human embryos. Detection of primitive streak molecular markers before 14 dpf suggests an earlier time for the onset of gastrulation upon extended culture in vitro. It is tempting to speculate that this event also occurs during human development in vivo, but without the precise staging of in vivo human samples, which is ethically and practically impossible, it is not possible to confirm directly. Additionally, in vitro culture might impact the timeline of primitive streak and mesoderm induction. Our data suggest that temporal staging of in vitro cultured human embryos and embryoids using molecular markers, in addition to morphological criteria, will provide a more comprehensive and precise understanding of the developmental potential of natural embryos and their models upon extended in vitro culture. This evidence is also supported by recent data obtained from in vitro cultured non-human primate embryos that displayed BRA expression and the onset of a primitive streak population around 11–13 dpf upon in vitro attachment (Niu et al., 2019; Ma et al., 2019).
Extended culture of in vitro attached human blastoids up to 10 dpa resulted in the self-organization of derivatives of the three germ layers, in the absence of exogenously presented BMP and WNT ligands or cell mixing with other extraembryonic cell types. Single-cell transcriptomics of self-organizing in vitro attached human blastoids allowed temporal and cellular matching with the dataset of the in vivo human gastrula at 16–19 dpf (Tyser et al., 2021). In vitro attached blastoids recapitulated most of the cellular populations present in the human gastrula; however, while mesodermal populations displayed proximal-distal organization from the epiblast territory, in vitro attached blastoids did not self-organize an amniotic cavity or the morphological features of an in vivo primitive streak. Interestingly, primitive streak and mesodermal derivatives can self-organize even in the absence of proper amnion and yolk sac cavitated structures.
Attachment on 2D plastic substrates may represent one of the limiting factors for further development in vitro. We anticipate that future models may take advantage of biocompatible polymers that can replicate implantation processes while facilitating 3D expansion, enabling the developmental progression of tissues and organs that resemble in vivo structures. Indeed, it has been recently shown that, by taking advantage of hydrogel embedding and optimized culture conditions, monkey embryos develop in vitro up to 25 dpf, recapitulating the cellular and molecular features of Carnegie stage 8 monkey embryos, including early organogenesis (Gong et al., 2023). Similarly, 3D cultured monkey blastocysts have been shown to progress in vitro, self-organizing derivatives of the three germ layers, including neural tube-like structures with anteroposterior and dorsoventral embryonic axes (Zhai et al., 2023). Interestingly, assembled 3D mouse embryoids derived from mouse naive PSCs are able to progress through stages of post-implantation development, including organogenesis, in the absence of physical attachment on a substrate (Tarazi et al., 2022; Amadei et al., 2022). Similarly, integrated embryo models derived from human naive PSCs have been recently shown to self-organize 3D structures that recapitulate the cytoarchitecture and the cellular populations of the human gastrula at 14 dpf (Weatherbee et al., 2023; Oldak et al., 2023). These model embryos self-assemble embryonic and extraembryonic derivatives upon cell mixing, bypassing a blastocyst-like stage (Weatherbee et al., 2023; Oldak et al., 2023). These findings suggest that physical attachment may not be essential for further developmental progression. Instead, it appears that the presence of extraembryonic tissues or the implementation of 3D culture conditions can provide the necessary support to natural embryos and embryoids for proper tissue morphogenesis. Insights from experimental investigations of stem cell models capable of recapitulating the chemical and/or mechanical inductive interactions, which guide embryonic tissue self-organization, will have a transformative impact on how we understand and engineer tissues in vitro.
Ascertaining the cellular and molecular logic of human gastrulation has tremendous implications for understanding the principles of body axes formation and potential causes of early miscarriages linked to embryonic failure. It has been estimated that up to 30% of total miscarriages occur after the second week of gestation (Ghimire et al., 2021), coincidentally aligning with the onset of gastrulation and possibly related to problems establishing axial organization during embryonic development. Our current knowledge of the mechanisms directing axis formation in the context of humans is limited and derives mostly from animal models. Important parameters such as embryo geometry, time of gestation, and gene-regulatory networks are species-specific attributes that must be considered when mechanisms of development are extrapolated from animal models. Studies on in vitro cultured natural human embryos and fetal samples provide an experimental framework to evaluate and interpret the cellular and molecular players involved in human gastrulation. Complementary to natural human embryos and fetal samples, models based on human stem cells offer virtually unlimited biological material, extensive possibilities for genetic manipulation, and experimental investigation. Although we trust that stem cell-derived models will become more representative of in vivo situations, we also believe that comparisons with actual fetal samples, in vitro cultured natural embryos, and cross-species evaluations are essential steps in evaluating stem cell-based models.
Limitations of the study
Upon in vitro attachment, our blastoids were unable to properly organize the cytoarchitecture of embryonic and extraembryonic tissues, although they self-organized derivatives from all germ layers. This limitation is likely due to our culture conditions; despite allowing extended in vitro culture, up to 10 dpa, they did not support the formation of in vivo-like structures. While in vitro attachment protocols have been instrumental in exploring the earliest events occurring in the pre-gastrulating embryo, our findings suggest that they inhibit proper morphogenesis, falling short of replicating the cytoarchitecture of implanted human embryos at the onset of gastrulation.
The lack of in vivo-like tissue architecture complicates the interpretation of the inductive interactions that led to the formation of the primitive streak and mesoderm populations in our blastoids. Consistent with previous studies, our work showed that the BMP and WNT signaling pathways control the induction of the primitive streak and mesoderm populations upon in vitro attachment. Due to the absence of the amniotic and yolk sac cavitated structures, which localize BMP and WNT signals in vivo, we cannot rule out the possibility that the blastoids’ primitive streak and mesoderm populations might have been induced in a non-physiological manner.
Experimental procedures
Resource availability
Corresponding author
Ali H. Brivanlou (brvnlou@rockefeller.edu).
Materials availability
The materials included in this study are available upon reasonable request to the corresponding author.
Data and code availability
The datasets generated in the current study are available from the corresponding author upon reasonable request. The accession number for the scRNA-seq data reported in this paper is GEO: GSE225893.
Human pluripotent stem cell culture and chemical reprogramming
RUES2 hESCs (NIHhESC-09-0013) were maintained in primed state using mouse embryonic fibroblast (MEF)-conditioned medium (CM) supplemented with 20 ng/mL fibroblast growth factor 2 (FGF2) on Geltrex-coated dishes (Thermo Fisher Scientific). CM-FGF2 medium was refreshed every day, and cells were passaged every 3–5 days using Accutase or ReleSR (Thermo Fisher Scientific).
Reprogramming to a naive state was performed according to Guo et al. (2017) and Bredenkamp et al. (2019) with minor modifications. In brief, undifferentiated prime RUES2 cells (in CM-FGF2) were dissociated as single cells with Accutase (STEMCELL Technologies), and 100,000 cells were plated on MEF plates with the addition of 10 μM ROCK inhibitor. The following day CM-FGF2 medium was refreshed, allowing cells to form colonies. Two days after seeding, reprogramming was started using N2B27 medium (50% DMEM-F12, 50% Neurobasal, 1× GlutaMAX, 55 mM β-mercaptoethanol, 0.5× N2, and 0.5× B27) supplemented with 10 ng/mL human leukemia inhibitory factor (LIF), 1 μM PD0325901, and 1 μM VPA (all from STEMCELL). Fifty percent of the medium was refreshed 2 days thereafter, and on day 3 the medium was switched to PXGL medium (N2B27 medium supplemented with 1 μM PD0325901, 2 μM XAV939, 2 μM Go6983, and 10 ng/mL LIF) and refreshed every day until day 10.
Human naive PSCs were maintained for up to 25 passages in PXGL medium. Naive cells were passaged on MEF plates upon single-cell dissociation with Accutase every 3–5 days in PXGL medium supplemented with 10 μM ROCK inhibitor and 1% Geltrex.
Blastoid differentiation in microwells
Naive hPSCs were differentiated to form blastoids according to Kagawa et al. (2022) with minor adjustments. In brief, naive PSCs were dissociated as single cells using Accutase, and MEFs were removed upon differential attachment on gelatin plates for 45 min. Unattached human naive cells were collected, counted, and seeded overnight on Aggrewells 400 at a density of 160 cells/well in N2B27 medium supplemented with 10 μM ROCK inhibitor. The next day, medium was switched to N2B27 medium supplemented with PALLY (1 μM PD0325901, 1 μM A83, 0.5 μM LPA, 10 ng/mL LIF, and 10 μM ROCK inhibitor) for 48 h. Following 48 h of PALLY medium, medium was refreshed for an additional 48 h with N2B27 medium supplemented with LY (0.5 μM LPA and 10 μM ROCK inhibitor). Based on morphological criteria, blastoids were picked manually for further experiments.
Human blastoids in vitro attachment protocol
Blastoids were selected based on morphological criteria and transferred on IBIDI 8 wells coated with laminin-521 (10 μg/mL) (Biolamina) in IVC1 medium adapted from Deglincerti et al. (2016) and Xiang et al. (2020). IVC1 medium was composed of 50% DMEM/F12 (Thermo Fisher Scientific) and 50% Neurobasal (Thermo Fisher Scientific) supplemented with 20% (v/v) heat-inactivated fetal bovine serum (R&D Systems, S1115050H), 1× GlutaMAX (Thermo Fisher Scientific), 1× ITS-X (Thermo Fisher Scientific), 8 nM β-estradiol, 200 ng/mL progesterone, and 25 μM N-acetyl-L-cysteine (all from Sigma-Aldrich). Two days post attachment, medium was switched to IVC2 medium: 50% DMEM/F12 (Thermo Fisher Scientific) and 50% Neurobasal (Thermo Fisher Scientific) supplemented with 30% KnockOut Serum Replacement (10828028, Thermo Fisher Scientific), 1× GlutaMAX (Thermo Fisher Scientific), 8 nM β-estradiol, 200 ng/mL progesterone, and 10 μM ROCK inhibitor. IVC2 medium was refreshed every other day. Extended culture up to day 10 was obtained upon extracellular matrix supplementation in IVC2 medium starting from 5 dpa with 5% Geltrex (Thermo Fisher Scientific). In the IVC2 minus KOSR medium, we replaced the KOSR serum with a 50% Neurobasal and 50% DMEM/F12 basal medium solution. For the IVC2-CRML medium, the Neurobasal and DMEM/F12 basal medium were replaced with the CMRL medium (Ma et al., 2019; Kagawa et al., 2022).
Human embryo thawing and in vitro attachment protocol
Cryopreserved IVF-derived human embryos (5–6 dpf) were thawed using the Kitazato kit (VT602US, Kitazato, USA) according to the manufacturer’s instructions. In brief, straws containing individual embryos were placed into TS1 solution for 1 min, passed twice in DS2 solution for 2 min, and twice in WS3 solution for 3 min. Embryos were cultured overnight in GLHP medium drops (GlobalHP) under mineral oil (FUJIFILM). The next day, hatched blastocysts were transferred on laminin-521-coated IBIDI 8 wells in IVC1 medium. After 48 h, 50% of the medium was replaced with IVC2 medium. Fifty percent of IVC2 medium was refreshed daily until conclusion of the experiment. The total number of human embryos used in this study is 21 from three rounds of experiments. Seven out of 21 embryos displayed OCT4+ epiblast cells and among those, 6 out of 7 embryos displayed OCT4+/BRA+ cells.
scRNA-seq sample preparation
Single cells were obtained upon enzymatic dissociation from day 7 and day 10 (Geltrex 5%) in vitro attached blastoids. Two different methods were used for sample preparation according to the presence of extracellular matrix in the medium. For day 7 blastoids, treatment with Accutase (STEMCELL) for 10 min at 37°C was sufficient to detach and isolate a single-cell suspension. To remove the extracellular matrix from the day-10 sample, we first applied Dispase solution (STEMCELL) for 7 min, washed it twice with PBS−/−, and applied Papain/DNase solution (Worthington) for 15 min at 37°C. Mechanical trituration followed by two washes with 0.04% BSA in PBS−/− was used to isolate single cells. Before Gem formation, samples were filtered with a 40-μm strainer. A Single Cell 3′ v.3 kit (10× Genomics) was used for library preparation according to the manufacturer’s instructions.
scRNA-seq analysis
10× Genomics libraries were sequenced using a NovaSeq 6000 (Illumina), and FASTQ files were aligned using Cell Ranger (7.0.0) against hg38 reference genome. Cell Ranger count matrices were processed in Python using Scanpy (Wolf et al., 2018) (scanpy = 1.9.1, https://pypi.org/project/scanpy/). Time points of analysis were 7 dpa and 10 dpa supplemented with extracellular matrix. Count matrices were filtered using the following criteria: minimum of 300 genes per cell and genes expressed in at least 3 cells. Cells with more than 20% of mitochondrial genes were discarded. After stringent filtering, 7,354 cells (7 dpa) and 5,489 cells (10 dpa) were selected for further analysis. Total counts were normalized to 10,000 reads per cell and log transformed. The top 3,000 highly variable genes were used for dimensionality reduction and further analysis. Leiden clustering and differentially expressed genes (Wilcoxon rank-sum teat) were used to annotate the clusters (Table S1). Data from the two time points (7 and 10 dpa) were integrated applying scanoroma (Hie et al., 2019) (scanorama.integrate_scanpy) using commonly detected highly variable genes. Pseudotime was calculated using the scanpy function “sc.tl.dpt” and plotted using PHATE dimensional reduction, which preserves the global structure of the data better than uniform manifold approximation and projection (UMAP) (Moon et al., 2019). The blastoids datasets (7 and 10 dpa) were integrated with in vivo human (Tyser et al., 2021; Xiang et al., 2020), mouse (Pijuan-Sala et al., 2019), and monkey (Ma et al., 2019; Zhai et al., 2022) datasets applying the scanoroma algorithm using commonly detected highly variable genes. Classification using the Embryogenesis Prediction tool was performed by submitting the raw counts matrix of 5,000 randomly sampled cells for each dataset (7 and 10 dpa) (Zhao et al., 2021) (https://petropoulos-lanner-labs.clintec.ki.se/app/shinyprediction). Classification results were plotted as individual colors in the UMAP space and are available in Table S3.
Raw count matrices and cell annotations are available in GEO under the accession number GEO: GSE225893.
Immunostaining
Samples were fixed with 4% paraformaldehyde in PBS+/+ at room temperature for 30 min, followed by three washes with PBS+/+. Samples were incubated with antibody solution (3% normal donkey serum, 0.2% Triton X-100 in PBS−/−) for 1 h before hybridization with the primary antibodies. Primary and secondary antibodies were incubated at room temperature for 1 h in antibody solution followed by three washes in 0.2% PBS−/−-Triton X-100. Samples were stored in IBIDI mounting solution at 4°C.
List of primary antibodies
OCT4: Santa Cruz Biotechnology (sc-5279), 1:200
BRA: R&D Systems (MAB20851-100), 1:500
BRA: R&D Systems (AF2085), 1:300
GATA6: R&D Systems (AF1700), 1:200
GATA6: Cell Signaling (D61E4), 1:200
GATA3: Thermo Fisher Scientific (14-9966-82), 1:100
SOX17: R&D Systems (MAB1924), 1:200
HAND1: R&D Systems (AF3168-SP), 1:200
KRT7: Abcam (ab209600), 1:100
ISL1: DSHB (39.4D5), 1:200
MIXL1: Sigma Prestige antibody (HPA005662), 1:200
SUSD2: Miltenyi Biotec (130-106-401), 1:100
SSEA4: Miltenyi Biotec (130-122-958), 1:100
KLF17: Atlas Antibodies (HPA024629), 1:200
SOX17: R&D Systems (AF2864), 1:200
GATA4: Thermo Fisher Scientific (14-9980-82), 1:100
FOXA2: Novus Biologicals (AF2400), 1:200
NR2F2: Abcam [EPR18442] (ab211776), 1:100
CD24: eBioscience (A5-2H10), 1:100
Vimentin: Abcam (ab8978), 1:200
Confocal imaging
Images were acquired on laser scanning confocal microscopes (Zeiss Inverted LSM 780 or Zeiss Inverted LSM 940) with ×10, ×20 dry, and ×63 oil objectives.
Image analysis
Images were shown as maximum-intensity projection (MIP) of z stacks over the z axis using ZEN black software or Fiji_V2 (version 2.1.0/1.53c). Fiji software was used for image processing, contrast, and export. The area of positive signal for individual channels was calculated on MIP images after Ilastick pixel classification (Berg et al., 2019). In brief, individual images were processed using Ilastik, and binarized images were used for area calculation. Values were scaled to the maximum value. Single-nuclei quantification was obtained by segmenting individual cells using Stardist (Weigert et al., 2020) and extracting the mean intensity for each channel within each nucleus (skimage). Plots were organized in Python using numpy (1.19.5), pandas (1.1.5), matplotlib (3.2.2), and seaborn (0.11.2) libraries.
Ethics statement
The research described in this paper was approved by The Rockefeller University Institutional Review Board according to university policy for research involving human embryonic stem cells and human embryos, and governed by a Tri-Institutional Stem Cell Initiative, Embryonic Stem Cell Oversight (Tri-SCI ESCRO) Committee that oversees human Embryo Research Oversight (EMRO) and serves The Rockefeller University, Memorial Sloan Kettering Cancer Center, and Weill Cornell Medicine. The ESCRO committee is an independent group charged with oversight of research involving hPSCs and embryos to ensure compliance with university policies. The Tri-SCI ESCRO committee is composed of members with scientific and bioethical expertise. Established ESCRO protocols are reviewed annually and adhere to ISSCR 2021 guidelines. Supernumerary IVF-derived human embryos donated for research were obtained from the Center for Human Reproduction and the Yale Fertility Center. Embryo donation was facilitated through an informed consent process according to NAS Guidelines for Human Embryonic Stem Cell Research and the Tri-Institutional Stem Cell Research Operating Procedures for ESCRO Reviewed Research.
Acknowledgments
We are grateful to members of the Brivanlou lab for their input and criticism. We would like to thank the Rockefeller Bio-Imaging Resource Center for imaging technical support and the NYU Genomic Core Facility for sequencing technical support. We also would like to thank Dr. Norbert Gleicher at the Center for Human Reproduction and Dr. Pasquale Patrizio at Yale Fertility Center for providing donated human embryos from patients for this study, and Dr. Emanuela Molinari for helping with embryo handling. We also would like to thank Dr. Ge Guo for sharing the niPSC75.2 line with us. This work was supported by the Robertson Therapeutic Development Fund and Rockefeller University private funds. R.D.S. was supported by EMBO-LTF-254-201. We also acknowledge the generosity of the people who donated their embryos to research.
Author contributions
R.D.S. conceived the project, performed human embryo and blastoid experiments, analyzed the data, and wrote the manuscript. E.R. contributed to hPSC culture, blastoid generation, and immunostaining. G.C. performed human embryo experiments. M.Y. recruited human embryos and contributed to human embryo experiments. E.A.R.-O. contributed to scRNA-seq analysis and revised the manuscript. A.H.B. conceived and coordinated the project, wrote the manuscript, and secured funding.
Declaration of interests
A.H.B. is the co-founder of RUMI Scientific and OvaNova. A.H.B. and E.A.R.-O. are shareholders of RUMI Scientific.
Published: December 14, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2023.11.005.
Supplemental information
References
- Amadei G., Handford C.E., Qiu C., De Jonghe J., Greenfeld H., Tran M., Martin B.K., Chen D.Y., Aguilera-Castrejon A., Hanna J.H., et al. Embryo model completes gastrulation to neurulation and organogenesis. Nature. 2022;610:143–153. doi: 10.1038/s41586-022-05246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold S.J., Robertson E.J. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat Rev Mol Cell Bio. 2009;10:91–103. doi: 10.1038/nrm2618. [DOI] [PubMed] [Google Scholar]
- Bredenkamp N., Stirparo G.G., Nichols J., Smith A., Guo G. The Cell-Surface Marker Sushi Containing Domain 2 Facilitates Establishment of Human naïve Pluripotent Stem Cells. Stem Cell Rep. 2019;12:1212–1222. doi: 10.1016/j.stemcr.2019.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg S., Kutra D., Kroeger T., Straehle C.N., Kausler B.X., Haubold C., Schiegg M., Ales J., Beier T., Rudy M., et al. ilastik: interactive machine learning for (bio)image analysis. Nat. Methods. 2019;16:1226–1232. doi: 10.1038/s41592-019-0582-9. [DOI] [PubMed] [Google Scholar]
- Deglincerti A., Croft G.F., Pietila L.N., Zernicka-Goetz M., Siggia E.D., Brivanlou A.H. Self-organization of the in vitro attached human embryo. Nature. 2016;533:251–254. doi: 10.1038/nature17948. [DOI] [PubMed] [Google Scholar]
- Ghimire S., Mantziou V., Moris N., Martinez Arias A. Human gastrulation: The embryo and its models. Dev. Biol. 2021;474:100–108. doi: 10.1016/j.ydbio.2021.01.006. [DOI] [PubMed] [Google Scholar]
- Gong Y., Bai B., Sun N., Ci B., Shao H., Zhang T., Yao H., Zhang Y., Niu Y., Liu L., et al. Ex utero monkey embryogenesis from blastocyst to early organogenesis. Cell. 2023;186:2092–2110.e23. doi: 10.1016/j.cell.2023.04.020. [DOI] [PubMed] [Google Scholar]
- Guo G., von Meyenn F., Rostovskaya M., Clarke J., Dietmann S., Baker D., Sahakyan A., Myers S., Bertone P., Reik W., et al. Epigenetic resetting of human pluripotency. Development. 2017;144:2748–2763. doi: 10.1242/dev.146811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hie B., Bryson B., Berger B. Efficient integration of heterogeneous single-cell transcriptomes using Scanorama. Nat. Biotechnol. 2019;37:685–691. doi: 10.1038/s41587-019-0113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa H., Javali A., Khoei H.H., Sommer T.M., Sestini G., Novatchkova M., Scholte Op Reimer Y., Castel G., Bruneau A., Maenhoudt N., et al. Human blastoids model blastocyst development and implantation. Nature. 2022;601:600–605. doi: 10.1038/s41586-021-04267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karvas R.M., Zemke J.E., Ali S.S., Upton E., Sane E., Fischer L.A., Dong C., Park K.M., Wang F., Park K., et al. 3D-cultured blastoids model human embryogenesis from pre-implantation to early gastrulation stages. Cell Stem Cell. 2023;30:1148–1165.e7. doi: 10.1016/j.stem.2023.08.005. [DOI] [PubMed] [Google Scholar]
- Ma H., Zhai J., Wan H., Jiang X., Wang X., Wang L., Xiang Y., He X., Zhao Z.A., Zhao B., et al. In vitro culture of cynomolgus monkey embryos beyond early gastrulation. Science. 2019;366 doi: 10.1126/science.aax7890. [DOI] [PubMed] [Google Scholar]
- Martyn I., Kanno T.Y., Ruzo A., Siggia E.D., Brivanlou A.H. Self-organization of a human organizer by combined Wnt and Nodal signalling. Nature. 2018;558:132–135. doi: 10.1038/s41586-018-0150-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyn I., Brivanlou A.H., Siggia E.D. A wave of WNT signaling balanced by secreted inhibitors controls primitive streak formation in micropattern colonies of human embryonic stem cells. Development. 2019;146:dev172791. doi: 10.1242/dev.172791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon K.R., van Dijk D., Wang Z., Gigante S., Burkhardt D.B., Chen W.S., Yim K., Elzen A.v.d., Hirn M.J., Coifman R.R., et al. Visualizing structure and transitions in high-dimensional biological data. Nat. Biotechnol. 2019;37:1482–1492. doi: 10.1038/s41587-019-0336-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgani S.M., Metzger J.J., Nichols J., Siggia E.D., Hadjantonakis A.-K. Micropattern differentiation of mouse pluripotent stem cells recapitulates embryo regionalized cell fate patterning. Elife. 2018;7 doi: 10.7554/eLife.32839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moris N., Anlas K., van den Brink S.C., Alemany A., Schröder J., Ghimire S., Balayo T., van Oudenaarden A., Martinez Arias A. An in vitro model of early anteroposterior organization during human development. Nature. 2020;582:410–415. doi: 10.1038/s41586-020-2383-9. [DOI] [PubMed] [Google Scholar]
- Niu Y., Sun N., Li C., Lei Y., Huang Z., Wu J., Si C., Dai X., Liu C., Wei J., et al. Dissecting primate early post-implantation development using long-term in vitro embryo culture. Science. 2019;366 doi: 10.1126/science.aaw5754. [DOI] [PubMed] [Google Scholar]
- Oldak B., Wildschutz E., Bondarenko V., Comar M.Y., Zhao C., Aguilera-Castrejon A., Tarazi S., Viukov S., Pham T.X.A., Ashouokhi S., et al. Complete human day 14 post-implantation embryo models from naïve ES cells. Nature. 2023;622:562–573. doi: 10.1038/s41586-023-06604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijuan-Sala B., Griffiths J.A., Guibentif C., Hiscock T.W., Jawaid W., Calero-Nieto F.J., Mulas C., Ibarra-Soria X., Tyser R.C.V., Ho D.L.L., et al. A single-cell molecular map of mouse gastrulation and early organogenesis. Nature. 2019;566:490–495. doi: 10.1038/s41586-019-0933-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham T.X.A., Panda A., Kagawa H., To S.K., Ertekin C., Georgolopoulos G., van Knippenberg S.S.F.A., Allsop R.N., Bruneau A., Chui J.S.H., et al. Modeling human extraembryonic mesoderm cells unaïvenaive pluripotent stem cells. Cell Stem Cell. 2022;29:1346–1365.e10. doi: 10.1016/j.stem.2022.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Pérez J.A., Magnuson T. Primitive streak formation in mice is preceded by localized activation of Brachyury and Wnt3. Dev. Biol. 2005;288:363–371. doi: 10.1016/j.ydbio.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Nakamura T., Okamoto I., Yabuta Y., Iwatani C., Tsuchiya H., Seita Y., Nakamura S., Shiraki N., Takakuwa T., et al. The Germ Cell Fate of Cynomolgus Monkeys Is Specified in the Nascent Amnion. Dev. Cell. 2016;39:169–185. doi: 10.1016/j.devcel.2016.09.007. [DOI] [PubMed] [Google Scholar]
- Shahbazi M.N. Mechanisms of human embryo development: from cell fate to tissue shape and back. Development. 2020;147:dev190629. doi: 10.1242/dev.190629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazi M.N., Jedrusik A., Vuoristo S., Recher G., Hupalowska A., Bolton V., Fogarty N.N.M., Campbell A., Devito L., Ilic D., et al. Self-organization of the human embryo in the absence of maternal tissues. Nat. Cell Biol. 2016;18:700–708. doi: 10.1038/ncb3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y., Taniguchi K., Townshend R.F., Miki T., Gumucio D.L., Fu J. A pluripotent stem cell-based model for post-implantation human amniotic sac development. Nat. Commun. 2017;8:208. doi: 10.1038/s41467-017-00236-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simunovic M., Metzger J.J., Etoc F., Yoney A., Ruzo A., Martyn I., Croft G., You D.S., Brivanlou A.H., Siggia E.D. A 3D model of a human epiblast reveals BMP4-driven symmetry breaking. Nat. Cell Biol. 2019;21:900–910. doi: 10.1038/s41556-019-0349-7. [DOI] [PubMed] [Google Scholar]
- Simunovic M., Siggia E.D., Brivanlou A.H. In vitro attachment and symmetry breaking of a human embryo model assembled from primed embryonic stem cells. Cell Stem Cell. 2022;29:962–972.e4. doi: 10.1016/j.stem.2022.05.001. [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L., Sepich D.S. Making and Shaping Germ Layers. Annu Rev Cell Dev Bi. 2012;28:687–717. doi: 10.1146/annurev-cellbio-092910-154043. [DOI] [PubMed] [Google Scholar]
- Tarazi S., Aguilera-Castrejon A., Joubran C., Ghanem N., Ashouokhi S., Roncato F., Wildschutz E., Haddad M., Oldak B., Gomez-Cesar E., et al. Post-gastrulation synthetic embryos generated ex utero from mnaïvenaive ESCs. Cell. 2022;185:3290–3306.e25. doi: 10.1016/j.cell.2022.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyser R.C.V., Mahammadov E., Nakanoh S., Vallier L., Scialdone A., Srinivas S. Single-cell transcriptomic characterization of a gastrulating human embryo. Nature. 2021;600:285–289. doi: 10.1038/s41586-021-04158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmflash A., Sorre B., Etoc F., Siggia E.D., Brivanlou A.H. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat. Methods. 2014;11:847–854. doi: 10.1038/nmeth.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherbee B.A.T., Gantner C.W., Iwamoto-Stohl L.K., Daza R.M., Hamazaki N., Shendure J., Zernicka-Goetz M. Pluripotent stem cell-derived model of the post-implantation human embryo. Nature. 2023;622:584–593. doi: 10.1038/s41586-023-06368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert M., Schmidt W., Haase R., Sugawara K., Myers G. IEEE Winter Conference on Applications of Computer Vision; WACV: 2020. Star-convex Polyhedra for 3D Object Detection and Segmentation in Microscopy; pp. 1–4. [Google Scholar]
- Wolf F.A., Angerer P., Theis F.J. large-scale single-cell gene expression data analysis. Genome Biol. 2018;19:15. doi: 10.1186/s13059-017-1382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang L., Yin Y., Zheng Y., Ma Y., Li Y., Zhao Z., Guo J., Ai Z., Niu Y., Duan K., et al. A developmental landscape of 3D-cultured human pre-gastrulation embryos. Nature. 2020;577:537–542. doi: 10.1038/s41586-019-1875-y. [DOI] [PubMed] [Google Scholar]
- Yanagida A., Spindlow D., Nichols J., Dattani A., Smith A., Guo G. Naive stem cell blastocyst model captures human embryo lineage segregation. Cell Stem Cell. 2021;28:1016–1022.e4. doi: 10.1016/j.stem.2021.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Goedel A., Kang Y., Si C., Chu C., Zheng Y., Chen Z., Gruber P.J., Xiao Y., Zhou C., et al. Amnion signals are essential for mesoderm formation in primates. Nat. Commun. 2021;12:5126. doi: 10.1038/s41467-021-25186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Wei Y., Duan J., Schmitz D.A., Sakurai M., Wang L., Wang K., Zhao S., Hon G.C., Wu J. Blastocyst-like structures generated from human pluripotent stem cells. Nature. 2021;591:620–626. doi: 10.1038/s41586-021-03356-y. [DOI] [PubMed] [Google Scholar]
- van den Brink S.C., van Oudenaarden A. 3D gastruloids: a novel frontier in stem cell-based in vitro modeling of mammalian gastrulation. Trends Cell Biol. 2021;31:747–759. doi: 10.1016/j.tcb.2021.06.007. [DOI] [PubMed] [Google Scholar]
- Zhai J., Guo J., Wan H., Qi L., Liu L., Xiao Z., Yan L., Schmitz D.A., Xu Y., Yu D., et al. Primate gastrulation and early organogenesis at single-cell resolution. Nature. 2022;612:732–738. doi: 10.1038/s41586-022-05526-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai J., Xu Y., Wan H., Yan R., Guo J., Skory R., Yan L., Wu X., Sun F., Chen G., et al. Neurulation of the cynomolgus monkey embryo achieved from 3D blastocyst culture. Cell. 2023;186:2078–2091.e18. doi: 10.1016/j.cell.2023.04.019. [DOI] [PubMed] [Google Scholar]
- Zhao C., Reyes A.P., Schell J.P., Weltner J., Ortega N.M., Zheng Y., Björklund A.K., Rossant J., Fu J., Petropoulos S., et al. Reprogrammed blastoids contain amnion-like cells but not trophectoderm. bioRxiv. 2021 doi: 10.1101/2021.05.07.442980. Preprint at. [DOI] [Google Scholar]
- Zheng Y., Xue X., Shao Y., Wang S., Esfahani S.N., Li Z., Muncie J.M., Lakins J.N., Weaver V.M., Gumucio D.L., Fu J. Controlled modelling of human epiblast and amnion development using stem cells. Nature. 2019;573:421–425. doi: 10.1038/s41586-019-1535-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated in the current study are available from the corresponding author upon reasonable request. The accession number for the scRNA-seq data reported in this paper is GEO: GSE225893.