Abstract
This study compares cytobrush and lavage techniques for the assessment of endometrial cytology (EC) in clinically normal postpartum dairy cows. The EC samples were collected from Holstein cows (n = 35) during visit 1 (V1) at 20 to 33 d in milk (DIM) and 2 wk later during visit 2 (V2) at 34 to 47 DIM by using both techniques. A minimum of 100 cells were counted to determine the percentage of cells that were neutrophils (%PMN). The mean %PMN was significantly different between the techniques at V1 (P = 0.001), but not at V2 (P = 0.474). Overall, the %PMN decreased with time postpartum (r2 = 0.36; P = 0.001), but not within V1 (P > 0.05) or V2 (P > 0.1). Uterine diameter was negatively correlated with fluid recovery by the lavage technique (r2 = 0.41; P = 0.002). The mean %PMN was not influenced by the volume of fluid recovered in successful attempts, but 17% (12/70) of attempts yielded no fluid. In conclusion, the cytobrush technique is a consistent and reliable method for obtaining endometrial samples for cystologic examination from postpartum dairy cows.
Abstract
Résumé — Comparaison des techniques de cytobrossage et de lavage utérin dans l’évaluation de la cytolgoie endométriale chez des vaches laitières cliniquement saines en post-partum. Cette étude compare les techniques de cytobrossage et de lavage lors de l’évaluation de la cytologie endométriale (CE) chez des vaches laitières cliniquement saines en post-partum. Les échantillons de CE ont été recueillis en utilisant les 2 techniques chez des vaches Holstein (n = 35) au cours de la première visite (VI) entre le 20ième et le 33ième jour en lait (JEL) et 2 semaines plus tard au cours de la visite 2 (V2) entre les 34ième et 47ième JEL. Un minimum de 100 cellules ont été comptées afin de déterminer le pourcentage de neutrophiles. Le pourcentage moyen de neutrophiles était significativement différent entre les 2 techniques à la V1 (P = 0,001) mais pas à la V2 (P = 0,474). Globalement, le pourcentage moyen de neutrophiles a diminué avec le temps suivant la mise bas (r2 = 0,36; P = 0,001) mais pas au cours de la VI (P > 0,05) ou de la V2 (P > 0,01). Le diamètre utérin avait une corrélation négative avec le liquide recueilli par la technique de lavage (r2 = 0,41; P = 0,002). Le pourcentage moyen de neutrophiles n’était pas influencé par le volume du liquide recueilli lors des tentatives réussies, mais 17 % (12/70) des tentatives n’ont pas réussi à recueillir du liquide. En conclusion, la technique de cyto-brossage est une méthode fidèle et efficace pour obtenir des échantillons d’endomètre pour examen cytologique chez les vaches laitières en post-partum.
(Traduit par Docteur André Blouin)
Introduction
Cytological examination of the reproductive tract is often used to evaluate possible reproductive lesions in humans and domestic animals. Cervical cytologic examination in women (1) and endometrial cytologic (EC) examination in mares (2) and cows (3,4) are accepted diagnostic techniques. Endometrial and inflammatory cells may be collected by a guarded cotton swab (5), uterine biopsy (6–8), uterine lavage (3,4,8), or cytobrush (1,5) techniques to evaluate EC, especially as an aid in the diagnosis of acute endometritis.
The susceptibility of the uterus to trauma and infection results in syndromes ranging from mild endometritis to toxic metritis. Endometritis in dairy cows is one of the most controversial topics discussed among practitioners due to the lack of a diagnostic gold standard. Numerous risk factors are involved in the causation of uterine disease in dairy cows, including retained placenta, dystocia, twins, parity, management, environment, and genetics (9). However, improved diagnostic procedures help to enhance diagnostic accuracy. A technique that yields well-preserved cells representative of a large uterine surface area without causing harm to the reproductive tract is required for consistent and reliable cytological results. The uterine lavage technique harvests cells from a larger uterine surface area and provides a more representative sample of luminal contents than does either a swab or a uterine biopsy (6–8), but it may cause irritation to the endometrium (10–12). Cotton swabs are more likely to distort cells and are not recommended in human cervical cytologic examination (13), as cancer diagnosis requires well-preserved cells to study cell details. Cotton swabs are routinely used to evaluate the vagina cytologically in the bitch. In both humans and the equine species, the cytobrush technique has been determined to be superior to other methods for the collection of cervical and endometrial cells (1,8).
Cytologic examination by uterine lavage with low volumes of saline to recover neutrophils has recently been studied in dairy cattle as a method to define sub-clinical endometritis (3) and endometritis (4).
The objective of this study was to compare the cytobrush and lavage techniques for assessment of EC in clinically normal postpartum dairy cows.
Materials and methods
The study population consisted of 35 Holstein cows at the Elora Dairy Research Station, University of Guelph. On visit (V)1, clinically normal cows at 20 to 33 d in milk (DIM), inclusive, and with no abnormal vaginal discharge, based on visual inspection and vaginoscopy, were selected. Samples were collected at V1 (20 to 33 DIM) and 2 wk later at V2 (34 to 47 DIM) by the same clinician. Endometrial samples for cytologic examination were collected from all cows by using the cytobrush technique followed by the lavage technique. A thorough examination of the reproductive tract, including transrectal ultrasonography (Aloka-500, Japan; Aloka Corometrics, Wallingford, Connecticut, USA) was performed on all cows before the collection of the EC samples.
Cytobrush technique
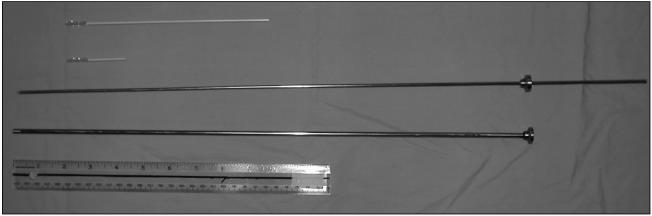
Endometrial samples for cytologic examination were collected by using a cytobrush (Fishers Scientific, Nepean, Ontario), modified for use in large animals (Figure 1). The normal cytobrush handle was cut to approximately 3 cm in length (Figure 2), threaded onto a 65-cm solid stainless steel rod (Figure 3), and placed in a stainless steel tube 50 cm in length and 5 mm in diameter for passage through the cervix. The instrument was placed in a sanitary plastic sleeve (Gencor, Guelph, Ontario) for protection from vaginal contamination. The vulva was cleansed with wet paper towels, after which the covered, lubricated (Muko lubricating jelly; Ingram & Bell, Mississauga, Ontario) instrument was passed through the vagina to the external cervical os; the sanitary sleeve was punctured and the instrument was advanced through the cervix into the base of the larger horn, at which point the stainless steel tube was retracted far enough to expose the cytobrush. Endometrial cytology samples were collected by rotating the cytobrush in a clockwise direction while in contact with the uterine wall. The cytobrush was retracted into the stainless steel tube prior to removal from the uterus. The stainless steel device was steam sterilized for 4 min between uses.
Figure 1.
Cytobrush and stainless steel instrument.
Figure 2.
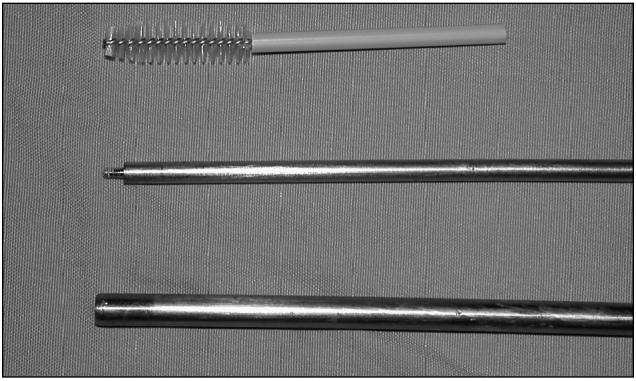
Cytobrush handle was cut to a length of 3 cm.
Figure 3.
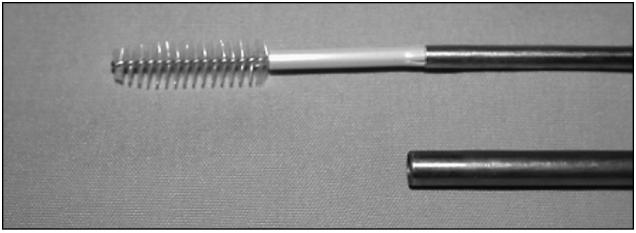
Cytobrush threaded on to the stylet.
Slides for cytologic examination were prepared by rolling the cytobrush onto a clean glass microscope slide (Figure 4) and fixing the sample with cytofixative (Cytoprep; Fishers Scientific). Slides were brought to the laboratory within 2 h and stained with modified Giemsa stain (Protocol-Hema3; Biochemical Sciences, Swedesboro, New Jersey, USA).
Figure 4.

Preparation of slide for cytologic examination.
Lavage technique
The uterine body was lavaged by infusing 60 mL of 0.9% sodium chloride solution (Baxter Corporation, Toronto, Ontario) into the uterine body with a 60-mL syringe (Monoject; Sherwood Medical, St. Louis, Missouri, USA) attached to a 52-cm disposable plastic infusion rod (Central Sales, Brampton, Ontario). The uterus was massaged and then retracted to recover the fluid. As much fluid as possible was recovered by negative pressure aspiration into the syringe and transferred to a 50-mL modified polystyrene centrifuge tube (Corning, Corning, New York, USA) without preservatives. Uterine lavage samples were brought to the laboratory within 2 h and centrifuged at 766 × g for 5 min. A drop of sediment was streaked on to a clean microscopic slide and airdried. All slides were fixed and stained in a similar way to the cytobrush samples.
Cytological assessment
Cytological assessment (Figures 5 and 6) was performed by counting a minimum of 100 cells at 400 × and 1000 × magnification (Leitz Labourlux-S, Wetzlar, Germany) to determine the percentage of neutrophils (%PMN). Initially the whole slide was assessed and a representative area was selected to determine the %PMN. Slides for cytologic examination were assessed twice by the clinician who collected the samples and once by a technician who was blinded regarding the sampling.
Figure 5.
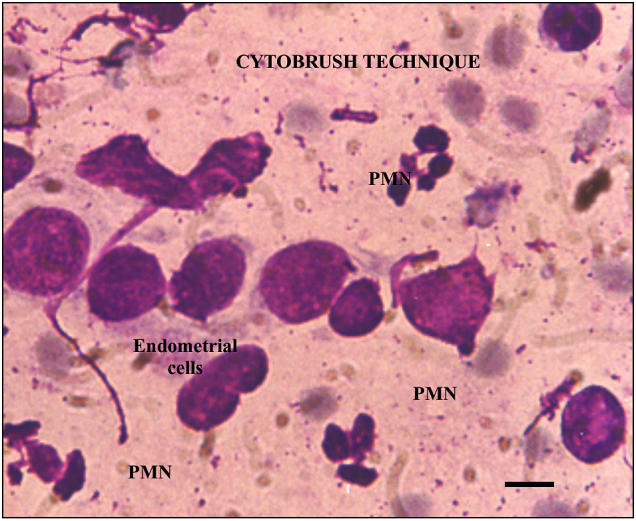
Slide from cytobrush technique. Bar = 10 μm.
Figure 6.
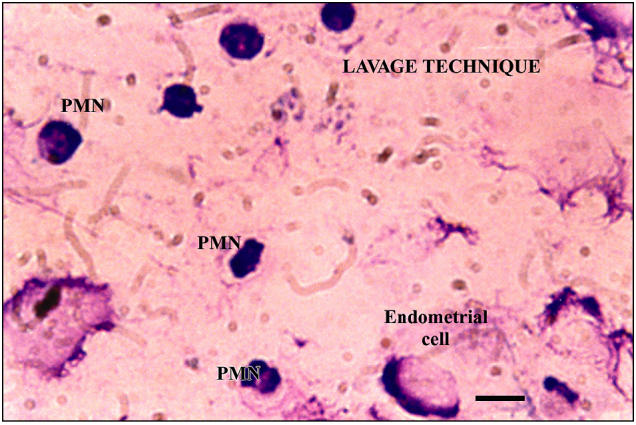
Slide from lavage technique. Bar = 10 μm.
Statistical analysis
Data were analyzed using a statistical program (SAS, version 6.12; SAS Institute, Cary, North Carolina, USA) and the α level was set at 0.05. The concordance correlation coefficient of Lin was used to analyze the intra- and inter-rater agreement in determining the %PMN. Mean %PMN for the 2 techniques was compared by using Student’s t-test. The differences between visits, methods, and their interactions were analyzed by using the General Linear Model. Regression analysis was performed to test the influence of uterine size on fluid recovery and %PMN by the lavage technique, and the association between %PMN and the independent variable DIM.
Results
Slides prepared for endometrial cytologic examination were all readable and assessed successfully. The agreement, within the clinician and between the clinician and technician, for counting the %PMN was excellent (Concordance correlation of Lin — 0.9). The %PMN assessed by the clinician were used in the analysis.
Figure 7 shows the cytologic results from the 2 collection techniques at V1 and V2. The %PMN means were significantly different between techniques at V1 (P = 0.001) but not at V2 (P = 0.474). No interaction was observed between the collection time and method (P > 0.05).
Figure 7.
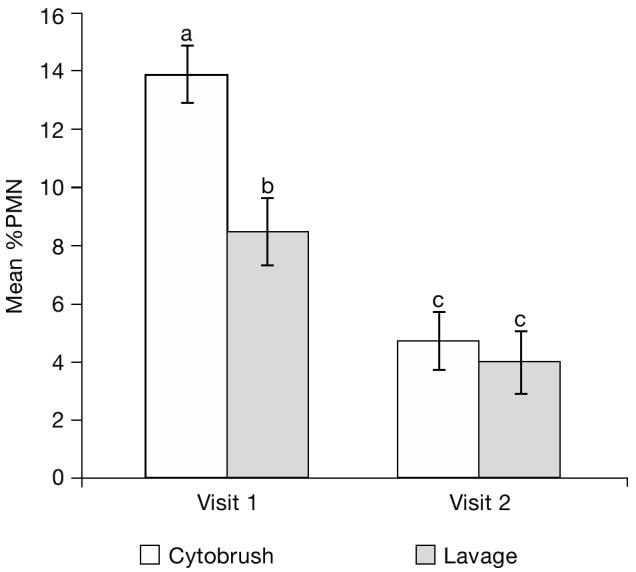
Endometrial cytology determined by the lavage and cytobrush techniques at visit 1 (20 to 33 DIM) and visit 2 (34 to 47 DIM). Bars with different superscripts were significantly different (P < 0.01).
Figure 8 shows the association between uterine diameter and fluid recovery by the lavage technique. The volume of fluid recovered is not normally distributed. Thus, the values are transformed into the square root of the volume of fluid recovered. Uterine diameter was negatively correlated with fluid recovery (Square root [Sqrt] Fluid Recovery [mL] = 7.24 − 0.1078 × uterine size [mm]; P = 0.002, r2 = 0.41). Of all uterine lavage attempts, 17% (12/70; V1 = 8; V2 = 4) failed to recover any fluid. The mean %PMN was not influenced by the volume of fluid recovered in successful attempts (P = 0.39) or by visit (V1, P = 0.52 and V2, P = 0.28).
Figure 8.
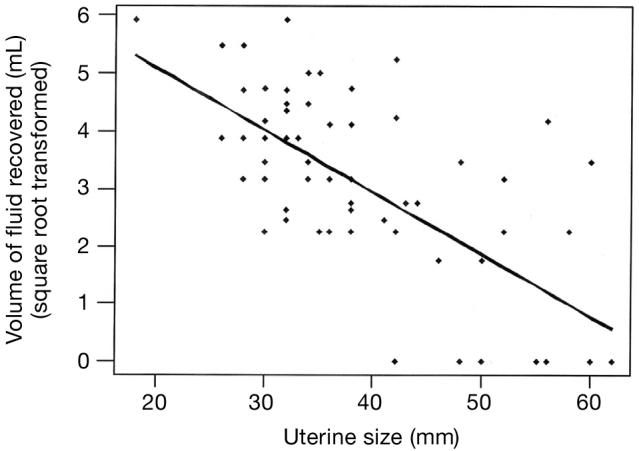
Association between uterine size (diameter) and fluid recovery by the lavage technique. Square root fluid recovery (mL) = 7.24 − 0.1078 × Uterine size (mm); P = 0.02; r2 = 0.41.
Overall, the %PMN decreased with time postpartum (Sqrt %PMN = 6.72 − 0.1117 DIM; r2 = 0.36; P = 0.001). The %PMN was not influenced by DIM withinV1 (P > 0.05) or V2 (P > 0.1).
Discussion
This study indicates that the cytobrush technique can be used successfully and reliably to obtain endometrial samples.
The cytobrush technique resulted in a significantly higher mean %PMN than did lavage technique at V1 (P = 0.001), but not at V2 (P = 0.474). In the later stages of the postpartum period, the lavage technique is appropriate for cytological evaluation, as the recovery of cells during V2 was not different from that of the cytobrush technique. The decrease in the uterine size during later stages of postpartum may help in the recovery of fluid from the uterus by the lavage technique, resulting in an increased recovery of cells compared with that in earlier stages of postpartum. This is in agreement with the findings of Gilbert et al (3), who used the lavage technique successfully for endometrial cytologic evaluation to diagnose endometritis between 40 and 60 d postpartum.
Endometrial cytologic samples were collected from all cows, first with the cytobrush technique, then by the lavage technique. The reason for performing the cytobrush technique first is to avoid irritation to the endometrium caused by the fluid from the lavage technique (10–12). It is arguable that the cytobrush technique may also cause irritation to the endometrium. The time required to obtain samples by the cytobrush technique was consistent and quick compared with that for lavage technique, in which there was considerable variation in the time required to obtain samples. Also, the volume of fluid left in the uterus after obtaining samples by the lavage technique varied between cows, which could affect the degree of irritation and thereby the result of cytobrush sampling.
Gilbert et al (3) did not report failure in attempts to recover fluid. In this study, 17% (12/70; V1 = 8; V2 = 4) of all attempts resulted in no fluid recovery, an unacceptably high rate of failure for making a diagnosis of endometritis. Gilbert et al (3) infused 20 mL of fluid in the uterus, whereas in this study, 60 mL was infused into the body, because manipulation of the uterus in order to pass the semi-rigid infusion rod to gain access to uterine horn might have resulted in trauma. Sixty milliliters of fluid was selected to enhance the chances of successful fluid recovery.
The mean %PMN at V1 was higher than at V2. The %PMN was negatively associated with DIM. This is in agreement with the findings of Bonnett et al (7) and Gilbert et al (14), who showed that there was a decrease in neutrophil number as the postpartum period approached the completion of histological involution, which occurs at 40 d postcalving (15,16).
The uterine diameter negatively influenced the volume of fluid recovered. The volume of fluid recovered did not influence the mean %PMN, excluding the failed fluid recovery attempts. The number of red blood cells in the sample tended to be higher in the lavage technique compared with the cytobrush technique. This may be related to trauma resulting from the manipulation of the uterus and infusion rod while attempting to recover fluid. The failure to recover fluid and possible trauma to the uterus indicate that the lavage technique is inconsistent and possibly detrimental.
In cytological studies, the number of cells recovered is critical, as it influences the threshold level used to define inflammation. The cytobrush technique yielded an in situ sample, which might represent the inflammatory nature of the endometrium, compared with the uterine lavage technique, which yielded diluted sample of luminal contents.
The cytobrush technique resulted in less distortion of cells compared with the lavage technique (Figures 5 and 6). In the lavage technique, the procedure, from sample collection to the preparation of the slide for cytologic examination, took a maximum of 2 h. It is possible that any delay in the cytologic evaluation will affect the total nucleated cell count and may alter the cytologic evaluation. Even though the cytobrush technique requires specialized equipment, sample collection by this method was easier, more consistent, and produced rapid results.
In conclusion, results from the cytobrush samples were superior in all parameters, indicating that the cytobrush technique is a move consistent and reliable method than the lavage method to obtain endometrial cytology samples from postpartum dairy cows.
References
- 1.Glenthoj A, Bostofte E, Rank F. Brush cytology from the uterine endocervix. Acta Obstet Gynecol Scand. 1986;65:689–691. [PubMed] [Google Scholar]
- 2.Wingfield-Digby NJ. The technique and clinical application of endometrial cytology in mares. Equine Vet J. 1987;10:176–180. doi: 10.1111/j.2042-3306.1978.tb02248.x. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert RO, Shin ST, Guard CL, Erb HN. Incidence of endometritis and effects on reproductive performance of dairy cows [Abstract] Theriogenology. 1998;49:251. doi: 10.1016/j.theriogenology.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Hammon DS, Holyoak GR, Jenson J, Bingham HR. Effects of endometritis at the beginning of the breeding period on reproductive performance in dairy cows [abstract]. Proc 34th Annu Conf Am Assoc Bov Pract, Vancouver, 2001:142–143.
- 5.Studer E, Morrow DA. Postpartum evaluation of bovine reproductive potential: Comparison of findings from genital tract examination per rectum, uterine culture and endometrial biopsy. J Am Vet Med Assoc. 1978;172:489–494. [PubMed] [Google Scholar]
- 6.Miller HV, Kimsey PB, Kendrick JW, et al. Endometritis of dairy cattle: Diagnosis, treatment and fertility. The Bov Pract. 1980;5:13–23. [Google Scholar]
- 7.Bonnett BN, Miller RB, Etherington WG, Martin SW, Johnson WH. Endometrial biopsy in Holstein-Friesian dairy cows-I. Technique, histological criteria and results. Can J Vet Res. 1991;55:155–161. [PMC free article] [PubMed] [Google Scholar]
- 8.Bourke M, Mills JN, Barnes AL. Collection of endometrial cells in the mare. Aust Vet J. 1997;75:755–758. doi: 10.1111/j.1751-0813.1997.tb12263.x. [DOI] [PubMed] [Google Scholar]
- 9.Coleman DA, Thayne WV, Dailey RA. Factors affecting reproductive performance of dairy cows. J Dairy Sci. 1985;68:1793–1803. doi: 10.3168/jds.S0022-0302(85)81029-8. [DOI] [PubMed] [Google Scholar]
- 10.Roszel JF, Freeman KP. Equine endometrial cytology. Vet Clin North Am Equine Pract. 1988;4:247–262. doi: 10.1016/s0749-0739(17)30640-5. [DOI] [PubMed] [Google Scholar]
- 11.Brook D. Uterine cytology. In: McKinnon AO, Voss JL, eds. Equine Reproduction. 2nd ed: London. Lea and Febiger, 1993:246–254.
- 12.Ball BA. Use of a low-volume uterine flush for microbiologic and cytologic examination of the mare’s endometrium. Theriogenology. 1988;29:1269–1283. [Google Scholar]
- 13.Kavak ZN, Eren F, Pekin S, Kullu S. A randomised comparison of the 3 Papanicolaou smear collection methods. Aust NZJ Obstet Gynaecol. 1995;65:689–691. doi: 10.1111/j.1479-828x.1995.tb02165.x. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert RO, Grohn YT, Miller PM, Hoffman DJ. Effect of parity on periparturient neutrophil function in dairy cows. Vet Immunol Immunopathol. 1993;36:75–82. doi: 10.1016/0165-2427(93)90007-q. [DOI] [PubMed] [Google Scholar]
- 15.Bondurant RH. Inflammation in the bovine female reproductive tract, Animal health 2: Inflammation and animal health. J Anim Sci. 1999;77:101–110. doi: 10.2527/1999.77suppl_2101x. [DOI] [PubMed] [Google Scholar]
- 16.Stevenson JS. Clinical reproductive physiology of the cow. In: Vounquist RS, ed. Current Therapy in Large Animal Theriogenology. Philadelphia: WB Saunders, 1997:257–267.