Abstract
Leukemia stem cells (LSC) represent a crucial and rare subset of cells present in acute myeloid leukemia (AML); they play a pivotal role in the initiation, maintenance, and relapse of this disease. Targeting LSC holds great promise for preventing AML relapse and improving long-term outcomes. However the precise molecular mechanisms governing LSC self-renewal are still poorly understood. Here, we present compelling evidence that the expression of miR-30e-5p, a potential tumor-suppressive microRNA, is significantly lower in AML samples than in healthy bone marrow samples. Forced expression of miR-30e effectively inhibits leukemogenesis, impairs LSC self-renewal, and delays leukemia progression. Mechanistically, Cyb561 acts as a direct target of miR-30e-5p in LSC, and its deficiency restricts the self-renewal of LSC by activating reactive oxygen series signaling and markedly prolongs recipients’ survival. Moreover, genetic or pharmacological overexpression of miR-30e-5p or knockdown of Cyb561 suppresses the growth of human AML cells. In conclusion, our findings establish the crucial role of the miR-30e-5p/Cyb561/ROS axis in finely regulating LSC self-renewal, highlighting Cyb561 as a potential therapeutic target for LSC-directed therapies.
Introduction
Acute myeloid leukemia (AML) is a highly aggressive and often fatal blood malignancy primarily caused by genetic mutations in hematopoietic stem cells (HSC) or committed progenitor compartments.1,2 Despite ongoing efforts, the standard therapy for most types of AML has not shown significant improvements, with a 5-year overall survival rate of approximately 24% in the USA.3 Leukemia stem cells (LSC), an indispensable subset of AML cells with a limitless capacity of self-renewal and differentiation block, play a key role in the initiation, maintenance, and propagation of AML.4 Targeting LSC has emerged as a promising strategy to address AML relapse and enhance long-term treatment outcomes.
MicroRNA (miRNA, miR), typically consisting of 18-24 nucle-otides, which function by binding to and cleaving mRNA or inhibiting their translation. Accumulating evidence supports the critical role of miRNA in regulating LSC self-renewal and differentiation.5-7 Among them, miR-30e-5p is implicated in many important biological regulation processes, such as cancer development and metastasis, epithelial-mesen-chymal transition, fatty acid metabolism, and osteogene-sis.8-12 Previous studies have documented lower levels of expression of miR-30e-5p in patients with chronic myeloid leukemia. Additionally, enforced expression of miR-30e-5p has demonstrated inhibitory effects on proliferation and induction of apoptosis in K562 cells.13 Another study highlighted the involvement of circPVT1, which regulates the miR-30e/DLL4 pathway, in suppressing the proliferation of T-cell acute lymphoblastic leukemia cells.14 However, the precise roles of miR-30e-5p in LSC initiation and maintenance and its therapeutic potential in AML remain largely unexplored.
In this study, we aimed to investigate the roles of miR-30e-5p in LSC initiation and maintenance in KMT2A::MLLT3-in-duced leukemia, as well as explore the potential of this microRNA as a therapeutic target in human AML cells. We demonstrated that miR-30e-5p is expressed at lower levels in human AML bone marrow (BM) samples compared to healthy samples. Forced expression of miR-30e delays the onset of KMT2A::MLLT3-driven leukemia by reducing cycling LSC and promoting LSC apoptosis. Using RNA-sequencing analysis, we found that endogenous Cyb561 is significantly suppressed in the LSC overexpressing miR-30e compared with LSC expressing empty retroviral miRVector. By quantitative PCR (qPCR), reporter assays, and functional assays, we identified Cyb561 as a direct downstream target of miR-30e-5p in leukemogenesis. Consistently, knockdown of Cyb561 resulted in reduced colony formation capacity in vitro, decreased LSC frequency, and delayed progression of mouse AML in vivo.
Moreover, the miR-30e-5p/Cyb561 signaling pathway was found to enhance intracellular reactive oxygen species (ROS) levels, while concurrently decreasing glutathione and ascorbate levels. The ROS scavenger N-acetyl-L-cysteine (NAC) impeded the roles of miR-30e-5p and Cyb561 in leukemia progression by regulation of cell cycle and apoptosis of LSC. In addition, both genetic and pharmacological up-regulation of miR-30e-5p hindered the growth of human AML cells. Consistent with these findings, we observed a significant upregulation of CYB561 expression in AML patients, and high-level expression of CYB561 correlated with shorter overall survival in AML patients. Inhibition of CYB561 had suppressive effects on human AML cell growth in vitro. Together, our study provides compelling evidence supporting the essential role of the miR-30e-5p/Cyb561/ ROS signaling pathway in the initiation and maintenance of LSC in KMT2A::MLLT3-induced leukemia. Furthermore, we highlight the potential of Cyb561 as a therapeutic target for LSC in AML.
Methods
Mice
C57BL/6J mice were purchased from VITALSTAR (Beijing, China). All the animal studies conducted in this research were approved by the Animal Care and Use Committee at Shanghai University.
Cell cultures
293T cells were cultured in DMEM (high glucose) containing 10% fetal bovine serum. THP-1, MONOMAC-6, NOMO-1, MV4-11, and NB-4 cells were cultured in RPMI1640 plus 10% fetal bovine serum. Human MA9.3 leukemic cells were cultured in IMDM plus 20% fetal bovine serum with defined cytokines.15 Enriched fresh hematopoietic stem and progenitor cells (HSPC) were cultured in vitro in StemSpan (STEMCELL Technologies, Vancouver, Canada) supplemented with 10 mg/mL heparin (Sigma, St Louis, MO, USA), 10 ng/mL murine stem cell factor, 20 ng/mL murine thrombopoietin, 20 ng/mL human insulin-like growth factor-II, 10 ng/mL murine fibroblast growth factor-1, and 100 ng/mL human angiopoietin-like protein 3. All recombinant proteins were purchased from PeproTech (Rock Hill, NJ, USA) or Genscript (Nanjing, China). All cell culture products were obtained from Jet Biofil (Guangzhou, China) unless otherwise specified in the text and figure legends.
Plasmids
KMT2A::MLLT3 (previously called MLL-AF9) was cloned into the retroviral vector pMIGR1 containing green fluorescent protein (GFP). The entire loci of mouse and human miR-30e, containing 100-bp upstream and downstream native flank sequences, were amplified by PCR from genomic DNA; then cloned into pMXs-miR-Puro (miRVector) retro-viral vector. To generate short hairpin (sh)RNA-expressing plasmids targeting mouse and human CYB561, we cloned shRNA control and CYB561 shRNA into Age I and EcoR I sites of Tet-pLKO-puro vector (Addgene, USA). To construct the luciferase reporters for identifying true targets of miR-30e-5p, the sequences of target 3’ untranslated regions (3’UTR) were amplified from genomic DNA of C57/ B6J BM cells by PCR using specific primers and cloned into the pGL3-control vector (Promega). To generate the mutant of Cyb561 3’UTR, point mutations in the miR-30e-5p binding site were introduced by PCR and cloned into the pGL3-control vector. The reporter plasmids were validated by DNA sequencing. For the ectopic expression of Cyb561, the coding sequence of Cyb561 was amplified from mouse BM cDNA and then cloned into a retroviral vector pMIBSD containing the selective antibiotic blasticidin. All the primers for plasmid cloning are listed in Online Supplementary Table S1.
Results
miR-30e-5p expression is downregulated in patients with acute myeloid leukemia
Previous clinical reports showed that miR-30e-5p is upregulated in patients with B-cell acute lymphoblastic leukemia and childhood acute lymphoblastic leukemia but down-regulated in patients with chronic myeloid leukemia.13,16,17 To assess the potential clinical relevance of miR-30e-5p expression levels in AML samples and healthy samples, we carried out qPCR analysis of the expression of mature miR-30e-5p and miR-30e-3p. Our findings revealed a significant downregulation of miR-30e-5p in AML BM samples compared to healthy BM samples (Figure 1A, Online Supplementary Table S2). No significant difference was observed in the expression of miR-30e-3p between AML samples and healthy samples (Online Supplementary Figure S1A). To assess the expression levels of miR-30e-5p in different subtypes of AML, defined according to the French-American-British (FAB) classification, and determine its prognostic value for overall survival, we searched clinical databases online (http://ualcan.path.uab.edu/index.html) and found that the expression of miR-30e-5p was lower in human AML-M3, -M4, and -M5 subtypes than in AML-M2 (Online Supplementary Figure S1B). The overall survival of AML patients was not significantly related to the expression level of miR-30e-5p (Online Supplementary Figure S1C). To provide more conclusive evidence for the decreased expression of miR-30e-5p in LSC, we examined a mouse AML model driven by the KMT2A::MLLT3 fusion oncogene. Our results demonstrated a reduction in miR-30e-5p expression in LSC-enriched granulocyte-monocyte progenitors (referred to as L-GMP, Lin–GFP+c-Kit+CD34+CD16/32+) compared to HSPC and granulocyte-monocyte progenitors (GMP) (Figure 1B). Furthermore, we observed the downregulation of endogenous miR-30e-5p in HSPC expressing various oncogenes (e.g., HOXA9, MESI1, and NUP98::HOXA9) (Figure 1C). These findings in the AML mouse model are consistent with the results obtained from human AML samples, suggesting a potentially significant role for miR-30e-5p in AML.
Overexpression of miR-30e delays the development of KMT2A::MLLT3-driven leukemia
To determine the role of miR-30e in AML cells, we enriched Lin–Sca-1+ HSPC by a single intraperitoneal dose of 5-flu-orouracil. These cells were subsequently co-transduced with retroviral particles containing KMT2A::MLLT3/miRVector and KMT2A::MLLT3/miR-30e. Overexpression of miR-30e in HSPC resulted in a 5.36-fold increase in miR-30e-5p expression compared to the level in miRVector-HSPC (Online Supplementary Figure S2A). Colony-forming/replating assays showed that forced expression of miR-30e suppressed KM-T2A::MLLT3-induced immortalization of mouse HSPC (Figure 2A, Online Supplementary Figure S2B). To explore the roles of miR-30e in AML in vivo, equal numbers of KMT2A::M-LLT3/miRVector- and KMT2A::MLLT3/miR-30e-transduced HSPC were transplanted into irradiated recipients. Both sets of HSPC developed AML in recipient mice with full penetrance; however, the onset was significantly delayed in miR-30e-overexpressing AML compared to miRVec-tor-AML (median survival, 78 days vs. 52 days, respectively; P<0.01) (Figure 2B, C). The expression level of miR-30e-5p was elevated 3.6-fold in miR-30e-overexpressing AML cells (Online Supplementary Figure S2C). Consistent with these findings, other parameters of AML severity, such as peripheral blood white cell count and spleen weight, were also reduced (Online Supplementary Figure S2D-F). In line with the primary BM transplantation results, the secondary recipients of miRVector developed AML significantly faster than the miR-30e-overexpressing secondary recipients (median survival, 41 days vs. 53 days, respectively; P<0.01) (Online Supplementary Figure S2G).
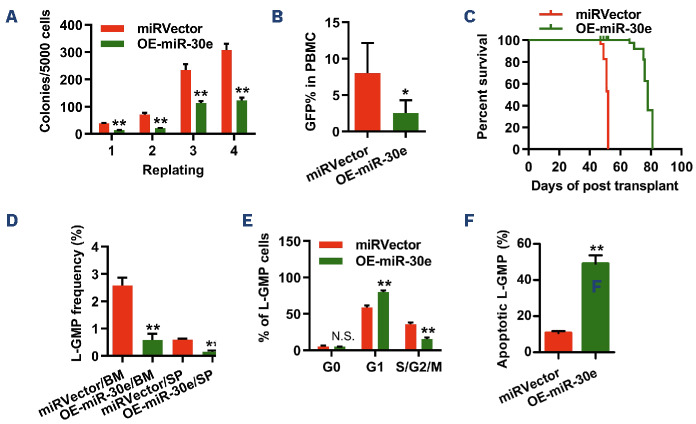
The frequency of LSC is thought to be associated with patients’ prognosis as well as leukemia progression in murine models. To further investigate the impact of miR-30e on LSC, we analyzed L-GMP (i.e., a LSC-enriched population) frequency in the secondary recipients overexpressing the miRVector or miR-30e. The results showed that forced expression of miR-30e resulted in a lower LSC frequency (Figure 2D, Online Supplementary Figure S3), indicating a potential role for miR-30e in modulating LSC self-renewal. Additionally, cell cycle analysis demonstrated a decreased proportion of miR-30e-overexpressing LSC in the S/G2/M phases, accompanied by a concomitant increase in the G1 phase, compared to miRVector LSC (Figure 2E, Online Supplementary Figure S3). Furthermore, miR-30e-overex-pressing LSC also showed heightened levels of apoptosis compared to miRVector-overexpressing LSC (Figure 2F). To directly evaluate the effect of miR-30e overexpression on the frequency of LSC, we conducted a limiting dilution assay. As expected, the estimated LSC frequency in the miR-30e-overexpressed group was 17-fold lower than that in the miRVector-group (1/529.9 vs. 1/31.1) (Online Supplementary Figure 2H, Online Supplememtary Table S3). Considering that the survival disparity between miRVector- and miR-30e-overexpressing AML recipients might be partly attributed to differences in homing efficiency, we determined the homing capability of secondary transplanted miRVector- and miR-30e-overexpressing AML cells. The results showed that homing efficiency was similar for both miRVector- and miR-30e-overexpressing AML cells at 16 h after BM transplantation (Online Supplementary Figure S2I). Taken together, these data demonstrated that miR-30e impaired cell cycle and enhanced apoptosis in LSC, thereby delaying AML progression in vivo.
Figure 1.
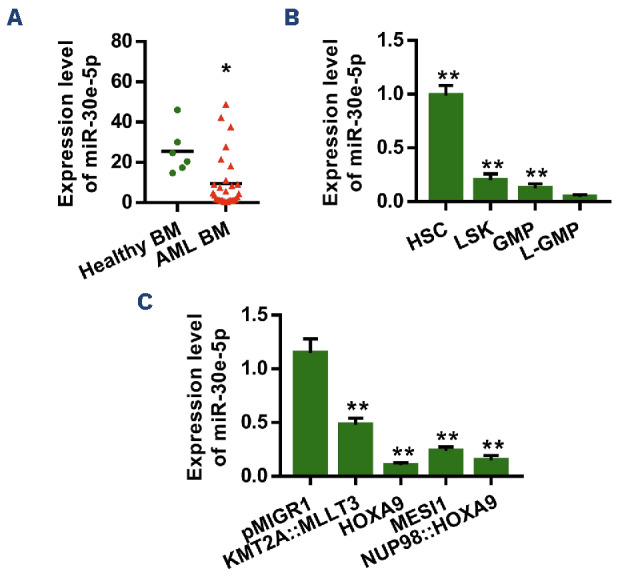
miR-30e-5p is repressed in both human and mouse acute myeloid leukemia cells. (A) Quantitative polymerase chain reaction (qPCR) analysis of the expression of miR-30e-5p between bone marrow cells of healthy controls and acute myeloid leukemia (AML) patients. Results are normalized to U6 expression and expressed relative to miR-30e-5p expression in the healthy group (healthy patients, N=6; AML patients, N=29). (B) qPCR analysis of the expression of endogenous miR-30e-5p in mouse HSC, LSK, GMP, and L-GMP. The indicated cells were sorted by flow cytometry. Results are normalized to U6 expression and expressed relative to miR-30e-5p expression in the L-GMP group (N=3). (C) qPCR analysis of the expression of endogenous miR-30e-5p in mouse hematopoietic stem and progenitor cells transduced with the retroviral vector pMIGR1 only or retrovirus expressing KMT2A::MLLT3, HOXA9, MESI1, NUP98::HOXA9. Results are normalized to U6 expression and expressed relative to miR-30e-5p expression in the retroviral empty vector group (N=3). All data are represented as mean ± standard deviation. Two-tailed Student t tests were used to assess statistical significance (*P<0.05; **P<0.01). BM: bone marrow; HSC: hematopoietic stem cells (Lin–Sca-1+c-Kit+C-D150+CD48–); LSK cells (Lin–Sca-1+c-Kit+); GMP: granulocyte-mono-cyte progenitors (Lin–c-Kit+CD34+CD16/32+); L-GMP: leukemia stem cell-enriched granulocyte-monocyte progenitors (GFP+Lin-lowc-Kit+CD34+CD16/32+) (N=3).
Figure 2.
Forced expression of miR-30e impairs leukemia stem cell self-renewal and delays the onset of KMT2A::MLLT3 leukemia. (A) Colony-forming assay of miRVector- or miR-30e-overexpressing acute myeloid leukemia (AML) cells (N=3). (B) Percentage green fluorescent protein positive cells in the peripheral blood at week 5 after primary bone marrow transplant with 5x104 AML cells (N=6). (C) Survival analysis of primary recipient mice. The post-transplant median survival was 52 versus 78 days for primary recipients of miRVector- or miR-30e-overexpressing AML cells, respectively (P<0.01, Mantel-Cox test, N=7). (D) Frequency of leukemia stem cell-enriched granulocyte-monocyte progenitors (L-GMP) in the bone marrow (BM) and spleen from primary recipients injected with 5x104 miRVector- or miR-30e-overexpressing AML cells (N=5). (E) Cell cycle phase distribution of L-GMP cells in BM from primary recipients of 5x104 with miRVector- or miR-30e-overexpressing AML cells at week 5 after transplantation (N=4). (F) Percentage of apoptotic L-GMP cells in the BM from primary recipients (N=5). Data are representative of two or three independent experiments. Excluding survival analysis, all data are represented as mean ± standard deviation. Two-tailed Student t tests were used to assess statistical significance (N.S.: not significant; *P<0.05; **P<0.01). miRVector: control; OE-miR-30e: cells with miR-30e overexpression; GFP: green fluorescent protein; PBMC: peripheral blood mononuclear cells; SP: spleen.
Since the miR-30e locus contains two mature miRNA (i.e., miR-30e-5p and miR-30e-3p), we next determined which mature miRNA affects AML colony formation. To this end, we transfected corresponding mature miRNA mimics into KMT2A::MLLT3 AML cells. The results revealed that over-expression of miR-30e-5p significantly suppressed AML colony formation. In contrast, forced expression of miR-30e-3p did not have a noticeable effect on colony formation (Online Supplementary Figure S2J). These findings suggest that miR-30e-5p, rather than miR-30e-3p, plays a pivotal regulatory role in KMT2A::MLLT3-driven leukemogenesis.
Cyb561 is a direct target of miR-30e-5p in KMT2A::MLLT3-driven leukemia stem cells
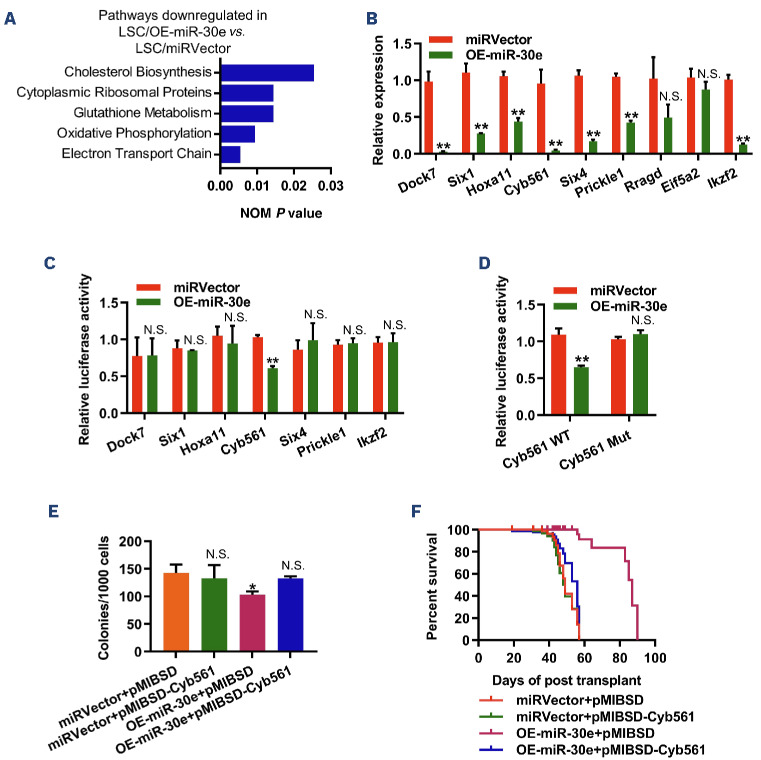
To gain insights into the underlying mechanisms by which miR-30e regulates the self-renewal of LSC, we sorted miRVector- and miR-30e-overexpressing LSC from two groups of recipients with leukemia driven by KMT2A:: MLLT3. We assessed the gene expression profiles of these cells using RNA-sequencing analysis (Online Supplementary Figure S4A). We found that 689 genes were upreg-ulated, and 531 genes were downregulated by >2.0-fold (P<0.01) in miR-30e-overexpressing LSC compared with LSC expressing miRVector (Online Supplementary Table S4). Through gene set enrichment analysis (GSEA), we found that the downregulated genes in miR-30e-overexpressing LSC were associated with electron transport chain, oxidative phosphorylation, and glutathione metabolism (Figure 3A, Online Supplementary Figure S4B, Online Supplementary Table S5). Further analysis of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways revealed that these differentially expressed genes could be classified into five functional groups, with more than 150 genes involved in signal transduction, including the MAPK pathway (Online Supplementary Figure S5, Online Supplementary Table S6). Considering that miRNA typically inhibit the translation of their mRNA by binding with the 3’UTR of their direct targets, we focused on the downregulated genes. Using the miRbase online database (www.mirbase.org) and qPCR, we validated seven genes as the most likely direct targets of miR-30e-5p, namely Dock7, Six1, Hoxa11, Cyb561, Six4, Prickle1, and Ikzf2 (Figure 3B, Online Supplementary Table S7). Among these seven miR-30e-5p-regulated genes, some (Ikzf2, Six1, and Hoxa11) have been shown to play important roles in LSC and AML.18-20 To confirm the true target of miR-30e-5p in LSC, we cloned the DNA fragment covering these genes’ 3’UTR into pGL3-control luciferase vector. Subsequently, we performed a luciferase reporter assay to validate this bioinformatic prediction. Our data revealed that miR-30e-5p inhibited the luciferase activity of the wild-type Cyb561 3’UTR construct by 45%, whereas the mutation in the binding site fully restored luciferase activity (Figure 3C, D, Online Supplementary Figure S6A). Additionally, the forced expression of miR-30e decreased the protein level of Cyb561 in mouse AML cells (Online Supplementary Figure S6B). These findings demonstrate that Cyb561 is a direct downstream target of miR-30e-5p in LSC. Consequently, a key question arises: does miR-30e-5p regulate LSC self-renewal through Cyb561? To address this question, we generated retroviral particles expressing Cyb561 and transduced the miRVector- and miR-30e-over-expressing AML cells. The expression level of Cyb561 was upregulated 9.86-fold in miRVector/pMIBSD-Cyb561 AML cells compared with miRVector/pMIBSD AML cells (Online Supplementary Figure S7A). Overexpression of Cyb561 rescued the colony-forming capacity of forced-expressed miR-30e LSC in vitro (Figure 3E). Furthermore, functional assays on LSC revealed that the overexpression of Cyb561 attenuated the effects of miR-30e on leukemia progression by regulating the cell cycle and apoptosis of LSC (Figure 3F, Online Supplementary Figure S7B-D). The limiting dilution assay showed that overexpression of Cyb561 resulted in an approximately 3.80-fold increase in LSC frequency (1/124.7) in AML mice overexpressing miR-30e compared to AML mice transplanted with overexpressing-miR-30e/ pMIBSD-AML cells (1/474.3) (Online Supplementary Table S8). Together, these findings demonstrate that Cyb561 is a direct functional target of miR-30e-5p in LSC.
Figure 3.
RNA-sequencing analysis identifies potential targets of miR-30e-5p in L-GMP cells from KMT2A::MLLT3-driven leukemia. (A) Enrichment plot of downregulated gene sets in miR-30e-overexpressing leukemia stem cells (LSC), as determined by gene set enrichment analysis. RNA-sequencing data for 27,359 transcripts were used for the analysis. (B) Quantitative polymerase chain reaction analysis of candidate target genes in LSC-enriched granulocyte-monocyte progenitors (L-GMP) sorted from recipients transplanted with miRVector- or miR-30e-overexpressing acute myeloid leukemia cells. Results are normalized to Hprt expression and expressed relative to the expression of target genes in miRVector L-GMP (N=3). (C) Luciferase reporter assay to identify the true target of miR-30e-5p (N=3). (D) Cyb561 3’UTR luciferase reporter assay to identify the binding site of miR-30e-5p (N=3). (E) Overexpression of Cyb561 impaired the suppressive function of miR-30e on LSC (N=3). (F) Forced expression of Cyb561 impeded the prolonged survival of miR-30e on KMT2A::MLLT3-driven leukemia (N=7). Data are representative of two or three independent experiments. All data are represented as mean ± standard deviation. Two-tailed Student t tests were used to assess statistical significance (N.S.: not significant; *P<0.05; **P<0.01). miRVector: control; OE-miR-30e: cells with miR-30e overexpression; NOM: nominal; WT: wild-type; mut: mutated.
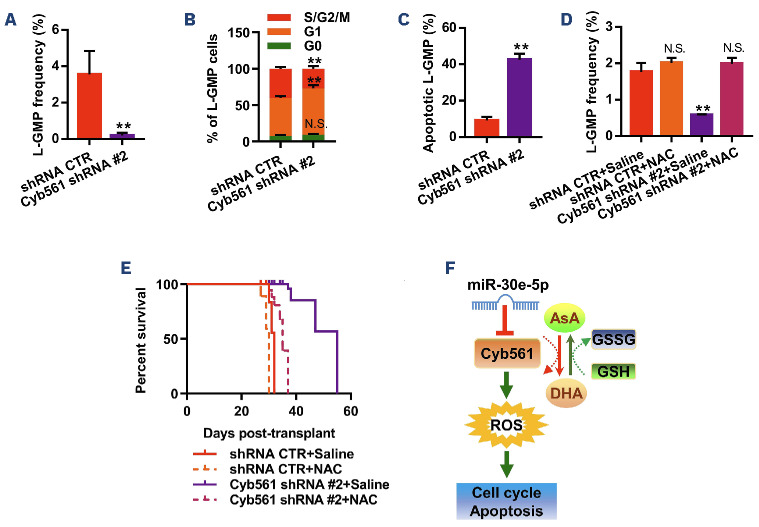
Cyb561 deficiency delays the development of KMT2A::MLLT3-driven acute myeloid leukemia by miR-30e-5p/ROS signaling pathway
Since miR-30e-5p suppressed the expression of endogenous Cyb561, we aimed to determine whether the knockdown of endogenous Cyb561 could impair AML development. To address this issue, we knocked down Cyb561 in KMT2A::MLLT3-driven LSC by inducible lentivirus-expressing shRNA. Our data showed that Cyb561 shRNA #1, #2 and #3 reduced the expression of Cyb561 in mouse AML cells by 57%, 80% and 31%, respectively (Online Supplementary Figure S8A). Subsequently, we conducted cell growth and colony-forming/replating assays using KMT2A::MLLT3-driven LSC transduced with scrambled shRNA (control) and two Cyb561 shRNA (#1 and #2). Knockdown of Cyb561 significantly inhibited the cell growth and reduced colony numbers compared to the control group (Online Supplementary Figure S8B, C). We then transplanted an equal number of shRNA-transduced LSC into irradiated recipients. Three to 5 weeks after BM transplantation, we analyzed AML cells in peripheral blood, spleen weight, LSC frequency, cell cycle, and apoptosis of LSC. The results showed that inhibition of Cyb561 decreased the frequency of GFP+ AML cells in peripheral blood, spleen weight, and LSC frequency in BM compared with the control group (Figure 4A, Online Supplementary Figure S8D, E). Suppression of Cyb561 expression also attenuated the cell cycle and promoted apoptosis of LSC (Figure 4B, C, Online Supplementary Figure S8F). The limiting dilution assay revealed that knockdown of endogenous Cyb561 resulted in an 11.8-fold reduction in LSC frequency (1/335.3) in AML mice transplanted with Cyb561 shRNA #2-AML cells compared to AML mice transplanted with shRNA control (1/28.4) (Online Supplementary Table S9). It has been reported that CYB561 is a type of transmembrane protein consisting of six transmembrane helices and two b-type hemes on each side of the membrane, and it plays a role in the ascorbate recycling process as dehydroascorbate (DHA) reductase.21 Ascorbate oxidation/ reduction is closely associated with ROS homeostasis.22 This suggests a possible link between CYB561 and intracellular ROS, which may be directly or indirectly involved in the cell cycle and apoptosis. Therefore, we investigated whether the knockdown of Cyb561 affects ROS production. Mean fluorescence intensity analysis revealed elevated intracellular ROS levels in Cyb561-knockdown LSC (Online Supplementary Figure S8G). Next, we examined the effect of miR-30e on the level of ROS in LSC from the recipients. As expected, overexpression of miR-30e increased intracellular ROS levels in LSC. However, overexpression of Cyb561 reduced intracellular ROS levels in ectopic miR-30e-expressing LSC (Online Supplementary Figure S7E, F). To further investigate whether miR-30e/Cyb561 impaired LSC by regulating intracellular ROS, we used the ROS scavenger NAC and Tiron to decrease ROS levels in the AML cells. Both NAC and Tiron rescued the colony-forming capacity of LSC overexpressing miR-30e and LSC with Cyb561 knockdown (Online Supplementary Figures S9A and S10A). LSC functional assays, showed that NAC treatment impaired the roles of miR-30e and Cyb561 in LSC and leukemia progression (Figure 4D, E, Online Supplementary Figures S9B-D and S10B-D). Given that CYB561 acts as a reductase in the ascorbate-DHA recycling process and there is an enriched gene set (i.e., glutathione metabolism) in LSC according to GSEA, we tested whether the miR-30e/Cyb561/ROS axis affects the levels of endogenous glutathione, ascorbate and DHA in AML cells. The results revealed a decrease in the levels of glutathione and ascorbate accompanied by an increase in DHA levels in AML cells overexpressing miR-30e and in AML cells with Cyb561 knockdown (Online Supplementary Figure S11). Furthermore, we examined the functions of miR-30e and Cyb561 in normal HSC, given the intriguing findings in LSC. As shown in Online Supplementary Figure S12, neither overexpression of miR-30e nor knockdown of Cyb561 influenced the repopulation capacity of normal HSC. Collectively, our data indicate that miR-30e-5p functions as a negative regulator of the Cyb561-ROS signaling pathway (Figure 4F).
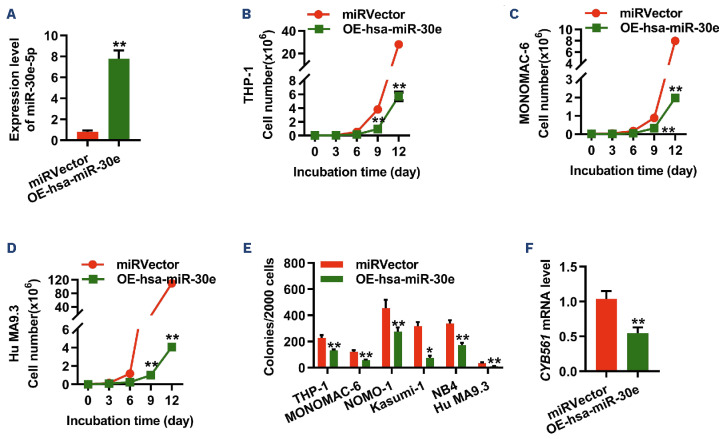
Overexpression of Homo sapiens-miR-30e and knockdown of CYB561 impairs human acute myeloid leukemias
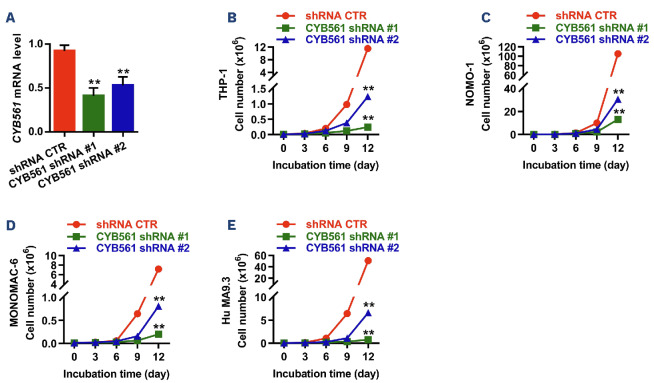
Although the results from the murine models provided robust evidence for the critical role of miR-30e-5p and Cyb561 in the initiation and maintenance of murine LSC induced by KMT2A::MLLT3, it remained unclear whether miR-30e-5p or Cyb561 also affects human leukemia. To address this question, we generated retroviral particles expressing Homo sapiens (hsa)-miR-30e and transduced them into six different human leukemic cells, namely THP-1, MONOMAC-6, NOMO-1, Kasumi-1, NB4, and human MA9.3 cells. Cell growth/proliferation assays demonstrated that forced expression of hsa-miR-30e inhibited the growth of all human leukemic cells (Figure 5A-D, Online Supplementary Figure S13A-C). Furthermore, overexpression of miR-30e induced extensive apoptosis in human AML cells without affecting their differentiation (Online Supplementary Figure S13D-F). In vitro colony-forming assays revealed reduced colony numbera in the hsa-miR-30e-overexpressing groups in comparison to the miRVector-expressing groups (Figure 5E). Moreover, ectopic expression of hsamiR-30e suppressed the expression level of CYB561 in human AML cells (Figure 5F). To further investigate whether miR-30e exerted similar effects on the functions of human LSC, we analyzed the cell cycle and apoptosis of human LSC (CD34+CD38–) in human MA9.3 cells, which closely resemble AML cell models containing the KMT2A::MLLT3 fusion found in patients’ AML samples.15 As depicted in Online Supplementary Figure S13G-H, overexpression of miR-30e increased the G1 phase while decreasing the S/ G2/M phases of LSC and promoted the apoptosis of LSC. To assess the relevance of CYB561 in human AML cells, we first examined the clinical significance of CYB561 expression levels in AML patients and the normal human population. The results showed that CYB561 expression was upregulated in BM mononuclear cells of FAB subtypes AML-M1, M2, M3, M4, M5 compared to normal BM mono-cytes (Figure 6A). Additionally, CYB561 expression was increased in LSC from AML patients compared to normal GMP (Figure 6B). Notably, AML patients with higher levels of CYB561 expression displayed a significantly shorter overall survival time, as observed through gene expression profiling interactive analysis (GEPIA: http://gepia.cancer-pku. cn/) (Figure 6C). Next, we examined functional potentials of CYB561 in human AML cells by doxycycline-inducible shRNA. As shown in Figure 7A, CYB561 shRNA #1 and #2 effectively reduced the expression of CYB561 in human leukemic cells by 56% and 45%, respectively. The knockdown of CYB561 suppressed the growth of all human leukemic cells and promoted apoptosis in vitro, while having no impact on the differentiation of human AML cells (Figure 7B-E, Online Supplementary Figure S14A-F). Moreover, inhibition of CYB561 increased the G1 phase while decreasing the S/G2/M phases of human LSC and promoted the apoptosis of human LSC (Online Supplementary Figure S14G, H).
Figure 4.
Knockdown of Cyb561 diminishes self-renewal of KMT2A::MLLT3-driven leukemia stem cells by enhancing intracellular levels of reactive oxygen species. (A) Frequency of leukemia stem cell-enriched granulocyte-monocyte progenitors (L-GMP) in the bone marrow (BM) from primary recipients injected with 1x105 acute myeloid leukemia (AML) cells transduced with control short hairpin (sh) RNA (shRNA CTR) or Cyb561 shRNA #2, at week 4 after transplantation (N=5). (B) Cell cycle phase distribution of L-GMP cells in BM from primary recipients injected with shRNA CTR or Cyb561 shRNA #2 AML cells, at week 5 after transplantation (N=4). (C) Percentage of apoptotic L-GMP cells in the BM from primary recipients transplanted with shRNA CTR or Cyb561 shRNA #2 AML cells (N=4). (D) Frequency of L-GMP in the BM from recipients receiving AML cells transduced with shRNA CTR or Cyb561 shRNA #2 following treatment with saline or N-acetyl-L-cysteine (NAC) (N=5). (E) Survival analysis of recipient mice receiving AML carrying shRNA CTR or Cyb561 shRNA #2 after treatment with saline or NAC. The median survival of the four groups of recipients was 32, 30, 55, and 35 days after transplantation (P<0.01, Mantel-Cox test, N=6). (F) Diagrammatic model of the miR-30e-5p/Cyb561/ROS signaling pathway. Data are representative of two or three independent experiments. All data are represented as mean ± standard deviation. Two-tailed Student t tests were used to assess statistical significance (N.S.: not significant; *P<0.05; **P<0.01). AsA: ascorbate; GSSG: oxidated glutathione; GSH: reduced glutathione; DHA: dehydroascorbate; ROS: reactive oxygen species.
Figure 5.
Overexpression of miR-30e impairs human acute myeloid leukemia cell growth. (A) Quantitative polymerase chain reaction (qPCR) analysis of miR-30e-5p expression in THP-1 cells. Results are normalized to U6 expression and expressed relative to miR-30e-5p expression in the miRVector group (N=3). (B-D) Ectopic expression of miR-30e suppresses the growth of THP-1 (B), MONOMAC-6 (C), and Hu MA9.3 (D) cells (N=3). (E) Colony-forming assay of human leukemia cells by overexpressed-miR-30e (N=3). (F) qPCR analysis of the expression level of CYB561 in THP-1 cells transduced with miRVector or miR-30e. Results are normalized to GADPH expression and expressed relative to CYB561 in the miRVector group (N=3). Data are representative of two or three independent experiments. All data are represented as mean ± standard deviation. Two-tailed Student t tests were used to assess statistical significance (*P<0.05; **P<0.01). miRVector: control; OE-hsa-miR-30e: cells with Homo sapiens miR-303 over-expression.
To explore the therapeutic potential of targeting miR-30e-5p and CYB561 in AML, we designed mimics of hsa-miR-30e-5p and siRNA corresponding to the CYB561 coding region. These molecules were delivered into various human leukemic cells. We observed inhibition of human leukemic cell growth upon treatment with these miRNA mimics and siRNA across all AML cell lines (Online Supplementary Figures S15 and S16). Taken together, these findings provide compelling evidence for the promising therapeutic potential of miR-30e-5p and CYB561 in human AML.
Discussion
LSC, a rare population of AML cells, play unique roles in the initiation, maintenance, and propagation of AML. Similar to normal HSC, LSC reside in the hypoxic BM niche and their biological functions are closely related to the intracellular ROS level and oxidative stress status.23 It has been observed that excessively elevated ROS levels can impair the self-renewal capacity of LSC.24 Therefore, targeting ROS and ROS-associated regulators in LSC represents a promising therapeutic strategy for improving long-term outcomes in AML. In this study, we have revealed that miR-30e-5p negatively regulates LSC self-renewal mainly through its direct target Cyb561, a transmembrane protein that functions as a reductase for regenerating ascorbate and providing reduced iron for transmembrane transport.21 The functional roles of Cyb561 in leukemogenesis have not been previously reported. However, in our investigations, we observed that miR-30e-5p/Cyb561 modulates LSC self-renewal by activating intracellular ROS. Intriguingly, both forced expression of miR-30e-5p and inhibition of Cyb561 have shown the ability to delay leukemia progression, suggesting that both miR-30e-5p and Cyb561 possess potential therapeutic effects on LSC in AML.
Figure 6.
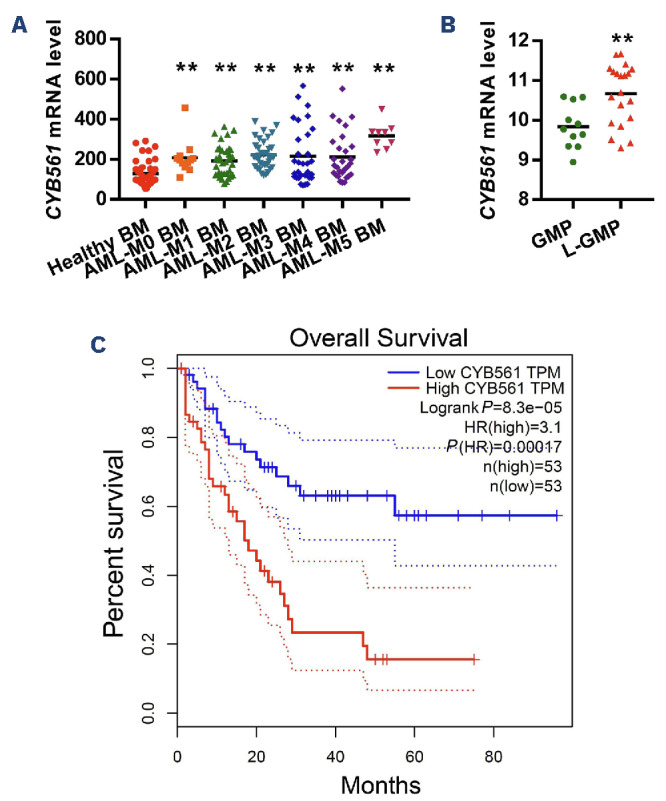
CYB561 is upregulated in acute myeloid leukemia patients. (A) The difference in expression of CYB561 between normal bone marrow cells from healthy controls and from patients with different subtypes of acute myeloid leukemia (AML) according to the French-American-British classification. The data were obtained from a public microarray database (access numbers: GSE11504, GSE19429, GSE10358) (normal patients, N=42; M0 patients, N=12; M1 patients, N=35; M2 patients, N=36; M3 patients, N=31; M4 patients, N=29; M5 patients, N=9). (B) The expression of CYB561 between normal granulocyte-monocyte progenitors (GMP) and leukemia stem cell-enriched granulocyte-monocyte progenitors (L-GMP) from patients with AML (normal patients, N=11; AML patients, N=20). (C) Correlation analysis between CYB561 expression level and overall survival in AML patients according to GEPIA. BM: bone marrow; FAB: French-American-British; TPM: transcripts per million; HR: hazards ratio.
miR-30e-5p is expressed at a high level in normal HSC and plays a significant role in various crucial biological processes. Previous studies have demonstrated its potential as a tumor suppressor in different types of cancer, such as bladder cancer, nasopharyngeal carcinoma, and liver cancer, and induces apoptosis of chronic myeloid leukemia cells.9,13,25,26 The level of expression of miR-30e-5p is elevated in patients with acute lymphoblastic leukemia and reduced in patients with chronic myeloid leukemia.13,16 Despite these notable findings regarding miR-30e-5p in cancer development and leukemia, to the best of our knowledge, there is no existing evidence demonstrating that overexpression of miR-30e-5p not only delays the onset of AML and targets LSC by regulating the cell cycle and apoptosis in vivo, but also acts as a negative regulator of the transmembrane reductase CYB561.
CYB561 is a di-heme transmembrane protein that plays a crucial role in ascorbate recycling and iron homeostasis and is associated with cellular senescence.21,27 It is expressed highly in metabolically active human tissues, such as the brain, kidney, and heart.28 Despite its clear physiological significance, the functions of Cyb561 in animal cells, particularly in LSC, remain unexplored. Previous studies have indicated that CYB561 mutations in patients result in a novel orthostatic hypotension syndrome, and that CYB561 could serve as a potential prognostic biomarker for breast cancer.28,29 In our current study, we found that miR-30e-5p downregulates the expression of Cyb561 in LSC. Intriguingly, the knockdown of Cyb561 in KMT2A::MLLT3-driven LSC prolongs the survival of recipient mice with a reduced frequency of LSC. Furthermore, we observed a negative correlation between overall survival and CYB561 expression level in AML patients. These findings suggest that Cyb561 acts as a potential suppressor of AML initiation and development and could serve as a biomarker for leukemia prognosis.
Through GSEA of downregulated gene sets in miR-30e-over-expressed LSC, we identified significant enrichment of gene sets associated with the electron transport chain, oxidative phosphorylation, and glutathione metabolism. These gene sets are highly relevant to intracellular ROS production and balance. Glutathione, as the most abundant endogenous antioxidant in mammalian cells, plays a crucial role in maintaining a “non-toxic” level of ROS.
Previous studies have demonstrated a significant reduction in glutathione levels in primary human CD34+ AML cells compared to CD34+ normal BM cells. Consistent with these findings, our study revealed a decrease in endogenous glutathione levels in miR-30e-overexpressing LSC, which was attributed to elevated levels of intracellular ROS, similar to the results observed in Cyb561-knockdown LSC. CYB561, functioning as a reductase, is involved in the reduction process from DHA to ascorbate, and its deficiency can disrupt this process, leading to intracellular ROS accumulation. Our data demonstrated that both overexpression of miR-30e-5p and knockdown of Cyb561 resulted in reduced intracellular ascorbate levels, accompanied by elevated DHA levels in LSC. Impaired ascorbate recycling due to endogenous Cyb561 knockdown consequently led to decreased glutathione levels and increased ROS levels in LSC. Integrating these findings with our results, we propose that miR-30e-5p regulates LSC self-renewal through the Cyb561/ROS signaling pathway, involving intracellular ascorbate/glutathione metabolism. In summary, our study unveils a previously unknown miR-30e-5p/Cyb561 axis that finely modulates an intracellular ROS signaling pathway in LSC. Based on our findings, pharmacological targeting of LSC through manipulation of miR-30e-5p or Cyb561, in combination with chemotherapy, holds great potential as a highly effective strategy to enhance therapeutic regimens.
Figure 7.
Knockdown of CYB561 suppresses the growth of human acute myeloid leukemia cells. (A) Quantitative polymerase chain reaction analysis of inducible CYB561 knockdown in NB4 cells. NB4 cells were transduced with lenitiviruses containing a scrambled short hairpin (sh) RNA control (CTR), or inducible CYB561 shRNA. After puromycin selection, 1 [ig/ imL of doxycycline was added to the medium for 72 h. Results are normalized to HPRT expression and expressed relative to the shRNA control group (N=3). (B-E) Knockdown of CYB561 suppresses the growth of THP-1 (B), NOMO-1 (C), MONOMAC-6 (D), and Hu MA9.3 (E) cells (N=3). Data are representative of two or three independent experiments. All data are represented as mean ± standard deviation. Two-tailed Student t tests were used to assess statistical significance (**P<0.01).
Supplementary Material
Funding Statement
Funding: This research was supported in part by the Natural Science Foundation of Shanghai (grant n. 18ZR1414900), the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning, Special Project of Science and Technology Plan of Shaoxing Science and Technology Bureau (grant n. 2020B33004), and the National Natural Science Foundation of China (grant n. 82070106).
References
- 1.Krivtsov AV, Twomey D, Feng Z, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442(7104):818-822. [DOI] [PubMed] [Google Scholar]
- 2.Wiseman DH, Greystoke BF, Somervaille TCP. The variety of leukemic stem cells in myeloid malignancy. Oncogene. 2014;33(24):3091-3098. [DOI] [PubMed] [Google Scholar]
- 3.Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev. 2019;36:70-87. [DOI] [PubMed] [Google Scholar]
- 4.Thomas D, Majeti R. Biology and relevance of human acute myeloid leukemia stem cells. Blood. 2017;129(12):1577-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Nguyen LXT, Chen YC, et al. Targeting miR-126 in inv(16) acute myeloid leukemia inhibits leukemia development and leukemia stem cell maintenance. Nat Commun. 2021;12(1):6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzales-Aloy E, Connerty P, Salik B, et al. miR-101 suppresses the development of MLL-rearranged acute myeloid leukemia. Haematologica. 2019;104(7):e296-e299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khalaj M, Woolthuis CM, Hu W, et al. miR-99 regulates normal and malignant hematopoietic stem cell self-renewal. J Exp Med. 2017;214(8):2453-2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma YX, Zhang H, Li XH, Liu YH. MiR-30e-5p inhibits proliferation and metastasis of nasopharyngeal carcinoma cells by targeting USP22. Eur Rev Med Pharmacol Sci. 2018;22(19):6342-6349. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Qin H, Jiang B, et al. miR-30e-5p suppresses cell proliferation and migration in bladder cancer through regulating metadherin. J Cell Biochem. 2019;120(9):15924-15932. [DOI] [PubMed] [Google Scholar]
- 10.Liang Z, Tang S, He R, Luo W, Qin S, Jiang H. The effect and mechanism of miR-30e-5p targeting SNAI1 to regulate epithelial-mesenchymal transition on pancreatic cancer. Bioengineered. 2022;13(4):8013-8028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z, Qiu H, Ma L, et al. miR-30e-5p and miR-15a synergistically regulate fatty acid metabolism in goat mammary epithelial cells via LRP6 and YAP1. Int J Mol Sci. 2016;17(11):1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding W, Li J, Singh J, et al. miR-30e targets IGF2-regulated osteogenesis in bone marrow-derived mesenchymal stem cells, aortic smooth muscle cells, and ApoE-/- mice. Cardiovasc Res. 2015;106(1):131-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hershkovitz-Rokah O, Modai S, Pasmanik-Chor M, et al. MiR-30e induces apoptosis and sensitizes K562 cells to imatinib treatment via regulation of the BCR-ABL protein. Cancer Lett. 2015;356(2 Pt B):597-605. [DOI] [PubMed] [Google Scholar]
- 14.Jia Y, Gu W. Up-regulation of circPVT1 in T cell acute lymphoblastic leukemia promoted cell proliferation via miR-30e/DLL4 induced activating NOTCH signaling. Pathol Res Pract. 2021;224:153536. [DOI] [PubMed] [Google Scholar]
- 15.Wei J, Wunderlich M, Fox C, et al. Microenvironment determines lineage fate in a human model of MLL-AF9 leukemia. Cancer Cell. 2008;13(6):483-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luna-Aguirre CM, de la Luz Martinez-Fierro M, Mar-Aguilar F, et al. Circulating microRNA expression profile in B-cell acute lymphoblastic leukemia. Cancer Biomark. 2015;15(3):299-310. [DOI] [PubMed] [Google Scholar]
- 17.Schotte D, Chau JC, Sylvester G, et al. Identification of new microRNA genes and aberrant microRNA profiles in childhood acute lymphoblastic leukemia. Leukemia. 2009;23(2):313-322. [DOI] [PubMed] [Google Scholar]
- 18.Park SM, Cho H, Thornton AM, et al. IKZF2 drives leukemia stem cell self-renewal and inhibits myeloid differentiation. Cell Stem Cell. 2019;24(1):153-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu Y, Chen Y, Li M, et al. Six1 regulates leukemia stem cell maintenance in acute myeloid leukemia. Cancer Sci. 2019;110(7):2200-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu JF, Shih LY, Yen TH. HOXA11 plays critical roles in disease progression and response to cytarabine in AML. Oncol Rep. 2021;46(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asard H, Barbaro R, Trost P, Bérczi A. Cytochromes b561: ascorbate-mediated trans-membrane electron transport. Antioxid Redox Signal. 2013;19(9):1026-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen J, Griffiths PT, Campbell SJ, Utinger B, Kalberer M, Paulson SE. Ascorbate oxidation by iron, copper and reactive oxygen species: review, model development, and derivation of key rate constants. Sci Rep. 2021;11(1):7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Liang Y, Luo X, Hu Q. Oxidative resistance of leukemic stem cells and oxidative damage to hematopoietic stem cells under pro-oxidative therapy. Cell Death Dis. 2020;11(4):291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herault O, Hope KJ, Deneault E, et al. A role for GPx3 in activity of normal and leukemia stem cells. J Exp Med. 2012;209(5):895-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma YX, Zhang H, Li XH, Liu YH. MiR-30e-5p inhibits proliferation and metastasis of nasopharyngeal carcinoma cells by target-ing USP22. Eur Rev Med Pharmacol Sci. 2018;22(19):6342-6349. [DOI] [PubMed] [Google Scholar]
- 26.Mao J, Hu X, Pang P, Zhou B, Li D, Shan H. miR-30e acts as a tumor suppressor in hepatocellular carcinoma partly via JAK1/ STAT3 pathway. Oncol Rep. 2017;38(1):393-401. [DOI] [PubMed] [Google Scholar]
- 27.Kang MK, Kameta A, Shin KH, Baluda MA, Kim HR, Park NH. Senescence-associated genes in normal human oral keratinocytes. Exp Cell Res. 2003;287(2):272-281. [DOI] [PubMed] [Google Scholar]
- 28.van den Berg MP, Almomani R, Biaggioni I, et al. Mutations in CYB561 causing a novel orthostatic hypotension syndrome. Circ Res. 2018;122(6):846-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang X, Zhao Y, Shao Q, Jiang G. Cytochrome b561 serves as a potential prognostic biomarker and target for breast cancer. Int J Gen Med. 2021;14:10447-10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szarka A, Tomasskovics B, Bánhegyi G. The ascorbate-glutathione-α-tocopherol triad in abiotic stress response. Int J Mol Sci. 2012;13(4):4458-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pei S, Minhajuddin M, Callahan KP, et al. Targeting aberrant glutathione metabolism to eradicate human acute myelogenous leukemia cells. J Biol Chem. 2013;288(47):33542-33558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.