Abstract
Legionella pneumophila causes Legionnaires’ disease by replication in alveolar macrophages and monocytes. The bacteria are internalized most efficiently by opsonin-dependent, CR3-mediated phagocytosis. This investigation focused on determining the role of actin polymerization and phosphorylation signals in this uptake mechanism. Uptake inhibition assays and confocal microscopic analysis indicated that entry of L. pneumophila activated tyrosine kinase (TK) and protein kinase C (PKC) and induced actin polymerization at the site of bacterial entry. Upon L. pneumophila entry, six major cellular proteins (75, 71, 59, 56, 53, and 52 kDa) were TK phosphorylated in soluble fractions of monocytes, and three of these proteins (52, 53, and 56 kDa) were consistently found in insoluble (i.e., cytoskeletal) fractions of monocytes as well. Tyrosine phosphorylation was suppressed when cells were pretreated with the kinase inhibitor genistein, tyrphostin, or staurosporine. A similar tyrosine-phosphorylated protein pattern was observed with CR3-mediated entry of avirulent L. pneumophila, Escherichia coli, or zymosan into monocytes. This study has shown that PKC and TK signals which activate actin polymerization during the process of phagocytosis are induced upon L. pneumophila entry. In addition, CR3 receptor-mediated phagocytosis into monocytes may involve tyrosine phosphorylation of similar proteins, regardless of the particle being phagocytosed. Therefore, the tyrosine-induced phosphorylation observed during opsonized L. pneumophila entry is not a virulence-associated event.
Legionella pneumophila is a gram-negative intracellular pathogen that causes Legionnaires’ disease, which is primarily a respiratory infection that may also involve the gastrointestinal tract and central nervous system (41, 45, 49). L. pneumophila infects human monocytes and enters these cells most efficiently by an opsonin-dependent, phagocytic mechanism (7, 22, 33), with bacteria binding to CR1 and CR3 integrin receptors on the surface of the host cell (23, 33). Microfilaments have been demonstrated to be involved in the phagocytosis of L. pneumophila into human macrophages since inhibition of uptake was observed following treatment of cells with cytochalasin D, a microfilament inhibitor (16). Horwitz demonstrated that following the entry of virulent L. pneumophila, the bacteria avoided phagolysosomal fusion and were able to replicate within the host cells (25). In contrast, an analysis of avirulent L. pneumophila showed that the phagosomes containing the bacteria continued on a normal endocytic route and fused with the lysosomes (24). These avirulent L. pneumophila bacteria were incapable of replication within the host cells.
The process of phagocytosis is initiated when a ligand on the surface of a particle becomes engaged with a receptor on the cell surface. Biochemical and mechanical signals then drive the polymerization of actin at the site of receptor-ligand interaction (35, 48), leading to phagocytic uptake. It is possible that some signals associated with the cytoskeleton may also provide the mechanical force required for phagolysosome fusion (53). In other gram-negative pathogens, actin and other cytoskeletal proteins are altered in conjunction with bacterial invasion. Enteropathogenic Escherichia coli entry into HeLa cells induces the assembly of a complex cytoskeletal structure (17, 29). Actin accumulation has also been associated with Shigella flexneri entry into HeLa cells (1, 11) and epithelial cells (12) and Salmonella entry into epithelial cells (18). Chlamydia trachomatis serovar E requires microfilament protein rearrangement upon entry into epithelial cells as well (42).
To activate the cytoskeletal rearrangement necessary for bacterial uptake, the interaction between the bacterium and the cell must induce a signal(s) to target the actin. Integrin receptors have recently been demonstrated to send signals to the cytoskeleton (35, 48). In addition, iC3b binding to CR3 (35, 56) and ligand binding to FcγRI (9, 32) have been shown to enhance the proximity of the receptors to cytoskeletal actin. Tyrosine-specific phosphorylation signals have previously been demonstrated to play a role in bacterial entry in many systems. C. trachomatis entry into HeLa cells was found to induce 64-, 97-, and 140-kDa tyrosine-specific proteins (8), while invasion by Mycoplasma penetrans led to tyrosine phosphorylation of a 145-kDa host protein in HeLa cells (5). A 44-kDa phosphotyrosine protein was induced upon Salmonella typhimurium entry into epithelial cells (39), and cortactin was phosphorylated upon S. flexneri entry into epithelial cells (14). Interestingly, during invasion of enteropathogenic E. coli, a 90-kDa bacterial protein was phosphorylated and then inserted into the host cell membrane (27). The role of protein kinase C (PKC)-specific phosphorylation in the promotion of bacterial entry has not been widely established. However, PKC-specific phosphorylation has been demonstrated in zymosan phagocytosis by macrophages (3) and Fc receptor-mediated phagocytosis by monocytes (55). PKC inhibitors have also been found to suppress ingestion by monocytes and polymorphonuclear neutrophils (4, 9, 21) but not in macrophages (9, 20).
This study investigated the hypothesis that L. pneumophila invasion of monocytes activates phosphorylation signals necessary to induce the cytoskeletal rearrangement required for the process of bacterial entry and the possibility that these signals differ between avirulent and virulent bacteria during the uptake event.
MATERIALS AND METHODS
Bacterial strains.
A clinical strain of L. pneumophila serogroup 1 (IDL-2V) was used for all experiments. Virulence was determined by the ability to replicate in human monocytes. An avirulent, isogenic strain (IDL-2A) was obtained by repeated passages of IDL-2V on BYCE agar (Difco) and additional passages on non-charcoal-containing GC-FC media (44). An E. coli reference isolate ATCC (25922) was used as a phagocytic control for comparison. Bacterial stock cultures were stored at −70°C and cultured on BCYE (Difco Laboratories, Detroit, Mich.) at 37°C for 2 to 3 days prior to experimentation.
Cell cultures.
Monocytes were isolated from 60 ml of fresh blood collected from healthy volunteers after informed consent had been obtained. The cells were separated from whole blood by layering on a Histopaque 1077 and 1119 gradient (Sigma Chemical Co., St. Louis, Mo.). This was centrifuged at 700 × g at room temperature for 30 min. The peripheral blood mononuclear cell band was removed and washed twice in 1× Hanks balanced salt solution (0.15 M NaCl plus 0.015 M sodium citrate; Gibco) containing 20 mM HEPES and 50 U of a penicillin-streptomycin mixture (BioWhittaker) per ml. Peripheral blood mononuclear cells were suspended in RPMI (Gibco) containing 20 mM HEPES and 0.1% heat-inactivated normal human serum (NHS) and incubated for 2 h in 24-well plates. Nonadherent cells were removed by washing with phosphate-buffered saline (PBS), and wells were refilled with RPMI containing 20 mM HEPES and 5% heat-inactivated NHS. The remaining monolayer consisted of 98% monocytes, as determined by nonspecific esterase staining.
Serum.
Whole blood was collected from volunteers, allowed to clot, and centrifuged to separate the serum. NHS was then stored at −70°C until needed. For experiments involving monocytes, L. pneumophila and E. coli were opsonized with complement C3b and C3bi. This was accomplished by rotating 108 bacteria/ml suspended in 5 ml of PBS, containing 20% NHS for 1 h at 37°C. The bacteria were washed three times in PBS prior to use. For use in cell culture, NHS was heat inactivated by incubation at 56°C for 30 min.
Inhibition assays.
Monocytes were pretreated with the tyrosine kinase inhibitor genistein (100, 200, 250, 300, or 400 μM) 15 min prior to infection or with tyrphostin-47 (100, 200, 300, or 400 μM) 60 min prior to infection. Genistein competitively inhibits the binding of ATP to the kinase, and tyrphostin competes with tyrosine residue binding to the kinase (2, 38). Although they inhibit by different mechanisms, both are considered strong tyrosine protein kinase (TPK) inhibitors (2, 19, 38, 51). Cells were treated with calphostin C (0.5, 0.7, and 1.0 μM) or staurosporine (0.5, 1.0, 1.5, and 2.0 μM) 1 h prior to infection. All inhibitors were purchased from Sigma. Although staurosporine is a potent inhibitor of PKC activity, it may also block some classes of TPK Src such as pp60c-src or serine/threonine protein kinases (38, 46). However, the drug acts as a poor inhibitor of other TPKs (46). Therefore, the use of a more specific PKC inhibitor, calphostin C, was included in this study although it is considered a less potent PKC inhibitor than staurosporine (46). Cells were infected at a multiplicity of infection (MOI) of 100:1 for 1 h, washed three times in PBS, treated with 100-μg/ml gentamicin for 90 min, rewashed, and lysed with 1 ml of sterile water. Samples were diluted and plated to determine the number of CFU per milliliter. Percent invasion for bacterium-infected cells that were untreated (control) was normalized to 100%. Percent invasion of inhibitor-treated, infected cells was determined by dividing the number of CFU per milliliter of these samples by the number of CFU per milliliter of the control, multiplied by 100.
To determine the effects of drugs on intracellular Legionella replication, cells were infected at an MOI of 100:1 for 2 h, treated with inhibitors, and gentamicin treated for 90 min. The cells were washed in PBS again, incubated with inhibitors for 2 h, and lysed at 0 and 48 h. Samples were plated to determine the number of CFU per milliliter, a measure of intracellular replication. During these experiments, monocyte viability was monitored by trypan blue dye exclusion.
When genistein is removed from the cell monolayer, its effects are quickly lost (31, 38). Based on this, it was possible to determine if the drug inhibits L. pneumophila adherence rather than internalization. Therefore, cells were pretreated with genistein, infected for 2 h, and washed to remove the drug and unbound bacteria. Cells were then treated with gentamicin at 0, 20, 40, and 60 min after genistein removal and plated for determination of the number of CFU per milliliter.
The long-term effects of staurosporine were utilized to determine if the inhibitors directly affect the ability of bacteria or monocytes to suppress invasion. The inhibitory effects of this compound are known to continue for long periods following its removal (37, 38). Cells were pretreated with the inhibitor 1 h prior to infection and then washed to remove the compound. The cells were infected and the inhibition assay was performed as described above.
Confocal microscopy.
To correlate actin polymerization with bacterial uptake, infected cells were double stained. Legionella cells were stained directly with fluorescein isothiocyanate (FITC) by rotating 5 ml of the bacteria (108/ml) suspended in PBS with FITC at 10 μg/ml for 1 h at 37°C. The bacterial suspension was then washed four times. Some samples were pretreated with inhibitors prior to infection. Cells were infected for 15 min, washed, removed with trypsin containing 1 mM Na3VO4, 0.4 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and 5 mM NaF (PENN), and pelleted. PENN was added to prevent dephosphorylation from occurring. Cells were simultaneously fixed, permeabilized, and stained for 25 min with 1 ml of a solution of 100-μg/ml lysopalmitidylphosphocholine, 3.7% formaldehyde, and 10-U/ml rhodamine phalloidin (Molecular Probes, Eugene, Oreg.). The cell suspension was washed four times with PBS and analyzed by a 486 33-MHz confocal argon laser microscope, the Olympus IMT-2. The argon laser was set at 50 mV. Cells were visualized with dual filters at 488/10 and 530/30 nm. Z scanning and quantitation of fluorescent actin were performed with Insight IQ software. Cells were selected manually for quantitation, and the fluorescent pixels were averaged for each selected cell.
Phosphotyrosine proteins were visualized in Legionella-infected cells by indirect fluorescent labeling. After 15 min of infection, cells were washed three times in PBS, trypsinized, pelleted, and fixed for 10 min with 100 μl of lysopalmitidylphosphocholine and 3.7% formaldehyde. The cells were then washed again and incubated with mouse antiphosphotyrosine antibody 4G10 (Upstate Biotechnology, Lake Placid, N.Y.) at a dilution of 1:1,000 in 0.2% bovine serum albumin (BSA) for 45 min. Cells were washed three times in PBS and incubated with tetramethyl rhodamine isocyanate-conjugated goat anti-mouse immunoglobulin G (IgG; Sigma) at a dilution 1:64 in 0.2% BSA (containing PENN). After washing, cells were analyzed by confocal microscopy with a 488/10-nm filter.
Preparation of lysates.
Approximately 4 × 106 monocytes were infected at an MOI of 100:1 for 15, 30, 60, or 120 min or incubated with 5-mg/ml nonopsonized zymosan (Sigma) for 15 min (53). There was no need to opsonize the zymosan since the β-glucan subunits can interact directly with CR3. Some samples were pretreated with TPK or PKC inhibitors prior to infection. Samples were washed, trypsinized, and pelleted. A 100-μl volume of lysis buffer (0.5% Triton X-100, 300 mM NaCl, 50 mM Tris-HCl [pH 7.5], 10-μg/ml leupeptin, 10-μg/ml aprotinin, 2 mM EDTA, 10 mM NaF, 1 mM phenylmethylsulfonyl fluoride) was immediately added to the pellet, and the mixture was placed on ice for 20 min with occasional shaking. Cells were microcentrifuged at 7,000 × g to separate the soluble and insoluble fractions. The latter contained host cytoskeletal and nuclear proteins, as well as bacterial proteins (39, 53), while the former contained host membrane and cytosolic proteins (39). Insoluble fractions were washed twice in PBS. An 80-μl volume of 2× sodium dodecyl sulfate (SDS) sample buffer was added to the soluble fraction, 40 μl was added to the insoluble fraction, and the samples were boiled for 6 min at 100°C. Samples were stored at −70°C.
Immunoblots.
SDS-polyacrylamide gel electrophoresis (PAGE) was performed on 7.5% separating gels and 4.0% stacking gels. The electrophoretic transfer of proteins to a 0.45-nm-pore-size nitrocellulose membrane was done with a Bio-Rad Mini Trans Blot Cell. For the molecular weight standard (Bio-Rad), 1 μl of standard was added to 20 μl of sample buffer. The membrane was blocked overnight in 1% BSA in PBS-Tween at room temperature with constant shaking. The membrane was then probed with 4G10 (1:1,000) in PBS-Tween for 2 h at room temperature with constant shaking. After four 15-min washes, it was probed with a goat anti-mouse IgG-peroxidase conjugate for 2 h at room temperature with constant shaking. Probed proteins were visualized with the use of an enhanced-chemiluminescence kit (Amersham Life Science, Amersham, Buckinghamshire, England) in accordance with the manufacturer’s instructions. The blot was exposed to Kodak X-Omat AR film for 1 min. Protein quantitation was assayed by the Bradford assay prior to loading onto gels. Additionally, Coomassie blue staining of the gel was done to ensure that equal amounts of protein were loaded into the wells.
Statistics.
Student’s t test was used to determine the significance of the average values for the experiments. A P value of <0.05 indicated significance.
RESULTS
TPK inhibitors block L. pneumophila entry into monocytes.
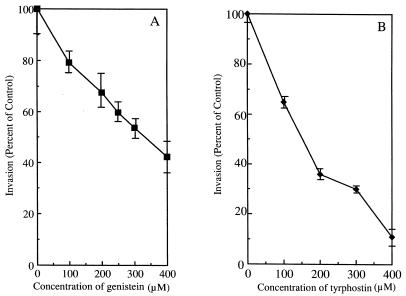
To determine the role of TPKs in L. pneumophila entry into monocytes, cells were pretreated with the TPK inhibitor genistein or tyrphostin prior to infection. Both compounds decreased invasion in a dose-dependent manner. However, tyrphostin was a more effective inhibitor of L. pneumophila entry into monocytes than was genistein (Fig. 1A and B). The former decreased invasion by 89% ± 2.5% (P < 0.05), while the latter only led to partial inhibition of bacterial entry into monocytes and decreased invasion by 61% ± 5.8% (P < 0.05) at 400 μM. The concentrations of genistein and tyrphostin used in the experiment whose results are shown in Fig. 1 had no effect on host cell viability (as measured by trypan blue dye exclusion). Concentrations above 400 μM were found to be slightly toxic to the monocytes and were not used in these experiments (data not shown).
FIG. 1.
Effects of genistein and tyrphostin on the internalization of L. pneumophila by human monocytes. (A) Genistein was added 15 min prior to infection. (B) Tyrphostin was added 1 h prior to infection. L. pneumophila entered 20% of untreated cells, and this was normalized to 100% invasion. Results are reported as means ± the standard errors of the means for three experiments.
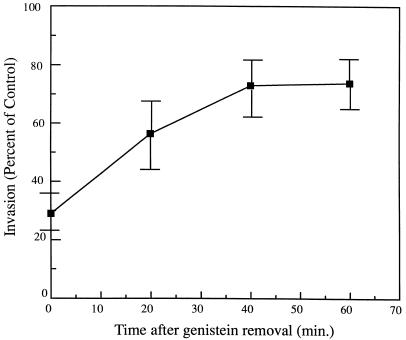
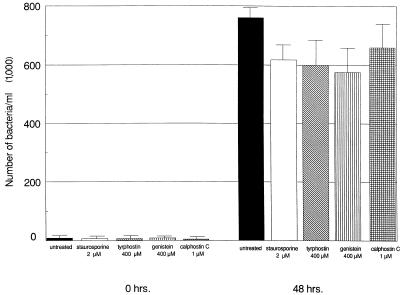
This study also examined the possibility that these inhibitors indirectly affect uptake by either blocking bacterial adhesion to the surface of the host cell or altering intracellular L. pneumophila viability. The rapid reversibility of genistein activity following its removal was used to determine if the inhibitors indeed affect internalization rather than bacterial adhesion (31, 38). If the inhibitory activity of genistein acts by blocking bacterial adhesion, invasion should not increase with time after its removal. However, bacterial invasion recovered from 30% ± 6.2% at 0 min to 74% ± 9.0% at 60 min following genistein removal (P < 0.05) (Fig. 2). The effects of genistein and tyrphostin on intracellular L. pneumophila viability were determined by testing the ability of the bacteria to replicate following exposure to the compounds after the entry process. If the inhibitors are lethal to intracellular L. pneumophila, then the bacteria should not replicate during the continued 48 h of infection. As shown in Fig. 3, neither genistein nor tyrphostin significantly reduced intracellular replication of L. pneumophila in monocytes compared to untreated cells following 48 h (P > 0.05). In all cases, intracellular Legionella increased 100-fold.
FIG. 2.
Invasion of monocytes by L. pneumophila after genistein removal. Cells were treated with 250 μM genistein 15 min prior to infection. Results are reported as means ± the standard errors of the means for three experiments.
FIG. 3.
Effects of kinase inhibitors on L. pneumophila replication. Following a 2-h bacterial uptake, cells were treated with kinase inhibitors. Cells were lysed, and CFU per milliliter were measured at 0 and 48 h to determine bacterial replication. Results are reported as means ± the standard errors of the means for three experiments.
PKC inhibitors block L. pneumophila entry into monocytes.
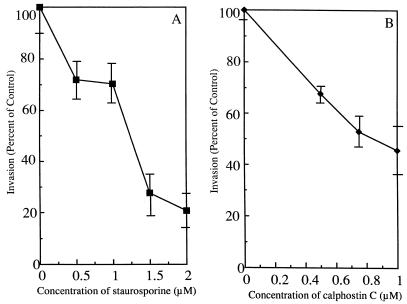
The role of PKC in L. pneumophila entry into host cells was investigated by pretreating the cells with the PKC inhibitors staurosporine and calphostin C prior to uptake (Fig. 4A and B). Both inhibitors suppressed L. pneumophila uptake by monocytes in a dose-dependent fashion, but staurosporine inhibited entry by 79% ± 4.7% (P < 0.05) at 2.0 μM, while calphostin C inhibited entry by only 54% ± 10% (P < 0.05) at a maximum concentration of 1.0 μM. As with the TPK inhibitors, the concentrations of calphostin C and staurosporine used in the experiment whose results are shown in Fig. 4 had no measurable effect on host cell viability. Higher concentrations were found to be slightly toxic to the monocytes and were not used in these experiments (data not shown).
FIG. 4.
Effects of staurosporine and calphostin C on internalization of L. pneumophila by human monocytes. (A) Staurosporine was added 1 h prior to infection and then removed by washing. (B) Calphostin C was added 60 min prior to infection. L. pneumophila entered 20% of untreated cells, and this was normalized to 100% invasion. Results are reported as means ± the standard errors of the means for three experiments.
Neither calphostin C at 1 μM nor staurosporine at 2 μM had detrimental effects on intracellular L. pneumophila viability since the bacteria were still capable of replication in monocytes following exposure to the inhibitors after the entry process (Fig. 3). Staurosporine and calphostin C slightly reduced the yield of L. pneumophila at 48 h compared to that of untreated cells, but the decrease was not statistically significant (P > 0.05).
To determine if the inhibitors acted directly on the bacterial cells to suppress invasion, the long-term effect of staurosporine was utilized (37, 38). As shown previously in Fig. 4, staurosporine was removed prior to bacterial infection and still led to a dose-dependent decrease, suggesting that the inhibitor acted not on L. pneumophila but on the host cell.
L. pneumophila entry into monocytes induces tyrosine protein phosphorylation.
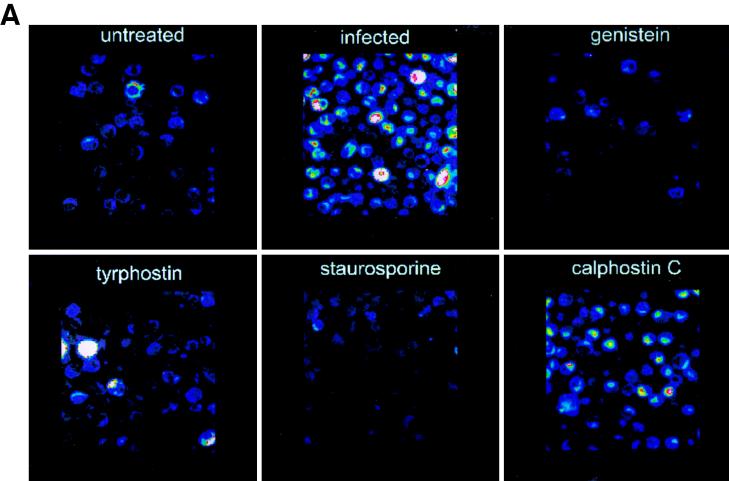
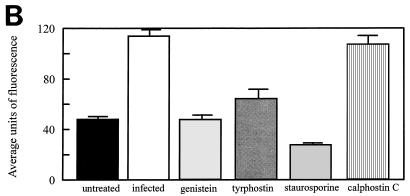
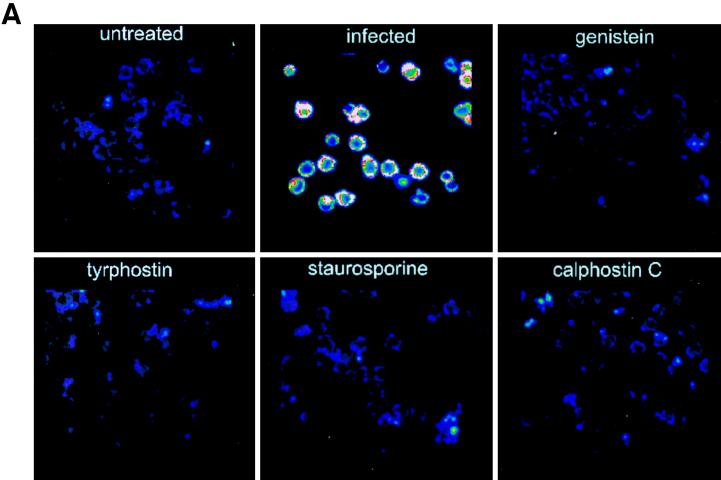
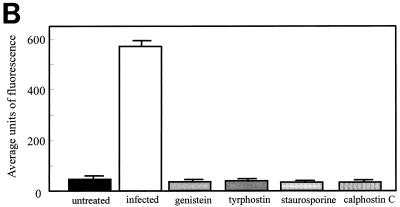
To directly investigate the role of TPK phosphorylation further, tyrosine-phosphorylated proteins were examined by immunofluorescent staining and confocal microscopy. Cells infected with L. pneumophila for 15 min had increased fluorescence of these tyrosine-phosphorylated proteins compared to noninfected, untreated monocytes (Fig. 5). The fluorescence of detectable proteins was twice that of noninfected monocytes (P < 0.0001). When cells were pretreated with the TPK inhibitor genistein or tyrphostin prior to infection, fluorescence returned to the baseline level of 50 U. Staurosporine was also able to inhibit phosphorylation of tyrosine proteins. However, the PKC inhibitor calphostin C did not prevent tyrosine phosphorylation of proteins and fluorescence was increased nearly threefold (P < 0.05). Similar results were observed during the entry of L. pneumophila IDL-2A into monocytes (data not shown).
FIG. 5.
Immunofluorescent staining of phosphotyrosine proteins present during L. pneumophila entry into human monocytes. (A) Observation was done by confocal microscopy. (B) Quantitation was done with Meridian software. Infected cells were exposed to L. pneumophila for 15 min. Cells were pretreated with genistein 15 min prior to infection or with tyrphostin, staurosporine, or calphostin C 60 min prior to infection. Cells were stained with antiphosphotyrosine antibody 4G10 and with a tetramethyl rhodamine isocyanate-conjugated goat anti-mouse antibody. Results are reported as means ± the standard errors of the means for three experiments.
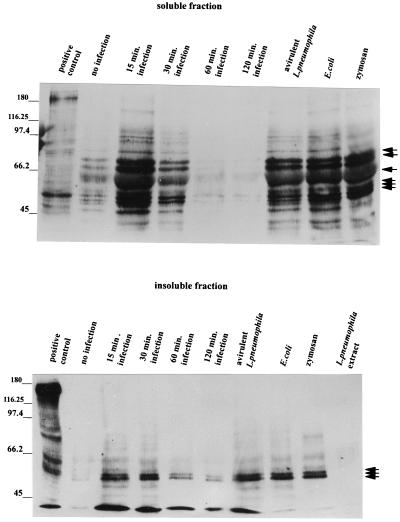
The detection of phosphotyrosine proteins was more specifically examined by immunoblot analysis of soluble and insoluble protein fractions from infected monocytes. The latter contains host cytoskeletal and nuclear proteins, as well as bacterial proteins (39, 53), while the former contains host membrane and cytosolic proteins (39). After 15 min of L. pneumophila infection, a pattern of tyrosine-phosphorylated proteins appeared in both fractions, as shown in Fig. 6. Many phosphotyrosine protein bands appeared in the soluble fraction, and there were six intensely stained protein bands at 75, 71, 59, 56, 53, and 52 kDa. The insoluble fraction yielded fewer bands, but the bands that were visible also appeared in the soluble fraction. The 52-, 53-, and 56-kDa bands consistently appeared in the insoluble fraction. The intensity of tyrosine phosphorylation reached a peak at 15 min of infection, began to decrease following 30 min, and returned to baseline levels following 60 min of infection.
FIG. 6.
Detection of phosphotyrosine proteins in L. pneumophila-infected cells. Cell lysates were prepared, resolved by SDS–7.5% PAGE, and immunoblotted with mouse antiphosphotyrosine antibody 4G10. The secondary antibody used was a goat anti-mouse IgG-peroxidase conjugate. The positive control was A431 cells which had been stimulated with epidermal growth factor for 5 min to induce phosphorylation of the receptor, as well as other cellular proteins. The values on the left are molecular sizes in kilodaltons. The arrows on the right indicate intensely stained protein bands.
E. coli was included as a positive control in the immunoblot studies. Because E. coli opsonizes complement better than L. pneumophila does (44), it may be more readily phagocytosed by monocytes. However, in comparing E. coli-infected cells with L. pneumophila-infected cells, no significant difference in the pattern of tyrosine-phosphorylated proteins was observed (Fig. 6). Avirulent L. pneumophila cells are also capable of internalization into monocytes. Nevertheless, no differences in the tyrosine phosphorylation pattern were found between virulent and avirulent L. pneumophila cells during entry into monocytes (Fig. 6). In addition, the tyrosine phosphorylation pattern of zymosan uptake was examined, since it has been reported to induce proteins similar in size in monocytes (53). Following 15 min of infection, similar patterns of phosphorylation in the insoluble and soluble fractions were observed (Fig. 6). In the absence of monocytes, bacterial tyrosine-phosphorylated proteins were not detected (Fig. 6).
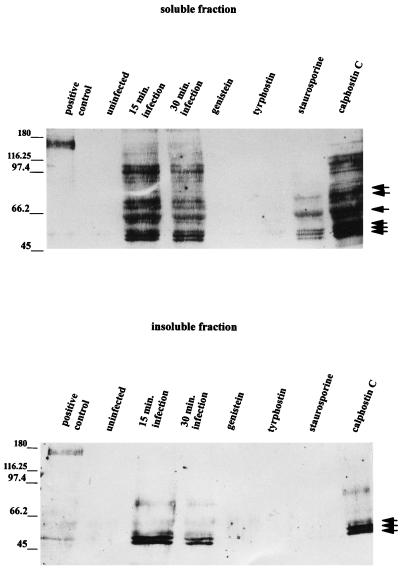
To further substantiate that specific tyrosine phosphorylation was induced by L. pneumophila interaction with monocytes, the cells were pretreated with TPK inhibitors prior to infection. Genistein and tyrphostin resulted in complete inhibition of tyrosine-phosphorylated bands (Fig. 7). Staurosporine also suppressed tyrosine phosphorylation, but calphostin C, a specific PKC inhibitor, did not (Fig. 7). These results are similar to those of the confocal-microscopy experiments.
FIG. 7.
Effects of kinase inhibitors on L. pneumophila-induced tyrosine phosphorylation in monocytes. Cell lysates were prepared and resolved by SDS–7.5% PAGE and immunoblotted with mouse antiphosphotyrosine antibody 4G10. The secondary antibody used was a goat anti-mouse IgG-peroxidase conjugate. The positive control was A431 cells which had been stimulated with epidermal growth factor for 5 min to induce phosphorylation of the receptor, as well as other cellular proteins. The values on the left are molecular sizes in kilodaltons. The arrows on the right indicate intensely stained protein bands.
Actin polymerization is associated with L. pneumophila internalization into monocytes.
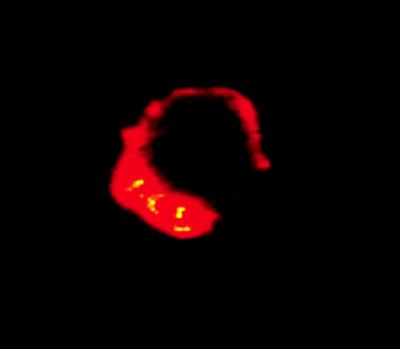
To correlate actin polymerization with the phagocytic entry of L. pneumophila, actin was immunofluorescently labeled with rhodamine phalloidin and L. pneumophila was directly labeled with FITC (Fig. 8). When monocytes were infected with bacteria for 15 min, there was a 10-fold increase in cellular actin compared with that in noninfected cells (P < 0.0001). However, when cells were pretreated with genistein, tyrphostin, staurosporine, or calphostin C prior to infection, the fluorescence intensity of actin remained at baseline levels, indicating a role for TPK and PKC signaling in the event of actin polymerization. The same results were observed when the entry of avirulent L. pneumophila was examined (data not shown). By direct labeling of L. pneumophila with FITC and cellular actin with rhodamine phalloidin, localization of actin could be visualized at sites of L. pneumophila entry at the cell periphery (Fig. 9).
FIG. 8.
Immunofluorescent staining of F-actin present during L. pneumophila entry into human monocytes. (A) Observation was done by confocal microscopy. (B) Quantitation was done with Meridian software. Infected cells were exposed to L. pneumophila for 15 min. Cells were pretreated with genistein 15 min prior to infection or with tyrphostin, staurosporine, or calphostin C 60 min prior to infection. Cells were stained with rhodamine phalloidin. Results are reported as means ± the standard errors of the means for three experiments.
FIG. 9.
Localization of actin at the site of L. pneumophila entry into monocytes. L. pneumophila cells were directly labeled with FITC prior to infection. Cells were infected with bacteria for 15 min, and actin was stained with rhodamine phalloidin. A sliced section of the infected cells were viewed by confocal microscopy under a 100× objective.
DISCUSSION
This investigation has demonstrated that TPK and PKC phosphorylation signals are involved in actin polymerization during phagocytic uptake of opsonized L. pneumophila into human monocytes. This conclusion is based on: (i) inhibition studies which showed that L. pneumophila entry can be suppressed by TPK and PKC inhibitors, (ii) confocal-microscopic studies that allowed active visualization of fluorescently labeled F-actin and phosphotyrosine proteins, and (iii) immunoblot analysis of phosphotyrosine proteins. Further investigation of the role of TPK indicated that virulent L. pneumophila uptake leads to the induction of a specific pattern of tyrosine-phosphorylated proteins. Avirulent L. pneumophila, E. coli, and zymosan entry into human monocytes also induced a pattern of tyrosine phosphorylation similar to that observed with virulent L. pneumophila. These data suggest that tyrosine phosphorylation, and perhaps PKC phosphorylation, of particular proteins is observed during CR3-mediated uptake into human monocytes and is unrelated to the ultimate virulence mechanism associated with intracellular replication of this pathogen.
In uptake inhibition studies, the TPK inhibitor tyrphostin effectively suppressed L. pneumophila entry at a concentration of 400 μM while the TPK inhibitor genistein failed to completely suppress uptake at this maximum usable concentration. These two TPK inhibitors have different mechanisms of action and thus may not be expected to demonstrate the same dose-response curves. The PKC inhibitor calphostin C suppressed bacterial uptake by only 54% ± 10%, while staurosporine suppressed bacterial entry by up to 79% ± 4.7%. Staurosporine is a known PKC inhibitor but has a broad range of inhibition (38, 46), which includes tyrosine kinases. Therefore, the greater inhibitory activity of staurosporine observed is probably due to its ability to suppress both TPK phosphorylation and PKC phosphorylation. Furthermore, the inhibitory effects of staurosporine on TPK activity were demonstrated to significantly reduce the levels of tyrosine-phosphorylated proteins normally observed during L. pneumophila infection of monocytes in immunofluorescence and immunoblotting studies. However, calphostin C specifically inhibits PKC activity only and was not observed to inhibit tyrosine phosphorylation in these experiments.
The ability of PKC and TPK inhibitors to suppress L. pneumophila entry suggests a role for both of these signals in phagocytosis by monocytes. The role of multiple signals for phagocytic uptake is not unusual and can be observed in Fcγ receptor-mediated phagocytosis, which requires PKC and calcium (4, 9, 30, 43). However complement receptors, of which CR3 is one, have been reported to generate one signal (43). The present study suggests that CR3-mediated uptake of L. pneumophila into monocytes may require at least two signals, both TPK phosphorylation and PKC phosphorylation. It is also possible that PKC and TPK activities are linked together in a signal cascade for the activation of phagocytosis. It should be emphasized that PKC and TPK inhibitors did not suppress the ability of L. pneumophila to replicate intracellularly, suggesting that protein phosphorylation is not required for bacterial replication once L. pneumophila has entered the cell.
Further investigation of the role of tyrosine phosphorylation was done by quantitating tyrosine-phosphorylated proteins detected by immunoblotting and immunofluorescence assays. These studies indicated a twofold increase in the phosphorylated product (averaged over the entire cell) in cells infected with L. pneumophila versus noninfected monocytes. Pretreatment of cells with genistein, tyrphostin, or staurosporine prior to infection lowered the amount of detectable phosphotyrosine proteins to baseline levels, confirming the specificity of these inhibitors for TPK activity. Other investigators have demonstrated similar roles for TPKs in the entry of enteropathogenic E. coli (37), M. penetrans (5), S. flexneri, (14), and C. trachomatis (8). However, TPK inhibitors were not observed to inhibit invasion by S. typhimurium (39).
Accumulation of F-actin was also correlated with L. pneumophila entry and was localized at the cell periphery near the site of bacterial entry. These observations are similar to those made with enteropathogenic E. coli (17, 29), S. flexneri (1, 11, 12), S. typhimurium (18), and C. trachomatis (42). L. pneumophila-infected cells showed a 10-fold increase in F-actin (averaged over the entire cell). However, pretreatment of cells with both PKC and TPK inhibitors returned detectable actin to baseline levels. Although calphostin C inhibited bacterial uptake by only 50% ± 10%, it was found to inhibit actin polymerization by 80% ± 12.5%. This apparent discrepancy could be due to the fact that different methods were used to measure bacterial uptake versus actin polymerization. The latter assay was done with cells in suspension, while the former was done with adherent cells. Another possible explanation is that some of the actin induced during these experiments may be related to other cellular activities and not directly involved in L. pneumophila uptake. Overall, these results confirm that internalization of opsonized L. pneumophila induces TPK and PKC to stimulate cytoskeletal reorganization.
To characterize tyrosine-phosphorylated protein patterns induced during L. pneumophila entry into monocytes, SDS-PAGE and immunoblot analysis were used to detect six major bands (75, 71, 59, 56, 53, and 52 kDa) with numerous minor bands in these infected cells. The latter three proteins are thought to be cytoskeleton associated due to their localization with the insoluble fraction in immunoblot analysis. The two bands that were detected at 56 and 53 kDa have molecular weights similar to those of 56lyn, a component of the p-53 Src tyrosine kinase. This Lyn kinase often appears as two bands at 56 and 53 kDa due to mRNA splicing. Others have observed the appearance of p53-56lyn during zymosan-triggered phagocytosis into human monocytes and have suggested that p53-56lyn may provide the molecular link between integrin receptors and the cytoskeleton (53). While our preliminary data have suggested a reaction of the 53- and 56-kDa protein bands with a monoclonal antibody to Lyn kinase, the identity of these proteins in our experiments has not been verified. We are currently investigating the identification of these and other proteins phosphorylated during Legionella uptake. Kinases have not been reported to exist in L. pneumophila, although L. micdadei is known to possess some kinase activity (6, 40). Thus, it is still not clear whether bacterial kinases are involved in the observed phosphorylations.
To determine if induced phosphotyrosine proteins were host or bacterial, a lane of L. pneumophila extract was included in the immunoblot. No protein bands similar in molecular weight to bands observed in bacterium-infected monocytes were detected. However, it could be argued that activation of tyrosine phosphorylation of bacterial proteins requires L. pneumophila interaction with the monocyte. To further resolve this question, the tyrosine-phosphorylated protein pattern of zymosan particle entry into monocytes was examined. Zymosan is a carbohydrate-rich cell wall preparation derived from Saccharomyces cerevisiae that interacts with the CR3 receptor for phagocytic uptake. This particle was found to induce a pattern of phosphorylation similar to that induced during L. pneumophila and E. coli entry. Taken together, these data strongly suggest that tyrosine-phosphorylated proteins observed during L. pneumophila entry are host and not bacterial in origin.
Opsonized, avirulent L. pneumophila cells are also capable of phagocytic entry into monocytes (24). However, there may be a difference in the tyrosine phosphorylation pattern (compared to virulent strains) because of the early divergence of their intracellular pathways. Specifically, phagosomes containing virulent strains do not fuse with lysosomes, in contrast to phagosomes containing avirulent strains (24, 25). A phosphorylation signal required at the onset of invasion may reorganize cytoskeletal components and provide mechanical means for phagolysosomal fusion. The lack of a signal to induce this event may lead to escape of the L. pneumophila phagosome from the normal endocytic route. Nevertheless, this study did not find any differences in the tyrosine phosphorylation pattern or in band intensity between avirulent and virulent L. pneumophila strains. In addition, uptake of opsonized E. coli and zymosan also generated similar tyrosine-phosphorylated proteins. These results indicate that TPK phosphorylation of specific proteins may be universal for CR-3-mediated phagocytosis into monocytes and that the mechanism is not directly virulence associated.
The fact that the present study did not find a virulence-associated relationship between tyrosine phosphorylation and L. pneumophila entry into monocytes does not eliminate the possibility that the presence or absence of a biochemical signal(s) leads to alteration of the pathway of an L. pneumophila-containing phagosome. First, a signal which alters the endocytic pathway of the bacterial phagosome may occur during binding (as opposed to uptake) of L. pneumophila into monocytes. One study determined that phosphorylation of a 76-kDa protein was induced upon virulent, but not avirulent, L. pneumophila binding to murine peritoneal macrophages (52). Recently, Venkataraman et al. (47) have reported that the attachment and invasion of the protozoan host Hartmanella vermiformis by L. pneumophila is associated with a dephosphorylation of multiple host cell proteins, including the potential lectin receptor used by L. pneumophila to invade the protozoa. Second, it is possible that the signal occurs after L. pneumophila has entered the monocyte and may be promoted by a bacterial protein. Third, other kinase or calcium signals that were not examined in this study may play a role in the divergence of phagolysosome fusion. Calcium has been associated with phagocytic events, but an increase in calcium is not always required to induce such an event (15, 26, 28, 36). The necessity for calcium in phagolysosome fusion has been demonstrated in neutrophils (26), and zymosan-triggered phagocytosis into monocytes was shown to associate with intracellular rises in calcium (28). Work in this laboratory has demonstrated that a virulent strain of L. pneumophila, compared to an E. coli control, suppressed calcium during entry into neutrophils (10). Current studies in this laboratory are focused on the possible role of calcium in the intracellular trafficking of the L. pneumophila phagosome.
Finally, a receptor that has not been identified may play a role in the activation of a signal that alters the intracellular pathway of L. pneumophila. The possibility that another receptor exists is suggested by evidence that L. pneumophila is still capable of nonopsonic entry and replication in macrophagelike cells in the absence of serum (34). A similar receptor may be responsible for the uptake and replication of L. pneumophila in a wide variety of nonprofessional phagocytes, such as HeLa cells (13), MRC-5 human embryonic lung fibroblasts (13, 50), and human endothelial cells (54). This may be analogous to the Gal/GalNAc lectin recently identified as a potential receptor for attachment to and invasion of the protozoan H. vermiformis (47). Future studies examining the protein phosphorylation patterns during nonopsonic uptake of L. pneumophila may provide evidence for the existence of such a virulence-associated signal.
ACKNOWLEDGMENTS
We thank Jon Klein for use of the confocal microscope and related equipment. In addition, we acknowledge Jeanine Mathiessen for her advice and aid in utilizing the confocal system.
REFERENCES
- 1.Adam T, Arpin M, Prevost M C, Gounon P, Sansonetti P J. Cytoskeletal rearrangements and the functional role of t-plastin during entry of Shigella flexneri into HeLa cells. J Cell Biol. 1995;129:367–381. doi: 10.1083/jcb.129.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- 3.Allen L H, Aderem A. A role for MARCKS, the isozyme of protein kinase C and myosin I, in zymosan phagocytosis by macrophages. J Exp Med. 1995;182:829–840. doi: 10.1084/jem.182.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson T, Fallman M, Lew D P, Stendahl O. Does protein kinase C control receptor-mediated phagocytosis in human neutrophils? FEBS Lett. 1988;239:371–375. doi: 10.1016/0014-5793(88)80954-2. [DOI] [PubMed] [Google Scholar]
- 5.Andreev J, Borovsky Z, Rosenshine I, Rottem S. Invasion of HeLa cells by Mycoplasma penetrans and the induction of tyrosine phosphorylation of a 145-kDa host cell protein. FEMS Microbiol Lett. 1995;132:189–194. doi: 10.1111/j.1574-6968.1995.tb07832.x. [DOI] [PubMed] [Google Scholar]
- 6.Bangsborg J M, Cianciotto N P, Hindersson P. Nucleotide sequence analysis of the Legionella micdadei mip gene, encoding a 30-kilodalton analog of the Legionella pneumophila Mip protein. Infect Immun. 1991;59:3836–3840. doi: 10.1128/iai.59.10.3836-3840.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellinger-Kawahara C G, Horwitz M A. Complement component C3 fixes selectively to major outer membrane protein (momp) of L. pneumophila and mediates phagocytosis of liposome-MOMP complexes by human monocytes. J Exp Med. 1987;172:1201–1210. doi: 10.1084/jem.172.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birkelund S, Johnsen H, Christiansen G. Chlamydia trachomatis serovar L2 induces protein tyrosine phosphorylation during uptake by HeLa cells. Infect Immun. 1994;62:4900–4908. doi: 10.1128/iai.62.11.4900-4908.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown E J. Phagocytosis. Bioessays. 1995;17:109–117. doi: 10.1002/bies.950170206. [DOI] [PubMed] [Google Scholar]
- 10.Buster B L, Summersgill J T, Miller R D. Abstracts of the 91st General Meeting of the American Society for Microbiology 1991. Washington, D.C: American Society for Microbiology; 1991. Intracellular free calcium responses in human neutrophils infected with Legionella pneumophila, abstr. B-72; p. 37. [Google Scholar]
- 11.Clerc P, Sansonetti P J. Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect Immun. 1987;55:2681–2688. doi: 10.1128/iai.55.11.2681-2688.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clerc P, Sansonetti P J. Entry of Shigella flexneri into epithelial cells: evidence for induced phagocytosis involving actin and myosin. In: Rouseet B F, editor. Structure and functions of the cytoskeleton. New York, N.Y: John Libbey Eurotext; 1988. p. 171. [Google Scholar]
- 13.Daisy J A, Benson C E, McKitrick J, Griedman H M. Intracellular replication of Legionella pneumophila. J Infect Dis. 1981;143:460–464. doi: 10.1093/infdis/143.3.460. [DOI] [PubMed] [Google Scholar]
- 14.Dehio C, Prevost M, Sansonetti P J. Invasion of epithelial cells by Shigella flexneri induces tyrosine phosphorylation of cortactin by a pp60c-src-mediated signaling pathway. EMBO J. 1995;11:2471–2482. doi: 10.1002/j.1460-2075.1995.tb07244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Vigilio F, Meyer B C, Greenberg S, Silverstein S C. Fc receptor-mediated phagocytosis occurs in macrophages at exceedingly low cytosolic Ca2+ levels. J Cell Biol. 1988;106:657–688. doi: 10.1083/jcb.106.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elliot J A, Winn W C., Jr Treatment of alveolar macrophages with cytochalasin D inhibits uptake and subsequent growth of Legionella pneumophila. Infect Immun. 1986;51:31–36. doi: 10.1128/iai.51.1.31-36.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finlay B B, Rosenshine I, Donnenberg M S, Kaper J B. Cytoskeletal composition of attaching and effacing lesions associated with enteropathogenic Escherichia coli adherence to HeLa cells. Infect Immun. 1992;60:2541–2543. doi: 10.1128/iai.60.6.2541-2543.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finlay B B, Ruschkowski S. Cytoskeletal rearrangements accompanying Salmonella entry into epithelial cells. J Cell Sci. 1991;99:283–296. doi: 10.1242/jcs.99.2.283. [DOI] [PubMed] [Google Scholar]
- 19.Gazit A, Yaish P, Gilon C, Levitzki A. Tyrphostins. I. synthesis and biological activity of protein kinase inhibitors. J Med Chem. 1989;32:2344–2352. doi: 10.1021/jm00130a020. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg S, Chang P, Silverstein S C. Tyrosine phosphorylation is required for Fc receptor-mediated phagocytosis in mouse macrophages. J Exp Med. 1993;177:529–534. doi: 10.1084/jem.177.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gresham H D, Zheleznyak A, Mormol J S, Brown E J. Studies on the molecular mechanism of human neutrophil Fc receptor-mediated phagocytosis: evidence that a distinct pathway for activation of the respiratory burst results in reactive oxygen metabolite-dependent amplification of infection. J Biol Chem. 1990;265:7819–7826. [PubMed] [Google Scholar]
- 22.Horwitz M A, Silverstein S C. Legionnaires’ disease bacterium (Legionella pneumophila) multiplies intracellularly in human monocytes. J Clin Invest. 1980;66:441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horwitz M A. Interaction between macrophages and Legionella pneumophila. Curr Top Microbiol Immunol. 1992;181:265–278. doi: 10.1007/978-3-642-77377-8_10. [DOI] [PubMed] [Google Scholar]
- 24.Horwitz M A. Characterization of avirulent mutant Legionella pneumophila that survive but do not multiply within human monocytes. J Exp Med. 1987;166:1310–1328. doi: 10.1084/jem.166.5.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horwitz M A. The Legionnaires’ disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983;158:2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaconi M E E, Lew D P, Carpentier J L, Magnusson K E, Sjogren M, Stendahl O. Cytosolic free calcium elevation mediates the phagosome-lysosome fusion during phagocytosis in human neutrophils. J Cell Biol. 1990;110:1555–1564. doi: 10.1083/jcb.110.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kenny B, DeVinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence to mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 28.Kim E, Enelow R I, Sullivan G W, Mandell G L. Regional and generalized changes in cytosolic free calcium in monocytes during phagocytosis. Infect Immun. 1992;60:1244–1248. doi: 10.1128/iai.60.3.1244-1248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Odin J A, Edberg J C, Painter J C, Kimberly R P, Unkeless J C. Regulation of phagocytosis and [Ca2+] flux by distinct regions of an Fc receptor. Science. 1991;254:1785–1788. doi: 10.1126/science.1837175. [DOI] [PubMed] [Google Scholar]
- 31.Ogawara H, Akiyama T, Ishiba J, Watanbe S, Suzuki K. A specific inhibitor of a tyrosine kinase from Pseudomonas. J Antibiot. 1986;39:606. doi: 10.7164/antibiotics.39.606. [DOI] [PubMed] [Google Scholar]
- 32.Ohta Y, Stossel T P, Hartwig J H. Ligand-sensitive binding of actin-binding protein to immunoglobulin G Fc receptor (FcγRI) Cell. 1991;67:275–282. doi: 10.1016/0092-8674(91)90179-3. [DOI] [PubMed] [Google Scholar]
- 33.Payne N R, Horwitz M A. Phagocytosis of Legionella pneumophila is mediated by human monocyte complement receptors. J Exp Med. 1987;166:1377–1389. doi: 10.1084/jem.166.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearlman E, Jiwa A J, Engleberg N C, Eisenstein B I. Growth of L. pneumophila in a human-like (U-937) cell line. Microbiol Pathol. 1988;5:87–95. doi: 10.1016/0882-4010(88)90011-3. [DOI] [PubMed] [Google Scholar]
- 35.Petty H R, Todd R F., III Receptor-receptor interactions of complement receptor type 3 in neutrophil membranes. J Leukocyte Biol. 1993;54:492–494. doi: 10.1002/jlb.54.5.492. [DOI] [PubMed] [Google Scholar]
- 36.Rosales C, Brown E J. Signal transduction by neutrophil immunoglobulin G Fc receptors. Dissociation of intracytoplasmic calcium concentration rise from inositol 1,4,5-trisphosphate. J Biol Chem. 1992;267:5267–5271. [PubMed] [Google Scholar]
- 37.Rosenshine I, Donnenberg M S, Kaper J B, Finlay B B. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992;11:3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roshenshine I, Duronio V, Ruschkowski S, Finlay B B. Inhibitors of cytoskeletal function and signal transduction to study bacterial invasion. Methods Enzymol. 1994;236:467–476. doi: 10.1016/0076-6879(94)36035-9. [DOI] [PubMed] [Google Scholar]
- 39.Rosenshine I, Ruschkowski S, Foubister V, Finlay B B. Salmonella typhimurium invasion of epithelial cells: role of induced host cell tyrosine protein phosphorylation. Infect Immun. 1994;62:4969–4974. doi: 10.1128/iai.62.11.4969-4974.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saha A K, Dowling J N, Mukhopadhyay N K, Glew R H. Legionella micdadei protein kinase catalyzes phosphorylation of tubulin and phosphatidylinositol. J Bacteriol. 1989;171:5103–5110. doi: 10.1128/jb.171.9.5103-5110.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanford J P. Legionionnaires’ disease—the first thousand days. N Engl J Med. 1979;300:654–656. doi: 10.1056/NEJM197903223001205. [DOI] [PubMed] [Google Scholar]
- 42.Schramm N, Wyrick P B. Cytoskeletal requirements in Chlamydia trachomatis infection of host cells. Infect Immun. 1995;63:324–332. doi: 10.1128/iai.63.1.324-332.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silverstein S C, Greenberg S, DiVirgilo F, Steinberg T H. Phagocytosis. In: Paul W E, editor. Fundamental immunology. New York, N.Y: Raven Press; 1989. pp. 703–720. [Google Scholar]
- 44.Summersgill J T, Raff M J, Miller R D. Interaction of virulent and avirulent Legionella pneumophila with human monocytes. J Leukocyte Biol. 1990;47:31–38. doi: 10.1002/jlb.47.1.31. [DOI] [PubMed] [Google Scholar]
- 45.Swartz M N. Clinical aspects of Legionnaires’ disease. Ann Intern Med. 1979;90:492–495. doi: 10.7326/0003-4819-90-4-492. [DOI] [PubMed] [Google Scholar]
- 46.Tamaoki T, Nakano H. Potent and specific inhibitors of protein kinase C of microbial origin. Bio/Technology. 1990;8:732–735. doi: 10.1038/nbt0890-732. [DOI] [PubMed] [Google Scholar]
- 47.Venkataraman C, Haack B J, Bondada S, Abu Kwaik Y. Identification of a gal/galNAc lectin in the protozoan Hartmannella vermiformis as a potential receptor for attachment and invasion of the Legionnaires’ disease bacterium. J Exp Med. 1997;186:537–547. doi: 10.1084/jem.186.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang N, Butler J P, Inberg D E. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 49.Whitehead J E M. Legionnellosis—doubts and certainties. J Infect. 1979;1:213–220. [Google Scholar]
- 50.Wong M C, Ewing E P, Jr, Callaway C S, Peacock W L., Jr Intracellular multiplication of Legionella pneumophila in cultured human embryonic lung fibroblasts. Infect Immun. 1990;28:1014–1018. doi: 10.1128/iai.28.3.1014-1018.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yaish P, Gazit A, Gilon C, Levitzki A. Blocking of EGF-dependent cell proliferation by EGF receptor kinase inhibitors. Science. 1988;242:933–935. doi: 10.1126/science.3263702. [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto T, Klein T W, Shinomiya H, Nakano M, Friedman H. Infection of macrophages with Legionella pneumophila induces phosphorylation of a 76-kilodalton protein. Infect Immun. 1992;60:3452–3455. doi: 10.1128/iai.60.8.3452-3455.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zaffran Y, Escallier J C, Ruta S, Capo C, Mege J L. Zymosan-triggered association of tyrosine phosphoproteins and lyn kinase with cytoskeleton in human monocytes. J Immunol. 1995;154:3488–3497. [PubMed] [Google Scholar]
- 54.Zhang Y, Powell L, Tse P, Justus D, Summersgill J, Ramirez J, Miller R D. Abstracts of the 94th General Meeting of the American Society for Microbiology 1994. Washington, D.C: American Society for Microbiology; 1994. Characterization of Legionella pneumophila replication in human pulmonary and umbilical endothelial cells, abstr. B-364; p. 93. [Google Scholar]
- 55.Zheleznyak A, Brown E J. Immunoglobulin-mediated phagocytosis by human monocytes requires protein kinase C activation. J Biol Chem. 1992;267:12042–12048. [PubMed] [Google Scholar]
- 56.Zhou M, Todd III R F, Petty H R. Detection of transmembrane linkages between immunoglobulin or complement receptors and the neutrophil’s cortical microfilaments by resonance energy transfer microscopy. J Mol Biol. 1991;218:263–268. doi: 10.1016/0022-2836(91)90709-f. [DOI] [PubMed] [Google Scholar]