Abstract
Anthocyanins are polyphenolic ring-based flavonoids, and are widespread in fruits and vegetables of red-blue color. Epidemiological investigations and animal experiments have indicated that anthocyanins may contribute to cancer chemoprevention. The studies on the mechanism have been done recently at molecular level. This review summarizes current molecular bases for anthocyanidins on several key steps involved in cancer chemoprevention: (i) inhibition of anthocyanidins in cell transformation through targeting mitogen-activated protein kinase (MAPK) pathway and activator protein 1 (AP-1) factor; (ii) suppression of anthocyanidins in inflammation and carcinogenesis through targeting nuclear factor kappa B (NF-κB) pathway and cyclooxygenase 2 (COX-2) gene; (iii) apoptotic induction of cancer cells by anthocyanidins through reactive oxygen species (ROS) / c-Jun NH2-terminal kinase (JNK)-mediated caspase activation. These data provide a first molecular view of anthocyanidins contributing to cancer chemoprevention.
INTRODUCTION
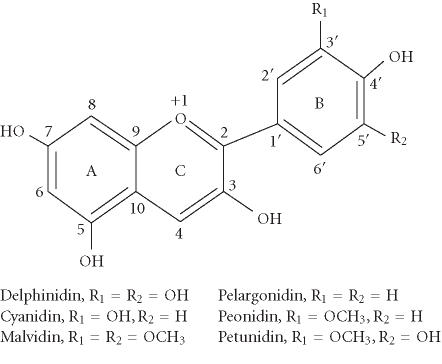
Anthocyanins are naturally occurring polyphenolic compounds that give the intense color to many fruits and vegetables such as berries, red grapes, purple sweet potato, and red cabbages [1, 2]. In contrast to the other flavonoids, anthocyanins carry a positive charge in the central ring structure and are thus cations. In plants, they are present exclusively as glycosidic compounds. The number and nature of the different attached sugar moieties are responsible for the high number of anthocyanins, more than 500 compounds [3]. The aglycone (named anthocyanidin) is a diphenylpropane-based polyphenolic ring structure, and is limited to a few structure variants including delphinidin, cyanidin, petunidin, pelargonidin, peonidin, and malvidin (Figure 1), that represent the aglycones of most anthocyanins in plants.
Figure 1.
Basic chemical structures of the major anthocyanidins.
Based on food composition data, we consume considerable amounts of anthocyanins from crops, fruits, and vegetable-based diets [4] although the range is from several milligrams to several hundred milligrams, depending on the nutrition customs. An enhanced intake of anthocyanin is now increasing because extracts with higher anthocyanin contents from bilberry or elderberry are commercially available. Epidemiological investigations have indicated that moderate consumption of anthocyanins through the intake of products such as red wine [5] or bilberry extract [6] is associated with a lower risk of coronary heart disease. Recent studies indicated that anthocyanins have strong free radical scavenging and antioxidant activities [7, 8, 9, 10, 11, 12, 13], and show inhibitory effects on the growth of some cancer cells [14, 15, 16, 17, 18]. Animal experiments showed that oral intake of anthocyanins from purple sweet potato (Ipomoea batatas L.) and red cabbage (Brassica oleracea L.) suppressed rat colon carcinogenesis induced by 1,2-dimethylhydrazine and 2-amino-1-methyl-6-phenylimidazo- [4,5-b] pyridine [19]. In addition, anthocyanins can be directly absorbed and distributed to the blood in human and rats after consumption of dietary anthocyanins [18, 19, 20, 21, 22, 23, 24]. These facts suggest that anthocyanins may play a role in cancer chemoprevention. Thus, mechanisms behind chemopreventive effects of anthocyanins need to be considered at molecular level.
ANTICARCINOGENESIS THROUGH TARGETING MAPK PATHWAY AND AP-1 FACTOR
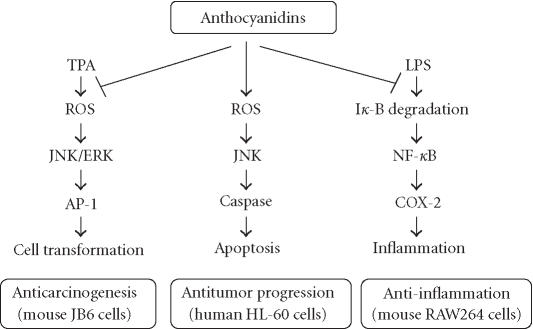
AP-1 is a transcription factor and has been shown to play a critical role in promoting carcinogenesis [25, 26]. In mouse epidermal cell line JB6 (+), tumor promoters such as 12-O-tetradecanoylphorbol-13-acetate (TPA), epidermal growth factor (EGF), and tumor necrosis factor (TNF) alpha can induce AP-1 activity and neoplastic transformation by activating MAPK including extracellular signal-regulated kinase (ERK), JNK, or p38 kinase [27, 28]. Delphinidin, cyanidin, and petunidin have been shown to inhibit TPA-induced AP-1 transcriptional activity and cell transformation in JB6 cells [29]. Structure-activity studies indicated that the ortho-dihydroxyphenyl structure on the B-ring of anthocyanidins seems essential for the inhibitory action because pelargonidin, peonidin, and malvidin, having no such ortho-dihydroxyphenyl structure, failed to show the inhibitory effects in both AP-1 activity and cell transformation. The results from signal transduction analysis indicated that delphinidin blocked ERK phosphorylation at early times and JNK phosphorylation at later times, but not p38 phosphorylation at any time [29]. Moreover, delphinidin blocked the phosphorylation of MAPK/ERK kinase (MEK, an ERK kinase), SAPK/ERK kinase (SEK, a JNK kinase), and c-Jun (a phosphorylation target of ERK and JNK). The data suggest that the inhibition of TPA-induced AP-1 activity and cell transformation by delphinidin involves the blockage of ERK and JNK signaling cascades (Figure 2). Furthermore, a greater inhibition was observed in combinations of superoxide dismutase (SOD) with anthocyanidins that have the ortho-dihydroxyphenyl structure on the B-ring. Multiplicative model analysis suggested that this greater inhibition between SOD and delphinidin is synergistic, not additive [29]. Thus, the inhibitory effects of anthocyanidins on AP-1 activation and cell transformation would be due in part to their potent scavenging activity for superoxide radicals and in part to MAPK blockage. SOD has been shown to selectively inhibit the TPA-induced activation of protein kinase Epsilon and to prevent subsequent activation of JNK2 in response to TPA, thereby delaying AP-1 activation and inhibiting mouse skin tumor promotion [30]. Thus, the signaling pathways blocked by delphinidin or SOD may differ in part although both are considered to be important in the cancer prevention activity of anthocyanidins.
Figure 2.
A schematic molecular view of cancer chemoprevention by anthocyanidins. Anthocyanidins may contribute to cancer chemoprevention through targeting three different signal transduction pathways and downstream genes. Abbreviations: AP-1, activator protein-1; ERK, extracellular signal-regulated kinase; JNK, c-Jun NH2-terminal kinase; LPS, lipopolysaccharide; NF-κB, nuclear factor κB; ROS, reactive oxygen species; TPA, 12-O-tetradecanoylphorbol-13-acetate.
ANTI-INFLAMMATION THROUGH TARGETING NF-κB PATHWAY AND COX-2 GENE
Cyclooxygenase (COX) is the rate-limiting enzyme for synthesis of dienoic eicosanoids such as prostaglandin (PG) E2. COX exists in three isoforms [31, 32]. COX-1 is expressed constitutively in many types of cells and is responsible for the production of PGs under physiological conditions. COX-3 is a COX-1 variant and is mainly expressed in cerebral cortex. Analgesic/antipyretic drugs such as acetaminophen, phenacetin, antipyrine, and dipyrone can selectively inhibit this enzyme. Thus, inhibition of COX-3 could represent a primary central mechanism by which these drugs decrease pain and possibly fever [31]. COX-2 is induced by proinflammatory stimuli, including mitogens, cytokines, and bacterial lipopolysaccharide (LPS) in cells in vitro and in inflamed sites in vivo [33]. Data indicate that COX-2 is involved in many inflammatory processes and induced in various carcinomas, suggesting that COX-2 plays a key role in tumorigenesis [34]. Interestingly, some antioxidants with chemopreventive effects inhibit the expression of COX-2 by interfering with the signaling mechanisms that regulate the COX-2 gene [35]. Wang et al [36] found that anthocyanins and their aglycone, cyanidin, from tart cherries could inhibit the activities of COX-1 and COX-2. Seeram et al [37] found that cyanidin showed superior inhibition of the cyclooxygenase activity in vitro. In our laboratory, we used mouse macrophage cell line RAW264 to demonstrate the molecular mechanism of anthocyanins in the inhibition of the COX-2 gene. We found that anthocyanin extracts from bilberry or purified delphinidin inhibited LPS-induced COX-2 expression at protein and transcriptional levels. Studies on signal pathway indicated that delphinidin at least blocked LPS-induced IκB degradation and then suppressed NF-κB activation and COX-2 gene expression (Hou et al, unpublished data). These data demonstrated that the blockage of NF-κB signaling pathway is involved in the inhibition of COX-2 gene expression by anthocyanins (Figure 2).
ANTITUMOR PROGRESSION THROUGH OXIDATIVE/JNK-MEDIATED APOPTOSIS
Apoptosis has been reported to play an important role in elimination of seriously damaged cells or tumor cells by chemopreventive agents [38, 39]. The cells that have undergone apoptosis have typically shown chromatin condensation and DNA fragmentation [40]. They are rapidly recognized by macrophages before cell lysis, and then can be removed without inducing inflammation [38, 41]. Therefore, apoptosis-inducing agents are expected to be ideal anticancer drugs of which human promyelocytic leukemia cell line (HL-60) provides a valid model for testing antileukemic or general antitumoral compounds [42]. Delphinidin, cyanidin, and petunidin induced apoptosis of HL-60 cells detected by morphological changes and by DNA fragmentation, whereas pelargonidin, peonidin, and malvidin showed no induction of apoptosis [43]. The anthocyanidin glycosides (anthocyanins) extracted from bilberry such as delphinidin glycosides and cyanidin glycosides also induced the apoptosis in HL-60 cells [44]. Structure-activity studies indicated that the potency of apoptosis induction of anthocyanidins is associated with the number of hydroxyl groups at the B-ring, and the ortho-dihydroxyphenyl structure at the B-ring appears essential for apoptosis actions [43]. It is noteworthy that anthocyanidins increased the levels of hydrogen peroxide in HL-60 cells with a structure-activity relationship that depends on the number of hydroxyl groups at the B-ring [43] and appears in the order of delphinidin > cyanidin, petunidin > pelargonidin, peonidin, and malvidin.
The mechanistic analysis indicates that the apoptosis induction by delphinidin may involve an oxidation/JNK-mediated caspase pathway. Delphinidin treatment increased the levels of intracellular ROS, which may be a sensor to activate JNK. Concomitant with the apoptosis, JNK pathway activation such as JNK phosphorylation, c-jun gene expression, and caspase-3 activation was observed in delphinidin-treated cells [43]. Antioxidants such as N-acetyl-L-cysteine (NAC) and catalase effectively blocked delphinidin-induced JNK phosphorylation, caspase 3 activation, and DNA fragmentation [43]. Thus, delphinidin may trigger an apoptotic death program in HL-60 cells through an oxidative stress-mediated JNK signaling cascades (Figure 2). It is interesting that anthocyanidins produced ROS, showing pro-oxidant activities, to induce apoptosis in HL-60 cells, contrary to the antioxidant activities of anthocyanidins in the inhibition of TPA-induced cell transformation in mouse skin JB6 cells [29].
It should be noted that anthocyanidins are the aglycons of the naturally occurring anthocyanins. Most of the molecular results on biological activities of anthocyanins were from anthocyanidins due to the fact that anthocyanidins are easier to be prepared than anthocyanins. Thus, it is not yet known whether the naturally occurring anthocyanins will also activate these molecular pathways. Accumulated results on structure-activity studies have shown that the biological activities of anthocyanins appear to increase with a decreasing number of sugar units and/or with an increasing number of hydroxyl groups at their aglycons [7]. For example, both antioxidant activity and cyclooxygenase inhibitory activity of cyanidin glycosides increased with a decreasing number of sugar units. Cyanidin-rutinose showed better activity than cyanidin-glucosylrutinose, and cyanidin aglycone showed the best activity at much lower concentrations [37]. In our laboratory, we found that the aglycons with ortho-dihydroxyphenyl structure at the B-ring, such as delphinidin and cyanidin [29, 43] and their glycosides (Hou et al, unpublished data), possessed the activities of anticarcinogenesis, anti-inflammation, and apoptosis induction. The ortho-dihydroxyphenyl structure on the B-ring appears essential for these actions, and the activities of aglycons such as delphinidin and cyanidin are stronger than that of their glycosides.
CONCLUSION
The molecular mechanisms of anticarcinogenesis, anti-inflammation, and apoptosis induction of malignant cells by anthocyanidins have been demonstrated at molecular level. These data provide a first molecular view of the chemopreventive effects of anthocyanidins. Based on many genes that may be associated with cancer chemoprevention, a genome-wide gene analysis by using microarray technology will be required to get the whole view of molecular mechanisms of cancer chemoprevention by anthocyanidins.
ACKNOWLEDGMENT
This work was partially supported by the Cooperation of Innovative Technology and Advanced Research in Evolutional Area (CITY AREA).
References
- 1.Harborne J.B, Grayer J. The anthocyanins. In: Harborne J.B, editor. The Flavonoids. London: Chapman and Hall; 1988. pp. 1–20. [Google Scholar]
- 2.Mazza G. Anthocyanins in grapes and grape products. Crit Rev Food Sci Nutr. 1995;35(4):341–371. doi: 10.1080/10408399509527704. [DOI] [PubMed] [Google Scholar]
- 3.Harborne J.B, Baxter H. vol 2. Chichester: John Wiley & Sons; 1999. The Handbook of Natural Flavonoids. [Google Scholar]
- 4.Kuhnau J. The flavonoids. A class of semi-essential food components: their role in human nutrition. World Rev Nutr Diet. 1976;24:117–191. [PubMed] [Google Scholar]
- 5.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339(8808):1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 6.Muth E.R, Laurent J.M, Jasper P. The effect of bilberry nutritional supplementation on night visual acuity and contrast sensitivity. Altern Med Rev. 2000;5(2):164–173. [PubMed] [Google Scholar]
- 7.Hou D.X. Potential mechanisms of cancer chemoprevention by anthocyanins. Curr Mol Med. 2003;2003(2):149–159. doi: 10.2174/1566524033361555. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Cao G, Prior R.L. Oxygen radical absorbing capacity of anthocyanins. J Agric Food Chem. 1997;45(2):304–309. [Google Scholar]
- 9.Wang H, Nair M.G, Strasburg G.M, et al. Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J Nat Prod. 1999;62(2):294–296. doi: 10.1021/np980501m. [DOI] [PubMed] [Google Scholar]
- 10.Noda Y, Kaneyuki T, Mori A, Packer L. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: delphinidin, cyanidin, and pelargonidin. J Agric Food Chem. 2002;50(1):166–171. doi: 10.1021/jf0108765. [DOI] [PubMed] [Google Scholar]
- 11.Tsuda T, Horio F, Osawa T. The role of anthocyanins as an antioxidant under oxidative stress in rats. Biofactors. 2000;13(1-4):133–139. doi: 10.1002/biof.5520130122. [DOI] [PubMed] [Google Scholar]
- 12.Tsuda T, Shiga K, Ohshima K, Kawakishi S, Osawa T. Inhibition of lipid peroxidation and the active oxygen radical scavenging effect of anthocyanin pigments isolated from Phaseolus vulgaris L. Biochem Pharmacol. 1996;52(7):1033–1039. doi: 10.1016/0006-2952(96)00421-2. [DOI] [PubMed] [Google Scholar]
- 13.Tsuda T, Horio F, Osawa T. Dietary cyanidin 3-O-beta-D-glucoside increases ex vivo oxidation resistance of serum in rats. Lipids. 1998;33(6):583–588. doi: 10.1007/s11745-998-0243-5. [DOI] [PubMed] [Google Scholar]
- 14.Kamei H, Kojima T, Hasegawa M, et al. Suppression of tumor cell growth by anthocyanins in vitro. Cancer Invest. 1995;13(6):590–594. doi: 10.3109/07357909509024927. [DOI] [PubMed] [Google Scholar]
- 15.Nagase H, Sasaki K, Kito H, Haga A, Sato T. Inhibitory effect of delphinidin from Solanum melongena on human fibrosarcoma HT-1080 invasiveness in vitro. Planta Med. 1998;64(3):216–219. doi: 10.1055/s-2006-957412. [DOI] [PubMed] [Google Scholar]
- 16.Meiers S, Kemeny M, Weyand U, Gastpar R, von Angerer E, Marko D. The anthocyanidins cyanidin and delphinidin are potent inhibitors of the epidermal growth-factor receptor. J Agric Food Chem. 2001;49(2):958–962. doi: 10.1021/jf0009100. [DOI] [PubMed] [Google Scholar]
- 17.Bomser J, Madhavi D.L, Singletary K, Smith M.A. In vitro anticancer activity of fruit extracts from Vaccinium species. Planta Med. 1996;62(3):212–216. doi: 10.1055/s-2006-957862. [DOI] [PubMed] [Google Scholar]
- 18.Bomser J.A, Singletary K.W, Wallig M.A, Smith M.A. Inhibition of TPA-induced tumor promotion in CD-1 mouse epidermis by a polyphenolic fraction from grape seeds. Cancer Lett. 1999;135(2):151–157. doi: 10.1016/s0304-3835(98)00289-4. [DOI] [PubMed] [Google Scholar]
- 19.Hagiwara A, Yoshino H, Ichihara T, et al. Prevention by natural food anthocyanins, purple sweet potato color and red cabbage color, of 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine (PhIP)-associated colorectal carcinogenesis in rats initiated with 1,2-dimethylhydrazine. J Toxicol Sci. 2002;27(1):57–68. doi: 10.2131/jts.27.57. [DOI] [PubMed] [Google Scholar]
- 20.Tsuda T, Horio F, Osawa T. Absorption and metabolism of cyanidin 3-O-beta-D-glucoside in rats. FEBS Lett. 1999;449(2-3):179–182. doi: 10.1016/s0014-5793(99)00407-x. [DOI] [PubMed] [Google Scholar]
- 21.Miyazawa T, Nakagawa K, Kudo M, Muraishi K, Someya K. Direct intestinal absorption of red fruit anthocyanins, cyanidin-3-glucoside and cyanidin-3,5-diglucoside, into rats and humans. J Agric Food Chem. 1999;47(3):1083–1091. doi: 10.1021/jf9809582. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto H, Inaba H, Kishi M, Tominaga S, Hirayama M, Tsuda T. Orally administered delphinidin 3-rutinoside and cyanidin 3-rutinoside are directly absorbed in rats and humans and appear in the blood as the intact forms. J Agric Food Chem. 2001;49(3):1546–1551. doi: 10.1021/jf001246q. [DOI] [PubMed] [Google Scholar]
- 23.Suda I, Oki T, Masuda M, et al. Direct absorption of acylated anthocyanin in purple-fleshed sweet potato into rats. J Agric Food Chem. 2002;50(6):1672–1676. doi: 10.1021/jf011162x. [DOI] [PubMed] [Google Scholar]
- 24.Cao G, Muccitelli H.U, Sanchez-Moreno C, Prior R.L. Anthocyanins are absorbed in glycated forms in elderly women: a pharmacokinetic study. Am J Clin Nutr. 2001;73(5):920–926. doi: 10.1093/ajcn/73.5.920. [DOI] [PubMed] [Google Scholar]
- 25.Hsu T.C, Young M.R, Cmarik J, Colburn N.H. Activator protein 1 (AP-1)- and nuclear factor kappaB (NF-kappaB)-dependent transcriptional events in carcinogenesis. Free Radic Biol Med. 2000;28(9):1338–1348. doi: 10.1016/s0891-5849(00)00220-3. [DOI] [PubMed] [Google Scholar]
- 26.Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072(2-3):129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 27.Dong Z, Birrer M.J, Watts R.G, Matrisian L.M, Colburn N.H. Blocking of tumor promoter-induced AP-1 activity inhibits induced transformation in JB6 mouse epidermal cells. Proc Natl Acad Sci USA. 1994;91(2):609–613. doi: 10.1073/pnas.91.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang C, Ma W.Y, Young M.R, Colburn N, Dong Z. Shortage of mitogen-activated protein kinase is responsible for resistance to AP-1 transactivation and transformation in mouse JB6 cells. Proc Natl Acad Sci USA. 1998;95(1):156–161. doi: 10.1073/pnas.95.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hou D.X, Kai K, Li J.J, et al. Anthocyanidins inhibit activator protein 1 activity and cell transformation: structure-activity relationship and molecular mechanisms. Carcinogenesis. 2004;25(1):29–36. doi: 10.1093/carcin/bgg184. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Y, Xue Y, Oberley T.D, et al. Overexpression of manganese superoxide dismutase suppresses tumor formation by modulation of activator protein-1 signaling in a multistage skin carcinogenesis model. Cancer Res. 2001;61(16):6082–6088. [PubMed] [Google Scholar]
- 31.Chandrasekharan N.V, Dai H, Roos K.L, et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci USA. 2002;99(21):13926–13931. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warner T.D, Mitchell J.A. Cyclooxygenase-3 (COX-3): filling in the gaps toward a COX continuum? Proc Natl Acad Sci USA. 2002;99(21):13371–13373. doi: 10.1073/pnas.222543099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell J.A, Belvisi M.G, Akarasereenont P, et al. Induction of cyclo-oxygenase-2 by cytokines in human pulmonary epithelial cells: regulation by dexamethasone. Br J Pharmacol. 1994;113(3):1008–1014. doi: 10.1111/j.1476-5381.1994.tb17093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mestre J.R, Chan G, Zhang F, et al. Inhibition of cyclooxygenase-2 expression. An approach to preventing head and neck cancer. Ann N Y Acad Sci. 1999;889:62–71. doi: 10.1111/j.1749-6632.1999.tb08724.x. [DOI] [PubMed] [Google Scholar]
- 35.Chinery R, Beauchamp R.D, Shyr Y, Kirkland S.C, Coffey R.J, Morrow J.D. Antioxidants reduce cyclooxygenase-2 expression, prostaglandin production, and proliferation in colorectal cancer cells. Cancer Res. 1998;58(11):2323–2327. [PubMed] [Google Scholar]
- 36.Wang H, Nair M.G, Strasburg G.M, et al. Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J Nat Prod. 1999;62(2):294–296. doi: 10.1021/np980501m. [DOI] [PubMed] [Google Scholar]
- 37.Seeram N.P, Momin R.A, Nair M.G, Bourquin L.D. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine. 2001;8(5):362–369. doi: 10.1078/0944-7113-00053. [DOI] [PubMed] [Google Scholar]
- 38.Thompson C.B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267(5203):1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 39.Galati G, Teng S, Moridani M.Y, Chan T.S, O'Brien P.J. Cancer chemoprevention and apoptosis mechanisms induced by dietary polyphenolics. Drug Metabol Drug Interact. 2000;17(1-2):311–349. doi: 10.1515/dmdi.2000.17.1-4.311. [DOI] [PubMed] [Google Scholar]
- 40.Kerr J.F.R, Wyllie A.H, Currie A.R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267(5203):1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- 42.Suh N, Luyengi L, Fong H.H, Kinghorn A.D, Pezzuto J.M. Discovery of natural product chemopreventive agents utilizing HL-60 cell differentiation as a model. Anticancer Res. 1995;15(2):233–239. [PubMed] [Google Scholar]
- 43.Hou D.X, Ose T, Lin S, et al. Anthocyanidins induce apoptosis in human promyelocytic leukemia cells: structure-activity relationship and mechanisms involved. Int J Oncol. 2003;23(3):705–712. [PubMed] [Google Scholar]
- 44.Katsube N, Iwashita K, Tsushida T, Yamaki K, Kobori M. Induction of apoptosis in cancer cells by bilberry (Vaccinium myrtillus) and the anthocyanins. J Agric Food Chem. 2003;51(1):68–75. doi: 10.1021/jf025781x. [DOI] [PubMed] [Google Scholar]