Abstract
Introduction
Despite radical prostatectomy (RP) and radiotherapy (RT) being established treatments for localised prostate cancer, a significant number of patients experience recurrent disease. While conventionally fractionated RT is still being used as a standard treatment in the postoperative setting, ultra-hypofractionated RT has emerged as a viable option with encouraging results in patients with localised disease in the primary setting. In addition, recent technological advancements in RT delivery and precise definition of isolated macroscopic recurrence within the prostate bed using prostate-specific membrane antigen-positron emission tomography (PSMA-PET) and multiparametric MRI (mpMRI) allow the exploration of ultra-hypofractionated schedules in the salvage setting using five fractions.
Methods and analysis
In this single-arm prospective phase II multicentre trial, 36 patients with node-negative prostate adenocarcinoma treated with RP at least 6 months before trial registration, tumour stage pT2a–3b, R0–1, pN0 or cN0 according to the UICC TNM 2009 and evidence of measurable local recurrence within the prostate bed detected by PSMA PET/CT and mpMRI within the last 3 months, will be included. The patients will undergo focal ultra-hypofractionated salvage RT with 34 Gy in five fractions every other day to the site of local recurrence in combination with 6 months of androgen deprivation therapy. The primary outcome of this study is biochemical relapse-free survival at 2 years. Secondary outcomes include acute side effects (until 90 days after the end of RT) of grade 3 or higher based on Common Terminology Criteria for Adverse Events V.5, progression-free survival, metastasis-free survival, late side effects and the quality of life (based on European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-C30, QLQ-PR25).
Ethics and dissemination
The study has received ethical approval from the Ethics Commission of the Canton of Bern (KEK-BE 2022-01026). Academic dissemination will occur through publications and conference presentations.
Trial registration number
Keywords: Radiation oncology, Prostatic Neoplasms, Urological tumours
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Innovative trial evaluating focal stereotactic radiotherapy combined with short-term androgen deprivation therapy for treating isolated local recurrence after radical prostatectomy.
Treatment planning is precisely defined based on prostate-specific membrane antigen-positron emission tomography imaging and multiparametric MRI.
Potential for improved efficacy and toxicity profile of salvage radiotherapy.
Non-randomised trial; further research will be required.
Small sample size.
Background
Radical prostatectomy (RP) and radiotherapy (RT) are cornerstones for the treatment of localised prostate cancer (PC).1 However, around 30%–60% of patients undergoing RP will develop recurrent disease.2 3 Various large randomised controlled studies have shown the effectiveness of postoperative RT in men who have a high risk of local recurrence following RP, such as pT3 tumour or positive resection margins 4–8. In the era of high-sensitivity prostate-specific antigen (PSA) and prostate-specific membrane antigen-positron emission tomography and CT (PSMA-PET/CT) as a standard staging examination in recurrent PC, new data suggest comparable oncological results if patients are treated early with salvage RT (sRT) compared with immediate adjuvant RT 9–12. Nevertheless, the aforementioned trials and those involving patients receiving sRT due to macroscopic tumour recurrence in the prostate bed were conducted with conventionally fractionated RT, typically 2 Gy per fraction 4–12.
Recently, ultra-hypofractionated RT, using usually >5 Gy or higher per fraction, was assessed as a valid therapeutic option in patients with low risk or intermediate risk as a definitive treatment. Published data with fair follow-up periods demonstrated excellent biochemical control management with a favourable toxicity profile 13–20. Moreover, the evidence on ultra-hypofractionated in high-risk individuals is emerging, and many significant studies have reported favourable findings 21–26. Ultra-hypofractionation is used to treat patients with PC due to its low α/β value which is thought to be around 1.5 Gy 27 28. It is anticipated that increasing the dose per fraction would increase the therapeutic ratio and, thus, the potential tumour control. Nevertheless, considering the low toxicity rates reported,29–37 using moderate hypofractionation in the postoperative setting with a daily RT dose of up to 3 Gy per fraction does not seem to corroborate this concern. However, the evidence on postoperative ultra-hypofractionated RT to the prostate bed is still in its early stages.
Further improvement in the oncological outcomes can be expected through technological developments in RT delivery and precise targeting of the local relapses in the prostate bed. An sRT using an ultra-hypofractionated schedule delivered in five fractions and limited only to the site of isolated macroscopic recurrence in the prostate bed as defined by PSMA-PET and multiparametric MRI (mpMRI) in combination with short-term androgen deprivation therapy for 6 months, may represent a valid treatment strategy to improve the therapeutic ratio in these patients (shorter overall treatment time, better sparing of organs at risk while delivering higher biological-equivalent dose into the target volume).
The main objective of this prospective single-arm trial is to assess the efficacy and safety of ultra-hypofractionated sRT delivered in five fractions to the site of local recurrence within the prostate bed with target delineation based on PSMA PET and MRI.
Methods/design
The Hypo Focal sRT Trial protocol was constructed using the SPIRIT reporting guidelines 29. Following permission from the regional ethics committees (KEK-BE 2022-01026), the research is registered with ClinicalTrials.gov (NCT05746806) and the Swiss National Clinical Trials Portal. Both the sponsor investigator and the trial statistician have given their approval to the protocol V.3.0 (dated 11 November 2022).
Study population
Inclusion criteria
Before registration and before any trial-specific procedures, written informed consent in accordance with ICH (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use)/Good Clinical Practice rules is required.
Minimum age to register is 18 years old.
Performance level 0–1 according to WHO.
Lymph node negative adenocarcinoma of the prostate treated with RP at least 6 months before trial registration. Tumour stage pT2a–3b, R0–1, pN0 or cN0 according to the UICC TNM 2009.
Evidence of measurable local recurrence at the prostate bed detected by PSMA PET/CT and mpMRI within the last 3 months. In case of unclear local recurrence, biopsy confirmation is recommended.
Patients must have non-metastatic (N0, M0) disease, as defined by no evidence of nodal or distant metastases seen on PSMA PET scan.
Patients must have a testosterone level >50 ng/dL.
Patients must not have had bilateral orchiectomy, luteinising hormone-releasing hormone (LHRH) agonists, antiandrogens or any combination of these in the past.
Absence of any psychological, family, sociological, or geographic situation that would make it difficult for the patient to adhere to the research protocol and follow-up plan; the patient should be informed of these factors before registering for the trial.
Exclusion criteria
PSA levels (>0.4 ng/mL) that persist 4–20 weeks after RP.
Previous diagnosis of haematological or primary solid malignancy during the preceding 3 years previous to registration, except for curatively managed localised non-melanoma skin cancer.
Use of substances known to alter PSA levels, such as androgen deprivation therapy and any kind of androgen suppression medication, within 4 weeks of the start of the trial treatment phase.
Bilateral hip prosthesis.
Comorbidities that are severe or active and that are likely to have an effect on whether or not sRT is advisable.
Treatment with any experimental treatment or involvement in a clinical trial within the last 30 days (with the exception of concurrent participation in the biobank research, which is allowed) is required for eligibility to register.
Study design and sample size
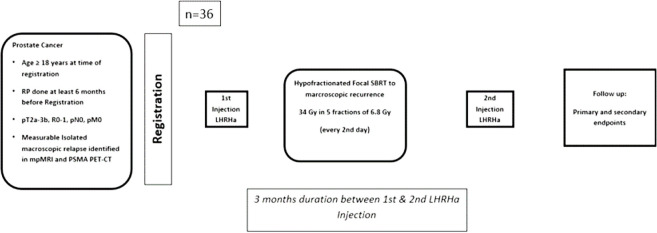
This is a single-arm, prospective, phase II multicentre study. According to the published prospective trials and retrospective series reporting the outcomes of the normo-fractionated sRT, we define biochemical relapse-free survival at 2 years of 60% as poor and of 80% as the promising outcome that would justify further investigation.30–33 We will, therefore, test the null hypothesis that the biochemical relapse-free survival at 2 years is lower than 60% against the alternative that it is at least 80%. Based on a one-sample binomial exact test with a one-sided alpha of 5%, 36 patients are required to reach a power of 80%, not taking into account patients lost to follow-up. We will control the safety of the intervention during the trial by assessing acute side effects (grade 3 or higher) at 90 days after 12 and 24 patients. The trial will be stopped if there is evidence that the proportion of patients with acute side effects (grade 3 or higher) is larger than 27%; the proportion observed would be tested using one-sample binomial exact tests with a one-sided alpha of 5%. Figure 1 shows a summary of the study design and schedule.
Figure 1.
Summary of the study design and schedule. LHRH, luteinising hormone-releasing hormone; mpMRI, multiparametric MRI; PET, positron emission tomography; PSMA, prostate-specific membrane antigen; SBRT, stereotactic radiotherapy.
Outcomes
Primary outcome
Biochemical relapse-free survival at 2 years.
Secondary outcome
Acute side effects (until 90 days after the end of RT) of grade 3 or higher based on Common Terminology Criteria for Adverse Events V.5.
Progression-free survival.
Metastasis-free survival.
Late side effects.
Quality of life (based on European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (QLQ)-C30, QLQ-PR25).
Study intervention
Preregistration imaging
Within 3 months prior to registration, PSMA PET/CT is mandatory to exclude regional or distant metastasis. Both 18F-PSMA and 68Ga-PSMA tracers are allowed. An mpMRI of the prostate bed is required within 3 months before registration is mandatory to define the extension of local recurrence.
Radiation treatment (stereotactic RT)
Patient’s positioning, immobilisation, data acquisition and simulation
Determining the gross tumour volume (GTV), the planned target volume (PTV) and the essential structures requires a treatment-planning CT scan with the patient in the same position as during treatment. The patients will be placed in the supine position for the entire process. Support for the knees and legs is strongly advised. On a flat table, each patient will be placed in the treatment position while being immobilised by a unique device. It is advised that patients be treated and scanned while having a comfortably full bladder. For prostate bed RT, it is advised to have an empty rectum. An example of a bladder and rectal protocol: An empty rectum is provided by using a rectal enema ±60 min before planning CT. After emptying the rectum and bladder, the patient is asked to drink the amount of 500–750 mL of water. The planning CT is then performed after 40 min. The patient repeats the bladder filling procedure during the entire treatment course. An endorectal balloon can be used for repositioning purposes as per local institutional standards.
Radiopaque fiducial markers (mandatory for robotic-based treatments) may be implanted in the prostate bed 1 week before the planning CT scan at the discretion of the treating centre. During the planning and performance of the treatment, the patient’s location will be reproduced employing skin markings and orthogonal laser beams. The pelvis should be scanned during the treatment planning CT scan, at least from the lower portion of the second lumbar vertebra (L2) to the lower half of the ischial tuberosities. The CT scan must cover the full target volume and all organs at risk (OAR). A CT slice should be no thicker than 2 mm. On every CT slice that shows the GTV, PTV and OAR, these structures must be highlighted. Morphological and topographical information given by clinical examination, mpMRI and PET/CT must be integrated to delineate the target volumes. Rigid or deformable coregistration is allowed.
Treatment volumes
Definition of target volume (refer to online supplemental material 1):
bmjopen-2023-075846supp001.pdf (929.2KB, pdf)
The GTV of the suspicious local recurrence is defined by the physician as all known gross disease before any treatment as defined by the CT/MRI images and PET scan using rigid or deformable fusion and/or clinical information.
The planning target volume (PTV) will provide the GTV a margin to account for daily treatment setup variations and internal motion brought on by breathing or movement during treatment. The PTV should surround the GTV with a 5 mm margin on all sides.
Organs at risk
The delineation of the OAR should be done following the RTOG guidelines; the normal pelvis atlas on the RTOG/NRG Oncology website provides examples of normal tissue contours.34
The bladder is defined by its external wall, with a thickness of 5 mm delineated on each slide, from the dome to the bladder neck and the start of the vesicourethral anastomosis (VUA).
The VUA and distal urethra are delineated from the bladder neck to the distal urethra using mpMRI sequences, and a 2 mm isotropic margin is added around these structures to create a planning organ at risk volume.
The rectum is defined by its external wall, with a thickness of 5 mm from the rectosigmoid junction to ischial tuberosities.
The femoral heads are delineated from the top of the hip joint to the small trochanter, while the bowel bag is delineated from the most inferior small or large bowel loop to 1 cm above the planning target volume (PTV) for coplanar beam plans, or more if non-coplanar beams or tomotherapy plans are used.
It is suggested that dose constraints be adhered to; however, if this is not practicable, the dose per fraction or target coverage may be adjusted to comply with the constraint. Table 1 shows the dose constraints for OARs.
Table 1.
Dose constraints for OARs
| Organ at risk | Dose constraint | Aim |
| Rectal Wall | V18.1 Gy V29 Gy V36 Gy |
<50% <20% <1 cc |
| Bladder Wall | V18.1 Gy V 37 Gy |
<40% <10 cc |
| PRV_VUA and distal Urethra | V36 Gy | <1 cc |
| Femoral heads | V14.5 Gy | <5% |
| Penile bulb | V29.5 Gy | <50% |
| Bowel | V18.1 Gy V30 Gy |
<5 cc <1 cc |
OAR, organs at risk; PRV, planning organ at risk volume; VUA, vesicourethral anastomosis.
Treatment techniques
It is required to apply rotating techniques or intensity-modulated RT (IMRT). Only dosimetry produced by inversed treatment planning is, by definition, regarded as IMRT. Step-and-shoot, sliding-window and volumetric modulated arc therapy, as well as MRI-guided radiation therapies (MRIdian or Elekta Unity), may be employed for performing IMRT. Treatment with Cyberknife is allowed.
Dose prescription
A total dose of 34 Gy (80% of the maximal dose) will be delivered in 5 fractions and fractions every second day (NTD2Gy 80 Gy α/β=1.5 Gy for tumour control and 66.6 Gy α/β=3 Gy for late toxicity). Treatment will be prescribed to the periphery of the target (80% of the dose (=34 Gy), should cover 90% of the PTV) covering the PTV. A maximal dose of 40 Gy is allowed to GTV. The priority will be given with respect to dose constraints over PTV coverage.
Androgen deprivation therapy
For a total of 6 months, each patient will be treated with a 3-monthly formulation of an LHRH agonist or antagonist. Prevention with an antiandrogen is indicated for at least 5 days before the initial injection of the agonist in the case of an LHRH agonist flare and should not be sustained for more than 15 days of the first-month duration.
Androgen deprivation therapy (ADT) should start no later than the first stereotactic RT (SBRT) fraction and no earlier than 2 weeks before the start of RT.
Palliative ADT should not be initiated for biochemical progression until clinical progression has been demonstrated. In the event of symptom progression, palliative ADT is required. In the event of asymptomatic clinical progression, men who are well informed are permitted to delay ADT until symptomatic progression occurs (EAU 2023 guidelines).35 Generally, we would only begin ADT in asymptomatic individuals if traditional imaging confirmed clinical progression. As a result, we would not advocate initiating ADT for PET positive lesions that do not seem suspicious on conventional imaging (CT/MRI/bone scintigraphy).
ADT-related toxicity should be managed, according to Nguyen et al.36
Study procedures
The study procedures and the schedule of assessments are presented in table 2.
Table 2.
Schedule of assessments
| Required investigation | Inclusion | Treatment | 1 month after RT | 3 months after RT | 6 months after RT | Every 6 months till the end of second year after RT, then once per year till 60 months | |
| Within 12 weeks prior to registration | Within 2 weeks prior to registration | Within 2 weeks prior to registration | |||||
| Eligibility check | x | ||||||
| Signed informed consent | x | ||||||
| Record prior history | x | ||||||
| Visits | |||||||
| Physical examination | x | x | x | x | x | ||
| Biochemistry (blood samples)* | |||||||
| PSA | x | x | x | x | x | ||
| Testosterone | x | x | x | x | x | ||
| Radiology | |||||||
| PSMA PET | x | ||||||
| MRI | x | ||||||
| Radiotherapy | |||||||
| Treatment planning | x | ||||||
| Record Planning results | x | ||||||
| Adverse Events | |||||||
| Baseline toxicity | x | ||||||
| Acute toxicity | x | x | x | ||||
| Late toxicity | x | x | |||||
| EORTC QoL questionnaire | |||||||
| QLQ-C30 | x | x | x | x | x | ||
| QLQ-PR25 | x | x | x | x | x | ||
*Blood samples: The obtained blood samples are used only for PSA and testosterone values. The measurement for this labs is conducted within the local hospital laboratory of each participating centre and the rest samples will be disposed afterwards. No blood will be collected or stored or used for other research purposes within the frame of this trial.
EORTC, European Organisation for Research and Treatment of Cancer; PSA, prostate-specific antigen; PSMA PET, prostate-specific membrane antigen-positron emission tomography; QLQ-C30, Quality of Life Questionnaire Core 30; QoL, quality of life; RT, radiotherapy.
Planned analysis
For descriptive statistics, the categorical variables will be presented as frequency and percentage, the normally distributed continuous variables will be presented as mean and SD, and the non-normally distributed continuous variables will be presented as median and IQR.
The time-to-event outcomes will be analysed using Kaplan-Meier-curves, the proportion of responders at 1 and 2 years, and the restricted mean survival time at 1 and 2 years with a 95% CI. Binary outcomes will be reported using absolute and relative frequencies with 95% CIs.
The probability of biochemical relapse-free survival and metastasis-free survival will be estimated using the Kaplan-Meier method. Cox proportional hazards models will be fit to assess the effects of treatment and baseline clinical and pathologic features (such as PSA, PSA doubling time, Gleason score) on biochemical relapse-free survival and metastasis-free survival).
Further subgroup analysis will follow after finalising the accrual (R0 vs R1), (pN0 vs cN0) and based the location of the recurrence.
Study status
Open and currently accruing since 20 February 2023.
The approximate recruitment will be completed by October 2024.
Patient and public involvement
Patients were not involved in the idea conception of this trial.
Patients were not involved in the design of this study nor in recruitment of the study.
Ethics and dissemination
The study has been submitted and approved by ethics commission of Canton of Bern. A written informed consent will be obtained from the study participants. Academic dissemination will occur through publication and conference presentations.
Discussion
External beam RT is a well-established treatment for organ-confined prostate cancer, with comparable cure rates to RP.37 Hypofractionation employs a higher dose-per-fraction while reducing the number of fractions offering a clinical benefit in terms of tumour control in tumours with a low alfa/beta ratio (eg, prostate cancer) and favourable toxicity, allowing for higher patient comfort.38 Based on the results of ten prior randomised trials, there is compelling evidence suggesting that moderate hypofractionation RT is not inferior to standard normofractionation RT schedules as a definitive treatment for primary PC.39 This evidence led to the integration of moderate hypofractionation schedules into the list of valid treatment options in the NCCN guidelines.40 In addition, recent advancements in the field of RT, including IMRT/rotational techniques, image-guided RT and SBRT, have permitted the gradual integration of ultra-hypofractionation in the treatment of localised PC. SBRT for PC has generated adequate data in terms of tumour control, patient-reported quality of life and minimal toxicity 14 16 25 to support its introduction in clinical practice. In addition, the prostate cancer-working group of the German Society of Oncology (DEGRO) and the NCCN Guidelines approve the use of SBRT in the treatment of localised low-risk and intermediate-risk prostate cancer and propose its use in clinical trials for patients with the localised high-risk disease.41 42
The evidence of ultra-hypofractionation has recently been supported by two randomised studies (HYPO RT-PC) 25, Prostate Advances in Comparative Evidence (PACE)-B trial,14 which compare its usage to conventional fractionation. Nevertheless, only HYPO-RT-PC provided information on the outcomes of long-term tumour and toxicity control. A randomised systematic review and meta-analysis of phase 3 studies evaluating SBRT with normofractionated and hypofractionated regimens were published in 2020. It was determined that the ultra-hypofractionated regimens had comparable 5-year disease-free survival outcomes, with late gastrointestinal and genitourinary (GU) toxicity of <15% and <21%, respectively, in comparison to hypofractionated regimens and conventional RT.43 In 2022, the toxicity outcome of the PACE B Trial was published, showing no significant differences between the five fractions of SBRT and conventional RT 44.
The use of moderate hypofractionation is gaining more popularity as a standard treatment in the postoperative setting.45 Retrospective and prospective single-arm studies support a safe toxicity profile and promising biochemical control rates with hypofractionation.45 According to newly released findings from the phase III clinical study NRG-GU003 evaluating hypofractionated postoperative prostate bed RT (HYPORT) to conventional postprostatectomy RT for men with prostate cancer, treatment with HYPORT did not cause a rise in patient-reported GI or GU toxicity for study subjects, with a comparable biochemical disease control at the 2-year follow-up.46
Parikh et al 47 did a theoretical feasibility study of SBRT following RP depending on the NTCP (normal tissue complication probability) model, using individuals who had been managed with conventional EBRT for biochemical recurrence after prostatectomy. The goal was to show that SBRT could be used safely and effectively in this clinical situation. A dose of 30 Gy was delivered to the PTV in five fractions, translating to an equivalent dose in 2 Gy fractions of 64.3 Gy, assuming an α/β value of 1.5 Gy, in accordance with RTOG standards to define postprostatectomy volumes. To predict the probability of late rectal and/or bladder toxicity, the NTCP model was used. According to the NTCP model, the average incidence of grade ≥2 late rectal toxicity was assessed to be 0.28%, and that of late grade 2 toxicity on the bladder neck was determined to be as low as 0.00013%, while the average incidence of late urinary symptoms exacerbation was calculated to be 4.81%. The author’s conclusion is that employing SBRT after surgery looks viable and may provide a safe, practical therapeutic alternative for individuals in both the adjuvant and salvage following biochemical failure, taking into account the limitations of the NTCP model.
Sampath et al examined the use of stereotactic dose-escalated RT on prostate beds in a prospective phase 1 research, which revealed a crude rate of biochemical control of 42% in the overall population.48 Patients received care using dose fractionation regimens of 35 Gy, 40 Gy and 45 Gy in five fractions each. The authors emphasised that raising the dosage to 45 Gy was possible without increasing the number of adverse events but that there was no observed improvement in PSA control when compared with 40 Gy in five fractions. Similarly, a recent propensity score study comparing salvage SBRT and conventional RT for macroscopic prostate bed recurrence revealed similar bRFS and PFS rates across the two modalities. On the other hand, a reduced incidence of toxicity was verified for patients receiving focal stereotactic sRT compared with conventionally fractionated sRT, with acute GI and GU adverse events recorded in 4.4% against 44.4% (p<0.001) and 28.9% against 46.7% (p=0.08) of participants, and late GI and GU side effects reported in 0% vs 13.3% (p=0.04) and 6.7% vs 22.2% (p=0.03) of patient populations, respectively.49 The authors argue that salvage SBRT is a desirable substitute for conventional sRT in this situation due to the approach’s favourable therapeutic ratio and the less number of required fractions. Additionally, the prospective phase 2 SCIMITAR trial reported the quality of life and toxicity outcome of 100 patients who received postoperative ultra-hypofractionated SBRT delivered in 5 fractions.50 Acute and late grade 2 GU toxicities were both 9%, while acute and late grade 2 GI toxicities were 5% and 0%, respectively. Three patients had grade 3 toxicity (n=1 GU, n=2 GI).50
The expected results from the Hypo-Focal sRT trial will provide the first prospective evidence for the focal hypofractionated RT in the salvage setting and can be used as a basis for a large multicentre phase 3 trial. In addition to the assumed improvement in efficacy and toxicity profile due to precise customisation of the treatment target volumes, the application of a focal hypofractionated RT is expected to achieve cost-effectiveness benefits. Due to the very short treatment course (unlike conventional RT treatments, which can take up to 7 weeks), hypofractionated focal sRT leads to greater patient convenience and comfortability.
Supplementary Material
Footnotes
Contributors: EM, AA, JL, CZ, BDB, DMA, MG and TZ contributed to the conception and design of the protocol, statistical design and initial drafting of the protocol. MS is the principal investigator. All authors contributed to the quality, conception, design of the protocol and establishment of study specific documents.TZ and MS are Equal contribution as last authors.
Funding: This work was funded by a grant from the Berger-Janser Stiftung (grant number 13/2021) and Debiopharm AG (grant number not applicable).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study received a permission 437 from the regional ethics committees (KEK-BE 2022-01026), the research is registered with 438 ClinicalTrials.gov (NCT05746806) and the Swiss National Clinical Trials Portal. Written, informed 439 consent to participate is and will be obtained from all participants before participating in the trial.
References
- 1.Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016;375:1415–24. 10.1056/NEJMoa1606220 [DOI] [PubMed] [Google Scholar]
- 2.Gandaglia G, Briganti A, Clarke N, et al. Adjuvant and salvage radiotherapy after radical Prostatectomy in prostate cancer patients. Eur Urol 2017;72:689–709. 10.1016/j.eururo.2017.01.039 [DOI] [PubMed] [Google Scholar]
- 3.Han M, Partin AW, Zahurak M, et al. Biochemical (prostate specific antigen) recurrence probability following radical Prostatectomy for clinically localized prostate cancer. J Urol 2003;169:517–23. 10.1097/01.ju.0000045749.90353.c7 [DOI] [PubMed] [Google Scholar]
- 4.Bolla M, van Poppel H, Collette L, et al. Postoperative radiotherapy after radical Prostatectomy: a randomised controlled trial [EORTC trial 22911]. Lancet 2005;366:572–8. 10.1016/S0140-6736(05)67101-2 [DOI] [PubMed] [Google Scholar]
- 5.Bolla M, van Poppel H, Tombal B, et al. Postoperative radiotherapy after radical Prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial. Lancet 2012;380:2018–27. 10.1016/S0140-6736(12)61253-7 [DOI] [PubMed] [Google Scholar]
- 6.Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for Pathologically advanced prostate cancer: a randomized clinical trial. JAMA 2006;296:2329–35. 10.1001/jama.296.19.2329 [DOI] [PubMed] [Google Scholar]
- 7.Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term follow-up of a randomized clinical trial. J Urol 2009;181:956–62. 10.1016/j.juro.2008.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiegel T, Bottke D, Steiner U, et al. Phase III postoperative adjuvant radiotherapy after radical Prostatectomy compared with radical Prostatectomy alone in Pt3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol 2009;27:2924–30. 10.1200/JCO.2008.18.9563 [DOI] [PubMed] [Google Scholar]
- 9.Kneebone A, Fraser-Browne C, Duchesne GM, et al. Adjuvant radiotherapy versus early salvage radiotherapy following radical Prostatectomy (TROG 08.03/ANZUP RAVES): a randomised, controlled, phase 3, non-inferiority trial. Lancet Oncol 2020;21:1331–40. 10.1016/S1470-2045(20)30456-3 [DOI] [PubMed] [Google Scholar]
- 10.Parker CC, Clarke NW, Cook AD, et al. Timing of radiotherapy after radical Prostatectomy (RADICALS-RT): a randomised, controlled phase 3 trial. Lancet 2020;396:1413–21. 10.1016/S0140-6736(20)31553-1 [DOI] [PubMed] [Google Scholar]
- 11.Vale CL, Fisher D, Kneebone A, et al. Adjuvant or early salvage radiotherapy for the treatment of Localised and locally advanced prostate cancer: a prospectively planned systematic review and meta-analysis of aggregate data. Lancet 2020;396:1422–31. 10.1016/S0140-6736(20)31952-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sargos P, Chabaud S, Latorzeff I, et al. Adjuvant radiotherapy versus early salvage radiotherapy plus short-term androgen deprivation therapy in men with Localised prostate cancer after radical Prostatectomy (GETUG-AFU 17): a randomised, phase 3 trial. Lancet Oncol 2020;21:1341–52. 10.1016/S1470-2045(20)30454-X [DOI] [PubMed] [Google Scholar]
- 13.Boike TP, Lotan Y, Cho LC, et al. Phase I dose-escalation study of stereotactic body radiation therapy for Low- and intermediate-risk prostate cancer. J Clin Oncol 2011;29:2020–6. 10.1200/JCO.2010.31.4377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brand DH, Tree AC, Ostler P, et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol 2019;20:1531–43. 10.1016/S1470-2045(19)30569-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen LN, Suy S, Uhm S, et al. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: the Georgetown University experience. Radiat Oncol 2013;8:58. 10.1186/1748-717X-8-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson WC, Silva J, Hartman HE, et al. Stereotactic body radiation therapy for localized prostate cancer: A systematic review and meta-analysis of over 6,000 patients treated on prospective studies. Int J Radiat Oncol Biol Phys 2019;104:778–89. 10.1016/j.ijrobp.2019.03.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King CR, Brooks JD, Gill H, et al. Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys 2012;82:877–82. 10.1016/j.ijrobp.2010.11.054 [DOI] [PubMed] [Google Scholar]
- 18.Kotecha R, Djemil T, Tendulkar RD, et al. Dose-escalated stereotactic body radiation therapy for patients with Intermediate- and high-risk prostate cancer: initial Dosimetry analysis and patient outcomes. Int J Radiat Oncol Biol Phys 2016;95:960–4. 10.1016/j.ijrobp.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 19.Meier RM, Bloch DA, Cotrutz C, et al. Multicenter trial of stereotactic body radiation therapy for Low- and intermediate-risk prostate cancer: survival and toxicity endpoints. Int J Radiat Oncol Biol Phys 2018;102:296–303. 10.1016/j.ijrobp.2018.05.040 [DOI] [PubMed] [Google Scholar]
- 20.Zilli T, Franzese C, Bottero M, et al. Single fraction urethra-sparing prostate cancer SBRT: phase I results of the ONE SHOT trial. Radiother Oncol 2019;139:83–6. 10.1016/j.radonc.2019.07.018 [DOI] [PubMed] [Google Scholar]
- 21.Bauman G, Ferguson M, Lock M, et al. A phase 1/2 trial of brief androgen suppression and stereotactic radiation therapy (FASTR) for high-risk prostate cancer. International Journal of Radiation Oncology*Biology*Physics 2015;92:856–62. 10.1016/j.ijrobp.2015.02.046 [DOI] [PubMed] [Google Scholar]
- 22.Callan L, Bauman G, Chen J, et al. A phase I/II trial of fairly brief androgen suppression and stereotactic radiation therapy for high-risk prostate cancer (FASTR-2): preliminary results and toxicity analysis. Advances in Radiation Oncology 2019;4:668–73. 10.1016/j.adro.2019.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foerster R, Zwahlen DR, Buchali A, et al. n.d. Stereotactic body radiotherapy for high-risk prostate cancer: A systematic review. Cancers;13:759. 10.3390/cancers13040759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murthy V, Gupta M, Mulye G, et al. Early results of extreme Hypofractionation using stereotactic body radiation therapy for high-risk, very high-risk and node-positive prostate cancer. Clinical Oncology 2018;30:442–7. 10.1016/j.clon.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 25.Widmark A, Gunnlaugsson A, Beckman L, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet 2019;394:385–95. 10.1016/S0140-6736(19)31131-6 [DOI] [PubMed] [Google Scholar]
- 26.Zilli T, Jorcano S, Bral S, et al. Once-a-week or every-other-day urethra-sparing prostate cancer stereotactic body radiotherapy, a randomized phase II trial: 18 months follow-up results. Cancer Med 2020;9:3097–106. 10.1002/cam4.2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brenner DJ, Martinez AA, Edmundson GK, et al. Direct evidence that prostate tumors show high sensitivity to fractionation (low Α/Β ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys 2002;52:6–13. 10.1016/s0360-3016(01)02664-5 [DOI] [PubMed] [Google Scholar]
- 28.Miralbell R, Roberts SA, Zubizarreta E, et al. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven International institutional Datasets: Α/Β = 1.4 (0.9-2.2) GY. Int J Radiat Oncol Biol Phys 2012;82:e17–24. 10.1016/j.ijrobp.2010.10.075 [DOI] [PubMed] [Google Scholar]
- 29.Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shelan M, Odermatt S, Bojaxhiu B, et al. Disease control with delayed salvage radiotherapy for macroscopic local recurrence following radical Prostatectomy. Front Oncol 2019;9:12. 10.3389/fonc.2019.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghadjar P, Hayoz S, Bernhard J, et al. Dose-intensified versus conventional-dose salvage radiotherapy for Biochemically recurrent prostate cancer after Prostatectomy: the SAKK 09/10 randomized phase 3 trial. Eur Urol 2021;80:306–15. 10.1016/j.eururo.2021.05.033 [DOI] [PubMed] [Google Scholar]
- 32.Zilli T, Jorcano S, Peguret N, et al. Results of dose-adapted salvage radiotherapy after radical Prostatectomy based on an Endorectal MRI target definition model. Am J Clin Oncol 2017;40:194–9. 10.1097/COC.0000000000000130 [DOI] [PubMed] [Google Scholar]
- 33.Benziane-Ouaritini N, Zilli T, Giraud A, et al. Prostatectomy bed image-guided dose-escalated salvage radiotherapy (SPIDER): an international multicenter retrospective study. Eur Urol Oncol 2023;6:390–8. 10.1016/j.euo.2023.02.013 [DOI] [PubMed] [Google Scholar]
- 34.NRG > about us > center for innovation in radiation oncology > male RTOG normal pelvis. Available: https://www.nrgoncology.org/About-Us/Center-for-Innovation-in-Radiation-Oncology/Male-RTOG-Normal-Pelvis [Accessed 19 Mar 2023].
- 35.Summaries and changes of the prostate cancer guideline - Uroweb. Available: https://uroweb.org/guidelines/prostate-cancer/summary-of-changes [Accessed 13 Apr 2023].
- 36.Nguyen PL, Alibhai SMH, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol 2015;67:825–36. 10.1016/j.eururo.2014.07.010 [DOI] [PubMed] [Google Scholar]
- 37.Hamdy FC, Donovan JL, Lane JA, et al. Fifteen-year outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 2023;388:1547–58. 10.1056/NEJMoa2214122 [DOI] [PubMed] [Google Scholar]
- 38.Fowler JF. The Radiobiology of prostate cancer including new aspects of fractionated radiotherapy. Acta Oncol 2005;44:265–76. 10.1080/02841860410002824 [DOI] [PubMed] [Google Scholar]
- 39.Hickey BE, James ML, Daly T, et al. Hypofractionation for clinically localized prostate cancer. Cochrane Database Syst Rev 2019;9:CD011462. 10.1002/14651858.CD011462.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.NCCN guidelines. Available: https://www.nccn.org/guidelines/nccn-guidelines [Accessed 13 Apr 2023].
- 41.Wolf F, Sedlmayer F, Aebersold D, et al. Ultrahypofractionation of localized prostate cancer: statement from the DEGRO working group prostate cancer. Strahlenther Onkol 2021;197:89–96. 10.1007/s00066-020-01723-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zerini D, Jereczek-Fossa BA, Fodor C, et al. Salvage image-guided intensity modulated or stereotactic body Reirradiation of local recurrence of prostate cancer. Br J Radiol 2015;88:20150197. 10.1259/bjr.20150197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scher N, Bauduceau O, Bollet M, et al. Stereotactic prostate focal Reirradiation therapy for local recurrence: preliminary results of Hartmann oncology radiotherapy group. BJR Open 2019;1:20180027. 10.1259/bjro.20180027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tree AC, Ostler P, van der Voet H, et al. Intensity-modulated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): 2-year toxicity results from an open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol 2022;23:1308–20. 10.1016/S1470-2045(22)00517-4 [DOI] [PubMed] [Google Scholar]
- 45.Picardi C, Perret I, Miralbell R, et al. Hypofractionated radiotherapy for prostate cancer in the postoperative setting: what is the evidence so far. Cancer Treat Rev 2018;62:91–6. 10.1016/j.ctrv.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 46.Tandberg DJ, Oyekunle T, Lee WR, et al. Postoperative radiation therapy for prostate cancer: comparison of conventional versus Hypofractionated radiation regimens. Int J Radiat Oncol Biol Phys 2018;101:396–405. 10.1016/j.ijrobp.2018.02.002 [DOI] [PubMed] [Google Scholar]
- 47.Parikh NR, Kishan AU, Kane N, et al. Phase 1 trial of stereotactic body radiation therapy Neoadjuvant to radical Prostatectomy for patients with high-risk prostate cancer. Int J Radiat Oncol Biol Phys 2020;108:930–5. 10.1016/j.ijrobp.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sampath S, Frankel P, Vecchio BD, et al. Stereotactic body radiation therapy to the prostate bed: results of a phase 1 dose-escalation trial. Int J Radiat Oncol Biol Phys 2020;106:537–45. 10.1016/j.ijrobp.2019.11.005 [DOI] [PubMed] [Google Scholar]
- 49.Francolini G, Jereczek-Fossa BA, Di Cataldo V, et al. Stereotactic or conventional radiotherapy for macroscopic prostate bed recurrence: a propensity score analysis. Radiol Med 2022;127:449–57. 10.1007/s11547-022-01465-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma TM, Ballas LK, Wilhalme H, et al. Quality-of-life outcomes and toxicity profile among patients with localized prostate cancer after radical Prostatectomy treated with stereotactic body radiation: the SCIMITAR multicenter phase 2 trial. Int J Radiat Oncol Biol Phys 2023;115:142–52. 10.1016/j.ijrobp.2022.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-075846supp001.pdf (929.2KB, pdf)