Abstract
Haemophilus ducreyi is the etiologic agent of the sexually transmitted genital ulcer disease chancroid. Predominantly a cutaneous pathogen, H. ducreyi is present in chancroid ulcers that are characterized by extensive neutrophil accumulation in intraepidermal lesions accompanied by a mononuclear infiltrate in the dermis. We used an in vitro human skin model composed of foreskin fibroblasts and keratinocytes to examine host skin cell interactions with H. ducreyi 35000. Bacteria replicated and persisted in artificial skin for at least 14 days. We observed H. ducreyi inside suprabasal keratinocytes using transmission electron microscopy. Although no bacteria were seen in the basal keratinocyte region, these cells were disrupted in infected cocultures. H. ducreyi infection stimulated increased secretion of interleukin-6 (IL-6) and IL-8 by skin cells. Conversely, tumor necrosis factor alpha and IL-1α levels were not elevated. IL-8 produced in response to H. ducreyi infection may be involved in recruiting polymorphonuclear leukocytes and other inflammatory cells, thereby contributing to the tissue necrosis and ulcer formation characteristic of chancroid.
Chancroid, an ulcerating cutaneous infection caused by Haemophilus ducreyi, is one of the most prevalent sexually transmitted diseases in developing countries (61) and is endemic in some areas of the United States (6, 8, 16, 19, 36). Chancroid is a recognized cofactor for human immunodeficiency virus transmission (33, 40), making H. ducreyi the focus of considerable recent research aimed at understanding the molecular mechanisms of pathogenesis. Chancroid ulcers contain a superficial zone of polymorphonuclear leukocytes (PMNs) and extracellular debris accompanied by a dermal infiltrate of T cells and macrophages (20, 38, 61). The mechanisms by which H. ducreyi causes or induces epithelial destruction during infection are not known.
Several potential H. ducreyi virulence factors have been identified, including lipooligosaccharide (LOS) (11), pili (9), and the production of extracellular cytotoxin(s) (30, 31, 44) and hemolysin (41, 60). Understanding the relative contributions of these factors and identifying other bacterial components essential for H. ducreyi pathogenesis require appropriate models of infection. Several useful animal models have been described, including rabbits (25, 43), primates (59), and pigs (27), and experimental human challenge studies employ the natural host for H. ducreyi infection (52, 53). Absent from the repertoire of experimental chancroid systems has been a relevant in vitro human skin cell model.
Human tissue culture monolayers have been used as in vitro substrates for adherence and invasion studies with a number of sexually transmitted pathogens, including Neisseria gonorrhoeae, Treponema pallidum, and Chlamydia trachomatis (28, 48, 57). Similar studies with H. ducreyi and cultured human foreskin fibroblasts have yielded confusing and often contradictory results. Several independent studies have shown that H. ducreyi adheres to fibroblasts; some indicate that the bacteria are invasive (32), and others suggest that the bacilli are not found within these host cells (2, 3). While fibroblasts are present in the dermis, the relevance of H. ducreyi attachment to and invasion of these cells has not been demonstrated in vivo. Keratinocytes are the predominant epithelial cell in the epidermis and are likely the first host cells with which H. ducreyi interacts during infection. Recent studies with purified human foreskin keratinocytes or primary human foreskin epithelial cells grown in monolayers suggest that H. ducreyi attaches to (10) and invades (58) these cells.
Cultured cells offer host tissue relevance and ease of growth and manipulation; however, cellular morphology and the distribution of cell surface components in monolayers may not accurately reproduce the environment encountered by an organism infecting the human host. By using alternative tissue culture substrates and techniques, it is possible to maintain polarized epithelial cell layers in vitro with differentiated apical and basolateral surfaces and well-developed tight junctions. Artificial tissue systems have been constructed by layering relevant cell types. An artificial skin model has been developed using neonatal foreskin fibroblasts and keratinocytes in a coculture system composed of dermal, epidermal, and stratum corneum layers anchored to a nylon mesh support (17, 55). Important features of the model include the development of a basal lamina and anchoring zone (13) supporting the stratified epithelium composed of keratinocytes expressing the K1 keratin differentiation marker (50). K1 is expressed by epithelial cells in human foreskin and adult skin, but not by keratinocytes cultured in monolayers (50).
In the present study, we examined the interactions of virulent H. ducreyi 35000 with human foreskin keratinocytes and fibroblasts in infected artificial skin. Bacteria were observed inside suprabasal keratinocytes, and structural changes in the epidermis were consistent with the pathology of chancroid. We characterized the cellular interleukin-1α (IL-1α), tumor necrosis factor alpha (TNF-α), IL-6, and IL-8 responses to H. ducreyi infection and herein describe an unusual pattern of proinflammatory cytokine induction.
MATERIALS AND METHODS
Human foreskin keratinocyte-fibroblast cocultures (KFCs).
Artificial skin samples (Skin2 model ZK 1300) were obtained from Advanced Tissue Sciences (ATS; LaJolla, Calif.). Culture conditions have been previously described in detail (39). Briefly, human neonatal foreskin fibroblasts and keratinocytes were isolated and expanded into monolayer cultures (13). Fibroblasts seeded onto an inert nylon mesh grew submerged for 26 days, during which time they secreted an extracellular matrix (ECM) composed of mature collagen fibrils, fibronectin, and both sulfated and nonsulfated glycosaminoglycans (51). Keratinocytes were seeded onto the dermal substrate, grown submerged for 1 week, and then raised to the air-liquid interface. Cocultures were maintained for 13 days at ATS before squares (9 by 9 mm) were laser cut and placed on agarose containing growth medium with antibiotics for shipment and overnight delivery.
Upon receipt in our laboratory, KFCs were placed with the dermal side resting on the permeable membrane of 25-mm MilliCell culture inserts (Millipore, Bedford, Mass.) inside 35-mm chambers of six-well tissue culture plates (Corning Costar, New York, N.Y.). Cells were maintained at 35°C in a humidified atmosphere with 5% (vol/vol) CO2 with 1 ml of tissue culture medium (Dulbecco’s modified Eagle’s medium containing 5% fetal calf serum and 100 μg of ascorbate and 0.5 μg of hydrocortisone per ml) supplied beneath the insert and replaced daily. In this system, liquid medium contacts the lower surface of the culture inserts, but the artificial skin squares and bacterial inocula are maintained at the air interface and are not immersed in fluid. KFCs were equilibrated with antibiotic-free medium for 3 days prior to inoculation to ensure that residual antibiotics were removed from the tissue.
Microscopic analysis of uninfected KFCs confirmed the presence of a well-developed epidermis overlaying a dermal equivalent of fibroblasts embedded in secreted ECM material (see Fig. 2). Basal keratinocytes formed a characteristic columnar layer with several suprabasal squamous cell layers covered by a thin stratum corneum. The extent of epidermal differentiation and functional properties of KFCs are detailed elsewhere (13, 50, 51, 56, 63).
FIG. 2.
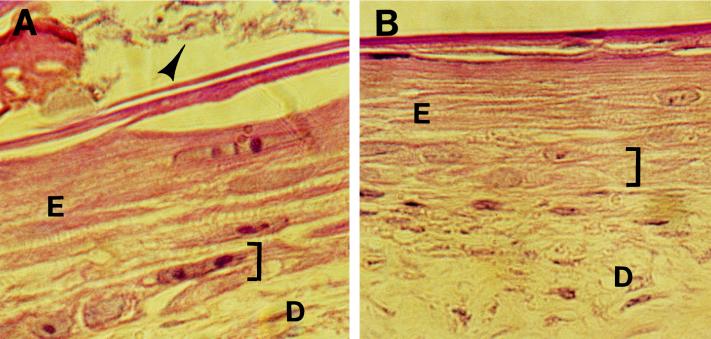
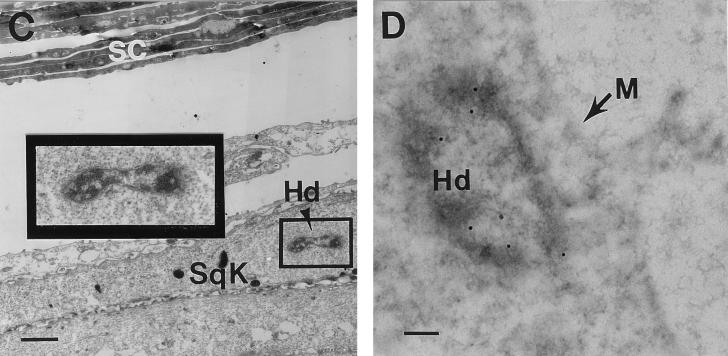
Localization of H. ducreyi in infected artificial skin; light micrographs of hematoxylin-and-eosin-stained artificial skin squares 18 h after abrasion and inoculation with live (A) or heat-killed (B) H. ducreyi 35000. E, epidermis; D, dermis; bracket, basal keratinocyte region; arrowhead in panel A, H. ducreyi aggregates. (C and D) TEM of H. ducreyi-infected artificial skin 18 h after inoculation. SC, terminally differentiated keratinocytes of the stratum corneum; SqK, squamous keratinocyte; Hd, H. ducreyi; rectangle in panel C, area enlarged in inset. Sample in panel D was probed with anti-H. ducreyi antiserum, followed by gold-labeled secondary antibody. M, membrane of a squamous keratinocyte, two to three layers deep in the epidermis. Original magnifications: ×400 (A and B), ×3,000 (C), and ×12,900 (D); bars, 2.5 mm (C) and 0.19 mm (D).
Bacterial cultures and inoculation procedures.
H. ducreyi 35000 (ATCC 33922) was isolated in Winnipeg, Canada, in the 1970s (24). The virulence of this isolate has been demonstrated in several animal models of infection (27, 43, 53, 59). Escherichia coli CC118 (34) and an uncharacterized isolate of Staphylococcus epidermidis were used as controls in some experiments. Bacteria were grown on chocolate agar (Difco GC agar base, 1% [wt/vol] IsoVitaleX [Becton Dickinson, Cockeysville, Md.], and 1% hemoglobin) at 35°C in a humidified atmosphere with 5% [vol/vol] CO2 and passaged daily up to three times from frozen stocks.
Inocula were prepared from log-phase cultures grown in brain heart infusion (BHI) broth (Difco Laboratories, Detroit, Mich.) supplemented with 1% IsoVitaleX and 50 μg of hemin per ml. Dilutions were made with filtered culture supernatants to maintain the concentration of potential bacterial virulence factors secreted during growth in vitro. Approximately 105 CFU in 10 μl were applied to the surface of KFCs. Filtered H. ducreyi culture supernatants and heat-killed inocula were applied to separate artificial foreskin squares to determine the relative contributions of secreted and heat-labile bacterial products in this model of infection. Controls for each experiment included BHI broth-inoculated and untreated but abraded samples. Infected and control cocultures were incubated at 35°C in a humidified atmosphere with 5% (vol/vol) CO2.
Preliminary experiments to determine the degree of abrasion required for H. ducreyi infection and productive interaction with foreskin cells in this model were conducted. Inocula were delivered by painting KFCs with a pipette tip as 10 μl was dispensed evenly over the surface of the square. In some experiments, inocula were dropped onto squares without mechanically perturbing the surface. Ultimately, uniform abrasion was achieved by using a sterile cytology brush with the tip bent at a right angle to the handle. The bristles of the brush were tapped lightly across the entire surface of each square, moving first horizontally and then vertically and along both diagonals. The inoculum was dropped onto the center of each square immediately after surface abrasion. The results presented here are from two independent experiments, with different lots of KFCs, abraded with cytology brushes before inoculation. Uninoculated control KFCs were also abraded. For each experiment, three to seven individual squares were included in each treatment or control group.
Bacterial recovery and sample collection.
At various times after inoculation, artificial foreskin samples were rinsed three times with 1 ml of phosphate-buffered saline (PBS) to remove loosely attached bacteria. To control for the effects of this procedure on subsequent microscopic and biochemical analyses, all samples, including uninoculated controls, were rinsed at the time of harvesting. Rinsed squares were quartered, and portions of each sample were fixed in 10% formalin for routine histology and 2% paraformaldehyde with 0.05% glutaraldehyde for electron microscopy, and these were frozen for future immunohistochemical analyses. The remaining quarter of samples infected with live H. ducreyi was incubated for 30 min in swelling buffer (1 mM EDTA, 10 mM Tris, 0.25 M sucrose) to render the eukaryotic cells osmotically fragile. Samples were then subjected to two 10-s bursts in a sonicating water bath, serially diluted in PBS, and plated on chocolate agar in duplicate. This procedure did not affect recovery from control H. ducreyi suspensions and resulted in lysis of the foreskin cells as judged by microscopic examination. Thus, potentially adherent and invasive bacteria would be recovered from these samples. Cell-associated counts recovered from one-fourth of a square (9 by 9 mm) were multiplied by 4 to normalize recovery to the entire sample. Likewise, the numbers of CFU per milliliter in PBS washes were multiplied by 3 for comparison with cell-associated counts.
At each sampling time, the 1 ml of culture medium beneath cell culture inserts was removed from all samples and replaced with fresh medium. Samples were aliquoted and stored at −70°C.
Cytokine assays.
Human TNF-α, IL-1α, IL-6, and IL-8 Quantikine kits (R&D Systems, Minneapolis, Minn.) were used with a slight modification of the manufacturer’s directions to determine cytokine concentrations in culture medium collected from KFCs. Sample and standard volumes (50 versus 100 μl) used were smaller than those suggested in product inserts for TNF-α and IL-1α. The minimum detectable concentrations were 4.4 and 0.5 pg/ml for TNF-α and IL-1α, respectively. Samples were diluted 1:100 and 1:1,000 to determine IL-6 and IL-8 concentrations, respectively. These assays were performed exactly as recommended by the manufacturer. The minimum detectable concentrations were 0.7 and 18 pg/ml for IL-6 and IL-8, respectively. Cytokine concentrations were expressed as picograms per milliliter for TNF-α and IL-1α and as nanograms per milliliter for IL-6 and IL-8.
Statistical analyses.
Data are presented as means ± standard deviations. H. ducreyi recovery data (Fig. 1) are from two separate experiments with a total of two to seven individual infected skin squares per time point, plated in duplicate. Cytokine concentrations (see Fig. 4) are from two separate experiments with a total of five to nine individual skin squares per time point, determined in duplicate. Since the data exhibited normal distributions, comparisons between treatment groups and controls were made by using the paired or unpaired Student’s t test, as indicated in the text.
FIG. 1.
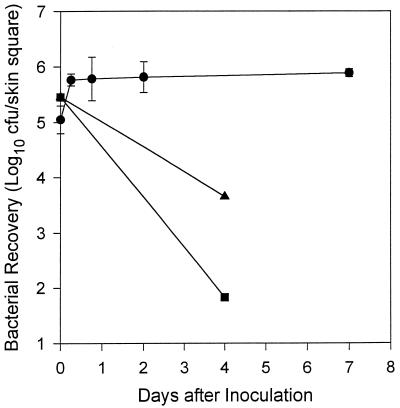
Time course of H. ducreyi recovery from infected artificial skin. Approximately 105 CFU of strain 35000 were inoculated onto the abraded surfaces of skin squares (9 by 9 mm). Total recovery from skin squares was calculated from quantitative cultures of rinsed plus adherent bacteria (circles). Data are from two independent experiments with a total of two to seven individual infected skin squares per time point, plated in duplicate. Data for H. ducreyi cells recovered from single samples of blank nylon mesh (squares) and cell culture medium without eukaryotic cells (triangles) are shown for comparison.
FIG. 4.
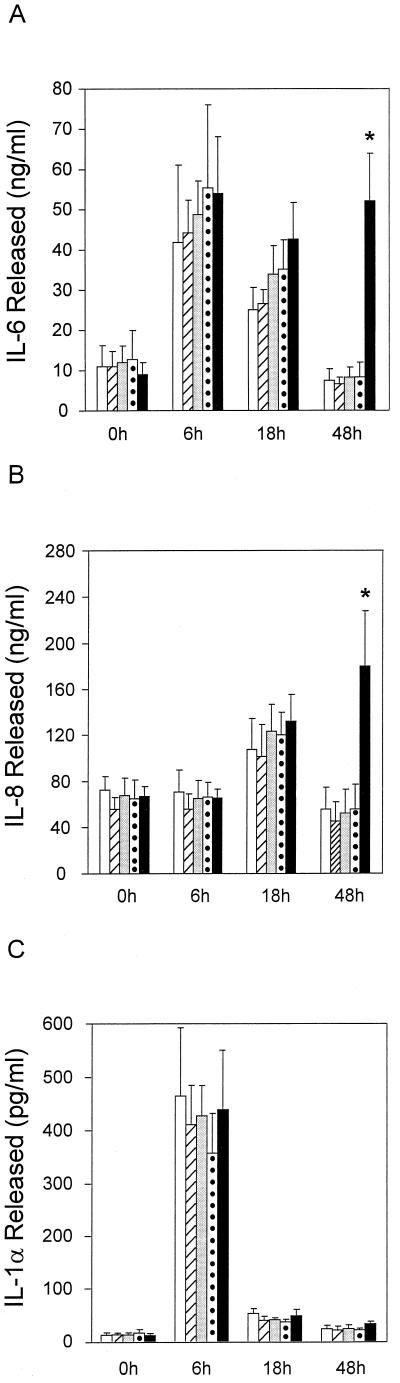
Cytokine secretion by artificial skin in response to H. ducreyi infection; kinetics of IL-6 (A), IL-8 (B), and IL-1α (C) secretion. Open bars, abraded, uninoculated skin squares; other bars, skin squares inoculated with BHI broth (hatched bars), H. ducreyi culture supernatant (lightly shaded bars), heat-killed H. ducreyi (dotted bars), and live H. ducreyi (dark bars) following abrasion. Data are from two independent experiments. Results are means ± standard deviations determined from a total of five to nine individual samples per time point, each assayed in duplicate. ∗, P < 0.01 compared to all other samples at 48 h (unpaired Student’s t test).
Microscopy.
For light microscopy, formalin-fixed samples were embedded in paraffin, processed for routine histology, and stained with hematoxylin and eosin. H. ducreyi were identified in these samples by visual comparison with pure cultures prepared in the same way. Fixed samples of KFCs inoculated with live or heat-killed H. ducreyi were embedded in Epon or Lowicryl resin as previously described (64) for transmission electron microscopy (TEM). Specimens were examined in a Zeiss electron microscope operating at 60 kV. For immunoelectron microscopy, grids containing sections of Lowicryl-embedded samples were probed with a 1:100 dilution of rabbit antiserum that had been raised against formalin-fixed, whole H. ducreyi 35000. Nonspecific antibody binding was blocked with 1% ovalbumin in PBS containing 0.01 M glycine. The secondary antibody was goat anti-rabbit immunoglobulin G conjugated to 15-nm gold particles. As a control, pure H. ducreyi cultures embedded in Lowicryl and labeled in this way routinely bound three to eight gold particles/bacterium (data not shown).
RESULTS
H. ducreyi replicate and persist in KFCs for 1 to 2 weeks.
We infected human foreskin KFCs with virulent H. ducreyi 35000. The kinetics of bacterial growth and recovery in the model are shown in Fig. 1. Approximately 5.0 log10 CFU of log-phase bacteria were inoculated onto the abraded surface of squares (9 by 9 mm) of artificial skin. H. ducreyi achieved a density of approximately 5.9 log10 CFU/square over the first 6 h. This culture was maintained with daily medium changes beneath the cell culture inserts for 7 days in these experiments (Fig. 1, circles). In a single experiment using an artificial skin square (11 by 11 mm) with a similar inoculum, 6.1 log10 CFU of H. ducreyi were recovered 14 days after inoculation. When 5.8 log10 CFU of H. ducreyi were inoculated onto nylon mesh squares without skin cells, 1.8 log10 CFU were recovered from the bare substrate at day 4 (Fig. 1, squares). Although bacteria were never immersed in fluid in the artificial skin system, we examined the growth of H. ducreyi in culture medium for comparison with growth in the intact system. In culture medium alone, without foreskin cells, H. ducreyi viability declined to 3.7 log10 CFU over 4 days (Fig. 1, triangles). Thus, human KFCs provided an environment that supported a steady-state bacterial culture that was not maintained in the absence of eukaryotic cells. H. ducreyi replicated and persisted in vitro in KFCs for up to 2 weeks, consistent with bacterial recovery from naturally occurring (37, 45) and experimental (52, 53) chancroid lesions of several days’ to several weeks’ duration.
In contrast to the moderate growth of H. ducreyi in artificial skin, E. coli grew vigorously, achieving a density of 8.2 log10 CFU/square 4 days after inoculation with 5.5 log10 CFU. Light microscopic inspection of E. coli-infected skin squares revealed massive cellular destruction with no intact foreskin cells remaining by day 4 (data not shown). S. epidermidis, often present on human skin, also grew well in artificial skin, reaching 8.7 log10 CFU 2 days after inoculation with 5.6 log10 CFU. Unlike KFCs infected with E. coli, foreskin cells appeared to remain grossly intact during infection with S. epidermidis (data not shown). Thus, H. ducreyi replication in the artificial skin model is modest by comparison with the other bacteria tested.
The data shown in Fig. 1 are total numbers of H. ducreyi cells recovered from infected KFCs. To examine bacterial adherence to artificial skin squares, each sample was rinsed three times with PBS, and H. ducreyi in the wash and cell-associated fractions were quantitated separately (see Materials and Methods). Six hours after inoculation, there were more cell-associated bacteria than were recovered in the wash (mean log10 CFU/square ± standard deviations = 5.7 ± 0.1 versus 5.05 ± 0.1; P <0.001 [paired Student’s t test]). At all later times, recoveries from cell-associated and wash fractions were equivalent (data not shown).
H. ducreyi can invade suprabasal keratinocytes and cause basal cell cytotoxicity.
Portions of artificial skin squares were prepared for light EM or 18 h after abrasion and inoculation with live or heat-killed H. ducreyi 35000. Using light microscopy, we saw aggregates of bacteria among the top layers of the stratum corneum in samples infected with live but not heat-killed bacteria (Fig. 2A and B). To further localize H. ducreyi within infected KFCs, we used TEM to examine the keratinocytes from the stratum corneum down to the basal layer and fibroblasts in the uppermost portion of the dermis. In samples embedded in Epon resin, occasional gram-negative bacteria were observed in suprabasal keratinocytes (Fig. 2C). The morphology of these bacteria was virtually identical to the morphology of control cultures of log-phase H. ducreyi processed and examined by EM (data not shown). In sections embedded in Lowicryl for immunoelectron microscopy, intracellular bacteria were labeled with anti-H. ducreyi rabbit antiserum (Fig. 2D) but not with normal rabbit serum (data not shown). Unlike the aggregates of bacteria observed in the uppermost layers of KFCs, intracellular H. ducreyi appeared as single cells. These organisms were rare (one or two organisms/tissue section) and did not appear to be contained in membrane-bound vacuoles.
In these studies, we did not identify H. ducreyi in or among basal keratinocytes, fibroblasts, or the extracellular material of the dermis 18 h after inoculation of abraded artificial skin. However, the architecture of the dermal-epidermal junction was dramatically altered as a result of infection with H. ducreyi 35000. We observed significant keratinocyte cytotoxicity in the basal cell region and in the lower layers of squamous epithelial cells 18 h after abraded KFCs were inoculated with live H. ducreyi, but not after abrasion and inoculation with heat-killed bacteria (Fig. 3).
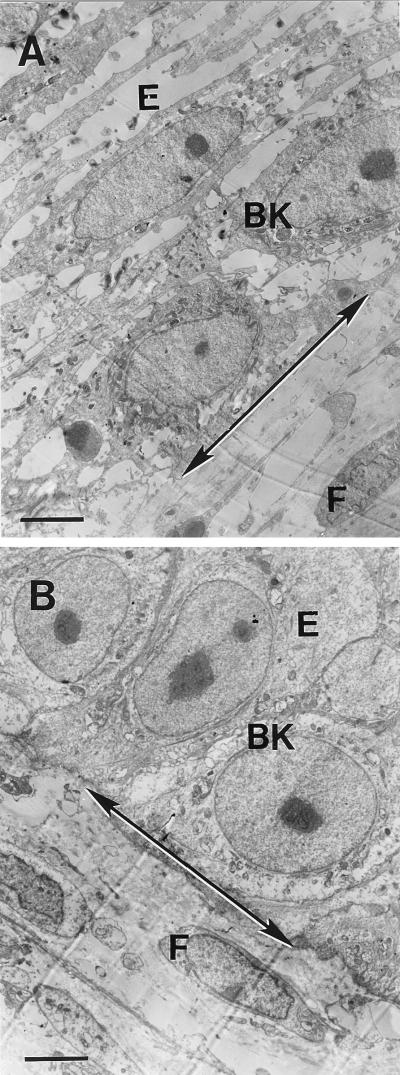
FIG. 3.
Dermal-epidermal junctions (arrows) of artificial skin 18 h after abrasion and inoculation with live (A) or heat-killed (B) H. ducreyi 35000. E, epidermis; BK, basal keratinocyte; F, fibroblast in dermis. Original magnification, ×2,550; bars, 3.9 mm.
Human foreskin cells secrete a unique pattern of cytokines in response to H. ducreyi infection in vitro.
We replaced the 1 ml of medium beneath cell culture inserts at various times after inoculation and determined the concentrations of TNF-α, IL-1α, IL-6, and IL-8 released by KFCs in response to H. ducreyi infection. Samples were collected from abraded artificial skin squares that had received live or heat-killed H. ducreyi, filtered supernatant from log-phase bacterial cultures, or BHI broth or were left untreated.
KFCs responded to H. ducreyi infection with increased secretion of IL-6 and IL-8 (Fig. 4A and B). The levels of secreted IL-6 were elevated in control and experimental samples 6 h after inoculation. IL-6 levels remained high in samples infected with live H. ducreyi, while they returned to baseline by 48 h in samples from KFCs that had received heat-killed H. ducreyi, filtered bacterial culture fluid, or BHI broth or from untreated controls (Fig. 4A). Forty-eight hours after inoculation, infected samples secreted at least 6.9 times more IL-6 than any of the controls (P < 0.01 [unpaired Student’s t test]).
The magnitude and kinetics of IL-8 secretion by infected KFCs were different from those of IL-6 secretion. IL-8 levels secreted at baseline were higher than IL-6 levels, and infected foreskin cells secreted three to five times more IL-8 than IL-6 at times ≥18 h after inoculation (notice the different y axis scales in Fig. 4A and B). IL-8 concentrations remained constant for all treatment groups and controls over the first 6 h and nearly doubled by 18 h after inoculation (Fig. 4B). IL-8 levels continued to increase in samples from KFCs infected with live H. ducreyi but returned to baseline by 48 h for all other samples. Infected foreskin cells secreted at least 3.2 times more IL-8 than any of the controls (P < 0.01 [unpaired Student’s t test]) 48 h after inoculation. Because the culture fluid beneath KFCs was removed and replaced with fresh medium at each time point, cytokine concentrations represent active secretion over the course of these experiments rather than accumulation and persistence of material released at early time points.
TNF-α levels in samples collected before inoculation and at 6, 18, or 48 h after inoculation ranged from ≤4.4 to 11.8 pg/ml, with no differences between experimental treatment groups and untreated abraded controls at any time point (n = 17). That KFCs were capable of TNF-α secretion was demonstrated by high levels of TNF-α in samples collected 24 h after inoculation with E. coli (273.2 pg/ml) and 48 h after inoculation with S. epidermidis (246.5 pg/ml).
KFCs released IL-1α over the first 6 h after inoculation in response to the abrasion technique; no experimental treatment elicited increased IL-1α secretion compared to untreated (but abraded) controls (Fig. 4C). By 18 h after inoculation, secreted IL-1α levels returned to baseline for all samples. The capacity for high level IL-1α secretion by foreskin cells in this model was demonstrated by their response to E. coli with 848.3 pg of IL-1α/ml 24 h after inoculation. Thus, KFCs released neither TNF-α nor IL-1α in response to H. ducreyi infection in vitro. However, the trauma of abrasion immediately prior to inoculation caused a peak of IL-1α secretion 6 h later.
DISCUSSION
Understanding the molecular mechanisms responsible for diseases caused by strict human pathogens such as H. ducreyi requires relevant in vitro models of infection. The development of increasingly sophisticated in vitro cell culture techniques has improved the approximation of human epithelial surfaces over standard cell monolayer cultures. We have used an artificial skin model, composed of normal human foreskin keratinocytes and fibroblasts, as a substrate for H. ducreyi infection. Artificial skin is a complex tissue with epidermal and dermal compartments separated by a well-developed basal lamina (13). Morphological and biochemical characterization indicate that this model recreates many of the structural and functional features of skin in vivo, including maintenance of a selective permeability barrier and cutaneous metabolic activity (50). Thus, the host cell cytokine responses to H. ducreyi infection in artificial skin and the behavior of the bacteria in this model may parallel critical steps in the pathogenesis of chancroid.
H. ducreyi infection of human foreskin KFCs stimulated increased secretion of the proinflammatory cytokines IL-6 and IL-8, but not IL-1α and TNF-α. The potent PMN chemoattractant activity of IL-8 is thought to be largely responsible for the local accumulation of inflammatory neutrophils, a hallmark of chancroid ulcers caused by H. ducreyi (20, 29). In psoriasis, an inflammatory skin disease, IL-8 production by keratinocytes is elevated in lesions in which it contributes to hyperproliferation of these cells (21). Similarly, acanthosis is a characteristic feature of the epidermis surrounding chancroid lesions (49). Our studies suggest that the chemoattractant and mitogenic properties of IL-8 produced by skin cells in response to H. ducreyi infection may be involved in chancroid ulcer development.
IL-6 is a pleiotropic cytokine produced by a variety of cells including keratinocytes and fibroblasts. Like IL-8, IL-6 stimulates keratinocyte proliferation, and it induces IL-2 and IL-2 receptor expression in T cells (33, 62). IL-2 expression is associated with a T-helper cell 1 (TH1)-type CD4 T-cell response; preliminary evidence from clinical observations and experimental H. ducreyi infection suggests that TH1 responses may predominate during the initial stages of chancroid. Soluble IL-2 receptors are elevated in the urine of chancroid patients (1), and gamma interferon mRNA, also a TH1-type cytokine, has been observed in experimental lesions in humans (52). Although the immune cells that produce these cytokines are absent from the artificial skin model used in the present studies, IL-6 and IL-8 released by keratinocytes and/or fibroblasts in response to H. ducreyi infection could be early signals resulting in the recruitment of PMNs and CD4 T cells to chancroid lesions.
The observation that differences between cytokines induced by live H. ducreyi and control preparations did not appear until 48 h after inoculation suggests that there may be environmental control of the H. ducreyi factors required for the cytokine induction seen. Future experiments comparing bacterial gene expression in the presence and absence of skin cells may identify important virulence factors required for chancroid ulcer production.
The relative lack of TNF-α and IL-1α responses in our studies was unexpected, since these two cytokines play a central role in initiating inflammatory responses to lipopolysaccharide (LPS) and microbial infection. IL-1α and TNF-α are potent inducers of IL-8, and increased production of both cytokines in keratinocyte-fibroblast cocultures in response to other stimuli (17) suggested that they could have been involved in skin cell responses to H. ducreyi infection. We did not observe TNF-α levels above baseline in response to inoculation with live or heat-killed H. ducreyi, bacterial culture supernatant, or BHI broth. Suppression of TNF-α expression in macrophages has recently been attributed to YopB, a plasmid-encoded outer membrane protein of Yersinia enterocolitica (7). H. ducreyi may utilize a similar evasion strategy against this component of the mucosal epithelial defense system. Alternatively, H. ducreyi may simply fail to trigger a TNF-α response in foreskin keratinocytes or fibroblasts. The H. ducreyi outer membrane contains LOS lacking the extensive saccharide side chains found in the LPS of gram-negative enteric bacteria (46). This structural difference may account for the lack of TNF-α secretion in our experiments. Indeed, E. coli dramatically stimulated TNF-α secretion in KFCs. Future studies with purified H. ducreyi LOS as a stimulus in the artificial skin model may address this issue.
In our studies of H. ducreyi infection of artificial skin, we noted a greater-than-20-fold increase in IL-1α secretion by KFCs over the first 6 h after inoculation (Fig. 4). This elevation did not require live H. ducreyi and appeared to be in response to the abrasion required to produce a productive infection in vitro. The nonspecific nature of increased IL-1α production in our studies does not rule out a role for this cytokine in the host response to chancroid. In naturally acquired and experimental chancroid, H. ducreyi requires breaks in the epidermis to enter the skin and cause infection (27, 38, 43, 53, 59).
Initially, we were uncertain of the barrier properties of artificial foreskin cell cultures with respect to H. ducreyi infection. In preliminary experiments, KFCs were painted with a pipette tip as the inoculum was dispensed evenly over the surface of the tissue square. Alternatively, inocula were allowed to drop onto squares without mechanically perturbing the surface. Cytokine responses of KFCs inoculated by the painting method were erratic, with discordant levels in replicate samples in the same treatment groups (data not shown). This result suggested that abrasion resulting from contact of the pipette tip with the surface of KFCs during inoculation was inadequate, since samples were not uniformly affected. That abrasion was required in the model was demonstrated by the relative lack of cytokine responses in KFCs inoculated by the drop method (data not shown).
The cytokine responses described herein resulted from uniform abrasion of the artificial skin surface immediately prior to inoculation, designed to approximate epidermal abrasions that occur during sexual intercourse. Our results suggest that relatively low levels of IL-1α may be present in genital skin as a result of such breaks in the epithelium. IL-1α induces physiologic responses at much lower concentrations (1 to 10 pg/ml) than other proinflammatory cytokines such as IL-8 (>10 ng/ml) (5). Thus, keratinocyte- or fibroblast-derived IL-1α may contribute to the cutaneous cytokine response to H. ducreyi infection in vivo.
The keratinocyte-fibroblast coculture system used in our studies recreates many of the structural and functional features of skin; however, it lacks key features of intact human skin that contribute to its immune functions in vivo. For example, Langerhans cells, potent antigen-presenting dendritic cells, are absent from the engineered epidermis and dermis. Langerhans cells play a critical role in cutaneous immune responses and have been shown to produce TNF, IL-1, IL-6, and IL-8 (12, 15, 18, 22, 54). The absence of peripheral blood-derived and resident skin leukocytes obviously reduces the complexity of the observable cytokine network, since PMNs, macrophages, and T cells are key components of the host immune response to H. ducreyi infection (38, 52). The cytokine responses documented here are only the potential contributions of foreskin keratinocytes and fibroblasts and thus represent a subset of the cutaneous immune response to H. ducreyi infection. Nevertheless, these are the predominant cell types in human skin, and keratinocytes are probably the first host cells with which H. ducreyi comes in contact during the initial stages of infection. Reconstitution of the current coculture system with dendritic cells, neutrophils, and/or lymphocytes may improve the model for use in defining and understanding the cytokine and ensuing cellular responses involved in chancroid ulcer formation.
The location of H. ducreyi within chancroid ulcers has not been well-characterized. Organisms can be recovered from clinical and experimental lesions of several days’ to several weeks’ duration (23, 43, 53) and are thus present in developing and mature lesions. Consistent with these observations, we recovered H. ducreyi from infected artificial skin at times ranging from 6 h to 2 weeks after inoculation. Early microscopic studies of chancroid biopsies occasionally demonstrated chains of bacilli located extracellularly at the base of ulcers (20, 35). We observed chains of H. ducreyi among the keratin layers on the surface of infected keratinocyte-fibroblast cocultures 18 h after inoculation. Many of these bacteria were only loosely associated with artificial skin, since we consistently recovered equivalent numbers of bacteria in the wash and cell-associated fractions ≥18 h after inoculation. These tangles of H. ducreyi are reminiscent of the microcolonies described by Alfa and colleagues (3, 4) and Lammel et al. (32) on the surface of infected fibroblast monolayers; however, we did not observe bacteria in the dermis, where fibroblasts are located in this model.
In addition to the surface bacteria, we observed H. ducreyi within suprabasal keratinocytes 18 h after inoculation. These bacteria were consistent in size and shape with reported characteristics of Ducrey’s bacillus. The range of variation in size is 0.5 to 2.5 mm long by 0.3 to 1.0 mm broad, and the organism is “frequently constricted in the middle” (26). The mottled interior of the organisms in artificial skin is typical of H. ducreyi (Fig. 2). Intracellular H. ducreyi cells were rare and were seen only as single longitudinal or cross-sections that appeared to be free in the keratinocyte cytoplasm (Fig. 2). The apparent paucity of H. ducreyi below the surface of infected artificial skin is consistent with the relatively low numbers of organisms recovered from lesions in experimental human infections (52, 53). Keratinocytes containing bacteria were typically three to four layers deep in the epidermis and two to three layers above the basal keratinocytes.
We did not see H. ducreyi in the basal keratinocyte region of infected artificial skin. Nevertheless, 18 h after abrasion and inoculation with live but not heat-killed bacteria, the architecture of the basal cell layer was disrupted and the keratinocytes appeared necrotic. This disruption was localized to the basal cell region, as suprabasal keratinocytes were grossly unaffected by infection and the overall architecture of the epidermal compartment remained intact (Fig. 2A and B). H. ducreyi elaborates several toxins that may be important virulence factors for ulcer formation. A bacterial cell-associated hemolysin belonging to the Proteus-Serratia family of pore-forming toxins has been characterized by Palmer et al. (41) and Totten et al. (60). Toxic activity of the H. ducreyi hemolysin requires bacterial contact with host target cells and is relatively specific for fibroblasts in vitro (41). The structural changes that we observed at the dermal-epidermal junction of H. ducreyi-infected artificial skin were probably not caused by the hemolysin, since bacteria were not evident in the zones of destruction. H. ducreyi also produces a secreted cytotoxin with homology to the cytolethal distending toxins (CDTs) of E. coli (47) and Campylobacter species (42). The H. ducreyi CDT is active against HeLa cells in vitro (14); its relative toxicity for keratinocytes and fibroblasts has not yet been described. Given its potential for activity at a distance from bacterial cells and the sensitivity of at least some epithelial cells to the toxin, the soluble H. ducreyi CDT may be responsible for the cytotoxicity that we noted at the basal keratinocyte layer of infected KFCs. Experiments with toxin-deficient mutants in the artificial foreskin model of infection should provide further information on the role of CDT in H. ducreyi virulence.
The artificial human skin model described here provides a relevant in vitro system for studying the pathogenesis of H. ducreyi infection. Using the in vitro skin model, we intend to characterize the pathogenic potential of defined H. ducreyi mutants to identify virulence factors important in the development of chancroid ulcers.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants 1F32 AI09496 to M.M.H. and U01 AI31496 to the North Carolina Sexually Transmitted Diseases Center and a University of North Carolina Faculty Research grant to T.H.K.
We are grateful to Vicki Madden and Robert Bagnell of the Pathology Electron Microscopy Core Facility for assistance with sample processing and use of the TEM and to Johnny Carson of the Department of Pediatrics Electron Microscopy Core Facility for use of the TEM. We thank Lani San Mateo and Myron S. Cohen for critically reviewing the manuscript.
REFERENCES
- 1.Abeck D, Korting H C, Zaba R, Dangor Y, Fehler G, Ballard R C. Soluble interleukin-2 receptors in serum and urine of patients with chancroid and their response to therapy. Int J Sex Transm Dis AIDS. 1990;1:282–284. doi: 10.1177/095646249000100411. [DOI] [PubMed] [Google Scholar]
- 2.Alfa M J. Cytopathic effect of Haemophilus ducreyi for human foreskin cell culture. J Med Microbiol. 1992;37:43–50. doi: 10.1099/00222615-37-1-43. [DOI] [PubMed] [Google Scholar]
- 3.Alfa M J, DeGagne P, Hollyer T. Haemophilus ducreyi adheres to but does not invade cultured human foreskin cells. Infect Immun. 1993;61:1735–1742. doi: 10.1128/iai.61.5.1735-1742.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alfa M J, Stevens M K, DeGagne P, Klesney-Tait J, Radolf J D, Hansen E J. Use of tissue culture and animal models to identify virulence-associated traits of Haemophilus ducreyi. Infect Immun. 1995;63:1754–1761. doi: 10.1128/iai.63.5.1754-1761.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baggliolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CxC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 6.Becker T M, DeWitt W, Van Dusen G. Haemophilus ducreyi infection in south Florida: a rare disease on the rise? South Med J. 1987;80:182–184. doi: 10.1097/00007611-198702000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Beuscher H U, Rodel F, Forsberg A, Rollinghoff M. Bacteria evasion of host immune defense: Yersinia enterocolitica encodes a supressor for tumor necrosis factor alpha expression. Infect Immun. 1995;63:1270–1277. doi: 10.1128/iai.63.4.1270-1277.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blackmore C A, Limpakarnjanarat K, Rigau-Perez J G, Albritton W L, Greenwood J R. An outbreak of chancroid in Orange County, California: descriptive epidemiology and disease-control measures. J Infect Dis. 1985;151:840–844. doi: 10.1093/infdis/151.5.840. [DOI] [PubMed] [Google Scholar]
- 9.Brentjens R J, Ketterer M, Apicella M A, Spinola S M. Fine tangled pili expressed by Haemophilus ducreyi are a novel class of pili. J Bacteriol. 1996;178:808–816. doi: 10.1128/jb.178.3.808-816.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brentjens R J, Spinola S M, Campagnari A A. Haemophilus ducreyi adheres to human keratinocytes. Microb Pathog. 1994;16:243–247. doi: 10.1006/mpat.1994.1025. [DOI] [PubMed] [Google Scholar]
- 11.Campagnari A A, Wild L M, Griffiths G E, Karalus R J, Wirth M A, Spinola S M. Role of lipooligosaccharides in experimental dermal lesions caused by Haemophilus ducreyi. Infect Immun. 1991;59:2601–2608. doi: 10.1128/iai.59.8.2601-2608.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caux C, Massacrier C, Vandervliet B, Dubois B, Van Kooten C, Durand I, Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Contard P, Bartel T L, Jacobs II L, Perlish J S, MacDonald II E D, Handler L, Cone D, Fleishmajer R. Culturing keratinocytes and fibroblasts in a three-dimensional mesh results in epidermal and formation of a basal lamina-anchoring zone. J Invest Dermatol. 1993;100:35–39. doi: 10.1111/1523-1747.ep12349952. [DOI] [PubMed] [Google Scholar]
- 14.Cope L D, Lumbley S, Latimer J L, Klesney-Tait J, Stevens M K, Johnson L S, Purven M, Munson R S, Jr, Lagergard T, Radolf J D, Hansen E J. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad Sci USA. 1997;94:4056–4061. doi: 10.1073/pnas.94.8.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cumberbatch M, Dearman R J, Kimber I. Constitutive and inducible expression of interleukin-6 by Langerhans cells and lymph node dendritic cells. Immunology. 1996;87:513–518. doi: 10.1046/j.1365-2567.1996.504577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiCarlo R P, Armentor B S, Martin D H. Chancroid epidemiology in New Orleans men. J Infect Dis. 1995;172:446–452. doi: 10.1093/infdis/172.2.446. [DOI] [PubMed] [Google Scholar]
- 17.Edwards S M, Donnelly T A, Sayre R M, Rheins L A, Spielmann H, Liebsch M. Quantitative in vitro assessment of phototoxicity using a human skin model, Skin2. Photodermatol Photoimmunol Photomed. 1994;10:111–117. [PubMed] [Google Scholar]
- 18.Enk A H, Angeloni V L, Udey M C, Katz S I. An essential role for Langerhans cell-derived IL-1 beta in the initiation of primary immune responses in skin. J Immunol. 1993;150:3698–3704. [PubMed] [Google Scholar]
- 19.Farris J R, Hutcheson D, Cartwright G, Glover J H. Chancroid in Dallas: new lessons from an old disease. Tex Med. 1991;87:78–81. [PubMed] [Google Scholar]
- 20.Freinkel A L. Histological aspects of sexually transmitted genital lesions. Histopathology. 1987;11:819–831. doi: 10.1111/j.1365-2559.1987.tb01885.x. [DOI] [PubMed] [Google Scholar]
- 21.Gearing A J H, Fincham N J, Bird C R, Wadhwa M, Meager A, Cartwright J E, Camp R D R. Cytokines in skin lesions of psoriasis. Cytokine. 1990;2:68–75. doi: 10.1016/1043-4666(90)90045-u. [DOI] [PubMed] [Google Scholar]
- 22.Groves R W, Allen M H, Ross E L, Barker J N, MacDonald D M. Tumour necrosis factor alpha is pro-inflammatory in normal human skin and modulates cutaneous adhesion molecule expression. Br J Dermatol. 1995;132:345–352. doi: 10.1111/j.1365-2133.1995.tb08666.x. [DOI] [PubMed] [Google Scholar]
- 23.Hammond G W. A history of the detection of Haemophilus ducreyi, 1889–1979. Sex Transm Dis. 1996;23:93–96. doi: 10.1097/00007435-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Hammond G W, Lian C J, Wilt J C, Ronald A R. Antimicrobial susceptibility of Haemophilus ducreyi. Antimicrob Agents Chemother. 1978;13:608–612. doi: 10.1128/aac.13.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen E J, Lumbley S R, Richardson J A, Purcell B K, Stevens M K, Cope L D, Datte J, Radolf J D. Induction of protective immunity to Haemophilus ducreyi in the temperature-dependent rabbit model of experimental chancroid. J Immunol. 1994;152:184–192. [PubMed] [Google Scholar]
- 26.Hewlett R T. Chancroid and Bacillus ducreyii and other organisms. In: Fildes P, Ledingham J C G, editors. A system of bacteriology in relation to medicine. Vol. 2. London, United Kingdom: His Majesty’s Stationery Office; 1929. pp. 411–416. [Google Scholar]
- 27.Hobbs M M, San Mateo L R, Orndorff P E, Almond G, Kawula T H. Swine model of Haemophilus ducreyi infection. Infect Immun. 1995;63:3094–3100. doi: 10.1128/iai.63.8.3094-3100.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joseph T D, Bose S K. A heat-labile protein of Chlamydia trachomatis binds to HeLa cells and inhibits the adherence of chlamydiae. Proc Natl Acad Sci USA. 1991;88:4054–4058. doi: 10.1073/pnas.88.9.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King R, Gough J, Ronald A, Nasio J, Ndinya-Achola J O, Plummer F, Wilkins J A. An immunohistochemical analysis of naturally occurring chancroid. J Infect Dis. 1996;174:427–430. doi: 10.1093/infdis/174.2.427. [DOI] [PubMed] [Google Scholar]
- 30.Lagergard T. The role of Haemophilus ducreyi bacteria, cytotoxin, endotoxin and antibodies in animal models for study of chancroid. Microb Pathog. 1992;13:203–217. doi: 10.1016/0882-4010(92)90021-f. [DOI] [PubMed] [Google Scholar]
- 31.Lagergard T, Purven M, Frisk A. Evidence of Haemophilus ducreyi adherence to and cytotoxin destruction of human epithelial cells. Microb Pathog. 1993;14:417–431. doi: 10.1006/mpat.1993.1041. [DOI] [PubMed] [Google Scholar]
- 32.Lammel C J, Dekker N P, Palefsky J, Brooks G F. In vitro model of Haemophilus ducreyi adherence to and entry into eukaryotic cells of genital origin. J Infect Dis. 1993;167:642–650. doi: 10.1093/infdis/167.3.642. [DOI] [PubMed] [Google Scholar]
- 33.Lotz M, Jirik F, Kabouridis P, Tsoukas C, Hirano T, Kishimoto T, Carson D A. B cell stimulating factor 2/interleukin 6 is a costimulant for human thymocytes and T lymphocytes. J Exp Med. 1988;167:1253–1258. doi: 10.1084/jem.167.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manoil C, Beckwith J. TnphoA; a transposon probe for protein export signals. Proc Natl Acad Sci USA. 1985;82:8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marsch W C, Haas N, Stuttgen G. Ultrastructural detection of Haemophilus ducreyi in biopsies of chancroid. Arch Dermatol Res. 1978;263:153–157. doi: 10.1007/BF00446436. [DOI] [PubMed] [Google Scholar]
- 36.Martin D H, DiCarlo R P. Recent changes in the epidemiology of genital ulcer disease in the United States. The crack cocaine connection. Sex Transm Dis. 1994;21:S76–S80. [PubMed] [Google Scholar]
- 37.Mauff A C, Ballard R C, Bilgeri Y R, Koornhof H J. Isolation of Haemophilus ducreyi from genital ulcerations in white men in Johannesburg. S Afr Med J. 1983;63:236–237. [PubMed] [Google Scholar]
- 38.Morse S A. Chancroid and Haemophilus ducreyi. Clin Microbiol Rev. 1989;2:137–157. doi: 10.1128/cmr.2.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naughton G K, Jacob L, Naughton B A. A physiological skin model for in vitro toxicity studies. In: Goldberg A M, editor. Alternative methods in toxicology. New York, N.Y: Mary Ann Leibert; 1989. pp. 183–187. [Google Scholar]
- 40.Nzila N, Laga M, Thiam M A, Mayimona K, Edidi B, Van Dyck E, Behets F, Hassig S, Nelson A, Mokwa K. HIV and other sexually transmitted diseases among female prostitutes in Kinshasa. AIDS. 1991;5:715–721. doi: 10.1097/00002030-199106000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Palmer K L, Goldman W E, Munson R S., Jr An isogenic haemolysin-deficient mutant of Haemophilus ducreyi lacks the ability to produce cytopathic effects on human foreskin fibroblasts. Mol Microbiol. 1996;21:13–19. doi: 10.1046/j.1365-2958.1996.00615.x. [DOI] [PubMed] [Google Scholar]
- 42.Pickett C L, Pesci E C, Cottle D L, Russell G, Erdem A N, Zeytin H. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter sp. cdtB gene. Infect Immun. 1996;6:2070–2078. doi: 10.1128/iai.64.6.2070-2078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Purcell B K, Richardson J A, Radolf J D, Hansen E J. A temperature-dependent rabbit model for production of dermal lesions by Haemophilus ducreyi. J Infect Dis. 1991;164:359–367. doi: 10.1093/infdis/164.2.359. [DOI] [PubMed] [Google Scholar]
- 44.Purven M, Falsen E, Lagergard T. Cytotoxin production in 100 strains of Haemophilus ducreyi from different geographic locations. FEMS Microbiol Lett. 1995;129:221–224. doi: 10.1111/j.1574-6968.1995.tb07583.x. [DOI] [PubMed] [Google Scholar]
- 45.Schmid G P, Faur Y C, Valu J A, Sikandar S A, McLaughlin M M. Enhanced recovery of Haemophilus ducreyi from clinical specimens by incubation at 33 versus 35 degrees C. J Clin Microbiol. 1995;33:3257–3259. doi: 10.1128/jcm.33.12.3257-3259.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schweda E K, Jonasson J A, Jansson P E. Structural studies of lipooligosaccharides from Haemophilus ducreyi ITM 5535, ITM 3147, and a fresh clinical isolate, ACY1: evidence for intrastrain heterogeneity with the production of mutually exclusive sialylated or elongated glycoforms. J Bacteriol. 1995;177:5316–5321. doi: 10.1128/jb.177.18.5316-5321.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scott D A, Kaper J B. Cloning and sequencing of the genes encoding Escherichia coli cytolethal distending toxin. Infect Immun. 1994;62:244–251. doi: 10.1128/iai.62.1.244-251.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaw J H, Falkow S. Model for invasion of human tissue culture cells by Neisseria gonorrhoeae. Infect Immun. 1988;56:1625–1632. doi: 10.1128/iai.56.6.1625-1632.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheldon W H, Heyman A. Studies on chancroid. Am J Pathol. 1945;22:415–425. [PubMed] [Google Scholar]
- 50.Slivka S R, Landeen L K, Zeigler F, Zimber M P, Bartel R L. Characterization, barrier functions, and drug metabolism of an in vitro skin model. J Invest Dermatol. 1993;100:40–46. doi: 10.1111/1523-1747.ep12354098. [DOI] [PubMed] [Google Scholar]
- 51.Slivka S R, Zeigler F, Landeen L, Bartel R L. Keratinocytes modulate extracellular matrix deposition in a novel dermal model. J Invest Dermatol. 1992;98:621A. [Google Scholar]
- 52.Spinola S M, Orazi A, Arno J N, Fortney K, Kotylo P, Chen C Y, Campagnari A A, Hood A F. Haemophilus ducreyi elicits a cutaneous infiltrate of CD4 cells during experimental human infection. J Infect Dis. 1996;173:394–402. doi: 10.1093/infdis/173.2.394. [DOI] [PubMed] [Google Scholar]
- 53.Spinola S M, Wild L M, Apicella M A, Gaspari A A, Campagnari A A. Experimental human infection with Haemophilus ducreyi. J Infect Dis. 1994;169:1146–1150. doi: 10.1093/infdis/169.5.1146. [DOI] [PubMed] [Google Scholar]
- 54.Srivastava B I, Srivastava A, Srivastava M D. Phenotype, genotype and cytokine production in acute leukemia involving progenitors of dendritic Langerhans’ cells. Leuk Res. 1994;18:499–511. doi: 10.1016/0145-2126(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 55.Stoppie P, Borghgraef P, De Wever B, Geysen J, Borgers M. The epidermal architecture of an in vitro reconstructed human skin equivalent (Advanced Tissue Sciences Skin2 models ZK 1300/2000) Eur J Morphol. 1993;31:26–29. [PubMed] [Google Scholar]
- 56.Taniguchi Y, Suzuki K, Nakajima K, Nakajima M, Miwa Y, Yamada Y, Satoh M, Takeyoshi M, Akie Y, Moriyasu M. Inter-laboratory validation study of the Skin2 Dermal model ZK1100 and MTT cytotoxicity assay kits. J Toxicol Sci. 1994;19:37–44. doi: 10.2131/jts.19.37. [DOI] [PubMed] [Google Scholar]
- 57.Thomas D D, Higbie L M. In vitro association of leptospires with host cells. Infect Immun. 1990;58:581–585. doi: 10.1128/iai.58.3.581-585.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Totten P A, Lara J C, Norn D V, Stamm W E. Haemophilus ducreyi attaches to and invades human epithelial cells in vitro. Infect Immun. 1994;62:5632–5640. doi: 10.1128/iai.62.12.5632-5640.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Totten P A, Morton W R, Knitter G H, Clark A M, Kiviat N B, Stamm W E. A primate model for chancroid. J Infect Dis. 1994;169:1284–1290. doi: 10.1093/infdis/169.6.1284. [DOI] [PubMed] [Google Scholar]
- 60.Totten P A, Norn D V, Stamm W E. Characterization of the hemolytic activity of Haemophilus ducreyi. Infect Immun. 1995;63:4409–4416. doi: 10.1128/iai.63.11.4409-4416.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trees D L, Morse S A. Chancroid and Haemophilus ducreyi: an update. Clin Microbiol Rev. 1995;8:357–375. doi: 10.1128/cmr.8.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Uyttenhove C, Coulie P G, Van Snick J. T cell growth and differentiation induced by interleukin-HP1/IL-6, the murine hybridoma/plasmacytoma growth factor. J Exp Med. 1988;167:1417–1427. doi: 10.1084/jem.167.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vickers A E, Biggi W A, Dannecker R, Fischer V. Uptake and metabolism of cyclosporin A and SDZ IMM 125 in the human in vitro skin2 dermal and barrier function models. Life Sci. 1995;57:215–224. doi: 10.1016/0024-3205(95)00265-8. [DOI] [PubMed] [Google Scholar]
- 64.Wyrick P B, Choong J, Knight S T, Goyeau D, Stuart E S, MacDonald A B. Chlamydia trachomatis antigens on the surface of infected human endometrial epithelial cells. Immunol Infect Dis. 1994;4:131–141. [Google Scholar]