Abstract
Bone marrow stromal cell antigen 2 (BST2) is a type II transmembrane protein that serves critical roles in antiretroviral defense in the innate immune response. In addition, it has been suggested that BST2 is highly expressed in various types of human cancer and high BST2 expression is related to different clinicopathological parameters in cancer. The molecular mechanism underlying BST2 as a potential tumor biomarker in human solid tumors has been reported on; however, to the best of our knowledge, there has been no review published on the molecular mechanism of BST2 in human solid tumors. The present review focuses on human BST2 expression, structure and functions; the molecular mechanisms of BST2 in breast cancer, hepatocellular carcinoma, gastrointestinal tumor and other solid tumors; the therapeutic potential of BST2; and the possibility of BST2 as a potential marker. BST2 is involved in cell membrane integrity and lipid raft formation, which can activate epidermal growth factor receptor signaling pathways, providing a potential mechanistic link between BST2 and tumorigenesis. Notably, BST2 may be considered a universal tumor biomarker and a potential therapeutical target.
Keywords: BST2, molecular mechanism, therapeutic target, cancer, lipid raft, EGFR
1. Introduction
Bone marrow stromal cell antigen 2 (BST2), which was originally named HM1.24, is a cell transmembrane protein that was initially reported to exhibit increased expression in multiple myeloma in 1994 (1). Later, BST2 was renamed tetherin and CD317, and was known as a potent antiviral host factor, due to its ability to inhibit the release of human immunodeficiency virus 1 (HIV-1) viral particles, which could be antagonized by the viral membrane HIV viral protein U (2). BST2 can regulate the response of hosts to viral infection either by restraining the generation of new viral particles or by inhibiting viral dissemination across viral synapses and in a monocyte-to-endothelial cell model (3). A decade later, the gene was renamed BST2, due to its expression on the membrane of bone marrow stromal cells, and it was revealed to be associated with the growth of pre-B cells through promoting interactions between cells (4). BST2 consists of 180 amino acids and is located on chromosome 19p13.2. The BST2 gene is widely expressed in numerous cells, including hepatocytes, plasma blast cells, early plasma cells, mature B cells, dendritic cells, pneumocytes, monocytes, pancreatic cells, kidney cells and vascular endothelial cells, suggesting that it plays vital roles in the innate immune response against viral infection and other physiological processes (2,5–7). An increasing number of studies have shown that BST2 upregulation is associated with numerous types of cancer, such as multiple myeloma (8,9), endometrial cancer (10), gastric cancer (11), glioblastoma multiforme (12), primary lung cancer (13), nasopharyngeal cancer (14), oral cavity cancer (15), oral squamous cell carcinoma (OSCC) (16), cervical cancer (17), breast cancer (18), pancreatic cancer (19), hepatocellular carcinoma (HCC) (20), colorectal cancer (21,22), head and neck squamous cell carcinoma (23), ovarian cancer (24), esophageal squamous cell carcinoma (22) and bladder cancer (25). The role and molecular mechanism of high BST2 expression have been investigated in several types of cancer; however, a review of the molecular mechanism of BST2 in these cancers not been reported.
The present review illuminates the structure and functions of the BST2 gene, and describes the association between high BST2 expression and clinicopathological parameters. Furthermore, the molecular mechanisms of high BST2 expression in breast cancer, HCC, gastrointestinal tumors and other solid tumors are considered, and whether BST2 could be used as a potential therapeutic target as a cell transmembrane protein is discussed.
2. Methods
Search strategy and study selection
The present review is a narrative review regarding the role of BST2 expression in cancer. To evaluate the clinical outcomes and prognostic significance of BST2 protein expression in cancer, eligible studies were searched for in the following databases: PubMed (https://pubmed.ncbi.nlm.nih.gov), Web of Knowledge (https://webofscience.clarivate.cn/wos/woscc/basic-search) and Cochrane Library (https://www.cochranelibrary.com/?contentLanguage=eng), between January 1970 and June 2023. The following key words were used for searching: ‘BST2’, ‘HM1.24’, ‘CD317’, ‘tetherin’, ‘Bone marrow stromal cell antigen 2’ and ‘human cancer’.
The inclusion criteria for the primary studies, which are shown in Table I, were as follows: i) Studies that investigated the relationship between BST2 gene expression and clinical characteristics in human cancer; ii) studies that measured BST2 expression via immunohistochemistry (IHC) and reverse transcription-quantitative (q)PCR; iii) studies that were published as full-text articles and in English. Finally, eight studies were collected to analyze the relationship between clinicopathological parameters and BST2 upregulation.
Table I.
Relationship between clinicopathological parameters and BST2 upregulation, as detected by immunohistochemistry.
| Association with high BST2 expression | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Type of cancer | Age | Sex | T stage | N stage | M stage | Lymphatic invasion | Vascular invasion | Tumor differentiation | Other outcomes | (Refs.) |
| Gastric cancer | - | NR | NR | + | + | + | NR | NR | Tumor size (−) Pathological grade (−) | (74) |
| Lymph node metastasis (+) | ||||||||||
| - | - | + | - | - | + | + | NR | Histological classification (+) HER2 expression (−) | (22) | |
| Oral squamous cell carcinoma | - | - | - | NR | NR | NR | NR | - | Tumor depth (+) Perineural invasion (+) Bone invasion (−) | (15) |
| Renal cell carcinoma | + | - | + | + | + | NR | NR | NR | Overall survival (+) Histological classification (−) | (87) |
| HCC | - | - | - | - | - | NR | NR | - | Tumor number (+) Overall survival (+) Tumor size (+) | (20) |
| Bladder cancer | - | - | + | NR | NR | - | - | NR | Cellular atypia classification (−) | (25) |
| Colorectal cancer | - | - | + | + | + | - | - | NR | Histological classification (−) | (22) |
| NR | NR | - | - | - | NR | NR | - | Microsatellite status (+) Histological type (−) | (79) | |
| - | - | + | + | + | NR | NR | NR | Overall survival (+) Pathological grade (−) Histological type (−) | (77) | |
| Esophageal squamous cell carcinoma | - | - | - | - | NR | + | - | NR | Histological classification (−) Poor survival (+) | (22) |
+, positive association; -, negative association; BST2, bone marrow stromal cell antigen 2; HCC, hepatocellular carcinoma; NR, not reported.
An independent search was carried out by HY and QB with the same search method. Differences were discussed with other authors until a consensus was reached on each item.
3. Structure and function of BST2
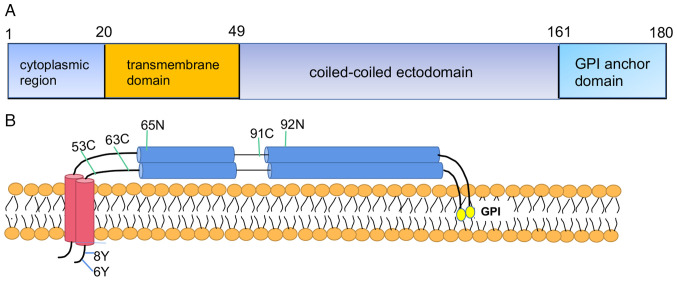
Structurally, BST2, as a type II transmembrane protein, is composed of four different regions, as follows: N-terminal cytoplasmic region, accompanied by a transmembrane region (TM), coiled-coiled ectodomain and C-terminal glycosylphosphatidylinositol (GPI)-anchor site (26). The cytoplasmic region of BST2 contains two highly conserved tyrosine domains, which might activate other signaling pathways (27). The BST2 cytoplasmic tail could also induce tumor cell invasion (28). The BST2 gene is expressed as a monomeric structure. When the BST2 gene is located on the cell membrane, two parallel monomer BST2 proteins undergo dimerization by disulfide-linking of three cysteine residues (29). The cysteine residues of human BST2 are located at positions 53, 63 and 91 (30). Dimerized BST2 is in an activated state and is essential for its antiviral activity (29,31). The glycosylation of the BST2 ectodomain is modified at two asparagine residues, which are located at positions 65 and 92 (32). Dimerized BST2 is anchored into the cell membrane and is responsible for virus/exosome tethering (33), signaling potential (34) and organization of membrane microdomains (35). BST2 contains a lipid raft in the GPI anchor motif and the TM domain (32,36). The GPI site can affect the transduction of cellular activation or inhibition signals, resulting in Ca2+ fluxes, protein tyrosine phosphorylation or cytokine secretion (37,38). The highly conserved YxY motif in the cytoplasmic region is important in nuclear factor (NF)-κB activation and for association with the actin cytoskeleton (34,39). The structure of BST2 is shown in Fig. 1. BST2 may interact with membrane proteins and activate signaling pathways through lipid raft flux. In addition, BST2 is located mainly on the cell membrane and is vital for protecting membrane integrity by modulating the cytoskeleton and protecting cancer cells from natural killer cell-mediated cytolysis (26,27); BST2 can also be located in the membrane of trans-Golgi and endosomes (27). Notably, BST2 is involved in cell membrane integrity and lipid raft formation, and can activate Ca2+ and NF-κB signaling pathways, providing a potential mechanistic link between BST2 and tumorigenesis.
Figure 1.
Schematic representation of human BST2 structure. (A) BST2 protein consists of four domains: N-terminal cytoplasmic region, transmembrane region, coiled-coiled ectodomain and C-terminal GPI-anchor domain. (B) Localization of BST2 on the cell membrane. Double tyrosine (Y) at positions 6 and 8 is vital for downstream factor activation. Cysteine (C) at positions 53, 63, and 91 is responsible for the formation of BST2 dimers. Asparagine (N) residues at positions 65 and 92 are glycosylation sites. The number represents the position of the amino acid. BST2, bone marrow stromal cell antigen 2; GPI, glycosylphosphatidylinositol.
4. BST2 and viral defense
BST2 is a restriction factor that can assist the host immune system against invading pathogens. Notably, BST2 was originally shown to prevent the release of HIV particles (2). In addition, BST2 can bind to other envelope proteins, and inhibit the release of several other viruses, including hepatitis C virus (HCV), hepatitis B virus (HBV), measles virus (MV) (40) and severe acute respiratory syndrome coronavirus 2 (41). Viral infection often stimulates interferon (IFN), which in turn can lead to high BST2 expression (42). There are various inflammatory cytokine binding sites in the BST2 gene promoter region, suggesting that BST2 expression can be regulated by inflammatory cytokines (18). After viral infection, plasmacytoid dendritic cells (pDCs) can quickly produce numerous type I IFNs, upregulating BST2 expression. Subsequently, increased BST2 expression on the membrane of pDCs has a cis interaction with immunoglobulin-like transcript 7 (ILT7) to protect cells from viral infection (43). BST2 may also inhibit replication and transmission of influenza A virus by enhancing endoplasmic reticulum stress-induced apoptosis signals (44). These findings indicated that BST2 is an important innate immune molecule against viral infections.
5. BST2: Roles in carcinogenesis
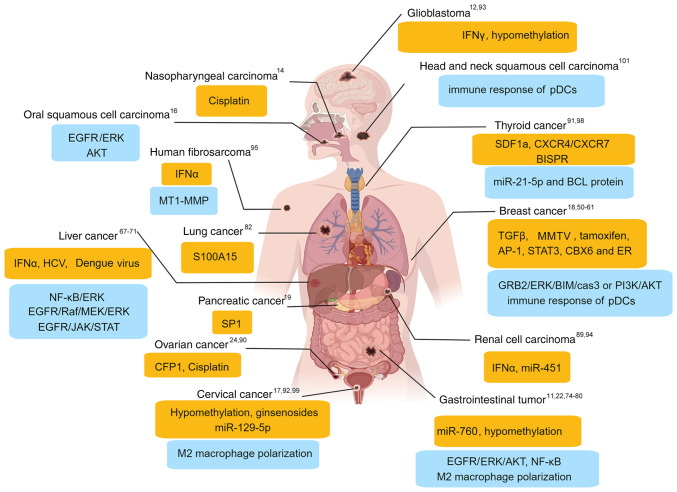
As well as the antiviral function of BST2, accumulating evidence has suggested that high BST2 expression is involved in several tumors, including hematological malignancies (such as multiple myeloma) (8), Down syndrome megakaryocytic leukemia (45) and solid tumors. The relationship between BST2 upregulation and clinicopathological characteristics is listed in Table I. The upstream factors and downstream signaling pathways of BST2 are summarized in Fig. 2. The present review focuses on the molecular mechanism of BST2 in human solid tumors.
Figure 2.
Schematic representation of molecular regulation of BST2. The upstream factors (yellow box) and downstream signaling factors or pathways (blue box) of BST2 in solid cancer are shown. The superscript numbers refer to the corresponding references. AKT, serine/threonine kinase; BISPR, BST2 IFN-stimulated positive regulator; BST2, bone marrow stromal cell antigen 2; cas3, caspase 3; CBX6, chromobox 6; ER, estrogen receptor; ERK, extracellular signal-regulated kinase; GRB2, growth factor receptor bound protein 2; HCV, hepatitis C virus; IFN, interferon; JAK, Janus kinase; miR, microRNA; MMTV, mouse mammary tumor virus; pDCs, plasmacytoid dendritic cells; PI3K, phosphoinositide 3-kinase; STAT3, signal transducer and activator of transcription 3; TGFβ, transforming growth factor-β.
BST2 and breast cancer
Breast carcinogenesis is generally accompanied by several genetic and epigenetic variations in normal or malignant cells. Over the last decade, therapy targeting specific biomolecules of breast cancer has been one of the directions of focus (46–48). Notably, the mRNA and protein expression levels of BST2 are elevated in breast cancer tissues and cells lines (4,18,28,42,46,49–60). In one study, the expression of BST2 was revealed to be significantly more increased than other known markers in breast cancer, such as human epidermal growth factor receptor (EGFR), estrogen receptor (ER), Myc or progesterone receptor (58).
Association between BST2 expression and clinical parameters in breast cancer
High BST2 expression is associated with some clinical parameters. BST2 has been shown to be significantly associated with tumor size in breast tumors and mammary cancer cells (54). BST2 can also increase cell proliferation, but is not associated with the percentage of apoptotic cells (4). BST2 has been reported to be highly expressed in grade 3 tumor cell lines (CCdl54, CCdl672 and CCdl675), as determined after analyzing 65 different breast cancer cells (18). Similar results have shown that BST2 mRNA expression is increased in high-grade luminal B tumors compared with that in low-grade luminal A tumors (54). BST2 also has a strong expression in preinvasive and invasive breast cancer cells (18) and tissues (49). Furthermore, BST2 upregulation increases the risk of bone metastasis. Another study confirmed that BST2 upregulation increases metastasis in a mouse model (54) and in bone metastatic breast tumor tissues (4). Moreover, Cox regression analysis has shown that increased BST2 expression is related to the prognosis of breast cancer (52). Notably, BST2 upregulation reduces survival and may be a predictor of distant metastasis (49,54). BST2 also promotes invadopodia formation and extracellular matrix degradation (49). These previous studies demonstrated that increased BST2 expression may be significantly associated with age, high grade, metastasis and survival rate.
Regulation of BST2 expression in breast cancer
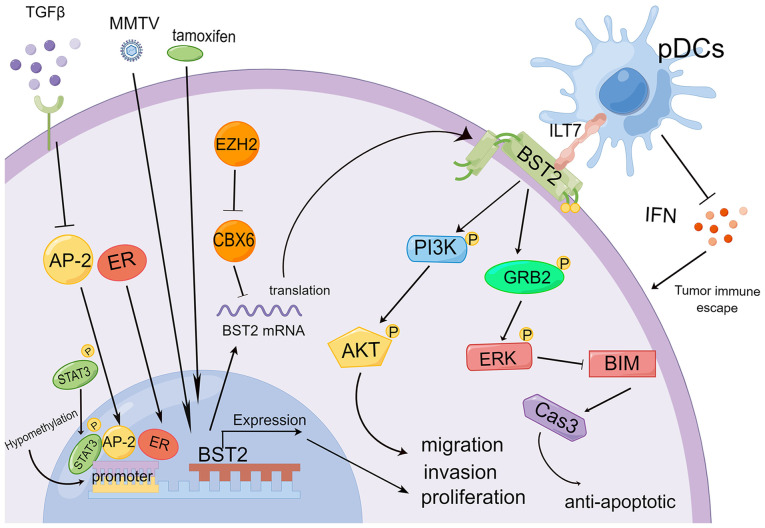
The abnormal expression of BST2 has been summarized in previously published studies (Fig. 3). BST2 expression can be induced by several factors. Mouse mammary tumor virus infection can induce BST2 upregulation in mammary tumor tissues, and, as an adhesion molecule, BST2 can contribute to the metastasis and progression of cancer (57). The binding of transcription factor AP2 to the BST2 promoter is weakened by inhibiting the transforming growth factor-β (TGFβ) pathway, thereby increasing the expression of BST2 in tumor cells. High BST2 expression can inhibit the induction of apoptosis and enhance cell proliferation (18). Tamoxifen-induced BST2 expression has been reported to be involved in the invasion and migration of breast cancer (56). Active signal transducer and activator of transcription 3 (STAT3) is known to promote the malignancy of breast cancer, and can promote the transcription and expression of the BST2 gene (56,61). Chromobox 6 (CBX6), as an RNA-binding protein, can be downregulated by enhancer of zeste homolog 2 (EZH2) and has decreased expression in breast cancer. Low expression of the CBX6 gene promotes BST2 expression, and enhances migration and invasion (51). Low expression of ER can also promote the expression of the BST2 gene, and high BST2 expression may be a potent predictor for poor patient survival and earlier activation of distant metastasis (50). Genome mutation analysis suggested that BST2 high mutation frequency is mainly induced by gene amplification, and increased BST2 expression is an independent prognostic indicator of breast cancer (52). Hypomethylation in the BST2 promoter region is correlated with high BST2 expression in breast cancer tissues and cell lines (55). Furthermore, increased BST2 expression can bind to the ILT7 ligand protein of pDCs and inhibit IFN release (42). Low levels of IFN further induce tumorigenesis through promoting tumor escape (62,63). Dimerization of BST2 may promote tumor cell proliferation via growth factor receptor bound protein 2 (GRB2)/extracellular signal-regulated kinase (ERK)/BIM/caspase 3 signaling pathway in vivo and in vitro (58). In addition, increased BST2 expression can activate phosphoinositide 3-kinase (PI3K)/serine/threonine kinase (AKT) in epithelial cells, gland cells and mammary tumors (57). These studies have suggested that BST2 gene expression is regulated by hypomethylation, viruses, and intracellular and extracellular factors (TGFβ, ER, AP2, STAT3, CBX6 and EZH2), and is involved in tumorigenesis via the GRB2/ERK/BIM/caspase 3 or PI3K/AKT signaling pathways in breast cancer.
Figure 3.
Molecular regulatory mechanism of high BST2 expression in breast cancer. Inflammatory microenvironment factors (TGFβ), viruses (MMTV) and drugs (tamoxifen) can regulate BST2 expression via activating transcription factors (AP-1, STAT3). Other factors (CBX6 and ER) can directly increase the expression of the BST2 gene. BST2 as a transmembrane protein can promote tumorigenesis, invasion and metastasis via the GRB2/ERK/BIM/cas3 or PI3K/AKT signaling pathways. BST2 upregulation can also inhibit the secretion of IFN in pDCs to enhance tumor escape. AKT, serine/threonine kinase; BST2, bone marrow stromal cell antigen 2; CBX6, chromobox 6; ER, estrogen receptor; ERK, extracellular signal-regulated kinase; EZH2, enhancer of zeste homolog 2; GRB2, growth factor receptor bound protein 2; IFN, interferon; ILT7, immunoglobulin-like transcript 7; MMTV, mouse mammary tumor virus; pDCs, plasmacytoid dendritic cells; PI3K, phosphoinositide 3-kinase; STAT3, signal transducer and activator of transcription 3; TGFβ, transforming growth factor-β.
BST2 and HCC
HCC is the most common type of primary liver cancer and the third most common cause of cancer-associated death worldwide (64). Numerous studies (65,66) have investigated the molecular biomarkers participating in HCC tumorigenesis; however, the intrinsic molecular mechanisms of the transmembrane protein BST2 in HCC are inadequately reported.
Expression and physiological functions of BST2 in HCC
Previous studies have shown that BST2 expression is significantly higher in HCC tissues than that in normal tissues (20,67,68). Similar results were confirmed in HCC cell lines, such as HepG2, Huh7, L02, HepAD38 and Huh7.5 (67,69,70). Xu et al (20) reported that BST2 upregulation is significantly associated with larger tumor size and overall survival, but is not related to sex, age, tumor differentiation, pathological grade and tumor-node-metastasis (TNM) stage, suggesting that BST2 could be an independent unfavorable prognosis factor (Table I). Another study revealed that high BST2 expression is also associated with HBV infection and overall survival (67). These studies have shown that higher expression of BST2 in patients with HCC is associated with a poorer prognosis.
Regulation of BST2 expression in HCC
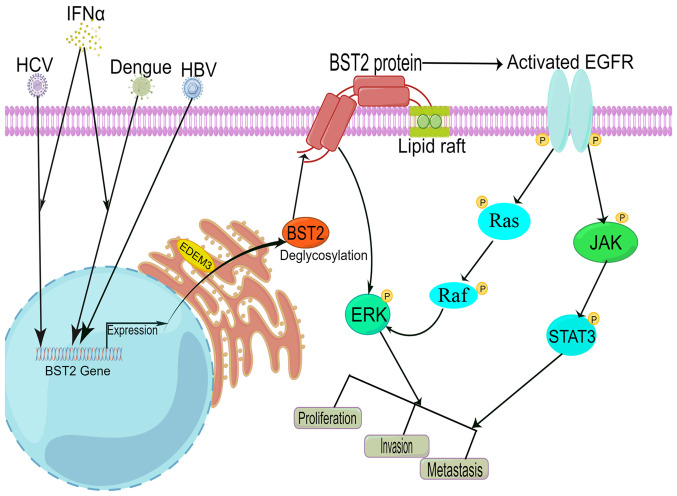
The molecular mechanism of BST2 in HCC is shown in Fig. 4. First, IFNα can induce BST2 expression in HCV-infected or Dengue virus-infected HCC tissues or cell lines (69–71). HBV can also upregulate the expression of the BST2 gene in HCC tissues or cell lines (67). These studies suggested that BST2 may be an essential factor for the virus-induced tumorigenesis of HCC. Second, the endoplasmic reticulum degradation-enhancing α-mannosidase-like protein 3 protein is increased and is responsible for the trimming of BST2 in HCC cells. Non-N-glycosylation of BST2 at the N65 site is essential for BST2 function, and can promote the proliferation, migration, invasion and colony-forming ability of HCC cells through activating the NF-κB/ERK pathway. Non-N-glycosylation of BST2 may be a novel factor of BST2 in regulating HCC tumorigenesis (67). BST2 can also activate EGFR in a lipid raft-dependent manner, not mediated by EGFR ligands. Lipid rafts are known as cholesterol/sphingolipid-rich membrane domains, and exhibit higher levels in cancer cells than non-tumorigenic controls (72). The activation of EGFR further initiates the downstream signaling pathways, including the Janus kinase/STAT3 and Ras/Raf/MEK/ERK pathways. This mode of EGFR activation may be an interesting and potential therapeutic direction for targeting EGFR-driven malignancies (68). These studies have demonstrated that viral infection and IFNα may upregulate BST2 expression, and that high BST2 expression can activate the EGFR and NF-κB/ERK pathways in a lipid raft-dependent manner in HCC.
Figure 4.
Molecular regulatory mechanism of high BST2 expression in HCC. IFNα can induce BST2 expression in HCV-infected or Dengue virus-infected HCC tissues or cell lines. HBV could upregulate the expression of the BST2 gene in HCC tissues or cell lines. Deglycosylation of BST2 enhances the proliferation, invasion and colony formation of hepatocytes in vivo via the NF-κB/ERK pathway. BST2 can activate EGFR via a lipid raft-dependent manner, not mediated by EGFR ligands. Subsequently, the activation of EGFR regulates downstream signaling pathways, including the Ras/Raf/MEK/ERK and JAK/STAT pathways. BST2, bone marrow stromal cell antigen 2; EDEM3, endoplasmic reticulum degradation-enhancing α-mannosidase-like protein 3; EGFR, epidermal growth factor receptor; ERK, extracellular signal-regulated kinase; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; IFN, interferon; JAK, Janus kinase; NF-κB, nuclear factor-κB; STAT3, signal transducer and activator of transcription 3.
BST2 and gastrointestinal tumors
Both gastric cancer and colorectal cancer are types of gastrointestinal cancer, which is one of the most common malignant tumors in the world, with 4.8 million new cases and 3.4 million deaths reported in 2018 (73). Therefore, it is necessary to explore effective biomarkers for the diagnosis of gastrointestinal cancer.
Expression and physiological functions of BST2 in gastrointestinal cancer
Several studies have reported that increased BST2 expression is detected in gastric cancer (11,22,74,75). Similar results have also been found in colorectal cancer by whole exome sequencing data, gene array and IHC (21,22,76,77). Combining 606 patients from The Cancer Genome Atlas (TCGA) and the Gene Expression Omnibus databases has shown that BST2 may be highly associated with prognosis (78). IHC and qPCR further confirmed that BST2 is mainly located in the cell membrane or cytoplasm, and high BST2 expression is detected in colorectal cancer compared with that in controls (78). The association between BST2 and clinical parameters in gastric and colorectal cancer has also been discussed in some previous studies. High BST2 expression has been shown to be significantly associated with histological classification, invasion, T classification and poor survival (Table I) (22). BST2 upregulation is also significantly related to lymph node metastasis and TNM stage (Table I) (74). In colorectal cancer, BST2 positive expression is closely associated with TNM stage and poorer survival (Table I) (22,77). However, high BST2 expression is not significantly linked to other clinicopathological parameters, including age, sex, histological grade, tumor location and distant metastasis (Table I) (77). These findings indicated that increased BST2 expression may be associated with the progression of gastric and colorectal cancer.
Regulation of BST2 expression in gastrointestinal cancer
The molecular mechanism of BST2 was summarized in this review according to the findings from previous studies (Fig. 5) (11,22,79,80). BST2 expression can be regulated by microRNAs (miRNAs/miRs) (11) and hypomethylation (79). BST2 has been reported to be regulated by miR-760, and to promote cell proliferation and migration by inducing NF-κB activation in gastric cancer (11). Upregulation of BST2 has also been revealed to be significantly associated with worse survival in patients with colorectal cancer (79). BST2 may also enhance cell proliferation and survival by activating EGFR/ERK/AKT (22). In addition, BST2 can promote the progression of colorectal cancer via inducing macrophage M2 polarization (80). These studies indicated that BST2 may enhance proliferation, survival and migration by activating the EGFR/ERK/AKT or NF-κB signaling pathways, and reducing immune surveillance of M2 macrophages.
Figure 5.
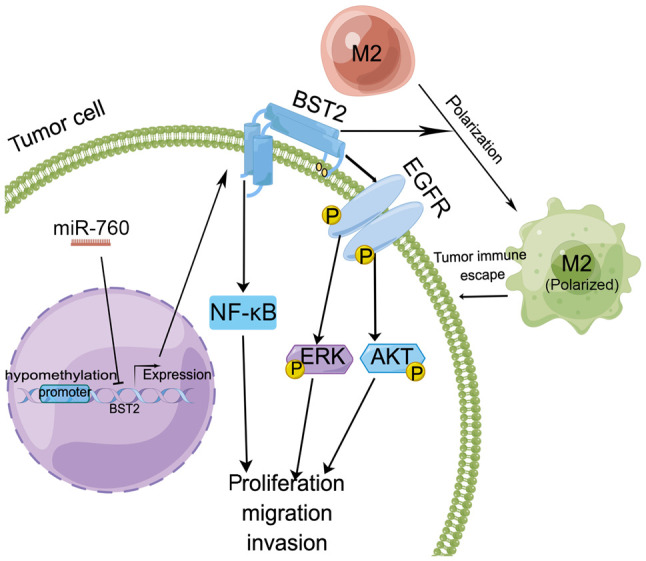
Molecular regulatory mechanism of high BST2 expression in gastrointestinal tumors. BST2 expression can be regulated by miRNAs and hypomethylation. BST2 induces macrophage M2 polarization to promote the progression of colorectal cancer to enhance tumor escape. BST2 may enhance proliferation, survival and migration via the EGFR/ERK/AKT or NF-κB signaling pathways. AKT, serine/threonine kinase; BST2, bone marrow stromal cell antigen 2; EGFR, epidermal growth factor receptor; ERK, extracellular signal-regulated kinase; miR/miRNA, microRNA; NF-κB, nuclear factor-κB.
BST2 and lung cancer
Wang et al (13) reported that BST2 expression is positive in 42% of non-small cell lung cancer cell lines and 57% of small cell lung cancer cell lines, and that BST2 overexpression can promote tumor growth. Additionally, a higher expression of BST2 has been detected in neoadjuvant-treated non-small cell lung cancer (81). RNA sequencing analysis has revealed that BST2 can promote lung cancer progression via the induction of S100A15 (82). Bioinformatics analysis has demonstrated that BST2 is significantly associated with progression-free survival in 34 lung cancer database samples (83). For therapeutic targeting of BST2 in lung cancer, antibodies and cytokine factors have been examined. Modified anti-BST2 monoclonal antibodies, such as chimeric or humanized antibodies, have the potential to be new therapeutic targets in lung cancer; moreover, it could be more effective to enhance antibody-dependent cell-mediated cytotoxicity when combining an anti-BST2 antibody with ILs/IFNs (84). Combination therapy with an anti-BST2 antibody and IFN-β has been shown to exhibit increased antitumor effects in lung cancer in a mouse model (13). Therefore, BST2 may promote cell proliferation and tumor progression in lung cancer, and combination therapy might be more effective than antibody therapy in lung cancer.
BST2 and other solid tumors
An increasing number of studies have shown that BST2 upregulation is associated with numerous types of cancer, including endometrial cancer (10), glioblastoma multiforme (12), nasopharyngeal cancer (14), oral cavity cancer (15), OSCC (16), cervical cancer (17), pancreatic cancer (19), head and neck squamous cell carcinoma (23), ovarian cancer (24), esophageal squamous cell carcinoma (22) and bladder cancer (25).
Expression and physiological functions of BST2 in other types of solid cancer
Abnormal BST2 expression is associated with different clinical parameters. In head and neck squamous cell carcinoma, Kaplan-Meier survival curve analysis has suggested that high BST2 expression is associated with a worse prognosis; however, tissue microarrays have shown that high BST2 expression is not significantly related with larger tumor size, higher histological grade and lymph node metastasis (23). In glioblastoma, BST2 exhibits high expression in high-grade tissues (85). Kong et al (12) reported that increased BST2 expression is associated with poor prognosis and higher grade gliomas. In esophageal squamous cell carcinoma, BST2 is associated with lymphatic invasion and significantly poorer survival, but is not significantly associated with age, sex, T classification, M classification, stage, histological classification and vascular invasion (Table I) (22). In bladder cancer, high BST2 expression is not significantly associated with age, sex, cellular atypia classification, lymphatic and vascular invasion, but is significantly related with T stage (Table I) (25). In endometrial cancer, authors have demonstrated that BST2 protein expression exhibits significantly positive staining in endometrial cancer compared with in the normal endometrium (P<0.0001); however, BST2 upregulation was not revealed to be associated with histological differentiation and pathological stage (86). In renal cell carcinoma, high BST2 expression is strongly related to age, TNM classification, stage and histological grade, but not to sex and histological classification (Table I) (87). An analysis of 530 patients with renal cell carcinoma from TCGA database has suggested that BST2 upregulation is closely associated with poor survival (88,89). These studies have suggested that BST2 upregulation could be associated with poor survival.
Regulation of BST2 expression in other types of solid cancer
BST2 is a transmembrane protein that can be regulated by viruses, antitumor drugs and cellular factors. First, drug resistance (such as to gefitinib, cisplatin and tamoxifen) can induce BST2 upregulation in OSCC cells (16), nasopharyngeal carcinoma cells (14) and in ovarian cancer cells (90). Second, FGD5 antisense RNA 1 downregulates miR-129-5p expression and further increases BST2 gene expression; BST2 can then promote cervical cancer progression via inducing M2 macrophage polarization (17). Notably, the expression levels of miR-451a have been shown to be strongly decreased in renal cell carcinoma. Notably, low miR-451a expression can induce the upregulation of BST2 expression, which is significantly associated with poor prognosis. Therefore, miRNAs may be a potential and effective target in the treatment of cancer (89). Third, the long non-coding RNA (lncRNA) BST2 IFN-stimulated positive regulator (BISPR) upregulates the expression of BST2 to promote the progression of thyroid papillary carcinoma (91). Fourth, hypomethylation is an important way to control BST2 expression in cervical cancer (92) and glioblastoma (93). The BST2 gene is significantly hypomethylated in cervical cancer tissue vs. in normal tissue (92). Fifth, type I IFN and MV infection can induce BST2 expression in neurons and mouse embryonic fibroblast cells (40). IFNα has also been reported to promote the expression of BST2 in a xenograft model of renal cell carcinoma (94), and BST2 is upregulated in human glioma and is associated with IFNγ response (12). In human fibrosarcoma cells, there is an opposite opinion that IFNα-induced BST2 could also interact with MT1-MMP to block cell proliferation and migration (95), thus suggesting that BST2 may be an inhibitor for cell proliferation and migration in human fibrosarcoma cells. IFNγ can strongly induce BST2 expression and BST upregulation can inhibit the adhesion of macrophages in tumor-draining lymph nodes and enhance tumor cell escape (96,97). Sixth, the transcription factor SP1 protein regulates the BST2 gene by binding to the BST2 promoter, and can promote cell proliferation and migration in pancreatic cancer (19). The CXXC zinc finger protein 1 can bind to the promoter of BST2 to directly regulate its transcription in ovarian cells (24). Seventh, the chemokine SDF1a interacts with CXCR4/CXCR7 to promote an invasive phenotype in the medullary thyroid by upregulating BST2 gene expression in thyroid cancer (98). These findings have suggested that BST2 expression can be regulated by viruses, antitumor drugs and cellular factors, and BST2 expression promotes the tumorigenesis of numerous types of cancer, with the exception of fibrosarcoma.
BST2 upregulation can induce cell proliferation and inhibit apoptosis. The BST2 gene promotes cell proliferation in OSCC (16), glioblastoma (12) and bladder cancer (25). BST2 elevates cell proliferation by upregulating the expression of cyclin A and cyclin D proteins, and by decreasing the expression of p21 in OSCC cells (16). By contrast, BST2 upregulation can inhibit cell apoptosis by inducing the anti-apoptotic BCL2 protein and decreasing the pro-apoptotic BAX protein (16). Furthermore, traditional medicinal plant ginsenosides can promote cell autophagy through decreasing the expression of BST2 in cervical cancer cells (99). BST2 small interfering RNA inhibits cell proliferation and invasive activities in renal cell carcinoma (87,88). By contrast, BST2 upregulation can contribute to the tumor migration of OSCC cells (15). BST2 may also promote the invasion of glioblastoma multiforme cells in vitro (12). The lncRNA BISPR upregulates BST2 and induces the progression of thyroid papillary carcinoma by regulating miR-21-5p and the anti-apoptotic BCL2 protein (91). Low BST2 expression induces calcium disorder, proteostasis breakdown and cell death (100). Thus, BST2 may promote cell proliferation and migration, and inhibit cell apoptosis in cancer.
BST2 upregulation has also been shown to promote tumor immune escape. BST2 upregulation is significantly associated with the high expression of several immune checkpoints (PD-L1, B7-H3) in the microenvironment of head and neck squamous cell carcinoma. BST2 can also interact with pDCs and regulate their immune response (101). In addition, BST2 upregulation is related to two tumor-associated macrophage markers (CD163 and CD68) in head and neck squamous cell carcinoma (23). High BST2 expression can induce macrophage polarization to the M2-like phenotype, and these cells can contribute to immunodepression and further promote tumor progression (17). Thus, these studies have demonstrated that BST2 may be an important immune-related factor involved in tumor progression.
6. Treatment potential and future direction
Inhibitors, antibodies and combination therapy that target BST2 have been used for the treatment of cancer. In recent years, antibodies have been used extensively for immunotherapy by targeting specific immune checkpoint pathways (102,103). Immunotherapy with antibodies or inhibitors that target the immune checkpoint pathway has been used in the treatment of drug-resistant cancer, distant metastases and cancer recurrence (104). A BST2 inhibitor (B49Mod1) disrupts cysteine-linked BST2-mediated cell-cell interaction and inhibits the proliferation of breast cancer cells (46). Modified anti-BST2 antibodies (including chimeric and humanized antibodies) can have an antitumor effect on lung cancer cells (84). A single chain of BST2-specific antibody has been shown to induce the apoptosis of multiple myeloma cells in an immunodeficiency mouse xenograft model (105). BST2 amino acid 22–30 peptide can activate CD8+ T cells to further kill multiple myeloma cells due to the highest probability of binding to HLA-A2 and may also be a suitable potential target for specific immunotherapy of multiple myeloma (106).
In addition, combination therapy with an antibody and cytokine/oligodeoxynucleotides may be more effective than using antibody alone. A combination of humanized anti-BST2 antibody with IFNα has been shown to exhibit a more effective antitumor ability in renal cell carcinoma xenograft models than antibody alone; thus, a humanized antibody accompanied by IFNα may be a potential therapy for renal cell carcinoma (94). CpG oligodeoxynucleotides are known to enhance tumor escape of macrophages and natural killer cells. In addition, combination therapy with an anti-BST2 antibody and oligodeoxynucleotides has been shown to exert an effective antitumor ability in a xenograft model (107). Therefore, combination therapy with an antibody and cytokine/oligodeoxynucleotides may be a future research direction for therapy.
7. Conclusions and limitations
Increased BST2 expression has been detected in various types of human cancer, including hematological tumors and solid tumors, especially in breast cancer, HCC, gastrointestinal cancer and lung cancer. In most types of cancer, high BST2 expression is associated with several tumor clinicopathological parameters, such as increased stage, invasion and overall survival. BST2 expression is regulated by viral infection, IFNs, transcription factors, miRNAs, lncRNAs, chemokines and methylation, and is involved in numerous types of cancer via activating signaling pathways, such as EGFR/AKT, NF-κB/ERK, GRB2/DIM/caspase 3. BST2 is also involved in cell membrane integrity and lipid raft formation, which can activate the EGFR signaling pathway via lipid rafts. Therefore, BST2 is considered a promising diagnostic marker and prognostic marker. The therapeutic effect of BST2 as an antitumor target has focused on inhibitors, antibodies or modified antibodies to BST2, and combination therapy with an antibody and cytokine/oligodeoxynucleotides. Notably, the aforementioned combination therapy may exhibit more profound antitumor activity.
To date, there is an abundance of in vitro evidence suggesting that high BST2 expression is significantly associated with tumorigenesis and progress. Further studies are still needed to investigate whether BST2 can be used as a novel biomarker in cancer. Finally, in combination with the structural, molecular functions and molecular mechanism of BST2 in various types of cancer, the present review may improve overall knowledge of tumorigenesis and progression in cancer types that exhibit BST2 upregulation induced by several factors, possibly leading to antibody therapeutic options for these types of cancer.
Acknowledgements
Fig. 3, Fig. 4, Fig. 5 were generated using the Figdraw platform (https://www.figdraw.com/#/).
Glossary
Abbreviations
- BST2
bone marrow stromal cell antigen 2
- HIV
human immunodeficiency virus 1
- OSCC
oral squamous cell carcinoma
- HCC
hepatocellular carcinoma
- TGFβ
transforming growth factor-β
- CBX
chromobox 6
- IFNα
interferon α
- EGFR
epidermal growth factor receptor
- ILT7
immunoglobulin-like transcript 7
- STAT3
signal transducer and activator of transcription 3
- EZH2
enhancer of zeste homolog 2
- GRB2
growth factor receptor bound protein 2
- ERK
extracellular signal-regulated kinase
- pDC
plasmacytoid dendritic cell
- PI3K
phosphoinositide 3-kinase
- AKT
serine/threonine kinase
- NF-κB
nuclear factor κB
- ER
estrogen receptor
Funding Statement
This research was funded by the Postdoctoral Program of Affiliated Hospital of Jining Medical University (grant no. JYGY322143), the China Postdoctoral Science Foundation (grant no. 2023M731308) and the Shandong Provincial Natural Science Foundation (grant nos. ZR2020MH078 and ZR2020MH070).
Availability of data and materials
Not applicable.
Authors' contributions
BB, ZKZ and HLY contributed to the conception and design of the study. HLY wrote the first draft of the manuscript. QB, ZCW, XW, XZW and LHL wrote sections of the manuscript. Data authentication is not applicable. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Goto T, Kennel SJ, Abe M, Takishita M, Kosaka M, Solomon A, Saito S. A novel membrane antigen selectively expressed on terminally differentiated human B cells. Blood. 1984;84:1922–1930. doi: 10.1182/blood.V84.6.1922.1922. [DOI] [PubMed] [Google Scholar]
- 2.Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 3.Mahauad-Fernandez WD, Okeoma CM. The role of BST-2/Tetherin in host protection and disease manifestation. Immun Inflamm Dis. 2015;4:4–23. doi: 10.1002/iid3.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai D, Cao J, Li Z, Zheng X, Yao Y, Li W, Yuan Z. Up-regulation of bone marrow stromal protein 2 (BST2) in breast cancer with bone metastasis. BMC Cancer. 2009;9:102. doi: 10.1186/1471-2407-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jourdan M, Caraux A, Caron G, Robert N, Fiol G, Reme T, Bolloré K, Vendrell JP, Le Gallou S, Mourcin F, et al. Characterization of a transitional preplasmablast population in the process of human B cell to plasma cell differentiation. J Immunol. 2011;187:3931–3941. doi: 10.4049/jimmunol.1101230. [DOI] [PubMed] [Google Scholar]
- 6.Jourdan M, Caraux A, De Vos J, Fiol G, Larroque M, Cognot C, Bret C, Duperray C, Hose D, Klein B. An in vitro model of differentiation of memory B cells into plasmablasts and plasma cells including detailed phenotypic and molecular characterization. Blood. 2009;114:5173–5181. doi: 10.1182/blood-2009-07-235960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erikson E, Adam T, Schmidt S, Lehmann-Koch J, Over B, Goffinet C, Harter C, Bekeredjian-Ding I, Sertel S, Lasitschka F, Keppler OT. In vivo expression profile of the antiviral restriction factor and tumor-targeting antigen CD317/BST-2/HM1.24/tetherin in humans. Proc Natl Acad Sci USA. 2011;108:13688–13693. doi: 10.1073/pnas.1101684108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohtomo T, Sugamata Y, Ozaki Y, Ono K, Yoshimura Y, Kawai S, Koishihara Y, Ozaki S, Kosaka M, Hirano T, Tsuchiya M. Molecular cloning and characterization of a surface antigen preferentially overexpressed on multiple myeloma cells. Biochem Biophys Res Commun. 1999;258:583–591. doi: 10.1006/bbrc.1999.0683. [DOI] [PubMed] [Google Scholar]
- 9.Rew SB, Peggs K, Sanjuan I, Pizzey AR, Koishihara Y, Kawai S, Kosaka M, Ozaki S, Chain B, Yong KL. Generation of potent antitumor CTL from patients with multiple myeloma directed against HM1.24. Clin Cancer Res. 2005;11:3377–3384. doi: 10.1158/1078-0432.CCR-04-0650. [DOI] [PubMed] [Google Scholar]
- 10.Wong YF, Cheung TH, Lo KW, Yim SF, Siu NS, Chan SC, Ho TW, Wong KW, Yu MY, Wang VW, et al. Identification of molecular markers and signaling pathway in endometrial cancer in Hong Kong Chinese women by genome-wide gene expression profiling. Oncogene. 2007;26:1971–1982. doi: 10.1038/sj.onc.1209986. [DOI] [PubMed] [Google Scholar]
- 11.Liu W, Li Y, Feng S, Guan Y, Cao Y. MicroRNA-760 inhibits cell viability and migration through down-regulating BST2 in gastric cancer. J Biochem. 2020;168:159–170. doi: 10.1093/jb/mvaa031. [DOI] [PubMed] [Google Scholar]
- 12.Kong Y, Xue Z, Wang H, Cui G, Chen A, Liu J, Wang J, Li X, Huang B. Identification of BST2 contributing to the development of glioblastoma based on bioinformatics analysis. Front Genet. 2022;13:890174. doi: 10.3389/fgene.2022.890174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W, Nishioka Y, Ozaki S, Jalili A, Abe S, Kakiuchi S, Kishuku M, Minakuchi K, Matsumoto T, Sone S. HM1.24 (CD317) is a novel target against lung cancer for immunotherapy using anti-HM1.24 antibody. Cancer Immunol Immunother. 2009;58:967–976. doi: 10.1007/s00262-008-0612-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuang CM, Fu X, Hua YJ, Shuai WD, Ye ZH, Li Y, Peng QH, Li YZ, Chen S, Qian CN, et al. BST2 confers cisplatin resistance via NF-kappaB signaling in nasopharyngeal cancer. Cell Death Dis. 2017;8:e2874. doi: 10.1038/cddis.2017.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang KH, Kao HK, Chi LM, Liang Y, Liu SC, Hseuh C, Liao CT, Yen TC, Yu JS, Chang KP. Overexpression of BST2 is associated with nodal metastasis and poorer prognosis in oral cavity cancer. Laryngoscope. 2014;124:E354–E360. doi: 10.1002/lary.24700. [DOI] [PubMed] [Google Scholar]
- 16.Jin H, Zhang L, Wang S, Qian L. BST2 promotes growth and induces gefitinib resistance in oral squamous cell carcinoma via regulating the EGFR pathway. Arch Med Sci. 2019;17:1772–1782. doi: 10.5114/aoms.2019.86183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu G, Du X, Xiao L, Zeng Q, Liu Q. Activation of FGD5-AS1 promotes progression of cervical cancer through regulating BST2 to inhibit macrophage M1 Polarization. J Immunol Res. 2021;2021:5857214. doi: 10.1155/2021/5857214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sayeed A, Luciani-Torres G, Meng Z, Bennington JL, Moore DH, Dairkee SH. Aberrant regulation of the BST2 (Tetherin) promoter enhances cell proliferation and apoptosis evasion in high grade breast cancer cells. PLoS One. 2013;8:e67191. doi: 10.1371/journal.pone.0067191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lei C, Hou Y, Chen J. Specificity protein 1-activated bone marrow stromal cell antigen 2 accelerates pancreatic cancer cell proliferation and migration. Exp Ther Med. 2021;22:1459. doi: 10.3892/etm.2021.10894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu X, Wang Y, Xue F, Guan E, Tian F, Xu J, Zhang H. BST2 promotes tumor growth via multiple pathways in hepatocellular carcinoma. Cancer Invest. 2020;38:329–337. doi: 10.1080/07357907.2020.1769125. [DOI] [PubMed] [Google Scholar]
- 21.Kim SC, Hong CW, Jang SG, Kim YA, Yoo BC, Shin YK, Jeong SY, Ku JL, Park JG. Establishment and characterization of paired primary and peritoneal seeding human colorectal cancer cell lines: Identification of genes that mediate metastatic potential. Transl Oncol. 2018;11:1232–1243. doi: 10.1016/j.tranon.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukai S, Oue N, Oshima T, Mukai R, Tatsumoto Y, Sakamoto N, Sentani K, Tanabe K, Egi H, Hinoi T, et al. Overexpression of Transmembrane Protein BST2 is associated with poor survival of patients with esophageal, gastric, or colorectal cancer. Ann Surg Oncol. 2017;24:594–602. doi: 10.1245/s10434-016-5100-z. [DOI] [PubMed] [Google Scholar]
- 23.Yang LL, Wu L, Yu GT, Zhang WF, Liu B, Sun ZJ. CD317 signature in head and neck cancer indicates poor prognosis. J Dent Res. 2018;97:787–794. doi: 10.1177/0022034518758604. [DOI] [PubMed] [Google Scholar]
- 24.Yang LQ, Hu HY, Han Y, Tang ZY, Gao J, Zhou QY, Liu YX, Chen HS, Xu TN, Ao L, et al. CpG-binding protein CFP1 promotes ovarian cancer cell proliferation by regulating BST2 transcription. Cancer Gene Ther. 2022;29:1895–1907. doi: 10.1038/s41417-022-00503-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shigematsu Y, Oue N, Nishioka Y, Sakamoto N, Sentani K, Sekino Y, Mukai S, Teishima J, Matsubara A, Yasui W. Overexpression of the transmembrane protein BST-2 induces Akt and Erk phosphorylation in bladder cancer. Oncol Lett. 2017;14:999–1004. doi: 10.3892/ol.2017.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng J, Liu Z, Deng T, Lu Z, Liu M, Lu X, Adeshakin FO, Yan D, Zhang G, Wan X. CD317 mediates immunocytolysis resistance by RICH2/cytoskeleton-dependent membrane protection. Mol Immunol. 2021;129:94–102. doi: 10.1016/j.molimm.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Masuyama N, Kuronita T, Tanaka R, Muto T, Hirota Y, Takigawa A, Fujita H, Aso Y, Amano J, Tanaka Y. HM1.24 is internalized from lipid rafts by clathrin-mediated endocytosis through interaction with alpha-adaptin. J Biol Chem. 2009;284:15927–15941. doi: 10.1074/jbc.M109.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naushad W, Mahauad-Fernandez WD, Okeoma CM. Structural determinant of BST-2-mediated regulation of breast cancer cell motility: A role for cytoplasmic tail tyrosine residues. Oncotarget. 2017;8:110221–110233. doi: 10.18632/oncotarget.22753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrew AJ, Miyagi E, Kao S, Strebel K. The formation of cysteine-linked dimers of BST-2/tetherin is important for inhibition of HIV-1 virus release but not for sensitivity to Vpu. Retrovirology. 2009;6:80. doi: 10.1186/1742-4690-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swiecki M, Scheaffer SM, Allaire M, Fremont DH, Colonna M, Brett TJ. Structural and biophysical analysis of BST-2/tetherin ectodomains reveals an evolutionary conserved design to inhibit virus release. J Biol Chem. 2011;286:2987–2997. doi: 10.1074/jbc.M110.190538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez-Caballero D, Zang T, Ebrahimi A, McNatt MW, Gregory DA, Johnson MC, Bieniasz PD. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell. 2009;139:499–511. doi: 10.1016/j.cell.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kupzig S, Korolchuk V, Rollason R, Sugden A, Wilde A, Banting G. BST-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic. 2003;4:694–709. doi: 10.1034/j.1600-0854.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- 33.Edgar JR, Manna PT, Nishimura S, Banting G, Robinson MS. Tetherin is an exosomal tether. Elife. 2016;5:e17180. doi: 10.7554/eLife.17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tokarev A, Suarez M, Kwan W, Fitzpatrick K, Singh R, Guatelli J. Stimulation of NF-kappaB activity by the HIV restriction factor BST2. J Virol. 2013;87:2046–2057. doi: 10.1128/JVI.02272-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Billcliff PG, Rollason R, Prior I, Owen DM, Gaus K, Banting G. CD317/tetherin is an organiser of membrane microdomains. J Cell Sci. 2013;126((Pt 7)):1553–1564. doi: 10.1242/jcs.112953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Billcliff PG, Gorleku OA, Chamberlain LH, Banting G. The cytosolic N-terminus of CD317/tetherin is a membrane microdomain exclusion motif. Biol Open. 2013;2:1253–1263. doi: 10.1242/bio.20135793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones DR, Varela-Nieto I. The role of glycosyl-phosphatidylinositol in signal transduction. Int J Biochem Cell Biol. 1998;30:313–326. doi: 10.1016/S1357-2725(97)00144-1. [DOI] [PubMed] [Google Scholar]
- 38.Muller GA, Muller TD. (Patho)physiology of glycosylphosphatidylinositol-anchored proteins I: Localization at plasma membranes and extracellular compartments. Biomolecules. 2023;13:855. doi: 10.3390/biom13050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galao RP, Le Tortorec A, Pickering S, Kueck T, Neil SJ. Innate sensing of HIV-1 assembly by Tetherin induces NF-kappaB-dependent proinflammatory responses. Cell Host Microbe. 2012;12:633–644. doi: 10.1016/j.chom.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holmgren AM, Miller KD, Cavanaugh SE, Rall GF. Bst2/tetherin is induced in neurons by type I interferon and viral infection but is dispensable for protection against neurotropic viral challenge. J Virol. 2015;89:11011–11018. doi: 10.1128/JVI.01745-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rahmani W, Chung H, Sinha S, Bui-Marinos MP, Arora R, Jaffer A, Corcoran JA, Biernaskie J, Chun J. Attenuation of SARS-CoV-2 infection by losartan in human kidney organoids. iScience. 2022;25:103818. doi: 10.1016/j.isci.2022.103818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao W, Bover L, Cho MW, Wen XX, Hanabuchi S, Bao MS, Rosen DB, Wang YH, Shaw JL, Du Q, et al. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J Exp Med. 2009;206:1603–1614. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao W, Bover L. Signaling and ligand interaction of ILT7: Receptor-mediated regulatory mechanisms for plasmacytoid dendritic cells. Immunol Rev. 2010;234:163–176. doi: 10.1111/j.0105-2896.2009.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yi E, Oh J, Kang HR, Song MJ, Park SH. BST2 inhibits infection of influenza A virus by promoting apoptosis of infected cells. Biochem Biophys Res Commun. 2019;509:414–420. doi: 10.1016/j.bbrc.2018.12.110. [DOI] [PubMed] [Google Scholar]
- 45.Ge Y, Dombkowski AA, LaFiura KM, Tatman D, Yedidi RS, Stout ML, Buck SA, Massey G, Becton DL, Weinstein HJ, et al. Differential gene expression, GATA1 target genes, and the chemotherapy sensitivity of Down syndrome megakaryocytic leukemia. Blood. 2006;107:1570–1581. doi: 10.1182/blood-2005-06-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahauad-Fernandez WD, Okeoma CM. B49, a BST-2-based peptide, inhibits adhesion and growth of breast cancer cells. Sci Rep. 2018;8:4305. doi: 10.1038/s41598-018-22364-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oue N, Sentani K, Sakamoto N, Uraoka N, Yasui W. Molecular carcinogenesis of gastric cancer: Lauren classification, mucin phenotype expression, and cancer stem cells. Int J Clin Oncol. 2019;24:771–778. doi: 10.1007/s10147-019-01443-9. [DOI] [PubMed] [Google Scholar]
- 48.Prat A, Pineda E, Adamo B, Galvan P, Fernandez A, Gaba L, Díez M, Viladot M, Arance A, Muñoz M. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24((Suppl 2)):S26–S35. doi: 10.1016/j.breast.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 49.Mahauad-Fernandez WD, Naushad W, Panzner TD, Bashir A, Lal G, Okeoma CM. BST-2 promotes survival in circulation and pulmonary metastatic seeding of breast cancer cells. Sci Rep. 2018;8:17608. doi: 10.1038/s41598-018-35710-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woodman N, Pinder SE, Tajadura V, Le Bourhis X, Gillett C, Delannoy P, Burchell JM, Julien S. Two E-selectin ligands, BST-2 and LGALS3BP, predict metastasis and poor survival of ER-negative breast cancer. Int J Oncol. 2016;49:265–275. doi: 10.3892/ijo.2016.3521. [DOI] [PubMed] [Google Scholar]
- 51.Deng H, Guan X, Gong L, Zeng J, Zhang H, Chen MY, Li G. CBX6 is negatively regulated by EZH2 and plays a potential tumor suppressor role in breast cancer. Sci Rep. 2019;9:197. doi: 10.1038/s41598-018-36560-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu S, Liu Y, Ma H, Fang S, Wei S, Li X, Lu Z, Zheng Y, Liu T, Zhu X, et al. A novel signature integrated of immunoglobulin, glycosylation and anti-viral genes to predict prognosis for breast cancer. Front Genet. 2022;13:834731. doi: 10.3389/fgene.2022.834731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Du Y, Yuan S, Zhuang X, Zhang Q, Qiao T. Multiomics differences in lung squamous cell carcinoma patients with high radiosensitivity index compared with those with low radiosensitivity index. Dis Markers. 2021;2021:3766659. doi: 10.1155/2021/3766659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mahauad-Fernandez WD, DeMali KA, Olivier AK, Okeoma CM. Bone marrow stromal antigen 2 expressed in cancer cells promotes mammary tumor growth and metastasis. Breast Cancer Res. 2014;16:493. doi: 10.1186/s13058-014-0493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahauad-Fernandez WD, Borcherding NC, Zhang W, Okeoma CM. Bone marrow stromal antigen 2 (BST-2) DNA is demethylated in breast tumors and breast cancer cells. PLoS One. 2015;10:e0123931. doi: 10.1371/journal.pone.0123931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yi EH, Yoo H, Noh KH, Han S, Lee H, Lee JK, Won C, Kim BH, Kim MH, Cho CH, Ye SK. BST-2 is a potential activator of invasion and migration in tamoxifen-resistant breast cancer cells. Biochem Biophys Res Commun. 2013;435:685–690. doi: 10.1016/j.bbrc.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 57.Jones PH, Mahauad-Fernandez WD, Madison MN, Okeoma CM. BST-2/tetherin is overexpressed in mammary gland and tumor tissues in MMTV-induced mammary cancer. Virology. 2013;444:124–139. doi: 10.1016/j.virol.2013.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mahauad-Fernandez WD, Okeoma CM. Cysteine-linked dimerization of BST-2 confers anoikis resistance to breast cancer cells by negating proapoptotic activities to promote tumor cell survival and growth. Cell Death Dis. 2017;8:e2687. doi: 10.1038/cddis.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shi Y, Castro-Gonzalez S, Chen Y, Serra-Moreno R. Effects of the SUMO Ligase BCA2 on metabolic activity, cell proliferation, cell migration, cell cycle, and the regulation of NF-kappaB and IRF1 in different breast epithelial cellular contexts. Front Cell Dev Biol. 2021;9:711481. doi: 10.3389/fcell.2021.711481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Becker M, Sommer A, Kratzschmar JR, Seidel H, Pohlenz HD, Fichtner I. Distinct gene expression patterns in a tamoxifen-sensitive human mammary carcinoma xenograft and its tamoxifen-resistant subline MaCa 3366/TAM. Mol Cancer Ther. 2005;4:151–168. doi: 10.1158/1535-7163.151.4.1. [DOI] [PubMed] [Google Scholar]
- 61.Ma JH, Qin L, Li X. Role of STAT3 signaling pathway in breast cancer. Cell Commun Signal. 2020;18:33. doi: 10.1186/s12964-020-0527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andzinski L, Kasnitz N, Stahnke S, Wu CF, Gereke M, von Kockritz-Blickwede M, Schilling B, Brandau S, Weiss S, Jablonska J. Type I IFNs induce anti-tumor polarization of tumor associated neutrophils in mice and human. Int J Cancer. 2016;138:1982–1993. doi: 10.1002/ijc.29945. [DOI] [PubMed] [Google Scholar]
- 63.Pylaeva E, Lang S, Jablonska J. The essential role of type I interferons in differentiation and activation of tumor-associated neutrophils. Front Immunol. 2016;7:629. doi: 10.3389/fimmu.2016.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.García-Pras E, Fernández-Iglesias A, Gracia-Sancho J, Pérez-Del-Pulgar S. Cell death in hepatocellular carcinoma: Pathogenesis and therapeutic opportunities. Cancers (Basel) 2021;14:48. doi: 10.3390/cancers14010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tayob N, Kanwal F, Alsarraj A, Hernaez R, El-Serag HB. The Performance of AFP, AFP-3, DCP as biomarkers for detection of hepatocellular carcinoma (HCC): A phase 3 biomarker study in the United States. Clin Gastroenterol Hepatol. 2023;21:415–423.e4. doi: 10.1016/j.cgh.2022.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y, Deng B. Hepatocellular carcinoma: Molecular mechanism, targeted therapy, and biomarkers. Cancer Metastasis Rev. 2023;42:629–652. doi: 10.1007/s10555-023-10084-4. [DOI] [PubMed] [Google Scholar]
- 67.Zhang J, Zheng B, Zhou X, Zheng T, Wang H, Wang Y, Zhang W. Increased BST-2 expression by HBV infection promotes HBV-associated HCC tumorigenesis. J Gastrointest Oncol. 2021;12:694–710. doi: 10.21037/jgo-20-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang G, Li X, Chen Q, Li J, Ruan Q, Chen YH, Yang X, Wan X. CD317 Activates EGFR by regulating its association with lipid rafts. Cancer Res. 2019;79:2220–2231. doi: 10.1158/0008-5472.CAN-18-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pan XB, Han JC, Cong X, Wei L. BST2/tetherin inhibits dengue virus release from human hepatoma cells. PLoS One. 2012;7:e51033. doi: 10.1371/journal.pone.0051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dafa-Berger A, Kuzmina A, Fassler M, Yitzhak-Asraf H, Shemer-Avni Y, Taube R. Modulation of hepatitis C virus release by the interferon-induced protein BST-2/tetherin. Virology. 2012;428:98–111. doi: 10.1016/j.virol.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 71.Pan XB, Qu XW, Jiang D, Zhao XL, Han JC, Wei L. BST2/Tetherin inhibits hepatitis C virus production in human hepatoma cells. Antiviral Res. 2013;98:54–60. doi: 10.1016/j.antiviral.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 72.Mollinedo F, Gajate C. Lipid rafts as signaling hubs in cancer cell survival/death and invasion: Implications in tumor progression and therapy. Thematic Review Series: Biology of Lipid Rafts. J Lipid Res. 2020;61:611–635. doi: 10.1194/jlr.TR119000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, Bray F. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020;159:335–349.e15. doi: 10.1053/j.gastro.2020.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu W, Cao Y, Guan Y, Zheng C. BST2 promotes cell proliferation, migration and induces NF-kappaB activation in gastric cancer. Biotechnol Lett. 2018;40:1015–1027. doi: 10.1007/s10529-018-2562-z. [DOI] [PubMed] [Google Scholar]
- 75.Anami K, Oue N, Noguchi T, Sakamoto N, Sentani K, Hayashi T, Hinoi T, Okajima M, Graff JM, Yasui W. Search for transmembrane protein in gastric cancer by the Escherichia coli ampicillin secretion trap: Expression of DSC2 in gastric cancer with intestinal phenotype. J Pathol. 2010;221:275–284. doi: 10.1002/path.2717. [DOI] [PubMed] [Google Scholar]
- 76.Rodriguez A, Corchete LA, Alcazar JA, Montero JC, Rodriguez M, Chinchilla-Tabora LM, Vidal Tocino R, Moyano C, Muñoz-Bravo S, Sayagués JM, Abad M. Dysregulated expression of three genes in colorectal cancer stratifies patients into three risk groups. Cancers (Basel) 2022;14:4076. doi: 10.3390/cancers14174076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chiang SF, Kan CY, Hsiao YC, Tang R, Hsieh LL, Chiang JM, Tsai WS, Yeh CY, Hsieh PS, Liang Y, et al. Bone marrow stromal antigen 2 is a novel plasma biomarker and prognosticator for colorectal carcinoma: A secretome-based verification study. Dis Markers. 2015;2015:874054. doi: 10.1155/2015/874054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shen C, Luo C, Xu Z, Liang Q, Cai Y, Peng B, Yan Y, Xia F. Molecular patterns based on immunogenomic signatures stratify the prognosis of colon cancer. Front Bioeng Biotechnol. 2022;10:820092. doi: 10.3389/fbioe.2022.820092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chu CH, Chang SC, Wang HH, Yang SH, Lai KC, Lee TC. Prognostic values of EPDR1 hypermethylation and its inhibitory function on tumor invasion in colorectal cancer. Cancers (Basel) 2018;10:393. doi: 10.3390/cancers10100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.He X, Chen H, Zhong X, Wang Y, Hu Z, Huang H, Zhao S, Wei P, Shi D, Li D. BST2 induced macrophage M2 polarization to promote the progression of colorectal cancer. Int J Biol Sci. 2023;19:331–345. doi: 10.7150/ijbs.72538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Casarrubios M, Provencio M, Nadal E, Insa A, Del Rosario Garcia-Campelo M, Lazaro-Quintela M, Dómine M, Majem M, Rodriguez-Abreu D, Martinez-Marti A, et al. Tumor microenvironment gene expression profiles associated to complete pathological response and disease progression in resectable NSCLC patients treated with neoadjuvant chemoimmunotherapy. J Immunother Cancer. 2022;10:e005320. doi: 10.1136/jitc-2022-005320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen YC, Lin MC, Hsiao CC, Zheng YX, Chen KD, Sung MT, Chen CJ, Wang TY, Lin YY, Chang HC, et al. Increased S100A15 expression and decreased DNA methylation of its gene promoter are involved in high metastasis potential and poor outcome of lung adenocarcinoma. Oncotarget. 2017;8:45710–45724. doi: 10.18632/oncotarget.17391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou Y, Tang L, Chen Y, Zhang Y, Zhuang W. An immune panel signature predicts prognosis of lung adenocarcinoma patients and correlates with immune microenvironment. Front Cell Dev Biol. 2021;9:797984. doi: 10.3389/fcell.2021.797984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang W, Nishioka Y, Ozaki S, Jalili A, Verma VK, Hanibuchi M, Abe S, Minakuchi K, Matsumoto T, Sone S. Chimeric and humanized anti-HM1.24 antibodies mediate antibody-dependent cellular cytotoxicity against lung cancer cells. Lung Cancer. 2009;63:23–31. doi: 10.1016/j.lungcan.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 85.Wainwright DA, Balyasnikova IV, Han Y, Lesniak MS. The expression of BST2 in human and experimental mouse brain tumors. Exp Mol Pathol. 2011;91:440–446. doi: 10.1016/j.yexmp.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yokoyama T, Enomoto T, Serada S, Morimoto A, Matsuzaki S, Ueda Y, Yoshino K, Fujita M, Kyo S, Iwahori K, et al. Plasma membrane proteomics identifies bone marrow stromal antigen 2 as a potential therapeutic target in endometrial cancer. Int J Cancer. 2013;132:472–484. doi: 10.1002/ijc.27679. [DOI] [PubMed] [Google Scholar]
- 87.Pham QT, Oue N, Yamamoto Y, Shigematsu Y, Sekino Y, Sakamoto N, Sentani K, Uraoka N, Tiwari M, Yasui W. The Expression of BTS-2 enhances cell growth and invasiveness in renal cell carcinoma. Anticancer Res. 2017;37:2853–2860. doi: 10.21873/anticanres.11637. [DOI] [PubMed] [Google Scholar]
- 88.Pan XQ, Huang W, Jin LW, Lin HZ, Xu XY. A novel pyroptosis-related prognostic signature for risk stratification and clinical prognosis in clear cell renal cell carcinoma. Dis Markers. 2022;2022:8093837. doi: 10.1155/2022/8093837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yamada Y, Arai T, Sugawara S, Okato A, Kato M, Kojima S, Yamazaki K, Naya Y, Ichikawa T, Seki N. Impact of novel oncogenic pathways regulated by antitumor miR-451a in renal cell carcinoma. Cancer Sci. 2018;109:1239–1253. doi: 10.1111/cas.13526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Januchowski R, Sterzynska K, Zawierucha P, Rucinski M, Swierczewska M, Partyka M, Bednarek-Rajewska K, Brązert M, Nowicki M, Zabel M, Klejewski A. Microarray-based detection and expression analysis of new genes associated with drug resistance in ovarian cancer cell lines. Oncotarget. 2017;8:49944–49958. doi: 10.18632/oncotarget.18278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang H, Cai Y, Zheng L, Zhang Z, Lin X, Jiang N. LncRNA BISPR promotes the progression of thyroid papillary carcinoma by regulating miR-21-5p. Int J Immunopathol Pharmacol. 2018;32:2058738418772652. doi: 10.1177/2058738418772652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Milutin Gasperov N, Farkas SA, Nilsson TK, Grce M. Epigenetic activation of immune genes in cervical cancer. Immunol Lett. 2014;162((2 Pt B)):256–257. doi: 10.1016/j.imlet.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 93.Etcheverry A, Aubry M, de Tayrac M, Vauleon E, Boniface R, Guenot F, Saikali S, Hamlat A, Riffaud L, Menei P, et al. DNA methylation in glioblastoma: Impact on gene expression and clinical outcome. BMC Genomics. 2010;11:701. doi: 10.1186/1471-2164-11-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kawai S, Azuma Y, Fujii E, Furugaki K, Ozaki S, Matsumoto T, Kosaka M, Yamada-Okabe H. Interferon-alpha enhances CD317 expression and the antitumor activity of anti-CD317 monoclonal antibody in renal cell carcinoma xenograft models. Cancer Sci. 2008;99:2461–2466. doi: 10.1111/j.1349-7006.2008.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gu G, Zhao D, Yin Z, Liu P. BST-2 binding with cellular MT1-MMP blocks cell growth and migration via decreasing MMP2 activity. J Cell Biochem. 2012;113:1013–1021. doi: 10.1002/jcb.23433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoo H, Park SH, Ye SK, Kim M. IFN-γ-induced BST2 mediates monocyte adhesion to human endothelial cells. Cell Immunol. 2011;267:23–29. doi: 10.1016/j.cellimm.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 97.Sibler E, He Y, Ducoli L, Rihs V, Sidler P, Puig-Moreno C, Frey J, Fujimoto N, Detmar M, Dieterich LC. Immunomodulatory responses of subcapsular sinus floor lymphatic endothelial cells in tumor-draining lymph nodes. Cancers (Basel) 2022;14:3602. doi: 10.3390/cancers14153602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Werner TA, Forster CM, Dizdar L, Verde PE, Raba K, Schott M, Knoefel WT, Krieg A. CXCR4/CXCR7/CXCL12 axis promotes an invasive phenotype in medullary thyroid carcinoma. Br J Cancer. 2017;117:1837–1845. doi: 10.1038/bjc.2017.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bian S, Zhao Y, Li F, Lu S, He Z, Wang S, Bai X, Zhao D, Liu M, Wang J. Total ginsenosides induce autophagic cell death in cervical cancer cells accompanied by downregulation of bone marrow stromal antigen-2. Exp Ther Med. 2021;22:667. doi: 10.3892/etm.2021.10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cheng J, Zhang G, Deng T, Liu Z, Zhang M, Zhang P, Adeshakin FO, Niu X, Yan D, Wan X, Yu G. CD317 maintains proteostasis and cell survival in response to proteasome inhibitors by targeting calnexin for RACK1-mediated autophagic degradation. Cell Death Dis. 2023;14:333. doi: 10.1038/s41419-023-05858-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang LL, Mao L, Wu H, Chen L, Deng WW, Xiao Y, Li H, Zhang L, Sun ZJ. pDC depletion induced by CD317 blockade drives the antitumor immune response in head and neck squamous cell carcinoma. Oral Oncol. 2019;96:131–139. doi: 10.1016/j.oraloncology.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 102.Jin S, Sun Y, Liang X, Gu X, Ning J, Xu Y, Chen S, Pan L. Emerging new therapeutic antibody derivatives for cancer treatment. Signal Transduct Target Ther. 2022;7:39. doi: 10.1038/s41392-021-00868-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu RQ, Lao XM, Chen DP, Qin H, Mu M, Cao WJ, Deng J, Wan CC, Zhan WY, Wang JC, et al. Immune checkpoint therapy-elicited sialylation of IgG antibodies impairs antitumorigenic type I interferon responses in hepatocellular carcinoma. Immunity. 2023;56:180–192.e11. doi: 10.1016/j.immuni.2022.11.014. [DOI] [PubMed] [Google Scholar]
- 104.Rimassa L, Finn RS, Sangro B. Combination immunotherapy for hepatocellular carcinoma. J Hepatol. 2023;79:506–515. doi: 10.1016/j.jhep.2023.03.003. [DOI] [PubMed] [Google Scholar]
- 105.Staudinger M, Glorius P, Burger R, Kellner C, Klausz K, Gunther A, Repp R, Klapper W, Gramatzki M, Peipp M. The novel immunotoxin HM1.24-ETA' induces apoptosis in multiple myeloma cells. Blood Cancer J. 2014;4:e219. doi: 10.1038/bcj.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hundemer M, Schmidt S, Condomines M, Lupu A, Hose D, Moos M, Cremer F, Kleist C, Terness P, Belle S, et al. Identification of a new HLA-A2-restricted T-cell, epitope within HM1.24 as immunotherapy target for multiple myeloma. Exp Hematol. 2006;34:486–496. doi: 10.1016/j.exphem.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hiramatsu K, Serada S, Kobiyama K, Nakagawa S, Morimoto A, Matsuzaki S, Ueda Y, Fujimoto M, Yoshino K, Ishii KJ, et al. CpG oligodeoxynucleotides potentiate the antitumor activity of anti-BST2 antibody. Cancer Sci. 2015;106:1474–1478. doi: 10.1111/cas.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.