Abstract
Accumulation of phenolic compounds has been monitored in a suspension culture of anthocyanin-accumulating sweet potato cell line grown under the conditions of modified Murashige and Skoog high-anthocyanin production medium (APM) over a period of 24 days. Tissue samples extracted with 15% acetic acid were analysed using HPLC at a detection wavelength of 326 nm. Among others, the following derivatives of caffeoylquinic acids were detected: 4,5-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, 3,4-dicaffeoylquinic acid, and 3,4,5-tricaffeoylquinic acid. Their total amount reached a maximum of 110 mg/gFW between the 4th and the 15th day of culture growth on APM. The major compound of the phenolic mixture was 3,5-dicaffeoylquinic acid with maximum accumulation level of 80 mg/100 gFW. The potential effects of targeted phenolic compounds on the nutraceutical qualities of in vitro produced anthocyanin-rich extracts are discussed.
INTRODUCTION
Free radicals are generated continuously in the human body as a byproduct of normal metabolism [1]. They are implicated in some diseases such as Alzheimer's disease [2], cancer [3], and vascular disorders (atherosclerosis, diabetes, hypertension) [4] and accelerate the aging process [5]. Increasing the level in foods of highly active radical scavenging compounds is important for maintaining a balance between oxidants and antioxidants in the body and for eliminating oxidative stress. The ability of phenolic compounds to scavenge free radicals and to contribute significantly towards antioxidant activities of vegetable and/or fruit extracts is well documented [6, 7] and a rapid screening method for relative antioxidant activities of flavonoids and phenolics has been developed [8]. Application of anthocyanin-based natural food colorants has been proposed [9] for increasing the antiradical activity of foodstuffs.
An extract prepared from the storage root of purple-fleshed sweet potato displayed an array of health-beneficial properties in in vitro studies such as radical scavenging [10, 11] and antimutagenic [12] activities and reduction of carbon tetrachloride-induced liver injury [13]. Phenolic acids and anthocyanins were identified to play a major role in these properties of sweet potato extract. Antihyperglycemic (antidiabetic) effect of sweet potato anthocyanins included into rat diet has also been reported [14].
Plant cell cultures have been successfully applied to produce secondary metabolites of interest, among them anthocyanins [15, 16]. We have established an anthocyanin-accumulating cell line (purple line (PL)) from the storage root of purple-fleshed sweet potato (Ipomoea batatas Lam cv Ayamurasaki, Convolvulaceae) [17]. Under the condition of a high-anthocyanin producing medium (APM) the sweet potato cell line accumulates 3 times more pigments than the field-grown storage root of Ayamurasaki cultivar [18] and is considered for a commercial production of natural food colorants. Evaluation of the potential chemopreventive properties of the cell line's anthocyanin-rich aqueous extracts in in vitro assays revealed enhanced radical scavenging, antimutagenic, and antiproliferation activities in comparison to the extract of field-grown Ayamurasaki storage root [19]. Enhanced accumulation of anthocyanins in the PL cell culture was suggested to contribute towards these activities. The biosynthetic pathway of phenolics is closely related to that of anthocyanins [20] and in an anthocyanin-accumulating cell line the presence of other phenolic compounds can be expected. Accumulation of two common phenolics, caffeic acid (CA) and chlorogenic acid (caffeoylquinic, CH), at low levels of maximum 10 mg/100 gFW in the PL cell line culture has been detected [21]. We also have observed that degradation of CH is concomitant with the appearance of new major compounds in the phenolic mixture [21]. Therefore the objective of the present study was to monitor the generation and degradation of caffeoylquinic acid derivatives induced under the conditions of high-APM. Cochromatography with standards has been employed to identify the major phenolic compounds. Their possible contributions toward chemopreventive properties of the PL cell line's extracts have been discussed.
MATERIALS AND METHODS
Plant material and culture conditions
Callus culture has been developed from the sweet potato storage root, cv Ayamurasaki, as described previously [17]. Suspended cell cultures were initiated by transferring about 1 g (fresh weight) of callus to 25 mL of liquid medium in 100 mL Erlenmeyer flasks. Basal Murashige and Skoog (MS) medium supplemented with 1.0 mg/L 2,4-dichlorophenoxyacetic acid (2,4-D) was used as a multiplication medium (MM). The cultures were incubated on a rotary shaker (130 rpm) at 25°C in the dark. The medium was changed weekly.
For the experiment, seven-day-old subcultures were transferred into a liquid high-APM which was a modified MS with 9.4 mM KNO3, without NH4NO3, with 5% sucrose and no growth regulators [18]. One hundred mg of cell aggregates were placed in 50 mL Erlenmeyer flasks containing 10 mL medium (pH 5.8 before autoclaving). The samples (6 replications per each sampling day) were harvested in 2–3 day intervals for a period of 24 days (growth period).
Extraction of phenolics and anthocyanins
Cell aggregates separated from the culture medium by vacuum filtration were ground and steeped in 15% acetic acid for 16 hours. The volume of acetic acid solution was calculated in the proportion of 20 mL/gfw of tissue. The samples were centrifuged at 10 000 rpm for 10 minutes and the phenolic compound in the supernatant identified.
Identification of phenolics and HPLC analysis
The supernatants were filtered through a 0.2 μm filter membrane (DISMIC-13cp, Advantec, Japan), injected (10 μL) into a YMC-Pack ODS-AM AM-302 column (150 × 4.6 mm, 5 μm; YMC, Kyoto, Japan) at 40°C. The HPLC system consisted of two LC-10AT pumps, an SIL-10AXL autoinjector, a CTO-10AC column oven, and an SPD-M10AVP photodiode array UV-VIS detector (Shimadzu, Kyoto, Japan) controlled by a CLASS-LC10 workstation (Shimadzu). The mobile phase consisted of mixtures of 0.2% (v/v) formic acid in water (solvent A) and methanol (solvent B). The elution profile was a linear gradient starting with 2% B from 0 to 15 minutes, 2% to 45% B from 15 to 50 minutes, and 45% B from 50 to 65 minutes. The flow rate was 1 mL/min [21]. CH and CA standards were purchased from Wako Pure Chemical Industries (Osaka, Japan). Because derivatives of caffeoylquinic acids are not commercially available, 4,5-dicaffeoylquinic acid (4,5-DCQA), 3,5-dicaffeoylquinic acid (3,5-DCQA), 3,4-dicaffeoylquinic acid (3,4-DCQA), and 3,4,5-tricaffeoylquinic acid (3,4,5-TCQA) standards were purified from the sweet potato leaves as described earlier [22]. Standard curves were prepared for calculations of the absolute concentration of compounds based on the peak areas. Peaks were detected at 326 nm wavelength and identified by co-chromatography.
RESULTS AND DISCUSSION
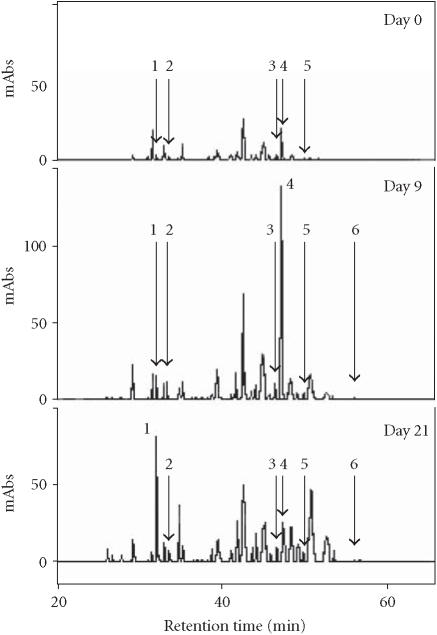
The extract of the PL cell line suspension culture, multiplied on standard MS enriched with 1.0 mg/L 2,4-D (multiplication medium), is rich in anthocyanins [17]. Tissue transfer into a high-APM resulted in further increase in accumulation of anthocyanins concomitant with a 3-fold increase in the total phenolics level [21]. Monitoring the generation of phenolics in the cell line’s tissue over one growth period of 24 days exhibited a mixture of about 30 different compounds (Figure 1). The reversed-phase HPLC chromatograms indicated dynamic changes in the phenolic pattern over time (Figure 1).
Figure 1.
HPLC chromatogram of phenolic compounds monitored in crude extract of the PL cell line suspension culture over one growth period of 24 days in a high-anthocyanin production medium. 1: caffeic acid (CA); 2: chlorogenic acid (CH); 3: 3,4-dicaffeoylquinic acid (3,4-DCQA); 4: 3,5-dicaffeoylquinic acid (3,5-DCQA); 5: 4,5-dicaffeoylquinic acid (4,5-DCQA); 6: 3,4,5-tricaffeoylquinic acid (3,4,5-TCQA).
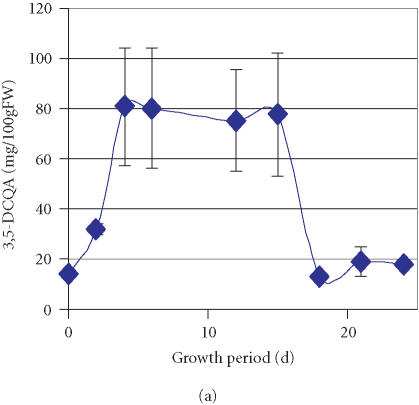
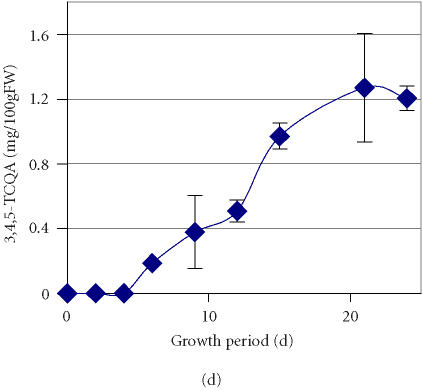
Further to the previous detection of two minor components, CA and CH, whose accumulation sharply increased from traces amounts to 10 mg/100 gFW during the first two days of culture growth and was followed by a gradual decrease over time [21], we have monitored an enhanced accumulation of a major compound represented by a peak that exhibited a retention time of 47.2 minutes (peak 4, Figure 1). Through comparison and cochromatography with standards of CH derivatives (caffeoylquinic acids) isolated from the sweet potato leaves, this peak was identified as 3,5-DCQA. Accumulation of 3,5-DCQA was concomitant with the decrease of CH level (Table 1). Between the 2nd and the 4th day of culture after transfer to high-APM the relative concentration of 3,5-DCQA in the mixture increased from 0.1% to 15.8% and remained constant till day 15 (Table 1). The total amount of 3,5-DCQA increased during the first 4 days after transfer to high-APM to 80 mg/100 gFW and decreased between the 15th and the 18th day to 20 mg/100 gFW (Figure 2a). 3,5-DCQA was the major compound of the phenolics mixture produced by the PL cell line and analysed under the described conditions (Table 1).
Table 1.
Relative concentrations* (%) of selected phenolic compounds in the extract of the PL cell line tissue grown on a high-anthocyanin producing medium during 24 days. (T: concentration less than 0.1%; ND: not detected.)
| Day | CA | CH | 3,5-DCQA | 4,5-DCQA | 3,4-DCQA | 3,4,5-TCQA | Total conc | Total amount (mg/gFW) |
|---|---|---|---|---|---|---|---|---|
| 0 | 4.7±0.0⧫ | 0.6±0.0 | 8.7±0.0 | 1.2±0.0 | 0.4±0.0 | ND | 15.2 | 20.2±0.0 |
| 2 | 6.0±1.4 | 3.2±0.3 | 0.1±0.1 | 0.1±0.1 | ND | ND | 9.4 | 21±3.8 |
| 4 | 3.8±1.3 | 1.2±0.4 | 15.8±5.2 | 1.8±0.1 | 0.6±0.1 | ND | 23.2 | 117±17.6 |
| 6 | 5.1±2.6 | 1.1±0.5 | 13.0±7.4 | 1.7±0.6 | 0.7±0.1 | T | 21.6 | 109±29.5 |
| 15 | 4.1±1.6 | 1.5±0.7 | 13.5±5.9 | 2.9±0.8 | 1.4±0.1 | 0.1±0.01 | 23.5 | 102±36.6 |
| 18 | 6.7±3.0 | 0.9±0.1 | 3.0±0.2 | 1.6±0.6 | — | 0.1±0.01 | 12.3 | 40±9.4 |
| 21 | 8.2±5.4 | 1.1±0.4 | 4.4±1.5 | 2.0±0.9 | 1.2±0.1 | 0.12±0.01 | 17.0 | 52±11.2 |
| 24 | 9.1±1.7 | 0.8±0.1 | 3.9±0.6 | 1.3±0.2 | 1.1±0.1 | 0.13±0.01 | 16.33 | 48±8.5 |
*The relative concentrations calculated based on the total peak area of phenolics detected at 326 nm (100%) and the area of the peaks of interest (%).
⧫Standard deviations of 6 independent determinations for each sampling point.
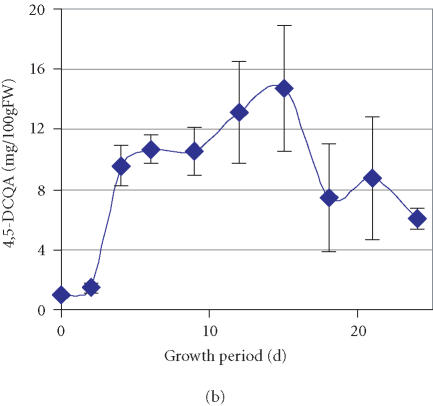
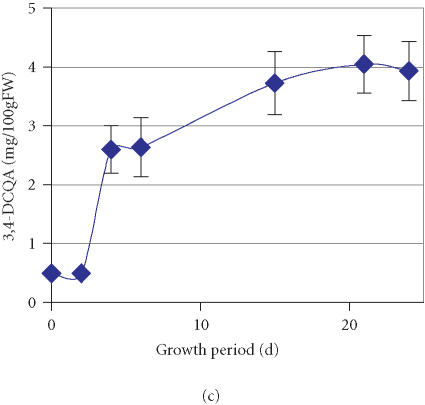
Figure 2.
Accumulation of (a) 3,5-dicaffeoylquinic acid (3,5-DCQA), (b) 4,5- dicaffeoylquinic acid (4,5-DCQA), (c) 3,4-dicaffeoylquinic acid (3,4-DCQA), (d) 3,4,5-tricaffeoylquinic acid (3,4,5-TCQA) in the PL suspension culture over one growth period in a high-anthocyanin producing medium (APM). Bars represent standard deviations of six replications.
The time course of accumulation for three other compounds represented by peaks with retention times of 46.7, 50.4, and 57.1 minutes (Figure 1) was also monitored. Through cochromatography with standards of caffeoylquinic acids, these compounds were identified as 4,5-DCQA, 3,4-DCQA, and 3,4,5-TCQA, respectively (Figure 3). The data suggests that active accumulation of both 4,5-DCQA and 3,4-DCQA was delayed compared to 3,5-DCQA and was at significantly lower levels (Figures 2b and 2c). The maximum concentration of 4,5-DCQA was 14.7 mg/100 gFW at the 15th day of culture. The highest level of 3,4-DCQA of 4.0 mg/100 gFW was observed at the stationary phase of growth period (day 21). Traces of the CH derivative with the most evolved molecular structure, 3,4,5-TCQA, were detected among the phenolic compounds at the 6th day of the growth period followed by a steady increase over time (Figure 2d). The highest level of accumulation (1.2 mg/100 gFW) was reached at a stationary phase. According to the relative concentrations calculated based on the peak area, the levels of accumulation of these selected phenolics in the tissue of PL cell line grown on APM were 3,5-DCQA > CA > 4,5-DCQA > CH > 3,4-DCQA > 3,4,5-TCQA.
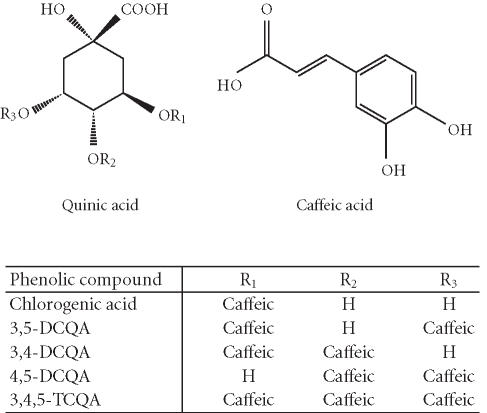
Figure 3.
Molecular structures of phenolic compounds in the PL cell line generated from the purple-fleshed sweet potato, cv Ayamurasaki.
Son et al [23] reported that the main phenolic acids identified in various sweet potato cultivars are CA, CH, and two isomers of DCQA. Detailed identification of the isomers of DCQA in extracts from sweet potato leaves of over 1389 varieties has been conducted by Islam et al [22]. These authors identified three isomers of DCQA: 4,5-DCQA, 3,5-DCQA, 3,4-DCQA, and 3,4,5-TCQA. Di- and tricaffeoylquinic acids are biosynthesised through conversion of CH, and isolation of an enzyme which catalyses the conversion of CH to 3,5-DCQA from sweet potato root has been reported [24]. Identification of the derivatives of CH in the callus culture established from the storage root of purple-fleshed sweet potato indicates that the biosynthetic pathway for the generation of these compounds is active in the callus culture and that their biosynthesis can be enhanced through modification of the culture’s medium. The highest level of the CH and derivatives in the PL cell line culture of about 110 mg/100 gFW was obtained between the 4th and the 15th day of growth on APM (Table1). The content of DCQA isomers alone was 93.0 mg/100 gFW with the contribution of CA and CH being 10.0 and 6.0 mg/gFW, respectively. Walter et al [25] reported that total phenolic content of sweet potato storage root (peels removed) ranged among cultivars from 14 to 51 mg/100 g FW, which is 8- to 2-fold lower than that obtained in our experiment. However, phenolics in sweet potato storage root are not equally distributed but localised in several tissues, including the periderm and tissue beneath it as the selected sides of accumulation [26]. A study of the chemical composition of the outer cortex of storage root revealed that within the external 3- mm layer the concentration of DCQA isomers varies between cultivars from 168 to 638 mg/100 gFW, that of CA from 33 to 138 mg/gFW, and CH from 22 to 173 mg/gFW [27].
The function of caffeoylquinic acid derivatives present in the outer cortex of sweet potato storage root is the protection against fungal diseases [27]. Yoshimoto et al [28] found that 3,4,5-TCQA, followed by the DCQA isomers, displayed superior antimutagenic activities to CH against cooked food mutagen Trp-P-1. We have evaluated antimutagenic activity of anthocyanin-rich aqueous extract of the PL cell line produced under the conditions of MM and high-APM medium and observed that the extract produced under high-APM conditions displayed stronger antimutagenic activity (73% inhibition of Trp-P-1-induced reverse mutation of Salmonella typhimurium TA98) compared to the cell line extract produced under the conditions of MM medium (54%) and the extract of field-grown sweet potato storage root, which was selected as the donor tissue for cell line development (36%) [19]. As described in the present paper, the accumulation of caffeoylquinic acids by the PL suspension culture under the conditions of APM might have been responsible for this result.
Caffeoylquinic acids exhibited some medical properties: Zhu et al [29] reported that DCQA specifically and irreversibly inhibited the replication of the human immunodeficiency virus, HIV-1. In contrary to the DCQA, their likely precursors, CH, CA, and quinic acid, were not active against HIV-1. The authors suggested that the DCQAs are promising lead compounds for developing new antiretroviral drugs. Under the conditions of high-APM the sweet potato cell line accumulates relatively high levels of DCQAs and therefore it might be considered as a source of their continuous production through plant cell culture-based technology.
Reports on the strong antioxidative and antimutagenic properties of the targeted phenolic compounds, CA, CH, and derivatives, by other researchers [28, 30] and our previous data on evaluation of the chemopreventive properties of the PL cell line extracts [19] suggest that inducing accumulation of caffeoylquinic acids in the sweet potato cell line may increase the nutraceutical properties of the anthocyanin-phenolic acid complex produced by the tissue of PL cell line. However the nutraceutical quality of this complex will entirely depend on the bioavailability and/or degradation during the digestion process. It has been reported that both CA and CH are absorbed at high levels in the thin intestine of humans: 97% and 33%, respectively [31]. It is of our further interest to evaluate the stability of generated phenolic compounds in food systems and their bioavailability in humans.
ACKNOWLEDGMENT
Founding of this project through JSPS postdoctoral fellowship to Izabela Konczak by the Japan International Science and Technology Exchange Centre is thankfully acknowledged.
References
- 1.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 2.Perry G, Cash A.D, Smith M.A. Alzheimer disease and oxidative stress. J Biomed Biotechnol. 2002;2(3):120–123. doi: 10.1155/S1110724302203010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Athar M. Oxidative stress and experimental carcinogenesis. Indian J Exp Biol. 2002;40(6):656–667. [PubMed] [Google Scholar]
- 4.Chen K, Thomas S.R, Keaney J.F., Jr. Beyond LDL oxidation: ROS in vascular signal transduction. Free Radic Biol Med. 2003;35(2):117–132. doi: 10.1016/s0891-5849(03)00239-9. [DOI] [PubMed] [Google Scholar]
- 5.Szweda P.A, Friguet B, Szweda L.I. Proteolysis, free radicals, and aging. Free Radic Biol Med. 2002;33(1):29–36. doi: 10.1016/s0891-5849(02)00837-7. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Cao G, Prior R.L. Oxygen radical absorbing capacity of anthocyanins. J Agric Food Chem. 1997;45(2):304–309. [Google Scholar]
- 7.Chu Y-H, Chang C-L, Hsu H-F. Flavonoid content of several vegetables and their antioxidant activity. J Sci Food Agric. 2000;80(5):561–566. [Google Scholar]
- 8.Pannala A.S, Rice-Evans C. Rapid screening method for relative antioxidant activities of flavonoids and phenolics. In: Packer L, editor. Flavonoids and Other Polyphenols. vol. 335 of Methods in Enzymology. New York, NY: Elsevier; 2001. pp. 266–272. [DOI] [PubMed] [Google Scholar]
- 9.Espin J.C, Soler-Rivas C, Wichers H.J, Garcia-Viguera C. Anthocyanin-based natural colorants: a new source of antiradical activity for foodstuff. J Agric Food Chem. 2000;48(5):1588–1592. doi: 10.1021/jf9911390. [DOI] [PubMed] [Google Scholar]
- 10.Cevallos-Casals B.A, Cisneros-Zevallos L. Stoichiometric and kinetic studies of phenolic antioxidants from Andean purple corn and red-fleshed sweetpotato. J Agric Food Chem. 2003;51(11):3313–3319. doi: 10.1021/jf034109c. [DOI] [PubMed] [Google Scholar]
- 11.Oki T, Masuda M, Furuta S, Nishiba Y, Terahara N, Suda I. Involvement of anthocyanins and other phenolic compounds in radical-scavenging activity of purple-fleshed sweet potato cultivars. J Food Sci. 2002;67(5):1752–1756. [Google Scholar]
- 12.Yoshimoto M, Okuno S, Yamaguchi M, Yamakawa O. Antimutagenicity of deacylated anthocyanins in purple-fleshed sweetpotato. Biosci Biotechnol Biochem. 2001;65(7):1652–1655. doi: 10.1271/bbb.65.1652. [DOI] [PubMed] [Google Scholar]
- 13.Suda I, Furuta S, Nishiba Y, Yamakawa O, Matsugano K, Sugita K. Hepato-protective activity of purple-colored sweetpotato juice. Sweetpotato Research Front. 1997;(4):3. [Google Scholar]
- 14.Matsui T, Ebuchi S, Kobayashi M, et al. Anti-hyperglycemic effect of diacylated anthocyanin derived from Ipomoea batatas cultivar Ayamurasaki can be achieved through the α-glucosidase inhibitory action. J Agric Food Chem. 2002;50(25):7244–7248. doi: 10.1021/jf025913m. [DOI] [PubMed] [Google Scholar]
- 15.Do C.B, Cormier F. Effects of high ammonium concentrations on growth and anthocyanin formation in grape (Vitis vinifera L.) cell suspension cultured in a production medium. Plant Cell Tiss Organ Cult. 1991;27:169–174. [Google Scholar]
- 16.Kobayashi Y, Akiita M, Sakamoto K, et al. Large-scale production of anthocyanin by Aralia cordata cell suspension cultures. Appl Microbiol Biotechnol. 1993;40(2-3):215–218. [Google Scholar]
- 17.Konczak-Islam I, Yoshinaga M, Nakatani M, Yamakawa O, Terahara N. Establishment and characteristics of an anthocyanin-producing cell line from sweet potato storage root. Plant Cell Reports. 2000;19(5):472–477. doi: 10.1007/s002990050758. [DOI] [PubMed] [Google Scholar]
- 18.Konczak-Islam I, Nakatani M, Yoshinaga M, Yamakawa O. Effect of ammonium ion and temperature on anthocyanin composition in sweet potato cell suspension culture. Plant Biotechnology. 2001;18(2):109–117. [Google Scholar]
- 19.Konczak-Islam I, Yoshimoto M, Hou D.X, Terahara N, Yamakawa O. Potential chemopreventive properties of anthocyanin-rich aqueous extracts from in vitro produced tissue of sweetpotato (Ipomoea batatas L.) J Agric Food Chem. 2003;51(20):5916–5922. doi: 10.1021/jf030066o. [DOI] [PubMed] [Google Scholar]
- 20.Haslam E. Cambridge, Mass, USA: Cambridge University Press; 1998. Practical Polyphenolics. From Structure to Molecular Recognition and Physiological Action. [Google Scholar]
- 21.Konczak-Islam I, Okuno S, Yoshimoto M, Yamakawa O. Composition of phenolics and anthocyanins in a sweet potato cell suspension culture. Biochem Eng J. 2003;14(3):155–161. [Google Scholar]
- 22.Islam M.S, Yoshimoto M, Yahara S, Okuno S, Ishiguro K, Yamakawa O. Identification and characterization of foliar polyphenolic composition in sweetpotato (Ipomoea batatas L.) genotypes. J Agric Food Chem. 2002;50(13):3718–3722. doi: 10.1021/jf020120l. [DOI] [PubMed] [Google Scholar]
- 23.Son K.C, Severson R.F, Snook M.E, Kays S.J. Root carbohydrate, organic acids and phenolic chemistry in relation to sweetpotato weevil resistance. HortScience. 1991;26:1305–1308. [Google Scholar]
- 24.Kojima M, Kondo T. An enzyme in sweet potato root which catalyzes the conversion of chlorogenic acid, 3-caffeoylquinic acid, to isochlorogenic acid, 3,5-dicaffeoylquinic acid. Agric Biol Chem. 1985;49:2467–2469. [Google Scholar]
- 25.Walter W.M., Jr., Purcell A.E, McCollum G.K. Use of high-pressure liquid chromatography for analysis of sweet potato phenolics. J Agric Food Chem. 1979;27(5):938–941. doi: 10.1021/jf60225a031. [DOI] [PubMed] [Google Scholar]
- 26.Shadel W.E, Walter W.M. Localization of phenols and polyphenol oxidase in ‘jewel’ sweet potatos (ipomoea batatas ‘jewel’) Can J Bot. 1981;59:1961–1967. [Google Scholar]
- 27.Stange R.R., Jr., Midland S.L, Holmes G.J, Sims J.J, Mayer R.T. Constituents from the periderm and outer cortex of Ipomoea batatas with antifungal activity against Rhizopus stolonifer. Postharvest Biol Technol. 2001;23(2):85–92. [Google Scholar]
- 28.Yoshimoto M, Yahara S, Okuno S, Islam M.S, Ishiguro K, Yamakawa O. Antimutagenicity of mono-, di-, and tricaffeoylquinic acid derivatives isolated from sweetpotato (Ipomoea batatas L.) leaf. Biosci Biotechnol Biochem. 2002;66(11):2336–2341. doi: 10.1271/bbb.66.2336. [DOI] [PubMed] [Google Scholar]
- 29.Zhu K, Cordeiro M.L, Atienza J, Robinson W.E., Jr., Chow S.A. Irreversible inhibition of human immunodeficiency virus type 1 integrase by dicaffeoylquinic acids. J Virol. 1999;73(4):3309–3316. doi: 10.1128/jvi.73.4.3309-3316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamada J, Tomita Y. Antimutagenic activity of caffeic acid and related compounds. Biosci Biotechnol Biochem. 1996;60(2):328–329. doi: 10.1271/bbb.60.328. [DOI] [PubMed] [Google Scholar]
- 31.Olthof M.R, Hollman P.C, Katan M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J Nutr. 2001;131(1):66–71. doi: 10.1093/jn/131.1.66. [DOI] [PubMed] [Google Scholar]