Abstract
With the coverage of COVID-19 vaccination, it has been possible to observe the potential side effects of SARS-CoV-2 vaccines, with the most common ones being fever, myalgia, headache, and fatigue. However, an association has been observed between new and recurrent kidney injuries, mainly glomerulonephritis and lupus nephritis associated with ANCA, with the Pfizer-BioNTech, Moderna, Sinovac, and AstraZeneca vaccines, although the relationship between them is not clear. We report a case of ANCA-related vasculitis and lupus glomerulonephritis after the second dose of the AstraZeneca vaccine. The elderly patient presented significant worsening of kidney function after immunosuppression and complications after a new onset COVID-19 infection that led to death. We provide a literature review about kidney damage related to ANCA vasculitis after COVID-19 vaccine, aiming for a better understanding of the pathophysiological mechanism of kidney injury, its presentation, and treatment.
Keywords: ANCA, glomerulonephritis, lupus nephritis, COVID-19, vaccine
1. Introduction
The most common side effects of the 2019 coronavirus vaccine (COVID-19) are fever, myalgia, headache, fatigue, local pain, and, rarely, anaphylactic reaction. However, case reports have demonstrated the occurrence of new and relapses cases of renal injury associated with SARS-CoV-2 immunization, including IgA nephropathy, membranous nephropathy, minimal change disease, anti-glomerular basement membrane disease, antineutrophil cytoplasmic antibody-associated vasculitis (ANCA), IgG4-related disease, lupus nephritis, scleroderma renal crisis, and thrombotic microangiopathy (1, 2). In these cases, such diseases had a temporal link with vaccination and a positive COVID-IgG response to SARS-CoV-2 (3).
Herein, we report a case from the Brazilian Consortium for the Study of COVID-19-Associated Renal Disease, which collects kidney biopsies from patients with kidney damage secondary to COVID-19 and its vaccine in 42 private and public hospitals (4, 5). This is a case report of ANCA-associated glomerulonephritis and lupus nephritis following COVID-19 vaccination, and a review of kidney manifestations related to this vaccine.
2. Case report
A 75-year-old woman presented to the emergency department with fever for 15 days, headache, adynamia, progressive dyspnea, polyarthralgia, macroscopic hematuria, foamy urine with no changes in urine volume. She had a previous diagnosis of hypothyroidism, hypertension and normal kidney function. She had been immunized against COVID-19 with the second dose AstraZeneca vaccine 15 days prior to admission. On physical examination, she was febrile (37.8 °C), normal blood pressure (130/80 mmHg), pulse rate 110 beats per minute, eupneic, and had an oxygen saturation of 98% in ambient air. The complementary physical examination was normal.
Three sequential RT-PCR tests for COVID-19 were negative, repeated every 2 days, and computed tomography (CT) of the chest showed no alterations. Laboratory tests on admission showed normocytic normochromic anemia, with hemoglobin (Hb) 10.4 g/dL and hematocrit (Ht): 30.1% and leukocytes: 10,810/mm3 with neutrophilia (9,015/mm3). Other laboratory tests were creatinine 2.9 mg/dL, sodium (Na): 128 mEq/L; potassium (K): 3.5 mEq/L, pH: 7.26, arterial partial oxygen pressure (PaO2): 81 mmHg; partial pressure of arterial carbon dioxide (PaCO2): 49 mmHg; bicarbonate: 20 mmol/L. Her urinalysis showed proteinuria, leukocyturia and hematuria, raising the hypothesis of nephritic syndrome. Her urine/protein/creatinine ratio was 2.5 mg/mg.
Although the RT-PCR test for COVID-19 was negative, the patient was admitted to the bed unit for COVID-19 cases for observation. Seven days after admission, her general condition worsened and her kidney function was compromised, with signs of hypervolemia such as peripheral edema, pulmonary congestion and dyspnea, requiring renal replacement therapy. At that time, laboratory tests showed Hb 7.5 g/dL; Ht 23.1%; platelets 104,000/mm3; D-dimer 3,250 ng/ml; ferritin 6,406 ng/ml; direct bilirubin 2.9 mg/dl; C-reactive protein 18.6 mg/dl; AST 230.6 U/L; ALT 139.4 U/L; urea: 105 mg/dl; Cr 2.7 mg/dl; procalcitonin: 0.98 ng/dl. Her c-ANCA serology was negative, with positive antinuclear antibody (ANA) (1:640) in a fine speckled pattern, as was p-ANCA (1:80). Considering the hypothesis of rapidly progressive glomerulonephritis, a kidney biopsy was performed five days after worsening (about 20 days after hospitalization). Laboratory findings at admission and seven days later are organized in Table 1 .
Table 1.
Laboratory findings on admission and seven days after admission.
| Test | Value at admission | Value after 7 days | Normal ranges (female) |
|---|---|---|---|
| Hemoglobin (g/dL) | 10.4 | 7.5 | 12.1 - 15.1 |
| Hematocrit (%) | 30.1 | 23.1 | 35 - 45 |
| Platelets (cells/mm³) | 104,000 | 250,000 - 260,000 | |
| Leukocytes (cells/mm³) | 10,810 | 4,500 - 11,000 | |
| Neutrophils (cells/mm³) | 9,015 | 1,500 - 7,000 | |
| Urea (mg/dL) | 105 | 21 - 43 | |
| Creatinine (mg/dL) | 2.9 | 2.7 | 0.6 - 1.1 |
| Sodium (mEq/L) | 128 | 135 - 145 | |
| Potassium (mEq/L) | 3.5 | 3.5 - 5.5 | |
| AST (IU/L) | 27 | 231 | 10 - 36 |
| ALT (IU/L) | 24 | 139 | 4 - 36 |
| GGT (IU/L) | 44 | 05 - 43 | |
| D-dimer (ng/mL) | 3,250 | < 250 | |
| Ferritin (ng/mL) | 6,406 | 11 - 306 | |
| Direct bilirubin (mg/dL) | 2.9 | < 1 | |
| Indirect bilirubin (mg/dL) | 1.0 | < 0.5 | |
| C-reactive protein (mg/dL) | 18.6 | < 0.3 | |
| Procalcitonin (ng/dL) | 0.98 | < 200 | |
| Blood pH level | 7.26 | 7.35 - 7.45 | |
| Arterial partial oxygen pressure (mmHg) | 81 | 80 - 100 | |
| Partial pressure of arterial carbon dioxide (mmHg) | 49 | 35 - 45 | |
| Bicarbonate (mmol/L) | 20 | 23–28 | |
| Urine/protein creatinine ratio (mg/g) | 2.5 | < 150 |
AST, Aspartate transaminase; ALT, Alanine transaminase; GGT, Gamma-glutamyl transferase
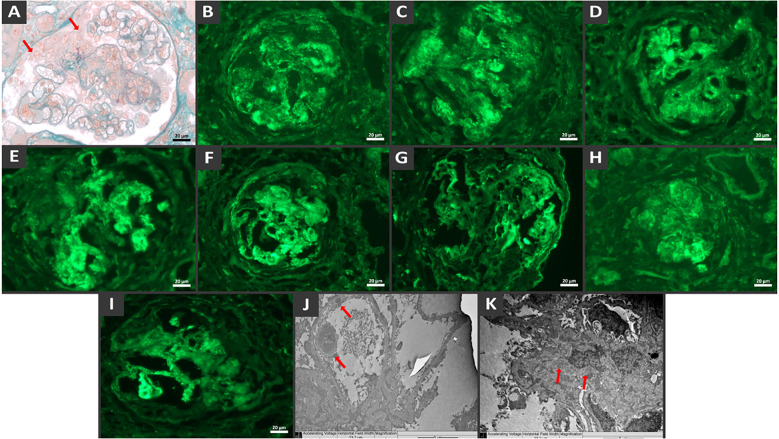
A renal biopsy revealed a proliferative and necrotizing glomerulonephritis with a full house immunofluorescence pattern, leading to the suspicion of lupus nephritis ( Figure 1 ). There was mild interstitial fibrosis, tubular atrophy and degeneration of the tubular epithelium. The blood vessels showed no significant changes. Because of the presence of diffuse crescents and the necrotizing appearance of the lesion, the histological hypothesis of lupus glomerulonephritis associated with ANCA-related vasculitis was confirmed.
Figure 1.
Histological findings show diffuse crescentic necrotizing glomerulonephritis with endocapillary proliferation and full-house immune complex deposition. Glomerulus with cellular crescent (arrow), endocapillary crescent and fibrinoid necrosis by light microscopy, Masson’s trichrome stain (A). Direct immunofluorescence demonstrates granular mesangial and capillary wall staining deposits of IgG (B), IgM (C), IgA (D), C3 (E), C1q (F), Fibrinogen (G), Kappa (H) e Lambda (I). Note the crescent surrounding the vascular tuft. Electron microscopy from paraffin embedded tissue reveals electron-dense deposits in the subendothelial (J) and mesangial (K) regions.
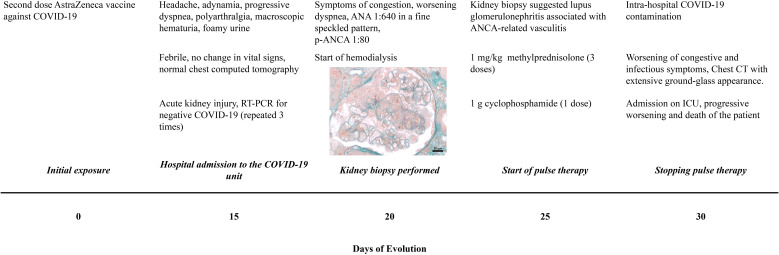
The patient was treated methylprednisolone 500mg for 3 days, followed by prednisone 1 mg/kg/day, and administered one dose of cyclophosphamide 1 g. After 30 days of the first symptoms, the RT-PCR test for COVID-19 was positive, possibly due to intra-hospital contamination. The imunossupression drugs were stopped. Immunoglobulin, plasmapheresis or remdesivir was not performed. Her general condition worsened rapidly, requiring intensive care due to dyspnea. Chest CT showed a extensive bilateral pleural effusion extensive with laminar consolidations, adjacent atelectasis, diffuse bilateral ground-glass opacities involving 75% of the parenchym, and signs of parapseptal and centrilobular emphysema. Unfortunately, the patient presented clinical worsening, with refractory hemodynamic instability, respiratory acidosis, and hypoxia, and evolved to death. The timeline of the patient’s evolution is shown in Figure 2 .
Figure 2.
Timeline of clinical evolution. The time and initial symptoms are specified from COVID-19 vaccination, investigation, treatment and patient outcome.
3. Discussion
The remarkable increase in the coverage of immunization against COVID-19 worldwide allows us to learn about the possible adverse effects of the vaccine, including those involving the renal system. Some vaccines have been previously linked to kidney injury. The influenza vaccine has been associated with nephrotic syndrome secondary to minimal change disease (6, 7), membranous nephropathy (8), vasculitis with pauci-immune glomerulonephritis (9), microscopic polyangiitis (10), ANCA-associated glomerulonephritis (9), rhabdomyolysis with acute kidney injury (11), vasculitis (12) and Henoch-Schonlein purpura (13, 14). The hepatitis B vaccine has been associated with nephrotic syndrome secondary to minimal change disease (15) and lupus nephritis (16). The pneumococcal vaccine was associated with glomerulonephritis (related to anti-glomerular basement membrane antibody) (17), the polio-diphtheria-tetanus vaccine with nephrotic syndrome (secondary to minimal change disease) (18), the varicella vaccine with nephrotic syndrome (19), and the measles vaccine with minimal change disease (20). The rabies vaccine was associated with possible relapse of nephrotic syndrome (21). The Bacillus Calmette-Guerin (BCG) vaccine used as intravesical immunotherapy to treat bladder cancer and not for immunization was associated with membranous nephropathy, interstitial nephritis, and formation of asymptomatic renal granulomas (22).
Several COVID-19 vaccines and their different mechanisms of action have been reported to have some renal injury side effects (4) ( Table 2 ). Lipid-based nanoparticle-mRNA vaccines cause a stimulatory response to CD4+ and CD8+ T lymphocytes and an increased production of B lymphocytes in germinal centers, resulting in the secretion of interferons (mainly gamma) and interleukin-2. DNA-adenovirus vaccines stimulate CD4+ and CD8+ cytotoxic T lymphocytes, in addition to increasing antibody production by B lymphocytes, mainly IgG1-IgG3 and to a lesser extent IGg2-IgG4 (2). Both vaccine classes have the same antigenic target, the viral spike protein, and have been associated with glomerular injury.
Table 2.
New onset and relapses cases of ANCA-associated glomerulonephritis and other glomerular lesions with ANCA-positive association reported after COVID-19 vaccination.
| Cases of renal involvement reported after COVID-19 vaccination | Total number of cases reported | Type of vaccine | Number of Cases per dose |
|---|---|---|---|
| ANCA-associated Glomerulonephritisa | 31* | mRNA Vaccine - 17 Viral Vector Vaccine - 10 Inactivated Vaccine - 4 |
1st dose: 9 2nd dose: 19 3nd dose: 3 |
| Lupus Nephritis (2* cases ANCA-positive)b | 6* | mRNA Vaccine - 3 Viral Vector Vaccine - 3 |
1st dose: 4 2nd dose: 2 |
| IgA Nephropathy (3 cases ANCA-positive)c |
30 | mRNA Vaccine - 28 Viral Vector Vaccine - 1 Inactivated Vaccine - 1 |
1st dose: 5 2nd dose: 25 |
| Anti-glomerular basement membrane nephritis (6 cases ANCA-positive)d | 14 | mRNA Vaccine - 13 Viral Vector Vaccine - 1 |
1st dose: 5 2nd dose: 8 3nd dose: 1 |
| Crescentic glomerulonephritis (6 cases ANCA-positive)e | 7 | mRNA Vaccine - 7 | 1st dose: 1 2nd dose: 6 |
| Membranous nephropathy (1 case ANCA-positive)f | 7 | mRNA Vaccine - 6 Viral Vector Vaccine - 1 |
1nd dose: 2 2nd dose: 5 |
| Other non-ANCa-positive glomerular lesions | |||
| Minimal change diseaseg | 22 | mRNA Vaccine - 20 Viral Vector Vaccine - 2 |
1st dose: 6 2nd dose: 8 |
| Collapsing glomerulopathyh | 4 | mRNA Vaccine - 2 Viral Vector Vaccine - 2 |
1st dose: 2 2nd dose: 2 |
The pathophysiological mechanism of post-vaccine glomerulonephritis remains unknown. However, the likely cause is mimicry of the remade viral proteins with the host’s proteins, thereby causing a type of “second wave infection” and subsequent renal damage (1). Infection by SARS-CoV-2 has been recognized as a trigger for the onset of several diseases and autoimmune reactions (48). The viral RNA is recognized by host receptors, binding to them and stimulating the production of type I interferon and inflammatory cytokines. In turn, type I interferon stimulates antibodies production, which are strictly associated with autoimmune diseases (1, 2).
The SARS-CoV-2 vaccine seems to be related to some cases of ANCA-associated glomerulonephritis and lupus nephritis, majority related after Pfizer-BioNTech, Moderna, Sinovac and AstraZeneca (23). The relationship between the vaccine and autoimmune diseases is still unclear. The mRNA vaccines can induce, for example, double-positive anti–glomerular basement membrane antibody and myeloperoxidase ANCA (62), subsequently nephritis and vasculitis. Proinflammatory status mediated by the vaccine containing lipid-based nanoparticles can lead to a subsequent loss of tolerance to self-antigens due to autoreactivity and immune system hyperactivation (88). It is suggested that the vaccines’ antigenic target, the viral spicule protein, stimulates antibody production.
The overlap of ANCA-associated glomerulonephritis and lupus nephritis is not uncommon (89). Although these diseases are easy to differentiate from each other by autoantibody profile and histopathological findings, some patients have shown an overlap of such findings (90). Crescentic glomerulonephritis is not rare in lupus nephritis, and the presence of ANCA antibodies is also well established in the literature (90, 91). ANCA antibodies are present more markedly in crescentic lupus nephritis than in lupus nephritis without crescents; such a finding favors the role of ANCA antibodies in renal crescent formation. Similarly, although associated p-ANCA glomerulonephritis is defined as pauci-immune on immunofluorescence, the presence of immune-complexes described in the present case is explained by the full house pattern of overlapping lupus nephritis (90, 91).
We present Supplementary Material with a detailed review of the literature on ANCA-related glomerulonephritis and other renal lesions in which ANCA positivity occurred after COVID-19 vaccination. The main conditions were ANCA glomerulonephritis (14%), IgA nephropathy (12.3%), anti-glomerular basement membrane glomerulonephritis (10.5%), crescentic glomerulonephritis (10.5%). In this review, 55.17% were women, 81.03% were vaccinated with the mRNA vaccine. The symptoms were nonspecific and started on average 18 days after the vaccine. Most patients used high doses of glucocorticoids and some type of immunosuppressant, and 18.96% required hemodialysis. In general, clinically significant chronic kidney disease may persist in more than 75% of ANCA-associated vasculitis in those with kidney impairment (24). The proportion of normal glomeruli appears to be associated with dialysis discontinuation, although some treatments such as plasmapheresis did not show significance with dialysis discontinuation (25).
4. Conclusion
The study of adverse effects, including renal involvement, of the COVID-19 vaccine, gives us the opportunity for early diagnosis and provision of assistance to patients with complications after autoimmune activation by the vaccine or direct contact with the SARS-CoV-2 virus.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Ethics statement
The requirement of ethical approval was waived by Comitê de Ética e Pesquisa do HUUFMA for the studies involving humans because Comitê de Ética e Pesquisa do HUUFMA. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MC: Conceptualization, Writing – original draft. TV: Data curation, Investigation, Writing – original draft. LM: Data curation, Investigation, Writing – original draft. LF: Data curation, Investigation, Writing – original draft. RM: Data curation, Investigation, Writing – original draft. DC: Writing – review & editing. NS: Resources, Writing – review & editing. RN: Conceptualization, Investigation, Writing – original draft. PN: Writing – review & editing. GS: Supervision, Writing – review & editing.
Acknowledgments
National Council for Scientific and Technological Development (CNPq in the Portuguese acronym) and the Foundation for the Support of Research and Scientific and Technological Development of Maranhão (FAPEMA in the Portuguese acronym).
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1298622/full#supplementary-material
References
- 1. Hassanzadeh S, Djamali A, Mostafavi L, Pezeshgi A. Kidney complications following COVID-19 vaccination; a review of the literature. J Nephropharmacol (2021) 11:e1–1. doi: 10.34172/npj.2022.01 [DOI] [Google Scholar]
- 2. Li NL, Coates PT, Rovin BH. COVID-19 vaccination followed by activation of glomerular diseases: does association equal causation? Kidney Int (2021) 100:959–65. doi: 10.1016/j.kint.2021.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carr EJ, Kronbichler A, Graham-Brown M, Abra G, Argyropoulos C, Harper L, et al. Review of early immune response to SARS-CoV-2 vaccination among patients with CKD. Kidney Int Rep (2021) 6:2292–304. doi: 10.1016/j.ekir.2021.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pacheco ICR, Costa DM do N, Sousa DS, Salgado Filho N, Silva GEB, Neves PDM de M. Kidney injury associated with COVID-19 infection and vaccine: A narrative review. Front Med (2022) 9:956158. doi: 10.3389/fmed.2022.956158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Teixeira Júnior AAL, Neves PDM de M, Lages JS, Cunha K de A, Muniz MPR, Brito DJ de A, et al. Brazilian consortium for the study on renal diseases associated with COVID-19: A multicentric effort to understand SARS-CoV-2-related nephropathy. Front Med (2020) 7:584235. doi: 10.3389/fmed.2020.584235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel C, Shah HH. Vaccine-associated kidney diseases: A narrative review of the literature. Saudi J Kidney Dis Transplant (2019) 30:1002–9. doi: 10.4103/1319-2442.270254 [DOI] [PubMed] [Google Scholar]
- 7. Kielstein JT, Termühlen L, Sohn J, Kliem V. Minimal change nephrotic syndrome in a 65-year-old patient following influenza vaccination. Clin Nephrol (2000) 54:246–8. [PubMed] [Google Scholar]
- 8. Kutlucan A, Gonen I, Yildizhan E, Aydin Y, Sav T, Yildirim U. Can influenza H1N1 vaccination lead to the membranous glomerulonephritis? Indian J Pathol Microbiol (2012) 55:239–41. doi: 10.4103/0377-4929.97893 [DOI] [PubMed] [Google Scholar]
- 9. Orbach H, Agmon-Levin N, Zandman-Goddard G. Vaccines and autoimmune diseases of the adult. Discovery Med (2010) 9:90–7. [PubMed] [Google Scholar]
- 10. Uji M, Matsushita H, Iwata S. Microscopic polyangiitis after influenza vaccination. Intern Med Tokyo Jpn (2005) 44:892–6. doi: 10.2169/internalmedicine.44.892 [DOI] [PubMed] [Google Scholar]
- 11. Plotkin E, Bernheim J, Ben-Chetrit S, Mor A, Korzets Z. Influenza vaccine–a possible trigger of rhabdomyolysis induced acute renal failure due to the combined use of cerivastatin and bezafibrate. Nephrol Dial Transplant (2000) 15:740–1. doi: 10.1093/ndt/15.5.740 [DOI] [PubMed] [Google Scholar]
- 12. Hyla-Klekot L, Kucharska G, Cieslak W. [Necrotizing glomerulonephritis in decursu vasculitis after vaccination against influenza]. Pol Merkur Lek Organ Pol Tow Lek (2005) 19:75–7. [PubMed] [Google Scholar]
- 13. Liu J-Y, Wu Q, Liu M, Wang L, Gao Y, Li S. HENOCH-SCHÖNLEIN PURPURA NEPHRITIS FOLLOWING INFLUENZA VACCINATION: A CASE REPORT AND REVIEW OF THE LITERATURE. Southeast Asian J Trop Med Public Health (2016) 47:945–50. [PubMed] [Google Scholar]
- 14. Damjanov J, Amato JA. Progression of renal disease in Henoch-Schönlein purpura after influenza vaccination. JAMA (1979) 242:2555–6. doi: 10.1001/jama.242.23.2555 [DOI] [PubMed] [Google Scholar]
- 15. Macário F, Freitas L, Correia J, Campos M, Marques A. Nephrotic syndrome after recombinant hepatitis B vaccine. Clin Nephrol (1995) 43:349. [PubMed] [Google Scholar]
- 16. Santoro D, Stella M, Montalto G, Castellino S. Lupus nephritis after hepatitis B vaccination: an uncommon complication. Clin Nephrol (2007) 67:61–3. doi: 10.5414/cnp67061 [DOI] [PubMed] [Google Scholar]
- 17. Tan SY, Cumming AD. Vaccine related glomerulonephritis. BMJ (1993) 306:248. doi: 10.1136/bmj.306.6872.248-b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clajus C, Spiegel J, Bröcker V, Chatzikyrkou C, Kielstein JT. Minimal change nephrotic syndrome in an 82 year old patient following a tetanus-diphteria-poliomyelitis-vaccination. BMC Nephrol (2009) 10:21. doi: 10.1186/1471-2369-10-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Angeletti A, Lugani F, La Porta E, Verrina E, Caridi G, Ghiggeri GM. Vaccines and nephrotic syndrome: efficacy and safety. Pediatr Nephrol Berl Ger (2022) 38(9):2915–28. doi: 10.1007/s00467-022-05835-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuzemko JA. Measles vaccination and the nephrotic syndrome. Br Med J (1972) 4:665–6. doi: 10.1136/bmj.4.5841.665-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singhal PC, Gupta VK, Nampoory MR, Lazar AI, Chugh KS. Case report: glomerulonephritis after immunization with antirabies vaccine. Ann Allergy (1981) 46:98–9. [PubMed] [Google Scholar]
- 22. Mohammed A, Arastu Z. Emerging concepts and spectrum of renal injury following Intravesical BCG for non-muscle invasive bladder cancer. BMC Urol (2017) 17:114. doi: 10.1186/s12894-017-0304-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thammathiwat T, Banjongjit A, Iampenkhae K, Townamchai N, Kanjanabuch T. ANCA associated glomerulonephritis following SARS-CoV-2 vaccination: A case series and systematic review. Vaccines (2023) 11:983. doi: 10.3390/vaccines11050983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hanberg JS, Cook C, Fu X, Choi HK, Zhang Y, Wallace ZS. Longitudinal patterns of renal function in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Care Res (2023) 75:2190–8. doi: 10.1002/acr.25100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee Y-J, Ahn S-M, Oh J-S, Kim Y-G, Lee C-K, Yoo B, et al. Recovery and long-term renal outcome of patients with anti-neutrophil cytoplasmic antibody-associated vasculitis who are on dialysis at presentation. J Rheum Dis (2023) 30:251–9. doi: 10.4078/jrd.2023.0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anderegg MA, Liu M, Saganas C, Montani M, Vogt B, Huynh-Do U, et al. De novo vasculitis after mRNA-1273 (Moderna) vaccination. Kidney Int (2021) 100:474–6. doi: 10.1016/j.kint.2021.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sekar A, Campbell R, Tabbara J, Rastogi P. ANCA glomerulonephritis after the Moderna COVID-19 vaccination. Kidney Int (2021) 100:473–4. doi: 10.1016/j.kint.2021.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dube GK, Benvenuto LJ, Batal I. Antineutrophil cytoplasmic autoantibody-associated glomerulonephritis following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int Rep (2021) 6:3087–9. doi: 10.1016/j.ekir.2021.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen C-C, Chen H-Y, Lu C-C, Lin S-H. Case report: anti-neutrophil cytoplasmic antibody-associated vasculitis with acute renal failure and pulmonary hemorrhage may occur after COVID-19 vaccination. Front Med (2021) 8:765447. doi: 10.3389/fmed.2021.765447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Davidovic T, Schimpf J, Sprenger-Mähr H, Abbassi-Nik A, Soleiman A, Zitt E, et al. De Novo and Relapsing Glomerulonephritis following SARS-CoV-2 mRNA Vaccination in Microscopic Polyangiitis. Case Rep Nephrol (2021) 2021:8400842. doi: 10.1155/2021/8400842 [DOI] [Google Scholar]
- 31. Feghali EJ, Zafar M, Abid S, Santoriello D, Mehta S. De-novo antineutrophil cytoplasmic antibody-associated vasculitis following the mRNA-1273 (Moderna) vaccine for COVID-19. Cureus (2021) 13:e19616. doi: 10.7759/cureus.19616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hakroush S, Tampe B. Case report: ANCA-associated vasculitis presenting with rhabdomyolysis and pauci-immune crescentic glomerulonephritis after Pfizer-BioNTech COVID-19 mRNA vaccination. Front Immunol (2021) 12:762006. doi: 10.3389/fimmu.2021.762006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schaubschlager T, Rajora N, Diep S, Kirtek T, Cai Q, Hendricks AR, et al. De novo or recurrent glomerulonephritis and acute tubulointerstitial nephritis after COVID-19 vaccination: A report of six cases from a single center. Clin Nephrol (2022) 97:289–97. doi: 10.5414/CN110794 [DOI] [PubMed] [Google Scholar]
- 34. Shakoor MT, Birkenbach MP, Lynch M. ANCA-associated vasculitis following Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis (2021) 78:611–3. doi: 10.1053/j.ajkd.2021.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Villa M, Díaz-Crespo F, Pérez de José A, Verdalles Ú, Verde E, Almeida Ruiz F, et al. A case of ANCA-associated vasculitis after AZD1222 (Oxford-AstraZeneca) SARS-CoV-2 vaccination: casualty or causality? Kidney Int (2021) 100:937–8. doi: 10.1016/j.kint.2021.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. David R, Hanna P, Lee K, Ritchie A. Relapsed ANCA associated vasculitis following Oxford AstraZeneca ChAdOx1-S COVID-19 vaccination: A case series of two patients. Nephrol Carlton Vic (2022) 27:109–10. doi: 10.1111/nep.13993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Garcia DS, Martins C, da Fonseca EO, de Carvalho VCP, de Rezende RPV. Clinical Images: Severe proteinase 3 antineutrophil cytoplasmic antibody glomerulonephritis temporally associated with Sinovac Biotech’s inactivated SARS-CoV-2 vaccine. ACR Open Rheumatol (2022) 4:277–8. doi: 10.1002/acr2.11397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. El Hasbani G, Uthman I. ANCA-associated vasculitis following the first dose of Pfizer-BioNTech COVID-19 vaccine. Nephron (2023) 147:103–7. doi: 10.1159/000525562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim BC, Kim HS, Han KH, Han SY, Jo HA. A case report of MPO-ANCA-associated vasculitis following heterologous mRNA1273 COVID-19 booster vaccination. J Korean Med Sci (2022) 37:e204. doi: 10.3346/jkms.2022.37.e204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Noel E, Rashid U, Rabbani R, Khan WA, Benjamin YS, Lee I. Antineutrophil cytoplasmic autoantibody-associated glomerulonephritis as a possible side effect of COVID-19 vaccination. Cureus (2022) 14:e30565. doi: 10.7759/cureus.30565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Obata S, Hidaka S, Yamano M, Yanai M, Ishioka K, Kobayashi S. MPO-ANCA-associated vasculitis after the Pfizer/BioNTech SARS-CoV-2 vaccination. Clin Kidney J (2021) 15:357–9. doi: 10.1093/ckj/sfab181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Prabhahar A, Naidu GSRSNK, Chauhan P, Sekar A, Sharma A, Sharma A, et al. ANCA-associated vasculitis following ChAdOx1 nCoV19 vaccination: case-based review. Rheumatol Int (2022) 42:749–58. doi: 10.1007/s00296-021-05069-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ramezanzade E, Ghanbari R, Yazdanipour T. Antineutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis in a 15-year-old patient after receiving the second dose of the BBIBP-CorV (Sinopharm) COVID-19 vaccine: A case report. Nephro-Urol Mon (2022) 14:e127124. doi: 10.5812/numonthly-127124 [DOI] [Google Scholar]
- 44. So D, Min K-W, Jung WY, Han S-W, Yu M-Y. Microscopic polyangiitis following mRNA COVID-19 vaccination: A case report. J Korean Med Sci (2022) 37:e154. doi: 10.3346/jkms.2022.37.e154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Suzuki M, Sekiguchi Y, Sasaki M, Inaba S, Oyama S, Inoue Y, et al. Antineutrophil cytoplasmic antibody-associated vasculitis after COVID-19 vaccination with Pfizer-BioNTech. Intern Med Tokyo Jpn (2022) 61:2925–9. doi: 10.2169/internalmedicine.9807-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yadav R, Shah S, Chhetri S. ANCA-associated vasculitis following Johnson and Johnson COVID-19 vaccine. Ann Med Surg (2022) 79:104123. doi: 10.1016/j.amsu.2022.104123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zamoner W, Scardini JB, De Dio BJ, Marques A de M, Silva VDS, Garcia AL, et al. ANCA-associated vasculitis following Oxford-AstraZeneca COVID-19 vaccine in Brazil: Is there a causal relationship? A case report. Front Med (2022) 9:1003332. doi: 10.3389/fmed.2022.1003332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Caza TN, Cassol CA, Messias N, Hannoudi A, Haun RS, Walker PD, et al. Glomerular disease in temporal association with SARS-CoV-2 vaccination: A series of 29 cases. Kidney360 (2021) 2:1770–80. doi: 10.34067/KID.0005372021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bansal SB, Rana AS, Manhas N, Rana A. Post COVID vaccination (COVAXINTM -BB152 V) pauci-immune crescentic glomerulonephritis. Indian J Nephrol (2022) 32:495–7. doi: 10.4103/ijn.ijn_352_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Klomjit N, Alexander MP, Fervenza FC, Zoghby Z, Garg A, Hogan MC, et al. COVID-19 vaccination and glomerulonephritis. Kidney Int Rep (2021) 6:2969–78. doi: 10.1016/j.ekir.2021.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tuschen K, Bräsen JH, Schmitz J, Vischedyk M, Weidemann A. Relapse of class V lupus nephritis after vaccination with COVID-19 mRNA vaccine. Kidney Int (2021) 100:941–4. doi: 10.1016/j.kint.2021.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sekar A. Lupus nephritis flare post Moderna mRNA-1273 coronavirus vaccine. QJM Mon J Assoc Physicians (2022) 114:882–3. doi: 10.1093/qjmed/hcab284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zavala-Miranda MF, González-Ibarra SG, Pérez-Arias AA, Uribe-Uribe NO, Mejia-Vilet JM. New-onset systemic lupus erythematosus beginning as class V lupus nephritis after COVID-19 vaccination. Kidney Int (2021) 100:1340–1. doi: 10.1016/j.kint.2021.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim HJ, Jung M, Lim BJ, Han SH. New-onset class III lupus nephritis with multi-organ involvement after COVID-19 vaccination. Kidney Int (2022) 101:826–8. doi: 10.1016/j.kint.2022.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Baskaran K, Cohen AWS, Weerasinghe N, Vilayur E. Report of two cases of minimal change disease following vaccination for COVID -19. Nephrol Carlton Vic (2022) 27:111–2. doi: 10.1111/nep.13995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fernández P, Alaye ML, Chiple MEG, Arteaga JD, Douthat W, Fuente JDL, et al. Glomerulopathies after vaccination against COVID-19. Four cases with three different vaccines in Argentina. Nefrol Publicacion Of Soc Espanola Nefrol (2021) 43(5):655–7. doi: 10.1016/j.nefro.2021.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hanna C, Herrera Hernandez LP, Bu L, Kizilbash S, Najera L, Rheault MN, et al. IgA nephropathy presenting as macroscopic hematuria in 2 pediatric patients after receiving the Pfizer COVID-19 vaccine. Kidney Int (2021) 100:705–6. doi: 10.1016/j.kint.2021.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Izzedine H, Bonilla M, Jhaveri KD. Nephrotic syndrome and vasculitis following SARS-CoV-2 vaccine: true association or circumstantial? Nephrol Dial Transplant (2021) 36:1565–9. doi: 10.1093/ndt/gfab215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kudose S, Friedmann P, Albajrami O, D’Agati VD. Histologic correlates of gross hematuria following Moderna COVID-19 vaccine in patients with IgA nephropathy. Kidney Int (2021) 100:468–9. doi: 10.1016/j.kint.2021.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lo WK, Chan KW. Gross haematuria after mRNA COVID-19 vaccination in two patients with histological and clinical diagnosis of IgA nephropathy. Nephrol Carlton Vic (2022) 27:110–1. doi: 10.1111/nep.13992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nakatani S, Mori K, Morioka F, Hirata C, Tsuda A, Uedono H, et al. New-onset kidney biopsy-proven IgA vasculitis after receiving mRNA-1273 COVID-19 vaccine: case report. CEN Case Rep (2022) 11:358–62. doi: 10.1007/s13730-021-00677-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ting JA, Barbir E-B, McRae SA, Schachter M, De Luca L, Riazy M, et al. Double-Positive Anti–Glomerular Basement Membrane Antibody and Myeloperoxidase Antineutrophil Cytoplasmic Autoantibody–Associated Glomerulonephritis Post COVID-19 mRNA vaccine: A Case Series of 4 Patients. Can J Kidney Health Dis (2023) 10:20543581231153217. doi: 10.1177/20543581231153217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sugita K, Kaneko S, Hisada R, Harano M, Anno E, Hagiwara S, et al. Development of IgA vasculitis with severe glomerulonephritis after COVID-19 vaccination: a case report and literature review. CEN Case Rep (2022) 11:436–41. doi: 10.1007/s13730-022-00695-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tan HZ, Tan RY, Choo JCJ, Lim CC, Tan CS, Loh AHL, et al. Is COVID-19 vaccination unmasking glomerulonephritis? Kidney Int (2021) 100:469–71. doi: 10.1016/j.kint.2021.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Coorey CP, Phua E, Chou A, Shen Y, Mather A. Anti-GBM Disease after Oxford-AstraZeneca ChAdOx1 nCoV-19 Vaccination: A Report of Two Cases. Case Rep Nephrol Dial (2022) 12:234–7. doi: 10.1159/000525737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Terakawa K, Niikura T, Katagiri D, Sugita A, Kikuchi T, Hayashi A, et al. A case of rapidly progressive glomerulonephritis with double-positive anti-GBM antibody and MPO-ANCA after SARS-CoV-2 vaccination and relapse during 1 year follow-up. CEN Case Rep (2023) 27:1–7. doi: 10.1007/s13730-023-00792-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sacker A, Kung V, Andeen N. Anti-GBM nephritis with mesangial IgA deposits after SARS-CoV-2 mRNA vaccination. Kidney Int (2021) 100:471–2. doi: 10.1016/j.kint.2021.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gupta RK, Ellis BK. Concurrent antiglomerular basement membrane nephritis and antineutrophil cytoplasmic autoantibody-mediated glomerulonephritis after second dose of SARS-CoV-2 mRNA vaccination. Kidney Int Rep (2022) 7:127–8. doi: 10.1016/j.ekir.2021.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hoi S, Ogawa M, Munemura C, Takata T, Isomoto H. Atypical anti-glomerular basement membrane nephritis after the first dose of the severe acute respiratory syndrome coronavirus 2 mRNA vaccine. Yonago Acta Med (2023) 66:300–5. doi: 10.33160/yam.2023.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Aydın MF, Yıldız A, Oruç A, Sezen M, Dilek K, Güllülü M, et al. Relapse of primary membranous nephropathy after inactivated SARS-CoV-2 virus vaccination. Kidney Int (2021) 100:464–5. doi: 10.1016/j.kint.2021.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nagai K, Iwase M, Ueda A. A case of anti-GBM nephritis following centipede bites and COVID-19 vaccination. CEN Case Rep (2022) 11:166–70. doi: 10.1007/s13730-021-00646-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kim S, Jung J, Cho H, Lee J, Go H, Lee JH. A child with crescentic glomerulonephritis following SARS-CoV-2 mRNA (Pfizer-BioNTech) vaccination. Pediatr Nephrol Berl Ger (2023) 38:299–302. doi: 10.1007/s00467-022-05681-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lebedev L, Sapojnikov M, Wechsler A, Varadi-Levi R, Zamir D, Tobar A, et al. Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis (2021) 78:142–5. doi: 10.1053/j.ajkd.2021.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to “potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases”. Clin Immunol Orlando Fla (2021) 224:108665. doi: 10.1016/j.clim.2021.108665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Al-Rasbi S, Al-Maqbali JS, Al-Farsi R, Al Shukaili MA, Al-Riyami MH, Al Falahi Z, et al. Myocarditis, pulmonary hemorrhage, and extensive myositis with rhabdomyolysis 12 days after first dose of Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine: A case report. Am J Case Rep (2022) 23:e934399. doi: 10.12659/AJCR.934399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gambella A, Barreca A, Biancone L, Roccatello D, Peruzzi L, Besso L, et al. Spectrum of kidney injury following COVID-19 disease: renal biopsy findings in a single Italian pathology service. Biomolecules (2022) 12:298. doi: 10.3390/biom12020298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Psyllaki A, Stavrakaki I, Androvitsanea A, Gakiopoulou H, Petrakis I, Stylianou K. Two cases of glomerular involvement after vaccination against COVID-19: epiphenomenon or causality? Clin Kidney J (2022) 15:574–5. doi: 10.1093/ckj/sfab252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Park HJ, An WS, Rha SH, Kim SE, Lee SM. Minimal change glomerulonephritis following the second dose of the Moderna COVID-19 vaccine. QJM Mon J Assoc Physicians (2022) 115:490–1. doi: 10.1093/qjmed/hcac094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Annicchiarico Petruzzelli L, Minale B, Serio V, De Luca A, Marino Marsilia G, Campione S, et al. Pediatric Minimal Change Disease and AKI following the Pfizer-BioNTech COVID-19 Vaccine: causal or incidental correlation? G Ital Nefrol Organo Uff Della Soc Ital Nefrol (2022) 39:2022–vol6. [PubMed] [Google Scholar]
- 80. D’Agati VD, Kudose S, Bomback AS, Adamidis A, Tartini A. Minimal change disease and acute kidney injury following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int (2021) 100:461–3. doi: 10.1016/j.kint.2021.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Maas RJ, Gianotten S, van der Meijden WAG. An additional case of minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis Off J Natl Kidney Found (2021) 78:312. doi: 10.1053/j.ajkd.2021.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Weijers J, Alvarez C, Hermans MMH. Post-vaccinal minimal change disease. Kidney Int (2021) 100:459–61. doi: 10.1016/j.kint.2021.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kervella D, Jacquemont L, Chapelet-Debout A, Deltombe C, Ville S. Minimal change disease relapse following SARS-CoV-2 mRNA vaccine. Kidney Int (2021) 100:457–8. doi: 10.1016/j.kint.2021.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Komaba H, Wada T, Fukagawa M. Relapse of minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis (2021) 78:469–70. doi: 10.1053/j.ajkd.2021.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Morlidge C, El-Kateb S, Jeevaratnam P, Thompson B. Relapse of minimal change disease following the AstraZeneca COVID-19 vaccine. Kidney Int (2021) 100:459. doi: 10.1016/j.kint.2021.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Holzworth A, Couchot P, Cruz-Knight W, Brucculeri M. Minimal change disease following the Moderna mRNA-1273 SARS-CoV-2 vaccine. Kidney Int (2021) 100:463–4. doi: 10.1016/j.kint.2021.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Neves PD, Caires RA, Guimarães MP, Costalonga EC, Cavalcante LB, Costa E Silva VT, et al. Collapsing glomerulopathy following SARS-CoV-2 adenovirus-vector-based vaccine: report of 2 cases. Kidney Int (2022) 101:637–9. doi: 10.1016/j.kint.2021.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ndeupen S, Qin Z, Jacobsen S, Bouteau A, Estanbouli H, Igyártó BZ. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience (2021) 24:103479. doi: 10.1016/j.isci.2021.103479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Turner-Stokes T, Wilson HR, Morreale M, Nunes A, Cairns T, Cook HT, et al. Positive antineutrophil cytoplasmic antibody serology in patients with lupus nephritis is associated with distinct histopathologic features on renal biopsy. Kidney Int (2017) 92:1223–31. doi: 10.1016/j.kint.2017.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nasr SH, D’Agati VD, Park H-R, Sterman PL, Goyzueta JD, Dressler RM, et al. Necrotizing and crescentic lupus nephritis with antineutrophil cytoplasmic antibody seropositivity. Clin J Am Soc Nephrol CJASN (2008) 3:682–90. doi: 10.2215/CJN.04391007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jarrot P-A, Chiche L, Hervier B, Daniel L, Vuiblet V, Bardin N, et al. Systemic lupus erythematosus and antineutrophil cytoplasmic antibody-associated vasculitis overlap syndrome in patients with biopsy-proven glomerulonephritis. Med (Baltimore) (2016) 95:e3748. doi: 10.1097/MD.0000000000003748 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.