Abstract
Babesia bigemina infection of mature bovine erythrocytes results in new proteins specifically exposed on the parasitized cell surface. Monoclonal antibody (MAb) 64/32 binds a protein, designated p94, on B. bigemina-infected erythrocytes but not on either uninfected or B. bovis-parasitized erythrocytes. However, p94 was not encoded by B. bigemina and was not a parasite-modified erythrocyte membrane protein. In contrast, we showed that p94 could be eluted from the infected erythrocyte surface and was identified as specifically bound immunoglobulin M (IgM) heavy chain for the following reasons: (i) MAb 64/32 bound a reduced molecule of 94 kDa in both infected erythrocyte lysates and normal bovine serum; (ii) MAb 64/32 bound a 94-kDa molecule in reduced preparations of purified IgM; (iii) an anti-bovine μ heavy-chain MAb, BIg73, reacted specifically with the surface of infected erythrocytes and bound the 94-kDa molecule in lysates of infected erythrocytes, normal bovine serum, and purified IgM; and (iv) immunoprecipitation of infected erythrocyte lysates with MAb 64/32 depleted the 94-kDa antigen bound by anti-μ MAb BIg73 and vice versa. Binding of IgM to the infected erythrocyte surface was detected in vivo early in acute parasitemia and occurred during both the trophozoite and merozoite stages of intraerythrocytic parasitism. The common feature of IgM binding to the parasitized erythrocyte surface among otherwise genetically and antigenically distinct B. bigemina strains is suggestive of an advantageous role in parasite survival in vivo.
Apicomplexan parasites in the genera Babesia and Plasmodium invade and replicate within erythrocytes, resulting in, respectively, babesiosis and malaria (13). Invasive stages bind specific receptors normally expressed on the surfaces of target erythrocytes (15, 27, 28, 40) and enter the cell via formation of a parasitophorous vacuole. The vacuole is later dissolved by babesial but not plasmodial parasites (11, 37). Inside the erythrocytes, the merozoite differentiates into a trophozoite, which undergoes asexual replication to produce daughter merozoites able to exit the host cell and invade additional erythrocytes (13). During this intracellular replicative cycle, the host erythrocyte membrane is altered (8). Changes required for intracellular growth are associated with active transport of nutrients from the serum (17), as well as elimination of catabolites from the parasitized erythrocytes (14). In addition to these metabolic functions, parasite-induced structural changes may alter the interaction of the infected erythrocyte with other host cells and molecules (2, 16). Erythrocytes infected with either Babesia bovis or Plasmodium falciparum are sequestered in the microvasculature as a result of parasite-induced adherence to endothelial cells (4, 48). Erythrocyte adhesion is mediated by parasite-encoded proteins, such as P. falciparum EMP-1, rosettin, and sequestrin (3, 9, 30), or, alternatively, by parasite modifications of host proteins (6). Importantly, both parasite-encoded proteins and modified host proteins may present new epitopes associated exclusively with infected cells and therefore may serve as targets of immunity as well as pathogenetic determinants (5, 18).
Similar to B. bovis and P. falciparum infections, B. bigemina infection results in new proteins specifically exposed on the erythrocyte surface (39). However, B. bigemina infection differs in that sequestration of parasitized erythrocytes and the resulting neurological signs do not occur and the clinical signs are referable principally to severe anemia. Correspondingly, we propose that the structural and functional modifications of the B. bigemina-infected cell surface are likely to be unique. Our research goal is to identify these modifications associated with B. bigemina infection and determine their pathogenetic significance. Using a monoclonal antibody (MAb) developed against a merozoite fraction (22), we identified a protein exposed on the surfaces of B. bigemina-infected bovine erythrocytes. This MAb, designated 64/32, reacts with the surfaces of erythrocytes infected with B. bigemina strains from Brazil, Mexico, Puerto Rico, and St. Croix but not with either uninfected or B. bovis-infected erythrocytes (47). Initial results indicated that MAb 64/32 bound a molecule of 94 kDa that was not metabolically labeled with [35S]methionine during in vitro culture of B. bigemina. Consequently, we hypothesized that the protein recognized by MAb 64/32 is not encoded by the parasite but represents a parasite-dependent modification. In this paper, we describe the identification of this protein and its binding to B. bigemina-infected erythrocytes.
MATERIALS AND METHODS
Parasites.
The Mexico strain of B. bigemina, its derivative the biological clone JG-29 (29), and the Mo7 clone from the Mexico strain of B. bovis (35) were maintained as cryopreserved stabilates (31, 47). Parasites were grown in vitro with bovine erythrocytes and normal bovine serum as described previously (21, 45).
Antibodies.
All MAbs used in this work were from twice-cloned hybridomas and were of the immunoglobulin G1 (IgG1) isotype. MAb 64/32 is reactive with the surfaces of B. bigemina-infected erythrocytes and binds a protein initially designated p94. MAb 14/1 specifically recognizes a 45-kDa glycoprotein, gp45, on the B. bigemina merozoite surface, and MAb 14/16 is reactive with a 58-kDa B. bigemina rhoptry protein, RAP-1 (24). MAb BIg73 is directed against the heavy chain of bovine IgM, and MAb BIg501 is specific for the bovine Ig λ light chain. MAb 23/8 is specific for a 225-kDa B. bovis spherical-body protein (33) and was used as the positive control for B. bovis-infected erythrocytes. MAb Tryp1E1, reactive with the Trypanosoma brucei variable surface glycoprotein, and MAbs 64/11 and ANA8A, reactive with a 220-kDa surface protein on normal and infected bovine erythrocytes, were used as negative controls. A bovine postinfection serum, B240, was obtained from a calf infected with B. bigemina (44). A murine polyclonal antibody was obtained by immunizing mice with p94. The p94 was purified from lysates of infected erythrocytes by MAb 64/32 affinity chromatography (see below).
B. bigemina infection.
A splenectomized 5-month-old calf was inoculated intravenously with 2 × 109 B. bigemina (Mexico strain) cells of a stabilate cryopreserved with 10% polyvinylpyrrolidone (46). Blood was collected daily in EDTA, and the reactivity of MAb 64/32 with infected erythrocytes was evaluated by live immunofluorescence.
IFA.
The fixed indirect immunofluorescence assay (IFA) was performed as previously described (26) with smears of washed erythrocytes infected with either B. bigemina JG-29 or B. bovis Mo7 and fixed with methanol. Smears of uninfected or Anaplasma marginale-infected erythrocytes were used as negative controls. Parasite nuclei were stained with 0.025% ethidium bromide diluted in PBS (pH 3.5). IFA with live parasites was performed as described previously (24). MAb 14/1, which specifically binds only the B. bigemina merozoite outer membrane, was used as a surface specificity control to confirm that the infected erythrocyte membrane was intact and impermeable to antibody (24).
Immunoaffinity chromatography.
The B. bigemina-infected erythrocyte surface antigen p94 was purified on a MAb 64/32 affinity column. Briefly, 25 mg of purified MAb 64/32 was suspended in 5 ml of 0.1 M phosphate buffer (pH 8.0) and coupled to an agarose matrix with 0.1 M NaCNBH3. B. bigemina-infected erythrocytes from in vitro cultures (10 ml of >95% parasitized erythrocytes after concentration on Percoll gradients) were solubilized in lysis buffer containing protease inhibitors. The lysate was incubated on the MAb-agarose-coupled column and eluted as described previously (25). A separate but identically prepared MAb 64/32 affinity matrix was used to isolate IgM from normal bovine serum. Eluted proteins were quantitated by the bicinchoninic acid technique (Pierce, Rockford, Ill.) and were electrophoresed in polyacrylamide gels with detection by silver staining or immunoblotting (43).
Electrophoresis and immunoblotting.
Proteins were solubilized in Laemmli sample buffer, electrophoresed in 7.5 to 17.5% or 1.5 to 15.0% gradient polyacrylamide gels with sodium dodecyl sulfate (SDS) (34), and transferred to nitrocellulose membranes (Schleicher & Schuell, Keene, N.H.). A solution of 3.0% bovine serum albumin in 10 mM Tris–150 mM NaCl–0.05% Tween 20 was used to block the membranes and for subsequent washes. The membranes were incubated at 23°C for 1 h in this buffer containing 2 μg of MAb per ml (32). Bound MAbs were detected with a 1:6,000 dilution of sheep anti-mouse antibody conjugated to horseradish peroxidase. The conjugate complexes were detected by enhanced chemiluminescence (Amersham Corp., Arlington Heights, Ill.). Protein standards in the range of 7.1 to 208 kDa (kaleidoscope prestained standards; Bio-Rad, Hercules, Calif.) were also electrophoresed and transferred to the nitrocellulose membranes.
Metabolic and surface labeling.
B. bigemina-encoded proteins were metabolically labeled, during in vitro culture, with [35S]methionine (200 μCi/well; NEG-072 EXPRE35S; Du Pont Co., Wilmington, Del.) or a mixture of 15 tritiated amino acids as described previously (10). The radiolabeled samples were immunoprecipitated with MAbs 64/32 and 14/16, a postinfection serum (from a B. bigemina-infected calf, B-240) (44), and the murine polyclonal antibody against affinity-purified p94. Proteins located on the surfaces of infected erythrocytes were labeled with biotin (19). Briefly, infected erythrocytes were suspended in a solution of 5.0 mM sulfosuccinimidyl-6-(biotinamido) hexanoate (NHS-LC-biotin; Pierce, Rockford, Ill.) in PBS (pH 7.4) to a final concentration of 2.8 mg of NHS-LC-biotin per 5 × 107 erythrocytes. After incubation at 4°C for 1 h, the succinimide esters were neutralized in a solution of 1 mM glycine. For immunoprecipitation, metabolically radiolabeled or biotin surface-labeled infected erythrocytes were solubilized as previously described (25).
In vitro translation of B. bigemina mRNA.
Briefly, RNA was isolated from 9 × 109 B. bigemina-infected erythrocytes by oligo(dT)-cellulose column chromatography. The mRNA was then translated by using a rabbit reticulocyte lysate with incorporation of biotin-labeled lysine (Boehringer Mannheim, Indianapolis, Ind.). Translation products were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and detected with streptavidin-horseradish peroxidase.
Depletion of IgM from infected erythrocyte lysate by immunoprecipitation.
Either MAb 64/32 or BIg73 (anti-bovine μ heavy chain) was incubated with a lysate of biotin-surface-labeled infected erythrocytes, and bound antigen-antibody complexes were precipitated with protein G (GammaBind G Plus Sepharose [Amersham]). This step was repeated once. The depleted supernatant was then incubated with a second MAb and precipitated with protein G-Sepharose. The immunoprecipitates were washed, separated by SDS-PAGE under reducing conditions, and blotted to nitrocellulose (34). Proteins were detected by using streptavidin-horseradish peroxidase and enhanced chemiluminescence (47).
RESULTS
Specificity of MAb 64/32 binding.
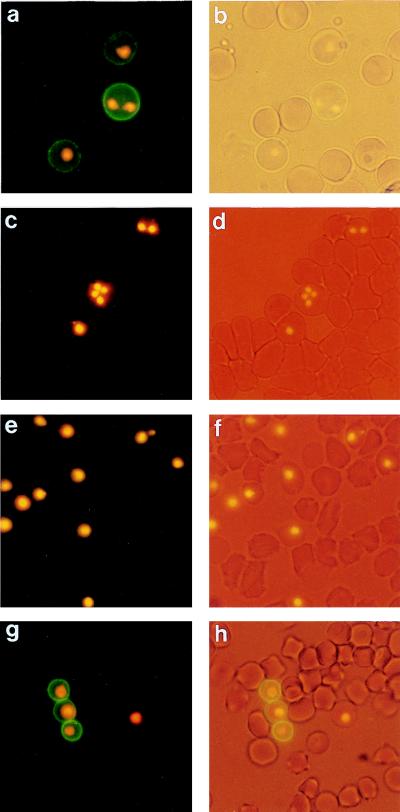
Immunofluorescence of live B. bigemina-infected erythrocytes from in vitro cultures showed that the reactivity of MAb 64/32 was localized to the surfaces of infected erythrocytes (Fig. 1a and b). Binding occurred on 87% ± 5.0% of infected erythrocytes and was similar whether the parasite stages were trophozoites or merozoites. MAb 14/1, which is specific for the B. bigemina merozoite outer membrane, was unable to bind, indicating that the infected erythrocyte membranes were intact and impermeable to antibody. MAb 64/32 showed no reactivity with B. bovis-infected erythrocytes (Fig. 1c and d) or uninfected erythrocytes. The isotype control MAb Tryp1E1 was unreactive with B. bigemina-infected erythrocytes (Fig. 1e and f).
FIG. 1.
Reactivity of MAbs with the surfaces of B. bigemina-infected erythrocytes assessed by live-cell IFA. (a to f) In vitro culture. (a) IFA reactivity of MAb 64/32; (b) light microscopy of the same field; (c) lack of reactivity of MAb 64/32 on the surfaces of B. bovis-infected erythrocytes; (d) light microscopy of the same field as in panel c; (e) lack of reactivity of the isotype control MAb Tryp1E1; (f) light microscopy of the same field as in panel e. (g and h) In vivo infection with B. bigemina (day 3). (g) IFA reactivity of MAb 64/32; (h) light microscopy of the same field as in panel g. Parasite nuclei were stained with ethidium bromide to detect infected erythrocytes.
To confirm that MAb 64/32 binding was not limited to in vitro-cultured parasites, a 5-month-old seronegative calf was infected with the Mexico strain of B. bigemina. Infected erythrocytes were initially observed on day 2 postinoculation, and their surfaces were bound by MAb 64/32 in the same pattern (Fig. 1g and h) as observed above on infected cells from in vitro culture (Fig. 1a and b). The binding pattern was unchanged on the subsequent 5 days of acute, increasing parasitemia (data not shown). Interestingly, in the infected calf, MAb 64/32 bound only 45% ± 4.6% of infected erythrocytes containing merozoites or trophozoites.
Immunoprecipitation of labeled proteins from infected erythrocytes.
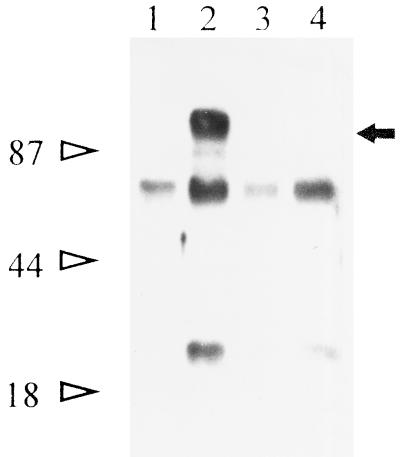
To identify the protein recognized on the surfaces of B. bigemina-infected erythrocytes by MAb 64/32, biotin-surface-labeled infected erythrocytes were lysed and immunoprecipitated and the proteins were separated by SDS-PAGE. MAb 64/32 specifically recognized an approximately 94-kDa protein (initially designated p94) from infected erythrocytes (Fig. 2, lane 2) but not from uninfected erythrocytes. Labeled proteins of about 50 and 25 kDa were also nonspecifically precipitated (lane 4).
FIG. 2.
Immunoprecipitation of antigens from the surfaces of B. bigemina-infected erythrocytes. Biotin-labeled surface proteins from infected erythrocytes were immunoprecipitated with MAb 64/32 (lane 2, arrow) or the negative control MAb 64/11 (lane 4). Surface-labeled uninfected erythrocytes were immunoprecipitated with either MAb 64/32 (lane 1) or MAb 64/11 (lane 3). Molecular size standards are designated on the left.
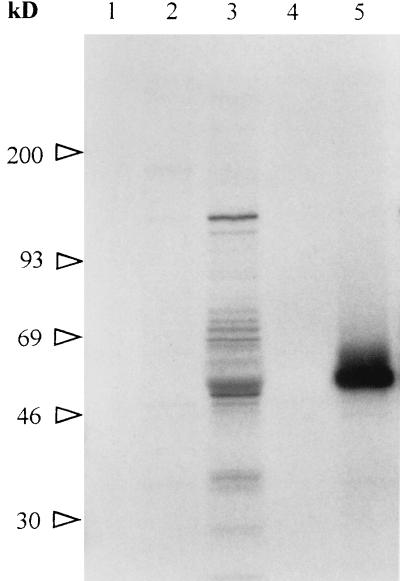
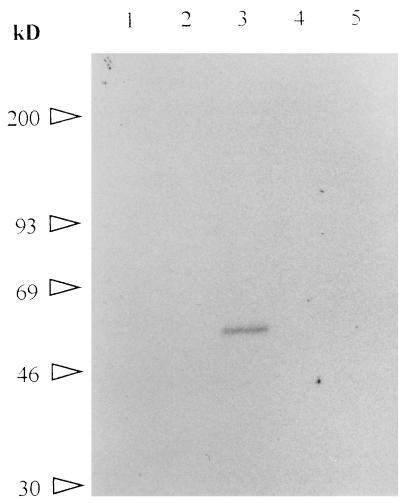
To test whether the polypeptide recognized by MAb 64/32 was B. bigemina encoded, proteins synthesized by the parasite during in vitro cultures were labeled with [35S]methionine, immunoprecipitated, and separated by SDS-PAGE. Autoradiography showed that neither the polyclonal antibody to p94 (from mice immunized with affinity-purified p94) nor MAb 64/32 was able to precipitate metabolically labeled p94 from uninfected (data not shown) or infected (Fig. 3, lanes 2 and 4) erythrocytes. The positive control MAb 14/16 precipitated labeled RAP-1 (lane 5), and the postinfection serum B240 (from a B. bigemina-infected calf) precipitated multiple labeled proteins including RAP-1 (lane 3). To test whether the failure to detect 35S-labeled p94 was attributable to a paucity of methionine residues, a mixture of 15 tritiated amino acids was used in a second in vitro labeling experiment. Neither MAb 64/32 nor the polyclonal antibody against p94 was able to precipitate any labeled protein (Fig. 4, lanes 1 and 5). The positive control MAb 14/16 immunoprecipitated 3H-RAP-1 (lane 3). To exclude the possibility that p94 translation was blocked in the in vitro cultures, purified mRNA from B. bigemina JG-29 was added to a reticulocyte translation system which included biotin-labeled lysine. The biotin-labeled translation products were transferred to a nitrocellulose membrane and detected with streptavidin-horseradish peroxidase. The lack of p94 translation (data not shown) supported the results of the metabolic labeling experiment.
FIG. 3.
Immunoprecipitation of 35S-labeled B. bigemina proteins. Proteins labeled with [35S]methionine during in vitro growth of B. bigemina were immunoprecipitated with MAb 64/32 (lane 4), MAb 14/16 (anti-p58, RAP-1) (lane 5), murine polyclonal antiserum to p94 (lane 2), bovine postinfection serum B240 (lane 3), or preinoculation murine serum (lane 1). Molecular size standards are designated on the left.
FIG. 4.
Immunoprecipitation of 3H-labeled B. bigemina proteins. Proteins labeled with a mixture of 15 tritiated amino acids during in vitro growth of B. bigemina were immunoprecipitated with MAb 64/32 (lane 1), MAb 14/16 (lane 3), murine polyclonal antiserum to p94 (lane 5), isotype control MAb Tryp1E1 (lane 2), or preinoculation murine serum (lane 4). Molecular size standards are designated on the left.
Host-encoded proteins on the infected erythrocyte surface.
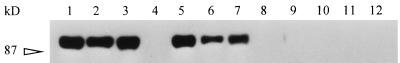
To identify host proteins on the surfaces of the erythrocytes, lysates of uninfected erythrocytes and normal bovine serum were separated by SDS-PAGE and probed with the polyclonal anti-p94 antibody. This polyclonal antibody was used in an attempt to identify p94 as a normal erythrocyte protein by binding multiple epitopes, since the single epitope recognized by MAb 64/32 could be a parasite-generated modification restricted to infected erythrocytes (6). Neither the polyclonal anti-p94 antibody nor MAb 64/32 was able to bind any protein from the uninfected erythrocytes (data not shown). However, the polyclonal antibody bound a protein from normal bovine serum which was abundant and had a molecular size similar to that of the p94 identified on the surfaces of infected erythrocytes (data not shown). Moreover, MAb 64/32 also recognized this approximately 94-kDa serum protein. This suggested that p94 was not an erythrocyte membrane protein modified by the parasite but could be a normal serum protein bound to B. bigemina-infected erythrocytes. Based on the abundance in serum and the molecular size, p94 was hypothesized to be the reduced μ chain of bovine IgM. When proteins from infected erythrocytes and normal bovine serum, both eluted from MAb 64/32 affinity chromatography columns, and purified bovine IgM were electrophoresed under reducing conditions and immunoblotted, MAb 64/32 bound an approximately 94-kDa protein in each preparation (data not shown). Subsequently, infected erythrocytes, normal bovine serum, purified IgM, and, as a negative control, purified IgG were electrophoresed and immunoblotted with either MAb 64/32 or MAb BIg73 (anti-bovine μ heavy chain). Both MAbs bound comigrating proteins from the infected erythrocytes, normal bovine serum, and purified IgM but not from purified IgG (Fig. 5). This strongly suggested that p94 was the IgM heavy chain.
FIG. 5.
Reactivity of MAb 64/32 with infected erythrocytes, normal bovine serum, and purified IgM. B. bigemina-infected erythrocytes (lanes 1, 5, and 9), normal bovine serum (lanes 2, 6, and 10), purified bovine IgM (lanes 3, 7, and 11), or purified bovine IgG (lanes 4, 8, and 12) were separated by SDS-PAGE under reducing conditions. The immunoblotted proteins were probed with MAb 64/32 (lanes 1 to 4), MAb BIg73 (anti-bovine μ heavy chain) (lanes 5 to 8), or isotype control MAb Tryp1E1 (lanes 9 to 12). The 87-kDa molecular size standard is indicated on the left.
Confirmation of MAb 64/32 reactivity with bovine IgM.
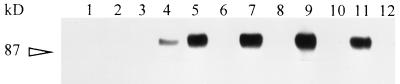
MAb 64/32 immunoprecipitation of biotin-labeled B. bigemina-infected erythrocyte proteins (Fig. 6, lane 11) depleted IgM from the lysate as detected with anti-μ heavy-chain MAb BIg73 (lane 12). As expected if both MAbs bound to the same antigen, immunoprecipitation of infected erythrocyte lysate with MAb BIg73 (lanes 7 and 9) also depleted all reactivity for MAb 64/32 (lane 10), as well as for itself (lane 8). Therefore, MAb 64/32 has the same antigen specificity as the anti-μ heavy-chain MAb, BIg73. Initial depletion by immunoprecipitation with an isotype control MAb (lane 3) did not block subsequent reactivity of MAb 64/32 with infected erythrocytes (lane 4). MAb 64/32 was unreactive with biotin-labeled uninfected erythrocytes (lane 2).
FIG. 6.
Binding of IgM from B. bigemina-infected erythrocytes by MAbs 64/32 and BIg73. Biotin-labeled infected erythrocyte lysates were specifically depleted by immunoprecipitation with the first MAb; the proteins remaining in the supernatant were then immunoprecipitated by the second MAb. The following six pairs of MAbs were used in a “first-second” order for the immunoprecipitations: for uninfected erythrocytes, ANA8-64/32 (lanes 1 and 2); for infected erythrocytes; 64/11-64/32 (lanes 3 and 4), 64/32-64/11 (lanes 5 and 6), BIg73-BIg73 (lanes 7 and 8), BIg73-64/32 (lanes 9 and 10), and 64/32-BIg73 (lanes 11 and 12). BIg73 is against the bovine μ heavy chain; MAbs ANA8 and 64/11 are isotype controls. The 87-kDa molecular size standard is indicated on the left.
DISCUSSION
The antigen defined by MAb 64/32, exposed on the surfaces of B. bigemina-infected erythrocytes, is present among otherwise antigenically and genetically distinct B. bigemina strains isolated from Mexico, Brazil, and the Caribbean (12, 23, 25, 36, 47). This conservation and reactivity during both trophozoite and merozoite stages is unique among the antigens previously identified on Babesia-infected erythrocytes (39, 47). Initial experiments involving MAb 64/32 immunoprecipitation of metabolically radiolabeled B. bigemina proteins failed to identify a parasite-encoded protein. In this study, polyclonal antibody to p94, produced in mice, also failed to precipitate either 35S- or 3H-p94, confirming the previous results obtained with MAb 64/32 (47). In addition, MAb 64/32 was unable to bind in vitro translation products of B. bigemina mRNA. These techniques, which take advantage of the inability of mature erythrocytes to synthesize proteins or mRNA, have been used successfully to identify parasite-encoded proteins of Babesia and Plasmodium (1, 10, 20, 24). Consequently, after these initial results, we directed our approach to the identification of host proteins specifically associated with intraerythrocytic B. bigemina parasitism.
Erythrocyte membrane proteins can be modified during invasion and intracellular parasitism, resulting in specific changes on the surfaces of infected erythrocytes. For example, P. falciparum cleaves the integral membrane protein band 3, a modification generating new surface-exposed epitopes (6). To determine whether MAb 64/32 bound to a new epitope resulting from parasite-mediated modification of a normal host protein, polyclonal anti-p94 was reacted with uninfected erythrocytes in an attempt to detect the normal unmodified host protein. However, this polyclonal antiserum retained specificity for infected erythrocytes. Although this did not totally preclude the possibility of a host protein modification as the source of infected erythrocyte specificity, we proceeded to address whether a host serum protein could specifically bind infected erythrocytes. In other parasitic infections, host antibodies bind the surface of either the parasite or the infected cell. Schistosoma mansoni parasites express tegumental IgG-Fc receptors in both schistosomula and adult stages (41, 42). Similarly, sequestered P. falciparum-infected erythrocytes have IgM bound between the erythrocyte membrane and the endothelial cells (38). Based on this rationale, we investigated and determined that MAb 64/32 recognizes bovine μ heavy chain, which consistently binds to the surfaces of B. bigemina-infected erythrocytes. The evidence includes the following: MAb 64/32 bound a reduced molecule of 94 kDa in both infected erythrocyte lysates and normal bovine serum; (ii) MAb 64/32 bound a 94-kDa molecule in reduced preparations of purified IgM; (iii) an anti-bovine μ heavy-chain MAb, BIg73, reacted specifically with the surfaces of infected erythrocytes and bound the 94-kDa molecule in lysates of infected erythrocytes, normal bovine serum, and purified IgM; and (iv) immunoprecipitation of infected erythrocyte lysates with MAb 64/32 depleted the 94-kDa antigen bound by anti-μ MAb BIg73 and vice versa. The 94-kDa apparent molecular size of the reduced bovine μ heavy chain is similar to the 85 kDa previously reported (7).
Importantly, both MAb 64/32 and the anti-bovine μ heavy-chain MAb BIg73 bound the surfaces of infected erythrocytes obtained from a calf during early acute B. bigemina infection. This indicated that IgM binding occurs in vivo and before the induction of a specific immune response. This observation is consistent with the hypothesis that the IgM binding is not antigen specific and is mediated by a receptor for the Fc region. This is supported by the binding of IgM from uninfected cattle to the surfaces of B. bigemina-parasitized erythrocytes. In addition, purified Fc5μ but not Fab fragments were able to bind B. bigemina-parasitized erythrocytes after removal of whole IgM by acid elution (data not shown). However, this rebinding of Fc5μ may not necessarily involve the same receptor used to bind whole IgM in vivo, since the acid elution procedure could alter the integrity of the receptor or the erythrocyte membrane.
IgM binding may be useful for parasite growth or survival, as indicated by its conservation among the otherwise antigenically and genetically distinct B. bigemina strains. Interestingly, not all infected erythrocytes observed in vivo had bound IgM, but the binding was not associated with any particular B. bigemina stage. These results are in contrast to those obtained with two other MAbs, specific for a B. bigemina-encoded protein of ∼200 kDa expressed on the erythrocyte surface, which bound 45% of B. bigemina-infected erythrocytes with reactivity determined by the parasite stage (39). Whether the variable IgM binding to B. bigemina-infected erythrocytes reflects variations among the host erythrocytes or in the parasite is unknown. Identification of the receptor on the infected erythrocytes to which the μ heavy chain binds is the next step in the determination of the significance of IgM binding during B. bigemina infection.
ACKNOWLEDGMENTS
This work was supported by U.S. Department of Agriculture NRICGP grant 96-35204-3667, U.S. Department of Agriculture BARD grant US-2496-94C, Washington State University, and the Instituto Nacional de Tecnologia Agropecuaria.
We appreciate the excellent technical assistance of Debra Alperin, Beverly Hunter, Emma Karel, and Carla Robertson.
REFERENCES
- 1.Adams J H, Hudson D E, Torii M, Ward G E, Wellems T E, Aikawa M, Miller L H. The Duffy receptor family of Plasmodium knowlesi is located within the micronemes of invasive malaria merozoites. Cell. 1990;63:141–153. doi: 10.1016/0092-8674(90)90295-p. [DOI] [PubMed] [Google Scholar]
- 2.Aikawa M, Rabbege J, Uni S, Ristic M, Miller L H. Structural alteration of the membrane of the erythrocytes infected with Babesia bovis. Am J Trop Med Hyg. 1985;34:45–49. doi: 10.4269/ajtmh.1985.34.45. [DOI] [PubMed] [Google Scholar]
- 3.Baruch D, Pasloske B L, Singh H B, Bi X, Ma X C, Feldman M, Taraschi T F, Howard R J. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 4.Berendt A R, Ferguson D J P, Gardner J, Turner G, Rowe A, McCormick C, Roberts D, Craig A, Pinches R, Elford B C, Newbold C I. Molecular mechanisms of sequestration in malaria. Parasitol. 1990;108:S19–S28. doi: 10.1017/s0031182000075685. [DOI] [PubMed] [Google Scholar]
- 5.Crandall I, Guthrie N, Sherman I W. Plasmodium falciparum: sera of individuals living in a malaria-endemic region recognize peptide motifs of the human erythrocyte anion transport protein. Am J Trop Med Hyg. 1995;52:450–452. doi: 10.4269/ajtmh.1995.52.450. [DOI] [PubMed] [Google Scholar]
- 6.Crandall I, Sherman I W. Antibodies to synthetic peptides based on band 3 motifs react specifically with Plasmodium falciparum (human malaria)-infected erythrocytes and block cytoadherence. Parasitology. 1994;108:389–396. doi: 10.1017/s0031182000075934. [DOI] [PubMed] [Google Scholar]
- 7.Davidson I, Ungar-Waron H, Katzav S, Haimovich J, Trainin Z. Membrane IgM of bovine lymphocytes. Vet Immunol Immunopathol. 1982;3:287–293. doi: 10.1016/0165-2427(82)90002-2. [DOI] [PubMed] [Google Scholar]
- 8.Elford B C, Cowan G M, Ferguson D J P. Parasite-regulated membrane transport processes and metabolic control in malaria-infected erythrocytes. Biochem J. 1995;308:361–374. doi: 10.1042/bj3080361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helmby H, Cavelier L, Pettersson U, Wahlgren M. Rosetting Plasmodium falciparum-infected erythrocytes express unique strain-specific antigens on their surface. Infect Immun. 1993;61:284–288. doi: 10.1128/iai.61.1.284-288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hines S A, McElwain T F, Buening G M, Palmer G H. Molecular characterization of Babesia bovis merozoite surface proteins bearing epitopes immunodominant in protected cattle. Mol Biochem Parasitol. 1989;37:1–10. doi: 10.1016/0166-6851(89)90096-0. [DOI] [PubMed] [Google Scholar]
- 11.Hines S A, Palmer G H, Brown W C, McElwain T F, Suarez C E, Vidotto O, Rice-Fitch A C. Genetic and antigenic characterization of Babesia bovis merozoite spherical body protein Bb-1. Mol Biochem Parasitol. 1995;69:149–159. doi: 10.1016/0166-6851(94)00200-7. [DOI] [PubMed] [Google Scholar]
- 12.Hötzel I, Suarez C E, McElwain T F, Palmer G H. Genetic variation in the dimorphic regions of rap-1 genes and rap-1 loci of Babesia bigemina. Mol Biochem Parasitol. 1998;90:479–489. doi: 10.1016/s0166-6851(97)00182-5. [DOI] [PubMed] [Google Scholar]
- 13.Igarashi I, Aikawa M, Kreier J P. Host cell parasite interactions in babesiosis. In: Ristic M, editor. Babesiosis of domestic animals and man. Boca Raton, Fla: CRC Press, Inc.; 1988. pp. 53–69. [Google Scholar]
- 14.Kanaani J, Ginsburg H. Transport of lactate in Plasmodium falciparum-infected human erythrocytes. J Cell Physiol. 1991;149:469–476. doi: 10.1002/jcp.1041490316. [DOI] [PubMed] [Google Scholar]
- 15.Kania S A, Allred D R, Barbet A F. Babesia bigemina: host factors affecting the invasion of erythrocytes. Exp Parasitol. 1995;80:76–84. doi: 10.1006/expr.1995.1009. [DOI] [PubMed] [Google Scholar]
- 16.Kilejian A. Characterization of a protein correlated with the production of knob-like protrusions on membranes of erythrocytes infected with Plasmodium falciparum. Proc Natl Acad Sci USA. 1979;76:4650–4653. doi: 10.1073/pnas.76.9.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirk K, Horner H A, Elford B C, Ellory J C, Newbold C I. Transport of diverse substrates into malaria-infected erythrocytes via a pathway showing functional characteristics of a chloride channel. J Biol Chem. 1994;269:3339–3347. [PubMed] [Google Scholar]
- 18.Land K M, Sherman I W, Gysin J, Crandall I. Anti-adhesive antibodies and peptides as potential therapeutics for Plasmodium falciparum malaria. Parasitol Today. 1995;11:19–23. [Google Scholar]
- 19.Lantz L M, Holmes K L. Improved non-radioactive cell surface labeling technique for immunoprecipitation. BioTechniques. 1995;18:58–60. [PubMed] [Google Scholar]
- 20.Leech J H, Barnwell J W, Miller L H, Howard R J. Identification of a strain-specific malarial antigen exposed on the surface of Plasmodium falciparum-infected erythrocytes. J Exp Med. 1984;159:1567–1575. doi: 10.1084/jem.159.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy M G, Ristic M. Babesia bovis: continuous in-vitro cultivation in microaerophilus stationary phase culture. Science. 1980;207:1218–1220. doi: 10.1126/science.7355284. [DOI] [PubMed] [Google Scholar]
- 22.Machado R Z, McElwain T F, Suarez C E, Hines S A, Palmer G H. Babesia bigemina: Isolation and characterization of merozoite rhoptries. Exp Parasitol. 1993;77:315–325. doi: 10.1006/expr.1993.1089. [DOI] [PubMed] [Google Scholar]
- 23.Madruga C R, Suarez C E, McElwain T F, Palmer G H. Conservation of merozoite membrane and apical complex B cell epitopes among Babesia bigemina and Babesia bovis strains isolated in Brazil. Vet Parasitol. 1996;61:21–30. doi: 10.1016/0304-4017(95)00809-8. [DOI] [PubMed] [Google Scholar]
- 24.McElwain T F, Perryman L E, Davis W C, McGuire T C. Antibodies define multiple proteins with epitopes exposed on the surface of live Babesia bigemina merozoites. J Immunol. 1987;138:2298–2304. [PubMed] [Google Scholar]
- 25.McElwain T F, Perryman L E, Musoke A J, McGuire T C. Molecular characterization and immunogenicity of neutralization-sensitive Babesia bigemina merozoite surface proteins. Mol Biochem Parasitol. 1991;47:213–222. doi: 10.1016/0166-6851(91)90181-5. [DOI] [PubMed] [Google Scholar]
- 26.McGuire T C, Palmer G H, Goff W L, Johnson M I, Davis W C. Common and isolate-restricted antigens of Anaplasma marginale detected with monoclonal antibodies. Infect Immun. 1984;45:697–700. doi: 10.1128/iai.45.3.697-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller L H, Mason S J, Dvora J A, McGinniss M H, Rothman I K. Erythrocyte receptor for (Plasmodium knowlesi) malaria: Duffy blood group determinant. Science. 1975;189:561–563. doi: 10.1126/science.1145213. [DOI] [PubMed] [Google Scholar]
- 28.Miller L H, Steven J M, Clyde D F, McGinniss M H. The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy. N Engl J Med. 1976;78:5829–5832. doi: 10.1056/NEJM197608052950602. [DOI] [PubMed] [Google Scholar]
- 29.Mishra V S, Stephens E B, Dame J B, Perryman L E, McGuire T C, McElwain T F. Immunogenicity and sequence analysis of recombinant p-58: a neutralization-sensitive, antigenically conserved Babesia bigemina merozoite surface protein. Mol Biochem Parasitol. 1991;47:207–212. doi: 10.1016/0166-6851(91)90180-e. [DOI] [PubMed] [Google Scholar]
- 30.Ockenhouse C F, Klotz F W, Tandon N N, Jamieson G A. Sequestrin, a CD36 recognition protein on Plasmodium falciparum malaria-infected erythrocytes identified by anti-idiotype antibodies. Proc Natl Acad Sci USA. 1991;88:3175–3179. doi: 10.1073/pnas.88.8.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmer D A, Buening G M, Carson C A. Cryopreservation of Babesia bovis for in-vitro cultivation. Parasitology. 1982;84:567–572. doi: 10.1017/s0031182000052835. [DOI] [PubMed] [Google Scholar]
- 32.Palmer G H, Barbet A F, Cantor G H, McGuire T C. Immunization of cattle with the MSP-1 surface protein complex induces protection against a structurally variant Anaplasma marginale isolate. Infect Immun. 1989;57:3666–3669. doi: 10.1128/iai.57.11.3666-3669.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmer G H, McElwain T F, Perryman L E, Davis W C, Reduker D W, Jasmer D P, Shkap V, Pipano E, Goff W L, McGuire T C. Strain variation of Babesia bovis merozoite surface-exposed epitopes. Infect Immun. 1991;59:3340–3342. doi: 10.1128/iai.59.9.3340-3342.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rickwood D, Hames B D, editors. Gel electrophoresis of proteins. A practical approach. 7th ed. Washington, D.C: IRL Press; 1987. pp. 26–27. [Google Scholar]
- 35.Rodriguez S D, Buening G M, Green T J, Carson C A. Cloning of Babesia bovis by in vitro cultivation. Infect Immun. 1983;42:15–18. doi: 10.1128/iai.42.1.15-18.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez S D, Palmer G H, McElwain T F, McGuire T C, Ruef B J, Chitko-McKown C G, Brown W C. CD4+ T-helper lymphocyte responses against Babesia bigemina rhoptry-associated protein 1. Infect Immun. 1996;46:2079–2087. doi: 10.1128/iai.64.6.2079-2087.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudzinska M A, Trager W, Lewengrub S J, Gubert E. An electron microscopic study of Babesia microti invading erythrocytes. Cell Tissue Res. 1976;169:323–334. doi: 10.1007/BF00219605. [DOI] [PubMed] [Google Scholar]
- 38.Scholander C, Treutiger C J, Hultenby K, Wahlgren M. Novel fibrillar structure confers adhesive property to malaria-infected erythrocytes. Nat Med. 1996;2:204–208. doi: 10.1038/nm0296-204. [DOI] [PubMed] [Google Scholar]
- 39.Shompole S, Perryman L E, Rurangirwa F R, McElwain T F, Jasmer D P, Musoke A J, Wells C W, McGuire T C. Monoclonal antibody to a conserved epitope on proteins encoded by Babesia bigemina and present on the surface of intact infected erythrocytes. Infect Immun. 1995;63:3507–3513. doi: 10.1128/iai.63.9.3507-3513.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sim B K, Chitnis C E, Wasniowska T J, Hadley T J, Miller L H. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science. 1994;264:1941–1944. doi: 10.1126/science.8009226. [DOI] [PubMed] [Google Scholar]
- 41.Tarleton R L, Kemp W M. Demonstration of IgG-Fc receptors and C3 receptors on adult Schistosoma mansoni. J Immunol. 1981;126:379–384. [PubMed] [Google Scholar]
- 42.Torpier G, Capron A, Ouaissi M A. Receptor for IgG (Fc) and beta 2-microglobulin on Schistosoma mansoni schistosomula. Nature. 1979;278:447–449. doi: 10.1038/278447a0. [DOI] [PubMed] [Google Scholar]
- 43.Towbin H, Gordon H. Immunoblotting and dot immunoblotting: current status and outlook. J Immunol Methods. 1984;72:313–340. doi: 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]
- 44.Ushe T C, Palmer G H, Sotomayor L, Figueroa J V, Buening G M, Perryman L E, McElwain T F. Antibody response to a Babesia bigemina rhoptry-associated protein 1 surface-exposed and neutralization-sensitive epitope in immune cattle. Infect Immun. 1994;62:5698–5701. doi: 10.1128/iai.62.12.5698-5701.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vega C A, Buening G M, Green T J, Carson C A. In-vitro cultivation of Babesia bigemina. Am J Vet Res. 1985;46:416–420. [PubMed] [Google Scholar]
- 46.Vega C A, Buening G M, Rodriguez S D, Carson C A, McLaughlin K. Cryopreservation of Babesia bigemina for in vitro cultivation. Am J Vet Res. 1985;46:421–423. [PubMed] [Google Scholar]
- 47.Vidotto O, McElwain T F, Machado R Z, Perryman L E, Suarez C E, Palmer G H. Babesia bigemina: identification of B cell epitopes associated with parasitized erythrocytes. Exp Parasitol. 1995;81:491–500. doi: 10.1006/expr.1995.1142. [DOI] [PubMed] [Google Scholar]
- 48.Wright I G. An electron microscopic study of intravascular agglutination in the cerebral cortex due to Babesia argentina infection. Int J Parasitol. 1972;2:209. doi: 10.1016/0020-7519(72)90008-2. [DOI] [PubMed] [Google Scholar]