Abstract
In 2016, the U.S. Food and Drug Administration (FDA), the Centers for Disease Control and Prevention (CDC), and state partners investigated nine Listeria monocytogenes infections linked to frozen vegetables. The investigation began with two environmental L. monocytogenes isolates recovered from Manufacturer A, primarily a processor of frozen onions, that were a match by whole genome sequencing (WGS) to eight clinical isolates and historical onion isolates with limited collection details. Epidemiologic information, product distribution, and laboratory evidence linked suspect food items, including products sourced from Manufacturer B, also a manufacturer of frozen vegetable/fruit products, with an additional illness. The environmental isolates were obtained during investigations at Manufacturers A and B. State and federal partners interviewed ill people, analyzed shopper card data, and collected household and retail samples. Nine ill persons between 2013 and 2016 were reported in four states. Of four ill people with information available, frozen vegetable consumption was reported by three, with shopper cards confirming purchases of Manufacturer B brands. Two identified outbreak strains of L. monocytogenes (Outbreak Strain 1 and Outbreak Strain 2) were a match to environmental isolates from Manufacturer A and/or isolates from frozen vegetables recovered from open and unopened product samples sourced from Manufacturer B; the investigation resulted in extensive voluntary recalls. The close genetic relationship between isolates helped investigators determine the source of the outbreak and take steps to protect public health. This is the first known multistate outbreak of listeriosis in the United States linked to frozen vegetables and highlights the significance of sampling and WGS analyses when there is limited epidemiologic information. Additionally, this investigation emphasizes the need for further research regarding food safety risks associated with frozen foods.
Keywords: Foodborne illness outbreaks, Individually quick-frozen vegetables, Listeria monocytogenes
Listeriosis is a rare but serious infection caused by Listeria monocytogenes (L. monocytogenes) commonly caused by eating contaminated food; invasive infections most often occur in pregnant women, older adults, and individuals with a weakened immune system and can result in severe illness or death, or during pregnancy, can cause miscarriage, stillbirth, and newborn death (Schlech, 2000). The Centers for Disease Control and Prevention (CDC) estimates that 1,600 listeriosis illnesses, 1,500 related hospitalizations, and 260 related deaths occur each year in the United States (US), with an annual average cost of $3.2 billion (Scallan et al., 2011). Most foodborne enteric pathogens grow poorly in low temperatures; however, L. monocytogenes can grow well at refrigeration temperatures (Chan & Wiedmann, 2009). This characteristic, as well as the ability to produce biofilms, allows L. monocytogenes to persist not only in refrigerated food but also in food processing and produce‐packing environments (especially when these are kept cold, such as in frozen food facilities) and equipment for extended periods of time.
Federal and state partners have made significant progress in efforts to enhance the detection and investigation of listeriosis outbreaks by standardizing surveillance activities, as well as epidemiologic data collection and sharing. Broader implementation of whole genome sequencing (WGS) has enhanced the ability to identify genetic associations between clinical and non‐clinical isolates, assisting in hypotheses development around outbreak sources (U.S. Food and Drug Administration, 2018b). Additionally, WGS has led to the identification of matching isolates that are collected and analyzed months to years apart from each other (Pightling et al., 2018). Investigations of clusters of foodborne illness are most often initiated with the detection of clinical isolates determined to be linked based on molecular subtyping and/or epidemiologic data (U.S. Centers for Disease Control and Prevention, 2018c). For listeriosis cases, food exposure histories are routinely obtained using the standardized Listeria Initiative (LI) case report form, with more focused interview questions developed, as needed, to determine a food or ingredient of interest to focus traceback and/or facility investigations conducted by federal and state regulatory partners (Irvin et al., 2021; U.S. Centers for Disease Control and Prevention, 2016a). However, increasing availability of WGS data for environmental and product samples collected outside of active outbreak investigations can shift the expected sequence of events once a possible cluster of illnesses is detected.
Federal and state health and regulatory partners linked this multistate outbreak of L. monocytogenes infections to the processing environment of Manufacturer A and frozen vegetables sourced from Manufacturer B (both frozen vegetable manufacturers). Based on investigational evidence, Manufacturers A and B conducted voluntary recalls of their products. FDA and CDC both issued public communications during the investigation, which included preliminary findings as well as advice for consumers and businesses to prevent additional illnesses (U.S. Centers for Disease Control and Prevention, 2016b; U.S. Food and Drug Administration, 2016a). This is the first known multistate L. monocytogenes outbreak in the U.S. linked to frozen vegetables and provides a powerful example of the impact WGS can have in foodborne illness outbreak investigations, particularly those that may have initially limited case exposure details.
Materials and Methods
FDA Facility Investigation
During March 8–11 and 14–17, 2016, in accordance with the domestic food safety compliance program, the U.S. Food and Drug Administration (FDA) initiated surveillance inspections at two independently owned frozen vegetable and fruit processing facilities (Manufacturer A and Manufacturer B), both located in Washington state (Fig. 1). Manufacturer A primarily processed frozen onions that were not sold directly to retail, while Manufacturer B processed a variety of frozen vegetable and fruit products sold at retail under several brand names. During each inspection, FDA investigators assessed facility compliance with current Good Manufacturing Practice (cGMP) requirements found in 21 CFR 110, including, but not limited to, plant construction and design; pest control procedures; building maintenance and design; safety of water; condition and cleanliness of food and non–food‐contact surfaces; and employee practices. In addition, FDA investigators collected environmental swabs and finished product samples of onions for Listeria species analysis and documents to support their inspectional observations (U.S. Food and Drug Administration, 2022a, 2022c).
Figure 1.
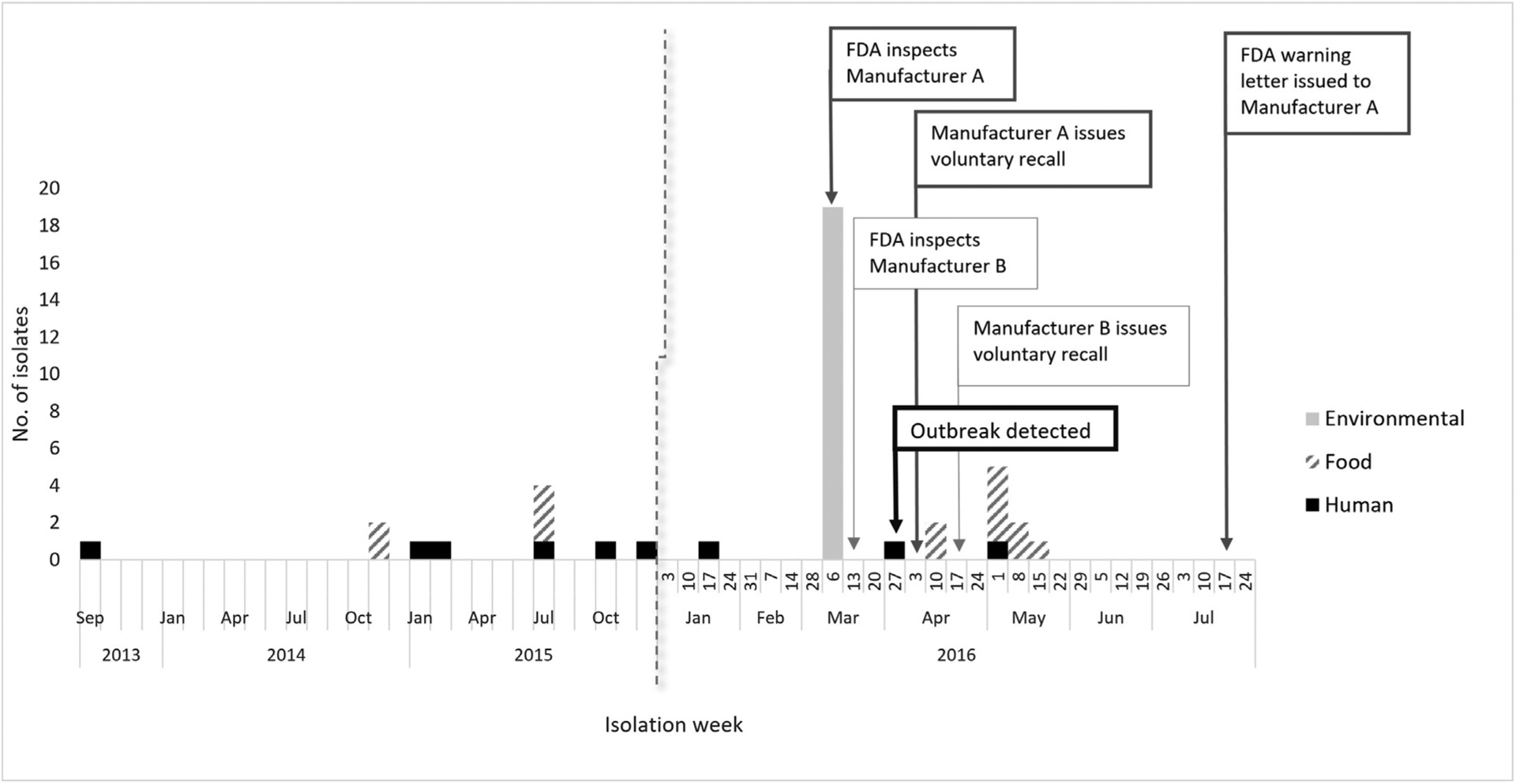
Confirmed human, food, and environmental isolates, by date of isolation for which information was reported as of July 18, 2016, and timeline of events that took place during the outbreak response including the outbreak detection, inspections, recalls, and regulatory actions taken.
Microbiological investigation
Environmental and product samples collected during FDA’s investigation of the manufacturing facilities were cultured for Listeria species at FDA laboratories using standard methods (Hitchins et al., 2011). Environmental samples were collected from Zone 1 (i.e., food‐contact surfaces), Zone 2 (i.e., areas directly adjacent to food‐contact surfaces), and Zone 3 (i.e., areas immediately surrounding Zone 2) locations within the facility environment per standard methods (U.S. Food and Drug Administration, 2019a, 2020a). Product samples were also collected at both manufacturing facilities. FDA performed pulsed‐field gel electrophoresis (PFGE) and WGS on L. monocytogenes isolates recovered from any product or environmental samples.
As part of the outbreak response, the California Department of Public Health (CDPH) and Idaho public health partners under the direction of the Idaho Department of Health and Welfare (IDHW) collected leftover product from the homes of ill people, when available, and performed PFGE and/or WGS on L. monocytogenes isolates recovered during product sample analyses. FDA also analyzed state samples, including WGS and/or the most‐probable‐number (MPN) method to enumerate L. monocytogenes (U.S. Food and Drug Administration, 2020a). Independent of the outbreak investigation, in April 2016, the Ohio Department of Agriculture (ODA) collected and tested two product samples of frozen corn and frozen green peas (consisting of one 10 oz. bag each) produced by Manufacturer B from a retail location in Ohio as part of routine surveillance sampling that included L. monocytogenes testing. WGS of non‐clinical isolates was performed at state public health and FDA laboratories, and WGS data were submitted to federal databases (U.S. Food and Drug Administration, 2020b).
Outbreak Detection
In 2013, CDC, FDA, the U.S. Department of Agriculture’s Food Safety and Inspection Service (USDA FSIS), and state health departments initiated real‐time WGS subtyping on all available clinical, food, and environmental L. monocytogenes isolates. The sequences and associated metadata of all isolates are uploaded to the Listeria PulseNet national database at CDC, and GenomeTrakr, a public genomic reference database of clinical, food, and environmental isolates from foodborne pathogens (Allard et al., 2016; Timme et al., 2019; Zitz et al., 2011). The sequence data and limited metadata are shared through public databases at the National Center for Biotechnology Information (NCBI) (Jackson et al., 2016; National Institute for Biotechnology Information). CDC’s PulseNet, the national molecular subtyping network for foodborne disease surveillance, uses the sequencing data to identify clusters of illness (U.S. Centers for Disease Control and Prevention, 2016c). On March 25, 2016, PulseNet detected a possible listeriosis outbreak comprised of ten isolates with a rare PFGE pattern combination; the cluster included isolates from eight ill people from four states, along with two historical onion isolates from 2014 with limited sample collection details (PFGE analysis was not initially performed). Subsequently, two environmental isolates obtained from Manufacturer A were found to be a match to this listeriosis cluster. All isolates were determined to match by WGS using Single Nucleotide Polymorphism (SNP) analysis carried out by CFSAN SNP Pipeline (Davis et al., 2015).
Epidemiologic investigation
Ill people or their surrogates were interviewed using CDC’s standard LI case report form, which collects information on the course of illness, demographics, and select food exposures during the month before illness began (U.S. Centers for Disease Control and Prevention, 2018a). Based on the close genetic relationship noted between clinical, historical onion, and the two environmental isolates from Manufacturer A, CDC and state partners developed a focused questionnaire that included detailed questions about onions, frozen foods (including frozen vegetables, fruits, and meals), and deli items. Due to the long shelf‐life of frozen food products, available shopper card data were collected for the three months before the person’s illness. Consent was obtained from ill people to share shopper card data with state and federal partners.
Results
Facility Investigation Findings
The March 8–11, 2016 inspection of Manufacturer A resulted in multiple observations of sources and routes of food contamination, including: failure to clean food‐contact surfaces as frequently as necessary to protect against contamination of food; facility construction not preventing condensate from contaminating food‐contact surfaces; food‐contact surfaces not adequately cleaned and sanitized to minimize accumulation of food particles and other organic matter that could provide conditions allowing growth of microorganisms; failure to maintain physical facilities in a sanitary condition; and facility construction not allowing adequate cleaning of floors and walls (U.S. Food and Drug Administration, 2016b).
During the March 14–17, 2016 inspection of Manufacturer B, FDA investigators observed that the materials and workmanship of equipment and utensils did not allow proper cleaning and maintenance, and therefore could be potential sources and/or routes of food contamination. There were no other significant observations from the inspection of Manufacturer B.
Microbiological Investigation Findings
Facility Environmental and Product Samples
Eighteen percent of the 106 environmental swabs (n = 19) collected by FDA investigators from Manufacturer A’s facility on March 8 and 9, 2016, were positive for L. monocytogenes (Table 1). Of these 19 positive environmental swabs, seven were collected from Zone 1 in the facility’s processing and packaging rooms. The seven Zone 1 positive environmental swabs were collected from direct food‐contact surfaces, including the chiller water and interior wall of the water chiller; a nylon strip in the tunnel discharge chute between the freezer and the finished product packaging room; and the metal arm on the chain conveyor belt between the freezer and packaging room. Two of the remaining 12 positive environmental swabs were collected from Zone 2 and ten from Zone 3 in the processing and packaging rooms that were in areas adjacent to food and non–food‐contact surfaces. One Zone 1 and one Zone 3 environmental isolate collected from Manufacturer A were subsequently found to match the PFGE pattern combination of Outbreak Strain 1 and were found to be a match by WGS to the eight clinical isolates included in this outbreak (see Case Definition and Epidemiologic Investigation). The 17 other L. monocytogenes environmental isolates from Manufacturer A were determined to be three different PFGE pattern combinations, with a distinct sequence that did not match any clinical isolates in GenomeTrakr or PulseNet (Table 1). Two product samples consisting of 20 (4 oz.) subsamples of finished frozen diced onions were also collected during the inspection; no Listeria species were isolated from the product samples or the remaining environmental swabs.
Table 1.
Clinical, food, and environmental samples associated with the outbreak investigation, including sample source, outbreak strain, and Whole Genome Sequencing Clade. Numbers in parentheses in the ‘Sample/Isolate Type’ column indicate the total number of isolates.
| Sample/Isolate Type | Sample/Isolate Year | Additional Details | Source | Collected By | Outbreak Strain | WGS Clade |
|---|---|---|---|---|---|---|
| Clinical (8) | 2013–2016 | CT (1), CA (4), MD (1), WA (1), VA (1) | Ill people | States | Strain 1 | Clade A |
| 1 Environmental Sample (2) | 2016 | Manufacturer A | FDA | |||
| 1 Food Sample (1) | 2016 | Frozen white sweet corn | Manufacturer B | ODA | ||
| 2 Food Samples (2)a | 2016 | Frozen mixed vegetables | Manufacturer B | CDPH | ||
| 2 Food Samples (7)b | 2016 | Frozen mixed vegetables | Manufacturer B | CDPH | ||
| 1 Food Sample (1)a | 2016 | Frozen baby peas | Manufacturer B | IDHW | ||
| Food (3)c | 2015 | Green beans | WA | Unk | Strain 1 | Clade A |
| Food (2)c | 2014 | Onions | WA | Unk | Strain 1 | Clade A |
| Clinical (1) | 2016 | CA (1) | Ill person | State | Strain 2 | Clade B |
| 1 Food Sample (1) | 2016 | Frozen petite green peas | Manufacturer B | ODA | Strain 2 | Clade B |
| 2 Food Samples (3)b | 2016 | Frozen mixed vegetables | Manufacturer B | CDPH | Strain 2 | Clade B |
| Food (1)c | 2015 | Green beans | WA | Unk | Strain 2 | Clade B |
| Food (1)c | 2010 | Potatoes | MN | Unk | Strain 2 | Clade B |
| Environmental (32)c | 2015 | WA | Unk | Strain 2 | Clade B | |
| 1 Environmental Sample (17) | 2016 | Manufacturer A | FDA | N/A | Clade C |
a Isolates analyzed by FDA.
b Isolates analyzed by CDPH.
c Isolates submitted to GenomeTrakr with minimal metadata.
Although none of the environmental swabs collected by FDA during the inspection of Manufacturer B’s facility on March 15 and 16, 2016 were positive for L. monocytogenes, 5% of 100 environmental swabs (n = 5) were positive for Listeria innocua, which indicates evidence of conditions that could be suitable for L. monocytogenes (Zitz et al., 2011). Of the five L. innocua positive swabs, two swabs were from Zone 1 and one swab from Zone 2 surfaces of the packing line (collected while the facility was repacking frozen corn), and two swabs were from Zone 3 surfaces of the processing line (collected while the facility was processing onions). A single product sample consisting of 20 subsamples of whole, peeled onions was also collected during the inspection; no Listeria species were isolated from the product samples or remaining environmental swabs collected during this inspection.
State Product Samples and Additional Whole Genome Sequencing (WGS) Analyses
Frozen corn and frozen green peas product samples sourced from Manufacturer B were collected from retail stores by ODA in April 2019 and determined to be contaminated with L. monocytogenes (Table 1). Additionally, one intact (5 lbs.) and one opened (5 lbs.) bag of frozen mixed vegetables, both sourced from Manufacturer B and collected by CDPH from the home of an ill person included in the outbreak, were determined to be positive for L. monocytogenes. The remainder of the CDPH product samples were submitted to FDA for analysis, including enumeration, and both samples were found positive for L. monocytogenes and L. innocua based on FDA analysis; enumeration results were below the detectable limits of the analysis (i.e., <0.3 MPN/g). A sample of frozen baby peas from an opened bag sourced from Manufacturer B and included in the subsequent recall was also collected by Idaho public health from a household of six family members with gastroenteritis compatible with noninvasive listeriosis (Ooi & Lorber, 2005). The sample was confirmed positive for L. monocytogenes; the remainder of this sample was submitted to FDA for WGS analysis (Table 1).
In addition to the eight clinical isolates matching Outbreak Strain 1, the two environmental isolates collected from Manufacturer A were found to be a match by WGS to 16 product isolates (SNP difference of ≤18 SNPs) (WGS Clade A; Fig. 2) (Davis et al., 2015). These product isolates included the frozen corn isolate collected by ODA, nine isolates from two household samples of frozen mixed vegetables collected by CDPH, and the household sample of frozen baby peas collected by Idaho public health. WGS Clade A also included the 2014 onion isolates; based upon further review of additional genomic data from GenomeTrakr, three isolates recovered from green beans in 2015 were also included. These sequences were submitted to GenomeTrakr by third‐party laboratories and had limited sample collection details.
Figure 2.
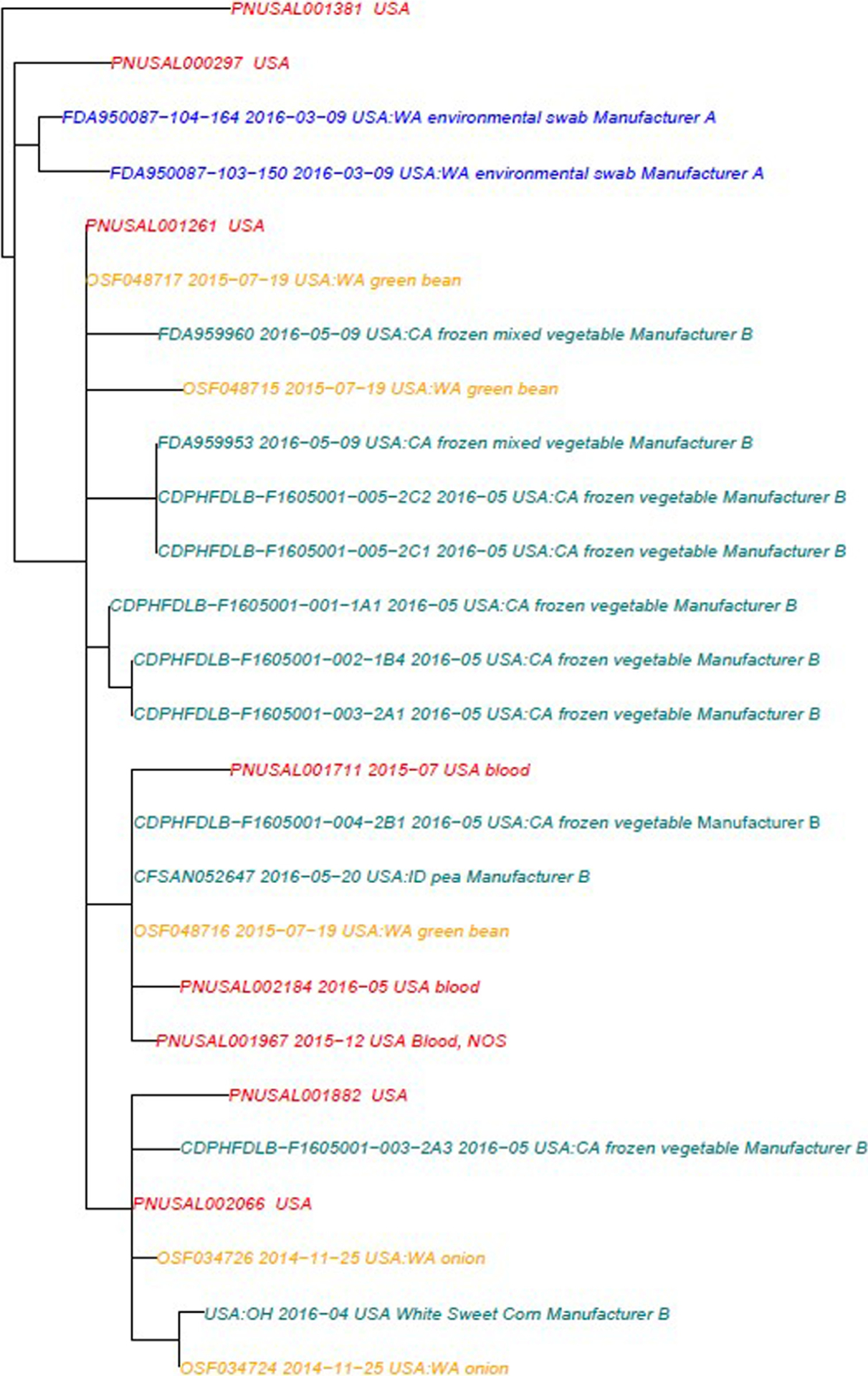
Whole Genome Sequencing Clade A Phylogenetic tree illustrating the analysis of eight clinical isolates included in this outbreak (red), 11 product isolates sourced from Manufacturer B (green), five product isolates from unknown sources (orange), and two environmental isolates from Manufacturer A (blue) matching Outbreak Strain 1. This tree represents isolates that were included in the database and subsequent analysis at the time of the investigation. (SNP Range 0:18, mean 6.46).
The isolate recovered from the frozen green peas sample also collected by ODA was a match by WGS to a single clinical isolate from 2016, six product isolates, and 32 environmental isolates (SNP difference of ≤21 SNPs) (WGS Clade B; Fig. 3). The product isolates included three isolates from the two household samples of frozen mixed vegetables collected by CDPH. A review of genomic data from GenomeTrakr identified the additional non‐clinical isolates that were included in WGS Clade B, namely green beans (one 2015 isolate), potatoes (one 2010 isolate), and environmental isolates (32 isolates from 2015). These sequences were submitted to the GenomeTrakr by third‐party laboratories and had limited sample collection details.
Figure 3.

Whole Genome Sequencing Clade B illustrating the analysis of a single clinical isolate from 2016 (red), four product isolates sourced from Manufacturer B (green), two product isolates from unknown sources (orange), and 32 environmental isolates from an unknown source (blue) that matched Outbreak Strain 2 (SNP range 0:21, mean 6.97). This tree represents isolates that were included in the database and subsequent analysis at the time of the investigation; isolates in black were not a match to Outbreak Strain 2, hence not determined to be of significance at the time of the investigation.
The 17 additional L. monocytogenes positive environmental isolates from Manufacturer A were not found to match any clinical isolates in PulseNet or the GenomeTrackr database but were found to be a match to each other by WGS (WGS Clade C; Fig. 4).
Figure 4.
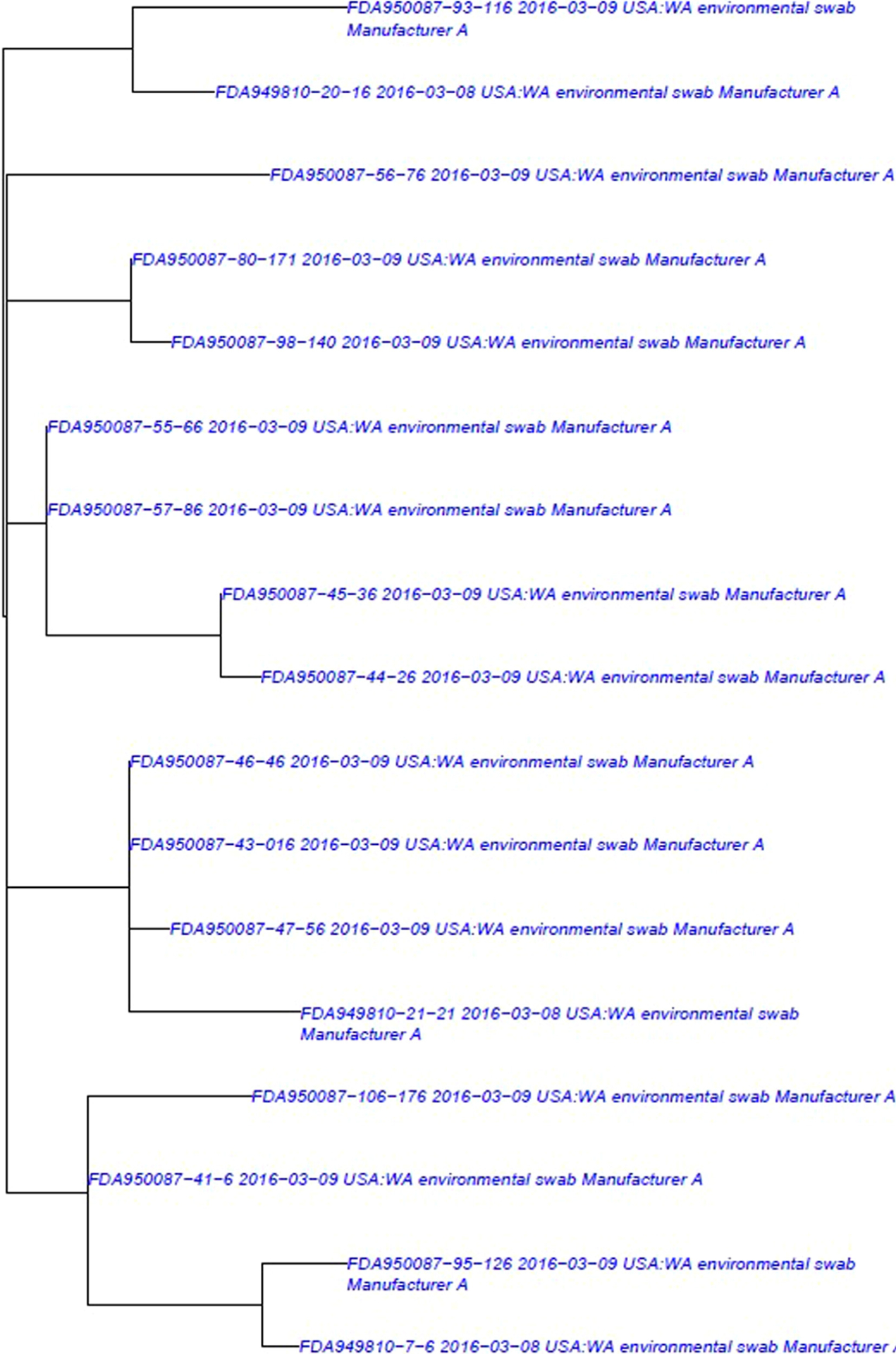
Whole Genome Sequencing Clade C Phylogenetic tree illustrating the analysis of 17 environmental isolates from Manufacturer A (blue) (SNP range 0:21, mean 6.97). This tree represents isolates that were included in the database and subsequent analysis at the time of the investigation.
Case Definition and Epidemiologic Investigation
An outbreak case was defined as infection with one of two outbreak strains of L. monocytogenes (Outbreak Strain 1 and Outbreak Strain 2), isolated from a normally sterile site from September 1, 2013, to May 15, 2016, and highly related by WGS (within 0–14 SNPs difference). A total of nine confirmed cases were identified in four states, including California (6), Connecticut (1), Maryland (1), and Washington (1). Ill people ranged in age from 56 to 91 years (median 76 years), and 78% were female. No illnesses were pregnancy‐associated. All nine ill people were hospitalized, and three deaths were reported; one death was considered attributable to listeriosis based on determination by state and local health officials. Eight ill people were infected with Outbreak Strain 1, and one with Outbreak Strain 2 (Table 1).
Exposure Information
At the start of the investigation, preliminary exposure information was available for six ill people; three were interviewed with the LI case report form, and three were interviewed with state‐developed forms. None of these standard case report forms included questions on exposure to onions, frozen fruits and vegetables, or frozen meals. To evaluate these potential exposures, CDC developed an outbreak‐specific focused questionnaire. Two ill people who had been interviewed with the LI case report form, and one ill person who had been interviewed with a state‐specific case report form, were reinterviewed with this focused questionnaire. A fourth patient was reinterviewed using an open‐ended approach where the interviewer asked about food items on the focused questionnaire. Among the four ill people interviewed with (or using content derived from) the focused questionnaire, three reported consuming frozen vegetables, three reported consuming frozen fruit, and two reported consuming fresh onions. One ill person in California reported cooking frozen vegetables on the stove and storing remaining uncooked product in the freezer; preparation information was not available for any other ill people. Shopper card records for foods purchased in the three months before illness onset were obtained for three ill people. Of these ill people, two purchased two brands of frozen vegetables sourced from Manufacturer B, while the third purchased a variety of frozen vegetable brands and products, including one brand sourced from Manufacturer B; two of the three ill people also purchased one of two brands each of frozen fruits associated with Manufacturer B.
An additional cluster of six illnesses in a family with gastroenteritis compatible with noninvasive listeriosis was identified by Idaho public health officials. Illness onset dates of family members ranged from April 24, 2016, to May 9, 2016. Five of the six ill people reported eating frozen baby peas sourced from, and recalled by, Manufacturer B before their illness began. The frozen baby peas were purchased on February 1, 2016, and the family ate them intermittently over a three‐month period, either uncooked or cooked. However, the individuals in this household did not meet the case definition and were not included as confirmed cases in this outbreak, as the only clinical sample collected (a less than ideal stool specimen from the person with the earliest onset date) was negative for L. monocytogenes.
Public Health Response Activities
After being informed by FDA and CDC of a cluster of illnesses that were closely related by WGS to FDA environmental isolates collected from their facility, on April 8, 2016 (within two weeks of the initial cluster detection), Manufacturer A promptly notified downstream customers of their voluntary recall of bulk frozen and fresh onion products manufactured between March 8 and April 8, 2016 (Figure 1). Based on the detection of L. monocytogenes in the retail samples collected and analyzed by ODA, Manufacturer B initiated a voluntary recall of 11 frozen products containing corn and green peas on April 22, 2016. This voluntary recall was expanded on May 2, 2016, to include products manufactured or processed at their facility since May 2014. Ultimately, Manufacturer B voluntarily recalled approximately 450 products related to this outbreak; the expansion of Manufacturer B’s recall resulted in one of the largest frozen vegetable recalls in the US, with at least 82,000 tons of FDA‐regulated and 23,000 tons of USDA‐regulated products removed from the market (U.S. Department of Agriculture Food Safety and Inspection Service, 2016, 2017a, 2017b; U.S. Food and Drug Administration, 2022b).
FDA, CDC, and state partners informed the public about investigational findings, public health actions that were taken in response to the outbreak, and measures for consumers to protect themselves through three FDA web posts, two CDC web posts, and a web page created on FoodSafety.gov listing the downstream recalls associated with the expanded recall issued by Manufacturer B. After reviewing inspectional information and initial corrective actions reported by the firm, an FDA warning letter was subsequently issued to Manufacturer A on July 15, 2016, which noted the presence of L. monocytogenes in the facility as being indicative of inadequate sanitation efforts to effectively control pathogens in the facility, and on processing equipment specifically, to prevent contamination of food (U.S. Food and Drug Administration, 2016b).
Discussion
This is the first reported multistate outbreak of L. monocytogenes illnesses associated with frozen vegetables in the United States. Routine and outbreak‐directed product sampling during the investigation led to the identification of the outbreak strains in a food product that otherwise would have been challenging to identify through epidemiologic investigation alone. Due to faster identification of a food source, swift public health action was able to be taken, likely saving lives and preventing additional illnesses. This investigation also demonstrates the significant role that environmental and/or product sampling during facility inspections can play as part of a comprehensive food safety system to not only help detect outbreaks but understand why they may have occurred and prevent future illness.
Previous outbreak investigations and studies of Listeria contamination of food processing plants suggest that the pathogen can establish itself in facilities with deficiencies in sanitation practices (Leong et al., 2014; U.S. Food and Drug Administration, 2014b; Vitas & Garcia‐Jalon, 2004). The FDA investigation of Manufacturer A provided evidence of multiple potential sources and routes of food contamination; most notably, the facility’s failure to clean food‐contact surfaces to protect against contamination of food, facility construction not preventing condensate from contaminating food‐contact surfaces, and an overall inadequacy of their sanitation practices. Although illnesses were not directly linked epidemiologically to Manufacturer A during the investigation, the sanitation deficiencies observed in the facility of Manufacturer A could have led to contamination of retail products sold by downstream customers of this manufacturer. In addition to detection of Outbreak Strain 1 (Clade A) in the environment of Manufacturer A, WGS analysis of 17 environmental swabs from various surfaces in Zones 1, 2, and 3 detected another strain of L. monocytogenes (Clade C), suggesting extensive contamination throughout the facility. Although this strain of L. monocytogenes was not found to match clinical cases, the investigation and subsequent recall of product by Manufacturer A may have prevented illnesses associated with this particular strain from occurring. The investigation of Manufacturer B also revealed sanitation deficiencies, specifically that the materials and workmanship of equipment and utensils did not allow proper cleaning and maintenance, which also could have resulted in potential sources and/or routes of food contamination. Even though the original source of the L. monocytogenes contamination is unknown, failure to control the pathogen in the processing environment of both Manufacturers A and B is believed to have played a role in contaminating food products. During this outbreak, illnesses occurred over the course of three years, further supporting the hypothesis that contamination persisting in the processing environment was likely an important contributing factor to the outbreak.
Following the completion of this investigation, an outbreak of L. monocytogenes infections linked to frozen corn and other frozen vegetables produced in a single facility occurred in five European Union (E. U.) member states, resulting in 53 reported cases and ten deaths during 2015 through 2018 (European Food Safety Authority, 2018; Koutsoumanis et al., 2020). Investigation findings for the E.U. outbreak also suggested that the pathogen persisted in the processing plant and was transferred to the final product, despite standard cleaning and sanitation practices being conducted, in combination with periods of inactivity in the plant and stock rotations (Koutsoumanis et al., 2020). L. monocytogenes can be recovered from processing equipment that may be difficult to clean and disinfect, and some strains have been found to persist for decades in some food processing environments, thus increasing the risk of contaminating the final product (Buchanan et al., 2017; Tompkin, 2002).
As described in this outbreak, investigations can follow a nontraditional sequence of events, with food and/or environmental isolates detected in tandem with, or prior to, the identification of a possible outbreak, providing early hypotheses about possible outbreak sources instead of hypothesis development about the source arising solely from interviews with ill people (Irvin et al., 2021; Jackson et al., 2016). Therefore, early information provided by genetic relationships between clinical and non‐clinical isolates could facilitate the progress of an outbreak investigation. Other investigations involving food or environmental isolates detected before or early in the investigation have been recently reported, including Salmonella spp. infections linked to mayonnaise made using raw eggs (U.S. Food and Drug Administration, 2019b), tahini (U.S. Food and Drug Administration, 2018a), and raw cake mix (Ladd‐Wilson et al., 2019), as well as L. monocytogenes infections linked to deli ham (U.S. Centers for Disease Control and Prevention, 2018b) and ice cream (U.S. Food and Drug Administration, 2015). In order to detect important linkages between clinical and non‐clinical isolates during an outbreak investigation, regulatory inspection and sampling efforts, as well as increasing publicly available sequence data (including contribution of food and environmental isolates by third parties, such as industry and academia) are critical. However, as the outbreak described here demonstrated, the WGS linkages to two distinct manufacturers alone are insufficient to indicate a causal relationship to clinical illness and should always be interpreted in the broader context of epidemiologic, traceback, and investigational data.
The use of WGS in outbreak surveillance and response can result in federal and state partners identifying more clusters of illnesses, in addition to linking seemingly temporally and geographically dispersed illnesses to contaminated food and facilities, due to the ability to determine genetic relatedness with greater certainty than with PFGE analysis alone (Jackson et al., 2016; U.S. Food and Drug Administration, 2016c). The strength of genetic relationships identified between food and/or environmental and clinical isolates has provided clues to inform epidemiological investigations, provided justification for further follow‐up, and has occasionally resulted in the initiation of outbreak investigations (Pightling et al., 2018). In addition, recent investigations of several other listeriosis outbreaks supported by WGS analysis have identified novel food vehicles, which, while known to be at risk for contamination by Listeria, have not traditionally been considered risks for outbreaks, such as stone fruit (Jackson et al., 2015), caramel apples (U.S. Food and Drug Administration, 2014a), and enoki mushrooms (U.S. Food and Drug Administration, 2020c). Genetic relationships between historical food and environmental and clinical isolates can also contribute to the refinement of questionnaires used for interviews of ill people (Jackson et al., 2016; U.S. Food and Drug Administration, 2016c).
Because frozen vegetables and fruits may be consumed without cooking, control of L. monocytogenes in frozen vegetable and fruit production environments is an important component of preventing potential product contamination. Once frozen foods, including frozen vegetables, are contaminated, preparation and/or manner of consumption of these foods may further influence whether infections occur. Although specific information indicating whether the implicated frozen products were cooked or not prior to consumption was not available for the 2015–2018 outbreak in the E.U., investigators suspected some ill people may have eaten thawed products without having cooked them properly or at all (Koutsoumanis et al., 2020). Frozen foods do not typically support the growth of L. monocytogenes, but can still contribute to the risk of listeriosis under certain conditions, such as when cooking instructions are not followed, or when frozen fruit or vegetables are consumed after thawing (e.g., added directly to smoothies or salads) (Zoellner et al., 2019). Frozen vegetables, such as green peas and corn, may be thawed and held refrigerated before consumption, and some people may eat them without cooking or heating, as was noted during this investigation based on follow‐up with the suspected cases of illness in Idaho. Holding these foods after thawing for extended periods may allow L. monocytogenes to grow to levels that present a public health concern (Kataoka et al., 2017). Based on the quantitative risk assessment model developed by the European Food Safety Authority (EFSA) following the listeriosis outbreak in E.U. member states, the probability of illness per serving of blanched frozen vegetables for females and males aged 65–74 years was found to be up to 3,600 times greater for products consumed uncooked rather than cooked (Koutsoumanis et al., 2020). Risk assessment modeling related to the contamination of frozen vegetables by Zoellner et al. also noted that for low‐level L. monocytogenes contamination of frozen foods that typically do not support bacterial growth, quantifying and understanding consumer handling practices becomes critical (Zoellner et al., 2019). In addition to measures to improve the safety of frozen foods, continued public messaging to raise awareness of the risk of listeriosis from foods, such as frozen vegetables, and consumer education on ways they can reduce that risk (e.g., through proper holding and cooking according to the manufacturer’s instructions), can further protect public health.
Analytical results of FDA environmental sampling provided a crucial clue to the possible source of the listeriosis illnesses. In addition, although limited during this investigation, the collection of product samples during listeriosis outbreak investigations for research aimed at enumerating the pathogen in product linked to illnesses can further inform risk and prevalence associated with particular commodities. The only product samples available for enumeration during this investigation indicated results below the detectable limits of the analysis (<0.3 MPN/g); there is insufficient information to indicate whether this finding reflects the dose consumed by those who became ill (which may be suggestive of low infective dose causing illness), as only two samples were enumerated, and consumers may have held products under conditions in which bacterial growth could have occurred. Recent research has demonstrated that low‐level contamination in food that may not support growth can still cause listeriosis in highly susceptible populations (Datta & Burall, 2018). A recent review of available literature related to L. monocytogenes prevalence in frozen vegetables noted that few studies reported enumeration of L. monocytogenes in frozen vegetables and, in most of the cases, the numbers were below the limit of enumeration of the plate count procedure applied in each of the studies (Koutsoumanis et al., 2020; Willis et al., 2020). In addition, occurrence of positive samples was noted to vary considerably among facilities (from 5.5% to 46.8%), which also supports the need for further research on prevalence and numbers of L. monocytogenes in frozen vegetables and fruit, but also more robust contamination prevention strategies for such foods.
Investigating outbreaks of listeriosis can be challenging because of difficulties in identifying suspected food items and exposures of ill people to these food items (Marshall et al., 2020). Investigators involved in this outbreak faced additional challenges because food items suspected early in the investigation (e.g., onions, frozen fruit and vegetables) were not included on the standard LI case report form or state questionnaire; this required reinterview of ill people, and five were not able to be contacted for reinterview. Second, these suspected food items are commonly consumed, and could also be ingredients in many frozen or ready‐to‐eat dishes; while three of four ill people reported or had purchase records indicating multiple exposures to frozen vegetables (including brands linked to Manufacturer B), it is still possible that some exposures may not have been remembered. Another challenge in this investigation was limited sample collection details for several non‐clinical isolates that were sequenced and submitted to GenomeTrakr before the outbreak was detected that were later found to be a match to the outbreak strains. While offering certain clues, limited information about the specific source and/or type (e.g., frozen, fresh‐cut) of non‐clinical isolates submitted to Genome-Trakr that are found to be a match to cases of illness can introduce additional complexity to outbreak investigations. In this particular investigation, however, while food isolates with limited collection details were identified early on, FDA and state sampling efforts provided the most compelling laboratory evidence that eventually led to product actions. Additional illnesses associated with this outbreak might have continued to occur without the availability of environmental and product isolates suggesting onions and frozen fruit and/or vegetables as suspect foods at the beginning of the investigation. The suspect food was supported by purchase records identifying specific frozen vegetable and/or fruit items purchased and eaten by ill people obtained later in the investigation.
There were important limitations of this investigation. First, investigators were ultimately unable to determine whether frozen fruit, in addition to frozen vegetables, was a source of illness for people linked to this outbreak. Although three ill people reported eating or had purchase records for frozen fruit, including an ill person who denied eating frozen vegetables, no leftover frozen fruit was available for microbiological testing to determine whether it could also have been contaminated with Outbreak Strains 1 and/or 2. Of note, frozen fruit brands reported by ill persons included two sourced from Manufacturer B, and Manufacturer A was known to process frozen blueberries at least once a month annually. Second, information on how ill people prepared and ate frozen vegetables and/or fruit, which could inform consumer‐focused prevention strategies, was extremely limited.
In conclusion, the FDA, CDC, and state and local health agencies collaborated successfully to identify and stop the first reported outbreak of listeriosis associated with frozen vegetables in the United States. Based on the findings of the facility inspections, failure to control the pathogen in the processing environment of both Manufacturers A and B is believed to have played a role, highlighting the importance of proper cleaning and sanitization of food‐contact and non–food‐contact surfaces to prevent the contamination of food and/or establishment of resident pathogens within the facility environment. Although manufacturers may consider frozen vegetables to be not ready‐to‐eat and provide cooking instructions, they should take steps to ensure these foods are not contaminated from the processing environment, especially since some consumers may use these products without cooking and/or with inadequate cooking. While labeling of frozen food products was not reviewed during this investigation, consumers may need to be informed to follow a manufacturer’s cooking instructions and that consuming undercooked or uncooked frozen vegetables could lead to foodborne illness. An assessment by manufacturers of cooking instructions on frozen food product labels, including accessibility, simplicity, and effectiveness, may also be warranted (Farber et al., 2021). Given evidence of low‐dose contamination causing listeriosis outbreaks in highly susceptible consumers (Pouillot et al., 2016), further research into the prevalence and risk of L. monocytogenes in food products such as frozen vegetables, including enumeration studies, is warranted. Finally, this investigation highlights how WGS has become an indispensable tool in outbreak investigations, with broader implementation enabling investigators, in some instances, to identify outbreaks that may not have been otherwise detected prior to when WGS was available as a tool; helping identify novel pathogen‐commodity pairs; identifying contamination in food production facilities that may be linked to illnesses over a broad timeframe (which could be suggestive of recurrent contamination); and allowing more efficient allocation of state and federal public health resources (Jackson et al., 2016).
Acknowledgments
The investigative team involved in the response to this Listeria monocytogenes outbreak included numerous public health and regulatory officials at local and state health departments, departments of agriculture and public health laboratories in the United States. The assistance of state partners in California, Connecticut, Ohio, Idaho, Maryland, and Washington are especially appreciated. Review and input from Jenny Scott was also greatly appreciated. The findings and conclusions in this article are those of the author(s) and do not necessarily represent the views or opinions of the agencies involved.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Allard MW, Strain E, Melka D, Bunning K, Musser SM, Brown EW, & Timme R (2016). Practical value of food pathogen traceability through building a whole-genome sequencing network and database. Journal of Clinical Microbiology, 54(8), 1975–1983. 10.1128/jcm.00081-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RL, Gorris LGM, Hayman MM, Jackson TC, & Whiting RC (2017). A review of Listeria monocytogenes: An update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control, 75, 1–13. 10.1016/j.foodcont.2016.12.016. [DOI] [Google Scholar]
- Chan YC, & Wiedmann M (2009). Physiology and genetics of Listeria monocytogenes survival and growth at cold temperatures. Critical Reviews in Food Science and Nutrition, 49(3), 237–253. 10.1080/10408390701856272. [DOI] [PubMed] [Google Scholar]
- Datta A, & Burall L (2018). Current trends in foodborne human listeriosis. Food Safety (Tokyo), 6(1), 1–6. 10.14252/foodsafetyfscj.2017020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Pettengill JB, Luo Y, Payne J, Shpuntoff A, Rand H, & Strain E (2015). CFSAN SNP pipeline: An automated method for constructing SNP matrices from next-generation sequence data. PeerJ Computer Science, 1, e20. [Google Scholar]
- European Food Safety Authority. (2018). Listeria monocytogenes: update on foodborne outbreak. Retrieved 5/5/2020 from https://www.efsa.europa.eu/en/press/news/180703 [Google Scholar]
- Farber JM, Zwietering M, Wiedmann M, Schaffner D, Hedberg CW, Harrison MA, Hartnett E, Chapman B, Donnelly CW, & Goodburn KE (2021). Alternative approaches to the risk management of Listeria monocytogenes in low risk foods. Food Control, 123 107601. [Google Scholar]
- Hitchins A, Jinneman K, & Chen Y (2011). Bacteriological analytical manual chapter 10: Detection and enumeration of listeria monocytogenes in foods. US Food and Drug Administration. [Google Scholar]
- Irvin K, Viazis S, Fields A, Seelman S, Blickenstaff K, Gee E, Wise M, Marshall K, Gieraltowski L, & Harris S (2021). An overview of traceback investigations and three case studies of recent outbreaks of Escherichia coli O157:H7 infections linked to romaine lettuce. Journal of Food Protection. 10.4315/jfp-21-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson BR, Salter M, Tarr C, Conrad A, Harvey E, Steinbock L, Saupe A, Sorenson A, Katz L, Stroika S, Jackson KA, Carleton H, Kucerova Z, Melka D, Strain E, Parish M, & Mody RK (2015). Notes from the field: Listeriosis associated with stone fruit–United States, 2014. MMWR. Morbidity and Mortality Weekly Report, 64(10), 282–283. [PMC free article] [PubMed] [Google Scholar]
- Jackson BR, Tarr C, Strain E, Jackson KA, Conrad A, Carleton H, Katz LS, Stroika S, Gould LH, Mody RK, Silk BJ, Beal J, Chen Y, Timme R, Doyle M, Fields A, Wise M, Tillman G, Defibaugh-Chavez S, … Gerner-Smidt, P. (2016). Implementation of nationwide real-time whole-genome sequencing to enhance listeriosis outbreak detection and investigation. Clinical Infectious Diseases, 63(3), 380–386. 10.1093/cid/ciw242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka A, Wang H, Elliott PH, Whiting RC, & Hayman MM (2017). Growth of Listeria monocytogenes in thawed frozen foods. Journal of Food Protection, 80(3), 447–453. 10.4315/0362-028x.Jfp-16-397r. [DOI] [PubMed] [Google Scholar]
- Koutsoumanis K, Alvarez-Ordóñez A, Bolton D, Bover-Cid S, Chemaly M, Davies R, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Jordan K, Sampers I, Wagner M, … Allende A (2020). Scientific Opinion on the public health risk posed by Listeria monocytogenes in frozen fruit and vegetables including herbs, blanched during processing. EFSA Journal, 18(4), 102. 10.2903/j.efsa.2020.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladd-Wilson SG, Morey K, Koske SE, Burkhalter B, Bottichio L, Brandenburg J, Fontana J, Tenney K, Kutumbaka KK, Samadpour M, Kreil K, & Cieslak PR (2019). Notes from the field: Multistate outbreak of Salmonella agbeni associated with consumption of raw cake mix – Five States, 2018. MMWR. Morbidity and Mortality Weekly Report, 68(34), 751–752. 10.15585/mmwr.mm6834a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong D, Alvarez-Ordóñez A, & Jordan K (2014). Monitoring occurrence and persistence of Listeria monocytogenes in foods and food processing environments in the Republic of Ireland. Frontiers in Microbiology, 5, 436. 10.3389/fmicb.2014.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall KE, Nguyen TA, Ablan M, Nichols MC, Robyn MP, Sundararaman P, Whitlock L, Wise ME, & Jhung MA (2020). Investigations of possible multistate outbreaks of salmonella, shiga toxin-producing Escherichia coli, and Listeria monocytogenes infections – United States, 2016. MMWR Surveillance Summaries, 69(6), 1–14. 10.15585/mmwr.ss6906a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Biotechnology Information. Bioproject. Retrieved December 14 from http://www.ncbi.nlm.nih.gov/bioproject/ [Google Scholar]
- Ooi ST, & Lorber B (2005). Gastroenteritis due to Listeria monocytogenes. Clinical Infectious Diseases, 40(9), 1327–1332. 10.1086/429324. [DOI] [PubMed] [Google Scholar]
- Pightling AW, Pettengill JB, Luo Y, Baugher JD, Rand H, & Strain E (2018). Interpreting whole-genome sequence analyses of foodborne bacteria for regulatory applications and outbreak investigations. Frontiers in Microbiology, 9, 1482. 10.3389/fmicb.2018.01482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouillot R, Klontz KC, Chen Y, Burall LS, Macarisin D, Doyle M, et al. (2016). infectious dose of listeria monocytogenes in outbreak linked to ice cream, United States, 2015. Emerging Infectious Diseases, 22(12), 2113–2119. 10.3201/eid2212.160165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, et al. (2011). Foodborne illness acquired in the United States–major pathogens. Emerging Infectious Diseases, 17(1), 7–15. 10.3201/eid1701.p11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlech WF 3rd., (2000). Foodborne listeriosis. Clinical Infectious Diseases, 31(3), 770–775. 10.1086/314008. [DOI] [PubMed] [Google Scholar]
- Timme RE, Sanchez Leon M, & Allard MW (2019). Correction to: Utilizing the public genometrakr database for foodborne pathogen traceback. Methods in Molecular Biology, 1918, C1. 10.1007/978-1-4939-9000-9_22. [DOI] [PubMed] [Google Scholar]
- Tompkin RB (2002). Control of Listeria monocytogenes in the food-processing environment. Journal of Food Protection, 65(4), 709–725. 10.4315/0362-028x-65.4.709. [DOI] [PubMed] [Google Scholar]
- U.S. Centers for Disease Control and Prevention. (2016a). Listeria Surveillance. Retrieved January 5, 2021 from https://www.cdc.gov/listeria/surveillance.html [Google Scholar]
- U.S. Centers for Disease Control and Prevention. (2016b). Multistate Outbreak of Listeriosis Linked to Frozen Vegetables (Final Update). Retrieved December 14 from https://www.cdc.gov/listeria/outbreaks/frozen-vegetables-05-16/index.html [Google Scholar]
- U.S. Centers for Disease Control and Prevention. (2016c). PulseNet. Retrieved November 11, 2020 from https://www.cdc.gov/pulsenet/index.html [Google Scholar]
- U.S. Centers for Disease Control and Prevention. (2018a). National Listeria Surveillance: Listeria Initiative. Retrieved May 5 from https://www.cdc.gov/nationalsurveillance/listeria-surveillance.html [Google Scholar]
- U.S. Centers for Disease Control and Prevention. (2018b). Outbreak of Listeria Infections Linked to Deli Ham (Final Update). Retrieved May 5 from https://www.cdc.gov/listeria/outbreaks/countryham-10-18/index.html [Google Scholar]
- U.S. Centers for Disease Control and Prevention. (2018c). Steps in a Foodborne Outbreak Investigation. Retrieved January 5 from http://www.cdc.gov/foodsafety/outbreaks/investigating-outbreaks/investigations/index.html [Google Scholar]
- U.S. Department of Agriculture Food Safety and Inspection Service. (2016). La Autentica Foods, LLC Firm Recalls Meat Tamale Products Due To Possible Listeria Contamination. Retrieved September 2 from https://www.fsis.usda.gov/recalls-alerts/la-autentica-foods-llc-firm-recalls-meat-tamale-products-due-possible-listeria [Google Scholar]
- U.S. Department of Agriculture Food Safety and Inspection Service. (2017a). Ajinomoto Windsor, Inc. Recalls Meat and Poultry Products Due to Possible Listeria Contamination. Retrieved September 2 from https://www.fsis.usda.gov/recalls-alerts/ajinomoto-windsor-inc.-recalls-meat-and-poultry-products-due-possible-listeria [Google Scholar]
- U.S. Department of Agriculture Food Safety and Inspection Service. (2017b). Garland Ventures LTD Recalls Poultry Products Due to Possible Listeria Contamination. Retrieved September 2 from https://www.fsis.usda.gov/recalls-alerts/garland-ventures-ltd-recalls-poultry-products-due-possible-listeria-contamination [Google Scholar]
- U.S. Food and Drug Administration. (2014a). Listeria linked to Caramel Apples. Retrieved May 5 from http://wayback.archive-it.org/7993/20171114154904/https://www.fda.gov/Food/RecallsOutbreaksEmergencies/Outbreaks/ucm427573.htm [Google Scholar]
- U.S. Food and Drug Administration. (2014b). Wholesome Soy Products, Inc., Chicago, IL, 483 Issued 10/31/2014. Retrieved May 5 from https://www.fda.gov/about-fda/orafoia-electronic-reading-room/wholesome-soy-products-inc [Google Scholar]
- U.S. Food and Drug Administration. (2015). Listeria in Ice Cream Products. Retrieved May 5 from http://wayback.archive-it.org/7993/20171114154904/https://www.fda.gov/Food/RecallsOutbreaksEmergencies/Outbreaks/ucm438104.htm [Google Scholar]
- U.S. Food and Drug Administration. (2016a). FDA Investigated Listeria Outbreak Linked to Frozen Vegetables. Retrieved May 5 from https://www.fda.gov/food/outbreaks-foodborne-illness/fda-investigated-listeria-outbreak-linked-frozen-vegetables [Google Scholar]
- U.S. Food and Drug Administration. (2016b). Warning Letter: Oregon Potato Company, July 15, 2016 Retrieved May 20 from https://wayback.archive-it.org/7993/20190424201928 https://www.fda.gov/ICECI/EnforcementActions/ WarningLetters/2016/ucm512776.htm [Google Scholar]
- U.S. Food and Drug Administration. (2016c). Whole Genome Sequencing: Cracking the Genetic Code for Foodborne Illness. Retrieved May 22 from https://www.fda.gov/consumers/consumer-updates/whole-genome-sequencing-cracking-genetic-code-foodborne-illness [Google Scholar]
- U.S. Food and Drug Administration. (2018a). Outbreak Investigation of Salmonella Concord: Tahini (November 2018). Retrieved May 5 from https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-salmonella-concord-tahini-november-2018 [Google Scholar]
- U.S. Food and Drug Administration. (2018b). Whole Genome Sequencing Program. Retrieved May 5 from https://www.fda.gov/food/science-research-food/whole-genome-sequencing-wgs-program [Google Scholar]
- U.S. Food and Drug Administration. (2019a). Investigations Operations Manual. Retrieved May 5 from https://www.fda.gov/media/75243/download [Google Scholar]
- U.S. Food and Drug Administration. (2019b). Outbreak Investigation of Listeria monocytogenes: Hard-Boiled Eggs (December 2019). Retrieved May 5 from https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-listeria-monocytogenes-hard-boiled-eggs-december-2019 [Google Scholar]
- U.S. Food and Drug Administration. (2020a). Bacteriological Analytical Manual (BAM). Retrieved May 5 from https://www.fda.gov/food/laboratory-methods-food/bacteriological-analytical-manual-bam [Google Scholar]
- U.S. Food and Drug Administration. (2020b). GenomeTrakr Network. Retrieved December 14, 2020 from https://www.fda.gov/food/whole-genome-sequencing-wgs-program/genometrakr-network [Google Scholar]
- U.S. Food and Drug Administration. (2020c). Outbreak Investigation of Listeria monocytogenes: Enoki Mushrooms (March 2020). Retrieved May 5 from https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-listeria-monocytogenes-enoki-mushrooms-march-2020 [Google Scholar]
- U.S. Food and Drug Administration. (2022a). Code of Federal Regulations Title 21 part 110. Retrieved November 2 from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRsearch.cfm?CFRPart=110 or https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-110 [Google Scholar]
- U.S. Food and Drug Administration. (2022b). Enforcement Report. U.S. Food and Drug Administration Recall Information Search. CRF Frozen Foods Retrieved February 8 from https://www.accessdata.fda.gov/scripts/ires/index.cfm#tabNav_advancedSearch [Google Scholar]
- U.S. Food and Drug Administration. (2022c). Investigations Operations Manual. Chapter 5: Establishment Inspections. Retrieved November 2 from https://www.fda.gov/media/76769/download [Google Scholar]
- Vitas AI, & Garcia-Jalon VA (2004). Occurrence of Listeria monocytogenes in fresh and processed foods in Navarra (Spain). International Journal of Food Microbiology, 90(3), 349–356. 10.1016/s0168-1605(03)00314-3. [DOI] [PubMed] [Google Scholar]
- Willis C, McLauchlin J, Aird H, Amar C, Barker C, Dallman T, et al. (2020). Occurrence of Listeria and Escherichia coli in frozen fruit and vegetables collected from retail and catering premises in England 2018–2019. International Journal of Food Microbiology, 334. 10.1016/j.ijfoodmicro.2020.108849108849. [DOI] [PubMed] [Google Scholar]
- Zitz U, Zunabovic M, Domig KJ, Wilrich PT, & Kneifel W (2011). Reduced detectability of Listeria monocytogenes in the presence of Listeria innocua. Journal of Food Protection, 74(8), 1282–1287. 10.4315/0362-028x.Jfp-11-045. [DOI] [PubMed] [Google Scholar]
- Zoellner C, Wiedmann M, & Ivanek R (2019). An Assessment of Listeriosis Risk Associated with a Contaminated Production Lot of Frozen Vegetables Consumed under Alternative Consumer Handling Scenarios. Journal of Food Protection, 82(12), 2174–2193. 10.4315/0362-028x.Jfp-19-092. [DOI] [PubMed] [Google Scholar]


