James Richards, DVM, Co-Chair
Director, Cornell Feline Health Center College of Veterinary Medicine Cornell University Ithaca, New York
Ilona Rodan, DVM, Diplomate ABVP (Feline Practice), Co-Chair
Cat Care Clinic Madison, Wisconsin
Thomas Elson, DVM, Diplomate ABVP (Feline Practice)
The Cat Hospital of Irvine Irvine, California
Duane Flemming, DVM, JD, Diplomate ACVO
Contra Costa Animal Eye Clinic Pleasant Hill, California
Richard Ford, DVM, MS, Diplomate ACVIM
Professor of Medicine College of Veterinary Medicine North Carolina State University Raleigh, North Carolina
Steven Henry, DVM
Chairman, American Veterinary Medical Association Drug Advisory Committee Council on Biologic and Therapeutic Agents Solomon, Kansas
David Hustead, DVM
Director, Professional Services Fort Dodge, Animal Health Overland Park Kansas
Michael Lappin, DVM, PhD, Diplomate ACVIM
Professor, Small Animal Internal Medicine College of Veterinary Medicine and Biomedical Sciences Colorado State University Ft. Collins, Colorado
Michael Paul, DVM
Immediate Past President, American Animal Hospital Association Concord, California
David Rosen, DVM, Diplomate ABVP (Feline Practice)
President, American Association of Feline Practitioners Kalamazoo, Michigan
Margie Scherk, DVM, Diplomate ABVP (Feline Practice)
Cats Only Veterinary Clinic Vancouver, BC, Canada
Fred Scott, DVM, PhD, Diplomate ACVM
Professor Emeritus
College of Veterinary Medicine Cornell University Ithaca, New York
Link Welborn, DVM, Diplomate ABVP (Canine and Feline Practice)
Secretary, American Animal Hospital Association The Cat Doctors Tampa, Florida
Reproduced with kind permission of the American Association of Feline Practitioners
Table of contents
Acknowledgements
The panel members thank the following individuals for their assistance:
James Richards, DVM, Co-Chair, for his diligence, patience and dedication to this project and for devoting numerous hours to writing and rewriting drafts of the guidelines. Jeanne Pitarri, DVM, Diplomate ABVP (Feline Practice) for assisting with the literature search. The following individuals assisted the panel by acting as reviewers:
Sue Cotter, DVM, Diplomate ACVIM
School of Veterinary Medicine Tufts University
North Grafton, Massachusetts
Philip Kass, DVM, PhD
Associate Professor of Epidemiology
Department of Population Health and Reproduction
School of Veterinary Medicine University of California Davis, California
Ronald Schultz, PhD, Honorary Diplomate, ACVM
Department of Pathobiological Sciences School of Veterinary Medicine
University of Wisconsin Madison, Wisconsin
Alice Wolf, DVM, Diplomate ACVIM (Internal Medicine)
Diplomate ABVP (Feline Practice)
Department of Small Animal Medicine and Surgery
College of Veterinary Medicine Texas A&M University College Station, Texas
Douglas DeBoer, DVM, Diplomate ACVD
School of Veterinary Medicine University of Wisconsin Madison, Wisconsin
Karen Moriello, DVM, Diplomate ACVD
School of Veterinary Medicine University of Wisconsin Madison, Wisconsin
The 2000 Report of the American Association of Feline Practitioners and Academy of Feline Medicine Advisory Panel on Feline Vaccines has received the endorsement of the Board of Regents of the American College of Veterinary Internal Medicine and the Board of Directors of the American Animal Hospital Association. The report was also reviewed and approved by the Feline Practice Guidelines Committee of the American Association of Feline Practitioners, the Academy of Feline Medicine Board of Directors, and the American Association of Feline Practitioners Board of Directors. Members include:
Jane Brunt, DVM
Deborah Edwards, DVM, Diplomate ABVP (Feline Practice)
Diane Eigner, VMD
William Folger, DVM, Diplomate ABVP (Feline Practice)
Joanna Guglielmino, DVM
Nicole Hird, DVM, Diplomate ABVP (Feline Practice)
Margaret Horstmeyer, DVM, Diplomate ABVP (Feline Practice)
Sandy Jamieson, DVM
Lorraine Jarboe, DVM, Diplomate ABVP (Feline Practice)
Cynthia McManis, DVM, Diplomate ABVP (Feline Practice)
Alice Mills, DVM
Jeanne Pitarri, DVM, Diplomate ABVP (Feline Practice)
Sara Stephens, DVM, Diplomate ABVP (Feline Practice)
Helen Tuzio, DVM
Drew Weigner, DVM, Diplomate ABVP (Feline Practice)
Elaine Wexler-Mitchell, DVM, Diplomate ABVP (Feline Practice)
The work of the Panel on Feline Vaccines was made possible by an educational grant from Fort Dodge Animal Health.
The guidelines incorporated in this report were developed on the basis of the best research information, clinical experience, and technical judgements available at the time of preparation. Although the information is intended to be accurate, thorough, and comprehensive, it is subject to change in light of developments in research, technology, and experience.
These guidelines are not exclusive, and other techniques and procedures may be available.
The AAFP and AFM expressly disclaim any warranties or guarantees, express or implied, and shall not be liable for any damages of any kind in connection with the material, information, techniques, or procedures set forth in these guidelines.
For more information regarding the American Association of Feline Practitioners and the Academy of Feline Medicine please contact them at the following address:
AAFP/AFM
530 Church Street, Suite 700 Nashville, TN 37219 USA (615) 254–3687
| Introduction | 49 |
| Vaccine selection and administration | 50 |
| Feline panleukopenia | 50 |
| Feline viral rhinotracheitis and feline calicivirus infection | 51 |
| Rabies | 51 |
| Feline leukaemia virus infection | 56 |
| Chlamydiosis | 56 |
| Feline infectious peritonitis | 57 |
| Dermatophytosis | 57 |
| Bordetella bronchiseptica infection | 57 |
| Giardiasis | 58 |
| Liability related to vaccination | 59 |
| Vaccine licensing | 61 |
| Vaccine labels | 62 |
| Adverse events and adverse event reporting | 63 |
| Variation among vaccines | 64 |
| Use of serologic testing to monitor immunity and assess the need for vaccination | 64 |
| Practice management considerations | 66 |
| Recommendations and future considerations | 67 |
| References | 68 |
| Appendices | |
| 1—Vaccination site recommendations | 70 |
| 2—Model consent forms | 70 |
| 3—USP Practitioners' Reporting Network adverse event reporting form | 71 |
| 4—Telephone numbers for reporting adverse events associated with vaccination | 71 |
| 5—Examples of examination reminder cards | 71 |
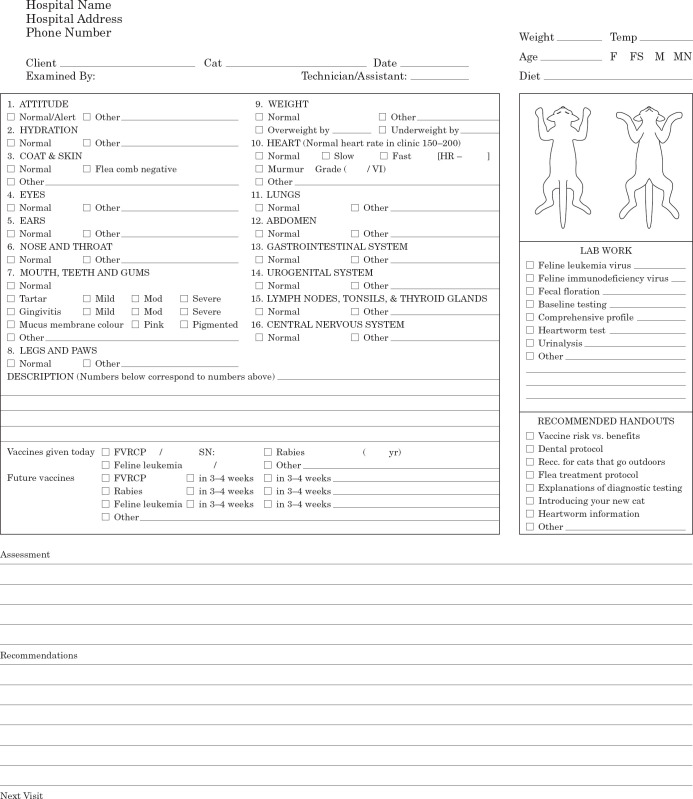
| 6—Example of dual-copy examination form | 72 |
Introduction
The ‘1998 Report of the American Association of Feline Practitioners and Academy of Feline Medicine Advisory Panel on Feline Vaccines’ 1 was developed to help veterinary practitioners formulate vaccination protocols for cats. The current panel report updates information, addresses questions, and speaks to concerns raised by the 1998 report. In addition, it reviews vaccine licensing, labelling, and liability issues and suggests ways to successfully incorporate vaccination protocol changes into a private practice setting. The material in the 1998 report is not fully reproduced here, and readers are referred to the 1998 report for more detailed information.
Vaccines play an important role in the control of infectious diseases. However, most vaccines do not induce complete protection from infection or disease, nor do they induce the same degree of protection in all animals. Factors that negatively affect an individual animal's ability to respond to vaccination include maternal antibody interference, congenital or acquired immunodeficiencies, concurrent disease, inadequate nutrition, immunosuppressive medication, and stress (eg, overcrowding and poor sanitation). 2 Every effort should be made to ensure that patients are healthy prior to vaccination. Because vaccination alone does not completely protect animals from infection and disease, environmental conditions should be addressed and exposure to infectious agents should be minimized.
The overall objectives of vaccination are to vaccinate the largest possible number of individuals in the population at risk, vaccinate each individual no more frequently than necessary, and vaccinate only against infectious agents to which individuals have a realistic risk of exposure and subsequent development of disease. Kittens younger than 16 weeks of age are generally more susceptible to infection than are adult cats and typically develop more severe disease. Thus, they represent the principal target population for vaccination. 3 Maternal antibody interference is the most common reason why some animals are not immunized following vaccination, and is the reason why a series of vaccinations is necessary for kittens younger than 12 weeks of age. 2 Vaccination needs of adult cats should be assessed at least once yearly, and if necessary, modified on the basis of an assessment of their risk.
Vaccine selection and administration (Table 1)
Table 1.
American Association of Feline Practitioners and Academy of Feline Medicine Feline Vaccine Panel recommended guidelines for vaccination of cats
| Primary vaccination | |||||
|---|---|---|---|---|---|
| Antigen | Vaccine types | Cats <12 weeks old | Cats ≥ 12 weeks old | Booster vaccination | Comments |
| Feline parvovirus * | MLV vaccine for parenteral administration MLV vaccine for topical administration Adjuvanted inactivated-virus vaccine for parenteral administration | If ≥ 6 weeks old, vaccinate at initial visit and every 3 to 4 weeks until ≥ 12 weeks old † | Administer 2 doses, 3 to 4 weeks apart | 1 year after primary vaccination, then no more frequently than every 3 years | Highly recommended for all cats; in most cats, protection derived following administration of booster vaccination 1 year after primary vaccination is sustained for at least 3 years and probably 5 to 6 years or more; MLV vaccines should not be administered to pregnant queens or kittens <4 weeks old |
| Feline herpesvirus-1 and feline calicivirus | Combined MLV vaccine for parenteral administration Combined adjuvanted inactivated-virus vaccine for parenteral administration | If ≥ 6 weeks old, vaccinate at initial visit and every 3 to 4 weeks until ≥ 12 weeks old † | Administer 2 doses, 3 to 4 weeks apart | 1 year after primary vaccination, then every 3 years | Highly recommended for all cats; MLV vaccines should not be administered to pregnant queens |
| Feline herpesvirus-1 and feline calicivirus | Combined MLV vaccine for topical administration | If ≥ 6 weeks old, vaccinate at initial visit and every 3 to 4 weeks until ≥ 12 weeks old § | Administer 1 dose | 1 year after primary vaccination, then every 3 years | Highly recommended for all cats; may be used as an alternative to the parenteral product; may be preferable to parenterally administered vaccines in cats reared in or entering environments in which viral upper respiratory tract disease is endemic (eg, some catteries, boarding facilities, shelters); MLV vaccine should not be administered to pregnant queens |
| Rabies | Adjuvented inactivated-virus vaccine for parenteral administration every year ‡ | Not eligible for vaccination | Administer 1 dose | 1 year after primary vaccination, then every year § | Rabies vaccination is highly recommended for all cats. Rabies vaccination of cats is required by law in some regions of the country, and veterinarians should comply with state and local statutes regarding type of vaccine to be used and vaccination interval |
| Rabies | Adjuvanted inactivated-virus vaccine for parenteral administration every 3 years ‡ | Not eligible for vaccination | Administer 1 dose | 1 year after primary vaccination, then every 3 years § | Rabies vaccination is highly recommended for all cats. Rabies vaccination of cats is required by law in some regions of the country, and veterinarians should comply with state and local statutes regarding type of vaccine to be used and vaccination interval |
| Rabies | Canarypox virus-vectored recombinant vaccine for parenteral administration | Administer 1 dose to cats as young as 8 weeks old | Administer 1 dose | 1 year after primary vaccination, then every year | Rabies vaccination is highly recommended for all cats. The recombinant rabies virus vaccine can be used as an alternative to products approved for annual use; this product does not contain an adjuvant |
| Feline leukemia virus | Adjuvanted and non-adjuvanted inactivated-virus vaccines for parenteral administration | Administer 2 doses, 3 to 4 weeks apart to cats as young as 8 weeks old ¶ | Administer 2 doses, 3 to 4 weeks apart ¶ | Annually | Recommended for cats that are not restricted to a closed, indoor, FeLV-negative environment; most important for cats <16 weeks old; not recommended for cats ≥ 16 weeks old with minimal to no risk of exposure to FeLV-infected cats |
| Chlamydia psittaci | Modified-live vaccine for parenteral administration Adjuvanted inactivated vaccine for parenteral administration | If ≥9 weeks old, administer 2 doses, 3 to 4 weeks apart | Administer 2 doses, 3 to 4 weeks apart | Annually | Not recommended for routine use; can be considered for use in cats in multiple-cat environments where C. psittaci infections associated with clinical disease have been documented |
| Feline infectious peritonitis virus | MLV vaccine for topical administration | Not approved for cats <16 weeks old | Administer 2 doses, 3 to 4 weeks apart to cats ≥16 weeks old | Annually | Not recommended for routine use; at this time, there is insufficient evidence to support the conclusion that the vaccine induces clinically relevant protection |
| Microsporum canis | Adjuvanted inactivated vaccine for parenteral administration | Not approved for cats <16 weeks old | First dose administered SC to cats ≥16 weeks old; second dose administered SC 12 to 16 days after the first dose; third dose administered SC 26 to 30 days after the second dose | Not stipulated | Not recommended for routine use; vaccination may be considered as one component of a comprehensive control programme in multiple-cat environments in which M. canis infection is endemic or as adjunctive treatment to hasten resolution of clinical signs in individual cats |
| Bordetella bronchiseptica | Modified-live vaccine for topical administrations # | Administer 1 dose (0.2 ml) intranasally to cats ≥4 weeks old | Administer 1 dose (0.2 ml) intranasally | Not stipulated | Not recommended for routine use; vaccination may be considered for cats entering or residing in multiple-cat environments where B. bronchiseptica infections associated with clinical disease have been documented |
| Giardia lamblia | Adjuvanted inactivated vaccine for parenteral administration | Administer the first dose to cats 8 weeks old and a second dose 3 to 4 weeks later | Administer 2 doses, 3 to 4 weeks apart | Annually | Not recommended for routine use; vaccination may be considered as one component of a comprehensive control programme in multiple-cat environments in which G. lamblia infections associated with clinical disease have been documented |
Cause of the feline panleukopenia.
For kittens that are orphaned or at high risk of exposure, vaccination when as young as 4 weeks old may be indicated.
For kittens that are orphaned or at high risk of exposure, vaccination when as young as 10–14 days old may be indicated.
A specific route of administration may be required; see product information for details.
Most often, the product approved for use annually is given for initial vaccination, followed 1 year later and every 3 years after that by administration of the product approved for use every 3 years; however, vaccination interval must comply with local and state statutes.
Feline leukaemia virus testing is recommended prior to vaccination; infected cats do not derive any benefit from vaccination.
This product is not the same as the B. bronchiseptica vaccine approved for use in dogs; the product approved for use in dogs should not be used in cats.
Parenteral vaccines should be administered subcutaneously or intramuscularly.
MLV=modified-live virus.
It is recommended that administration sites for parenteral vaccines be chosen in accordance with the guidelines established by the AAFP and adopted by the Vaccine-Associated Feline Sarcoma Task Force 4 (Appendix 1). Use of multiple-dose vials is discouraged, because inadequate mixing may result in unequal distribution of antigen and adjuvant, possibly resulting in decreased efficacy or an increased likelihood of adverse events; iatrogenic contamination is an additional risk. The panel discourages the use of polyvalent vaccines other than those containing combinations of feline panleukopenia virus, feline herpesvirus-1, and feline calicivirus, exclusively. This opinion is based on the belief that as the number of antigens in a vaccine increases, so too does the probability of associated adverse events. Additionally, use of polyvalent vaccines may force practitioners to administer vaccine antigens not needed by the patient.
Feline panleukopenia. Feline panleukopenia is caused by feline parvovirus (FPV). The virus remains infectious for months to years in the environment and is primarily spread via the faecal-oral route. Fomites (eg, cages, food bowls, litter boxes, and health care workers) play an important role in the transmission of the organism. Clinical signs of infection include lethargy, anorexia, vomiting, diarrhoea, fever, and profound panleukopenia; mortality rates are highest in young, susceptible cats. 5 In utero infection with FPV is a common cause of cerebellar hypoplasia. 6
Vaccination against FPV is highly recommended for all cats. Immunity to feline panleukopenia is primarily through antibody response to natural infection, vaccination, or passive transfer of maternal antibodies from queen to kittens. Maternal antibody may interfere with immunisation when antibody titres are high during the neonatal period. Maternal antibody titres generally wane sufficiently to allow immunisation by 12 weeks of age. 7 Immunity conferred by feline panleukopenia vaccines is considered to be excellent, and most vaccinated animals are completely protected from infection and clinical disease. Both serologic and challenge exposure data indicate that a parenteral FPV vaccine induces immunity that is sustained for at least 7 years. 8,9 Therefore, following the initial series of vaccinations and re-vaccination, 1 year later, cats should be vaccinated no more frequently than once every 3 years.
Modified-live virus (MLV) vaccines and adjuvanted inactivated virus vaccines for parenteral administration and a MLV vaccine for topical (intranasal) administration are available and effective. Experimental studies have shown that intranasal administration of canine parvovirus-2 vaccines to puppies is less effective than parenteral administration in overcoming maternal antibody interference (Ron Schultz, personal communication). The most likely reason is that fewer virus particles reach lymphoid tissue when the product is given intranasally, as compared with parenteral administration, and viral replication in lymphoid tissue is required for immunisation with MLV parvovirus vaccines. Although studies have not been performed in cats, the same phenomenon may occur in this species as well. Therefore, caution is appropriate when contemplating the use of intranasal FPV vaccines for primary immunisation of kittens, especially those residing in environments where exposure to FPV is likely.
It has recently been found that some cats with panleukopenia-like disease were infected with canine parvovirus-2b (CPV-2b). Studies show that FPV vaccines provide excellent protection not only from FPV but also from CPV-2b; thus, canine parvovirus infection should not be a concern for cats immunised as a result of vaccination with FPV vaccines. 10
Serious adverse events associated with FPV vaccines are rare. Tumour formation at the site of a topically administered vaccine has not been reported. Vaccination of pregnant queens with modified-live FPV vaccines may possibly result in neurologic disease in developing fetuses; 11 the same concern applies to kittens vaccinated at less than 4 weeks of age. Therefore, the use of MLV vaccines should be avoided in pregnant queens and kittens less than 1 month of age. 11,12
Feline viral rhinotracheitis and feline calicivirus infection . Feline viral rhinotracheitis, caused by feline herpesvirus-1 (FHV-1), and feline calicivirus (FCV) infection account for up to 90% of all cases of infectious upper respiratory tract disease in cats. 13 Both viruses are shed in ocular, nasal, and pharyngeal secretions of infected cats. 14 Organisms are transmitted from cat to cat directly, through sneezed macro-droplets, or indirectly, via contaminated fomites. 13 The disease is self-limiting; however, infected cats may develop chronic oculonasal disease. Latent infection is lifelong for cats infected with FHV-1; reactivation can occur during periods of stress or following corticosteroid administration. Some cats infected with FCV become persistently infected and shed virus for prolonged periods (months to years). Although rarely serious in adult cats, disease caused by these viruses may be severe, and sometimes fatal, in kittens. Lameness and chronic oral inflammatory syndromes have been linked to calicivirus infection and vaccination with modified-live calcicivirus vaccines. 15 –20 Risk of exposure to either FHV-1 or FCV is high, because both organisms are widespread in the feline population.
Vaccination against FHV-1 and FCV is highly recommended for all cats. Immunity is through humoral and cell-mediated immune responses to natural infection or vaccination or through passive transfer of maternal antibodies from queen to kittens. Maternal antibody may interfere with induction of a systemic immune response; however, by 12 weeks of age, maternal antibody titres wane sufficiently to allow parenteral immunisation. Topically administered (intranasal, conjunctival) vaccines are capable of inducing a local immune response in the face of high maternal antibody titres. 21 Serologic and challenge exposure data indicate that a parenteral FHV-1 and FCV vaccine induces protection that lasts at least 3 years. 8,9 Therefore, following the initial series of vaccinations and re-vaccination 1 year later, cats should be vaccinated once every 3 years.
Regardless of the route of administration, FHV-1 and FCV vaccines induce only relative, not complete, protection. At best, these vaccines induce an immune response that lessens the severity of disease; vaccinates are not immune to infection, nor are they protected from all signs of disease. 2 Currently available FCV vaccines probably do not induce protection from all isolates of the virus. 22
Modified-live virus and inactivated virus vaccines for parenteral administration and MLV vaccines for topical intranasal and conjunctival administration are available. If a susceptible cat is born into or is entering an environment in which viral upper respiratory tract disease is endemic (eg, some catteries, boarding facilities, and shelters), the use of a topical product may be advantageous. Administration of such products to kittens as young as 10 to 14 days of age could be considered in these situations; however, products that also contain modified-live FPV antigens should not be administered to kittens younger than 4 weeks of age. 12 Adverse events associated with vaccination against FHV-1 and FCV include mild transient fever, sneezing, conjunctivitis, oculonasal discharge, lameness, and, for parenteral products, pain at the injection site. 16,22 Sneezing, conjunctivitis, oculonasal discharge, and ulceration of the nasal philtrum are believed to occur more frequently with vaccines licensed for topical use. Tumour formation at the site of a topically administered vaccine has not been reported.
Rabies . Rabies is transmitted mainly through bite wounds of infected mammals. More cats than dogs develop rabies in the United States, 23 and although relatively resistant to rabies, both species serve as potential sources of infection for human beings. 23,24 Treatment is ineffective in cats that develop clinical signs and should not be attempted because of the high potential for zoonotic infection. 24 All instances of suspected or known rabies virus infection must be reported to local health department officials. Proper precautions and quarantine procedures as outlined by local regulations and described in the ‘Compendium of Animal Rabies Prevention and Control’ should be followed. 25
Although vaccine-associated sarcomas have been reported to develop in association with administration of a variety of vaccines, current data suggests they are more frequently associated with administration of feline leukaemia virus vaccines and adjuvanted rabies vaccines. 26 Inflammatory reactions are commonly observed at sites where adjuvanted rabies virus vaccines have been administered, and concern has arisen regarding the possible association between these reactions and vaccine-associated sarcomas. 27 With the exception of a recently approved canarypox virus-vectored recombinant feline rabies virus vaccine (Pure Vax Rabies Vaccine, Merial Ltd.), all rabies virus vaccines currently on the market contain adjuvants. In rats, inflammation induced by the recombinant product appears to be minimal, 28 but whether the use of this vaccine will be associated with a reduced likelihood of vaccine-associated sarcoma formation in cats is not yet known. The recombinant product is currently licensed only for annual administration.
Rabies virus vaccination is highly recommended for all cats, and is required by law in some states and municipalities. Manufacturers are required by the USDA to establish, by means of experimental challenge exposure studies, the minimum duration of immunity for rabies virus vaccines they sell, and products approved for use every year or every 3 years are available. Statutes governing the administration of rabies virus vaccines vary considerably throughout the United States; veterinarians should comply with the legal requirements of their area.
Feline leukaemia virus infection. Feline leukaemia virus (FeLV) infects domestic cats throughout the world. Transmission is through transfer of virus in the saliva or nasal secretions resulting from prolonged intimate contact (eg, mutual grooming), biting, or sharing of food and water utensils. The virus may also be transmitted by transfusion of blood from an infected cat, in utero, or through the milk. 29 Exposure to virus persisting in the environment on fomites, or in aerosolised secretion is not an efficient means of viral transmission. Clinical signs of FeLV infection are primarily related to neoplasia, anaemia, and diseases resulting from immunosuppression.
Kittens are the most susceptible to infection; resistance increases with maturity. Experimental data demonstrate that kittens younger than 16 weeks of age are most susceptible to infection, with cats older than this being relatively resistant. 30 Cats at greatest risk include outdoor cats (free-roaming pets, stray cats, and feral cats). Also at risk are cats residing in open, multiple-cat environments, cats living with FeLV-infected cats, and cats residing in households with unknown FeLV status.
The decision to vaccinate an individual cat against FeLV infection should be based on the cat's age and its risk of exposure. Vaccination against FeLV is recommended for cats at risk of exposure (i.e., cats not restricted to a closed, FeLV-negative, indoor environment), especially those younger than 4 months of age. Vaccination is not recommended for cats with minimal to no risk of exposure, especially those older than 4 months of age. The ability of a particular vaccine brand to induce an immune response sufficient to resist persistent viremia varies from study to study. 31 Because protection is not induced in all vaccinates, preventing exposure to infected cats remains the single best way to prevent FeLV infection. Vaccination against FeLV does not diminish the importance of testing cats to identify those that are viremic. It is of critical importance that viremic cats not be in contact with other cats, especially those younger than 4 months of age. Therefore, the FeLV infection status of all cats should be determined. 32 Adverse events associated with vaccination against FeLV include local swelling or pain, transient lethargy or fever, and post-vaccination granuloma formation. Although vaccine-associated sarcomas have been reported to develop in association with administration of other vaccines, current data suggests they are more frequently associated with administration of FeLV vaccines and adjuvanted rabies virus vaccines. 26 If vaccination is deemed appropriate, annual revaccination is recommended. Cats should be tested for FeLV infection before initial vaccination and when there is a possibility that they have been exposed to FeLV since they were vaccinated. The ELISA is the preferred screening test; the IFA is the preferred confirmatory test. 32 Individuals confirmed to be infected with FeLV need not receive FeLV vaccines but they should be segregated from uninfected cats.
Chlamydiosis . Chlamydia psittaci is a bacterial pathogen of the conjunctiva and respiratory tract of cats. Transmission is through direct cat-to-cat contact; fomite transmission is less likely because the organism is unstable in the environment. Serous conjunctivitis, which may initially affect only one eye, is the most common clinical sign. Sneezing or nasal discharge may develop, but if so, are usually mild. Clinical signs are usually evident 5 to 10 days after infection and resolve with appropriate antimicrobial treatment. 33 Isolation rates have been reported to range from approximately 1% for cats without signs of respiratory tract disease to approximately 14% for cats with concurrent upper respiratory tract disease. 34 Highest rates of infection are reported for cats between 5 weeks and 9 months of age. 35 Immunity conferred by C. psittaci vaccines is similar to that conferred by FHV-1 and FCV vaccines, in that vaccinates are protected from severe clinical disease but not from infection. 2 The frequency of adverse systemic events associated with C. psittaci vaccines is higher than that associated with other commonly used vaccines; reactions include lethargy, depression, anorexia, lameness, and fever 7 to 21 days after vaccination. 36 Because signs of disease associated with C. psittaci infection are comparatively mild and respond favourably to treatment and because adverse events associated with use of C. psittaci vaccines are of greater concern than adverse events associated with use of many other products, routine vaccination against C. psittaci infection is not recommended. Vaccination may be considered for cats in multiple-cat environments where infections associated with clinical disease have been confirmed. If vaccination is deemed appropriate, annual revaccination is recommended.
Feline infectious peritonitis . Feline coronaviruses (FCoV) vary considerably in pathogenic potential and have historically been grouped into two biotypes: feline enteric coronaviruses (FECV) that typically cause subclinical to mild enteric infections, and feline infectious peritonitis viruses (FIPV) that cause feline infectious peritonitis (FIP). Currently, FIPV are believed to be generated as mutant variants in FECV-infected cats. 37,38 FCoV are widespread in feline populations worldwide, with seropositivity rates highest in crowded multiple-cat environments. 39 Transmission of the virus is mainly via the fecal-oral route. In environments in which FCoV infection is endemic (eg, most multiple-cat environments), 35 to 70% of cats will be shedding FCoV in the stool at any given time. 40,41 Most infected cats remain healthy, although a few— usually between 1 and 5%—ultimately develop FIP. Affected cats rarely survive regardless of treatment. 39 Kittens are most often affected with FIP, but the disease reportedly can develop in cats of all ages. A genetic predisposition has been suggested, with higher disease incidence in certain lines. 39,42
Considerable controversy surrounds the ability of the currently available FIP vaccine (Primucell-FIP, Pfizer Animal Health) to prevent disease. Some studies demonstrate protection from disease; 43,44 others show little benefit from vaccination. 45,46 Antibody-dependent enhancement (ADE) of disease in vaccinates has been demonstrated in experimental challenge exposure studies, 47 but it is uncertain whether ADE occurs in a natural setting. Discrepancies between study results are probably attributable to differences in test methodology (eg, strain and dose of challenge virus, genetic predisposition of the test animals). Protection from disease has not been demonstrated in animals vaccinated when younger than 16 weeks of age. However, most kittens born and reared in environments in which FCoV infection is endemic are infected prior to reaching this age. 41,48 In these instances, vaccination of infected cats has not proven beneficial. At this time, there is no evidence that the vaccine induces clinically relevant protection, and its use is not recommended.
Dermatophytosis . Dermatophytosis in cats is primarily caused by infection with Microsporum canis. A variety of clinical manifestations, including transitory clinical disease and chronic infection with or without clinical signs, have been reported. Although successful treatment of individual cats is usually straightforward, elimination of endemic infection from multiple-cat environments is expensive, labour intensive, and time consuming. 49
An M. canis vaccine (Fel-O-Vax MC-K, Fort Dodge Animal Health) is approved for use as an aid in the prevention and treatment of clinical signs associated with M. canis infection. Vaccination has not been demonstrated to prevent infection or to eliminate M. canis organisms from infected cats. Therefore, routine vaccination against M. canis infection is not recommended. At the time of this writing, the product has not been independently evaluated for efficacy. Based on studies conducted by the manufacturer, it is reasonable to consider vaccination as adjunctive treatment for individual infected cats 4 months of age or older to hasten resolution of clinical signs. If the vaccine induces an immune response that accelerates lesion resolution, then the number of infectious fungal spores produced by vaccinates may be reduced as well; therefore, it is reasonable to consider vaccination as one component of a comprehensive treatment programme in multiple-cat environments in which M. canis infection is endemic. Nonetheless, the ability of this product to hasten elimination of endemic infections from such environments has not been evaluated. The revaccination interval is not stipulated on the label. Major adverse events reportedly associated with the use of this product are pain, temporary hair loss, and formation of sterile abscesses or granulomas at the vaccine site. 49
Bordetella bronchiseptica infection . Bordetella bronchiseptica is a small, aerobic, gram-negative coccobaccilus long recognised as a respiratory tract pathogen of several species of animals. The natural route of transmission in cats is believed to be via the aerosol or intranasal route. 50 Experimental challenge exposure studies have shown that B. bronchiseptica can act as a primary pathogen in cats; inoculation of specific-pathogen-free (SPF) kittens results in self-limiting disease characterised by variable degrees of fever, nasal or ocular discharge, sneezing, induced or spontaneous coughing, pulmonary rales, and submandibular lymphadenopathy. 50 Bronchopneumonia associated with naturally occurring B. bronchiseptica infection has been reported in both kittens and adult cats. 51 Other factors, including nutritional status, overcrowding, co-infection with other agents such as FCV or FHV-1, and suboptimal hygiene, may influence the outcome of exposure. 52,53
Seroprevalence surveys suggest that exposure to the organism is common, with infection rates varying from population to population. The highest rates of seropositivity (often >80%) are found among cats in rescue shelters and multiple-cat households, especially when there is a history of respiratory tract disease. Lowest rates are found among cats in households with few cats and no history of respiratory tract disease. 54,55 Similarly, isolation rates vary. Bordetella bronchiseptica was isolated from the oropharynx of 19 of 614 (3.1%) and from the distal trachea in six of 614 (1%) of asymptomatic cats from shelters in Louisiana. 56 In a recent survey of 740 cats in the United Kingdom, none of the household cats were found to be infected, but 9% of cats from breeding colonies and 19% of cats from rescue shelters were found to be carrying the organism. 57 In the same survey, 9% of healthy cats and 14% of cats with respiratory tract disease tested positive for the organism. An additional finding was a strong positive association between oropharyngeal isolation of B. bronchiseptica and residence in households containing dogs with a recent history of respiratory tract disease.
Definitive diagnosis of disease associated with B. bronchiseptica infection may be difficult, in part because signs of infection often mimic those associated with FHV-1 or FCV infection. Isolation of B. bronchiseptica from a cat with respiratory tract disease is supportive of the diagnosis, but carriage of the organism in asymptomatic cats precludes establishing a direct cause-and-effect relationship. Resolution of disease with appropriately chosen antimicrobial medication might suggest a causative role for B. bronchiseptica, but the self-limiting nature of many cases of viral upper respiratory tract disease prevents attributing disease resolution solely to antimicrobial treatment.
A vaccine (Protex-Bb, Intervet Inc.) to prevent disease caused by infection with B. bronchiseptica has recently been licensed. The product contains a live, reduced-virulence culture of B. bronchiseptica and is licensed for administration via the intranasal route to cats 4 weeks of age and older. Efficacy of the vaccine has been independently evaluated, but in studies conducted by the manufacturer to gain vaccine licensure, vaccinated 4-week-old SPF cats experienced less severe signs of disease than did unvaccinated controls when challenge exposed 3 weeks after vaccination. Similar results were obtained when 8-week-old kittens were challenge exposed 72 h after vaccination. As of this writing, studies to evaluate the duration of protection induced by the vaccine have not been completed, and the revaccination interval is not yet stipulated on the label. Routine use of this vaccine is not recommended. It is reasonable to consider vaccinating cats entering or residing in multiple-cat environments (eg, shelters, catteries, or boarding facilities) where disease associated with B. bronchiseptica infection has been confirmed. However, the ability of the product to reduce the prevalence of infection or the severity of disease in such environments has not been evaluated.
Giardiasis . Infection of cats with the protozoan Giardia lamblia is associated with acute or chronic gastrointestinal disease ranging in severity from subclinical to severe. 58,59 Because infected cats shed cysts intermittently, diagnosis of G. lamblia infection is often cumbersome and usually requires multiple fecal examinations. Several methods of diagnosis are available, including examination of a faecal smear, the zinc sulfate centrifugation method, and use of an ELISA to test faeces. 59 There are currently no approved treatment methods for cats, and although treatment commonly controls signs of disease, it is uncertain that it clears infection. 60 Treatment effectiveness is highly variable, and resistant organisms are commonly encountered. 60,61 Giardia lamblia is transmitted via the faecal-oral route; cysts may be ingested from contaminated water, from direct cat-to-cat transmission especially in crowded environments (eg, through mutual grooming), from exposure to contaminated litter boxes, and from consuming prey. 61,62 Giardiasis is a recognised zoonotic disease, but the role of cats in transmission of the organism is not well established. 59,63,64
A vaccine has recently been licensed by the USDA (Fel-O-Vax Giardia, Fort Dodge Animal Health) as an aid in the prevention of disease associated with G. lamblia infection and reduction in the severity of shedding of cysts. This vaccine is composed of quantified, homogenated, and chemically inactivated G. lamblia trophozoites, and contains an adjuvant commonly found in other feline products from the manufacturer, but different from the adjuvant in the manufacturer's canine product. The vaccine is approved for use in cats 8 weeks of age and older. At the time of this writing, the vaccine has not been dependently evaluated for efficacy, but in studies conducted by the manufacturer to gain vaccine licensure, vaccinates had a statistically significant reduction in severity of clinical signs (diarrhoea), duration of cyst shedding, and prevalence of infection (percentage of cats with trophozoites at the end of the trial), compared with control animals. Protection was demonstrated to persist for at least 1 year after vaccination.
Routine use of this vaccine is not recommended, but because vaccinates had less severe clinical disease and shed cysts for a shorter time, it is reasonable to consider vaccination as part of a comprehensive control programme in environments where exposure to G. lamblia is clinically significant. When parasite exposure is on-going, revaccination at annual intervals is recommended. Some vaccinates may shed cysts subsequent to G. lamblia exposure; therefore, proper hygiene and sanitation practices should be implemented even with vaccinated cats. The ability of this product to aid in hastening elimination of endemic infection from multiple-cat environments has not been evaluated.
Liability related to vaccination
In the United States, licensed vaccines are subject to the Virus, Serum, and Toxin Act (VSTA) of 1913 (9 CFR §101.2(w) [1991]). Consequently, use of animal vaccines is regulated by the United States Department of Agriculture (USDA), not the Food and Drug Agency (FDA). Regulations incorporated in the Animal Medicinal Drug use Clarification Act (AMDUCA) do not apply to animal vaccines, so using a vaccine in a manner other than stated on the package insert is not considered extralabel use; a more appropriate term is ‘discretionary’ use. The VSTA applies only to the preparation, sale, barter, exchange, or shipment of biologics. a It does not regulate use of vaccines by veterinarians. Although these are usage guidelines within specific state or federal eradication and control programmes and perhaps as isolated rules within some state practice acts, there are no overarching federal regulations concerning the after-sale use of licensed animal vaccines by veterinarians or lay persons in the United States.
Even so, many veterinarians rely on the vaccine label to protect them. In the past, this was not an unreasonable approach, because by adhering to label instructions, veterinarians could, in most cases, shift the focus of litigation to the vaccine manufacturer. However, in 1996 the United States Supreme Court refused to review the Seventh Circuit Court's decision in Lynbrook Farms vs SmithKline Beecham Corp (117 S.Ct. 178). In that decision, the Circuit Court upheld the contention by the USDA Animal and Plant Health Inspection Service (APHIS) that the VSTA preempted all state court tort remedies that would have the effect of imposing requirements different from or in addition to those imposed by the USDA regarding the safety, efficacy, potency, or purity of a product. In effect, this action eliminated vaccine manufacturers as defendants in all state vaccine tort cases unless it was alleged that the vaccine was improperly manufactured. b,c However, professional negligence and breach of warranty claims against veterinarians using these products were not preempted. As a result, future consumer claims involving vaccines will, in all likelihood, be centred around veterinary malpractice or the failure of veterinarians to adhere to prevailing standards of practice in selecting and administering vaccines as well as claims that vaccines were given without the proper informed consent.
If, in a court of law, the quality of care provided by a practitioner is being called into question, the practitioner's actions will likely be compared with the prevailing ‘standard of care’, a legal term of art that, simply defined, is the care a practitioner of equal experience and training would deliver under the same or similar circumstances. The prevailing standard of care regarding the use of vaccines is in a state of flux, as exemplified by the recommendation of an increasing number of veterinary virologists, veterinary colleges, professional organisations, and practitioners to extend the revaccination interval for certain vaccine antigens. However, by and of themselves, a few published articles or stated opinions of recognised experts do not define a new standard of care; rather, it is their adoption and utilisation by a substantial portion of the veterinary community. Vigorous debate within the profession will undoubtedly result in a new standard of care in the selection and use of vaccines. Although many veterinarians will, for various reasons, resist and delay adoption of new protocols, they should know that adherence to old protocols may, in the light of new knowledge, not protect them as ‘… conformity to custom is not in itself an exercise of care as a matter of law’ (30 AmJur2nd Evidence § 1123). In this uncertain atmosphere, questions about a veterinarian's actions will likely focus on the following types of inquiry: Did the animal need the vaccine? If so, did the veterinarian select the proper agent? Was it in the proper form? Was is given in the proper manner and location? Was the vaccine handled properly? Was it administered aseptically? Was it administered at the proper interval? Did the client give informed consent before the veterinarian vaccinated the animal? Except in the case of herd or population medicine, the answers to these kinds of questions will be unique to the animal being treated.
The current informed consent standard is the ‘reasonable patient standard’. Under this standard, the scope of disclosure is not measured by the physician's standards, but rather by the patient's needs and whether the information is material to the patient's decision (material information is that which a reasonable person in the client's position would use to make an intelligent decision to accept or reject vaccination). d Under this standard, a veterinarian should disclose the nature of the condition being vaccinated against along with any reasonable dangers within the veterinarian's knowledge that are incident to or may result from vaccination. When vaccination inherently involves a known risk of death or serious harm to an animal, it is the veterinarian's duty to disclose to the client the possibility of such outcomes and to explain in lay terms any significant potential complications that might occur. The veterinarian is also expected to provide information to the client regarding all reasonable alternatives to vaccination. It is the client's decision, not the veterinarian's, to approve or disapprove of vaccination. Once the veterinarian has provided the appropriate information and effectively communicated it to the client, he or she should specifically ask for and obtain the client's consent to the proposed vaccination. In fact, the failure to specifically obtain the client's informed consent could itself be negligent and result in legal liability. For this reason, veterinarians should consider developing consent forms to be signed by owners prior to vaccination of their animals (Appendix 2).
Veterinarians should be cautious in their statements regarding the safety or effectiveness of vaccines. If a veterinarian guarantees that a particular vaccine product is safe or effective, the veterinarian, not the manufacturer, may be liable for breech of warranty. e This cause of action may not be covered by veterinary malpractice insurance.
The lack of specific rules regarding use of animal vaccines by veterinarians leaves them especially vulnerable to litigation. A veterinarian's exposure to legal liability will be specific to the facts of the case, and though there is not absolute safeguard from litigation, practitioners can go a long way towards protecting themselves by conforming to the standards of practice as they apply to the use of vaccines, by closely adhering to the doctrine of informed consent, and by not providing undue warranty regarding the vaccines they administer.
Vaccine licensing
The VSTA grants authority to the USDA to approve animal vaccines for interstate sale. To be approved, a vaccine must meet requirements for efficacy, purity, potency, and safety. 65
Efficacy . Efficacy is a measure of a vaccine's ability to stimulate a protective immune response. Vaccine efficacy is an in vivo measurement, and depending on USDA policy for the disease of interest, it is usually determined by direct challenge exposure of test animals or by measuring serologic responses to vaccination. The USDA has published its approved efficacy determination procedures in the Code of Federal Regulations (9 CFR § 113). The manufacturer must follow USDA codified procedures whenever they exist. The procedures are usually quite specific, regulating the number and species of animals involved in the test, and the method of challenge exposure and evaluation of efficacy. 66
Codified procedures for evaluating efficacy of different products are similar in many regards. In general, for vaccines to be approved on the basis of measurement of serologic responses, at least 75% of vaccinates must have an antibody titre greater than a set limit when measured a short time (usually 2 weeks) after vaccine administration. For vaccines approved on the basis of challenge exposure studies, in most cases at least 80% of the non-vaccinated controls must develop evidence of disease after challenge exposure, whereas 80% of vaccines must have evidence of protection (the 80:80 efficacy guideline). 66 Animals are usually challenge exposed 3 to 4 weeks after vaccination. In addition, the number of animals required by either method of efficacy assessment is usually small (eg, at least 20 vaccinates and five controls for modified-live FPV vaccines).
The use of codified procedures has the potential to simplify comparisons of the efficacy of vaccines, but unfortunately the USDA does not have codified standards for all of the currently available feline vaccines (eg, FeLV vaccines). If a manufacturer desires to produce a vaccine for which there are no codified efficacy standards, it must submit to the USDA a test procedure it believes adequately demonstrates effectiveness; if the test procedure is approved, the manufacturer may then use that procedure to demonstrate vaccine efficacy. Although the flexibility of this method allows new and novel vaccines to enter the marketplace more quickly than might otherwise be the case, it hampers comparisons of vaccine efficacy, because different manufacturers may have gained approval using different test procedures.
How closely do results of vaccine efficacy trials reflect real-world effectiveness? For most diseases, experimental results compare favourably with what veterinarians experience in practice. As examples, efficacy tests of FPV vaccines indicate that vaccine-induced immunity is sufficient to completely protect most cats against challenge exposure. Similarly, tests of the efficacy of FHV-1 and FCV vaccines demonstrate protection from serious disease in most vaccinated cats. Both of these results parallel the experience of most practitioners. However, many variables influence a cat's response to vaccination, so efficacy trials may not tell users how vaccination will affect a specific animal or population of animals.
Purity . Pure cultures of an infectious agent (‘master seed stocks’) are used to produce a vaccine. An extensive array of tests are conducted to be as certain as possible that the organism in these cultures is indeed the intended agent and that no adventitious agents are present. The cells used in establishing and manufacturing the master seeds (‘master cell stocks’) undergo similar stringent testing to ensure that they have been correctly identified and are themselves free of contamination. Once a manufacturer has established a master cell or master seed stock, the USDA performs its own confirmatory testing; if results are acceptable, the USDA releases the master stock for use by the manufacturer. To produce a vaccine, the manufacturer then creates working cells and seeds from the master stocks, which subsequently are frozen and stored in liquid nitrogen.
Although purity testing is extensive, it is not without potential error. Contaminants that are closely related to the intended infectious agent are occasionally missed, and adventitious agents that are present at levels below the threshold of detection may not be identified. This is particularly important if an adventitious agent is pathogenic—a major risk associated with manufacturing of MLV vaccines. Improvements in test methodologies have made creation of master stocks more difficult but also more precise, and have allowed detection of contaminates missed by previous testing methods.
Potency . Potency testing determines the quantity of antigen in a vaccine. Potency and efficacy are closely related, but there are important differences. Potency is usually an in vitro assessment made during the manufacturing process, whereas efficacy is an in vivo assessment of how a vaccine performs in animals. The USDA must approve all potency test procedures, and requires that the manufacturer demonstrate a correlation between potency test results and vaccine efficacy. Each batch of vaccine manufactured is tested for potency, and once the potency exceeds a predetermined limit, the vaccine can be sold.
One factor that makes in vitro potency testing attractive is that prior to use of potency testing, each batch of vaccine had to be tested for efficacy—an expensive requirement that cost the lives of many thousands of animals. Unfortunately, the correlation between potency and efficacy is not always strong. First, potency tests are usually comparisons between production batches of vaccine and a reference vaccine. Because of the way reference vaccines are made and approved, subsequent reference vaccines may contain more antigen mass than previous batches, with a resulting upward shift in the potency of manufactured vaccines. Increased potency may raise safety concerns. Second, vaccines of unequal efficacy may receive equivalent potency test results. For instance, although a heated or frozen vaccine may maintain potency, its efficacy may be compromised. Third, potency tests tend to ignore the role that an adjuvant plays in vaccine efficacy. As an example, a vaccine adjuvant may be adversely affected by storage, yet potency test results may remain unaffected. For these reasons, potency test results parallel efficacy only under the limited set of conditions under which they were originally approved.
Safety . Vaccine safety is demonstrated by monitoring vaccinates for clinically significant problems. Both laboratory safety data (eg, reversion-to-virulence studies, evaluation for local or systemic reactions, and shedding of live vaccine antigens) and field safety data must be generated. A standard field safety test must include a number of animals vaccinated at various geographic locations, usually multiple veterinary practices. Historically the requisite number of test animals has been relatively small (no fewer than 300 animals), but recently the number has been increased, with 1000 animals now being the common standard. In most instances, test animals are vaccinated by a veterinarian and observed for a brief period, usually 30 min. The owners are then instructed to monitor the animals at home and to report any unusual signs to the veterinarian. Other vaccines or medications are often administered simultaneously with the test vaccine, a practice that often complicates data analysis, but which more accurately reflects the way the product will be used.
Safety testing of this nature is likely to demonstrate problems that occur with considerable frequency during the immediate post-vaccination period; it is less likely to reveal rare or subtle vaccine problems, or those that occur a long time after vaccination. Therefore, safety testing should be considered exclusionary. In other words, if safety problems are encountered during the test period, then the vaccine will probably be unsafe in practice as well. But having successfully completed safety tests does not necessarily ensure that a vaccine will be completely safe—or even adequately safe—in a clinical setting. Safety is never absolute; rather, it is a subjective balance between frequency and severity of adverse events on the one hand and the benefits of disease reduction or prevention on the other.
Vaccine labels
The set of rules under which a vaccine was developed influences the amount and type of information included on the label. When comparing vaccines, it is important to understand how the information presented on the label was obtained.
The label contains information about the disease that the vaccine is intended to prevent. If the disease produces many clinical syndromes, usually efficacy of the vaccine for only a single syndrome has been tested. Precisely which syndrome for which the vaccine was tested may not be stated on the label of older products, but the USDA now requires that specific syndromes be stated on the label of novel vaccines (ie, vaccines with an antigen or antigens not contained in any previously licensed products).
Vaccine labels contain one of three common wordings describing the level of protection afforded by vaccination. The wording ‘… prevents infection with (certain microorganisms)’ may be placed on the label if data demonstrates that the product is able to prevent all colonisation or replication of the challenge microorganisms in vaccinated-and-challenged animals. The wording ‘… indicated for the prevention of disease’ normally applies to vaccines that have produced results consistent with the 80:80 efficacy guidelines. The wording ‘… indicated as an aid in the prevention of disease’ is found on vaccines for which efficacy testing demonstrated a statistically significant difference between vaccinates and controls, but not of the level required for the stronger wording. There are several reasons why a reduced level of efficacy might be observed: the vaccine may be less effective, the challenge exposure may have been less severe, or the disease the vaccine attempts to attenuate may create only mild or subtle clinical signs. At any efficacy level, the manufacturer need not demonstrate that protection induced by the vaccine is clinically apparent or relevant to an individual animal, or in the case of the latter two levels, that use of the vaccine will reduce the prevalence of disease in a population. There is also no requirement that the label state how the vaccine is best used in a preventive medicine programme. For additional information on vaccine efficacy studies, see USDA-APHIS CVB Veterinary Services Memorandum No. 800.2000 (http://www/aphis/usda/gov/vs/cvb/lpd/memos/VSMemo800.200.PDF).
Label directions usually reflect the way the vaccine was used during the required safety and efficacy testing. For example, the label may contain the following directions: ‘Administer intramuscularly 1 ml dose of vaccine. Repeat in 2–3 weeks. Annual revaccination is recommended’. There is no requirement to demonstrate that both doses are necessary or that 2 to 3 weeks is the optimal revaccination interval, nor is there a requirement to indicate how to proceed if the second dose is administered more than 3 weeks after the first.
Approximately three decades ago, the paucity of data regarding the duration of protection induced by canine vaccines led experts to recommend annual administration as an attempt to ensure maintenance of protection from disease throughout the life of an animal and to maintain long-term population immunity. 67,68 However, for the vast majority of animal vaccines currently available, the USDA does not require manufacturers to provide observational data on the label to support the recommendation for annual revaccination. The USDA does require manufacturers introducing vaccines containing novel antigens (ie, vaccines with an antigen or antigens not contained in any previously licensed products) to provide data demonstrating duration of immunity claims stated on the product label, but there is no requirements to determine the maximal or optimal revaccination interval.
The route of administration and dose volume indicated on the label should be carefully heeded, because they were probably the only ones tested for safety and efficacy during the licensing process. The practice of reducing the vaccine dose in an effort to reduce adverse post-vaccination events is unlikely to improve vaccine safety and may compromise effectiveness.
Vaccine labels often indicate the ages of animals to which the product may be administered. Age restrictions may exist for safety reasons, as a consequence of regulatory policy, or both. Unfortunately there is no way for the reader of the label to know under which set of rules the vaccine was approved, or why an age restriction is or is not indicated on the label. When in doubt, practitioners should consult with the vaccine manufacturer's technical assistance staff.
Other than warning of the possibility of anaphylactic reactions, vaccine labels have historically provided little safety information. The USDA is beginning to require that manufacturers list vaccine-mediated events (eg, fever, lethargy, or swelling at the injection site) observed during safety testing, but this requirement only applies to newly approved products or to older products for which the manufacturer is submitting changes to the USDA. Currently it is not possible for a reader to know why the label for one vaccine contains safety information not included on the label of a competitor's product. Consequently, labels of products that are nearly identical may list markedly different safety information; the converse is also true. 66 Vaccine users can attempt to clarify the confusion by contacting the manufacturer's technical assistance staff.
Adverse events and adverse event reporting
Despite the admirable safety record of animal vaccines, adverse events do occur. They may be local or systemic; mild, severe, or even fatal; or peracute, acute, subacute, or chronic; and may include vaccine-induced disease or failure to confer immunity. However, even when vaccination immediately precedes an adverse event, it may be difficult to determine with certainty whether the vaccine was responsible. There are many confounding factors that make it difficult to establish a cause-and-effect relationship between vaccination and subsequent illness or death (eg, simultaneous administration of more than one vaccine from the same or different manufacturers, concurrent administration of non-vaccine products, pre-existing disease, or prior exposure to the organism and incubation of disease at the time of vaccination).
Although reporting of adverse events associated with vaccination is not mandatory, it is helpful for all vaccine users to assist in development of databases of adverse events. Receiving reports of real or suspected adverse events is the only way manufacturers can economically obtain the data necessary to evaluate the safety and efficacy of their products in clinical settings. Suspected or real adverse events should be reported to the manufacturer of the product and the United States Pharmacopeia (USP; Appendices 3 and 4). If more than one manufacturers' product was used concurrently, all manufacturers should be contacted. Even though the USP forwards all adverse event reports it receives to the manufacturer, a veterinarian may be able to obtain technical assistance by directly contacting the company. Veterinarians may also choose to report adverse events associated with vaccines to the United States Department of Agriculture Center for Veterinary Biologics (USDA-CVB), adverse events associated with pesticides to the Environmental Protection Agency (EPA), and adverse events associated with pharmaceuticals to the Food and Drug Administration (FDA). Reports sent to the USP are automatically forwarded to the appropriate governmental agency and to the American Veterinary Medical Association.
Calculating the rate of adverse events associated with a vaccine requires knowing both the number of such events and the number of vaccines administered during the same period. Because many adverse events go unreported, the calculated rate should be considered a minimum value; the actual rate is probably higher. And, because the total number of doses administered is not known, caution must be exercised when evaluating the number of adverse events associated with a particular product. If the numbers of adverse events reported for two products are the same, but one vaccine has half the sales of the other, the rate of adverse events for the less popular product is actually double that for the more popular one.
Variation among vaccines
Unlike the FDA's licensing process for human vaccines, the USDA does not stipulate the strain or isolate of organism used to develop an animal vaccine, or the cell line used for vaccine licensure and production. Veterinary biologics manufacturers are free to develop vaccines by any means, so long as they are able to demonstrate a consistent manufacturing process that results in consistent purity, potency, efficacy, and safety. Although perhaps differing fundamentally in composition, vaccines for a particular antigen produced by various manufacturers are usually a great deal more similar than they are different.
Veterinary biologics manufacturers do not evaluate the ability of their vaccines to boost immunity conferred by a competitor's products, nor do they attempt to determine whether their vaccines interfere with a competitor's product. Not surprisingly, there is an absence of data demonstrating efficacy when vaccines from one manufacturer are used interchangeably with those of another. However, temporal patterns of immunologic responses in kittens and adult cats suggest that a satisfactory immune response is achieved when a similar vaccine antigen from one manufacturer is used interchangeably with that of another (eg, an FCV vaccine from one manufacturer may be used as a booster vaccine for a cat originally immunised with the FCV vaccine from a different manufacturer).
Use of serologic testing to monitor immunity and assess the need for vaccination
Specific immunity to infectious agents comprises both cell-mediated and humoral responses. In general, both are important in resistance to infection or disease, but whether cell-mediated or humoral responses are most important for mediation of protection varies with the pathogen and the vaccination status of the animal. Antibodies are generally most effective against pathogens that are extracellular; cell-mediated immune responses are generally most effective against pathogens that are intracellular, because antibodies do not readily enter infected cells. For some pathogens, antibodies produced as a result of vaccination may be effective in preventing infection; for example, cats vaccinated with an FPV vaccine generally are completely protected from infection as a result of antibodies induced by vaccination. For other pathogens, even when antibodies do not prevent infection, they can limit or prevent disease by reducing the amount of infectious agent. Vaccination is designed to stimulate immunologic memory; that is, to expand populations of antigen-specific T- and B-lymphocytes that can respond if the animal is exposed to the organism at a later date.
In many respiratory or gastrointestinal tract infections, mucosal immune responses, particularly IgA antibody, are most effective. Measurement of specific immune responses to an infectious agent could potentially be used to predict whether vaccination is required in an individual cat, provided the appropriate immune response can be accurately measured. 69 Unfortunately, cell-mediated and mucosal immune responses cannot be directly determined in a clinical setting.
Determination of serum antibody responses is technically easy and can be used clinically in some situations to predict resistance to infection or disease. 69 Detection of serum antibodies against an infectious agent can also be used as an indirect measure of cell-mediated immune responses, because T-lymphocyte functions are required for maintenance of B-lymphocyte functions. 70 The presence of serum antibodies to an infectious agent—even if detected months or years after vaccination—indicates that the animal has the memory cells required for a rapid anamnestic cell-mediated and antibody response if the animal is exposed to the same infectious agent at a later time. Serum antibody titres can be correlated with sterile immunity (protection from infection) for a few pathogens (eg, FPV); however, in general, antibody titre is not directly correlated with protection, and the presence of antibody should be considered, rather, an indicator of immunologic memory. 71
Information correlating vaccine-induced serum antibody responses with resistance to infection has been collected primarily for FPV, FHV-1, and FCV. For FPV, serum antibody titres, determined by use of a validated virus neutralisation assay (VN) or enzyme-linked immunosorbent assay (ELISA), can be used to predict resistance to infection and disease. 8,9,72,73 in two published studies, 9,73 all cats positive for FPV antibody that had been vaccinated within the previous seven years were protected against the USDA challenge strain of FPV.
Vaccination against FCV and FHV-1 does not prevent infection, but only lessens the severity of clinical disease among cats subsequently exposed to virulent virus. Thus, immunologic tests will never be completely accurate in predicting whether disease will occur following exposure to either of these two agents. One study correlated serum FCV and FHV-1 antibody titres (VN and ELISA) with severity of clinical signs after exposure to the USDA challenge strains of FCV and FHV-1 in cats vaccinated between six and 36 months previously. 73 All cats with detectable FCV antibodies and most cats with detectable FHV-1 antibodies had greater than 50% reduction in severity of clinical signs, compared with control cats.
Virus neutralisation antibodies against FeLV can be detected. 74 However, studies correlating antibody titres in FeLV-vaccinated cats with protection over time are not available. Additionally, humoral immune responses do not always correlate with immunity to this virus.
Studies correlating antibody titres with protection in vaccinated cats have assessed only small numbers of cats to date. Additionally, only a few vaccines have been evaluated, and results with other products may not be equivalent. Accordingly, the panel recommends the use of set revaccination intervals, as described in this report, for most cats. However, if serologic testing is being considered in lieu of using set revaccination intervals, the following points should be kept in mind:
If a vaccination history is unavailable, vaccines should be administered.
Serologic test results from different laboratories cannot be assumed to be equivalent; practitioners are cautioned to use only laboratories that have validated their test results (ie, they have correlated antibody titres with protection).
Virus neutralisation assays document in vitro inactivation of the specific virus by serum antibodies. ELISA can be designed to measure antibodies against viral antigens, but positive results do not necessarily document that the antibodies detected are protective. Thus, only ELISA for which results have been shown to correlate with protection should be used.
Because maternal antibodies can be detected by VN assays and ELISA and may not indicate long-term protection, serologic testing for assessment of vaccination needs should be reserved for adult, previously vaccinated cats. If circumstances require measurement of antibody in kittens younger than 16 weeks of age, a sample should be collected on the day of vaccination and a second sample should be collected two or more weeks after vaccination. A significant increase in antibody titre indicates that vaccination induced an immune response.
Serologic testing should not be used to assess vaccination needs in cats with proven or suspected immunosuppressive diseases.
Detection of serum antibodies against FPV, FCV, and FHV-1 by validated VN assays and ELISA appear to correlate to resistance to infection in most cats, but failure to detect serum antibodies does not correlate to susceptibility. Thus, if serologic testing is used to assess vaccination needs in individual vaccinated cats, some seronegative cats will be vaccinated needlessly.
Practice management considerations
For many years, cats and veterinarians have benefited from annual administration of vaccines. By encouraging cat owners to bring their pets in yearly for vaccination, veterinarians have been enabled to recognise and treat disease earlier than might otherwise be the case. The annual visit has provided an opportunity to inform clients of important aspects of feline health care, and annual vaccine administration has bolstered the financial strength of many practices.
Unfortunately, many clients have come to believe that vaccination is the only reason—or at least the most important reason—for the annual visit. Veterinarians are justifiably concerned that a reduction in vaccination frequency will cause clients to forego annual examination of their cats, and that the quality of care they deliver will be degraded. To avoid this consequence, it is vital that veterinarians stress the importance of all aspects of a comprehensive health care programme. In addition to vaccination, ways to diminish the impact of infectious disease (eg, reducing overcrowding, improving nutrition, and restricting access to infected animals) should be emphasised. Clients should be informed that cats with serious disease often appear healthy, and that regularly scheduled health evaluations facilitate early detection. The importance of dental care, proper nutrition, appropriate diagnostic testing, control of parasites, and control of zoonotic diseases should be emphasised during each patient evaluation. Behaviour concerns should be discussed, as should the necessity for more frequent examination of kittens and older cats. Examination reminder cards (Appendix 5) or calls and on-hold message systems can inform clients that all aspects of preventative health care, not just vaccinations, are important and will be addressed during each appointment.
Each patient's vaccination needs should be assessed at least yearly and, if necessary, vaccination schedules should be modified on the basis of changes in the cat's age or health, the environment in which it resides, and its risk of exposure to infectious agents. As the number of vaccines patients receive is reduced, clients should not be led to erroneously conclude that vaccination is no longer important. They should be informed that vaccination is a medical procedure with value as well as risk, and will be performed only after considering the needs of the individual patient. Even though the judgement and counsel of their veterinarian will guide them, clients should understand that the decision to vaccinate their pets remains with them. Newsletters and client educational brochures provided before or during scheduled appointments can inform clients about specific infectious diseases, vaccines, risk factors, and adverse events associated with vaccination.
Staff training and education . Staff members should understand the practice's vaccination protocols and other aspects of patient care. An informed staff is able to play a large role in client education, allowing for better time management and staff utilisation. As an added benefit, job satisfaction of staff members increases when they view themselves as important members of the health care team.
Medical record documentation . At the time of vaccine administration, the following information should be recorded in the patient's permanent medical record: the date the vaccine was administered; the name of the person administering the vaccine; the vaccine name, lot or serial number, expiration date, and manufacturer; and the site and route of vaccine administration. Use of peel-off vaccine labels facilitates this type of record keeping. Serologic test results and adverse events associated with vaccination should also be recorded in the patient's permanent medical record. A signed consent form maintained in the record is the best documentation that relevant information was provided to the client and that the client consented to the procedure. At the very least, a notation indicating that a discussion of vaccine risks and benefits took place prior to vaccination should be included in the record. Suspected vaccine failures and adverse events should be recorded in a manner that will alert all staff members.
Methods to emphasise the importance of regularly scheduled physical examinations . The following methods may be used to convince clients of the importance of bringing their cats in for regularly scheduled examination:
On the invoice, the cost of vaccinations should be separated from the cost of the office visit and physical examination, so clients can appreciate the value of all the services rendered. Each service should be invoiced appropriately. Clients will often be willing to pay for additional diagnostic testing, dental care, and other services if they haven't paid for unnecessary vaccines.
Dual-copy physical examination forms can be used to increase the client's perception of the value of the examination (Appendix 6).
Reminders should be sent to clients at least annually for adult cats and semiannually for older cats (Appendix 5).
Having a technician administer vaccines (when allowed by law) at the completion of the physical examination emphasises the primary importance of the examination and consultation.
Recommendations and future considerations
To provide the best possible vaccination advice to clients, veterinarians should have an understanding of the epidemiology of the disease in question and the impact vaccination has on disease prevalence and severity. Regrettably, objective information on the impact of methods to reduce infection rate and disease severity is usually unavailable. The panel encourages the collection of objective information on the epidemiology of infectious diseases in cats as well as on the efficacy of various prevention strategies.
Information on vaccine labels should be standardised to allow more accurate comparison of different products. Efficacy testing protocols should be standardised, and vaccine challenge exposure studies should include cats of various ages and more closely parallel natural exposure. In addition, safety testing protocols should be expanded, and results should be presented more clearly on vaccine labels.
Manufacturers should continue to develop and market monovalent vaccines. It is logical to have multiple vaccine antigens in a single product when the target population and duration of protection induced by the antigens is similar, the route of administration is identical, and the product's efficacy and safety is not compromised (eg, FHV-1 and FCV). However, as veterinarians formulate vaccination protocols specific to the needs of each cat, vaccines containing more than one antigen become less useful. Additionally, monovalent products facilitate staggering of vaccinations to encourage annual client visits. From the manufacturers' standpoint, gaining USDA approval of monovalent vaccines is simpler and less expensive.
The duration of immunity induced by most feline vaccines is not known. Adjustments in human vaccination protols, including revaccination intervals, are determined in part by evaluation of disease incidence data collected by the Centers for Disease Control and Prevention. However, with the exception of rabies, there is no requirement that feline infectious diseases be reported to any agency, so it is unlikely that optimal revaccination intervals in cats will ever be determined in a similar manner. The duration of protection induced by feline vaccines must therefore be determined by other means. The panel encourages investigation of the actual duration of immunity induced by feline vaccines and provision of that information on vaccine labels.
Administration of rabies virus vaccines to cats is subject to inconsistent state and local statutes. In some cases, the requirements fail to consider the duration of protection such vaccines induce; annual administration of rabies virus vaccines approved for triennial administration is required in many locales. Veterinary organisations should continue to work with state and local governing bodies to ensure that rabies virus vaccine regulations are consistent with the known duration of immunity of available vaccines.
Interest in vaccine-related issues has increased in the last several years, fueled in part by safety concerns and questions regarding duration of immunity. These issues are being addressed by national veterinary organisations, scientists in academic settings, and vaccine manufacturers. The individual and cooperative efforts of these groups should be applauded and encouraged. Nonetheless, the quest to more completely understand the etiopathogenesis, immune response, and epidemiology of feline infectious diseases must continue if practitioners are to develop safer and more effective infectious disease control programmes.
Although traditional methods of vaccine testing and production are still viable, the future impact of novel technologies (eg, recombinant techniques) on vaccine safety, production, and, possibly, duration of protection, cannot be overestimated. The manner by which recombinant vaccines invoke immunity and the methods used to evaluate the patient's response often differ from those of traditional products, and it will be increasingly important for practitioners to familiarise themselves with this emerging technology. Animal vaccine manufacturers will inevitably continue to develop new and safer vaccines, and if used properly, these products have the potential to improve the quality of care veterinarians deliver to their patients. There is every indication that new products will be introduced at an unprecedented rate, and veterinary practitioners must arm themselves with information to enable them to make the most appropriate vaccination recommendations.
Acknowledgments
Appendix 1
Vaccination site recommendations
The following guidelines for recommended vaccine administration sites were developed by the American Association of Feline Practitioners and the Academy of Feline Medicine and adopted by the Vaccine-Associated Feline Sarcoma Task Force:
Vaccines containing antigens limited to feline parvovirus, feline herpesvirus-1, and feline calicivirus (with or without Chlamydia psittaci) should be administered over the right shoulder (avoiding the midline), as distally as possible.
Vaccines containing rabies virus antigen (plus any other antigen) should be administered on the right rear limb, as distally as possible.
Vaccines containing feline leukaemia virus antigen (plus any other antigen except rabies virus antigen) should be administered on the left rear limb, as distally as possible.
Injection sites of other medications should be recorded.
Appendix 2
Model consent forms
The consent form should be entitled ‘Consent to Vaccinate’ or something similar to clearly express the purpose of the document. The body of the document should describe the diseases being vaccinated against, an estimate of the odds of the cat contracting the disease (if known), benefits to the cat of vaccination against the disease, the expected adverse effects of vaccination, and odds of the cat developing any particular adverse effect. The document should also include the veterinarian's name, the clinic's name and address, the client's name, and the name of the animal being vaccinated. The document should conclude with a statement of understanding and consent, and areas for the client and veterinarian to sign and date. If necessary, additional educational materials may be provided to clients in advance to assist them in making their decision. Materials provided to clients at the time the informed consent form is signed are not considered part of the information required for providing informed consent.
Two examples of informed consent forms are provided below; they are for educational purposes only, and should not be considered legal advice. Veterinarians should discuss development of an informed consent form with an attorney familiar with local informed consent laws prior to using such forms in their practice.
Example 1—long form
Consent to Vaccination
The goal of animal vaccination is to effectively reduce the extent and severity of infectious diseases in our pets.
In granting this consent to vaccinate I hereby state that:
I understand that (cat's name) may be exposed to (disease).
I understand that (cat's name) has a (state current best estimate of odds) chance of contracting (disease).
I understand that vaccination of (cat's name) with (name and type of vaccine) will substantially reduce but may not completely eliminate his/her chances of contracting (disease).
I understand that (cat's name) may develop (list adverse effects) within (state time frame) of vaccination. I understand that these adverse effects are usually minor and will usually resolve without the need for additional veterinary care. I understand that should (cat's name) develop any severe or unanticipated reaction to the vaccination, I should contact (veterinarian's name and how he or she may be reached) immediately for instructions.
I understand that (cat's name) has a (current best estimate of odds) chance of developing a sarcoma (a type of tumour) at the vaccination site. I understand that this type of tumour, should it occur, is life-threatening and may require extensive medical or surgical treatment.
I understand that Dr (veterinarian's name) makes no warranty, either express or implied, as to the safety or efficacy of the vaccine being used.
I have had an opportunity to ask any questions I have concerning this vaccination. All such questions have been answered to my satisfaction.
Date: Owner's signature:
Date: Witness' signature:
Example 2—short form
Consent to Vaccination
I hereby consent to have my animal vaccinated for (disease). I have read and understood to my satisfaction the materials provided to me by Dr (veterinarian's name). He/she has answered, to my satisfaction, all of my questions. I am aware of the potential risks and benefits of vaccinating (cat's name) against (disease). I understand that Dr (veterinarian's name) makes no warranty, either express or implied, as to the safety of efficacy of the vaccine being used.
Date: Owner's signature:
Date: Witness' signature:
Appendices 3 and 4
Appendices 3 and 4 contain information specific to veterinarians in the USA only and are therefore not reproduced here.
Appendix 5
Examples of examination reminder cards
Annual Examination Reminder
Clinic Name:
Clinic Address:
Clinic Phone Number:
Dear (cat's name),
Our records indicate that it's time for your annual check-up. This check-up includes:
-
*
a physical examination for early disease detection
-
*
a yearly assessment of your vaccination needs
-
*
a plan for dental health
-
*
diagnostic testing individualised for your needs
-
*
parasite control
-
*
nutritional counselling customised for you
-
*
answering your person's questions to prevent problems
Please have your people call our people to set up an appointment to help us keep you in ‘purrfect’ shape. We look forward to seeing you soon.
Semiannual Examination Reminder (for older cats)
Clinic Name:
Clinic Address:
Clinic Phone Number:
Dear (cat's name),
Because you are in your ‘golden years’, it is important to take care of yourself in order to maintain a healthy, youthful lifestyle.
To help make this possible, your friends at the (clinic name) want to remind you that it is time for your semi-annual physical examination.
Please have your family call or stop into the clinic to make an appointment.
Appendix 6
Footnotes
The Virus, Serum, and Toxin Act of 1913 (21 USC § 151–158) in part provides that ‘… it shall be unlawful for any person, firm, or corporation to prepare, sell, barter, or exchange in any place under the jurisdiction of the United States, or to ship or deliver for shipment from one State or Territory or the District of Columbia, any worthless, contaminated, dangerous, or harmful virus, serum, toxin, or analogous product intended for use in the treatment of domestic animals, and that no person, firm or corporation shall prepare, sell, barter, exchange, or ship as aforesaid any virus, serum, toxin, or analogous product manufactured within the United States and intended for use in the treatment of domestic animals, unless and until the said virus, serum, toxin, or analogous product shall have been prepared, under and in compliance with regulations prescribed by the Secretary of Agriculture, at an establishment holding an un-suspended and unrevoked license issued by the Secretary of Agriculture as hereinafter authorised.’
Veterinarian and vaccine manufacturer liability after Smith– Kline: implications for both sectors (a panel presentation), in Proceedings. AVMLA, 1997.
Federal preemption of vaccine product liability litigation— rationale and result, in Proceedings. AVMLA, 1998.
A more complete discussion of the informed consent doctrine as it applies to veterinarians can be found in ‘The informed consent doctrine: what you should tell your clients’. Calif Vet 1997; 51(5): 12–13.
California Jury Verdicts Volume 41, No. 27, Page 28: John Shelby and Don Fullerton dba Fulbor Cattle Company et al. vs Grand Labs; Veterinary Pharmaceuticals, Inc; Thomas Worthington, DVS; and Chino Corona Veterinary Service. Number RCV 65023 consolidated with 01521. Plaintiff award for $1,541,948 or negligent administration of vaccine, in part due to breech of warranty that the vaccine was safe for use in cattle under 3 months of age.
References
- 1. Elston T, Rodan I, Flemming D, et al. (1998) Report of the American Association of Feline Practitioners and Academy of Feline Medicine Advisory Panel on Feline Vaccines. Journal of the American Veterinary Medical Association 212, 227–241. [PubMed] [Google Scholar]
- 2. Greene CE. (1998) Immunoprophylaxis and immunotherapy. In: Infectious diseases of the dog and cat, 2nd ed. Greene CE, ed. Philadelphia: WB Saunders Co, 717–750. [Google Scholar]
- 3. Pedersen NC. (1987) Basic and clinical immunology. In: Diseases of the cat: medicine and surgery. Holzworth J, ed. Philadelphia: WB Saunders Co, 146–181. [Google Scholar]
- 4. Richards JR. (1997) Feline sarcoma task-force meets. Journal of the American Veterinary Medical Association 210, 310–311. [PubMed] [Google Scholar]
- 5. Pollock RVH, Postorino NC. (1994) Feline panleukopenia and other enteric viral diseases. In: The cat: diseases and clinical management. Sherding RG, ed. New York: Churchill Livingstone, 479–487. [Google Scholar]
- 6. de Lahunta A. (1971) Comments on cerebellar ataxia and its congenital transmission in cats by feline panleukopenia virus. Journal of the American Veterinary Medical Association 158, 901–906. [PubMed] [Google Scholar]
- 7. Scott FW. (1987) Viral diseases: Panleukopenia. In: Diseases of the cat: medicine and surgery. Holzworth J, ed. Philadelphia: WB Saunders Co, 182–193. [Google Scholar]
- 8. Scott FW, Geissinger C. (1997) Duration of immunity in cats vaccinated with an inactivated feline panleukopenia, herpesvirus, and calicivirus vaccine. Feline Practice 25 (4), 12–19. [Google Scholar]
- 9. Scott FW, Geissiner GM. (1999) Long-term immunity in cats vaccinated with an inactivated trivalent vaccine. American Journal of Veterinary Research 60, 652–658. [PubMed] [Google Scholar]
- 10. Olson L, Larson L, Schultz R. (1998) Canine parvovirus (CPV-2b) infection in cats, in Proceedings. Conf Res Workers Anim Dis, 79.
- 11. Sharp NJH, Davis BJ, Guy JS, Cullen JM, Steingold SF, Kornegay JN. (1999) Hydranencephaly and cerebellar hypoplasia in two kittens attributed to intrauterine parvovirus infection. Journal of Competitive Pathology 121, 39–53. [DOI] [PubMed] [Google Scholar]
- 12. Wolf AM. (2000) Other feline viral diseases. In: Textbook of veterinary internal medicine, 5th ed. Ettinger SJ, Feldman EC, eds. Philadelphia: WB Saunders Co, 444–453. [Google Scholar]
- 13. Ford RB. (1991) Viral upper respiratory infection in cats. Compendium on Continuing Education for the Practising Veterinarian 13, 593–602. [Google Scholar]
- 14. Pedersen NC. (1988) Feline calicivirus infection. In: Feline infectious diseases. Pedersen NC, ed. Goleta, California: American Veterinary Publications, 61–67. [Google Scholar]
- 15. Church R. (1989) Lameness in kittens after vaccination. Veterinary Record 125, 609. [PubMed] [Google Scholar]
- 16. Dawson S, McArdle F, Bennett D, et al. (1993) Investigation of vaccine reactions and breakdowns after feline calicivirus vaccination. Veterinary Record 132, 346–350. [DOI] [PubMed] [Google Scholar]
- 17. Tenorio AP, Franti CE, Madewell BR, Pedersen NC. (1991) Chronic oral infections of cats and their relationship to persistent oral carriage of feline calici-, immunodeficiency, or leukemia viruses. Veterinary Immunology and Immunopathology 29, 1–14. [DOI] [PubMed] [Google Scholar]
- 18. Bennett D, Gaskell RM, Mills A, Knowles J, Carter S, McArdle F. (1989) Detection of feline calicivirus antigens in the joints of infected cats. Veterinary Record 124, 329–332. [DOI] [PubMed] [Google Scholar]
- 19. Reubel GH, Hoffmann DE, Pedersen NC. (1992) Acute and chronic faucitis of domestic cats. A feline calicivirus-induced disease. Vet Clin North Am (Small Anim Pract) 22, 1347–1360. [DOI] [PubMed] [Google Scholar]
- 20. Dawson S, Bennett D, Carter SD, et al. (1994) Acute arthritis of cats associated with feline calicivirus infection. Res Vet Sci 56, 133–143. [DOI] [PubMed] [Google Scholar]
- 21. Johnson RP, Povey RC. (1985) Vaccination against feline viral rhinotracheitis in kittens with maternally derived feline viral rhinotracheitis antibodies. Journal of the American Veterinary Medical Association 186, 149–152. [PubMed] [Google Scholar]
- 22. Dawson S, Gaskell RM. (1993) Problems with respiratory virus vaccination in cats. Compendium on Continuing Education for the Practising Veterinarian 15, 1347–1354. [Google Scholar]
- 23. Krebs JW, Smith JS, Rupprecht CE, Childs JE. (1998) Rabies surveillance in the United States during 1997. Journal of the American Veterinary Medical Association 213, 1713–1728. [PubMed] [Google Scholar]
- 24. Greene CE, Dreesen DW. (1998) Rabies. In: Infectious diseases of the dog and cat, 2nd ed. Greene CE, ed. Philadelphia: WB Saunders Co, 114–126. [Google Scholar]
- 25. Jenkins SR, Auslander M, Conti L, Johnson RH, Leslie MJ, Sorhage FE. (2000) Compendium of animal rabies prevention and control, 2000. Journal of the American Veterinary Medical Association 216, 338–343. [DOI] [PubMed] [Google Scholar]
- 26. Kass PH, Barnes WG, Spangler WL, Chomel BB, Culbertson MR. (1993) Epidemiologic evidence for a causal relation between vaccination and fibrosarcoma tumorigenesis in cats. Journal of the American Veterinary Medical Association 203, 396–405. [PubMed] [Google Scholar]
- 27. Macy DW, Hendrick MJ. (1996) The potential role of inflammation in the development of postvaccinal sarcomas in cats. Vet Clin North Am Small Animal Pract 26, 103–109. [DOI] [PubMed] [Google Scholar]
- 28. Macy DW, Chretin J. (1999) Local postvaccinal reactions of a recombinant rabies vaccine. Veterinary Forum 16, 44–49. [Google Scholar]
- 29. Levy JK. (2000) FeLV and non-neoplastic FeLV-related disease. In: Textbook of veterinary internal medicine, 5th ed. Ettinger SJ, Feldman EC, eds. Philadelphia: WB Saunders Co, 424–432. [Google Scholar]
- 30. Hoover EA, Olsen RG, Hardy WD, Jr, Schaller JP, Mathes LE. (1976) Feline leukemia virus infection: Age-related variation in response of cats to experimental infection. Journal of the National Cancer Institute 57, 365–369. [DOI] [PubMed] [Google Scholar]
- 31. Sparkes AH. (1997) Feline leukemia virus—a review of immunity and vaccination. Journal of Small Animal Practice 38, 187–194. [DOI] [PubMed] [Google Scholar]
- 32. Edwards D, Elston T, Loar A, et al. (1996) American Association of Feline Practitioners and the Academy of Feline Medicine recommendations for feline leukemia virus testing and recommendations for feline immunodeficiency virus testing. Albuquerque: American Association of Feline Practitioners. [Google Scholar]
- 33. Ford RB, Levy JK. (1994) Infectious diseases of the respiratory tract. In: The cat: diseases and clinical management. Sherding RG, ed. New York: Churchill Livingstone, 489–500. [Google Scholar]
- 34. Sykes JE, Anderson GA, Studdert VP, Browning GF. (1999) Prevalence of feline Chlamydia psittaci and feline herpesvirus 1 in cats with upper respiratory tract disease. Journal of Veterinary Internal Medicine 13, 153–162. [DOI] [PubMed] [Google Scholar]
- 35. Wills J, Howard P, Gruffydd-Jones T, Wathes C. (1988) Prevalence of Chlamydia psittaci in different cat populations in Britain. Journal of Small Animal Practice 29, 327–339. [Google Scholar]
- 36. Starr RM. (1993) Reaction rate in cats vaccinated with a new controlled-titre feline panleukopenia-rhinotracheitis-calicivirus-Chlamydia psittaci vaccine. Cornell Vet 83, 311–323. [PubMed] [Google Scholar]
- 37. Vennema H, Poland A, Hawkins KF, Pedersen NC. (1995) A comparison of the genomes of FECVs and FIPVs and what they tell us about the relationships between feline coronaviruses and their evolution. Feline Practice 23, 40–44. [Google Scholar]
- 38. Vennema H, Poland A, Foley J, Pedersen NC. (1998) Feline infectious peritonitis viruses arise by mutation from endemic feline enteric coronaviruses. Virology 243, 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pedersen NC. (1995) An overview of feline enteric coronavirus and infectious peritonitis virus-infections. Feline Practice 23, 7–20. [Google Scholar]
- 40. Foley JE, Poland A, Carlson J, Pedersen NC. (1997) Patterns of feline coronavirus infection and fecal shedding from cats in multiple-cat environments. Journal of the American Veterinary Medical Association 210, 1307–1312. [PubMed] [Google Scholar]
- 41. Harpold LM, Legendre AM, Kennedy MA, Plummer PJ, Millsaps K, Rohrbach B. (1999) Fecal shedding of feline coronavirus in adult cats and kittens in an Abyssinian cattery. Journal of the American Veterinary Medical Association 51, 948–951. [PubMed] [Google Scholar]
- 42. Foley JE, Pedersen NC. (1996) The inheritance of susceptibility to feline infectious peritonitis in purebred catteries. Feline Practice 24, 14–22. [Google Scholar]
- 43. Gerber JD, Ingersoll JD, Gast AM, et al. (1990) Protection against feline infectious peritonitis by intranasal inoculation of temperature-sensitive FIPV vaccine. Vaccine 8, 536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hoskins JD, Taylor HW, Lomax TL. (1995) Independent evaluation of a modified-live feline infectious peritonitis virus-vaccine under experimental conditions (Louisiana experience). Feline Practice 23, 72–73. [Google Scholar]
- 45. McArdle F, Tennant B, Bennett M, Kelly DF, Gaskell CJ, Gaskell RM. (1995) Independent evaluation of a modified-live FIPV vaccine under experimental conditions (University of Liverpool experience). Feline Practice 23, 67–71. [Google Scholar]
- 46. Scott FW, Corapi WV, Olsen CW. (1995) Independent evaluation of a modified-live FIPV Vaccine under experimental conditions (Cornell experience). Feline Practice 23, 74–76. [Google Scholar]
- 47. Scott FW, Olsen CW, Corapi WV. (1995) Antibody-dependent enhancement of feline infectious peritonitis virus infection. Feline Practice 23, 77–80. [Google Scholar]
- 48. Addie DD, Jarrett O. (1992) A study of naturally occurring feline coronavirus infections in kittens. Veterinary Record 130, 133–137. [DOI] [PubMed] [Google Scholar]
- 49. Moriello KA, DeBoer DJ. (1997) Dermatophytosis: Advances in therapy and control. In: Consultations in feline internal medicine, 3rd ed. August JR, ed. Philadelphia: WB Saunders Co, 177–190. [Google Scholar]
- 50. Coutts AJ, Dawson S, Binns S, Hart CA, Gaskell CJ, Gaskell RM. (1996) Studies on natural transmission of Bordetella bronchiseptica in cats. Veterinary Microbiology 48, 19–27. [DOI] [PubMed] [Google Scholar]
- 51. Welsh RD. (1996) Bordetella bronchiseptica infections in cats. Journal of American Animal Hospital Association 32, 153–158. [DOI] [PubMed] [Google Scholar]
- 52. Speakman AJ, Dawson S, Binns SH, Gaskell CJ, Hart CA, Gaskell RM. (1999) Bordetella bronchiseptica infection in the cat. Journal of Small Animal Practice 40, 252–256. [DOI] [PubMed] [Google Scholar]
- 53. Pedersen NC. (1991) Common infectious diseases of multi-cat environments. In: Feline husbandry: diseases and management in the multi-cat environment. Pratt PW, ed. Goleta, California: American Veterinary Publications, 163–288. [Google Scholar]
- 54. Bergman JE, Vernooij J, Zegers EM. (1997) Prevalence of antibodies against Bordetella bronchiseptica in cats with a history of respiratory disease. Veterinary Quarterly 19, S50–S51. [DOI] [PubMed] [Google Scholar]
- 55. McArdle HC, Dawson S, Couttes AJ, et al. (1994) Seroprevalence and isolation rate of Bordetella bronchiseptica in cats in the UK. Veterinary Record 135, 506–507. [DOI] [PubMed] [Google Scholar]
- 56. Hoskins JD, Williams J, Roy AF, Peters JC, McDonough P. (1998) Isolation and characterization of Bordetella bronchiseptica from cats in southern Louisiana. Veterinary Immunology and Immunopathology 65, 173–176. [DOI] [PubMed] [Google Scholar]
- 57. Binns SH, Dawson S, Speakman AJ, et al. (1999) Prevalence and risk factors for feline Bordetella bronchiseptica infection. Veterinary Record 144, 575–580. [DOI] [PubMed] [Google Scholar]
- 58. Leib MS, Zajac AM. (1999) Giardiasis in dogs and cats. Veterinary Medicine 94, 793. [Google Scholar]
- 59. Zajac AM. (1994) Giardiasis. In: Consultations in feline internal medicine, 2nd ed. August JR, ed. Philadelphia: WB Saunders Co, 83–86. [Google Scholar]
- 60. Zajac AM. (1992) Giardiasis. Compendium on Continuing Education for the Practising Veterinarian 14, 604–611. [Google Scholar]
- 61. Leib MS, Zajac AM. (1995) Giardia: Diagnosis and treatment. In: Current veterinary therapy XII. Bonagura JD, Kirk RW, eds. Philadelphia: WB Saunders Co, 716–720. [Google Scholar]
- 62. Lappin MR. (2000) Protozoal and miscellaneous infections. In: Textbook of veterinary internal medicine, 5th ed. Ettinger SJ, Feldman EC, eds. Philadelphia: WB Saunders Co, 408–417. [Google Scholar]
- 63. Barr SC. (1998) Enteric protozoal infections. In: Infectious diseases of the dog and cat, 2nd ed. Greene CE, ed. Philadelphia: WB Saunders Co, 482–491. [Google Scholar]
- 64. Bowman D. (1994) Feline giardiasis. CFHC Information Bulletin 13, 1–3. [Google Scholar]
- 65. Espeseth DA, Myers TJ. (1999) Authorities and procedures for licensing veterinary biological products in the United States. In: Veterinary vaccines and diagnostics. Schultz RD, ed. San Diego, California: Academic Press, 585–593. [DOI] [PubMed] [Google Scholar]
- 66. Hustead DR. (1999) Why do vaccine labels say the funny things they do? In: Veterinary vaccines and diagnostics. Schultz RD, ed. San Diego, California: Academic Press, 633–641. [DOI] [PubMed] [Google Scholar]
- 67. Baker JA. (1959) Annual report of the Veterinary Virus Research Institute. Ithaca, NY: Cornell University. [Google Scholar]
- 68. Piercy SE. (1961) An appraisal of the value and method of use of living attenuated canine distemper vaccines. Veterinary Record 73, 944–949. [Google Scholar]
- 69. Tizard I, Ni YW. (1998) Use of serologic testing to assess immune status of companion animals. Journal of the American Veterinary Medical Association 213, 54–60. [PubMed] [Google Scholar]
- 70. Tizard I. (1992) Veterinary immunology: an introduction. Philadelphia: WB Saunders Co, 135–150. [Google Scholar]
- 71. Schultz R, Conklin S. (1998) The immune system and vaccines. Compendium on Continuing Education for the Practising Veterinarian 20, 5–8. [Google Scholar]
- 72. Povey RC, Koones H, Hays MB. (1980) Immunogenicity and safety of an inactivated vaccine for the prevention of rhinotracheitis, caliciviral disease, and panleukopenia in cats. Journal of the American Veterinary Medical Association 177, 347–350. [PubMed] [Google Scholar]
- 73. Lappin MR, Jensen W, Andrew J, et al. (2000) Prediction of resistance to panleukopenia, herpesvirus 1 and calicivirus infections utilizing serology. Journal of the American Veterinary Medical Association submitted for publication. [Google Scholar]
- 74. Macy DW. (1994) Vaccination against feline retroviruses. In: Consultations in feline internal medicine, 2nd ed. August JR, ed. Philadelphia: WB Saunders Co, 33–40. [Google Scholar]