Abstract
Non-ocular melanoma is considered to be a rare neoplasm in cats; however, more than 150 cases have been reported in the literature since 1961. The objective of this study was to characterise this tumour better by evaluating case outcome and survival data for cats with melanoma and to compare clinical and histopathological findings with those of previous reports. Twenty-three feline non-ocular melanomas were identified, the most common locations being the nose, digit and pinna. Cats with digital melanomas had survival rates similar to their canine counterparts. Histological assignation of benignity, malignancy or junctional activity was not found to be an accurate predictor of clinical behaviour. Melanoma should be considered as a differential diagnosis for cats presenting with pigmented or non-pigmented masses and histopathology is essential for definitive diagnosis, as other tumours may clinically appear quite similar. Regular follow-up examinations are recommended indefinitely for benign or malignant feline melanomas.
Melanomas are tumours of melanocytes and melanoblasts, which may be benign or malignant in nature. A melanoblast is the precursor cell to the melanocyte, which is the mature, melanin-synthesising dendritic cell located at the epidermal-dermal junction between the cells of the basal layer of the epidermis (Moulton 1990). These tumours are relatively common in dogs, horses and pigs, but occur less frequently in the cow and goat (Moulton 1990).
Although typically considered a rare neoplasm of cats, at least 161 feline dermal melanocytic tumours have been reported in the literature since 1961 (Cotchin 1957, Cotchin 1961, Schmidt et al 1967, Whitehead 1967, Engle & Bradley 1969, Patnaik et al 1975, Macy et al 1981, Patnaik & Mooney 1988, Miller et al 1991, Day et al 1995, Van Der Linde-Sipman et al 1997). In this retrospective study, we provide case outcome and survival data and correlate our findings with those of previous reports in an attempt to understand better the clinical behaviour of this tumour in cats.
Materials and methods
The databases of four veterinary college diagnostic laboratories and/or teaching hospitals in the USA (University of Missouri, Iowa State University, Auburn University, and Tufts University) were searched to identify cases of feline non-ocular melanoma between January 1991 and June 1999. One pathologist (SET) confirmed diagnoses through histological evaluation of specimens. When available, necropsy specimens were also reviewed histologically.
For all confirmed cases, data regarding signalment, presenting complaint, site of origin, tumour staging, histological type, blood work and urinalysis abnormalities, treatment and case outcome were collected by review of medical records and telephone interview of referring veterinarians, when applicable.
Results
Case records and histopathology specimens were reviewed for 25 cats. One case was reclassified as a pigmented basal cell tumour and another could not be confirmed as a melanoma on re-evaluation. Both were eliminated from the study. A diagnosis of feline melanoma was confirmed in 23 cases. A data summary is provided in Table 1. The age of the cats ranged from 2 to 18 years (mean, 10.8; median, 11.5). The majority of cats (n = 16) were female, 12 spayed and four intact. Five cats were neutered males and one was an intact male. The gender of one cat was not recorded in the medical record. Although there was a trend towards a female sex predisposition, this could not be determined, as complete patient population data was not available from all participating sites to confirm a 50:50 sex ratio. Breeds represented included domestic short hair (DSH) (n=12), domestic long hair (DLH) (n=4), Maine coon (n=2), silver tabby (n=1), Siamese (n=1) and unknown (n=3).
Table 1.
Data summary for 23 cats with non-ocular melanoma
| Case | Breed | Sex | Age (years) | Site of origin | Presenting complaint | Benign/malignant | Histologic type | Treatment | Survival (days) | Cause of death | Necropsy | Recurrence or mets | Junctional activity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | DSH | FS | 4 | Ventral to left nares | Dark, pigmented, Firm, solid | Benign | Epithelial | SX | 368 | NA—alive | NA—alive | No | No |
| 2 | Siam | M | 13 | Dorsal neck | Small black tumour, non-tender, fast growing | Benign | Epithelial | SX | LTF@181 | LTF | LTF | No | No |
| 3 | NK | NK | 10 | Right pinna | Acute onset of growth | Benign | Spindle | SX | 564 | NA—alive | NA—alive | No | No |
| 4 | DSH | F | NK | Right dorsolateral lumbar region | Unknown | Benign | Epithelial | SX | 725 | NA—alive | NA—alive | No | Yes |
| 5 | DHC | F | 17 | Back of right ear | Growth came up in last 3–4 months | Benign | Spindle | SX | LTF | LTF | LTF | LTF | Yes |
| 6 | DSH | MC | 6 | Left pinna | Small skin growth noted at time of dental | Benign | Epithelial | SX | 364 | Sudden death unrelated to tumour | Myocardial-endocardial fibrosis | No | Yes |
| 7 | Silver Tabby | FS | 15 | Dorsal muzzle, caudal to nose | Previous diagnosis of apocrine cysts, recurrent | Benign | Epithelial | SX | 1003 | Euthanasia unrelated to tumour | Not done | No | Yes |
| 8 | DLH | FS | 14 | Left front 5th digit | Erythema and alopecia over digit | Benign | Spindle | SX, carboplatin | 356 | Euthanasia related to tumour | Not done | Suspected recurrence and solitary lung met; no histopath confirmation | Yes |
| 9 | DSH | F | 12 | Dorsum of head | Slow-growing black dermal mass | Benign | Spindle | SX | 969 | Euthanasia unrelated to tumour | Not done | No | No |
| 10 | DSH | MC | 11 | Plantar surface of left rear limb | Fast growing subcutaneous mass | Malignant | Spindle | Mid-femoral amputation | 577 | NA–alive | NA—alive | No | Yes |
| 11 | DSH | F | 17 | Digit, left front paw | Non-weight bearing left forelimb, lytic lesion on X-rays, painful | Malignant | Balloon cell | Euthanasia | 0 | Euthanasia related to tumour | Hepatic nodules and uterine polyps | Metastasis to liver | Cannot be determined |
| 12 | DSH | FS | 12 | Right ear margin | Lump on ear | Malignant | Spindle | SX | 422 | Euthanasia related to tumour | Not done | Recurrence and lymph node metastasis | Cannot be determined |
| 13 | DSH | FS | 3 | Palmar surface of left front foot | Owner noticed blood on bed | Malignant | Epithelial | SX | LTF@275 | LTF | LTF | LTF | Yes |
| 14 | DSH | FS | 9.5 | Dorsum, above shoulder | 1 month duration with slow growth | Malignant | Epithelial | SX | 674 | NA—alive | NA—alive | No | Yes |
| 15 | DLH | MC | 11 | Left external nares | Inflammatory lesion of several months duration | Malignant | Epithelial | SX | 40 | Euthanasia | Not taken | No | Yes |
| 16 | DSH | FS | 18 | Lateral thorax | Large mass on thorax | Malignant | Spindle | SX | 16 | NA—alive | NA—alive | No | Yes |
| 17 | DSH | FS | 18 | Nasal planum | Rapidly growing and bleeding mass | Malignant | Spindle | SX | LTF@205 | LTF | LTF | Local recurrence at 4 and 6 months | Yes |
| 18 | Maine coon | FS | 2 | Right lateral thigh | Recurrent after excision | Malignant | Epithelial | SX, RTX d0,7,21, doxorubicin, carboplatin, melphalan | 122 | Euthanasia—progressive dz | Not done | Multiple skin masses and inguinal lymphadenopathy | No |
| 19 | DLH | FS | 12 | Right forepaw large pad | Recurrent after sx, prescap lymph node metastasis, anorexia, lethargy | Malignant | Epithelial | SX originally, carboplatin approx. 140 d after sx | Approx. 150 | Euthanasia—progressive dz | Mets to prescap In, liver, spleen, no evidence of dz on foot pad | Mets to prescap In, liver, spleen, recurrent after original sx | No |
| 20 | DLH | FS | 10 | Oral mass-maxillary | Recurrence and pulmonary metastasis | Malignant | Epithelial | SX originally, carboplatin approx 195 d after sx; Taxol® | Approx. 365 | Progressive dz | Not done | Multiple skin and lung masses, palpable renal masses, midabd mass palpable | Yes |
| 21 | NK | MC | 7 | Unknown skin location | Skin mass found during grooming | Benign | Epithelial | SX | Alive | NA—alive | NA—alive | No | Yes |
| 22 | Maine coon | FS | 12 | Oral mass-maxillary | Mass detected at routine dental prophylaxis | Benign | Epithelial | Radiation therapy, chemotherapy pending | Alive | NA—alive | NA—alive | No | No |
| 23 | DSH | MC | 10 | Oral mass-maxillary | Mass detected at routine dental prophylaxis | Malignant | Spindle | Radiation therapy | Alive | NA—alive | NA—alive | Large recurrent/progressive mass | Yes |
DSH, domestic short hair. DLH, domestic long hair. Siam, Siamese. NK, not known. F, female. M, male. LTF, lost to follow-up. NA, not applicable. SX, surgery
In 16 cases, cats were presented for mass evaluation. One was presented for non-weight bearing lameness, and tumours in three other cats were detected during routine examination prior to dental prophylaxis. Presenting complaint of three cases was not recorded in the medical record.
Gross features of the lesions included dark pigmentation (n=2), haemorrhage (n=3), ulceration (n=1), erythema and alopecia (n=1), and bone lysis (n=1). Gross features were not recorded for the remaining cases.
All cases in the present study were classified as either dermal or oral in location. Ocular melanoma was excluded. Sites of origin included the digit (n=5), pinna (n=5), nose (n=4), oral cavity (n=3), dosum of head (n=1), dorsum of neck (n=1), shoulder (n=1), lateral thorax (n=1), hip (n=1) and unknown skin location (n=1).
Serum biochemistry profile results were available for 11 cats, complete blood count for 10, and urinalysis for four. No single abnormality was found to occur in all cases.
Two cats (3 and 9) were additionally diagnosed with diabetes mellitus. Cat 9 was diagnosed at the time of melanoma diagnosis and cat 3 was diagnosed 1 year after melanoma diagnosis. Cat 16 had concurrent squamous cell carcinoma overlying the elbow joint. Cat 12 had a previous diagnosis of malignant fibrohistiocytoma of the skin overlying the hock joint. Cat 23 had a mandibulectomy performed 46 months earlier as treatment for osteoma.
Treatment included surgical excision alone (n=16), radiation therapy alone (n=1), surgery and chemotherapy (n=3), radiation therapy and chemotherapy (n=1) with chemotherapy in progress at the time of this writing, surgery, radiation therapy, and chemotherapy (n=1), and euthanasia (n=1).
Histological evaluation resulted in classification of 11 benign and 12 malignant tumours. The benign tumours ranged from 0.1 to 0.6 cm and the malignant tumours exceeded 0.6 cm in size. Size was not recorded for two malignant type tumours. The melanomas were of three histological types: epithelioid, spindle and balloon. No signet-ring types were recorded and classification was based upon predominant histological type.
Epithelioid tumours
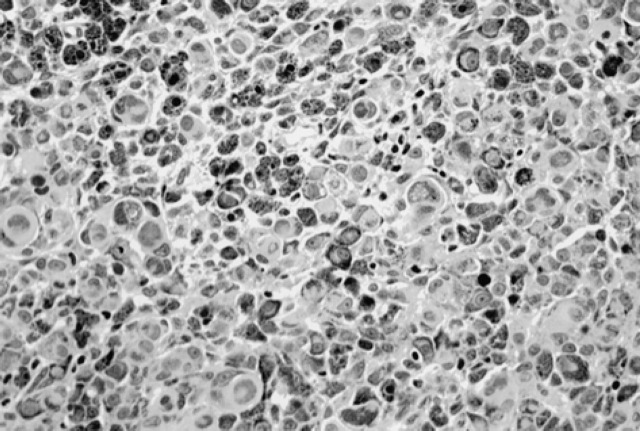
Epithelioid tumours (n=13) had rounded cells, round to oval nuclei, variably distinct nucleoli and moderate to abundant amounts of eosinophilic cytoplasm. Six of the epithelioid tumours were malignant and seven were benign. Four of the malignant epithelioid tumours were sparsely pigmented. Malignant epithelioid tumours exhibited greater nuclear pleomorphism with moderate to marked anisokaryosis, prominent nucleoli and high mitotic index (Fig 1).
Fig 1.
Epithelioid tumour.
One of the six cats with a malignant epithelioid tumour had confirmed metastasis while two had suspected metastasis. Radicality of surgeries performed for these cases was not available to the authors. Cat 18 had recurrence 6 weeks after initial local excision with three separate secondary masses. Inguinal lymph node metastasis was also suspected at the time of death 4 months after initial surgery but confirmation was not obtained. Cat 19 presented due to recurrence of the original mass and lymph node metastasis 4 months after initial local excision. Metastatic disease in the lymph node, liver and spleen were confirmed on necropsy 5 months after initial excision. Cat 20 had an oral mass that had local recurrence and pulmonary nodules 6 months after non-radical excision. Twelve months post excision, multiple skin, pulmonary and renal masses were present, as well as a mid-abdominal mass. None of these masses were histopathologically or cytologically evaluated to confirm metastasis; however, none of these lesions were present prior to treatment.
Spindle tumours
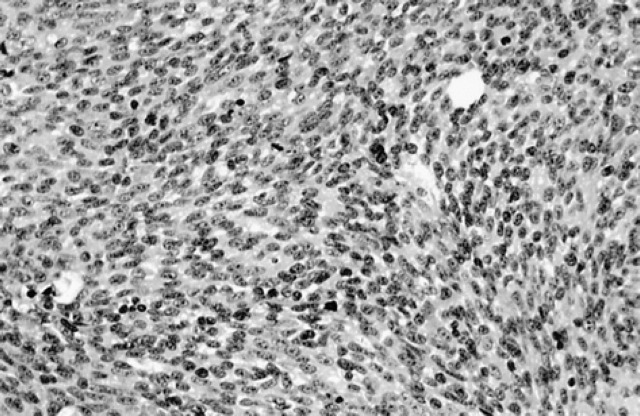
Spindle tumours (n=9) were composed of spindle-shaped cells usually arranged in nests or cords. Five of these tumours were histologically malignant and contained scant amounts of melanin pigment (Fig 2).
Fig 2.
Spindle tumour.
Four of the spindle tumours had recurrence and/or metastasis. Three of these were histologically classified as malignant and one as benign. Cat 12 had metastasis to a regional lymph node. Surgical excision of this tumour had appeared complete in the initial biopsy specimen. One tumour (cat 17) recurred twice. At the first recurrence, 4 months after diagnosis, the lesion was surgically excised again. A second recurrence was noted 2 months later. Surgical excision of this tumour was incomplete both times. Cat 23 also had a recurrence of the malignant spindle tumour. One of the benign tumours (cat 8) had suspected recurrence and a solitary lung metastasis, neither of which were confirmed histologically leaving the possibility of another tumour type with metastasis or other primary lung tumour.
Balloon tumour
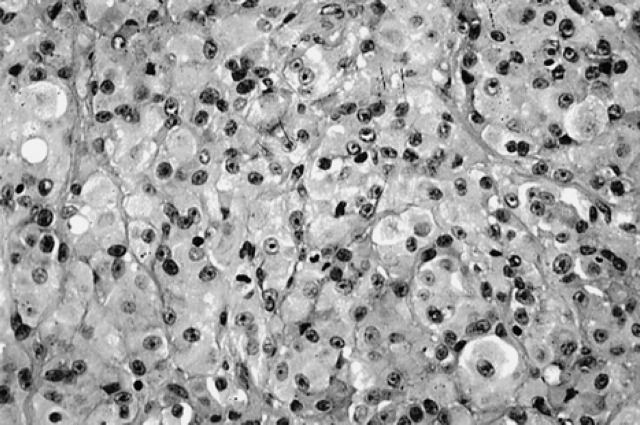
One balloon type tumour was diagnosed. The tumour was malignant and had rounded to polygonal cells with marked nuclear pleomorphism, abundant eosinophilic cytoplasm, a few multinucleated giant cells and rare pigment granules. The cells were subdivided into nests by fine fibrous connective tissue septa (Fig 3).
Fig 3.
Balloon tumour.
Radiographs at the time of presentation showed bony lysis of a digit. Metastatic lesions were present in the liver at necropsy.
Histologic appearance and clinical behaviour
The mitotic rate of the benign tumours ranged from 0 to 3 mitotic figures per 10 high power fields, while the malignant tumours ranged from 11 to 73 mitoses per 10 high power fields. Two of the benign tumours were too heavily pigmented for mitotic rate determination. Junctional activity, defined as proliferation of tumour cells along the dermoepidermal junction, was noted in six of the 11 benign tumours. It was noted in eight of 12 malignant tumours, could not be assessed in two and was not present in two cases.
Tumour recurrence at the primary site was noted in seven of the cats, all within 9 months of initial presentation. Three cats had confirmed metastasis. Of these, one had a malignant balloon cell tumour, one a malignant spindle cell tumour, and one a malignant epithelioid tumour. One benign spindle cell tumour and two malignant epithelioid tumours had evidence of metastasis without confirmation. Two other malignant spindle cell tumours had local recurrence only. Metastatic sites included the lungs, liver, regional and distant lymph nodes, integument, muscle fascia and spleen.
Melanoma of the digit was diagnosed in five of our cases. At the time of publication for this study, one case was free of disease, three had been euthanised because of disease progression and one was lost to follow-up. Cat 8 was treated with digital amputation, had suspected recurrence and possible lung metastasis, and was euthanised 356 days after initial diagnosis with progressive disease. Cat 10 had a mid-femoral amputation and later had suspected metastases to the lumbar vertebrae and lungs. The cat, however, is alive with a 577+ day survival time. Cat 11 was euthanised at presentation due to non-weight bearing lameness of the left front limb. Radiographic evidence of bone lysis was present. The fourth cat with digital melanoma (13) was lost to follow-up at 275 days. Cat 19 had tumour recurrence and prescapular lymph node metastasis. The tumour was resected a second time and treated with carboplatin. Euthanasia was performed approximately 150 days after initial diagnosis due to progressive disease. Metastasis was confirmed to the prescapular lymph node, liver and spleen. No evidence of disease was noted at the original tumour site at the time of death.
Three cases of oral melanoma, all originating from the maxilla, were identified in our study. Two of three were classified as malignant. Cat 20 had pulmonary metastasis at the time of presentation to the referral institution. Surgery was initially performed, followed by chemotherapy. Survival time was 365 days. Cat 22 was treated with radiation therapy and was to be followed with chemotherapy. Cat 23 was treated with radiation therapy alone and had a progressive mass in spite of therapy. The metastatic potential of feline oral melanomas cannot be adequately evaluated in this study due to the early stage of the disease in two of the cases; however, one cat had metastasis and another had evidence of local progressive disease. Survival times ranged from 76–365 days. Median survival time has not been reached, as two of the cats were still alive at the time of publication.
Chemotherapy was administered in four cats. Agents used included carboplatin (134–210 mg/m2 iv), melphalan (2 mg/m2/day × 10 days PO), doxorubicin (25 mg/m2 iv), and Taxol® (80 mg/m2 iv). Disease stabilisation occurred in two cats; however, progressive disease occurred 170 and 72 days following the onset of therapy.
Discussion
Feline melanoma is typically considered a rare neoplasm in cats. It is important, however, for the veterinary practitioner to recognise its existence and include it in the differentials for feline neoplasia. The data from the present study provides information concerning diagnosis and prognosis for non-ocular melanoma in cats. The tumour typically presents in older cats, although in this study cats as young as 2 years presented with melanoma. A sex predisposition trend towards female cats was found; however, a predisposition could not be confirmed without complete patient population information for all sites participating in the study to ensure a 50:50 sex ratio.
In the present study, presenting complaint and gross features were associated with a mass of variable appearance. While the most common sites were the nose, digits and pinna, other sites were noted. This site distribution is consistent with other studies of feline neoplasms (Holzworth 1987, Patnaik & Mooney 1988).
The pinna, nose and eyelids are also common sites for squamous cell carcinoma (SCC) in cats (Ogilvie & Moore 1995, Vail & Withrow 1996). These areas lack cutaneous pigment and SCC is often sunlight-induced in skin with little or no pigment. A definitive link between sun exposure and development of cutaneous melanomas has not been proven in cats. Holzworth (1987) suggested a possible predisposition to melanoma in cats with hair in the sex-linked colour spectrum of red, black, blue and tricolour. The present study did not provide data that would substantiate or refute a colour pattern predisposition. Due to the similar site predisposition for SCC and melanoma, histopathology is necessary to differentiate the two.
Basal cell tumours originating from basal cells of the epidermis, hair follicles, sweat glands or sebaceous glands are most commonly located on the head, neck and shoulders of dogs and cats, with a more common occurrence in cats (Ogilvie & Moore 1995, Vail & Withrow 1996). As these tumours can be pigmented, they may be misdiagnosed as melanomas. Basal cell tumours may have a relatively high mitotic rate for benign tumours. It is important to distinguish between basal cell tumours and melanoma histologically, as most basal cell tumours are benign and slow growing with rare recurrence or metastasis and favourable long-term prognosis. However, according to Miller et al (1991), up to 10% of basal cell tumours are carcinomas.
For lesions located on the digit, amelanotic melanomas are the primary differential (Yager & Wilcock 1994) although a diagnosis of plasmacytoma should also be considered. Plasmacytomas are solitary round cell tumours that are well circumscribed and are non-encapsulated and rarely occur in the cat. They can have strong criteria for malignancy in spite of a usual benign course of disease, including mononuclear giant cells, a high mitotic index and marked cellular pleomorphism. Areas most commonly affected include the digits, oral cavity, and ear canal (Yager & Wilcock 1994).
In dogs, up to half of subungual melanomas develop distant metastasis (Aronsohn & Carpenter 1990, Brewer 1999). Patnaik & Mooney (1988) reported two cases, one with evidence of recurrence and metastasis to the lymph nodes, lungs and liver and the second resected with no evidence of disease 365 days after surgery. Based on the finding of confirmed metastatic disease in two of five cases and suspected in one other case reported here and those reported previously (Patnaik & Mooney 1988) the tumour appears to have similar behaviour in cats.
The clinical behaviour of pinnal melanomas in cats has been disputed in the literature. While some authors have suggested that these are benign lesions (Holzworth 1987, Goldschmidt & Shofer 1992), Miller et al (1991) reported that they can exhibit malignant behaviour. In the present study, cat 12 had a pinnal melanoma with tumour recurrence and metastasis, supporting the conclusions of Miller et al (1991).
Also of interest is an apparent discrepancy between histological findings and clinical behaviour. Holzworth (1987) observed that microscopic evidence of malignancy did not necessarily predict clinical behaviour. Conversely, two other reports indicated a strong correlation between histological characteristics and clinical behaviour (Goldschmidt & Shofer 1992, Miller et al 1993). One of seven histologically benign tumours with complete follow-up in this study had suspected recurrence and metastasis, however, we cannot use this information for comparison as this was not confirmed histologically. For 10 malignant tumours with complete follow-up, six had recurrence and/or metastasis. Therefore, histological determination of benign or malignant classification should be regarded as helpful, but not an entirely accurate predictor of clinical behaviour.
Epithelioid type tumours have been reported more likely to be malignant and spindle cell type more likely to be benign (Goldschmidt et al 1993). In that study, 80% of the epithelioid tumours were malignant while 71% of the spindle cell tumours were benign. A retrospective study performed by Miller et al (1993) disagrees with the findings of Goldschmidt as only 32% of epithelioid tumours were malignant and 50% of spindle cell tumours were benign. Our study correlates with Miller in that six of 13 epithelioid tumours have been characterised as malignant and four of nine spindle cells as benign. Malignancy is considered unrelated to predominant cell type in canine cutaneous melanomas according to Yager & Scott (1993) with which the findings of both Miller and the current study agree.
Although previous reports indicate that junctional activity is predictive of benign behaviour (Goldschmidt & Shofer 1992, Miller et al 1993, Thomas & Fox 1998), eight of 12 cases of malignant melanoma were characterised by junctional activity while six of 11 benign tumours had junctional activity in our study. Four of the cases with junctional activity exhibited clinical recurrence or metastasis. Therefore, this study suggests that junctional activity does not predict clinical behaviour. According to Moulton (1990), in humans, junctional activity, or location of the tumour cells at the epidermal-dermal location, occurs in melanocytomas or benign tumours initially, and as these tumours grow and mature the cells migrate to a more dermal location. However, some benign melanomas can arise from the dermis itself and have no junctional activity. Malignant melanomas can arise from normal melanocytes in the epidermis or by transformation from benign melanomas of either junctional or dermal location (Moulton 1990). Discerning benign from malignant melanoma can be difficult because malignant as well as benign tumours can arise from the epidermaldermal junction. Also, malignant transformation of benign tumours can occur, and if they do so early in the disease process, it may be difficult to assign histopathological behaviour. Because of these difficulties, it is necessary to consider all general indicators of malignancy before assigning this behaviour (Moulton 1990).
Wide surgical excision has been suggested for treatment of non-ocular melanoma in both dogs and cats (Weinstock et al 1993). Monitoring for metastasis is crucial, as surgery will only control localised disease. The literature suggests that aggressive surgery (Vernon & Helphrey 1983, Withrow & Holmberg 1983) or large doseper-fraction protocols of radiation therapy (Overgaard et al 1985, Meleo 1997) are treatment options in dogs (King et al 1997). No studies to date have critically evaluated the response of feline melanomas to treatment. Based on the findings in our limited number of cases, feline non-ocular melanomas seem to respond much like canine melanomas and have similar biological tendencies to their canine counterparts. Surgery, radiation therapy and chemotherapy are treatment options. With the high potential for metastatic disease, chemotherapy would seem a viable option, although studies in dogs evaluating mitoxantrone (Ogilvie et al 1991), doxorubicin (Ogilvie et al 1989), melphalan (Page et al 1991) and cisplatin (Kitchell 1994), have shown that complete remissions are rare (Thomas et al 1998). Partial remission or disease stabilisation is more likely (Thomas et al 1998). Treatment modalities are dependent upon the extent of tumour spread and invasion. It is important to consider the nature of the melanoma (benign vs malignant), as well as tumour location (ie digit, pinna, oral cavity etc) when determining treatment recommendations as surgical capabilities may be limited due to the location.
Metastatic rates for cutaneous melanomas in cats have been reported to range from 5 to 25% (Holzworth 1987, Patnaik & Mooney 1988). Of the cats for which follow-up information was available in this study (n=19), three had confirmed metastatic disease, three had suspected metastatic disease, and two others had recurrent, progressive disease without evidence of metastasis. Our findings regarding metastatic potential are similar to those of Patnaik & Mooney (1988).
In the dog, melanoma is the most common malignant oral tumour (Conroy 1967, Bostock 1979, Patnaik & Mooney 1988). Oral melanomas are uncommon in the cat and carry a guarded prognosis much like their canine counterpart (Patnaik & Mooney 1988). In the study conducted by Patnaik & Mooney (1988), five cats were diagnosed with oral melanomas. The mean survival time for four cats was 61 days (range, 1–135 days) and 80% had documented metastasis. Three cases were diagnosed in our study with an undetermined mean survival time as two are still alive. However, one case died of progressive disease with recurrence and suspected metastasis and another had progressive disease.
Conclusion
Non-ocular melanoma should be considered as a differential diagnosis for cats presenting with pigmented or non-pigmented masses, especially on the pinnae, digits and nares or the oral cavity. Histopathology is essential for differentiating this tumour from SCC, pigmented basal cell tumours and plasmacytomas as well as for discerning benign from malignant lesions. Although surgical excision provided long survival times for several of the cats in this study, recurrence or metastasis was known or suspected in eight cats, including one with an initial diagnosis of benign melanoma. Predominant cell type and junctional activity were not found to be predictors of clinical behaviour. As metastasis may occur a year or longer after initial diagnosis, regular follow-up examinations are recommended. The efficacy of chemotherapy, radiation therapy and immunotherapy is unknown for this tumour in cats but with the potential for recurrence and metastasis, further clinical investigation is warranted in order to determine the best treatment modalities for feline melanomas.
References
- Aronsohn MG, Carpenter JL. (1990) Distal extremity melanocytic nevi and malignant melanomas in dogs. Journal American Animal Hospital Association 26, 605–612. [Google Scholar]
- Bostock DE. (1979) Prognosis after surgical excision of canine melanomas. Veterinary Pathology 16, 32–40. [DOI] [PubMed] [Google Scholar]
- Brewer WG. Personal Communication, VCOG study coordinator for subungual neoplasms project, Auburn University, AL, USA. June 7, 1999. [Google Scholar]
- Conroy JD. (1967) Melanocytic tumors of domestic animals with special reference to dogs. Archives of Dermatology 96, 372–380. [PubMed] [Google Scholar]
- Cotchin E. (1957) Neoplasia in the cat. The Veterinary Record 69, 425–434. [Google Scholar]
- Cotchin E. (1961) Skin tumours of cats. Research Veterinary Science 2, 353–361. [Google Scholar]
- Day MY, Lucke VM. (1995) Melanocytic neoplasia in the cat. Journal of Small Animal Practice 36, 207–213. [DOI] [PubMed] [Google Scholar]
- Engle GC, Brodey RS. (1969) A retrospective study of 395 feline neoplasms. Journal American Animal Hospital Association 5, 21–31. [Google Scholar]
- Goldschmidt MH, Shofer FS. (1992) Melanomas. In: Skin Tumours in the Dog and Cat. Pergamon Press, Oxford, pp 131–141. [Google Scholar]
- Goldschmidt MH, Liu SM, Shofer FS. (1993) Feline dermal melanomas. A retrospective study. In: Advances in Veterinary Dermatology Vol II, Ihrke PJ, Mason IS, White SD. (eds). Baillière Tindall, London, pp 285–291. [Google Scholar]
- Holzworth J. (1987) Diseases of the Cat: Medicine and Surgery. W B Saunders Company, Philadelphia, pp 579–583. [Google Scholar]
- King GK, Bergman PJ, Harris D. (1997) Radiation oncology of head and neck tumours. In: The Veterinary Clinics of North America Small Animal Practice Vol 27, Burk RL, King GK. (eds), pp 101–113. [DOI] [PubMed] [Google Scholar]
- Kitchell BE, Brown DM, Luck EE, Woods LL, Orenberg EK, Block DA. (1994) Intralesional implant for treatment of primary oral malignant melanoma in dogs. Journal of the American Veterinary Medical Association 204, 229–236. [PubMed] [Google Scholar]
- Macy DW, Reynolds HA. (1981) The incidence, characteristics and clinical management of skin tumours of cats. Journal of American Animal Hospital Association 17, 1026–1032. [Google Scholar]
- Meleo KA. (1997) Tumors of the skin and associated structures. In: The Veterinary Clinics of North America Small Animal Practice. Vol 27. Burk RL, King GK. (eds), pp 73–94. [DOI] [PubMed] [Google Scholar]
- Miller MA, Nelson SL, Turk JR, Pace LW, Brown TP, Shaw DP, Fischer JR, Gosser HS. (1991) Cutaneous neoplasia in 340 cats. Veterinary Pathology 28, 389–395. [DOI] [PubMed] [Google Scholar]
- Miller WH, Scott DW, Anderson WI. (1993) Feline cutaneous melanocytic neoplasms: A retrospective analysis of 43 cases (1979–1991). Veterinary Dermatology 4, 19–26. [Google Scholar]
- Moulton JE. (1990) Tumours in Domestic Animals, 3rd edn. University of California Press, Berkeley, USA, pp 75–82. [Google Scholar]
- Ogilvie GK, Moore AS. (1995) Managing the Veterinary Cancer Patient. Veterinary Learning Systems, Trenton, NJ, USA. pp 492–493. [Google Scholar]
- Ogilvie GK, Obradovich JE, Elmslie RE, et al. (1991) Efficacy of mitoxantrone against various neoplasms in dogs. Journal of the American Veterinary Medical Association 198, 1618–1621. [PubMed] [Google Scholar]
- Ogilvie GK, Reynolds HA, Richardson RC, et al. (1989) Phase II evaluation of doxorubicin for treatment of various canine neoplasms. Journal of the American Veterinary Medical Association 195, 1580–1583. [PubMed] [Google Scholar]
- Overgaard J, von der Maase H, Overgaard M. (1985) A randomized study comparing two high dose per fraction radiation schedules in recurrent or metastatic malignant melanoma. International Journal of Radiation Oncology, Biology and Physiology 11, 1837–1839. [DOI] [PubMed] [Google Scholar]
- Page RL, Thrall DE, Dewhirst MW, et al. (1991) Phase I study of melphalan alone and melphalan plus whole body hyperthermia in dogs with malignant melanoma. International Journal of Hyperthermia 7, 559–566. [DOI] [PubMed] [Google Scholar]
- Patnaik AK, Liu SK, Hurvitz AI, et al. (1975) Nonhematopoietic neoplasms in the cat. Journal of National Cancer Institute 54, 855–860. [PubMed] [Google Scholar]
- Patnaik AK, Mooney S. (1988) Feline melanoma: A comparative study of ocular, oral and dermal neoplasms. Veterinary Pathology 25, 105–112. [DOI] [PubMed] [Google Scholar]
- Schmidt RE, Langham RF. (1967) A survey of feline neoplasms. Journal of American Veterinary Medical Associations 151, 1325–1328. [Google Scholar]
- Stebbins KE, Morse CE, Goldschmidt MH. (1989) Feline oral neoplasia: A 10 year study. Veterinary Pathology 23, 121–128. [DOI] [PubMed] [Google Scholar]
- Thomas RC, Fox LE. (1998) Tumors of the skin and subcutis. In: Cancer in Dogs and Cats: Medical and Surgical Management. Morrison WB. (ed). Williams and Wilkins, Baltimore, USA, pp 499–500. [Google Scholar]
- Van der Linde-Sipman JS, de Wit MML, van Garderen E, Molenbeek RF, van der Velde-Zimmerman D, de Weger RA. (1997) Cutaneous malignant melanomas in 57 cats: Identification of signet-ring and balloon cell types and verification of their origin by immunohistochemistry, electron microscopy and in situ hybridization. Veterinary Pathology 34, 31–38. [DOI] [PubMed] [Google Scholar]
- Vail DM, Withrow SJ. (1996) Tumours of the skin and subcutaneous tissues. Small Animal Clinical Oncology. (2nd edn). W B Saunders Company, Philadelphia, USA, pp 183–185. [Google Scholar]
- Vernon FF, Helphrey M. (1983) Rostral mandibulectomy: Three case reports in dogs. Veterinary Surgery 12, 26–29. [Google Scholar]
- Weinstock MA, Clark JW, Calabresi P. (1993) Melanoma. In: Medical Oncology, (2nd edn). Calabresi P, Sehein PS. (eds). McGraw-Hill, St. Louis, USA, pp 545–563. [Google Scholar]
- Whitehead JE. (1967) Neoplasia in the cat. Veterinary Medicine Small Animal Clinics 62, 357–359. [PubMed] [Google Scholar]
- Withrow SJ, Holmberg DL. (1983) Mandibulectomy in the treatment of oral cancer. Journal of the American Animal Hospital Association 19, 273–286. [Google Scholar]
- Yager FA, Scott DW. (1993) The skin and appendages. In: Pathology of Domestic Animals, Vol 1, (4th edn). Jubb KVF, Kennedy PC, Palmer N. (eds). Academic Press, London, UK, pp 719–722. [Google Scholar]
- Yager JA, Wilcock BP. (1994) Surgical pathology of the dog and cat: dermatopathology and skin tumors. Mosby Yearbook Ltd, London, UK, pp 304–307. [Google Scholar]