Abstract
The aims of our study were to determine a reference range for plasma leptin in healthy, normal-weight cats and to measure the effect of weight gain on plasma leptin levels. To increase our understanding of the association between leptin and feline obesity, we investigated the relationship between plasma leptin and measures of adiposity in cats. Twenty-six normal-weight cats were used to determine the reference range for feline leptin using a multispecies radioimmunoassay. In the second part of the study, plasma leptin concentrations were determined in 16 cats before and after approximately 10 months of spontaneous weight gain. Dual energy X-ray absorptiometry scans (DEXA) were performed after weight gain. The tolerance interval for plasma leptin concentrations was 0.92–11.9 ng/ml Human Equivalent (HE) with a mean concentration of 6.41 ± 2.19 ng/ml HE. In part two of the study, 16 cats gained on average 44.2% bodyweight over 10 months. The percentage of body fat in obese cats ranged from 34.2 to 48.7%. Mean plasma leptin concentrations increased from 7.88 ± 4.02 ng/ml HE before weight gain to 24.5 ± 12.1 ng/ml HE after weight gain, (P < 0.001). Total body fat and body fat per cent were the strongest predictors of plasma leptin in obese cats (r = 0.8 and r = 0.78, P < 0.001, respectively). In conclusion, plasma leptin concentrations increased three-fold in cats as a result of weight gain and were strongly correlated with the amount of adipose tissue present. Despite elevated leptin levels, cats continued to eat and gain weight, suggesting decreased sensitivity to leptin. This investigation into the biology of leptin in cats may aid the overall understanding of the role of leptin and the development of future treatments to help prevent and manage feline obesity.
Introduction
Regulation of food intake involves a complex balance between long-term control of fat mass and short-term control of individual meals. The central nervous system determines long-term food intake and energy expenditure in response to afferent signals including leptin, insulin and the adrenal hormones, which are secreted in response to the size of fat stores (Kaiyala et al 1995).
The product of the ob gene leptin, is a hormone secreted exclusively by white adipose tissue (Zhang et al 1994). When administered to mice, leptin is known to induce loss of weight and fat tissue by reducing food intake and increasing energy expenditure (Campfield et al 1995, Halaas 1995, Pellymounter et al 1995). Ob/ob mice which lack endogenous leptin and db/db mice which lack functioning leptin receptors become massively obese (Zhang et al 1994, Chen et al 1996, Lee et al 1996). Both the expression of the ob gene and/or circulating leptin levels correlate with body fat content in rodents and humans (Lönnqvist et al 1995, Maffei et al 1995, Masuzaki 1995, Considine et al 1996). Leptin concentrations are higher in obese compared with normal-weight individuals (Maffei et al 1995, Considine et al 1996, Havel et al 1996b) and so, it is postulated that the role of leptin is to inform the brain of the mass of stored adipose reserves. Although leptin is secreted in proportion to the total amount of body fat, synthesis may be stimulated by increased insulin and glucocorticoid concentrations (Cusin et al 1995, Meier 1995). After release into the circulation, leptin is transported through the blood brain barrier via a saturable transport system (Tartaglia et al 1995). Evidence suggests that the hypothalamus may be a critical site of action for the central appetite-controlling effects of leptin. Neuropeptide-Y and/or corticotrophin-releasing hormone have been implicated as mediators of leptin's actions (Campfield et al 1995, Stephens et al 1995, Schwartz & Seeley 1997). Hypothalamic neuropeptide-Y is a potent stimulator of food intake and inhibitor of energy expenditure and leptin has been shown to decrease neuropeptide-Y synthesis and release in normal animals (Stephens et al 1995, Campfield et al 1996). In contrast, corticotrophin-releasing hormone inhibits food intake and increases energy expenditure (Rothwell 1989). Leptin has been shown to act in the central nervous system to stimulate corticotrophin-releasing hormone gene expression (Schwartz et al 1996b).
Leptin receptors are not present in the hypothalamus alone, but are also widely expressed in peripheral tissues including adipocytes, skeletal muscle, hepatocytes and pancreatic beta-cells, suggesting a broader role for the hormone (Tartaglia et al 1995, Chen et al 1996, Kieffer et al 1996, Lee 1996, Scarpace & Matheny 1998). One mechanism by which leptin acts to regulate fat mass, is by increasing energy expenditure. Current data suggest that the ability of leptin to increase energy expenditure may involve uncoupling mitochondrial oxidative metabolism in brown adipose tissue and perhaps in white adipose tissue and skeletal muscle (Scarpace et al 1997, Boss et al 1998, Scarpace & Matheny 1998). Uncoupling proteins are able to partially uncouple mitochondrial respiration, releasing ingested energy which is excess to the body's needs as heat, rather than capturing it as high energy ATP and depositing it as fat (Klingenberg 1990). This acts like a safety valve, helping to prevent accumulation of excess energy in the body.
There has been little work reported on leptin in cats. Recently, a leptin radioimmunoassay was validated for use in cats (Backus et al 2000). The study found a relationship between plasma leptin and body fat as determined using the isotopic dilution method in 19 intact male cats with a range of body weights (Backus et al 2000). It did not examine the effect of increasing body weight on leptin concentrations in the same cats, nor did the study include female cats, neutered cats or extremely obese cats.
The aims of our study were to report reference values for leptin, and to examine its association with adiposity in cats. To determine reference values, we measured leptin concentrations in 26 neutered, mixed-gender cats, with a normal or ‘ideal’ body condition, selected from the general population. To further our understanding of the association between leptin and feline obesity, we examined the magnitude of the leptin increase that occurred after 16 cats spontaneously gained weight over 10 months. Our study also investigated the relationship between plasma leptin concentrations and simple measures of adiposity determined in cats before and after weight gain, as well as with DEXA-derived body composition measurements in overweight and obese cats. The effect of gender on leptin concentrations was also examined.
Materials and methods
Animals
The study was divided into two parts. Twenty-six cats were used in part one, 16 of which were randomly selected to participate in the second part of the study. All cats were neutered and assessed as healthy by clinical examination and routine haematological and serum biochemical analyses. Accurate ages of the cats were unknown; however, all were estimated by visual assessment and examination of dentition to be young adults between 1 and 5 years of age. The protocol for this study and the care and handling of these animals were approved by the Animal Experimentation Ethics Committee of the University of Queensland.
In the first part of the study, 26 healthy cats (11 males and 15 females) were used to determine the reference range for plasma leptin. Body-weight was measured in all cats using scales with a precision of 0.1 kg. Body mass index was calculated using the formula:
Bodyweight (kg)/(body length (m)×height (m))
(Nelson et al 1990). Body condition scores were determined using a scale from one to five (Sunvold & Bouchard 1998). All cats were considered to have a normal or ‘ideal’ body weight based on having a body condition score of three (Sunvold & Bouchard 1998). For a minimum of 2 weeks prior to commencement of testing, cats were individually housed and acclimatised to the test holding facilities. After an overnight fast, jugular catheters (18 g×8 cm polyurethane jugular catheters; Cook Veterinary products) were placed under general anaesthesia with propofol (Diprivan, Zeneca Limited). Blood was immediately drawn from each cat via the catheter and submitted for routine haematology and biochemistry profiles. Cats were allowed to recover and were fed. A minimum of 24 h after anesthesia, and after a 12-h fast, blood samples were drawn via the catheter for plasma leptin determination.
In the second part of the study, body weight, body mass index and body condition scores were determined in 16 healthy cats (6 males and 10 females). General methods for housing and sample collection were identical to part one of the study. Routine haematology and biochemistry analyses were performed and baseline plasma leptin concentrations were determined as described above. Cats were then transferred to a group housing facility and offered two high energy density extruded foods ad libitum (450 and 490 kcal/100g metabolisable energy), for a period of approximately 10 months, to promote weight gain. After weight gain, the cats were transferred back to individual housing and acclimatised to the test holding facilities for a minimum of four weeks. Routine biochemical and haematological analyses were repeated. Body weights, body mass indexes, body condition scores and basal plasma leptin concentrations were determined in cats after weight gain. In addition, lean body mass, fat mass and percentage of body fat were measured after weight gain by DEXA (Dual Energy X-ray Absorptiometry). A fan beam X-ray bone densometer (Hologic QDR-4500A; Hologic Inc.) with human adult software version 9.1 was used. Whole-body scans were performed, with cats placed in ventrodorsal recumbency using a technique previously validated for dogs and cats (Sunvold & Bouchard 1998). The adiposity ratio was defined as the ratio between total body fat and total lean tissue (fat-to-lean ratio) (Walton et al 1995). Cats were classified overweight or obese based on having a body condition score of 4 or 5 respectively, and a DEXA-derived percentage fat content above an arbitrary cut-off point of 25% (Butterwick et al 1994).
Sample handling and analysis
Samples from both parts of the study were handled similarly. Blood samples were placed into sterile EDTA vacuettes containing the proteinase inhibitor, aprotinin (Trasylol; Bayer Australia Ltd) added to the vacuettes at 0.05 ml per ml of blood. After centrifugation for 8 min, plasma samples for leptin measurements were placed in 500 μl vials and stored at −70°C until assayed.
Plasma leptin concentrations were determined with a radioimmunoassay kit (Multispecies Leptin RIA Kit, Cat #XL-85K, Linco Research Inc.). This kit was developed to quantitate leptin in plasma or serum from several species and has been validated for use in cats (Backus et al 2000). The RIA is based on a guinea pig-derived leptin antibody, which was raised against human leptin but displays broad cross-reactivity to leptin in other species.
Statistical analysis
The reference range for leptin in 26 normal cats was established by calculation of a tolerance interval containing 95% of the population with a probability of 0.90 (Lumsden & Mullen 1978). The tolerance interval was calculated as mean ± 2.5027 SD (Lumsden & Mullen 1978). Data were analysed for normality by the use of the Kolmogorov–Smirnov test of normality (Sigmastat Version 2.0, SPSS Inc.). Where necessary, data was logarithmically transformed to achieve a normal distribution prior to parametric analysis (Lumsden & Mullen 1978).
To investigate the differences in leptin concentrations before and after weight gain, statistical analysis was performed using the one-way repeated measures analysis of variance (ANOVA) with SPSS software (Sigmastat Version 2.0, SPSS Inc). For non-normally distributed data, a repeated measures ANOVA on ranks was executed.
Pearson product moment correlations and linear regression analysis were used to measure the strength of association of plasma leptin concentrations with adiposity variables. Measures of adiposity included bodyweight and body mass index before weight gain and body mass index, body weight, percentage weight gain, fat percentage, total fat mass and the adiposity ratio (ie, fat-to-lean ratio) after weight gain. Student's t-test was performed to compare leptin concentrations between male and female cats. To determine if gender differences were still present after adjusting for adiposity, analysis of covariance was performed using SAS (GLM Procedure, Version 8.0 for windows, SAS Institute Inc.)
All data are reported as mean ± 1 SD, followed by range in parentheses. A P-value of <0.05 was considered significant.
Results
Reference values for plasma leptin concentrations
Reference values for plasma leptin were established in 26 cats considered to have an ‘ideal’ bodyweight based on a body condition score of three out of five. Mean bodyweight was 4.37 ± 0.64 kg and ranged from 3.35 kg to 6.0 kg. The body mass index of these cats averaged 60 ± 5.51 kg/m2 and ranged from 51.2 to 75.0 kg/m2. Mean plasma leptin concentration measured after a 12-h fast for 26 normal-weight cats was 6.41 ± 2.19 ng/ml HE with upper and lower tolerance intervals for the population of 0.92 and 11.9 ng/ml HE
There was no relationship between mean plasma leptin concentrations and bodyweight or body mass index in 26 normal-weight cats. There were no significant differences in leptin concentration in 11 male cats compared with 15 female cats with normal body weights (6.54 ± 2.18 vs 6.31 ± 2.27 ng/ml HE), indicating that gender is not a significant determinant of leptin concentrations in normal-weight, neutered cats.
Comparison of plasma leptin concentrations before and after weight gain
Based on body condition scores, the 16 cats used in the weight gain part of the study were classed as normal weight (n = 14, score 3), underweight (n = 1, score 2) and overweight (n = 1, score 4) prior to weight gain. A highly significant increase (P<0.001) in bodyweight, body mass index and body condition score occurred following an average of 10 months weight gain (Table 1). Mean bodyweight of the cats after weight gain was 6.28 ± 1.26 kg (range, 4.4–8.6), representing a mean gain of 44.2% (range, 8.51–74.7%), or 1.91 kg per cat. Based on the body condition score, six cats became overweight (score 4) and 10 became obese (score 5). The DEXA-derived fat percentage of cats after weight gain ranged from 34.2 to 48.7% with a mean value of 41.3%.
Table 1.
Adiposity measurements (mean ± SD, range) in 16 cats before and after weight gain
| Before weight gain | After weight gain | |
|---|---|---|
| Bodyweight (kg) | 4.37 ± 0.76 (3.35–6.25) | 6.28** ± 1.26 (4.4–8.6) |
| Body condition score | 3.0 ± 0.37 (2–4) | 4.63** ± 0.5 (4.0–5.0) |
| Body mass index (kg/m2) | 60.8 ± 7.72 (51–75) | 76.5** ± 7.43 (64.4–89.1) |
| Fat per cent (%) | n/a | 41.3 ± 3.96 (34.2–48.7) |
| Total fat mass (kg) | n/a | 2.68 ± 0.78 (1.54–4.25) |
| Lean body mass (kg) | n/a | 3.63 ± 0.52 (2.63–4.33) |
| Adiposity ratio (fat-to-lean ratio) | n/a | 0.728 ± 0.125 (0.528–0.983) |
Values in obese cats are significantly
P < 0.001) different from lean cats.
n/a=not available.
When weight gain was analysed separately on a gender basis, male cats gained a significantly greater amount of weight (P = 0.03) than female cats, although their initial weights were not significantly different (Table 2). This resulted in male cats weighing significantly (P = 0.01) more than female cats after weight gain. Male cats also had significantly greater total fat and lean body mass than female cats after weight gain (Table 2).
Table 2.
Adiposity measurements for six male and 10 female cats before and after weight gain
| Before weight gain | After weight gain | |||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Body weight (kg) | 4.78 ± 1.01 | 4.12 ± 0.45 | 7.26* ± 1.19 | 5.69 ± 0.9 |
| Body condition score | 3.17 ± 0.41 | 2.9 ± 0.32 | 4.56 ± 0.52 | 4.45 ± 0.55 |
| Body mass index (kg/m2) | 63.7 ± 8.98 | 59.0 ± 6.73 | 80.2 ± 9.16 | 74.2 ± 5.52 |
| Fat per cent (%) | n/a | n/a | 43.1 ± 4.82 | 40.2 ± 3.14 |
| Total fat mass (kg) | n/a | n/a | 3.23* ± 0.86 | 2.35 ± 0.52 |
| Lean body mass (kg) | n/a | n/a | 4.06* ± 0.34 | 3.37 ± 0.44 |
| Adiposity ratio (fat-to-lean ratio) | n/a | n/a | 0.79 ± 0.16 | 0.69 ± 0.09 |
Values in male cats are significantly
(P<0.05) different from values in female cats.
n/a=not available.
The mean fasting plasma leptin concentration for 16 overweight and obese cats was 24.5 ± 12.1 ng/ml HE (range, 8.4–54.8 ng/ml HE), compared with 7.88 ± 4.02 (range, 3.2–19.8 ng/ml HE) when they were normal-weight (P<0.001). There was a wide variation in plasma leptin concentrations for a given fat mass. For example, two cats with a similar fat mass of 4.2 kg after weight gain, had fasting leptin concentrations of 30.9 ng/ml HE and 54.8 ng/ml HE, respectively (Table 3).
Table 3.
Body fat mass measured by dual energy X-ray absorptiometry (DEXA), and basal plasma leptin concentrations in 16 cats after weight gain
| Cat | Body fat mass (kg) | Plasma leptin concentration (ng/ml HE) |
|---|---|---|
| 1 | 4.15 | 30.95 |
| 2 | 3.28 | 20.19 |
| 3 | 2.88 | 24.44 |
| 4 | 2.66 | 24.79 |
| 5 | 2.42 | 16.71 |
| 6 | 2.16 | 21.83 |
| 7 | 2.10 | 12.44 |
| 8 | 1.54 | 8.40 |
| 9 | 4.25 | 54.76 |
| 10 | 3.12 | 47.04 |
| 11 | 3.23 | 29.65 |
| 12 | 2.57 | 27.55 |
| 13 | 2.17 | 18.93 |
| 14 | 2.55 | 22.96 |
| 15 | 1.79 | 17.40 |
| 16 | 2.00 | 14.23 |
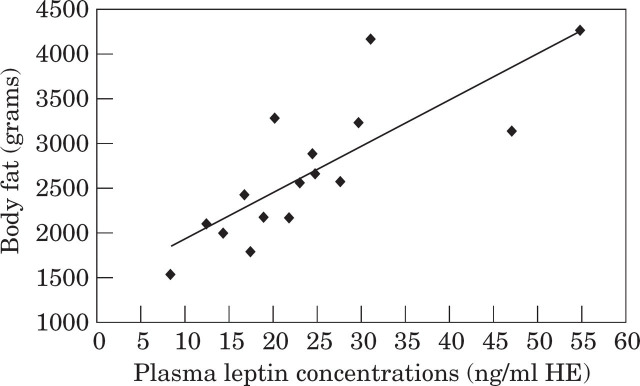
There was a strong positive correlation between serum leptin concentrations and DEXA-derived fat mass in obese cats (r = 0.80; P < 0.001) (Fig. 1). The adiposity ratio, percentage body fat, lean body mass, bodyweight, body condition score and body mass index were also related to plasma leptin concentrations (Table 4). These results are analogous with other findings in cats where percentage of body fat and total body fat, determined by the deuterium dilution method were the strongest predictors of plasma leptin (Backus et al 2000).
Fig 1.
Correlation of plasma leptin concentrations with DEXA-derived total body fat in 16 obese cats. The correlation coefficient was 0.80 (P<0.001).
Table 4.
Pearson correlation coefficients for leptin concentrations and adiposity measurements in cats, before and after weight gain
| Leptin ng/ml HE | |
|---|---|
| Lean cats | |
| Body weight (kg) | 0.69** |
| Body condition score | 0.64** |
| Body mass index (kg/m2) | 0.6* |
| Obese cats | |
| Body weight (kg) | 0.76*** |
| Body condition score | 0.66** |
| Body mass index (kg/m2) | 0.63** |
| Fat per cent (%) | 0.78*** |
| Total fat mass (kg) | 0.80*** |
| Lean body mass (kg) | 0.64** |
| Adiposity ratio (fat-to-lean ratio) | 0.79*** |
P<0.05,
P<0.01,
P<0.001.
In obese cats, the mean fasting plasma leptin concentrations tended to be higher in the six male cats (31.4 ± 16.1 ng/ml HE), than in the 10 female cats (20.4 ± 6.95 ng/ml HE), (P=0.07). This trend for higher mean leptin concentrations in obese male cats in part reflected their statistically greater weight and fat mass than in obese female cats (Table 2). When measures of adiposity were adjusted for, the trend was weaker but still observable, although the analyses did not reach significance for gender when any independent adiposity measure was used as a covariate.
Discussion
The findings in our study are similar to the results of several studies in humans that have shown that leptin concentrations are higher in obese than in normal-weight individuals and are highly correlated with body fat (Maffei et al 1995, Considine et al 1996, Havel et al 1996b). Our study found a three-fold increase in plasma leptin concentrations in cats after weight gain with mean absolute values corresponding to those reported in humans of 7.5 ± 9.3 ng/ml HE in lean subjects and 31.3 ± 24.1 ng/ml HE in obese subjects (Considine et al 1996). This suggests that obesity is not caused by an absolute deficiency in leptin levels per se. It has been hypothesised in other species that leptin acts as an adipocytederived signaling molecule, informing the brain of the mass of fat reserves in a negative feedback loop serving to regulate energy balance (Zhang et al 1994, Caro et al 1996). If this is true for cats, one would expect that as the cats in our study increased their caloric intake and gained weight, subsequent elevations in their plasma leptin concentrations would result in a feedback mechanism to reduce food intake and increase energy expenditure. This would result in a relatively stable body fat mass controlled by minimal fluctuations in leptin concentrations. Despite body weight increasing by an average of 44.2% and leptin levels increasing three-fold, cats continued to overeat and gain weight. This paradox of increased leptin concentrations in obesity has been observed in other species and is hypothesised to be a consequence of ‘leptin resistance’ (Frederich et al 1995, Maffei et al 1995, Considine et al 1996). Our data suggest that leptin resistance is also present in obese cats. Several explanations have been proposed for the mechanism of this resistance. These include intravascular defects, defects in the transport of leptin into the central nervous system, a failure of leptin to activate the leptin receptor or post-receptor defects in the leptin signal transduction pathway (Caro et al 1996, Schwartz et al 1996a, Havel 1998).
There was a substantial variation in plasma leptin concentrations between individual obese cats with similar body fat mass. This suggests that leptin production and secretion may be regulated by factors other than the level of adipose tissue. Factors which have been shown to influence leptin levels in other species are related to the availability of energy and include dietary macronutrient content (Havel et al 1999), energy restriction (Dubuc et al 1998), fasting (Boden et al 1996, Weigle et al 1997), and re-feeding (Weigle et al 1997). These factors are unlikely to explain the differences between our cats; however, as all cats were individually housed and fed the same diet ad libitum prior to commencement of data collection. Several hormonal influences including glucocorticoids (Larsson and Ahrén 1996, Bradley and Cheatham 1999), lipolysis (Dubuc et al 1998), glucose (Mueller et al 1998), and insulin (Leroy et al 1996, Utriainen et al 1996, Wabitsch et al 1996) have also been shown to influence leptin secretion in humans and rodents. Further studies are required to determine what factors influence leptin production and secretion in cats.
Factors identified as predisposing to feline obesity include lack of physical activity, extended sleeping periods, apartment dwelling, male gender, neutering, aging, mixed breed ancestry as well as environmental, social and behavioural factors (Sloth 1992, Scarlett et al 1994, Scarlett and Donoghue 1996, Robertson 1999). Dietary factors may influence the risk of obesity in cats and other species. Cats fed some types of highly palatable, prescribed and speciality foods are more at risk of developing obesity than cats fed typical commercial grocery store products (Scarlett et al 1994). It has been postulated that the stimulus of highly palatable foods, overrides the effects of physiological satiety signals (Havel 1998). Interestingly, central leptin administration in rats with diet-induced obesity has been shown to reduce the intake of a standard diet but not that of a more palatable, energy-dense diet (Widdowson et al 1997). The degree of weight gain achieved by cats in our study may, in part, be due to the highly palatable and energy dense nature of the foods being offered free-choice, resulting in overriding of normal satiety signals. This, coupled with leptin resistance and physical inactivity, may have resulted in cats becoming overweight and in most cases, obese.
There was no significant differences in leptin concentrations between male and female cats, indicating that at least in normal-weight, neutered cats, gender is not a significant determinant of leptin concentrations. Obese male cats tended to have higher leptin levels than female cats. Male cats gained an average of 58% more weight, however, and had a significantly greater amount of fat tissue compared with female cats, consistent with reports that male cats are more likely to be overweight than females (Scarlett & Donoghue 1996). After adjusting for body fat percentage, the trend towards higher leptin levels in male cats was weakened, but nevertheless still remained (27.6 ng/ml vs 22.7 ng/ml HE, respectively). In humans, men tend to store adipose abdominally whereas women tend to deposit fat peripherally on the hips and thighs (Krotkiewski et al 1983). Plasma leptin has been shown to correlate with subcutaneous fat at the umbilical level in humans (Takahashi et al 1996). Although, in both male and female cats, excess adipose tissue tends to collect intra-abdominally and subcutaneously over the thorax and abdomen rather than peripherally (Hand et al 1989), neutered male cats particularly tend to store fat in the fold of skin just cranial to the inguinal area (Hand et al 1989). It is possible that the increased propensity for neutered male cats to store fat subcutanously in this ‘inguinal apron’ may contribute to the trend for higher leptin levels in male cats. Cats in our study were all neutered, thus eliminating any gender differences due to the influence of reproductive hormones.
Gender differences appear to influence plasma leptin concentrations in humans (Maffei et al 1995, Considine et al 1996, Havel et al 1996a, Ho et al 1999). Testosterone is negatively correlated with plasma leptin concentrations in human males suffering insulin-dependent diabetes mellitus, suggesting that androgens may exert an inhibitory effect on leptin secretion (Tuominen et al 1997). Inhibition of leptin by testosterone may explain the findings of the previous study in cats where the maximum plasma leptin concentration was only 4.9 ng/ml HE, despite the heaviest cat weighing 7.1 kg (Backus et al 2000). However, the absolute body fat percent of this individual cat was not reported. An intact male cat weighing 7.1 kg may not necessarily be overweight or obese.
Conclusion
In conclusion, our results document increased circulating plasma leptin concentrations associated with spontaneous weight gain in cats. Plasma leptin correlated strongly with all measures of adiposity in obese cats, with the highest correlation for body fat. The results of this study are consistent with a postulated lipostat role of leptin. After adjusting for adiposity, there were no significant differences in plasma leptin concentrations between neutered male and female cats, though there was a non-significant trend for higher leptin in males. Despite having high leptin concentrations, cats continued to overeat and gain weight suggesting that obese cats may be resistant to the action of leptin in controlling fat mass.
Further understanding of leptin production and its mechanism of action in cats may assist the development of future obesity treatments. These therapies may involve influencing leptin production or expression of the uncoupling protein gene, possibly through dietary manipulations. Further understanding of leptin resistance and the leptin signaling pathway may also result in therapies directed towards enhancing ob gene transcription, influencing leptin transport, improving leptin receptor activation or addressing post-receptor defects.
Acknowledgements
Appreciation is expressed to Rachael Price, Emma Bennett and Rebekah Wilson for their assistance with animal care and data collection, to Lyn Knott for her help with sample analysis and to Jan Priest for her statistical advice.
References
- Backus RC, Havel PJ, Gingerich RL, Rogers QR. (2000) Relationship between serum leptin immunoreactivity and body fat mass as estimated by use of a novel gas-phase Fourier transform infrared spectroscopy deuterium dilution method in cats. American Journal of Veterinary Research 61, 796–801. [DOI] [PubMed] [Google Scholar]
- Boden G, Chen X, Mozzoli M, Ryan I. (1996) Effect of fasting on serum leptin in normal human subjects. Journal of Clinical Endocrinology and Metabolism 81, 3419–3423. [DOI] [PubMed] [Google Scholar]
- Boss O, Muzzin P, Giacobino JP. (1998) The uncoupling proteins, a review. European Journal of Endocrinology 139, 1–9. [DOI] [PubMed] [Google Scholar]
- Bradley RL, Cheatham B. (1999) Regulation of ob gene expression and leptin secretion by insulin and dexamethasone in rats. Diabetes 48, 272–278. [DOI] [PubMed] [Google Scholar]
- Butterwick RF, Wills JM, Sloth C, Markwell PJ. (1994) Astudy of obese cats on a calorie-controlled weight reduction program. Veterinary Record 134, 372–377. [DOI] [PubMed] [Google Scholar]
- Campfield LA, Smith FJ, Burn P. (1996) The OB protein (Leptin) pathway—A link between adipose tissue mass and central neural networks. Hormone and Metabolic Research 28, 619–632. [DOI] [PubMed] [Google Scholar]
- Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. (1995) Recombinant mouse ob protein: Evidence for a peripheral signal linking adiposity and central neural networks. Science 269, 546–549. [DOI] [PubMed] [Google Scholar]
- Caro J, Sinha MH, Kolaczynski JW, Zhang PL, Considine RV. (1996) Leptin: The tale of an obesity gene. Diabetes 45, 1455–1462. [DOI] [PubMed] [Google Scholar]
- Chen H, Charlat O, Tartaglia LA, et al. (1996) Evidence that the diabetes gene encodes the leptin receptor: Identification of a mutation in the leptin receptor gene in db/db mice. Cell 84, 491–495. [DOI] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, et al. (1996) Serum immuno-reactive leptin concentrations in normal-weight and obese humans. New England Journal of Medicine 334, 292–295. [DOI] [PubMed] [Google Scholar]
- Cusin I, Sainsbury A, Doyle P, Rohner-Jeanrenaud F, Jeanrenaud B. (1995) The ob gene and insulin. Diabetes 44, 1467–1470. [DOI] [PubMed] [Google Scholar]
- Dubuc GR, Phinney SD, Stern JS, Havel PJ. (1998) Changes of serum leptin and endocrine and metabolic parameters after 7 days of energy restriction in men and women. Metabolism: Clinical and Experimental 47, 429–434. [DOI] [PubMed] [Google Scholar]
- Frederich RC, Hamann A, Anderson S, Lollman B, Lowell BB, Flier JS. (1995) Leptin levels reflect body lipid content in mice: Evidence for diet-induced resistance to leptin action. Nature Medicine 1, 1311–1314. [DOI] [PubMed] [Google Scholar]
- Halaas JL. (1995) Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269, 543–546. [DOI] [PubMed] [Google Scholar]
- Hand MS, Armstrong JP, Allen TA. (1989) Obesity: Occurrence, treatment and prevention. Veterinary Clinics of North America. Small Animal Practice 19, 447–474. [DOI] [PubMed] [Google Scholar]
- Havel PJ. (1998) Leptin production and action: Relevance to energy balance in humans. American Journal of Clinical Nutrition 67, 355–356. [DOI] [PubMed] [Google Scholar]
- Havel PJ, Kasim-Karakas S, Dubuc GR, Mueller W, Phinney SD. (1996a) Gender differences in plasma leptin concentrations. Nature Medicine 2, 949–950. [DOI] [PubMed] [Google Scholar]
- Havel PJ, Kasim-Karakas S, Mueller W, Johnson PR, Gingerich RL, Stern JS. (1996b) Relationship of plasma leptin to plasma insulin and adiposity in normal weight and overweight women: Effects of dietary fat content and sustained weight loss. Journal of Clinical Endocrinology and Metabolism 81, 4406–4413. [DOI] [PubMed] [Google Scholar]
- Havel PJ, Townsend R, Chaump L, Teff K. (1999) High-fat meals reduce circulating leptin concentrations in women. Diabetes 48, 334–341. [DOI] [PubMed] [Google Scholar]
- Ho SC, Tai ES, Eng PHK, Ramli A, Tan CE, Fok ACK. (1999) A study in the relationships between insulin, and body fat in Asian subjects. International Journal of Obesity 23, 246–252. [DOI] [PubMed] [Google Scholar]
- Kaiyala KW, Woods SCMWS. (1995) A new model for the regulation of energy balance by the central nervous system. American Journal of Clinical Nutrition 62, 1123S–1134S. [DOI] [PubMed] [Google Scholar]
- Kieffer TJ, Heller RS, Habener JF. (1996) Leptin receptors expressed on pancreatic β-cells. Biochemical and Biophysical Research Communications 224, 522–527. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. (1990) Mechanism and evolution of the uncoupling protein of brown adipose tissue. Trends in Biochemical Sciences 15, 108–112. [DOI] [PubMed] [Google Scholar]
- Krotkiewski M, Björntorp P, Sjöstström L, Smith U. (1983) Impact of obesity on metabolism in men and women: Importance of regional adipose tissue distribution. Journal of Clinical Investigation 72, 1150–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson H, Ahrén B. (1996) Short-term dexamethasone treatment increases plasma independently of changes in insulin sensitivity in healthy women. Journal of Clinical Endocrinology and Metabolism 81, 4428–4432. [DOI] [PubMed] [Google Scholar]
- Lee GH, Proenca R, Montez JM, et al. (1996) Abnormal splicing of the leptin receptor in diabetic mice. Nature 379, 632–635. [DOI] [PubMed] [Google Scholar]
- Leroy P, Dessolin S, Villageois P, Moon BC, Friedman JM, Ailhaud G, Dani C. (1996) Expression of ob gene in adipose cells. Regulation by insulin. Journal of Biological Chemistry 271, 2365–2368. [DOI] [PubMed] [Google Scholar]
- Lönnqvist F, Arner P, Nordfors L, et al. (1995) Overexpression of the obese (ob) gene in adipose tissue of human subjects. Nature Medicine 44, 855–858. [DOI] [PubMed] [Google Scholar]
- Lumsden JH, Mullen K. (1978) On establishing reference values. Canadian Journal of Comparative Medicine 42, 293–301. [PMC free article] [PubMed] [Google Scholar]
- Maffei M, Halaas J, Ravussin E, et al. (1995) Leptin levels in human and rodent: Measurement of plasma leptin and obRNA in obese and weight-reduced subjects. Nature Medicine 1, 1155–1161. [DOI] [PubMed] [Google Scholar]
- Masuzaki H, Ogawa Y, Isse N, Satoh N, et al. (1995) Human obese gene expression: Adipocyte-specific expression and regional differences in the adipose tissue. Diabetes 44, 855–858. [DOI] [PubMed] [Google Scholar]
- Meier CA. (1995) Advances in the understanding of the molecular basis of obesity. European Journal of Endocrinology 133, 761–763. [DOI] [PubMed] [Google Scholar]
- Mueller WM, Gregoire FM, Stanhope KL, et al. (1998) Evidence that glucose metabolism regulates plasma leptin secretion from cultured rat adipocytes. Endocrinology 139, 551–558. [DOI] [PubMed] [Google Scholar]
- Nelson RW, Himsel CA, Feldman EC, Bottoms GD. (1990) Glucose tolerance and insulin response in normal-weight and obese cats. American Journal of Veterinary Research 51, 1357–1362. [PubMed] [Google Scholar]
- Pellymounter M, Cullen M, Baker M, Hecht R, Winters D, Boone T, Collins F. (1995) Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269, 540–543. [DOI] [PubMed] [Google Scholar]
- Robertson ID. (1999) The influence of diet and other factors on owner-perceived obesity in privately owned cats from metropolitan Perth, Western Australia. Preventative Veterinary Medicine 40, 75–85. [DOI] [PubMed] [Google Scholar]
- Rothwell N. (1989) Central effects of CRF on metabolism and energy balance. Neuroscience and Biobehavioral Reviews 14, 263–271. [DOI] [PubMed] [Google Scholar]
- Scarlett JM, Donoghue S. (1996) Obesity in cats: Prevalence and prognosis. Veterinary Clinical Nutrition 3, 128–132. [Google Scholar]
- Scarlett JM, Donoghue S, Daidla J, Wills J. (1994) Overweight cats: Prevalence and risk factors. International Journal of Obesity 18, S22–S28. [PubMed] [Google Scholar]
- Scarpace PJ, Matheny M. (1998) Leptin induction of UCP1 is dependent on sympathetic innervation. American Journal of Physiology 275, E259–E264. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Matheny M, Pollock BH, Tümer N. (1997) Leptin increases uncoupling protein expression and energy expenditure. American Journal of Physiology 273, E226–E230. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D., Jr (1996a) Cerebrospinal fluid leptin levels: Relationship to plasma levels and to adiposity in humans. Nature Medicine 2, 589–593. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ. (1997) The new biology of weight reduction. Journal of the American Dietetic Association 97, 54–58. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seely RJ, Campfield LA, Burn P, Baskin DG. (1996b) Identification of targets of leptin action in rat hypothalamus. Journal of Clinical Investigation 98, 1101–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloth C. (1992) Practical management of obesity in dogs and cats. Journal of Small Animal Practice 33, 178–182. [Google Scholar]
- Stephens TW, Basinski M, Bristow PK, et al. (1995) The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature 377, 530–532. [DOI] [PubMed] [Google Scholar]
- Sunvold GD, Bouchard GF. (1998) Assessment of obesity and associated metabolic disorders. Proceedings of the Iams Nutrition Symposium. Recent Advances in Canine and Feline Nutrition, pp 135–148.
- Takahashi M, Funahashi T, Shimomura I, Miyaoka K, Matsuzawa Y. (1996) Plasma leptin levels and body fat distribution. Hormone and Metabolic Research 28, 751–752. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Dembski M, Weng X, et al. (1995) Identification and expression cloning of a leptin receptor, OB-R. Cell 83, 1263–1271. [DOI] [PubMed] [Google Scholar]
- Tuominen U, Ebeling P, Stenmen U, Heiman M, Stephens TW, Koivisto VA. (1997) Leptin synthesis is resistant to acute effects of insulin in insulin-dependent diabetes mellitus patients. Journal of Clinical Endocrinology and Metabolism 81, 381–382. [DOI] [PubMed] [Google Scholar]
- Utriainen T, Malmström R, Mäkimattila S, Yki-Järvinen H. (1996) Supraphysiological hyperinsulinaemia increases plasma leptin concentrations after 4h in normal subjects. Diabetes 45, 1364–1366. [DOI] [PubMed] [Google Scholar]
- Wabitsch M, Jensen PB, Blum WF, et al. (1996) Insulin and cortisol promote leptin production in cultured human fat cells. Diabetes 45, 1435–1438. [DOI] [PubMed] [Google Scholar]
- Walton C, Lees B, Crook D, Godsland IF, Stevenson JC. (1995) Relationships between insulin metabolism serum lipid profile body fat distribution and blood pressure in healthy men. Arteriosclerosis 118, 35–43. [DOI] [PubMed] [Google Scholar]
- Weigle DS, Barton Duell P, Connor WE, Steiner RA, Soules MR, Kuijper JL. (1997) Effect of fasting, refeeding and dietary fat restriction on plasma leptin levels. Journal of Clinical Endocrinology and Metabolism 81, 561–565. [DOI] [PubMed] [Google Scholar]
- Widdowson PS, Upton R, Buckingham R, Arch J, Williams G. (1997) Inhibition of food response to intracerebroventricular injection of leptin is attenuated in rats with diet-induced obesity. Diabetes 46, 1782–1785. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J. (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432. [DOI] [PubMed] [Google Scholar]