Abstract
Haemobartonella felis is a pleomorphic uncultivated wall-less haemotrophic bacterial parasite. Phylogenetic analysis of 16S rRNA gene sequences from a number of isolates of H felis has demonstrated that these bacteria are most closely related to species in the genus Mycoplasma, and Haemobartonella and related organisms are currently being reclassified as Mollicutes. Diagnosis by cytological examination of blood smears has been problematic, but recent molecular studies have led to the development of sensitive polymerase chain reaction (PCR) assays for diagnosis. Such studies have also resulted in the recognition of two distinct strains of H felis, which are divided into different groups based on phylogenetic analysis. This evolutionary divergence between strains is accompanied by differences in pathogenecity. This review discusses new developments in the diagnosis and treatment of H felis, focusing on the use of, and interpretation of, PCR assays.
In 1942 Clark described an eperythrozoon infection in an anaemic cat in South Africa naming the species Eperythrozoon felis. Flint & Moss (1953) later described a similar organism causing an infectious anaemia in cats in the USA. In 1955, Flint & McKelvie suggested that this organism be named Haemobartonella felis.
Haemobartonella felis is now recognised worldwide (Anderson & Charleston 1967, Bedford 1970, Bobade & Akinyemi 1981, Clark 1942, Espada et al 1991, Flagstad & Larsen 1969, Hatakka 1972, Maede et al 1974, Manusu 1961, Mrljak et al 1995, Prieur 1960, Seamer & Douglas 1959, Théry 1966) although studies describing the prevalence of infections in cats in different parts of the world have been sparse. Despite it being a major cause of anaemia in cats there is still a paucity of information available regarding its epidemiology and the disease it causes. One of the major factors limiting the investigation of this organism is that it has not yet been successfully cultivated outside the host.
However, recent molecular studies have advanced our understanding of the nature and pathogenicity of this organism. Analysis of the 16S rRNA gene of several H felis isolates has led to the discovery of its evolutionary relatedness to members of the Mollicutes rather than the Rickettsiales (Johansson et al 1999, Neimark & Kocan 1997, Rikihisa et al 1997) and reclassification of the organism has been recommended. In situ hybridisation studies (Berent et al 2000) have physically linked H felis DNA sequences to areas of known cellular pathology on feline erythrocytes. Similarly the presence of H felis DNA coincides with experimental infection (Berent et al 1998) and the amount of DNA is correlated with the number of organisms present in the blood (Cooper et al 1999). These molecular findings have proven that H felis is the cause of feline infectious anaemia. Recent molecular work also documents the existence of different strains (Berent et al 1998, Foley et al 1998, Messick et al 1998, Rikihisa et al 1997) that may ultimately prove to be different Haemobartonella species.
Since H felis cannot be cultured, diagnosis of infection until recently was primarily based on cytological identification of organisms on the surface of red blood cells. However, there are various factors resulting in false positive and false negative results with this method. The polymerase chain reaction (PCR) is now the test of choice for the diagnosis of H felis infection and is commercially available in several laboratories in North America. The availability of a sensitive diagnostic test has enabled more accurate studies to be performed to assess success of treatment as well. This review will focus on recent advances in characterisation of the H felis strains as well as the diagnosis and treatment of haemobartonellosis.
Diagnosis of H felis infection
Examination of blood smears
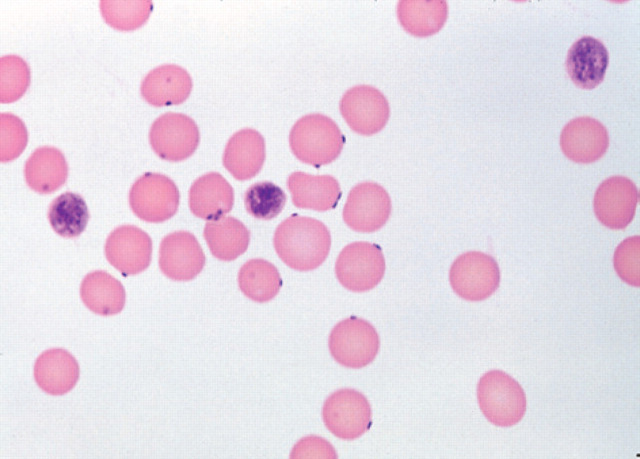
The most commonly used method to definitively diagnose H felis is demonstration of organisms on the surface of erythrocytes by microscopic examination of a good quality blood smear. Butt provides an excellent review in the preparation and staining of diagnostic blood smears (Butt 1990). A number of Romanowsky-type blood stains can be used including Giemsa, May–Grunwald–Giemsa, Wright, and Wright–Giemsa stains. The organism is typically found around the periphery of the erythrocyte, and may be found singly, in pairs, or in chains with severe infestation (Figs 1 and 2). Very occasionally organisms may lie free of the erythrocytes because of detachment from cells.
Fig 1.
Wright–Giemsa stained blood smear (original magnification ×1000) showing small epicellular coccoid and rod shaped H felis bodies on approximately 50% of erythrocytes.
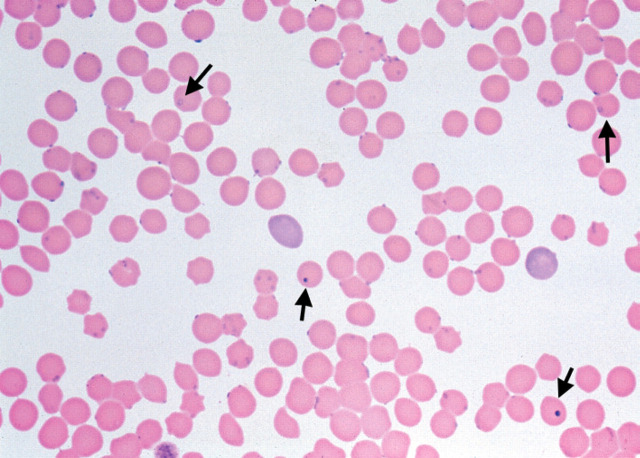
Fig 2.
Wright-Giemsa stained blood smear (original magnification ×400) showing H felis bodies on approximately 65% of erythrocytes. Ring forms of H felis are visible (large arrows). Larger and more densely staining Howell–Jolly bodies can be seen (small arrows) for comparision.
False positive diagnoses are sometimes made when artefacts caused by improper drying or fixation and stain precipitation are confused with the organism. The use of fresh filtered stain solutions is imperative. Stain precipitate is usually found above the plane of focus of the erythrocytes and is more densely staining and larger than H felis organisms. Refractile artefacts occur when moisture adheres to the cells on the film and these tend to have irregular borders and appear colourless when the erythrocytes are in focus. H felis must also be distinguished from other feline erythrocytic inclusions. Howell–Jolly bodies (Fig 2) are erythrocytic nuclear remnants and can be differentiated from H felis by their larger size (around 1–2 μm in diameter). Pappenheimer bodies are aggregates of iron accumulation and appear as lightly staining small blue granules. Acridine orange (AO) and direct fluorescent antibody staining methods are reported to be more sensitive than standard Romanowsky stains for demonstrating H felis (Berent et al 1998, Bobade & Nash 1987, Small & Ristic 1967). However, both these techniques are limited by the need for a fluorescent microscope. AO combines specifically with nucleic acids, and organisms appear bright orange with an undertone of yellow green. Non-specific fluorescence can also give false positive results and Howell–Jolly bodies, which also stain with AO, must be differentiated from H felis (Harvey & Gaskin 1977).
False negative diagnoses are also a major problem when blood smears are relied upon for the diagnosis of H felis infection. While parasitaemia can be extremely heavy during acute infection, the clearance of parasites from the blood can be rapid resulting in negative cytological findings within a few hours (Alleman et al 1999, Harvey & Gaskin 1977). This cyclical parasitaemia means that the absence of organisms on examination of a blood smear does not definitively exclude a diagnosis of haemobartonellosis. These factors contribute to the poor sensitivity of cytopathology in the diagnosis of haemobartonellosis. In a recent experimental study that compared cytopathology and PCR in experimentally inoculated cats, the detection rates were 37.5% and 100% respectively in samples collected after inoculation (Westfall et al 2001). The preparation of multiple fresh blood smears over a period of 24 h or over the course of a few days may increase the chance of obtaining a positive diagnosis. Sample handling is also important. High concentrations of EDTA anticoagulant have been reported to detach organisms from the erythrocyte surface within a couple of hours (Alleman et al 1999), making identification of organisms on blood smears extremely difficult. It would therefore seem prudent to prepare blood smears immediately after blood collection using non-anticoagulated blood or use heparin which is not thought to result in the organisms being dislodged (Alleman et al 1999).
Serology
Results of Western immunoblotting showed that some H felis antigens are recognised as early as 14 days after infection (Alleman et al 1999). In one study of cats experimentally inoculated with two H felis strains, antibodies were usually detected by indirect immunofluorescent antibody assay by 21 days post-infection and persisted for at least 6 months, well after clinical disease had resolved (Foley et al 1998). Since antibodies can be demonstrated in subclinically infected carrier cats as well as in anaemic cats, a positive serological test does not equate with the presence of clinical disease. Sera generated against one strain of H felis did not detect organisms of another strain suggesting that antigenic variation exists (Foley et al 1998), and such antigenic variation may actually play a role in the development of cyclical parasitaemia in infected cats. If an antibody assay could be developed that detected only antibodies against pathogenic strains, it may have diagnostic value. Serological testing is not currently available commercially.
Polymerase chain reaction
The polymerase chain reaction (PCR) is a powerful and sensitive molecular technique whereby low numbers of specific fragments of DNA are amplified exponentially to detectable levels. A review of the principals of the PCR test has recently been published (Lutz et al 1999). It is claimed that as few as 52 H felis organisms can be detected by PCR on blood (Cooper et al 1999).
Various American studies have amplified H felis 16S rRNA DNA sequences from infected cats and these PCR products have been sequenced (Berent et al 1998, Foley et al 1998, Messick et al 1998, Rikihisa et al 1997) resulting in the recognition of genetically distinct strains of H felis. The Ohio (large) strain and the California (small) strain have been most extensively investigated by molecular methods (Berent et al 1998, Foley et al 1998, Jensen et al 2001, Westfall et al 2001). Recently sequencing of a United Kingdom strain of H felis (named the Birmingham strain) revealed near identity to the California small strain (Tasker et al 2001a) confirming that strains similar to this American strain are present in the Old World. Recently work in the UK (Tasker et al 2001b) has also confirmed the presence of H felis strains very similar to the American large form (Figure 3). Further work is required to determine whether other strains exist and to document any worldwide geographical variation. Phylogenetic analysis of the 16S rRNA DNA sequences of the H felis strains currently available reveals the evolutionary divergence of these organisms (Johansson et al 1999, Neimark & Kocan 1997, Rikihisa et al 1997, Tasker et al 2001a). Figure 4 shows a phylogenetic tree constructed from 1000 sets of bootstrapped data by the neighbour-joining method of Saitou & Nei (1987) following alignment of 16S rRNA gene sequences using CLUSTAL W (Version 1.6, EMBL) according to the method of Thompson et al (1994). The California and Birmingham H felis strains form a monophyletic group that is more closely related to the Eperythrozoon organisms E suis and E wenyonii than other Haemobartonella spp. Conversely, the Ohio/Florida, Illinois and Oklahoma H felis strains all share close homology and are more closely related as a group to H muris and H canis than to the California and Birmingham strains. The division, by phylogeny, of H felis strains into two divergent groups suggests at least two different species of Haemobartonella exist. The divergence determined among H felis strains is reflected in phenotypic differences between them. Morphologically, on Wright–Giemsa stained blood smears, the large/Ohio form is said to be approximately twice as large and substantially darker than the small/California form (Foley et al 1998), although the strains cannot be reliably distinguished by appearance alone.
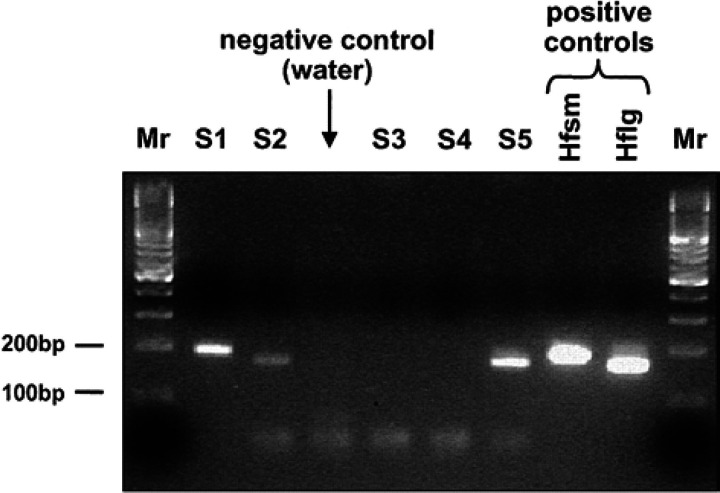
Fig 3.
Four percent agarose gel showing PCR products obtained by amplification of DNA extracted from feline blood samples. PCR protocol used courtesy of Heska Corporation, Fort Collins, Colorado, USA. S, suspected case from United Kingdom; Hfsm, positive control sample infected with H felis small form; Hflg, positive control sample infected with H felis large form; Mr, DNA molecular weight markers; S1 is infected with H felis small form and S2 and S5 are infected with H felis large form. Cases S3 and S4 are PCR negative for H felis.
Fig 4.
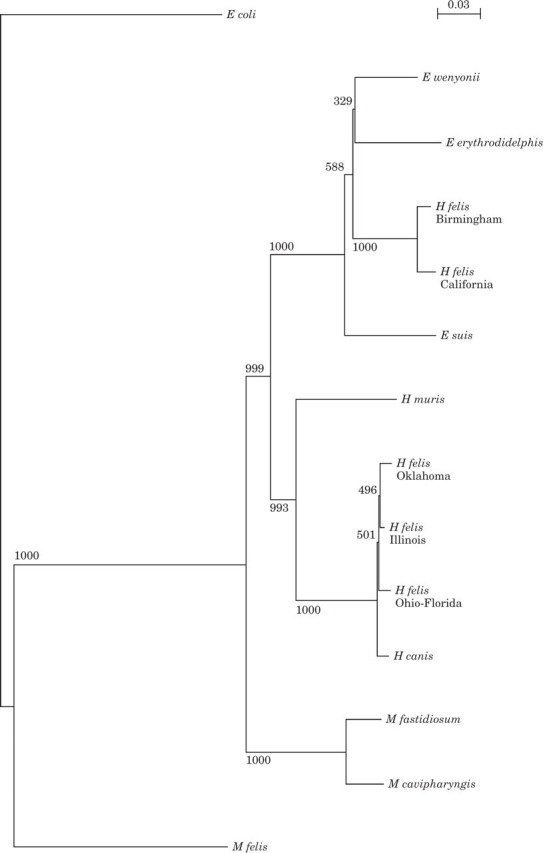
Phylogenetic tree comparing the available Haemobartonella felis sequences and the related organisms Eperythrozoon wenyonii, Eperythrozoon erythrodidelphis, Eperythrozoon suis, Haemobartonella muris, Haemobartonella canis, Mycoplasma fastidiosum, Mycoplasma cavipharyngis and Mycoplasma felis. Escherichia coli is shown for reference. The numbers at each node indicate the number of times the groups to the right of the node occurred among 1000 trees. The bar represents the mean number of differences per site.
Important from a clinical standpoint are the differences in pathogenicity apparent between the strains in the few studies performed to date. Experimental infection of cats with two different isolates of the California strain (Foley et al 1998, Westfall et al 2001) resulted in minimal clinical signs and anaemia was not usually induced. Preliminary findings suggest that the genetically related Birmingham strain is also of low pathogenicity (Tasker et al 2001a). Conversely experimental infection with the Ohio strain often results in a severe, sometimes fatal, haemolytic anaemia (Berent et al 1998, Foley et al 1998, Westfall et al 2001). Additionally, experimental inoculation of large form of H felis in cats already infected with the small form appeared to result in more severe clinical disease than the large form alone (Westfall et al 2001). This finding may be because prior infection with one Haemobartonella strain predisposes to immune-mediated disease when cats become infected with another strain. The nature of such super-infections and characterisation of the host's immune response to infection have not yet been investigated.
At this time, it is impossible to make definitive statements concerning pathogenicity of the H felis strains. Since H felis cannot be cultivated or accurately counted, it cannot be determined if experimentally inoculated cats received similar doses of organism which may have influenced clinical findings. Additionally, most of the experimental infections with defined strains to date have used intravenous inoculation (Berent et al 1998, Messick et al 1998, Westfall et al 2001). Natural routes of transmission of H felis have not yet been defined and it is possible that route of transmission (including possible vectors) may affect pathogenicity.
Recently, Jensen and colleagues (Jensen et al 2001) developed a PCR assay that is able to detect and distinguish both the small and large strains of H felis and applied the assay to blood from 220 American cats. The overall prevalence of H felis infection was 19.5%. Anaemic cats were more likely to be infected with either the large strain or both the small and large strains, than non-anaemic cats. This supports the experimental findings that infection with the large strain or dual infection results in more severe clinical disease (Westfall et al 2001). A recent UK study (Tasker et al 2001b) based on the same PCR assay reported an similar prevalence of H felis infection (18%) but very few cats were infected with the large strain precluding statistical evaluation of an association between the large strain and anaemia. Small strain infection in the UK was common and was not associated with the presence of anaemia. Further studies are required to determine whether geographical variation in the prevalence of H felis infection, including the different strains, exists.
PCR was shown to be more sensitive than examination of blood smears in the detection of H felis in several studies. As previously discussed, Westfall et al (2001) found that only 37.5% of samples were positive on cytopathology after experimental infection compared to 100% positivity with PCR. H felis can be detected by PCR as early as 8 days post-infection and cats remain PCR positive until antibiotic treatment is started. Cats then remain negative by PCR for the duration of antibiotic treatment, but become positive again between 3 days and 5 weeks after completion of the antibiotic course (Berent et al 1998, Foley et al 1998). Blood samples for H felis PCR should therefore not be submitted during antibiotic treatment. Subclinically infected cats remain PCR positive for at least 6 months, long after clinical signs of disease have resolved. Additionally, 14.5% of normal, naturally exposed cats in a US study (Jensen et al 2001) and 10% of healthy cats in a UK study (Tasker et al 2001b) were positive for H felis by PCR. Thus, it is apparent that a positive PCR result does not equate with clinical disease, making interpretation of results problematic. A positive PCR result should be interpreted in line with the strain identified, the haematological findings and clinical signs reported.
Clinical signs and haematology features
Previous reports that uncomplicated H felis infection caused few or no clinical signs (Bobade et al 1988, Seamer & Douglas 1959) may have reflected infection with an H felis small strain of low pathogenicity (Foley et al 1998). Chronically infected cats are usually clinically normal (Berent et al 1998). In other studies, a severe haemolytic anaemia occurred in all infected cats (Flint et al 1958, Foley et al 1998), most probably due to a pathogenic large strain. Clinical signs seen in ill cats are non-specific but include those typically associated with anaemia such as pallor and lethargy, as well as anorexia, weight loss and depression. Intermittent pyrexia is often seen, particularly in the acute stages of the disease. Splenomegaly and lymphadenopathy may occur, reflecting extramedullary haematopoiesis. Icterus, due to haemolysis, is occasionally seen. H felis infection typically causes a regenerative anaemia with reticulocytosis, anisocytosis, macrocytosis and polychromasia. The severity of anaemia depends on the stage of infection but the haematocrit usually falls to less than 20%, with average values of 15–18% reported (Foley et al 1998, VanSteenhouse et al 1993). Normoblasts may also be visible and, although these can be a feature of diseases which are not associated with regenerative anaemias, H felis was found to be the most common cause of normoblastaemia in a recent retrospective study (Hammer & Wellman 1999). Non-regenerative anaemias due to haemobartonellosis have also been reported (VanSteenhouse et al 1993).
Treatment
Haemobartonella spp are sensitive to tetracyclines which specifically inhibit protein synthesis in procaryotes. Tetracycline and oxytetracycline can both cause drug-induced pyrexia (Wilkinson 1968) in cats and require three times daily dosing, which may be problematic. The preferred tetracycline derivative used is doxycycline due to fewer side effects and less frequent dosing. The recommended doxycycline dose is 5–10 mg/kg per os once daily. Therapy should be continued for 14 to 21 days depending on response to treatment. As mentioned above, PCR has demonstrated that although the tetracyclines are effective for the treatment of anaemia, treatment does not eliminate the causal organism (Berent et al 1998, Foley et al 1998). Enrofloxacin has been recommended by some (Winter 1993) for the treatment of haemobartonellosis at a dose of 10 mg/kg per os daily for at least 14 days. Fluoroquinolones are said to have activity against mycoplasmal organisms but further study into their effectiveness against H felis is warranted before a recommendation can be made. The macrolide azithromycin, which is effective for the treatment of several Mycoplasma-associated syndromes in humans, was recently found to be ineffective in the treatment of haemobartonellosis at one dose regime (15 mg/kg per os twice daily) (Westfall et al 2001).
The anaemia induced by H felis is thought to be, in part, immune-mediated so glucocorticoids may be indicated (VanSteenhouse et al 1993). It is important that concurrent diseases such as toxoplasmosis, which could be exacerbated by the use of glucocorticoids, are ruled out first (Hoskins & Barta 1984). Some advocate the use of glucocorticoids only if the anaemia is pronounced or acute in onset and/or autoagglutination is present (Carney & England 1993). The recommended dose of prednisolone is 2 mg/kg per day per os, alongside antibiotic therapy, with a tapering of dosage over 3 weeks. The value of glucocorticoids in the treatment of H felis has not yet been evaluated. However, cats with experimentally induced haemobartonellosis have apparently responded to tetracycline derivatives (with or without supportive treatment) without the use of glucocorticoids (Berent et al 1998, Foley et al 1998).
Supportive care including whole blood transfusion may be required in severely anaemic cats. Cats tolerate anaemia well but if the anaemia is severe (haematocrit less than 12%) or if the PCV has dropped rapidly, a blood transfusion may be necessary. Feline blood transfusions should only be performed with typed or cross-matched recipient and donor blood to avoid potentially fatal blood transfusion reactions.
It has not been definitely documented that H felis is transmitted by fleas but it has been suggested that they are a major vector for H felis transmission. Since it is known that H felis can be transmitted by inoculation of infected blood, it seems wise to recommend thorough flea control to potentially lessen the risk of a cat acquiring haemobartonellosis.
Based on studies to date, several recommendations concerning testing methods and when to administer antibiotics with anti-Haemobartonella activity can be made.
If a cat has clinical findings consistent with haemobartonellosis and H felis is detected cytologically or DNA is detected by PCR (either strain), treatment is indicated.
If cytology is the only test available and is negative in a cat with a high index of suspicion for haemobartonellosis (such as unexplained haemolytic anaemia), the cat should be treated due to a high incidence of false negative cytological results.
In experimental studies, DNA can usually be detected by PCR prior to clinical signs of disease. Thus, if PCR results on blood collected prior to treatment are negative, the cat probably does not have haemobartonellosis and other differential diagnoses should be considered.
Since H felis can be transmitted by blood, and cytology is commonly falsely negative in chronically infected cats, all blood donor cats from areas where haemobartonellosis occurs, should be screened by PCR if possible.
Treatment of healthy cats is not recommended at this time since no drug or protocol have been shown to clear the body of the organism.
The currently recommended first line treatment is doxycycline at a dose of 5–10 mg/kg per os once daily for 14 to 21 days depending on response to treatment.
Conclusions
In recent years molecular studies have suggested reclassification of H felis into the Mollicutes family and enabled the development of diagnostic PCR assays. The discovery of different H felis strains which vary so greatly in pathogenicity means that the interpretation of a positive PCR is not straight forward, and confirming that H felis is the cause of clinical disease is difficult. In the future, research should focus on looking into the existence of additional H felis strains and how effective PCR is in detecting different strains, and determining whether geographical variation in prevalence of different strains exists. The pathogenesis of such strains also needs further investigation, in particular with respect to interaction with other feline diseases, and the host response to H felis infection.
Acknowledgements
Dr CR Helps of the University of Bristol is thanked for his help with the construction of the phylogenetic tree. ST is part funded by the Royal College of Veterinary Surgeons Trust Fund.
Addendum
Since this article was submitted for publication (26 February 2001), the official reclassification of H felis within the genus Mycoplasma has been proposed in two reports. It is suggested that the small strains (California and Birmingham) are renamed as ‘Candidatus Mycoplasma haemominutum’ whilst the large strains (Ohio/Florida, Illinois and Oklahoma) are renamed as ‘Candidatus Mycoplasma haemofelisf’. The Candidatus classification indicates that this is a provisional status for the organism until further phenotypic and genotypic information is available.
Foley JE, Pedersen NC (2001) ‘Candidatus Mycoplasma haemominutum’, a low-virulence epierythrocytic parasite of cats. International Journal of Systematic and Evolutionary Microbiology 51, 815–817.
Neimark H, Johansson KE, Rikihisa Y, Tully JG (2001) Proposal to transfer some members of the genera Haemobartonella and Eperythrozoon to the genus Mycoplasma with descriptions of ‘Candidatus Mycoplasma haemofelis’, ‘Candidatus Mycoplasma haemomuris’, ‘Candidatus Mycoplasma haemosuis’ and ‘Candidatus Mycoplasma wenyonii’. International Journal of Systematic and Evolutionary Microbiology, 51, 891–899.
References
- Alleman AR, Pate MG, Harvey JW, Gaskin JM, Barbet AF. (1999) Western immunoblot analysis of the antigens of Haemobartonella felis with sera from experimentally infected cats. Journal of Clinical Microbiology 37, 1474–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DC, Charleston WAG. (1967) Haemobartonella felis. New Zealand Veterinary Journal 15, 47. [DOI] [PubMed] [Google Scholar]
- Bedford PG. (1970) Feline infectious anaemia in the London area. Veterinary Record 87, 305–310. [DOI] [PubMed] [Google Scholar]
- Berent LM, Messick JB, Cooper SK. (1998) Detection of Haemobartonella felis in cats with experimentally induced acute and chronic infections, using a polymerase chain reaction assay. American Journal of Veterinary Research 59, 1215–1220. [PubMed] [Google Scholar]
- Berent LM, Messick JB, Cooper SK, Cusick PK. (2000) Specific in situ hybridisation of Haemobartonella felis with a DNA probe and tyramide signal amplification. Veterinary Pathology 37, 47–53. [DOI] [PubMed] [Google Scholar]
- Bobade PA, Akinyemi JO. (1981) A case of haemobartonellosis in a cat in Ibadan. Nigerian Veterinary Journal 10, 23–25. [Google Scholar]
- Bobade PA, Nash AS. (1987) A comparative study of the efficiency of acridine orange and some Romanowsky staining procedures in the demonstration of Haemobartonella felis in feline blood. Veterinary Parasitology 26, 169–172. [DOI] [PubMed] [Google Scholar]
- Bobade PA, Nash AS, Rogerson P. (1988) Feline haemobartonellosis: clinical, haematological and pathological studies in natural infections and the relationship to infection with feline leukaemia virus. Veterinary Record 122, 32–36. [DOI] [PubMed] [Google Scholar]
- Butt MT. (1990) Diagnosing erythrocytic parasitic diseases in cats. Compendium of Continuing Education for the Practising Veterinarian 12, 628–638. [Google Scholar]
- Carney HC, England JJ. (1993) Feline hemobartonellosis. Veterinary Clinics of North America—Small Animal Practice 23, 79–90. [DOI] [PubMed] [Google Scholar]
- Clark R. (1942) Eperythrozoon felis (sp. Nov) in a cat. Journal of the South African Veterinary Medical Association 13, 15–16. [Google Scholar]
- Cooper SK, Berent LM, Messick JB. (1999) Competitive, quantitative PCR analysis of Haemobartonella felis in the blood of experimentally infected cats. Journal of Microbiological Methods 34, 235–243. [Google Scholar]
- Espada Y, Prats A, Albo F. (1991) Feline haemobartonellosis. Veterinary International 3, 35–40. [Google Scholar]
- Flagstad A, Larsen SA. (1969) The occurrence of feline infectious anaemia in Denmark. Nordisk Veterinaer Medicin 21, 129–141. [PubMed] [Google Scholar]
- Flint JC, McKelvie DH. (1955) Feline Infectious Anaemia—Diagnosis and Treatment. In 92nd Annual Meeting of the American Veterinary Medical Association, pp. 240–242. Minneapolis.
- Flint JC, Moss LC. (1953) Infectious anaemia in cats. Journal of the American Veterinary Medical Association 122, 45–48. [PubMed] [Google Scholar]
- Flint JC, Roepke MH, Jensen R. (1958) Feline infectious anaemia. I. Clinical aspects. American Journal of Veterinary Research 19, 164–168. [PubMed] [Google Scholar]
- Foley JE, Harrus S, Poland A, Chomel B, Pedersen NC. (1998) Molecular, clinical, and pathologic comparison of two distinct strains of Haemobartonella felis in domestic cats. American Journal of Veterinary Research 59, 1581–1588. [PubMed] [Google Scholar]
- Hammer AS, Wellman M. (1999) Leukoerythroblastosis and normoblastemia in the cat. Journal of the American Animal Hospital Association 35, 471–473. [DOI] [PubMed] [Google Scholar]
- Harvey JW, Gaskin JM. (1977) Experimental feline haemobartonellosis. Journal of the American Animal Hospital Association 13, 28–38. [Google Scholar]
- Hatakka M. (1972) Haemobartonellosis in the domestic cat. Acta Veterinaria Scandanavica 13, 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins JD, Barta O. (1984) Concurrent Haemobartonella felis and Toxoplasma gondii infections in a cat. Veterinary Medicine: Small Animal Clinician 79, 633–637. [Google Scholar]
- Jensen WA, Lappin MR, Kamkar S, Reagen WJ. (2001) Use of a polymerase chain reaction assay to detect and differentiate two strains of Haemobartonella felis infection in naturally infected cats. American Journal of Veterinary Research 62, 604–608. [DOI] [PubMed] [Google Scholar]
- Johansson KE, Tully JG, Bolske G, Pettersson B. (1999) Mycoplasma cavipharyngis and Mycoplasma fastidiosum, the closest relatives to Eperythrozoon spp. and Haemobartonella spp. FEMS Microbiology Letters 174, 321–326. [DOI] [PubMed] [Google Scholar]
- Lutz H, Leutenegger C, Hofmann-Lehmann R. (1999) The role of polymerase chain reaction and its newer developments in feline medicine. Journal of Feline Medicine and Surgery 1, 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maede Y, Hata R, Shibata H, Niiyama M, Too K, Sonoda M. (1974) Clinical observation on 6 cases of feline infectious anaemia. Journal of the Japanese Veterinary Medical Association 27, 267–272. [Google Scholar]
- Manusu HP. (1961) Infectious feline anaemia in Australia. Australian Veterinary Journal 37, 405. [Google Scholar]
- Messick JB, Berent LM, Cooper SK. (1998) Development and evaluation of a PCR-based assay for detection of Haemobartonella felis in cats and differentiation of H felis from related bacteria by restriction fragment length polymorphism analysis. Journal of Clinical Microbiology 36, 462–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrljak V, Modric Z, Bedrica L, Tudja M, Curic S, Stanin D. (1995) Haemobartonella felis as a cause of infectious anaemia in a cat in Croatia. Kleintierpraxis, 403–408. [Google Scholar]
- Neimark H, Kocan KM. (1997) The cell wall-less rickettsia Eperythrozoon wenyonii is a Mycoplasma. FEMS Microbiology Letters 156, 287–291. [DOI] [PubMed] [Google Scholar]
- Prieur WD. (1960) Beitrag zur infektiösen anämie der Katze. Kleintier praxis 5, 87–89. [Google Scholar]
- Rikihisa Y, Kawahara M, Wen B, Kociba G, Fuerst P, Kawamori F, Suto C, Shibata S, Futohashi M. (1997) Western immunoblot analysis of Haemobartonella muris and comparison of 16S rRNA gene sequences of H. muris, H felis, and Eperythrozoon suis. Journal of Clinical Microbiology 35, 823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. (1987) The neighbour-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology & Evolution 4, 406–425. [DOI] [PubMed] [Google Scholar]
- Seamer J, Douglas SW. (1959) A new blood parasite of British cats. Veterinary Record 71, 405–408. [Google Scholar]
- Small E, Ristic M. (1967) Morphologic features of Haemobartonella felis. American Journal of Veterinary Research 28, 845–851. [PubMed] [Google Scholar]
- Tasker S, Helps CR, Belford CJ, Birtles RJ, Day MJ, Sparkes AH, Gruffydd-Jones TJ, Harbour DA. (2001a) 16S rDNA comparison demonstrates near identity between a United Kingdom Haemobartonella felis strain and the American California strain. Veterinary Microbiology 81, 73–78. [DOI] [PubMed] [Google Scholar]
- Tasker S, Gruffydd-Jones TJ, Jensen WA, Lappin MR. (2001b) Prevalence and Molecular Analysis of Haemobartonella felis strains in the United Kingdom. In 11th Congress of the European Society of Veterinary Internal Medicine, pp. 112. Dublin, Ireland.
- Théry A. (1966) Un foyer d'hémobartonellose féline à Paris. Recueil de Medecine Veterinaire 142, 1163–1166. [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research 22, 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanSteenhouse JL, Millard JR, Taboada J. (1993) Feline haemobartonellosis. Compendium of Continuing Education for the Practising Veterinarian 15, 535–545. [Google Scholar]
- Westfall DS, Jensen WA, Reagan WJ, Radecki SV, Lappin MR. (2001) Inoculation of two genotypes of Haemobartonella felis (California and Ohio variants) to induce infection in cats and the response to treatment with azithromycin. American Journal of Veterinary Research 62, 687–681. [DOI] [PubMed] [Google Scholar]
- Wilkinson GT. (1968) A review of drug toxicity in the cat. Journal of Small Animal Practice 9, 21–32. [DOI] [PubMed] [Google Scholar]
- Winter RB. (1993) Using quinolones to treat haemobartonellosis. Veterinary Medicine, 306–308. [Google Scholar]