Abstract
Feline leprosy refers to a condition in which cats develop granulomas of the subcutis and skin in association with intracellular acid-fast bacilli that do not grow on routine laboratory media. In this study the definition was extended to include cases not cultured, but in which the polymerase chain reaction (PCR) identified amplicons characteristic of mycobacteria. Tissue specimens from 13 such cases from eastern Australia were obtained between 1988 and 2000. This cohort of cats could be divided into two groups on the basis of the patients' age, histology of lesions, clinical course and the sequence of 16S rRNA PCR amplicons.
One group consisted of four young cats (less than 4 years) which initially developed localised nodular disease affecting the limbs. Lesions progressed rapidly and sometimes ulcerated. Sparse to moderate numbers of acid-fast bacilli were identified using cytology and/or histology, typically in areas of caseous necrosis and surrounded by pyogranulomatous inflammation. Organisms did not stain with haematoxylin and ranged from 2 to 6 μm (usually 2 to 4 μm). Mycobacterium lepraemurium was diagnosed in two cases based on the sequence of a 446 bp fragment encompassing the V2 and V3 hypervariable regions of the 16S rRNA gene a different sequence was obtained from one additional case, while no PCR product could be obtained from the remaining case. The clinical course was considered aggressive, with a tendency towards local spread, recurrence following surgery and development of widespread lesions over several weeks. The cats resided in suburban or rural environments.
A second group consisted of nine old cats (greater than 9 years) with generalised skin involvement, multibacillary histology and a slowly progressive clinical course. Seven cats initially had localised disease which subsequently became widespread, while two cats allegedly had generalised disease from the outset. Disease progression was protracted (compared to the first group of cats), typically taking months to years, and skin nodules did not ulcerate. Microscopically, lesions consisted of sheets of epithelioid cells containing large to enormous numbers of acid-fast bacilli 2 to 8 μm (mostly 4 to 6 μm) which stained also with haematoxylin. A single unique sequence spanning a 557 bp fragment of the 16S rRNA gene was identified in six of seven cases in which it was attempted. Formalin-fixed paraffin-embedded material was utilised by one laboratory, while fresh tissue was used in another. The same unique sequence was identified despite the use of different primers and PCR methodologies in the two laboratories. A very slow, pure growth of a mycobacteria species was observed on Lowenstein-Jensen medium (supplemented with iron) and semi-solid agar in one of three cases in which culture was attempted at a reference laboratory. Affected cats were domicile in rural or semi-rural environments. These infections could generally be cured using two or three of rifampicin (10–15 mg/kg once a day), clofazimine (25 to 50 mg once a day or 50 mg every other day) and clarithromycin (62.5 mg per cat every 12 h).
These findings suggest that feline leprosy comprises two different clinical syndromes, one tending to occur in young cats and caused typically by M lepraemurium and another in old cats caused by a single novel mycobacterial species.
Feline leprosy refers to a mycobacterial infection in which single or multiple granulomas form in the skin or subcutis in association with acid-fast bacilli (AFB) which are non-culturable using standard mycobacteriological methods (Pedersen 1988). The condition was first recorded by New Zealand and Australian researchers, in 1962 and 1963, respectively, although Carne had encountered cases as early as 1934 (Brown et al 1962, Lawrence & Wickham 1963). The disease has since been reported in Western Canada (McIntosh 1982), the Netherlands (Poelema & Leiker 1974), Britain (Wilkinson 1964, Robinson 1975) and the United States (Frye et al 1974) and is well documented in texts (Wilkinson 1977, Wilkinson & Mason 1991, Lewis & Kunkle 1998).
Although the causal organism(s) most likely have a world-wide distribution, the infection seems particularly common in certain geographical locations such as the North Island of New Zealand, the Netherlands and British Columbia. In these places the disease is well recognized and series of 44 and 179 cases have been reported (Scheifer et al 1974, Thompson et al 1979). Based on available data, feline leprosy would appear to be more common in temperate coastal areas and port cities, rather than in inland or tropical habitats (Pedersen 1988).
The causative agent of feline leprosy is purported to be Mycobacterium lepraemurium (Lewis & Kunkle 1998). This bacterium causes murine leprosy, a systemic mycobacterial infection of rats (Lowe 1937, Pattyn 1984). Cats are thought to contract M lepraemurium following bites from infected rodents (Lawrence & Wickham 1963). M lepraemurium is a fastidious, slow-growing organism which, with difficulty, can be cultured from large inocula on Ogawa's enriched egg yolk medium under strictly controlled conditions of temperature (35°C) and atmosphere (Ogawa & Motomura 1970) or in liquid medium with a pH of 6.0 to 6.2 (Nakamura 1999). As few investigators have successfully grown M lepraemurium from lesions from infected cats (Pattyn & Portaels 1980), the basis of ascribing this bacterium as the aetiological agent of feline leprosy has been historically dependent on transmission studies and the results of delayed-type hypersensitivity reactions (in cats) to intradermally administered tissue extracts from infected rats (Leiker & Poelema 1974, Pedersen 1988). Several groups have shown that material obtained from feline leprosy lesions can be used to transmit disease experimentally to rats and mice, and subsequently back to cats (Lawrence & Wickham 1963, Leiker & Poelema 1974, Allan & Wickham 1976, Schiefer & Middleton 1983, Mori & Kohsaka 1986). In such studies, the incubation period varied from 2 months to 1 year or more. Interestingly, some cats appeared more susceptible to infection than others and experimental cats tended to develop more localised and self-limiting disease than spontaneous cases. Cats that had recovered from natural infections were immune to experimental reinfection (Schiefer & Middleton 1983, Pedersen 1988). Guinea pigs injected with fresh material from spontaneous cases of feline leprosy show self-limiting lesions (Lawrence & Wickham 1963, Allan & Wickham 1976), while rats, mice and hamsters develop localised lesions which subsequently disseminate widely to internal organs and the bone marrow (Lawrence & Wickham 1963, Poelma & Leiker 1974, Allan & Wickham 1976, Schiefer & Middleton 1983).
According to the literature, cats with feline leprosy are generally less than 5 years of age, perhaps with a preponderance of males among reported cases. Presumably these demographic considerations reflect the need for the cat to interact with a rat to become infected. The initial lesion is said to be a focal granuloma involving the subcutis and skin (Fig 1). Owners become aware of solitary, or more commonly multiple, painless, raised, fleshy, tumour-like lesions, from a few millimetres up to 4 cm in diameter. Lesions are freely movable over underlying tissues, develop rapidly and, when large, may ulcerate (Pedersen 1988, Wilkinson & Mason 1991, Lewis & Kunkle 1998). Infection spreads to adjacent areas of the integument and lesions may encroach on underlying local tissues such as muscle and fascia. Histologically, organisms may be detected in regional lymph nodes.
Fig 1.
Localised feline leprosy lesions on the distal forelimb of a cat from North America (photograph courtesy of Dr Peter Ihrke). Note the ulcerated surface of the lesions.
Nodules can occur anywhere on the body, but tend to be concentrated on the head and limbs. Small lesions are occasionally found on the tongue, lips and nasal planum. Lesions, even if multiple, tend to be concentrated initially in one region and have the propensity to recur following attempted surgical excision. Affected cats are usually in good general health, at first. In some cases, however, the infection may become generalised with the development of large numbers of skin lesions of different sizes over the entire body, presumably as a result of haematogenous spread subsequent to local lymphatic drainage (Figs 2 to 5). These cats suffer malaise, depression, poor appetite and wasting and further investigation or necropsy may reveal a systemic infection with granulomatous lesions in internal organs (especially liver, spleen or lungs), lymph nodes and bone marrow (Pedersen 1988, Wilkinson & Mason 1991, Lewis & Kunkle 1998).
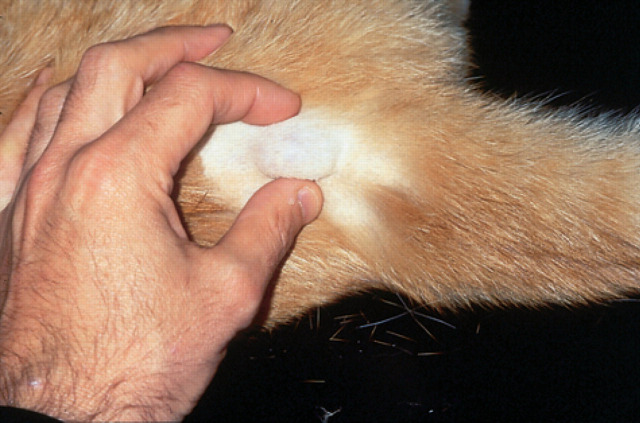
Fig 5.
A single subcutaneous mass is evident in the subcutis over the sacrum of a 13-year-old cat (case 16) infected with the novel mycobacterial species. Several similar lesions had been resected previously from the right thoracic limb.
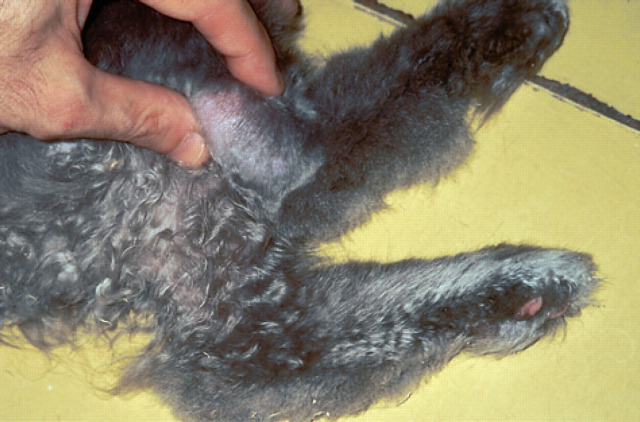
Fig 3.
A large subcutaneous nodule is evident in the subcutis over the lateral aspect of the hock in a 10.5-year-old cat (case 12) infected with the novel species of mycobacteria. Similar, but smaller, lesions were present in numerous other locations.
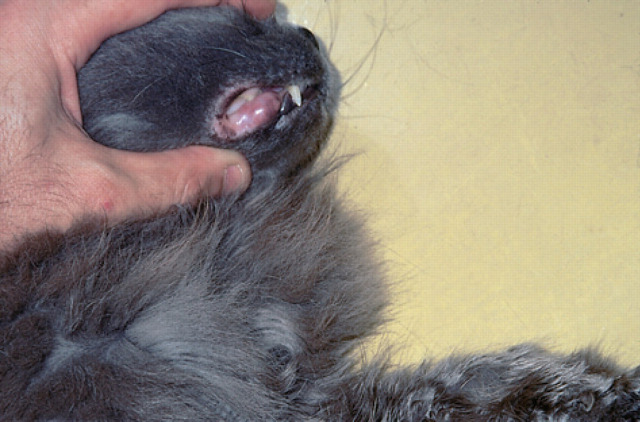
Fig 4.
A small lesion is present in the gingival tissues of the cat illustrated in Fig 3.
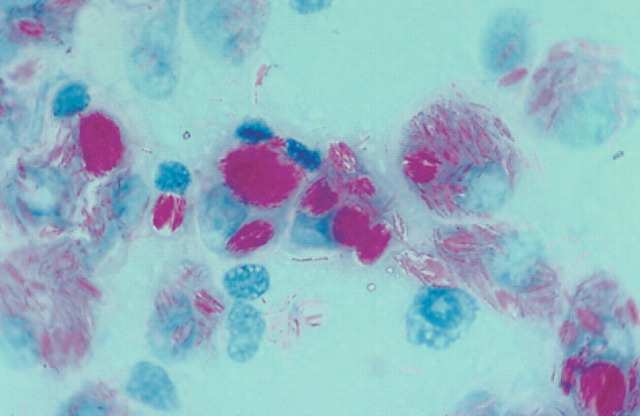
Pathologically, feline leprosy has been subdivided into ‘lepromatous’ or ‘tuberculoid’ forms on the basis of the number of AFB present and the immunological response of the host (Schiefer & Middleton 1983). Because the causal mycobacteria are slow-growing organisms capable of intracellular survival, the clinicohistological manifestations of disease are said to depend on the host's cell-mediated immune response to the parasite (Wolinsky 1973, Youmans 1980, Grange & Yates 1986, Kaufman 1993). When the immune response is poor, lepromatous (or multibacillary) disease develops with infiltration of the dermis with large sheets of foamy macrophages that contain enormous numbers of organisms. Macrophages loaded with mycobacteria (‘virchowcytes’) are sometimes considered to be ‘incompetent’, although recent work suggests they may actually be ‘bacterial cemeteries’ (Abulafia & Vignale 1999). In feline leprosy, AFB are usually arranged in the cytoplasm of macrophages as dense parallel accumulations known as globi, which displace the nucleus to an eccentric position within the cell (Figs 6–8) (Wilkinson 1977, Pedersen 1988). If the host's immune response is more effective, multiplication of the organism is limited by the granulomatous response in the dermis. This tuberculoid response consists of epithelioid histiocytic cells, moderate numbers of lymphoid cells, plasma cells and regions of caseous necrosis. This form of feline leprosy has accounted for a substantial proportion of cases in Western Canada, New Zealand and Holland (Schiefer et al 1974, Thompson et al 1979, McIntosh 1982). Granulomas are not encapsulated and tend to spread into adjacent tissues. Invasion of local nerves, a prominent feature of human leprosy, is rarely observed in feline leprosy, although a recent case report described a cat (without cutaneous lesions) which presented for mycobacterial infiltration of one sciatic nerve (Paulsen et al 2000).
Fig 6.
DiffQuik-stained smear made from biopsy material obtained from case 16. Negatively-stained bacilli are evident, individually and in bundles, predominantly within macrophages. ×726.
Fig 8.
Smear made from biopsy material from case 16 stained with Burke's modification of the Gram stain. Large number of bacilli are evident within macrophages; organisms have Gram-positive, Gram-negative and Gram-variable staining. ×726.
In smears and tissue sections from feline leprosy cases, AFB appear as long slender rods, 3 to 6 μm in length (Wilkinson 1977). In smears stained with Romanowsky stains such as DiffQuik, organisms appear as negative-staining bacilli (Fig 6) (Maygarden & Flanders 1990). In smears or sections stained with acid-fast stains such as Ziehl-Neelsen (ZN) or Fite's stain, organisms are acid/alcohol fast and retain the carbol fuschin stain (Fig 7). M lepraemurium is said to have a characteristic morphology both in vitro and in lesions, with pleomorphic AFB including very long filamentous forms, beaded forms, swollen ends and branching (Lowe 1937, Pattyn 1984).
Fig 7.
Acid-fast stain of biopsy material from case 16. Macrophages laden with abundant intracellular AFB (‘virchowcytes’) appear pink as a result of taking up the carbol fuschin stain, which is acid/alcohol fast. The AFB are often grouped into ovoid bundles (‘globi’). ×726.
Recently, molecular methodologies have been applied to the investigation of feline mycobacterial diseases. Of eight cases of invasive or disseminated cutaneous mycobacterial disease investigated by Hughes et al (1997) in Belfast using material collected largely from New Zealand cats, four cases were shown to have M lepraemurium infections. Of the remaining cases, one cat had a disseminated M avium infection (confirmed by culture), the aetiology in one cat was undetermined and in two cases infection was attributed to a novel mycobacterial species, which shared close nucleotide sequence identity with M malmoense (Hughes et al 1997). This suggested that at least one other fastidious or non-culturable mycobacterial species was involved in a proportion of feline leprosy cases. Given the current state of knowledge, the term feline leprosy is thus probably best considered to be a syndrome rather than a specific infection. Similar situations exist in human mycobacteriology, where, for example, the ‘Scrofula syndrome’ of localised cervical pyogranulomatous lymphadenitis in children can be caused by a large variety of mycobacteria including M scrofulaceum, M avium complex (MAC), M genavense and M interjectum (Grange & Yates 1986, Lotti & Hautmann 1993, Grange 1996).
Data from earlier molecular studies (Hughes et al 1997) encouraged us to re-appraise cases of feline leprosy studied previously and examine as many new cases as possible prospectively. The conclusion from our analysis is that feline leprosy actually comprises two syndromes referable to different mycobacterial species, that produce distinct disease manifestations in separate cohorts of cats.
Materials and methods
Clinical data and tissue specimens were obtained from cats with presumptive feline leprosy over a 13-year period. Most specimens were obtained during the prospective phase of the study, subsequent to the publication of new molecular information by Hughes et al (1997). Cases were initially defined as cats in which nodular lesions of the subcutis and skin were associated with AFB (detected cytologically or histologically) that could not be cultured on routine media. Subsequently, the definition was extended to include cases not cultured, but in which PCR identified characteristic amplicons indicative of either M lepraemurium or a novel mycobacterial species. Four of these were examined and treated at the University Veterinary Centre Sydney (UVCS). Tissue specimens from the remaining cats were submitted to the UVCS Diagnostic Laboratory for cytology and attempted culture, or were obtained at our request from commercial veterinary laboratories in Australia.
For cases examined at UCVS, a tentative clinical diagnosis of feline leprosy was confirmed by analyses of aseptically-collected deep tissue specimens for cytology, histopathology, culture and PCR. When fresh material was submitted from lesions, smears were made from crush preparations and stained with DiffQuik (Lab Aids Pty Ltd, 3 Gondola Road, Narrabeen 2101), Burkes modification of the Gram stain and an acid-fast stain (decolourisation with 6% sulphuric acid for 3 min). The morphology of bacteria in the smears was recorded, as was the cytological features of the host's response. After dissection of any adherent overlying skin, fresh material from the lesion was triturated in brain heart infusion broth using sterile sand in a sterile mortar and pestle. The resulting tissue homogenate was streaked onto duplicate 5% sheep blood agar plates and 1% Ogawa egg yolk medium slants (Ogawa & Motomura 1970) and incubated aerobically at 37°C for at least 2 months. Cases diagnosed cytologically or histologically by commercial laboratories were sometimes subjected to routine culture.
In five cases, fresh material was submitted to the mycobacteria reference laboratory at Westmead Hospital for attempted culture at 33°C and 37°C on a range of routine mycobacterial media, including Lowenstein-Jensen medium (with and without supplementary pyruvate or iron), Middlebrook semi-solid medium, medium supplemented with mycobactin used to culture M avium subsp paratuberculosis and BACTEC 12B medium. Fresh biopsy material was used to amplify the 16S rRNA gene using a semi-nested polymerase chain reaction (PCR) similar to that described previously and standard sample preparation methods (Boddinghaus et al 1990, Greenfield & White 1993, Hughes et al 2000). For many of the external cases, only paraffin-embedded, formalin-fixed material was available. For these cases, one portion of the block was used for histology, while another was sent to Belfast for DNA capture, PCR and sequencing of the 16S rRNA gene (Hughes et al 1999, 2000). In one case, a portion of fresh biopsy material was freeze dried and processed in Belfast by methods described previously (Hughes et al 2000).
For all specimens for which formalin-fixed material was available, haematoxylin and eosin (H&E), Brown and Brenn (Gram) and ZN-stained sections were prepared. These sections were used to obtain qualitative information concerning the mycobacteria in the sections (numbers, morphology, length, Gram staining reaction, acid-fastness) and the accompanying host response (granulomatous, pyogranulomatous, presence of caseous necrosis). In some cases, paraffin blocks could not be obtained from cases for which limited fresh tissue had been submitted; in these cases we relied on the histological description provided by the veterinary pathologist who reported on the sections. In some cases the presence in serum of FeLV antigen or FIV antibodies were determined using commercial ELISA test kits (Table 1).
Table 1.
Clinical and laboratory data concerning 19 cats with feline leprosy
| Case number/name of patient | Age (yrs) | Breed/FIV status | Sex (neuter/intact) | Domicile/state | Urban/suburban/rural | Date lesions first detected | Distribution of lesions | Clinical course | Treatment | Response | Cytology/AFB morphology/culture | Species ID/place of ID/EMBL accession number |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (A) Feline leprosy in young cats associated with tuberculoid histology necrosis, rapidly progressive clinical course and usually attributable to M lepraemurium | ||||||||||||
| Case 1 | 1.5 | DSH/FIV negative | M(E)* | Narraweena/NSW | Suburban | 2/98 | Left and right axillae and antebrachium, then generalised skin with ulceration | Aggressive, progressive | Surgery; clofazimine, clarithromycin, rifampicin | No response to therapy; euthanased | Pyogranulomatous; large areas of necrosis containing sparse to moderate no. AFB (1 to 2+); culture negative at W | M lepraemurium (NI & W); AJ279017 |
| Case 2 | 2 | DSH/FIV negative | F(N) | South Gippsland/Victoria | Rural/near coast | 9/99 | Caudal right hock; then left front lateral digit | Indeterminate | Surgery; clofazimine | Cured | Numerous AFB, branching morphology; no necrosis; routine culture negative | Mycobacterium sp but not M lepraemurium or the novel species (W) |
| Case 3 | 3 | DSH/N/A | M(N) | Winston Hills/NSW | Suburban | N/A | Localised | Aggressive | Extensive surgery; doxycycline | Lost to follow-up; thought to be cured | Pyogranulomatous; sparse AFB in areas of necrosis (1+) culture negative at UVCS and Queensland Mycobacteria Reference Laboratory | M. lepraemurium (NI); AJ279017 |
| Case 4 | 3 | DSH/FIV negative | M(E)* | Avalon/NSW | Suburban/semi-rural | 9/88 | Left antebrachium; then generalised skin | Aggressive; disseminated widely within two months | Drainage, amoxicillin, trimeth/sulpha | Euthanased | Pyogranulomatous; moderate no. AFB in skin, subutis and lymph nodes (2+); AFB moderate length, branching; culture negative at UVCS | No PCR product obtained (NI) |
| Case 5 Hughes et al–case 3† | 3 | DSH | M(N?) | New Zealand | N/A | N/A | ‘tumour’ | N/A | N/A | N/A | Granulomatous; 4+ | M lepraemurium (NI); AJ279017 |
| Case 6 Hughes et al–case 4† | 8 | N/A | F(N?) | New Zealand | N/A | N/A | Domed forehead | N/A | N/A | N/A | Granulomatous; 3+ | M lepraemurium (NI); AJ279017 |
| Case 7 Hughes et al–case 5† | 1 | DSH | F(N?) | New Zealand | N/A | N/A | Left shoulder | N/A | N/A | N/A | Pyogranulomatous; central necrosis; 2+ | M lepraemurium (NI); AJ279017 |
| Case 8 Hughes et al–case 7† | 2 | DSH | M(N?) | New Zealand | N/A | N/A | Generalised skin | N/A | N/A | N/A | Pyogranulomatous; 2+ | M lepraemurium (NI); AJ279017 |
| (B) Feline leprosy in old cats with lepromatous histology, a slowly progressive clinical course and attributable to a single novel mycobacterial species | ||||||||||||
| Case 9 | 9.5 | DSH/N/A | F(N) | Terrey Hills/NSW | Suburban/semi-rural | 10/95 | Left antebrachium; then generalised skin and nose | Indolent; infection present for 4 years | Surgery, doxycycline; surgery, enroflox | Partial control; eventually euthanased | Granulomatous; enormous no. AFB (4+) | Novel (NI); AJ294741 |
| Case 10 | 10 | DSH/FIV positive | M(N) | Hilltop/Southern Highlands NSW | Rural | 3/96 | Tail base, distal hind limb, head, possibly face | Indolent; biopsied 8/97, euthanased 3/2000 | Doxycycline; then enrofloxacin | Partial control | Pyogranulomatous; moderate to marked no. AFB (3 to 4+) | Novel (NI); AJ294743 |
| Case 11 | >10 | DSH/FIV negative | F(N) | Wyee Point Central Coast NSW | Suburban/rural | 8/96 | Tail and left hock; then generalised skin | Indolent; progressed slowly over 5 months | Surgery, clofazimine (toxic?), rifampicin | Much improved; died of renal failure | Granulomatous; enormous no. AFB (4+); culture negative at UVCS and W | Novel (W); AJ294742 |
| Case 12‖ | 10.5 | Persian/FIV-negative | M(N) | Terrey Hills/NSW | Suburban/rural | 7/97 | Tail base and left hock; then generalised skin and? liver | Indolent; progressed slowly over 2 months | Surgery, clofazimine, doxycycline, ciprofioxacin | Cured | Granulomatous; enormous no. AFB (3 to 4+); culture negative at UVCS and W | Novel (W); AJ294740 |
| Case 13 | 13 | DSH/N/A | M(N) | Maclean/NSW | Rural | 11/98 | Generalised skin | Indeterminate | Rifampicin, clofazimine, clarithromycin | Cured; developed megestrol-induced diabetes and euthanased 6/99 | Numerous AFB; routine culture negative | No PCR product obtained (W) |
| Case 14 | 13 | DSH/ | ||||||||||
| N/A | M(N) | Balgownie/NSW | Suburban | 4/99 | Left forelimb; then generalised skin with ulceration | Indeterminate | Rifampicin, clofazimine, clarithromycin (toxicity?) | Cured; later died of renal failure with Trichosporon beigelii in urine | Granulomatous; numerous AFB; Routine culture negative | Not done | ||
| Case 15 | 14 | DLH/N/A | F(N) | Williamstown/Victoria | Suburban/rural | 1/98 | Nasal lesion; then, after 1 year, generalised skin, especially limbs | Indolent | Clofazimine monotherapy; later rifampicin, clofazimine, clarithromycin | Partial response; photo-sensitivity to clofazimine | Granulomatous; numerous beaded AFB; routine culture negative | Not done |
| Case 16 | ∼13 | DSH/FIV-positive | M (N) | Rankin Park/Central Coast NSW | Suburban/rural | 9/2000 | Right forelimb, skin over sacrum, ear | Uninterpretable | Surgery, rifampicin, clarithromycin | Cured | Pyogranulomatous; moderate to numerous AFB (2 to 3+); positive culture at W | Novel (W) |
| Case 17 | 14 | DSH/N/A | F(N?) | Gordon/NSW | Suburban | N/A | Forehead | N/A | N/A | N/A | Pyogranulomatous; sparse to moderate no. AFB (1 to 3+) | Novel (NI); AJ294744 |
| Case 18 Hughes et al–case 1† | 9 | DLH | M(N?) | New Zealand | N/A | N/A | Generalised skin | N/A | N/A | N/A | Granulomatous; 4+ | Novel (NI); AJ294745 |
| Case 19 Hughes et al–case 2† | 9 | Persian | M(N?) | New Zealand | N/A | N/A | Forelimb | N/A | N/A | N/A | Pyogranulomatous; 4+ | Novel (NI); AJ294746 |
both these cats were entire males at the time the diagnosis of mycobacteriosis was made; both were subsequently castrated.
Hughes et al J Clin Micro 35, 2464–2471, 1997; limited clinical information only available for these cases.
Barrs et al Aust Vet Practit 29, 159–164, 1999.
AFB, acid fast bacilli; DSH, domestic short hair cat; DLH, domestic long hair cat; F(N), speyed female; ID, identification; M(E), Male entire i.e. Tom cat; M(N), castrated male; N/A, not available; NI, Northern Ireland; NSW, New South Wales; trimeth/sulpha, trimethoprim/sulphadiazine; UVCS, University Veterinary Centre Sydney; W, Centre for Infectious Diseases and Microbiology, Institute for Clinical Pathology and Research, Westmead Hospital, Westmead, NSW 2145, Australia.
Clinical information, laboratory data and follow-up were often incomplete for external cases included in this study. One of the authors (RM) consulted with the primary clinician concerning the treatment of several external cases. In the prospective study, this was facilitated by the donation of clarithromycin for this project by Abbott Australasia.
Pertinent information concerning 13 Australian cases was tabulated into a spreadsheet, along with limited information concerning six additional cases from New Zealand reported previously by Hughes et al (1997) (Table 1). Data from all these cases are considered in the analysis which follows.
Results
Tissue specimens from 13 cats with feline leprosy were obtained between 1988 and 2000. All but two of the cats were domicile in New South Wales, presumably reflecting the tendency of veterinarians to consult with their local University Veterinary Centre. The remaining two cases were from Victoria. Cats ranged in age from 1.5 to 14 years; eight were male (two entire; six castrated) and five were female (all spayed); 12 were domestic crossbred cats (11 short hair, one long hair) and one was a Persian (Table 1).
Preliminary analysis of these cases indicated that the data set could be partitioned into two groups based on the patient's age, histology of lesions, clinical course and the sequence of PCR amplicons: (1) young cats, with initially localised but rapidly progressive disease associated with pyogranulomatous inflammation and necrosis; amplicons characteristic of M lepraemurium (Hughes et al 1997) were typically obtained from these cases using PCR, and (2) old cats with localised or generalised skin involvement, lepromatous pathology and a slowly progressive clinical course; PCR amplicons of a single novel species of mycobacterium were obtained from these cases.
Feline leprosy in young cats associated with tuberculoid histology, necrosis, rapidly progressive clinical course and usually attributable to M lepraemurium
This group comprised four young cats (1.5 to 3 years) from Australia and a further four previously cited from New Zealand (Table 1). Of the Australian cats, three were male (two entire at diagnosis) and one was a spayed female. All resided in suburban/semirural (three) or rural (one) environments and initially had localised disease affecting the proximal (3) or distal (1) limbs. In at least one case the lesions were ulcerated.
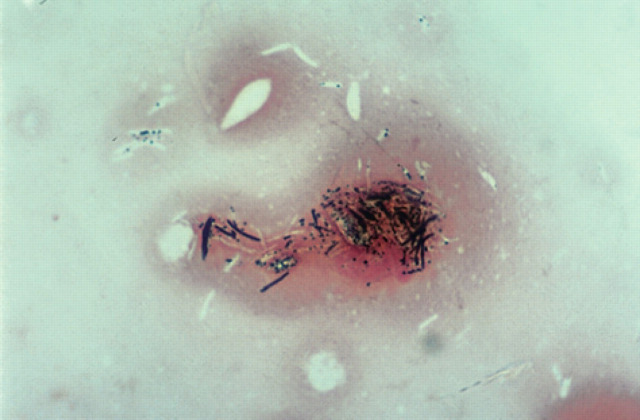
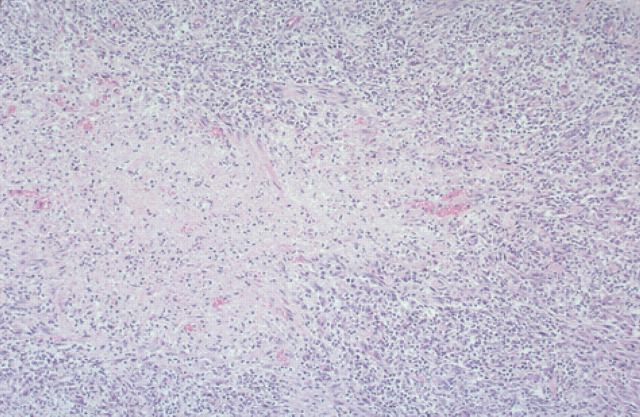
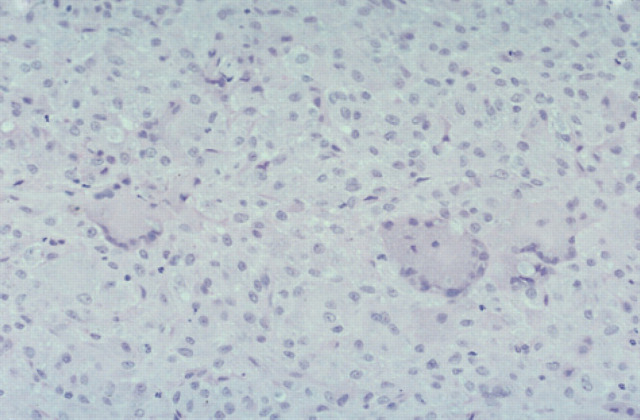
Sparse to moderate numbers of AFB were identified microscopically in affected tissues. Lesions were not well circumscribed because of the numerous lymphocytes and plasma cells surrounding and dispersed through the nodules. Neutrophils were numerous but well dispersed and giant cells were evident also. Because of the heavy and diffuse infiltration with lymphoid cells and neutrophils, it was difficult to visualise epithelioid cells clearly. A prominent feature in these specimens was the large areas of necrosis (Fig 9). Bacterial filaments and rods were not evident in H&E stained sections, but ZN staining demonstrated AFB primarily in the necrotic areas. Organisms ranged from 2 to 6 μm, but most were 2–4 μm. They had a beaded appearance and there was some suggestion of a branching morphology. In Brown and Brenn-stained sections, organisms showed Gram-variable staining although the numbers observed were small. There was no indication of dissemination to internal organs clinically, or in the two cases necropsied, despite widespread cutaneous (Fig 2) and regional lymph node involvement present in the worse cases. Despite using Ogawa egg medium and special growth conditions described by Ogawa, Portaels and their collaborators (Ogawa & Motomura 1970, Pattyn & Portaels 1980), we were unsuccessful in isolating mycobacteria from the cases (1, 3 and 4) in which this was attempted. Utilisation of liquid medium with a pH of 6.0 to 6.2 (Nakamura 1999) was not undertaken, however.
Fig 9.
Histological section (6 μm) of a biopsy specimen from a case of feline leprosy from which gene fragments typical of M lepraemurium were amplified (case 3). There is a central area of necrosis surrounded by numerous lymphocytes and neutrophils that overlay macrophages. H&E, original magnification ×110.
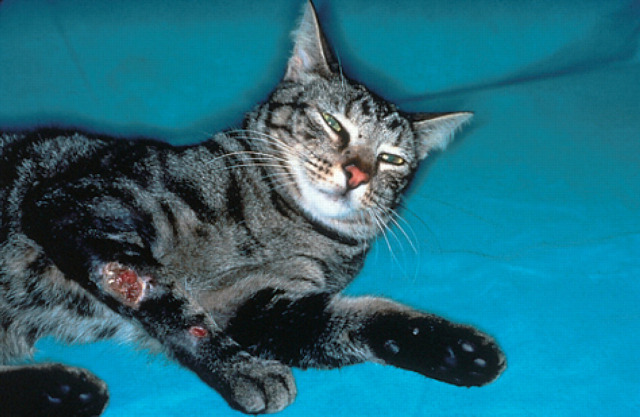
Fig 2.
Multiple subcutaneous nodules affecting the distal forelimb of a 3-year-old cat (case 4) with feline leprosy. This cat had similar nodular lesions over virtually the entire integument.
Freeze dried (case 1), fresh (case 2) or formalin-fixed paraffin-embedded (case 3 and 4) tissues were available for PCR of the 16S rRNA gene and sequence analysis. M lepraemurium was diagnosed in two cases (cases 1 and 3) based on the sequence of a 446 bp fragment encompassing the V2 and V3 hypervariable regions (EMBL Accession Number AJ279017). A different 16S rRNA sequence (at least 8 bp differences from M lepraemurium over the V2 and V3 regions) was obtained from case 2 (which differed also from the other cases histologically in having numerous organisms but no necrosis), while no PCR product was amplified from the paraffin blocks from case 4.
The clinical course was considered aggressive in three of the four cats, with a tendency towards local spread, recurrence following surgery and rapid (within 2 months) development of widespread cutaneous involvement. One of these cats failed to improve despite surgery and subsequent combination therapy with rifampicin, clofazimine and clarithromycin; one was lost to follow-up although thought to be cured following extensive surgery and doxycycline therapy, whilst treatment was not attempted in one cat referred following development of widespread cutaneous disease following surgical drainage and routine antibacterial therapy. The cat (case 2) in which PCR demonstrated a sequence other than M lepraemurium was cured by wide surgical excision of a two distal lesions followed by eight weeks of clofazimine (25 to 50 mg daily).
The four additional cases from New Zealand conformed with the proposed profile for this cohort of patients, apart from one cat (case 6) that was older than usual (8 years) at diagnosis. All specimens were submitted from the Upper Hutt region of the Northern Island, a cold climate area where feline leprosy is prevalent. In each case, PCR confirmed an aetiologic role for M lepraemurium. Lesions were localised in two of three cases for which data were available.
Feline leprosy in old cats with lepromatous histology, a slowly progressive clinical course and attributable to a single novel mycobacterial species
This group comprised nine aged cats (9.5 to 14 years) from Australia and a further two cats from New Zealand (Table 1). Of the Australian cats, five were castrated males and four were spayed females. Of four cats tested, two had FIV antibodies detected using ELISA, but none were FeLV-positive. There was a noticable tendency for cats to live outside urban/suburban metropolitan areas. Seven of the nine cats had well documented localised disease (affecting the head, tail or limbs), which in most cases subsequently became generalised. The progression of disease was protracted, typically taking many months or years. Although two cats were allegedly presented with widespread cutaneous disease it was not possible to determine whether they had localised lesions initially that had not been detected. Lesions in these cats were never ulcerated.
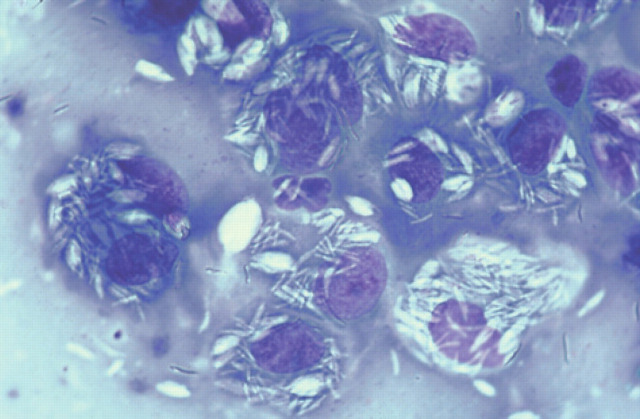
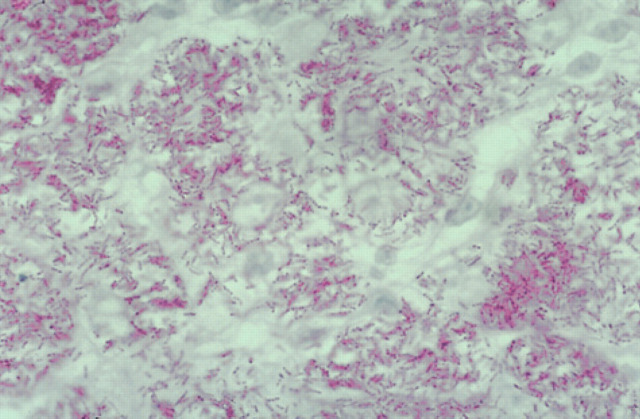
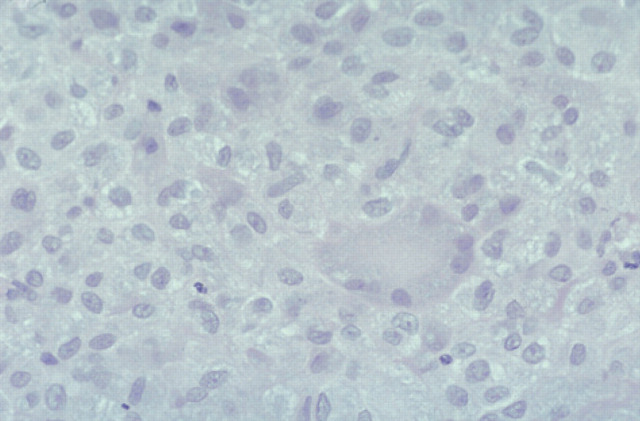
The histological changes were similar in all cases, with moderate to enormous numbers of AFB in tissue specimens (Figs 6–8; 10–12). Lesions consisted of discrete or coalesced nodules composed primarily of sheets of epithelioid cells (Figs 10 and 11). Nodules were highly vascular but contained little new connective tissue, although some collagen from the dermis had been surrounded by the epithelioid cells. Lesions involved the subcutis and deep dermis primarily, and hair follicles were often surrounded by the pathologic process. In some specimens, where nodules had coalesced, epithelioid cells extended to just below the epidermis. The epithelioid cells were often very plump and polygonal, although in parts of some sections they appeared spindle-shaped. Giant cells varied from sparse to numerous. Neutrophils were usually sparse and well dispersed, but some small clusters were present within more extensive lesions. Lymphocytes were sparse and well dispersed, but perivascular nodules did occur and were sometimes numerous in advanced lesions. Plasma cells were variably admixed with the lymphocytes. In H&E stained sections, lightly staining blue-grey filaments and rods were often apparent within epithelioid macrophages and giant cells (Fig 11). Brown and Brenn-stained sections showed Gram-variable rods present in very large numbers. ZN-stained sections demonstrated these were AFB, 2–8 μm in length (but mostly 4–6 μm) with variable amounts of beading and possible branching, and they were often tightly packed in parallel arrays within epithelioid cells (Fig 12). Partial staining of organisms with the ZN method occurred in one skin specimen obtained subsequent to effective antimicrobial therapy. Dissemination to the liver may have occurred in one cat (case 12), which was icteric and had moderately elevated activities of alanine aminotransferase and alkaline phosphatase; these indices of hepatic involvement normalised during successful drug therapy.
Fig 10.
Histological section (6 μm) of a biopsy obtained from a case of feline leprosy caused by the novel mycobacteria species (case 11). Note the sheets of epithelioid cells and some scattered multinucleate giant cells. Occasional small lymphocytes are evident also. H&E, original magnification ×220.
Fig 12.
Histological section (6 μm) of a biopsy obtained from a case of feline leprosy caused by the novel mycobacteria species. Numerous beaded acid-fast rods and filaments are present in clusters within the cytoplasm of macrophages. ZN, original magnification ×1100.
Fig 11.
Histological section (6 μm) of a biopsy obtained from the same case as Fig 10. Clusters of grey/blue filaments and rods are visible in the cytoplasm of the multinucleate giant cell and epithelioid cells. H&E, original magnification ×440.
Fresh (cases 11, 12, 13, 16) or paraffin-embedded formalin-fixed (cases 9, 10, 17) tissues were subjected to 16S rRNA PCR and sequence analysis. PCR was performed in Belfast on freeze-dried or paraffin-embedded tissues and at Westmead on fresh tissue specimens. A single unique sequence spanning a 557 bp fragment of the 16S rRNA gene was identified in cases 9, 10, 11, 12, 16 and 17 (accession numbers have been deposited at EMBL Database for five of these cases; Table 1). No PCR product was obtained from the sparse tissue available from case 13, and although paraffin blocks were available from cases 14 and 15, limited resources precluded further PCR studies. The two additional cases from New Zealand conformed with stated profile for this cohort of patients, namely cats older than nine years, with numerous AFB in lesions and the same unique 16S rRNA sequence reported, in part, previously (Table 1; Hughes et al 1997).
Mycobacterial culture was attempted for three of the cases seen at the UVCS, both in our laboratory and at a reference laboratory. Despite abundant AFB in the biopsy specimens, culture was unsuccessful, except for case 16 in which a scant, pure growth of a very slow growing mycobacteria species was observed on Lowenstein-Jensen medium (supplemented with iron) and in semi-solid agar. This specimen was from a large solitary lesion containing enormous numbers of mycobacteria, resected at UCVS and sent promptly to the reference laboratory. 1 It could be that as with M lepraemurium, successful culture hinges on using an extremely large inoculum onto solid and semi-solid medium (Pattyn & Portaels 1980). 16S rRNA PCR amplicons from colonial material grown in vitro had an identical sequence to the AFB-containing tissue specimen from which the culture was obtained.
We have detailed information concerning therapy for the cases managed at the UVCS or in consultation with the authors. In the remaining cases, limited information was available from clinical notes obtained retrospectively. Six cats were treated using drug regimens with likely efficacy against slow-growing, saprophytic, mycobacterial species, sometimes combined with surgery designed to debulk large accessible lesions. It was considered that optimal therapy constituted two or three orally-administered agents including rifampicin (10–15 mg/kg once a day; typically 50 to 75 mg per cat once a day), clofazimine (25 to 50 mg per cat once a day or 50 mg every second day) and clarithromycin (62.5 mg per cat every 12 h). Sometimes additional agents were also used, including doxycycline (5 mg/kg every 12 h) or ciprofloxacin (62.5 mg to 125 mg per cat every 12 h) (Broughton & Lloyd 1987, Mundell 1990, Rapp et al 1994, Kaufman et al 1995, Gunn-Moore et al 1996, Greene & Watson 1998, Lewis & Kunkle 1998, Malik et al 1998a, Malik et al 2000).
Four cases (12, 13, 14, 16) were considered to be cured based on complete resolution of lesions. The treatment of one of these (case 12) has been recorded (Barrs et al 1999). Another (case 14) had perinephric pseudocysts at the time of diagnosis, and eventually died seven months later of renal failure, in association with a Trichosporon beigelii urinary tract infection. One cat (case 11) also developed terminal renal failure during therapy, at a time when its subcutaneous lesions were resolving. As azotaemia was not initially evident, renal failure may have resulted from the drugs administered or effects secondary to successful antibacterial therapy. In case 15 combination therapy was thought probably to have cured the mycobacterial infection; clofazimine was considered to be the most effective agent, although its use was associated with photosensitivity. The response to treatment was hard to gauge in two patients treated using surgery and doxycycline, and later surgery and enrofloxacin, while insufficient data was obtainable for the remaining patient (case 17).
Discussion
Information from this study and the seminal paper by Hughes et al (1997) establish feline leprosy as a clinical condition comprising at least two different syndromes. One occurs principally in young cats and is usually caused by the rat leprosy bacillus M lepraemurium, while another occurs predominantly in old cats and is caused by a single novel species of mycobacterium. Both probably begin as localised lesion(s) that have the propensity to spread locally and to become widespread, although progression of lesions is often more rapid for infections caused by M lepraemurium. It should be mentioned also that other mycobacterial species, such as M tuberculosis (Wilkinson & Mason 1991), M microti (Gunn-Moore et al 1996), MAC (Hughes et al 1997) and M genavense (Hughes et al 1999, Lucas et al 2000) can produce disseminated disease in small animals, sometimes with cutaneous involvement, although these infections tend to present with conspicuous involvement of liver, spleen, lymph nodes, lungs or bone marrow, rather than primarily for disease of the subcutis and skin.
The molecular insights obtained in this study were clarifying, as we would have otherwise been puzzled as to why many feline leprosy cases did not conform with the expected textbook picture. Instead of a localised lesion or group of lesions on the head or extremity of a young cat, the majority of cats in our investigation were elderly and had typically developed widespread cutaneous disease by the time they were referred for a second opinion. Interestingly, close examination of the original data concerning feline leprosy cases from New Zealand shows a biphasic age distribution, with most infections occurring in cats about three years of age, but with a second smaller peak in cats about 10 years old (Thompson et al 1979). The existence of two syndromes, rather than one, clearly has important implications for prevention, diagnosis and therapy. For example, most authors recommend wide surgical excision as the treatment of choice for localised feline leprosy cases (Pedersen 1988, Wilkinson & Mason 1991, Lewis & Kunkle 1998). This is a practical option in infections caused by M lepraemurium, at least when cats are presented for treatment in a timely manner and when the primary clinician has a high index of suspicion for a mycobacterial aetiology. On the other hand, although surgery may have a place in cytoreducing particularly large lesions in infections caused by the novel mycobacterial species, numerous small lesions will almost invariably develop elsewhere in these cats despite radical excision of the likely primary focus.
The division of feline leprosy into two syndromes should be considered in relation to observations by veterinary pathologists (Richard Miller, personal communication) that this condition is more common in temperate climates (Victoria and South Australia) than in the warmer regions (New South Wales and Queensland). Murine leprosy is common in these regions in part because there are sufficient rodent vectors, but also presumably because the cool climate facilitates the development of disease. Temperature per se may have some bearing on the pathogenesis and incidence of M lepraemurium infections, as not only is disease more common in cooler environments, but lesions most commonly develop in the coolest months of the year (eg, in June/July in New Zealand; Thompson et al 1979). Furthermore, there are reports of disease regression with increasing ambient temperature. It could be that, like M marinum (Grange 1996) and the canine leproid granuloma syndrome organism (Malik et al 1998b), M lepraemurium may prefer to grow at cooler temperatures, such as those encountered in the integument, oral mucosa and upper respiratory tract. This would explain why dissemination to internal organs was not seen in case 4, despite extensive and widespread involvement of virtually the entire skin surface and draining regional lymph nodes. It would also provide a reason why M lepraemurium infections are not more common in rat-infested tropical environments near the cane fields in far north Queensland, where diseases related to rats such as leptospirosis are common.
We suspect this requirement for cool temperature is less a feature of the novel mycobacterial species and thus dissemination to internal organs is possible. Case 12, for example, had biochemical evidence of hepatic involvement which resolved during therapy. Widely disseminated disease with internal organ involvement has been reported occasionally as a sequela of feline leprosy (Pedersen 1988). However, except for the occasional detailed case report concerning an elderly cat (Donnelly et al 1982), it is not possible to determine whether, as we suspect, these cases were attributable to the novel mycobacterial species.
Based on our findings, M lepraemurium infections have a number of distinguishing features that suggest this aetiology even where molecular testing is not practicable. A ‘tuberculoid’ pyogranulomatous response with prominent involvement of lymphoid cells and neutrophils, in association with regions of necrosis containing sparse to moderate numbers of AFB is strongly suggestive of this aetiology. The propensity for AFB to be located preferentially in necrotic areas of lepromas has been reported previously (Mori & Kohsaka 1986). Ulceration of gross lesions, presumably corresponding to the areas of caseous necrosis visible microscopically, is probably also suggestive of M lepraemurium infection. Failure of sufficient organisms to take up the haematoxylin stain and become visible in H&E stained sections is another distinguishing feature. Finally, M lepraemurium infections are more common in certain temperate geographical areas.
In contrast, cases caused by the novel species lack caseous necrosis and do not develop ulcerated lesions. Furthermore, the pathology is invariably lepromatous, with very large numbers of AFB, which are visible also in H&E stained sections. The environmental niche of the novel mycobacterium species has yet to be determined. However, the preponderance of cases from rural or semi-rural areas suggests the organism is a saprophyte found more commonly in these locations than in metropolitan environments (Wolinsky 1973, Kazda et al 1980, Youmans 1980, Collins et al 1984, Grange & Yates 1986, Grange 1996). To speculate, the organism may normally reside in soil or stagnant watery environments that favour the proliferation of saprophytic mycobacteria, and subsequently become inoculated into the subcutis or skin through contamination of traumatic injuries (from cats, or another mammalian vector, such as the brush-tailed possum), or via a biting insect vector (Lowe 1937, Malik et al 1998b). Interestingly, a case of widely disseminated disease in an old cat reported previously (Donnelly et al 1982) resided in the same New South Wales Central Coast district where two of our cats were domicile.
The establishment or spread of infection with the novel mycobacterial species may have a requirement for decreased effective immunological surveillance, which may account for the preponderance of older cats in this series. Case 14 was of great interest in this regard, as when this cat was re-presented in renal failure seven months after initial diagnosis, at a time when mycobacterial disease was no longer evident, the fungus Trichosporon beigelii was visualised and cultured from a urine sample obtained by cystocentesis. The finding of sequential opportunistic infections with unusual pathogens is suggestive of immune deficiency. We have limited information concerning the involvement of underlying retroviral infections in these older patients, although neither cases 11 nor 12 was positive for FeLV antigen or FIV antibodies using commercial ELISA. In contrast, cases 10 and 16 were FIV-positive. Evaluation of a larger number of these cases using more sensitive techniques such as Western blot analysis, viral isolation or lymphocyte subset analysis (Walker et al 1995) is appropriate to confirm or exclude the involvement of FIV in these patients. It is conceivable that feline leprosy caused by the novel mycobacterial species may represent a manifestation of deteriorating immune competence in elderly cats with longstanding FIV infection, as has been reported recently for M genavense (Hughes et al 1999).
The presence of renal disease in some of the cats infected with the novel mycobacterial species deserves some comment. The presence of kidney dysfunction in such an old cohort of cats may represent an epiphenomenon, reflecting the commonness of intercurrent renal disease in elderly Australian cats. On the other hand, decreased cellular immunity associated with azotaemia may predispose cats to the development of disease with saprophytic mycobacteria of limited virulence. It seems necessary to implicate some phenomenon such as this to account for the absence of young cats amongst the cohort of patients infected with the novel mycobacterial species. Alternately, renal disease may occur as a consequence of the mycobacterial infection. Glomerulonephritis occurs in a proportion of rats with disseminated M lepraemurium infection and has been reported as a necropsy finding in cats with feline leprosy (Lawrence & Wickham 1963). Perhaps immune complex disease occurs as a consequence of long-standing infection, resulting in secondary renal dysfunction. There is a well known association between hypercalcaemia and widespread granulomatous disease due to mycobacteria, and hypercalcaemia could conceivably contribute to nephrotoxicity in this setting (Mealey et al 1999). However of the four cats (cases 4, 11, 12, 16) in which the serum calcium concentration was determined, a slight elevation was present in case 4 only (3.1 mmol/l [reference range 2.1 to 2.9 mmol/l]).
Clinical, histological and molecular findings in case 2 were problematic, in so far as the observations did not permit this cat to be classified confidently into either of the two defined clinical syndromes. This patient was young and had localised disease (limited to two digits), and was cured by surgical excision and adjunctive clofazimine therapy — features typical of recorded M lepraemurium infections. However, mycobacteria were present in enormous numbers within lesions, necrosis was absent and the sequence of the PCR amplicon was different to both M lepraemurium and the novel species encountered in older cats. Findings in this cat therefore suggest yet another mycobacterial species may give rise to feline leprosy lesions, albeit rarely.
Recommendations for diagnosis of feline leprosy syndromes
Diagnosis of feline leprosy is usually straightforward, provided that the clinician has a high index of suspicion. Needle aspirates, crush preparations of biopsy material and histological sections stained with ZN or similar methods demonstrate granulomatous or pyogranulomatous inflammation and variable numbers of AFB (Gross et al 1992). In DiffQuik stained smears mycobacteria can be recognized by their negative-staining appearance and location within macrophages and giant cells. Aseptically obtained tissue specimens should be submitted for culture, because occasionally slowly-growing species such as M microti or MAC can produce an identical clinical presentation (Gunn-Moore et al 1996; Hughes et al 1997) and in these cases optimal antibacterial therapy can be selected more rationally on the basis of in vitro susceptibility results and published data. However, in the majority of cases conventional culture is negative due to the fastidious nature of causal organisms and a mycobacterial aetiology can only be confirmed using molecular techniques such as nucleotide sequence determination of PCR amplicons (Rogall et al 1990, Kim et al 1999). Fresh (frozen) tissue delivered to a mycobacteria reference laboratory with PCR facilities provides the optimal sample for such diagnoses. Sometimes PCR can be performed successfully on formalin-fixed paraffin-embedded material, although fixation conditions invariably cause some DNA degradation and may limit the success of the procedure (Hughes et al 2000), as in some of the present cases.
Recommendations for therapy
Too few cases with a documented aetiology have been reported to provide accurate guidelines for treatment. Although M lepraemurium and the novel species can be cultured in vitro, it is currently not routine or reliable to isolate these organisms due to their slow growth and fastidious requirements. Determination of in vitro susceptibility data for individual isolates is therefore not possible. Only limited experimental studies have been undertaken to determine effective drug therapy for M lepraemurium in vitro or in vivo (Portaels et al 1982, Ji et al 1986) and as yet we have no data for the novel mycobacterial species. Portaels et al (1982) found the minimum inhibitory concentration for rifampicin of two strains of M lepraemurium to be 4 and 8 μg/ml, levels that should be just obtainable in vivo based on extrapolation from pharmacokinetic studies in humans and dogs (Frank 1990). Other drugs shown to have activity against M lepraemurium in vitro include ansamycin compounds and sulpha drugs (Portaels et al 1982, Ji et al 1986).
Where a high index of suspicion for M lepraemurium infection exists and cases are diagnosed while disease is localised, there is sufficient literature to suggest that wide surgical excision of infected tissues provides the best chance to simply and rapidly effect a cure (Pedersen 1988, Wilkinson & Mason 1991, Lewis & Kunkle 1998). Reconstructive surgical techniques applicable to the head and limbs (Swaim & Henderson 1997) permit en bloc resection of even extensive lesions and reconstruction of the resulting tissue deficits. Such an approach should be combined with adjunctive antimicrobial therapy, beginning a few days prior to surgery, to obtain therapeutic drug levels in the blood and tissues intra- and post-operatively to ensure clean wound margins and primary intention healing. The drug clofazimine (up to 10 mg/kg once daily orally; Mundell 1990, Paulsen et al 2000) has the best reported success rate, although it is likely that combination therapy using two or more drugs (see below) will prove to be a superior approach. Interestingly, there has been one report of spontaneous resolution in a cat with a single lesion on its distal forelimb 3.5 months following diagnosis (Roccabianca et al 1996).
Drugs likely to have broad antibacterial activity against slow-growing mycobacteria such as the novel mycobacterial species include rifampicin (Portaels et al 1982, Ji et al 1986, Frank 1990, Larsson et al 1994), clofazimine (Mundell 1990, Kaufman et al 1995, Malik et al 1998a) and clarithromycin (Peters & Clissold 1992, Tartglione 1997, Alvarez-Elcoro & Enzler 1999) although doxycycline, the fluoroquinolones and aminoglycosides may also prove to be useful. We believe that therapy using two or three of the drugs clofazimine (25 to 50 mg per cat orally every day or every other day), clarithromycin (62.5 mg twice daily) or rifampicin (10 to 15 mg/kg per day) represents optimal therapy. Based on our limited experience, we are unsure which component of therapy is the most efficacious, although we currently recommend combination therapy using rifampicin and clarithromycin.
Clofazimine capsules, which contain 50 mg of the dye, can be cut into halves using a scalpel blade while wearing disposable gloves, and the two portions placed into gelatin capsules to facilitate dosing. Rifampicin is prepared by dividing the contents of a 150 mg capsule and reformulating the approximate dose in a gelatin capsule. As clofazimine and rifampicin both can produce reversible hepatotoxicity, biochemical monitoring of cats regularly during therapy is appropriate and vomiting and/or inappetence suggest the need for dosage reduction or temporary discontinuation of therapy. Our experience is that of these different agents, clarithromycin is the least likely to cause troublesome side effects. Monotherapy with this agent, however, is not recommended because of the possibility of resistance developing during the course of treatment. Guidelines for duration of therapy are hard to define, although generally speaking mycobacterial infections should be treated for several months and typically therapy should be continued for at least 2 months (the lifespan of a macrophage in the tissues) after disappearance of lesions.
Concluding remarks
This study has established beyond doubt that feline leprosy is not a disease caused by a single mycobacterial species. Clinical, epidemiological, histological and molecular observations have demonstrated that at least two mycobacterial species are responsible for feline leprosy cases in Australia and New Zealand. Furthermore, the involvement of different aetiologic agents accounts, at least in part, for the different clinical and pathological features observed in individual affected cats. Outside Australia, infections with other mycobacterial species (including fastidious or slow growing species such as M bovis and M microti) may give rise to cutaneous lesions clinically indistinguishable from feline leprosy, although further investigations will generally demonstrate concurrent involvement of other body systems. Thus, in order to make a definitive diagnosis in cats with cutaneous mycobacterial lesions, molecular studies (such as PCR and sequence analysis) are required in addition to a complete clinical investigation and mycobacterial culture at a reference laboratory.
Acknowledgements
Clarithromycin used in this study was generously donated by Abbott Australasia Pty Ltd. The study was further supported by a grant in aid from The Canine Research and Veterinary Foundation (New South Wales) and a grant from the Australian Companion Animal Health Foundation. Richard Malik is supported by the Valentine Charlton Bequest administered by the Post Graduate Foundation of Veterinary Science of the University of Sydney. Keith Ellis provided expert technical assistance with photography and computer support. Karen Barnes performed excellent histological processing. This paper is dedicated to the memory of Daria Love.
Footnotes
A similar isolate was obtained from feline leprosy material cultured on Ogawa egg yolk medium by one of the authors (DNL) in the 1970s following several weeks of incubation.
Addendum
Recently we have used clarithromycin at a dose of 125 mg every 12 h to successfully treat a cat with mycobacterial panniculitis that failed to respond to 62.5 mg every 12 h. Such high doses may prove useful also for treating refractory feline leprosy cases in concert with other drugs.
Interestingly, a recent study of human patients with non-tuberculous cutaneous mycobacterial infections suggested the presence of a foamy histiocytic infiltrate of the dermis and subcutis (resembling that of lepromatous leprosy) seems to be observed exclusively in patients with profound immunodeficiency (Bartralot et al 2000).
References
- Abulafia J, Vignale RA. (1999) Leprosy: pathogenesis updated. International Journal of Dermatology 38, 321–334. [DOI] [PubMed] [Google Scholar]
- Allan GS, Wickham N. (1976) Mycobacterial granulomas in a cat diagnosed as leprosy. Feline Practice 6, 34–36. [Google Scholar]
- Alvarez-Elcoro S, Enzler MJ. (1999) The Macrolides: Erythromycin, Clarithromycin and Azithromycin. Mayo Clinic Proceedings 74, 613–634. [DOI] [PubMed] [Google Scholar]
- Barrs VB, Martin P, James G, Chen S, Love DN, Malik R. (1999) Feline leprosy in a cat due to infection with novel mycobacterial species. Australian Veterinary Practitioner 29, 159–164. [Google Scholar]
- Bartralot R, Pujol RM, Garcia-Patos V, Sitjas D, Martin-Casabona N, Coll P, Alomar A, Castells A. (2000) Cutaneous infections due to nontuberculous mycobacteria: histopathological review of 28 cases. Comparative study between lesions observed in immunosuppressed patients and normal hosts. Journal of Cutaneous Pathology 27, 124–129. [DOI] [PubMed] [Google Scholar]
- Boddinghaus B, Rogall T, Flohr T, Blocker H, Böttger EC. (1990) Detection and identification of mycobacteria by amplification of rRNA. Journal of Clinical Microbiology 28, 1751–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LR, May CD, Williams SE. (1962) A non-tuberculous granuloma of cats. New Zealand Veterinary Journal 10, 7–9. [Google Scholar]
- Broughton CR, Lloyd AR. (1987) The management of leprosy. Medical Journal of Australia 146, 593–598. [DOI] [PubMed] [Google Scholar]
- Collins CH, Grange J M, Yates MD. (1984) Mycobacteria in water. Journal of Applied Bacteriology 57, 193–211. [DOI] [PubMed] [Google Scholar]
- Donnelly T, Jones MR, Wickham N. (1982) Diffuse cutaneous granulomatous lesions associated with acid-fast bacilli in a cat. Journal of Small Animal Practice 23, 99–105. [Google Scholar]
- Frank LA. (1990) Clinical pharmacology of rifampin. Journal of the American Veterinary Medical Association 197, 114–117. [PubMed] [Google Scholar]
- Frye FL, Carney JD, Loughman WD. (1974) Feline lepromatous leprosy. Veterinary Medicine/Small Animal Clinician 69, 1272–1273. [PubMed] [Google Scholar]
- Gross TE, Ihrke PJ, Walder EJ. (1992) Infectious nodular and diffuse granulomatous and pyogranulomatous diseases of the dermis. In: Veterinary Dermatopathology. A macroscopic and microscopic evaluation of canine and feline skin disease. Mosby, St Louis, pp. 169–171. [Google Scholar]
- Grange JM. (1996) The biology of the genus Mycobacterium. Journal of Applied Bacteriology Symposium Supplement 81, 1S–9S. [PubMed] [Google Scholar]
- Grange JM, Yates MD. (1986) Infections caused by opportunistic mycobacteria: a review. Journal of the Royal Society of Medicine 79, 226–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene CE, Watson ADJ. (1998) Appendix 8. Antimicrobial Drug Formulary. In: Infectious Diseases of the Dog and Cat. (2nd edn). Greene CE. (ed). Saunders, Philadelphia, p. 899. [Google Scholar]
- Greenfield L, White TJ. (1993) Sample preparation methods. In: Diagnostic Molecular Microbiology: Principles and Applications. Persing DH, Smith TF, Tenover FC, White TJ. (eds), American Society of Microbiology, Washington DC, pp. 122–137. [Google Scholar]
- Gunn-Moore DA, Jenkins PA, Lucke VM. (1996) Feline tuberculosis: a literature review and discussion of 19 cases caused by an unusual mycobacterial variant. Veterinary Record 138, 53–58. [DOI] [PubMed] [Google Scholar]
- Hughes MS, Ball NW, Beck L-A, de Lisle GW, Skuce RA, Neill SD. (1997) Determination of the etiology of presumptive feline leprosy by 16S rRNA gene analysis. Journal of Clinical Microbiology 35, 2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MS, Ball NW, Love DN, Canfield PJ, Wigney DI, Dawson D, Davis PE, Malik R. (1999) Disseminated Mycobacterium genavense infection in an FIV-positive cat. Journal of Feline Medicine and Surgery 1, 23–30. [DOI] [PubMed] [Google Scholar]
- Hughes MS, James G, Ball N, Scally M, Malik R, Wigney DI, Martin P, Chen S, Mitchell D, Love DN. (2000) Identification by 16S rRNA gene analysis of a potential novel mycobacterial species as an aetiological agent of canine leproid granuloma syndrome. Journal of Clinical Microbiology 38, 953–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji BH, Chen JK, Lu XZ, Wang SY, Ni GX, Hou YH, Zhou DH, Tang QK. (1986) Antimycobacterial activities of two newer ansamycins, R-76–1 and DL 473. International Journal of Leprosy and other Mycobacterial Diseases 54, 563–577. [PubMed] [Google Scholar]
- Kaufman AC, Greene CE, Rakich PM, Weigner DD. (1995) Treatment of localized Mycobacterium avium complex infection with clofazimine and doxycycline in a cat. Journal of the American Veterinary Medical Association 207, 457–459. [PubMed] [Google Scholar]
- Kaufman SHE. (1993) Immunity to intracellular bacteria. Annual Review of Immunology, pp. 129–163. [DOI] [PubMed] [Google Scholar]
- Kazda J, Irgens LM, Müller K. (1980) Isolation of non-cultivable acid-fast bacilli in sphagnum and moss vegetation by foot pad technique in mice. International Journal of Leprosy 48, 1–6. [PubMed] [Google Scholar]
- Kim BJ, Lee SH, Lyu MA, Kim SJ, Bai GH, Chae GT, Kim EC, Cha CY, Kook YH. (1999) Identification of mycobacterial species by comparative sequence analysis of the RNA polymerase gene. Journal of Clinical Microbiology 37, 1714–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson CE, Michalany NS, Pinheiro SR, Ledon ALBP, Vasconcellos SA. (1994) Mycobacteriosis in domestic dogs. Report of two cases in Sao Paulo. Revista Da Faculdade de Medicina Veterinaria e Zootecnia Da Universidade de Sao Paulo 31, 35–41. [Google Scholar]
- Lawrence WE, Wickham N. (1963) Cat leprosy: infection by a bacillus resembling Mycobacterium lepraemurium. Australian Veterinary Journal 39, 390–393. [Google Scholar]
- Leiker DA, Poelma FG. (1974) On the etiology of cat leprosy. International Journal of Leprosy 42, 312–315. [PubMed] [Google Scholar]
- Lewis DT, Kunkle GA. (1998) Feline leprosy. In: Infectious Diseases of the Dog and Cat (3rd edn), Greene CE. (ed). WB Saunders, Philadelphia, pp. 321–324. [Google Scholar]
- Lotti T, Hautmann G. (1993) Atypical mycobacterial infections: a difficult and emerging group of infectious dermatoses. International Journal of Dermatology 32, 499–501. [DOI] [PubMed] [Google Scholar]
- Lowe J. (1937) Rat leprosy. A critical review of the literature. International Journal of Leprosy 5, 311–328; 463–482. [Google Scholar]
- Lucas J, Lucas A, Furber H, James G, Hughes MS, Martin P, Chen SCA, Mitchell DH, Love DN, Malik R. (2000) Mycobacterium genavense infection in two aged ferrets with conjunctival lesions. Australian Veterinary Journal 78, 685–689. [DOI] [PubMed] [Google Scholar]
- Maygarden SJ, Flanders EL. (1990) Mycobacteria can be seen as ‘negative images’ in cytology smears from patients with acquired immunodeficiency syndrome. Modern Pathology 2, 239–243. [PubMed] [Google Scholar]
- Malik R, Gabor LJ, Martin P, Mitchell DH, Dawson DJ. (1998a) Subcutaneous granuloma due to Mycobacterium avium complex infection in a cat. Australian Veterinary Journal 76, 604–607. [DOI] [PubMed] [Google Scholar]
- Malik R, Love DN, Wigney DI, Martin P. (1998b) Mycobacterial nodular granulomas affecting the subcutis and skin of dogs (canine leproid granuloma syndrome). Australian Veterinary Journal 76, 403–407. [DOI] [PubMed] [Google Scholar]
- Malik R, Wigney DI, Dawson D, Martin P, Hunt GB, Love DN. (2000) Infection of the subcutis and skin of cats with rapidly-growing mycobacteria: A review of microbiological and clinical findings. Journal of Feline Medicine and Surgery 2, 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh DW. (1982) Feline leprosy: A review of forty-four cases from Western Canada. Canadian Veterinary Journal 23, 291–295. [PMC free article] [PubMed] [Google Scholar]
- Mealey KL, Willard MD, Nagode LA, Helman RG. (1999) Hypercalcemia associated with granulomatous disease in a cat. Journal of the American Veterinary Medical Association 215, 959–962. [PubMed] [Google Scholar]
- Mori T, Kohsaka K. (1986) Identification of cat leprosy bacillus grown in mice. International Journal of Leprosy 54, 584–595. [PubMed] [Google Scholar]
- Mundell AC. (1990) New therapeutic agents in veterinary dermatology. Veterinary Clinics of North America: Small Animal Practice 20, 1544–1545. [DOI] [PubMed] [Google Scholar]
- Nakamura M. (1999) For the growth of Mycobacterium lepraemurium in cell-free liquid medium, the key essential factor may be the pH (optimal 6.0 to 6.2) of the culture medium, rather than the presence of α-ketoglutaric acid. Japanese Journal of Leprosy 68, 157–163. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Motomura K. (1970) Studies on murine leprosy bacillus. 1. Attempt to cultivate in vitro the Hawaiian strain of Mycobacterium lepraemurium. Kitasato Archives of Experimental Medicine 43, 21–36. [PubMed] [Google Scholar]
- Paulsen DB, Kern MR, Weigand CM. (2000) Mycobacterial neuritis in a cat. Journal of the American Veterinary Medical Association 216, 1589–1591. [DOI] [PubMed] [Google Scholar]
- Pattyn SR, Portaels F. (1980) In vitro cultivation and characterization of Mycobacterium lepraemurium. International Journal of Leprosy 48, 7–14. [PubMed] [Google Scholar]
- Pattyn SR. (1984) Mycobacterium lepraemurium. In: The mycobacteria: a sourcebook, Kubica GP, Wayne LG. (ed). Marcel Dekker Inc, New York, pp. 1277. [Google Scholar]
- Pedersen NC. (1988) Atypical Mycobacteriosis. In: Feline Infectious Diseases. American Veterinary Publications, Goleta, p. 197. [Google Scholar]
- Peters DH, Clissold SP. (1992) Clarithromycin. A review of its antimicrobial activity, pharmacokinetic properties and therapeutic potential. Drugs 44, 117–164. [DOI] [PubMed] [Google Scholar]
- Poelma FG, Leiker D. (1974) Cat leprosy in the Netherlands. International Journal of Leprosy 42, 307–311. [PubMed] [Google Scholar]
- Portaels F, Pattyn SR, Francken A. (1982) In vitro sensitivity of Mycobacterium lepraemurium for antimycobacterial drugs. Arzneimittel-Forschlung 32, 1123–1124. [PubMed] [Google Scholar]
- Rapp RP, McCraney SA, Goodman NL, Shaddick DJ. (1994) New macrolide antibiotics: usefulness in infections caused by mycobacteria other than Mycobacterium tuberculosis. Annals of Pharmacotherapy 28, 1255–1263. [DOI] [PubMed] [Google Scholar]
- Robinson M. (1975) Skin granuloma of cats associated with acid-fast bacilli. Journal of Small Animal Practice 16, 563–567. [DOI] [PubMed] [Google Scholar]
- Roccabianca P, Caniatti M, Scanziani E, Penati (1996) Feline leprosy: spontaneous remission in a cat. Journal of the American Animal Hospital Association 32, 189–193. [DOI] [PubMed] [Google Scholar]
- Rogall T, Flohr T, Böttger EC. (1990) Differentiation of Mycobacterium species by direct sequencing of amplified DNA. Journal of General Microbiology 136, 1915–1920. [DOI] [PubMed] [Google Scholar]
- Schiefer HB, Gee BR, Ward GE. (1974) A disease resembling feline leprosy in Western Canada. Journal of the American veterinary Medical Association 165, 1085–1087. [PubMed] [Google Scholar]
- Schiefer HB, Middleton DM. (1983) Experimental transmission of a feline mycobacterial skin disease (feline leprosy). Veterinary Pathology 20, 460–471. [DOI] [PubMed] [Google Scholar]
- Swaim SF, Henderson RA. (1997) Wounds of the Head. In: Small Animal Wound Management (2nd edn) Williams and Wilkins, Baltimore, pp. 191–234. [Google Scholar]
- Tartglione T. (1997) Treatment of nontuberculous mycobacterial infections: role of clarithromycin and azithromycin. Clinical Therapeutics 19, 626–638. [DOI] [PubMed] [Google Scholar]
- Thompson EJ, Little PB, Cordes DO. (1979) Observations of cat leprosy. New Zealand Veterinary Journal 27, 233–235. [DOI] [PubMed] [Google Scholar]
- Walker C, Malik R, Canfield PJ. (1995) Analysis of leucocytes and lymphocyte subsets in cats with naturally occurring cryptococcosis but differing feline immunodeficiency virus status. Australian Veterinary Journal 72, 93–96. [DOI] [PubMed] [Google Scholar]
- Wilkinson GT, Mason KV. (1991) Clinical Aspects of Mycobacterial Infections of the Skin. In: Consultations in Feline Internal Medicine, August JR. (ed). WB Saunders, Philadelphia, pp. 129–136. [Google Scholar]
- Wilkinson GT. (1964) A non-tuberculous granuloma of the cat associated with an acid-fast bacillus. Veterinary Record 76, 777–778. [Google Scholar]
- Wilkinson GT. (1977) Feline Leprosy. In: Current Veterinary Therapy IV, Kirk RW. (ed). WB Saunders, Philadelphia, pp. 569–571. [Google Scholar]
- Wolinsky E. (1973) Mycobacteria. In: Microbiology Davis BD, Dulbecco R, Eisen HN, Ginsberg HS, Wood WB. (eds). Harper and Row, Hagerstown, p. 884. [Google Scholar]
- Youmans GP. (1980) General characteristics of Mycobacteria. In: The Biologic and Clinical Basis of Infectious Diseases (2nd edn), Youmans GP, Paterson PY, Sommers HM. (eds). WB Saunders, Philadelphia, p. 367. [Google Scholar]