Abstract
Gastroduodenal ulceration (GU) and blood loss was diagnosed in eight cats and compared with 25 previously reported cases of feline GU. Cats with GU presented in a critical condition. Clinical signs consistent with gastrointestinal bleeding were infrequently identified although anaemia was a common finding. Non-neoplastic causes of feline GU tended to have a shorter clinical course with ulcers confined to the stomach. Conversely, cats with tumour-associated GU usually had a more protracted clinical course, weight loss, and ulcers located in the stomach for gastric tumours and the duodenum for extra-intestinal tumours. In this series, definitive diagnosis was possible for cats with neoplasia (gastric tumours and gastrinoma), however, it was difficult to precisely identify the underlying aetiology in cats with non-neoplastic GU. Prompt stabilisation with a compatible blood transfusion, surgical debridement or resection, antibiotic and antiulcer therapy, and treatment of the underlying disease, if identified, was successful in the majority of cases. The prognosis for cats with appropriately managed GU depended on the underlying aetiology, but even cats with neoplasia could be successfully palliated for prolonged periods.
Introduction
Gastroduodenal ulcers (GU) are mucosal defects which expose the submucosa or deeper layers of the stomach to gastric acid (Guilford & Strombeck 1996b). Any condition which compromises the normal physiological defence mechanisms of the gastric mucosa, such as increased secretion of hydrochloric acid, reduced mucosal blood flow and alteration of the mucus-bicarbonate barrier, can result in GU (Guilford & Strombeck 1996a, 1996b, Hall 2000).
Ulcers of the stomach and duodenum are uncommon in cats, with less than 30 cases reported (Seawright & Grono 1964, Schalm et al 1975, Wrigley 1977, Hasler & van den Ingh 1978, Liska et al 1979, Weller & Hornof 1979, Evans & Biery 1980, Gordon 1980, Jones & Hunt 1983, Middleton et al 1983, McEwen et al 1985, Sheikh-Omar & Abdullah 1985, van der Gaag et al 1988, Holmberg et al 1989, Thilgar et al 1989, Aoki et al 1990, Bortnowski & Rosenthal 1992, Eng et al 1992, Jergens et al 1992, Curtsinger et al 1993, Twedt 1994, Mai & Bouhoula 1998, Muller et al 1998, Runk et al 1999). Cats with GU frequently present with life-threatening gastrointestinal haemorrhage. However, definitive diagnosis can be difficult as clinical signs identifying the gastrointestinal tract as the site of blood loss, such as haematemesis and melaena, are often inapparent or obscured by hypovolaemia, sepsis or more obvious signs referable to anaemia.
In dogs, neoplasia, non-steroidal antiinflammatory drugs (NSAIDS), hepatic disease and inflammatory bowel disease (IBD) are common causes of GU (Stanton & Bright 1989, Hall 2000). The aetiology of GU in cats, however, has not well characterised. The aim of this report, by presenting a series of eight cats with GU and reviewing 25 cases reported in the literature, is to further elucidate the aetiology and management of cats with GU.
Materials and methods
Gastric and/or duodenal ulcers were diagnosed in eight cats at the University Veterinary Centre Sydney (UVCS) between February 1998 and January 2001. A definitive diagnosis of GU was made at either surgery or necropsy and confirmed histologically Signalment, clinical and laboratory findings, treatment, ulcer location and outcome were determined from case records. The literature was reviewed for cases of feline GU and the findings from these reports were tabulated when sufficient information was available, otherwise the diagnosis only was recorded.
Results
Case reports
Details of these cases are summarised in Table 1.
Table 1.
Summary of cats with gastroduodenal ulcers seen at the University Veterinary Centre Sydney from 1998 to 2001
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| Breed | DSH | Manx cross | Abyssinian | DSH | DSH | DSH | DSH | Chinchilla |
| Age (years) | 2 | 11 | 0.6 | 10 | 14 | 7 | 13 | 12 |
| Sex | MN | MN | MN | FS | FS | FS | FS | FS |
| Duration (weeks) | 0.86 | 1.3 | 0.07 | 8 | 52 | 3 | 0.14 | 35 |
| Vomiting | Y | Y | Y | Y | Y | Y | Y | |
| Haematemesis | Y | Y | ||||||
| Melaena | Y | Y | Y | |||||
| Inappetence | Y | Y | Y | Y | Y | |||
| Weight loss | Y | Y | Y | Y | Y | |||
| Polydipsia | Y | Y | Y | |||||
| Abdominal pain | Y | Y | Y | |||||
| Anaemia | Y | Y | Y | Y | Y | Y | Y | Y |
| Hypoproteinaemia | Y | Y | Y | |||||
| Lymphopaenia | Y | Y | Y | |||||
| Neutrophilia | Y | Y | Y | Y | Y | |||
| Left shift | Y | Y | ||||||
| Hypokalaemia | Y | Y | Y | |||||
| Ulcer location | Pylorus (1) | Pylorus (3) | Fundus (1) | Pylorus (1) | Duodenum (1) | Fundus (NR) | Pylorus (NR) | Fundus (1) |
| Pylorus (1) | Duodenum (2) | Pylorus (NR) | ||||||
| Perforated | Y | Y | Y | |||||
| Treatment | Suture | None/Died | Suture | Suture | Suture | Billroth I | Billroth I | Euthanasia |
| Cause | Unknown | Unknown | Unknown | Gastrinoma | Pancreatic AC | Gastric LSA | Gastric LSA | Gastric AC |
| Survival (months) | >16 | 0 | >30 | 18 | 12 | >20 | >13 | 0 |
DSH, domestic short hair; MN, male neuter; FS, female spayed; Y, yes; AC, adenocarcinoma; LSA, lymphosarcoma; NR, not recorded.
Case 1. A 2-year-old, castrated domestic short-hair (DSH) (4.4 kg) presented to the UVCS with a 6-day history of vomiting, malaena and polydipsia. The cat was tachycardic [260 beats per min (bpm)] and had pale mucous membranes. Haematology, biochemistry and urinalysis revealed a poorly regenerative, macrocytic, normochromic anaemia [packed cell volume (PCV) 0.09 l/l, mean corpuscular volume (MCV) 50 fl, mean corpuscular haemoglobin (MCH) 15 pg/l, reticulocytes 63×109/l], marked azotaemia [urea 52.2 mmol/l, creatinine 580 μmol/l and urine specific gravity (USG) 1.012], hypokalaemia (2.3 mmol/l) and hyponatraemia (127 mmol/l) (reference ranges; Table 2). The total plasma protein concentration was 59 g/l. A blood lead concentration (0.09 μmol/l) was within normal limits. Abdominal radiographs revealed distension of the stomach and some loops of small intestine with gas. A fresh, cross-matched whole blood transfusion was administered and an exploratory celiotomy performed.
Table 2.
Reference ranges for haematology, biochemistry, analytes, thyroxine and lead
| Parameter | Reference range |
|---|---|
| Packed cell volume (l/l) | 0.30–0.45 |
| Mean corpuscular volume (fl) | 40–45 |
| Mean corpuscular haemoglobin (pg) | 13–17 |
| Reticulocytes (×10 /l) | <60 |
| Total plasma protein (g/l) | 59–78 |
| Leucocytes (×109l) | 8–14 |
| Lymphocytes (×109l) | 1.6–7.0 |
| Neutrophils (×109l) | 3.76–10.08 |
| Bands (×109l) | 0.0–0.42 |
| Monocytes (×109l) | 0.08–0.56 |
| Urea (mmol/l) | 7.2–10.7 |
| Creatinine (mmol/l) | 90–180 |
| Inorganic phosphate (mmol/l) | 1.3–2.3 |
| Urine specific gravity | 1.035–1.080 |
| Alanine aminotransferase (u/l) | <60 |
| Alkaline phosphatase (u/l) | <50 |
| Aspartate aminotransferase (u/l) | <40 |
| Total bilirubin (μmol/l) | 2.5–3.5 |
| Creatine kinase (u/l) | <200 |
| Potassium (mmol/l) | 4.0–4.6 |
| Sodium (mmol/l) | 147–156 |
| Chloride (mmol/l) | 115–130 |
| Bicarbonate (mmol/l) | 17–21 |
| Thyroxine (nmol/l) | 15–45 |
| Lead (μmol/l) | <0.50 |
| Gastrin (pg/ml) | 44–64 |
Note that the reference range for gastrin was based on the results of six clinically normal cats assayed simultaneously with cases 3 and 4.
Abnormalities detected at surgery included gastric serosal discolouration at the angular incisure, reflux of bile into the pancreatic ducts and oedema of the portal lymphatics. Gastrotomy revealed a 10 mm diameter ulcer in the pyloric antrum. The ulcer was resected and the resultant defect sutured. The bile duct was catheterised and flushed with saline through a duodenotomy Firm, dark faeces were removed through a colotomy. There was no evidence of hair or bones in the faeces. The small intestine, colon, pancreas, liver and a mesenteric lymph node were biopsied.
Histologically, the gastric ulcer consisted of a layer of fibrin, cellular debris and necrotic material. There was coagulative necrosis and neutrophilic infiltration of the hepatic parenchyma compatible with either acute hypoxia or exposure to a toxin. The pancreas, small intestine, colon and lymph node were unremarkable.
Initially, the cat was fasted for 36 h and treated with cimetidine [(Tagamet; SmithKline Beecham) 44 mg subcutaneously (SC) every 8 h] and enrofloxacin [(Baytril; Bayer) 20 mg SC every 12 h]. Two days post-operatively, oral feeding was commenced and the medication changed to cimetidine [50 mg per os (PO) every 8 h], sucralfate [(Carafate; Hoechst Marion Roussel) 250 mg PO every 12 h] and enrofloxacin (25 mg PO every 12 h) for 14 days.
A cause for the gastric ulcer was not found although a toxic agent may have been responsible for both the gastric ulcer and hepatic necrosis. Acute renal failure could not be excluded but chronic renal failure was considered an unlikely explanation for the gastric ulcer, due to the absence of ulcerative stomatitis and continuing polyuria and polydipsia, but could not be excluded. The azotaemia and inappropriately dilute urine may have been secondary to hyponatraemia and/or hypokalaemia as both can interfere with normal urine-concentrating mechanisms (DiBartola 2000; DiBartola & De Morais 2000) and the hypovolaemia may have contributed to reduced mucosal perfusion.
Case 2. An 11-year-old, castrated Manx (8.7 kg) presented with a 9-day history of respiratory distress and coughing, followed by vomiting. It had received aggressive frusemide therapy from the referring veterinarian. The cat was obese and lethargic with bruising over venepuncture sites (cephalic and jugular veins), a grade II/VI systolic heart murmur, increased inspiratory effort with an expiratory stridor, and marked abdominal pain. Haematology revealed a poorly regenerative, macrocytic, normochromic anaemia (PCV 0.16 l/l, MCV 54 fl, MCH 16.6 pg/l, reticulocytes 90×109/l), leucocytosis with neutrophilia (17.75×109/l) and lymphocytosis (7.83×109/l). A coagulation profile was not performed but platelet numbers were adequate on examination of a blood smear. Lymphocyte morphology was atypical. Total plasma protein level was normal. Biochemistry revealed a moderately increased creatine kinase (5253 u/l) and total bilirubin (18.0 μmol/l), mildly increased alanine aminotransferase [(ALT) 310 u/l], moderate azotaemia (urea 24.2 mmol/l and creatinine 225 μmol/l), hypokalaemia (2.5 mmol/l), hyponatraemia (131.3 mmol/l) and hypochloraemia (100.1 mmol/l). Abdominal radiography and ultrasonography were unremarkable.
The cat died unexpectedly. Necropsy revealed three strips of ulceration (4 mm by 10–30 mm) in the pyloric antrum with numerous smaller erosions on the mucosal surface of the stomach. Histologically, the gastric lesions were characterised by deep ulceration, fibroplasia and infiltration with inflammatory cells. The stomach and colon were filled with digested blood while the small intestine contained a large amount of fresh blood. Ulceration was not detected in either the small or large intestine. Other findings included myocarditis, adrenocortical necrosis and chronic interstitial pancreatitis. Toxoplasmosis was considered a diagnostic possibility although zoites were not detected. The precise aetiology of the gastric ulceration was not determined although stress secondary to severe illness, hypovolaemia and azotaeima due to frusemide administration and poor cardiac output associated with myocarditis may have compromised gastric mucosal perfusion (Vleet & van Ferrans 1986, Kiss & Szilagyi 1988).
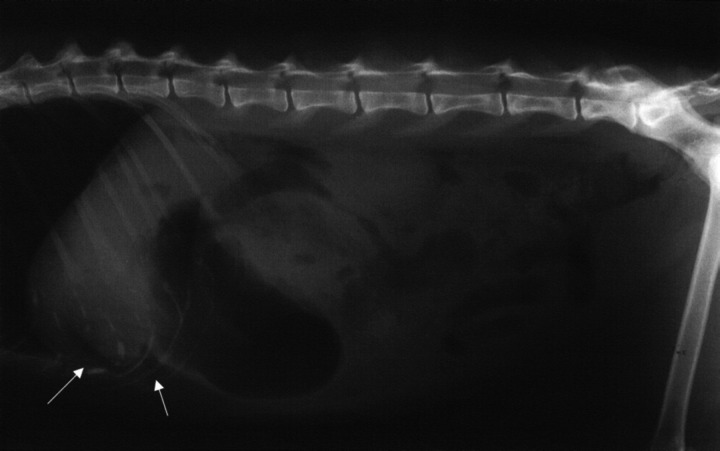
Case 3. A 7-month-old, castrated Abyssinian cross (3.2 kg) presented with a 12 h history of vomiting and lethargy 36 h after eating part of a cooked chicken carcass. The cat had marked, generalised abdominal pain. Haematology, serum biochemistry and urinalysis were performed. Abnormalities included neutrophilia (17.38×109/l) with a left shift (1.15×109/l) and increased creatine kinase (2580 u/l) and ALT (308 u/l) activities. Abdominal radiographs were considered normal, however, barium-impregnated polyethylene spheres (BIPS, Chemstock Animal Health) were administered due to the suspicion of an intestinal foreign body. The cat initially responded to supportive treatment with intravenous crystalloids [Hartmann's Solution (Baxter Healthcare)] and fasting. However, vomiting and lethargy recurred following cessation of intravenous fluid therapy and resumption of feeding. Abdominal pain and an equivocal mass were detected during palpation of the cranial abdomen. Repeat abdominal radiographs revealed retention of BIPS in the stomach and generalised loss of serosal detail (Fig 1). The PCV had dropped from 0.39 l/l to 0.23 l/l and an exploratory celiotomy was therefore scheduled to investigate the acute abdomen and occult blood loss.
Fig 1.
Lateral (A) and ventrodorsal (B) abdominal radiographs of a cat with perforated gastric ulcers of undetermined cause (case 3). Note the loss of serosal detail indicative of peritoneal fluid accumulation secondary to generalised peritonitis. Retention of the barium-impregnated polyethylene spheres in the stomach 36 h after administration was suggestive of delayed gastric emptying or a pyloric outflow obstruction.
During preparation for surgery subcutaneous bruising of the ventral abdomen was noted. Exploration of the abdominal cavity revealed perforated ulcers in the gastric fundus and pyloric antrum. Ingesta was present in the peritoneal cavity. The perforated ulcer in the fundus was associated with adhesions to the visceral surface of the liver. Adhesions and an abscess had formed between the ulcer in the pyloric antrum and the left limb of the pancreas. The gastric ulcers were debrided and sutured, and the abscess debrided, lavaged and omentalised. The abdominal cavity was copiously lavaged with warm isotonic saline and closed routinely.
Histologically, the gastric ulcer consisted of haemorrhage, fibrinous exudate and immature granulation tissue with erythrophagocytosis in the submucosa and interstitium of the muscle layers. Areas of necrosis were associated with hair and foreign material. The lamina propria contained low to moderate numbers of neutrophils with occasional eosinophils. Further blood tests were performed. Gastrin concentration (45 pg/ml) was within the reference range and blood lead levels were suggestive of exposure but not toxicity (0.53 μmol/l). A definitive cause was not determined although increased creatine kinase activity and bruising of the ventral abdomen was compatible with a traumatic aetiology.
Initial treatment consisted of cimetidine [32 mg intravenously (IV) every 6 h], cephazolin [(Kefzol; Eli Lilly) 100 mg IV every 6 h] and gentamicin [(Gentapex-50; Apex Laboratories) 6.4 mg IV every 8 h] until oral feeding resumed 48 h post-operatively The cat recovered uneventfully and was treated successfully with enrofloxacin (50 mg PO every 24 h), metronidazole [(Metrogyl; Alphapharm) 25 mg PO every 12 h], amoxycillin [(Apex Laboratories) 50 mg PO every 12 h], ranitidine [(Zantac; Glaxo-Wellcome) 18.75 mg PO every 12 h] and sucralfate (250 mg PO every 12 h) for 14 days. Thirty months after surgery, the cat remains in excellent health.
Case 4. A 10-year-old, spayed DSH (3.3 kg) presented to the UVCS with an 8-week history of vomiting and polydipsia. The cat vomited dark brown fluid daily unrelated to eating, and was losing weight despite a normal appetite. Vomition was unresponsive to medical management with cimetidine and dietary manipulation.
The cat was emaciated with palpably enlarged thyroid glands. Haematology demonstrated a macrocytic, normochromic anaemia (PCV 0.16 l/l, MCV 52 fl, MCH16 pg/l), neutrophilia (13.2×109/l) with a left shift (bands 1.1×109/l) and monocytosis (1.1×109/l). Serum biochemistry showed marginal hypoproteinaemia (52 g/l), mildly increased aspartate aminotransferase [(AST) 70 u/l] and ALT (267 u/l) activities, hypokalaemia (2.0 mmol/l), and urea and creatinine within the reference range. The plasma thyroxine concentration (37 nmol/l) was within the upper limits of the reference range.
Abdominal ultrasonography revealed mild thickening of the stomach and small intestine and enlargement of the aortic and mesenteric lymph nodes. Barium-impregnated polyethylene spheres were administered to evaluate gastrointestinal motility and the possibility of a luminal obstruction. During the BIPS study, there was radiographic evidence of free abdominal gas.
The blood type was determined (type B) and an exploratory celiotomy performed. Pneumoperitoneum was confirmed. The liver was large and infiltrated by fat. The common bile duct was tortuous and dilated proximal to a lobulated mass in the body of the pancreas. A perforated ulcer, 20 mm in diameter, was found in the pyloric antrum. Two additional perforated ulcers were detected in the proximal duodenum. The ulcers were debrided and sutured. The liver, stomach, mesenteric lymph node and mass in the body of the pancreas were biopsied. The cat was hypotensive throughout surgery and received inotropic support [continuous rate infusion of dopamine (David Bull Laboratories) at 6 μg/min] and an intraoperative transfusion of fresh, type B blood.
Histology demonstrated necrosis and granulation of the pyloric and duodenal ulcers with helicobacter-like organisms in moderate numbers in the crypts surrounding the duodenal ulcers. However, the relative lack of an inflammatory response adjacent to the ulcers suggested that a non-infectious process caused the ulcers. The liver showed moderate fibrosis and congestion and the mass obstructing the bile duct was considered a normal lymph node.
The cat was treated with cimetidine (30 mg IV every 6 h), enrofloxacin (20 mg SC every 12 h) and amoxycillin-clavulanic acid [(Clavulox; Pfizer) 50 mg SC every 12 h] until oral feeding was resumed 48 h post-operatively. The cat required a second fresh, typed blood transfusion three days after surgery as the PCV had dropped from 0.18 l/l to 0.11 l/l. A fasting serum gastrin level was performed on the basis of exploratory celiotomy findings and on-going gastrointestinal blood loss. The marked increase in gastrin (404 pg/ml), in the absence of azotaemia, was supportive of a diagnosis of gastrinoma. The pancreatic mass and enlarged mesenteric lymph node may have been the primary gastrinoma with lymph node metastases as the samples submitted for cytological and histological analysis, respectively, may not have been representative of the disease process.
Treatment was changed to omeprazole [(Losec; Astra) 10 mg PO every 24 h] and ranitidine (18.75 mg every 12 h) for four weeks. Omeprazole was continued for 18 months. The cat had gained weight and was clinically normal during this time. However, the cat died three days after the owner discontinued omeprazole therapy. Necropsy was not permitted but, following death, fluid collected by abdominocentesis confirmed haemoperitoneum.
Case 5. A 14-year-old, spayed DSH (3.9 kg) presented with a 12-month history of vomiting, inappetence, weight loss and polydipsia. Vomiting occurred daily and was unrelated to eating. The cat was emaciated but no other abnormal physical findings were detected. Haematology, serum biochemistry and plasma thyroxine concentration were unremarkable. Management with dietary manipulation was unsuccessful and the cat returned six weeks later with continued vomiting. The cat was then anaemic (PCV 0.25 l/l) and hypoproteinaemic (50 g/l) and a proximal gastrointestinal obstruction was suspected as the cat was hyponatraemic (136 mmol/l), hypochloraemic (93 mmol/l) and alkalotic (bicarbonate 27.8 mmol/l).
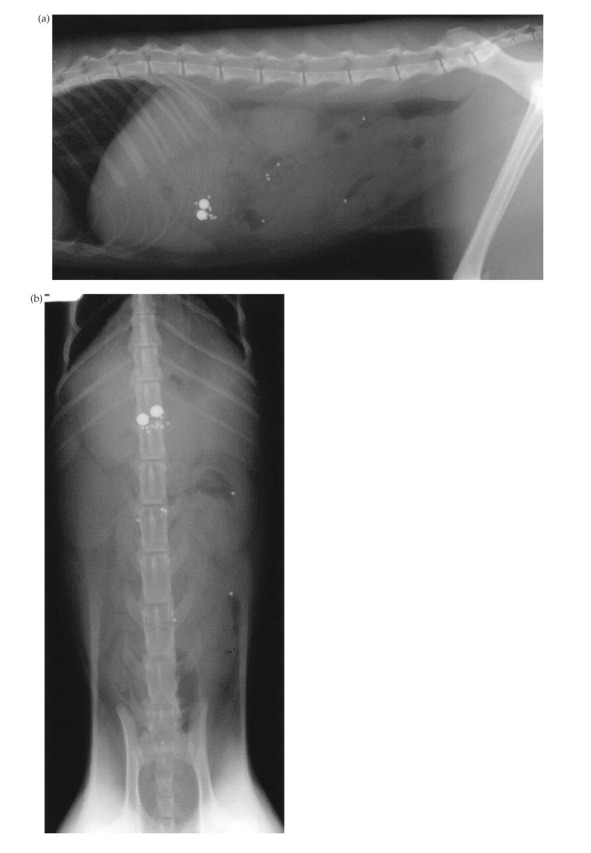
Abdominal radiography showed free peritoneal gas, gastric dilation and loss of serosal detail of the abdominal viscera (Fig 2). A thickened pancreas surrounded by fluid, gas and hyperechoic fat, and moderate thickening of the gastric and small intestinal wall were noted during abdominal ultrasonography. Cytological examination of fluid collected by abdominocentesis was diagnostic for septic peritonitis with 90% neutrophils and intracellular bacteria such as Gram-positive rods and cocci and Gram-negative rods.
Fig 2.
Lateral abdominal radiograph of a cat with a perforated duodenal ulcer and pancreatic carcinoma (case 5). Pneumoperitoneum (arrows) and loss of serosal detail is suggestive of gastrointestinal perforation and generalised peritonitis.
A ventral midline exploratory celiotomy was performed. There was a large amount of turbid peritoneal fluid and the stomach was distended. A tube was passed to decompress the stomach. A perforated ulcer, 8 mm in diameter, on the serosal surface of the proximal duodenum surrounded by gastrointestinal contents and inflamed, discoloured omentum. The duodenal ulcer was debrided and enlarged to examine the duodenal papilla and mucosa. The papilla appeared normal although the mucosal surface of the antimesenteric duodenal border was polypoid. The debrided ulcer was sutured. Tissue samples of the gastric fundus, duodenal mucosa, liver and right pancreatic limb were obtained for microscopic examination. The abdomen was copiously lavaged and closed routinely.
The most significant histological finding was a ductal carcinoma of presumed pancreatic origin. The epithelial cells on the pancreatic biopsy showed moderate pleomorphism and mitosis and were arranged in tightly packed papillary structures. There was no evidence of extension of the tumour into the duodenum or stomach. The duodenal ulcer was characterised by dense granulation tissue with necrotic edges. There were non-specific changes in the liver.
The cat was initially treated with cimetidine (10 mg IV every 8 h), enrofloxacin (20 mg SC every 12 h) and flucloxacillin [(Flucil; Rhône-Poulenc Rorer) 80 mg IV every 6 h]. The cat started eating three days post-operatively and was discharged with instructions to administer ranitidine (18.75 mg PO every 12 h), clindamycin [(Antirobe; Pharmacia & Upjohn) 25 mg PO every 12 h] and enrofloxacin (25 mg PO every 24 h). Long-term management with ranitidine was effective although vomiting resumed nine months post-operatively and the cat was euthanased 12 months after surgery. Necropsy was not performed.
Case 6. A 7-year-old, spayed DSH (2.5 kg) presented with a 3-week history of inappetence and intermittent vomiting. Blood tests revealed a microcytic, hypochromic anaemia (PCV 0.12 l/l, MCV 39 fl, MCH 9.0 pg/l). An occult faecal blood test was positive. The cat improved clinically and haemodynamically following an unmatched, fresh whole blood transfusion and was referred to the UVCS.
On physical examination, the cat was thin, tachycardic (210 bpm) and tachypnoeic (60 breaths per min). The poorly regenerative, microcytic, hypochromic anaemia (PCV 0.17 l/l, MCV 41 fl, MCH 12.1 pg/l, reticulocytes 67×109/l) was confirmed on repeat blood tests. The cat was observed for 6 days and treated with cimetidine (19 mg PO every 12 h). During this period, she became progressively inappetent and developed melaena and haematemesis. As a result, the cat was scheduled for exploratory surgery and her blood type determined (type A).
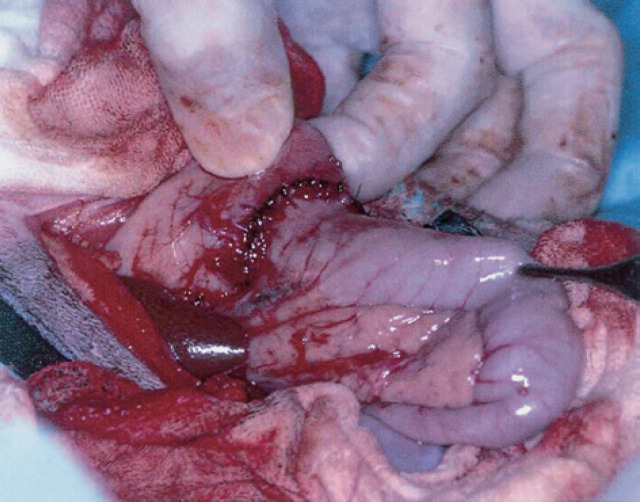
At celiotomy, the caudal fundus and antrum of the stomach were thickened and a mesenteric lymph node was enlarged. Crush preparations of the thickened gastric mucosa and lymph node were suggestive of gastric lymphosarcoma (LSA) and a reactive hyperplasia, respectively. A partial gastrectomy and gastroduodenal anastomosis (Billroth I) were performed (Fig 3). Histopathology confirmed the diagnosis of gastric LSA with no evidence of LSA in the mesenteric lymph node. Fresh, type A blood was administered pre- (20 ml) and intra-operatively (80 ml).
Fig 3.
A gastroduodenal anastomosis (Billroth I) performed after resection of a gastric lymphosarcoma (case 6).
The cat was treated with antibiotics (amoxycillin-clavulanic acid, 0.5 ml SC every 12 h; gentamicin, 5 mg every 12 h) for 48 h, cimetidine (19 mg every 8 h) for seven days, and recovered uneventfully from surgery. A sequential, multiagent chemotherapy protocol (Malik et al 2001) was administered for six months. The cat is alive and well 20 months after surgery with no evidence of recurrent gastric LSA.
Case 7. A 13-year-old, spayed DSH (4.3 kg) presented with an acute onset of lethargy and inappetence. A mass was palpable in the cranial abdomen. Serum biochemistry was within normal limits but a regenerative normocytic, hypochromic anaemia (PCV 0.14 l/l, MCV 44 fl, MCH 11.3 pg/l, reticulocytes 333×109/l) and lymphopenia (0.3×109/l) were detected. Abdominal ultrasound revealed a 30×40 mm mass in the cranioventral abdomen with anechoic material in the gastric lumen. Blood type was determined (type B) and an exploratory celiotomy performed. The cat received a fresh transfusion of type B blood (100 ml) during the perioperative period. At celiotomy, a mass was detected in the pyloric antrum of the stomach. The pylorus and proximal duodenum were resected and a gastroduodenal anastomosis performed. The gastric lymph node was also biopsied.
Histopathology revealed gastric LSA with involvement of the regional lymph node. The mucosal surface of the tumour was extensively ulcerated. The cat was maintained on intravenous fluids [0.45% sodium chloride and 2.5% glucose (Baxter Healthcare) with an additional 20 mmol/l potassium chloride], cimetidine (8.5 mg IV every 8 h), amoxycillin-clavulanic acid (0.5 ml every 12 h) and gentamicin (10 mg every 8 h). All treatment was ceased when the cat resumed eating two days after surgery. The sequential multiagent chemotherapy protocol was begun at suture removal 10 days post-operatively and continued for a total of seven months. The cat is currently doing well 13 months post-operatively with occasional vomiting but no ultrasonographic evidence of recurrence.
Case 8. A 12-year-old, spayed Chinchilla cat (5.4 kg) had a 4-day history of inappetence, polydipsia and haematochezia. She had presented twice in the preceding eight months with vomiting, diarrhoea and weight loss (0.4 kg). Physical findings included tachycardia (250 bpm), pale mucous membranes and caudal abdominal pain. Haematology, serum biochemistry and urinalysis were performed. Abnormalities included a regenerative, macrocytic, normochromic anaemia (PCV 0.14 l/l, MCV 47 fl, MCH 14.0 pg/l, reticulocytes 300×109/l), neutrophilia (32.7×109/l) and lymphopenia (0.79×109/l).
Abdominal ultrasonography revealed an intramural gastric mass with thickening of the stomach wall in the body and pyloric antrum and anechoic material in the gastric lumen. An anechoic cystic structure (20 mm×30 mm), containing echogenic particles, was detected in the right kidney.
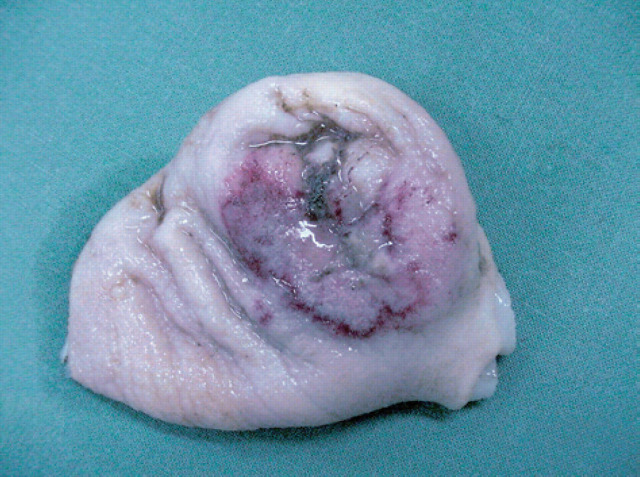
The cat was euthanased and a necropsy performed. A 30×30 mm mass, with an extensively ulcerated mucosal surface, was present along the lesser curvature of the stomach (Fig 4). The contents of the stomach and proximal small intestine were dark-coloured and voluminous. Histologically, the gastric mass was a poorly differentiated adenocarcinoma. Polycystic kidney disease, with haemorrhage into a large cyst in the right kidney, was also confirmed.
Fig 4.
The mucosal surface of a gastric adenocarcinoma (case 8). Note the extensive ulceration.
Literature review
A review of the literature revealed a further 25 cases of GU in cats. Gastroduodenal ulcers were associated with neoplasia in 11 cases with mast cell tumour (n=7) (Seawright & Grono 1964, Schalm et al 1975, Hasler & van den Ingh 1978, Liska et al 1979, Gordon 1980, Jones & Hunt 1983, Bortnowski & Rosenthal 1992, Mai & Bouhoula 1998) the most common diagnosis followed by gastrinoma (n=3) (Middleton et al 1983, van der Gaag et al 1988, Eng et al 1992) and LSA (n=1) (Weller & Hornof 1979). Zwahlen et al (1998) described two additional cases of intestinal perforation associated with alimentary LSA although the location of perforation were not recorded and hence were not included in this metanalysis.
A variety of other causes for GU were diagnosed in the remaining cats. Two cases referable to parasitic disease, Aonchotheca putorii (n=1) (Curtsinger et al 1993) and Toxocara cati (n=1) (Aoki et al 1990), have been reported. Inflammatory bowel disease has been reported as a cause of gastroduodenal erosion and ulceration in cats (Jergens et al 1992). Gastroduodenal ulceration has also been associated with hypereosinophilic syndrome (McEwen et al 1985), staphylococcal granuloma (Sheikh-Omar & Abdullah 1985), toxicity to Dieffenbachia leaves (Muller et al 1998), carprofen administration (Runk et al 1999), and following surgery (Evans & Biery 1980, Holmberg et al 1989). One case of gastric rupture was excluded from review as the presentation was not consistent with gastric ulceration and histopathology of the gastric lesion was not performed (Wright 1983). Interestingly, a cause for ulceration was not identified in the remaining four cases (Wrigley 1977, Gordon 1980, Thilgar et al 1989, Twedt 1994).
Detailed information was available for 14 of the reviewed cases (six with neoplasia and eight with non-neoplastic causes of GU). Table 3 lists the causes of GU and Table 4 summarises the pertinent clinical findings of neoplastic and non-neoplastic causes of feline GU in the present series and previously recorded cases. The location of ulcers was almost exclusively pyloroantral or fundic in cats with non-neoplastic GU. Only one cat with non-neoplastic GU, due to carprofen administration, had a duodenal ulcer (Runk et al 1999). Conversely, duodenal ulceration was more common in cats with extra-intestinal neoplasia. There was a relatively equal distribution of single and multiple ulcers irrespective of the aetiology.
Table 3.
Causes of gastric and duodenal ulcers reported in cats
| Causes of ulcers | Types |
|---|---|
| Drugs | Nonsteroidal antiinflammatory drugs |
| Primary gastric disease | Neoplasia (lymphosarcoma and adenocarcinoma), inflammatory bowel disease |
| Stress | Surgery |
| Gastric hyperacidity | Systemic mastocytosis, gastrinoma |
| Systemic disorders | Renal failure, hypovolaemia |
| Miscellaneous | Endoparasites, bacterial granuloma, hypereosinophilic syndrome, toxicity, pancreatic adenocarcinoma |
Discussion
Gastroduodenal ulceration is uncommonly reported in cats. The present series describes the clinical features of eight cats with gastrointestinal disease and blood loss due to GU. By including a further 25 cases from the literature, a greater range of causes for feline GU were detected (Table 3). Gastroduodenal ulceration was arbitrarily divided into neoplastic and non-neoplastic causes, however, a definitive aetiology could not be identified in some cats with non-neoplastic GU. Most cats responded well to appropriate management, including several cases with neoplasia, despite often presenting in, or progressing to, a critical condition.
The presenting signs, physical findings and laboratory results in cats with GU were either non-specific or consistent with upper gastrointestinal disease. The duration of clinical signs was notably greater in cats with tumour-associated GU and these cats were more likely to present with weight loss due to prolonged inappetence or cancer cachexia. Haematemesis and melaena provided evidence for blood loss into the gastrointestinal tract but were present in less than a third of cats with GU (Table 4). Examination of the oral cavity is important in the investigation of cases with occult gastrointestinal blood loss as it may reveal a linear foreign body anchored to the base of the tongue or ulcerative stomatitis as an accompaniment to uraemic gastritis. Furthermore, bleeding palatine ulcers (Wildgoose 1990, Menrath & Miller 1995), which may mimic GU, can be excluded.
Table 4.
Summary of signalment, presenting signs, physical examination and laboratory findings, and ulcer characteristics in cats with gastroduodenal ulcers from the University Veterinary Centre Sydney and literature review
| Tumour UVCS | Non-tumour UVCS | Tumour review | Non-tumour review | Tumour total | Non-tumour total | Total | |
|---|---|---|---|---|---|---|---|
| Breed | 80% DSH (4/5) | 33% DSH (1/3) | 60% DSH (3/5) | 83% DSH (5/6) | 70% DSH (7/10) | 67% DSH (6/9) | 68% DSH (13/19) |
| Age (years) | 11.2 | 4.5 | 9.7 | 9.1 | 10.4 | 7.2 | 9.1 |
| Sex | 100% F/FS (5/5) | 0% F/FS (0/3) | 50% F/FS (3/6) | 60% F/FS (3/5) | 73% F/FS (8/11) | 38% F/FS (3/8) | 58% F/FS (11/19) |
| Duration (weeks) | 19.6 | 0.7 | 25.4 | 1.5 | 22.5 | 1.3 | 12.4 |
| Vomiting | 80% (4/5) | 100% (3/3) | 100% (5/5) | 29% (2/7) | 90% (9/10) | 50% (5/10) | 70% (14/20) |
| Haematemesis | 40% (2/5) | 0% (0/3) | 25% (1/4) | 17% (1/6) | 33% (3/9) | 11% (1/9) | 22% (4/18) |
| Melena | 40% (2/5) | 33% (1/3) | 0% (0/4) | 33% (2/6) | 22% (2/9) | 33% (3/9) | 28% (5/18) |
| Inappetence | 80% (4/5) | 33% (1/3) | 60% (3/5) | 60% (3/5) | 70% (7/10) | 50% (4/8) | 61% (11/18) |
| Weight loss | 100% (5/5) | 0% (0/3) | 100% (4/4) | 29% (2/7) | 100% (9/9) | 20% (2/10) | 58% (11/19) |
| Polydipsia | 40% (2/5) | 33% (1/3) | 0% (0/4) | 0% (0/7) | 22% (2/9) | 10% (1/10) | 16% (3/19) |
| Abdominal pain | 20% (1/5) | 67% (2/3) | 0% (0/3) | 29% (2/7) | 13% (1/8) | 40% (4/10) | 28% (5/18) |
| Anaemia | 100% (5/5) | 100% (3/3) | 50% (2/4) | 40% (2/5) | 78% (7/9) | 63% (5/8) | 71% (12/17) |
| Hypoproteinaemia | 60% (3/5) | 0% (0/3) | 0% (0/4) | 40% (2/5) | 33% (3/9) | 25% (2/8) | 29% (5/17) |
| Lymphopaenia | 60% (3/5) | 0% (0/3) | 0% (0/4) | 40% (2/5) | 33% (3/9) | 25% (2/8) | 29% (5/17) |
| Neutrophilia | 60% (3/5) | 67% (2/3) | 25% (1/4) | 20% (1/5) | 44% (4/9) | 38% (3/8) | 41% (7/17) |
| Left shift | 20% (1/5) | 33% (1/3) | 25% (1/4) | 40% (2/5) | 22% (2/9) | 38% (3/8) | 29% (5/17) |
| Hypokalaemia | 20% (1/5) | 67% (2/3) | 0% (0/4) | 20% (1/5) | 11% (1/9) | 38% (3/8) | 24% (4/17) |
| Renal failure | 0% (0/5) | 0% (0/3) | 0% (0/3) | 0% (0/5) | 0% (0/8) | 0% (0/8) | 0% (0/16) |
| Hepatic failure | 0% (0/5) | 0% (0/3) | 0% (0/3) | 0% (0/5) | 0% (0/8) | 0% (0/8) | 0% (0/16) |
| Ulcer location | 30% Fundus | 17% Fundus | 17% Fundus | 69% Fundus | 23% Fundus | 54% Fundus | 39% Fundus |
| 40% Pylorus | 83% Pylorus | 0% Pylorus | 19% Pylorus | 18% Pylorus | 36% Pylorus | 27% Pylorus | |
| 30% Duodenum | 0% Duodenum | 83% Duodenum | 13% Duodenum | 59% Duodenum | 9% Duodenum | 34% Duodenum | |
| Perforated | 40% (2/5) | 33% (1/3) | 50% (2/4) | 44% (4/9) | 44% (4/9) | 42% (5/12) | 43% (9/21) |
| Survival (months) | 12.2 | 14.7 | 6.8 | 2.3 | 9.2 | 6.4 | 8.0 |
UVCS, University Veterinary Centre Sydney; DSH, domestic short hair; F, female; FS, female spayed.
Anaemia was detected in all cats with GU in the present series. Thus, GU should be considered in any cat presenting with anaemia in which the cause of blood loss cannot be readily identified. Other laboratory findings were nonspecific and reflected changes associated with vomiting, blood loss, inflammation and stress (Guilford & Strombeck 1996b).
A variety of imaging techniques have been described in the diagnosis of GU including endoscopy, ultrasonography, plain and contrast radiography, and scintigraphy (Guilford & Strombeck 1996b, Penninck & Tidwell 1997, Hall 2000). In the present series, simple imaging techniques, such as abdominal radiography and ultrasonography, provided pertinent information rapidly and without the need for chemical restraint. In cats with a perforated ulcer, evidence of pneumoperitoneum or peritonitis on plain abdominal radiographs signalled the need for urgent exploratory surgery. Abdominal ultrasonography was performed if plain radiography was unrewarding. In dogs, the ultrasonographic features of gastric ulceration include local mural thickening, loss of the five-layer structure, presence of a defect or crater in the gastric wall, fluid accumulation in the lumen of the stomach and diminished gastric motility (Penninck & Tidwell 1997). However, of the seven cases in which abdominal ultrasonography was performed in this study, these findings were only recognised in the three cats with gastric tumours. Although endoscopy is the preferred imaging modality for detecting gastric and duodenal ulcers (Guilford & Strombeck 1996b, Hall 2000), the majority of cats with GU presented in a critical condition which precluded its use due to time constraints, anaesthetic risk and possibility of iatrogenic ulcer perforation.
The management of cats with gastrointestinal disease and blood loss can be complicated by their critical nature and lack of specific clinical signs and physical findings. In general, following blood tests and abdominal imaging, cats with GU were stabilised with a transfusion of fresh, whole blood. All cats receiving blood transfusions in the present series were either cross-matched or blood-typed to ensure compatibility. Transfusion reactions are common in cats, ranging from decreased erythrocyte survival time in type A cats receiving type B blood to life-threatening incompatibility reactions in type B cats receiving type A blood (Hohenhaus & Rentko 2000). Hence, cross-matching or blood-typing is mandatory prior to administration of fresh, whole blood, especially in Australia where approximately 25% of DSH are type B (Auer & Bell 1981).
In the present series, surgery was performed in all cases that subsequently survived. Life-threatening-haemorrhage, failure to respond to medical management and perforation are indications for surgical intervention (van Sluys 1993, Hall 2000). In humans, non-surgical management of peptic ulcers is common as prospective studies comparing conservative and surgical treatment of perforated peptic ulcers show no significant difference in morbidity and mortality (Zittel et al 2000). However, in our experience, prompt surgical intervention is preferred as cats with GU often present in a critical condition with perforation and spillage of intestinal contents and bacteria into the peritoneal cavity.
A thorough exploration of the abdominal cavity should be performed as extra-intestinal neoplasms may cause GU and unperforated ulcers can be difficult to locate (Hall 2000). Intraoperative endoscopy or ultrasonography has been recommended to localise unperforated ulcers (Hall 2000). However, in the present series, unperforated GU was easily identified as the ulcers were associated with either adhesions or a gastric mass. Since multiple lesions are relatively common in cats with either neoplastic and non-neoplastic GU, a complete examination of the gastrointestinal tract should be performed (Gordon 1980, Middleton et al 1983, van der Gaag et al 1988, Aoki et al 1990, Jergens et al 1992).
In humans, conservative management of hyperacidity with H2-receptor antagonists and proton pump inhibitors has replaced the need for radical surgery (Guilford & Strombeck 1996b, Hall 2000, Zittel et al 2000). The current recommendation for surgical management of GU, as employed in the majority of the present cases, is debridement and suturing of the ulcer (Zittel et al 2000). The risk of disruption to the major duodenal papilla is minimised by using such conservative surgical techniques in the treatment of duodenal ulcers. However, more extensive surgery, such as a Billroth I, may be required for the resection of gastrointestinal masses causing GU (van Sluys 1993).
Extra-intestinal tumours that can cause GU in cats include systemic mastocytosis, pancreatic adenocarcinoma and gastrinoma (Seawright & Grono 1964, Schalm et al 1975, Hasler & van den Ingh 1978, Liska et al 1979, Jones & Hunt 1983, Middleton et al 1983, van der Gaag et al 1988, Eng et al 1992, Mai & Bouhoula 1998). Splenectomy is recommended in cats with GU secondary to systemic mastocytosis with splenic involvement (Vail 1996). Partial pancreatectomy should be performed in cats with resectable pancreatic masses (Simpson & Dykes 1997). However, tumours of the pancreas may either be inapparent, such as the suspected gastrinoma in case 4, or diffuse, as illustrated by the adenocarcinoma in case 5, and hence not amenable to partial pancreatectomy. In dogs with Zollinger-Ellison syndrome, resection of the right limb of the pancreas has been recommended if the pancreatic mass is not visible grossly (Simpson & Dykes 1997) but this should not be extrapolated to cats as little information is available on the distribution of gastrinomas in the feline pancreas due to a paucity of reported cases (Eng et al 1992, van der Gaag et al 1988, Middleton et al 1983).
Adjunctive medical therapy is essential in the management of cats with GU. Antibiotics have significantly reduced the morbidity and mortality associated with perforated ulcers in humans (Zittel et al 2000). The oral cavity is the most common source of gastric bacteria and recent studies have shown that healthy cats harbour high numbers of gram-positive cocci, coliforms, and facultative and strict anaerobes in the proximal small intestine (Guilford & Strombeck 1996a, Papasouliotis et al 1998, Johnston et al 2001). Hence, a broad spectrum antibiotic, such as amoxycillin-clavulanic acid, or combination of antibiotics, such as amoxycillin-clavulanic acid, enrofloxacin and metronidazole, is recommended.
Antisecretory agents, such as H2-receptor antagonists and proton pump inhibitors, are important in the management of cats with GU. In the present series, H2-receptor antagonists, such as cimetidine and ranitidine, were used in the immediate post-operative period to aid the healing of sutured gastroduodenal defects as they were the only preparations available in both parenteral and oral preparations at the time. Ranitidine is considered superior to cimetidine as it results in greater suppression of gastric acid secretion and a longer duration of action, thus requiring less frequent administration (Hall 2000, Guilford & Strombeck 1996b). Omeprazole, a proton pump inhibitor, is preferred for cats with unremitting gastric hyperacidity, such as with systemic mastocytosis and gastrinoma, as it has a more profound suppression of gastric acid secretion than H2-receptor antagonists, inhibits gastric acid secretion regardless of the secretagogue, and only requires once daily administration (Guilford & Strombeck 1996b). In the present series, one cat with duodenal ulceration due to a suspected gastrinoma (case 4) survived 18 months with once daily omeprazole administration. Other possible treatments include sucralfate, which protect ulcers from gastric acid, and misoprostol, a prostaglandin E1 analogue, which is preferred for the prevention and treatment of NSAIDS-induced GU (Hall 2000).
Causes of GU were divided into neoplastic and non-neoplastic in the present series and literature review. Non-neoplastic GU tended to have a short clinical history, minimal weight loss and ulcers located in the gastric fundus, body, antrum or pylorus. However, diagnosing the underlying cause of GU was difficult in these cases. Gastroduodenal ulcers in cats do not appear to be strongly associated with renal or hepatic dysfunction. Hepatic disease, although a common cause of GU in dogs (Stanton & Bright 1989), was not identified in cats with GU. Renal dysfunction may have contributed to GU in case 1, but has not been identified as a potential cause in previous reports. However, a strong association has been reported between chronic renal failure and iron deficiency anaemia, presumably due to gastrointestinal blood loss (Cowgill et al 1998). Gastric erosions and ulceration have been diagnosed in cats with IBD, however, these lesions are usually subclinical and rarely cause significant gastrointestinal blood loss (Jergens et al 1993, Baez et al 1999, Willard 1999). The administration of NSAIDS is a common cause of GU in dogs (Stanton & Bright 1989), however, despite numerous experimental reports of the ulcerogenic effects of NSAIDS in cats (Twedt 1994), NSAIDS-induced GU has only been reported in one clinical case (Runk et al 1999). The incidence of NSAIDS-induced gastric ulcers may increase as more NSAIDS are registered for management of pain and treatment of osteoarthritis in cats (Runk et al 1999). Hypovolaemia or stress may also play a role in the development of GU as perforated gastric ulcers have been reported in two cats following surgery (Evans & Biery 1980, Holmberg et al 1989) and either could have contributed in case 2 of the present series.
Parasitic infestation, bacterial infection, toxins and foreign bodies are reported causes of localised, non-neoplastic GU in cats (Sheikh-Omar & Abdullah 1985, Aoki et al 1990, Curtsinger et al 1993, Muller et al 1998, Hall 2000). Parasites associated with feline GU include Toxocara cati (Aoki et al 1990) and Aonchotheca putorii (Curtsinger et al 1993). Ollulanus tricuspis and Gnathostoma spp also have the potential to cause gastric ulceration (Trueman & Ferris 1977, Guilford & Strombeck 1996b, Hall 2000). Infection with Helicobacter spp is the most important cause of chronic gastritis, peptic ulceration and gastric neoplasia in people (Neiger & Simpson 2000). The role of Helicobacter spp in the pathogenesis of feline GU is uncertain, especially as Helicobacter-like organisms are commonly found in normal cats (Guilford & Strombeck 1996b, Neiger & Simpson 2000). Spiral-shaped bacteria were not considered to be the primary causative agent in any of our cats with GU. Other potential causes of feline GU include external trauma and intraluminal trauma from a foreign body or trichobezoar. However, identification of a definitive cause of non-neoplastic GU was uncommon in this series and previously reported cases (Wrigley 1977, Gordon 1980, Thilgar et al 1989, Twedt 1994). Regardless of the cause, the prognosis is good for non-neoplastic GU following prompt stabilisation and surgical intervention.
Tumour-associated GU tended to present with a protracted history of vomiting, inappetence and weight loss. A number of different tumours have been reported to cause GU (Seawright & Grono 1964, Schalm et al 1975, Hasler & van den Ingh 1978, Liska et al 1979, Weller & Hornof 1979, Jones & Hunt 1983, Middleton et al 1983, van der Gaag et al 1988, Eng et al 1992, Mai & Bouhoula 1998). Gastric tumours, such as LSA and adenocarcinoma, cause gastric ulceration by altering mucosal integrity and blood flow (van Sluys 1993, Hall 2000). However, ulceration is apparently rare in cats with proximal gastrointestinal tumours (Weller & Hornof 1979, Mahony et al 1995, Zwahlen et al 1998, Cribb 1988, Kosovsky et al 1988, Turk et al 1981, Patnaik et al 1976). Gastric LSA may behave differently to other types of feline alimentary LSA as survival greater than 12 months was reported in both of the present cases following surgical resection and a brief but intensive course of adjunctive multiagent chemotherapy. This survival time is greater than typically reported for alimentary LSA (Mahony et al 1995, Zwahlen et al 1998) and, although case numbers are too low for a meaningful comparison, similar conclusions have been made by other investigators (Willard 2000).
Systemic mastocytosis is the most frequently reported cause of neoplastic GU in cats (Seawright & Grono 1964, Schalm et al 1975, Hasler & van den Ingh 1978, Liska et al 1979, Jones & Hunt 1983, Bortnowski & Rosenthal 1992, Mai & Bouhoula 1998). Intestinal mast cell tumours have not been reported to cause ulceration in cats (Alroy et al 1975), despite being a common cause of GU in dogs (Takahashi et al 2000), although a perforated gastric ulcer was reported in one cat with a gastric mast cell tumour (Bortnowski & Rosenthal 1992).
Gastrinomas are functional non-islet cell tumours of the pancreas resulting in hypergastrinaemia, hypersecretion of gastric acid and peptic ulceration (Zerbe & Washabau 2000). A gastrinoma was suspected in case 4 on the basis of markedly elevated serum gastrin levels. The prognosis is guarded for cats with gastrinoma, as metastases are frequently detected at diagnosis, although surgical resection of the primary lesion and adjunctive treatment with antisecretory drugs, including omeprazole, may result in survival times greater than 12 months (Eng et al 1992).
Pancreatic adenocarcinoma has not previously been reported to cause GU. Ulceration may have been caused by either local invasion or the local release of activated pancreatic enzymes (Steiner & Williams 1997). Pancreatitis may also have contributed in case 2. The prognosis for pancreatic adenocarcinoma is usually poor due to regional extension, distant metastases at the time of diagnosis or the development of paraneoplastic syndromes (Pascal-Tenorio et al 1997, Steiner & Williams 1997), although case 5 in the present series survived 12 months following surgery.
Conclusion
Cats with GU often presented in a critical condition. Clinical signs consistent with gastrointestinal bleeding were uncommon although anaemia was a frequent laboratory finding. Appropriate management of cats with GU involves prompt stabilisation with a compatible blood transfusion, surgical debridement or resection of the ulcer, antibiotic and antiulcer therapy, and treatment of the underlying disease, if identified.
Non-neoplastic causes of feline GU tended to have a shorter clinical course with ulcers confined to the stomach. The causative factor was difficult to identify. However, with appropriate management, cats with non-neoplastic GU had an excellent prognosis.
Cats with tumour-associated GU usually had a more protracted clinical course, weight loss, and ulcers located in the stomach for gastric tumours and the duodenum for extra-intestinal tumours. In both the present series and previously published case reports, the prognosis for cats with GU secondary to gastric LSA, gastrinoma and systemic mastocytosis is good.
Acknowledgements
The authors would like to thank clinicians, anaesthetists and technical staff at the University Veterinary Centre Sydney, and referring veterinarians for their assistance with case management.
Addendum
Since writing this report a 20-year-old, spayed DSH died acutely in our hospital. Necropsy revealed proximal duodenal ulceration with significant intraluminal haemorrhage, generalised splenomegaly and mild hepatomegaly. A diagnosis of duodenal ulceration secondary to systemic mastocytosis was made on the basis of splenic and hepatic histopathology.
References
- Auer L, Bell K. (1981) The AB blood group system of cats. Animal Blood Groups & Biochemical Genetics 12, 287–297. [DOI] [PubMed] [Google Scholar]
- Alroy J, Leav I, DeLellis RA, Weinstein RS. (1975) Distinctive intestinal mast cell neoplasms of domestic cats. Laboratory Investigation 33, 159–167. [PubMed] [Google Scholar]
- Aoki S, Yamagami T, Saeki H, Washizu M. (1990) Perforated gastric ulcer caused by Toxocara cati in a cat. Journal of the Japanese Veterinary Medical Association 43, 207–210. [Google Scholar]
- Baez JL, Hendrick MJ, Walker LM, Washabau RJ. (1999) Radiographic, ultrasonographic, and endoscopic findings in cats with inflammatory bowel disease of the stomach and small intestine: 33 cases (1990–1997). Journal of the American Veterinary Medical Association 215, 349–354. [PubMed] [Google Scholar]
- Bortnowski HB, Rosenthal RC. (1992) Gastrointestinal mast cell tumors and eosinophilia in two cats. Journal of the American Animal Hospital Association 28, 271–275. [Google Scholar]
- Cribb AE. (1988) Feline gastrointestinal adenocarcinoma: a review and retrospective study. Canadian Veterinary Journal 29, 709–712. [PMC free article] [PubMed] [Google Scholar]
- Cowgill LD, James KM, Levy JK, Browne JK, Miller A, Lobingier RT, Egrie JC. (1998) Use of recombinant human erythropoietin for management of anemia in dogs and cats with renal failure. Journal of the American Veterinary Medical Association 212, 521–528. [PubMed] [Google Scholar]
- Curtsinger DK, Carpenter JL, Turner JL. (1993) Gastritis caused by Aonchotheca putorii in a domestic cat. Journal of the American Veterinary Medical Association 203, 1153–1154. [PubMed] [Google Scholar]
- DiBartola SP. (2000) Disorders of sodium and water. In: Fluid Therapy in Small Animal Practice, 2nd edn. DiBartola SP, ed. Philadelphia: WB Saunders, pp. 45–72. [Google Scholar]
- DiBartola SP, De Morais HA. (2000) Disorders of potassium. In: Fluid Therapy in Small Animal Practice, 2nd edn. Di Bartola SP, ed. Philadelphia: WB Saunders, pp. 83–107. [Google Scholar]
- Eng J, Du BH, Johnson GF, Kanakamedala S, Samuel S, Raufman JP, Straus E. (1992) Cat gastrinoma and the sequence of cat gastrins. Regulatory Peptides 37, 9–13. [DOI] [PubMed] [Google Scholar]
- Evans SM, Biery DN. (1980) Congenital peritoneopericardial diaphragmatic hernia in the dog and cat: a literature review and 17 additional case histories. Veterinary Radiology 21, 108–116. [Google Scholar]
- Gordon RN. (1980) A perforating gastric ulcer in a cat. Australian Veterinary Journal 56, 41. [DOI] [PubMed] [Google Scholar]
- Guilford WG, Strombeck DR. (1996a) Acute gastritis. In: Strombeck's Small Animal Gastroenterology, 3rd edn. Guilford WG, Center SA, Strombeck DR, Williams DA, Meyer DJ, eds. Philadelphia: WB Saunders, pp. 264–273. [Google Scholar]
- Guilford WG, Strombeck DR. (1996b) Chronic gastric diseases. In: Strombeck's Small Animal Gastroenterology, 3rd edn. Guilford WG, Center SA, Strombeck DR, Williams DA, Meyer DJ, eds. Philadelphia: WB Saunders, pp. 284–291. [Google Scholar]
- Hall JA. (2000) Diseases of the stomach. In: Textbook of Veterinary Internal Medicine: Diseases of the Dog and Cat, 5th edn. Ettinger SJ, Feldman EC, eds. Philadelphia: WB Saunders, pp. 1154–1168. [Google Scholar]
- Hasler UC, van den Ingh TS. (1978) Malignant mastocytosis and duodenal ulceration in a cat. Schweizer Archiv fur Tierheilkunde 120, 263–268. [PubMed] [Google Scholar]
- Hohenhaus AE, Rentko V. (2000) Blood transfusions and blood substitutes. In: Fluid Therapy in Small Animal Practice, 2nd edn. DiBartola SP, ed. Philadelphia: WB Saunders, pp. 451–464. [Google Scholar]
- Holmberg DL, Fries C, Cockshutt, Van Pelt D. (1989) Ventral rhinotomy in the dog and cat. Veterinary Surgery 18, 446–449. [DOI] [PubMed] [Google Scholar]
- Jergens AE, Moore FM, March P, Miles KG. (1992) Idiopathic inflammatory bowel disease associated with gastroduodenal ulceration-erosion: a report of nine cases in the dog and cat. Journal of the American Animal Hospital Association 28, 21–26. [Google Scholar]
- Johnston KL, Swift NC, Forster-van Hijfte M, Rutgers HC, Lamport A, Ballevre O, Batt RM. (2001) Comparison of the bacterial flora of the duodenum in healthy cats and cats with signs of gastrointestinal disease. Journal of the American Veterinary Medical Association 218, 48–51. [DOI] [PubMed] [Google Scholar]
- Jones TC, Hunt TD. (1983) In: Veterinary Pathology, 5th edn. Philadelphia: Lea & Febiger, p. 1127. [Google Scholar]
- Kiss G, Szilagyi T. (1988) Cardiogenic shock in domestic cats. I. Inflammatory lesions in the heart. Magyar Allatorvosok Lapja 43, 729–732. [Google Scholar]
- Kosovsky JE, Matthiesen DT, Patnaik AK. (1988) Small intestinal adenocarcinoma in cats: 32 cases (1978–1985). Journal of the American Veterinary Medical Association 192, 233–235. [PubMed] [Google Scholar]
- Liska WD, MacEwan EG, Zaki F, Garvey M. (1979) Feline systemic mastocytosis: a review and results of splenectomy in seven cases. Journal of the American Animal Hospital Association 15, 589–597. [Google Scholar]
- McEwen SA, Valli VEO, Hulland TJ. (1985) Hypereosinophilic syndrome in cats: a report of three cases. Canadian Journal of Comparative Medicine 49, 248–253. [PMC free article] [PubMed] [Google Scholar]
- Mahony OM, Moore AS, Cotter SM, Engler SJ, Brown D, Pennick DG. (1995) Alimentary lymphoma in cats: 28 cases (1988–1993). Journal of the American Veterinary Medical Association 207, 1593–1597. [PubMed] [Google Scholar]
- Mai W, Bouhoula L. (1998) Mastocytose systemique associee a une mastocytose sanguine et a un ulcere gastrique chez un chat. Le Point Veterinaire 29, 1161–1165. [Google Scholar]
- Malik R, Gabor LJ, Foster SF, McCorkell BE, Canfield PJ. (2001) Therapy for Australian cats with lymphosarcoma. Australian Veterinary Journal 79, 808–817. [DOI] [PubMed] [Google Scholar]
- Menrath VH, Miller R. (1995) The repair and prevention of bleeding palatine erosive lesions in the cat. Australian Veterinary Practitioner 25, 202–206. [Google Scholar]
- Middleton DJ, Watson ADJ, Vasak E, Culvenor JE. (1983) Duodenal ulceration associated with a gastrin-secreting pancreatic tumor in a cat. Journal of the American Veterinary Medical Association 183, 461–462. [PubMed] [Google Scholar]
- Muller N, Glaus T, Gardelle O. (1998) Ausgedehnte magenulzera durch Dieffenbachia-intoxikation bei einer katze. Tierarztliche Praxis 26, 404–407. [PubMed] [Google Scholar]
- Neiger R, Simpson KW. (2000) Helicobacter infection in dogs and cats: facts and fiction. Journal of Veterinary Internal Medicine 14, 125–133. [DOI] [PubMed] [Google Scholar]
- Papasouliotis K, Sparkes AH, Werrett G, Egan K, Gruffydd-Jones EA, Gruffydd-Jones TJ. (1998) Assessment of the bacterial flora of the proximal small intestine in healthy cats, and the effect of sample collection method. American Journal of Veterinary Research 59, 48–51. [PubMed] [Google Scholar]
- Pascal-Tenorio A, Olivry T, Gross TL, Atlee BA, Ihrke PJ. (1997) Paraneoplastic alopecia associated with internal malignancies in the cat. Veterinary Dermatology 8, 47–52. [DOI] [PubMed] [Google Scholar]
- Patnaik AK, Liu SK, Johnson GF. (1976) Feline intestinal adenocarcinoma: a clinicopathologic study of 22 cases. Veterinary Pathology 13, 1–10. [DOI] [PubMed] [Google Scholar]
- Penninck D, Tidwell A. (1997) Ultrasonography of gastric ulceration in the dog. Veterinary Radiology and Ultrasound 38, 308–312. [DOI] [PubMed] [Google Scholar]
- Runk A, Kyles AE, Downs MO. (1999) Duodenal perforation in a cat following the administration of nonsteroidal anti-inflammatory medication. Journal of the American Animal Hospital Association 35, 52–55. [DOI] [PubMed] [Google Scholar]
- Schalm OW, Jain NC, Carroll EJ. (eds) (1975) Veterinary Hematology, 3rd edn. Philadelphia: Lea & Febiger, pp. 565–591. [Google Scholar]
- Seawright AA, Grono LR. (1964) Malignant mast cell tumour in a cat with perforating duodenal ulcer. Journal of Pathology and Bacteriology 87, 107–111. [DOI] [PubMed] [Google Scholar]
- Sheikh-Omar AR, Abdullah AS. (1985) Perforated gastric ulcer associated with disseminated staphylococcal granuloma (botryomycosis) in a cat. Veterinary Record 117, 131. [DOI] [PubMed] [Google Scholar]
- Simpson KW, Dykes NL. (1997) Diagnosis and treatment of gastrinoma. Seminars in Veterinary Medicine and Surgery (Small Animal) 12, 274–281. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Bright RM. (1989) Gastroduodenal ulceration in dogs: retrospective study of 43 cases and literature review. Journal of Veterinary Internal Medicine 3, 238–244. [DOI] [PubMed] [Google Scholar]
- Steiner JM, Williams DA. (1997) Feline exocrine pancreatic disorders: insufficiency, neoplasia, and uncommon conditions. Compendium on the Continuing Education of the Practicing Veterinarian 19, 836–848. [Google Scholar]
- Takahashi T, Kadosawa T, Nagase M, Matsunaga S, Mochizuki M, Nishimura R, Sasaki N. (2000) Visceral mast cell tumors in dogs: 10 cases (1982–1997). Journal of the American Veterinary Medical Association 216, 222–226. [DOI] [PubMed] [Google Scholar]
- Thilgar S, David A, Balasubramanian NN. (1989) Gastric ulceration with perforation and pneumoperitoneum in a cat—a case report. Indian Veterinary Journal 66, 1068. [Google Scholar]
- Trueman KF, Ferris PBC. (1977) Gnathostomiasis in three cats. Australian Veterinary Journal 53, 498–499. [DOI] [PubMed] [Google Scholar]
- Turk MAM, Gallina AM, Russell TS. (1981) Nonhematopoietic gastrointestinal neoplasia in cats: a retrospective study of 44 cases. Veterinary Pathology 18, 614–620. [DOI] [PubMed] [Google Scholar]
- Twedt DC. (1994) Diseases of the stomach. In: The Cat: Diseases and Clinical Management, 2nd edn. Sherding RG, ed. New York: Churchill Livingstone, pp. 1199–1201. [Google Scholar]
- Vail DM. (1996) Mast cell tumors. In: Small Animal Clinical Oncology, 2nd edn. Withrow SJ, MacEwen EG, eds. Philadelphia: WB Saunders, pp. 192–210. [Google Scholar]
- van der Gaag I, van den Ingh TSGAM, Lamers CBHW, Lindeman J. (1988) Zollinger-Ellison syndrome in a cat. Veterinary Quarterly 10, 151–155. [DOI] [PubMed] [Google Scholar]
- van Sluys FJ. (1993) Upper gastrointestinal bleeding. In: Textbook of Small Animal Surgery, 2nd edn. Slatter DH, ed. Philadelphia: WB Saunders, pp. 576–580. [Google Scholar]
- Vleet JF, van Ferrans VJ. (1986) Myocardial diseases of animals. American Journal of Pathology 124, 98–178. [PMC free article] [PubMed] [Google Scholar]
- Weller RE, Hornof WJ. (1979) Gastric malignant lymphoma in two cats. Modern Veterinary Practice 60, 701–704. [PubMed] [Google Scholar]
- Wildgoose WH. (1990) Palatine arterial haemorrhage in a cat. Veterinary Record 126, 273. [PubMed] [Google Scholar]
- Willard MD. (1999) Feline inflammatory bowel disease: a review. Journal of Feline Medicine and Surgery 1, 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard MD. (2000) Diarrhoea: stopping the unstoppable flood. Post Graduate Foundation in Veterinary Science University of Sydney Proceedings 328, pp. 43–73.
- Wright RP. (1983) An unusual case of feline gastrorrhexis. Veterinary Medicine Small Animal Clinician 78, 921–923. [Google Scholar]
- Wrigley R. (1977) A perforating gastric ulcer in a cat. Australian Veterinary Practitioner 7, 173–176. [Google Scholar]
- Zerbe CA, Washabau RJ. (2000) Gastrointestinal endocrine disease. In: Textbook of Veterinary Internal Medicine: Diseases of the Dog and Cat, 5th edn. Ettinger SJ, Feldman EC, eds. Philadelphia: WB Saunders, pp. 1502–1506. [Google Scholar]
- Zittel TT, Jehle EC, Becker HD. (2000) Surgical management of peptic ulcer disease today—indication, technique and outcome. Langenbeck's Archives of Surgery 385, 84–96. [DOI] [PubMed] [Google Scholar]
- Zwahlen CH, Lucroy MD, Kraegel SA, Madewell BR. (1998) Results of chemotherapy for cats with alimentary malignant lymphoma: 21 cases (1993–1997). Journal of the American Veterinary Medical Association 213, 1144–1149. [PubMed] [Google Scholar]