Abstract

Practical relevance Long-term pain in cats is an important welfare issue but is often overlooked and undertreated.
Audience All practitioners are faced with cats that require analgesic intervention to improve their quality of life.
Patient group Any cat may potentially experience long-term pain and discomfort. Degenerative joint disease and diabetic-related pain is more common in middle-aged or older individuals, whereas persistent postsurgical pain can occur at any age and is seen in young cats following onychectomy.
Evidence base Robust evidence on long-term pain issues in cats — specifically, relating to prevalence, etiology, and treatment protocols and outcomes — is missing from the veterinary literature. The aim of this review is to summarise the current state of knowledge. In doing so, it takes a practical approach, highlighting the obvious, and some not so obvious, causes of long-term pain in cats; some aspects that warrant closer attention; our ability to recognize pain and monitor how this impacts on quality of life; and today's treatment options.
Djd-Associated Pain
A sister article addressing the clinical challenges of recognising, assessing and treating pain associated with degenerative joint disease in cats appears on pages 200––212 of this issue of J Feline Med Surg, and at: doi:10.1016/j.jfms.2010.01.003
Defining pain is not a simple matter
Pain is a multifactorial experience, with sensory (‘ouch’), affective/emotional (how it makes the cat feel) and functional (can the cat still jump up on a window ledge?) components. It can result from obvious causes (eg, a fracture) and last an expected period of time. However, in many cases, for example post-amputation, the pain persists after the original injury or wound appears to have healed. In diseases such as interstitial cystitis the underlying mechanisms that cause pain are poorly understood, there is no clear path to resolution and classic analgesics such as opioids and anti-inflammatory agents do not consistently result in relief. In some cases pain has no obvious cause and is sometimes classified as ‘idiopathic’ or dysfunctional. 1
In the past, pain has often been categorized as acute or chronic based solely on duration — the latter arbitrarily being pain that lasts more than 3–6 months. However, it is now accepted that this may not be a helpful classification, and it is suggested that the terms adaptive and maladaptive be adopted instead (see page 189).
Ideally, pain should be classified by the underlying mechanism; 2 for example, inflammatory or neuropathic. Taken one step further, pain occurring in different diseases or conditions would be classified according to the underlying mechanisms in the individual patient. For the practitioner, knowledge of this so-called ‘neurobiological signature of pain’ in a particular disease would better guide the choice of treatment. For example, a diagnosis of ‘cancer’ pain is not very helpful since the cause could be mechanical compression of a nerve, inflammation from tissue necrosis, or mechanical distension of an organ. However, a diagnosis of ‘osteosarcoma bone pain’, with the associated knowledge of the upregulation of peripheral TRPV1 and ASIC receptors, and central COX-2 enzyme, is far more informative in terms of guiding clinical treatment. Without this information, treatment is empirical — or perhaps better termed ‘hit or miss’.
Types of Pain
NOCICEPTIVE PAIN
Example: touching something hot
No inflammation or nervous system damage
Adaptive
Inflammatory Pain
Example: surgical incision
Active inflammation
Adaptive
Protective during healing
Reversible
Neuropathic Pain
Example: post-amputation pain secondary to surgical damage to nerves
Nervous system damage
Maladaptive
Dysfuctional-does not protect and does not support healing
Often persistent
Adaptive pain infers a normal response to tissue damage (eg, a surgical incision) and involves an inflammatory component. It is reversible over an expected, relatively short time period.
Maladaptive pain results from changes in the spinal cord and brain that lead to abnormal sensory processing. It is usually persistent. Maladaptive pain may develop from poorly treated adaptive pain, and can arise quickly in some circumstances.
That said, the underlying cause may be complex, and some mechanisms of pain can coexist and require several different treatment strategies. This approach is discussed in pain management guidelines developed jointly by the American Animal Hospital Association and American Association of Feline Practitioners. 3 It is akin to identifying the bacteria and/or virus that is causing an infection so that the correct drug(s) can be chosen for treatment.
Even if the underlying cause or mechanism is identified, the disease process and associated pain may not be static, and this presents an additional-challenge for the practitioner. Acute ‘flare-ups’ are common with feline degenerative joint disease (DJD) (ie, the ‘good’ days and ‘bad’ days) and a cat that is receiving good pain management for a tumor can still experience acute exacerbations if the tumor grows rapidly or becomes necrotic. Concurrent disease conditions, such as inflammatory bowel disease, may also alter the rocessing of noxious signals and, thus, the perceived pain.
Even if the underlying cause or mechanism is identified, the disease process and associated pain may not be static.
The underlying cause may be complex, and some mechanisms of pain can coexist and require several different treatment strategies.
Potential causes of long-term pain
The clinical conditions that are likely to result in long-term pain and discomfort in cats include DJD, interstitial cystitis, various cancers, many dermatological diseases (Fig 1), dental and oral diseases such as gingivostomatitis (Fig 2), slow healing wounds (Fig 3), burns (Figs 4 and 5) and diabetic neuropathy (Fig 6). In these various examples pain can be correlated with a disease or injury. Other potential long-term pain issues are treatment-related, including persistent postsurgical pain, radiation burns (Fig 5) and chemotherapy-induced neuropathy.
Fig 1.
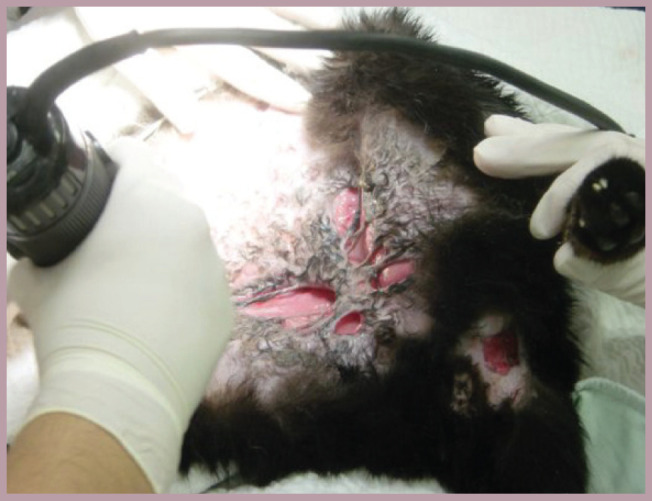
Cat with a longstanding cutaneous mycobacterial infection that was being specifically managed with antibiotics and surgical debridement. This disease can be difficult to treat and it can take up to a year to achieve resolution. Treatment of the accompanying pain is often overlooked in such cases. The owner of this cat reported a significant improvement in its demeanour when nonsteroidal anti-inflammatory therapy (meloxicam given every other day at 0.05 mg/kg) was added to the treatment plan
Fig 2.
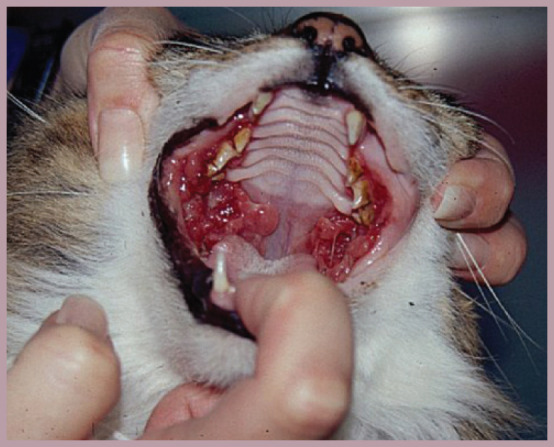
A case of severe gingivostomatitis that had necessitated multiple tooth extractions and resection of proliferative lesions over a period of months. The foundation of the pain management plan in this cat was meloxicam, but oral transmucosal buprenorphine was required for break-through pain
Fig 3.
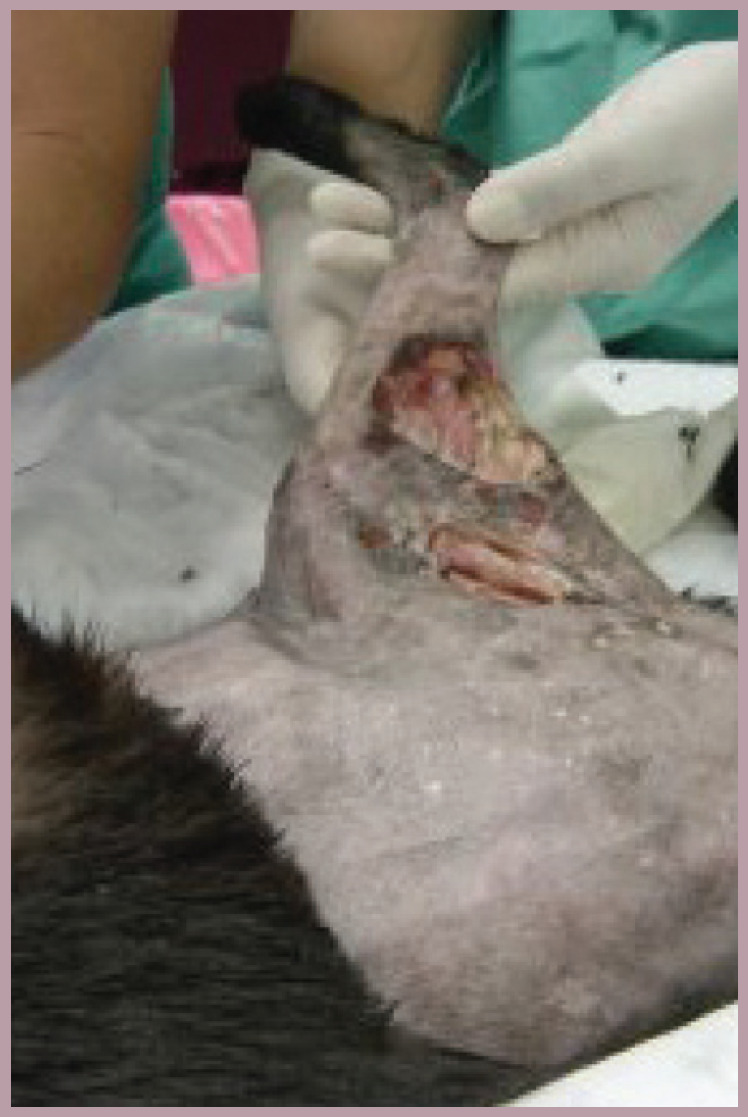
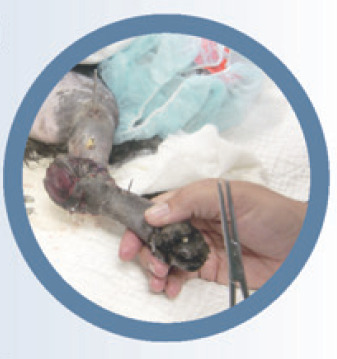
This cat was referred for definitive treatment of a long-standing (3 months' duration) wound. The inciting cause was unknown but there was extensive osteomyelitis in the underlying femur. The cat was lame, and resented even gentle palpation over its hindquarters. It had also lost 0.9 kg in the previous 6 weeks. A decision was made to amputate the limb. Perioperative pain management included a fentanyl and ketamine constant rate infusion (CRI) during and for 24 h after surgery, and epidural administration of morphine and bupivacaine. Oral transmucosal buprenorphine was initiated the day after surgery and continued for 1 week. Gabapentin was started the day before surgery and continued for a month following surgery. No NSAIDs were used in this case because of underlying chronic renal disease
Fig 4.
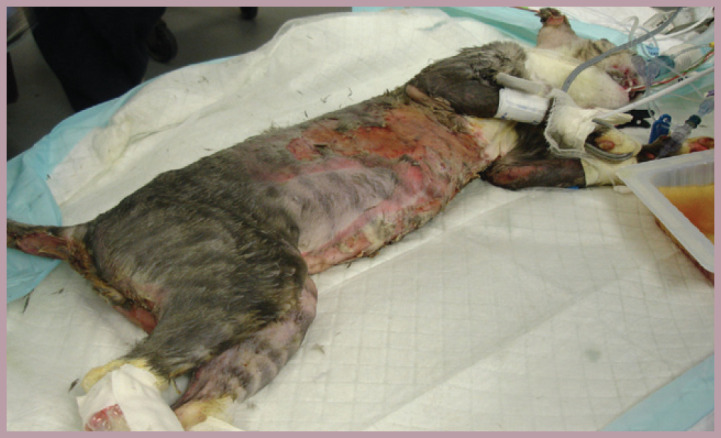
This cat's injuries were the result of a malicious attack; it was set on fire and suffered first to third degree burns over approximately 60% of its body. This case highlights the importance of immediate and aggressive pain management of an acute injury to prevent progression to long-standing maladaptive pain. The patient received CRIs of remifentanil and ketamine, and general anesthesia was induced (higher doses of ketamine and diazepam) for each bandage change and wound debridement session. Supportive care was also essential in this case and included total parenteral nutrition and antibiotic therapy. The cat was nursed on a warm water bed. Although it succumbed to overwhelming infection and died 8 days after initial presentation, the authors considered that the pain management was good — the cat remained friendly and interactive, and would eat voluntarily
Fig 5.
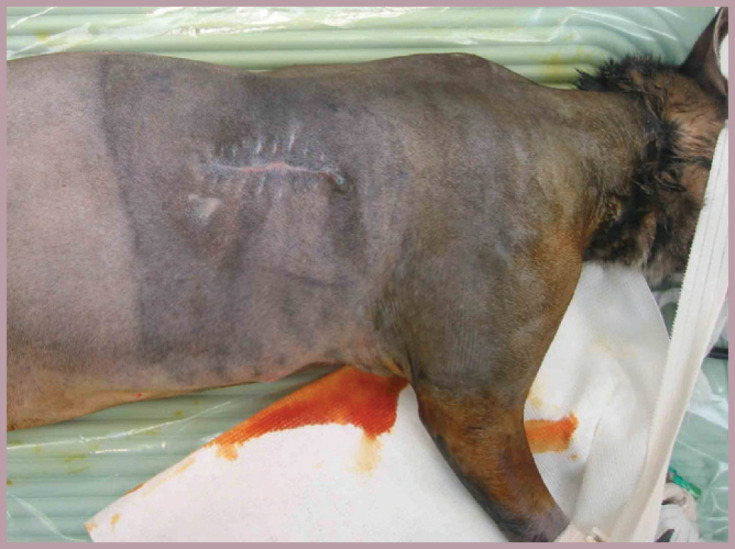
Radiation-induced skin toxicity is not as dramatic or obvious in cats as it is in dogs, manifesting initially as slight erythema and progressing to dry desquamation (in dogs, there is progression to moist desquamation). Skin side effects can persist for several weeks after treatment, with the treated area usually healed 3–6 weeks after completion of therapy. This cat had received 57 Gy, divided into 19 daily fractions, as preoperative radiation therapy for a recurrent injection site sarcoma. The area of radiation is obvious by the change in color of the skin. The cat became very hyperesthetic to light touch over the irradiated area towards the end of, and following, the course of radiation treatment. The authors consider this to be a common effect following radiation; although there are few ‘dramatic’ skin changes, the radiation appears to induce some form of maladaptive pain. The cat was treated with a combination of amantadine and gabapentin towards the end of radiation treatment and during the 2 weeks following radiation and prior to surgery being performed (moderate kidney disease precluded use of an NSAID). The response was only moderate, and so the perioperative analgesic regimen was aggressive, comprising ketamine and fentanyl CRIs during surgery and for 3 days following surgery. An analgesic catheter was also placed at the time of surgery and used to instill local anesthetic into the wound every 6 h for 3 days following surgery
Fig 6.

Severe neuropathy (plantigrade stance) developed in a 12-year-old male cat within a week of diabetes mellitus being diagnosed. Nerve conduction studies were performed and were abnormal. A year later the cat was showing no obvious neurologic signs and nerve conduction velocities had returned to within the normal range. Based on regular fructosamine assessment and urine glucose testing, glucose control was good. However, the cat began licking his feet and this resulted in staining of the fur with saliva, as pictured here. The owner also reported that the cat had stopped grooming and did not like to be brushed — something it had ‘enjoyed’ before. This patient was treated with gabapentin, with excellent results. After 3 months of treatment gabapentin was slowly tapered off and, at the time of writing, the cat had not resumed this behavior
Assessment of pain and discomfort
Due to the nature of long-term pain, which is sometimes slow and insidious in onset, the accompanying behavioral changes can be subtle and easily missed. This is especially true for cats with DJD. The assessment of pain in cats thus continues to present a significant challenge.
In people, questionnaire-based outcome measures have been developed, validated and reasonably well accepted. By contrast, subjective outcome measures are less well developed in the veterinary field and validation studies are lacking in feline medicine. Owner assessment questionnaires (instruments) have been developed in dogs for the evaluation of DJD-associated pain,4–8 and it is very likely that the same approach (that is, using owners) will be effective in assessing long-term pain in cats. However, client education on what to look for will be important. The signs thought to be associated with different types of long-term pain are discussed in the relevant sections below, and in the accompanying article on DJD-associated pain.
Clinical examination remains an important part of the assessment process, and a few pointers for optimizing the examination are listed below.
In some cases, however, long-term pain can lead to overt behavioral changes, as two video clips accompanying this article show. The first shows a cat with an injection site sarcoma of 2 months' duration. The cat had not received analgesic agents during that time. It tolerated being petted around its head and showed normal response to this interaction; but, when gently stroked over the tumor, there was a dramatic reaction accompanied by dilated pupils and licking of the lips. This is an example of maladaptive pain, and in this cat there is an obvious cause for the pain. The second clip shows a cat that had been missing for 2 days and returned home covered in matted hair. It was clipped and there were no visible injuries. The cat spontaneously vocalized and even light touch provoked more vocalizing. It became clear that the warm air from the heating unit was provoking this response. This is an example of allodynia (pain due to a stimulus that does not normally provoke pain).
Multimedia
Two video recordings of cats showing a variety of behavioral responses to pain are included in the online version of this article at doi:10.1016/j.jfms.2010.01.002
There is no evidence to suggest that animals can conceptualize future benefits or understand that ‘tomorrow might be better’. They live in the present - and what is important is how they feel ‘now’.
Tips for optimizing the clinical examination
Be prepared to put the required time in
Be willing to perform the examination in stages - ie, start then come back to complete it later.
Approach the cat calmly but confidently.
-
Choose a room that is:
quiet
away from barking dogs
does not have ‘hiding places’ that a cat can get lodged in
Use a surface that is soft, so that the cat will not slip around (eg, a soft pad over a piece of thin yoga matting)
Minimize restraint, unless absolutely necessary
Perform the examination in the position the cat is most comfortable (eg, standing, lying, or in the owner's arms)
Try to keep your hands in contact with the cat continuously
In some cases having the owner present will facilitate the examination; however, in other cases, if the examination is proving difficult, it may be beneficial to have the owner leave
Management goals and priorities
At the outset, some clear treatment goals should be established. In many cases — as, for example, in cats with DJD — we are not dealing with a curable disease. The aim should be to make the cat comfortable. Woolf makes an excellent point that the aim of treatment is to normalize pain sensitivity, not to remove it. 2 Another important principle of pain management is that the level of treatment should be matched to the level and complexity of the pain present. For example, long-term low-level pain due to DJD in one cat may be eminently treatable with simple dietary modification, whereas the long-term debilitating pain experienced by another cat may require a complex and aggressive treatment regimen to achieve the required goals.
As mentioned earlier, many diseases such as DJD or cancer are not static, and neither is the pain associated with them, which adds to the challenge. Frequent modification of the treatment plan is required to maintain a constant level of comfort.
As treatment may be for a prolonged period of time, it is important to have a discussion with the owner at the outset about the financial, time and emotional commitments required. It needs to be emphasised that treatment may require considerable trial and error, with associated disappointments and frustrations. And it is important to be realistic about what can be achieved and not to give false hope or allow the owner to embark on treatment pathways that have no scientific basis and offer little chance of success.
Because many owners treat their pets as a family member or best friend, and some have no financial restrictions on treatment, it is helpful to sensitively discuss the differences between animals and humans when dealing with long-term pain or illness. Rollin explains this eloquently by pointing out that human cognition allows us to endure short-term unpleasant experiences (eg, chemotherapy, major surgery) so we can achieve long-term future goals (perhaps a cure or prolongation of life). 9 By contrast there is no evidence to suggest that animals can conceptualize future benefits (what Rollin calls ‘extra life’) or understand that ‘tomorrow might be better’ — they live in the present and what is important is how they feel ‘now’ and how pleasurable that time is. In many ways this places a bigger responsibility on veterinarians for alleviating pain in these populations.
It is important to be realistic about what can be achieved and not to give false hope or allow the owner to embark on treatment pathways that have no scientific basis and offer little chance of success.
Euthanasia may be a difficult subject to broach, but should be discussed as an ultimate treatment option, and not as a last resort measure or result of ‘failure’.
Treatment options and strategies
Analgesic trials
Sometimes it can be difficult to be certain that a cat is painful based on a clinical assessment, or an owner may be convinced that some of the behavioral changes seen are just part of ‘slowing down and getting old’. In these cases an analgesic trial can be helpful. For example, a 1-month course of meloxicam treatment in a cat with a putative diagnosis of DJD may result in the owner realising they had indeed seen a change in behavior, and reporting that the cat is now ‘back to its old self’.
Drug therapy
The following discussion outlines what is known about various classes of drugs for pain control in cats. A table in the accompanying article on DJD-associated pain lists drug information and suggested doses that may be useful in managing long-term pain in cats. It should be noted that, in the majority of cases, this constitutes ‘off label’ use.
Psychoactive drugs
Drugs that come under this umbrella include the selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors (MAOs) and tricyclic antidepressants (TCAs). These act variously to alter the reuptake and release of, and to deactivate, catecholamines and serotonin, which are neurotransmitters known to be involved in pain transmission in the spinal cord. Psychoactive drugs, in particular the TCAs, are used in humans for the treatment of chronic and neuropathic pain, often at doses lower than those used in the treatment of depression.
There are no reports on the use of clomipramine for pain control, but a dose of clomipramine for the control of urine marking in cats has been reported. 13 However, recent work has indicated that the pharmacokinetics of clomipramine in cats are very variable.14–15 Amitryptiline has been used successfully in cats with interstitial cystitis, 16 a disease that to a large extent is considered a pain syndrome. Based on this, doses for pain control in the cat have been suggested. 17 One study indicated that transcutaneous absorption of amitryptiline was poor compared with the oral route when the drug was delivered in a transdermal organogel containing pluronic and lecithin (PLO gel). 18 The TCAs should not be used concurrently with drugs that also modify the serotonergic system (eg, tramadol).
Is the treatment working?
Monitoring the efficacy of treatment is important. It is all too common for owners to be unaware of, or in denial about, the decline in their cat's quality of life. As animal advocates we must promote quality not quantity of life; we can prolong life but should always ask ‘at what cost to the animal’? Of course quality of life is difficult to define and measure scientifically in animals, and currently there are no validated quality of life scoring systems for cats living with potentially long-term painful conditions.
One approach is to encourage the owner to keep a diary, noting things such as appetite, playing, hiding, socialization or interaction with other pets, and to compare current with previous entries, or with how the cat was before the onset of pain. Having a written account may show, for example, that the bad days are outnumbering the good days and help the owner decide on whether to continue with treatment. In his book ‘Unlocking the animal mind’, 10 and his article ‘Quality of life in animals’, 11 McMillan offers both veterinarians and pet owners some useful tools for approaching these complex and difficult decisions.
For cats with DJD, the concept of ‘client-specific outcome measures’ has been used to monitor the response to treatment. 12 This approach encompasses having clients monitor ‘time and place specific’ activities, and using these to evaluate treatment. This is discussed further in the accompanying article on DJD-associated pain.
As animal advocates we must promote quality not quantity of life.
Antiepileptics (alpha-2-delta ligand drugs)
Calcium channels modulate nociceptive transmission at the level of the neuronal synapse in the central nervous system (CNS). The role of L, N and P/Q type voltage-gated calcium channels varies with the nature of the neural injury. Calcium channel antagonists can reduce pain, allodynia and hyperalgesia. A growing body of evidence points to a distinct pattern of calcium channel expression in animal models of neuropathic pain — in particular, the expression of the alpha-2-delta subunit on calcium channels. 19 Gabapentin and pregabalin interact with the alpha-2-delta subunit of the voltage-gated calcium channel, 20 and both have been effective in various neuropathic pain states in humans. Although there is considerable information on gabapentin disposition in dogs,21–22 and some information on its use as an anticonvulsant in dogs, 23 there is, as yet, no information on its use for pain control.
The authors find gabapentin a particularly useful drug for so-called neuropathic or neurogenic pain in cats.
Very recently, information on the kinetics of gabapentin in cats has become available, 24 but there are no scientific publications demonstrating its efficacy for long-term pain in this species. However, the authors find gabapentin a particularly useful drug for so-called neuropathic or neurogenic pain, at starting doses of 10 mg/kg q12h. If no response is seen in a week, the dose should be increased as there is considerable variation in the effective dose. Sedation and ataxia are the main side effects we have seen in cats.
Sodium channel blockers
Alterations in the level of expression, cellular localization and distribution of sodium channels are strongly associated with neuropathic pain.25–27 Although not a convenient mode of delivery for most patients with neuropathic pain, intravenous lidocaine has proven effective for neuropathic pain in humans, with single treatments sometimes offering long-term relief. 28 The authors have used this drug as part of an intravenous cocktail for the treatment of neurogenic pain, such as nerve root entrapment and lumbosacral pain. The intravenous administration of lidocaine to cats (at rates to target plasma concentrations of 0, 3, 5, 7, 9 and 11 μg/ml) was associated with cardiovascular depression. 29 These same plasma concentrations did not alter nociceptive thresholds in a thermal threshold testing model in healthy cats, 30 but this model is probably not an appropriate test of the analgesic action of drugs that may work best in pathological or maladaptive pain states.
There is increasing interest in transdermal lidocaine patches. A recent study reported on the absorption and kinetics of lidocaine administered via transdermal patches in cats; 31 plasma lidocaine concentrations remained well below systemically toxic concentrations. There are no reports evaluating the efficacy of lidocaine patches for pain control in cats, and it is not known if the plasma concentrations reached may prove to be an algesic in maladaptive pain states. However, in the above-mentioned study, 31 skin concentrations of lidocaine were reported to be high, and the transdermal technique may provide cutaneous analgesia and prove useful for alleviating pain originating from wounds and scar tissue.
Transdermal lidocaine may provide cutaneous analgesia and prove useful for alleviating pain originating from wounds and scar tissue.
Ketamine, as a bolus or CRI, may have a role to play in the ‘resetting’ of the CNS in maladaptive pain states.
NMDA inhibitors
Preclinical evidence indicates that hyperalgesia and allodynia following peripheral tissue or nerve injury depends on N-methyl-D-aspartate (NMDA) receptor-mediated central changes in synaptic excitability, and also shows quite clearly that NMDA antagonists can attenuate hyperalgesia and allodynia in animal models of neuropathic pain. Memantine, amantadine, ketamine and dextromethorphan are non-competitive NMDA antagonists that have been used clinically in humans with neuropathic pain. Amantadine (3–5 mg/kg PO q24h), as an adjunct to NSAID use, has been shown to be effective in dogs with osteoarthritis. 32 The doses used in the study were based on published work on amantadine kinetics and toxicity in the dog.33–34 No work has yet been performed in the cat.
The authors have noticed a marked decrease in pain-related behaviors in cats suffering from maladaptive pain that were sedated with ketamine for diagnostics. Ketamine, as a bolus or CRI, may have a role to play in the ‘resetting’ of the CNS in such pain states.
Mixed analgesics (tramadol)
Although not classified as a true opioid, tramadol has weak binding affinity at mu-receptors and is thought to activate monoaminergic spinal inhibition of pain, although this may not apply to non-primate species. It can be administered by multiple routes and is effective for chronic pain in humans.
There has been considerable interest in evaluating tramadol in cats. Subcutaneous administration (1 mg/kg) to normal cats had limited effect on thermal and mechanical thresholds. 35 However, pharmacokinetic evaluation of intravenous (2 mg/kg) and oral (5 mg/kg) tramadol demonstrated a relatively long half-life, and rapid and significant formation of the M1 metabolite (O-desmethyltramadol) when compared with dogs. 36 The M1 metabolite is considered to be the active metabolite responsible for analgesia. No clinically apparent adverse effects were noted in this study following the single doses. 36 In another study, the administration of a single dose of approximately 10 mg/kg was found to reduce the minimum alveolar concentration of isoflurane in anesthetized cats by an amount between that caused by butorphanol and that produced by hydromorphone. 37
In a clinical study of cats undergoing ovariohysterectomy, tramadol (2 mg/kg SC q8h) appeared to provide some analgesia, but the best effect was seen when it was combined with the NSAID vedaprofen. 38 Using a tail-clamp stimulus, other investigators found 1 mg/kg of tramadol delivered epidurally provided measurable antinociception for up to 6 h. 39 From this work, it appears that tramadol can provide analgesia in the cat, at least in the acute setting. As yet, there is no work evaluating the analgesic action of tramadol in chronic or maladaptive scenarios.
One of the biggest drawbacks with oral tramadol is its bitter taste; even with flavoring, this drug can be difficult to administer to cats. The development of palatable formulations that allow high compliance will be essential to future studies.
Opioids
Opioids have variable efficacy in the treatment of maladaptive pain in humans, and their role is far from being defined in cats with long-standing pain. Transdermal fentanyl can be an effective way of providing analgesia in the cat,40–41 but there are no reports of this method being used for chronic pain control in this species. Transmucosal buprenorphine is highly bioavailable, easy to administer and antinociceptive in the cat. 42 Potentially, this route of administration could be used to provide longer term pain control, although no clinical reports of this exist.
NSAIDs
Non-steroidal anti-inflammatory drugs often form the foundation of a treatment plan for long-term pain patients. The fact that they are generally fairly effective in a variety of conditions is a reflection of their multiple mechanisms of action. They interact with the cyclooxygenase enzymes (COX-1 and/or COX-2), or cyclooxygenase and lipoxygenase (LOX) enzymes (dual inhibitors), inhibiting the production of many prostanoids (and leu-cotrienes [dual inhibitors]) involved in facilitating pain transmission. NSAIDs also act on other COX- or LOX-independent systems to help inhibit the transmission of pain.
The use of NSAIDs in cats has been comprehensively reviewed. 12 There have been two pertinent developments in the 3 years since that publication. The first has been the approval in Europe and other countries, including Australia, of meloxicam to treat chronic musculoskeletal pain. The approval is for an unlimited time at a dose of 0.05 mg/kg. An evaluation of 40 cats with DJD-associated pain suggested that a dose of 0.01–0.03 mg/kg q24h, with a mean treatment duration of 5.8 months, was well tolerated. 43 Gastrointestinal upset in 4% of cats was the only adverse effect noted. The second development is that the first coxib class of NSAIDs, robenacoxib,44–45 has been granted approval in Europe for use in cats. In cats with musculoskeletal pain it is labeled for up to 6 days of therapy, at a dose of 1–2 mg/kg q24h.
Environmental enrichment
Environmental enrichment is important for all cats, especially those confined indoors. For cats with long-standing disease it may be a critical component of treatment. Cats with DJD-associated pain will benefit from exercise, which can be increased by, for example, using cat towers, providing toys, and hiding food to encourage foraging and hunting behavior. 46 A stimulating environment or being engaged in activities distracts human patients from focusing on their pain and these techniques could be applied to animals.
A prerequisite when designing a suitable environment is to know what cats normally do, 47 and for how long they perform each activity over a 24 h period; this process involves developing an ethogram and documenting time budgets. There is surprisingly little published information on this but one study reported that cats spent approximately 40% of the day sleeping, 22% resting, 15% hunting, 14% grooming, 3% traveling, 2.3% eating and 2.4% hiding. 48 Some of these activities are obviously missing from an indoor cat's repertoire, especially the time spent hunting. In debilitated cats or those with DJD-associated pain, grooming may be absent, yet it occupied a significant time budget in the healthy cats studied; owners should, therefore, try to perform this activity for their cats.
When designing an environment for cats we should allow them to ‘choose’ things (rather than assume we know what they want), and be creative.
Perhaps not surprisingly, cats don't always do what we think they do! They use windows and seek sunlight less that we might assume; in one study, indoor cats spent 5 h per day looking out of windows. 49 But this is not to say that access to both is not important, and there are individual differences. A source of heat appears to be important in the choice of a resting place. 48 When designing an environment for cats we should, therefore, allow them to ‘choose’ things (rather than assume we know what they want), and be creative.
Multimodal environmental modification (MEMO) shows promise as an adjunctive therapy for cats with idiopathic cystitis,50–51 and avoiding stressors such as changes in diet and housing is also important to help prevent clinical exacerbations of disease.50–51 Westropp et al's study showed differences in plasma catecholamine levels and sympathetic nervous system function in affected cats, and these may be linked to exacerbation of pain. 51
Physical therapies
Controlled exercise, passive range of motion (ROM) exercises and massage are all physical rehabilitation techniques that can be incorporated into a treatment plan. When used as an adjunct to conventional therapy, massage reduced pain, discomfort, tension and stress, and improved mood, in human pediatric patients with chronic pain. 52 The benefit of such therapies is just beginning to be defined in canine medicine, but has not yet been evaluated in feline medicine. However, it is likely that the same basic principles and benefits will apply to feline patients. ROM and massage techniques can be taught to owners and both can help to alleviate muscle pain; they also contribute to environmental enrichment for the cat — there is more interaction between owner and cat, and this can fill otherwise empty time budgets.
Similar to massage and ROM exercises, other modalities such as shock wave therapy, laser therapy (see right), and heat and cold therapy may well be of benefit in certain circumstances in feline patients, but no well controlled scientific studies of these therapies in cats have been published.
Potential of laser therapy in healing and analgesia
Gingivostomatitis is challenging to treat in cats and may require multiple approaches including tooth extractions, steroids, NSAIDs, antiviral and immunomodulator drugs, and laser resection.53 Pain and a poor quality of life is a feature of this disease in many cats. Although lasers are widely used for oral surgery, 54 they may also have a potential role in healing and analgesia. In humans with recurrent stomatitis, CO2 laser irradiation provided excellent and rapid pain relief.55 In cancer patients with mucositis, specific laser photo - therapy protocols had a positive effect on pain and healing.56–57
Complementary therapies
In recent years, the popularity of more ‘holistic’ or ‘natural’ approaches to medicine for both humans and pets has increased. The legitimacy of acupuncture has been questioned due to a lack of well controlled scientific and clinical trials. Although the evidence base is still a concern, in a review of the animal-specific acupuncture literature Habacher et al stated that there were enough promising results to support pursuing acupuncture as a viable treatment in veterinary patients. 58 However, none of these studies have involved cats.
Diabetic-related pain
The prevalence of diabetes mellitus in domestic cats is increasing as a consequence of sedentary lifestyles and obesity, with older neutered males being affected most frequently. The diagnosis, treatment and monitoring of this disease has recently been reviewed. 59 The focus here is on the neurological complications and potential for pain to develop in affected cats.
In many ways feline diabetes mellitus closely resembles the disease in humans and the cat is now regarded as a good model for the human disease.60–63 Functional consequences of diabetic neuropathy, including tetraparesis, plantigrade and palmigrade stance, pelvic limb weakness and difficulty in ambulating, are well described in cats, along with changes in nerve conduction velocity and histological changes in nerve biopsies.60,62
Painful diabetic neuropathy occurs in some, but not all, human diabetics and can be seen at variable times after diagnosis. It may resolve as nerves degenerate but, when present, is distressing and has a significant impact on quality of life.64,65 This component of the disease is less well described in cats. However, ‘irritability, especially when touching the feet’ is reported in the literature, 60 and by many owners and veterinarians. In humans, sensory changes start in the feet and hands. Unlike humans, changes in sensation to thermal and tactile stimuli have not yet been documented in cats, 63 but only a very small number of cats have been tested, and only at one time point in the disease process.
Clues as to whether diabetic cats are painful include reports by the owner that the cat dislikes being touched, especially around the distal extremities. Licking of the feet, leading to discoloration of the fur, can sometimes be seen in light-colored cats (Fig 6) and suggests tingling or other sensory changes.
Treatment
Treatment is aimed firstly at tight glucose control, which can be challenging. In humans a wide range of drugs has also been used in an attempt to alleviate painful diabetic neuropathy, with inconsistent and variable success.65 Classes of drugs include TCAs (amitriptyline, imipramine), selective serotonin noradrenaline reuptake inhibitors (duloxetine), anticonvulsants (gabapentin and pregabalin), opioids and tramadol, and there are reports of success with acupuncture.66 There is little scientific information on how to approach the issue of diabetic-related pain in cats, but gabapentin would be a logical choice.
Persistent postsurgical pain
In 10–50% of human patients, acute postoperative pain associated with common surgeries, such as inguinal hernia repair and breast surgery, transitions into persistent pain, lasting for more than 3–6 months, and can be severe in up to 10% of patients. 67 Despite these alarming figures persistent postsurgical pain has not been vigorously addressed in the medical arena. Why it arises in some but not all patients is largely unknown and has not been scientifically studied, but it may have a genetic basis. In one small study, 68 preoperative thermal nociceptive testing predicted postoperative pain, suggesting that patients could be screened for risk. It is proposed that iatrogenic neuropathic pain is the primary contributor and that surgeons should strive to develop surgical techniques that avoid or minimize nerve damage. Ongoing inflammatory pain is another possible, but less common, perpetuator of persistent postsurgical pain. Because the risk of developing persistent pain correlates with pain intensity in the immediate postoperative period, aggressive multimodal analgesic therapy is advocated in the perioperative period. 66
In cats, persistent postsurgical pain occurs but it is likely to be under-recognized and is not widely reported. One example is persistent pain following onychectomy (‘declaw’), a controversial procedure that is still commonly performed in the United States, despite growing ethical and welfare concerns. 69 In his review of complications associated with the procedure, Patronek highlights the lack of definitive data on the issue. 69 Although short-term complications are well described, long-term issues are not. Long-standing lameness (3–96 months) is reported, 70 but whether this is a result of biomechanical changes and/or pain has not been addressed.
Iatrogenic neuropathic pain is the primary contributor and surgeons should strive to develop surgical techniques that avoid or minimize nerve damage.
Several cats have been seen by the authors that fit the description of persistent postsurgical pain reported in humans. These cats can present weeks to months following onychectomy with a history of persistent or intermittent lameness. On observation and careful history taking these cats also show some of the behaviors listed below. In some cases cats received minimal perioperative analgesia (often only one treatment with a short-acting opioid), but, in others, the pain management protocol was quite robust.
Behavioral signs suggesting post-onychectomy pain
Licking and chewing at the feet
‘Walking as if on hot coals’
Shaking and ‘flicking’ of the paws
Aversion to the feet being touched
Reluctance to use the feet to play with toys or to cover urine/feces with litter
Periods of suddenly sitting still
Spontaneously vocalizing ‘for no apparent reason’
‘Running around as if stung by a bee’
Because of the nature of the surgical procedure, these behaviors are most likely to be the result of persistent inflammation and/or neuropathic pain. Humans with neuropathic pain report tingling sensations, shooting pain, allodynia and feeling as if they are walking on hot sand. 65 Although in danger of being anthropomorphic, it is hard not to believe that this is what these cats are experiencing.
Clinical approach and treatment
The clinical approach to cats with suspected neuropathic pain post-onychectomy includes careful examination and imaging of the feet to rule in/out residual inflammation, underlying infection or remaining bony fragments to determine if antibiotics, NSAIDs or further surgery may be indicated. It may be difficult to rule out inflammation and in many cases these cats are given NSAIDs to see if there is a response to treatment. In the absence of obvious pathological findings, a presumptive diagnosis of neuropathic pain directs the course of treatment. There are anecdotal reports of success with amitriptyline and gabapentin (see relevant sections in the text). In the authors' experience, most cats do respond to treatment although it may take several weeks or even months to see a significant improvement and adjustments to the dose or drug(s) used may be required. In many cases the cat can, after varying durations of treatment, be slowly weaned off medication
There is a perception that cats may not tolerate acupuncture techniques as well as dogs. In a large university-based acupuncture clinic (Veterinary Medical Center, College of Veterinary Medicine, University of Florida), only 4–6% of all patients seen over the past 5 years were cats, albeit the majority of these did have a long-term disease with a pain component.
Clinical approach in specific scenarios
Recently, more information on DJD and its impact on feline patients has become available. The clinical aspects of diagnosing and treating DJD-associated pain are reviewed in the accompanying article.
The clinical impact and treatment of diabetic neuropathy and persistent postsurgical pain are discussed in the boxes on page 195 and above.
Prevention of long-term pain in cats
As the oft-used adage ‘prevention is better than cure’ suggests, we should take a fresh look at the causes of long-term pain in cats and see if any are preventable. Things that we can promote are good dental hygiene and check-ups to prevent oral and dental pain, and tight control of diabetes to minimize the adverse effects of diabetic neuropathy. There is enormous interest in the relationship between diet and disease in both humans and animals, 71 and the discipline of nutrigenomics, which studies the relationship between diet, genetics and disease, may transform pet nutrition especially in relation to the prevention or alleviation of DJD.
The development of less invasive surgical techniques (eg, laparoscopy, thoracoscopy, natural orifice translumenal endoscopic surgery [NOTES] and laser surgery) should reduce the incidence of persistent postsurgical pain. For the meantime, careful assessment of pain in the acute postoperative period is advocated, along with more aggressive treatment protocols that incorporate pre-emptive use of analgesic agents and a multimodal approach (eg, a combination of NSAIDs, opioids and local anesthetics).
Future directions for long-term pain control in cats
Stem cell therapy
The potential of ‘regenerative medicine’ for managing degenerative diseases such as DJD is covered in the accompanying article.
Neurotrophic agents
Prosaptide T×14(A) is an exogenous neurotrophic agent that has undergone clinical trials in cats with naturally occurring diabetes mellitus. Enrolled cats underwent a full clinical examination, nerve conduction testing and collection of a nerve, muscle and skin biopsy prior to and following 6 months of treatment with either a placebo or prosaptide injection. Results of this study are expected in the next year. 63
Neurotoxins
Resiniferatoxin, a capsaicin analog, has been injected intrathecally in dogs with severe pain related to osteosarcoma and provided good analgesia. 72 The proposed mechanism of action is a selective effect at the vallinoid receptors in nociceptive neurons, primarily C-fiber afferents of the dorsal horn; motor function and normal nociception is left intact. Other agents, such as substance P-saporin combinations, have been investigated in dogs as potential selective neuroablative agents for managing severe pain. 73 No reports of the use of these agents in cats currently exist.
Key Points
Long-term pain in cats is an important welfare issue but is often overlooked and therefore goes undertreated.
Any cat may potentially experience long-term pain and discomfort. DJD and diabetic-related pain affects middle-aged or older cats, whereas persistent postsurgical pain can occur at any age.
Recognizing and assessing pain in cats is a challenge. Careful observation and a history of changes in behavior are key factors and owner input is essential.
Our understanding of the underlying mechanisms of persistent pain is incomplete, making the rational choice of treatments) a challenge.
Treatment may involve drugs, non-pharmacologic modalities such as acupuncture, or combinations of these.
There are very few studies related to long-term pain management in the cat that would fall into a grade I or II classification of level of evidence. Unfortunately many reports of ‘success’ fall into the grade IV category.
There is considerable work to be done in this area of feline medicine, but progress is being made. Notably, knowledge of the incidence, pathophysiology and treatment of DJD in cats has advanced greatly in the past 5 years.
References
- 1.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: A maladaptive response of the nervous system to damage. Annu Rev Neurosci 2009; 32: 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woolf CJ. Pain: Moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med 2004; 140: 441–51. [DOI] [PubMed] [Google Scholar]
- 3.Hellyer P, Rodan I, Brunt J, Downing R, Hagedorn JE, Robertson SA. AAHA/AAFP pain management guidelines for dogs and cats. J Feline Med Surg 2007; 9: 466–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown DC, Boston RC, Coyne JC, Farrar JT. Ability of the canine brief pain inventory to detect response to treatment in dogs with osteoarthritis. J Am Vet Med Assoc 2008; 15: 1278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hielm-Bjorkman AK, Kuusela E, Liman A, et al. Evaluation of methods for assessment of pain associated with chronic osteoarthritis in dogs. J Am Vet Med Assoc 2003; 222: 1552–58. [DOI] [PubMed] [Google Scholar]
- 6.Hielm-Bjorkman AK, Rita H, Tulamo RM. Psychometric testing of the Helsinki chronic pain index by completion of a questionnaire in Finnish by owners of dogs with chronic signs of pain caused by osteoarthritis. Am J Vet Res 2009; 70: 727–34. [DOI] [PubMed] [Google Scholar]
- 7.Wiseman-Orr ML, Nolan AM, Reid J, Scott EM. Development of a questionnaire to measure the effects of chronic pain on health-related quality of life in dogs. Am J Vet Res 2004; 65: 1077–84. [DOI] [PubMed] [Google Scholar]
- 8.Wiseman-Orr ML, Scott EM, Reid J, Nolan AM. Validation of a structured questionnaire as an instrument to measure chronic pain in dogs on the basis of effects on health-related quality of life. Am J Vet Res 2006; 67: 1826–36. [DOI] [PubMed] [Google Scholar]
- 9.Rollin BE. Preserving quality of life in oncology. In: Lascelles BDX, ed. BSAVA manual of oncology. 3rd edn. Gloucester: BSAVA, 2010. [Google Scholar]
- 10.McMillan FD. Unlocking the animal mind. Emmaus, PA, USA: Rodale Inc, 2004. [Google Scholar]
- 11.McMillan FD. Quality of life in animals. J Am Vet Med Assoc 2000; 216: 1904–10. [DOI] [PubMed] [Google Scholar]
- 12.Lascelles BD, Court MH, Hardie EM, Robertson SA. Nonsteroidal anti-inflammatory drugs in cats: A review. Vet Anaesth Analg 2007; 34: 228–50. [DOI] [PubMed] [Google Scholar]
- 13.King JN, Steffan J, Heath SE, et al. Determination of the dosage of clomipramine for the treatment of urine spraying in cats. J Am Vet Med Assoc 2004; 225: 881–87. [DOI] [PubMed] [Google Scholar]
- 14.Lainesse C, Frank D, Beaudry F, Doucet M. Effects of physiological covariables on pharmacokinetic parameters of clomipramine in a large population of cats after a single oral administration. J Vet Pharmacol Ther 2007; 30: 116–26. [DOI] [PubMed] [Google Scholar]
- 15.Lainesse C, Frank D, Meucci V, Intorre L, Soldani G, Doucet M. Pharmacokinetics of clomipramine and desmethylclomipramine after single-dose intravenous and oral administrations in cats. J Vet Pharmacol Ther 2006; 29: 271–78. [DOI] [PubMed] [Google Scholar]
- 16.Chew DJ, Buffington CA, Kendall MS, DiBartola SP, Woodworth BE. Amitriptyline treatment for severe recurrent idiopathic cystitis in cats. J Am Vet Med Assoc 1998; 213: 1282–86. [PubMed] [Google Scholar]
- 17.Robertson SA. Managing pain in feline patients. Vet Clin North Am Small Anim Pract 2005; 35: 129–46. [DOI] [PubMed] [Google Scholar]
- 18.Mealey KL, Peck KE, Bennett BS, et al. Systemic absorption of amitriptyline and buspirone after oral and transdermal administration to healthy cats. J Vet Intern Med 2004; 18: 43–46. [DOI] [PubMed] [Google Scholar]
- 19.Luo ZD, Chaplan SR, Higuera ES, et al. Upregulation of dorsal root ganglion (alpha)2(delta) calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J Neurosci 2001; 21: 1868–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies A, Hendrich J, Van Minh AT, Wratten J, Douglas L, Dolphin AC. Functional biology of the alpha(2)delta subunits of voltage-gated calcium channels. Trends Pharmacol Sci 2007; 28: 220–28. [DOI] [PubMed] [Google Scholar]
- 21.Radulovic LL, Turck D, von Hodenberg A, et al. Disposition of gabapentin (neurontin) in mice, rats, dogs, and monkeys. Drug Metab Dispos 1995; 23: 441–48. [PubMed] [Google Scholar]
- 22.Vollmer KO, von Hodenberg A, Kolle EU. Pharmacokinetics and metabolism of gabapentin in rat, dog and man. Arzneimittelforschung 1986; 36: 830–39. [PubMed] [Google Scholar]
- 23.Platt SR, Adams V, Garosi LS, et al. Treatment with gabapentin of 11 dogs with refractory idiopathic epilepsy. Vet Rec 2006; 159: 881–84. [PubMed] [Google Scholar]
- 24.Siao KT, Pypendop BH, Ilkiw JE, eds. Pharmacokinetics of gabapentin in cats. 10th World Congress of Veterinary Anaesthesia; 2009; Glasgow, Scotland.
- 25.Attal N, Bouhassira D. Translating basic research on sodium channels in human neuropathic pain. J Pain 2006; 7 (Suppl 1): S31–7. [DOI] [PubMed] [Google Scholar]
- 26.Lindia JA, Kohler MG, Martin WJ, Abbadie C. Relationship between sodium channel NaV1.3 expression and neuropathic pain behavior in rats. Pain 2005; 117: 145–53. [DOI] [PubMed] [Google Scholar]
- 27.Devor M, Govrin-Lippmann R, Angelides K. Na+ channel immunolocalization in peripheral mammalian axons and changes following nerve injury and neuroma formation. J Neurosci 1993; 13: 1976–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Challapalli V, Tremont-Lukats IW, McNicol ED, Lau J, Carr DB. Systemic administration of local anesthetic agents to relieve neuropathic pain. Cochrane Database Syst Rev 2005; 4: CD003345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pypendop BH, Ilkiw JE. Assessment of the hemodynamic effects of lidocaine administered IV in isoflurane-anesthetized cats. Am J Vet Res 2005; 66: 661–68. [DOI] [PubMed] [Google Scholar]
- 30.Pypendop BH, Ilkiw JE, Robertson SA. Effects of intravenous administration of lidocaine on the thermal threshold in cats. Am J Vet Res 2006; 67: 16–20. [DOI] [PubMed] [Google Scholar]
- 31.Ko JC, Maxwell LK, Abbo LA, Weil AB. Pharmacokinetics of lidocaine following the application of 5% lidocaine patches to cats. J Vet Pharmacol Ther 2008; 31: 359–67. [DOI] [PubMed] [Google Scholar]
- 32.Lascelles BD, Gaynor JS, Smith ES, et al. Amantadine in a multimodal analgesic regimen for alleviation of refractory osteoarthritis pain in dogs. J Vet Intern Med 2008; 22: 53–59. [DOI] [PubMed] [Google Scholar]
- 33.Bleidner WE, Harmon JB, Hewes WE, Lynes TE, Hermann EC. Absorption, distribution and excretion of amantadine hydrochlo-ride. J Pharmacol Exp Ther 1965; 150: 484–90. [PubMed] [Google Scholar]
- 34.Vernier VG, Harmon JB, Stump JM, Lynes TE, Marvel JP, Smith DH. The toxicologic and pharmacologic properties of amantadine hydrochloride. Toxicol Appl Pharmacol 1969; 15: 642–65. [DOI] [PubMed] [Google Scholar]
- 35.Steagall PV, Taylor PM, Brondani JT, Luna S P, Dixon MJ. Antinociceptive effects of tramadol and acepromazine in cats. J Feline Med Surg 2008; 10: 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pypendop BH, Ilkiw JE. Pharmacokinetics of tramadol, and its metabolite O-desmethyl-tramadol, in cats. J Vet Pharmacol Ther 2008; 31: 52–59. [DOI] [PubMed] [Google Scholar]
- 37.Ko JC, Abbo LA, Weil AB, Johnson BM, Inoue T, Payton ME. Effect of orally administered tramadol alone or with an intravenously administered opioid on minimum alveolar concentration of sevoflurane in cats. J Am Vet Med Assoc 2008; 232: 1834–40. [DOI] [PubMed] [Google Scholar]
- 38.Brondani JT, Luna Loureiro SP, Beier SL, Minto BW, Padovani CR. Analgesic efficacy of perioperative use of vedaprofen, tramadol or their combination in cats undergoing ovariohysterectomy. J Feline Med Surg 2009; 11: 420–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castro DS, Silva MF, Shih AC, Motta PP, Pires MV, Scherer PO. Comparison between the analgesic effects of morphine and tramadol delivered epidurally in cats receiving a standardized noxious stimulation. J Feline Med Surg 2009; 11: 948–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franks JN, Boothe HW, Taylor L, et al. Evaluation of transdermal fentanyl patches for analgesia in cats undergoing onychectomy. J Am Vet Med Assoc 2000; 217: 1013–20. [DOI] [PubMed] [Google Scholar]
- 41.Glerum LE, Egger CM, Allen SW, Haag M. Analgesic effect of the transdermal fentanyl patch during and after feline ovariohysterectomy. Vet Surg 2001; 30: 351–58. [DOI] [PubMed] [Google Scholar]
- 42.Robertson SA, Lascelles BD, Taylor PM, Sear JW. PK-PD modeling of buprenorphine in cats: Intravenous and oral transmucosal administration. J Vet Pharmacol Ther 2005; 28: 453–60. [DOI] [PubMed] [Google Scholar]
- 43.Gunew MN, Menrath VH, Marshall RD. Long-term safety, efficacy and palatability of oral meloxicam at 0.01–0.03 mg/kg for treatment of osteoarthritic pain in cats. J Feline Med Surg 2008; 10: 235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giraudel JM, Toutain PL, King JN, Lees P. Differential inhibition of cyclooxygenase isoenzymes in the cat by the NSAID robena-coxib. J Vet Pharmacol Ther 2009; 32: 31–40. [DOI] [PubMed] [Google Scholar]
- 45.Giraudel JM, King JN, Jeunesse EC, Lees P, Toutain PL. Use of a pharmacokinetic/pharmacodynamic approach in the cat to determine a dosage regimen for the COX-2 selective drug robenacoxib. J Vet Pharmacol Ther 2009; 32: 18–30. [DOI] [PubMed] [Google Scholar]
- 46.Ellis S. Environmental enrichment. Practical strategies for improving feline welfare. J Feline Med Surg 2009; 11: 901–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Curtis TM. Environmental enrichment for indoor cats. Compend Contin Educ Pract Vet 2007; 29: 104–6. [PubMed] [Google Scholar]
- 48.Panaman R. Behaviour and ecology of free-ranging female farm cats (Felis catus L.). Z Tierpsychol 1981; 56: 59–73. [Google Scholar]
- 49.Shyan-Norwalt MR. Caregiver perceptions of what indoor cats do ‘for fun’. J Appl Anim Welfare Sci 2005; 8: 199–209. [DOI] [PubMed] [Google Scholar]
- 50.Buffington CA, Westropp JL, Chew DJ, Bolus RR. Clinical evaluation of multimodal environmental modification (MEMO) in the management of cats with idiopathic cystitis. J Feline Med Surg 2006; 8: 261–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Westropp JL, Kass PH, Buffington CA. Evaluation of the effects of stress in cats with idiopathic cystitis. Am J Vet Res 2006; 67: 731–36. [DOI] [PubMed] [Google Scholar]
- 52.Suresh S, Wang S, Porfyris S, Kamasinski-Sol R, Steinhorn DM. Massage therapy in outpatient pediatric chronic pain patients: Do they facilitate significant reductions in levels of distress, pain, tension, discomfort, and mood alterations? Paediatr Anaesth 2008; 18: 884–87. [DOI] [PubMed] [Google Scholar]
- 53.Lyon KF. Gingivostomatitis. Vet Clin North Am Small Anim Pract 2005; 35: 891–911, vii. [DOI] [PubMed] [Google Scholar]
- 54.Lewis JR, Tsugawa AJ, Reiter AM. Use of CO2 laser as an adjunctive treatment for caudal stomatitis in a cat. J Vet Dent 2007; 24: 240–49. [DOI] [PubMed] [Google Scholar]
- 55.Zand N, Ataie-Fashtami L, Djavid GE, et al. Relieving pain in minor aphthous stomatitis by a single session of non-thermal carbon dioxide laser irradiation. Lasers Med Sci 2009; 24: 515–20. [DOI] [PubMed] [Google Scholar]
- 56.Simoes A, Eduardo F P, Luiz AC, et al. Laser phototherapy as topical prophylaxis against head and neck cancer radiotherapy-induced oral mucositis: Comparison between low and high/low power lasers. Lasers Surg Med 2009; 41: 264–70. [DOI] [PubMed] [Google Scholar]
- 57.Kuhn A, Porto FA, Miraglia P, Brunetto AL. Low-level infrared laser therapy in chemotherapy-induced oral mucositis: A randomized placebo-controlled trial in children. J Pediatr Hematol Oncol 2009; 31: 33–37. [DOI] [PubMed] [Google Scholar]
- 58.Habacher G, Pittler MH, Ernst E. Effectiveness of acupuncture in veterinary medicine: Systematic review. J Vet Intern Med 2006; 20: 480–88. [DOI] [PubMed] [Google Scholar]
- 59.Rios L, Ward C. Feline diabetes mellitus: Diagnosis, treatment, and monitoring. Compend Contin Educ Pract Vet 2008; 30: 626–39. [PubMed] [Google Scholar]
- 60.Mizisin AP, Shelton GD, Burgers ML, Powell HC, Cuddon PA. Neurological complications associated with spontaneously occurring feline diabetes mellitus. J Neuropathol Exp Neurol 2002; 61: 872–84. [DOI] [PubMed] [Google Scholar]
- 61.Henson MS, O'Brien TD. Feline models of type 2 diabetes mellitus. ILAR J 2006; 47: 234–42. [DOI] [PubMed] [Google Scholar]
- 62.Mizisin AP, Nelson RW, Sturges BK, et al. Comparable myelinated nerve pathology in feline and human diabetes mellitus. Acta Neuropathol 2007; 113: 431–42. [DOI] [PubMed] [Google Scholar]
- 63.Calcutt NA, Mizisin AP, Shelton GD, eds. The diabetic cat as a model for diabetic neuropathy — developing therapeutics for neuropathy in companion animals. IASP Special Interest Group: measuring nociception and pain in non-human species: beyond the hot plate and paw pressure test; 2008 August 14–15; Glasgow, Scotland.
- 64.Veves A, Backonja M, Malik RA. Painful diabetic neuropathy: Epidemiology, natural history, early diagnosis, and treatment options. Pain Med 2008; 9: 660–74. [DOI] [PubMed] [Google Scholar]
- 65.Tesfaye S. Advances in the management of diabetic peripheral neuropathy. Curr Opin Support Palliat Care 2009; 3: 136–43. [DOI] [PubMed] [Google Scholar]
- 66.Abuaisha BB, Costanzi JB, Boulton AJ. Acupuncture for the treatment of chronic painful peripheral diabetic neuropathy: A long-term study. Diabetes Res Clin Pract 1998; 39: 115–21. [DOI] [PubMed] [Google Scholar]
- 67.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: Risk factors and prevention. Lancet 2006; 367: 1618–25. [DOI] [PubMed] [Google Scholar]
- 68.Werner MU, Duun P, Kehlet H. Prediction of postoperative pain by preoperative nociceptive responses to heat stimulation. Anesthesiology 2004; 100: 115–19. [DOI] [PubMed] [Google Scholar]
- 69.Patronek GJ. Assessment of claims of short- and long-term complications associated with onychectomy in cats. J Am Vet Med Assoc 2001; 219: 932–37. [DOI] [PubMed] [Google Scholar]
- 70.Tobias KS. Feline onychectomy at a teaching institution: A retrospective study of 163 cases. Vet Surg 1994; 23: 274–80. [DOI] [PubMed] [Google Scholar]
- 71.Innes J. Diet and disease: Exploring the link through nutrigenomics. Can Vet J 2006; 47: 68–70. [PMC free article] [PubMed] [Google Scholar]
- 72.Brown DC, Iadarola MJ, Perkowski SZ, et al. Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. Anesthesiology 2005; 103: 1052–59. [DOI] [PubMed] [Google Scholar]
- 73.Allen JW, Mantyh PW, Horais K, et al. Safety evaluation of intrathecal substance P-saporin, a targeted neurotoxin, in dogs. Toxicol Sci 2006; 1: 286–98. [DOI] [PubMed] [Google Scholar]


