Abstract
Aims This article reviews the incidence, etiology, diagnosis, treatment and prognosis of mammary tumors in cats.
Practical relevance Approximately 80% of feline mammary masses are malignant, with adenocarcinoma being the most common tumor type. Early diagnosis is, therefore, essential to improve the prognosis and quality of life of affected cats.
Treatment approaches Surgery is the most widely used treatment for malignant tumors. However, as mammary tumors are often advanced and metastasis has already occurred by the time of diagnosis, surgery routinely does not provide a cure. Ovariohysterectomy or hormonal therapy are the treatments of choice for fibroadenomatous hyperplasia (the most common benign mass) and usually lead to a successful outcome.
Feline mammary gland tumors are the third most common neoplasm in domestic cats (Felis catus) after skin and lymphohematopoi-etic tumors.1–4 Because most feline mammary tumors are malignant, early detection and aggressive therapy have a significant influence on survival time. 5
Owners are increasingly requesting the use of the most advanced therapeutic tools to improve survival time and quality of life of their companion animals. In order to provide clients with accurate information about pathology therapeutic options and prognosis, it is important to have a thorough understanding of this disease.
Anatomy of the feline mammary gland
The queen has four pairs of mammary glands, 6 two pairs of thoracic (T1, T2) and two pairs of abdominal glands (A1, A2) (Fig 1a). In some cats, additional rudimentary glands may be present in the inguinal region. 7
Fig 1.
(a) The four pairs of mammary glands in the cat, with their associated lymph nodes and lymphatic drainage. (b) Venous drainage of the mammary glands
There is general consensus that mammary glands in cats drain cranially towards the axillary lymph center and caudally towards the superficial inguinal lymph center. 6 However, Raharison and Sautet described T2 as consistently draining cranially, and sometimes in both directions, and A1 as consistently draining caudally, and often in both directions. 7 In the same study, they described that T1, T2 and A1 very often drain towards the cranial sternal lymph node in the female cat (Fig 1a). 7 Unlike Mailot et al, who reported lymphatic connections between T1 and T2, and between A1 and A2, 8 the above-mentioned authors did not find connections between adjacent glands (T1 and T2, T2 and A1, A1 and A2), or between left and right glands.7,9
The arterial supply to T1 and T2 is provided by the lateral thoracic vessels, the intercostal vessels (laterally) and the internal thoracic vessels (medially). The A1 mammary glands receive blood from the cranial superficial epigastric artery, while A2 receive blood from the caudal superficial epigastric artery (a directed branch from the pudendoepigastric trunk). 6 The veins of the feline mammary glands closely follow the arteries, except for some small veins that cross midline; it is thought that these may be responsible for the spread of malignant mammary tumors between a pair of glands.6–10 Similarly, venous drainage of glands A1 and A2 through the chest wall, via the internal thoracic or intercostal veins, may allow the spread of mammary tumor to the thoracic cavity (Fig 1b).6,11
Benign mammary lesions
Classification
Even though benign mammary lesions are much less common in cats than malignant lesions, they are an important consideration in the differential diagnosis of feline mammary gland masses. 11 Benign mammary lesions in cats include both neoplastic and non-neoplastic lesions. The former comprise duct papilloma, simple and complex adenomas, fibroadenomas and benign mixed tumors. The latter include cysts, duct ectasia, focal fibrosis, and two types of non-inflammatory hyperplasia (ductular and lobular). 12 Lobular hyperplasia is further categorised into three subtypes — epithelial hyperplasia, adenosis and fibroadenomatous hyperplasia (see right).
Morphological classification
Feline benign mammary lesions
Benign neoplasms
Duct papilloma
Simple adenoma
Complex adenoma
Fibroadenoma*
Benign mixed tumors
Non-neoplastic lesions
Cysts
Duct ectasia*
Focal fibrosis*
-
Non-inflammatory hyperplasia:
Ductal
Lobular (epithelial hyperplasia, adenosis, fibroadenomatous hyperplasia*)
*Most frequently seen lesions in the domestic cat Adapted from Misdorp et al (1999) 12
Benign neoplasms
Prevalence, incidence and signalment
Approximately 10–20% of feline mammary masses are benign.13,14 In a large-scale review of feline diagnoses over a 10-year period, five out of 55 feline mammary tumors were benign neoplasms. 10 The affected animals were all intact females; two were 1 year old, and the others were 4, 7 and 9.5 years old.
Unless the clinician has a strong suspicion of fibroadenomatous hyperplasia, female cats with nodular and non-nodular masses must receive a complete work-up.
Pathology and natural behavior
Fibroadenoma is a common benign lesion in cats and can involve one, several or all of the mammary glands. 15 This tumor is composed of a mixture of luminal epithelial cells and stromal cells, sometimes together with myoepithelial cells. There are two subtypes of fibroadenoma — pericanalicular and intra-canalicular.12,15
Duct papilloma, adenomas and benign mixed tumors are rare benign neoplasms in cats.12,15
Non-neoplastic lesions
Prevalence, incidence and signalment
Lobular hyperplasia (notably fibroadenomatous hyperplasia) and duct ectasia are the non-neoplastic lesions that arise most frequently in the feline mammary gland.
Fibroadenomatous hyperplasia is usually seen in young, sexually intact queens at the time of puberty or in pregnant cats that are under the influence of luteal progesterone (see box below). 16 However, it has been described in male and female cats, neutered or intact and of any age, as well as in older pregnant queens (9–12 years old).17–20
Cysts are non-neoplastic lesions that are rarely seen in cats. Focal fibrosis, another type of non-neoplastic lesion, is usually seen in association with lobular hyperplasia and duct ectasia. 12
Pathology and natural behavior
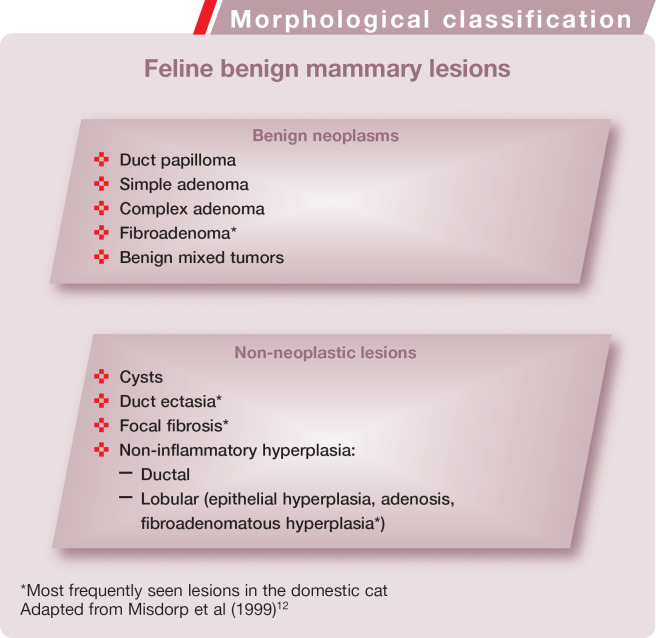
Fibroadenomatous hyperplasia is histological-ly characterized by rapid and abnormal proliferation of stroma and duct epithelium of one or more mammary glands (Fig 2).16,23 In cases of u ectasia there is total transformation of the mammary gland into a spongy mass. The ectasia can affect the ducts as well as the terminal ductules, intralobular ductules and acini. 15 This lesion may sometimes be accompanied by lobular hyperplasia.
Fig 2.
Histopathologic appearance of feline fibroadenomatous hyperplasia. There are sparse branching ducts within an abundant fibrous stroma of the mammary gland. Hematoxylin and eosin stain. Bar = 500 μm
Treatment of benign mammary lesions
Fibroadenomatous hyperplasia
Fibroadenomatous hyperplasia is characterized by aggressive and rapid swelling of the mammary glands, with progression to necrosis if left untreated. However, in some cases, fibroadenomatous hyperplasia in pregnant or non-pregnant luteal phase queens can regress after parturition or luteolysis. 30
Ovariectomy is an option that will be curative in queens with fibroadenomatous hyperplasia, but has the obvious disadvantage of irreversible loss of fertility. The hyperplastic lesions usually regress within 3–4 weeks of surgery, but regression can take up to 5–6 months.20,26,27 Adjunctive treatments include systemic broad spectrum antibiotics and analgesics in animals with extensive ulcerative skin lesions over the mammary glands. 18 Care should be taken not to use steroidal drugs as analgesics.
Where lesions have been caused by prolonged administration of megestrol acetate or medroxyprogesterone, the medication must be discontinued.20,26 Occasionally, removal of the progesterone source or withdrawal of progestagens does not result in regression of fibroadenomatous hyperplasia and, in these cases, partial or total mastectomy is recommended. 20 However, this surgery is invasive and, depending on the size of the mammary masses, may be difficult to perform. For these reasons, surgery is generally not recommended in young queens.
Antiprogestins have been proposed as an alternative treatment. 25 The basis for their therapeutic activity is antagonism of the action of progesterone at its intracellular receptor, thus inhibiting its growth-stimulating effect. 31 When treating intact cats, pregnancy must first be ruled out because of the abortive effects of these drugs.32,33 Two different protocols using the antiprogestin aglepristone (Alizine; Virbac) have been reported. In a study by Wehrend et al, 25 10 mg/kg aglepristone (Alizine) was administered subcutaneously for 4–5 consecutive days. Five days after the first injection, mammary tissue mass was significantly reduced, and involution was complete after 3–4 weeks. However, this treatment was not effective in those animals in which fibroadenomatous hyperplasia developed as a result of the use of depot progestins. 25 Gorlinger et al reported that administration of aglepristone (Alizine), at 20 mg/kg once a week until complete remission was achieved, was effective for cats that developed fibro adenomatous hyperplasia as a result not only of endogenous progestagens but also of exogenous progestagens. 27 Due to the prolonged biological effect of depot progestins, longer antiprogestin treatment is required until the effects of exogenous progestins subside. Thus treatment of fibroadenomatous hyperplasia with antiprogestins has proven to be a valuable, well-tolerated alternative to ovariectomy with the major advantage of preserving fertility.
Other benign lesions
For any other type of benign mammary lesion, the mass should be surgically removed. It is important to be aware that benign lesions can subsequently undergo malignant transformation, and so clean margins must be obtained. After surgery the mass should be submitted for histopathologic examination.
Treatment of fibroadenomatous hyperplasia with antiprogestins has proven to be a valuable, well-tolerated alternative to ovariectomy, with the major advantage of preserving fertility.
Etiology of fibroadenomatous hyperplasia.
Fibroadenomatous hyperplasia is the only non-tumoral lesion in cats for which the etiology and pathogenesis has been studied. 13 The presence of progesterone and estrogen receptors in feline hyperplastic mammary glands suggests that both hormones are involved.16,21,22 The lesion, which represents an exaggerated proliferative response of mammary glandular tissue, may develop:
In pregnant or pseudopregnant queens. In these animals, the mammary tissue shows an exaggerated response to endogenous progesterone. 21
In cats receiving prolonged megestrol acetate or medroxyprogesterone acetate therapy. These synthetic progestational compounds, which have an activity 25 times greater than that of endogenous progesterone, 17 are used as contraceptives in females, and for skin or behavioral problems in both sexes. If these hormones are administered when endogenous levels of progesterone are increased, such as during pregnancy or pseudopregnancy, the response of progestin-sensitive mammary tissue can be exacerbated, resulting in fibroadenomatous hyperplasia. 18
In cats receiving progestational compounds when endogenous levels of estrogen are increased. As estrogen induces the synthesis of intracellular receptors for progesterone, high estrogen levels (including during the follicular phase of the estrous cycle and puberty) increase the sensitivity of mammary tissues to progestational hormones, generating an exaggerated response in the mammary gland. 18
Clinical presentation
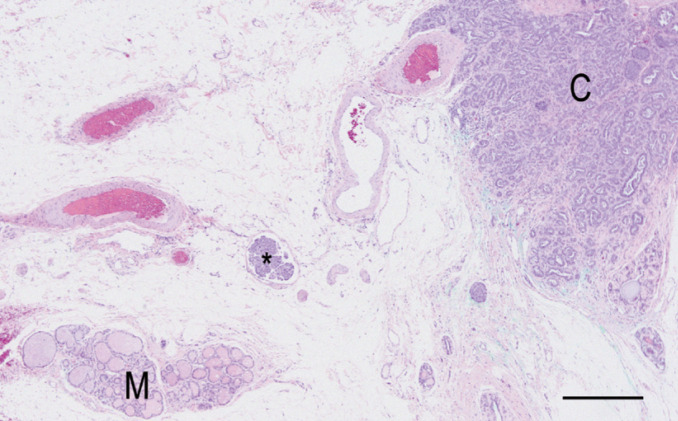
Some benign lesions have a characteristic presentation in cats; others present as mammary enlargement that can be differentiated only through histological examination. Adenomas in the cat manifest as small, solitary, circumscribed, firm nodules, while cysts may be felt in one or more glands as finely beaded or nodular areas.14,24 However, cats with fibroadenomatous hyperplasia have the most typical presentation of one or multiple enlarged mammary glands, without milk production. 25 The mammary glands may be edematous, painful and sometimes so large that they impair walking (Fig 3). Ulceration can also occur. 26 Possible systemic signs include tachycardia, lethargy and anorexia. 27
Fig 3.
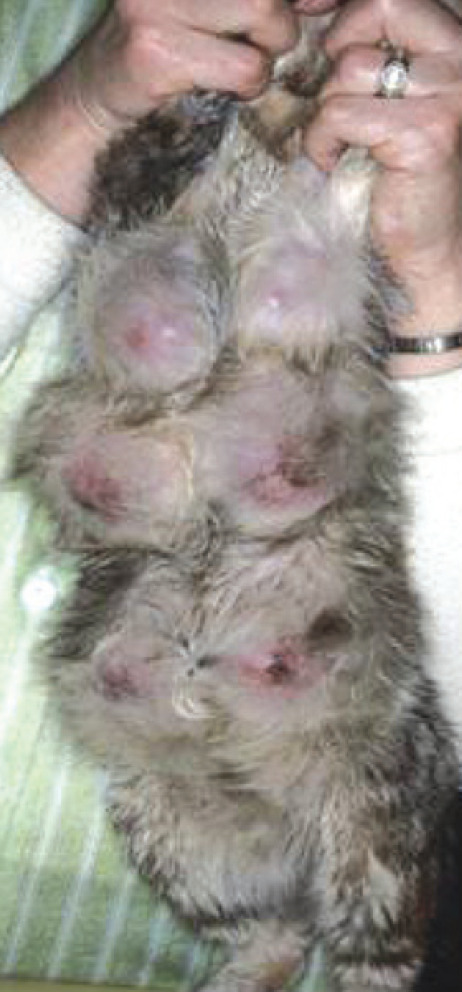
Clinical appearance of mammary hyperplasia. Courtesy of Stein Memorial Feline Image Collection and Washington State University, College of Veterinary Medicine. Database: http://imagedb.vetmed.wsu.edu/
Diagnosis
Unless the clinician has a strong suspicion of fibroadenomatous hyperplasia, female cats with nodular and non-nodular masses must receive a complete work-up (see later discussion on the diagnosis of malignant mammary tumors).
By contrast, the diagnosis of fibroadenomatous hyperplasia in the cat is based on gross appearance, patient signalment and history. 28 On presentation of a young cat with mammary enlargement, palpation and/or abdominal ultrasound should be undertaken to rule out pregnancy. 25 If the queen is not pregnant, hormone assays are helpful as they will reveal high levels of progesterone, consistent with fibroadenomatous hyperplasia. 18 In spayed female cats with mammary hyperplasia the possibility of ovarian remnant syndrome should be investigated (see right).
If fibroadenomatous hyperplasia cannot be diagnosed, further consideration should be given to a malignant process and a complete work-up undertaken as soon as possible (see later).
Treatment of benign mammary lesions.
Fibroadenomatous hyperplasia
Fibroadenomatous hyperplasia is characterized by aggressive and rapid swelling of the mammary glands, with progression to necrosis if left untreated. However, in some cases, fibroadenomatous hyperplasia in pregnant or non-pregnant luteal phase queens can regress after parturition or luteolysis. 30
Ovariectomy is an option that will be curative in queens with fibroadenomatous hyperplasia, but has the obvious disadvantage of irreversible loss of fertility. The hyperplastic lesions usually regress within 3–4 weeks of surgery, but regression can take up to 5–6 months.20,26,27 Adjunctive treatments include systemic broad spectrum antibiotics and analgesics in animals with extensive ulcerative skin lesions over the mammary glands. 18 Care should be taken not to use steroidal drugs as analgesics.
Where lesions have been caused by prolonged administration of megestrol acetate or medroxyprogesterone, the medication must be discontinued.20,26 Occasionally, removal of the progesterone source or withdrawal of progestagens does not result in regression of fibroadenomatous hyperplasia and, in these cases, partial or total mastectomy is recommended. 20 However, this surgery is invasive and, depending on the size of the mammary masses, may be difficult to perform. For these reasons, surgery is generally not recommended in young queens.
Antiprogestins have been proposed as an alternative treatment. 25 The basis for their therapeutic activity is antagonism of the action of progesterone at its intracellular receptor, thus inhibiting its growth-stimulating effect. 31 When treating intact cats, pregnancy must first be ruled out because of the abortive effects of these drugs.32,33 Two different protocols using the antiprogestin aglepristone (Alizine; Virbac) have been reported. In a study by Wehrend et al, 25 10 mg/kg aglepristone (Alizine) was administered subcutaneously for 4–5 consecutive days. Five days after the first injection, mammary tissue mass was significantly reduced, and involution was complete after 3–4 weeks. However, this treatment was not effective in those animals in which fibroadenomatous hyperplasia developed as a result of the use of depot progestins. 25 Gorlinger et al reported that administration of aglepristone (Alizine), at 20 mg/kg once a week until complete remission was achieved, was effective for cats that developed fibro adenomatous hyperplasia as a result not only of endogenous progestagens but also of exogenous progestagens. 27 Due to the prolonged biological effect of depot progestins, longer antiprogestin treatment is required until the effects of exogenous progestins subside. Thus treatment of fibroadenomatous hyperplasia with antiprogestins has proven to be a valuable, well-tolerated alternative to ovariectomy with the major advantage of preserving fertility.
Other benign lesions
For any other type of benign mammary lesion, the mass should be surgically removed. It is important to be aware that benign lesions can subsequently undergo malignant transformation, and so clean margins must be obtained. After surgery the mass should be submitted for histopathologic examination.
Treatment of fibroadenomatous hyperplasia with antiprogestins has proven to be a valuable, well-tolerated alternative to ovariectomy, with the major advantage of preserving fertility.
The incidence of malignant tumours increases dramatically after 6 years, and peaks at 10–11 years of age.
Malignant tumors
Prevalence, incidence and signalment
Most mammary masses in domestic cats are malignant.34,35 Although the majority of mammary tumors occur in female cats, approximately 1–5% of feline mammary neoplasms affect male cats.3,36,37 The average age at diagnosis of malignant mammary tumors is 10–12 years.4,20,38 The incidence increases dramatically after 6 years, and peaks at 10–11 years of age, after which it declines.1,36
There are breed-associated risks for the development of mammary neoplasia, with higher incidence rates reported for Siamese and shorthaired cats.3,39 In addition, the mean age at diagnosis seems to be lower in Siamese female cats (average 9 years old) than in other breeds, suggesting a genetic predisposition in this breed.5,39
Intact cats are predisposed to the development of mammary neoplasia. 40 In a study by Misdorp, intact females were overrepresented by a factor of seven times among cats diagnosed with malignant mammary tumors compared with a control population. 41 Overley et al later showed that cats spayed before 1 year of age have a significantly decreased risk of developing mammary carcinoma. 40 In that study, cats spayed prior to 6 months of age or between 6 and 12 months of age had only 9% and 14%, respectively, the risk of mammary carcinoma development compared with intact cats. Nevertheless, malignant mammary gland tumors do occur in queens ovariectomized at less than 1 year of age, so early neutering does not completely eliminate the risk of mammary carcinoma. 5
Etiology
The etiology of malignant mammary tumors in cats is uncertain. 35 Older studies suggested that viruses could play a role; 42 however, there are no further studies to support a viral etiology for mammary carcinoma.
Steroid hormones have been implicated in the development of mammary carcinoma because neutered animals are less likely to develop tumors than intact cats. 40 In the study by Misdorp, 41 progestagens used for estrous prevention or treatment of dermatological problems in cats considerably increased the risk of mammary carcinoma if given regularly, but not if given intermittently. The study concluded that there is a dose-related tumori-genic effect of progestagens in the development of mammary tumors in cats.41,42
The effect of parity on the development of mammary tumors in cats has been evaluated and no association was found. 42
Classification, pathology and natural behavior
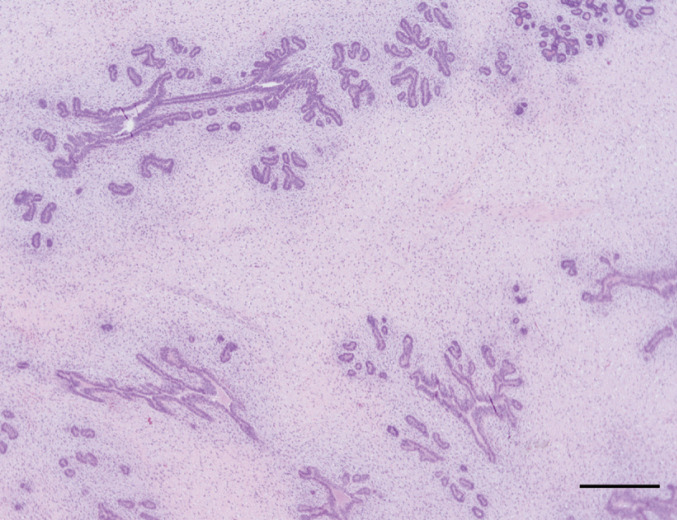
Most malignant feline mammary tumors are classified as adenocarcinomas (Fig 4). 4 Further classification between specific types of adenocarcinoma differs slightly between pathologists, but most agree that non-infiltrating (in situ), tubulopapillary, solid and cribriform are the most common forms. 4 Other subtypes of feline simple carcinomas include squamous cell carcinomas, mucinous carcinoma, carcinosarcomas and adenosquamous carcinomas.4,12,43
Fig 4.
Histopathologic appearance of a tubular feline mammary carcinoma (C) with a raft of neoplastic cells within a lymphatic vessel (*). Normal adjacent mammary gland (M) is also present. Hematoxylin and eosin stain. Bar = 500 μm
Inflammatory mammary carcinoma and lymphangiosarcoma have also been described in cats.44,45
Feline malignant tumors grow rapidly, and metastases are reported to occur in 50–90% of affected animals. 10 Metastasis to regional lymph nodes (83%), lungs (83%) (Fig 5), pleura (22%) and liver (25%) are most common. 35 However, various studies have also documented widespread metastases to the adrenal glands, diaphragm and kidneys.3,34,46
Fig 5.
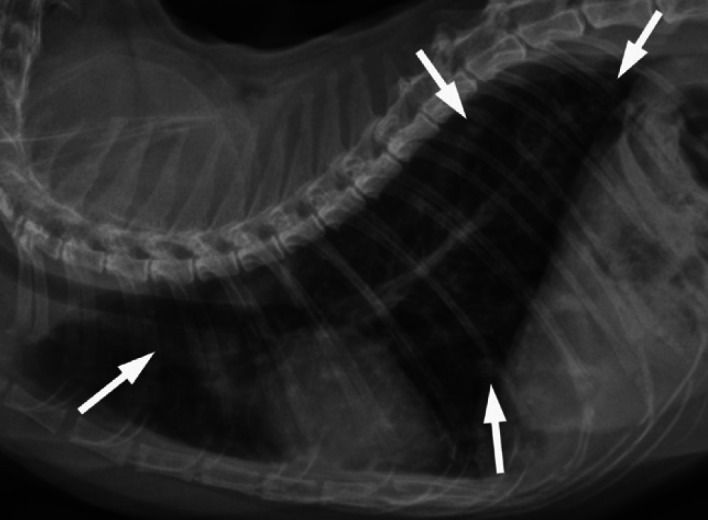
Left lateral radiograph of the thorax of an 11-year-old female spayed domestic shorthair cat presented for staging of mammary adenocarcinoma of the left caudal mammary chain. Numerous ill-defined rounded to amorphous soft tissue opacities of up to 4 mm are scattered throughout the lung fields, consistent with pulmonary metastases (arrows). A generalized unstructured interstitial and bronchial pattern is also present, of uncertain significance at this point. Cardiovascular, mediastinal and thoracic wall structures are within normal limits for a cat of this age
Malignant mammary gland tumors do occur in queens ovariectomized at less than 1 year of age, so early neutering does not completely eliminate the risk of mammary carcinoma.
In addition to a full diagnostic work-up, staging of the tumour is imperative.
Clinical presentation
Mammary carcinoma is often advanced, and metastases may already have occurred by the time the veterinarian is consulted.26,20 Feline malignant mammary tumors occur as discrete, palpable and moveable masses; 25% of affected cats show ulceration in association with extensive tumor necrosis (Fig 6).14,46 Swelling due to tumor thrombi in femoral arteries or decreased vascular return from femoral veins can cause discomfort, edema and a decrease in the temperature in the pelvic limbs. The involved nipples are often red and swollen and may exude tan- or yellow-colored fluid. 28
Fig 6.
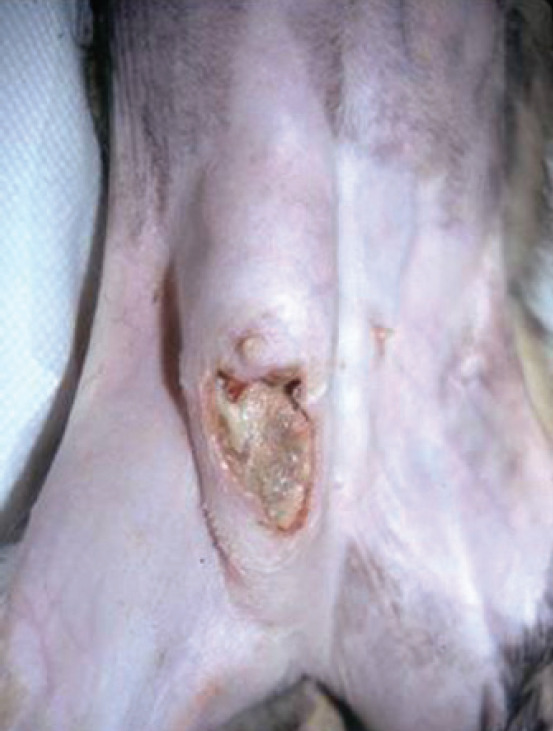
Clinical appearance of mammary carcinoma. Courtesy of Stein Memorial Feline Image Collection and Washington State University, College of Veterinary Medicine. Database: http://imagedb.vetmed.wsu.edu/
Some studies suggest that the caudal glands are more frequently involved,14,36 while Hayden and Nielsen reported that the cranial glands were more frequently affected. 10 However, Viste et al described a high prevalence in both cranial and caudal mammary glands. 47
Diagnosis
If the owner of a cat with suspected mammary carcinoma is considering treatment, a full diagnostic work-up must be performed, including a physical examination, complete blood count, serum chemistry profile and urinalysis. 28 Thoracic radiographs, including ventrodorsal and right and left lateral views, should be obtained to identify potential metastases, 28 and abdominal ultrasonography performed to identify hepatic, splenic or renal metastases and involvement of intra-abdominal lymph nodes. 28 Fine-needle aspiration, scraping of ulcerated lesions, or cytology of fluids expressed from affected glands may yield a diagnosis, which could be helpful to rule out skin and subcutaneous non-mammary malignancies and/or differentiate mammary carcinoma from fibroadenomatous hyperplasia.4,20,26
If the above diagnostic methods do not allow a definitive diagnosis, excisional biopsy is recommended. An informal review of feline mammary masses received during 2008 by the Surgical Pathology Service at the University of Tennessee identified one-third of the masses as being benign lesions (unpublished data). Therefore, malignancy should be confirmed before mastectomy.
Staging and grading of tumors
In addition to a full diagnostic work-up, staging of the tumor is imperative. Tumors in domestic animals are staged based essentially on the World Health Organization's TNM classification scheme for malignant tumors in humans. 10 T represents the extent of the primary tumor (size, fixation to skin or fascia). N describes the condition of the regional lymph nodes. 4 It is important to remember that lymphadenopathy is not a common finding at first presentation and that normal-sized lymph nodes may still contain tumor cells. 48 Thus, the regional lymph nodes (axillary and inguinal) should be examined carefully, and fine-needle aspiration may be necessary to help categorize N status.4,28 Finally, M designates the presence or absence of distant metastases. 4 Using these criteria tumors are classified from stage I to IV (Table 1).
Table 1.
Clinical staging of feline mammary tumors based on the TNM scheme
| Stage I | T1 | N0 | M0 |
| Stage II | T2 | N0 | M0 |
| Stage III | T1–2 | N1 | M0 |
| T3 | N0–1 | M0 | |
| Stage IV | Any T | Any N1 | M0 |
T1 = tumor <2 cm diameter, T2 = tumor 2–3 cm diameter, T3 = tumor >3 cm diameter. N0 = no histologic/cytologic metastasis in regional lymph nodes, N1 = histologic/ cytologic metastasis in regional lymph nodes.
M0 = no evidence of distant metastasis, M1 = evidence of distant metastasis.
System modified from Owen LN. Classification of tumors in domestic animals. Geneva, World Health Organization: 1980
Lymphadenopathy is not a common finding at first presentation and normal-sized lymph nodes may still contain tumor cells.
The most important prognostic factor in cats with mammary gland neoplasia is tumor size, which significantly affects both disease-free interval and survival time.
Histological grading systems for feline mammary tumors have been published49,50 based on: cellular differentiation and degree of tubule formation; nuclear pleomorphism; and mitotic frequency. Mammary tumors are graded as well differentiated, moderately differentiated or poorly differentiated (Tables 2 and 3). 49
Table 2.
Histological grading of feline mammary tumors
| Feature | Grade |
|---|---|
| Degree of tubule formation | |
| High (>75%) | Well differentiated |
| Moderate (10–75%) | Moderately differentiated |
| Little or none (<10%) | Poorly differentiated |
| Nuclear/cellular pleomorphism | |
| Small, regular, uniform cells/nuclei | Well differentiated |
| Moderate increase in size and variability | Moderately differentiated |
| Marked variation in size and variability | Poorly differentiated |
| Mitotic count (see Table 3) | Well differentiated to poorly differentiated |
From Jeglum et al (1985) 48
Table 3.
Assignment of points for mitotic count
| Number of mitoses per field area | Mitotic count score |
|---|---|
| 0–7 | Well differentiated |
| 8–14 | Moderately differentiated |
| >15 | Poorly differentiated |
Based on the fact that, like the staging system, the grading system is a human classification system applied to cats, and that most feline mammary tumors have been classified as moderately or poorly differentiated, grading is not usually performed by pathologists.49,51 However, it can be performed upon request.
Treatment options
Treatment options that have been studied for feline mammary neoplasia are surgical excision, chemotherapy, immunotherapy and radiation therapy. 20 These differ in terms of clinical outcome — the box on page 221 discusses the various modalities and the evidence that currently exists to support them.
While tamoxifen is a successful therapy in women with estrogen receptor tumors, it does not appear to be beneficial in queens,20,24,52 the reason being that, in cats, most of these malignant lesions express estrogen receptors in only a few cells or are estrogen-receptor negative.24,52–54
Prognostic factors
Tumor size The most important prognostic factor in cats with mammary gland neoplasia is tumor size, which significantly affects both disease-free interval and survival time. 61 MacEwen et al reported that cats diagnosed with mammary tumors smaller than 2 cm survived for an average of 54 months, while those with tumors 2–3 cm in diameter survived for an average of 24 months. 60 Given this association between tumor size and survival time, early diagnosis and treatment have an important bearing on the prognosis for cats with malignant mammary tumors. 4
Histopathology grade An association between survival time and histopathology grade has been demonstrated. The rate of death 1 year after surgery was 0% in cats with well-differentiated carcinoma, and 100% in those with poorly differentiated carcinoma. However, there was not a good correlation between moderate differentiation and survival time. 48
Mitotic count The number of mitotic figures found in tumor tissue has been shown to be of prognostic value. Longer survival times were seen in animals with tumors exhibiting fewer than two mitotic figures per high power field. 36
Disease stage Clinical stage at presentation is another factor that has been shown to be significantly associated with survival time. Median survival times of cats with stage I, II, III and IV disease were 29, 12.5, 9 and 1 month(s), respectively. 39
Surgical approach MacEwen et al compared the results of conservative surgery and radical mastectomy in cats with malignant mammary adenocarcinoma and found that cats that had undergone radical mastectomy had a significantly reduced rate of local recurrence compared with cats that had undergone conservative surgery. 61
Molecular markers
The expression of some genes, receptors or proteins can be altered during the malignant process in feline mammary tumors. These so-called molecular markers can be detected by immunohistochemistry and yield useful prognostic information in some cases.
Cyclin A
Cyclin A is a gene that regulates the cell cycle and it is commonly targeted in canine malignant mammary tumors as well as in feline mammary carcinomas. 62 In a small study, cyclin A gene abnormalities were found in 7/8 mammary carcinomas. 62 However, in a larger study, the involvement of cyclin A in feline mammary tumors was detected in only 48.6% of feline mammary carcinomas. 63 These results suggest that cyclin A may be associated with tumorigenesis in cases of feline mammary carcinoma.
Treatment of malignant mammary lesions.
Surgery
Even though surgical removal remains the most widely accepted treatment option for feline mammary tumors, it is usually not curative. Complete removal of neoplastic tissue is hampered by the degree of invasion and ulceration. 26 In contrast to the dog, in which more conservative resections may be appropriate in carefully selected cases, most cats require unilateral or bilateral chain mastectomy with removal of draining lymph nodes. 55 While more radical procedures are associated with longer disease-free intervals, they do not have a significant effect on overall survival rates.24,55 In cases requiring bilateral mastectomy the procedure should be performed in two stages, with a 2-week interval between surgeries.11,46 The inguinal lymph node is always removed with the A2 gland, whereas the axillary lymph node is removed only if it is enlarged or cytologically positive for tumor. Prophylactic removal of axillary lymph nodes is unlikely to have a therapeutic benefit. 4
Malignant mammary neoplasms in the cat often invade lymphatics and veins; therefore, early vessel ligation is essential when performing radical unilateral or bilateral chain mastectomy. 35 All damaged tissue must be handled gently, with copious flushing of the surgical area to help eliminate exfoliated neoplastic cells. 35 Because the histological completeness of resection has been correlated with survival, the surgeon should submit the entire specimen with the surgical margins inked for histological review to ensure that complete margins have been obtained. 36
The question of whether to perform ovariohysterectomy at the same time as a radical mastectomy is a controversial one. Whereas some authors believe that spaying cats with mammary carcinoma has little therapeutic value, others recommend spaying at the time of tumor removal.3,14,23
Survival times for cats with mammary gland adenocarcinomas treated with surgery alone depend on the stage of disease when the cat is presented. Stage is directly related to tumor size and the presence or absence of metastases (see prognostic factors). 46 The average time between detection of primary tumor and death in untreated cats is 12 months. 35
Chemotherapy
Opinions differ among veterinarians regarding the value of postsurgical chemotherapy. While some recommend it as adjunct therapy in cats with tumors showing evidence of invasion into the blood vessels or lymphatic vessels, others recommend chemotherapy in all cases. Regardless, it is important to note that the response of mammary tumors to chemotherapy is usually poor once metastases have occurred.
Results of in vitro studies using cell lines derived from primary feline malignant mammary tumors indicate sensitivity to similar antineoplastic drugs as used for women with breast cancer (eg, 5-fluorouracil, methotrexate, cyclophosphamide, prednisone, vincristine, doxorubicin). 55 However, it should be noted that 5-fluorouracil is associated with lethal neurotoxicity in cats. 35 In a study by Jeglum et al, 48 14 cats with non-resectable mammary carcinoma were treated with a combination of doxorubicin and cyclophosphamide. Results showed that 45% of the patients responded to the treatment, with an increased survival time. Mauldin et al treated 14 cats with mammary adenocarcinoma with the same combination of drugs and noted that the treatment induced short-term partial or complete response in 50% of cats with metastatic or non-resectable local disease. 56 However, results of this study indicated that survival time was related to tumor volume rather than to treatment. In more recent studies by Novosad et al, adjunctive doxorubicin postsurgery (1 mg/kg IV every 3 weeks for a maximum of five treatments, or until the cat developed progressive disease or concurrent illness) produced longer survival times in cats with advanced-stage disease than historically reported with surgery alone. 46 Despite favorable results, there were some patients in which the treatment had to be discontinued due to doxorubicin side effects (anorexia, moderate myelosuppression and nephrotoxicity). 46 Reducing the dose of doxorubicin or substituting mitox-antrone (5 mg/m 2 every 3 weeks) may limit toxicity to an acceptable level. 4 It is important to note that even though the results reported by Novosad et al sound promising, the study did not include a control group, so further investigation is needed in this area. 46
Thus, on the basis of results reported to date, more clinical trials need to be conducted to assess which chemotherapeutic doses and combinations are the most effective in increasing survival time.
Immunotherapy
Immunotherapy is a method of cancer treatment that entails administration of immunomodulators to stimulate the host immune response to tumor cells. 57
Treatment with biologic adjuvants like bacillus Calmette-Guerin (BCG) or Corynebacterium parvum vaccine, administered either intratumorously or subcutaneously in combination with surgery, was not successful. 58 The biologic response modifier liposome-encapsulated muramyl tripeptide phosphatidyl ethanolamine (L-MTP-PE) used to stimulate monocyte cytotoxic activity following mastectomy also did not improve the disease-free interval or survival time of cats when compared with placebo-treated cats. 59 MacEwen et al reported that the treatment of feline malignant mammary tumors with oral levamisole (5 mg/kg, 3 days a week) as an adjuvant to surgery was ineffective in altering the recurrence rate of the disease and did not increase the survival time of treated cats compared with control groups. 60 Therefore, thus far, immunomodulators have proven unsuccessful in the treatment of mammary neoplasia in cats.
Radiation therapy
Radiation therapy is seldom used in the treatment of feline mammary tumors due to the lack of evidence to support increased survival rates in this species.4,55
In contrast to the dog, in which more conservative resections may be appropriate in carefully selected cases, most cats require unilateral or bilateral chain mastectomy with removal of draining lymph nodes.
The response of mammary tumors to chemotherapy is usually poor once metastases have occurred.
Protein p53
Protein p53 is another gene that regulates the cell cycle and functions as a tumor suppressor. The latter is due to the main role of this gene, which is conserving genome stability, preventing its mutation. 64 Over half of human cancers have a mutation in the p53 protein or one of its partners. 64 Protein p53 mutation and nuclear accumulation has been detected immunohistochemically in 18.9% of feline mammary carcinomas, and it was shown that p53 immunohistochemical reactivity was significantly lower in adenomas compared with adenocarcinomas.63,65 Other studies demonstrated overexpression of p53 in 32.5–33% of carcinomas.66,67
RON
Macrophage-stimulating protein receptor (RON) is a member of the family of receptor tyrosine kinases that stimulate invasive properties of carcinoma cells. 68 As it has been shown that there is a remarkable increase in expression of the RON gene in human breast cancer, RON/stem cell-derived tyrosine kinase (stk) homologue gene in cats has been identified and its expression in feline mammary carcinoma has been studied. RON/stk was found to be expressed at a very high level in 20% of feline mammary carcinomas.68,69
c-erbB-2
Another gene frequently targeted in feline breast cancers is the human epidermal receptor type 2 (HER-2), alias c-erbB-2, which is normally involved in the signal transduction pathways that lead to cell growth and differ-entiation. 70 In humans, the overexpression of HER-2 protein correlates with more aggressive clinicopathologic features. 71 The feline orthologue of HER2 has been characterized and its expression studied; f-HER2 was found to be barely detectable in the normal cat mammary gland, increased in mammary benign tumors, and elevated in a high percentage of carcinoma samples.70,72 A study that measured the correlation between HER-2 expression and overall survival showed that cats with tumors associated with HER-2 overexpression had shorter overall survival times. 73 Together, these findings suggest that HER-2 status could provide valuable prognostic and predictive information.
Vegf
Vascular endothelial growth factor (VEGF) is an angiogenic factor released by tumors that is involved in the growth, invasion and metastasis of tumors. 71 In human breast cancer it has been shown that tumors with high levels of VEGF are biologically more aggressive. 74 This factor has also been studied in feline mammary tumors, and its expression was higher in poorly differentiated carcinomas. This finding suggests that VEGF could be a useful marker for predicting clinical outcome in cats with mammary tumors. 75
Cox
Cyclo-oxygenase (COX), also known as prostaglandin endoperoxide synthase, is a key enzyme in the biosynthetic pathway leading to the conversion of arachidonic acid into prostaglandins. Two structurally different forms of COX have been identified and characterized, COX-1 and COX-2. Upregulated expression of COX-2 has been documented in many cancers in humans. 75 In a large proportion of feline mammary tumors COX-2 is also expressed, and its expression is associated with a poorer prognosis.54,76
TopBP1
Topoisomerase IIβ binding protein 1 (TopBP1) is a nuclear protein that has structural and functional similarities to the familial gene associated with breast cancer in humans, BRCA2. The expression of TopBP1 was examined immunohistochemically in neoplastic and non-neoplastic feline mammary lesions and a tendency towards increased expression was found with increasing grade of malignancy. 66
Key Points
Malignant feline mammary masses are the rule rather than the exception, and require early diagnosis and radical surgical intervention to improve survival time and quality of life in affected cats.
In order to optimise early diagnosis, clients should be warned about the high rate of malignant mammary tumors and veterinarians should always include palpation of the mammary I glands as part of the routine physical examination of cats.
If an abnormality is found, unless there is a strong suspicion of fibroadenomatous hyperplasia, a full diagnostic work-up must be undertaken.
Given the possibility of a benign mass, malignancy should always be confirmed before mastectomy.
Acknowledgements
We wish to thank Dr Cheryl R Dhein, University of Washington State, for allowing us to use the pictures from the Washington State College of Veterinary Medicine Image Database. We also thank the original image source, Dr Barb Stein, who was an excellent teacher and an accomplished photographer, as evidenced by the pictures in this collection.
References
- 1.Dorn CR, Taylor DO, Frye FL, Hibbard HH. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. I. Methodology and description of cases. J Natl Cancer Inst 1968; 40: 295–305. [PubMed] [Google Scholar]
- 2.Dorn CR, Taylor DO, Schneider R, Hibbard HH, Klauber MR. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. II. Cancer morbidity in dogs and cats from Alameda County. J Natl Cancer Inst 1968; 40: 307–18. [PubMed] [Google Scholar]
- 3.Hayes HM, Jr, Milne KL, Mandell CP. Epidemiological features of feline mammary carcinoma. Vet Rec 1981; 108: 476–79. [DOI] [PubMed] [Google Scholar]
- 4.Lana SE, Rutteman GR, Withrow SJ. Tumors of the mammary gland. In: Withrow SJ, Vail DM, eds. Small animal clinical oncology. 4th edn. Canada: Saunders Elsevier, 2001: 628–36. [Google Scholar]
- 5.Hayes AA, Mooney S. Feline mammary tumors. Vet Clin North Am Small Anim Pract 1985; 15: 513–20. [DOI] [PubMed] [Google Scholar]
- 6.Silver IA. The anatomy of the mammary gland of the dog and cat. J Small Anim Pract 1966; 7: 689–96. [DOI] [PubMed] [Google Scholar]
- 7.Raharison F, Sautet J. Lymph drainage of the mammary glands in female cats. J Morphol 2006; 267: 292–99. [DOI] [PubMed] [Google Scholar]
- 8.Mailot JP, Lagneau F, Parodi AL, Andre F. Particularite's des tumeurs mammaires de la chatte. Point Veterin 1980; 11: 107–8. [Google Scholar]
- 9.Raharison F, Sautet J. The topography of the lymph vessels of mammary glands in female cats. Anat Histol Embryol 2007; 36: 442–52. [DOI] [PubMed] [Google Scholar]
- 10.Hayden DW, Nielsen SW. Feline mammary tumours. J Small Anim Pract 1971; 12: 687–98. [DOI] [PubMed] [Google Scholar]
- 11.Ogilvie GK. Feline mammary neoplasia. Compend Contin Educ Pract Vet 1983; 5: 384–91. [Google Scholar]
- 12.Misdorp W, Else RW, Hellmen E, Lipscomb TP. Histological classification of mammary tumors of the dog and the cat. Washington DC: Armed Forces Institute of Pathology in cooperation with the American Registry of Pathology and The World Health Organization Collaborating Center Worldwide Reference on Comparative Oncology, 1999. [Google Scholar]
- 13.Allen HL. Feline mammary hypertrophy. Vet Pathol 1973; 10: 501–8. [DOI] [PubMed] [Google Scholar]
- 14.Carpenter J. Tumor and tumor-like lesions. In: Holzworth J, ed. Diseases of the cat: medicine and surgery. Philadelphia: WB Saunders; 1987: 406–596. [Google Scholar]
- 15.Hampe JF, Misdorp W. Tumours and dysplasias of the mammary gland. Bull World Health Organ 1974; 50: 111–33. [PMC free article] [PubMed] [Google Scholar]
- 16.Hayden DW, Johnston SD, Kiang DT, Johnson KH, Barnes DM. Feline mammary hypertrophy/fibroadenoma complex: Clinical and hormonal aspects. Am J Vet Res 1981; 42: 1699–703. [PubMed] [Google Scholar]
- 17.Hayden DW, Barnes DM, Johnson KH. Morphologic changes in the mammary gland of megestrol acetate-treated and untreated cats: A retrospective study. Vet Pathol 1989; 26: 104–13. [DOI] [PubMed] [Google Scholar]
- 18.Loretti AP, Ilha MR, Ordas J, de las Mulas Martin J. Clinical, pathological and immunohistochemical study of feline mammary fibro epithelial hyperplasia following a single injection of depot medroxyprogesterone acetate. J Feline Med Surg 2005; 7: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pukay BP, Stevenson DA. Mammary hypertrophy in an ovario-hysterectomized cat. Can Vet J 1983; 24: 143–44. [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston SD, Kustritz MVR, Olson PNS. Disorders of the mammary gland of the queen. In: Johnston SD, Kustritz MVR, Olson PNS, eds. Canine and feline theriogenology. Philadelphia: WB Saunders, 2001: 474–85. [Google Scholar]
- 21.Hinton M, Gaskell CJ. Non-neoplastic mammary hypertrophy in the cat associated either with pregnancy or with oral progestagen therapy. Vet Rec 1977; 100: 277–80. [DOI] [PubMed] [Google Scholar]
- 22.De Las Mulas Martin J, Millan Y, Bautista MJ, Perez J, Carrasco L. Oestrogen and progesterone receptors in feline fibroadenomatous change: An immunohistochemical study. Res Vet Sci 2000; 68: 15–21. [DOI] [PubMed] [Google Scholar]
- 23.Rutteman GR, Misdorp W. Hormonal background of canine and feline mammary tumours. J Reprod Fertil Suppl 1993; 47: 483–87. [PubMed] [Google Scholar]
- 24.Hahn KA, Bravo L, Avenell JS. Feline breast carcinoma as a pathologic and therapeutic model for human breast cancer. In Vivo 1994; 8: 825–28. [PubMed] [Google Scholar]
- 25.Wehrend A, Hospes R, Gruber AD. Treatment of feline mammary fibroadenomatous hyperplasia with a progesterone-antagonist. Vet Rec 2001; 148: 346–47. [DOI] [PubMed] [Google Scholar]
- 26.Argyle DJ. The mammary gland. In: Simpson GM, England GCW, Harvey M, eds. Manual of small animal reproduction and neonatology. 1st edn. Gloucester: British Small Animal Veterinary Association, 1998: 53–59. [Google Scholar]
- 27.Gorlinger S, Kooistra HS, van den Broek A, Okkens AC. Treatment of fibroadenomatous hyperplasia in cats with aglepristone. J Vet Intern Med 2002; 16: 710–13. [DOI] [PubMed] [Google Scholar]
- 28.Moore AS, Ogilvie GK. Mammary tumors. In: Stecher Y, ed. Feline oncology. 1st edn. United States: Veterinary Learning Systems, 2001: 355–67. [Google Scholar]
- 29.Johnston SD, Kustritz MVR, Olson PNS. Disorders of the feline ovaries. In: Johnston SD, Kustritz MVR, Olson PNS, eds. Canine and feline theriogenology. 1st edn. Philadelphia: WB Saunders, 2001: 453–62. [Google Scholar]
- 30.Mandel M. Spontaneous remission of feline benign mammary hypertrophy. Vet Med Small Anim Clin 1975; 70: 846–47. [PubMed] [Google Scholar]
- 31.Hoffmann B, Schuler G. Receptor blockers — general aspects with respect to their use in domestic animal reproduction. Anim Reprod Sci 2000; 60–61: 295–312. [DOI] [PubMed] [Google Scholar]
- 32.Fieni F, Martal J, Marnet PG, Siliart B, Guittot F. Clinical, biological and hormonal study of mid-pregnancy termination in cats with aglepristone. Theriogenol 2006; 66: 1721–28. [DOI] [PubMed] [Google Scholar]
- 33.Georgiev P, Wehrend A. Mid-gestation pregnancy termination by the progesterone antagonist aglepristone in queens. Theriogenol 2006; 65: 1401–6. [DOI] [PubMed] [Google Scholar]
- 34.Moulton JE. Mammary tumors of the cat. In: Moulton JE, ed. Tumors in domestic animals. 3rd edn. Berkeley: University of California Press, 1990: 547–52. [Google Scholar]
- 35.Hahn KA, Adams WH. Feline mammary neoplasia: Biological behavior, diagnosis, and treatment alternatives. Feline Pract 1997; 25: 5–11. [Google Scholar]
- 36.Weijer K, Hart AA. Prognostic factors in feline mammary carcinoma. J Natl Cancer Inst 1983; 70: 709–16. [PubMed] [Google Scholar]
- 37.Skorupski KA, Overley B, Shofer FS, Goldschmidt MH, Miller CA, Sorenmo KU. Clinical characteristics of mammary carcinoma in male cats. J Vet Intern Med 2005; 19: 52–55. [DOI] [PubMed] [Google Scholar]
- 38.Kustritz MV. Determining the optimal age for gonadectomy of dogs and cats. J Am Vet Med Assoc 2007; 231: 1665–75. [DOI] [PubMed] [Google Scholar]
- 39.Ito T, Kadosawa T, Mochizuki M, Matsunaga S, Nishimura R, Sasaki N. Prognosis of malignant mammary tumor in 53 cats. J Vet Med Sci 1996; 58: 723–26. [DOI] [PubMed] [Google Scholar]
- 40.Overley B, Shofer FS, Goldschmidt MH, Sherer D, Sorenmo KU. Association between ovarihysterectomy and feline mammary carcinoma. J Vet Intern Med 2005; 19: 560–63. [DOI] [PubMed] [Google Scholar]
- 41.Misdorp W. Progestagens and mammary tumours in dogs and cats. Acta Endocrinol (Copenh) 1991; 125 (Suppl 1): 27–31. [PubMed] [Google Scholar]
- 42.Misdorp W, Romijn A, Hart AA. Feline mammary tumors: A case-control study of hormonal factors. Anticancer Res 1991; 11: 1793–97. [PubMed] [Google Scholar]
- 43.Sarli G, Brunetti B, Benazzi C. Mammary mucinous carcinoma in the cat. Vet Pathol 2006; 43: 667–73. [DOI] [PubMed] [Google Scholar]
- 44.Sugiyama A, Takeuchi T, Morita T, et al. Lymphangiosarcoma in a cat. J Comp Pathol 2007; 137: 174–78. [DOI] [PubMed] [Google Scholar]
- 45.Perez-Alenza MD, Jimenez A, Nieto AI, Pena L. First description of feline inflammatory mammary carcinoma: Clinicopathological and immunohistochemical characteristics of three cases. Breast Cancer Res 2004; 6: R300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Novosad CA, Bergman PJ, O'Brien MG, et al. Retrospective evaluation of adjunctive doxorubicin for the treatment of feline mammary gland adenocarcinoma: 67 cases. J Am Anim Hosp Assoc 2006; 42: 110–20. [DOI] [PubMed] [Google Scholar]
- 47.Viste JR, Myers SL, Singh B, Simko E. Feline mammary adenocarcinoma: Tumor size as a prognostic indicator. Can Vet J 2002; 43: 33–37. [PMC free article] [PubMed] [Google Scholar]
- 48.Jeglum KA, de Guzman E, Young KM. Chemotherapy of advanced mammary adenocarcinoma in 14 cats. J Am Vet Med Assoc 1985; 187: 157–60. [PubMed] [Google Scholar]
- 49.Castagnaro M, Casalone C, Bozzetta E, De Maria R, Biolatti B, Caramelli M. Tumour grading and the one-year post-surgical prognosis in feline mammary carcinomas. J Comp Pathol 1998; 119: 263–75. [DOI] [PubMed] [Google Scholar]
- 50.Weijer K, Head KW, Misdorp W, Hampe JF. Feline malignant mammary tumors. I. Morphology and biology: Some comparisons with human and canine mammary carcinomas. J Natl Cancer Inst 1972; 49: 1697–704. [DOI] [PubMed] [Google Scholar]
- 51.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathol 1991; 19: 403–10. [DOI] [PubMed] [Google Scholar]
- 52.Johnston SD, Hayden DW, Kiang DT, Handschin B, Johnson KH. Progesterone receptors in feline mammary adenocarcinomas. Am J Vet Res 1984; 45: 379–82. [PubMed] [Google Scholar]
- 53.Rutteman GR, Blankenstein MA, Minke J, Misdorp W. Steroid receptors in mammary tumours of the cat. Acta Endocrinol (Copenh) 1991; 125 (Suppl 1): 32–37. [PubMed] [Google Scholar]
- 54.Millanta F, Calandrella M, Vannozzi I, Poli A. Steroid hormone receptors in normal, dysplastic and neoplastic feline mammary tissues and their prognostic significance. Vet Rec 2006; 158: 821–24. [DOI] [PubMed] [Google Scholar]
- 55.Novosad CA. Principles of treatment for mammary gland tumors. Clin Tech Small Anim Pract 2003; 18: 107–9. [DOI] [PubMed] [Google Scholar]
- 56.Mauldin GN, Matus RE, Patnaik AK, Bond BR, Mooney SC. Efficacy and toxicity of doxorubicin and cyclophosphamide used in the treatment of selected malignant tumors in 23 cats. J Vet Intern Med 1988; 2: 60–65. [DOI] [PubMed] [Google Scholar]
- 57.MacEwen EG. An immunologic approach to the treatment of cancer. Vet Clin North Am 1977; 7: 65–75. [DOI] [PubMed] [Google Scholar]
- 58.Rutten VP, Misdorp W, Gauthier A, et al. Immunological aspects of mammary tumors in dogs and cats: A survey including own studies and pertinent literature. Vet Immunol Immunopathol 1990; 26: 211–25. [DOI] [PubMed] [Google Scholar]
- 59.Fox LE, MacEwen EG, Kurzman ID, et al. Liposome-encapsulated muramyl tripeptide phosphatidylethanolamine for the treatment of feline mammary adenocarcinoma — a multicenter randomized double-blind study. Cancer Biother 1995; 10: 125–30. [DOI] [PubMed] [Google Scholar]
- 60.MacEwen EG, Hayes AA, Mooney S, et al. Evaluation of effect of levamisole on feline mammary cancer. J Biol Response Mod 1984; 3: 541–46. [PubMed] [Google Scholar]
- 61.MacEwen EG, Hayes AA, Harvey HJ, Patnaik AK, Mooney S, Passe S. Prognostic factors for feline mammary tumors. J Am Vet Med Assoc 1984; 185: 201–4. [PubMed] [Google Scholar]
- 62.Murakami Y, Tateyama S, Rungsipipat A, Uchida K, Yamaguchi R. Amplification of the cyclin A gene in canine and feline mammary tumors. J Vet Med Sci 2000; 62: 783–87. [DOI] [PubMed] [Google Scholar]
- 63.Murakami Y, Tateyama S, Rungsipipat A, Uchida K, Yamaguchi R. Immunohistochemical analysis of cyclin A, cyclin D1 and P53 in mammary tumors, squamous cell carcinomas and basal cell tumors of dogs and cats. J Vet Med Sci 2000; 62: 743–50. [DOI] [PubMed] [Google Scholar]
- 64.Horvath MM, Wang X, Resnick MA, Bell DA. Divergent evolution of human p53 binding sites: Cell cycle versus apoptosis. PLoS Genet 2007; 3: e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakano M, Wu H, Taura Y, Inoue M. Immunohistochemical detection of Mdm2 and p53 in feline mammary gland tumors. J Vet Med Sci 2006; 68: 421–25. [DOI] [PubMed] [Google Scholar]
- 66.Morris JS, Nixon C, Bruck A, Nasir L, Morgan IM, Philbey AW. Immunohistochemical expression of TopBP1 in feline mammary neoplasia in relation to histological grade, Ki67, ERalpha and p53. Vet J 2008; 175: 218–26. [DOI] [PubMed] [Google Scholar]
- 67.Nasir L, Krasner H, Argyle DJ, Williams A. Immunocytochemical analysis of the tumour suppressor protein (p53) in feline neoplasia. Cancer Lett 2000; 155: 1–7. [DOI] [PubMed] [Google Scholar]
- 68.De Maria R, Maggiora P, Biolatti B, et al. Feline STK gene expression in mammary carcinomas. Oncogene 2002; 21: 1785–90. [DOI] [PubMed] [Google Scholar]
- 69.Maggiora P, Marchio S, Stella MC, et al. Overexpression of the RON gene in human breast carcinoma. Oncogene 1998; 16: 2927–33. [DOI] [PubMed] [Google Scholar]
- 70.Ordas J, Millan Y, Dios R, Reymundo C, de Las Mulas JM. Protooncogene HER-2 in normal, dysplastic and tumorous feline mammary glands: An immunohistochemical and chromogenic in situ hybridization study. BMC Cancer 2007; 7: 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sahin AA. Biologic and clinical significance of HER-2/neu (cerbB-2) in breast cancer. Adv Anat Pathol 2000; 7: 158–66. [DOI] [PubMed] [Google Scholar]
- 72.De Maria R, Olivero M, Iussich S, et al. Spontaneous feline mammary carcinoma is a model of HER2 overexpressing poor prognosis human breast cancer. Cancer Res 2005; 65: 907–12. [PubMed] [Google Scholar]
- 73.Millanta F, Calandrella M, Citi S, Della Santa D, Poli A. Overexpression of HER-2 in feline invasive mammary carcinomas: An immunohistochemical survey and evaluation of its prognostic potential. Vet Pathol 2005; 42: 30–34. [DOI] [PubMed] [Google Scholar]
- 74.Gasparini G. Prognostic value of vascular endothelial growth factor in breast cancer. Oncologist 2000; 5 (Suppl 1): 37–44. [DOI] [PubMed] [Google Scholar]
- 75.Millanta F, Lazzeri G, Vannozzi I, Viacava P, Poli A. Correlation of vascular endothelial growth factor expression to overall survival in feline invasive mammary carcinomas. Vet Pathol 2002; 39: 690–96. [DOI] [PubMed] [Google Scholar]
- 76.Sayasith K, Sirois J, Dore M. Molecular characterization of feline COX-2 and expression in feline mammary carcinomas. Vet Pathol 2009; 46: 423–29. [DOI] [PubMed] [Google Scholar]