Abstract

Practical relevance The control of nausea and vomiting in cats is important in order to prevent the development of food aversion, anorexia (with its associated complications of weight loss and dehydration), and hepatic lipidosis.

Clinical challenges There are several antiemetic drugs that are clinically effective in cats. Making a rational choice from the available options requires knowledge of the likely cause of the vomiting, and the mechanisms of action and side effects of each drug. For example, a drug such as prochlorperazine, which can cause sedation, may be a useful first-line choice in a hospitalized cat that requires mild sedation to be handled, but would be undesirable in a critically ill cat.
Audience For companion animal and feline practitioners, the vomiting cat is a common presentation.
Evidence base The guidance provided in this review draws on the findings of clinical trials in humans, experimental studies in cats, some clinical trials in cats, and clinical experience.
First-line antiemetics
Metoclopramide
Metoclopramide is a prokinetic and central antiemetic drug (Fig 1). It exerts its prokinetic effects through the release of acetylcholine in gastric and small intestinal smooth muscle,1,2 leading to increased gastric emptying and net ‘downstream’ intestinal motility. In humans, it also increases tone in the lower esophageal sphincter, reducing reflux. 3
Fig 1.
In cats, metoclopramide is a prokinetic drug, but it may not have direct central antiemetic effects. The dosage should be reduced (eg, by 50% or more) for cats in renal failure. Tremors are a possible side effect, especially in combination with phenothiazine antiemetics
Metoclopramide's central antiemetic action is due to antagonism of dopamine receptors at the chemoreceptor trigger zone. In cats, however, metoclopramide is not an effective antagonist of the emetic effects of dopamine. 4 Emesis from dopamine appears to be mediated through α2-adrenergic receptors rather than dopamine receptors in cats. 4 This is consistent with the finding that cats are sensitive to emesis from xylazine (an α2-agonist). 5
Despite this, metoclopramide appears to be clinically effective as an antiemetic in cats, perhaps via its prokinetic effects that increase gastric emptying and decrease gastric atony.6,7 Metoclopramide may also be useful to reduce reflux esophagitis in cats with megaesophagus, although its effects on lower esophageal sphincter tone appear to be weak in cats (study performed in kittens). 6 It is unclear whether metoclopramide is effective as a central antiemetic for cats with uremia, intoxications or pancreatitis, or for those undergoing chemotherapy. Other central antiemetics may be more effective in such cases.
Because metoclopramide is a prokinetic drug, intestinal obstruction should be ruled out, based on physical examination and abdominal radiography (Fig 2), before therapy is instituted. Metoclopramide is dosed empirically in cats at 0.2–0.4 mg/kg SC or PO qid. Because of a short elimination half-life in other species, metoclopramide may be most effective when given by continuous rate infusion (CRI) at 1–2 mg/kg/day. The dosage should be reduced (eg, by 50% or more) in cats with renal failure, as clearance of the drug is impaired in renal failure, at least in humans. 8
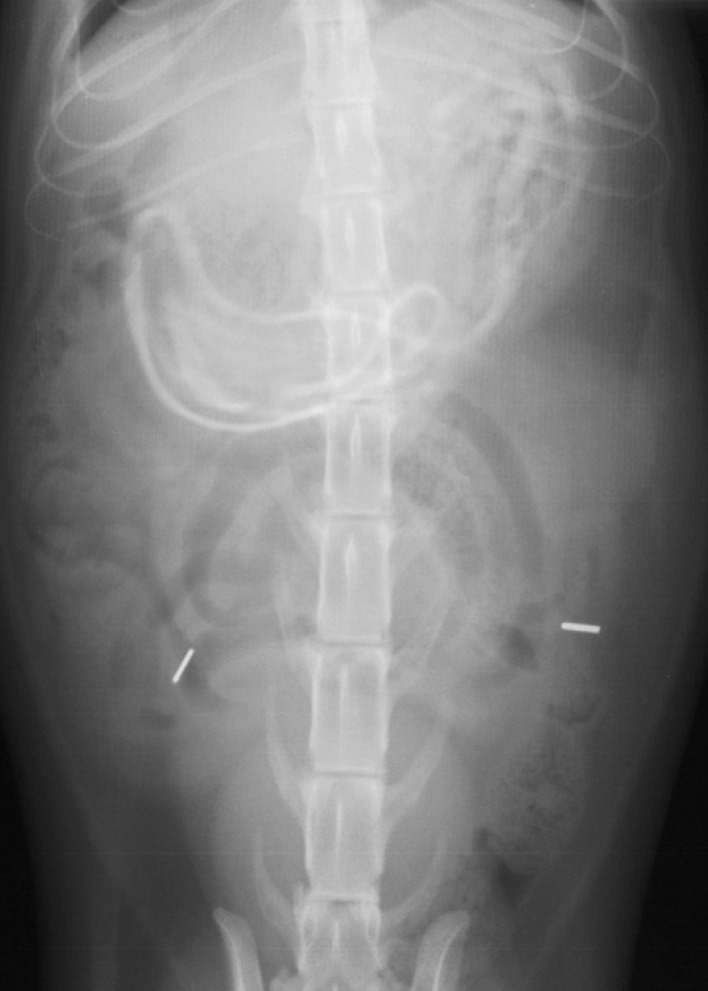
Fig 2.
This radiograph of a 2-year-old female spayed cat, which presented with a 3-week history of vomiting and inappetence, shows linear foreign bodies in the stomach. Multiple hair elastics were removed by endoscopy, and the cat recovered uneventfully. Courtesy of the Section of Radiology, University of Wisconsin-Madison, School of Veterinary Medicine
Metoclopramide dosing in cats
0.2–0.4 mg/kg SC or PO qid
Most effective when given by CRI (1–2 mg/kg/day)
It is unclear whether metoclopramide is effective as a central antiemetic in cats.
Abdominal radiography should be performed in all vomiting cats before antiemetics are considered.
High plasma concentrations of metoclopramide can lead to frenzied behavior or tremors (Parkinson's-like). If either occur, the drug should be discontinued until signs resolve. If signs were mild, the infusion can then be started again at half of the previous dosage.
Ondansetron and dolasetron
Ondansetron (Zofran; GlaxoSmithKline) and dolasetron (Anzemet; Sanofi Aventis) are antiemetics with 5-HT3 receptor antagonist activity, both in the central nervous system (CNS) and gastrointestinal (GI) tract. These drugs are effective anecdotally for prophylaxis of vomiting associated with chemotherapy in cats and dogs. 9 They also appear to be useful for refractory vomiting in hospitalized cats with pancreatitis, hepatic lipidosis, severe inflammatory bowel disease, GI neoplasia and cholangitis.
Ondansetron dosing in cats
-
0.5 mg/kg IV or PO bid
Dolasetron dosing in cats
0.6–1.0 mg/kg IV or PO sid
Ondansetron is available as a 2 mg/ml injectable, 4 mg tablets and an oral solution (4 mg per 5 ml). The empirical dosage in cats is 0.5 mg/kg bid. Dolasetron is supplied as an injectable and as 50 mg tablets, which must be reformulated for use in cats. The empirical dosage is 0.6–1.0 mg/kg IV or PO sid. Oral dolasteron is approximately twice as expensive per mg as ondansetron, excluding reformulation costs.
Significant side effects have not to date been reported in cats for either ondansetron or dolasteron. In humans, headache and diarrhea are reported most commonly. 10 Ondansteron, more so than dolasteron, may cause CNS side effects such as dizziness in humans. 10 If this is also true in cats, then ondansetron may not be the best choice in patients with vomiting due to vestibular disease. Dolasetron has been associated with prolongation of the QT interval in humans, with hypokalemia as a risk factor. 11 This electrocardiographical change may lead to life-threatening arrhythmias, and has also been reported in humans receiving cisapride.
Prochlorperazine and chlorpromazine
Prochlorperazine (Compazine; GlaxoSmith-Kline) and chlorpromazine are phenothiazine central antiemetics with multiple mechanisms of action, including antagonism of dopamine, histamine type 1, α2-adrenergic and mus carinic receptors. These drugs inhibit vomiting at the chemoreceptor trigger zone and directly at the emetic center.
Cats appear to be more sensitive than dogs to the emetic effects of α2-agonists, such as xylazine; 12 they may, therefore, respond well to α2-antagonist antiemetics such as pheno-thiazines. Phenothiazines are useful for refractory vomiting in patients with diagnosed underlying disease (eg, pancreatitis, GI neoplasia, chemotherapy), in which intravenous fluid support can be provided. The empirical dosage of either prochlorperazine or chlorpromazine in cats is 0.1–0.5 mg/kg SC tid. Due to their antihistamine effects, these drugs can cause sedation. Therefore, despite requiring frequent dosing, either drug may be useful as a first-line antiemetic in hospitalized cats that also require mild sedation to be handled.
As well as their sedative effects, phenothiazine antiemetics can also cause hypotension (due to α-adrenergic blockade). They are not, therefore, recommended for empirical outpatient use or in dehydrated animals.
Prochlorperazine/chlorpromazine dosing in cats
0.1–0.5 mg/kg SC tid
It may be wise to avoid concurrent use of metoclopramide and phenothiazine emetics, due to the risk of tremors.
Adjunctive fluid support is strongly recommended. There is additionally a risk of tremors due to dopamine antagonism; this may be more likely to occur if the drug is used in combination with metoclopramide, 13 and it may be wise, therefore, to avoid concurrent use of metoclopramide and phenothiazine antiemetics. Formulations containing anticholinergics such as isopropamide should be avoided, as they will lead to ileus.
Maropitant
Maropitant (Cerenia; Pfizer) is a neurokinin-1 (NK-1) receptor antagonist that was recently approved as an antiemetic in dogs in the US. Together with its related drugs it inhibits substance P binding to NK-1 receptors, which are located in the emetic center, chemoreceptor trigger zone and the enteric plexus of the gut, 14 and otherwise lead to vomiting when stimulated.
NK-1 receptor antagonists are gaining popularity for prevention of chemotherapy-associated vomiting in humans. 15 In dogs, maropitant is effective in preventing chemotherapy-induced vomiting, motion sickness and non-specific vomiting.16,17 In cats, maropitant has been proven effective for both xylazine-induced emesis and motion sickness. 18 Anecdotally, it also appears to be effective in cats for a variety of other causes of nausea and vomiting, as shown in dogs.
An initial dosage estimate in cats is 1 mg/kg IV, SC or PO sid, based on preliminary studies and a long elimination half-life (13–17 h) in this species. 18 Experience with maropitant is still emerging in cats, so any common side effects remain to be clearly defined. Pain at the injection site has been reported anecdotally. NK-1 receptor antagonists do not interact with dopaminergic or serotonergic receptors, 14 and should not cause cumulative side effects in combination with metoclopramide or 5-HT3 antagonists.
Maropitant dosing in cats
1 mg/kg IV, SC or PO sid
Adjunctive drugs for specific indications
Famotidine
Famotidine (Pepcid; Merck Pharmaceuticals) is an H2 blocker that reduces gastric acid secretion. It is more potent than ranitidine in cats, but has a similar duration of action. 19 Unlike cimetidine, famotidine does not inhibit cytochrome P450-mediated clearance of other drugs; therefore, in humans it causes relatively few drug interactions. 20
Famotidine is not an antiemetic, and appears to be overused in vomiting animals, as hyperacidity is probably a relatively uncommon cause of vomiting in cats. It may be useful in cases of severe or persistent vomiting, where secondary reflux and esophagitis are of concern (Fig 3), and is indicated for vomiting due to hyperacidity caused by high gastrin levels in renal failure 21 or by histamine released from mast cell tumors.
Fig 3.
Esophagitis, such as that caused by clindamycin capsules in this 12-year-old male neutered Persian (a), may initially manifest as vomiting and then progress to regurgitation. Esophagitis should be treated aggressively to prevent progression to esophageal stricture, as occurred in this case (b). The cat was treated successfully with balloon dilation of the esophageal stricture, and was discharged on a short course of famotidine and sucralfate treatment
Famotidine is not an antiemetic and appears to be overused in vomiting animals, as hyperacidity is probably a relatively uncommon cause of vomiting in cats.
Table 1.
Recommended drugs for specific indications in vomiting cats
| Underlying disease | Clinical approach to vomiting |
|---|---|
| Renal failure | Famotidine or ranitidine for hyperacidity Hydration; treat hypokalemia if present Maropitant if still vomiting |
| Cholangitis Hepatic lipidosis | Famotidine or ranitidine for hyperacidity Treat encephalopathy (lactulose, hydration) Treat hypokalemia |
| Avoid metronidazole if GI upset already present, or use metronidazole benzoate Maropitant if still vomiting | |
| Inflammatory bowel disease | Treat underlying inflammation (novel protein diet, glucocorticoids) If no response, metoclopramide or cisapride if gastroparesis suspected Famotidine or ranitidine if gastric reflux suspected |
| Pancreatitis | Metoclopramide, maropitant, ondansetron, or prochlorperazine (if mild sedation desired) If abdominal pain present, buprenorphine or fentanyl CRI |
| Intoxication | Gastric lavage; then consider ondansetron or maropitant Prokinetics may enhance intestinal absorption of toxin, so not recommended |
| Hairballs | Petrolatum products If no response, cisapride, metoclopramide or ranitidine |
| Motion sickness | Maropitant (dimenhydrinate or maropitant in dogs) |
| Megacolon with vomiting | Ranitidine or cisapride |
CRI = constant rate infusion
The empirical dosage of famotidine in cats is 0.5–1.0 mg/kg sid to bid. The drug is generally well tolerated. Prior anecdotal reports of hemolysis after intravenous administration were unsupported in a recent study, which showed that famotidine did not cause hemolysis in any of 56 hospitalized cats when given by slow bolus injection over 5 mins. 22 In humans, a dose reduction is recommended in renal failure patients, 20 as high plasma famotidine concentrations can lead to mental status abnormalities such as confusion or delirium. 23
Famotidine dosing in cats
0.5–1.0 mg/kg
IV, PO or SC sid to bid
Ranitidine
Ranitidine (Zantac; GlaxoSmithKline) is also an H2 blocker and has been shown to decrease gastric hydrochloric acid secretion in cats. 24 In addition, ranitidine has prokinetic effects on the GI tract, including the colon, as a result of its weak acetylcholinesterase activity.25,26 As with famotidine, ranitidine may be useful for severe persistent vomiting where esophageal reflux or esophagitis is a concern, as well as for vomiting due to hyperacidity (renal failure, mast cell disease). Ranitidine may be preferable to famotidine in cats with both suspected hyperacidity or reflux, and gastric atony or megacolon.
Ranitidine dosing in cats
2.5 mg/kg IV bid or
3.5 mg/kg PO bid
Ranitidine is available without prescription in the USA as 75 mg tablets. The recommended dosage, based on pharmacokinetic data in cats, is 2.5 mg/kg IV bid or 3.5 mg/kg PO bid. 27 Similar to famotidine, a dose reduction of ranitidine is recommended in human renal failure patients. No clinically significant cytochrome P450 enzyme inhibition is seen with ranitidine at therapeutic concentrations. 28 Ranitidine by intravenous bolus may cause transient hypotension in cats.2,3
Omeprazole
Omeprazole (Prilosec; AstraZeneca) and related proton pump blockers inhibit the H+/K+ ATPase pump at the luminal surface of gastric parietal cells. This prevents the final step in gastric hydrochloric acid secretion, and these drugs are more potent antacids than H2 blockers. Omeprazole has been shown to reduce gastric acid secretion in cats. 24
Omeprazole is useful for significant or refractory gastroduodenal ulceration or erosive esophagitis. It has no direct antiemetic effects. Although there is a delay of 1–3 days before omeprazole exerts its maximal effect, adding H2 blockers for the first few days of treatment provides little or no improvement in efficacy in humans, and is not necessary.
Omeprazole is available in 10 and 20 mg capsules. An empirical dosage in cats (and dogs) is 0.5–1.0 mg/kg PO sid. The drug is enteric-coated to prevent degradation. If reformulated, enteric-coated granules can be measured and placed in a gelatin capsule. The equine preparation (Gastrogard; Merial) is much too concentrated to use safely in cats (or dogs).
Omeprazole is a cytochrome P450 enzyme inhibitor in humans, but without the wide range of enzyme inhibition seen with cimetidine. Omeprazole can decrease the clearance of diazepam in humans, 29 and, depending on the drug, omeprazole can also act as a P450 inducer. Because of the potential for drug interactions, omeprazole may not be an ideal long-term antacid in cats being treated with multiple drugs.
The safety of omeprazole for long-term administration has not been established in cats. Because of effective acid suppression, pump blockers lead to a feedback increase in gastrin release, which has been associated with parietal cell hyperplasia in dogs, and, at very high doses, with gastric carcinoids in rats. 30 Long term (>1 year) use of omeprazole is associated with an increased incidence of gastric polyps in humans, although dysplastic changes are rare, 31 and an increase in gastric cancer has not been observed in humans.
Omeprazole dosing in cats
0.5–1.0 mg/kg PO sid
The most important complication of sucralfate use is its potential for drug interactions.
Sucralfate
Sucralfate (Carafate; Aventis Pharmaceuticals) is a disaccharide complexed to aluminium hydroxide. In the acidic environment of the stomach it becomes cross-linked and forms a gel that adheres to gastric epithelium, especially that of exposed ulcer beds. Sucralfate also binds pepsin and bile salts, which can otherwise contribute to ulcer formation. In addition, it can act as a phosphate binder. Sucralfate may speed ulcer healing by enhancing the production of cytoprotective prostaglandins by the gastric mucosa; however, this effect has not been studied in cats, and could not be demonstrated in canine gastric mucosa. 32
Sucralfate dosing in cats
1/4 of a 1 g tablet per cat tid to qid
Sucralfate is indicated for esophagitis, such as that caused by doxycycline or clindamycin capsules in cats,33,34 gastric or duodenal ulceration, and uremic gastritis. It may be helpful after endoscopic retrieval of gastric or esophageal foreign bodies. Sucralfate has been shown experimentally to prevent acid-induced esophagitis in cats, 35 and, therefore, may be useful prophylactically prior to surgery when reflux is anticipated (eg, a recent meal, presence of megaesophagus or a hiatal hernia). Sucralfate has been advocated for radiation mucositis, but its efficacy has been disappointing in humans. 36
The empirical dosage of sucralfate is one-quarter of a 1 g tablet per cat tid to qid. Sucralfate may be crushed and suspended in water; it is stable for 14 days in the refrigerator as a 200 mg/ml suspension. 37 A gel formulation of sucralfate is as effective as the suspension and can be given less frequently (twice daily in humans); 38 sucralfate gel is marketed in Europe for human use.
Sucralfate can lead to constipation, and has a chalky, unpalatable taste, but is a safe drug overall. The most important complication of treatment is the potential for drug interactions. The aluminium in sucralfate binds other drugs such as doxycycline, digoxin and all fluoroquinolones, and markedly impairs their absorption. If sucralfate must be given orally with any of these drugs, it is best to give the other drug 2 h before sucralfate. Reversing the order will not prevent an interaction, as sucralfate remains in the stomach for many hours after administration. Sucralfate can be given concomitantly with H2 blockers without affecting their overall absorption;39,40 therefore, no separation of dosing times is necessary.
Cisapride
Cisapride is a prokinetic drug in the same family as metoclopramide. It enhances the release of acetylcholine in the smooth muscle of the GI tract, via effects on serotonin receptors in the myenteric plexus. Like metoclopramide, cisapride increases lower esophageal sphincter pressure, gastric emptying and small intestinal motility. Unlike metoclopramide, however, it also increases colonic motility 41 Cisapride is a more potent gastric prokinetic than metoclopramide (as shown in dogs). 42
While it has no direct efficacy as a central antiemetic, cisapride may be an effective treatment for vomiting due to gastroparesis (eg, from postoperative ileus or infiltrative gastric disease) or associated with recurrent constipation. Cisapride may also be useful for gastroesophageal reflux that is unresponsive to H2 blockers or omeprazole alone. The recommended dosage of cisapride, based on pharmacokinetics in cats, is 1.5 mg/kg PO bid. 43
Being a prokinetic, cisapride is contra-indicated for cats with intestinal obstruction. Side effects of diarrhea and cramping have been reported in humans. Unlike metoclopramide, cisapride is not a dopaminergic antagonist, so tremors and other CNS side effects are not observed. Cisapride exhibits drug interactions with ketoconazole or itraconazole in humans; these azole antifungals inhibit cisapride metabolism and the resulting high plasma cisapride concentrations can lead to QT prolongation and ventricular arrhythmias. This adverse effect has led to the removal of cisapride from the US market for use in humans. In cats, cisapride can also lead to QT prolongation, but at dosages 20 times higher than those used clinically 44 These same ECG changes (QT prolongation) have been reported for dolasetron. Until more is known in cats, the combination of cisapride and dolasetron may best be avoided.
Cisapride dosing in cats
1.5 mg/kg PO bid
Key Points
Clinically effective antiemetics in cats target α2-adrenergic, neurokinin-1 and 5-HT3 receptors.
Dopaminergic antagonists such as metoclopramide may be primarily effective as prokinetic agents rather than as central antiemetics in cats.
Phenothiazine antiemetics such as prochlorperazine or chlorpromazine should not be used in dehydrated or depressed patients.
Sucralfate should not be given concomitantly with oral fluoroquinolones or doxycycline.
References
- 1.Hay AM, Man WK. Effect of metoclopramide on guinea pig stomach: Critical dependence on intrinsic stores of acetylcholine. Gastroenterol 1979; 76: 492–96. [PubMed] [Google Scholar]
- 2.Johnson AG. The effect of metoclopramide on gastroduodenal and gallbladder contractions. Gut 1971; 12: 158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durazo FA, Valenzuela JE. Effect of single and repeated doses of metoclopramide on the mechanisms of gastroesophageal reflux. Am J Gastroenterol 1993; 88: 1657–62. [PubMed] [Google Scholar]
- 4.Jovanovic-Micic D, Samardzic R, Beleslin DB. The role of alpha-adrenergic mechanisms within the area postrema in dopamine-induced emesis. Eur J Pharmacol 1995; 272: 21–30. [DOI] [PubMed] [Google Scholar]
- 5.Lucot JB, Crampton GH. Xylazine emesis, yohimbine and motion sickness susceptibility in the cat. J Pharmacol Exp Ther 1986; 237: 450–55. [PubMed] [Google Scholar]
- 6.Hillemeier C, McCallum R, Oertel R, et al. Effect of bethanechol and metoclopramide on upper gastrointestinal motility in the kitten. J Pediatr Gastroenterol Nutr 1986; 5: 134–37. [DOI] [PubMed] [Google Scholar]
- 7.Mangel AW, Stavorski JR, Pendleton RG. Effects of bethanechol, metoclopramide, and domperidone on antral contractions in cats and dogs. Digestion 1983; 28: 205–9. [DOI] [PubMed] [Google Scholar]
- 8.Lehmann CR, Heironimus JD, Collins CB, et al. Metoclopramide kinetics in patients with impaired renal function and clearance by hemodialysis. Clin Pharmacol Ther 1985; 37: 284–89. [DOI] [PubMed] [Google Scholar]
- 9.Ogilvie GK. Dolasetron: A new option for nausea and vomiting. J Am Anim Hosp Assoc 2000; 36: 481–83. [DOI] [PubMed] [Google Scholar]
- 10.Goodin S, Cunningham R. 5-HT(3)-receptor antagonists for the treatment of nausea and vomiting: A reappraisal of their side-effect profile. Oncologist 2002; 7: 424–36. [DOI] [PubMed] [Google Scholar]
- 11.Cubeddu LX. QT prolongation and fatal arrhythmias: A review of clinical implications and effects of drugs. Am J Ther 2003; 10: 452–57. [DOI] [PubMed] [Google Scholar]
- 12.Hikasa Y, Takase K, Ogasawara S. Evidence for the involvement of alpha 2-adrenoceptors in the emetic action of xylazine in cats. Am J Vet Res 1989; 50: 1348–51. [PubMed] [Google Scholar]
- 13.Montastruc JL, Llau ME, Rascol O, et al. Drug-induced parkinsonism: A review. Fundam Clin Pharmacol 1994; 8: 293–306. [DOI] [PubMed] [Google Scholar]
- 14.Prommer E. Aprepitant (EMEND): The role of substance P in nausea and vomiting. J Pain Palliat Care Pharmacother 2005; 19: 31–39. [PubMed] [Google Scholar]
- 15.Trigg ME, Inverso DM. Nausea and vomiting with high-dose chemotherapy and stem cell rescue therapy: A review of antiemetic regimens. Bone Marrow Transplant 2008; 42: 501–6. [DOI] [PubMed] [Google Scholar]
- 16.Benchaoui HA, Siedek EM, De La Puente-Redondo VA, et al. Efficacy of maropitant for preventing vomiting associated with motion sickness in dogs. Vet Rec 2007; 161: 444–47. [DOI] [PubMed] [Google Scholar]
- 17.de la Puente-Redondo VA, Siedek EM, Benchaoui HA, et al. The anti-emetic efficacy of maropitant (Cerenia) in the treatment of ongoing emesis caused by a wide range of underlying clinical aetiologies in canine patients in Europe. J Small Anim Pract 2007; 48: 93–98. [DOI] [PubMed] [Google Scholar]
- 18.Hickman MA, Cox SR, Mahabir S, et al. Safety, pharmacokinetics and use of the novel NK-1 receptor antagonist maropitant (Cerenia) for the prevention of emesis and motion sickness in cats. J Vet Pharmacol Ther 2008; 31: 220–29. [DOI] [PubMed] [Google Scholar]
- 19.Coruzzi G, Bertaccini G, Noci MT, et al. Inhibitory effect of famotidine on cat gastric secretion. Agents Actions 1986; 19: 188–93. [DOI] [PubMed] [Google Scholar]
- 20.Echizen H, Ishizaki T. Clinical pharmacokinetics of famotidine. Clin Pharmacokinet 1991; 21: 178–94. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein RE, Marks SL, Kass PH, et al. Gastrin concentrations in plasma of cats with chronic renal failure. J Am Vet Med Assoc 1998; 213: 826–28. [PubMed] [Google Scholar]
- 22.de Brito Galvao JF, Trepanier LA. Risk of hemolytic anemia with intravenous administration of famotidine to hospitalized cats. J Vet Intern Med 2008; 22: 325–29. [DOI] [PubMed] [Google Scholar]
- 23.Catalano G, Catalano MC, Alberts VA. Famotidine-associated delirium. A series of six cases. Psychosomatics 1996; 37: 349–55. [DOI] [PubMed] [Google Scholar]
- 24.Fandriks L, Jonson C. Effects of acute administration of omeprazole or ranitidine on basal and vagally stimulated gastric acid secretion and alkalinization of the duodenum in anaesthetized cats. Acta Physiol Scand 1990; 138: 181–86. [DOI] [PubMed] [Google Scholar]
- 25.Kounenis G, Koutsoviti-Papadopoulou M, Elezoglou A, et al. Comparative study of the H2-receptor antagonists cimetidine, ranitidine, famotidine and nizatidine on the rabbit stomach fundus and sigmoid colon. J Pharmacobiodyn 1992; 15: 561–65. [DOI] [PubMed] [Google Scholar]
- 26.Petroianu GA, Arafat K, Schmitt A, et al. Weak inhibitors protect cholinesterases from strong inhibitors (paraoxon): In vitro effect of ranitidine. J Appl Toxicol 2005; 25: 60–67. [DOI] [PubMed] [Google Scholar]
- 27.Duran S, Jernigan A, Ravis W, et al. Pharmacokinetics of oral and intravenous ranitidine in cats [abstract]. 9th Annual ACVIM Forum 1991; 902.
- 28.Martinez C, Albet C, Agundez JA, et al. Comparative in vitro and in vivo inhibition of cytochrome P450 CYP1A2, CYP2D6, and CYP3A by H2-receptor antagonists. Clin Pharmacol Ther 1999; 65: 369–76. [DOI] [PubMed] [Google Scholar]
- 29.Andersson T. Omeprazole drug interaction studies. Clin Pharmacokinet 1991; 21: 195–212. [DOI] [PubMed] [Google Scholar]
- 30.Carlsson E. A review of the effects of long-term acid inhibition in animals. Scand J Gastroenterol Suppl 1989; 166: 19–23. [DOI] [PubMed] [Google Scholar]
- 31.Jalving M, Koornstra JJ, Wesseling J, et al. Increased risk of fundic gland polyps during long-term proton pump inhibitor therapy. Aliment Pharmacol Ther 2006; 24: 1341–48. [DOI] [PubMed] [Google Scholar]
- 32.Scheiman JM, Kraus ER, Yoshimura K, et al. Effect of sucralfate on components of mucosal barrier produced by cultured canine epithelial cells in vitro. Dig Dis Sci 1992; 37: 1853–59. [DOI] [PubMed] [Google Scholar]
- 33.Beatty JA, Swift N, Foster DJ, et al. Suspected clindamycin-associated oesophageal injury in cats: Five cases. J Feline Med Surg 2006; 8: 412–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.German AJ, Cannon MJ, Dye C, et al. Oesophageal strictures in cats associated with doxycycline therapy. J Feline Med Surg 2005; 7: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katz PO, Ginsberg GG, Hoyle PE, et al. Relationship between intragastric acid control and healing status in the treatment of moderate to severe erosive oesophagitis. Aliment Pharmacol Ther 2007; 25: 617–28. [DOI] [PubMed] [Google Scholar]
- 36.Keefe DM, Schubert MM, Elting LS, et al. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 2007; 109: 820–31. [DOI] [PubMed] [Google Scholar]
- 37.Nahata M, Hipple T. Pediatric drug formulations. 2nd edn. Cincinnati: Harvey Whitney Books, 1992. [Google Scholar]
- 38.Guslandi M, Ferrero S, Fusillo M. Sucralfate gel for symptomatic chronic gastritis: Multicentre comparative trial versus sucralfate suspension. Ital J Gastroenterol 1994; 26: 442–45. [PubMed] [Google Scholar]
- 39.Albin H, Vincon G, Lalague MC, et al. Effect of sucralfate on the bioavailability of cimetidine. Eur J Clin Pharmacol 1986; 30: 493–94. [DOI] [PubMed] [Google Scholar]
- 40.Mullersman G, Gotz VP, Russell WL, et al. Lack of clinically significant in vitro and in vivo interactions between ranitidine and sucralfate. J Pharm Sci 1986; 75: 995–98. [DOI] [PubMed] [Google Scholar]
- 41.Hasler AH, Washabau RJ. Cisapride stimulates contraction of idiopathic megacolonic smooth muscle in cats. J Vet Intern Med 1997; 11: 313–18. [DOI] [PubMed] [Google Scholar]
- 42.Gullikson GW, Loeffler RF, Virina MA. Relationship of serotonin-3 receptor antagonist activity to gastric emptying and motor-stimulating actions of prokinetic drugs in dogs. J Pharmacol Exp Ther 1991; 258: 103–10. [PubMed] [Google Scholar]
- 43.LeGrange SN, Boothe DM, Herndon S, et al. Pharmacokinetics and suggested oral dosing regimen of cisapride: A study in healthy cats. J Am Anim Hosp Assoc 1997; 33: 517–23. [DOI] [PubMed] [Google Scholar]
- 44.Kii Y, Nakatsuji K, Nose I, et al. Effects of 5-HT(4) receptor agonists, cisapride and mosapride citrate on electrocardiogram in anaesthetized rats and guinea-pigs and conscious cats. Pharmacol Toxicol 2001; 89: 96–103. [DOI] [PubMed] [Google Scholar]