Abstract
Background
Increasing physical activity (PA) is an effective strategy to slow reductions in cortical volume and maintain cognitive function in older adulthood. However, PA does not exist in isolation, but coexists with sleep and sedentary behaviour to make up the 24-hour day. We investigated how the balance of all three behaviours (24-hour time-use composition) is associated with grey matter volume in healthy older adults, and whether grey matter volume influences the relationship between 24-hour time-use composition and cognitive function.
Methods
This cross-sectional study included 378 older adults (65.6 ± 3.0 years old, 123 male) from the ACTIVate study across two Australian sites (Adelaide and Newcastle). Time-use composition was captured using 7-day accelerometry, and T1-weighted magnetic resonance imaging was used to measure grey matter volume both globally and across regions of interest (ROI: frontal lobe, temporal lobe, hippocampi, and lateral ventricles). Pairwise correlations were used to explore univariate associations between time-use variables, grey matter volumes and cognitive outcomes. Compositional data analysis linear regression models were used to quantify associations between ROI volumes and time-use composition, and explore potential associations between the interaction between ROI volumes and time-use composition with cognitive outcomes.
Results
After adjusting for covariates (age, sex, education), there were no significant associations between time-use composition and any volumetric outcomes. There were significant interactions between time-use composition and frontal lobe volume for long-term memory (p = 0.018) and executive function (p = 0.018), and between time-use composition and total grey matter volume for executive function (p = 0.028). Spending more time in moderate-vigorous PA was associated with better long-term memory scores, but only for those with smaller frontal lobe volume (below the sample mean). Conversely, spending more time in sleep and less time in sedentary behaviour was associated with better executive function in those with smaller total grey matter volume.
Conclusions
Although 24-hour time use was not associated with total or regional grey matter independently, total grey matter and frontal lobe grey matter volume moderated the relationship between time-use composition and several cognitive outcomes. Future studies should investigate these relationships longitudinally to assess whether changes in time-use composition correspond to changes in grey matter volume and cognition.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12966-023-01557-4.
Keywords: Time use, Physical activity, Sleep, Sedentary behaviour, Brain volume, Cognitive function, Aging
Background
Healthy ageing is associated with changes in cortical volume and cognitive function [1, 2]. For example, older adults without diagnosed cognitive impairment may still present with cognitive challenges in vocational and interpersonal domains. Effective strategies which slow reductions in brain volume may have additional benefits for maintaining healthy cognitive functioning in older age and preventing or delaying future cognitive decline and dementia. One strategy is through achieving and maintaining sufficient physical activity. Physical activity has been identified as a modifiable risk factor for dementia in older adulthood [3] and is positively associated with both brain volume and cognitive function in healthy older adults. Cross-sectional and longitudinal magnetic resonance imaging (MRI) studies suggest that older adults who engage in higher levels of PA have greater global brain volume [4–8] and greater volume in medial temporal [9, 10] and frontal regions [11]. Similarly there is evidence that habitual physical activity is positively associated with cognitive function in healthy older adults across several cognitive domains [12]. The mechanisms underlying this relationship are not well understood, however it is likely that slowing reductions in cortical volume contributes to the positive association between physical activity and cognitive function.
Although physical activity is positively related to both brain volume and cognitive function in older adults, an important but often overlooked consideration is that physical activity does not take place in isolation. Physical activity must fit within the 24-hour day. In fact, the 24-hour day can be broadly divided into three time-use behaviours: physical activity, sedentary behaviour, and sleep, which are each related to brain health. Sedentary behaviours, defined as time spent in a sitting or reclined position and expending < 1.5 metabolic equivalents (METs), may impact brain health through mechanisms that differ from those involved in physical inactivity, typically defined as achieving insufficient amounts of physical activity at higher intensities (based on physical activity guidelines) [13, 14]. To date, few studies have investigated the relationship between sedentary behaviour and grey matter volume in older adults. A recent study found no differences in total grey matter volume between high and low sedentary behaviour groups (< 8 h or > 8 h per day), but reported a trend of lower hippocampal volumes in the high sedentary behaviour group [15]. Two additional studies reported a relationship between high levels of sedentary behaviour and cortical thinning in hippocampal sub-regions (i.e., parahippocampal cortex, entorhinal cortex, and subiculum) [15, 16]. Similarly, the associations between sedentary behaviour and cognitive function in older adults are not clear [12]. Several mechanisms, including modulation of cerebral blood flow, neurotrophic factors, and brain structure [17, 18], have been postulated to mediate links between sedentary behaviour and cognitive function. However, it is hypothesized that grey matter volume may be less sensitive to the cardiovascular health effects imposed by excessive sedentary behaviour compared to other aspects of brain structure such as the volume and composition of white matter [19].
There is also mixed evidence on associations between sleep, brain structure and cognitive function in older adults. Some studies have indicated that short or long sleep durations (i.e., <6 h or > 9 h per night) are associated with loss of cortical volume in frontal and temporal regions [20] and ventricular enlargement [21] in older adults. Similarly, short or long sleep duration has been negatively associated with cognitive function in older adults [22, 23]. Conversely, a recent 28-year longitudinal study found no significant differences in grey matter volume or cognitive changes between older adults who slept for 5, 6, 7 or 8 h per night. However, the null findings in that study may have been driven by the small number of participants in ‘extreme’ groups (i.e., most participants slept 6–7 h per night which more closely aligns with sleep guidelines) [24]. It is not well understood whether poor sleep causes reduced cortical volume, whether reduced cortical volume causes poor sleep, or whether the relationship is bi-directional [25]. In summary, the mechanisms underlying the relationship between sleep and cognitive function in older age remain unclear, and it is hard to disentangle direct effects from a wide range of possible confounders.
To date, physical activity, sleep, and sedentary behaviour have been studied independently in relation to grey matter volume, or while considering only two of the three behaviours together. However, physical activity, sleep and sedentary behaviour interact to make up the 24-hour day, such that increasing time in one behaviour will lead to a reduction in one or both remaining behaviours [26]. It is therefore more meaningful to consider the effects of time-use composition (or the proportion of time spent in each time-use behaviour within the 24-hour period) on brain structure. A recent study by Maasakkers et al. [27] found that sedentary participants had smaller hippocampal volumes, but this relationship was attenuated when controlling for levels of moderate-vigorous physical activity (MVPA). Thus, the interactions between time-use behaviours may be important for grey matter volume beyond the independent effects of each time-use behaviour alone.
Understanding the interactive effects of physical activity, sedentary behaviour and sleep on grey matter volume may help inform 24-hour movement guidelines for healthy ageing and dementia prevention in older adults. Further, understanding whether the association between 24-hour time-use composition and cognitive function is moderated by grey matter volume will provide further insight into the mechanisms by which optimal time use benefits cognitive functioning in older adulthood. To our knowledge, no studies have investigated how 24-hour time-use composition is associated with grey matter volume in healthy older adults. Our previous study found weak associations between independent time-use behaviours (but not 24-hour time-use composition) and cognitive function in a similar sample of healthy older adults [28] but did not explore neurophysiological mechanisms underlying these relationships. To address these important gaps in knowledge, cross-sectional data from the baseline phase of the ACTIVate study were used to investigate (a) whether 24-hour time-use composition is associated with grey matter volume, and (b) whether grey matter volume moderates the relationship between 24-hour time-use composition and cognitive function.
Methods
Ethics
The ACTIVate study was registered with the Australia New Zealand Clinical Trials Registry (ACTRN12619001659190) on November 27, 2019. Ethics approval was obtained from the University of South Australia and University of Newcastle Human Research Ethics Committee (202639). All procedures were conducted in accordance with the Declaration of Helsinki.
Participant recruitment and screening
Eligibility criteria for the ACTIVate study have been reported in detail elsewhere [29]. Briefly, participants were eligible if they were aged 60–70 years, fluent in English, had no clinical diagnoses of dementia or any other neurological or psychiatric disorders, did not have an intellectual or major physical disability, and presented no contraindications to transcranial magnetic stimulation or MRI screening tools [30].
Participants were recruited for the ACTIVate study using a rolling convenience sampling strategy [29]. Those who met initial eligibility criteria were further screened against cognitive impairment using the Montreal Cognitive Assessment (blind) via phone interview. Participants who scored < 13 (out of a potential 22) were excluded from the study.
Power calculations were used to determine the required sample size for the larger ACTIVate study (based on cognitive outcomes), which have been detailed extensively elsewhere [29]. Briefly, aiming for 80% power, allowing for attrition and response rate at recruitment and accounting for the longitudinal design of the study, the final sample size of 448 participants was determined.
Study measures
Device-measured time-use patterns
Data were collected between August 2020 and February 2022. Measures of daily time-use patterns (time spent in physical activity, sedentary behaviour, and sleep) were derived from triaxial accelerometers. Participants wore an Axivity AX3 monitor on their non-dominant wrist for 7 consecutive days and were asked to complete a sleep log for each day of wear (time they got out of bed, time they went to bed, time spent napping during the waking day, and reasons for removal of monitor during the day). Accelerations were sampled at 100 Hz. Raw acceleration data were downloaded using the Open Movement GUI software (OmGUI; Newcastle, UK), converted to.CSV files and processed using a custom MATLAB graphic user interface developed by researchers at the University of South Australia (COBRA; MATLAB R2018B).
Time spent in sleep was classified manually by marking the wake and sleep times for each 24-hour recording period while cross-checking participants’ sleep logs against the accelerometry trace. Brief nighttime awakenings were not captured unless waking periods were explicitly reported by participants. Waking day behaviours were classified as time spent in MVPA (> 93 mg), light intensity PA (LPA; >48 mg) or sedentary behaviour (< 48 mg) using previously published cut points adjusted for sampling frequency [31–33]. ‘Valid wear days’ were classified if accelerometers were worn for at least 10 waking hours [34], and the participant accrued less than six hours of non-wear time (thus, a minimum of 18 hours average wear time per 24-hour recording period was required). To be included in final analyses, participants were required to have at least three valid weekdays and one valid weekend day. Total time spent in each time-use behaviour was averaged across the recording period, providing values reflecting the average time spent in MVPA, LPA, sedentary behaviour, and sleep per day (in minutes).
Brain imaging and MRI processing
MRI acquisition was performed on a Siemens Skyra 3T scanner in Adelaide, and a Siemens Prisma 3T scanner in Newcastle, both using a 64-channel head and neck coil. T1-weighted magnetization prepared rapid gradient echo (MPRAGE) images were acquired for volumetric quantification of brain structures, and T2-weighted fluid-attenuated inversion recovery (FLAIR) images were used to assess white matter hyperintensity burden. The acquisition parameters for MRI sequences are described elsewhere [29].
3D T1 MPRAGE images were first segmented into grey matter, white matter and cerebrospinal fluid tissues using an in-house implementation of the expectation maximization algorithm [35]. The brain parcellation was performed using the NeuroMorphometrics parcellation atlas and Learning Embeddings for Atlas Propagation method [36], allowing measurement of cortical and subcortical grey matter volumes. White matter hyperintensities were quantified from T2 FLAIR images using the HyperIntensity Segmentation Tool [37]. For the purpose of this study, white matter hyperintensity volume data were only used to characterize the sample and were not included in main analyses.
Volumetric measures of grey matter regions of interest (ROIs) were derived from the brain segmentation and the NeuroMorphometrics parcellation, which included total grey matter, lateral ventricle, bilateral frontal lobe, bilateral temporal lobe and bilateral hippocampus volumes.
Cognitive function measures
Cognitive function was measured using a series of tests from the Cambridge Automated Neuropsychological Test Automated Battery (CANTAB). Using the Cattell-Horn-Carroll-Miyake taxonomy [38] as a guiding framework, tests were z-scored and then combined to create three cognitive composites: long-term memory (Verbal Recognition Memory test); executive function (Multitasking and One Touch Stockings of Cambridge tests); and processing speed (Reaction Time test). The average z-score of cognitive tests within each composite (i.e., a single z-score value for each cognitive domain) was used in final statistical models. Higher z-scores indicated better cognitive performance. The methods used to create these composites have been described in detail elsewhere [28].
Covariates
Age (years), sex (male, female) and education (total years) were entered as covariates in linear regression models, based on previous evidence of their associations with grey matter volume [39–41] and cognitive function [3, 42]. Age and sex data were derived from a demographics questionnaire, whilst total years of education (including primary, secondary, and tertiary education) was derived from the Australian National University Alzheimer’s Disease Risk Index collected for the larger study [43].
Statistical analysis
All inferential statistics were conducted in R version 4.2.2 and the code used for data analysis is available at https://github.com/MaddisonMellow/time-use-brainvol-paper. To account for total brain size and MRI scanner/protocol differences, all ROI volumes were adjusted for total intracranial volume, scanner site (Adelaide or Newcastle), and use of the distortion correction option during scanning (on or off) using linear regression models. Next, outcome variables (ROI volumes) were inspected for normality and extreme skewness. At this stage of analysis, no transformations were performed as data were normally distributed, but data were further inspected and transformed later to improve model fit as needed.
Pairwise correlations
To explore relationships between individual time-use variables (minutes/day in sleep, sedentary behaviour, LPA and MVPA), brain volume measures (total grey matter, lateral ventricle, frontal lobe, temporal lobe, and bilateral hippocampus volumes), cognitive outcomes (long-term memory, executive function, and processing speed) and continuous/binary covariates (age, sex, and education), pairwise correlation coefficients were calculated. Pearson correlations were used for all pairwise correlations outside of the time-use composition (i.e., all correlations except for those between time-use behaviours). Because time-use behaviours are components of a composition, and therefore have a constant sum constraint (1440 min of the day), applying traditional correlation analysis between time-use variables may result in spurious correlations [44]. Intuitively, because of the fixed sum constraint, an increase in one compositional part will result in lower values in remaining compositional parts which, when using a traditional Pearson’s correlation coefficient, would impose a negative correlation. To overcome this, symmetric balanced isometric log ratio coordinates are used [44]. In essence, these symmetric balanced coordinates focus on the pairwise changes in two compositional parts (e.g., sleep and MVPA) compared to a representative value of the average of the remaining parts, using two sets of sensibly chosen isometric log ratio coordinates that are bisected (and length normalized) for each pairwise comparison of compositional parts. Thus, traditional correlation coefficients can be calculated on these transformed values, but the interpretation is not the strength of the linear relationship between the two variables/parts as with Pearson’s correlation coefficient. Rather, correlation coefficients of pairwise compositional parts expressed in symmetric balanced coordinates are interpreted as the “dominance” of one compositional part over another (similarly, values between − 1 and 1): coefficients are positive when the two compositional parts increase simultaneously compared to a representative value of the average of the remaining parts, whereas coefficients are negative when the increase of one compositional part (e.g., MVPA) is associated with a decrease in the other compositional part (e.g., sleep) compared to a representative value of the average of the remaining parts. This method was applied to calculate correlations between time-use behaviours (i.e., within the 24-hour composition) using the corCoDa function in the robCompositions package [45].
Compositional data analysis (CoDA)
All compositional analyses were conducted using the R compositions package version 1.4 [46]. Daily time-use compositions were created for each participant, representing the average proportion of time spent in MVPA, LPA, sedentary behaviour and sleep each day. As in previous studies using compositional data analysis [26, 47], the closure function (‘clo()’ in the compositions package) was applied to proportionally re-scale time-use components to sum to 1440 min [46]. Time-use compositions were then isometric log-ratio transformed to be included in statistical analyses as predictors (see Dumuid et al. [26] for overview of this method).
First, linear regression models were used to derive the associations between 24-hour time-use composition (predictor) and each ROI volume (outcome). Model 1 included covariates only (age, sex, education), whilst Model 2 included covariates and the predictor of interest (time-use composition). Model fit was examined using the performance package in R [48]. To improve the model fit, lateral ventricle volumes were log-transformed, whilst all other model fit diagnostics passed assumption checks and variables were therefore not transformed. To account for the possibility that associations between time-use composition and ROI volume outcomes may be non-linear (e.g., inverted U-shaped relationships between sleep and ROI volume), an additional model (Model 3) which was identical to Model 2 but expressed time-use composition using quadratic (squared) terms was fit. We used an F-test to explore whether quadratic terms (Model 3) improved model fit compared to Models 1 and 2. Expressing time-use composition using quadratic terms did not improve the model fit for any ROI volumes compared to the standard linear regressions (at an alpha of 0.05) and are not discussed further.
Next, we investigated whether the associations between time-use composition and the cognitive function outcomes were moderated by grey matter volume, frontal lobe volume, temporal lobe volume or hippocampus volume. For each cognitive outcome (long-term memory, executive function, processing speed), a series of linear regression models were fit, with each incorporating main effects of ROI volume and time-use composition, as well as the interaction between time-use composition and the respective ROI volume. All models were adjusted for covariates (age, sex, education).
Type II F-tests were used in determining variable significance (assessing variable effects after adjusting for other variables while adhering to the principle of marginality [49, 50]). To account for multiple comparisons, p-values within each of the final regression models were Benjamini-Hochberg false discovery rate adjusted [51, 52].
Modelling reallocations of time
In the instance that 24-hour time-use composition was significantly associated with a volumetric outcome (following false discovery rate adjustment), we planned to plot model-generated predictive response curves to demonstrate how volumetric measures were associated with meaningful reallocations of time, using one-for-remaining swaps (e.g., increasing MVPA by 30 min at the expense of all other behaviours equally) and one-for-one swaps (e.g., increasing MVPA by 30 min whilst taking that time directly from sleep) [26]. Similarly, where an interaction between 24-hour time-use composition and a volumetric outcome was significantly associated with a cognitive outcome, we planned to plot model-generated predictive response curves to demonstrate how cognitive function was associated with meaningful reallocations of time across different brain volume levels (dichotomized to above or below the mean ROI volume) using one-for-remaining and one-for-one swaps.
Results
Participant demographics
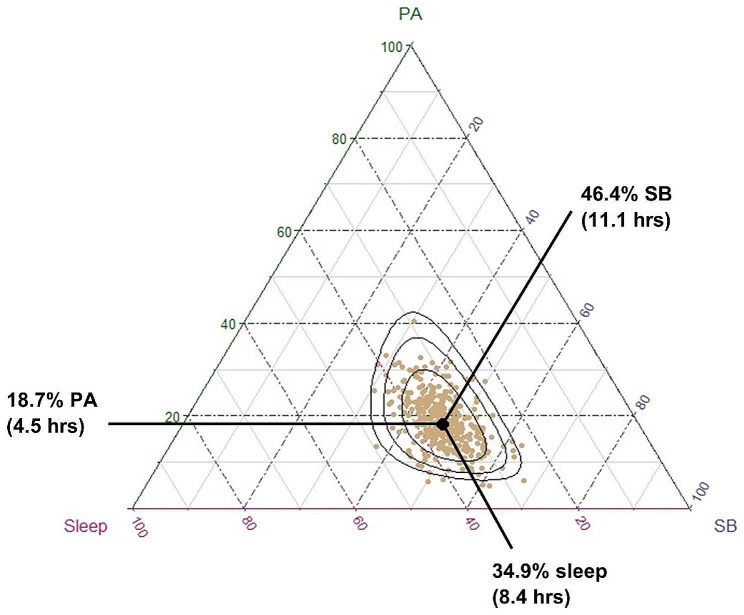
Of the original 426 participants recruited in the baseline phase of the ACTIVate study, 395 participants completed both T1 MPRAGE and T2 FLAIR imaging protocols. Seventeen participants were removed from the dataset as they did not have valid accelerometry data: 7 did not meet minimum criteria for a valid accelerometry dataset (i.e., less than minimum required days of recording); 8 were missing accelerometry data; and two had > 1500 min of recorded time use per day. The overall final sample included 378 older adults (65.6 ± 3.0 years old, 123 males). Means, standard deviations and range (minimum and maximum) of key continuous variables are presented in Table 1. Participants had low white matter hyperintensity burden (mean = 2 ml) and were highly active, spending approximately 4.5 h per day in physical activity (1.5 h in MVPA; 3 h in LPA), 11.1 h in sedentary behaviour, and 8.4 h sleeping. Participants’ time-use compositions are displayed in Fig. 1.
Table 1.
Participant demographics
| Total (n = 378) | |||
|---|---|---|---|
| Mean ± SD | Range (min, max) | ||
| Age | 65.6 ± 3.0 | 60.1, 71.2 | |
| Sex (%) | Female | 255 (67%) | - |
| Male | 123 (33%) | - | |
| Education (years) | 16.6 ± 3.2 | 7, 30 | |
| Accelerometer waking wear time (valid files only; mins) | 936.6 ± 56.5 | 756.5, 1106.8 | |
| Arithmetic means of time-use behaviours (min/day) | MVPA | 90.6 ± 47.0 | 3.4, 301.7 |
| LPA | 178.6 ± 50.6 | 57.9, 342.6 | |
| SB | 667.4 ± 91.5 | 410.3, 982.3 | |
| Sleep | 501.8 ± 58.5 | 330.8, 741.0 | |
| Compositional means of time-use behaviours (min/day) | MVPA | 90.7 ± 47.1 | 3.4, 301.7 |
| LPA | 178.8 ± 50.7 | 57.8, 346.6 | |
| SB | 668.2 ± 92.2 | 410.8, 968.1 | |
| Sleep | 502.2 ± 57.5 | 331.4, 712.5 | |
| Brain volume (ml) | TIV | 1551.0 ± 141.2 | 1202.0, 2279.0 |
| Global GM | 596.0 ± 50.8 | 466.3, 843.0 | |
| Temporal lobe | 95.3 ± 9.2 | 67.4, 130.9 | |
| Hippocampus | 6.2 ± 0.6 | 4.6, 8.5 | |
| Lateral ventricles | 25.0 ± 12.5 | 6.0, 84.5 | |
| Frontal lobe volume | 168.0 ± 16.9 | 127.0, 240.0 | |
| WMH | 1.9 ± 3.4 | 0.0, 33.7 | |
Note: Values are presented in the “Mean ± SD” column as either mean ± SD for numeric variables or count (percentage) for categorical variables. Range data represents minimum and maximum values. MVPA = moderate-vigorous physical activity; LPA = light physical activity; SB = sedentary behaviour; TIV = total intracranial volume; GM = grey matter; WMH = white matter hyperintensities. Volumetric data are presented in raw form (i.e., prior to adjusting for site, total intracranial volume and use of distortion correction during imaging). ‘Arithmetic means’ represent the average minutes of time spent in each behaviour before applying the closure function. ‘Compositional means’ represent the geometric average of time use values after applying the closure function (closing composition to sum up to 1440 minutes).
Fig. 1.
Distribution of participants’ time-use compositions. Each gold dot represents a single participant’s 24-hour time-use composition, whereas the black dot represents the average time-use composition of the entire sample (calculated as the compositional mean). On average, participants spent 18.7% of their day in physical activity (moderate-vigorous and light intensity, summed), 34.9% of their day in sleep, and 46.4% of their day in sedentary behaviour. Black ellipses represent 75%, 95% and 99% density contours assuming compositional normality (normal distribution on the simplex [46]
Associations between 24-hour time-use composition and brain structure
Pairwise correlations
Pairwise Pearson correlations and their respective 95% confidence intervals are displayed in Table 2. There were no significant correlations between individual time-use behaviours and any volumetric outcomes. Several relationships were observed between individual time-use behaviours and cognitive outcomes: more time spent in MVPA was associated with faster processing speed (r = 0.18, 95% CI [0.07, 0.27]); more time spent in sedentary behaviour was associated with slower processing speed (r=-0.14, 95% CI [-0.24, -0.04]); and more time spent in sleep was associated with poorer long-term memory performance (r=-0.12, 95% CI [-0.23, -0.02]). Similarly, several ROI volumes were related to cognitive function: long-term memory was positively correlated with hippocampus volume (r = 0.13, 95% CI [0.03, 0.23]); and executive function was positively correlated with total grey matter volume (r = 0.22, 95% CI [0.12, 0.32]), temporal lobe volume (r = 0.14, 95% CI [0.03, 0.24]), hippocampus volume (r = 0.12, 95% CI [0.02, 0.22]) and frontal lobe volume (r = 0.15, 95% CI [0.04, 0.25]) and negatively correlated with ventricle volume (r=-0.15, 95% CI [-0.25, -0.05]). Higher age was associated with lower total grey matter (r=-0.28, 95% CI [-0.37, -0.19]), temporal lobe (r=-0.20, 95% CI [-0.29. -0.10]) and hippocampus volume (r=-0.16, 95% CI [-0.25, -0.06]), greater lateral ventricle volume (r = 0.21, 95% CI [0.11, 0.30]), slower processing speed (r=-0.14, 95% CI [-0.24, -0.04]) and worse executive function (r=-0.26, 95% CI [-0.35, -0.16]). Point biserial correlations showed that female sex (female = 1, male = 0) was negatively correlated with time in MVPA (r=-0.20, 95%CI [-0.30, -0.10]) and executive function (r=-0.17, 95%CI [-0.27, -0.07]), and positively correlated with time in sleep (r = 0.14, 95%CI [0.04, 0.23]) and frontal lobe volume (r = 0.12, 95%CI [0.02, 0.22]).
Table 2.
Pairwise correlations between continuous covariates, predictors, and outcome variables
| Sex | Education (years) | LPA (min) | MVPA (min) | SB (min) | Sleep (min) | Total GM vol | Temporal vol | Hippocamp. vol | Ventricle vol | Frontal lobe vol | Processing speed | Executive function | Long-term memory | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) |
-0.10 (-0.20,-0.00) |
0.01 (-0.09, 0.11) |
0.05 (-0.05, 0.15) |
-0.08 (-0.18, 0.02) |
-0.00 (-0.11, 0.10) |
0.03 (-0.07, 0.14) |
-0.28 (-0.37, -0.19) |
-0.20 (-0.29, -0.10) |
-0.16 (-0.25, -0.06) |
0.21 (0.11, 0.30) |
-0.10 (-0.20, 0.00) |
-0.14 (-0.24, -0.04) |
-0.26 (-0.35, -0.16) |
-0.07 (-0.25, 0.03) |
| Sex |
-0.07 (-0.17, 0.03) |
0.03 (-0.07, 0.13) |
-0.20 (-0.30, -0.10) |
0.00 (-0.10, 0.10) |
0.14 (0.04, 0.23) |
0.02 (-0.08, 0.12) |
-0.01 (-0.11, 0.09) |
-0.08 (-0.18, 0.02) |
-0.01 (0.11, 0.09) |
0.12 (0.02, 0.22) |
0.01 (-0.10, 0.10) |
-0.17 (-0.27, -0.07) |
0.06 (-0.04, 0.16) |
|
| Education (years) |
-0.05 (-0.15, 0.05) |
-0.01 (-0.11, 0.09) |
0.09 (-0.01, 0.19) |
-0.10 (-0.20, 0.00) |
0.05 (-0.05, 0.15) |
0.03 (-0.07, 0.13) |
0.02 (-0.08, 0.12) |
-0.03 (-0.13, 0.08) |
0.02 (-0.08, 0.12) |
-0.01 (-0.12, 0.09) |
0.12 (0.02, 0.22) |
0.08 (-0.02, 0.19) |
||
| LPA (min) |
0.29 a (0.19, 0.38) |
0.06a (-0.04, 0.16) |
0.10 a (0.00, 0.19) |
-0.04 (-0.14, 0.06) |
-0.04 (-0.14, 0.06) |
-0.00 (-0.10, 0.10) |
0.02 (-0.07, 0.13) |
-0.01 (-0.11, 0.09) |
0.07 (-0.03, 0.18) |
-0.05 (-0.15, 0.06) |
0.02 (-0.09, 0.12) |
|||
| MVPA (min) |
-0.67 a (-0.73, -0.59) |
-0.55 a (-0.63, -0.46) |
0.04 (-0.06, 0.14) |
0.05 (-0.05, 0.15) |
0.06 (-0.04, 0.16) |
-0.05 (-0.15, 0.05) |
-0.07 (-0.17, 0.03) |
0.18 (0.07, 0.27) |
0.02 (-0.08, 0.12) |
0.05 (-0.05, 0.15) |
||||
| SB (min) |
0.81 a (0.77, 0.85) |
-0.02 (-0.12, 0.08) |
-0.02 (-0.12, 0.08) |
-0.00 (-0.10, 0.10) |
0.01 (-0.09, 0.11) |
0.07 (-0.03, 0.17) |
-0.14 (-0.24, -0.04) |
0.01 (-0.09, 0.12) |
0.04 (-0.06, 0.14) |
|||||
| Sleep (min) |
0.04 (-0.06, 0.14) |
0.03 (-0.08, 0.13) |
-0.04 (-0.14, 0.06) |
0.00 (-0.10, 0.10) |
-0.04 (-0.14, 0.06) |
0.01 (-0.09, 0.12) |
0.01 (-0.09, 0.11) |
-0.12 (-0.23, -0.02) |
||||||
| Total GM vol |
0.70 (0.65, 0.75) |
0.19 (0.10, 0.29) |
-0.24 (-0.33, -0.14) |
0.67 (0.60, 0.72) |
0.00 (-0.10, 0.11) |
0.22 (0.12, 0.32) |
0.07 (-0.04, 0.17) |
|||||||
| Temporal vol |
0.18 (0.08, 0.27) |
-0.32 (-0.41, -0.23) |
0.46 (0.38, 0.54) |
0.01 (-0.10, 0.11) |
0.14 (0.03, 0.24) |
0.04 (-0.06, 0.15) |
||||||||
| Hippocamp. vol |
-0.21 (-0.30, -0.11) |
0.05 (-0.05, 0.15) |
0.06 (-0.06, 0.16) |
0.12 (0.02, 0.22) |
0.13 (0.03, 0.23) |
|||||||||
| Ventricle vol |
-0.15 (-0.25, -0.05) |
-0.06 (-0.17, 0.04) |
-0.15 (-0.25, -0.05) |
-0.09 (-0.19, 0.02) |
||||||||||
| Frontal lobe vol |
-0.06 (-0.16, 0.05) |
0.15 (0.04, 0.25) |
0.10 (-0.00, 0.20) |
|||||||||||
| Processing speed |
0.01 (-0.09, 0.11) |
0.07 (-0.04, 0.17) |
||||||||||||
| Executive function |
0.24 (0.13, 0.33) |
Note: Coefficients, unless stated otherwise, are Pearson correlation coefficients (with 95% confidence intervals using Fisher’s z-transformation) [53].a Denotes coefficients within the 24-hour time-use composition using pairwise symmetric balance coordinates (with non-parametric bootstrap 95% confidence intervals on 1000 bootstrap samples). LPA = light physical activity; MVPA = moderate-vigorous physical activity; SB = sedentary behaviour; vol = volume (ml); GM = grey matter; WMH = white matter hyperintensity. Correlations between sex (male = 0, female = 1) and other variables were explored using point biserial correlations, with positive correlations reflecting a positive relationship between female sex and the other variable. Bold denotes that p-value is statistically significant (p < 0.05)
Associations between 24-hour time-use composition and brain volume
Table 3 displays outcomes of regression models testing the associations between 24-hour time-use composition and ROI volumes after adjusting for covariates (age, sex, education) and false discovery rate. In covariate-adjusted models, 24-hour time-use composition was not associated with total grey matter volume, frontal lobe volume, temporal lobe volume, hippocampal volume, or lateral ventricle volume both before and after adjusting for false discovery rate.
Table 3.
Statistical output of ANOVA Type II F-tests for volumetric outcomes
| Model | Variable | GM vol | Temporal lobe vol | Hippocampal vol | Frontal lobe vol | Ventricle vol | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | adj.p | F | p | adj.p | F | p | adj.p | F | p | adj.p | F | p | adj.p | ||
| 1 | Age | 32.44 | < 0.001 | < 0.001 | 15.41 | < 0.001 | < 0.001 | 10.46 | 0.001 | 0.004 | 2.79 | 0.095 | 0.143 | 16.84 | < 0.001 | < 0.001 |
| Sex | 0.03 | 0.861 | 0.861 | 0.41 | 0.521 | 0.534 | 3.38 | 0.067 | 0.100 | 5.01 | 0.026 | 0.078 | 0.07 | 0.791 | 0.791 | |
| Education | 1.13 | 0.288 | 0.431 | 0.39 | 0.534 | 0.534 | 0.12 | 0.730 | 0.730 | 0.33 | 0.565 | 0.565 | 0.31 | 0.575 | 0.791 | |
| 2 | Age | 30.17 | < 0.001 | < 0.001 | 13.83 | < 0.001 | < 0.001 | 8.73 | 0.003 | 0.013 | 2.75 | 0.098 | 0.196 | 14.15 | < 0.001 | < 0.001 |
| Sex | 0.01 | 0.909 | 0.909 | 0.25 | 0.621 | 0.792 | 2.18 | 0.140 | 0.281 | 4.69 | 0.031 | 0.124 | 0.00 | 0.945 | 0.945 | |
| Education | 1.31 | 0.253 | 0.507 | 0.45 | 0.506 | 0.792 | 0.07 | 0.795 | 0.785 | 0.19 | 0.665 | 0.665 | 0.28 | 0.599 | 0.859 | |
| Time-use composition | 0.46 | 0.713 | 0.909 | 0.35 | 0.792 | 0.792 | 0.35 | 0.785 | 0.785 | 0.67 | 0.571 | 0.665 | 0.55 | 0.645 | 0.859 | |
Note: F = F statistic (numerator and denominator degrees of freedom are 1 and 360, respectively, for all outcomes); adj.p = p-value adjusted for false discovery rate; vol = volume (ml); GM = grey matter. Bold denotes p-values that remained statistically significant after false discovery rate adjustment (p < 0.05)
Cognitive function, brain volume and time-use composition
Regression outputs investigating interactions between 24-hour time-use composition and ROI volume for cognitive outcomes are displayed in Table 4. The interaction between time-use composition and total grey matter volume was associated with long-term memory and executive function outcomes prior to adjusting for false discovery rate. Similarly, the interaction between time-use composition and frontal lobe volume was associated with long-term memory and executive function outcomes. After false discovery rate adjustment, several interactions remained statistically significant: executive function was associated with the interaction between time-use composition and total grey matter volume (padj=0.028) and the interaction between time-use composition and frontal lobe volume (padj=0.018); and long-term memory remained significantly associated with the interaction between time-use composition and frontal lobe volume (padj=0.018).
Table 4.
Linear regression outputs
| Cognitive outcome | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Long-term memory | Executive function | Processing speed | ||||||||
| ROI outcome: total grey matter volume | ||||||||||
| F | p | adj.p | F | p | adj.p | F | p | adj.p | ||
| Model | Age | 0.10 | 0.747 | 0.747 | 17.95 | 0.000 | 0.000 | 5.29 | 0.022 | 0.066 |
| Sex | 2.96 | 0.086 | 0.172 | 15.33 | 0.000 | 0.000 | 0.23 | 0.631 | 0.814 | |
| Education | 1.35 | 0.248 | 0.372 | 4.92 | 0.027 | 0.033 | 0.00 | 0.944 | 0.944 | |
| Time-use composition | 2.88 | 0.036 | 0.107 | 0.17 | 0.917 | 0.917 | 4.09 | 0.007 | 0.043 | |
| ROI volume | 0.79 | 0.374 | 0.449 | 8.66 | 0.003 | 0.007 | 0.92 | 0.337 | 0.675 | |
| Time-use composition * ROI volume | 3.79 | 0.011 | 0.064 | 3.37 | 0.018 | 0.028 | 0.51 | 0.678 | 0.813 | |
| ROI outcome: temporal lobe volume | ||||||||||
| F | p | adj.p | F | p | adj.p | F | p | adj.p | ||
| Model | Age | 0.21 | 0.647 | 0.693 | 25.19 | 0.000 | 0.000 | 5.40 | 0.021 | 0.062 |
| Sex | 2.99 | 0.085 | 0.255 | 13.90 | 0.000 | 0.000 | 0.24 | 0.623 | 0.935 | |
| Education | 1.43 | 0.233 | 0.467 | 4.73 | 0.030 | 0.061 | 0.00 | 0.944 | 0.944 | |
| Time-use composition | 2.77 | 0.041 | 0.248 | 0.20 | 0.898 | 0.898 | 4.03 | 0.007 | 0.046 | |
| ROI volume | 0.39 | 0.535 | 0.693 | 2.47 | 0.117 | 0.176 | 0.27 | 0.600 | 0.935 | |
| Time-use composition * ROI volume | 0.48 | 0.693 | 0.693 | 0.84 | 0.472 | 0.566 | 0.24 | 0.869 | 0.944 | |
| ROI outcome: frontal lobe volume | ||||||||||
| F | p | adj.p | F | p | adj.p | F | p | adj.p | ||
| Model | Age | 0.01 | 0.933 | 0.933 | 22.69 | 0.000 | 0.000 | 6.09 | 0.014 | 0.042 |
| Sex | 3.33 | 0.069 | 0.138 | 17.71 | 0.000 | 0.000 | 0.34 | 0.557 | 0.836 | |
| Education | 1.85 | 0.174 | 0.209 | 4.07 | 0.044 | 0.053 | 0.01 | 0.922 | 0.922 | |
| Time-use composition | 2.70 | 0.045 | 0.136 | 0.29 | 0.832 | 0.832 | 3.90 | 0.009 | 0.042 | |
| ROI volume | 1.99 | 0.159 | 0.209 | 7.64 | 0.006 | 0.012 | 1.72 | 0.190 | 0.380 | |
| Time-use composition * ROI volume | 4.74 | 0.003 | 0.018 | 3.70 | 0.012 | 0.018 | 0.23 | 0.878 | 0.921 | |
| ROI outcome: hippocampus volume | ||||||||||
| F | p | adj.p | F | p | adj.p | F | p | adj.p | ||
| Model | Age | 0.03 | 0.871 | 0.871 | 23.91 | 0.000 | 0.000 | 4.89 | 0.027 | 0.083 |
| Sex | 4.21 | 0.041 | 0.094 | 11.35 | 0.000 | 0.003 | 0.14 | 0.704 | 0.864 | |
| Education | 1.44 | 0.231 | 0.277 | 4.61 | 0.032 | 0.065 | 0.03 | 0.864 | 0.864 | |
| Time-use composition | 2.67 | 0.047 | 0.094 | 0.21 | 0.886 | 0.886 | 3.96 | 0.008 | 0.051 | |
| ROI volume | 4.90 | 0.028 | 0.094 | 1.17 | 0.279 | 0.336 | 0.12 | 0.729 | 0.864 | |
| Time-use composition * ROI volume | 2.32 | 0.076 | 0.113 | 1.97 | 0.117 | 0.177 | 0.37 | 0.776 | 0.864 | |
Note: Linear regression outputs investigating main effects of time-use composition, ROI volume and covariates, and interaction effects on cognitive outcomes. adj.p = p-value adjusted for false discovery rate. Bold denotes p-values that remained significant after false discovery rate adjustment (p < 0.05). Sample sizes for each cognitive outcome varied due to missing data: long-term memory, n = 360; executive function, n = 363; processing speed, n = 368
To further investigate these interactions, we plotted a series of model-estimated cognitive response curves which demonstrate the estimated associations of time reallocations with long-term memory and executive function outcomes, across high and low brain volume groups in the frontal lobe and total grey matter, respectively. Additionally, post-hoc multiple linear regression analyses between time-use composition and each cognitive outcome within high and low volume brain volume groups can be viewed in Additional File 2. High and low volume groups were quantified as those above and below the mean frontal lobe volume (168 ml) and total grey matter volume (596 ml) in the sample, as the data were normally distributed (therefore a median split achieved similar data separation). Before creating the response plots, regression models containing the significant interactions were replicated with frontal lobe volume and total grey matter volume included as categorical variables (two levels, upper and lower volume group, rather than as a continuous variable) to ensure that the interaction between time-use composition and each ROI volume remained significant. Interestingly, the interaction between time-use composition and frontal lobe volume (as a categorical variable) did not remain significant for executive function (padj=0.80). This remained true when frontal lobe volume was split into quartiles (padj=0.31). For this reason, the time-use composition by frontal lobe volume interaction for the executive function outcome was not further explored here. Figures 2 and 3 display the predicted differences in long-term memory z-score and executive function z-score associated with reallocations of time from the reference mean time-use composition towards and away from each time-use behaviour (positive and negative reallocations on the x-axis), whilst drawing time spent in the remaining behaviours in the 24-hour day pro-rata (i.e., one-to-remaining reallocations). One-for-one reallocation models are displayed in Additional File 1. To illustrate how brain structure interacts with these relationships, predictions were plotted separately for those above and below the mean frontal lobe and total grey matter volume, respectively.
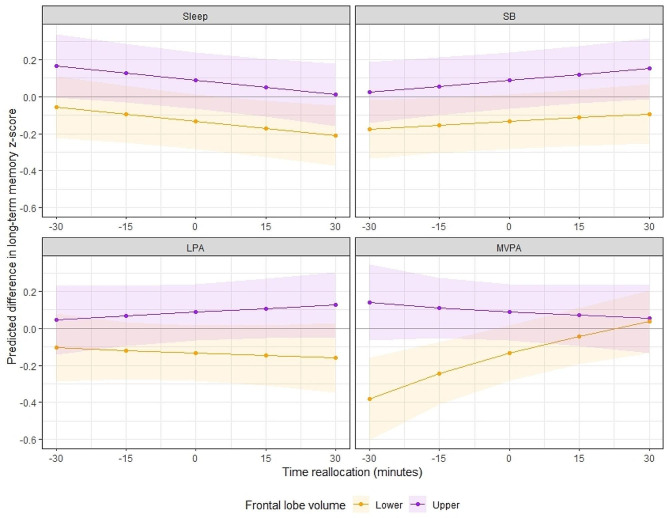
Fig. 2.
The model-predicted difference in long-term memory z-score (y-axis) associated with reallocations of time towards or away from each time-use behaviour (displayed in the header of each panel), in 15-minute increments from the reference mean time-use composition. Purple lines represent participants with greater than the sample mean frontal lobe volume (‘Upper’); orange lines represent participants with less than the sample mean frontal lobe volume (‘Lower’). Shading represents 95% confidence intervals. Mean frontal lobe volume (corrected) in the ‘upper’ group = 174.8 ± 5.0, range = 168.3, 193.0. Mean frontal lobe volume (corrected) in the ‘lower’ group = 161.2 ± 5.0, range = 143.3, 168.3.
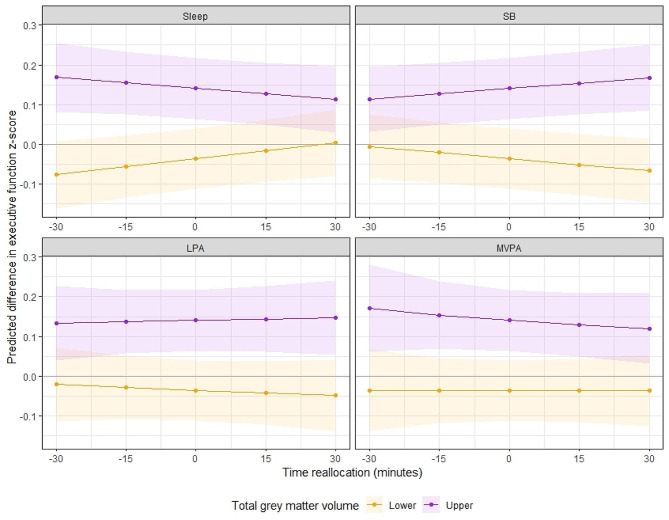
Fig. 3.
The model-predicted difference in executive function z-score (y-axis) associated with reallocations of time towards or away from each time-use behaviour (displayed in the header of each panel) in 15-minute increments from the reference mean time-use composition. Purple lines represent participants with greater than the sample mean total grey matter volume (‘Upper’); orange lines represent participants with less than the sample mean total grey matter volume (‘Lower’). Shading represents 95% confidence intervals. Mean total grey matter volume (corrected) in the ‘upper’ group = 611.4 ± 10.6, range = 596.7, 647.9. Mean total grey matter volume (corrected) in the ‘lower’ group = 580.7 ± 12.9, range = 519.8, 596.7
Figure 2 (displaying one-for-remaining reallocations) suggests that for those with smaller frontal lobe volume (below the sample mean), more time in MVPA was associated with better long-term memory performance. One-for-one reallocation plots (Supplementary Fig. 1, Additional File 1) supported that this reallocation was most beneficial when time was taken from either sleep or sedentary behaviour, whilst taking time from LPA had positive but non-significant associations with long-term memory. Contrary to this, reallocating time towards MVPA had little predicted benefit for those with greater frontal lobe volume (above the sample mean). Spending more time in LPA at the equal expense of other behaviours (Fig. 2) was associated with small unfavorable differences in long-term memory performance for those with smaller frontal lobe volume, and slight but favorable differences in performance for those with greater frontal lobe volume. Finally, reallocating time towards or away from sleep and sedentary behaviour (at the equal expense of remaining behaviours) had similar (minimal) associations with long-term memory performance for both high and low frontal lobe volume groups. However, one-for-one reallocations suggested that for those with greater frontal lobe volume, increasing time in sedentary behaviour at the expense of sleep was positively associated with long-term memory.
Figure 3 shows that the executive function response curves for sleep and sedentary behaviour reallocations differ by total grey matter volume. Although supplementary regression analyses demonstrated that there were no statistically significant associations between time-use composition and executive function within total grey matter volume groups (Additional File 2), the data are described further here for completeness. Figure 3 suggests that for those with smaller total grey matter volume, more time in sleep and less time in sedentary behaviour was favorably associated with executive function, while these reallocations were negatively associated with executive function in those with greater total grey matter volume. One-for-one reallocations (Supplementary Fig. 2, Additional File 1) confirmed that increasing time in sleep at the direct expense of sedentary behaviour was beneficial for executive function in those with smaller total grey matter volume, whilst no other reallocations towards sleep were associated with executive function (i.e., from LPA or MVPA). Reallocating time towards or away from LPA had minimal associations with executive function across both high and low total grey matter volume groups. This was consistently observed across both proportional and one-for-one reallocations (i.e., regardless of which other compositional part time was reallocated from or towards). Finally, spending more time in MVPA at the equal expense of all other behaviours had small unfavorable associations with executive function in the higher total grey matter volume group, whilst reallocations towards or away from MVPA had minimal associations in the lower total grey matter group. One-for-one swaps supported these observations, suggesting that increasing or decreasing time in MVPA at the expense of sleep, LPA or sedentary behaviour had minimal associations with executive function.
Discussion
Twenty-four-hour time-use composition and grey matter volume in healthy older adults
The primary aim of this study was to explore whether 24-hour time-use composition of MVPA, LPA, sedentary behaviour and sleep was associated with total and regional grey matter volume (temporal lobe, frontal lobe, hippocampus, and lateral ventricle volume) in healthy older adults. Our main finding was that there were no associations between 24-hour time-use composition and any volumetric outcomes, after adjustment for age, sex, and education. This finding is likely not a result of accounting for all four time-use behaviours together (i.e., the effects of one time-use behaviour “canceling out” or altering the effect of another) as there were also no significant pairwise correlations between independent time-use behaviours with any volumetric outcomes. It is challenging to contextualize the findings of this study with previous research for several reasons. To our knowledge, this study was the first to investigate associations between 24-hour time-use composition and grey matter volume in older adults. Additionally, although several previous studies in older adults have reported associations between grey matter volume and physical activity [4, 6, 7, 11], sedentary behaviour [16] or sleep [20, 21] (when considered as independent behaviours), it is difficult to draw comparisons with these studies as they also adjusted their analyses for covariates (e.g., age, sex), whilst univariate analyses in the current study (correlations between independent time-use behaviours and brain volume outcomes) did not adjust for covariates.
In addition to the apparent differences in data analysis approaches, there are several factors that may have contributed to the null findings in the primary analysis of this study, and the contrast of these findings to several previous studies. First, the sample used in the current study was recruited for a longitudinal study which aims to map differences in lifestyle (time use and diet) against changes in cognitive function, therefore at baseline participants were required to be healthy with no cognitive impairment. Despite best efforts to recruit participants across diverse lifestyle profiles, the sample had high cognitive function (mean score on Addenbrooke’s Cognitive Examination III at baseline was approximately 95/100 in the ACTIVate sample [28]), participants were physically active (mean total PA per day = 4.5 h), and there was little variability in time-use composition at baseline across the sample (see Fig. 1). Conversely, several previous studies which did observe associations between physical activity or sleep and grey matter volume were conducted in populations with greater variability in time use profiles [4, 9]. For example, Bugg and Head [9] reported that physical exercise engagement (measured as self-reported engagement in running, walking or jogging over the past 10 years) was associated with volume in the superior frontal cortex. Due to extreme skewness in their data, participants were categorized in to low or high engagement groups, whereby the mean levels of physical exercise engagement were 0.63 and 7.76, respectively (sample range = 0.00-29.72). The authors reported that those in the low engagement group engaged in moderate intensity activity less than 2 days per week for 10 min, whereas the average amount of moderate activity for the high engagement group was approximately 5 days per week for 30 min each [9]. Similarly in a longitudinal study, Tan and colleagues [4] reported that Physical Activity Index (PAI) scores at baseline were significantly associated with total cerebral and hippocampal volumes at follow-up (~ 10 years). PAI scores were generated by asking participants to report the number of hours per day spent sleeping (weighting factor = 1), sedentary (weighting factor = 1.1), or in light (weighting factor = 1.5), moderate (weighting factor = 2.4) and heavy activities (weighting factor = 5). Thus, a score of 24 reflects a day spent sleeping continually [4]. The range of PAI scores in their sample ranged from 24.2 to 65.7 in females, and 24.6–74.5 in males, with median PAI scores of 34.4 and 35.4, respectively. On balance, a much wider range of time-use profiles were included in their study and it could be that the observed relationships between volumetric outcomes and activity levels were driven by extreme cases (e.g., highest quintiles relative to lowest) [4]. Similarly, other studies that have reported associations between physical activity or sleep and grey matter volume observed differences between extreme sub-groups only (i.e., lowest VS. highest quintile of physical activity) [6, 20].
Although there are few studies investigating associations between sedentary behaviour and grey matter volume in older adults, the null findings of the our study align with that of a recent review by Maasakkers et al. [19] who reported inconclusive evidence for the relationship between sedentary behaviour and grey matter volume. As hypothesized by Maasakkers et al. [19], it may be that white matter volume is more sensitive to the physiological effects of sedentary behaviour compared to grey matter. It should also be noted that several other studies have reported no significant associations between sedentary behaviour and total grey matter volume (in line with the current study), but did find significant differences between low and high sedentary behaviour groups in smaller sub-regions, such as hippocampal sub-fields [15, 16]. Given that we found no association between sedentary behaviour and total hippocampal volume in this study, it may be possible that the ROIs investigated in this study were not sensitive enough to detect differences which may be present in sub-regions.
Another key difference between the current study and previous studies which may contribute to discrepancies in findings is the age of the sample. Participants in the ACTIVate study were aged 60–70 years at baseline (mean age = 66 ± 3 years), a narrow age range chosen in order to capture healthy older adults who may be in pre-clinical stages of dementia (i.e., where brain changes are beginning to occur with no behavioural or cognitive symptoms). Several studies that have reported associations between independent time-use behaviours and grey matter volume were conducted in older samples (e.g., 70–80 years) which may have more progressed brain atrophy in comparison [4, 7], or in samples with a wider age range (e.g., including middle-age and older adults in sample). It is plausible that the observed differences in these studies may have been driven by older participants.
Finally, as is typical for time-use research, there are considerable inconsistencies in the measures used to estimate physical activity, sedentary behaviour, and sleep across studies (i.e., subjective recall versus accelerometry), which limits comparability with the current study. To our knowledge, the current study was the first to assess 24-hour time-use composition against grey matter volume in older adults using accelerometry, whereby participants’ activity patterns were monitored by a wrist-worn device for 24-hours per day. Comparatively, previous studies which have reported relationships between independent time-use behaviours and grey matter volume outcomes have mostly used self-report measures of duration [20] or validated questionnaires such as the Pittsburgh Sleep Quality Index [21], PAI [4], or activity compendiums [11]. Moreover, studies which have used accelerometry to derive activity patterns have not taken a 24-hour approach, and have quantified physical activity outcomes using different metrics including total physical activity (irrespective of intensity) [7] or classification into low and high levels of MVPA [6]. Taken together, the variability in measures used to capture activity patterns, as well as the absence of studies that have taken a 24-hour compositional approach, limits the comparability of the current study to previous studies.
Grey matter volume as a mechanism linking 24-hour time use and cognitive function
Several mechanisms likely underlie the relationship between lifestyle and cognitive function in older adults, including maintenance of grey matter volume. This relationship has only been investigated when considering physical activity, sedentary behaviour and sleep independently, rather than as a 24-hour composition. In our secondary analysis, we found that long-term memory was associated with the interaction between 24-hour time-use composition and frontal lobe volume, and that executive function was associated with the interaction between 24-hour time-use composition and both total grey matter volume and frontal lobe volume (although, the frontal lobe by executive function interaction was not further explored). It should be noted that after separating the dataset by mean frontal lobe and total grey matter volumes (Additional File 2), only the association between time-use composition and long-term memory in the smaller frontal lobe sub-group remained statistically significant.
Predictive modelling allowed further understanding of significant interactions in the total sample, while separating participants by ROI volume (above or below the sample mean). For long-term memory outcomes, reallocating time towards or away from LPA, sedentary behaviour or sleep (at the equal expense of all other behaviours) had minimal predicted associations with performance regardless of frontal lobe volume. However, reallocating time towards or away from MVPA was associated with long-term memory performance, and this relationship was more pronounced for those with smaller frontal lobe volume. The same reallocation, e.g., spending 30 min less time in MVPA (at the equal expense of other behaviours), was associated with a ~ 0.25 SD lower long-term memory z-score for those with smaller frontal lobe volume, but only a slight difference (~ 0.05 SD higher) in long-term memory z-score for those with larger frontal lobe volumes. One-for-one reallocation modelling demonstrated that increasing time in MVPA at the expense of either sleep or sedentary behaviour had the greatest predicted benefit for long-term memory, with similar but non-significant trends observed for LPA. Together, these findings suggest that benefits from spending more time in MVPA (or more so, the deficits from reducing time spent in MVPA) on memory performance may differ by frontal lobe volume.
There are several key points that could be considered to contextualize these findings. First, evidence suggests that performing physical activity at higher intensities is positively associated with concentrations of neurochemicals which may enhance memory and learning [54, 55]. Second, evidence from both animal and human studies suggests that effects of physical activity on the brain appear to be specific, in that particular brain regions are more sensitive to the benefits of physical activity than others [56]. One such brain region is the frontal cortex, which along with the hippocampus, also typically shows marked age-related atrophy compared to other regions [56]. On balance, it has been suggested that brain regions with more age-related atrophy may yield the most benefits from physical activity [56]. This may explain why the predicted impact of increasing or decreasing time in MVPA on long-term memory performance was more prominent in participants with smaller frontal lobe volumes.
Interestingly, our secondary analysis did not infer the same importance for MVPA in those with lower total grey matter volume in the context of executive function outcomes. Reallocating time towards or away from LPA or MVPA had minimal predicted associations with performance regardless of total grey matter volume (increasing MVPA had slight unfavorable associations in those with greater volume). However, reallocating time towards sleep or away from sedentary behaviour at the equal expense of other behaviours appeared favorably associated with executive function (+ 0.03SD for each 30-minute reallocation) in older adults with smaller total grey matter volume, whilst the opposite was true for those with greater total grey matter volumes (less sleep and more sedentary behaviour = better executive function). One-for-one reallocation modelling confirmed that increasing time in sleep at the expense of sedentary behaviour was associated with better executive function performance in those with smaller total grey matter volume, whilst no one-for-one reallocations were statistically significant for those with greater total grey matter volume. Whilst these findings somewhat align with a recent study by Tai et al. [57] who suggested brain volume mediates the relationship between sleep duration and executive function, they are difficult to contextualize due to the cross-sectional nature of the study and the novelty of our analytical approach. We propose several potential contributors to these findings. For example, it is possible that those with smaller grey matter volume were achieving good quality sleep at baseline, so increasing time in this behaviour would be beneficial for executive function (whilst the opposite may have been true for those with greater volume). Alternatively, it may be that those with smaller total grey matter volume were engaging in sedentary behaviours which are not beneficial for cognitive function (i.e., TV watching), whilst those with greater total grey matter volume were engaging in cognitively stimulating sedentary behaviours (i.e., computer use, reading), so reallocating time away from or towards sedentary behaviour, respectively, would be beneficial for executive function [17]. It should be noted that a previous study found no impact of sedentary behaviour context or sleep quality ratings on associations between 24-hour time-use composition and executive function in the ACTIVate cohort, but these analyses were conducted in the total sample only (and were not stratified by grey matter volume) [28]. Above all, it is important to note that the predicted differences in executive function resulting from each reallocation in this study were much smaller compared to those observed for long-term memory, so these findings should be interpreted with caution. This is likely because the range in executive function z-scores in this cohort were much narrower (range = -1.77, 1.14) than long-term memory z-scores (range = -3.59, 1.58), so smaller differences in executive function z-score (resulting from each reallocation) were likely needed to achieve statistical significance.
Importantly, results in these secondary analyses were derived from cross-sectional data and the predicted associations were modest in scale, and therefore should be interpreted with some caution. Despite correcting each ROI volume for total intracranial volume in this study, we cannot deduce that smaller ROI volumes reflect accelerated atrophy due to the cross-sectional nature of the study. If each of these findings are upheld in longitudinal studies, they may indicate that lifestyle interventions which aim to maintain or improve cognitive functions such as memory and executive function (as a means to reduce dementia risk or delay dementia onset) could be tailored based on individual differences in brain volume (i.e., prescribing higher intensity physical activity for those with more progressed brain atrophy in the frontal lobe, or targeting sleep duration in those with more progressed total grey matter atrophy).
Strengths, limitations, and future directions for research
The current study was the first to investigate associations between 24-hour time use and grey matter volume using a compositional data analysis approach. We used reliable cognitive tests and device-based measures of time use which may be less susceptible to recall bias in older adults, and took a conservative approach to our data analysis by accounting for technical differences in scanning across the cohort (scanner type and use of distortion correction function) and by adjusting for false discovery rate which is not typically done in exploratory research [52]. However, there are several limitations that should be noted. As outlined in a previous study using the same dataset [28], the recruited sample were highly active and highly educated despite best efforts to recruit participants across a variety of activity and dietary patterns. The cross-sectional nature of the study limits the inferences that can be made about causal relationships between variables. Due to the exploratory nature of the study, a number of additional modifiable dementia risk factors and other important indicators of health status which may relate to inter-individual variability in time use, brain volume and cognitive function were not included as covariates in this study (e.g., adiposity, depression, smoking, alcohol consumption) or were not measured as part of the wider ACTIVate study (e.g., aerobic fitness). Thus, observed associations may have been attenuated by accounting for other health outcomes and these should be considered in future research. There are a number of limitations associated with the use of wrist-worn accelerometers to delineate time-use behaviours which should be acknowledged. Wrist-worn accelerometers are less sensitive in detecting sitting time compared to other wear locations (e.g., thigh) as they are unable to differentiate lower body postures (e.g., sitting versus standing), and so it is possible that periods of standing were incorrectly classified as sedentary time rather than LPA, and on the contrary, it is possible that high intensity activities such as stationary cycling may have been captured as sedentary behaviour due to the limited movement of the upper limb during the activity. The cut points used to differentiate time-use behaviours in this study were derived from a validation study conducted in a sample of younger adults, and thus the distribution of time spent in different intensity bands in this sample may differ in older adults. Taken together, it is possible that the use of wrist-worn accelerometers and the chosen cut points resulted in an overestimation of sedentary time, and therefore the findings surrounding sedentary behaviour should be interpreted with caution. Finally, the handling of non-wear time and subsequent imputation methods (i.e., proportional re-scaling of time-use data across all components using closure function) may have resulted in the over-estimation of time spent in sleep. Non-wear time is not typically accrued during sleep, and thus increasing time in sleep as a result of the proportional rescaling across all time-use components may have resulted in the overestimation of time spent in this behaviour (see Haszard et al. [58] for applied example).
Conclusions
The current study found no associations between 24-hour time-use composition and measures of global and regional grey matter volume in a sample of healthy older adults without dementia. We found some evidence that grey matter volume (globally, and in the frontal lobe) may moderate the relationship between 24-hour time-use composition and specific cognitive functions. These relationships should be explored longitudinally and in a more diverse sample to better understand the directionality and temporal effects of 24-hour time use on grey matter volume and cognitive function in healthy older adults.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Strengthening the reporting of observational studies in epidemiology (STROBE) checklist
Supplementary Material 2: Additional file 1 (Supplementary Figures 1 and 2; one-for-one reallocation modelling)
Supplementary Material 3: Additional file 2 (Supplementary analyses within volumetric sub-groups)
Acknowledgements
We firstly thank the imaging teams at the Hunter Medical Research Institute and the Clinical Research Imaging Centre (SAHMRI site) for your invaluable assistance in collecting MRI data for our study. We thank Montana Hunter, Louise Massie, Kate Dyer, Johanna Paddick, Felicity Simpson, Ashlee Harvey, Nicholas Ware, Leon Day, Elena Milochis, Alannah Graziano, Elizabeth Taddeo, Karen Wilson, Jenna Johnson, Nathan Tran, Gemma Mieko Furuhashi, Helen Nicholas, Riley Jackson, Teigan Cotterill, Mahmoud Abdolhoseini, Fayeem Aziz, and David Metherell for their valued contribution towards data collection and study coordination. We also thank the HMRI Research Volunteer Register for partial recruitment of participants at the Newcastle site.
Abbreviations
- MRI
magnetic resonance imaging
- METs
metabolic equivalents
- >MVPA
moderate-vigorous physical activity
- LPA
light physical activity
- SB
sedentary behaviour
- MPRAGE
magnetization-prepared rapid gradient-echo
- FLAIR
fluid-attenuated inversion recovery
- CANTAB
Cambridge Neuropsychological Test Automated Battery
- CoDA
compositional data analysis
- ROI
region of interest
- PAI
Physical Activity Index
- SD
standard deviation
Author contributions
MM contributed to the study design, data analysis, interpretation of results, writing, and drafting of the manuscript. DD, TS, JF and YX contributed to the data analysis, interpretation of results, and drafting of the manuscript. AW and FK contributed to the study design, interpretation of results, and drafting of the manuscript. TO, MG, JD and MB contributed to interpretation of results and drafting of the manuscript. AS contributed to study design, data collection, interpretation of results and drafting of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
MM was supported by a Dementia Australia Research Foundation PhD scholarship. The ACTIVate study is funded by an NHMRC Boosting Dementia Research Priority Round 5 grant (GNT1171313, $1.23 m). DD was supported by an NHMRC Early Career Fellowship (GNT1162166, 2019–2022) and an ARC Discovery Early Career Award (DE230101174, 2023–2025). MG was supported by an Australian Research Council (ARC) fellowship (DE200100575). TS was supported by a Hospital Research Foundation grant (C-PJ-008-Transl-2020) awarded to AS and DD. AS was supported by a Dementia Australia Henry Brodaty Mid-Career Fellowship. The funding bodies were not involved the design or conduct of the study; collection, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for peer-reviewed publication.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to the longitudinal nature of the larger ACTIVate study (ongoing at the time of publication) but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Ethics approval was obtained from the University of South Australia and University of Newcastle Human Research Ethics Committee (202639).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bunce D, Anstey KJ, Christensen H, Dear K, Wen W, Sachdev P. White matter hyperintensities and within-person variability in community-dwelling adults aged 60–64 years. Neuropsychologia. 2007;45(9):2009–15. doi: 10.1016/j.neuropsychologia.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Fox NC, Schott JM. Imaging cerebral atrophy: normal ageing to Alzheimer’s Disease. The Lancet. 2004;363(9406):392–4. doi: 10.1016/S0140-6736(04)15441-X. [DOI] [PubMed] [Google Scholar]
- 3.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet (London England) 2020;396(10248):413–46. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan ZS, Spartano NL, Beiser AS, DeCarli C, Auerbach SH, Vasan RS, et al. Physical activity, brain volume, and Dementia Risk: the Framingham Study. The Journals of Gerontology: Series A. 2016;72(6):789–95. doi: 10.1093/gerona/glw130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arenaza-Urquijo EM, de Flores R, Gonneaud J, Wirth M, Ourry V, Callewaert W, et al. Distinct effects of late adulthood cognitive and physical activities on gray matter volume. Brain Imaging and Behavior. 2017;11(2):346–56. doi: 10.1007/s11682-016-9617-3. [DOI] [PubMed] [Google Scholar]
- 6.Melo Neves L, Ritti-Dias R, Juday V, Marquesini R, Mendes Gerage A, Cândido Laurentino G et al. Objective Physical Activity Accumulation and Brain Volume in Older Adults: An MRI and Whole-Brain Volume Study. The Journals of Gerontology: Series A. 2022:glac150. [DOI] [PubMed]
- 7.Halloway S, Arfanakis K, Wilbur J, Schoeny ME, Pressler SJ. Accelerometer Physical Activity is Associated with Greater Gray Matter volumes in older adults without Dementia or mild cognitive impairment. The Journals of Gerontology: Series B. 2019;74(7):1142–51. doi: 10.1093/geronb/gby010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamer M, Sharma N, Batty GD. Association of objectively measured physical activity with brain structure: UK Biobank study. J Intern Med. 2018;284(4):439–43. doi: 10.1111/joim.12772. [DOI] [PubMed] [Google Scholar]
- 9.Bugg JM, Head D. Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiol Aging. 2011;32(3):506–14. doi: 10.1016/j.neurobiolaging.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dougherty RJ, Ellingson LD, Schultz SA, Boots EA, Meyer JD, Lindheimer JB et al. Meeting physical activity recommendations may be protective against temporal lobe atrophy in older adults at risk for Alzheimer’s disease. 2016;4(1):14–7. [DOI] [PMC free article] [PubMed]
- 11.Flöel A, Ruscheweyh R, Krüger K, Willemer C, Winter B, Völker K et al. Physical activity and memory functions: are neurotrophins and cerebral gray matter volume the missing link? NeuroImage. 2010;49(3):2756–63. [DOI] [PubMed]
- 12.Mellow ML, Crozier AJ, Dumuid D, Wade AT, Goldsworthy MR, Dorrian J, et al. How are combinations of physical activity, sedentary behaviour and sleep related to cognitive function in older adults? A systematic review. Exp Gerontol. 2022;159:111698. doi: 10.1016/j.exger.2022.111698. [DOI] [PubMed] [Google Scholar]
- 13.Voss MW, Carr LJ, Clark R, Weng T. Revenge of the sit II: does lifestyle impact neuronal and cognitive health through distinct mechanisms associated with sedentary behavior and physical activity? Ment Health Phys Act. 2014;7(1):9–24. doi: 10.1016/j.mhpa.2014.01.001. [DOI] [Google Scholar]
- 14.van der Ploeg HP, Hillsdon M. Is sedentary behaviour just Physical Inactivity by another name? Int J Behav Nutr Phys Activity. 2017;14(1):142. doi: 10.1186/s12966-017-0601-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maasakkers CM, Thijssen DHJ, Knight SP, Newman L, O’Connor JD, Scarlett S, et al. Hemodynamic and structural brain measures in high and low sedentary older adults. J Cereb Blood Flow Metabolism. 2021;41(10):2607–16. doi: 10.1177/0271678X211009382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siddarth P, Burggren AC, Eyre HA, Small GW, Merrill DA. Sedentary behavior associated with reduced medial temporal lobe thickness in middle-aged and older adults. PLoS ONE. 2018;13(4):e0195549. doi: 10.1371/journal.pone.0195549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olanrewaju O, Stockwell S, Stubbs B, Smith L. Sedentary behaviours, cognitive function, and possible mechanisms in older adults: a systematic review. Aging Clin Exp Res. 2020;32(6):969–84. doi: 10.1007/s40520-019-01457-3. [DOI] [PubMed] [Google Scholar]
- 18.Mellow ML, Dumuid D, Thacker JS, Dorrian J, Smith AE. Building your best day for healthy brain aging—the neuroprotective effects of optimal time use. Maturitas. 2019;125:33–40. doi: 10.1016/j.maturitas.2019.04.204. [DOI] [PubMed] [Google Scholar]
- 19.Maasakkers CM, Weijs RWJ, Dekkers C, Gardiner PA, Ottens R, Olde Rikkert MGM, et al. Sedentary behaviour and brain health in middle-aged and older adults: a systematic review. Neurosci Biobehavioral Reviews. 2022;140:104802. doi: 10.1016/j.neubiorev.2022.104802. [DOI] [PubMed] [Google Scholar]
- 20.Spira AP, Gonzalez CE, Venkatraman VK, Wu MN, Pacheco J, Simonsick EM, et al. Sleep duration and subsequent cortical thinning in cognitively normal older adults. Sleep. 2016;39(5):1121–8. doi: 10.5665/sleep.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo JC, Loh KK, Zheng H, Sim SKY, Chee MWL. Sleep duration and age-related changes in Brain structure and cognitive performance. Sleep. 2014;37(7):821. doi: 10.5665/sleep.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura N, Aso Y, Yabuuchi K, Ishibashi M, Hori D, Sasaki Y et al. Modifiable Lifestyle Factors and Cognitive Function in Older People: A Cross-Sectional Observational Study. 2019;10. [DOI] [PMC free article] [PubMed]
- 23.Wei J, Hou R, Xie L, Chandrasekar EK, Lu H, Wang T, et al. Sleep, sedentary activity, physical activity, and cognitive function among older adults: the National Health and Nutrition Examination Survey, 2011–2014. J Sci Med Sport. 2021;24(2):189–94. doi: 10.1016/j.jsams.2020.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Zitser J, Anatürk M, Zsoldos E, Mahmood A, Filippini N, Suri S, et al. Sleep duration over 28 years, cognition, gray matter volume, and white matter microstructure: a prospective cohort study. Sleep. 2020;43(5):zsz290. doi: 10.1093/sleep/zsz290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y-R, Fan D-Q, Gui W-J, Long Z-L, Lei X, Yu J. Sleep-related brain atrophy and disrupted functional connectivity in older adults. Behav Brain Res. 2018;347:292–9. doi: 10.1016/j.bbr.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 26.Dumuid D, Stanford TE, Martin-Fernández J-A, Pedišić Ž, Maher CA, Lewis LK, et al. Compositional data analysis for physical activity, sedentary time and sleep research. Stat Methods Med Res. 2017;27(12):3726–38. doi: 10.1177/0962280217710835. [DOI] [PubMed] [Google Scholar]
- 27.Maasakkers CM, Claassen JAHR, Gardiner PA, Olde Rikkert MGM, Lipnicki DM, Scarmeas N, et al. The Association of sedentary behaviour and cognitive function in people without Dementia: a coordinated analysis across five Cohort studies from COSMIC. Sports Med. 2020;50(2):403–13. doi: 10.1007/s40279-019-01186-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mellow ML, Dumuid D, Wade AT, Stanford T, Olds TS, Karayanidis F, et al. Twenty-four-hour time-use composition and cognitive function in older adults: cross-sectional findings of the ACTIVate study. Front Hum Neurosci. 2022;16:1051793. doi: 10.3389/fnhum.2022.1051793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith AE, Wade AT, Olds T, Dumuid D, Breakspear MJ, Laver K, et al. Characterising activity and diet compositions for Dementia prevention: protocol for the ACTIVate prospective longitudinal cohort study. BMJ Open. 2022;12(1):e047888. doi: 10.1136/bmjopen-2020-047888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hildebrand M, Van Hees VT, Hansen BH, Ekelund U. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med Sci Sports Exerc. 2014;46(9):9. doi: 10.1249/MSS.0000000000000289. [DOI] [PubMed] [Google Scholar]
- 32.da Silva ICM, van Hees VT, Ramires VV, Knuth AG, Bielemann RM, Ekelund U, et al. Physical activity levels in three Brazilian birth cohorts as assessed with raw triaxial wrist accelerometry. Int J Epidemiol. 2014;43(6):1959–68. doi: 10.1093/ije/dyu203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cassidy S, Fuller H, Chau J, Catt M, Bauman A, Trenell MI. Accelerometer-derived physical activity in those with cardio-metabolic Disease compared to healthy adults: a UK Biobank study of 52,556 participants. Acta Diabetol. 2018;55(9):975–9. doi: 10.1007/s00592-018-1161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 35.Leemput KV, Maes F, Vandermeulen D, Suetens P. Automated model-based tissue classification of MR images of the brain. IEEE Trans Med Imaging. 1999;18(10):897–908. doi: 10.1109/42.811270. [DOI] [PubMed] [Google Scholar]
- 36.Wolz R, Aljabar P, Hajnal JV, Hammers A, Rueckert D. LEAP: learning embeddings for atlas propagation. NeuroImage. 2010;49(2):1316–25. doi: 10.1016/j.neuroimage.2009.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manjón JV, Coupé P, Raniga P, Xia Y, Desmond P, Fripp J, et al. MRI white matter lesion segmentation using an ensemble of neural networks and overcomplete patch-based voting. Comput Med Imaging Graph. 2018;69:43–51. doi: 10.1016/j.compmedimag.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Webb SL, Loh V, Lampit A, Bateman JE, Birney DP. Meta-analysis of the effects of Computerized Cognitive Training on Executive functions: a cross-disciplinary taxonomy for classifying Outcome cognitive factors. Neuropsychol Rev. 2018;28(2):232–50. doi: 10.1007/s11065-018-9374-8. [DOI] [PubMed] [Google Scholar]
- 39.Boller B, Mellah S, Ducharme-Laliberté G, Belleville S. Relationships between years of education, regional grey matter volumes, and working memory-related brain activity in healthy older adults. Brain Imaging and Behavior. 2017;11(2):304–17. doi: 10.1007/s11682-016-9621-7. [DOI] [PubMed] [Google Scholar]
- 40.Coffey CE, Lucke JF, Saxton JA, Ratcliff G, Unitas LJ, Billig B, et al. Sex differences in Brain Aging: a quantitative magnetic resonance imaging study. Arch Neurol. 1998;55(2):169–79. doi: 10.1001/archneur.55.2.169. [DOI] [PubMed] [Google Scholar]
- 41.Crivello F, Tzourio-Mazoyer N, Tzourio C, Mazoyer B. Longitudinal Assessment of Global and Regional Rate of Grey Matter Atrophy in 1,172 healthy older adults: modulation by sex and age. PLoS ONE. 2014;9(12):e114478. doi: 10.1371/journal.pone.0114478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoogendam YY, Hofman A, van der Geest JN, van der Lugt A, Ikram MA. Patterns of cognitive function in aging: the Rotterdam Study. Eur J Epidemiol. 2014;29(2):133–40. doi: 10.1007/s10654-014-9885-4. [DOI] [PubMed] [Google Scholar]
- 43.Anstey KJ, Cherbuin N, Herath PM, Qiu C, Kuller LH, Lopez OL, et al. A self-report risk index to predict occurrence of Dementia in three Independent cohorts of older adults: the ANU-ADRI. PLoS ONE. 2014;9(1):e86141. doi: 10.1371/journal.pone.0086141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kynčlová P, Hron K, Filzmoser P. Correlation between compositional parts based on symmetric balances. Math Geosci. 2017;49(6):777–96. doi: 10.1007/s11004-016-9669-3. [DOI] [Google Scholar]
- 45.Templ M. Robust Methods for Compositional Data. R Documentation2021.
- 46.van den Boogaart KG, Tolosana-Delgado R. Compositions: a unified R package to analyze compositional data. Comput Geosci. 2008;34(4):320–38. doi: 10.1016/j.cageo.2006.11.017. [DOI] [Google Scholar]
- 47.Chastin SF, Palarea-Albaladejo J, Dontje ML, Skelton DA. Combined effects of Time spent in physical activity, sedentary behaviors and sleep on obesity and cardio-metabolic health markers: a Novel Compositional Data Analysis Approach. PLoS ONE. 2015;10(10):e0139984. doi: 10.1371/journal.pone.0139984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lüdecke D, Ben-Shachar MS, Patil I, Waggoner P, Makowski D. Performance: an R package for assessment, comparison and testing of statistical models. J Open Source Softw. 2021;6(60).
- 49.Langsrud Ø. ANOVA for unbalanced data: use type II instead of type III sums of squares. Stat Comput. 2003;13(2):163–7. doi: 10.1023/A:1023260610025. [DOI] [Google Scholar]
- 50.Fox J, Weisberg S. An R companion to applied regression. Sage publications; 2011.
- 51.Benjamini Y, Hochberg YJJRB. Controlling the false discovery rate: a practical and powerful approach to multiple testing. 1995;57(1):289–300.
- 52.Jafari M, Ansari-Pour N, Why When and how to adjust your P values? Cell J. 2019;20(4):604–7. doi: 10.22074/cellj.2019.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hotelling H. New Light on the correlation coefficient and its transforms. J Roy Stat Soc: Ser B (Methodol) 1953;15(2):193–225. [Google Scholar]
- 54.Voss MW, Erickson KI, Prakash RS, Chaddock L, Kim JS, Alves H, et al. Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav Immun. 2013;28:90–9. doi: 10.1016/j.bbi.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piepmeier AT, Etnier JL. Brain-derived neurotrophic factor (BDNF) as a potential mechanism of the effects of acute exercise on cognitive performance. J Sport Health Sci. 2015;4(1):14–23. doi: 10.1016/j.jshs.2014.11.001. [DOI] [Google Scholar]
- 56.Erickson KI, Gildengers AG, Butters MA. Physical activity and brain plasticity in late adulthood. Dialog Clin Neurosci. 2013;15(1):99–108. doi: 10.31887/DCNS.2013.15.1/kerickson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tai XY, Chen C, Manohar S, Husain M. Impact of sleep duration on executive function and brain structure. Commun Biology. 2022;5(1):201. doi: 10.1038/s42003-022-03123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haszard JJ, Meredith-Jones K, Farmer V, Williams S, Galland B, Taylor R. Non-wear Time and Presentation of compositional 24-Hour time-use analyses influence conclusions about Sleep and Body Mass Index in Children. J Meas Phys Behav. 2020;3(3):204–10. doi: 10.1123/jmpb.2019-0048. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Strengthening the reporting of observational studies in epidemiology (STROBE) checklist
Supplementary Material 2: Additional file 1 (Supplementary Figures 1 and 2; one-for-one reallocation modelling)
Supplementary Material 3: Additional file 2 (Supplementary analyses within volumetric sub-groups)
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to the longitudinal nature of the larger ACTIVate study (ongoing at the time of publication) but are available from the corresponding author on reasonable request.